Abstract
Environmentally friendly extraction technologies for biologically active substances (BASs) are an actively developing and important industry. In recent years, the development of this area has been associated with the use of low-melting mixtures, which are most often referred to as “deep eutectic solvents”. Vaccinium vitis-idaea L. is a valuable source of phenolic biologically active compounds. However, to date, there are limited studies devoted to the use of such solvents for the extraction of biologically active substances from V. vitis-idaea. This study introduces the use of low-melting mixtures of choline chloride or betaine with lactic acid and water for the ultrasonic extraction of phenolic secondary metabolites from V. vitis-idaea leaves for the first time. The kinetics of extraction have been studied, and the extraction conditions have been optimized using a Box–Behnken design. It was found that the optimal extraction conditions are follow: the most suitable mixture is betaine with lactic acid and water at a molar ratio of 1:10:5, the optimal temperature is 33 °C, and the optimal ratio of the mass of plant material to the volume of the solvent is 1:20. Under these conditions, the yield of total phenolic compounds was 744.3 ± 1.2 mg GAE/g, and total flavonoids reached 24.4 ± 0.2 mg RE/g. The IC50 values of the obtained extract were 2.45 mg/mL for free radical scavenging with DPPH and 3.47 mg/mL for ABTS. The data obtained can be used in the development of green technologies for the extraction of biologically active substances from the leaves of V. vitis-idaea.
1. Introduction
Technologies for the environmentally safe extraction of biologically active substances (BASs) are actively developing and play an important role in industry. One of the key challenges in the field of green chemistry is to replace toxic and volatile solvents with safer and more environmentally friendly alternatives. In recent years, considerable attention has been paid to the use of low-melting mixtures known as “deep eutectic solvents” (DESs) [1,2,3]. These mixtures, consisting of two or more components, form a mixture with a low melting point. Most of such mixtures are considered to have a number of advantages, such as biodegradability, low toxicity, and relatively low cost, which makes them promising for use in various industries, including the extraction of BASs from various plants [4,5,6,7]. However, the term “eutectic mixture” should strictly refer to a specific composition determined by methods such as differential scanning calorimetry. At the same time, many studies use various formulations of mixtures that are liquid at room temperature [8,9,10,11,12]. In addition, DESs are often diluted with water for extraction. However, in this case, the mixture becomes multicomponent, and water participates in the formation of a network of hydrogen bonds [12]. Therefore, it is more correct to use the term, for example, “low-melting mixtures” (LMMs) [13]. From the point of view of practical application, it is not so important whether the eutectic mixture was formed or it is just a mixture with a low melting point. Extraction efficiency is much more important.
Mixtures of choline chloride or betaine with lactic acid are convenient, as they are easy to prepare and homogenize. Various compositions of mixtures of choline chloride or betaine with lactic acid are known in the literature, and the molar ratios turn out to be quite nontrivial, for example, 1:1.3 [14]. In addition, mixtures of various hydrogen bond acceptors with lactic acid have a fairly low glass transition temperature, for example, a 1:2 betaine to lactic acid mixture has a glass transition temperature of −46.86 °C. Mixtures of choline chloride with lactic acid at a ratio of 1:10 have a glass transition temperature of −66.3, for 1:1.3 it is −75.75, and for 1:2 it is −77.32 °C [14].
Mixtures of choline chloride with lactic acid in molar ratios were used in reference [15], including 1:1, 1:2, and 1:3, as well as mixtures of betaine with lactic acid and water at a ratio of 1:1:2, along with water additives of 30–50% by weight. In the work [16], a mixture of the same composition was used to extract phlorotannins from Fucus vesiculosus. At the same time, water was added in an amount of 30–50% by weight. The high antioxidant activity of the obtained extracts was shown in comparison with ethanolic extracts. Mixtures of choline chloride and lactic acid at ratios of 1:1–1:4 were used to extract biologically active substances from tea leaves [10,17]. Mixtures based on choline chloride and lactic acid at a ratio of 1:3 showed a fairly high efficiency in extracting antioxidants from dittany (Origanum dictamnus), fennel (Foeniculum vulgare), marjoram (Origanum majorana), sage (Salvia officinalis), and mint (Mentha spicata) in comparison with water and 60% (v/v) aqueous ethanol. In this case, 80% aqueous solutions of the prepared mixtures were used. However, phenolic compounds and flavonoids were not extracted as well.
