Abstract
Wheat (Triticum aestivum L.) is a major global staple crop, widely consumed in processed forms such as bread and pasta. As consumer demand for healthier food options increases, organic wheat production is gaining importance. However, organic farming excludes the use of synthetic pesticides and fungicides, potentially increasing the risk of fungal contamination and mycotoxin presence. At the same time, questions remain about whether organically grown wheat can match the grain quality needed for industrial processing, particularly in terms of protein content, gluten strength, and dough properties. This study aims to evaluate grain and flour quality parameters, as well as the occurrence of selected mycotoxins, in eight winter wheat cultivars grown under both organic and conventional farming systems in northern Serbia during the 2023/2024 season. Cultivars included modern premium varieties recommended for organic production, as well as one traditional and one conventional cultivar. Despite unfavourable weather conditions in the early stages of the vegetation in 2024, favourable conditions during grain ripening contributed to the good quality of organically produced varieties. The quality parameters of most varieties from organic production (PC > 17%, WG > 49%, WA > 62%) were significantly higher than the standard for organic wheat. NS Epoha stood out as the variety with the highest yield in organic production. As expected, varieties from the organic system had a higher incidence of mycotoxin contamination, but their concentrations were low.
1. Introduction
Common wheat (Triticum aestivum L.), along with other cereal grains, represents a vital source of nutrients and energy in the human diet. It is the second most produced cereal globally, following maize, and the predominant cereal crop in Europe, accounting for nearly half of the total cereal harvest [1]. In Serbia, wheat accounts for a similarly important share of the total cereal production, with an average annual output of 3.3 million tons during 2021–2023 [2]. Wheat serves as the main ingredient in a wide variety of food products, including bread, pasta, pastries, and numerous other processed foods. Globally, it ranked second in 2018 in terms of food supply available for human consumption, with an estimated per capita availability of 78.4 kg per year [1].
Globally, the majority of wheat is produced through conventional farming, while organic production accounts for a significantly smaller share. In Serbia, organic production has been expanding since the 2010s, with cereals representing the second most prominent category within organic crop production. In 2017, organic cereals were grown on 4161 hectares, representing approximately 31% of all certified organic agricultural land in Serbia [3]. However, the area cultivated with organic cereals in Serbia remains considerably smaller compared to neighbouring Hungary, where 33,246 hectares were cultivated in 2018 [4]. This is significantly less compared with 14.8 million hectares in 2020, organic farming accounted for 9.1% of the European Union’s total agricultural area of which cereals represent 16% [5].
The rise in organic production is driven by growing consumer awareness of the health benefits associated with organic foods, including reduced exposure to pesticides and a higher intake of beneficial nutrients and bioactive compounds [6]. As a result, many major retail chains now include organic products their assortments [7]. Organic production prohibits synthetic pesticides, mineral fertilizers, plant growth regulators, and genetically modified cultivars, while emphasizing the use of organic fertilizers, such as manure and compost, to maintain soil fertility and plant health [8,9,10]. However, the absence of fungicide use allows certain fungal strains to develop on cereals, producing secondary metabolites known as mycotoxins [11,12]. Consumption of cereals contaminated with mycotoxins above regulatory limits can cause serious health problems in humans, including gastrointestinal disorders, hormonal imbalances, weakened immune function, and potential mutagenic and carcinogenic effects [13,14]. The key mycotoxins in wheat are deoxynivalenol (DON), zearalenone (ZEA), and T-2/HT-2 toxins, which are produced by Fusarium species infecting cereal grains in the field [11,13,14]. In addition, Fusarium species can also produce the mycotoxin moniliformin (MON) in cereals [15,16]. Among mycotoxins produced by Alternaria fungi, tentoxin (TEN) is among the four most frequently detected in wheat grains [17,18], while α-ergocryptine is one of the six principal alkaloids of Claviceps purpurea that may also occur in cereal grains [19,20]. On the other hand, several studies [21,22] confirm that organic cereal production enhances antioxidant and polyphenolic activity. Dietary intake of polyphenols may play a crucial role in promoting health by regulating metabolism, body weight, the progression of chronic diseases, and cell proliferation [23]. As one of the major subclasses of polyphenols, flavonoids significantly contribute to the overall antioxidant capacity of cereals, protecting biological molecules from oxidative damage by neutralizing free radicals [24].
In addition to the positive impact of organic wheat on human health, the end-use quality of the wheat itself is also very important. Wheat quality is a complex trait defined by various parameters that provide a comprehensive understanding of its suitability for different end-use applications in the food industry. Among these, protein content is the most important parameter, as it largely determines the market value of wheat [25]. Another key indicator of wheat quality is the test weight. A low test weight reflects a higher proportion of small and shrivelled grains in wheat bulk, which are associated with a higher bran content, and, consequently, reduced milling yield [26]. Furthermore, the viscoelastic properties of wheat dough-crucial for its performance-are determined by proteins in the grain endosperm, specifically monomeric prolamins and polymeric glutenins [27]. These proteins constitute the main components of gluten, the complex polymer responsible for dough functionality. While glutenins account for about 70% of dough elasticity [28], gliadins primarily contribute to its extensibility [29]. Both elasticity and extensibility are critical properties in bread production. During fermentation, elastic dough can retain the gases produced without collapsing, while dough with high extensibility contributes to improving bread volume [30]. Therefore, developing a wheat cultivar that combines these desirable properties remains a challenging task for breeders.
The specific objectives of this study were to: (a) evaluate the end-use quality of different wheat cultivars intended for organic production and compare them with those cultivated under conventional farming, (b) assess the impact of production systems (organic vs. conventional) on antioxidant activity, and (c) compare the levels of mycotoxin in wheat grain from different cultivars grown under organic and conventional practices.
2. Materials and Methods
2.1. Materials
A total of eight winter wheat (Triticum aestivum L.) cultivars were included in the study, cultivated under both organic and conventional conditions. The conventional cultivars included the domestic varieties Zvezdana and NS Epoha (Table 1). Zvezdana is one of the five most commercially widespread varieties, valued for its consistent performance and adaptability in standard farming systems, while NS Epoha is a modern high-yielding milling-quality variety and well-balanced end-use characteristics [31]. The organic cultivars included Bánkúti 1201, an old Hungarian heritage variety known for its distinctive quality traits as well as one of the most widely used varieties in organic production in Hungary [32,33]. Also, five premium Hungarian and Austrian wheat varieties (Genotype 1, Genotype 2, Genotype 3, Genotype 4, and Genotype 5) specifically recommended for organic production and recently introduced to Serbia were included (Table 1). NS Epoha was cultivated under both organic and conventional systems to enable a direct comparison of how different growing practices influence its end-use quality, antioxidative properties, and mycotoxin contamination. All cultivars were sown in the autumn of 2023 on the same organic farm, together with the standard varieties NS Epoha and Zvezdana.

