Abstract
Residual oil saturation in reservoirs is primarily influenced by viscous and capillary forces, with interfacial tension (IFT) being a critical factor in fluid distribution due to capillary pressure. Adjusting IFT is essential for enhancing oil recovery, particularly in waterflooding, which is the most common secondary recovery technique after primary production. The salinity of injected water directly affects the IFT between crude oil and brine, making it a crucial factor in optimizing recovery. However, limited studies have examined IFT using live oil samples under actual reservoir conditions. In this study, a high-pressure, high-temperature (HPHT) drop shape analyzer was used to measure the IFT between live oil and brine under reservoir conditions. Five live oil samples and two sodium chloride (NaCl) brine concentrations (30,000 and 100,000 ppm) were tested at a reservoir temperature of 180 °F. Measurements were conducted above the bubble points of the oils, replicating undersaturated reservoir conditions. The results revealed that the impact of pressure on IFT was more complex than that of salinity. IFT generally decreased with increasing pressure but showed mixed behavior across different samples. Conversely, IFT consistently increased with higher salinity. These findings enhance the understanding of IFT behavior under reservoir conditions, supporting improved reservoir simulations and oil recovery strategies.
1. Introduction
After primary recovery mechanisms, significant amounts of oil (up to 90% in some reservoirs) remained trapped in porous media due to the capillary and viscous forces [1,2,3,4]. A thorough understanding of the parameters influencing these forces is essential, as they directly affect fluid distribution, residual oil saturation, and the mobility of trapped hydrocarbons. Accurately analyzing these parameters can help to optimize recovery strategies, improve hydrocarbon mobilization, and enhance overall reservoir management practices.
In reservoir engineering, the capillary number is used to understand fluid displacement processes, such as oil displacement by water or gas in a reservoir. It is a dimensionless group that characterizes the relative importance of viscous forces compared to capillary forces. This concept helps in assessing whether capillary forces, which act to retain fluids within the pores of the rock, or viscous forces, which facilitate the displacement of fluids across the rock matrix, dominate in controlling flow dynamics [5,6,7]. The capillary number defined by [8] can be calculated using Equation (1).
where Nc is the capillary number, μ is the viscosity of the displacing fluid, ν is the velocity of the displacing fluid, γ is the interfacial tension (IFT) between two immiscible fluids, and θ is the contact angle between the rock and the fluid.
Maximizing the capillary number is critical for improving displacement efficiency, which can be achieved by either increasing the viscosity of the displacing fluid or decreasing the capillary forces through a reduction in interfacial tension or the contact angle [9,10]. IFT, a critical thermophysical parameter, has a wide range of implications for various reservoir and rock–fluid properties, such as relative permeability, wettability, transition column length, and fluid displacement efficiency [11,12,13]. Thus, understanding the impact of IFT is crucial for mobilizing trapped hydrocarbons and optimizing reservoir management after primary or secondary recovery processes.
IFT refers to the boundary or surface energy between two or more immiscible fluids [14] and helps quantify the mass transfer and solubility between participating fluids [15]. IFT values depend strongly on several factors, such as oil composition (including interactions with brine, and thus, brine chemistry), whether the fluid is undersaturated or saturated, in situ reservoir conditions like temperature, pressure, brine salinity, or pH of the formation water, aging time, and the presence of surfactants or acidic and basic compounds in the reservoir systems [14,15,16,17,18,19].
The interfacial tension in oil/brine systems at reservoir conditions significantly affects oil mobility and recovery, particularly since only about one-third of the oil in reservoirs has been produced, requiring waterflooding or gas enhanced oil recovery (EOR) methods to improve recovery. Many researchers have investigated the effects of various parameters on IFT in oil/brine systems due to the presence of water in reservoirs, with waterflooding being the most common and well-known EOR method [20,21].
Over the last century, many techniques for measuring interfacial tension (IFT) have been developed, with significant advancements. Drelich et al. [22] classified these techniques into categories, including direct measurements using a microbalance (e.g., Du Nouy ring), capillary pressure measurement (e.g., growing drop), analysis of capillary–gravity forces (e.g., capillary rise), gravity-distorted drops (e.g., pendant drop), and reinforced distortion of drops (e.g., spinning drop). Among these techniques, the pendant drop method has been found to be the most effective for measuring IFT, even under high-pressure and high-temperature (HPHT) conditions [23,24].
For instance, Firoozabadi and Ramey [25] used reservoir oil/brine systems in a high-pressure drop shape apparatus and with a pendant drop method (PDM), concluding that the IFT decreased slightly with increasing pressure but increased with temperatures between 185–195 °F. Cai et al. [26] reported that IFT decreased with increasing temperatures but increased slightly with pressure, using pure components and a ternary system of n-C6, n-C10, n-C16 mixtures with distilled water and various types of brine. In addition, researchers [20,21,27] have further investigated the effect of salinity, surfactants, and pressure on IFT, often finding conflicting trends showing that IFT increased with brine dilution or decreasing salinity beyond a certain threshold, while it generally decreased with increasing pressure and temperature. In their studies, Kumar [17] and Lashkarbolooki et al. [18] used pure hydrocarbons and mixtures of them, as well as crude oils with various densities, bitumen, and aqueous phases of different salinities, concluding that the IFT trend depends on the oil type and brine.
While many studies have focused on IFT between dead oils and synthetic/formation brines, fewer have examined live oil/brine systems, which are critical for understanding IFT under actual reservoir conditions. Xu [28] investigated the effects of temperature, pressure, brine composition, and salinity on the dynamic IFT of live oil/brine systems and observed that live oil IFT values increased with decreasing salinity and temperature, but the trends differed with pressure. Some studies [19,29] found different trends with pressure and salinity; the IFT values increased with pressure and synthetic formation salinity; however, the IFT trend varied for different oil samples. In their studies, Ratnakar et al. [26,27] investigated the effect of asphaltene stability on live oil/water IFT and observed that the live oil IFT values decreased with increasing pressure for some samples, emphasizing the effect of oil composition on IFT. More recent studies [30,31] underscore the complexity of IFT behavior, highlighting its dependence on hydrocarbon types and reservoir conditions, such as pressure, temperature, and brine salinity.
Given the variability in IFT trends for complex live oil/brine systems, further studies are essential to understand IFT and its effect on oil recovery. While the impact of temperature on IFT is well-established, typically decreasing with rising temperature, the effects of pressure and salinity remain less clear, especially for live oils under reservoir conditions. This study addresses this gap by investigating IFT at 180 °F under pressures above the bubble point, simulating undersaturated reservoir conditions. We used five distinct live oil samples with varying GOR, API gravity, and bubble points, offering a broader perspective than previous studies. Notably, these oils were lost water-soluble components, which resulted in unique IFT behavior with the brine samples. This comprehensive analysis provides new insights into IFT dynamics that are critical for enhanced oil recovery.
2. Experimental Method
2.1. Fluid Properties
For the hydrocarbon phase, five live oil samples were obtained from the reservoirs using high-pressure sample cylinders to accurately capture the in-situ characteristics of the oil. The properties of the oil samples are summarized in Table 1. The API gravity of the oil samples ranged from 16.7° to 33.1°, indicating significant variations in oil density and viscosity across the samples. The gas/oil ratio (GOR) for the samples ranged from 338 to 789 scf/stb, reflecting different levels of gas content in the oil, which is critical for understanding the reservoir’s performance and gas recovery potential. Additionally, the bubble point pressures of the samples varied between 2110 psi and 5333 psi.

