Abstract
Research on cannabis oil has evolved to encompass the pharmaceutical industry for the therapeutic potential of the active compounds for pathologies such as Alzheimer, auto-immune disorders, and cancer. These debilitating diseases are best treated with cannabinoids such as tetrahydrocannabinol (∆9-THC), cannabigerol (CBG), and cannabinol (CBN), which relieve neuropathic pain and stimulate the immune system. We extracted cannabinoids from plants with supercritical CO2 and produced an extract with a total yield close to 26%. The three-level Box–Behnken experimental design considered four factors: Temperature, pressure, CO2 flow rate, and processing time, with predetermined parameters at low, medium, and high levels. The mathematical model was evaluated by regression analysis. The yield of ∆9-THC and CBG reached a maximum after 2 h and 15 g/min of CO2, 235 bar, 55 °C (64.3 g THC/100 g of raw material and 4.6 g CBG/100 g of raw material). After another 2 h of extraction time, the yield of CBN reached 2.4 g/100 g. The regression analysis identified pressure and time as the only significant factors for total yield while pressure was the only significant factor for ∆9-THC and CBG. Time, temperature, pressure, and flow rate were all significant factors for CBN.
1. Introduction
The cannabis plant was originally used in central Asia during the Neolithic period. Western medicine adopted medicinal cannabis in the 1800s, when the Irish physician William O’Shaughnessy and French psychiatrist Jacques-Joseph Moreau attempted to treat tetanus, rabies, and mental disorders with it [1]. For example, dronabinol, a synthetic tetrahydro-cannabinoid (∆9-THC), is effective for anorexia, nausea, and vomiting, and was initially approved by the FDA on 31 May 1985 [2]. The interest in cannabis interest grew, especially for the psychoactive ingredients of the plant in other medical contexts, such as weight gain in HIV-positive patients [3].
Chronic pain affects 30% of the adult population, with prognostic factors of age, baseline pain, mental health complaints, and genetic factors. In fact, patients experience the same pain at multiple sites in the body [4]. Ointments and tinctures were the first forms of medical cannabis applied to relieve soldier’s pain during the American civil war [1] but they also came as oils and resins [5]. In addition to these medicinal forms, people smoke cannabis for recreational purposes or apply it as a wax [6].
In 2019, cannabis plant seeds and female flowers were recognized as pharmaceuticals and nutraceuticals by Health Canada [7]. Results from clinical trials published in 2022 confirmed the safety, tolerance, and pain reduction properties of medical cannabis for older adults [8].
Pain costs society billions of dollars in lower productivity and affects millions of people [9]. However, the scale and standardization of cannabis-based product is still a challenge. There is an urgent need to establish the efficacy of medical cannabis and standardize its properties for adjunctive therapy [10]. The European Pain Federation advises patients to use medical cannabis as an oil extract [11].
For the cannabis industry, choosing a technology depends on the composition of the natural plant, but also on the end use (recreational vs. pharmaceutical, for example). Supercritical fluid extraction (SFE) is a technology already commercialized for cannabis oil extraction for recreational purposes. It is considered a green technique because of its minimal environmental impact and the absence of non-hazardous solvents. However, the lack of data concerning the interactions between the operational parameters and biomass source remains an active area of research.
Recent studies focus on pressure and temperature, but the experimental design must also consider objectives beyond extraction yield end use, cost, and time. Several studies use supercritical CO2 to produce pharmaceutical-grade extracts with varying pressure and temperature while maintaining the same CO2 densities [12]: For 100 g of CBD-dominant biomass, the total extraction yield was 8.8%. Qamar and co-workers [13] reported among the highest total yields (29.7%), using 500 g of the same type of biomass, which was double their earlier studies with 1 g of biomass [14]. On a similar Indica plant, Pattnaik et al. [15] reported a 7.3% total yield for 100 g feed after 1.7 h processing time, while Rochfort et al. [5] added a decarboxylation step and processed 1 kg of the same biomass type for 10 h and obtained a similar total yield of 7.1%.
Adding ethanol as a co-solvent increases the polarity of CO2 and yield. Kargili and Aytaç [16] increased the total yield to 9.7%, with 2% ethanol on 100 g of CBD biomass after 2 h, but decarboxylated the biomass before the extraction. Grijo [17] and Fernandez [18] also added ethanol as a co-solvent, decarboxylated, and reported 31% and 18% total yield, respectively.
