Abstract
Recently, vegetable oil-derived monounsaturated fatty acids (MUFAs) have predominantly been utilized in producing bio-based surfactants, resulting in low bioresource utilization and high separation costs. Although polyunsaturated fatty acids (PUFAs) are abundant and often co-exist with MUFAs, bio-based surfactants synthesized from PUFA-rich feedstocks have been less researched due to concerns regarding their interfacial performance. In this study, a novel series of PUFA-based zwitterionic surfactants with strong interfacial activity was synthesized from waste vegetable oils via an eco-friendly three-step process, optimized through an orthogonal experimental design. The structures and conversion rates of the surfactants were confirmed using GC-MS, LC-MS, and ESI-MS. At 0.5 g/L and 3.0 g/L (typical concentrations often used in most oil fields), the bio-based surfactants derived from waste soybean oil (PUFA-to-MUFA ratio ≈ 2.11, C18:2, and C18:1 in large contents) could reduce the interfacial tension between Daqing crude oil and simulated formation groundwater to an ultra-low level of ~10−3 mN/m. These results confirm our hypothesis that bio-based zwitterionic surfactants derived from PUFA-rich feedstocks possess excellent interfacial activity, providing a potential sustainable option to be considered for chemically enhanced oil recovery.
1. Introduction
Enhanced oil recovery (EOR) aims to improve crude oil extraction beyond primary and secondary methods. Among various EOR approaches, chemically EOR—through injection of alkalis, surfactants, or polymers—is widely applied to reduce oil–water interfacial tension and mobilize residual oil [1]. Currently, petroleum-based surfactants, such as petroleum sulfonates and alkyl benzene sulfonates, are commonly used in chemically EOR [2]. However, these surfactants present several challenges in practical applications, as petroleum sulfonate surfactants have poor stability, and it is hard to reach an ultra-low oil–water interfacial tension in alkali-free or polymer systems, which involve significant increases in costs and a tedious preparation process. The use of alkalis also has long-term adverse effects on the reservoir. Importantly, there is an urgent need to find alternative surfactants with an improved performance that do not rely on alkaline agents, are environmentally friendly, and have a stable performance [3,4,5,6,7,8].
In recent years, bio-based surfactants have garnered increasing attention as potential alternatives to petroleum-based surfactants. The raw materials for bio-based surfactants come from renewable biological resources, offering higher sustainability, a stable supply, and a lower environmental impact. Since the 1990s, with the growing focus on renewable resources, bio-based surfactants have gradually become a research hotspot [8,9,10,11,12]. Their primary raw materials include proteins, carbohydrates, natural oils, and their fatty acid derivatives. Vegetable oils, especially waste cooking oils, have emerged as significant raw materials for the potential replacement of petroleum-based resources for surfactant production due to their low cost, wide availability, and sustainability [8,11,13,14]. Vegetable oils contain a substantial amount of both saturated and unsaturated fatty acids, with unsaturated fatty acids—such as oleic acid, linoleic acid, and linolenic acid—being among the main components [15,16,17]. Previous studies have primarily focused on utilizing monounsaturated fatty acids (MUFAs) or single fatty acids derived from vegetable oils. For example, oleic acid has been used as a raw material to synthesize betaine-type amphoteric surfactants via alkylation or epoxidation reactions, demonstrating promising potential in alkali-free enhanced oil recovery systems [18,19,20,21,22]. However, the separation of single fatty acids is challenging, leading to a low oil utilization efficiency and high production costs. To enhance bioresource utilization, researchers have explored the synthesis of sulfonated Gemini surfactants from polyunsaturated fatty acids (PUFAs), such as linoleic acid [23], and the development of novel surfactants from non-edible vegetable oils [24,25] and waste oils [26]. Despite these advancements, challenges remain in improving interfacial performance.
PUFA-rich feedstocks have traditionally been regarded as unsuitable for high-performance bio-based surfactant synthesis, which results in the waste of a vast amount of bioresource feedstock [14]. The PUFA/MUFA ratio range and the constant saturated fatty acid (SFA) content have been studied based on global and Chinese vegetable oil production, import, and consumption data [14] (Figure 1) and the fatty acid composition [15,16,17,27,28,29] of common vegetable oils (Table 1). In China, major vegetable oils include soybean, rapeseed, peanut, and imported palm oil, exhibiting distinct fatty acid composition ranges and varying significantly in their import and export proportions. Differences in the use of these oils lead to significant variations in the PUFA/MUFA ratio of waste oils. By treating the production and import volumes of major vegetable oils as indicators of consumption and performing a weighted average based on their respective proportions and fatty acid compositions, this study estimates that the average PUFA/MUFA ratio in waste cooking oil falls within the range of approximately 0.53 to 1.21, which is considered a moderate level. It should be noted that this estimate is only used to represent the typical composition of feedstocks and does not affect the control of variables in the experiments. Specifically, waste oils with a higher PUFA/MUFA ratio primarily originate from waste soybean oil, while those with a lower ratio are mainly derived from waste rapeseed oil. In addition, based on the fatty acid composition of common vegetable oils and production and consumption (import and export) data in Figure 1c,d, the average palmitic acid content in vegetable oils is estimated to be approximately 11%.
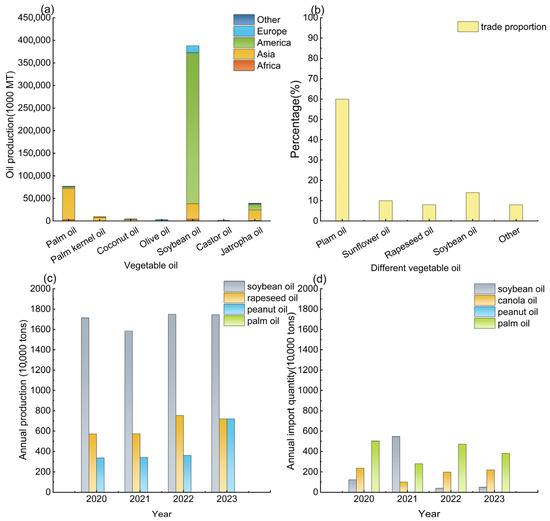
Figure 1.
