Abstract
The demand for strategic elements, including nickel and cobalt, increases each year due to rapid technological advancements. However, due to their scarcity and environmental concerns, the development of sustainable recycling processes supported by green-energy technologies is becoming essential. In this study, a process relying on indirect bioleaching was used to recover nickel and cobalt from three different superalloy residue powders as a second source of metals, as part of a wider study to recycle superalloys within a waste process. A comparison between the three methods was carried out to analyse the bioleaching mechanisms of the target metals. Acidolysis was selected for further study due to its set-up simplicity and superior recovery rates. Variations in agitation speed of the lixiviant processing the Ni 30167 superalloy revealed that 270 rpm achieved the optimal active metal surface–oxidising agent interaction, with 60% and 70% dissolution rates after 24 h for nickel and cobalt, respectively. For the Re 30168 superalloy, extraction rates of 60% and 50% were obtained in 48 h for nickel and cobalt, respectively. The effect of hydrogen peroxide as an additive to improve metal solubilisation and overcome passivation, are discussed together with the challenges posed by the presence of iron, the materials’ elemental complexity, and its interaction with different oxidising agents.
1. Introduction
Rapid technological advancements have driven increased metal mining from primary sources, with demand expected to double [1], leading to more waste, emissions, and energy consumption. The metallurgical industry significantly impacts the climate, biodiversity, and metal availability, with CO2 emissions, mainly from carbothermic reduction using fossil fuels, being a key driver of the climate crisis [2,3].
The scale of these challenges calls for collaboration between scientists and industry leaders to develop a circular manufacturing system with recycling at its core. However, global metal recycling is increasing, driven by sustainability goals, cost-efficiency, and technological advancements, with the market projected to grow significantly in the coming years [4]. With new regulations, metal producers are required to adopt sustainable methods to produce high-quality materials with the same properties but a lower carbon footprint, using renewable energy and recycled metals [5].
To reduce gas emissions, renewable energy technologies have received significant investment, research, and development in recent decades. However, producing green energy systems like solar panels and wind turbines requires base and critical metals, adding pressure on metal availability. For instance, the U.S. Geological Survey [6] reports that 3.6 million tons of nickel (Ni) were mined in 2023, 9.1% more than the previous year, while cobalt (Co) production reached 230,000 tons, a 14.3% increase.
This rebound effect highlights the need for increased consumption of key metals like nickel and cobalt to enhance process sustainability. However, long-term solutions lie in combining sustainable energy technologies with green recycling of secondary resources, such as recycling scraps and dust. Currently, dust from the production and cutting of superalloy bars is wrongly treated as waste, leading to the loss of valuable metals, only for more to be mined, chemically processed, and transported globally using fossil fuel-powered vehicles. Therefore, the extraction of metals from the scraps/residues and their reintegration as raw materials into the alloy and superalloy manufacturing cycle is an attractive strategy as an alternative to mining from primary sources, reducing the energy expenditure, minimising the gas emissions, and bringing benefits to the economy [5,7].
The knowledge of superalloy microstructures is essential for understanding element distributions and improving the efficiency of metal recovery during leaching. A study by Shi et al. (2020) [8] on the Inconel 718 superalloy showed that inclusions like Al2O3 and TiN were evenly distributed throughout the material and could act as nucleation points for subsequent precipitate development. These microstructural features, identified using SEM-EDS and confirmed by XRD, are critical in understanding the behaviour of superalloys under leaching conditions. In the context of leaching, the presence, distribution, and composition of such inclusions can significantly influence dissolution rates, leachability of specific elements, and overall efficiency of metal recovery. For example, oxide inclusions such as Al2O3 may exhibit high chemical stability, resisting leaching, while surrounding phases may dissolve more readily. Therefore, characterisation of the original structure of superalloys, including their inclusions, is essential for correlating microstructural properties with leaching behaviours and optimising the selective extraction of valuable metals.
Different methods for recovering metals from superalloys have been investigated, including hydrometallurgical processes, each with its own advantages and disadvantages, such as high temperature requirements and significant acid consumption. Fan et al., 2013 [9] investigated the recovery of metals from superalloys by chemical leaching using sulphuric acid. The optimal conditions for leaching Ni and Co from superalloys using sulphuric acid were determined as 85 °C, 40 wt% acid concentration, 5 h leaching time, 250 r/min stirring speed, and 80–120 mesh particle size, achieving at least 96% leaching efficiency. The residual Ni and Co in the slag were 6.77% and 0.96%, respectively, and the resulting leachate can be used for Ni and Co product recovery. Wang et al., 2022 [10] explored ultrasonic leaching with a two-stage separation process for recovering rare metals from superalloy scraps. Optimal conditions (600 W ultrasonic power, 80 °C, 60 min, 10:1 liquid–solid ratio, 8 M HCl, and 4.8 vol% H2O2) resulted in high leaching efficiencies: Re (92.3%), Ni (95.2%), Co (98.5%), Al (98.7%), and Cr (97.5%). The two-stage separation effectively removed 94.6% of Al and 82.1% of Cr at pH 4.5, and 99.5% of Ni and 98.3% of Co at pH 7.5, leaving an Re-rich solution for further processing, all without acid or alkali waste.
In the last decades, bioleaching/bio-hydrometallurgy has attracted more interest due to its cost-effectiveness and its environmental-friendly nature [11]. While bioleaching has shown promise in recovering metals from e-waste like PCBs [12,13], LCD screens [14], and batteries [15], to our knowledge it has not yet been tested on superalloys. Superalloys are known for their exceptional properties, such as their high tensile strength, and their heat, pressure, and corrosion resistance [16], which are due to the addition of small amounts of critical metals (up to 30–40 elements), typically mixed with one or two dominant metals, to enhance their physical and chemical properties [17]. Bioleaching is the process of solubilising metals from insoluble substrates using a leaching environment created by microorganisms naturally found in places like mines [18]. Different microorganisms, such as fungi, algae, and bacteria, produce oxidants that are necessary for bio-oxidation during their growth cycle. Acidithiobacillus thiooxidans (A. thiooxidans), alongside Acidithiobacillus ferrooxidans, is one of the most commonly used microbes in bioleaching [19]. These chemolithoautotrophic, mesophilic bacteria synthesize cellular material using atmospheric CO2, with Acidithiobacillus ferrooxidans deriving energy from the oxidation of reduced iron and sulphur (Equations (1) and (2)), while A. thiooxidans, a sulphur-oxidising bacterium, converts sulphur compounds to sulphuric acid, which aids in metal solubilisation (Equation (1)) [20].
A recent study explored the bioleaching potential of six thermoacidophilic archaea from the waste of the steel industry, with Acidianus manzaensis showing the highest efficiency. Microscopy confirmed its ability to solubilize the mineral matrix, while operational refinements indicated that the pH affects element-specific solubilisation, and an increased dust load enhances metal release. These findings emphasize the role of thermoacidophilic archaea in sustainable metal recycling and circular economy strategies [21].
Key parameters such as agitation speed and hydrogen peroxide (H2O2) concentration play a crucial role in enhancing metal mobilisation during leaching. Peng et al., 2023 [22] investigated the effect of shaking speed on the recovery of Co and Ni from Li-ion batteries. Mixing at 400 r/min or higher eliminated boundary layer diffusion, significantly enhancing Ni and Co leaching rates with increasing temperatures. H2O2 is a strong oxidising agent with a high reduction potential, making it a promising additive for enhancing metal mobilisation in bioleaching [23]. Tran et al., 2022 [24] found that nickel and cobalt dissolution reached 90–100% in one hour at 60 °C using a sulphuric acid and hydrogen peroxide mixture. Han et al., 2022 [25] achieved 80% nickel dissolution from sintered alloy in 100 min at 80 °C with 1.2 M sulphuric acid and 20% H2O2. Wanta et al., 2021 [26] reported a 95% nickel extraction efficiency from a spent catalyst in 4 h at 30 °C and 200 rpm, using 9% H2O2 in 1 M sulphuric acid.
This study investigates the application of bioleaching as a green, economically viable strategy for the recovery of nickel and cobalt from nickel-based superalloy residue powders, which represent an underutilized secondary resource rich in critical metals. While bioleaching has been extensively studied for primary ores and some electronic wastes like PCBs, batteries, and catalysts, its use for superalloy residues remains largely unexplored, despite their growing availability from the aerospace and power generation industries. This study not only addresses that research gap but also focuses on optimising key operational parameters to enhance the efficiency and scalability of the process, thereby paving the way for its potential integration into industrial metal recovery and recycling systems. It tackles challenges like material density, elemental complexity, and interactions with oxidising agents. By testing various agitation speeds, bioleaching methods (Bio-acid, Bio-ferric, and Bio-acid/Bio-ferric), and hydrogen peroxide as an additive, the study aims to improve metal dissolution. Three different superalloys were used to assess the potential for large-scale recycling systems for Ni-based superalloys with varying compositions. This study suggests that low temperatures and less concentrated reagents can achieve effective metal mobilisation at minimal costs.
2. Materials and Methods
2.1. Superalloy Residue (SAR) Source, Preparation, and Characterisation
The three superalloy residues (SAR), including SAR-Ni 30167, SAR-In 32083, and SAR-Re 30168, were sourced from nickel and cobalt-based superalloy bars provided by AAS Ltd. (Sheffield, UK). Due to the heterogeneity of the samples, approximately 35 g of each material was processed using a planetary ball mill containing eight stainless steel balls with a diameter of 20 mm at 300 rpm for 10 min. After milling, the powder was sieved, and the portion with a particle size of less than 250 µm was collected for metal analysis and bioleaching experiments. X-ray fluorescence (XRF) analysis was conducted using a Rigaku NEX-DE system to determine the metal abundance and composition of the powder samples semi quantitatively. The samples were digested with aqua regia in a molar ratio of 1:3. This digestion process took place in 50 mL Erlenmeyer flasks, where 10 mL of acid and 5% (w/v) solid powder of SAR were added and refluxed for 4 h. After digestion, the solutions were filtered using Whatman filters with a pore size of 0.2 µm. The filtered solutions were then analysed for metal concentrations using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) with an Optima 8300 system from PerkinElmer, UK. The results are reported as mean ± SEM (n = 3).
2.2. Microorganisms Cultivation and Biological Metabolite Production
The culture of A. thiooxidans and a microbial consortium of sulphur and iron-oxidising bacteria were obtained from the Acidophile Culture Collection at Bangor University in Wales, UK. A. thiooxidans was utilized to produce a Bio-acid lixiviant, while the consortium was employed to create a Bio-ferric lixiviant, as well as a mixture of both Bio-acid and Bio-ferric lixiviants. An inoculum of 2% (v/v) containing at least 1 × 108 cells/mL of the microorganism was added to a solution consisting of 2% (v/v) ABS, 0.1% (v/v) TE, 0.5% (w/v) sulphur, and 45 mM ferrous iron in deionized water [27]. This was done under sterile conditions in 250 mL Erlenmeyer flasks, with a working volume of 100 mL.
The pH of the solution was adjusted to 1.8 using 25% sulphuric acid. For Bio-acid production, the medium was supplemented with 0.5% sterile sulphur powder and inoculated with 2% (v/v) A. thiooxidans. The Bio-ferric production medium was supplemented with 45 mM ferrous iron from a 1 M sterile stock solution and 2% (v/v) of a microbial consortium. For the combined Bio-ferric and Bio-acid production, the medium contained 0.5% (w/v) sulphur powder, 45 mM ferrous iron, and 2% (v/v) of the consortium. The flasks were then placed in an orbital shaking incubator set to 30 °C and 130 rpm. Once the cultures reached the desired pH and/or concentration of ferric iron, the lixiviant was filtered using a Corning bottle-top vacuum filter with a membrane pore size of 0.2 µm and then the spent culture media (without microorganisms) was used for bioleaching experiments.
