Drug Formulation of Securigera securidaca Seed Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of S. securidaca
2.2. Extraction Process
2.3. Formulation and Evaluation of S. securidaca Tablets
2.3.1. Appearance, Average Weight, and Uniformity of Weight
2.3.2. Compaction Processes
2.3.3. Coating Process
2.3.4. Hardness
2.3.5. Friability
2.3.6. Disintegration Time
2.3.7. Water Content
2.3.8. HPLC Detection
HPLC Assay
Preparation of a Standard Solution
Preparation of the Sample Solution
Procedure
Evaluation of System Suitability
2.3.9. HPLC Dissolution
Preparation of Dissolution Media
Preparation of Standard Solution
Preparation of the Sample Solution
Procedure
Evaluation of System Suitability
3. Results and Discussion
3.1. Formulation Process
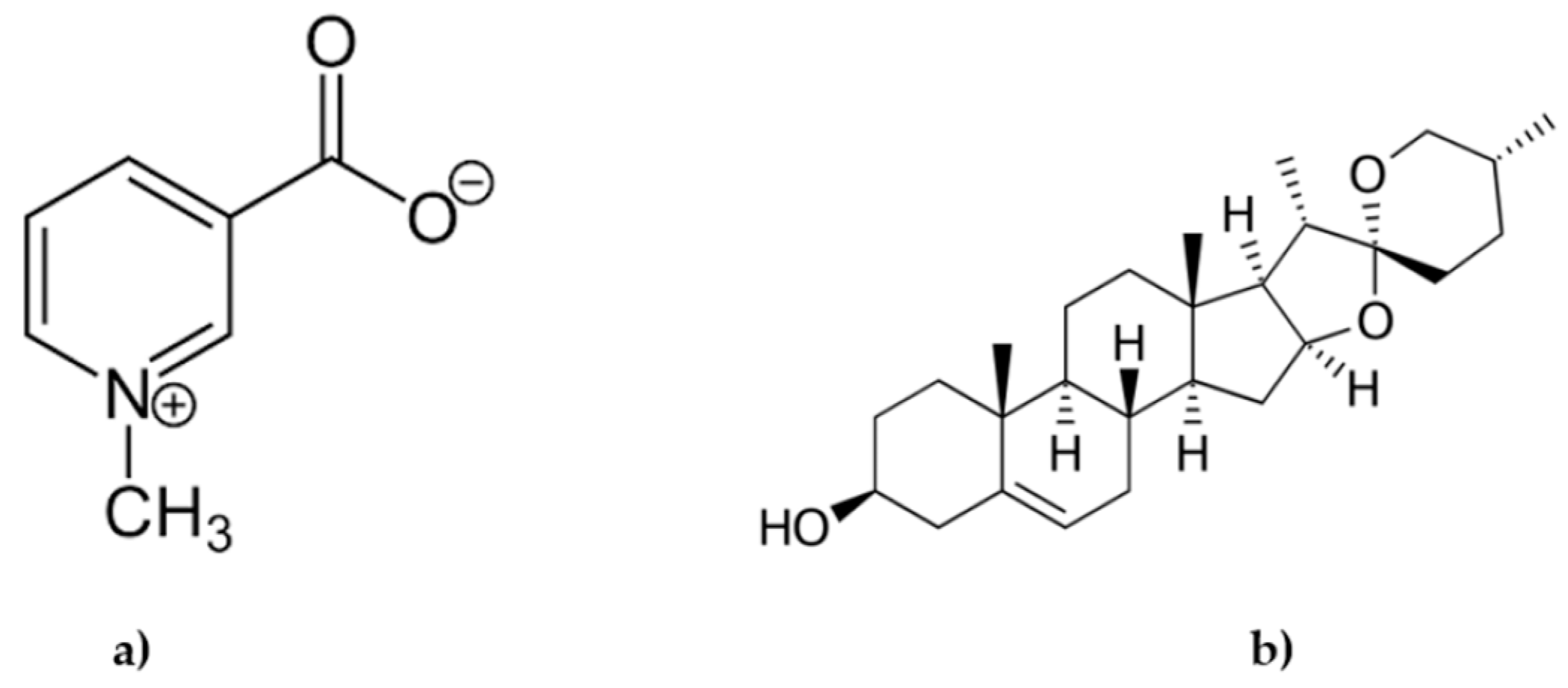
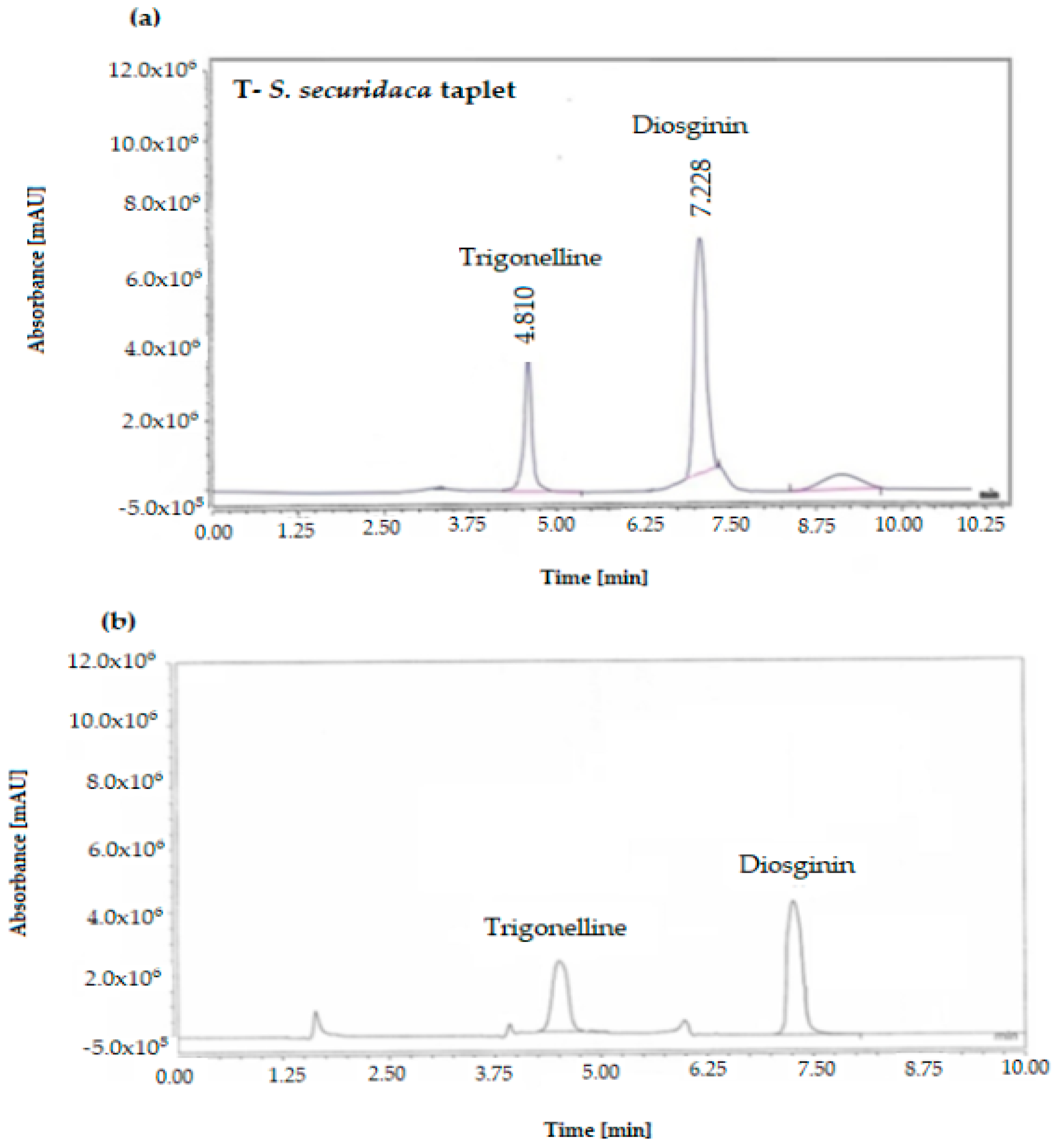
3.2. Analysis of Trigonelline and Diosgenin in the Formula
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, A.; Khalili, M.; Margedari, S.; Nahri-Niknafs, B. Antidiabetic and Antilipidemic Effects of Some Polar and Nonpolar Extracts of Securigera securidaca Flowers. Pharm. Chem. J. 2016, 49, 753–759. [Google Scholar] [CrossRef]
- Nik, A.B.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019, 3, 243–250. [Google Scholar] [CrossRef]
- Behnamnik, A.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. Evaluation of physicochemical, structural, and antioxidant properties of microencapsulated seed extract from Securigera securidaca by co-crystallization method during storage time. Biocatal. Agric. Biotechnol. 2021, 35, 102090. [Google Scholar] [CrossRef]
- Nurcahyanti, A.D.R.; Jap, A.; Lady, J.; Prismawan, D.; Sharopov, F.; Daoud, R.; Wink, M.; Sobeh, M. Function of selected natural antidiabetic compounds with potential against cancer via modulation of the PI3K/AKT/mTOR cascade. Biomed. Pharm. 2021, 144, 112138. [Google Scholar] [CrossRef]
