Abstract
This study examines the UVB-induced (Ultraviolet B radiation) degradation of carbon black-filled polyvinyl chloride (CB-PVC) composites. After 500 h of exposure, the material exhibited a 30.13% drop in dielectric strength, a 27.6% increase in surface roughness, and significant pit formation, indicating substantial physicochemical deterioration. Degradation followed a triphasic kinetic pattern: an initial induction phase, an autocatalytic acceleration, and a stabilization phase, driven by radical propagation and photo-oxidation. These findings highlight the complex role of UVB in the photodegradation of cable sheeting.
1. Introduction
Low-voltage aerial branching cables are essential components in modern electrical grids, transmitting power over short distances safely and efficiently. However, their long-term performance is significantly influenced by environmental factors [1], particularly exposure to ultraviolet (UV) radiation.
Ultraviolet radiation is composed of three types based on wavelength: ultraviolet A (UVA, 315–400 nm), ultraviolet B (UVB, 280–315 nm), and ultraviolet C (UVC, 100–280 nm). While UVC is mostly absorbed by the atmosphere, UVA and UVB reach the Earth’s surface and can significantly degrade polymeric insulation materials [2]. This degradation, driven by photo-oxidation and chain scission, leads to embrittlement, cracking, and material failure—potentially compromising cable safety and reliability.
The insulation used in power cables is typically made of polymers such as polyvinyl chloride (PVC), polyethylene (PE), or cross-linked polyethylene (XLPE) [3]. Among these, PVC is widely adopted due to its cost-effectiveness, chemical resistance, and versatility. It can be manufactured as a rigid, durable material or as a flexible compound with lower crystallinity [4,5].
Despite the availability of stabilized formulations, UVB radiation remains a significant degradation factor. This study specifically focuses on carbon black (CB)-reinforced PVC composites (CB-PVC) due to carbon black’s established role as a UV stabilizer. However, limited research has explored the behavior of CB-PVC under prolonged UVB exposure, particularly in the context of electrical cable insulation. Understanding the degradation behavior of CB-PVC is crucial for improving the design of durable outdoor-rated materials.
The rationale for focusing on CB-PVC lies in its dual role as both a conductive and stabilizing filler. While carbon black is known to absorb and scatter UV radiation, thereby reducing penetration depth and photodegradation, its effectiveness in long-term exposure, especially under UVB-specific spectra, requires further investigation.
Recent climatological studies provide context for this research focus. Herman (2009) [6] reported significant increases in UV irradiance at most latitudes due to ozone depletion, particularly in the Southern Hemisphere. Other research indicates that elevated UV levels, especially UVB, will persist in both hemispheres throughout the summer months [7]. Although some models predict that UV exposure may return to pre-1980 levels by the mid-21st century [8], regional case studies show that daily maximum UV Index (UVImax) continues to rise by approximately 3.2% per decade [9]. In regions like Southern Europe, UVB radiant exposure annually ranges from 5408 to 7802 kJ·m−2 [10], levels that are highly relevant for cable degradation analysis. Studies in the Swiss Alps [11] have shown that global erythemal UV radiation increases by approximately 10.7% per 1000 m of altitude, a rise primarily attributed to the increase in UVB radiation rather than UVA, due to the differential atmospheric absorption of these wavelengths.
To simulate these environmental conditions, accelerated weathering chambers are commonly used [12,13], incorporating Xenon arc, fluorescent UV, or metal halide lamps. These sources provide controlled and intensified UV spectra to expedite degradation [14,15,16,17,18,19]. In our study, a fluorescent UVB lamp-based exposure chamber was used.
Although previous studies have reported photodegradation effects in other polymers like cross-linked polyethylene XLPE [20,21,22] or polyvinyl chloride (PVC) composites [23,24,25,26], the degradation kinetics specific to CB-PVC under UVB remain poorly understood. This scarcity highlights the novelty and importance of the present study, which employs a fluorescent UVB lamp to simulate and accelerate polymer degradation under controlled conditions. This paper proposes a triphasic kinetic degradation mechanism based on observed changes in dielectric strength and surface morphology. However, it is acknowledged that additional validation, either through further experimentation or reference to spectroscopic and kinetic modeling studies, is required to fully support this mechanism.
2. Materials and Methods
2.1. Photodegradation of PVC
Polyvinyl chloride (PVC) is highly sensitive to ultraviolet (UV) radiation, which can trigger significant photodegradation upon prolonged exposure [25]. The photodegradation process begins with the absorption of UV light by specific chromophoric sites in the polymer, including allylic chlorine and structural defects. When polyvinyl chloride (PVC) is exposed to ultraviolet (UV) radiation, specific molecular sites, such as structural irregularities or allylic chlorine atoms, absorb the incoming energy. The UV photons, particularly those below 300 nm, possess energy levels exceeding 3.5 eV, which is sufficient to excite electrons and promote bond cleavage. This absorption typically results in homolytic scission of carbon–chlorine (C–Cl) or carbon–carbon (C–C) bonds in the polymer backbone. Although the bond dissociation energies (BDEs) of C–Cl (approximately 3.42–3.63 eV) and C–C (3.58–3.83 eV) bonds in PVC are relatively close, C–Cl bonds are more susceptible to photolytic cleavage under UVB irradiation due to their higher absorption efficiency in the UVB range and the polar nature of the bond. This facilitates n → σ* transitions that selectively promote homolytic scission of C–Cl bonds, leading to the formation of chlorine radicals (Cl•), which are relatively stable and capable of initiating radical chain degradation. Consequently, C–Cl cleavage is considered the primary initiation step in the photooxidative degradation pathway of PVC. The resulting carbon-centered radicals (e.g., •CH2) are not free radicals in the gas phase sense but remain covalently tethered to the polymer backbone, forming in-chain radical sites such as –CH2–CH•–Cl.