Global annual consumption of vegetable oils. (a) Annual production data of vegetable oils worldwide; (b) 2022 vegetable oil trade data; (c,d) annual production and import data of vegetable oils in China in recent years [30].

Table 1.
The fatty acid composition of vegetable oils with high annual consumption.
In this work, linoleic acid, oleic acid, and palmitic acid were selected as representatives of polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), and saturated fatty acids (SFAs), respectively, to simulate the typical fatty acid composition of waste vegetable oils. Based on differences in the PUFA/MUFA ratio, we designed three groups of mixed fatty acid methyl esters with a relatively constant SFA content: the low-PUFA group (PUFA/MUFA ≈ 0.236, corresponding to waste rapeseed oil) with a molar ratio of linoleic acid methyl ester, oleic acid methyl ester, and palmitic acid methyl ester of 0.17:0.72:0.11; the medium-PUFA group (PUFA/MUFA ≈ 0.534, corresponding to waste cooking oil) with a ratio of 0.31:0.58:0.11; and the high-PUFA group (PUFA/MUFA ≈ 2.56, corresponding to waste soybean oil) with a ratio of 0.64:0.25:0.11. Based on these three representative compositions, a green and environmentally friendly three-step synthesis route was employed, and reaction conditions were optimized via an orthogonal experimental design to successfully prepare a series of bio-based zwitterionic surfactants with excellent interfacial properties. The influence of varying PUFA/MUFA ratios on the interfacial performance of the resulting surfactants was systematically evaluated to explore their potential application in EOR. Furthermore, synthesis verification using actual waste rapeseed oil, waste cooking oil, and waste soybean oil as raw materials demonstrated the feasibility and practical value of this method.
2. Materials and Methods
2.1. Materials
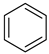
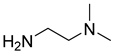
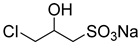
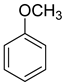
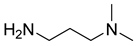
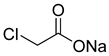
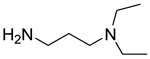
The instruments used in this study included a DF101S magnetic stirrer (Henan Yuhua Instrument Co., Ltd., Zhengzhou, China), GC9790Plus gas chromatograph (Zhejiang Fuli Instrument Co., Ltd., Hangzhou, China), GC-MS (Agilent 6890N, Agilent Technologies, Santa Clara, CA, USA), GC-MS-TQ8040 NX (Shimadzu Corporation, Kyoto, Japan), LC-MS (Shimadzu LC-MS8045, Shimadzu Corporation, Kyoto, Japan), XEVO G2 TOF electrospray mass spectrometer (Waters Corporation, Milford, MA, USA), and TX-500C rotating droplet interfacial tensiometer (Shanghai Geoscience Instrument Co., Ltd., Shanghai, China). The reagents used were of analytical grade, including benzene, methyl oleate, methyl linoleate, methyl palmitate, toluene, benzyl ether, N, N-dimethylethylamine, N, N-dimethylpropane-1, 3-diamine, N, N-diethylpropane-1, 3-diamine, sodium 3-chloro-2-hydroxypropane sulfonate, sodium chloroacetate, and butanesulfonic lactone (all purchased from Aladdin Industrial Corporation, Shanghai, China, or Titan Technology Co., Ltd., Shanghai, China). The waste soybean oil and waste rapeseed oil used in this study were edible oils beyond their shelf life, obtained from Yihai Kerry Oils & Grains Industries Co., Ltd., Shanghai, China, while the waste cooking oil was supplied by Daqing Huali Biotechnology Co., Ltd., Daqing, China. Data processing and plotting were performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) and Origin 2018 64-bit (OriginLab Corporation, Northampton, MA, USA). HPLC-MS data were acquired and processed using LabSolutions software (Shimadzu Corporation, Kyoto, Japan); however, the software version was not available. ESI-MS and GC-MS data were provided by the Analytical Testing Center of East China University of Science and Technology, and the corresponding software and version information were not accessible.
2.2. Orthogonal Experimental Design and Preparation of Surfactants
Mixed fatty acid methyl esters with different PUFA/MUFA ratios were used as raw materials. An orthogonal experimental design (Table 2) was employed to systematically investigate the effects of different reaction types (alkylation, amidation, and quaternization), various reagents, and different PUFA contents in the raw materials on the oil–water interfacial tension of the synthesized surfactants [31,32,33,34]. A series of amphoteric surfactants were synthesized according to the orthogonal experimental scheme (Table 3), where alkylation was conducted at 65 °C for 6 h with a molar ratio of raw material to reagent of 5:1, catalyzed by methanesulfonic acid; amidation was performed at 160 °C for 6 h with a molar ratio of 2:1, catalyzed by TiCl4; and quaternization was carried out at 75 °C for 8 h with a molar ratio of 2:1, catalyzed by Na2CO3, using an ethanol–water (7:3, v/v) solution as the solvent. The best reagent combination optimized through the orthogonal experiment was used to synthesize amphoteric surfactants, with mixed fatty acid methyl esters and different waste oils having different PUFA/MUFA ratios as raw materials, to validate the results of the orthogonal optimization and their practical applicability.

Table 2.
The orthogonal experiment factors (reaction steps) and levels (reagents).

Table 3.
Orthogonal experimental design table.
2.3. Analysis of Intermediates and Products in Surfactant Synthesis
The raw materials and the products of alkylation and amidation were analyzed by gas chromatography–mass spectrometry. The concentration was 2.0%, with a sample volume of 1 μL and a split ratio of 20:1 (the initial temperature was 260 °C, held for 2 min, then heated to 290 °C at a rate of 20 °C/min). The quaternization products were analyzed by liquid chromatography–mass spectrometry with a flow rate of 0.40 mL/min, a sample volume of 10 μL, and methanol/water as the mobile phase (80% methanol for the initial gradient for 8 min, then the gradient was increased from 8 to 28 min to 100% methanol over 20 min). Finally, the products were verified by electrospray ionization mass spectrometry.