2.3. Bioleaching Studies
Metals Bioleaching by Different Biological Reagent
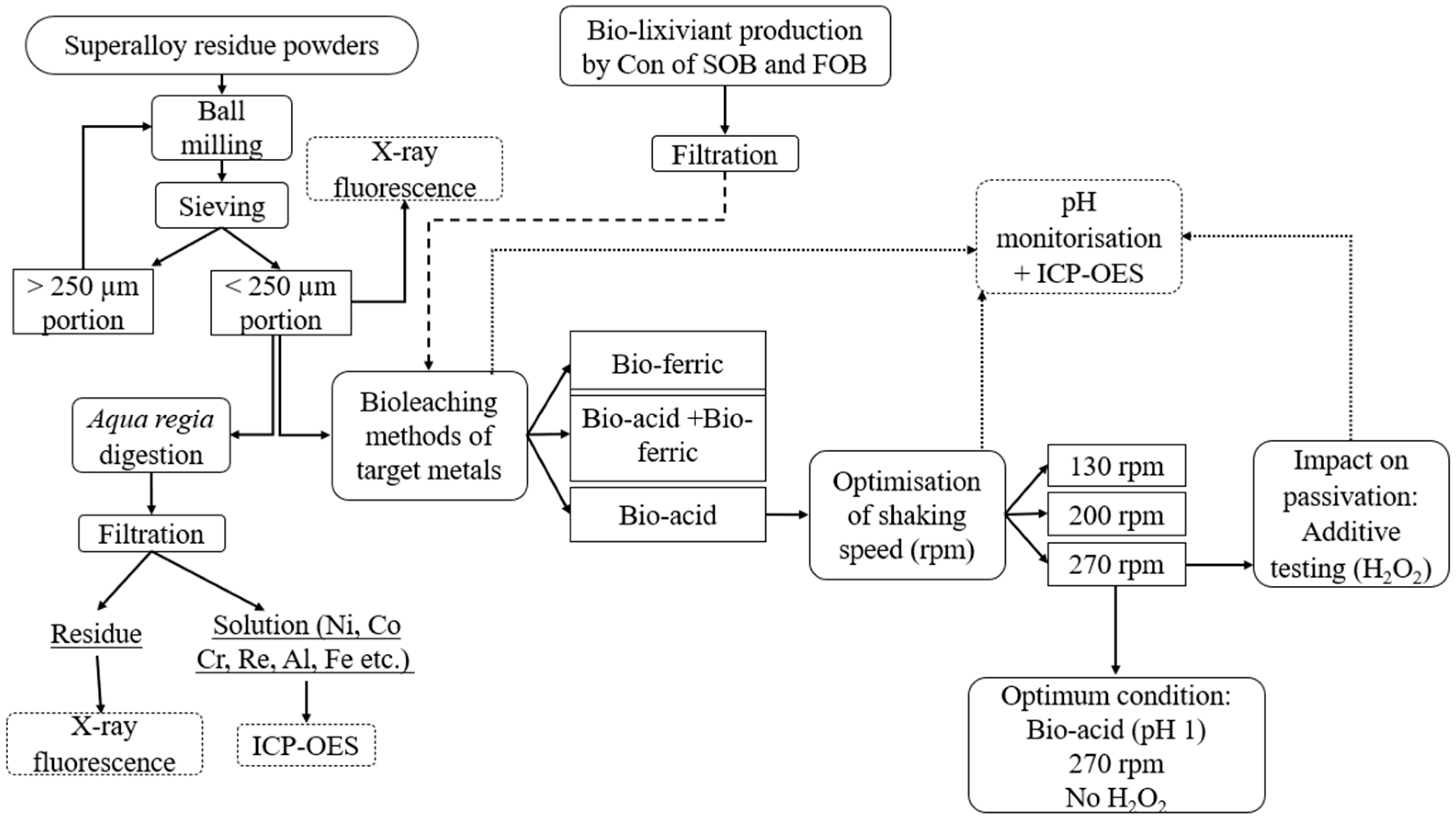
One of the objectives of this study was to assess the impact of bioleaching metabolites on the recovery of Co and Ni from superalloy residue (SAR) powders. Three different spent medium cultures were utilized to bioleach Co and Ni from various SARs, including SAR- Ni 30167, SAR-In 32083, and SAR-Re 30168. A density of 1% (w/v) of the SAR powders was added to 100 mL of Bio-acid leaching (pH 1.0), Bio-ferric leaching (which contained 45 mM ferric ions, pH 1.8), and a combination of both Bio-acid and Bio-ferric leaching (pH 1.0 and 45 mM ferric iron). The flasks were then placed in an orbital shaking incubator at a temperature of 30 °C and 130 rpm for 7 days. Figure 1 presents an overview of the experimental procedure.
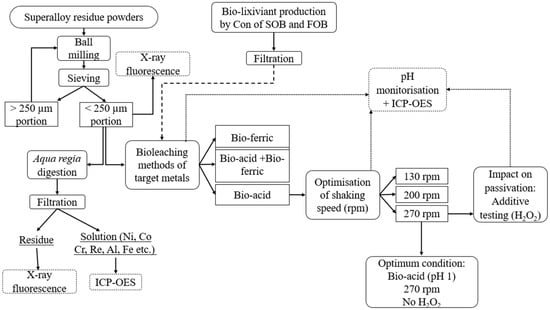
Figure 1.
Schematic diagram of SAR residue preparation and bioleaching process.
To enhance metal recovery from SAR powders via indirect bioleaching with a Bio-acid, the agitation speed and oxidising agent were optimized using a one-factor-at-a-time approach. Optimising agitating speed is crucial to maintaining suspension, enhancing attrition, and preventing pulp deposition, ensuring effective lixiviant diffusion [28]. Also, H2O2 was selected to address passivation issues and improve metal extraction.
The experiments were conducted under sterile conditions in 250 mL Erlenmeyer flasks, containing 100 mL of a chosen biological metabolite (Bio-acid) mixed with 1% (w/v) SAR powders (SAR-Ni 30167, SAR-In 32083, and SAR-Re 30168) at various agitation speeds of 130, 200, and 270 rpm at 30 °C for 7 days.
After optimising the agitation speed, the effects of H2O2 were investigated for metal dissolution enhancement. A density of 1% (w/v) of SAR powders was added to 100 mL of Bio-acid at pH 1, varying the volume of H2O2 (30%) from 1 to 7 mL (100–700 mM). The solutions were shaken at an agitation speed of 270 rpm and maintained at 30 °C for 10 days.
All experiments were performed in triplicates and sampling of bioleachate was performed after compensating for the evaporation that occurred during incubation time by adding sterile deionized water on each measurement day. The metal ion concentrations, pH, ORP, and Fe2+/Fe3+/total iron concentrations were measured over time. Metal dissolution composition was measured by ICP-OES through standard procedures after filtering the samples with a 0.22 µm syringe filter (Sartorius Minisart, Göttingen, Germany). The bioleaching yields were determined based on following Equation (3) [29].
where cf is concentration of element in bioleachate (mg/L), V the volume of the bioleaching solution (L), Cf the metal concentration in the superalloy residue sample (mg/g), and mf is the mass of the superalloy added (g) into the bioleaching system.
2.4. Analytical Methods
The pH and ORP were analysed using a pH (Orion™ ROSS™ Sure-Flow™, Thermo Scientific™, Oxford, UK) and an Eh electrode (Orion™ Sure-Flow™ Combi Redox/ORP, Thermo Scientific™, Oxford, UK) equipped with Ag/AgCl reference electrode, both connected to a multiparameter benchtop meter (Orion™ Versa Star Pro™, Thermo Scientific™, Oxford, UK). Ferrozine assay [30] was used to determine the ferrous iron and total iron concentrations in the solution. The difference between the total and ferrous iron was calculated to estimate the ferric iron concentration for each measurement day.
3. Results and Discussion
3.1. SAR Powder Characterisation
To determine metal concentrations, the materials were analysed using ICP-OES after chemical digestion with aqua regia. The ICP analysis of the resulting solutions (Table 1) indicated that nickel was the most abundant element across all materials, ranging from 612.56 ± 40.33 to 784.64 ± 44.62 g/kg, followed by cobalt, chromium, and aluminium in SAR-Ni 30167 and SAR-Re 30168. In SAR-In 32083, chromium, aluminium, iron, and molybdenum were the predominant metals, while SAR-Re 30168 contained a significant amount of rhenium at 48.50 ± 0.14 g/kg.

Table 1.
Elemental composition of superalloys resulting from aqua regia treatment (g/kg). Data shows mean ± SEM.