Vaccinium vitis-idaea (lingonberry) leaves are used in Chinese medicine as an anti-inflammatory treatment for respiratory tract infections. The Encyclopedia of Traditional Chinese Medicines indicates that raw materials from lingonberry are used as a diuretic and detoxifying agent for infectious diseases. In the Russian State Pharmacopoeia (XIV edition), lingonberry leaves are included in the list of medicinal products and are traditionally used as an antiseptic and diuretic for urinary tract infections, as well as for stomach pain, diarrhea, and rheumatism. In modern Russian medicine, a decoction of V. vitis-idaea leaves is recommended as a diuretic, choleretic, antiseptic, and astringent for the treatment of kidney and bladder diseases, gastroenteritis, diarrhea, rheumatism, gout, and arthritis. In folk medicine, lingonberry leaves and fruits are used to relieve pain and relieve inflammation, especially in gastrointestinal disorders, diseases of the urinary system, and rheumatoid arthritis [18,19,20]. The medicinal properties of this evergreen shrub, which is widely distributed in northern temperate forests, are due to its phytochemical composition, including anthocyanins, proanthocyanidins, flavonols, phenolic acids, and simple phenols [21,22,23]. V. vitis-idaea leaves contain various triterpenoids [24] that exhibit anti-cancer activity, for example, against HT-29, IGR39, and CaKi-1 cancer cells [25].
Among the various extraction methods used to extract phenolic compounds, acetone, ethanol [22], and methanol [26] extraction are found. Deep eutectic solvents, or mixtures like them, may be eco-friendly alternatives for the extraction of BASs from V. vitis-idaea. However, no studies have been found on the use of deep eutectic solvents or low-melting organic mixtures from lingonberry leaves. Therefore, the presented study is devoted to eliminating this gap concerning the development of methods for the use of low-melting organic mixtures for the extraction of BASs from V. vitis-idaea, as well as evaluating the effect of solvent composition on extraction efficiency.
This study aimed to investigate ultrasound-assisted extraction of phenolic compounds from Vaccinium vitis-idaea leaves using low-melting mixtures of choline chloride or betaine with lactic acid and water and to evaluate the impact of mixture composition on the yield of target compounds.
2. Materials and Methods
2.1. Plant Material and Reagents
The commercially available plant raw materials of V. vitis-idaea leaves (Pharmatsvet, Russia) were used in the work. The leaves were ground and sifted through a sieve with holes 1 mm in diameter.
Choline chloride (99%, Rongsheng Biotech, Shanghai, China), betaine (>95%, Himfarmproduct, St. Petersburg, Russia), and lactic acid (80%, Vekton, St. Petersburg, Russia) were used for LMM preparation. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (99%, Sigma-Aldrich, Rockville, MD, USA); diammonium-2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) (>98%, Toronto Research Chemicals, Inc., Vaughan, ON, Canada); ammonium orthomolybdate; potassium dihydrogen phosphate; aluminum chloride (>99%, Vekton, Russia); ethanol (96%, RFK Company, Moscow, Russia); concentrated sulfuric and hydrochloric acids (>94%, Nevareactiv, St. Petersburg, Russia); glacial acetic acid (Vekton, Russia); ascorbic acid (>99.7%, Hugestone, Nanjing, China); rutin, myricetin, and quercetin analytical standards (>98%, Sigma-Aldrich, USA); and HPLC-grade acetonitrile (Component Reactive, Moscow, Russia) were used for chemical analysis. Deionized water was produced via the “Millipore Element” water purification system (Millipore, Burlington, MA, USA).
2.2. Low-Melting Mixture Preparation and Characterization
Based on the data available in the literature, several ratios of choline chloride (or betaine) and lactic acid were selected for testing: 1:1, 1:2, and 1:10. Often, to reduce the viscosity, a certain amount of water is added to the LMM, based on the mass content. A significant decrease in viscosity is observed when more than 20% by weight of water is added. At the same time, water participates in the formation of a network of hydrogen bonds between the components. Therefore, water should be considered not only as a diluent, but as a component of a complex formed due to the formation of hydrogen bonds. Therefore, it is more correct to calculate the addition of water not from the mass, but from the stoichiometric ratio. In the presented work, the molar ratios of choline chloride (or betaine) and water were 1:1, 1:3, and 1:5. Thus, the compositions of the mixtures used in the work are written as [ChCl][Lac]x[H2O]y or [Bet][Lac]x[H2O]y (where ChCl—choline chloride; Bet—betaine; Lac—lactic acid; x = 1, 2, or 10; y = 1, 3, or 5).
To prepare the mixtures, the required mass of the components were measured using an CAS CAUW 120D Ver. NO 2.3.3 analytical balance (CAS Corporation, Yangju, Republic of Korea), mixed in a closed flask, and heated to form a homogeneous liquid at 80 °C. An 80% aqueous lactic acid solution was used for preparation, and, in some cases, water was evaporated after mixing to achieve the desired mixture composition.