- BehnamNik, A.; Vazifedoost, M. Optimizing the formulation of the functional beverage from the co-crystalized powder of Securigera securidaca seed extract. J. Food Sci. Technol. 2020, 57, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Fanalou, S.; Babaei, M.; Hosseini, A.; Azadi, N.; Nazarizadeh, A.; Shojaii, A.; Borji, M.; Malekinejad, H.; Bahreini, E. Effects of Securigera securidaca seed extract in combination with glibenclamide on antioxidant capacity, fibroblast growth factor 21 and insulin resistance in hyperglycemic rats. J. Ethnopharmacol. 2020, 248, 112331. [Google Scholar] [CrossRef] [PubMed]
- Mazaideh, G.M.A.; Quran, S.A.A. Effect of Methanolic Extract of Securigera securidaca as Antioxidant and Antibacterial Activities. J. Pharm. Res. Int. 2020, 32, 10–17. [Google Scholar] [CrossRef]
- Aldal’in, H.K.; Al-Mazaideh, G.; Al-Nadaf, A.H.; Al-Rimawi, F.; Afaneh, A.T.; Marashdeh, A.; Abu-Lafi, S.; Alakhras, F.; Ayyal-Salman, H.; Jamhour, R.M. Phytochemical constituents of securigera securidaca seed extract using gs-ms and hplc. Trop. J. Nat. Prod. Res. 2020, 4, 540–544. [Google Scholar]
- Hoodgar, F.; Nasri, S.; Amin, G. Investigation of Antinociceptive and Anti-inflammatory Effects of Hydro-alcoholic Extract of Securigera securidaca L. Intern. Med. Today 2011, 17, 12–19. [Google Scholar]
- Ibrahim, R.M.; El-Halawany, A.M.; Saleh, D.O.; Naggar, E.M.B.E.; El-Shabrawy, A.E.-R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. Farmacogn. 2015, 25, 134–141. [Google Scholar] [CrossRef]
- El-Suhaibani, M.; Ahmed, M.A.; Osman, M.A. Study of germination, soaking and cooking effects on the nutritional quality of goat pea (Securigera securidaca L.). J. King Saud. Univ. Sci. 2020, 32, 2029–2033. [Google Scholar] [CrossRef]
- Al-Zereini, W.A.; Al-Rimawi, F.; Abu-Lafi, S.; Alakhras, F.; Al-Mazaideh, G.M.; Salman, H.J.A.; Jamhour, R.M. Identification and Antibacterial Evaluation of some Selected Jordanian Medicinal Plants. Orient. J. Chem. 2018, 34, 2456–2463. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Alakhras, F.; Al-Zereini, W.A.; Aldal’in, H.K.; Abu-Lafi, S.; Al-Mazaideh, G.M.; Salman, H.J.A. HPLC Analysis of Chemical Composition of Selected Jordanian Medicinal Plants and Their Bioactive Properties. Orient. J. Chem. 2018, 34, 2397–2403. [Google Scholar] [CrossRef]
