Abstract
The rising trend in the consumption of healthy, safe, and functional foods has motivated studies on cold-pressed specialty oils, including macadamia nut oil. Cold-pressed macadamia nut oil (CPMO) is given preference by consumers over solvent extracted and refined oil because of its exceptional quality attributes and safety. This review contains a detailed presentation of the chemical properties, health benefits, and applications of CPMO. The monounsaturated fatty acids (oleic acid and palmitoleic acid) rich oil also contains a significant concentration of bioactive phytochemicals including, β-sitosterol, α-tocopherol, α-tocotrienols, ρ-hydroxybenzoic acid, and caffeic acid. Moreover, the oil has good oxidative stability. The highlighted properties offer CPMO health benefits related to the prevention of cardiovascular diseases, diabetes, cancer, high blood pressure, and neurodegenerative diseases. The fatty acid composition of CPMO allows for its diverse application in the food, cosmetic, nutraceutical, and pharmaceutical industries.
1. Introduction
Vegetable oils and fats are a vital part of the human diet as a source of essential fatty acids and lipid-soluble bioactive compounds. Globally, the predominant edible vegetable oils are soybean oil, palm oil, rapeseed oil, and sunflower seed oil [1]. The increased consumer demand for healthy and nutrient foods has increased the interest in alternative sources of vegetable oils. Researchers have focused on tree nuts as an alternative source of vegetable oil to meet the future demand for healthy foods. Tree nuts are defined as single-seeded dry fruits with a hard overall at maturity stage [2]. They are not only a rich source of macronutrients (carbohydrates, proteins, and fat) and micronutrients (minerals and vitamins), but the oil is a treasure of health benefitting compounds that could prevent cardiovascular diseases, type-2 diabetes, cancer, high blood pressure, and neurodegenerative diseases [3]. In this view, tree nuts have been recognized as ‘heart healthy’ foods by the Food and Drug Administration (FDA) [4]. According to the FDA, consumption of about 43 g of tree nuts per day may reduce the risk of heart diseases [2].
The commonest tree nuts include almond (Prunus spp.), Brazil nut (Bertholletia excelsa), cashew (Anacardium occidentale), hazelnut (Corylus avellana), macadamia (Macadamia spp.), pecan (Carya illinoinensis), pine nut (Pinus spp.), pistachio (Pistacia vera), and walnut (Juglans regia) [5]. Due to the acclaimed health benefits, the economic importance of tree nuts continues to grow. For instance, in 2017, tree nuts recorded an annual global trade in excess of USD 32 billion [6]. During the 2019/2020 season, the global tree nut production reached about 4.6 million metric tons, representing a 2% crop growth from the previous season (2018/2019) [7]. The primary producers of tree nuts are the United States, Turkey, and China, contributing 59% of the world share in the 2018/2019 season [7]. In most parts of the world, tree nuts are cultivated for use as oil crops and healthy snack foods [8].
Oil from tree nuts can be extracted either using pressure systems (expelling by screw press or pressing by hydraulic presses) or organic solvents. Mechanical oil extraction is generally regarded as a cold extraction method since only pressure is applied, notwithstanding that the screw press system requires preheating of the barrel to enhance oil extraction efficiency [9,10]. From a nutrition viewpoint, consumers prefer oil extracted by cold pressing because of the high concentration of bioactive compounds such as essential fatty acids, tocopherols, and phytosterols in the oil [11,12]. Cold-pressed oil is obtained without applying solvents or chemical treatments, which makes the process environmentally friendly and the extracted oil safer for human consumption [11]. Therefore, there is growing consumer demand for cold-pressed oil, such as cold-pressed macadamia nut oil (CPMO). CPMO has a clear golden yellow color, distinctive nutty aroma, and flavor [13]. The oil is rich in monounsaturated fatty acids (MUFA), predominantly oleic (60%) and palmitoleic (~20%) acids, which are vital in reducing plasma low-density lipoprotein (LDL) cholesterol, prevention of cancer, and atherosclerosis [10,14]. In addition, CPMO contains other bioactive constituents such as tocopherols, phytosterols, and squalene, which have antioxidant properties.
This paper provides an overview of the current research on CPMO. The chemical properties, health benefits, and application of CPMO are thoroughly reviewed to incite future studies and further developments in the application of CPMO.
2. Methodology
Relevant articles used in this review were searched on the Google Scholar database. Boolean operators “AND” and “OR” were used to widen the search. The keywords used for searching were macadamia nut, chemical properties, health benefits, and application of CPMO. The search focused on scientific research articles (most of them scientific indexed papers) and reports. The scientific articles and reports were published between 2000 and 2020, with three-quarters of the publications being less than five years old. The key words ‘cold-pressed macadamia nut oil’; ‘palmitoleic acid’; ‘oleic acid’; ‘phytosterols’; ‘tocotrienols’; and ‘health benefits’ appear in the title and abstract.
3. Description of Macadamia Species
The macadamia nut, which belongs to the Proteaceae family, is native to Australia [15]. At the maturity stage, the nut is approximately 2.6 cm in length and 2.7 cm in width, and it consists of the husk, shell, and kernel [13,16]. The kernel, an edible part of the nut, contains oil (69–78%) [17]. About nine species of macadamia have been discovered to date; however, for commercial production, only two species, which are Macadamia integrifolia and Macadamia tetraphylla and their hybrids, are common [13]. The physical differences between the two species are that Macadamia integrifolia is characterized by a round shape with a smooth shell, whilst Macadamia tetraphylla is identified as spindle-shaped with a rough shell [18]. Some macadamia species are not edible because they contain toxic cyanogen compounds. In a previous study, Navarro et al. [10] reported that Macadamia ternifolia (another species) contained about 9.6 mmol/g of cyanogenic glycosides that was 60 times higher than those found in Macadamia integrifolia and Macadamia tetraphylla. The level is poisonous and imparts a bitter taste to the species. For that reason, it is important that macadamia nut oil be extracted from non-toxic species.
4. Economic Importance of Macadamia Nut and Oil
Driven by its health benefits, the global macadamia nut production has steadily increased in the past decade, and it reached 60.057 Metric tons (kernel basis) in 2019, which represented a 2% increase from the previous year (Figure 1) [7].
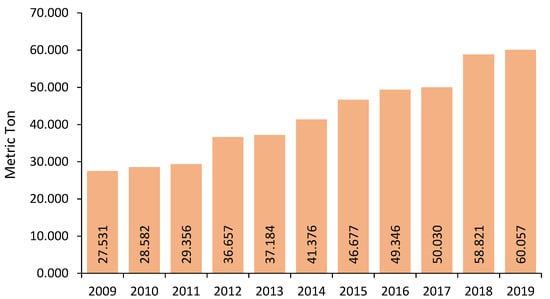
Figure 1.
Global production of macadamia nut (2009–2019). Adapted from: International Nut and Dried Fruit Council [7].
The largest producers of macadamia nuts are South Africa (29%) and Australia (22%), which contribute 51% of the global production (Figure 2A). Other significant producers of macadamia nuts are Kenya (12%), China (11%), the United States (7%), Guatemala (5%), and Malawi (3%) [7]. Although the area of macadamia nut production slightly increased (18%) in Australia from 2010 to 2016, the industry production almost doubled within the same period, recording an average industry production rate of 2.8 tons per hectare [19]. In China, macadamia nuts were introduced in the 1970s from Australia, and to date, it is one of the fastest producers of nuts globally [15]. Macadamia nut is a lucrative crop in Malawi, which is ranked the seventh top producer of nuts, valued at USD 35 million. In the past two decades, the total production area in Malawi has remarkably increased by 83% [20]. The current world-leading macadamia exporters are South Africa (29%) and Australia (24%); however, the chief importers of macadamia nuts are the United States, Germany, Netherlands, China, and Japan, which import over 70% of the global share. Macadamia nuts are most consumed in United States (37%), China (18%), Germany (10%), Australia (10%), and Japan (7%) (Figure 2B) [7]. Despite being the world leader in macadamia production, the local market in South Africa only consumes 2% of the nut, with the United States and Europe together taking up more than 44% of the total production [21].
