Solid Waste Management: Degradation of Commercial and Newly Fabricated Cellulose Acetate Ultrafiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions, Materials, and Cultures
2.2. Fabrication of Membranes
| S/N | Membrane | CA (% w/v) | PCL (% w/v) | DMF (% v/v) | DCM (% v/v) | PEG (% v/v) | DS |
|---|---|---|---|---|---|---|---|
| 01 | UFM | 70 | 30 | 63 | 27 | 10 | 1.8 |
| 02 | UFM-T * | 70 | 30 | 63 | 27 | 10 | 1.8 |
2.3. Fabrication of Photo-Assisted Self-Cleaning Membranes
2.4. Ozonation
2.5. Study System Setup
- (1)
- Pretreatment stage: This chamber serves to achieve an adequate HRT, which in turn decreases membrane structural stability while promoting membrane biocompatibility for the attachment of fungal biomass. Additionally, it offers flexibility in combining various treatment methods.
- (2)
- Bioreactor: Designed to ensure an adequate HRT for biodegradation, but not excessively long, as prolonged HRT can reduce the efficiency of the process while restricting its potential for commercial scalability. The bioreactor was operated under presumed aerobic conditions (although dissolved oxygen (D.O.) was not measured), using two air diffusers for aeration. The pH of the bioreactor medium ranged between 6.5 and 7.5, with a temperature of 30 °C. Within this treatment setup, two distinct retention times were identified as follows: (1) the solids retention time (SRT) of the membrane fragments, encompassing both the pretreatment stage and the bioreactor, and (2) the hydraulic retention time (HRT) specific to the bioreactor. The separate HRT for pretreatment and bioreactor was set at 15 days, while solids (membrane particles) retention time within the treatment system was 30 days. Both are considered relatively short. In contrast, CA degradation studies typically involve much longer HRT, ranging up to 365 days (Table 3).