- Khalid, M.; Amayreh, M.; Sanduka, S.; Salah, Z.; Al-Rimawi, F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Wedian, F.; Alasmari, F.; Faris Shalayel, M.H. Assessment of antioxidant, antimicrobial, and anticancer activities of Sisymbrium officinale plant extract. Heliyon 2022, 8, e10477. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Jamhour, R.; Al-Nadaf, A.H.; Wedian, F.; Al-Mazaideh, G.M.; Mustafa, M.; Huneif, M.A.; Mahmoud, S.Y.; Farrag, E.S.; Al-Rimawi, F.; Salman, H.A.; et al. Phytochemicals As a Potential Inhibitor of COVID-19: An In-Silico Perspective. Russ. J. Phys. Chem. A 2022, 96, 1589–1597. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Al-Mazaideh, G.M.; Alakhras, F.; Huneif, M.A.; Ali Shah, S.I.; Ahmad, A.; Ayyal Salman, H.; Helmy Faris Shalayel, M. Monoclonal Antibodies as Potential Treatment Drugs for Nsp15 and 3CLpro Proteins. Phys. Chem. Res. 2023, 11, 623–629. [Google Scholar] [CrossRef]
- Al-Mazaideh, G.M.; Al-Mustafa, A.H.; Alnasser, S.M.A.; Nassir-Allah, I.; Tarawneh, K.A.; Al-Rimawi, F.; Mohamad Ibrahim, M.N.; Huneif, M.A.; Alshammari, S.O.; Yaqoob, A.A.; et al. Phytochemical composition and bioactivities of Crataegus aronia as antioxidant, antibacterial and antioxidative stress in red blood cells—Is it a window of hope for children with glucose-6-phosphate dehydrogenase deficiency. Heliyon 2022, 8, e11516. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.A.; Yaakop, A.S.; Al-Mustafa, A.; Tarawneh, K.; Aladaileh, S.; Al-Rimawi, F.; Alakhras, F.; Abu-Lafi, S.; Zarzour, R.A.; Wahab, H. The dual impact of Jordanian Ephedra alte for inhibiting pepsin and treating microbial infections. Saudi J. Biol. Sci. 2021, 28, 6245–6253. [Google Scholar] [CrossRef]
- Mustafa, M.; Wedian, F.; Aldal’in, H.K.; Al-Mazaideh, G.M.; Mahmoud, S.Y.; Farrag, E.S.; Gharaibeh, M.; Hijawi, T.; Al-Rimawi, F.; Abbadi, J.; et al. The efficiency of some active ingredients of Arum palaestinum as inhibitiors to 3clpro and nsp15 proteins. Acta Pol. Pharm. Drug Res. 2021, 78, 657–665. [Google Scholar]
- Al-Mazaideh, G.M.; Al-Swailmi, F.K.; Parrey, M.U.R. Molecular Docking Evaluation of the Desert Truffles as Potent Antifungal Inhibitors. J. Pharm. Res. Int. 2021, 33, 36–48. [Google Scholar] [CrossRef]