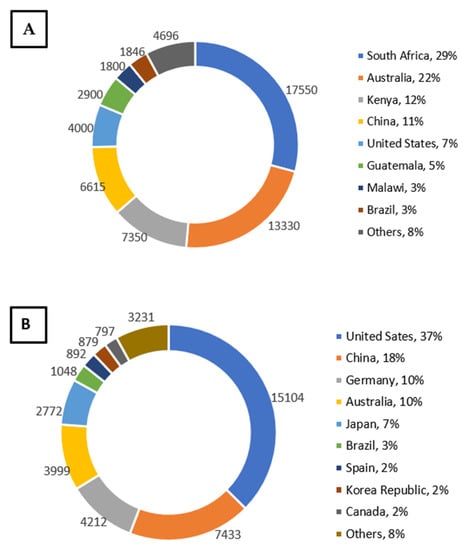
Figure 2.
(A) World estimated macadamia nut production (metric ton) and (B) consumption (metric ton) in 2018. Adapted from: International Nut and Dried Fruit Council [7].
The continued increase in demand for unrefined oil and consumers’ health consciousness has driven the processing of macadamia nuts into oil. Depending on the extraction method employed, macadamia nut oil can be marketed as cold-pressed or virgin oil. Unlike in virgin oil, the application of heat in cold-pressed oil during processing is not allowed [22], which makes CPMO a desirable raw material for functional food, pharmaceutical, and cosmetics formulations [23,24]. In addition, to maximize the economic benefits, a variety of value-added macadamia nut by-products are produced, which are in high demand in the food industry for making snack and confectionery products [25].
5. Cold Pressing and Seed Pretreatment
The literature has shown that macadamia cultivar, growing conditions, harvesting time, degree of ripeness, storage conditions, and oil extraction technique are some of the major quality determinants of oil [11,12].
Typically, at the commercial level, two oil extraction methods are available, which are solvent extraction and pressing. Whilst solvent extraction is more suitable for seeds with lower oil content (<20%), pressing is suitable for seeds with higher oil content, such as macadamia nuts. Nevertheless, a significant amount of oil remains trapped in the seed cold-pressed oil cake and therefore, the method produces a low oil yield. Rodriguez et al. [26] extracted macadamia nut oil using a cold press and reported oil yield (30–40%), which was 25–50% lower than that of solvent extracted oil (40–60%). The results suggested that the pressed meal still contained a significant quantity of oil. In another study, Sarkis et al. [27] determined a residual oil content of approximately 52% in a defatted macadamia meal. Despite the high oil content in macadamia nuts (69–78%), by using a cold press, Phosa [28] and Skenjana [29] only managed to extract oil ranging between 23 and 26%. Moreover, the pressed meal contains substantial amounts of bioactive compounds such as phenolics (approx. 0.5 mg/g on a dry basis) [27]. To extract the residual oil, the pressed meal can be further subjected to solvent extraction. Nonetheless, the use of organic solvents such as hexane, petroleum ether, benzene, or pentane in food extractions is not recommended due to their negative health effects on humans and the environment [12]. In addition, plant security issues, higher operational costs, poor quality products due to high temperature, and residual solvent are some of the disadvantages of using organic solvents in extracting vegetable oil [11]. In comparison, mechanical pressing is simpler, safer, and requires less capital investment than solvent extraction [30].
Two types of mechanical pressing methods are common, and these are cold pressing and hot-pressing. The literature has reported that the maximum temperature of cold-pressed oil ranges between 30 and 50 °C, which preserves its nutritional quality [13,31]. Three main stages are involved in cold pressing, including seed defatting, seed fragmentation, and seed conditioning. Seed defatting though uncommon at the commercial level due to technical issues enhances the quality of the oil by reducing the concentration of chlorophyll and other substances such as metals and pesticides. This is to produce oil with brighter color and high resistance to oxidation [11]. Seed fragmentation enlarges the oil spillage by destroying seed structures and hulls. It is noteworthy to indicate that this process should be immediately followed by pressing as it may expose the oil to oxidation. Seed conditioning, which involves heat pretreatment at 100 °C and moisturizing it to the optimal humidity, is vital to improving the oil yield [10]. Seed condition is essential for seeds with low fiber content, such as macadamia nuts, to prevent the machine from producing an oily paste instead of oil and cake. CPMO may be physically purified through filtration, sedimentation, or centrifugation processes. The packed oil may be further flushed with nitrogen to prevent oxidation and the generation of odoriferous compounds. To this effect, the nutty flavor, and minor nutrients like vitamin E, phytosterols, and polyphenols are well preserved. The shelf life of CPMO stored under appropriate storage conditions (25 °C and low oxygen) ranges from 9–15 months [26,32].
Cold-pressed oils are produced using either a hydraulic or screw press. The hydraulic press operates in a non-continuous mode at a pressure ranging from 11.376 to 12.755 kPa and uses liquid (oil) as a medium to squeeze out the oil from the seed. This method produces a lower oil yield but of higher quality than screw press [33]. On the other hand, the screw press operates by applying a high shear force to the material to press out the oil. As a result of frictional heat that may be produced during pressing, a cooling system may be attached to it, to avoid degradation of heat-labile bioactive phytochemicals [34]. The critical parameters that affect cold-pressed oil quality include the seed characteristics (moisture content, oil content, and type of seed), feed rate, temperature, rotation speed, die diameter, and seed pretreatment. To circumvent the low oil yield problem in cold pressing, seed pretreatment has become an appropriate and valuable step in cold pressing. Conventionally pretreatment of seeds prior to oil extraction includes dehulling, size reduction, breaking, grinding, and thermal and non-thermal treatment to debilitate the cell coats and enhance oil extraction during pressing. The application of microwaves to macadamia nuts prior to oil extraction has gained research interests due to its technical and economic benefits. The microwaves generate high-frequency waves, which break the cells by shock induction. The waves penetrate the material and vibrate the molecules, provoking a rapid heating and subsequent rupture of the cells, which may help to improve oil extraction efficiency. In the study of Ribeiro et al. [33], microwave pretreatment of macadamia nut improved the oil extraction efficiency of a hydraulic press by more than 50%. The authors further highlighted that combining microwave heating with hydraulic pressing produced macadamia nut oil with better oxidative stability.
6. Lipidomics, Chemical and Antioxidant Properties of Cold-Pressed Macadamia Nut Oil
6.1. Fatty Acid and Triacylglycerol Profile
Triacylglycerol is the major form of dietary lipid in plant or animal fats and oils. In CPMO, they form the major component of neutral lipids, accounting for 87% of total neutral lipids [35]. As shown in Figure 3, Triolein (OOO: 24.0%), dioleoyl-palmitoleoyl-glycerol (OOPo: 21.6%), and dioleoyl-palmitoyl-glycerol (OOP: 14.7%) are the primary triacylglycerol components present in CPMO. Other triacylglycerols present in CPMO, but in small quantities are dioleoyl-dipalmitoyl-glycerol (OPPO) (8.51%), oleoyl-dipalmitoleoyl-glycerol (OPoPo) (6.7%), dioleoyl-arachidoyl-glycerol (OOA) (3.69%), dioleoyl-linoleoyl-glycerol (OOL) (2.31%), palmitoyl-dipalmitoleoyl-glycerol (PPoPo) (1.78%), palmitoyl-oleoyl-stearoyl-glycerol (POS) (1.51%) and palmitoyl-oleoyl- arachidoyl-glycerol (POA) (1.1%) [26]. Monoacylglycerols and polar lipids are found in minor quantities, representing 1 and 3%, respectively [35]. The profiles and relative concentration of the triacylglycerols from CPMO did not vary much from that of commercial macadamia nut oil [13].