The process generates highly reactive radical species, such as carbon-centered radicals (•CH2) and chlorine radicals (Cl•), which act as the primary initiators of degradation.
Once free radicals are formed, they interact with atmospheric oxygen, producing peroxyl radicals, which continue to degrade the PVC through photo-oxidation. The peroxyl radicals abstract hydrogen atoms from adjacent polymer chains, resulting in the formation of hydroperoxides (ROOH) and the regeneration of alkyl radicals (R•) shown in Figure 1. These peroxyl radicals further propagate the degradation process by abstracting hydrogen atoms from neighboring polymer chains. The resulting hydroperoxide and alkyl radical products contribute to the formation of oxidation products such as alcohols, carbonyls, and carboxylic acids, which affect the physical properties of PVC [27].
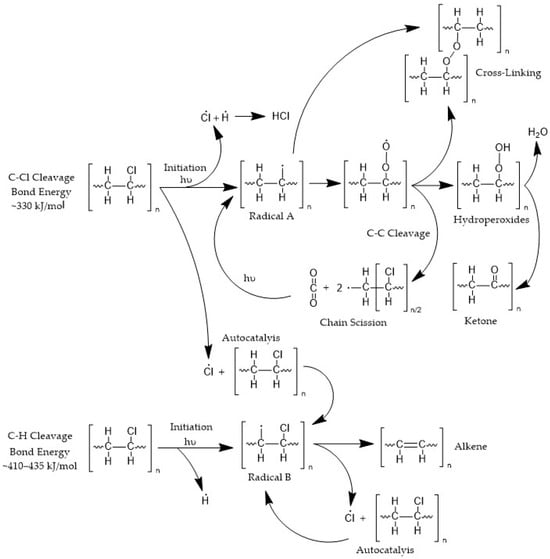
Figure 1.
Photodegradation of PVC.
During UV exposure, the accumulated free radicals cause chain scission (the breaking of polymer chains) and cross-linking (the formation of covalent bonds between chains), both of which disrupt the PVC structure. The balance between these two competing processes results in a loss of flexibility, tensile strength, and impact resistance, leading to embrittlement and cracking of the material [28].
UV-induced dehydrochlorination [29] is a photochemical degradation mechanism in poly (vinyl chloride) (PVC) and related chlorinated polymers, whereby absorption of ultraviolet radiation induces homolytic cleavage of C-Cl. This generates chlorine radicals (Cl•) that abstract adjacent hydrogen atoms via a chain reaction mechanism, yielding hydrogen chloride (HCl) as a gaseous byproduct, while simultaneously reducing molecular weight through chain scission events. This dual degradation pathway significantly compromises mechanical properties, as evidenced by up to 70% reduction in tensile strength upon 500 h UV exposure [30], while the liberated HCl accelerates corrosion of adjacent materials [31].
2.2. Material Details
The PVC tested comes from the sheathing of an Aerial Bundle Stranded aluminum cable with PVC insulation, type TYIR, a Romanian cable classification, indicating a power distribution cable, 3 × 16 mm2 + 25 mm2, provided by Schrack Technik Oradea Branch, manufactured by Electroplast (Bistrița, Romania). The cable is equivalent to NAYY 3 × 16 +25 mm2 in IEC Standard Equivalent, H07V-K 3 × 16 +25 mm2 in European Harmonized (HAR) Designation, and 6491X 3 × 16 +25 mm2 in British Standard. The cable PVC sheathing follows the IEC 60502-1 [32] and the EN 50525 standards [33]. The cable rated voltage is 0.6/1 kV with the maximum operational voltage of 1.2 kV, and the composition of the PVC sheathing given by the manufacturer can be seen in Table 1, and the sizes of the cable are shown in Figure 2.

Table 1.
Composition of the PVC sheathing.

Figure 2.
Cable representation with sizes.
2.3. Equipment Used
The surface was analyzed using a Keyence VK-X3000 3D Laser Scanning Microscope (Mechelen, Belgium), with images captured using the manufacturer’s software (Version 1.5.7), Keyence VK300 Multifile Analyzer (Mechelen, Belgium). For the atomic force microscopy, we used an Agilent Technologies 5500 AFM (Keysight Technologies Inc., Santa Rosa, CA, USA); the images were captured using the software Pico-Image (Version 6.2.7106). For determining the dielectric breakdown test, a setup of an HV AC Test Set (variable transformer + step-up HV transformer, voltage regulator, current limiter, breakdown detection), which starts at 1000 V and gradually increases the voltage by 500 V/s (as per IEC 60243 standard [34]). The tests were performed on multiple samples, as samples are destroyed after performing the test. The test setup for the dielectric breakdown can be seen in Figure 3.
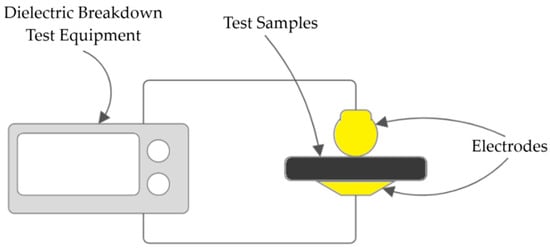
Figure 3.
Setup for the dielectric breakdown.