- Akdag, Y.; Gulsun, T.; Izat, N.; Cetin, M.; Oner, L.; Sahin, S. Characterization and comparison of deferasirox fast disintegrating tablets prepared by direct compression and lyophilization methods. J. Drug Deliv. Sci. Technol. 2020, 57, 101760. [Google Scholar] [CrossRef]
- Akasha, A.A. Comparative physical studies of atenolol brands available in Libyan drug market. Innoriginal Int. J. Sci. 2016, 3, 1–3. [Google Scholar]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression Physics in the Formulation Development of Tablets. Crit. Rev. TM Ther. Drug Carr. Syst. 2006, 23, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; O’Brien, D.P.; Croker, D.M.; Walker, G.M. Chapter 2—The development of a pharmaceutical oral solid dosage forms. In Computer Aided Chemical Engineering; Singh, R., Yuan, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 41, pp. 27–65. [Google Scholar]
- Nagar, P.; Singh, K.; Chauhan, I.; Verma, M.; Yasir, M.; Khan, A.; Sharma, R.; Gupta, N. Orally disintegrating tablets: Formulation, preparation techniques and evaluation. J. Appl. Pharm. Sci. 2011, 1, 35–45. [Google Scholar]
- Acheampong, A.; Gyasi, W.O.; Darko, G.; Apau, J.; Addai-Arhin, S. Validated RP-HPLC method for simultaneous determination and quantification of chlorpheniramine maleate, paracetamol and caffeine in tablet formulation. SpringerPlus 2016, 5, 625. [Google Scholar] [CrossRef]
- Ceresole, R.; Rosasco, M.A.; Segall, A.I. HPLC Assay for Meprednisone in Tablets. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2795–2804. [Google Scholar] [CrossRef]
- Barbara, K.; Mohammed, L.K.; Piotr, M.; Messaoud, B.; Mirosława, K. HPTLC determination of diosgenin in fenugreek seeds. Acta Pharm. Croat. Pharm. Soc. 2018, 68, 97–107. [Google Scholar] [CrossRef]
- <1094> Capsules—Dissolution testing and related quality attri-butes. In USP-NF; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2020.
- <1092> The Dissolution Procedure: Development and Validation. In USP-NF; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2020.