Table 1.
Type of farming, end-use quality and originate of wheat varieties.
A field experiment was conducted during the 2023/24 growing season in Kanjiža, northern Vojvodina, Serbia (46°00′ N, 19°54′ E), representing a typical Pannonian environment. The soil at the experimental site was classified as a haplic chernozem aric [34]. Prior to sowing, soil samples were collected from the 0–30 cm layer for analysis, and the basic soil properties are presented in Table 2.

Table 2.
Basic soil properties of the organic production field in 2023.
The production fields follow a long-term crop rotation of small-grain cereals, maize, and soybeans, with one of the field certified for organic production. The preceding crop for winter wheat was maize in both organic and conventional production fields. Selected winter wheat cultivars were sown at the optimal regional time (13–14 October 2023) at a density of 400–500 viable seeds per m2, as recommended for large-scale production.
In the organic system, 346 kg per ha of organic fertilizer derived from composted and heat-treated poultry manure (Aminor; NPK 4/3/3 + 10 CaO + 60% OM + 30% C + SE) was applied before ploughing. No additional fertilizers or pesticides were used in organic production. In the conventional production system, a total of 120 kg N, 30 kg P2O5, and 30 kg K2O per ha was applied. Weeds, diseases, and pests were controlled using recommended herbicides, fungicides, and insecticides (tribenuron-methyl + fluroxypyr; cyproconazole + picoxystrobin; deltamethrin). Harvest was carried out on 3 July 2024.
2.2. Grain and Flour Quality Traits
The protein content (PC) and moisture content of the grain were measured using a handheld GrainSense Analyzer (Oulu, Finland). Prior to milling, wheat samples were tempered to 13.5% moisture for 24 h. Thirty min before milling, the samples were further adjusted to a final moisture content of 15% and then milled using a Bühler MLU-202 laboratory mill (Uzwil, Switzerland). Also, the test weight (TW) was determined according to ISO 7971-3:2009 [35].
Wet gluten content (WG) and gluten index (GI) were determined following ICC Method 137/1 [36], using a Glutomatic 2200 system (Perten Instruments, Huddinge, Sweden). Dough properties, including water absorption (WA), farinograph resistance (FR), and softening degree (SD), were evaluated with a farinograph (Brabender OHG, Duisburg, Germany) following the Hungarian Standard Method 6369/653 [37]. Alveograph parameters, including deformation energy (W), extensibility (L), and the over-pressure/extensibility ratio (P/L), were assessed using a Chopin alveograph (Paris, France), according to ICC Method 121 [38].
2.2.1. Total Phenolic Content
The total phenolic content (TPC) was determined using a modified Folin–Ciocalteu assay [39]. Briefly, 0.5 mL of the wheat extract was transferred into a 10 mL volumetric flask containing 6.0 mL of double-distilled water and 0.5 mL of Folin–Ciocalteu reagent. After 60 s of reaction, 2.0 mL of 15% (w/v) sodium carbonate solution was added, and the mixture was stirred for 30 s. The volume was then adjusted to 10 mL with double-distilled water. The solution was incubated in the dark for 45 min, after which the absorbance was measured at 760 nm using a UV–Vis spectrophotometer (UV-2100, Unico Instrument Co., Shanghai, China). A calibration curve was prepared using gallic acid, and the results were expressed as milligrams of gallic acid equivalents per 100 g of dry matter (mg GAE/100 g DM).
2.2.2. Total Flavonoid Content
The total flavonoid content (TFC) was determined using the aluminum chloride colorimetric method [40]. In a 10 mL volumetric flask, 1.0 mL of wheat extract was mixed with 4.0 mL of double-distilled water, followed by the addition of 0.3 mL of 5% (w/v) sodium nitrite solution. After 5 min, 0.3 mL of 10% (w/v) aluminum chloride was added. One minute later, 2.0 mL of 1 mol/L sodium hydroxide was added, and the volume was adjusted to 10 mL with double-distilled water. The mixture was incubated in the dark at room temperature for 15 min, and the absorbance was measured at 510 nm. Quercetin was used as the calibration standard, and the results were expressed as milligrams of quercetin equivalents per 100 g of dry matter (mg QE/100 g DM).
2.3. Mycotoxins Analysis
The modified liquid chromatography tandem mass spectrometry (LC-MS/MS) method established by Hofmann and Scheibner [41] was used for the quantification of mycotoxins in the collected wheat cultivars. Briefly, this method enabled the simultaneous monitoring of 22 mycotoxins. For this purpose, the following chemicals were used: methanol (MeOH) (Carlo Erba, Val de Reuil, France), water (Carlo Erba, Val de Reuil, France), and formic acid (Fluka Analytical, Sigma-Aldrich, Steinheim, Germany) of LC-MS/MS grade, and acetonitrile (ACN) (Fisher Scientific, Geel, Belgium) of HPLC grade. With the exception of T-2 toxin (100 μg/mL), which was acquired from LGC Standards (Wesel, Germany), mycotoxin standards were obtained as follows: DON (100 μg/mL), fumonisins (FUs) (FB1 and FB2, each 50 μg/mL), HT-2 toxin (100 μg/mL), ZEN (100 μg/mL), TEN (100 μg/mL), alternariol (5 mg) and alternariol monomethyl ether (5 mg) from Fluka Analytical (Steinheim, Germany); MON (100.4 μg/mL), mixed standards of six ergot alkaloids (100 μg/mL of each), and a mixed aflatoxins standard (Afs) (2 μg/mL of AFB1 and AFG1 and 0.5 μg/mL of AFB2 and AFG2) from Biopure, Romerlab (Tuln Austria). MeOH/water (50/50, v/v) was used to dissolve a mixed standard stock solution of 15 distinct mycotoxin standards. Calibration solutions were prepared by spiking matrix extracts with a mixture of standards. The individual standards were dried down and reconstituted in MeOH/water (50/50, v/v). Eleven calibration levels were used, ranging from 0.01 to 200 ug/kg, with correlation coefficients (r2) greater than 0.9915. All mycotoxin standards and stock standard solutions were stored at −18 °C until analysis.