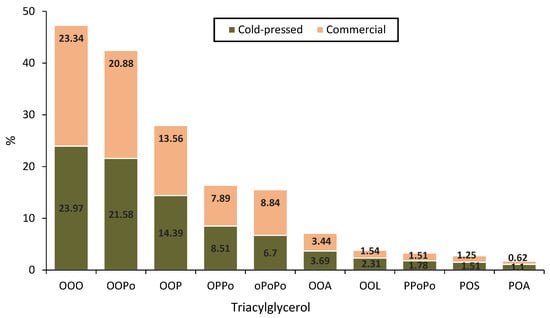
Figure 3.
The primary triacylglycerol components (%) of cold-pressed and commercial macadamia nut oils.OOO-dioleoyl-palmitoleoyl-glycerol, OOPo-dioleoyl-palmitoleoyl-glycerol, OOP-dioleoyl-palmitoyl-glycerol, OPPo-oleoyl-palmitoyl-palmitoleoyl-glycerol, OPoPo-oleoyl-dipalmitoleoyl-glycerol, OOA-dioleoyl-arachidoyl-glycerol, OOL-dioleoyl-linoleoyl-glycerol, PPoPo-palmitoyl-dipalmitoleoyl-glycerol, POS-palmitoyl-oleoyl-stearoyl-glycerol, POA-palmitoyl-oleoyl-arachidoyl-glycerol. Adapted from Tan et al. [13].
Macadamia nut oil is acknowledged as one of the healthiest tree nut oils due to its unique fatty acid profile, characterized by higher monounsaturated fatty acids (MUFAs) accounting for more than 70% of the total fatty acids. CPMO is lower in saturated fatty acids (SFA) (<16%) and polyunsaturated fatty acid (PUFA) (<5%), and therefore, the oil has great oxidative stability and longer shelf life [36]. A review of the fatty acid profiles of CPMO in presented in Table 1. PUFAs and saturated fatty acids (SFAs) in CPMO range from 3–4% and 12–16%, respectively. However, Cicero et al. [37] reported relatively higher levels of PUFAs (23.22%) in CPMO. This contrasts with the results reported in other studies [33,34,38,39,40]. The differences could be attributed to variation in cultivar, growing conditions, harvesting time, degree of ripeness, among other factors. The unsaturated fatty acids (UFAs) to SFAs (5–7%) ratio in CPMO is similar to other cold-pressed oils such as grapeseed oil (6%); however, the quality of the unsaturated fatty acids varies. Oleic acid (51–64%) represents the most abundant fatty acid in CPMO, followed by palmitoleic acid (10–21%) and palmitic acid (7–12%). The literature reports that a higher intake of MUFA is associated with low incidences of coronary heart diseases, colon, breast, and skin cancer. Therefore, from a health and nutrition perspective, high levels of oleic acid in vegetable oils are desirable. Structurally, oleic acid is a more stable unsaturated fatty acid compared with other unsaturated fatty acids such as linoleic acid [41]. Other fatty acids in CPMO include stearic acid, linoleic acid, linolenic acid, arachidic acid, malic acid, behenic acid, gadoleic acid, heptadecanoic acid, lignoceric acid, which are present in smaller amounts (0.1–2.5%)

Table 1.
Fatty acid composition (%) of cold-pressed macadamia nut oil.
Linoleic acid and linolenic acid also referred to as omega-6 fatty acid (ɷ6) and omega-3 fatty acid (ɷ3), respectively, are two essential fatty acids crucial in the maintenance of physiological functions of the human body. The ɷ6/ɷ3 ratio is valuable in explaining the effect of dietary PUFAs on the pathogenesis of cardiovascular diseases, cancer, inflammatory and autoimmune disorders [37]. Generally, a high ɷ6/ɷ3 ratio is regarded as harmful to human health, whereas a value around 1 is considered healthier and protective against such chronic diseases. An unbalanced ɷ6/ɷ3 ratio that is in favor of omega-6 PUFAs contributes to the prevalence of atherosclerosis, obesity, and diabetes [42]. Cicero et al. [37] reported a ɷ6/ɷ3 ratio of 1.69 in CPMO, indicating a healthy ratio, important in the prevention and management of obesity. Stearic acid is a saturated fatty acid with unique functional properties related to the reduction of LDL cholesterol levels in humans. Behenic acid, another saturated fatty acid present in trace amounts in CPMO, has low bioavailability and, therefore, causes less effect on cholesterol content in humans.
Gong et al. [34] compared the fatty acid composition of CPMO with cold-pressed oil from almond, hazelnut, pecan, pine nut, pistachio, and walnut. The authors reported that CPMO contained higher palmitoleic acid and stearic acid levels than other tree nut oils. Except for cold-pressed oil from walnut (14%) and pecan (32%), all the other cold-pressed tree nut oils contained high levels of oleic acid (>50%). Similar findings were reported in the studies of Madawala et al. [38], Gliszczynska-Swigło et al. [39], Yuenyong et al. [40], and Prescha et al. [43] (Table 2). This indicates that tree nut oils are good sources of oleic acid, a suitable raw material for formulating plasma cholesterol reduction diets. CPMO contains lower linoleic acid and linolenic acid levels compared with cold-pressed oil from other tree nuts. Among the cold-pressed tree nut oils, walnut exhibited the best concentration of these essential fatty acids (Table 2). Ribeiro et al. [33] investigated the effect of seed microwave heating (maximum power, 2450 MHz, 1 min) on CPMO and reported an insignificant effect on the linoleic and linolenic. The results suggest increasing CPMO yield through microwave pretreatment of the seed for financial benefit without altering the essential fatty acid profiles.

Table 2.
Comparison of fatty acid composition (%) of cold-pressed macadamia nut oil with cold-pressed oil from selected tree nuts.
It is generally agreed among researchers that the extraction method may not significantly affect the fatty acid profile of seed oil [12]. The fatty acid composition of CPMO was compared with that of macadamia nut oil obtained by solvent extraction (Table 3). The relative percentages of SFAs, MUFAs, PUFAs, and individual fatty acids of CPMO did not differ much from the solvent-extracted macadamia nut oil, indicating that the fatty acids profile of macadamia nut oil may not be affected by the extraction method [26,34,38,43,44,45,46].

Table 3.
Comparison of fatty acid composition (%) of cold-pressed, solvent, and supercritical carbon dioxide extracted macadamia nut oil.
6.2. Tocochromanol
Tocopherols and tocotrienols, collectively known as tocochromanols, are lipid-soluble molecules that belong to the group of vitamin E compounds and are essential in the human diet [49]. Whilst tocopherols are characterized by a saturated isoprenoid side chain, tocotrienols have an unsaturated side chain. The antioxidant activity of tocochromanols is mainly due to the capacity of their heterocyclic chromanol ring system to donate the phenolic hydrogen to lipid-free radicals [49].