Table 1.
Properties of live oil samples.
The densities of the oil samples at each specific pressure were determined using constant composition expansion (CCE) at reservoir temperature of 180 °F. This method provides an accurate measure of oil volume changes with pressure reductions and is essential for determining the behavior of the oil phase in reservoir simulations. The GOR values were determined using a single-stage flash separation process, in which the oil was depressurized from 10,000 psi and 180 °F to atmospheric pressure and 80 °F, allowing for the efficient separation of gas from the liquid phase. This process allows for the precise quantification of the gas content in the oil under standard reservoir conditions, contributing to a comprehensive understanding of the fluid dynamics within the reservoir.
For the aqueous phase, two concentrations of synthetic brine were prepared by dissolving sodium chloride (NaCl, ACS reagent, ≥99.0%) in deionized water: 30,000 ppm (3 wt.%) and 100,000 ppm (10 wt.%). These concentrations were selected based on formation water analysis, which indicated a minimum of 81% NaCl among the dissolved salts. The brine solutions were carefully prepared in a flask using a magnetic stirrer and left for at least a minimum of one hour to ensure complete dissolution of the salt. The density of the brine samples at each salinity and at 180 °F under varying pressures was obtained from PHREEQC [32], and the resulting values are provided in Table 2.

Table 2.
Density of brine with respect to pressure at reservoir temperature (180 °F).
2.2. Equipment
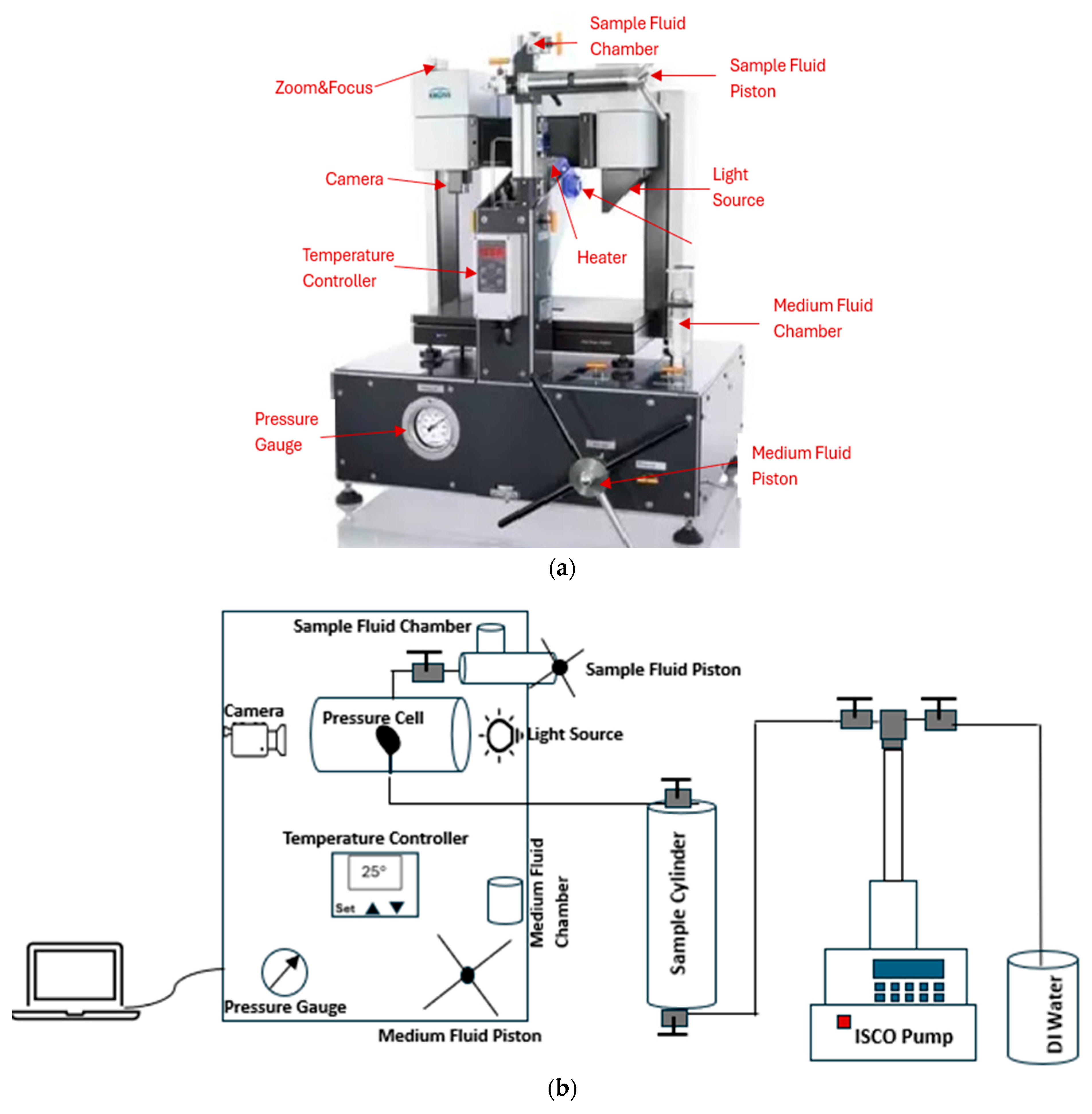
In this study, we utilized the pendant drop method with a drop shape analyzer to measure interfacial tension (IFT) in live oil/brine systems under reservoir conditions. A high-pressure, high-temperature (HPHT) drop shape analyzer (Model: DSA100) manufactured by Kruss and Eurotechnica, made in Germany, was used for these experiments. This equipment is ideal for surface and interfacial characterization under HPHT conditions. The integration of the pressure cell for IFT, surface tension, or contact angle measurements allows operation at pressures up to 10,000 psi and temperatures up to 356 °F (180 °C), as shown in Figure 1a. The software and the camera enable real-time measurements while recording data and calculating IFT values based on the pendant drop method (PDM). Since the density of the oil is lower than the density of the surrounding brine, the IFT can be determined from the shape of the rising oil drop at the needle tip. The schematic of the DSA and the experimental set up is shown in Figure 1b.
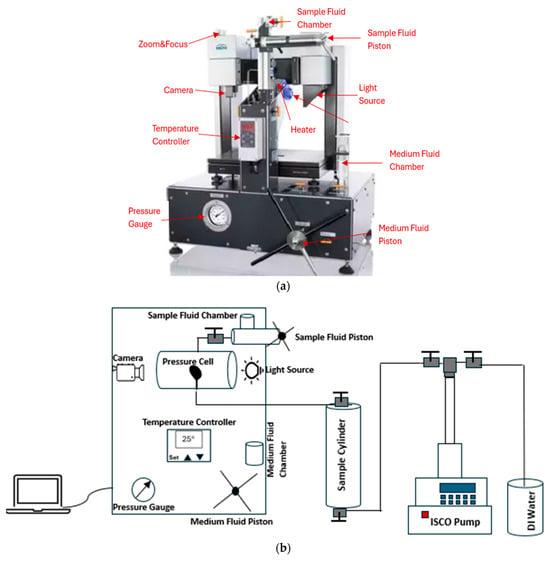
Figure 1.
(a) HPHT drop shape analyzer (DSA) [33], (b) The schematic of the DSA and the experimental set up.