2.3.1. Sample Preparation for LC-MS/MS Analysis
Sample preparation for LC-MS/MS analysis followed the protocol of Hofmann and Scheibner [41] with slight modifications. HPLC-grade acetonitrile purchased from Fisher Scientific (Geel, Belgium) and water purified using an Adrona Water Purification system (Riga, Latvia) were used for sample preparation. In brief, 5 g of ground wheat sample was placed into a 50 mL polypropylene tube and extracted with acetonitrile/water (80/20, v/v). During extraction, samples were shaken on a horizontal shaker (Biosan, Latvia) at 350 rpm for 60 min and centrifuged (Boeco, Hamburg, Germany) at 4000 rpm for 5 min. A 400 μL aliquot of the supernatant was diluted with 600 μL MeOH/water (50/50, v/v) and filtered through a 0.2 µm polytetrafluoroethylene (PTFE) disposable syringe filter into vials.
2.3.2. LC-MS/MS Analysis
An HPLC Vanquish Core system equipped with the Hypersil GOLD C18 Selectivity HPLC column (100 × 2.1 mm i.d., 1.9 μm particle size), coupled to a TSQ Quantis Triple Quadrupole mass spectrometer with a heated electrospray ionization (HESI) source (all from Thermo Fisher Scientific, Waltham, MA, USA), was used for the detection and quantification. The analysis was carried out as described in the ThermoFisher Scientific Application Note 65,969 [41], with several modifications. Briefly, the mobile phase consisted of water (95%) and methanol (5%), each containing 0.1% formic acid. The autosampler tray was maintained at 20 °C, and the column was set to 40 °C, with an injection volume of 10 μL. The retention times and the SRM transitions for the analyzed compounds are summarized in Table 3. Nitrogen was used as the sheath, auxiliary, and sweep gas, set to 30, 6, and 1 arbitrary units (Arb), respectively. Argon at 1.5 mTorr served as the collision-induced dissociation (CID) gas. The ion transfer tube and vaporizer temperatures were adjusted to 325 °C and 350 °C, respectively. The duration of each cycle was 0.5 s. Data acquisition and processing were carried out using TSQ Quantis 3.2 Tune and TraceFinder 5.1 (ThermoFisher Scientific, Waltham, MA, USA).

Table 3.
Retention times and SRM transitions for the different mycotoxins.
2.3.3. Method Validation
Method validation was performed in accordance with the Technical Report CEN/TR 16,059 [42] and the European Regulation, No 2002/657/EC [43]. Therefore, the performance of the applied LC-MS/MS method was evaluated in terms of linearity, recovery, repeatability, reproducibility, limit of quantification (LOQ), and matrix effects. The matrix effect was calculated as the signal suppression/enhancement ratio (SSE), expressed as the slope ratio of the matrix-matched calibration curve to solvent-based calibration curve. To distinguish between extraction efficiency and matrix-induced signal suppression/enhancement, the slope ratios of the linear calibration functions were computed to determine the apparent recovery (RA), representing the overall method recovery, and the signal suppression/enhancement (SSE) attributable to matrix effects. The extraction recovery (RE), or sample preparation recovery, was calculated by dividing the overall recovery by the matrix effect (Table 4). The repeatability and reproducibility were assessed for six detected mycotoxins by analyzing blank wheat samples spiked at six concentration levels, each in six replicates (Table 5). Limits of detection (LOD) and quantification (LOQ) were estimated by injecting decreasing concentrations of ma-trix-matched standards and measuring signal-to-noise (S/N) ratios of ≥3 and ≥10, respectively. The established LOD and LOQ fulfil the validation criteria for recovery and repeatability (Table 5).

Table 4.
Matrix effect (SSE) and Recovery data of the employed analytical method based on the solvent (RA) and matrix-matched (RE) calibration curves.

Table 5.
Precision data of the employed LC-MS/MS method for detected mycotoxins.
2.4. Statistical Analysis
Experimental data related to technological parameters and antioxidant activity were subjected to one-way analysis of variance (ANOVA), supplemented by Tukey’s Honestly Significant Difference (HSD) test, to discern notable differences at a significance level of p < 0.05. ANOVA analyses were performed using InfoStat software, Version 2016e (UNC, Córdoba, Argentina; trial version). The relationship among the studied parameters was further evaluated using Pearson’s correlation analysis, with correlations considered statistically significant at p < 0.05. Principal Component Analysis (PCA) was also performed to identify patterns and groupings among the variables. These were conducted using XLSTAT BASIC, version 5.1, 2022 software (Addinsoft, Paris, France).
3. Results
The 2023/24 growing season was the warmest on record in Serbia. According to data from the local meteorological station in Kanjiža, the autumn-winter period (October–December) was characterized by temperatures considerably above the long-term average (Figure 1). The total amount of precipitation during this period was about 10% lower than the multiannual average (378 mm vs. 410 mm), with substantial variability in distribution. Precipitation in October was markedly below average, hindering soil tillage and crop emergence, whereas November recorded the highest precipitation total in recent decades, replenishing soil moisture reserves. Unusually warm weather persisted throughout the spring vegetation period in 2024, accompanied by below-average precipitation, particularly in February and March.
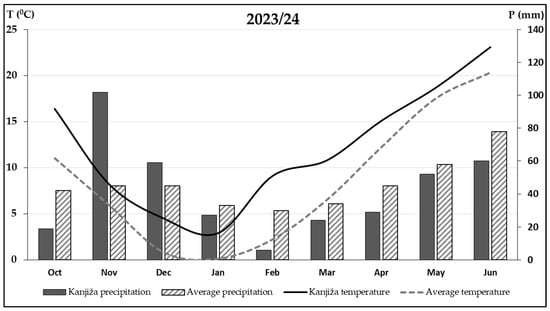
Figure 1.
Monthly temperature and precipitation in 2023/24 vs. long-term average.