2.4. Measurement of Dynamic Interfacial Tension of Surfactants
Surfactant solutions with concentrations of 3.0 g/L and 0.50 g/L were prepared using Daqing simulated formation water (composition shown in Table 4). The dynamic interfacial tension between these solutions and Daqing crude oil (composition shown in Table 5) was measured at 45 °C for 2 h using a rotating drop tensiometer. The focus of the tests was on the equilibrium interfacial tension values of the surfactant solutions at both concentrations with Daqing crude oil.

Table 4.
Composition of Daqing simulated formation water.

Table 5.
Characteristics of Daqing crude oil measured at 45 °C.
2.5. Utilization of Waste Oil as Feedstock for Surfactant Preparation
In this study, the waste soybean oil, waste rapeseed oil, and waste cooking oil (dehydrated before use) were first subjected to acid-catalyzed esterification (1 wt% H2SO4 in methanol, oil-to-methanol molar ratio 1:10, 65 °C for 1 h) to reduce the free fatty acid content. After pretreatment, the oils underwent a base-catalyzed transesterification with methanol (1:6 molar ratio) under 1 wt% KOH at 65 °C for 2 h to obtain mixed fatty acid methyl esters. The mixed fatty acid methyl esters obtained from the transesterification were then directly used as raw materials without separation and reacted with the optimized alkylation, amidation, and quaternization reagents to prepare the corresponding amphoteric surfactants.
3. Results and Discussion
3.1. Characterization and Conversion Rates of Synthesized Compounds
In the orthogonal experiment, mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios were used as raw materials. A total of 27 bio-based zwitterionic surfactants were successfully synthesized via alkylation, amidation, and quaternization, using mixed fatty acid methyl esters with different PUFA/MUFA ratios and the modification reagents listed in Table 3. The GC-MS characterization results of the intermediates, along with the HPLC-MS and ESI-MS characterization results of the target products, as well as the molecular weight data of the intermediates and target products, are provided in the Supplementary Materials (Figures S1–S201 and Tables S1–S109, where M1, M2, and M3 correspond to the molecular weights of the intermediates or target products derived from oleic acid methyl ester, linoleic acid methyl ester, and palmitic acid methyl ester, respectively). In both the alkylation and quaternization reactions, palmitic acid methyl ester remained unchanged before and after the reaction. As a result, it was used as an internal standard to calculate the alkylation conversion rate (the formula for the internal standard method is provided in the Supporting Information, Equation (S1.1)). In the quaternization reaction, the alkylation product did not participate in the reaction and was therefore also used as an internal standard to calculate the quaternization conversion rate. The conversion rate for the amidation reaction was determined using the peak area normalization method (the specific formula is shown in the Supporting Information, Equation (S1.2)). As summarized in Table 6, the conversion rates of all intermediate reaction steps exceeded 90%, indicating excellent synthetic efficiency.

Table 6.
A summary of the conversion percentages (%) for all intermediate and final reaction steps.
Surfactants were synthesized from mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios using the optimal reagent combination obtained from the orthogonal experiment. The intermediate products were characterized by GC-MS, and the final products were analyzed by HPLC-MS and ESI-MS (detailed results are provided in the Supplementary Information, Figures S202–S216). Additionally, further validation was performed using actual waste oils (waste rapeseed oil, waste cooking oil, and waste soybean oil) as raw materials. The GC-MS results of the intermediate products, along with the HPLC-MS and ESI-MS results of the target products, are provided in the Supplementary Information (Figures S217–S241). The HPLC and ESI spectra of the target products are shown in Figure 2 and Figure 3. In Figure 3, M1, M2, M3, and M4 correspond to the molecular weights of products derived from oleic acid methyl ester, linoleic acid methyl ester, palmitic acid methyl ester, and stearic acid methyl ester in waste oils, respectively. With an increase in linoleic acid content in the raw materials, the proportion of disubstituted products increased, as can be seen in the ESI-MS spectra in Figure 3.
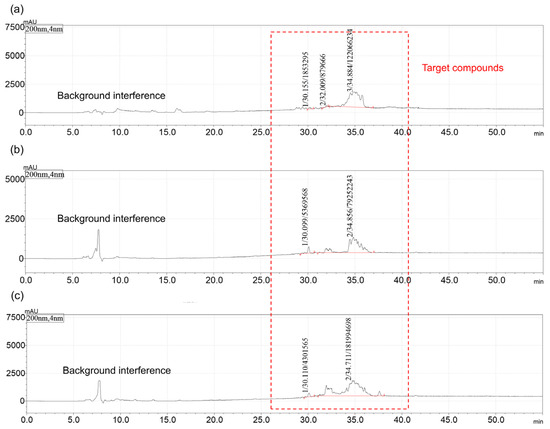
Figure 2.
HPLC spectra of bio-based zwitterionic surfactants synthesized using waste oils as raw materials, under the optimal reagent combination. (a–c) Waste rapeseed oil, cooking oil, and soybean oil, respectively.
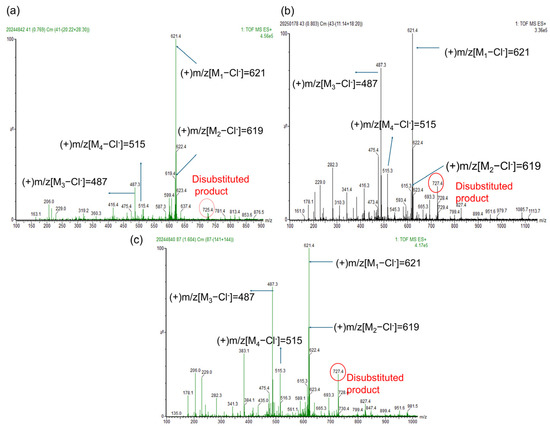
Figure 3.
ESI-MS of bio-based zwitterionic surfactants synthesized using waste oils as raw materials, under the optimal reagent combination. (a–c) Rapeseed oil, waste cooking oil, and soybean oil, respectively.