2.3. Procedure
Since IFT is an interfacial property that is highly sensitive to impurities, segregated phases, particles, and any changes in pressure or temperature, extra precautions are necessary to ensure accurate measurements. Additionally, as the measurements are directly captured and processed through the glass window by a camera, proper cleaning of the contacted surfaces is crucial. Before each series of IFT measurements, the entire system underwent a thorough cleaning process, which included sequential treatment with toluene, acetone, isopropanol alcohol, deionized water, and pressurized air flushing. Also, prior to any measurement, the equipment was calibrated to ensure accuracy and repeatability.
To measure interfacial tension (IFT), sample fluids were first homogenized at reservoir temperature (180 °F) for 24 h using a rocker. The DSA100 pressure cell was then filled with brine, flushed of air, and heated to the test temperature. After reaching thermal equilibrium, the system was pressurized to the target level. Prior to each measurement, multiple droplets were introduced to eliminate impurities from the capillary. Data were continuously logged, and equilibrium stage measurements are reported in this study.
The experimental matrix closely follows a similar structure to the density matrix in Table 1. For each oil sample (except Sample A), experiments were conducted at three pressure conditions: 4000, 6000, and 8000 psi, all at a reservoir temperature of 180 °F. Due to the high bubble point pressure of Sample A (5000 psi), tests for this sample were conducted only at 6000 and 8000 psi. Each test was performed twice at each pressure level to ensure data quality, and only data with an error margin of less than 5% is presented in this study based on the uncertainty in temperature, pressure, and density, and the standard deviation of two repeated experiments. The biggest factor affecting the uncertainty in the results is the standard deviation of the IFT measurements. Therefore, the authors suggest repeating the tests at least three times to minimize the error on the IFT.
2.4. Methodology
The analysis methodology implemented in the DSA using the PDM calculates dynamic IFT at the liquid interface based on the shape of the oil droplet image. The image of the droplet, shown in Figure 2, is matched with a theoretical profile obtained through numerical integration of the Young−Laplace equation:
where
γ: The interfacial tension, mN/m (dyne/cm),
Δρ: The density difference between the oil and brine, g/mL,
g: The gravitational acceleration, m/s2,
β: The Bond number,
Ro: Measured radius of curvature at the apex of the drop, cm.

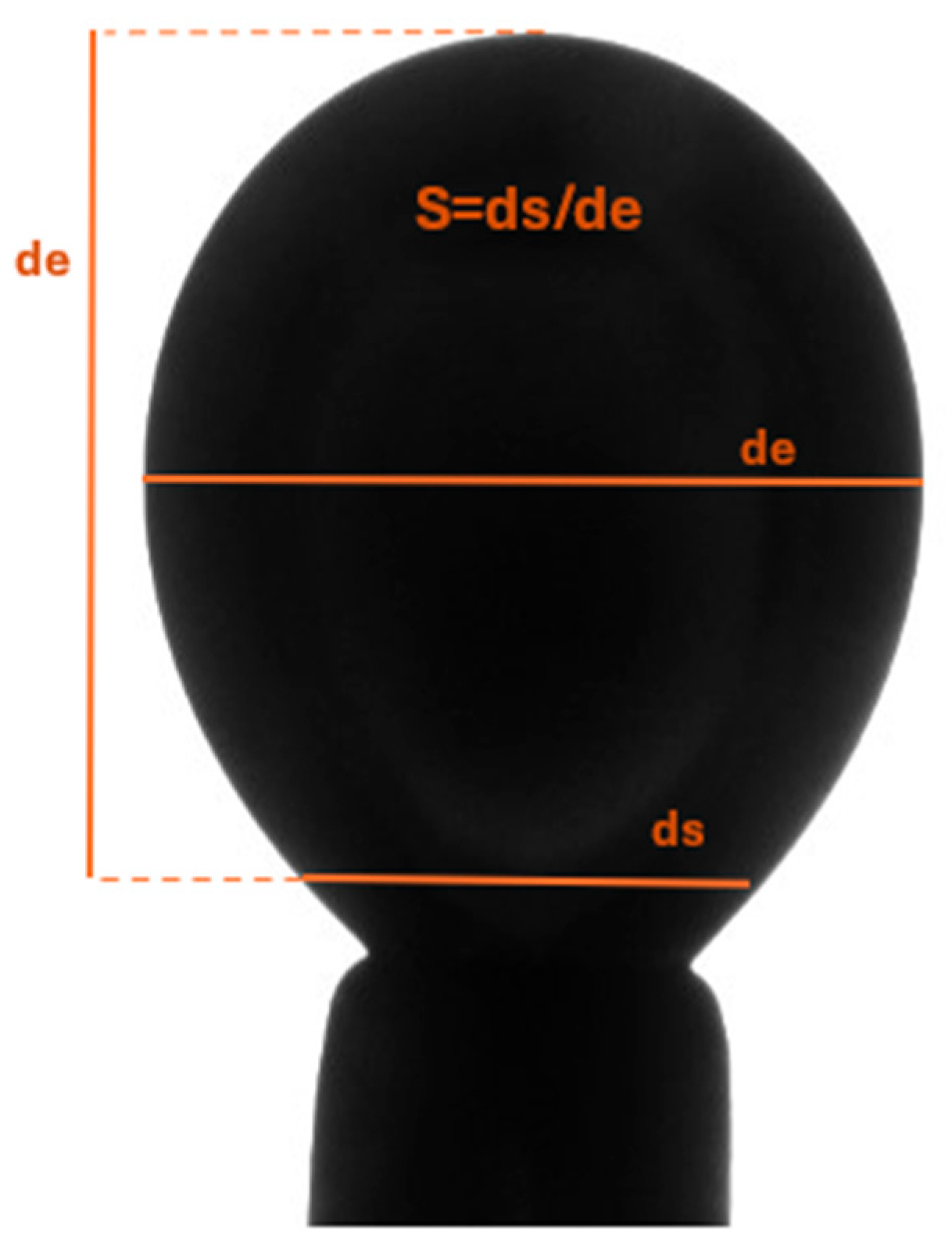
Figure 2.
Calculation of key dimensions (diameters at specified locations) of the droplet) (After Andreas et al [8]).
Figure 2.
Calculation of key dimensions (diameters at specified locations) of the droplet) (After Andreas et al [8]).
The Bond number, β, and the radius of curvature at the apex of the drop, Ro, can be calculated with Equations (3) and (4) [23,34].
where S is the shape factor (S = ds/de), ds is diameter at an arbitrarily selected plane, de is diameter at the equator as shown in Figure 2 and explained in detail by Andreas et al [23].
2.5. Measurement of Interfacial Tension
In this study, we experimentally investigated the effects of salinity and pressure on the interfacial tension (IFT) of reservoir (live) oil/brine systems. Five undersaturated live oil samples were used, with API gravities ranging from 16.7° to 33.1° and bubble point pressures between 2110 and 5333 psi. The surrounding phase for these experiments consisted of sodium chloride (NaCl) brine at concentrations of 3 wt.% and 10 wt.% NaCl (30,000–100,000 ppm). All IFT measurements were conducted at a constant temperature of 180 °F and pressures ranging from 4000 to 8000 psi. The density values for the oil and brine samples are given in Table 1 and Table 2. To ensure data quality and repeatability, at least three consecutive bubbles were generated for each test, and each test was repeated at least twice.