Physical, chemical, and functional characteristics of the investigated wheat cultivars are summarized in Table 6. The NS Epoha cultivar exhibited nearly a twofold statistically higher yield under conventional than under organic production, which was an expected outcome (Table 6). Among the tested cultivars, NS Epoha achieved the highest GY in organic production (3.14 t ha−1), with values ranging between 1.95 and 3.14 t ha−1. This is in line with the findings of Földi et al. [33], who reported similar levels in 2018. Despite a lower yield, organic production resulted in wheat with superior quality traits. The PC of five other wheat varieties grown organically reached 17% or more and was statistically higher than other two cultivars from organic production, which is considered high even for organic production, given that Földi et al. [33] reported PC levels of about 15% in both examined years. Only one wheat variety of premium quality from organic production (Genotype 2) showed slightly lower PC—15.5%. The PC of organically produced premium wheat varieties was higher than average PC of bread wheat conventionally produced in Martonvásár and Serbia; 15.2 and 12.9%, respectively [44]. A similar trend was observed for WG and WA among wheat cultivars from organic production, with five varieties exhibiting values above 49% and 62%, respectively. The values of these parameters with five organically grown varieties were statistically higher from other cultivars. Among organically grown wheat cultivars, FR was markedly higher in six cultivars, whereas NS Epoha O was an exception, exhibiting a value of 3.5 min, closer to the values observed in the remaining cultivars from conventional production. The value of this parameter in NS Epoha O did not differ significantly from that of conventionally grown varieties. SD of Genotype 2 O and Genotype 4 O from organic production was significantly lower that of other organically and conventionally grown varieties. Five organic cultivars exhibited a GI below 80%, that was significantly lower than organic cultivars NS Epoha and Genotype 2 showed values of 100% and 96.7%, respectively. Conventional cultivars had a GI higher than 80% significantly lower than organic cultivars NS Epoha and Genotype 2. W was generally higher for organic wheat cultivars, ranging from 184 × 10−4 J to 296 × 10−4 J, whereas cultivars from conventional farming showed values below 172 × 10−4 J. Regarding extensibility, organic cultivars generally exhibited higher values, with three cultivars exceeding 100 mm, while the others ranged from 71 to 98 mm. Among the conventionally grown cultivars, Zvezdana showed the highest extensibility (90 mm), whereas NS Epoha C had lower value of 65 mm. There was no statistical differentiation of this parameter between varieties from organic and conventional production. The P/L for almost all wheat varieties was between 0.6 and 1, which, according to the Italian quality regulation, classifies these varieties between good and improvers [45]. Considering antioxidant activity, organic cultivars generally had higher polyphenol and flavonoid content. Among them, the organic cultivar Genotype 4 was particularly prominent, with total phenolic content (TPC) and total flavonoid content (TFC) of 154.48 and 122.22 mg/100 g DM, respectively, indicating a particularly strong antioxidant potential. There was no statistical differentiation of these two parameters between varieties from organic and conventional production.

Table 6.
Physical, Chemical and Functional Properties of Wheat Cultivars.
The correlations among the physicochemical properties of wheat grains, their rheological behaviour, and antioxidant activity are presented in Table 7. The study revealed that the two key parameters of wheat, GY and PC, were strongly negatively correlated, as previously reported by Silva et al. [46] and Živančev et al. [30]. GY also showed a strong negative correlation with WG, consistent with the findings of Soorninia et al. [47], as well as with farinograph parameters, WA and FR. In contrast, PC was expectedly strongly positively correlated with end-use quality parameters WG and WA, which themselves exhibited a statistically significant positive correlation. WA was strongly negatively correlated with GI, indicating that wheat varieties with high WA values tend to have softer or weaker WG, whereas TW showed a statistically significant positive correlation with FR and P. As expected, alveograph parameters P and L were strongly correlated with the P/L ratio, with P positively and L negatively associated with it. Finally, TFC exhibited a strong positive correlation with PC and a negative correlation with GY.

Table 7.
Correlation among wheat grains’ physicochemical properties, rheological behaviour with antioxidant and polyphenolic activity. Correlation matrix (Pearson).
To investigate the influence of key physical, chemical, functional, and antioxidant properties of wheat cultivars grown under organic and conventional farming systems, a comprehensive principal component analysis (PCA) was conducted (Figure 2). The first principal component (PC1) accounted for more than 45% of the total variability, while the second principal component (PC2) explained over 24%.
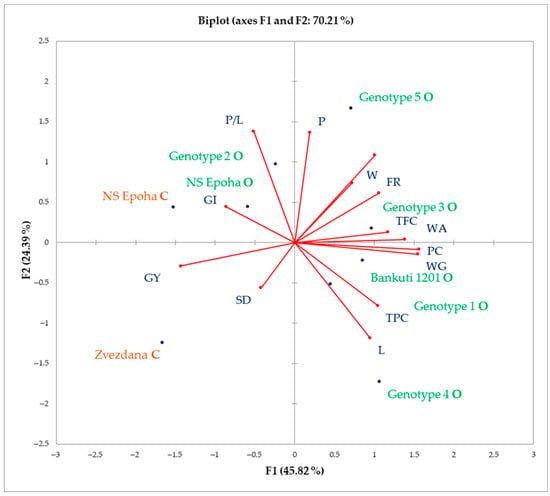
Figure 2.
PCA biplot of the physicochemical, rheological behaviour, and antioxidant properties of wheat grains. Red dots depict examined properties whereas blue dots depict wheat genotypes. The names of wheat genotypes written in green are from organic farming, whereas the names of wheat genotypes written in orange are from conventional farming.
PC was strongly positivelly correlated with WG, WA, and TFC, while strongly negatively correlated with GY, which confirm values of F1 factor (Table 8). TPC and L were positively correlated with each other but negatively correlated with GI and P/L ratio. Also, W exhibited a positive correlation with FR and TW, while displaying a negative correlation with farinograph SD. Furthermore, TPC and showed no association with W, TW, and FR, whereas GY was unrelated to the P/L ratio.

Table 8.
Loading values of first two factors from PCA biplot of the physicochemical, rheological behaviour, and antioxidant properties of wheat grains.