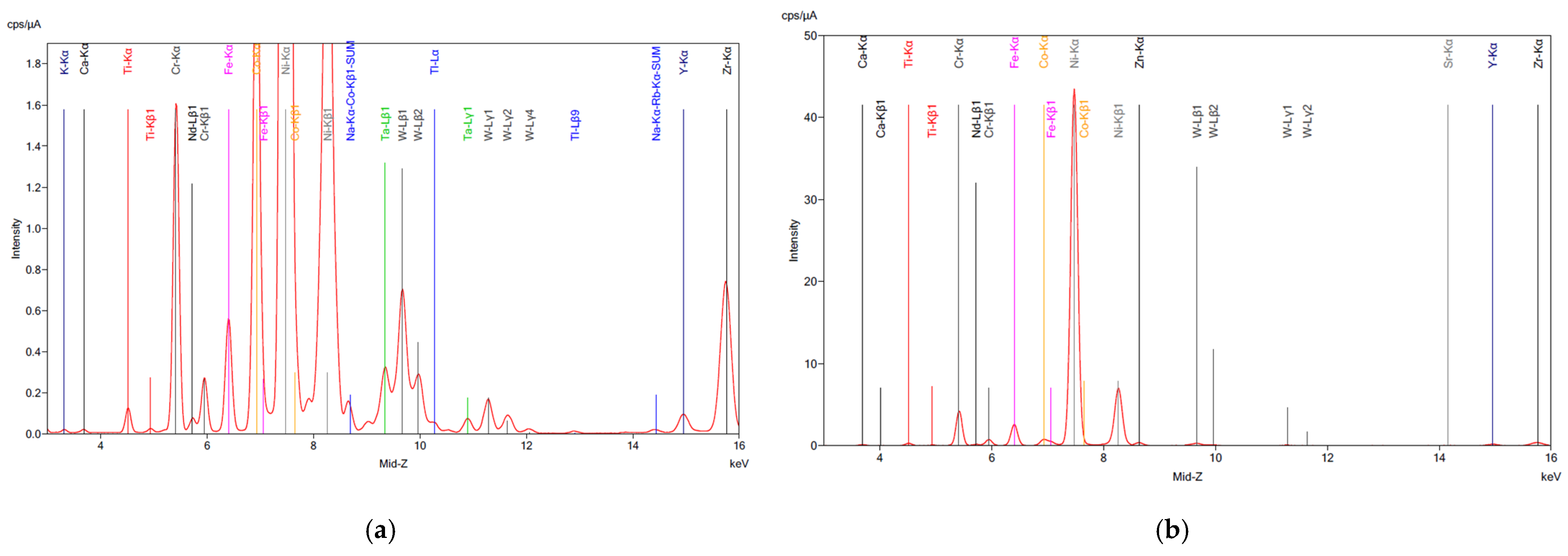
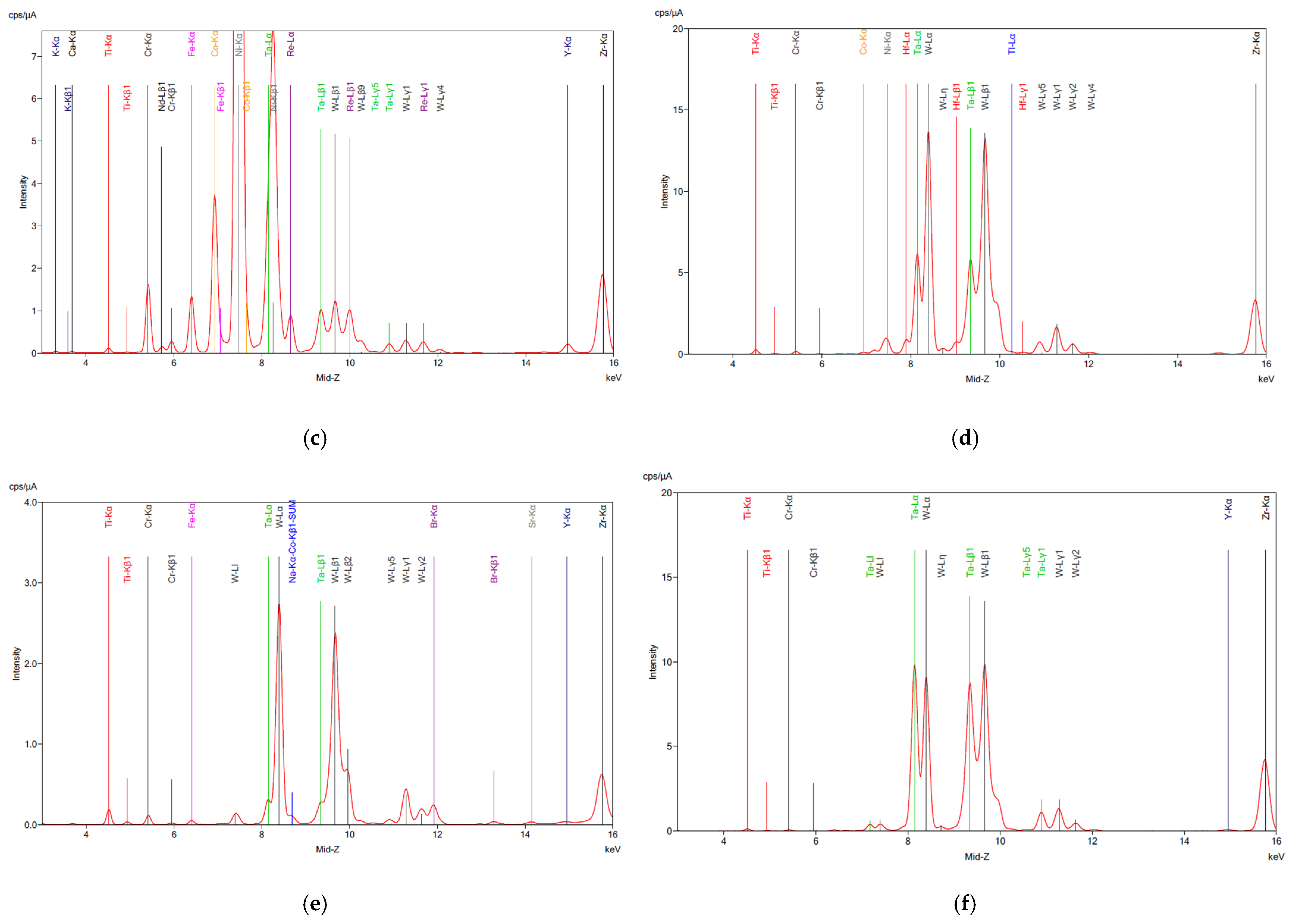
The XRF analysis of the SAR samples before and after digestion is shown in Figure 1, confirming the ICP results for all samples. As anticipated, partial dissolution of the material occurred during digestion, likely due to the corrosion-resistant nature of superalloys. This resistance is attributed to the presence of tungsten (W), tantalum (Ta), and hafnium (Hf), which remained in the residue according to the XRF analysis (Figure 2d–f), along with traces of base metals. The high concentrations of these critical metals highlight the potential of superalloy recycling as a valuable source of high-value elements, particularly given the growing demand for rare earth elements (REEs) in the aerospace and energy industries.
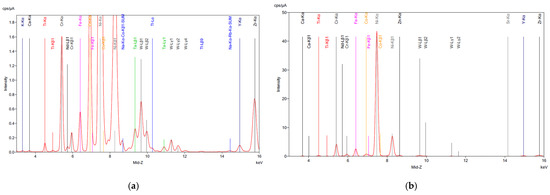
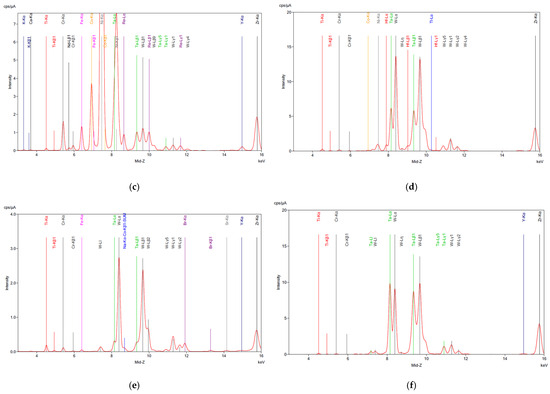
Figure 2.
XRF analysis on superalloy powders (a) SAR-Ni 30167; (b) SAR-In 32083; and (c) SAR-Re 30168 before processing. (d) SAR-Ni 30167; (e) SAR-In 32083; and (f) SAR-Re 30168 residue after aqua regia. The elements detected along with their corresponding symbols and colour coding are presented as follows: Calcium (Ca), Titanium (Ti), Chromium (Cr), Neodymium (Nd), Iron (Fe), Cobalt (Co), Nickel (Ni), Zinc (Zn), Tungsten (W), Strontium (Sr), Yttrium (Y), Zirconium (Zr), Tantalum (Ta), Bromine (Br), Rhenium (Re), Potassium (K), and Na-Kα-Co-Kβ1-SUM.
3.2. Effect of Bioleaching Methods on Ni and Co Recovery
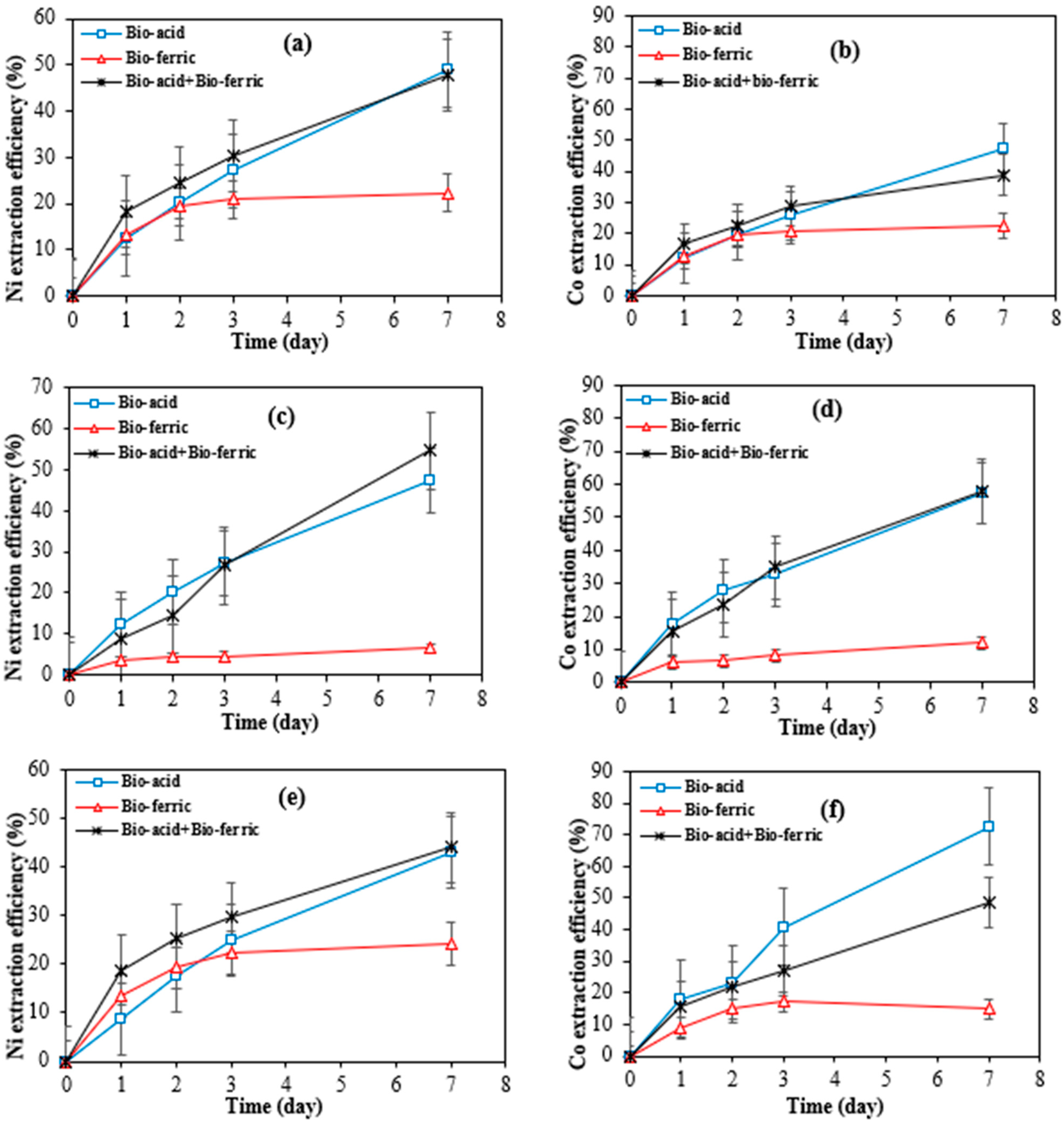
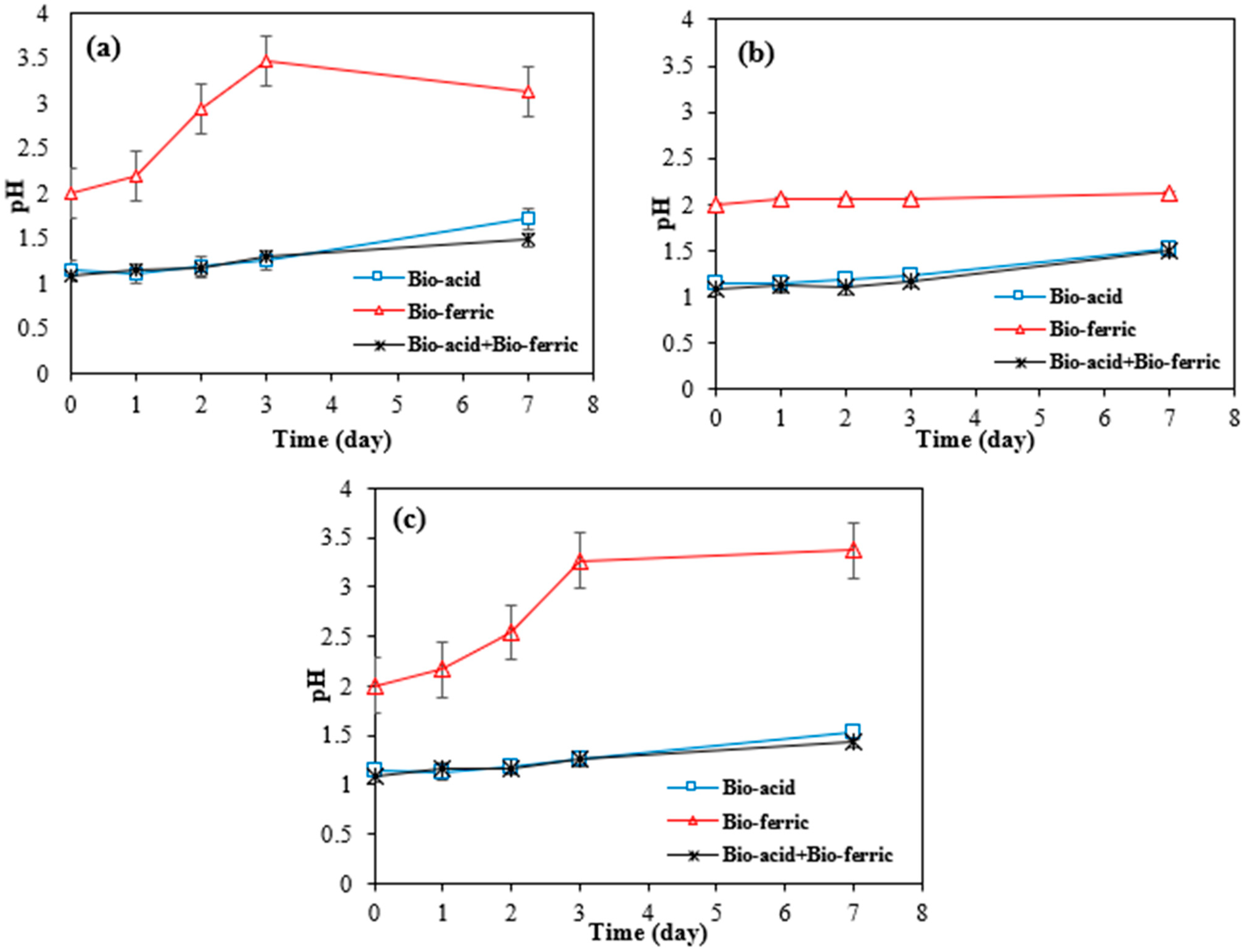
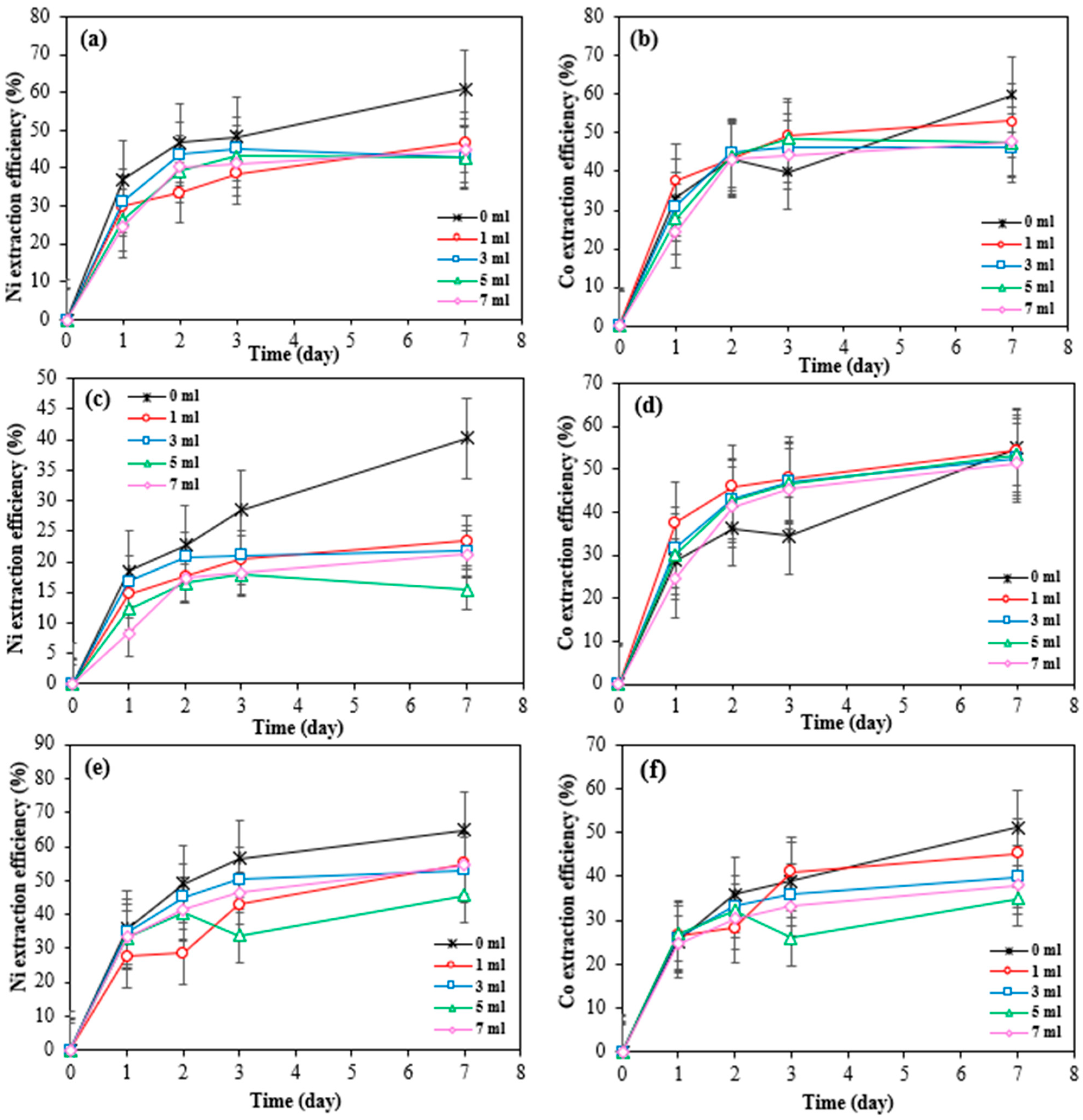
Figure 3a–f shows the cobalt and nickel extraction efficiency over time from SAR samples by three different bioleaching methods including Bio-acid leaching, Bio-ferric leaching, and a combination of Bio-acid and Bio-ferric leaching.
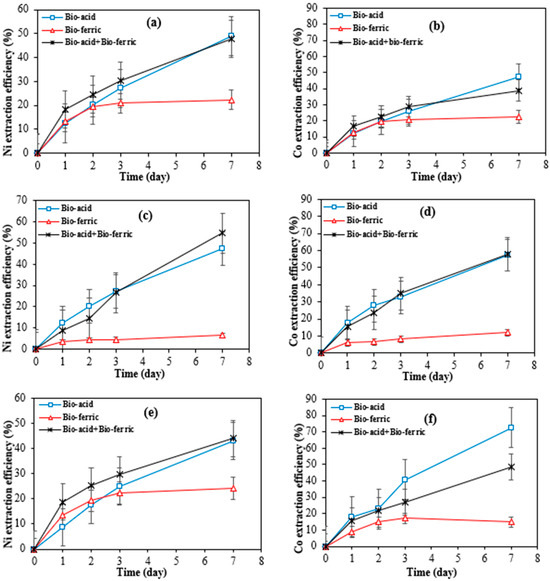
Figure 3.
Extraction efficiency of nickel and cobalt by different bioleaching methods from SAR-Ni 30167 (a,b), SAR-In 32083 (c,d), and SAR-Re 30168 (e,f). Data are shown as mean ± SEM (n = 3).
Equations (4)–(6) illustrate the metal solubilisation processes through acidolysis and redoxolysis. In acidolysis, hydrogen protons interact with the elements (Equation (4)), while the sulphate ion serves as a ligand for the metal cation in the process (Equation (5)) [31]. On the other hand, in redoxolysis, ferric iron oxidizes the metal, converting it into a cation (Equation (6)) [32].