Collectively, these results demonstrate the successful synthesis of 27 surfactants through the modification of three raw materials with varying PUFA contents, using different functional group modifications in the orthogonal experiment. These characterization results collectively confirm that, under the modified reagent combination, surfactants were successfully synthesized from mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios, as well as oils such as waste rapeseed oil, waste cooking oil, and waste soybean oil.
3.2. Interfacial Activity of the Synthesized Surfactants
The dynamic interfacial tension between Daqing crude oil and simulated formation groundwater with different concentrations (3.0 g/L and 0.50 g/L) of bio-based zwitterionic surfactant systems (made up with feedstocks of different PUFA/MUFA ratios under different reaction reagents, as shown in Table 3) at 45 °C is shown in Figure 4. Orthogonal experiments were conducted by controlling various reaction reagents of alkylation, amidation, and quaternization reaction types, with the average equilibrium interfacial tension (as shown in Table 7) of the surfactant as the indicator, to optimize the best reagent combination. The data in Table 7 represent the average equilibrium interfacial tension measured from triplicate experiments.
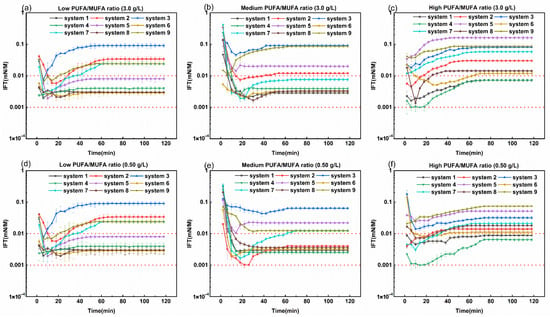
Figure 4.
Dynamic interfacial tension between Daqing crude oil and simulated formation groundwater with different concentrations of bio-based zwitterionic surfactant systems (made up of feedstocks of different PUFA/MUFA ratios under different reaction reagents as shown in Table 3) at 45 °C. (a–c) Low, medium, and high PUFA/MUFA ratios at a concentration of 3.0 g/L, respectively. (d–f) Low, medium, and high PUFA/MUFA ratios at a concentration of 0.50 g/L, respectively.

Table 7.
Summary of the equilibrium interfacial tension of bio-based zwitterionic betaine surfactant systems at 0.50 g/L and 3.0 g/L after 2 h at 45 °C.
3.2.1. The Effects of Different Reaction Types and Reactants on the Equilibrium Interfacial Tension of Surfactants
We performed a range analysis of the data to evaluate the impact of each factor and level on the interfacial tension. However, the factor with the largest R-value has the most significant effect [31,35,36]. Since all IFTequ values were below 1 mN/m, we used 1/IFTequ as the new response variable for the convenience of the range analysis. A higher 1/IFTequ value indicates a stronger ability to reduce the oil–water interfacial tension. Regarding the influence of different reaction types on the ability to reduce the oil–water interfacial tension of surfactants, the range analysis results (as shown in Table 8) indicate that factor C, the hydrophilicity of the head group, has the most significant impact. The second most influential factor is factor B, which pertains to the linking group between the head group and the hydrophobic tail chain. Among these, the polarity of the amide group helps it form hydrogen bonds with water molecules, thereby enhancing the overall hydrophilicity of the surfactant. This effect is particularly pronounced in long-chain phenyl fatty acid amide betaine-type surfactants [37]. Factor A, which refers to the difference in phenyl substituents on the hydrophobic tail chain, has the least impact on oil–water interfacial tension, despite the generally significant influence of the hydrophobic tail chain on surfactant performance. It is noteworthy that when using raw materials with a high content of linoleic acid, Factor B has a more significant effect on oil–water interfacial tension at low concentrations compared to Factor C. In contrast, when using raw materials with a low linoleic acid content, the effect of Factor A exceeds that of Factor B at high concentrations. In conclusion, the results of this study indicate that, compared to the hydrophobic phenyl substituents, the hydrophilic portion of betaine-type surfactants plays a more significant role in reducing oil–water interfacial tension, which may be related to the larger space occupied by this portion at the interface [38].

Table 8.
Range analysis of 1/IFTequ of bio-based zwitterionic betaine surfactants synthesized from mixed fatty acid methyl esters with varying PUFA/MUFA ratios (low, medium, and high).
Regarding different reactants, the effect of different types of head groups in reducing oil–water interfacial tension (as shown in Table 8) is as follows: hydroxysulfonic acid > carboxyl > butylsulfonic acid. The introduction of hydroxyl groups enhances hydrogen bonding with water molecules, thereby increasing hydrophilicity [1,24,39]. On the other hand, butylsulfonic acid, compared to sulfonamide betaine, has additional carbon atoms that enhance hydrophobicity, thereby reducing hydrophilicity. Carboxyl betaine also has stronger hydrophilicity than sulfonamide betaine [1]. Therefore, for phenyl long-chain fatty acid amide betaine-type surfactants, stronger hydrophilicity contributes to more effectively reducing the oil–water interfacial tension. Next, when analyzing factor B (linking group) at three levels, the effect in reducing oil–water interfacial tension is as follows: N, N-dimethylethanamine > N, N-dimethylpropane-1,2-diamine > N, N-diethylpropane-1,2-diamine. This is due to the hydrophilicity of the amide group [37]. The introduction of additional carbon atoms reduces the hydrophilicity of the amide group [21], which is unfavorable given its ability to reduce oil–water interfacial tension. Additionally, the volume of the head group is increased by larger side chains, leading to less compact packing of surfactant molecules at the interface, which hinders the ability to reduce oil–water interfacial tension. Finally, for factor A (phenyl substituent), the results show that methoxy-substituted phenyl groups achieve a better interfacial performance, which may be related to their polarity [40]. Adding polar substituents or shortening the alkyl chain can enhance the hydrophilicity of surfactants. Methoxy groups, by forming hydrogen bonds with water molecules, enhance the hydrophilicity of phenyl betaine surfactants, thereby optimally reducing oil–water interfacial tension. In contrast, methylphenyl substituents increase hydrophobicity, leading to a poorer interfacial performance.