- (3)
- The system is designed in a continuous flow configuration that allows for the accumulation of membrane solids, including biomass and membrane particles, over time. Therefore, the approach involves the use of SBP technology to control the accumulated immobilized biomass. The bioreactor was inoculated with 50 SBP capsules.
- (4)
- Phase separation: In this phase, there is an effluent separation chamber that facilitates the refreshment of the growth medium and solids (membrane fragments) circulated back to the bioreactor.
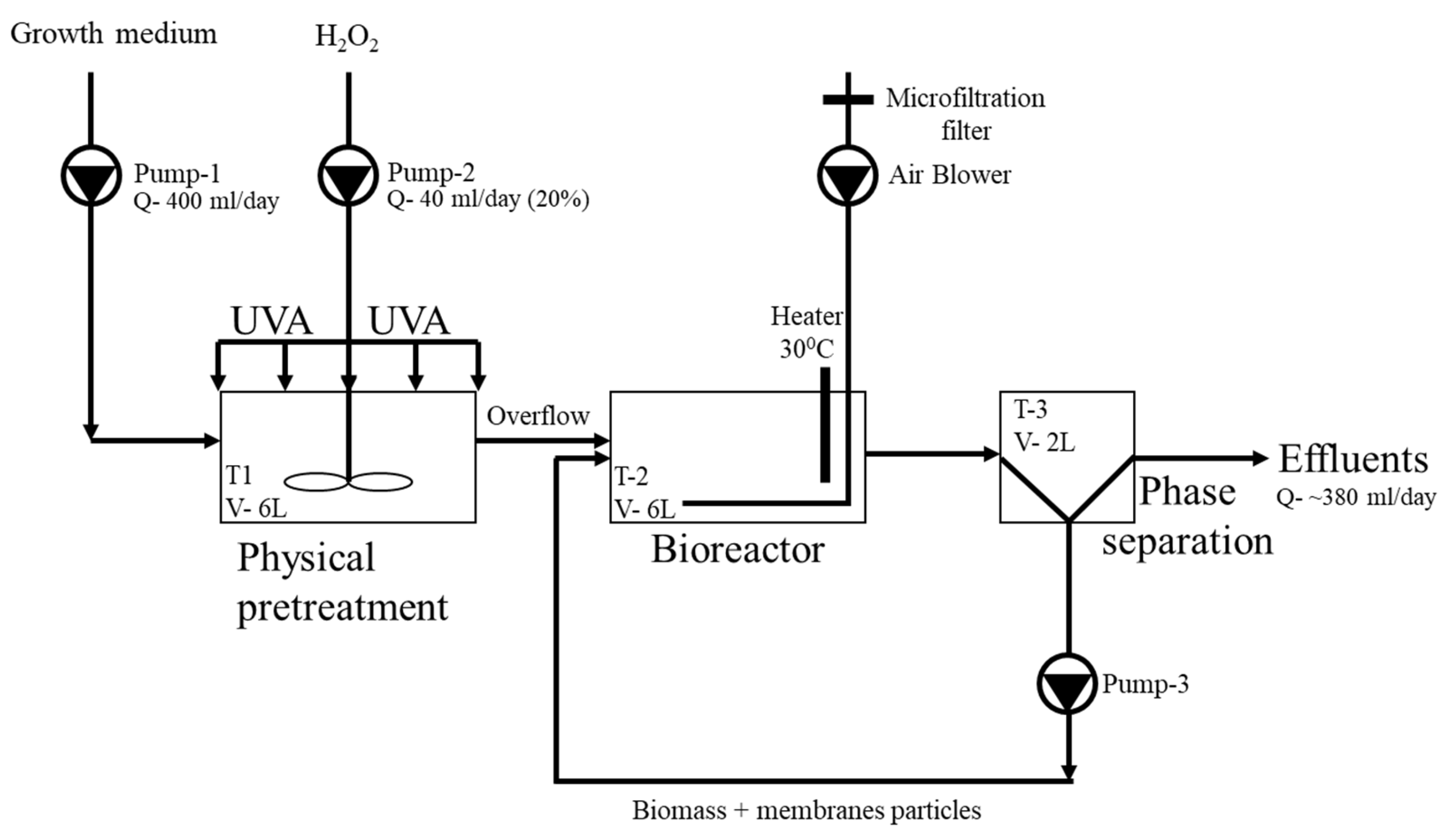
2.6. Membrane Degradation Analysis
2.7. Statistics
3. Results and Discussion
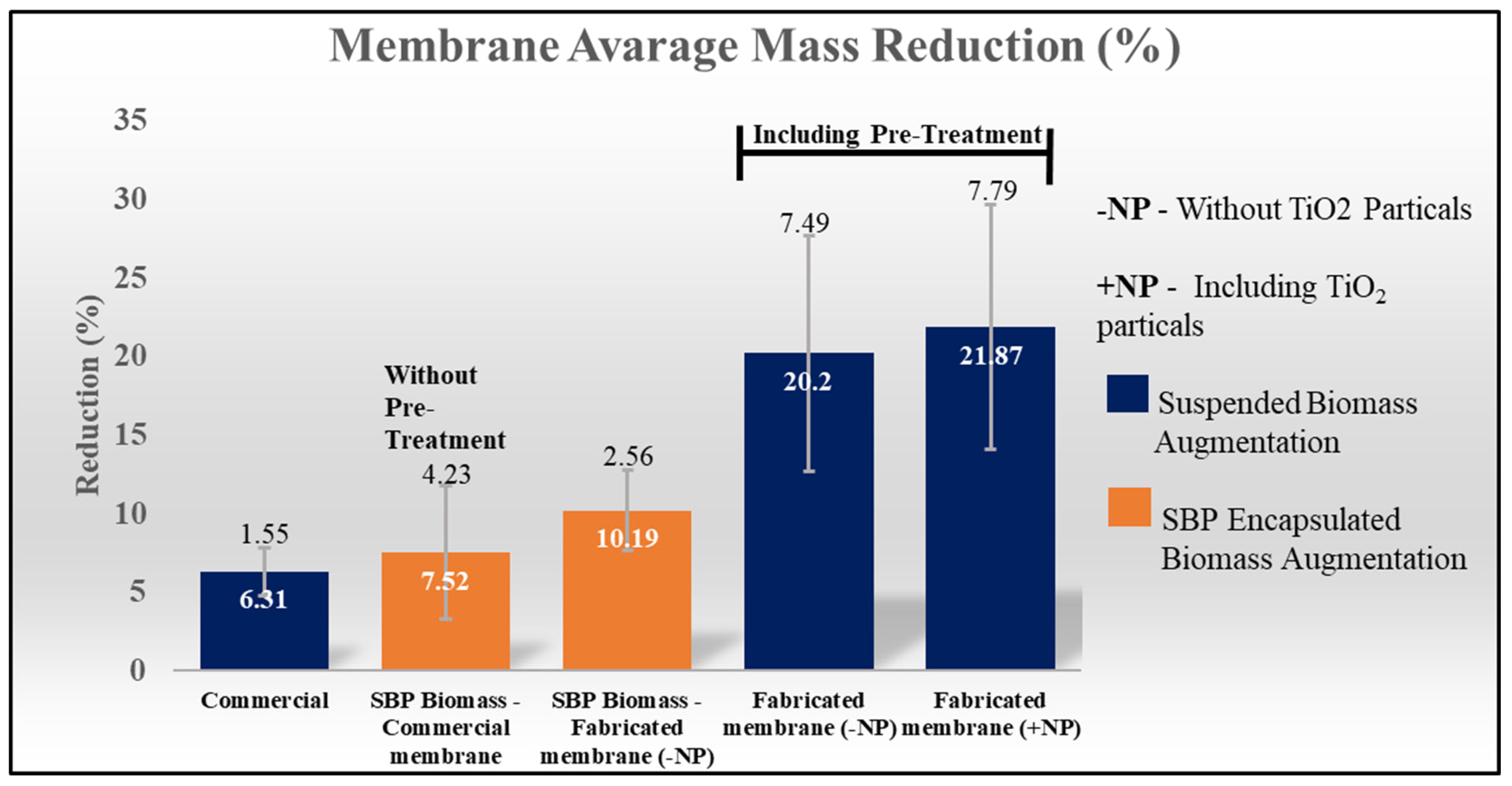
3.1. Membrane Degradation by Pretreatment and Suspended Culture
| Pretreatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study # | Membrane Type | Trametes versicolor Culture Type | Additional Carbon * | Nanoparticles | UVA | H2O2 | Ozone | Initial Weight | Weight Change | % Weight Loss |
| 1A | Commercial CA membrane | Suspended | - | + | + | + | - | 5.08 | 0.3048 | 6 |
| 1B | - | + | + | - | - | 4.8187 | 0.3855 | 8 | ||
| 1C | + | - | - | - | - | 5.014 | 4.7523 | 4.94 | ||
| Average (±SD) | 6.31 (±1.55) | |||||||||
| 2A | UFM-T | Suspended | - | + | + | + | - | 4.1984 | 1.0496 | 25 |
| 2B | - | + | + | + | - | 3.7545 | 0.2889 | 13 | ||
| 2C | - | + | + | + | + | 2.4553 | 0.6553 | 26.7 | ||
| Average (±SD) | 21.87 (±7.79) | |||||||||
| 3A | UFM | Suspended | - | - | + | + | - | 3.7035 | 0.6185 | 16.7 |
| 3B | - | - | + | + | - | 4.2206 | 0.6379 | 15.1 | ||
| 3C | - | - | + | + | + | 1.2833 | 0.3691 | 28.8 | ||