According to the PCA biplot, two wheat varieties from conventional production (Zvezdana and NS Epoha) clustered closely with GY. In contrast, NS Epoha O and Genotype 2 wheat varieties from organic production demonstrated close associations with GI and P/L ratio. The old Hungarian variety Bánkúti 1201 and the Austrian variety Genotype 3 were strongly associated with PC with WG, WA, and TFC, while Genotype 5 was closely linked to P, TW, and W. Additionally, the PCA biplot revealed that the varieties Genotype 1 and Genotype 4 were characterized by high values of L parameter. Mycotoxin contamination levels in the examined wheat samples are presented in Table 9. To minimize matrix effects on the detected mycotoxin concentrations, quantification was performed using an external matrix-matched calibration procedure. Among the 22 mycotoxins analyzed, only six were detected and/or quantified in selected wheat varieties from both organic and conventional farming systems. The warm and dry conditions observed in February and March 2024 (elevated temperatures and below-average precipitationled to wheat flowering (heading) occurring 15–20 days earlier than usual (in the first half of April instead of early May). These weather conditions werelikely unfavourable for the development of toxigenic filamentous fungi in wheat. Consequently, among Fusarium toxins, only ZEA, T-2, HT-2, and MON were detected and/or quantified, while among Alternaria toxins, only TEN was quantified. Regarding ergot alkaloids (Claviceps toxins), only ergocryptine was quantified in wheat from the organic farming system, whereas no ergot alkaloids were detected in conventionally produced wheat. Wheat varieties Zvezdana C and Epoha C from conventional production were contaminated with only one of the examined mycotoxins (TEN and MON, respectively). On the other hand, wheat varieties from the organic farming system were contaminated with at least two of the examined mycotoxins. The results indicate that the Austrian variety Genotype 3 was contaminated with all detected mycotoxins (Table 9), indicating a higher susceptibility to fungal infections under organic farming conditions, leading to increased mycotoxin contamination.

Table 9.
Mycotoxins in wheat cultivars from organic production and conventional production.
To comprehensively assess mycotoxin content across various wheat cultivars grown under organic and conventional farming systems, the results were subjected to a robust compositional PCA. The first principal component (PC1) accounted for over 43% of the total variability, while the second principal component (PC2) nealry 29% (Figure 3).
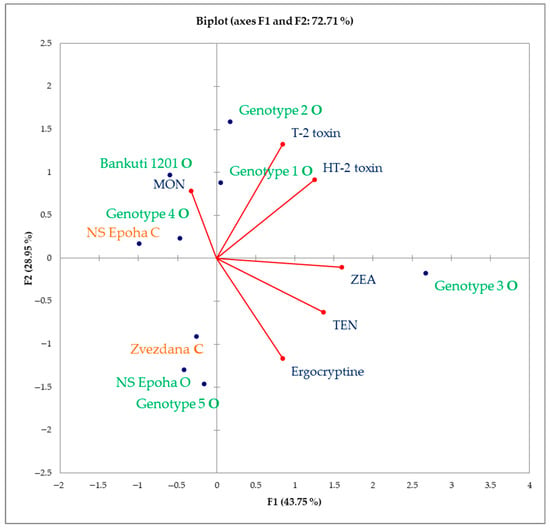
Figure 3.
PCA biplot of wheat cultivars based on mycotoxin profiles. Red dots depict mycotoxins, whereas blue dots depict wheat genotypes. The names of wheat genotypes written in green are from organic farming, whereas the names of wheat genotypes written in orange are from conventional farming.
T-2 toxin exhibited a positive correlation with HT-2 toxin confirming the values of the F1 factor (Table 10). This relationship is expected, as both toxins are primarily produced by Fusarium sporotrichioides [14]. TEN (an Alternaria toxin) showed a positive correlation with ZEA, that confirm values of F1 factor, (a Fusarium toxin) and ergocryptine (a Claviceps toxin), while ergocryptine displayed a negative correlation with MON (a Fusarium toxin). Furthermore, T-2 toxin was corelated with TEN, while HT-2 toxin showed no association with MON or ergocryptine.

Table 10.
Loading values of first two factors from PCA biplot on mycotoxin profiles.
Two wheat varieties from organic production (Genotype 5 and NS Epoha O) demonstrated close associations with ergocryptine, while one conventional variety (Zvezdana C) was closely associated with TEN. Further, the old Hungarian variety Bánkúti 1201, the Austrian variety Genotype 2, and Genotype 4 from organic production, as well as NS Epoha from conventional production, were closely associated with MON. Conversely, the Hungarian variety Genotype 1 and the Austrian variety Genotype 2 demonstrated close associations with T-2 and HT-2 toxins, while Genotype 3 was closely linked to ZEA and TEN.
4. Discussion
Our preliminary survey conducted in Serbia highlights the potential of organic wheat farming in terms of end-use quality. This observation contrasts with the findings of Takač et al. [44], who reported that protein content, wet gluten content, Zeleny sedimentation value, and farinograph parameters of bread wheat cultivars were superior in conventional production compared to organic farming in Hungary. This suggests that the relative performance of organic versus conventional wheat may be strongly influenced by regional agroclimatic conditions and local management practices. Under organic farming conditions in 2024, the Serbian control variety NS Epoha demonstrated broad adaptability in both yield and quality, despite being primarily classified as a milling-quality wheat cultivar. The average GY in 2024 was comparable to the values reported by Földi et al. [33] for the dry season of 2018. This outcome may be explained by significant variability in weather conditions, particularly high temperatures during the growing season, the early onset of key phenological stages (stem elongation and heading), and the late frost events, which collectively resulted in yields lower than the long-term average for the studied agroecological region. These conditions accelerated phenological development and strongly influenced the final grain yield. Elevated temperatures resulted in an unusually early onset of stem elongation (early March instead of early April) and heading (first half of April instead of early May), a shift not previously recorded in recent years. Consequently, heading occurred 15–20 days earlier, and plants displayed greater vigour and more advanced development compared to typical seasons. Between 20 and 26 April, minimum night temperatures dropped below 0 °C on three occasions, creating a substantial risk of partial spike (flower) damage, which may have further contributed to yield reductions. This stress period coincided with a slight soil moisture deficit, compounding the negative effects on reproductive development. Favourable warm and rainy conditions returned in late May and persisted through June, promoting grain filling and ripening; however, these improvements were insufficient to fully offset the earlier cold-induced stress events. Generally, the protein and wet gluten contents from organic production were similar to those reported by Takač et al. [44] for spelt wheat cultivars. The excessively high WG content observed in most wheat varieties from organic production led to a significant shift in the glutenin-to-gliadin ratio, favouring gliadin [48]. Although these varieties exhibited good dough mixing properties according to farinograph measurements, the altered gluten composition negatively affected their alveograph performance. This can be explained by the fact that glutenins primarily contribute to dough elasticity, while gliadins are responsible for extensibility, as previously mentioned. This observation was further confirmed by the PCA biplot (Figure 2), where WG clustered closely with FR and showed a strong negative correlation with the GI, a parameter reflecting the proportion of weak versus strong components of wet gluten. The high WG content can likely be attributed to the type of fertilizer used in organic production, as well as to favourable soil nutrient availability, particularly nitrogen (Table 2). Moreover, the bread-making quality of two wheat varieties from conventional production was below the three-year average reported by Živančev et al. [31]. Contrary to the findings of Wang et al. [22], who reported significant differences in TPC and TFC between organic and conventional wheat in the UK and Germany, no such differences were observed in this study, except in one organic variety. However, the relatively stable antioxidant activity across production systems in our study suggests that environmental factors, such as seasonal fluctuations in temperature and precipitation, may exert a stronger influence on phenolic accumulation than the farming system itself. This observation aligns with conclusions of Baranski et al. [21], who emphasized that year-to-year climatic variability often outweigh production practices in determining polyphenol levels. It is also noteworthy that one organic wheat variety exhibited slightly higher TPC and TFC values, highlighting the influence of genetic background in modulating antioxidant capacity. Nevertheless, our findings do not exclude the potential of organic farming to enhance antioxidant levels, as certain varieties may benefit more from organic practices under specific conditions.