Wall et al. [14] studied the tocochromanol composition of CPMO from different cultivars harvested in two different seasons (2006 and 2007). The total tocochromanol varied between 30.15 and 91.59 µg/g. In the study, tocopherols; delta (δ), gamma (γ), alpha (α) were not detected in most of the CPMO samples, except for low amounts of γ-tocopherol and α-tocopherol detected in only two cultivars (HAES 856 in 2006 and HAES 294 in 2007, respectively). The α-tocopherol, which is the primary tocopherol in CPMO varied from 2.25–3.12 µg/g, whilst γ-tocopherol a minor tocopherol, ranged between 0.59 and 2.03 µg/g. The α-tocopherol is the most biologically active form of vitamin E. Epidemiological studies have established that α-tocopherol supplementation is associated with a reduction in cardiovascular events in patients with coronary disease. To this effect, the United States has recommended dietary allowance (RDA) for vitamin E based only on α-tocopherol, as it is the most biologically active homolog. However, CPMO contains lower amounts of α-tocopherol, therefore, may not meet the RDA for vitamin E [14]. Although found in lower concentrations in CPMO, γ-tocopherol is more effective than α-tocopherol in inhibiting prostate cancer cell growth, reducing oxidative deoxyribonucleic acid (DNA) damage, and anti-inflammatory activities [50]. Tocotrienols are found in higher concentrations in CPMO than tocopherols. Moreover, they are more powerful antioxidants than tocopherols and show better cholesterol-lowering and anti-cancer properties [15]. The α-tocotrienol (15.91–46.83 µg/g) was the most abundant followed by γ-tocotrienol (9.04–22.23 µg/g) and δ-tocotrienol (3.00–17.66 µg/g) [14]. The study also demonstrated the variation of CPMO tocotrienols with cultivar and season, which is important for CPMO processors. Meanwhile, Madawala et al. [38] reported levels of α-tocopherol and γ-tocopherol that were more than double the level reported by Wall [14]. The differences could be explained by variation in macadamia cultivars, growing conditions, harvesting time, degree of ripeness, and storage conditions. Although it can be largely accepted that CPMO has lower levels of tocopherols, extracting the oil using petroleum ether resulted in non-detection of the tocopherol components [51]. In all the studies, no β-tocopherol was identified in the CPMO (Table 4). Madawala et al. [38] and Ying et al. [52] reported α-tocotrienol ranging from 14 to 20 µg/g. There were also discrepancies in the concentrations and compositional profiles of tocochromanols in CPMO from the different studies. Other studies that investigated the levels of tocochromanols in CPMO are reported in Table 3.

Table 4.
Tocopherols, tocotrienols, and phytosterols content in cold-pressed macadamia nut oil.
6.3. Phytosterol and Squalene
Phytosterols are plant-based structures analogous to mammalian cholesterol [54]. They contain an identical ring structure as cholesterol but differ in the side chain at the carbon 24 positions of unsaturated double bonds and differ in stereochemistry around chiral carbons (Figure 4). It has been reported that phytosterols can reduce intestinal cholesterol absorption due to the displacement of cholesterol in micelles. Their consumption at doses between 1.5 and 3 g/day can efficiently decrease LDL cholesterol by up to 15%. Cold-pressed plant oil contains a substantial amount of phytosterols, while most of the phytosterols in seeds are degraded during the refining process. Kumar et al. [54] reported that more than 40% of phytosterols could be lost during the oil refining processes; therefore, cold-pressed oil could be an excellent source of phytosterols. Table 4 shows the phytosterols content of CPMO reported in the previous studies. The main phytosterol was β-sitosterol (0.66–2.11 mg/g) followed by campesterol (0.07.0–0.16 mg/g), avenasterol (0.04–0.29 mg/g), and stigmasterol (0.001–0.02 mg/g) [34,38,40,51]. The total phytosterol content ranged between 0.78 and 2.57 mg/g oil and was higher than the total phytosterol content of cold-pressed almond, walnut, and hazelnut oil (0.55 and 2.05 mg/g oil). Nevertheless, the total phytosterol content was lower than that of other cold-pressed tree nut oils (pecan, pine nut, and pistachio), ranging between 3.49 and 4.14 mg/g oil [34]. The β-sitosterol content of CPMO was 1.1 to 3.4 times higher than pine nut oil, black currant seed oil, hemp seed oil, dill seed oil, parsley seed oil, milk thistle oil, and black cumin seed oil. Being the predominant phytosterol, most of the health benefits of phytosterols are contributed by β-sitosterol. Bioactive phytosterol has been reported to be effective in reducing serum cholesterol and LDL cholesterol levels [55].
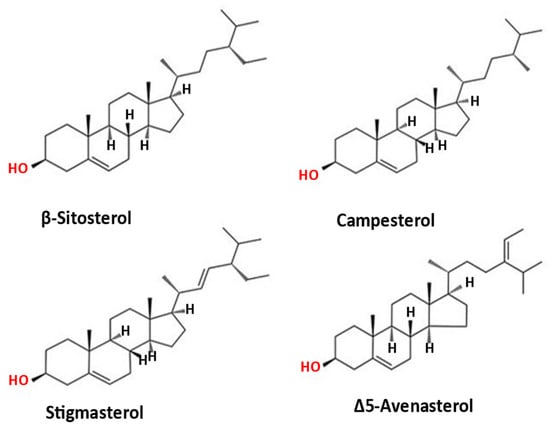
Figure 4.
Chemical structures of the predominant phytosterols in cold-pressed macadamia nut oil.
Squalene is a precursor in the phytosterol biosynthetic pathway. In vitro and in vivo studies have demonstrated the antioxidant effects of squalene. In addition, triterpene has been reported to provide beneficial effects in chemoprevention and reduction of serum cholesterol and triacylglycerol levels [40]. Macadamia nut is not a good source of dietary squalene. Cicero et al. [37] reported that squalene content of CPMO (23 µg/g) was lower than that of other cold-pressed plant oils (olive, Brazil nut, grapeseed, and palm oil), which ranges from 88 to 1046 µg/g. Recently, Yuenyong et al. [40] reported a higher concentration of squalene in CPMO (549 µg/g). Large variation exists in the concentration of squalene between the two studies; therefore, more studies are required to have informative data on the levels of squalene in CPMO. Cold-pressed oil from coconut, almond, hazelnut, pistachio, and walnut showed a better concentration of squalene ranging between 702 and 2568 µg/g [40]. It seems solvent extraction of macadamia nut oil is a more efficient oil extraction method for maximum recovery of squalene (72–171 mg/g) than cold pressing [14].
6.4. Polyphenol
Phenolic compounds contribute not only to the taste of vegetable oil but also to its oxidative stability. Moreover, they have been reported to have multiple biological effects, including antioxidant activity through breaking the oxidation chain reaction or scavenging the free radicals depending on their chemical structures [56]. The total phenolic content of CPMO analyzed using ultra-high-performance liquid chromatography-mass spectrometry (UHPLC) was 2.36 µg/g [37]. Although the UHPLC-MS analysis revealed a more exiguous polyphenol profile, the method is more accurate. However, using ultraviolet-visible (UV-Vis) spectroscopy and the Folin-Ciocalteu assay (FC), Castelo-Branco et al. [53], reported that the total phenolic content in CPMO was 22.5 mg of gallic acid equivalents (GAE)/100 g. However, lack of selectivity is the primary disadvantage of the FC method, which may contribute to high values of total phenolic compounds. The total phenolic content in CPMO was 3 to 24 times higher than that of cold-pressed oil from almond nut, hazelnut, Brazil nut, and pecan nut. In the same study, CPMO showed significantly higher levels of total phenolic compounds (2–15 fold) than refined oil from canola, corn, soya bean, and sunflower) [53]. Cicero et al. [37] studied the phenolic profile of CPMO and reported four types of phenolic compounds (flavonoids and phenolic acids), namely, apigenin 7-glucoside, luteolin, caffeic acid, and p-hydroxybenzoic acid ranging between 0.43 and 0.80 µg/g. Figure 5 shows the chemical structures of these phenolic compounds. The free radical attack aims at the phenolic compound carbon atom with the highest electron density and subsequently abstracts the hydrogen atom from the hydroxyl group (OH) and form stable compounds, subsequently breaking the oxidation chain reaction [56]. The concentration of phenolic compounds in CPMO was inferior to that of cold-pressed oil from palm seed, grapeseed, olives, canola seed, and coconut (Figure 6).
Figure 5.
Chemical structures of the predominant phenolic compounds in cold-pressed macadamia nut oil.