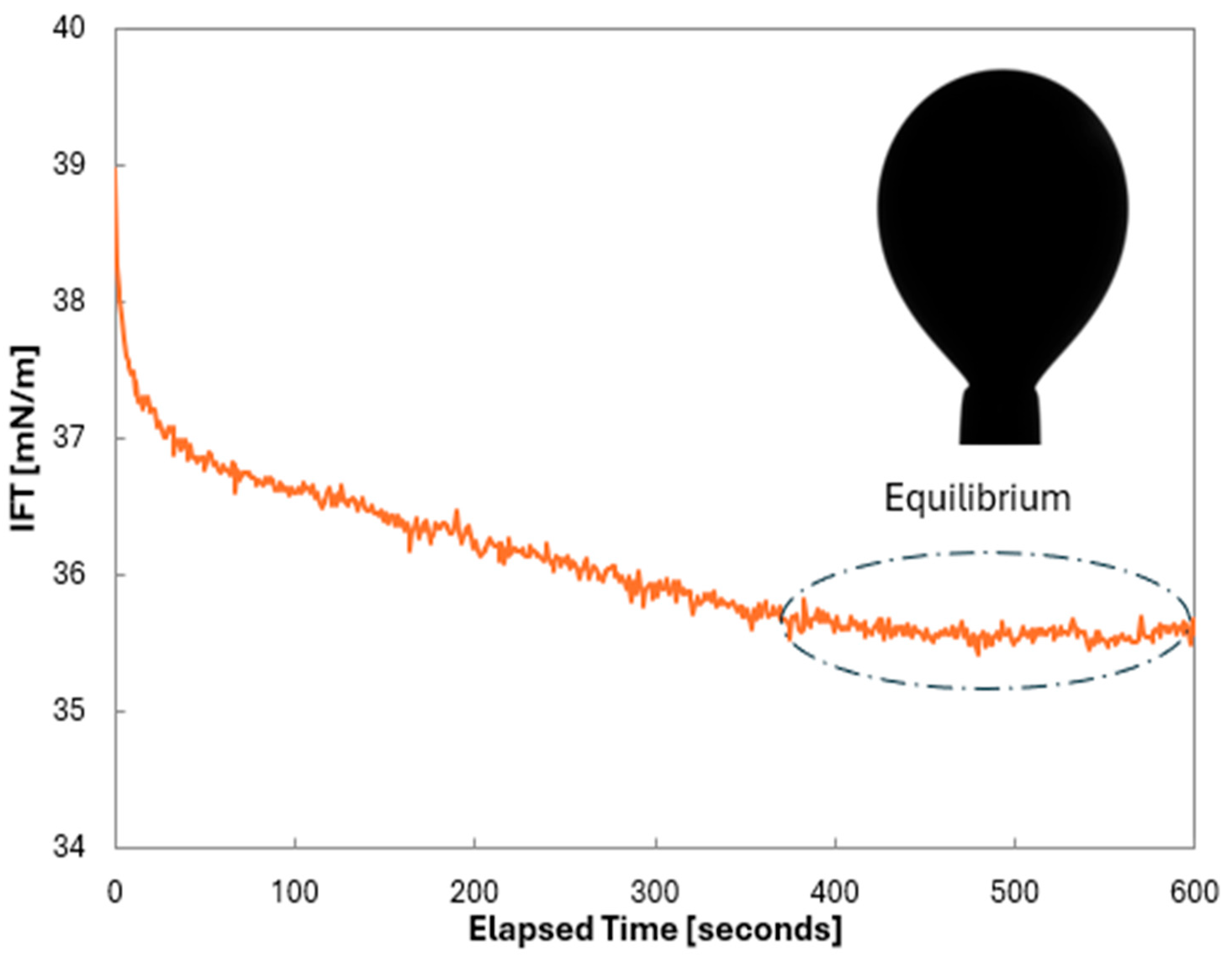
Using the Young–Laplace equation applied to the pendant drop method, real-time IFT data were obtained via embedded software, as illustrated in Figure 3. The IFT of oil/brine systems typically decreases sharply at early times due to the movement of the polar component at the equilibrium. Each test lasted 30–45 min (excluding repetitions), depending on the time required to reach equilibrium. Once equilibrium was reached, the IFT values for the respective oil/brine system under the specified temperature and pressure conditions were recorded.

Figure 3.
Real-time IFT measurements by utilizing pendant drop of reservoir oil in brine.
It is important to note that small changes in pressure or temperature, as well as potential vibrations around the equipment, can cause fluctuations in the IFT data. To minimize such effects, we continuously monitored the experimental setup to ensure stability throughout the measurements.
3. Results and Discussion
In this study, the IFT of live oil/synthetic brine systems was investigated at 180 °F and pressures of 4000, 6000, and 8000 psi using the pendant drop method. All the experiment results are summarized in Table 3.

Table 3.
Measured IFT values of live oil/brine systems with respect to salinity and pressure.
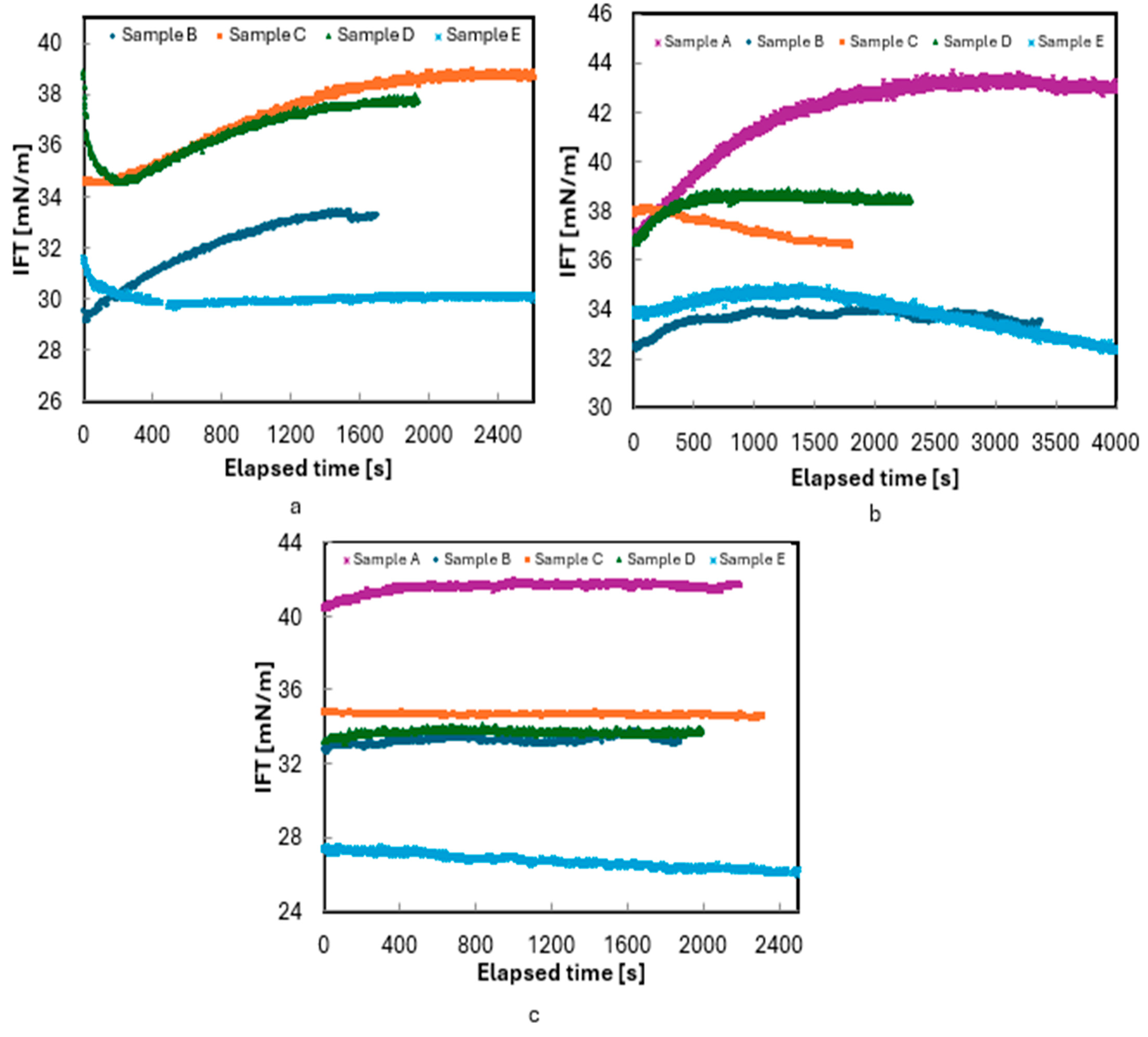
The initial experiments were conducted using 3 wt.% NaCl. Figure 4 represents the IFT measurements of all five live oil/3wt.% brine (30,000 ppm) samples with respect to time at these pressures. Sample A has a bubble point pressure of 5333 psi, so the measurements for Sample A were carried out only at 6000 and 8000 psi. As shown in Figure 4, IFT measurements were conducted for 30 to 60 min to ensure an equilibrium was reached between the oil and brine. At equilibrium, the IFT readings stabilized with fluctuations of less than 1mN/m (dyne/cm). These minor fluctuations were likely caused by vibrations during the measurements.