Studies comparing mycotoxin levels in organic and conventional wheat have shown mixed results, although most findings are favourable for organic production. Several investigations have indicated that organic wheat generally contains considerably lower levels of Fusarium mycotoxins compared to conventional wheat. Remža et al. [49] found that organic wheat samples in Slovakia contained substantially lower concentration of DON and ZEA than conventional samples. Similarly, Schneweis et al. [50] reported that conventionally cultivated wheat in Germany exhibited a higher frequency of Fusarium contamination and elevated concentrations of ZEA and DON compared to organic wheat. Rossi et al. [51] likewise confirmed that organic wheat produced in Italy had lower DON levels than conventional wheat, while ochratoxin A concentration were low and did not differ significantly between production systems. Edwards [52] observed no significant disparity in DON and ZEA concentrations between organic and conventional wheat samples in the UK; however, organic samples exhibited markedly lower levels of HT-2 and T-2 toxins. On the other hand, Pussemier et al. [53] investigated the DON, OTA, and ZEN content in organic and conventional Belgian wheat samples, as well as wholemeal wheat flour samples from Belgian retail shops. The authors emphasized that the conventional wheat tended to contain higher levels of the examined mycotoxins compared to organic wheat, while the opposite trend was observed for wholemeal wheat flour, where organic samples were more frequently contaminated with DON and OTA than conventional ones. A review by Bernhoft et al. [54] examines the effects of organic versus conventional cereal production methods on Fusarium head blight and mycotoxin contamination. The authors reported that organic cereals contained lower levels of Fusarium mycotoxins, with DON, ZEA, and T-2/HT-2 concentration being 62%, 110%, and 180% higher, respectively, in conventional cereals. Wang et al. [22] reported that organic flour exhibited ZEA concentrations about 9% higher than conventional flour, while the Fusarium mycotoxin levels were roughly ten-fold lower than the EU maximum limits, implying that both production systems meet acceptable safety standards. In our study, wheat varieties from organic farming systems were more frequently contaminated with various mycotoxins than those from conventional farming systems; however, the detected concentrations of mycotoxins remained relatively low (Table 8). These findings likely reflect the combined effects of the wheat varietal characteristics, the implemented agricultural practice, and, most importantly, the weather conditions during the growing season. In addition to the temperature variation described above, an analysis of precipitation during May—the critical period for wheat anthesis and potential Fusarium infection—showed that in several seasons (2022–2025) rainfall remained below or close to the long-term average (Figure S1). No excessive or prolonged wet periods were recorded during flowering, which substantially reduces the likelihood of fungal infection in these years. Although intra-seasonal variability was pronounced, the absence of high humidity during the sensitive phenological stage suggests that natural infection pressure was relatively low. Future multi-year trials including seasons with wetter flowering periods will allow a more comprehensive assessment of genotype × environment interactions related to fungal infection. A major limitation of previously published data has been related to the difficulty of isolating the influence of the production system from other confounding factors, such as genotype, climate, and storage conditions. Consequently, the reported results are often contradictory or lack general applicability. Moreover, a truly interdisciplinary approach is still lacking-agronomy, microbiology, toxicology, and food technology need to be more closely integrated to enable a comprehensive assessment of mycotoxin occurrence and risk in cereal production.
5. Conclusions
The results indicate that the weather conditions were a decisive factor influencing wheat development and yield. Organic production resulted in lower yields but superior technological and grain quality characteristics. Yields in organic production were approximately two-fold lower than in conventional production, but the quality parameters of five organically grown varieties (PC > 17%, WG > 49%, WA > 62%) were significantly higher than the standard for organic wheat. The elevated WG content in organic varieties was accompanied by lower GI, indicating a higher proportion of the gliadin fraction in gluten. No consistent differences in TPC and TFC were observed between organic and conventional systems, except for one organic variety. This suggests that seasonal climatic factors (e.g., temperature and precipitation) exert a stronger influence on the accumulation of phenolic compounds than the production system itself. Contamination with mycotoxins was more frequent in organic production but remained at low concentrations. The absence of DON and the generally low mycotoxin levels were most likely due to unfavourable conditions for Fusarium development during early spring (warm and dry during weather coinciding with wheat anthesis).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13123938/s1, Figure S1: The amount of precipitation (mm) at the Kanjiža location over several years and compared to the long-term average.