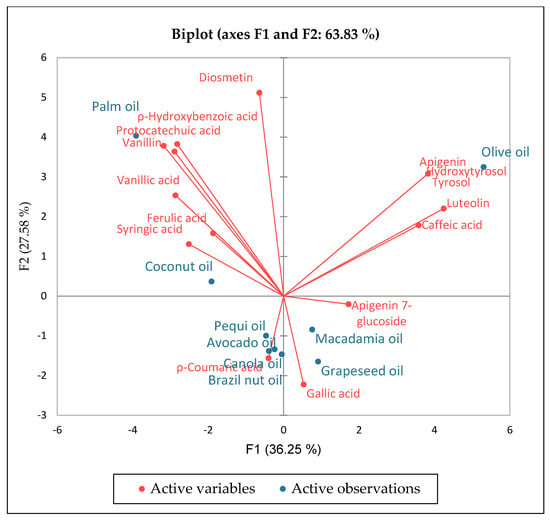
Figure 6.
Principal component analysis showing a comparison of phenolic compounds in coldpressed macadamia nut oil and other selected cold-pressed oils. Adapted from Cicero et al. [37].
6.5. Antioxidant Activity
In vitro studies on the antioxidant activity of CPMO have been mainly expressed as Trolox equivalent antioxidant capacity (TEAC). Wall et al. [14] reported cultivar and seasonal variation in antioxidant activity of CPMO. The findings were supported by the agglomerative hierarchical clustering (Figure 7). The antioxidant activity values ranged between 37.48 and 65.78 (nmolTE/g oil), with HAES 294, and HAES 835 cultivars harvested in both 2006 and 2007 exhibiting higher antioxidant potency than the rest of the cultivars (clustered together). The authors attributed the antioxidant activity of the CPMO to tocopherols, tocotrienols, phenolics, phytosterols, squalene, and phospholipids. However, based on the low concentration of phenolics in CPMO, their contribution to antioxidant activity should be minimal [14]. Notwithstanding the relatively high concentration of tocopherols, tocotrienols, and squalene in CPMO, no statistical correlations were observed between these bioactive compounds and antioxidant activity. Thus, more studies on the correlations between the bioactive compounds and antioxidant activities of CPMO are warranted. In another study, Prescha et al. [43] reported that the antioxidant activity of CPMO was 0.17 mM TAEC/kg a value which was lower than that of other cold-pressed oils (avocado, sesame, pumpkin, safflower, rosehip, walnut, flaxseed, hemp, poppy, milk thistle and linola). It appears that researchers agree to the conclusion that CPMO is not a good source of antioxidant activity. In their study, Castelo-Branco et al. [53] reported that cold-pressed oil from Brazil nut, almond, and pecan (1.02–2.04 mmol TE/kg) had superior antioxidant potency than CPMO (0.87 mmol TE/kg). Even refined oil from canola, corn, sunflower, and soyabean showed greater antioxidant activity than CPMO (1.73–4.35 mmol TE/kg) [53].
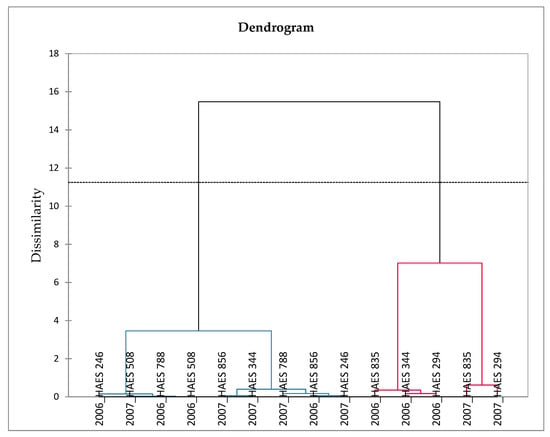
Figure 7.
Agglomerative hierarchical clustering showing the variation of oxidative stability and antioxidant properties of cold-pressed macadamia nut oil with cultivar (from Hawaii) and season. HAES: Hawaii Agricultural Experiment Station. Adapted from Wall et al. [14].
6.6. Oxidative Stability
The oxidative stability of seed oil may be measured using the Rancimat system and expressed as the induction time (hours) that precede a rapid increase in autooxidation [12]. Alternatively, the oxidative stability of oil can be evaluated using the oxidation indices such as peroxide value, acid value, anisidine value, and total oxidation value. It depends on the degree of unsaturation and the level of available antioxidative components in the oil. Castelo-Branco et al. [53] compared the oxidative stability of CPMO with other cold-pressed tree nut oils (almond, Brazil nut, hazelnut, and pecan). The authors observed that the induction time, peroxide value, and acid value of CPMO were 7.38 h, 7.15 mEqO2/kg, and 3.39 mg KOH/g, respectively. Other cold-pressed oils, including pecan, hazelnut, and Brazil nut, showed a higher induction period (8.24–9.27 h). Similar values of CPMO induction time (6.82–10.08 h) were reported by Wall et al. [14], who further highlighted that the oxidative stability of CPMO may vary with season and cultivar (Figure 7). The study also revealed the positive correlation between antioxidant activity and oxidative stability of CPMO. Cultivars exhibiting higher antioxidant activity showed higher oxidative stability (HAES 835, HAES 344, HAES 294), and were thus clustered together (Figure 7). In terms of peroxide value, CPMO exhibited higher values than pecan and Brazil nut (1.4 and 2.5-fold, respectively), while hazelnut and almond showed greater values (1.2 and 1.8-fold, respectively). Likewise, the acid value was higher in the CPMO than the rest of the cold-pressed oils studied. Considerable variation in the CPMO oxidative stability exists among studies found in the literature. Therefore, it is important to consider factors, including cultivar, growing conditions, and season when interpreting the oxidative stability of CPMO. In the study of Madawala et al. [38], the induction time, peroxide value, anisidine value, and total oxidation value of CPMO were 37.0 h, 0.5 mEqO2/kg oil, 0.8, and 1.8, respectively. In this study, CPMO showed better oxidative stability than other cold-pressed oils studied (almond, hazelnut, walnut, argan, avocado, grapeseed, sesame, rice bran, and rapeseed). The authors explained that the high level of MUFAs (78.8%), low level of PUFAs (3.7%), and minor antioxidant compounds in CPMO contributed to greater oxidative stability.
6.7. Mineral Content
Despite the possible contamination of vegetable oil during processing, information on the mineral content of CPMO in the literature is limited. The factors, including agronomic practices, metal contamination during production and storage processes, have a significant role in determining the elemental profile of edible oil [37]. Furthermore, some elements significantly contribute to human health, whilst others can be correlated to some oil quality parameters. For instance, high levels of copper, iron, and manganese in edible oil accelerate oxidation processes, negatively affecting quality and storability. On the other hand, elements such as selenium, sodium, magnesium, potassium, and calcium are important to the well-being of humans. However, Cicero et al. [37] compared the mineral content of CPMO with cold-pressed oil from olive, Brazil nut, grapeseed, canola, avocado, coconut, pequi, and palm seed (Figure 8). CPMO exhibited higher sodium, magnesium, potassium, and calcium levels, which ranged between 1.11 to 13.91 μg/kg. These elements play crucial roles in many physiological functions of the human body, including preventing diseases such as hypertension, heart attack, and various gastrointestinal cancers. With cold-pressed oil from grapeseed, CPMO was high in iron (10.20–11.48 µg/kg), a prooxidant element, which was 2 to 5-fold higher than the rest of cold-pressed oils. Nonetheless, the reported iron levels were within the maximum permissible limits (5.0 mg/kg) of the Codex Alimentarius Commission standard for cold-pressed vegetable oil (Codex-Stan 210–1999) [22]. The levels were considered too low to cause significant oil deterioration [37].
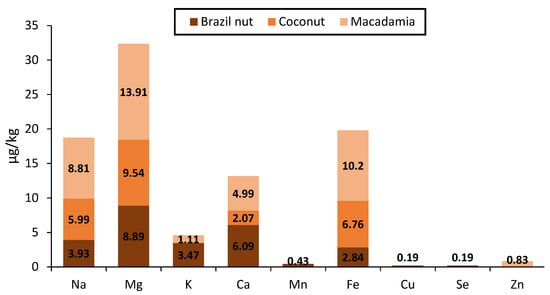
Figure 8.