Ethanol increases total yield even without the decarboxylation step [19,20,21] and reaches up to 22%. However, decarboxylation activates the pharmacological properties of cannabinoids [22], which is missing in many studies.
From the above-mentioned studies, only four (Grijo, Rovetto, Lewis, and Fernandez) tested ∆9-THC-dominant biomass, reporting 37%, 17%, 26%, and 21% total yields, respectively, with correspondingly large differences in the mass treated (6 g, 500 g, 0.25–3.75 g, and 3.7–5.1 g). Ethanol was the co-solvent in all cases. Although the extraction conditions were similar, two factors that account for the differences in total yield are the CO2-to-feed ratio and the CO2 density. The yield from 600 g of THC dominant biomass was 7.2% with 17% ethanol and 1 h processing time [23], while for 8 g of biomass, it reached 26% with 5% ethanol and 4 h processing time [24], both without decarboxylation. The yield from decarboxylated THC-CBD balanced biomass was 18% with 5% ethanol and 16% in the absence of ethanol [25]. Ethanol increases the yield and concentration of cannabinoids but considering the International Council for Harmonisation threshold for solvent residue in the pharmaceutical formulations, which is 0.005 v/v, the quality of the product should be prioritized on the quantity and total yield.
Here, we optimize the supercritical CO2 conditions to extract ∆9-THC, CBG, and CBN from 15 g of LSD-balanced hybrid, THC-rich cannabis biomass from Quebec, targeted by a Canadian company to initiate standardization of a nutraceutical grade natural extract, with the perspective of providing products to meet the increasing demand of the Canadian market. This contribution to standardization is an important step in the Goods Manufacturing Practices (GMP) compliance process.
2. Materials and Methods
2.1. Plant Material
We purchased 1 kg of dried and crushed Quebec LSD-type Cannabis flowers (18–28% THC) from QCGold TECH, (Saint-André-Avellin, QC, Canada). The cannabis was stored in the dark, at room temperature.
2.2. Chemicals
CO2 (99% purity) used for the supercritical extraction was from Oxymed (Montréal, QC, Canada). HPLC solvents, methanol (MeOH), and formic acid (HCOOH) were of analytical grade from Techni Science (Oisterwijk, The Netherlands). The standards used for the HPLC quantification are THCA, ∆9-THC, CBD, CBG, and CBN, of 1.0 mg/mL in methanol, bought from Sigma Aldrich (Oakville, ON, Canada).
2.3. Experimental Method
2.3.1. Decarboxylation
The cannabis plants were decarboxylated at 120 °C for 90 min in a Thermo Electron Corp. (Waltham, MA, USA) oven (model 6520 series) that employs gravity convection as a method of heat transfer.
2.3.2. Supercritical Fluid Extraction (SFE) Protocol
A half-liter Thar Technologies extraction vessel was equipped with a removable basket that facilitated the transfer of material to and from the system. The basket was 230 mL. The reactor was also equipped with a controlled heating element.
In each run, 15 g of dried decarboxylated cannabis was placed in the extraction vessel (Figure 1). The CO2 was filled from a 50 L cylinder and compressed to the desired pressure by a model P50 CO2 pump from Thar Technologies (Pittsburgh, PA, USA). A manual backpressure regulator maintained the pressure at the prescribed settings (BPRV, model BP66-1A11QEQ151, 0–10,000 PSIG, GO Regulator, Wajax, Montreal, QC, Canada). The extract was separated from the solvent when the pressure dropped to 50 bar in the receiver vessel.
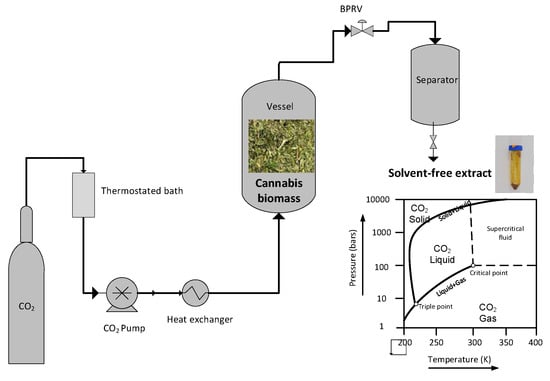
Figure 1.
Supercritical carbon dioxide extraction unit.