- <711> Dissolution. In USP-NF; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2022.
- FDA Guidance for Industry, Analytical Procedures and Method Validation for Drugs and Biologics. July 2015. Available online: https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf (accessed on 14 January 2023).
- Available online: https://hmc.USP.org/about/general-chapters (accessed on 14 January 2023).
- Abdelbagi, M.E.M.; Al-Mazaideh, G.M.; Ahmed, A.E.; Al-Rimawi, F.; Ayyal Salman, H.; Almutairi, A.; Abuilaiwi, F.A.; Wedian, F. Exploring Securigera securidaca Seeds as a Source of Potential CDK1 Inhibitors: Identification of Hippeastrine and Naringenin as Promising Hit Candidates. Processes 2023, 11, 1478. [Google Scholar] [CrossRef]
| Ingredients | Composition/mg | Total Weight |
|---|---|---|
| S. securidaca extract | 500 mg | 946.8 mg |
| Lactose monohydrate | 250 mg | |
| Starch powder | 120 mg | |
| Talc (3%) | 28.4 mg | |
| Magnesium stearate (3%) | 28.4 mg | |
| Sodium starch glycolate | 20 mg |
| Test | Specification | Result | Reference |
|---|---|---|---|
| Appearance | Extended-release film coated tablet with yellow to pale yellow color | Conformed | U.S.P * |
| Color | Yellow to pale yellow | Conformed | U.S.P * |
| Average weight of tablet | 946.8 ± 7.5% (875.79 mg to 1017.81 mg) | 921.02 | U.S.P * |
| Thickness | 3.15 mm | 2.5–5.5 mm | U.S.P * |
| Compression pressure | 22 MPa | 5–65 MPa | U.S.P * |
| Solubility | Met requirements | Particularly soluble in water (pH: 6.5–7.0) | U.S.P * |
| Hardness | Average hardness N.L.T. 3.0 Kp | 3.433 KP | U.S.P * |
| Friability | N.M.T. 1.0% | 0.71% | U.S.P * |
| Water content (K.F) | N.M.T. 5.0 % | 2.38% | U.S.P * |
| Dissolution: 45 min dissolved diosgenin or trigonelline (HPLC) (USP Test 2) [30,32] | N.L.T. 80 % of amount based on assay by HPLC | 94.05% | FDA modified in-house * |
| Disintegration Time | N.M.T 15 min | 4.11 min | U.S.P * |
| Quantitative analysis of Diosgenin Trigonelline by high-performance liquid chromatography (HPLC) | Conformed Conformed | Conformed Conformed | U.S.P * |
| Assay of Diosgenin Trigonelline by high-performance liquid chromatography (HPLC) | 7.31–8.5 mg/mL 3.30–3.8 mg/mL | 7.65 mg/mL 3.51 mg/mL | FDA modified in-house * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbagi, M.E.M.; Al-Mazaideh, G.M.; Ahmed, A.E.; Al-Rimawi, F.; Ayyal Salman, H.; Almutairi, A.; Abuilaiwi, F.A.; Wedian, F. Drug Formulation of Securigera securidaca Seed Extracts. Processes 2023, 11, 1955. https://doi.org/10.3390/pr11071955
Abdelbagi MEM, Al-Mazaideh GM, Ahmed AE, Al-Rimawi F, Ayyal Salman H, Almutairi A, Abuilaiwi FA, Wedian F. Drug Formulation of Securigera securidaca Seed Extracts. Processes. 2023; 11(7):1955. https://doi.org/10.3390/pr11071955
Chicago/Turabian StyleAbdelbagi, Mohamed E. M., Ghassab M. Al-Mazaideh, Adil Elhag Ahmed, Fuad Al-Rimawi, Haya Ayyal Salman, Abdulrahman Almutairi, Faraj Ahmad Abuilaiwi, and Fadel Wedian. 2023. "Drug Formulation of Securigera securidaca Seed Extracts" Processes 11, no. 7: 1955. https://doi.org/10.3390/pr11071955
APA StyleAbdelbagi, M. E. M., Al-Mazaideh, G. M., Ahmed, A. E., Al-Rimawi, F., Ayyal Salman, H., Almutairi, A., Abuilaiwi, F. A., & Wedian, F. (2023). Drug Formulation of Securigera securidaca Seed Extracts. Processes, 11(7), 1955. https://doi.org/10.3390/pr11071955