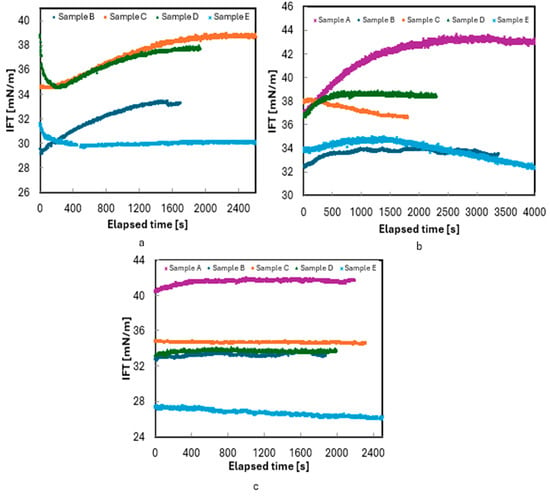
Figure 4.
Measurement of IFT of live oils/3 wt.% NaCl brine at 180 °F: (a) 4000, (b) 6000, and (c) 8000 psi.
The high GOR make the oil more volatile, and the lighter components of the oil migrate towards the oil–brine interface and cause a lower surface energy at the interface. Therefore, in Figure 4, the initial rapid decay in IFT can be attributed to the adsorption of polar species at the interface, forming a film between the two phases. The subsequent increase in IFT is likely due to the diffusion of surface-active species back into their bulk phases, as described by Saad et al. [35] and Okasha et al. [30]. However, with increasing pressure, this nonmonotonic transient behavior was replaced by monotonic IFT decay, as observed in Samples B and D in Figure 4.
This behavior is consistent with the transient accumulation of surface-active components at the interface, as described by Hjelmeland and Larrondo [16]. However, despite repeating the experiments at least twice, a nonmonotonic transient regime was observed before equilibrium in some cases, as shown in Figure 4a, b. This behavior, which aligns with observations in previous studies [29,30,35,36], involves a rapid decay in IFT during the early stages, followed by an increase and then a gradual decline toward equilibrium.
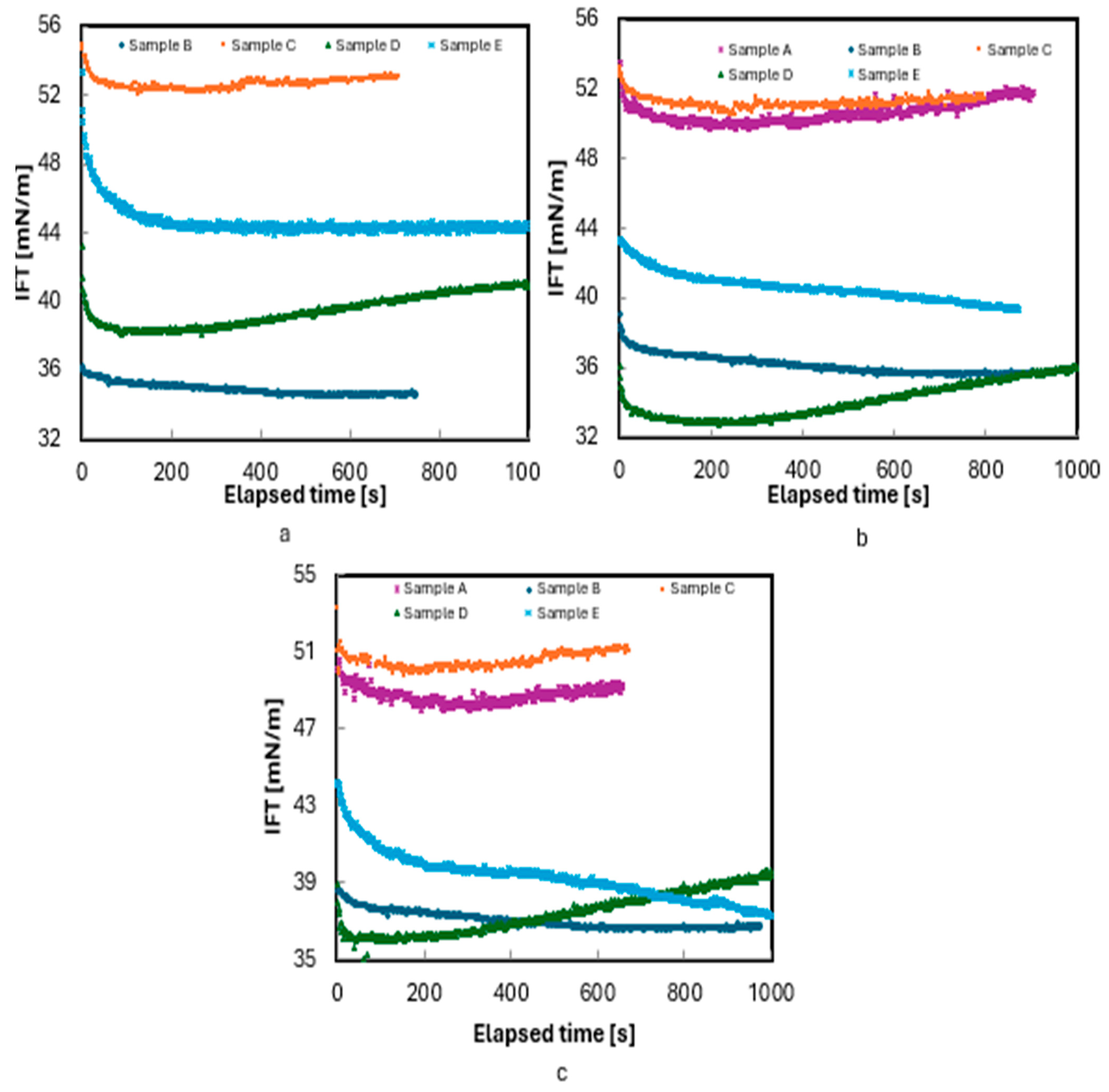
A separate set of experiments was conducted using a 10 wt.% NaCl brine at 180 °F and pressures of 4000, 6000, and 8000 psi. Since Sample A has a bubble point pressure of 5333 psi, measurements for this sample were only performed at 6000 and 8000 psi. As shown in Figure 5, the time required to reach the equilibrium between the phases was shorter compared to the experiments with 3 wt.% brine. Interestingly, only Sample D exhibited distinct nonmonotonic transient behavior with increasing salinity, indicating partitioning of Sample D into the brine. However, for this sample, pressure influenced only the IFT values and did not alter the trend. To better understand the effect of pressure on the partitioning of the samples, additional tests are needed using oils with known compositions.