The densities of the solvents were measured using a DMA 5000 M density meter (Anton Paar GmbH, Graz, Austria); the measurement uncertainty was 0.00001 g·cm−3. The viscosities were determined using a Modular Compact Rheometer MCR 702 (Anton Paar GmbH, Austria); the measurement uncertainty was 0.08 mPa·s.
2.3. Ultrasound-Assisted Extraction, Kinetics, and Box–Behnken Design Optimization
Based on methods described in [27], ultrasound-assisted extraction was performed using a Vilitek VBS 3-DP ultrasound bath (Vilitek, Moscow, Russia, 2018) at 120 W and 40 kHz. Kinetic studies were conducted at 45 °C with a volume-to-mass ratio of 20:1 over a time range of 10–60 min at 10-min intervals. Data were fitted using a second-order kinetic model [27,28]. A Box–Behnken design (BBD) with five replicates of the center point, combined with response surface methodology (RSM), was used to optimize extraction conditions. The temperature ranged from 30 to 60 °C, volume-to-mass ratio from 10:1 to 20:1, and the molar ratio of water from 1 to 3. BBD parameters are presented in Table 1.

Table 1.
Combinations of parameters for optimization of extraction conditions for the BBD.
2.4. Chemical Analysis
The total phenolic content (TPC) was measured using the Folin–Ciocalteu method [27] with a DEL-100 96-well microplate reader (Miulab, Hangzhou, China). Briefly, 100 µL of 0.2 M Folin–Ciocalteu reagent was mixed with 20 µL of extract diluted 100 times. After this, 80 µL of 5% sodium carbonate was added. The mixture was incubated at 25 °C for 1 h. Absorbance was measured at 750 nm. The TPC is expressed in terms of mg gallic acid equivalent per 1 g of plant material (mg GAE/g).
The total flavonoid content (TFC) was measured using aluminum chloride complexation reaction. Briefly, 100 µL of 2% aluminum chloride solution in 90% ethanol were mixed with 100 µL of extract diluted 10 times. The mixture was incubated at 25 °C for 1 h. Absorbance was measured at 415 nm using a DEL-100 microplate reader. The TFC is expressed in terms of mg rutin equivalent per 1 g of plant material (mg RE/g).
LC-UV analysis was performed with a Milichrom A-02 liquid chromatograph with a ProntoSil-120-5-C18 AQ column, 75 × 2 mm, with a particle size of 5 μm (Econova, Novosibirsk, Russia, 2022). The method is described in detail in [27]. The calibration was performed for myricetin in the concentration range of 125–1000 μg/mL (C = 20.5 × S + 119.1, R2 = 0.9997) and quercetin in the concentration range of 62.5–1000 μg/mL (C = 11.8 × S + 26.9, R2 = 0.9996).
2.5. Antioxidant Activity Measurements
The total antioxidant content (TAC) was measured using the phosphomolybdate method described in detail in [28,29]. Absorbance was measured at 750 nm. TAC was expressed in terms of mg ascorbic acid equivalent per 1 g of plant material (mg AAE/g).
To measure free radical scavenging using the DPPH method [30], 100 µL of DPPH (4 mg in 25 mL of 90% ethanol) was mixed with diluted extract in a 96-well microplate. The concentration of extract was in the range 11.25–0.6925 mg/mL. The concentration was obtained by dissolving a sample of the extract (measured using an analytical balance) in 1 mL of water. After that, a series of binary dilutions was performed. Measurements were carried out for three series of dilutions of different initial concentrations. Absorbance was measured at 520 nm.
For the ABTS method [31], 7.2 mg of ABTS was dissolved in 2 mL of K2S2O8 solution (3.31 mg of K2S2O8 in 5 mL of deionized water), and it was left in the dark at room temperature for 12–24 h before use. After this, the ABTS solution was dissolved 16 times. For the measurements, 100 mL of diluted ABTS solution was mixed with 100 mL of extract in a concentration range of 63.29–3.22 mg/mL in a 96-well microplate. Absorbance was measured at 630 nm.
Experimental data on free radical scavenging were approximated with Boltzmann’s sigmoidal equation:
where I—inhibition (%), IC50—half maximal inhibitory concentration, C—concentration of extract (mg/mL), and dC—a constant.
2.6. Statistical Analysis
All measurements were taken three times for each analysis. The statistical analysis was conducted using factorial analysis of variance (ANOVA) and a post hoc Tukey’s HSD test with a significance level of p ≤ 0.05. The calculations were performed using Microsoft Excel 2010. The design and optimization of BBD were carried out using Design Expert 11 (StatEase, Minneapolis, MN, USA).