2.4. UVA—UVB Photodegradation Chamber
The UV aging is carried out using a cubic chamber (30 × 30 × 20 cm), designed and built specially for this study. The chamber is fabricated with steel panels, made from a repurposed IP66 steel electrical box, covered with aluminum foil (reflection coefficient of about 0.9) to avoid any loss of radiation emitted by the UV sources. The samples’ irradiation is accomplished using four low-pressure metal vapor 15-watt fluorescent lamps. The sample at 10 cm receives 5.484 mW/cm2 of UVA and 1.1 mW/cm2 of UVB, determined by two radiometers a Model 5.0 UVA-Meter and a Model 6.0–UVB Meter by Solar Light (Orlando, FL, USA). The samples-to-lamp distance is 10 cm at a 90-degree angle. Only one side of the samples is subjected to radiation. To minimize location-based bias, sample placement was randomized across replicates.
The total duration of exposure is 500 h. The aging is performed under a temperature of 40 ± 5 °C in a relative (±5%) constant humidity of 50. Prior to the experiments, we measured the total UVA and UVB irradiance across the 280–400 nm spectrum using the radiometers, and we changed the light sources at 250 h, as the total UV irradiance of the lamps had declined by 10%. The chamber schematic is shown in Figure 4. Under clear summer conditions in Eastern Europe, solar UV radiation comprises ~95% UVA (315–400 nm; 4–5 mW/cm2) and ~5% UVB (280–315 nm; 50–150 µW/cm2), with negligible UVC due to atmospheric ozone absorption according to Copernicus Atmosphere Monitoring Service.
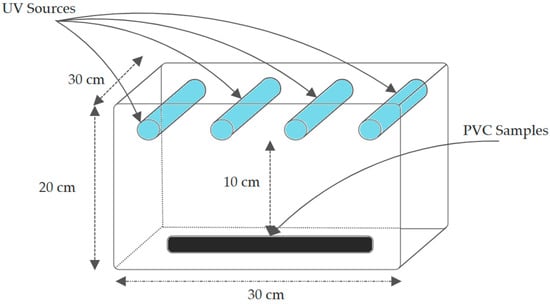
Figure 4.
UVB exposure chamber.
Reference [19] highlights the importance of accelerated aging tests in predicting the long-term behavior of polymers, but also cautions that these tests must be carefully designed to avoid unrealistic degradation scenarios. The UVB Exposure chamber was built similarly to the setup found in [35], which had a 2 mW/cm2 of UVB, or [36], which used a QUV Weatherometer of 1550 µW/cm2.
3. Results
Before applying any UV treatment to the samples, they were wiped with isopropanol to remove dust, mold release agents, or contaminants, and a test sample was examined under the Laser Microscope and AFM, in the middle to avoid edge degradation from dominating results.
3.1. Dielectric Breakdown Test
The dielectric breakdown strength of carbon black-stabilized polyvinyl chloride (CB-PVC) composites is influenced by several parameters, including the carbon black (CB) loading, dispersion uniformity, and environmental aging conditions. In accordance with IEC 60502 [32] and ASTM G154 [37] standards, a dielectric breakdown strength reduction of approximately 10–15% after 500 h of accelerated aging is considered typical for well-stabilized CB-PVC systems. Conversely, in cases where stabilization is insufficient or poorly implemented, degradation levels may reach up to 40% under identical aging conditions, representing a worst-case scenario.
Based on these reference benchmarks, two predictive degradation models—representing optimal (best-case) and suboptimal (worst-case) scenarios—were developed to assess dielectric loss over time. These theoretical models were subsequently compared with the experimentally obtained data, as presented in Table 2.

Table 2.
Dielectric breakdown of the samples.
The dielectric breakdown strength of carbon black-stabilized polyvinyl chloride (CB-PVC) composites exhibited a time-dependent degradation under UVB accelerated aging conditions. Quantitative analysis revealed a progressive decline in dielectric strength from an initial value of 15.6 kV/mm (unaged) to 10.9 kV/mm after 500 h of exposure, corresponding to an overall reduction of approximately 30.13%. The degradation trend was non-linear, with the rate of decline decreasing over time, as indicated by the slope of the breakdown strength loss shown in Figure 5.
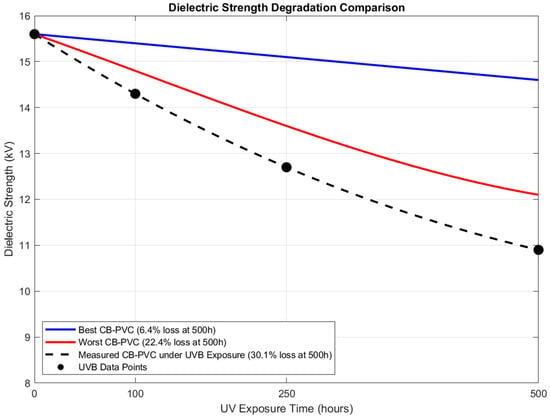
Figure 5.
UV Exposure Demonstration.
3.2. Surface Morphology of PVC Samples
Performing a side-by-side surface analysis of the samples at 0 h and 100 h of UV exposure, shown in Figure 6, we can observe that at no UV exposure the surface appears smoother with large, slightly elevated patches fewer sharp depressions, and with a low count of pitting while at 250 h we can observe high frequency of small-scale peaks and pits, suggesting greater surface irregularity.