In analysis of variance (ANOVA), the p-value is used to quantitatively assess the influence of each factor and determine the significance of differences between groups. The smaller the p-value for a factor, the more significant its effect on the results [41]. Under all experimental conditions shown in Table 9, the p-value for factor C (hydrophilic head group) was consistently significant and typically had the highest F-value, indicating that it had the greatest effect on the experimental results. Factor B (the linking group between the hydrophilic head and hydrophobic tail) had the next most significant effect, with its p-value also showing significance, but its influence was smaller than that of factor C. The p-value for factor A (the variation in the phenyl substituent group on the hydrophobic chain) was less than 0.05 in some cases, indicating a significant effect on the results, while in other cases, the p-value ranged between 0.05 and 0.10, showing marginal significance. This could be due to experimental error, an insufficient sample size, or other uncontrolled interfering factors. Overall, most factors (A, B, and C) showed significant differences, and the experimental results were statistically significant. The influence of the factors was consistently ranked as C > B > A, which is consistent with the conclusions of the range analysis, indicating that the impact of the hydrophilic portion is greater than that of the hydrophobic phenyl substituent group.

Table 9.
ANOVA results of factors affecting the interfacial activity of surfactants synthesized from mixed fatty acid methyl esters with varying PUFA/MUFA ratios (low, medium, and high).
In conclusion, the results indicate that the factors affecting the reduction of oil–water interfacial tension follow the order C > B > A. This means that the effect of the quaternary ammonium group on the interfacial performance of the surfactant is greater than that of the amide group, which in turn is greater than that of the alkylation group with a phenyl substituent. The optimal reagent combination is A2B1C1, where the alkylation reagent is benzyl ether, the amidation reagent is N, N-dimethylethanolamine, and the quaternization reagent is sodium 3-chloro-2-hydroxypropyl sulfonate. All surfactants synthesized in this study are betaine-type amphoteric surfactants, with hydrophobic tail chains composed of long-chain fatty acids substituted with different phenyl groups. Enhancing the hydrophilicity of the head group and the polarity of the hydrophobic chain contributes to improving the interfacial performance of the surfactant.
3.2.2. Effect of the PUFA Content in Raw Materials on Surfactant Performance
The effect of the polyunsaturated fatty acid (PUFA) content in raw materials on the oil–water interfacial performance of phenyl-substituted betaine-type amphoteric surfactants was investigated in this study. The effect of different reaction reagents in various reaction types on the interfacial performance of surfactants derived from mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios (as shown in Figure 5) also shows that as the content of PUFA methyl esters in the raw materials increases, the mean value of the vertical coordinate 1/IFT gradually decreases, indicating a significant decline in its ability to reduce interfacial tension. This is primarily attributed to the structural characteristics of the products and the presence of multiple reactive sites in PUFAs: (1) Spatial structural effects of the products: Compared to monounsaturated fatty acids (MUFAs), PUFAs exhibit a higher degree of unsaturation [14,42,43]. The products obtained from polyunsaturated fatty acids are typically monophenyl-substituted, retaining one double bond, which leads to spatial distortion and the formation of a rigid structure, thereby increasing steric hindrance. This structure prevents the surfactant molecules from packing tightly at the oil–water interface, reducing the cohesion of the monolayer and weakening their ability to reduce interfacial tension. In contrast, MUFA-derived products are entirely monosubstituted and do not contain additional double bonds, making it easier to form tightly packed monolayers that effectively lower interfacial tension. (2) Multiple reactive sites in PUFAs: The PUFA molecules contain two reactive sites. When used to prepare surfactants, monophenyl-substituted products are typically formed. However, diphenyl-substituted products may also be produced. This can be observed in the electrospray mass spectra provided in Figure 3. Products containing two phenyl groups occupy more space, hindering their packing at the interface, which in turn affects their interfacial performance. In conclusion, as the PUFA content in the raw materials increases, the ability of the surfactant to reduce interfacial tension gradually decreases.
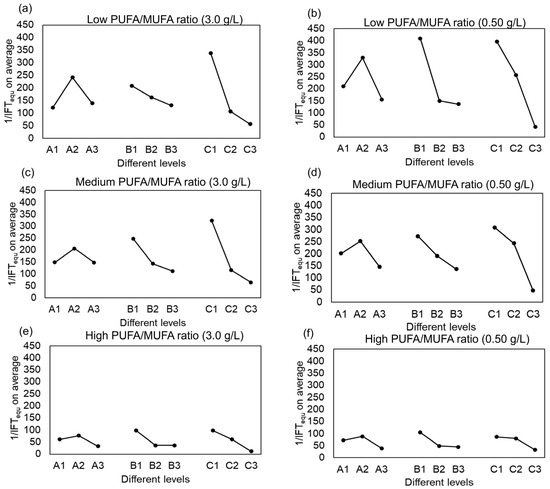
Figure 5.
The effect of different reaction reagents in various reaction types on the interfacial performance of surfactants derived from mixed fatty acid methyl esters with varying PUFA contents at 3.0 g/L and 0.50 g/L concentrations and 45 °C. (a,c,e) Surfactants synthesized from mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios at a concentration of 3.0 g/L, respectively. (b,d,f) Those synthesized from the same raw materials at a concentration of 0.50 g/L.
3.3. The Interfacial Performance of Surfactants Prepared from Mixed Fatty Acid Methyl Esters with a High PUFA Content
The orthogonal experiment determined the optimal reagent combination as A2B1C1, where the alkylation reagent was phenyl methyl ether, the amidation reagent was N, N-dimethylethylenediamine, and the quaternization reagent was sodium 3-chloro-2-hydroxypropylsulfonate. Verification experiments were conducted using three different PUFA/MUFA ratio mixed fatty acid materials. Figure 6 demonstrates that at 45 °C and concentrations of 3.0 g/L and 0.50 g/L, the surfactant solution and Daqing crude oil achieved and maintained ultra-low interfacial tension for up to 2 h, without the necessity of alkali addition.