A high degree of similarity in nickel and cobalt dissolution rates was observed when Bio-acid reagents or the combination of Bio-ferric with Bio-acid reagents were utilised as the bioleaching reagents. In both cases, the nickel extraction reached 50, 45, and 47–54% after 7 days of bioleaching from SAR-Ni 30167, SAR-Re 30168, and SAR-In32083, respectively. Also, for cobalt extraction, 40–50 and 60% efficiency were achieved for SAR-Ni 30167 and SAR-In 32083, respectively. However, for SAR-Re 30168, Bio-acid leaching achieved the highest Co efficiency (~70%).
The high parallel between the dissolution rate of the metals coupled with the pH profiles of the Bio-acid method and the mixture of Bio-acid and Bio-ferric methods, indicates that the proton action in the acidolysis mechanism was the main one responsible for the bioleaching process.
As can be seen in Figure 4a–c, in bioleaching with Bio-ferric leaching, the number of available protons was lower than in bioleaching with other methods, suggesting that the main mechanism for metal dissolution by Bio-ferric leaching was redoxolysis.
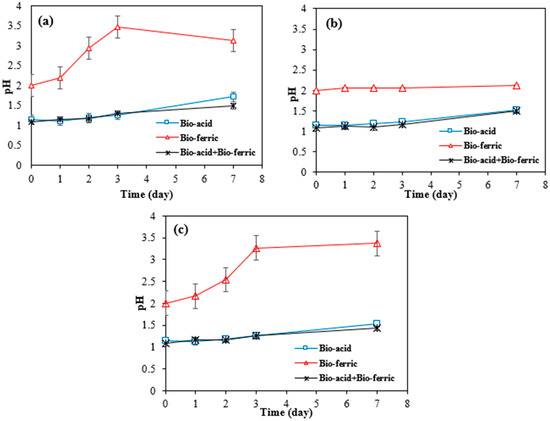
Figure 4.
Variation in pH over time with different bioleaching methods for (a) SAR-Ni 30167; (b) SAR-In 32083; and (c) SAR-Re 30168. Data are shown as mean ± SEM (n = 3).
According to Figure 3, the low efficiency of the Bio-ferric reagent in recovering Ni and Co particularly in the case of SAR-In 32083 can be attributed to differences in the morphology and elemental composition of the SAR samples. As shown in Table 1, SAR-In 32083 has a higher iron content and the lowest concentrations of Co and Ni compared to the other two SAR samples. A high iron content may also lead to precipitation phenomena; these precipitated iron compounds can form a coating or layer on the surface of the SAR materials, leading to a passivation layer that hinders the accessibility or solubility of target metals like Ni and Co. Furthermore, morphological differences such as particle size, surface area, and texture could affect bio-reagent penetration and reactivity with the mineral matrix. SAR-In 32083 was observed to be less responsive and resistant to any treatments when compared to the other two materials, which can be attributed to the difference in chemical composition, as well as a different mixture and ratio of elemental phases. This is confirmed by the partial metal dissolution, coupled with the low proton consumption and partial ferric iron reduction (Figure 4 and Figure 5). In combination, these factors may explain the reduced metal recovery efficiency observed for SAR-In 32083.