After extraction, an analytical balance weighed the estimated yield, which was expressed as a mass percentage on a dry basis (% d.b.).
The total yield (Y) is the ratio of the mass of extract, Me, to the mass of raw material loaded to the reaction, Mrm,
The yield for each cannabinoid in g/100 g raw material, Yi, is the ratio of the mass percentage of the cannabinoid, Mi (Xi Me/100), to Mrm
where:
- Xi: Mass percentage of the cannabinoid i, in g/100 g of extract.
- Ci: Concentration of cannabinoid i, measured by HPLC.
- Cs: Concentration of the analyzed solution of cannabinoid i.
- Ma: Mass of analyzed cannabinoid i.
- Vs: Volume of analyzed solution of cannabinoid i.
2.3.3. Box–Behnken Experimental Design
We applied a Box–Behnken experimental design with 4 factors at 3 levels (Table 1). Ranges for the 4 factors were chosen based on literature data [26]. A randomization factor was added to the experimental plan to restrict the variability of the response due to external factors, for a total of 27 experimental runs. The central point was repeated three times. All responses are expressed in a second-order polynomial equation, as a function of independent variables, according to Equation (5):
where Z is the response, a0, a1, a2, a3, and a4 are the linear coefficients, a11, a22, a33, and a44 are the quadratic coefficients, and a12, a13, a14, a23, a24, and a34 are the interaction coefficients for the independent variables X1 (temperature), X2 (pressure), X3 (flowrate), and X4 (time). Statistical analysis was performed using Statistica® software (14.0.0.15). Analysis of an experiment with three level factors for Box–Behnken designs (α < 0.05) and a model of 2-way interactions (linear.quadratic.) were used to evaluate the model’s fit (the block effect was excluded).
Z = a0 + a1X1 + a2X2 + a3X3 + a4X4 + a11X12 + a22X22 + a33X32 + a44X42 + a12X1X2 + a13X1X3 + a14X1X4 + a23X2X3 +
a24X2X4 + a34X3X4
a24X2X4 + a34X3X4

Table 1.
Range and variables for the experimental design.
The correlation between the predicted and observed data was established using the coefficient of determination (R2). The accuracy of the model was evaluated using the values of R2 and R2 adjusted. The model equation can be used for interpolation in the experimental domain because it defines the true behavior of the system and has acceptable values of R2 [27].
2.3.4. HPLC Analysis
All chromatographic analyses were performed using an Agilent 1260 Infinity Quaternary HPLC (Agilent Technologies Canada, Montreal, QC, Canada), including a quaternary pump, a solvent degasser, an autosampler, and a column temperature regulation module. A 1260 Agilent photodiode-array detector (DAD) with a Phenomenex Kinetex® (Torrance, CA, USA) C18 100 Å column (50 × 2.1 mm ID and 2.6 µm particle size) measured the concentration of the extract at a wavelength of 220 nm. Data acquisition and integration were performed with MassHunter Quantitative Analysis Software (6.0.388.0).
The mobile phase A was a mixture of 5% MeOH, 94.9% H2O, and 0.1% HCOOH. Mobile phase B was 99.9% MeOH and 0.1% HCOOH. The column temperature was maintained at 40 °C with a mobile phase flow rate of 0.4 mL/min.
We injected 7 μL and the total run time was 26 min. A variable gradient was used, starting with 48% B, gradually increasing to 88% B over 18 min, then to 100% B after 1 min, and decreasing to 48% B after 7 min.
Standards at 100, 50, 25, 12.5, 6.25, and 3.125 mg/L were prepared for the calibration curve, for each cannabinoid (THCA, ∆9-THC, CBDA, CBD, CBN, and CBG).
The 7 μL sample was drawn from a solution of 25 mg of extract charged to a 25 mL flask with 20 mL of methanol. All samples were filtered and loaded into the sample vial, then injected into the HPLC. For ∆9-THC analysis, we diluted the sample by a factor of 10 with methanol, since LSD cannabis is a THC-rich plant.
3. Results
3.1. Effects of Decarboxylation
Decarboxylation of the cannabis biomass was performed to convert the acidic cannabinoids into their neutral forms, making them more extractable because of their lower polarity [20] and biopharmacologically active [22]. According to a study that compared SC-CO2 extraction with and without decarboxylation, the first extract contained 5- to 10-fold higher CBD and ∆9-THC content [25].
