Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Extraction of Polyphenols from Oilseed Cakes
2.3. HPTLC Method
2.4. DPPH˙ Scavenging Assay
2.5. Antimicrobial Aliivibrio Fischeri Bioassay
2.6. Enzymatic AChE Inhibitory Assay
2.7. Antimicrobial Bacillus Subtilis Bioassay
2.8. pYES Bioassay
2.9. Characterization of Bioactive Compounds Using Mass Spectrometry
3. Results and Discussion
3.1. Selection of the Mobile Phase on Silica Gel Phases
3.1.1. Hemp and Canola Seed Cakes
| # | Solvent mixture | Ratio (V/V) | Elution power |
|---|---|---|---|
| Hemp seed cake extract (also for canola seed cake extract) | |||
| 1 | n-hexane, ethyl acetate and acetic acid (6 mL HCl, 37%applied on a filter paper in the opposite trough) | 5:3:1 | Too weak |
| 2 | toluene, ethyl acetate and acetic acid | 80:25:4 | |
| 3 | ethyl acetate, formic acid, acetic acid and water | 100:11:11:26 | Too high |
| 4 | toluene, ethyl acetate, formic acid and water | 15:30:5:3 | Medium |
| Flax seed cake extract | |||
| 5 | ethyl acetate, formic acid, acetic acid and water | 40:10:10:13 | |
| 6 | ethyl acetate, formic acid, acetic acid and water | 40:20:20:13 | |
| 7 | ethyl acetate, methanol, water and acetic acid | 9:6:3:2 | |
| 8 | ethyl acetate, methanol and ammonia 25% | 10:7:3 | |
| 9 | ethyl acetate and ethanol | 1:19 | |