Author Contributions
Conceptualization, D.Ž., E.J.H. and I.D.; methodology, D.Ž., E.J.H., Z.S. and A.Đ.; software, D.Ž. and D.M.; validation, D.Ž., E.J.H., N.G., Z.S. and A.Đ.; formal analysis, D.Ž., E.J.H., N.G., Z.S., A.Đ., V.A. and D.M.; investigation, D.Ž., E.J.H., N.G., Z.S., A.Đ., V.A. and D.M.; resources, D.Ž., I.D., E.J.H., N.G., Z.S. and M.N.; data curation, I.D., M.N., E.J.H., D.M. and A.Đ.; writing—original draft preparation, D.Ž., E.J.H., Z.S., A.Đ. and V.A.; writing—review and editing, D.Ž., E.J.H., Z.S., A.Đ., V.A., N.G., I.D., D.M. and M.N.; visualization, D.Ž., E.J.H., N.G. and Z.S.; supervision, I.D. and D.M.; project administration, D.Ž. and I.D.; funding acquisition, D.Ž., E.J.H., N.G. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia grant number 451-03-66/2025-03/200032, 451-03-136/2025-03/200222 and 451-03-65/2025-03/200134 and the Provincial Secretariat for Science and Technological Development of Vojvodina, project “Innovative strategies for improving maize production in AP Vojvodina” for the project cycle 2025–2028.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge Mihály Fõldy from Research Institute of Organic Agriculture, Budapest, Hungary, for providing seeds of the wheat varieties for organic production, János Farago from Kanjiža, on whose estate the field trial experiment was performed and Robert Nemeš from Institute of Field and Vegetable Crops who collected the wheat samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Górska-Warsewicz, H.; Rejman, K.; Ganczewski, G.; Kwiatkowski, B. Chapter 18—Economic importance of nutritional and healthy cereals and/or cereal products. In Developing Sustainable and Health Promoting Cereals and Pseudocereals; Rakszegi, M., Papageorgiou, M., Rocha, J.M., Eds.; Elsevier EBooks; Elsevier: Amsterdam, The Netherlands, 2023; pp. 433–450. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. FAOSTAT. 2025. Available online: http://www.fao.org/faostat/ (accessed on 27 June 2025).
- Organic Production Annual Report 2017. Available online: https://www.dnrl.minpolj.gov.rs/en/o_nama/organska/organska_proizvodnja_u_srbiji.html (accessed on 1 July 2025).
- KSH-Hungarian Central Statistical Office. Annual Agricultural Report. Data in Time Series, Agriculture. 2019. Available online: https://www.ksh.hu/stadat_eves_4_1 (accessed on 27 June 2025).
- Organic Farming in EU. Agricaltural Market Brief N°20. 2023. Available online: https://agriculture.ec.europa.eu/document/download/df01a3c7-c0fb-48f1-8eca-ce452ea4b8c2_en?filename=agri-market-brief-20-organic-farming-eu_en.pdf (accessed on 20 November 2025).
- Rahman, S.M.E.; Mele, M.A.; Lee, Y.-T.; Islam, M.Z. Consumer Preference, Quality, and Safety of Organic and Conventional Fresh Fruits, Vegetables, and Cereals. Foods 2021, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Escarnot, E.; Dornez, E.; Verspreet, J.; Agneessens, R.; Courtin, C.M. Quantification and Visualization of Dietary Fibre Components in Spelt and Wheat Kernels. J. Cereal Sci. 2015, 62, 124–133. [Google Scholar] [CrossRef]
- Baker, B.P.; Benbrook, C.M.; III, E.G.; Benbrook, K.L. Pesticide Residues in Conventional, Integrated Pest Management (IPM)-Grown and Organic Foods: Insights from Three US Data Sets. Food Addit. Contam. 2002, 19, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Commission implementation regulation (EU). Commission Implementation Regulation (EU) 2016/673 of 29 April 2016 Amending Regulation (EC) No 889/2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control; Commission implementation regulation (EU): Brussels, Belgium, 2016. [Google Scholar]
- Murphy, K.M.; Campbell, K.G.; Lyon, S.R.; Jones, S.S. Evidence of Varietal Adaptation to Organic Farming Systems. Field Crops Res. 2007, 102, 172–177. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Tangni, E.K.; Pussemier, L.; Schneider, Y.-J.; Larondelle, Y. Mycotoxins in Organic and Conventional Cereals and Derived Products from Europe: A Review. Cah. Agric. 2013, 22, 152–164. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of T-2 and HT-2 Toxin in Food and Feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs Official Journal of the European Union; European Commission: Brussels, Belgium, 2006; Volume 364, pp. 5–24. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks to Human and Animal Health Related to the Presence of Moniliformin in Food and Feed. EFSA J. 2018, 16, 5082. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Giorni, P.; Rastelli, S.; Vaccino, P.; Lanzanova, C.; Locatelli, S. Co-Occurrence of Moniliformin and Regulated Fusarium Toxins in Maize and Wheat Grown in Italy. Molecules 2020, 25, 2440. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence OfAlternariatoxins in Feed and Food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural Occurrence of Alternaria Toxins in the 2015 Wheat from Anhui Province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef]
- Debegnach, F.; Patriarca, S.; Brera, C.; Gregori, E.; Sonego, E.; Moracci, G.; De Santis, B. Ergot Alkaloids in Wheat and Rye Derived Products in Italy. Foods 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for Animal Health Related to the Presence of Ergot Alkaloids in Feed. EFSA J. 2024, 22, e8496. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher Antioxidant and Lower Cadmium Concentrations and Lower Incidence of Pesticide Residues in Organically Grown Crops: A Systematic Literature Review and Meta-Analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef]
- Wang, J.; Chatzidimitriou, E.; Wood, L.; Hasanalieva, G.; Markelou, E.; Iversen, P.O.; Seal, C.; Baranski, M.; Vigar, V.; Ernst, L.; et al. Effect of Wheat Species (Triticum Aestivum vs. T. Spelta), Farming System (Organic vs Conventional) and Flour Type (Wholegrain vs. White) on Composition of Wheat Flour—Results of a Retail Survey in the UK and Germany—2. Antioxidant Activity, and Phenolic and Mineral Content. Food Chem. X 2020, 6, 100091. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 370438. [Google Scholar] [CrossRef]
- Atmani, D.; Chaher, N.; Atmani, D.; Berboucha, M.; Debbache, N.; Boudaoud, H. Flavonoids in Human Health: From Structure to Biological Activity. Curr. Nutr. Food Sci. 2009, 5, 225–237. [Google Scholar] [CrossRef]
- Pure Nutrient Fact 10 Wheat Quality. Available online: https://www.yara.es/globalassets/country-websites/campaign-assets/nbs-campaign/sub-pages/profit-page/crop-performance/pure-nutrient-fact-10-wheat-quality.pdf (accessed on 1 July 2025).