Decarboxylation increased ∆9-THC yield and reduced total THCA (Table 2). Moreover, the quantity of CBN increased due to the oxidation of CBNA and ∆9-THC. CBG increased as a product of CBGA oxidation.

Table 2.
Cannabinoids concentration before and after decarboxylation.
In the three natural synthetic pathways for the most studied cannabinoids [12,22,28], (Figure 2), (1) CBGA decarboxylates to CBG and biosynthesizes CBDA, CBCA, and THCA; (2) CBCA decarboxylates to CBC, CBDA to CBD, and THCA to ∆9-THC, but also oxidizes to CBNA; and (3) CBNA potentially decarboxylates to CBN, and finally, ∆9-THC also oxidizes to CBN.
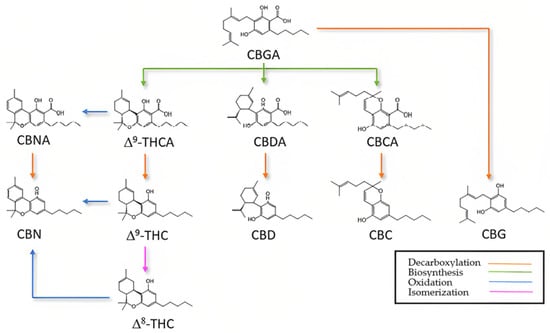
Figure 2.
Simplified cannabinoid synthetic pathway: Decarboxylation, biosynthesis [29].
3.2. Supercritical Fluid Extraction Yields
In this study, the effects of four parameters (pressure, temperature, time, and CO2 flow rate) on cannabinoid extraction are investigated. Table 3 summarizes all extraction results. The yield is the total extract weight recovery, and cannabinoids (THC, CBG, and CBN) content is expressed as a weight percentage of each one in the dry extract.

Table 3.
Extraction yield and cannabinoid content.
The conditions of run 8 (235 bar, 55 °C, and a CO2 flow rate of 15 g/min for 4 h) had the highest yield at 25.9%. This yield is comparable to the maximum yield obtained by Gallo-Molina and co-workers (26.4%) [24], with ethanol as a co-solvent. These conditions demonstrate the effectiveness of the supercritical solvent, attributed to the elevated extraction pressure. Lower temperatures can enhance the extraction yield [30] due to the increase in SC-CO2 solubility by increasing its density [16], but this impact becomes less significant at high pressure. This phenomenon, called the cross-over region, also affects the selectivity of the SC-CO2 extractions [25], thus increasing the cannabinoids’ volatility at higher temperatures [16], which leads us to question if variations of the extraction conditions affect the recovery of cannabinoids of interest in the range of optimal CO2 density since it affects its polarity [25].
The yield of run 26 was also high (24.9%) at 320 bar, 55 °C, and 10 g/min of CO2 flow rate for 2 h. Similarly, run 6 achieved a high yield of 22.7% using the same pressure, temperature, and CO2 flow rate as run 8, but with a shorter extraction time of 2 h instead of 4 h.
Previous studies [18,21] achieved comparable yields under similar pressure conditions, but with different biomass compositions (CBD-rich and THC-rich, respectively), and employed ethanol as a co-solvent in the latter case. Gallo-Molina previously explained how the raw material composition and extraction conditions impact cannabinoid content [24]. These similar recoveries, despite the use of co-solvent and shorter processing time, could be due to the variation of the CO2-to-feed ratio, considering the CO2 density is the same (same pressure and temperature).
The highest extraction yield of 25.9% was achieved by employing a combination of the longest extraction time (4 h), the highest flow rate (15 g/min), and a medium pressure of 235 bar. These conditions also resulted in high ∆9-THC recoveries. However, due to the limited number of studies focusing on cannabinoid content using neat CO2, it is premature to draw definitive conclusions about the optimal ∆9-THC recovery conditions for different biomass. Fernandez et al. quantified ∆9-THC at 63.6 g/100 g extract, using similar biomass [18], but the inclusion of ethanol as a co-solvent enhances CO2 solubility, compromising a direct comparison. In contrast, the lowest extraction yields (2.4%, 6.7%, and 7.4%) were obtained using lower to medium flow rates, shorter extraction times of 2–3 h, and pressures in the low to medium range.