| Average (±SD) | 20.2 (±7.49) | |||||||||
| 4A | Commercial CA membrane | SBP-encapsulated | + | - | - | - | - | 5.033 | 0.6244 | 12.4 |
| 4B | - | - | - | - | - | 4.8889 | 0.2567 | 5.25 | ||
| 4C | - | - | - | - | - | 5.0696 | 0.2486 | 4.9 | ||
| Average (±SD) | 7.52 (±4.23) | |||||||||
| 5A | UFM | SBP-encapsulated | + | - | - | - | - | 5.0808 | 0.4059 | 8 |
| 5B | + | - | - | - | - | 5.0808 | 0.4866 | 9.58 | ||
| 5C | + | - | - | - | + | 5.0457 | 0.6561 | 13 | ||
| Average (±SD) | 10.19 (±2.56) | |||||||||
3.2. Membrane Degradation by SBP-Encapsulated Culture
| Culture | Type | DS ** | CA Weight Loss (%) | Incubation Time (Days) | References |
|---|---|---|---|---|---|
| Rhizobium meliloti | Bacteria | 0.4–0.8 | 34 | 150 | [28] |
| Neisseria sicc SB | Bacteria | 1.81 | 51 & 40 * | 20 | [11] |
| Neisseria sicc SC | Bacteria | 2.3 | 60 & 45 * | 20 | [11] |
| Alcaligens xylosoxidans | Bacteria | 0.4–0.8 | 23 | 150 | [28] |
| Various biota from natural water sources | 2.2 | 10 | 365 | [29] | |
| Trametes versicolor | Fungi | 2.6 | Up to 28.8 | 30 | Present study |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, L.; Zhang, Y.; Nei, L.; Feng, X. A novel loosely structured nanofiltration membrane bioreactor for wastewater treatment: Process performance and membrane fouling. J. Membr. Sci. 2022, 644, 120128. [Google Scholar] [CrossRef]
- Markets and Markets, Membranes Market by Material (Polymeric, Ceramic), Technology (RO, MF, UF, NF), Application (Water & Wastewater Treatment, Industrial Processing), & Region (North America, EU, APAC, Middle East & Africa, South America)—Global forecast 2027. 2022. Available online: https://www.marketsandmarkets.com/Market-Reports/membranes-market-1176.html (accessed on 3 December 2024).