3. Results
3.1. LMM Characterization
Figure 1a,c show that the density of mixtures increases with [ChCl][Lac] or [Bet][Lac] molar ratios of 1:1, 1:2, and 1:10. As water content increases, mixtures with [ChCl][Lac] or [Bet][Lac] ratios of 1:1 and 1:2 exhibit similar behavior, with density decreasing consistently. However, for mixtures with a [ChCl][Lac]10 or [Bet][Lac]10 ratio, water content has a minimal effect on density. Notably, the density of [Bet][Lac]10[H2O]5 is markedly lower than that of mixtures with other water contents. Mixtures with [ChCl][Lac]2 or [Bet][Lac]2 ratios exhibit higher viscosity than those with other molar ratios of the components. For [ChCl][Lac] mixtures, viscosity decreases steadily with increasing water content. The viscosities of [Bet][Lac]10[H2O] and [Bet][Lac]10[H2O]3 are similar, whereas that of [Bet][Lac]10[H2O]5 is markedly lower.
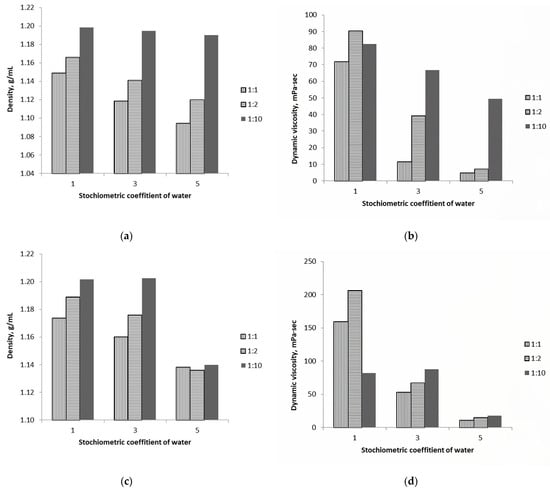
Figure 1.
Densities and dynamic viscosities for [ChCl][Lac]x[H2O]y (a,b) and [Bet][Lac]x[H2O]y (c,d) with various molar ratios of ChCl (or Bet) and Lac. The densities measurement uncertainty was 0.00001 g·cm−3, and the dynamic viscosities measurement uncertainty was 0.08 mPa·s.
3.2. Kinetical Analysis
The TPC data are well approximated by the second-order reaction equation (Table 2). The values of the coefficients of determination are more than 0.9 in most cases. The TFC data are approximated well without exceptions. The rate constants of the extraction of phenolic compounds in mixtures with betaine are lower than with choline chloride. At the same time, there is no correlation with the densities or viscosities of the mixtures. The rate constants of flavonoid extraction, on the contrary, are higher for mixtures with betaine than for mixtures with choline chloride. Similar patterns are found for the values of the output times of the process to the regular regime.

Table 2.
Parameters from fitting the experimental data on the dependence of the TPC and TFC yields on time using a second-order reaction equation.
It was found that 30 min is sufficient for extraction. At this time, a comparative extraction was performed. It has been shown (Figure 2) that a mixture of [Bet][Lac]10[H2O]3 is the most effective for both phenolic compounds and flavonoids.
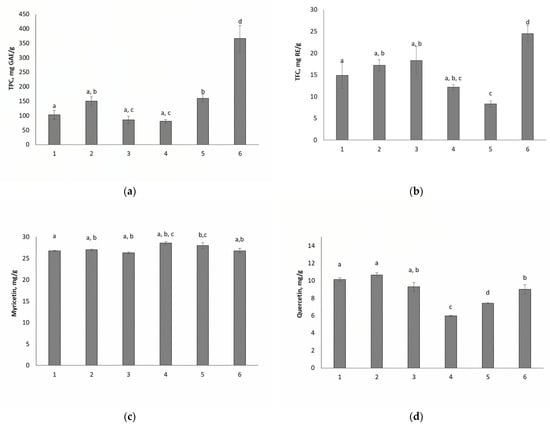
Figure 2.
Comparison of the effectiveness of mixtures of different compositions in relation to the extraction of TPC (a) and TFC (b) as well as the yield of myricetin (c) and quercetin (d). 1—[ChCl][Lac][H2O]3, 2—[ChCl][Lac]2[H2O]3, 3—[ChCl][Lac]10[H2O]3, 4—[Bet][Lac][H2O]3, 5—[Bet][Lac]2[H2O]3, 6—[Bet][Lac]10[H2O]3. The same letters denote the values, the difference between which is not statistically significant at p < 0.05.
3.3. Optimization
A mixture of [Bet][Lac]10[H2O]3 was used to further optimize the extraction conditions according to the BBD. TPC and TFC responses were satisfactory approximated with the following equation:
where A—temperature, B—volume-to-mass ratio, and C—stoichiometric coefficients of water in the complex.