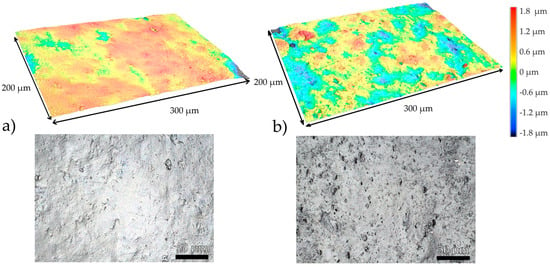
Figure 6.
Laser Confocal Microscopy Side-by-Side Surface analysis of the sample at (a) 0 h of UV exposure and at (b) 100 h of UV exposure with 2D laser scan and 3D representation.
Performing a side-by-side surface analysis of the samples at 250 h and 500 h seen in Figure 7, we can observe that at 250 UV exposure, the surface appears a light gray matrix with scattered dark inclusions, some of which are irregularly shaped. Rough-looking with noticeable surface inhomogeneity, while at 500 h, we can observe a high frequency of small, uniformly distributed dark spots. and pits, and a higher count of small pits, which may suggest a more advanced stage of photodegradation. The pit sizes are shown in Table 3.
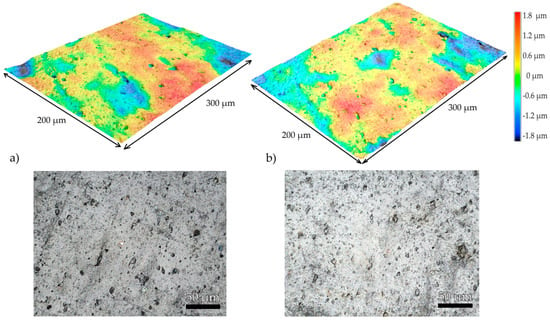
Figure 7.
Laser confocal microscopy side-by-side surface analysis of the sample at (a) 250 h of UV exposure and at (b) 500 h of UV exposure with 2D laser scan and 3D representation.

Table 3.
Average Pit size in the examined samples.
The photodegradation of polyvinyl chloride (PVC) under prolonged UV irradiation exhibits a marked increase in surface pit formation, with average pit dimensions expanding from an initial 0.29 ± 0.11 µm (0 h control) to 0.86 ± 0.33 µm after 500 h of exposure—representing a 197% increase in surface cavity development. This non-linear progression occurs through distinct kinetic phases: an initial induction period (0–250 h, +38% growth) dominated by photo-oxidative chain scission at tertiary carbon sites, followed by an autocatalytic acceleration phase (100–250 h, +46%) where newly formed radicals propagate pit coalescence, ultimately reaching a stabilization threshold (250–500 h, +47%) as the depletion of labile chlorine groups and conjugated polyene formation reduces further photoactivity [31,36].
3.3. Atomic Force Microscopy (AFM) of PVC Samples
AFM provides information about the surface roughness and pore sizes of polymers. Previous reports have shown that non-irradiated PVC films have smooth surfaces that contain a limited number of holes, as seen in Figure 8a. After irradiation, the 2D and 3D AFM images showed that the PVC has gained a significant number of holes and the surface has undergone changes.
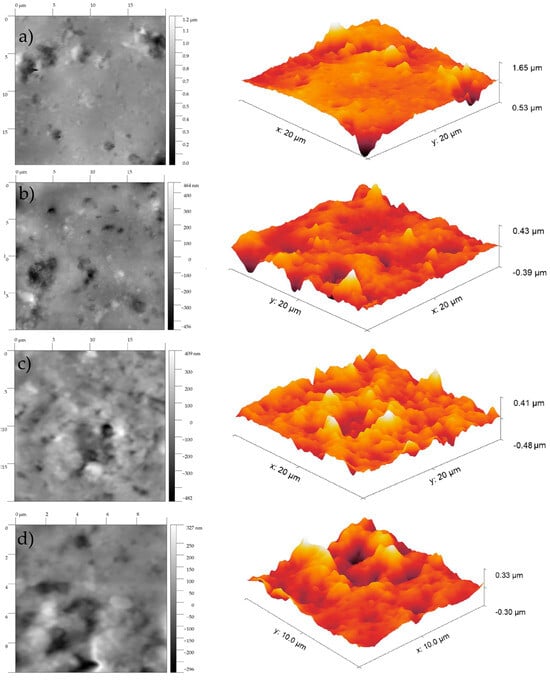
Figure 8.
AFM images of the PVC after (a) 0 h, (b) 100 h, (c) 250 h, and (d) 500 h exposure to UV.
The roughness changes based on exposure time can be seen in Table 4. The roughness factor measures the changes in physical properties, due to either cleavage of the C–C or C–Cl bonds or photo-oxidation process within the polymeric chain.

Table 4.
Surface roughness of the PVC.
Photodegradation of PVC under UV irradiation induces a progressive increase in surface roughness, exhibiting a 27.6% rise after 500 h of exposure (from 89.7 nm to 114.5 nm), with the most significant increase (16.5%) occurring within the first 100 h, indicative of rapid initial chain scission and oxidative surface modification followed by a plateauing effect due to reduced availability of susceptible polymer bonds.
Systematic UV irradiation of PVC surfaces induces progressive morphological degradation characterized by three distinct regimes. Figure 9a shows an initial surface activation phase (250 h) producing localized undulations (±0.15 μm amplitude) through radical-mediated chain scission at allylic chlorine sites, Figure 9b an intermediate cooperative degradation stage (500 h) exhibiting broader roughness features (±0.2 μm) due to micro void coalescence and oxidative pit propagation, and Figure 9c terminal surface erosion (1000 h) with severe peak-trough development (±0.25 μm) resulting from synergistic photo-oxidative weight loss and mechanical weakening, as demonstrated through quantitative profile analysis [38] and consistent with established models of polymer photodegradation kinetics [32,39,40]. This hierarchical damage progression highlights critical exposure thresholds beyond which surface integrity becomes compromised, with direct implications for predicting service life in outdoor applications [41].