- Maalige, N.R.; Mruthunjayappa, M.H.; Nataraj, S.K. Sustainable polymer-based materials for energy and environmental applications. In Polymer-Based Advanced Functional Materials for Energy and Environmental Applications; Subramani, N.K., Nataraj, S.K., Patel, C., Shivanna, S., Eds.; Springer: Singapore, 2022; pp. 9–30. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Blanco, A.; Garcia-Pacheco, R.; Landaburu-Aguirre, J.; Garcia-Calvo, E. Prospective Life Cycle Assessment and economic analysis of direct recycling of end-of-life reverse osmosis membranes based on Geographic Information Systems. J. Clean. Prod. 2021, 282, 124400. [Google Scholar] [CrossRef]
- Bandehali, S.; Sanaeepur, H.; Ebadi Amooghin, A.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Guo, Y.; Hu, J.; Lin, S.; Tu, Y.; Chen, L.; Ni, Y.; Huang, L. Recent advances on cellulose-based nanofiltration membranes and their applications in drinking water purification: A review. J. Clean. Prod. 2022, 333, 130171. [Google Scholar] [CrossRef]
- Arockiasamy, D.L.; Nagendran, A.; Shobana, K.H.; Mohan, D. Preparation and characterization of cellulose acetate/aminated polysulfone blend ultrafiltration membranes and their application studies. Sep. Sci. Technol. 2009, 44, 398–421. [Google Scholar] [CrossRef]
- Han, B.; Zhang, D.; Shao, Z.; Kong, L.; Lv, S. Preparation and characterization of cellulose acetate/carboxymethyl cellulose acetate blend ultrafiltration membranes. Desalination 2013, 311, 80–89. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose regeneration and chemical recycling: Closing the “cellulose gap” using environmentally benign solvents. Macromol. Mater. Eng. 2020, 305, 1900832. [Google Scholar] [CrossRef]
- Sakai, K.; Yamauchi, T.; Nakasu, F.; Ohe, T. Biodegradation of cellulose acetate by Neisseria sicca. Biosci. Biotech. Biochem. 1996, 60, 1617–1622. [Google Scholar] [CrossRef]
- Mruthunjayappa, M.H.; Shachar, C.; Imbar, A.; Menashe, O.A.; Mamane, H. Cellulose acetate and polycaprolactone based photoactive ultrafiltration membrane: A novel approach with UV-switchable photocatalytic activity. Sep. Purif. Technol. 2023, 329, 125102. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, F.; Jia, L.; Zhang, X.; Deng, S.; Luo, B.; Zhou, Y.; Fan, M.; Xia, Y. Fungal selectivity and biodegradation effects by white and brown rot fungi for wood biomass pretreatment. Polymers 2023, 15, 1957. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.; Malakahmad, A.; Isa, M.H. Refinery wastewater biological treatment: A short review. J. Sci. Ind. Res. 2012, 71, 251–256. [Google Scholar]
- Menashe, O.; Kurzbaum, E. Small-bioreactor platform technology as a municipal wastewater additive treatment. Water Sci. Technol. 2014, 69, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Menashe, O.; Rosen-Kligvasser, J.; Kurzbaum, E.; Suckeveriene, R.Y. Structural properties of a biotechnological capsule confined by a 3D-cellulose acetate membrane. Polym. Adv. Technol. 2020, 32, 681–689. [Google Scholar] [CrossRef]
- Machado, A.S.; Valadares, F.; Silva, T.F.; Milagres, A.M.F.; Segato, F.; Ferraz, A. The secretome of Phanerochaete chrysosporium and Trametes versicolor grown in microcrystalline cellulose and use of the enzymes for hydrolysis of lignocellulosic materials. Front. Bioeng. Biotechnol. 2020, 8, 826. [Google Scholar] [CrossRef]