Y = a0 +a1A + a2B + a3C + a4AB + a5AC + a6BC + a7A2 + a8B2 + a9C2 + a10A2B+ a11A2C+ a12AB2
The coefficients for the equations are presented in Table 3. The coefficients of correlation (R2) are 0.999 for TPC and 0.998 for TFC. Lack-of-fit values are not significant for all responses. Thus, the quality of the data approximation can be considered quite good. It should also be noted that the term BC is insignificant for TPC, and the exclusion of its equation leads to the best approximation quality. For TFC, the combined influence of volume-to-mass ratio (B) and stoichiometric coefficients of water (C) is significant. Data approximation is often limited to 10 parameters [32]. However, adding additional terms such as A2B, A2C, and AB2 improves the quality of the approximation and increases R2. Excluding additional terms from the equation gives a worse description. Response surfaces for TPC and TFC are presented in Figure 3 and Figure 4.

Table 3.
Coefficients of models for the fitting of response surface methodology results and coefficients of determination (R2).
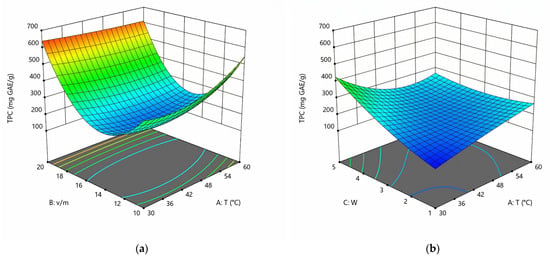
Figure 3.
Response surfaces displaying the effect of extraction temperature (T), volume-to-mass ratio (v/m), and water content (w) on the extraction yield of TPC at w = 3 (a) and v/m = 15 (b).
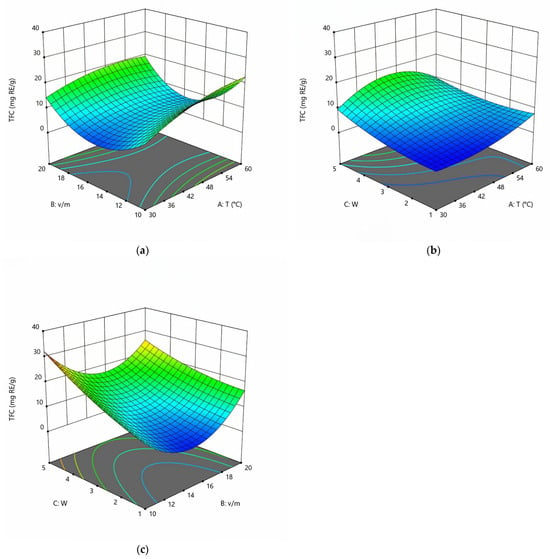
Figure 4.
Response surfaces displaying the effect of extraction temperature (T), volume-to-mass ratio (v/m), and water content (w) on the extraction yield of TFC at w = 3 (a), v/m = 15 (b), and T = 45 °C (c).
Based on obtained equations described TPC and TFC, optimal extraction conditions were calculated to obtain the maximum TPC and TFC yields. It was found that the optimal LMM composition is [Bet][Lac]10[H2O]5, temperature is 33 °C, and volume-to-mass ratio is 20. To confirm the calculations, extraction was performed under the calculated optimal conditions. The experimental value of TPC was 744.3 ± 1.2 mg GAE/g (calculated value is 788.2), and that for TFC was 24.4 ± 0.2 mg RE/g (calculated value is 20.1). Thus, we can talk about the good quality of optimization and predictive capabilities of the model.
3.4. Antioxidant Activity
Various types of antioxidant activity were measured for the extract obtained under optimal conditions: the ability to reduce metal ions (TAC) as well as the ability to inhibit neutral (DPPH) and charged (ABTS) free radicals. The TAC value was 89.9 ± 4.0 AAE/g. From approximated data on free radical scavenging (Figure 5), it was found that the IC50 for DPPH was 2.45 mg/mL, and that for ABTS was 3.47 mg/mL. In general, the IC values are quite close, but the ABTS radical is somewhat less inhibited by the substances contained in the extract.
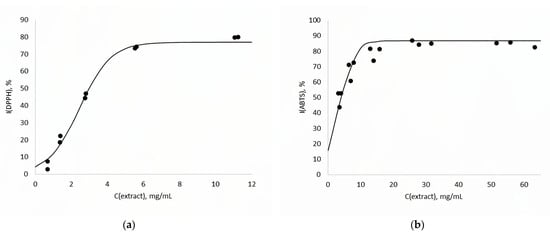
Figure 5.