Interestingly, the three center point runs (9-18-27) resulted in low extraction yields but high ∆9-THC content, suggesting potential medicinal value under these specific process conditions. Thus, we confirm that CO2 density may have a limited impact on the cannabinoid’s solubility, even at the optimal CO2 density of 818 kg/m3 as reported by Qamar [25].
ANOVA analyses and effect estimates confirmed that pressure and time have a significant impact (p < 0.05) on extraction yield (Table 4). The fitted equation for ∆9-THC content in the cannabis extract shows that only pressure positively affected the content, while temperature, flow rate, and time had no effect. However, a median temperature (55 °C) and pressure (235 bar) with high flow rate and low time were associated with high ∆9-THC and CBG yields. When time increases, ∆9-THC and CBG yields decrease, whereas CBN yield increases.

Table 4.
Effect estimates for the total yield.
Moreover, according to the three best extraction conditions for CBN yield, high temperature increases CBN yield, while, conversely, the ∆9-THC and CBG yield decreases (Table 3). This leads us to choose a point where the extract contains the maximum of the three cannabinoids simultaneously.
The optimal SC-CO2 runs were selected using the desirability value after varying the constraints. Run 6 (235 bar, 55 °C, 15 g/min, 2 h) was optimal and was used for verification. Conditions in run 8 (235 bar, 55 °C, 15 g/min, 4 h) are the same as run 6, but with an extended processing time.
The first-order term of temperature (X1), flow rate (X3), and time (X4) had a significant effect (p < 0.05) on CBN in raw extract (Table 5). The first-order term of pressure (X2) had a significant effect (p < 0.05) on ∆9-THC, CBG, and CBN extraction yields. The second-order interactions between time and flowrate (X3 X4) and pressure and flowrate (X2 X3) also had a significant effect (p < 0.05) on CBN content in raw extract.

Table 5.
Analysis of variance.
The impact of pressure was linear on the ∆9-THC content, while temperature had no significant effect based on the statistical model (Table 6). ∆9-THC recovery does change with temperature, which is likely due to differences in temperature-induced polarity. This observation leads us to investigate the impact of CO2 density and the CO2-to-biomass feed ratio to identify the optimal CO2 density, maximizing cannabinoid extraction [25], and determine if it is independent of the solvent-to-feed ratio.

Table 6.
Regression model.
4. Discussion
Pressure is a significant factor in both the total extraction yield and the quantity of bioactive compounds extracted. Increasing the pressure from 150 to 320 bar increases the yield of cannabis extract. However, it increases total yield at the expense of ∆9-THC, CBN, and CBG content. Higher pressures increase solvent strength and decrease extraction selectivity [24]. Recent studies [5,16,24,31,32] indicate that pressure impacts the extraction process, regardless of whether or not ethanol is a co-solvent. This impact is significant when the objective is the extraction of cannabinoids, particularly ∆9-THC. Temperature affects extraction yield but not cannabinoid content [32].
The cannabinoid yield is influenced by the operating conditions, which depend on their respective solubilities. Perrotin et al. determined the solubility of four cannabinoids in supercritical carbon dioxide [33,34]. Molar solubility for ∆9-THC lies between 0.20 and 2.95 × 10−4, 42 and 72 °C, and 130 and 250 bar. The molar solubility of CBN (1.26 to 4.16 × 10−4) and CBG (1.17 to 1.91 × 10−4) was determined in the range of 41 to 61 °C and 113 to 206 bars.
The optimal extraction parameters (235 bar, 55 °C, and 15 g/min) produce the most ∆9-THC and CBG at 2 h processing, but at 4 h, the CBN yield increases at the expense of ∆9-THC and CBG. This trend agrees with kinetic models suggesting that both CBG and ∆9-THC degrade to CBN [22]. Therefore, ∆9-THC and CBG formulations should not exceed 2 h, while if CBN is the target molecule, longer times are better.
In our attempt to establish a correlation among the studied parameters (pressure, temperature, time, and flowrate), we hypothesize that the combination of temperature and pressure can be effectively represented by the density, while the combination of time and flowrate can be represented by the CO2-to-feed ratio. Further investigation is needed to validate this hypothesis and explore the precise relationships between these parameters.
Our research specifically targets the extraction of THC, CBG, and CBN for medical purposes, considering their complementary therapeutic effects [28] and similar solubilities, which facilitate their simultaneous extraction [20,34]. However, during this study, our focus is primarily on THC, as a preliminary test, when examining the CO2 density and solvent-to-feed ratio.