Comparison of mineral content of cold-pressed macadamia nut oil with cold-pressed oil from selected tree nuts. Adapted from Cicero et al. [37].
7. Health Promoting Effects of Macadamia Nut Oil
Data regarding the health effects of CPMO is limited. Nevertheless, based on the high levels of MUFAs (oleic acid and palmitoleic acid) the potential health-promoting benefits of CPMO have been extensively emphasized [13,34,36,37,43]. CPMO is envisaged to possess better health properties than refined or commercial macadamia nut oil [54]. Despite the lack of data on CPMO, in vivo studies carried out using commercial macadamia nut oil have demonstrated that the oil lowers serum lipids or lipoproteins, thereby reducing cardiovascular disease risk [57] (Table 5). Therefore, macadamia nut oil can be used as a supplement to offer a cholesterol-lowering effect. The influence of commercial macadamia nut oil (Baxter Healthcare, Deerfield, IL, USA) on the plasma and liver fatty acid level, hepatic lipogenesis activity, hepatic stearoyl-CoA-1 desaturase activity, and hepatic elongase activity was evaluated using mice [58]. C57BL/6 male mice, which were 6 weeks old were fed with macadamia nut oil after diets that varied in the type of carbohydrates (starch, sucrose, and fructose). The authors reported that macadamia nut oil prevented steatosis, prevented an increase in saturated fatty acids and lipogenesis and stearoyl-CoA-1 desaturase activity. Similar findings were reported by Poudyal et al. [59] after feeding 96 male Wistar rats (9–10 weeks old; 336 ± 2 g) with commercial macadamia nut oil (3%) (Proteco Gold Pty. Ltd., Kingaroy, Queensland, Australia) enriched diets for 8 weeks. The decrease in plasma total cholesterol, plasma markers of liver damage, trans fatty acids in the heart, liver, and skeletal muscle, stearoyl-CoA desaturase-1 activity index, and normalization of systolic blood pressure was attributed to oleic acid from the macadamia nut oil. Stearoyl CoA-1 desaturase catalyzes the rate-limiting step in the production of MUFA in the human body. It has been reported that high stearoyl-CoA-1 desaturase expression may cause metabolic diseases such as obesity and insulin resistance. In addition, a complete loss of stearoyl-CoA-1 desaturase expression has been implicated in liver dysfunction and inflammatory diseases such as dermatitis, atherosclerosis, and intestinal colitis. Attenuation of the increase in inflammation and adipocyte hypertrophy in obese mice (8-week-old C57BL/6 male mice) fed with commercial macadamia nut oil (Vital Atman, Uchoa, SP, Brazil) was also reported [60]. Based on the reported health benefits from commercial macadamia nut oil and the health-promoting compounds present in CPMO, clinical and epidemiological studies using CPMO are essential.

Table 5.
Health benefits of commercial macadamia nut oil (in vivo studies with animal participation).
8. Application of Cold-Pressed Macadamia Nut Oil
As shown in Table 6, macadamia nut oil has various food and non-food applications that include food fortification, the development of skin, hair, and health care products. Zhong et al. [63] studied the effect of coating CPMO with macadamia protein isolate, and chitosan hydrochloride on microencapsulation efficiency and the properties of the spray-dried powders. The authors highlighted that macadamia protein isolate and chitosan hydrochloride mixed with CPMO at a ratio of 5:1 gave the highest encapsulation efficiency (94.2%) and the best oxidation stability (<4 meqO2/kg oil), and good storage stability after rehydration. The authors concluded that converting CPMO into powder may enhance its application as a healthy lipid in food and non-food products. The ability of CPMO to improve the oxidative stability of palm olein oil during deep-fat frying was investigated [36]. Palm olein oil was blended with 25–75% CPMO and the oil blends were subjected to smoking and oxidative degradation. It was established that the oil blends improved the induction period and inhibited primary and secondary oxidation products formation, a phenomenon that was attributed to the higher oxidative stability of MUFA and natural antioxidants present in macadamia nut oil. Besides applying CPMO in the formulation of oil blends with improved oxidative stability, the approach may be applied to formulate healthier and functional diets higher in oleic acid and palmitoleic acid. Although it was acknowledged that the price of oil blends could be higher, costs could be lowered by decreasing the number of frying oil replenishment during the frying cycles by taking advantage of the enhanced stability [36].
The oil fatty acid composition is crucial in developing cosmetic and skincare products. The literature reports that fatty acids similar to those found in the human body, such as palmitic acid, oleic acid, and linoleic acid, are crucial for the biological functions of the body. Palmitoleic acid, one of the predominant monounsaturated fatty acids in CPMO, forms part of the sebum of human skin [10]. To this effect, the composition of CPMO makes it a desirable ingredient in the formulation of skincare and anti-aging creams. Syed et al. [64] established that CPMO could be successfully applied in the production of esters and surfactants, valuable in the development of hair and skincare products. The authors reported that the CPMO-based esters and surfactants showed good ease of emulsification, good refractive index, emolliency with good after-feel, lack of greasiness, low cloud point, and pour point and high spreading coefficient, characteristics desired in hair and skincare products. Moreover, macadamia nut oil easily penetrates, softens, and influences the condition and proper functioning of the skin. Gu et al. [65] developed solid-lipid microparticles with 40–55% CPMO and corn oil, which enhanced the absorption of vitamin C by the skin. Therefore, CPMO based solid-liquid microparticles may be used in the drug delivery system for hydrophilic active ingredients with low permeability. A skincare product containing at least 25% CPMO was reported to contribute to the prevention or reduction of dryness, irritation, and skin lacerations in the elderly [66]. Moreover, Blin and Gaillard [67] patented the use of CPMO in lipstick production and claimed that the oil assists in the maintenance of lip moisture. Other applications of CPMO are reported in Table 6.

Table 6.
Food and non-food applications of cold-pressed macadamia nut oil.
Table 6.
Food and non-food applications of cold-pressed macadamia nut oil.
| Product and Application | Technique Used | Amount of Macadamia Oil Used | Key Parameters Evaluated | Remark | Reference |
|---|---|---|---|---|---|
| Rehydrated emulsion: food fortification | Encapsulation and spray drying | 8 g of macadamia oil | Encapsulation efficiency, hygroscopicity, wettability and solubility, bulk density and flowability, oxidative stability | Macadamia protein isolate and chitosan hydrochloride mixed with macadamia oil at a ratio of 5:1 gave higher encapsulation efficiency and stronger protection against lipid oxidation | Zhong et al. [63] |
| Solid-lipid microparticles: drug delivery system for hydrophilic active ingredients with low permeability | Emulsification | 40–55% macadamia and corn oil | Entrapment efficiency and retention rate, droplet size, viscosity, stability, skin penetration | The solid-lipid microparticles resulted in 5.52-fold increase in absorption of vitamin C. | Gu et al. [65] |
| Palm olein oil and macadamia oil blend: stabilize vegetable oils during deep fat frying | Blending | 25–75% macadamia oil | Smoke points and oxidative stability | Blended oils showed improved induction period and inhibited primary and secondary oxidation products formation. | Koohikamali and Alam [36] |
| Esters and surfactants: emulsifiers in the development of hair and skin care products | Esterification | 187–881g of macadamia oil | Skin feel, emolliency, slip, stickiness, moisturizing effect, viscosity, refractive index, spreading coefficient | The macadamia-based esters and surfactants showed good ease of emulsification, good refractive index, emolliency with good after-feel, lack of greasiness, low cloud point and pour point and high spreading coefficient. | Syed et al. [64] |
| Lipstick: cosmetic for making up or caring for the lips | Blending and cooling | 18.7% macadamia oil | Hardness, organoleptic tests | Good organoleptic properties and lip moisture maintenance | Blin and Guillard [67] |
9. Conclusions
This paper has reviewed the chemical properties, health benefits, and application of CPMO. CPMO is primarily composed of oleic acid, palmitoleic acid, and palmitic acid, the top three fatty acids. The oil is also a good source of minor bioactive compounds including, β-sitosterol, α-tocopherol, and α-tocotrienols. The high concentration of MUFAs (>70%) and the presence of bioactive compounds are implicated in the CPMO therapeutic properties. In vivo studies on rats, mice and humans showed that macadamia nut oil lower total cholesterol, LDL cholesterol, body weight, body mass index, inhibit the development of sucrose/fructose-induced hepatic steatosis, attenuate hypertrophy of adipocytes and inflammation in the adipose tissue and macrophages. Considering the richness of CPMO, more studies on the health benefits of the oil are warranted. The oil potential application has been confirmed in food, cosmetic, and pharmaceutical products, which is a desirable development from a nutrition and health viewpoint. Overall, the findings of this review may incite further studies on the health benefits and application of CPMO.