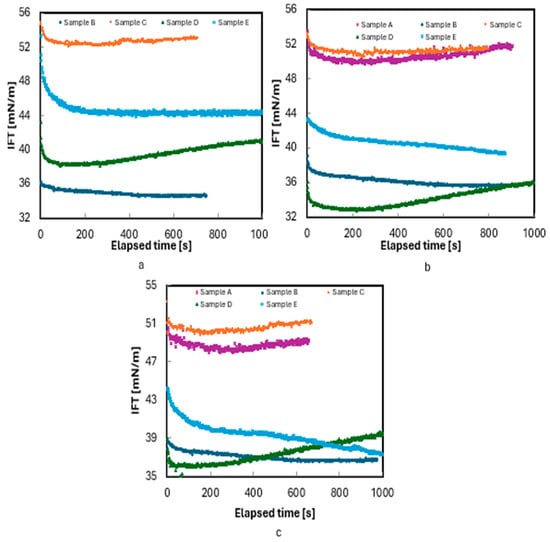
Figure 5.
Measurement of IFT of live oils/10 wt.% NaCl brine at 180 °F: (a) 4000, (b) 6000, and (c) 8000 psi.
The effect of salinity on the IFT trend is more obvious than the behavior at the interface with aging time. We used 3 wt.% and 10 wt.% brine to compare the effects, and overall, the IFT values of each live oil sample increased with higher salinity, as shown in Figure 6 and Figure 7. Since the density of the 10 wt.% brine is higher than the density of 3 wt.% brine, the IFT values increased, as also predicted by the Young–Laplace equation. This behavior aligns with previously reported data [19,21,26,31]. The results are summarized in Table 3.

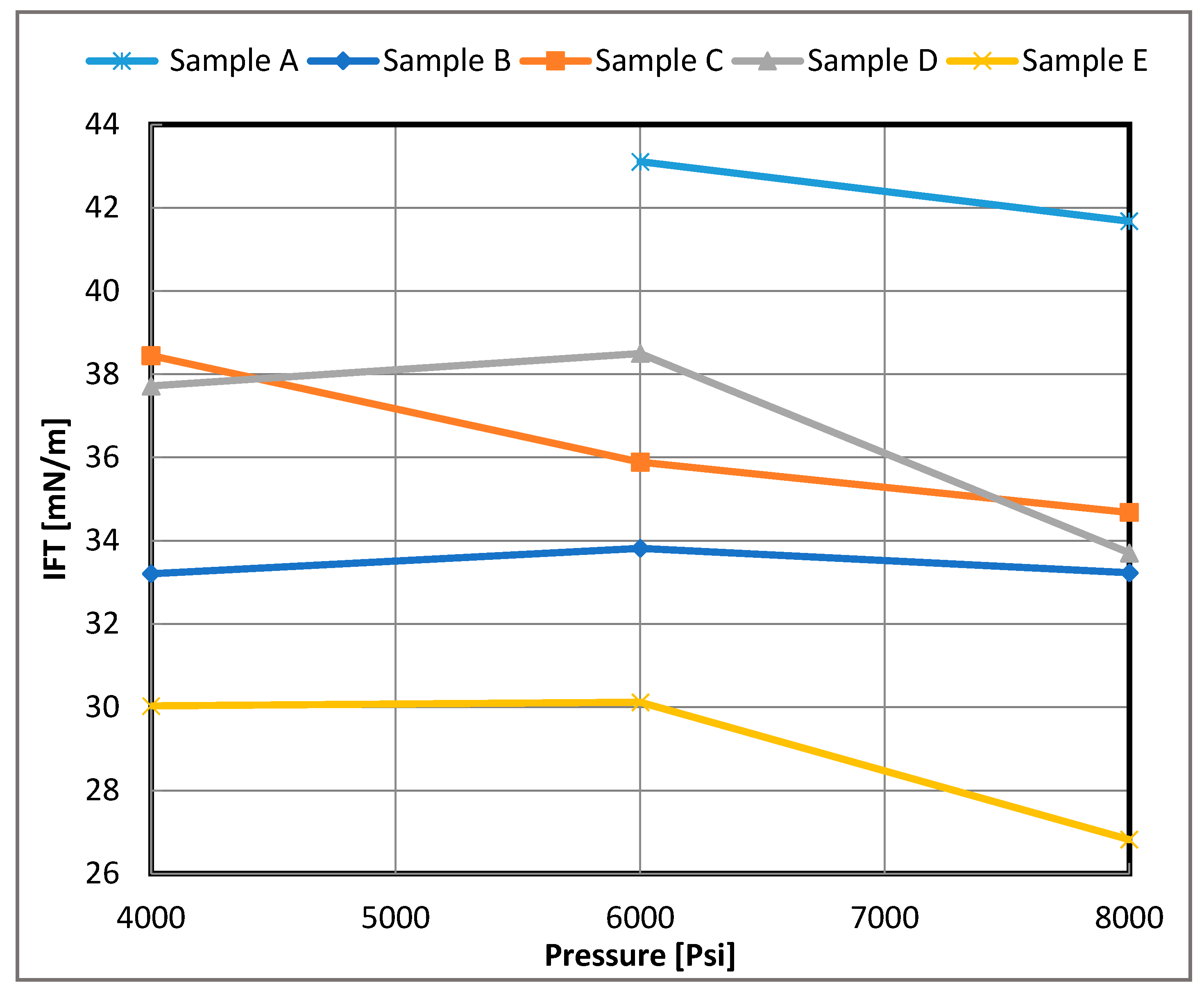
Figure 6.
Measured IFT of live oil/brine systems versus pressure for 3 wt.% brine for each sample, A–E.