- Marshall, D.R.; Ellison, F.W.; Mares, D.J. Effects of Grain Shape and Size on Milling Yields in Wheat. I. Theoretical Analysis Based on Simple Geometric Models. Aust. J. Agric. Res. 1984, 35, 619–630. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Batey, I.L.; MacRitchie, F. Relationships between protein composition and functional properties of wheat flours. Cereal Chem. 1992, 69, 125–131. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R Soc. Lond. B. Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzmán, C.; Ibba, M.I.; Álvarez, J.B.; Sissons, M.; Morris, C. Wheat Quality. In Wheat Improvement; Reynolds, M.P., Braun, J.H., Eds.; Springer: Cham, Switzerland, 2022; pp. 177–193. [Google Scholar] [CrossRef]
- Živančev, D.; Mirosavljević, M.; Aćin, V.; Momčilović, V.; Mikić, S.; Torbica, A.; Jocković, B. Variation in Quality Traits of Newly Developed Serbian Wheat Cultivars under Different Environmental Conditions of Pannonian Plain. Ital. J. Agron. 2021, 17, 1911. [Google Scholar] [CrossRef]
- Juhász, A.; Larroque, O.R.; Tamás, L.; Hsam, S.L.K.; Zeller, F.J.; Békés, F.; Bedő, Z. Bánkúti 1201—An Old Hungarian Wheat Variety with Special Storage Protein Composition. Theor. Appl. Genet. 2003, 107, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Földi, M.; Bencze, S.; Hertelendy, P.; Veszter, S.; Kovács, T.; Drexler, D. Farmer Involvement in Agro-Ecological Research: Organic On-Farm Wheat Variety Trials in Hungary and the Slovakian Upland. Org. Agric. 2021, 12, 293–305. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- ISO 7971-3:2009; Cereals—Determination of Bulk Density, Called Mass per Hectolitre—Part 3: Routine method. International Organization for Standardization: Geneva, Switzerland, 2009.
- ICC Method 137/1; Mechanical Determination of the Wet Gluten Content of Wheat Flour (Perten Glutomatic). International Association for Cereal Science and Technology Press: Vienna, Austria, 2011.
- Method MSZ 6369/6; Lisztvizsgálati Módszerek. A Vízfelvevő Képesség és a Sütőipari Érték Vizsgálata [Flour Testing Methods. Determination of Water Absorption Capacity and Baking Quality]. MSZT: Budapest, Hungary, 1988; Hungarian Standard.
- ICC Method 121/2; Method for using the Chopin Alveograph. International Association for Cereal Science and Technology Press: Vienna, Austria, 2011.
- Stojanović, Z.S.; Uletilović, D.D.; Kravić, S.Ž.; Kevrešan, Ž.S.; Grahovac, N.L.; Lončarević, I.S.; Ðurović, A.D.; Marjanović Jeromela, A.M. Comparative Study of the Nutritional and Chemical Composition of New Oil Rape, Safflower and Mustard Seed Varieties Developed and Grown in Serbia. Plants 2023, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.-H.; Yi, G.; Go, Y.S.; Ha, J.Y.; Choi, Y.-C.; Son, J.-H.; Shin, S.-H.; Jung, T.; Lee, S.-W. Measuring Antioxidant Activity in Yellow Corn (Zea mays L.) Inbreds from Three Different Geographic Regions. Appl. Biol. Chem. 2021, 64, 56. [Google Scholar] [CrossRef]
- Hofmann, S.; Scheibner, O. Quantification of 48 Myco- and Phytoxins in Cereal Using Liquid Chromotography-Triple Quadrupole Mass Spectrometry; Application Note 65969; ThermoFisher Scientific: Waltham, MA, USA, 2021; pp. 1–9. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-65969-mycotoxin-phytotoxins-cereal-tsq-quantis-an65969-en.pdf (accessed on 27 June 2025).
- CEN/TR 16059; Food Analysis-Performance Criteria for Single Laboratory Validated Methods of Analysis for the Determination of Mycotoxins. European Committee for Standardization, Management Centre: Brussels, Belgium, 2012; pp. 1–14.
- European Commission. Commission decision 2002/657/EC of 12 August 2002, Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Comm. 2002, L221, 8–36. [Google Scholar]
- Takač, V.; Tóth, V.; Rakszegi, M.; Mikić, S.; Mirosavljević, M.; Kondić-Špika, A. Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production. Foods 2021, 10, 156. [Google Scholar] [CrossRef]
- Lásztity, R.; Salgó, A. Quality assurance of cereals—Past, present, future. Period. Polytech. Chem. Eng. 2002, 46, 5–13. [Google Scholar]
- Silva, C.L.; da Benin, G.; Bornhofen, E.; Todeschini, M.H.; Dallo, S.C.; Sassi, L.H.S. Characterization of Brazilian Wheat Cultivars in Terms of Nitrogen Use Efficiency. Bragantia 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Soorninia, F.; Najaphy, A.; Kahrizi, D.; Mostafaei, A. Yield Attributes and Qualitative Characters of Durum Wheat as Affected by Terminal Drought Stress. Int. J. Plant Prod. 2023, 17, 309–322. [Google Scholar] [CrossRef]
- Borghi, B. Nitrogen As Determinant of Wheat Growth and Yield. In Wheat Ecology and Physiology of Yield Determination; Satorre, E.H., Slafer, G.A., Eds.; CRC Press eBooks; CRC Press: Boca Raton, FL, USA, 2024; pp. 67–84. [Google Scholar] [CrossRef]
- Remža, J.; Lacko-Bartošová, M.; Kosík, T. Fusarium Mycotoxin Content of Slovakian Organic and Conventional Cereals. JCEA 2016, 17, 164–175. [Google Scholar] [CrossRef]
- Schneweis, I.; Meyer, K.; Ritzmann, M.; Hoffmann, P.; Dempfle, L.; Bauer, J. Influence of Organically or Conventionally Produced Wheat on Health, Performance and Mycotoxin Residues in Tissues and Bile of Growing Pigs. Arch. Anim. Nutr. 2005, 59, 155–163. [Google Scholar] [CrossRef]
- Rossi, F.; Bertuzzi, T.; Comizzoli, S.; Turconi, G.; Roggi, C.; Pagani, M.; Cravedi, P.; Pietri, A. Preliminary survey on composition and quality of conventional and organic wheat. Ital. J. Food Sci. 2006, 18, 355–366. [Google Scholar]
- Edwards, S.G. Fusariummycotoxin Content of UK Organic and Conventional Wheat. Food Addit. Contam. Part A 2009, 26, 496–506. [Google Scholar] [CrossRef]
- Pussemier, L.; Piérard, J.-Y.; Anselme, M.; Tangni, E.K.; Motte, J.-C.; Larondelle, Y. Development and Application of Analytical Methods for the Determination of Mycotoxins in Organic and Conventional Wheat. Food Addit. Contam. 2006, 23, 1208–1218. [Google Scholar] [CrossRef]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).