Figure 3 illustrates that, regardless of the CO2 density (796.94 kg/m3), the solvent’s ability to extract THC varies, resulting in both maximum and minimum THC yield. This observation holds true for all other tested densities. This can be attributed to the increase in vapor pressure, where the temperature has a greater influence on solubility compared to density, particularly at pressures above 200 bar [25]. Consequently, we conclude that CO2 density only plays a minor role to optimize cannabinoid extraction.
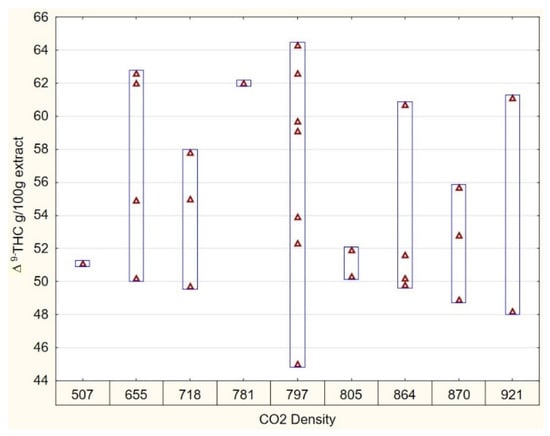
Figure 3.
Variability plot of ∆9-THC as a function of CO2 density.
Similar to density, the CO2-to-feed ratio as a single factor is incapable of accounting for the variance in the data (Figure 4). Specifically, for a fixed ratio of 120, the CO2 extraction yields both the maximum and minimum ∆9-THC concentrations. Grijo et al. [17] reported a similar crossover behavior in their investigation of solvent-to-feed ratios for cannabinoid-rich plants, indicating that higher densities may result in lower ∆9-THC concentrations. Rovetto et al. [20] explained that while consuming the same amount of CO2, higher pressures are associated with an expected increase in yield. Therefore, in cannabinoid extraction, the interplay between the solvent-to-feed ratio, density, and pressure should be considered. Including these factors, the extraction conditions for our Quebec LSD-type cannabis flowers of maximum ∆9-THC, CBG, and CBN are 235 bar, 55 °C, 15 g/min, 4 h, 797 kg/m3 of CO2 density, and a 120 solvent-to-feed ratio.
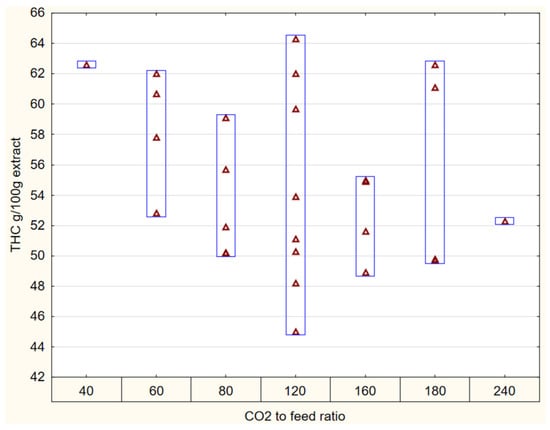
Figure 4.
∆9-THC as a function of CO2-to-feed ratio.
Rochfort et al. [5] conducted a medicinal cannabis extraction study and emphasized the complexity of ∆9-THC recoveries compared to CBD, indicating that the interaction between time and pressure plays a role. Additionally, longer extraction times may not always lead to increased total yield under the same pressure and temperature conditions. These findings support our approach of prioritizing the balance between CO2 density and the solvent-to-feed ratio over the initial parameters studied.
Prior to undertaking any process development, it is crucial to thoroughly test and optimize the extraction conditions specific to the composition of different cannabis plants, including cannabinoids and other compounds [33]. Consequently, we present this experimental research focusing on the LSD cannabis indica type from Quebec, aiming to support the industry in gathering data and establishing Good Manufacturing Practices (GMPs) for their facilities. This study not only sheds light on the impact of varying extraction conditions on ∆9-THC, CBG, and CBN cannabinoids but also examines their interrelation. While the optimization primarily emphasizes ∆9-THC yield, it can serve as a valuable starting point for investigations targeting the extraction of CBG or CBN.