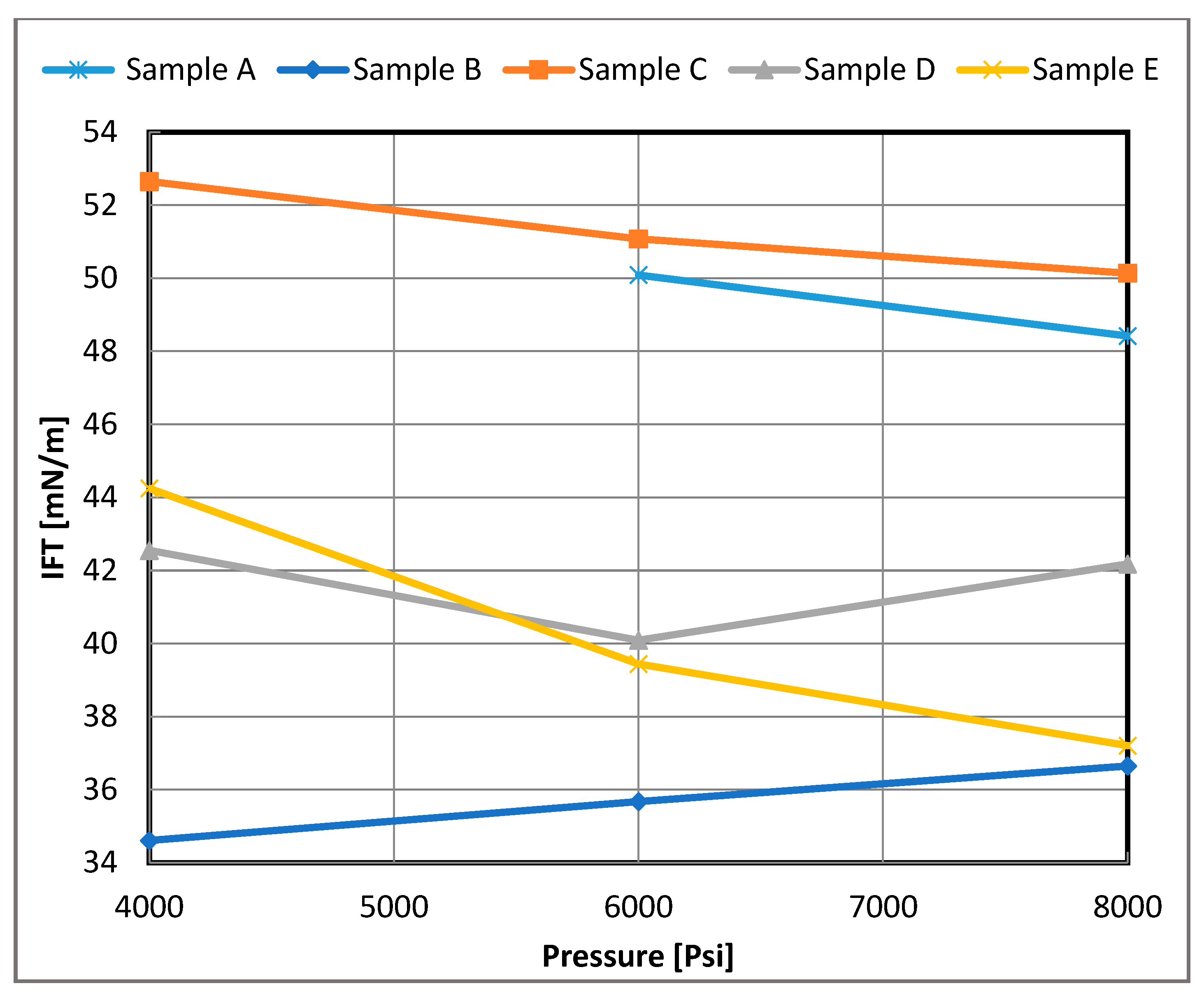
Figure 7.
Measured IFT of live oil/brine systems versus pressure for 10 wt.% brine for each sample, A–E.
The IFT values with respect to pressure for each live oil/brine system are shown in Figure 6 and Figure 7. The IFT values exhibited mixed behavior with increasing pressure. For the low salinity system, most measurements indicated that the IFT was relatively insensitive to pressures up to approximately 6000 psi for each sample. Beyond 6000 psi, a decline in IFT values was observed, which can be attributed to a broadening of the interface and reduced density differences between the oil and brine phases.
In contrast, for the high salinity system, the effect of pressure was more consistent, with IFT values generally decreasing as pressure increased, except in the case of Sample B. This trend can be explained by the faster movement of surface-active components due to the greater density differences between oil and brine at higher pressures. For Samples B and D, the higher IFT values could be attributed to the presence of longer hydrocarbon chains. These chains may have migrated to the interface, creating a larger density difference at the boundary and contributing to the observed behavior.
4. Conclusions
We performed detailed IFT measurements under reservoir conditions (reservoir P&T) using real reservoir oils and synthesized brines at different salinities, mimicking reservoir brines. Based on our study, we reached the following key conclusions:
The influence of pressure on IFT exhibited a more complex pattern than that of salinity, with inconsistent behavior observed across both systems as pressure increased. This complexity may be attributed to factors such as the oil composition, gas–oil ratio, acid number, and asphaltene content of the oil samples. Further experimental investigations, supported by additional data, are essential to establish clearer correlations among these factors.
As expected, brine salinity had a significant impact on IFT values. Our results revealed that IFT values increased with salinity due to higher density differences between the oil and brine phases. To explain the effect of ions and their concentrations at the interface, more tests with various salinities should be conducted along with various types of salt in future studies.
Nonmonotonic behavior was more pronounced in low salinity systems, with the pressure effect on IFT showing variability. Further studies with a broader spectrum of oil chemistries are needed to better understand and clarify this phenomenon.
Understanding IFT at equilibrium is crucial for planning enhanced oil recovery (EOR) or improved oil recovery (IOR) projects aimed at mobilizing trapped oil within the reservoir.
Author Contributions
Conceptualization, B.D., L.B. and R.R.R.; Methodology, D.M.P., S.S., H.S. and P.J.; Software, H.S. and P.J.; Validation, D.M.P., S.S. and B.D.; Formal analysis, D.M.P. and B.D.; Investigation, D.M.P. and B.D.; Resources, B.D., L.B. and R.R.R.; Data curation, D.M.P. and S.S.; Writing—original draft, D.M.P.; Writing—review & editing, D.M.P., B.D. and R.R.R.; Visualization, D.M.P.; Supervision, B.D.; Funding acquisition, B.D. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Acknowledgments
We would like to thank the members of the Interactions of Phase Behavior and Flow (IPB&F) Consortium, Shell International Exploration and Production Inc., the University of Houston, Kruss, and Eurotechnica for providing funding and equipment support. This work was conducted entirely at the University of Houston.
Conflicts of Interest
Authors Ram R. Ratnakar and Leslie Baksmaty are/were employed by Shell International Exploration and Production Inc.; however, their contributions are voluntary, and no Shell resources (other than funding) have been used to conduct this work. The research was conducted in the absence of any commercial relationships that could be construed as a potential conflict of interest.