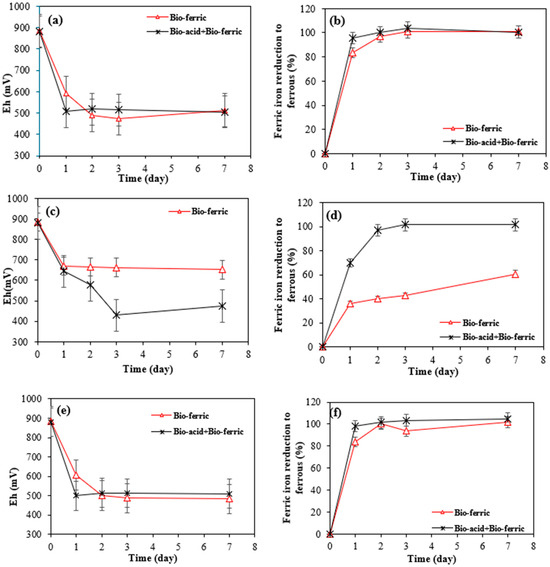
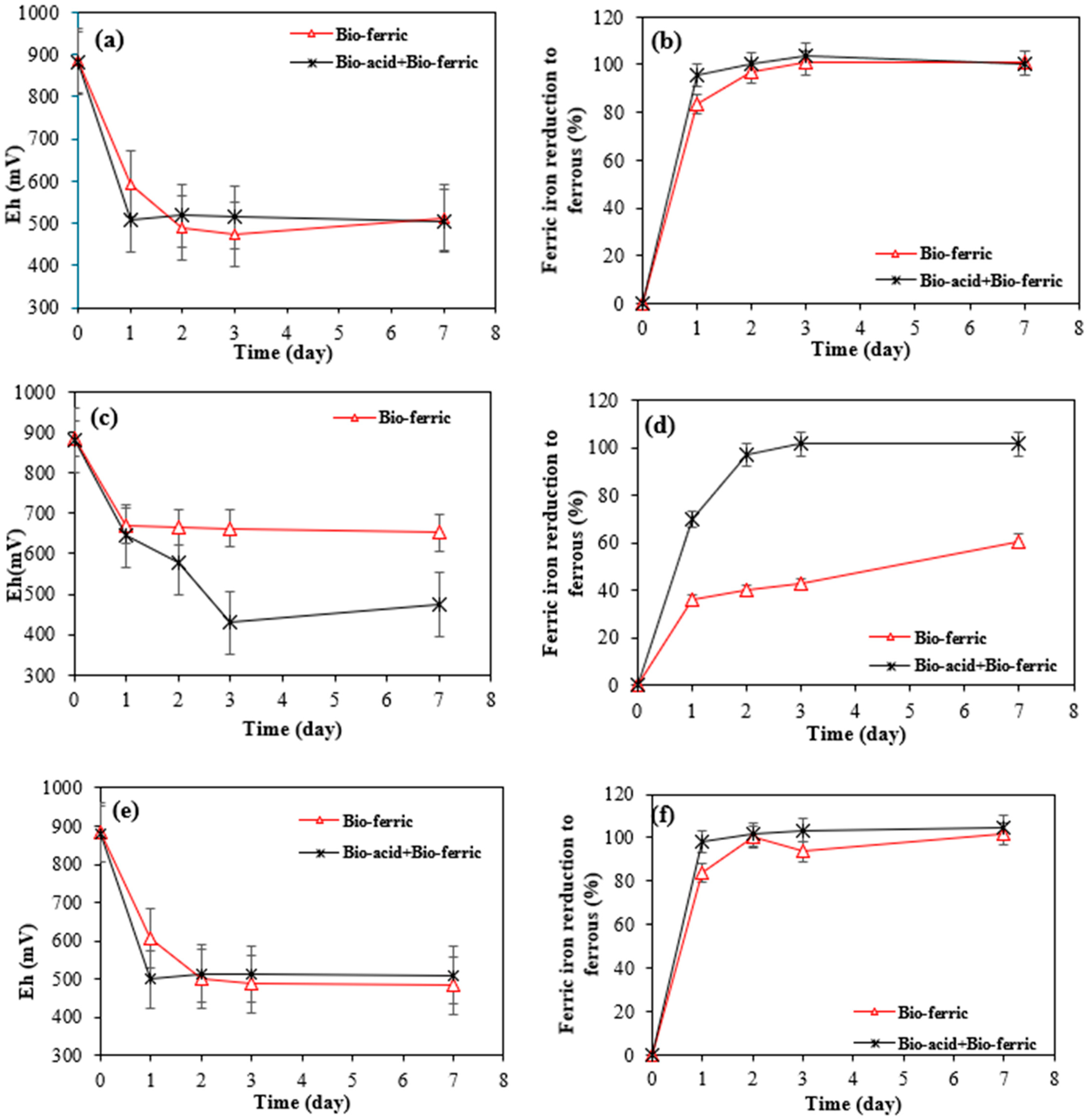
Figure 5.
Variation in (a) Eh and (b) ferric ion reduction over time for SAR-Ni 30167; (c) variation in Eh and (d) ferric ion reduction over time for SAR-In 32083; and variation in (e) Eh and (f) ferric ion reduction over time for SAR-Re 30168.
The sharp increase in pH for the Bio-ferric lixiviant tested can be attributed to a series of reactions that take place in the system for SAR-Ni 30167 (a) and SAR-Re 30168 (c). Firstly, the metals are dissolved by the available protons and the ferric iron. This leads to an increase in pH due to the consumption of protons and reduction of ferric iron to ferrous iron. However, due to the rapid rise in pH after 3 days, many dissolved elements begin to precipitate in the form of hydroxides. This precipitation process accounts for the limited dissolution or the apparent stabilisation of Ni and Co after 3 days. However, in the Bio-acid + Bio-ferric system, there is an excess of protons, which allows the metal ions to remain in the solution as sulphates without triggering the cycle of oxidative reactions, and the pH remains mostly stable for the entirety of the experiment due to the improper interaction between the active surface area and the lixiviant.
Figure 5a–f shows a significant decrease in the ORP (approx. 500 mV) and a full reduction of ferric iron to ferrous iron was observed after only 2 days of bioleaching with Bio-acid + Bio-ferric and Bio-ferric leaching alone for all three SAR samples, which suggests partial metal oxidation through ferric iron action that is done in conjunction with a proton attack mechanism. This theory is confirmed and supported by the matching patterns in the ORP and ferric iron reduction profiles for the combination of Bio-ferric and Bio-acid leaching, and the Bio-ferric leaching coupled with the lower nickel and cobalt extraction rates where ferric iron was the only oxidiser present. Moreover, this mechanism is the most visible in the behaviour of the SAR-In 32083 material in contact with the Bio-ferric-only system where nickel and cobalt recovery were the lowest, but the oxidising agent was only partially reduced (60%) by the end of the experiment (Figure 5d), which suggests the need for a second oxidiser like Bio-sulphuric acid to continue the reaction.
The results potentially suggest that indirect bioleaching methods can be employed for achieving superior recovery rates. However, for an indirect bioleaching approach, it was concluded that acidolysis mechanisms are best suited to extract the metals of interest where the protons attack the exposed superalloy surface and displace the metal through electron transfer and release it as cations, which form sulphate complexes with the available sulphate anions in the solution. Therefore, the series of reactions are interconnected and play a crucial role in dissolving nickel and cobalt from all the samples, along with other factors that establish a suitable oxidising environment like gas dispersion, particle diffusion through agitation, and finally proton and ligand availability.
Indirect bioleaching using the Bio-acid method was selected as the most suitable biological agent for extracting Ni and Co from SAR for the following reasons: (1) From an industrial implementation perspective, Bio-acid-based bioleaching is more practical, as it delivers similar extraction performance to the combined Bio-ferric and Bio-acid method without requiring additional reagents, such as ferrous iron sulphate, for Bio-ferric production. (2) The use of ferric ions in the bioleaching process introduces operational challenges, including the risk of jarosite or other iron precipitate formation, which requires additional monitoring and control. (3) The high concentrations of iron in the leach solution reduce the efficiency of Ni and Co recovery, as the iron must first be removed before metal extraction can proceed effectively. Therefore, the impact of agitation speed on nickel and cobalt recovery was further studied only in the context of indirect bioleaching by the Bio-acid system.
3.3. Effect of Shaking Speed
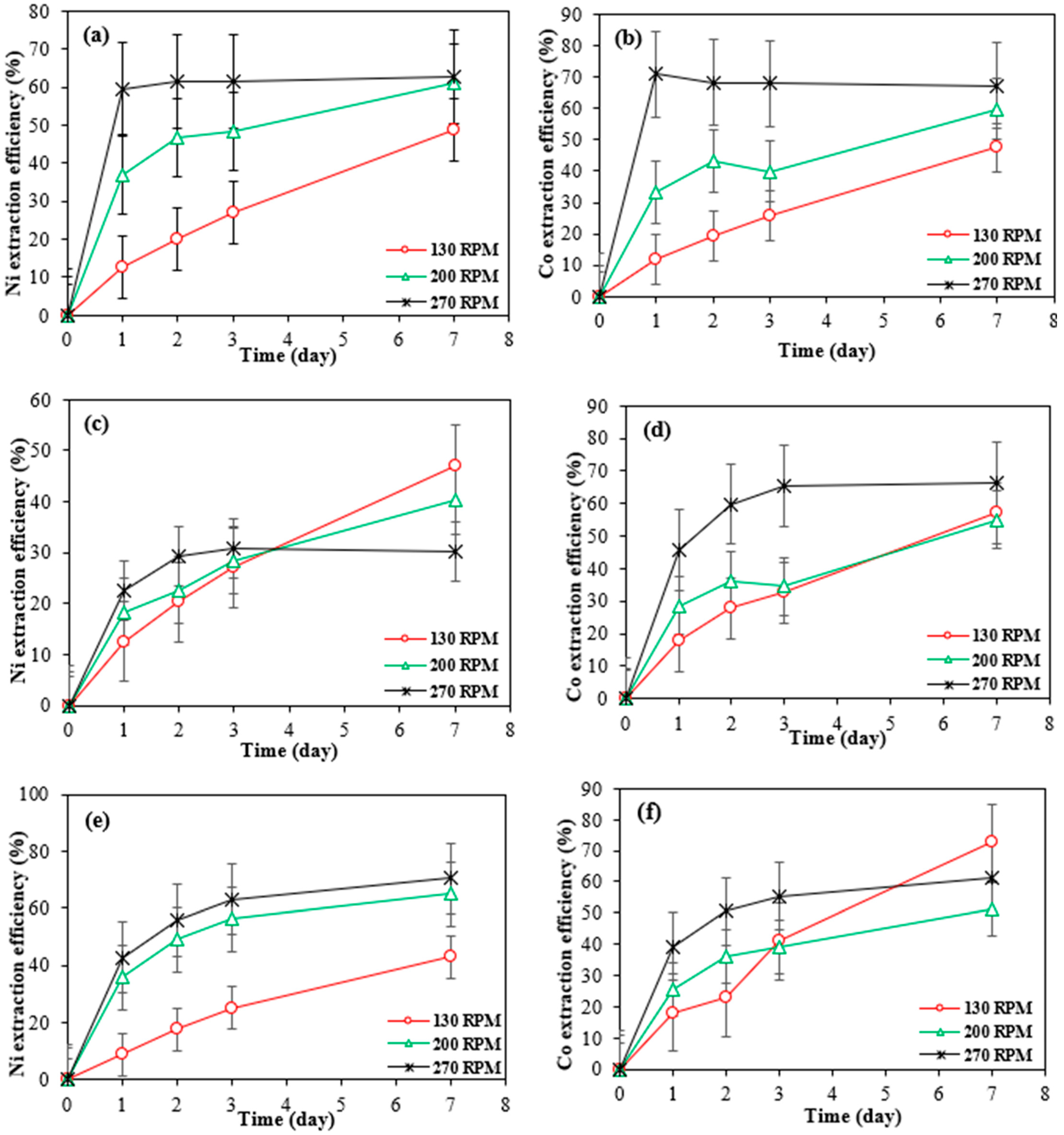
Figure 6 illustrates the impact of agitation speed on the extraction of cobalt and nickel from three different SAR powders, each at a concentration of 1% (w/v), using Bio-acid leaching at speeds of 130, 200, and 270 rpm.
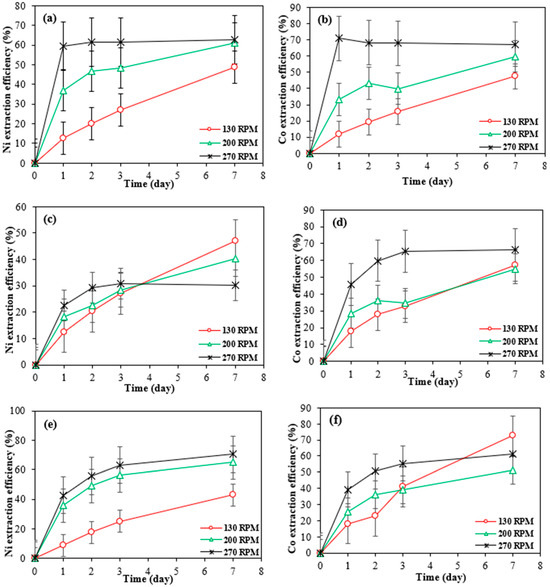
Figure 6.