Author Contributions
Conceptualisation, T.K. and O.A.F.; methodology, T.K. and O.A.F.; investigations, T.K.; writing—original draft preparation, T.K.; writing—review and editing, T.K., O.A.F. and U.L.O.; visualisation, T.K., O.A.F. and U.L.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based on the research supported wholly or partially by the National Research Foundation of South Africa (Grant Numbers: 129295). The authors are grateful to the University Research Committee at the University of Johannesburg for financial support.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| BMI | Body mass index |
| CPMO | Cold-pressed macadamia nut oil |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| MUFA | Monounsaturated fatty acid |
| ND | Not detected |
| PUFA | Polyunsaturated fatty acid |
| SFA | Saturated fatty acid |
| TEAC | Trolox equivalence antioxidant capacity |
| UFA | Unsaturated fatty acid |
| UHPLC | Ultra-high-performance liquid chromatography-mass spectrometry |
References
- Serraa, J.L.; Rodrigues, A.M.C.; de Freitas, R.V.; Meirelles, A.J.A.; Darnete, S.H.; da Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Vlase, L.; Popa, D.S. Anti-aging potential of tree nuts with a focus on the phytochemical composition, molecular mechanisms, and thermal stability of major bioactive compounds. Food Funct. 2018, 9, 2554–2575. [Google Scholar] [CrossRef] [PubMed]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality, and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients. 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Shahidi, F. Tree Nuts: Composition, Phytochemicals, and Health Effects; CRC Press: Boca Raton, CA, USA, 2009. [Google Scholar]
- International Nut and Dried Fruit Council (INC). Nuts and Dried Fruits Statistical Yearbook, 2016/2017; INC: Reus, Spain, 2017; Available online: https://www.nutfruit.org/industry/technical-resources (accessed on 31 July 2021).
- International Nut and Dried Fruit Council (INC). Nuts and Dried Fruits Statistical Yearbook, 2019/2020; INC: Reus, Spain, 2020; Available online: https://www.nutfruit.org/industry/technical-resources (accessed on 31 July 2021).
- Shahidi, F.; Miraliakbari, H. Tree nut oils. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 175–193. [Google Scholar]
- Siger, A.; Józefiak, M.; Górnaś, P. Cold-pressed and hot-pressed rapeseed oil: The effects of roasting and seed moisture on the antioxidant activity, canolol, and tocopherol level. Acta Sci. Pol. Technol. Aliment. 2017, 16, 69–81. [Google Scholar] [CrossRef]
- Navarro, S.L.B.; Rodrigues, C.E.C. Macadamia oil extraction methods and uses for the defatted meal byproduct. Trends Food Sci. Technol. 2016, 54, 148–154. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Fatty acid composition, bioactive phytochemicals, antioxidant properties and oxidative stability of edible fruit seed oil: Effect of preharvest and processing factors. Heliyon 2020, 6, e04962. [Google Scholar] [CrossRef]
- Tan, C.X.; Tan, S.S.; Tan, S.T. Chapter 52—Cold pressed macadamia oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramadan, M.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 587–595. [Google Scholar]
- Wall, M.M. Functional lipid characteristics, oxidative stability, and antioxidant activity of macadamia nut (Macadamia integrifolia) cultivars. Food Chem. 2010, 121, 1103–1108. [Google Scholar] [CrossRef]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative study of chemical compositions and antioxidant capacities of oils obtained from 15 macadamia (Macadamia integrifolia) Cultivars in China. Foods 2021, 10, 1031. [Google Scholar] [CrossRef]
- Tu, X.; Chen, H.; Du, L. Preparation and physicochemical of microemulsion based on macadamia nut oil. AIP Conf. Proc. 2018, 1944, 020038. [Google Scholar]
- Phatanayindee, S.; Borompichaichartkul, C.; Srzednicki, G.; Craske, J.; Wootton, M. Changes of chemical and physical quality attributes of macadamia nuts during hybrid drying and processing. Dry. Technol. 2012, 30, 1870–1880. [Google Scholar] [CrossRef]
- Maestri, D.; Cittadini, M.C.; Bodoira, R.; Martinez, M. Tree nut oils: Chemical profiles, extraction, stability, and quality concerns. Eur. J. Lipid Sci. Technol. 2020, 122, 1900450. [Google Scholar] [CrossRef]
- Australian Macadamia Industry. Information for New and Potential Growers and Investors; Australian Macadamia Society: Lismore, Australia, 2017; pp. 1–20. [Google Scholar]
- Zuza, E.J.; Bhagwat, S.; Emmott, A.; Rawes, W.; Maseyk, K.; Araya, Y.N. Review of macadamia production in Malawi: Focusing on what, where how much is produced and major constraints. Agriculture 2021, 11, 152. [Google Scholar] [CrossRef]
- SADC Macadamias. Offering Rewarding Opportunities for SADC. 2020. Available online: https://themacadamia.co.za/wp-content/uploads/2020/03/Mac-SADC-Digest-web-compressed.pdf. (accessed on 7 December 2021).
- Codex Alimentarius. Codex Standard for Named Vegetable Oils. Codex-Stan 210. 1999. Available online: http://www.fao.org/docrep/004/y2774e/y2774e04.htm#bm4.1 (accessed on 20 October 2020).