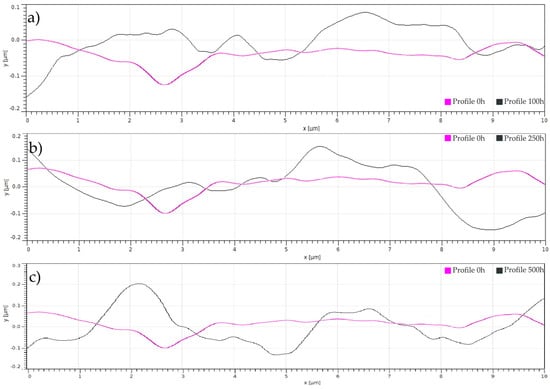
Figure 9.
Profile analysis provided by AFM of the PVC after (a) 100 h, (b) 250 h, and (c) 500 h.
4. Discussion
The present study reveals that carbon black-stabilized polyvinyl chloride (CB-PVC) composites undergo substantial physicochemical degradation under accelerated UVB irradiation, as evidenced by a marked reduction in dielectric strength, significant surface morphological evolution, and increased surface roughness. These findings align with and expand upon the existing literature on the degradation kinetics and mechanisms of PVC under UV-rich environments.
A 30.13% decrease in dielectric breakdown strength, from 15.6 to 10.9 kV/mm after 500 h, suggests progressive deterioration in the insulating capabilities of the CB-PVC matrix. This trend is consistent with prior research on UV-irradiated PVC, where prolonged exposure leads to chain scission and radical formation that disrupt the polymer’s structural and electrical integrity [42,43]. Similar patterns were observed by [25], who found that carbon black-filled polymers exhibited a decline in dielectric performance when subjected to photothermal aging, particularly when filler dispersion was non-uniform. The degradation can be attributed to the accumulation of conjugated double bonds and polyene sequences that create localized charge-trapping sites, exacerbating dielectric failure.
Winslow [44] detailed the photooxidation mechanisms in high polymers, emphasizing a triphasic kinetic model comprising initiation, propagation, and termination phases. Similarly, Wiles and Scott [45] described the photo-oxidative degradation of polypropylene as proceeding through distinct stages: initial radical-induced chain scission, followed by radical diffusion, and culminating in stabilization as reactive species are depleted. In our study, the observed increase in the average pit size from 0.29 ± 0.11 µm to 0.86 ± 0.33 µm reflects advanced surface photodegradation that aligns with this triphasic model. The degradation initiates with photo-oxidative cleavage at tertiary carbon centers, progresses through an autocatalytic phase driven by radical propagation leading to rapid pit expansion, and concludes with a stabilization phase likely due to the depletion of labile chlorine atoms and UV-absorbing polyenes. This progression mirrors the established degradation kinetics in polymers [46,47,48,49] systems undergoing UV-induced oxidative stress.
The photodegradation of CB-PVC composites under UVB exposure follows a distinct triphasic degradation behavior, characterized by three kinetic stages commonly observed in halogenated polymers. In the induction phase (0–100 h), degradation proceeds slowly as initial chain scission and photo-oxidative dehydrochlorination occur, primarily at tertiary carbon sites. This phase involves the formation of hydrogen chloride and polymeric radicals but shows limited macroscopic damage, reflected in a moderate 38% increase in surface pit size. The process then transitions into an autocatalytic acceleration phase (100–250 h), marked by the rapid propagation of radical-induced reactions, leading to significant coalescence of surface pits and a steep reduction in dielectric strength. During this stage, degradation is intensified by the formation of conjugated polyenes and oxygenated species that further destabilize the polymer matrix. Finally, the system enters a stabilization phase (250–500 h), in which the depletion of labile chlorine groups and accumulation of UV-absorbing degradation products (e.g., polyenes and carbonyls) reduce further photo-reactivity. This is evidenced by the relative plateau in pit size growth and roughness evolution, suggesting a self-limiting process as oxidized surface layers form and absorb incoming UVB radiation. These phases are consistent with degradation models reported by [42,43], affirming the universal nature of triphasic photodegradation in PVC and related polymers.
Such pit formation is further supported by literature on UV-weathered microplastics, where pitting and surface erosion were linked to chain oxidation and hydroperoxide formation [25,45,50]. The increased standard deviation in pit size after 500 h (σ = 0.33 µm vs. 0.11 µm at 0 h) reflects spatial heterogeneity in degradation, likely driven by differences in crystallinity and additive migration, factors well-documented in studies of PVC aging. The broadening of the pit size distribution over time suggests that degradation does not occur uniformly across the PVC surface. This heterogeneity in degradation kinetics may arise from localized differences in crystallinity, which affect how UV radiation is absorbed and how radicals propagate according to [38]. The data suggest that carbon black’s effectiveness as a UV stabilizer in PVC is highly dependent not only on its inherent absorption capability, but also on how evenly it is distributed within the matrix. Poor dispersion can paradoxically introduce degradation heterogeneity, even as CB generally delays bulk degradation.
The 27.6% increase in surface roughness after 500 h correlates with oxidative chain scission and the emergence of surface voids. The rapid early-stage increase (+16.5% within 100 h) underscores the susceptibility of surface-exposed chains to initial UV attack, while the subsequent plateau suggests the formation of a passive, oxidized outer layer that limits further degradation. Similar effects have been reported in polymer degradation studies [51,52] where early rapid roughening transitions into a slower kinetic regime due to surface saturation with photo-products that inhibit further UV penetration.