Dependence of free radical inhibition of DPPH (a) and ABTS (b) on extract concentration, C(extract). •—experimental data, ―—approximation curve.
4. Discussion
The addition of water significantly affects the density and viscosity of the mixtures, consistent with the literature data for other systems [33,34,35,36]. Notably, the mass fraction of water varies depending on the molar ratio of choline chloride (or betaine) to lactic acid. For the [ChCl][Lac][H2O]x complexes, the water mass fraction ranges from 7.3% to 28.2% as x increases from 1 to 5. For [ChCl][Lac]2[H2O]x, it ranges from 5.3% to 22.0%. For [ChCl][Lac]10[H2O]x, it ranges from 1.7% to 8.0%. This likely explains why changes in water content have a less pronounced effect on the density and viscosity of [ChCl][Lac]10[H2O]x compared to complexes with choline chloride-to-lactic acid ratios of 1:1 and 1:2. Similarly, for [Bet][Lac][H2O]x complexes, the water mass fraction ranges from 8.0% to 30.3%. For [Bet][Lac]2[H2O]x complexes, it ranges from 5.7% to 23.2%. For [Bet][Lac]10[H2O]x complexes, it ranges from 1.7% to 8.1%. However, the [Bet][Lac]10[H2O]5 mixture exhibits a marked decrease in density, which is not observed in the corresponding choline chloride-based mixture. This mixture also demonstrated superior extraction efficiency, suggesting unique structural properties that enhance its performance compared to the general trend.
The rate constants obtained as a result of this work are generally consistent with values that are known from other studies for other solvent compositions. For example, in [27], for mixtures of polybasic carboxylic acids and glycerol (or propylene glycol), the maximum value of k = 65.6 × 10−3 g/mg×min (for a mixture of malonic acid and propylene glycol) was obtained for the extraction of flavonoids, and the minimum value was k = 4.4 × 10−3 g/mg×min (for a mixture of citric acid and glycerin). In the case of phenolic compounds, the k values ranged from 0.6 to 17 × 10−3 g/mg×min. In [28], mixtures of choline chloride and polybasic carboxylic acids were considered. The extraction rate constants of phenolic compounds ranged from 0.3 to 2.5 × 10−3 g/mg×min, and that of flavonoids ranged from 1.1 to 3.6 × 10−3 g/mg×min. In this work, the rate constants of extraction of phenolic compounds range from 1.1 to 54.8 × 10−3 g/mg×min, and that of flavonoids range from 5.4 to 44.6 × 10−3 g/mg×min. It can also be noted that, on average, the extraction rate constants of polyphenols are lower than those of flavonoids. This may be due to the fact that the sum of phenolic compounds includes various classes of metabolites, some of which are obviously extracted more slowly. The lower R2 value for [ChCl][Lac]10[H2O]3 in TPC extraction kinetics may be attributed to unique intermolecular interactions or higher viscosity affecting mass transfer, warranting further structural and kinetic studies.
The relative effectiveness of the mixtures in relation to TPC and TFC does not correlate with each other. It can be noted that TPC and TFC values for mixtures with choline chloride have practically no statistical differences. However, the ratio of betaine and lactic acid strongly affects the yield of the target groups of substances. Interestingly, the yield dependence of the total amount of glycosides of myricetin and quercetin does not depend so much on the composition of the mixtures. The yield of myricetin glycosides for all extracts is almost the same and varies between 25 and 30 mg/g. The yield of the total amount of quercetin glycosides is the same for mixtures with choline chloride (about 10 mg/g) and depends more on the composition in the case of mixtures of betaine with lactic acid (6–9 mg/g).
Comparing the yield of the sum of phenolic compounds with the values known in the literature, it can be found that lingonberry juice contains 3.64–6.60 mg GAE/g of phenolic compounds [21]. The literature mentions a different content of phenolic components in extracts of lingonberry leaves. For example, in [37], it was found that the ethanol extract contains 130–140 mg GAE/g phenolic compounds and 499.418 ± 8.03 mg QE/plant material (QE—quercetin equivalent). In another study, acidified aqueous ethanol was used to extract 85.95 ± 0.99 mg GAE/g [38]. In [39], TPC ranged from 468 to 661 mg GAE/100 g fresh weight. At the same time, it is quite difficult to compare the results obtained in different studies for different raw materials, since the content of certain substances depends on the place of collection and the season [40,41].
Among the flavonoids in lingonberry leaves, mainly glycosides of quercetin and kaempferol were found [23,37]. In addition, the content of astragalin, avicularin, hyperoside, and quercitrin was evaluated in the work [42]. The quercetin content varied from 1676.31 ± 68.43 to 7826.68 ± 319.52 µg/g for cultivars or lower taxa using ethanolic extraction. In the present work, the quercetin content is from about 6.0 ± 0.1 to 10.7 ± 0.3 mg/g. However, no kaempferol compounds were found in the extracts obtained in this work.