Extraction efficiency of nickel and cobalt at different agitating speeds by Bio-acid from 10 g/L of Ni 30167 (a,b), In 32083 (c,d), and Re 30168 (e,f). Data are shown as mean ± SEM (n = 3).
The results show that increasing the shaking speed from 130 rpm to 270 rpm significantly improved the bioleaching rates of both nickel and cobalt from the SAR-Ni 30167 and SAR-Re 30168 samples. For the SAR-Ni 30167 sample, the highest dissolution rates for nickel and cobalt were achieved within one day, reaching 60% and 70%, respectively. In contrast, the SAR-Re 30168 sample required two days to reach optimal bioleaching, achieving extraction rates of 60% for nickel and 50% for cobalt.
This improvement can be attributed to the enhanced diffusion rates at higher agitation speeds, which facilitated greater surface contact and interactions between the lixiviant and superalloy particles. The increased turbulence likely reduced mass transfer limitations, ensuring more efficient exposure of the active materials and accelerating the leaching process [28]. Additionally, as shown in Figure 6, the improved lixiviant diffusion led to increased acid action, resulting in faster and more effective metal mobilisation, along with higher proton consumption.
Notably, after the 6th day, the extraction efficiency of Co and Ni at the lower mixing speed (130 rpm) was higher than the higher mixing speed 270 rpm. This might because of that at lower mixing speeds, the bio-generated acids in the spent medium are more stable over time. It is likely that higher agitation may enhance the formation of secondary precipitates, especially in the presence of metal ions and sulphate. These precipitates can reduce Co and Ni, concentration in the leachate over time. Also, mixing may cause degradation or dilution effects for Bio-acids or oxidants in the spent medium. This could reduce leaching efficiency in later stages. Lower rpm may allow more prolonged interaction between the spent medium and the solid residue (settled particles), enhancing diffusion-controlled dissolution in the later stages. In contrast, high rpm keeps particles suspended but may reduce effective contact time due to turbulent flow.
Despite this, from an industrial viewpoint, the higher early-stage extraction efficiency at 270 rpm (50% Co in 2 days) (Figure 6f) remains more desirable than the slightly higher yield at 130 rpm after 7 days. The 10% improvement at 130 rpm corresponds to only ~7.45 g/kg Co, which is considered a minor increase in large-scale applications.
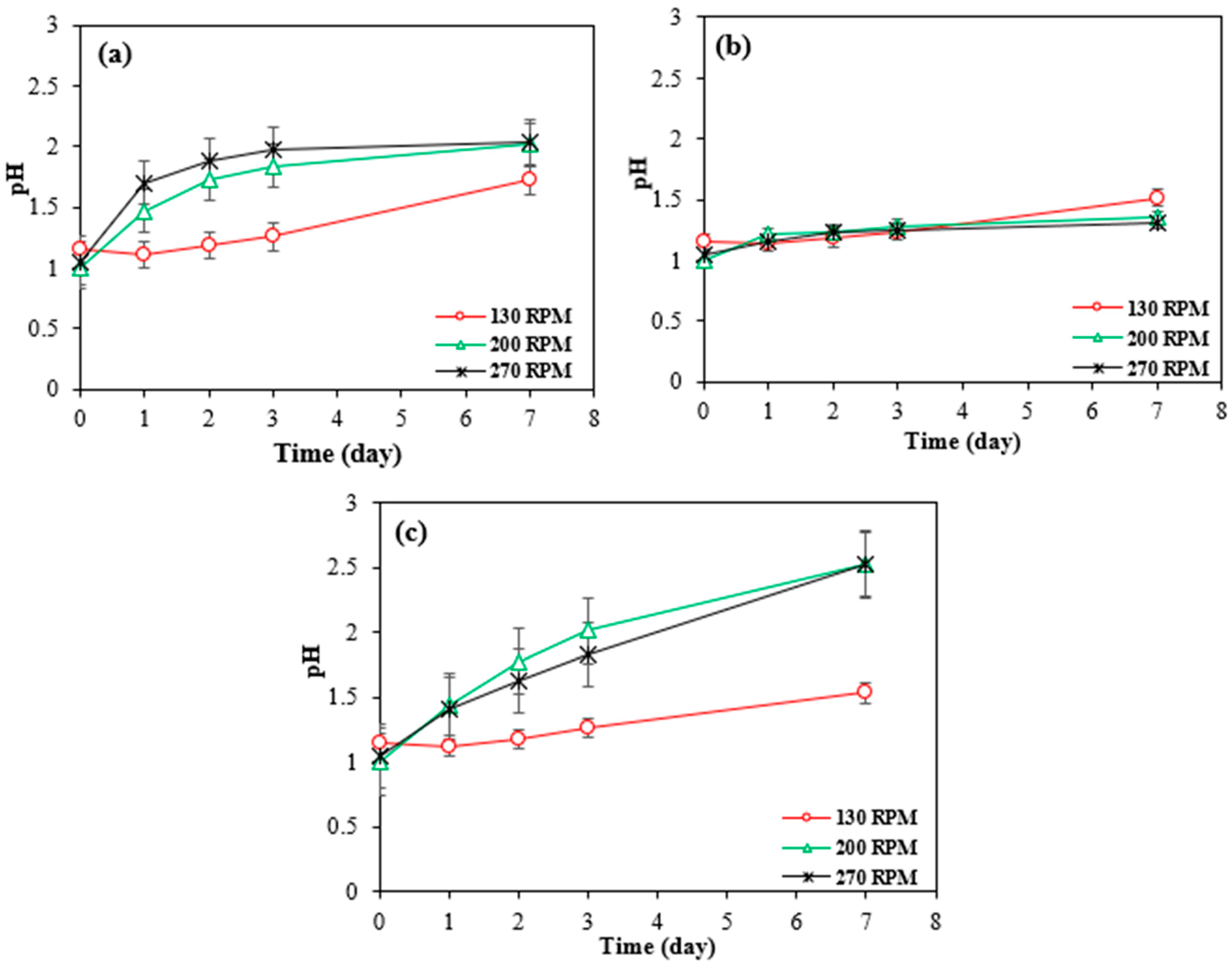
Although according to the Figure 7c, the pH was lower at 130 rpm, indicating a more acidic environment, this did not result in higher metal dissolution. Instead, the extraction efficiencies were higher at 200 and 270 rpm.
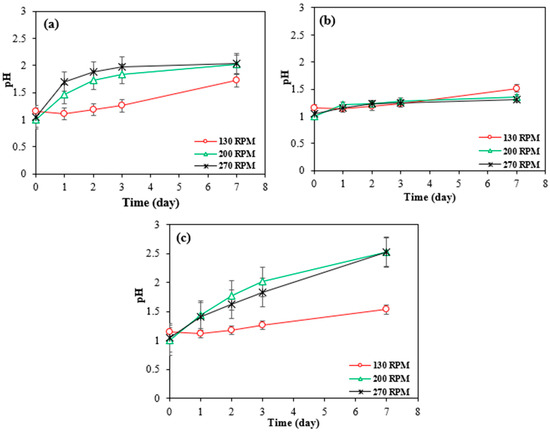
Figure 7.
Changes in pH over time with bioleaching by Bio-acid from 10 g/L of (a) SAR- Ni 30167; (b) SAR- In 32083; and (c) SAR- Re 30168 at different agitation speeds. Data are shown as mean ± SEM (n = 3).
At higher agitation rates (200 and 270 rpm), the mixing enhances particle suspension, lixiviant dispersion, and contact between the leaching agents and the metal-bearing particles. This leads to more effective and rapid leaching in the early stages (2 days). In contrast, at 130 rpm, even though the environment is more acidic (lower pH), the reduced agitation limits particle-lixiviant interactions, resulting in slower metal dissolution in the initial day, as can be seen in Figure 6f, the highest Co extraction efficiency was achieved in 7 days.
Moreover, in all cases, the pH increased and stabilized near ~2 after the first day, indicating a consumption of protons and oxidation of metals. However, due to better initial access of the lixiviant to reactive surface sites at higher rpms, most of the leachable material was extracted early, explaining the higher cumulative extraction despite similar or even higher pH values later on.
Therefore, efficient leaching is not solely dependent on acidity but also on how effectively the lixiviant reaches and reacts with the target particles. Higher rpms facilitate this interaction, leading to greater metal recovery, even when the overall acidity (pH) is slightly less favourable.
For SAR-In 32083, higher agitation speeds did not lead to increased extraction rates for nickel and cobalt. By the end of the experiment, between 40% and 47% of nickel was dissolved at lower shaking speeds. For cobalt, although the extraction rate reached 65% at 270 rpm, the bioleaching process slowed significantly after day 3 and eventually reached equilibrium. It is important to note that the cobalt content in this material is 7 to 10 times lower than in the other two residue powders (Table 1). The maximum difference in cobalt dissolved from this material between the various shaking speeds was only 3 g/kg. Hence, the effect of different agitation speeds on the cobalt dissolution rate was considered insignificant.
This phenomenon can be explained by two potential mechanisms. The metals within the material may exist as a mixture of elemental forms, oxides, and/or hydroxides, as all the superalloys tested have different origins and come from various manufacturing processes, and the target metals as well as Al, and Cr are notoriously known to form oxides or hydroxides as protective layers which provide excellent protection against temperature and corrosion [10,25,33,34]. Nickel and cobalt, when in oxide or hydroxide forms, exhibit greater resistance to corrosion caused by proton attack due to their high stability and more structured network. This characteristic results in lower metal dissolution rates and a stable trend of proton concentration throughout the entire experiment, as illustrated in Figure 7b. Additional energy, time, and chemical processes are required to break the bonds between the metal cations and anions. In contrast, metals in their elemental state are more readily available to undergo oxidation in an acidic environment.
Another factor that may have influenced the dissolution rate of the target metals in this superalloy material is the standard reduction potential, coupled with the higher iron content. SAR-In 32083 contains 26.64 g/kg of iron (Table 1), which is 2.5 to 3 times more than the other two materials. As illustrated in Table 2, iron has a greater tendency to oxidize as ferrous iron, as indicated by its more negative standard potential value in comparison to other metals like nickel and cobalt, which have a lower propensity to lose electrons.

Table 2.
Nickel, cobalt, and iron quantities found in SAR-In 32083 along with corresponding standard electrode potentials and half-reactions.