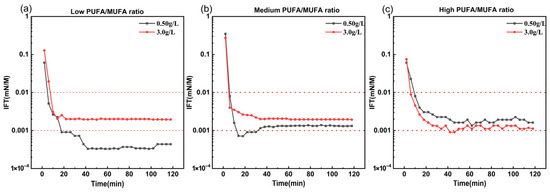
Figure 6.
Dynamic interfacial tension between Daqing crude oil and mixed fatty acid methyl ester-derived bio-based zwitterionic surfactants with varying PUFA contents under optimized modification reagents, at 45 °C. (a–c) Mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios, respectively.
The excellent interfacial performance was superior to other orthogonal combinations, indicating the success of orthogonal optimization. Additionally, raw materials with high PUFA contents (Figure 6c) also yielded surfactants with high interfacial activity, which has significant implications. The surfactant synthesized from the optimal reagent combination not only effectively reduced the oil–water interfacial tension but also showed an enhanced tolerance to variations in the PUFA content.
3.4. The Interfacial Performance of Surfactants Prepared from Different Waste Oils
Waste rapeseed oil, waste cooking oil, and waste soybean oil were used as raw materials to prepare a mixed fatty acid methyl ester via transesterification. The compositions of the resulting methyl esters are summarized in Table 10, with PUFA/MUFA ratios of 0.158, 0.423, and 2.105. Subsequently, phenyl ether, N, N-dimethyl ethylenediamine, and 3-chloro-2-hydroxypropane sulfonate were used to synthesize surfactants (the results of the reaction intermediates and products are provided in Supplementary Information Figures S217–S241). As shown in Figure 7, surfactants derived from all three oil sources achieved ultra-low interfacial tension after 2 h without the addition of alkali, demonstrating their potential application in alkali-free oil-flooding systems.

Table 10.
Fatty acid composition of three types of oils.
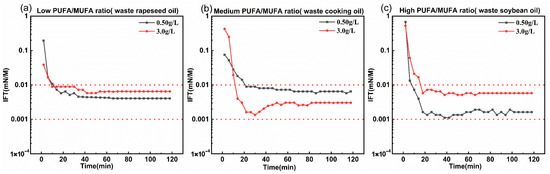
Figure 7.
The dynamic interfacial tension between Daqing crude and waste vegetable oil-derived bio-based zwitterionic surfactants with varying PUFA contents under optimized modification reagents, at 45 °C. (a–c) Waste rapeseed oil, cooking oil, and soybean oil, respectively.
Although their interfacial performance was slightly lower than that of surfactants prepared from purified mixed fatty acid methyl esters, they still exhibited good activity. This difference may be related to the complex composition of waste oils. Furthermore, waste oils are widely available, low-cost, and require no further purification, offering significant potential for industrial application in alkali-free oil-flooding systems. In terms of sustainability, this work emphasizes the utilization of waste oils as renewable feedstocks. While this represents a key advantage, we acknowledge that the use of methanesulfonic acid and the relatively high temperature required for amidation may raise environmental concerns. Future efforts will aim to explore greener catalysts and milder conditions to improve the overall environmental profile of the synthesis.
It should be noted that this study primarily focused on the synthesis and interfacial performance of PUFA-based bio-surfactants, without addressing the potential effects of microorganisms present in oil reservoirs on the stability and efficacy of these surfactants. Considering that microbial degradation has been reported to impact the field performance of some biopolymers, future research will aim to investigate the microbial stability and performance of these surfactants under reservoir-like conditions to further evaluate their practical applicability.
4. Conclusions
In summary, chemical-enhanced oil recovery using surfactant flooding has gained significant interest; however, most surfactants used are petrochemical derivatives that pose severe environmental concerns. To promote environmental and material sustainability, the industry is shifting toward bio-based surfactants due to their renewability, biodegradability, and lower toxicity. In this study, a novel series of PUFA-based zwitterionic surfactants was synthesized using an orthogonal experimental design to systematically investigate the effects of different reaction types (alkylation, amidation, and quaternization) and various reagents on the interfacial activity of products derived from fatty acid methyl esters with varying PUFA to MUFA ratios. The ratios cover dominant waste vegetable oils derived from the major plant oils (PUFA/MUFA ≈ 0.236, 0.534, and 2.56, the ratios corresponding to waste rapeseed oil, cooking oil, and soybean oil, respectively). The optimal combinations of modifying reagents in the three reaction types were identified as phenyl methyl ether for alkylation, N, N-dimethylethylenediamine for amidation, and sodium 3-chloro-2-hydroxypropylsulfonate for quaternization. Ultra-low interfacial tensions (~10−3 mN/m) could be achieved by the synthesized surfactants without extra alkaline addition. For instance, at 0.50 g/L and 3.0 g/L (typical concentrations used in most oil fields), the bio-based surfactants derived from waste soybean oil with a PUFA-to-MUFA ratio of approximately 2.11 could reduce the interfacial tension between Daqing crude oil and simulated formation water to 1.62 × 10−3 and 5.78 × 10−3 mN/m, respectively. This approach of bio-based surfactants from PUFA-rich mixed fatty acids and waste vegetable oils expands upon previous studies of bio-based surfactants, which primarily relied on monounsaturated fatty acid raw materials, and contributes to improving the comprehensive utilization of oil resources. Furthermore, the synthesized surfactants exhibited excellent interfacial activity under alkaline-free conditions, highlighting their potential for applications in environmentally friendly, alkaline-free oil displacement systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13072159/s1, Conversion rate formulas, molecular weights, and GC-MS, HPLC-MS, and ESI-MS data for intermediates and final 27 surfactant products from the orthogonal experiment, as well as surfactants synthesized from mixed fatty acid methyl esters with low, medium, and high PUFA/MUFA ratios, and from waste rapeseed oil, waste cooking oil, and waste soybean oil using the optimal reagent combination.
Author Contributions
X.-M.Z.: data curation, investigation, software, writing—original draft preparation. H.I.M.: writing—review and editing, formal analysis. S.-Z.Y. and B.-Z.M.: funding acquisition, project administration, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (Grant No. 2022YFC2105200).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request due to privacy concerns.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Key Research and Development Program of China under Grant No. 2022YFC2105200.