Carbon black, while traditionally added as a UV stabilizer due to its ability to absorb and dissipate UV radiation, appears to play a dual role in this system. While some studies confirm its radical-scavenging ability [53], others highlight that poor dispersion or aggregation can create localized thermal hotspots that accelerate degradation [54]. The present data, particularly the broadening of pit distributions and increased surface roughness, suggest nonuniform protection across the surface, implicating filler-induced heterogeneity as a potential accelerator of photodegradation.
5. Conclusions
This study demonstrates that carbon black-stabilized PVC (CB-PVC) composites undergo significant photodegradation under prolonged UVB exposure, as evidenced by reductions in dielectric breakdown strength, increased surface roughness, and pronounced morphological deterioration. These degradation behaviors are consistent with previously reported UV-aging mechanisms in PVC systems and offer deeper insight into the role of carbon black in modifying degradation kinetics.
- The observed ~30% decline in dielectric strength aligns closely with the findings of [55] who reported 25–35% losses under similar UV stress conditions, emphasizing the susceptibility of electrical performance to environmental exposure.
- Moreover, the triphasic degradation behavior—comprising an induction phase, autocatalytic acceleration, and eventual stabilization—confirms a pattern well-documented by [56] halogenated polymers, indicating a universal sequence of degradation stages driven by radical formation and photo-oxidation.
- Importantly, the magnitude of surface roughening and pit development in CB-PVC exceeds that of unfilled PVC reported in prior studies, such as those by [46], suggesting that the incorporation of carbon black, while traditionally serving as a UV stabilizer, may also introduce heterogeneous degradation pathways through localized thermal effects or radical propagation sites.
Collectively, these findings underscore the complex interplay between filler type, UVB exposure, and degradation mechanisms. They highlight the need for optimized filler dispersion and synergistic stabilizer systems in media where UVB exposure is high. While FTIR spectroscopy was not employed in the present study, its integration in future work would provide valuable complementary insights into chemical bond changes and oxidation products, thereby enhancing the mechanistic interpretation of the photodegradation process. Further research should focus on these aspects.
Author Contributions
Conceptualization, C.-O.S. and A.-D.T.; methodology, C.-O.S.; software, A.-D.T. and T.O.C.; validation, C.-O.S., A.-D.T., T.O.C. and M.-N.A.; formal analysis, C.-O.S.; investigation, A.-D.T. and T.O.C.; resources, M.-N.A. and F.-I.H.; data curation, A.-D.T.; writing—original draft preparation, C.-O.S. and A.-D.T.; writing—review and editing, C.-O.S.; visualization, M.-N.A. and F.-I.H.; supervision, C.-O.S. and L.B.; project administration, M.-N.A. and L.B.; funding acquisition, M.-N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the University of Oradea and the project: Developing solutions and proposing recommendations to prevent premature degradation of electrical equipment and cables during use, to improve long-term durability and reliability. PREDELCAB 08/01.04.2025, Research Fund of the University of Oradea.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Czapp, S.; Szultka, S.; Tomaszewski, A. Design of Power Cable Lines Partially Exposed to Direct Solar Radiation—Special Aspects. Energies 2020, 13, 2650. [Google Scholar] [CrossRef]
- Patrick, S.G. Practical Guide to Polyvinyl Chloride; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-Term Effect of Ultraviolet Irradiation on Poly(vinyl chloride) Films Containing Naproxen Diorganotin(IV) Complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [PubMed]
- Hedir, A.; Moudoud, M. Effect of Ultraviolet Radiations on Medium and High Voltage Cables Insulation Properties. Int. J. Eng. Technol. 2016, 8, 2308–2317. [Google Scholar] [CrossRef]
- Islam, I.; Sultana, S.; Ray, S.K.; Nur, H.P.; Hossain, M.T.; Ajmotgir, W.M. Electrical and Tensile Properties of Carbon Black Reinforced Polyvinyl Chloride Conductive Composites. C 2018, 4, 15. [Google Scholar] [CrossRef]
- Herman, J.R. Global Increase in UV Irradiance During the Past 30 Years (1979–2008) Estimated from Satellite Data. Geophys. Res. Lett. 2009, 36, L05805. [Google Scholar] [CrossRef]
- Yamamoto, A.L.C.; Corrêa, M.P.; Torres, R.R.; Martins, F.B.; Godin-Beekmann, S. Projected Changes in Ultraviolet Index and UV Doses Over the Twenty-First Century: Impacts of Ozone and Aerosols from CMIP6. Photochem. Photobiol. Sci. 2024, 23, 1234–1245. [Google Scholar] [CrossRef]
- Bais, A.F.; Tourpali, K.; Kazantzidis, A.; Akiyoshi, H.; Bekki, S.; Braesicke, P.; Chipperfield, M.P.; Dameris, M.; Eyring, V.; Garny, H.; et al. Projections of UV Radiation Changes in the 21st Century: Impact of Ozone Recovery and Cloud Effects. Atmos. Chem. Phys. 2011, 11, 7533–7545. [Google Scholar] [CrossRef]