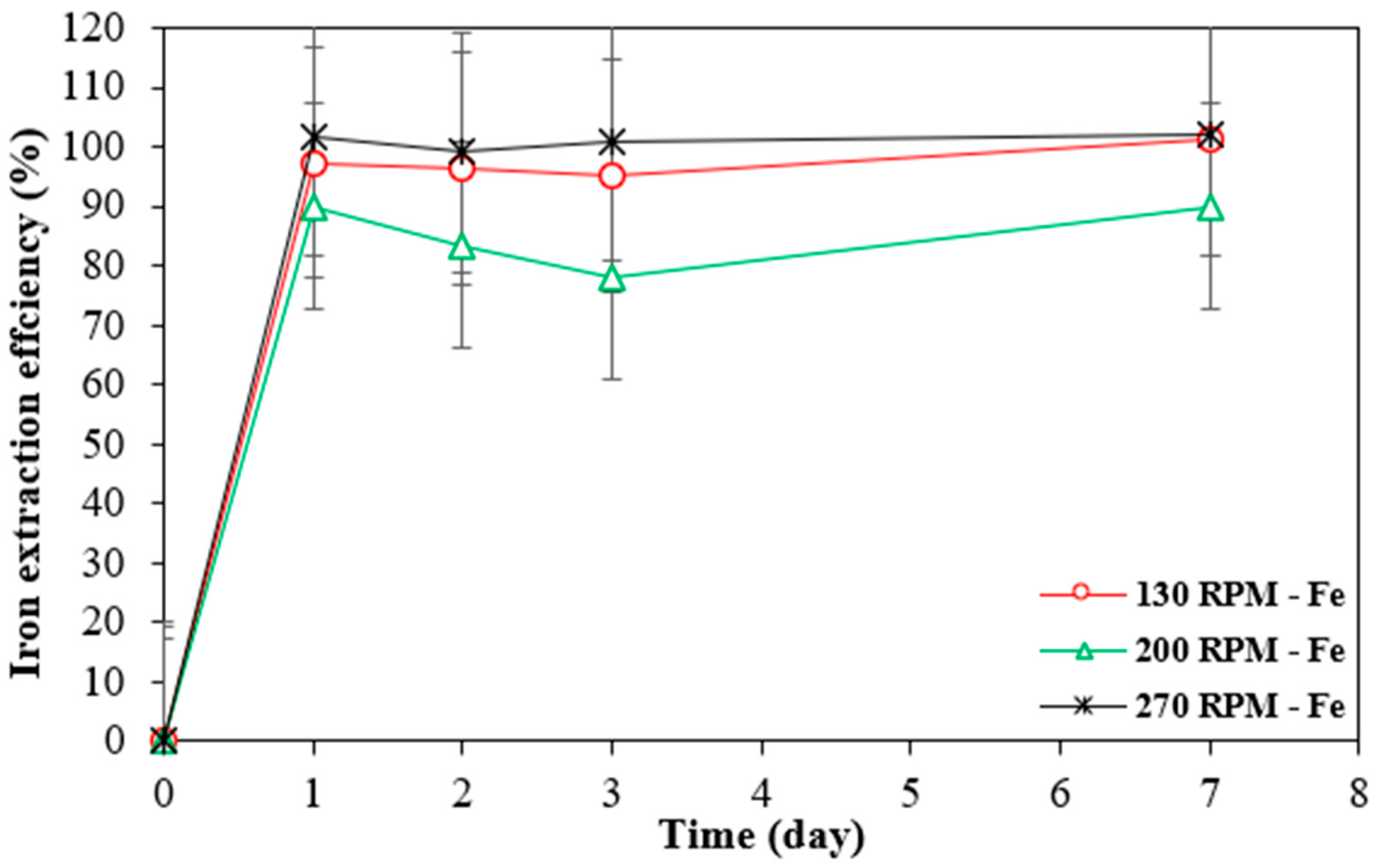
Iron is typically mobilized as ferrous iron in leaching systems under mildly acidic or dilute conditions, where strong oxidising agents and high temperatures, which could facilitate further oxidation to ferric iron, are absent. This theory is supported by the extraction rate trends observed for all three metals, which indicate that iron was preferentially dissolved over the metals of interest, regardless of the shaking speed applied (Figure 8). The same trend was noted with the other two materials, where 100% of the iron was dissolved within a day, independent of the shaking speed.
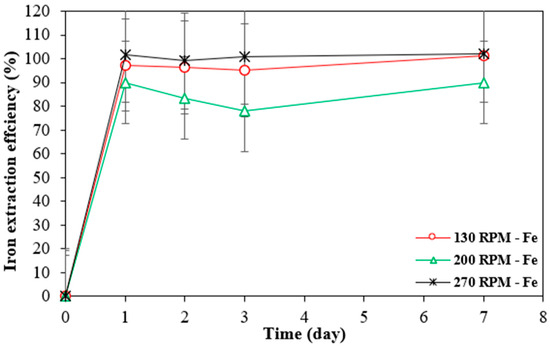
Figure 8.
Extraction rates of iron by Bio-acid from 10 g/L SAR-In 32083 powder at different agitation speeds. Data are shown as mean ± SEM (n = 3).
By analysing the data collectively and considering all possible mechanisms, it can be concluded that a combination of factors contributed to the chemical kinetics occurring in the bioleaching system for the SAR-In 32083 material.
3.4. Effect of H2O2 Addition Concentration
Figure 9 shows the effect of H2O2 concentrations of 1, 3, 5, and 7 mL on the nickel and cobalt extraction efficiency from different SAR powders by the Bio-acid bioleaching method at 270 rpm.
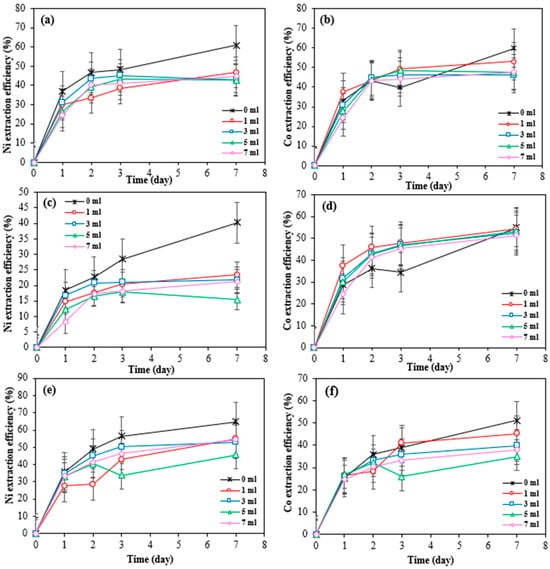
Figure 9.
Extraction efficiency of nickel and cobalt by Bio-acid at 270 rpm in the context of hydrogen peroxide addition at different dosages from 10 g/L SAR-Ni 30167 (a,b), SAR-In 32083 (c,d), and SAR-Re 30168 (e,f). Data are shown as mean ± SEM (n = 3).
As previously mentioned, metals like Al, Cr, Ni, Co and others form a passivation oxide layer on the superalloy surface, which provides a high level of protection against corrosion and heat; therefore, the leaching efficiency of target metals is hindered by the presence of this layer.
Chiang et al., 2006 [35] demonstrated that increasing the aluminium content in Fe–Al alloys enhances passivation in sulphuric acid, forming stable oxide layers that inhibit corrosion. In the context of metal leaching from superalloy residues, such aluminium-induced passivation can reduce the leaching efficiency by creating protective barriers that limit access of the acid to valuable metals.
Park et al., 2012 [36] explored how varying chromium (Cr) contents influence the corrosion resistance of low alloy steels in sulphuric acid environments. The study finds that increasing the Cr content enhances corrosion resistance by promoting the formation of dense, protective oxide layers, such as Cr2O3, which act as barriers against further acid attacks.
This research is pertinent to the leaching of metals from superalloy residues using sulphuric acid, as many superalloys contain chromium. During leaching, the formation of Cr-rich passive films can impede the dissolution of valuable metals, thereby reducing the leaching efficiency. Understanding the passivation behaviour induced by chromium helps in developing strategies to mitigate these effects, such as pre-treatment processes or additive use, to improve metal recovery from superalloy residues. The present work investigated the possibility of penetrating and breaking down the passivation layer using hydrogen peroxide as demonstrated in previous studies [10,24,25].
The addition of hydrogen peroxide at any concentration did not improve the nickel extraction efficiency in the bioleaching system for any of the superalloy residues. This can be attributed to the limited concentrations of the additive tested and its interaction with the material’s elemental complexity. In a leaching environment where H2O2 is present, metals like iron and aluminium are more readily oxidised due to their more negative redox potentials compared to nickel. This results in the dissolution of these metals over nickel, reducing the available active lixiviant to interact with and dissolve Ni.
Additionally, according to Nicol (2020) [23], the presence of certain cations (e.g., iron) in the aqueous solution act as catalysts for triggering the rapid dismutation of peroxide due to the intense oxidative reaction between peroxide and iron based on the 1 V difference in the standard electrode potential. Moreover, ferrous iron can also be further oxidised by peroxide to ferric iron, which, at higher pHs (above 1), is interacting with the remaining hydrogen peroxide molecules in a Fenton-like reaction generating the Fe(III)-hydroperoxy complex, which is formulated as Fe(III)(HO2)2+ [37]. This reaction not only depletes H2O2 but also reduces its effectiveness as an oxidising agent for nickel, which consequently inhibits any significant extraction of nickel. Therefore, the combined effects of competition for the lixiviant and the rapid breakdown of H2O2 due to the presence of more reactive metals, particularly iron, obscured any beneficial effects of hydrogen peroxide on nickel extraction.
This theory is confirmed by the registered high iron mobilisation and the difference in aluminium dissolution for all three materials. Between 50 and 100% of iron was dissolved within one hour of bioleaching and an increase of ~10–17% for aluminium dissolution was observed when hydrogen peroxide was added in any concentration within the first 3 days of bioleaching.
For cobalt extraction from SAR-Re 30168, the addition of H2O2 at any concentration did not enhance bio-dissolution. However, for SAR-Ni 30167 and SAR-In 32083, hydrogen peroxide improved the extraction efficiency by 14–16% after three days of bioleaching.
Figure 9b shows that the addition of H2O2 led to a measurable increase in cobalt extraction from SAR-Ni 30167, with a 10% improvement observed when 1 mL of H2O2 was added. However, the absolute gain in Co recovery (9 g/kg of processed material) was considered industrially insignificant.
However, the effectiveness of H2O2 was not consistently observed across all materials, possibly due to differences in mineralogy and metal speciation among the SAR residues. Varying Fe2+ contents in SAR samples strongly influences the rate of H2O2 decomposition, particularly when coupled with differences in particle morphology or surface oxidation layers that impact lixiviant access. These are key factors that impacted the effectiveness of the additive tested.
SAR-Ni 30167 contains the lowest concentration of iron (8 g/kg) in comparison with other two SAR samples: SAR-In 32083 (26.4 g/Kg) and SAR-Re 30,168 (10 g/Kg). Furthermore, the presence of iron (particularly Fe2+) accelerates the decomposition of hydrogen peroxide through Fenton-type reactions, where Fe2+ catalyses the breakdown of H2O2 into hydroxyl radicals (•OH) and oxygen. This reaction depletes the H2O2 concentration and reduces the Co extraction efficiency in samples with higher iron concentrations.
In the case of SAR-In 32083, a similar increase in Co extraction was observed (from 34% to 50% with 2 mL H2O2), yet the actual increase in mass was minimal (1.47 g/kg), highlighting that statistical improvements in recovery may not translate to industrial significance.
The Bio acid-H2O2 system, with its higher oxidative strength, enhances the dissolution of metals such as Ti, Al, and Fe. These metals, which have more negative redox potentials than the target metals, are more readily oxidized and are dissolved in the presence of hydrogen peroxide. This leads to the rapid depletion of these metals in the system during the initial stages of the reaction. As the dissolution of these metals progresses, hydrogen ions (H+) are consumed in the oxidation and dissolution reactions. The consumption of H+ leads to a decrease in the acidity of the leachate, which is crucial for maintaining optimal dissolution conditions for the target metals, such as Ni. With the depletion of H+ ions and the exhaustion of hydrogen peroxide over time, the environment becomes less acidic, which in turn reduces the efficiency of further metal dissolution. This results in the stabilisation of the extraction efficiency as the process reaches equilibrium. In the absence of significant acidity, the dissolution rate of the target metals decreases, leading to stabilized bio-dissolution in the H2SO4-H2O2 system.
In contrast, when no H2O2 is used (0 mL), the extraction efficiency may initially increase as the system relies solely on the sulphuric acid, and the leaching process is more controlled by the acid concentration and the dissolution kinetics of the metals, without the added oxidative influence of hydrogen peroxide.
3.5. Comparison to Hydrometallurgy Approaches and Scale-Up Considerations
Table 3 summarizes the research on leaching Ni from different wastes using hydrometallurgical methods. Previous studies have reported the full dissolution of Ni and Co in up to 5 h from different waste types undergoing sulphuric acid leaching at different concentrations ranging from 1 to 7.5 M with or without the usage of H2O2 at varied temperatures.

Table 3.
Comparison of nickel leaching from wastes in previous studies.