- Matthaus, B.; Spener, F. What we know and what we should know about virgin oils-a general introduction. Eur. J. Lipid Sci. Technol. 2008, 110, 597–601. [Google Scholar] [CrossRef]
- Michalak, M.; Kiełtyka-Dadasiewicz, A. Nut oils and their dietetic and cosmetic significance: A review. J. Oleo Sci. 2019, 68, 111–120. [Google Scholar]
- Munro, I.A.; Garg, M.L. Chapter 15: Nutrient composition and health beneficial effects of macadamia nuts. In Tree Nuts Composition, Phytochemicals, and Health Effects; Alasalvar, C., Shahidi, F., Eds.; Taylor Francis Group: New York, NY, USA, 2009; pp. 249–258. [Google Scholar]
- Rodrigues, C.E.C.; Silva, F.A.; Marsaioli, A.; Meirelles, A.J.A. Deacidification of Brazil nut and macadamia nut oils by solvent extraction: Liquid-liquid equilibrium data at 298.2 K. J. Chem. Eng. Data 2005, 50, 517–523. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Correa, A.P.; Michel, I.; Brandeli, A.C.I.; Tessaro, I.C. Evaluation of the phenolic content and antioxidant activity of different seed and nut cakes from the edible oil industry. J. Am. Oil Chem. Soc. 2014, 91, 1773–1782. [Google Scholar] [CrossRef]
- Phosa, M.A. The Nutritive Value of Macadamia Oil Cake Meal and Wood Ash as Alternative Feed Ingredients for Chickens in Rural Areas. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2009. [Google Scholar]
- Skenjana, A. The Potential Nutritive Value of Waste Products from the Sub-Tropical Fruit Processing Industry as Livestock Feed. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2011. [Google Scholar]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, J.; Achachlouei, B.F. Effect of pretreeatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Rabrenovic, B.B.; Dimic, E.B.; Novakovic, M.M.; Tesevic, V.V.; Basic, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT-Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Kochhar, S.P.; Henry, C.J.K. Oxidative stability, and shelf-life evaluation of selected culinary oils. International Food Sci. Nutr. 2009, 60, 289–296. [Google Scholar] [CrossRef]
- Ribeiro, A.P.L.; Haddada, F.F.; Tavares, T.S.; Magalhães, K.T.; Pimenta, C.J.; Nunesa, C.A. Characterization of macadamia oil (Macadamia integrifolia) obtained under different extraction conditions. Emir. J. Food Agric. 2020, 32, 295–302. [Google Scholar] [CrossRef]
- Gong, Y.; Pegg, R.B.; Carr, E.C.; Parrish, D.R.; Kellett, M.E.; Kerrihard, A.L. Chemical and nutritive characteristics of tree nut oils available in the US market. Eur. J. Lipid Sci. Technol. 2017, 119, 1600520. [Google Scholar] [CrossRef]
- Koaze, H.; Karanja, P.N.; Kojima, M.; Baba, N.; Ishibashi, K.I. Lipid accumulation of macadamia nuts during kernel development. Food Preserv. Sci. 2002, 28, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Koohikamali, S.; Alam, M.S. Improvement in nutritional quality and thermal stability of palm olein blended with macadamia oil for deep-fat frying application. J. Food Sci. Technol. 2019, 56, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.D.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; Di Stefano, V.; Di Bella, G.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef]
- Madawala, S.R.P.; Kochhar, S.P.; Dutta, P.C. Lipid components and oxidative status of selected specialty oils. Grasas y Aceites 2012, 63, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Gliszczynska-Swigło, A.; Jajor, Z.; Kmiecik, D. Fourier-transform near infrared spectroscopy and chemometrics for discrimination of cold-pressed oils and determination of their chemical parameters. J. Near Infrared Spec. 2018, 24, 262–272. [Google Scholar] [CrossRef]
- Yuenyong, J.; Pokkanta, P.; Phuangsaijai, C.; Kittiwachana, S.; Mahatheeranont, S.; Sookwong, P. GC-MS and HPLC-DAD analysis of fatty acid profile and functional phytochemicals in fifty cold-pressed plant oils in Thailand. Heliyon 2021, 7, e06304. [Google Scholar] [CrossRef]
- Prabakaran, M.; Lee, K.J.; An, Y.; Kwon, C.; Kim, S.; Yang, Y.; Ahmad, A.; Kim, S.H.; Chung, M. Changes in soybean (Glycine max L.) flour fatty-acid content based on storage temperature and duration. Molecules 2018, 23, 2713. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Derewiaka, D.; Szwed, E.; Wołosiak, R. Physicochemical properties and composition of lipid fraction of selected edible nuts. Pak. J. Bot. 2014, 46, 337–343. [Google Scholar]
- Kaijser, A.; Dutta, P.; Savage, G. Oxidative stability and lipid composition of macadamia nuts grown in New Zealand. Food Chem. 2000, 71, 67–70. [Google Scholar] [CrossRef]
- Carrillo, W.; Carpio, C.; Morales, D.; Vilcacundo, E.; A’lvarez, M. Fatty acids composition in Macadamia seed oil (Macadamia integrifolia) from Ecuador. Asian J. Pharm. Clin. Res. 2017, 10, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Jiang, L.; Du, F.; Gao, Z.; Sun, G. Chemical Classification on Animals and Plants for Exploitation Based on Analysis of Fatty-Acid Compositions of their oils. Int. Conf. Environ. Chem. Biol. 2012, 49, 100–104. [Google Scholar]
- Zhu, B.Q.; Lin, L.J.; Li, J.H.; Lv, G.T.; Huang, M.F. Comparison of four different extraction methods of oil from Macadamia integrifolia. Adv. Mat. Res. 2013, 610, 3382–3386. [Google Scholar] [CrossRef]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Appel, L.J. Supplementation of diets with α-tocopherol reduces serum concentrations of γ- and δ-tocopherol in humans. J. Nutr. 2003, 133, 3137–3140. [Google Scholar] [CrossRef] [Green Version]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzinska, M. Phytochemical content, oxidative stability, and nutritional properties of unconventional cold-pressed edible oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Kumar, M.S.S.; Mawlong, I.; Singh, D. Phytosterol recovery from oilseeds: Recent advances. J. Food Process. Eng. 2016, 40, e12466. [Google Scholar] [CrossRef]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effect of blanching pomegranate seed on physicochemical attributes, bioactive compounds, and antioxidant activity of extracted oil. Molecules 2020, 25, 2554. [Google Scholar] [CrossRef]
- Griel, A.E.; Cao, Y.; Bagshaw, D.D.; Cifelli, A.M.; Holub, B.; Kris-Etherton, P.M. A Macadamia nut-rich diet reduces total and LDL-Cholesterol in mildly hypercholesterolemic men and women. J. Nutr. Nutr. Dis. 2008, 138, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, R.A.; Xu, Z.; Harvey, K.A.; Pavlina, T.M.; Becker, M.J.; Zaloga, G.P. Comparative study of the modulation of fructose/sucrose-induced hepatic steatosis by mixed lipid formulations varying in unsaturated fatty acid content. Nutr. Metab. 2015, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudyal, H.; Kumar, S.A.; Iyer, A.; Waanders, J.; Ward, L.C.; Brown, L. Responses to oleic, linoleic, and α-linolenic acids in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1381–1392. [Google Scholar] [CrossRef]
- Lima, E.A.; Silveira, L.S.; Masi, L.N.; Crisma, L.R.; Davanso, M.R.; Souza, G.I.G.; Santamarina, A.B.; Moreira, R.G.; Martins, A.R.; de Sousa, L.G.O.; et al. Macadamia oil supplementation attenuates inflammation and adipocyte hypertrophy in obese mice. Mediat. Inflamm. 2014, 2014, 870634. [Google Scholar] [CrossRef] [Green Version]
- Matthan, N.R.; Dillard, A.; Lecker, J.L.; Blanche, I.; Lichtenstein, A.H. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B Golden Syrian Hamster. J. Nutr. 2008, 139, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Malvestiti, R.; Borges, L.D.S.; Weimann, E.; Junior, E.P.D.S.; Levada-Pires, A.C.; Dermargos, A.; Lambertucci, R.H.; Hatanaka, E. The effect of macadamia oil intake on muscular inflammation and oxidative profile kinetics after exhaustive exercise. Eur. J. Lipid Sci. Technol. 2017, 119, 1600382. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiang, X.; Wang, X.; Zhang, Y.; Hu, M.; Chen, T.; Liu, C. Fabrication and characterization of oil-in-water emulsions stabilized by macadamia protein isolate/chitosan hydrochloride composite polymers. Food Hydrocoll. 2020, 103, 105655. [Google Scholar] [CrossRef]
- Syed, S.; Walele, I. Macadamia Lipid Based Surfactants and Derivatives and Process for Preparing Same. U.S. Patent US20060210520, 21 September 2006. [Google Scholar]
- Gu, C.; Hu, C.; Ma, C.; Fang, O.; Xing, T.; Xia, Q. Development, and characterization of solid lipid microparticles containing vitamin C for topical and cosmetic use. Eur. J. Lipid Sci. Technol. 2016, 118, 1093–1103. [Google Scholar] [CrossRef]
- Lindbeck, K.; Wait, W. Cosmetic Composition. International Patent Application No. PCT/AU2013/000243, 19 September 2013. [Google Scholar]
- Blin, X.; Guillard, S. Cosmetic Composition Comprising Macadamia Oil and Wax. EU Patent Application No. EP2224899A2, 8 September 2010. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).