In general, based on the results of the work, it can be seen how strongly the composition of LMMs can affect the yield of target groups of substances, while the most suitable component ratio is far from equimolar (which is often used in the preparation of “DES”). The use of [Bet][Lac]10[H2O]5 offers a scalable, environmentally friendly alternative to traditional solvents like ethanol. However, challenges such as higher viscosity and the cost of betaine may require optimization for industrial applications. The components included in the resulting extract allow it to be used to develop formulations of biologically active additives, although it is necessary to follow the recommendations regarding the use of food additives, taking into account the antibacterial activity of substances contained in lingonberry leaves [43].
5. Conclusions
The study demonstrated the effectiveness of using environmentally friendly technologies for the extraction of biologically active substances (BASs) from plant raw materials, which is a relevant area in modern science and industry. Particular attention is paid to the use of low-melting mixtures known as “deep eutectic solvents” (DESs) for the extraction of phenolic secondary metabolites from lingonberry leaves (Vaccinium vitis-idaea L.).
The kinetics of ultrasonic extraction of phenolic compounds from lingonberry leaves using mixtures of choline chloride, lactic acid, and water as well as betaine, lactic acid, and water were studied for the first time. The extraction conditions, including mixture selection, temperature, and the ratio of plant material mass to solvent volume, were optimized using the experimental planning method (Box–Behnken design). It was found that the optimal mixture for extraction is betaine with lactic acid and water at a molar ratio of 1:10:5. The optimal extraction temperature is 33 °C, and the optimal ratio of plant material mass to solvent volume is 1:20. The obtained extracts have a high antioxidant activity, which is confirmed by the results of tests with the free radicals DPPH and ABTS.
This study significantly contributes to the development of environmentally friendly technologies for extracting BASs from Vaccinium vitis-idaea leaves. This study provides novel insights into the use of betaine-based LMMs for ultrasonic extraction of phenolic compounds from Vaccinium vitis-idaea leaves, offering a sustainable alternative to traditional solvents for applications in cosmetics, functional nutrition, and pharmaceuticals.
Author Contributions
Conceptualization, N.T. and O.M.; methodology, N.T. and A.K.; software, N.T.; validation, A.A. and A.K.; formal analysis, N.T.; investigation, A.A.; resources, A.K. and A.S.; data curation, A.A., A.K. and A.S.; writing—original draft preparation, N.T.; writing—review and editing, N.T., A.A., A.K., A.S. and O.M.; visualization, N.T. and A.K.; supervision, N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 25-25-00287.
Data Availability Statement
Raw data are available upon reasonable request from the corresponding author, subject to institutional restrictions.
Acknowledgments
The experimental work was facilitated by the equipment of Centre for Diagnostics of Functional Materials for Medicine, Pharmacology and Nanoelectronics, St. Petersburg State University Research Park.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Dzhavakhyan, M.A.; Prozhogina, Y.E. Deep Eutectic Solvents: History, Properties, and Prospects. Pharm. Chem. J. 2023, 57, 296–299. [Google Scholar] [CrossRef]
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojčić Redovniković, I.; Duarte, A.R.C. Review of Deep Eutectic Systems from Laboratory to Industry, Taking the Application in the Cosmetics Industry as an Example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Zuo, J.; Geng, S.; Kong, Y.; Ma, P.; Fan, Z.; Zhang, Y.; Dong, A. Current Progress in Natural Deep Eutectic Solvents for the Extraction of Active Components from Plants. Crit. Rev. Anal. Chem. 2023, 53, 177–198. [Google Scholar] [CrossRef]
- Wawoczny, A.; Gillner, D. The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (Nades): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Ho Row, K. Extraction and Determination of Quercetin from Ginkgo Biloba by DESs-Based Polymer Monolithic Cartridge. J. Chromatogr. Sci. 2017, 55, 866–871. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of New Natural Deep Eutectic Solvents for the Extraction of Isoflavones from Soy Products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhou, Y.; Zhang, M.; Xia, Q.; Bi, W.; Chen, D.D.Y. Ecofriendly Mechanochemical Extraction of Bioactive Compounds from Plants with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 6297–6303. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ruß, C.; König, B. Low Melting Mixtures in Organic Synthesis—An Alternative to Ionic Liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar] [CrossRef]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. New Natural and Renewable Low Transition Temperature Mixtures (LTTMs): Screening as Solvents for Lignocellulosic Biomass Processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 21–24. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel Lactic Acid-Based Natural Deep Eutectic Solvents: Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Common Native Greek Medicinal Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Pukalskas, A.; Sipailiene, A.; Raudone, L. Exploring Vaccinium vitis-idaea L. as a Potential Source of Therapeutic Agents: Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Extracts and Fractions. J. Ethnopharmacol. 2022, 292, 115207. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; Their History and Applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal Plants from the 14th Edition of the Russian Pharmacopoeia, Recent Updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Kostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Stojanov, L.; Veličkovska, S.K.; Winterhalter, P.; Esatbeyoglu, T. Bioactive Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.): Extraction, Chemical Characterization, Fractionation and Cellular Antioxidant Activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, A.A.; Bubenchikova, V.N.; Chernikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium vitis-idaea L.: Chemical Contents, Pharmacological Activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Optimization, Validation and Application of HPLC-PDA Methods for Quantification of Triterpenoids in Vaccinium vitis-idaea L. Molecules 2021, 26, 1645. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Marksa, M.; Ivanauskas, L.; Jakstas, V.; Raudone, L. Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants 2023, 12, 465. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L. Development, Validation, and Application of UPLC-PDA Method for Anthocyanins Profiling in Vaccinium L. Berries. J. Berry Res. 2021, 11, 583–599. [Google Scholar] [CrossRef]
- Koigerova, A.; Gosteva, A.; Samarov, A.; Tsvetov, N. Deep Eutectic Solvents Based on Carboxylic Acids and Glycerol or Propylene Glycol as Green Media for Extraction of Bioactive Substances from Chamaenerion angustifolium (L.) Scop. Molecules 2023, 28, 6978. [Google Scholar] [CrossRef]
- Tsvetov, N.; Pasichnik, E.; Korovkina, A.; Gosteva, A. Extraction of Bioactive Components from Chamaenerion angustifolium (L.) Scop. with Choline Chloride and Organic Acids Natural Deep Eutectic Solvents. Molecules 2022, 27, 4216. [Google Scholar] [CrossRef]
- Tsvetov, N.; Sereda, L.; Korovkina, A.; Artemkina, N.; Kozerozhets, I.; Samarov, A. Ultrasound-Assisted Extraction of Phytochemicals from Empetrum Hermafroditum Hager. Using Acid-Based Deep Eutectic Solvent: Kinetics and Optimization. Biomass Convers. Biorefin. 2022, 12, 145–156. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M. Optimization of Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polysaccharides from Dioscorea Opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, M.M.; Pedro, S.N.; Vilela, C.; Vaz, P.D.; Ribeiro-Claro, P.; Rudić, S.; Parker, S.F.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Water in Deep Eutectic Solvents: New Insights From Inelastic Neutron Scattering Spectroscopy. Front. Phys. 2022, 10, 834571. [Google Scholar] [CrossRef]
- Ninayan, R.; Levshakova, A.S.; Khairullina, E.M.; Vezo, O.S.; Tumkin, I.I.; Ostendorf, A.; Logunov, L.S.; Manshina, A.A.; Shishov, A.Y. Water-Induced Changes in Choline Chloride-Carboxylic Acid Deep Eutectic Solvents Properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132543. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M. The Formulation and Physicochemical Properties of Betaine-Based Natural Deep Eutectic Solvent. J. Mol. Liq. 2022, 360, 119392. [Google Scholar] [CrossRef]
- Sánchez, P.B.; González, B.; Salgado, J.; José Parajó, J.; Domínguez, Á. Physical Properties of Seven Deep Eutectic Solvents Based on L-Proline or Betaine. J. Chem. Thermodyn. 2019, 131, 517–523. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Drózdz, P.; Sežiene, V.; Wójcik, J.; Pyrzyńska, K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules 2018, 23, 53. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Phenological and Geographical Effects on Phenolic and Triterpenoid Content in Vaccinium vitis-idaea L. Leaves. Plants 2021, 10, 1986. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Vaccinium Vitis-idaea L. Fruits: Chromatographic Analysis of Seasonal and Geographical Variation in Bioactive Compounds. Foods 2021, 10, 2243. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef] [PubMed]
- Ivashkin, V.T.; Maev, I.V.; Abdulganieva, D.I.; Alekseenko, S.A.; Gorelov, A.V.; Zakharova, I.N.; Zolnikova, O.Y.; Ivashkina, N.Y.; Korochanskaya, N.V.; Mammayev, S.N.; et al. Practical Recommendations of Scientific Society for the Study of Human Microbiome and the Russian Gastroenterological Association on Use of Probiotics, Prebiotics, Synbiotics and Functional Foods in Treatment and Prevention of Gastroenterological Diseases. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2021, 31, 65–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).