References
- Ferer, M.; Anna, S.L.; Kadambi, J.R.; Oliver, M.; Bromhal, G.S.; Smith, D.H. Two-Phase Flow in Porous Media: Predicting Its Dependence on Capillary Number and Viscosity Ratio. Transp. Porous Med. 2011, 86, 243–259. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998. [Google Scholar]
- Lake, L.W. Enhanced Oil Recovery; Prentice Hall: Hoboken, NJ, USA, 1989; ISBN 0132816016. [Google Scholar]
- Lake, L.W.; Johns, R.T.; Rossen, W.R.; Pope, G.A. Fundamentals of Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014; ISBN 978-1-61399-885-4. [Google Scholar] [CrossRef]
- Buckley, J.S.; Morrow, N.R. Characterization of Crude Oil Wetting Behavior by Adhesion Tests. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 22–25 April 1990. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Jain, V.; Sharma, M.M. Effect of Capillary Pressure, Salinity, and Aging on Wettability Alteration in Sandstones and Limestones. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar] [CrossRef]
- Ahmadyar, S.; Samara, H. The effect of gas and liquid phase composition on miscibility through interfacial tension measurements of model oils in compressed CO2. J. Petrol. Explor. Prod. Technol. 2025, 15, 31. [Google Scholar] [CrossRef]
- Moore, T.F.; Slobod, R.L. Displacement of oil by water-effect of wettability, rate, and viscosity on recovery. In Proceedings of the Fall Meeting of the Petroleum Branch of AIME, New Orleans, LA, USA, 2–5 October 1955; p. SPE-502-G. [Google Scholar] [CrossRef]
- Stern, D. Mechanisms of Miscible Oil Recovery: Effects of Pore-Level Fluid Distribution. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. [Google Scholar] [CrossRef]
- Taber, J.J. Dynamic and Static Forces Required to Remove a Discontinuous Oil Phase from Porous Media Containing Both Oil and Water. SPE J. 1969, 9, 3–12. [Google Scholar] [CrossRef]
- Benson, S.; Pini, R.; Reynolds, C.; Krevor, S. Relative Permeability Analyses to describe Multi-Phase Flow in CO2 Storage Reservoirs. Glob. CCS Inst. 2015, 1–49. [Google Scholar]
- Ratnakar, R.R.; Mantilla, C.A.; Dindoruk, B. Experimental and Numerical Investigation of the Impact of Asphaltene Stability on Interfacial Properties and the Hysteresis Behavior at the Interfaces. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018; p. SPE-190385-MS. [Google Scholar] [CrossRef]
- Yildiz, H.O.; Valat, M.; Morrow, N.R. Effect of Brine Composition on Wettability and Oil Recovery of a Prudhoe Bay Crude Oil. J. Can. Pet. Technol. 1999, 38. [Google Scholar] [CrossRef]
- Buckley, J.S.; Fan, T. Crude Oil/Brine Interfacial Tensions1. Petrophysics 2007, 48, SPWLA-2007-v48n3a1. [Google Scholar]
- Jaeger, P.T.; Schnitzler, J.V.; Eggers, R. Interfacial tension of fluid system considering the nonstationary case with respect of mass transfer. Chem. Eng. Technol. 1996, 19, 197–202. [Google Scholar] [CrossRef]
- Hjelmeland, O.S.; Larrondo, L.E. Experimental Investigation of the Effects of Temperature, Pressure, and Crude Oil Composition on Interfacial Properties. SPE Reserv. Eng. 1986, 1, 321–328. [Google Scholar] [CrossRef]
- Kumar, B. Effect of Salinity on the Interfacial Tension of Model and Crude Oil Systems. Master’s Thesis, University of Calgary, Department of Chemical and Petroleum Engineering, Calgary, AB, USA, 2012. [Google Scholar]
- Lashkarbolooki, M.; Riazi, M.; Ayatollahi, S.; Zeinolabedini, H.A. Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel 2016, 165, 75–85. [Google Scholar] [CrossRef]
- Okasha, T.; Al-Shiwaish, A.J. Effect of Brine Salinity on Interfacial Tension in Arab-D Carbonate Reservoir, Saudi Arabia. In Proceedings of the 2009 SPE Middle East Oil & Gas Show and Conference held in the Bahrain International Exhibition Centre, Manama, Bahrain, 15–18 March 2009; p. SPE-119600-MS. [Google Scholar]
- Alotaibi, M.B.; Nasr-El-Din, H.A. Effect of Brine Salinity on Reservoir Fluids Interfacial Tension. In Proceedings of the 2009 SPE EUROPEC/EAGE Annual Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar] [CrossRef]
- Vijapurapu, C.S.; Rao, D.N. Effect of Brine Dilution and Surfactant Concentration on Spreading and Wettability. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar] [CrossRef]
- Drelich, J.; Fang, C.; White, C.L. Measurement of Interfacial Tension in Fluid-Fluid Systems. Encycl. Surf. Colloid Sci. 2002, 3, 3158–3163. [Google Scholar]
- Andreas, J.M.; Hauser, E.A.; Tucker, W.B. Boundary Tension by Pendant Drops. J. Phys. Chem. 1938, 42, 1001–1019. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Mantilla, C.A.; Dindoruk, B. Experimental Investigation of the Effects of Asphaltene Stability on Interfacial Behavior of Live-Reservoir-Fluid Systems. SPE J. 2019, 24, 21–31. [Google Scholar] [CrossRef]
- Firoozabadi, A.; Ramey, H.J., Jr. Surface Tension of Water-Hydrocarbon Systems at Reservoir Conditions. J. Can. Pet. Technol. 1988, 27. [Google Scholar] [CrossRef]
- Cai, B.Y.; Yang, J.T.; Guo, T.M. Interfacial Tension of Hydrocarbon + water/brine Systems under High Pressure. J. Chem. Eng Data 1996, 41, 493–496. [Google Scholar] [CrossRef]
- Serrona-Saldana, E.; Dominguez-Ortiz, A.; Perez-Aguilar, H.; Kornhauser-Strauss, I.; Rojas-Gonzales, F. Wettability of solid/brine/n-dodecane systems: Experimental study of the effects of ionic strength and surfactant concentration. Colloids Surf. A Physicochem. Eng. Aspects 2004, 241, 343–349. [Google Scholar] [CrossRef]
- Xu, W. Experimental Investigation of Dynamic Interfacial Interactions at Reservoir Conditions. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2005. Available online: https://repository.lsu.edu/gradschool_theses/968 (accessed on 18 August 2024).
- Sauerer, B.; Stukan, M.; Abdallah, W.; Buiting, J. Toward Determining Interfacial Tension at Reservoir Conditions Based on Dead Oil Measurements. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 6–9 March 2017. [Google Scholar] [CrossRef]
- Okasha, T.; Al-Hamad, M.; Sauerer, B.; Abdallah, W. Accurate Live Interfacial Tension for Improved Reservoir Engineering Practices. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 28 November–1 December 2021; p. SPE-204615-MS. [Google Scholar]
- Soleymanzadeh, A.; Rahmati, A.; Yoisefi, M.; Roshani, B. Theoretical and experimental investigation of effect of salinity and asphaltene on IFT of brine and live oil samples. J. Pet. Explor. Prod. Technol. 2021, 11, 769–781. [Google Scholar] [CrossRef]
- PHREEQC Version 3. USGS Asset Identifier Service (AIS). 2021. Available online: https://www.usgs.gov/software/phreeqc-version-3 (accessed on 5 April 2024).
- Kruss-Scientific. Drop Shape Anlayzer-DSA100HP690. 2024. Available online: https://www.kruss-scientific.com/en/products-services/products/dsa100hp690?_gl=1*180h4qp*_up*MQ..*_gs*MQ..&gclid=Cj0KCQiAgJa6BhCOARIsAMiL7V-oC9nbLBelnbxSl2rnaMKfoZEPFJrLXGryurxqtu_xOYznEOM0xW8aAoDeEALw_wcB (accessed on 15 April 2024).
- Herd, M.D.; Lassahn, G.D.; Thomas, C.P.; Bala, G.A.; Eastman, S.L. Interfacial Tension of Microbial Surfactants Determined by Real-Time Video Imaging of Pendant Drops. In Proceedings of the SPE/DOE Eight Symposium on EOR, Tulsa, OK, USA, 22–24 April 1992; p. SPE-24206-MS. [Google Scholar] [CrossRef]
- Saad, A.M.; Aime, S.; Mahavadi, S.C.; Song, Y.; Yutkin, M.P.; Weitz, D.; Patzek, T.W. Adsorption of Polar Species at Crude Oil–Water Interfaces: The Chemoelastic Behavior. Langmuir 2022, 38, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Nasr-El-Din, H.A.; Taylor, K.C. Dynamic interfacial tension of crude oil/alkali/surfactant systems. Colloids Surf. 1992, 66, 23–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).