Conflicts of Interest
The authors declare no competing interests.
References
- Wang, Y.J.; Zhang, Y.M.; Liu, X.L.; Wang, J.Y.; Wei, L.M.; Feng, Y.J. Effect of a Hydrophilic Head Group on Krafft Temperature, Surface Activities and Rheological Behaviors of Erucyl Amidobetaines. J. Surfactants Deterg. 2014, 17, 295–301. [Google Scholar] [CrossRef]
- Gbadamosi, A.; Hussai, S.M.S.; Kamal, M.S.; Patil, S.; Solling, T.; Hassan, S.F.; Wang, J.X. Evaluating the Potential of Zwitterionic Surfactants for Enhanced Oil Recovery: Effect of Headgroups and Unsaturation. Energy Fuels 2023, 37, 5078–5086. [Google Scholar] [CrossRef]
- Al-Azani, K.; Abu-Khamsin, S.; Al-Abdrabalnabi, R.; Kamal, M.S.; Patil, S.; Zhou, X.M.; Hussain, S.M.S.; Al Shalabi, E. Oil Recovery Performance by Surfactant Flooding: A Perspective on Multiscale Evaluation Methods. Energy Fuels 2022, 36, 13451–13478. [Google Scholar] [CrossRef]
- Chowdhury, S.; Shrivastava, S.; Kakati, A.; Sangwai, J.S. Comprehensive Review on the Role of Surfactants in the Chemical Enhanced Oil Recovery Process. Ind. Eng. Chem. Res. 2022, 61, 21–64. [Google Scholar] [CrossRef]
- Lu, J.; Liyanage, P.J.; Solairaj, S.; Adkins, S.; Arachchilage, G.P.; Kim, D.H.; Britton, C.; Weerasooriya, U.; Pope, G.A. New surfactant developments for chemical enhanced oil recovery. J. Pet. Sci. Eng. 2014, 120, 94–101. [Google Scholar] [CrossRef]
- Liang, T.B.; Zhao, X.R.; Yuan, S.; Zhu, J.W.; Liang, X.Y.; Li, X.H.; Zhou, F.J. Surfactant-EOR in tight oil reservoirs: Current status and a systematic surfactant screening method with field experiments. J. Pet. Sci. Eng. 2021, 196, 108097. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Zhou, Y.; Lu, Y.; Chen, B.; Zhang, J. Systematic investigation of the physicochemical properties of eco-friendly biobased anionic-nonionic surfactants for enhanced oil recovery. J. Mol. Liq. 2021, 323, 114628. [Google Scholar] [CrossRef]
- Ahn, C.; Morya, V.K.; Kim, E.-K. Tuning surface-active properties of bio-surfactant sophorolipids by varying fatty-acid chain lengths. Korean J. Chem. Eng. 2016, 33, 2127–2133. [Google Scholar] [CrossRef]
- An, Y.; Yao, X.; Zhong, J.; Pang, S.; Xie, H. Enhancement of oil recovery by surfactant-polymer synergy flooding: A review. Polym. Polym. Compos. 2022, 30. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Han, Y.; Kim, Y.W.; Lee, Y.W.; Hong, S.; Hwang, I.T. Feasibility of unsaturated fatty acid feedstocks as green alternatives in bio-oil refinery. Biofuels Bioprod. Biorefining-Biofpr 2019, 13, 690–722. [Google Scholar] [CrossRef]
- Rajput, C.V.; Sastry, N.V.; Chikhaliya, N.P. Vegetable oils based precursors: Modifications and scope for futuristic bio-based polymeric materials. J. Polym. Res. 2023, 30, 159. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Ahmed, M.N.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.; Maggi, F.; Caprioli, G.; Sakar, E. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Wang, S.F.; Furuno, T.; Cheng, Z. Synthesis of new betaine-type amphoteric surfactants from tall oil fatty acid. J. Wood Sci. 2002, 48, 419–424. [Google Scholar] [CrossRef]
- Mohammed, S.; Ikiensikimama, S.S. Vegetable oils as surfactant feedstocks for enhanced oil recovery: A review. Chem. Eng. Res. Des. 2023, 200, 693–705. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Martínez-Klimov, M.; Murzin, D.Y. Hydroconversion of fatty acids and vegetable oils for production of jet fuels. Fuel 2021, 306, 121673. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Cai, B.X.; Gang, H.Z.; Yang, S.Z.; Mu, B.Z. A family of novel bio-based zwitterionic surfactants derived from oleic acid. Rsc Adv. 2014, 4, 38393–38396. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Gang, H.Z.; Liu, J.F.; Mu, B.Z.; Yang, S.Z. A thermal-stable and salt-tolerant biobased zwitterionic surfactant with ultralow interfacial tension between crude oil and formation brine. J. Pet. Sci. Eng. 2019, 181, 106181. [Google Scholar] [CrossRef]
- Lang, J.Q.; Mtui, H.I.; Gang, H.Z.; Mu, B.Z.; Yang, S.Z. Highly Ca2+-Ion-Tolerant Biobased Zwitterionic Surfactant with High Interfacial Activity. Acs Omega 2022, 7, 32775–32783. [Google Scholar] [CrossRef]
- Wang, W.; Liang, M.Y.; Lang, J.Q.; Homely Isaya, M.; Yang, S.Z.; Mu, B.Z. A new thermal-tolerant bio-based zwitterionic surfactant for enhanced oil recovery. Green Mater. 2023, 12, 50–59. [Google Scholar] [CrossRef]
- Liu, F.H.; Gao, C.L.; Zhang, C.C.; Gang, H.Z.; Mu, B.Z.; Yang, S.Z. A new zwitterionic surfactant with high interfacial activity and high salt tolerance derived from methyl oleate through an eco-friendly aryl-introducing method. J. Surfactants Deterg. 2023, 26, 135–144. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Jia, Z.; Yang, L.; Dong, J. Renewable dissymmetric sulfonate gemini surfactants from addition of sodium hydrogensulfite to alkyl linoleate. Aiche J. 2023, 69, e17898. [Google Scholar] [CrossRef]