5. Conclusions
Supercritical carbon dioxide is a selective extraction method for cannabinoids. Literature supports the observation that the concentration of cannabinol (CBN) increases over time due to the degradation of delta-9-tetrahydrocannabinol (∆9-THC) and cannabigerol (CBG), demonstrating that reaction kinetics are involved. The optimal conditions for extracting ∆9-THC and CBG from the Quebec LSD cannabis plant, were 55 °C, 235 bar, 15 g/min CO2, and 2 h. The optimal conditions for CBN extraction were the same but with a longer duration of 4 h. When targeting cannabinoid extraction, it is important to consider the CO2 density (818 kg/m3) and the CO2-to-feed ratio (120). The pressure was identified as the primary factor influencing ∆9-THC yield, while time was found to be a limiting factor due to ∆9-THC degradation into CBN. Additionally, time is a significant factor in increasing total yield. The results of our experimental work, based on the Box–Behnken design of experiments, align with the existing literature: SC-CO2 can achieve maximum extract yields of 24% (w/w) and a ∆9-THC content of 64 g/100 g of extract. The CO2-to-feed ratio and CO2 density are also two key factors to balance in order to extract the highest concentrations of cannabinoids.
Author Contributions
H.B., Y.B., M.S., G.S.P., D.C.B. and X.B., conceptualization; H.B., Y.B. and M.S., methodology; Y.B. and M.S., validation; H.B., formal analysis; H.B., investigation; Y.B., resources; H.B. and Y.B., data curation; H.B., writing—original draft preparation; Y.B., G.S.P. and D.C.B., writing—review and editing; H.B., visualization; Y.B., supervision; H.B. and Y.B., project administration; Y.B. and D.C.B., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
NSERC College and Community Innovation program—Innovation Enhancement grants (CÉPROCQ, 544301-2019) the hosting laboratory, and Zollaris Laboratories Corporation the industrial partner, are both gratefully acknowledged for the financial support for this project. This project was also supported by Mitacs through the Mitacs Accelerate program (IT25903).
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (stipend allocated to Hinane Boumghar via the NSERC-CREATE PrEEmiuM program). This research was undertaken, in part, thanks to funding from the Canada Research Chair program.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Song, Y.X.; Furtose, A.; Fuoco, D.; Boumghar, Y.; Patience, G.S. Meta-analysis and review of cannabinoids extraction and purification techniques. Can. J. Chem. Eng. 2023, 101, 3108–3131. [Google Scholar] [CrossRef]
- Schedules of Controlled Substances: Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)—[DELTA] Less than 9 Greater than—(Trans)-Tetrahydrocannabinol] in Sesame Oil and Encapsulated in Soft Gelatin Capsules from Schedule II to Schedule III; Final rule; Federal Register; Department of Justice (DOJ): Washington, DC, USA; Drug Enforcement Administration (DEA): Springfield, VA, USA, 1999; Volume 64, pp. 35928–35930.
- Tibbo, P.; Crocker, C.E.; Lam, R.W.; Meyer, J.; Sareen, J.; Aitchison, K.J. Implications of cannabis legalization on youth and young adults. Can. J. Psychiatry 2018, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Landmark, T.; Dale, O.; Romundstad, P.; Woodhouse, A.; Kaasa, S.; Borchgrevink, P.C. Development and course of chronic pain over 4 years in the general population: The HUNT pain study. Eur. J. Pain 2018, 22, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Isbel, A.; Ezernieks, V.; Elkins, A.; Vincent, D.; Deseo, M.A.; Spangenberg, G.C. Utilisation of design of experiments approach to optimise supercritical fluid extraction of medicinal cannabis. Sci. Rep. 2020, 10, 9124. [Google Scholar] [CrossRef]
- Goodman, S.; Wadsworth, E.; Leos-Toro, C.; Hammond, D. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int. J. Drug Policy 2020, 76, 102658. [Google Scholar] [CrossRef]
- Classifying Cannabis in the Canadian Statistical System. 2019. Available online: https://www150.statcan.gc.ca/n1/pub/11-621-m/11-621-m2018105-eng.htm (accessed on 13 October 2022).