- Qiu, Y.; Ouyang, F. Fabrication of TiO2 hierarchical architecture assembled by nanowires with anastase/TiO2(B) phase-junctions for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2017, 403, 691–698. [Google Scholar] [CrossRef]
- Zhao1, K.; Wu1, Y.W.; Young, S.; Chen, X.J. Biological Treatment of Dairy Wastewater: A Mini Review. J. Environ. Inform. Lett. 2020, 4, 22–31. [Google Scholar] [CrossRef]
- Im, J.H.; Woo, H.J.; Choi, M.W.; Han, K.B.; Kim, C.W. Simultaneous organic and nitrogen removal from municipal landfill leachate using an anaerobic–aerobic system. Water Res. 2001, 35, 2403–2410. [Google Scholar] [CrossRef]
- Schirp, A.; Wolcott, M.P. Fungal degradation of wood-plastic composites and evaluation using dynamic mechanical analysis. J. Appl. Polym. Sci. 2006, 99, 3138–3146. [Google Scholar] [CrossRef]
- Komarek, R.J.; Gardner, R.M.; Buchanan, C.M.; Gedon, S. Biodegradation of radiolabeled cellulose acetate and cellulose propionate. J. Appl. Polym. Sci. 1993, 50, 1739–1746. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of cellulose acetate–based materials: A review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Khalaf, A.; Alqader Ibrahim, A.; Halaweh, G. Cellulose Acetate Membranes: Fouling Types and Antifouling Strategies: A Brief Review. Processes 2023, 11, 489. [Google Scholar] [CrossRef]
- Andrady, A.L.; Harnid, S.H.; Hu, X.; Torikai, A. Effects of increased solar ultraviolet radiation on materials. J. Photochem. Photobiol. B Biol. 1998, 46, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bar Oz, Y.; Mamane, H.; Menashe, O.; Cohen-Yaniv, V.; Kumar, R.; Iasur Kruh, L.; Kurzbaum, E. Treatment of olive mill wastewater using ozonation followed by an encapsulated acclimated biomass. J. Environ. Chem. Eng. 2018, 6, 5014–5023. [Google Scholar] [CrossRef]
- Gu, J.-D.; Eberiel, D.; McCarthy, S.P.; Gross, R.A. Degradation and mineralization of cellulose acetate in simulated thermophilic compost environments. J. Environ. Polym. Degrad. 1993, 1, 281–291. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradation of cellulose acetate in simulated aqueous environments: One-year study. Mater. Eng. 2002, 307, 2100951. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shachar, C.; Mamane, H.; Mruthunjayappa, M.H.; Halpern, B.; Menashe, O.A. Solid Waste Management: Degradation of Commercial and Newly Fabricated Cellulose Acetate Ultrafiltration Membranes. Processes 2025, 13, 3580. https://doi.org/10.3390/pr13113580
Shachar C, Mamane H, Mruthunjayappa MH, Halpern B, Menashe OA. Solid Waste Management: Degradation of Commercial and Newly Fabricated Cellulose Acetate Ultrafiltration Membranes. Processes. 2025; 13(11):3580. https://doi.org/10.3390/pr13113580
Chicago/Turabian StyleShachar, Cliff, Hadas Mamane, Manohara Halanur Mruthunjayappa, Barak Halpern, and Ofir Aslan Menashe. 2025. "Solid Waste Management: Degradation of Commercial and Newly Fabricated Cellulose Acetate Ultrafiltration Membranes" Processes 13, no. 11: 3580. https://doi.org/10.3390/pr13113580
APA StyleShachar, C., Mamane, H., Mruthunjayappa, M. H., Halpern, B., & Menashe, O. A. (2025). Solid Waste Management: Degradation of Commercial and Newly Fabricated Cellulose Acetate Ultrafiltration Membranes. Processes, 13(11), 3580. https://doi.org/10.3390/pr13113580