- Xu, Y.M.; Zhang, X.X.; Zhao, H.X.; Chen, W.; Yan, X.D.; Liu, H.Q.; Liu, C.Y.; Xu, B.C. Synthesis, Characterization, and Surface-Active Properties of Carboxylbetaine and Sulfobetaine Surfactants based on Vegetable Oil. J. Surfactants Deterg. 2019, 22, 103–114. [Google Scholar] [CrossRef]
- Gao, C.L.; Gang, H.Z.; Liu, J.F.; Mu, B.Z.; Yang, S.Z. A New Benzylated Fatty Acid Amide Amphoteric Surfactant Derived from Hydrogenated Castor Oil with Ultra-Low Interfacial Tension between Crude Oil and Brine. J. Surfactants Deterg. 2021, 24, 511–515. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Cai, B.X.; Xu, W.J.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Mu, B.Z. The Rebirth of Waste Cooking Oil to Novel Bio-based Surfactants. Sci. Rep. 2015, 5, 9971. [Google Scholar] [CrossRef]
- da Silva, E.K.A.; Rial, R.C. Fatty acid profile, nutritional and therapeutic properties of vegetable oils from the Brazilian Cerrado. J. Food Compos. Anal. 2025, 137, 106819. [Google Scholar] [CrossRef]
- Kamyab, B.; Beims, R.; Chambers, D.W.; Bassi, A.S.; Xu, C.B. Sustainable production of high-performance bio-based hydraulic fluids from vegetable oils: Recent advances, current challenges, and future perspectives. Biomass Bioenergy 2024, 183, 107160. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Analysis of the Supply and Demand Situation of Agricultural Products in China. Available online: http://www.scs.moa.gov.cn/jcyj/202407/t20240712_6458930.htm (accessed on 24 July 2024).
- Hou, P.Q.; Yang, L.; Wang, Y.F.; Wang, L.X.; Li, S.N.; Lu, S.H. Optimize hydrothermal synthesis and electrochemical performance of Li2FeTiO4 composite cathode materials by using orthogonal experimental design method. Ionics 2020, 26, 1657–1662. [Google Scholar] [CrossRef]
- Huang, L.J.; Wang, Y.A.; Wei, Z.H.; Han, X.X.; Mo, Q.; Wang, X.Y.; Li, Y.S. Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide. Polymers 2022, 14, 1959. [Google Scholar] [CrossRef]
- Tyagi, R.; Sharma, P.; Nautiyal, R.; Lakhera, A.K.; Kumar, V. Synthesis of quaternised guar gum using Taguchi L(16) orthogonal array. Carbohydr. Polym. 2020, 237, 116136. [Google Scholar] [CrossRef]
- Chen, D.M.; Tang, W.J.; Wang, H.; Sheng, Y.Q.; Tan, X.; Shi, Y.; Fan, W.; Ge, S.B. Phosphoric acid pretreatment of poplar to optimize fermentable sugars production based on orthogonal experimental design. Front. Chem. 2023, 11, 1119215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Jia, L. Variables Affecting Biodiesel Production from Zanthoxylum bungeanum Seed Oil with High Free Fatty Acids. Ind. Eng. Chem. Res. 2012, 51, 3525–3530. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, J.; Tian, Z.; Yang, K.; Yao, Z. Optimization of Spent Cathode Carbon Purification Process under Ultrasonic Action Using Taguchi Method. Ind. Eng. Chem. Res. 2018, 57, 7700–7710. [Google Scholar] [CrossRef]
- Butler, C.S.G.; Kelleppan-Meaney, V.T.; Williams, A.P.; Giles, L.W.; Vidallon, M.L.P.; Sokolova, A.; de Campo, L.; Tuck, K.L.; Tabor, R.F. Influence of tail group length, amide functionality and added salt ion identity on the behaviour of betaine surfactants. J. Colloid Interface Sci. 2024, 653, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Zhang, Q.; Liu, Y.; Wang, H.Z.; Cai, H.Y.; Zhang, F.; Tian, M.Z.; Liu, Z.Y.; Zhang, L.; Zhang, L. Effect of Fatty Acids on Interfacial Tensions of Novel Sulfobetaines Solutions. Energy Fuels 2014, 28, 1020–1027. [Google Scholar] [CrossRef]
- Gao, S.F.; Song, Z.Z.; Lan, F.; Li, P.; Zhang, A.H.; Hu, J.J.; Jiang, Q.Z. Studies on Physicochemical Properties and Aggregation Behavior of Two Pairs of Betaine Surfactants. Ind. Eng. Chem. Res. 2019, 58, 22260–22272. [Google Scholar] [CrossRef]
- Verma, C.; Rhee, K.Y.; Quraishi, M.A. Hydrophilicity and hydrophobicity consideration of organic surfactant compounds: Effect of alkyl chain length on corrosion protection. Adv. Colloid Interface Sci. 2022, 306, 102723. [Google Scholar] [CrossRef]
- Gok, Z.; Kucukcongar, S.; Turkyilmaz, M.; Oksuz, S.T. Treatment of strained yoghurt wastewater by electrochemical oxidation method using Taguchi experimental design. J. Appl. Electrochem. 2023, 53, 1595–1607. [Google Scholar] [CrossRef]
- Sekhar, K.P.C.; Patel, D.; Holey, S.A.; Kanjilal, S.; Nayak, R.R. Tail-group unsaturation tailors the surface and self-assembly behavior of C18-fatty acid-based glycolipids. J. Mol. Liq. 2022, 351, 118585. [Google Scholar] [CrossRef]
- Wang, Y.X.; Jiang, L.; Shen, Q.K.; Shen, J.; Han, Y.W.; Zhang, H.M. Investigation on the self-assembled behaviors of C18 unsaturated fatty acids in arginine aqueous solution. RSC Adv. 2017, 7, 41561–41572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).