- MacNair, L.; Kalaba, M.; Peters, E.N.; Feldner, M.T.; Eglit, G.M.L.; Rapin, L.; El Hage, C.; Prosk, E.; Ware, M.A. Medical cannabis authorization patterns, safety, and associated effects in older adults. J. Cannabis Res. 2022, 4, 50. [Google Scholar] [CrossRef]
- Mackey, S. Future directions for pain management: Lessons from the institute of medicine pain report and the national pain strategy. Hand Clin. 2016, 32, 91–98. [Google Scholar] [CrossRef]
- Blake, A.; Wan, B.A.; Malek, L.; De Angelis, C.; Diaz, P.; Lao, N.; Chow, E.; O’Hearn, S. A selective review of medical cannabis in cancer pain management. Ann. Palliat. Med. 2017, 6, S215–S222. [Google Scholar] [CrossRef]
- Häuser, W.; Finn, D.P.; Kalso, E.; Krcevski-Skvarc, N.; Kress, H.G.; Morlion, B.; Perrot, S.; Schäfer, M.; Wells, C.; Brill, S. European pain federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur. J. Pain 2018, 22, 1547–1564. [Google Scholar] [CrossRef]
- Jokic, S.; Jerkovic, I.; Pavic, V.; Aladic, K.; Molnar, M.; Kovac, M.J.; Vladimir-Knezevic, S. Terpenes and cannabinoids in supercritical CO2 extracts of industrial hemp inflorescences: Optimization of extraction, antiradical and antibacterial activity. Pharmaceuticals 2022, 15, 1117. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Fractional factorial design study for the extraction of cannabinoids from CBD-dominant cannabis flowers by supercritical carbon dioxide. Processes 2022, 10, 93. [Google Scholar] [CrossRef]
- Qamar, S.; Manrique, Y.J.; Parekh, H.S.; Falconer, J.R. Development and optimization of supercritical fluid extraction setup leading to quantification of 11 cannabinoids derived from medicinal cannabis. Biology 2021, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, F.; Hans, N.; Patra, B.R.; Nanda, S.; Kumar, V.; Naik, S.N.; Dalai, A.K. Valorization of wild-type Cannabis indica by supercritical CO2 extraction and insights into the utilization of raffinate biomass. Molecules 2023, 28, 207. [Google Scholar] [CrossRef]
- Karğılı, U.; Aytaç, E. Supercritical fluid extraction of cannabinoids (∆9-THC and CBD) from four different strains of cannabis grown in different regions. J. Supercrit. Fluids 2022, 179, 105410. [Google Scholar] [CrossRef]
- Ribeiro Grijó, D.; Vieitez Osorio, I.A.; Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. CO2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Fernández, S.; Carreras, T.; Castro, R.; Perelmuter, K.; Giorgi, V.; Vila, A.; Rosales, A.; Pazos, M.; Moyna, G.; Carrera, I.; et al. A comparative study of supercritical fluid and ethanol extracts of cannabis inflorescences: Chemical profile and biological activity. J. Supercrit. Fluids 2022, 179, 105385. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extraction of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef]
- Da Porto, C.; Voinovich, D.; Decorti, D.; Natolino, A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Monton, C.; Chankana, N.; Leelawat, S.; Suksaeree, J.; Songsak, T. Optimization of supercritical carbon dioxide fluid extraction of seized cannabis and self-emulsifying drug delivery system for enhancing the dissolution of cannabis extract. J. Supercrit. Fluids 2022, 179, 105423. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Martínez Ramírez, J.A.; Rivera Monroy, Z.J.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Effects of ethanol on the supercritical carbon dioxide extraction of cannabinoids from near equimolar (∆9-THC and CBD balanced) cannabis flowers. Separations 2021, 8, 154. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Quinino, R.C.; Reis, E.A.; Bessegato, L.F. Using the coefficient of determination R2 to test the significance of multiple linear regression. Teach. Stat. 2013, 35, 84–88. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Moreno, T.; Dyer, P.; Tallon, S. Cannabinoid decarboxylation: A comparative kinetic study. Ind. Eng. Chem. Res. 2020, 59, 20307–20315. [Google Scholar] [CrossRef]
- Gaspar, F. Extraction of essential oils and cuticular waxes with compressed CO2: Effect of extraction pressure and temperature. Ind. Eng. Chem. Res. 2002, 41, 2497–2503. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, R.J. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Perez, P.C.; Van Roosmalen, M.J.E.; Van Spronsen, J.; Witkamp, G.J.; Peters, C.J. Solubility of Δ9-tetrahydrocannabinol in supercritical carbon dioxide: Experiments and modeling. J. Supercrit. Fluids 2010, 52, 6–10. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H. Sustainable Production of Cannabinoids with Supercritical Carbon Dioxide Technologies. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).