- Lorenz, S.; Heinzl, F.; Bauer, S.; Janßen, M.; De Bock, V.; Mangold, A.; Scholz-Kreisel, P.; Weiskopf, D. Increasing Solar UV Radiation in Dortmund, Germany: Data and Trend Analyses and Comparison to Uccle, Belgium. Photochem. Photobiol. Sci. 2024, 23, 2173–2199. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, J.A.; Utrillas, M.P.; Núñez, J.A.; Esteve, A.R.; Gómez-Amo, J.L.; Estellés, V.; Pedrós, R. Measurement and Analysis of Broadband UVB Solar Radiation in Spain. Photochem. Photobiol. 2012, 88, 1489–1496. [Google Scholar] [CrossRef]
- Schmucki, D.A.; Philipona, R. UV Radiation in the Alps: The Altitude Effect. Atmosphere 2002, 13, 15. [Google Scholar] [CrossRef]
- Waqas, H.; Naz, S.; Khan, T.; Ahmed, M.; Khan, F. Case Study of PVC Cables Exposed in Accelerated Thermal and Radiation Environment. Defect Diffus. Forum 2022, 418, 161–168. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C.; Antonie, S.R.; Chiriac, M.; Precup, M.; Yang, J.; Roy, C. Study of the Natural Ageing of PVC Insulation for Electrical Cables. Polym. Eng. Sci. 2000, 67, 209–221. [Google Scholar] [CrossRef]
- Hoseini, M.; Stead, J.; Bond, T. Ranking the Accelerated Weathering of Plastic Polymers. Environ. Sci. Process. Impacts 2023, 25, 2081–2091. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, S.H.; Hu, X.; Torikai, A. Effects of Increased Solar Ultraviolet Radiation on Materials. J. Photochem. Photobiol. B Biol. 1998, 46, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, O.; Avadhani, C.V.; Singh, R. Effect of UV Rays on Degradation and Stability of High Performance Polymer Membranes. Adv. Mater. Lett. 2014, 5, 272–279. [Google Scholar] [CrossRef]
- Briassoulis, D. Mechanical Behaviour of Biodegradable Agricultural Films under Real Field Conditions. Polym. Degrad. Stab. 2006, 91, 1256–1272. [Google Scholar] [CrossRef]
- Diepens, M.; Gijsman, P. Outdoor and Accelerated Weathering Studies of Bisphenol A Polycarbonate. Polym. Degrad. Stab. 2011, 96, 649–652. [Google Scholar] [CrossRef]
- Frigione, M. Assessment of the Ageing and Durability of Polymers. Polymers 2022, 14, 1934. [Google Scholar] [CrossRef]
- Torikai, A.; Hasegawa, H. Accelerated Photodegradation of Poly(vinyl chloride). Polym. Degrad. Stab. 1999, 63, 441–445. [Google Scholar] [CrossRef]
- Hedir, A.; Bechouche, A.; Moudoud, M.; Teguar, M.; Lamrous, O.; Rondot, S. Experimental and Predicted XLPE Cable Insulation Properties under UV Radiation. Turk. J. Electr. Eng. Comput. Sci. 2020, 28, 1763–1775. [Google Scholar] [CrossRef]
- Zaharescu, T.; Marinescu, M.; Lungulescu, E.M.; Scagliusi, S.R.; Lugão, A.B. Characterization of Ethylene-Propylene-Diene Terpolymer Based Electrical Insulation. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1061–1067. [Google Scholar] [CrossRef]
- Nóbrega, A.M.; Martinez, M.L.B.; de Queiroz, A.A.A. Investigation and Analysis of Electrical Aging of XLPE Insulation for Medium Voltage Covered Conductors Manufactured in Brazil. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 628–635. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; Ahmed, A.A.; Hameed, A.; Al-Amiery, A.; Kadhum, A.A.H.; Mohamad, A.B. The Effect of High UV Radiation Exposure Environment on the Novel PVC Polymers. Environ. Sci. Pollut. Res. 2019, 26, 10345–10354. [Google Scholar] [CrossRef]
- Nasrat, L.S.; Ibrahim, A.A.A.; El-Shazly, A.A.H. Carbon Black Effect on Electrical Performance of Semi-Conducting Layers for Power Cables. Int. J. Sci. Eng. Sci. 2017, 1, 47–50. [Google Scholar]
- Tang, C.-C.; Chen, H.-I.; Brimblecombe, P.; Lee, C.-L. Textural, Surface and Chemical Properties of Polyvinyl Chloride Particles Degraded in a Simulated Environment. Mar. Pollut. Bull. 2018, 133, 392–401. [Google Scholar] [CrossRef]
- Lacoste, J.; Carlsson, D.J.; Falicki, S.; Wiles, D.M. Polyethylene Hydroperoxide Decomposition Products. Polym. Degrad. Stab. 1991, 309–323. [Google Scholar] [CrossRef]
- Arias, G.; Benavides, R.; Garza, E.; Téllez-Rosas, M. Crosslinking of PVC Formulations Treated with UV Light. J. Vinyl Addit. Technol. 2006, 49–54. [Google Scholar] [CrossRef]
- Lipik, V.T.; Martsul, V.N.; Abadie, M.J.M. Dehydrochlorination of PVC Compositions During Thermal Degradation. Eurasian Chem.-Technol. J. 2002, 4, 25–29. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Ultra-Violet Spectra Studies of Photodegradation of PVC Films in Presence of Fe(III) Chelate Complex. Eur. J. Chem. 2013, 4, 178–182. [Google Scholar] [CrossRef]
- Wypych, G. Chapter 7, Corrosion of Adjacent Materials. In PVC Degradation and Stabilization; ChemTec Publishing: Toronto, ON, Canada, 2008; pp. 210–230. [Google Scholar]
- IEC 60502-1; Power Cables with Extruded Insulation and Their Accessories for Rated Voltages from 1 kV (Um = 1.2 kV) up to 30 kV (Um = 36 kV)—Part 1: Cables for Rated Voltages of 1 kV (Um = 1.2 kV) and 3 kV (Um = 3.6 kV). International Electrotechnical Commission (IEC): Geneva, Switzerland, 2009.