In comparison, the present study demonstrates that high extraction efficiencies can be generated with minimum equipment and without generating by-products that can cause harm to the environment or the workers, while using conditions that are associated with a reduction in energy consumption and costs, making the entire process more energy-efficient and attractive for industrial implementation. By establishing a semi-continuous system with conditions that can complement the consortium/bacterial culture growth, lixiviant production is more efficient, however, great care needs to be taken when choosing the right microorganism based on the nature and concentration of the lixiviant desired, as well as the implemented growth conditions like, for example, choosing only Fe-oxidising bacteria if ferric iron is the desired lixiviant, or if the conditions used involve the usage of higher temperatures, then thermophilic bacteria is a better choice than a mesophilic species.
A bioleaching system is not necessarily cost-saving at the build-up stage in an industrial setting compared to a hydro- or pyrometallurgical system. However, long term, this system requires lower expenditures as the bacteria can be grown at room temperature successfully and the bioleaching process takes place at 30 °C and atmospheric pressure, which are conditions that are not only attractive from an economic point of view, but also are safer for the workers. Concomitantly, any by-products generated (e.g., water) at the end of the process can be reintroduced into the system making the bioleaching process extremely versatile and fully green with no gas emissions or chemical waste generation.
As a comparison, the traditional methods achieve faster dissolution within hours, however the disadvantages posed by these techniques cannot be ignored in the present global context and the slight sluggishness of bioleaching, which, as demonstrated, can achieve promising results within a day, can be overcome by increasing the efficiency of the metal recovery methods post-dissolution to decrease the timeframe between material processing and the final product stage.
4. Conclusions
This work confirms that bioleaching can be successfully used as a sustainable method to extract nickel and cobalt at a low cost and energy expenditure from superalloy residue powders, which are known to be highly corrosion resistant. It was found that among the three different bioleaching methods employed, the acidolysis method resulted in the highest nickel and cobalt extraction from all three samples tested. Due to the high-density nature of the material, different mixing speeds were tested to examine the impact of the lixiviant diffusion rate on metal mobilisation. The highest agitation speed tested (270 rpm) resulted in the highest extraction efficiency for cobalt and nickel within 24 h. Nickel dissolution reached 60% in SAR-Ni 30167 within one day and in SAR-Re 30168 within two days. For cobalt, dissolution rates of 70% and 50% were obtained after one and two days of bioleaching, respectively. The high content of iron in SAR-In 32083 presented a challenge in the bioleaching of the target metals from this material, as it was preferentially and fully dissolved over other elements within one day of bioleaching. This element also hindered the effect of hydrogen peroxide, employed as an additive, in solving the passivation issue to improve the extraction efficiency. Therefore, prior to the bioleaching of the target metals, with or without hydrogen peroxide, strategies for iron dissolution and selective removal should be considered.
Author Contributions
A.D.C.: Writing—original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. S.H.: Funding acquisi-tion, Resources. F.P.: Writing—original draft, Validation, Data curation, Concep-tualization. S.F.: Writing—review & editing, Supervision, Resources, Project admin-istration, Funding acquisition, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed through a collaborative funding arrangement between Ad-vanced Alloy Services Ltd. (UK) and Coventry University (UK).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The first author gratefully acknowledges Roman Haraburda for his invaluable contributions to the experimental work and his overall support in the laboratory. Special thanks also go to Raluca Barza for her expert assistance with material characterisation on the ICP-OES.
Conflicts of Interest
Author Stephen Hall is employed by the company Advanced Alloys Services Ltd. who provided the material. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ni | Nickel |
| Co | Cobalt |
| A. thiooxidans | Acidithiobacillus thiooxidans |
| H2O2 | Hydrogen peroxide |
| SAR | Superalloy residue |
| XRF | X-ray fluorescence |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| SAs | Superalloy powders |
| W | Tungsten |
| Ta | Tantalum |
| Hf | Hafnium |
| REEs | Rare earth elements |
References
- OECD. OECD Economic Outlook; OED Publishing: Paris, France, 2019; Issue 2. [Google Scholar] [CrossRef]
- Liu, T.; Du, X.; Li, S.; Wu, Q.; Guo, Q.; Liu, Z.; Zhao, J.; Liu, F. Carbothermal redox reaction in constructing defective carbon as superior oxygen reduction catalysts. Nanoscale 2022, 14, 14248–14254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Matsuura, H.; Tsukihashi, F. Chapter 4.4—Processes for Recycling. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 1507–1561. [Google Scholar] [CrossRef]
- Grand View Research. Metal Recycling Market Size, Share & Trends Analysis Report by Metal (Steel, Copper, Aluminum), By Sector (Construction, Automotive, Consumer Goods, Industrial Goods), By Region, And Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/metal-recycling-market#:~:text=Metal%20Recycling%20Market%20Size%20&%20Trends,augmenting%20scrap%20generation%20activities%20worldwide (accessed on 1 April 2025).
- Raabe, D. The materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey, Mineral Commodity Summaries. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-cobalt.pdf (accessed on 10 January 2025).
- Hein, G.A.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Shi, A.; Wang, Z.; Shi, C.; Guo, L.; Guo, C.; Guo, Z. Supergravity-Induced Separation of Oxide and Nitride Inclusions from Inconel 718 Superalloy Melt. ISIJ Int. 2020, 60, 205–211. [Google Scholar] [CrossRef]
- Fan, X.; Xing, W.; Dong, H.; Zhao, J.; Wu, Y.; Li, B.; Tong, W.; Wu, X. Factors research on the influence of leaching rate of nickel and cobalt from waste superalloys with sulfuric acid. Int. J. Nonferrous Metall. 2013, 2, 63–67. [Google Scholar] [CrossRef]
- Wang, L.; Lu, S.; Fan, J.; Ma, Y.; Zhang, J.; Wang, S.; Pei, X.; Sun, Y.; Lv, G.; Zhang, T. Recovery of rare metals from superalloy scraps by an ultrasonic leaching method with a two-stage separation process. Separations 2022, 9, 184. [Google Scholar] [CrossRef]
- Golzar-Ahmadi, M.; Bahaloo-Horeh, N.; Pourhossein, F.; Norouzi, F.; Schoenberger, N.; Hintersatz, C.; Chakankar, M.; Holuszko, M.; Kaksonen, A.H. Pathway to industrial application of heterotrophic organisms in critical metals recycling from e-waste. Biotechnol. Adv. 2024, 77, 108438. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.E.; Ray, D.A.; De Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-loop recycling of copper from waste printed circuit boards using bioleaching and electrowinning processes. Waste Biomass Valorization 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Constantin, A.; Pourhossein, F.; Ray, D.; Farnaud, S. Investigating the acidophilic microbial community’s adaptation for enhancement indium bioleaching from high pulp density shredded discarded LCD panels. J. Environ. Manag. 2024, 365, 121521. [Google Scholar] [CrossRef]
- Panda, S.; Dembele, S.; Mishra, S.; Akcil, A.; Agcasulu, I.; Hazrati, E.; Tuncuk, A.; Malavasi, P.; Gaydardzhiev, S. Small-scale and scale-up bioleaching of Li, Co, Ni and Mn from spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2024, 99, 1069–1082. [Google Scholar] [CrossRef]
- Asmatulu, R. Chapter 14—Nanocoatings for corrosion protection of aerospace alloys. In Corrosion Protection and Control Using Nanomaterials; Woodhead Publishing: Sawston, UK, 2012; pp. 357–374. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Wu, X.; Lin, X.; Yao, T.; Peng, L. Multi-alloying effect of Ti, Mn, Cr, Zr, Er on the cast Al-Zn-Mg-Cu alloys. Mater. Charact. 2023, 201, 112984. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Etteieb, S.; Zolfaghari, M.; Fathollahzadeh, H.; Calugaru, L.; Komtchou, S.P.; Tanabene, R.; Brar, S.K. Integrated bioleaching-electrometallurgy for copper recovery-A critical review. J. Clean. Prod. 2021, 291, 125257. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H.; Xin, B. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 114927. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Köelbl, D.; Memic, A.; Schnideritsch, H.; Wohlmuth, D.; Klösch, G.; Albu, M.; Giester, G.; Bujdoš, M.; Milojevic, T. Thermoacidophilic bioleaching of industrial metallic steel waste product. Front. Microbiol. 2022, 13, 864411. [Google Scholar] [CrossRef]
- Peng, X.; Shi, L.; Qu, T.; Yang, Z.; Lin, L.; Xie, G.; Xu, B. Kinetics of Ni and Co Recovery via Oxygen-Enriched Pressure Leaching from Waste Lithium-Ion Batteries. Separations 2023, 10, 64. [Google Scholar] [CrossRef]
- Nicol, M.J. The role and use of hydrogen peroxide as an oxidant in the leaching of minerals. 1. acid solutions. Hydrometallurgy 2020, 193, 105328. [Google Scholar] [CrossRef]
- Tran, T.T.; Moon, H.S.; Lee, M.S. Separation of Cobalt, Nickel, and Copper from Synthetic Metallic Alloy by Selective Dissolution with Acid Solutions Containing Oxidizing Agent. Miner. Process. Extr. Metall. Rev. 2022, 43, 313–325. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Mao, X.; Chang, X.; Zeng, H.; Qin, W. Efficient extraction of nickel from sintered alloy by stepwise leaching: Thermodynamic and kinetic studies. Miner. Eng. 2022, 187, 107776. [Google Scholar] [CrossRef]
- Wanta, K.C.; Natapraja, E.Y.; Susanti, R.F.; Gemilar, G.P.; Astuti, W.; Petrus, H.T.B.M. Increasing of metal recovery in leaching process of spent catalyst at low temperature: The addition of hydrogen peroxide and sodium chloride. Metalurgi 2021, 36, 77–86. [Google Scholar] [CrossRef]
- Ňancucheo, I.; Rowe, O.; Hedrich, S.; Johnson, D.B. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol Lett. 2016, 363, fnw083. [Google Scholar] [CrossRef]
- Petersen, J. Modelling of bioleach processes: Connection between science and engineering. Hydrometallurgy 2010, 104, 404–409. [Google Scholar] [CrossRef]
- Beiki, V.; Mousavi, S.M.; Naseri, T. Ecofriendly recovery of copper from spent telecommunication printed circuit boards using an indigenous cyanogenic bacterium. J. Environ. Manag. 2023, 344, 118399. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M.; Beolchini, F. Innovative bio-acid leaching method for high recovery of critical metals from end-of-life light emitting diodes. Resour. Conserv. Recycl. 2022, 182, 106306. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of copper, nickel, and gallium recovery from LED waste by adaptation of Acidithiobacillus ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Zhai, Y.; Chen, Y.; Guo, Y.; Luo, Y.; Lu, H.; Zhao, J.; Long, H.; Li, A. Selective oxidation of nanoscale nickel-based superalloys revealed by multi-dimensional electron tomography. Mater. Charact. 2021, 178, 111219. [Google Scholar] [CrossRef]
- Meng, X.; Lyu, S.; Xie, X.; Tang, C.; Yu, W.; Hou, W.; Wang, C.; Qu, J.; Du, J. Hot Corrosion Behavior and Damage Mechanism on Yield Property of Nickel-Based Superalloy. Materials 2025, 18, 1749. [Google Scholar] [CrossRef]
- Chiang, W.C.; Luu, W.C.; Wu, J.K. Effect of aluminum content on the passivation behavior of Fe–Al alloys in sulfuric acid solution. J. Mater. Sci. 2006, 41, 3041–3044. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.H.; Kim, J.G. Effect of chromium on the corrosion behavior of low alloy steel in sulfuric acid. Met. Mater. Int. 2012, 18, 975–987. [Google Scholar] [CrossRef]
- Gallard, H.; Laat, J.D.; Legube, B. Spectrophotometric study of the formation of iron(III)-hydroperoxyexes in homogeneous aqueous solutions. Water Res. 1999, 33, 2929–2936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).