- EN 50525-1; Electric Cables—Low Voltage Energy Cables of Rated Voltages up to and Including 450/750 V (U0/U)—Part 1: General Requirements. European Committee for Electrotechnical Standardization (CENELEC): Brussels, Belgium, 2011.
- IEC 60243-1; Electrical Strength of Insulating Materials—Test Methods—Part 1: Tests at Power Frequencies. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013.
- Mergos, J.A.; Athanassopoulou, M.D.; Argyropoulos, T.G.; Dervos, C.T.; Vassiliou, P. The Effect of Accelerated UV-Ageing on the Dielectric Properties of PVC, PTFE and HDPE. In Proceedings of the 10th IEEE International Conference on Solid Dielectrics (ICSD), Potsdam, Germany, 4–9 July 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Hu, J.; Wang, F.; Hu, C. Relationship between physical and mechanical properties of accelerated weathering and outdoor weathering of PVC-coated membrane material under tensile stress. J. Ind. Text. 2017, 47, 417–429. [Google Scholar] [CrossRef]
- ASTM D3826-18; Standard Practice for Determining Degradation End Point in Degradable Polyethylene and Polypropylene Using a Tensile Test. ASTM International: West Conshohocken, PA, USA, 2018. Available online: https://www.astm.org/d3826-18.html (accessed on 12 February 2025).
- Rabie, S.; Nada, A. Glucoside/(UV Absorber) Mixtures as Photostabilizers for Rigid PVC. J. Vinyl Addit. Technol. 2008, 79–83. [Google Scholar] [CrossRef]
- Al-Ali, M.; Madi, N.K.; Thani, N.A.; El-Muraikhi, M.; Turos, A. Mechanical and thermal properties of gamma-ray irradiated polyethylene blends. Vacuum 2003, 227–236. [Google Scholar] [CrossRef]
- Domingos, A.; Duarte, L.; Pinheiro, A.; Moura, F.; Vasconcelos, L.; Carvalho, D.; Fadel, F.; Sakai, P. Comparative Analysis of Insulation Aging in Cross-Linked Polyethylene and Ethylene-Propylene Rubber Cables Through the Progression Rate of Partial Discharge. Energies 2025, 18, 2653. [Google Scholar] [CrossRef]
- Lambert, S.; Sinclair, C.J.; Boxall, A.B.A. Occurrence, Degradation, and Effect of Polymer-Based Materials in the Environment. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Ieda, M. Dielectric Breakdown Process of Polymers. IEEE Trans. Electr. Insul. 1980, EI-15, 206–224. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Sheena, H. Stabilization of Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Winslow, F.H. Photooxidation of High Polymers. Pure Appl. Chem. 1977, 49, 495–502. [Google Scholar] [CrossRef][Green Version]
- Wiles, D.M.; Scott, J.R. Polyolefins with controlled environmental degradability. Polym. Degrad. Stab. 2006, 91, 1220–1230. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef]
- Pinto, J.J.C.; Carvalho, M.E.S.; Ferreira, J.F.A. The kinetics and mechanism of polyethylene photo-oxidation. Angew. Makromol. Chem. 2003, 216, 113–133. [Google Scholar] [CrossRef]
- Mehmandoust, S.; Alizadeh, R.; Babaluo, A.A. Kinetic Study of the Poly(vinyl chloride)/Titanium Dioxide Nanocomposites Photodegradation under Accelerated Ultraviolet and Visible Light Exposure. Polym. Adv. Technol. 2014, 25, 1000–1008. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride)—Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Smith, L.M.; Aitken, H.M.; Coote, M.L. The Fate of the Peroxyl Radical in Autoxidation: How Does Polymer Degradation Really Occur? Acc. Chem. Res. 2018, 51, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.R.J.; Balbinot, L.; Poppi, R.J.; De Paoli, M.-A. Effect of Conducting Carbon Black on the Photostabilization of Injection Molded Poly(propylene-co-ethylene) Containing TiO2. Polym. Degrad. Stab. 2003, 82, 89–98. [Google Scholar] [CrossRef]
- Gugumus, F. Mechanisms of Photooxidation of Polyolefins. J. Appl. Polym. Sci. 1990, 51, 1387–1397. [Google Scholar] [CrossRef]
- Liu, M.; Horrocks, A.R. Effect of Carbon Black on UV Stability of LLDPE Films under Artificial Weathering Conditions. Polym. Degrad. Stab. 2002, 77, 485–499. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Mwila, J.; Miraftab, M.; Liu, M.; Chohan, S.S. The Influence of Carbon Black on Properties of Oriented Polypropylene 2. Thermal and Photodegradation. Polym. Degrad. Stab. 1999, 97, 430–438. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Liu, X.; Li, Y.; Tang, S.; Liu, J.; Xu, Z.; Zhang, C. Synergistic Effect of TNPP and Carbon Black in Weathered XLPE Materials. Polymers 2009, 1, 56–65. [Google Scholar] [CrossRef]
- Celina, M.; Gillen, K.T.; Assink, R.A. Accelerated Aging and Lifetime Prediction: Review of Non-Arrhenius Behavior Due to Two Competing Processes. Polym. Degrad. Stab. 2005, 90, 395–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).