| 10 | ethyl acetate, ethanol, formic acid and water | 77:13:5:10 | |
| 11 | propanol, ammonia 25% and water | 8:1:1 | |
| 12 | ethyl acetate, methanol and water | 4:3:3 | Too high |
| 13 | ethyl acetate, methanol, formic acid and water | 14:3:1:2 | Medium |
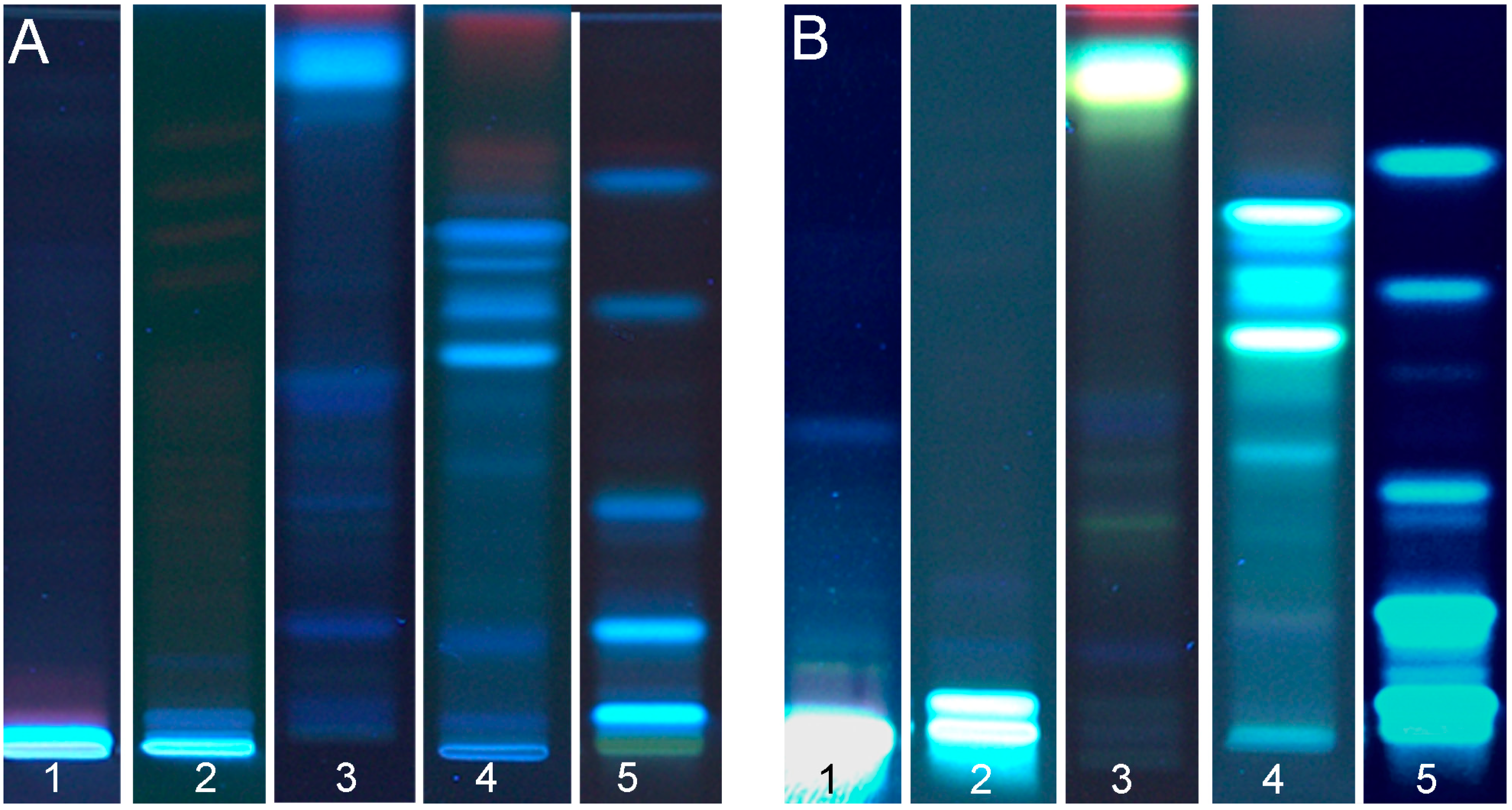
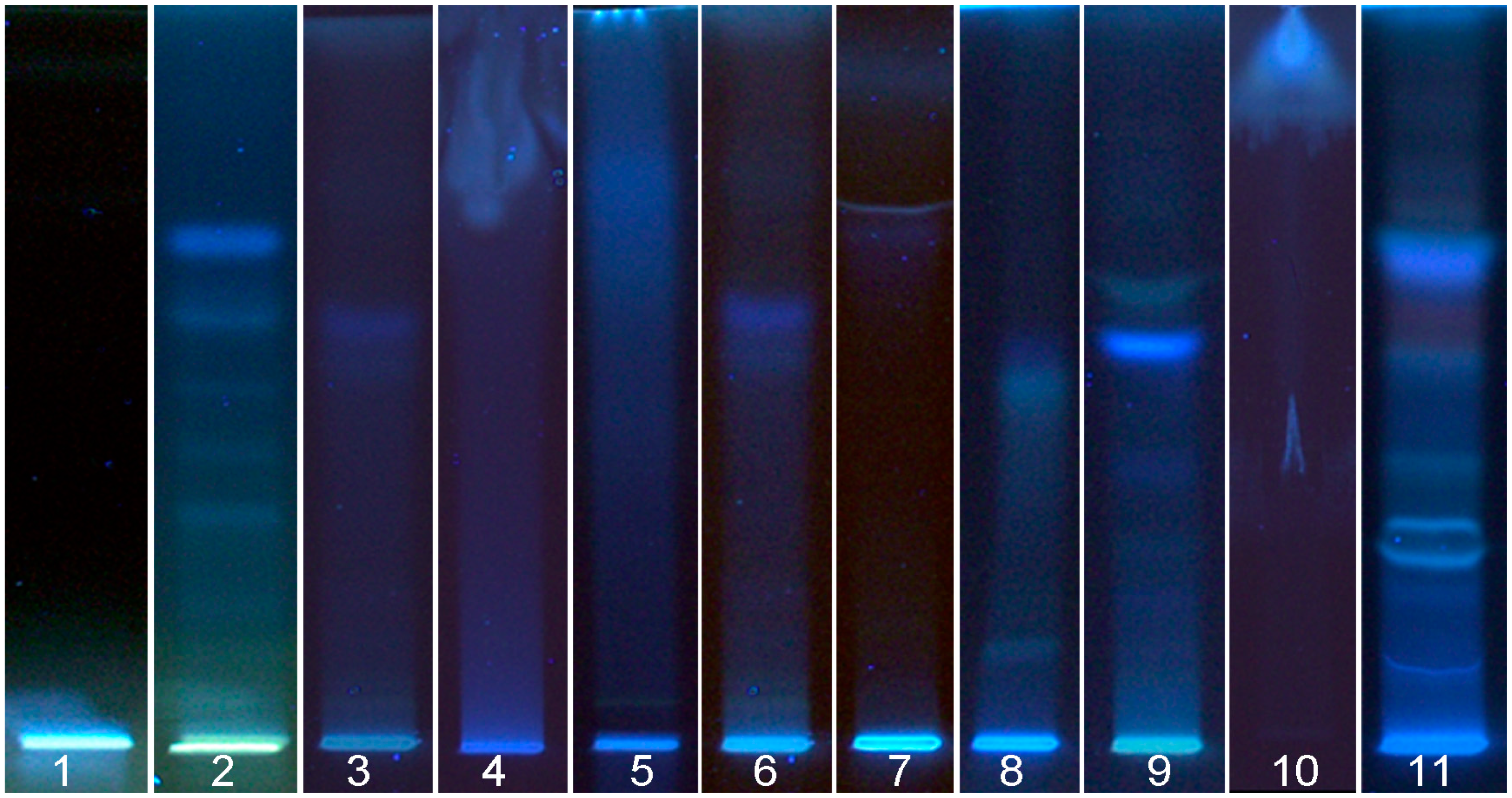
3.1.2. Flax Seed Cake
3.2. Detection of DPPH˙ Scavenging Activity
3.3. Detection of Antimicrobial Aliivibrio Fischeri-Effective Compounds
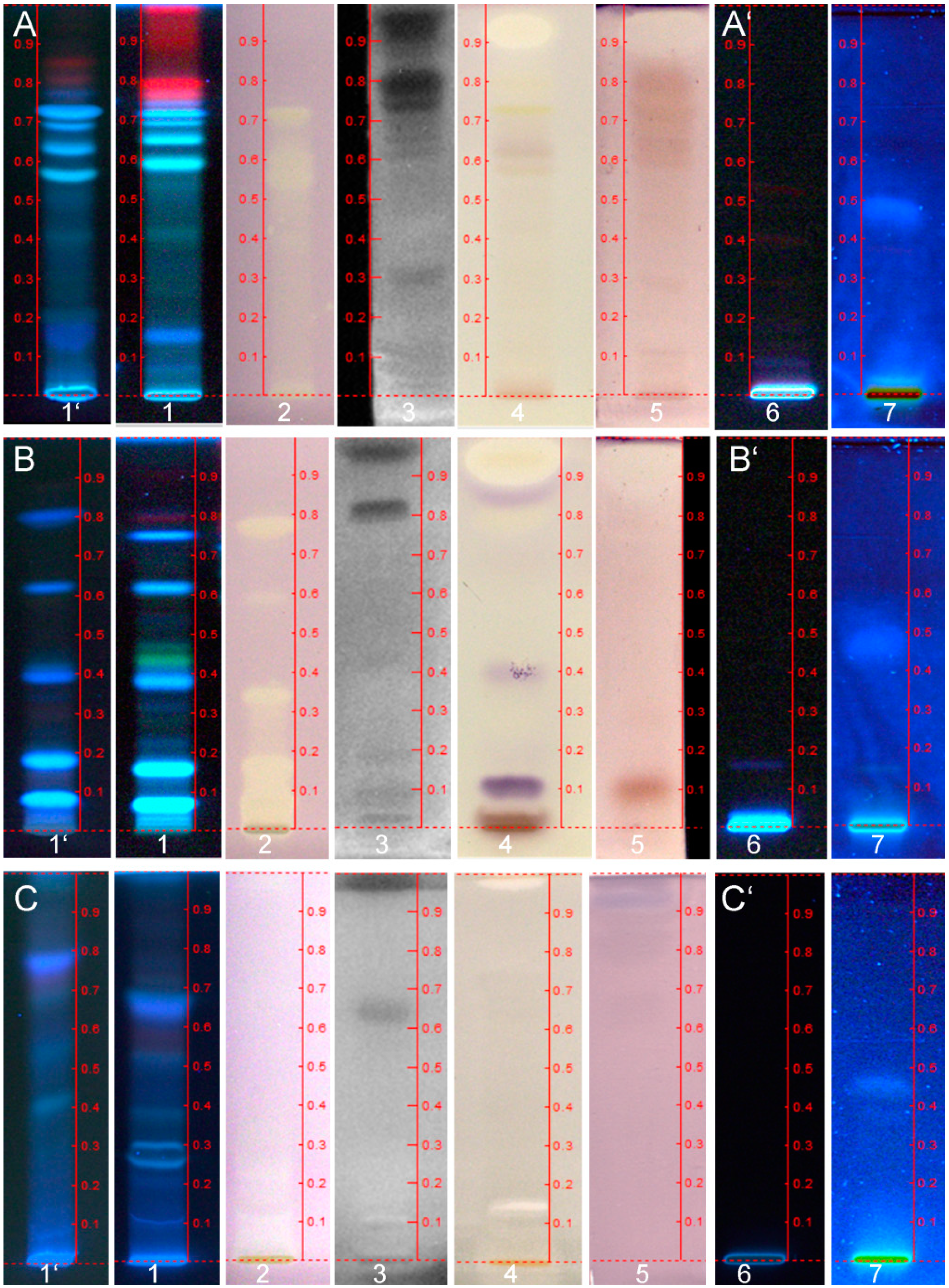
3.4. Detection of AChE Inhibitory Compounds
3.5. Detection of Antimicrobial Bacillus Subtilis-Effective Compounds
3.6. Detection of Estrogen-Effective Compounds
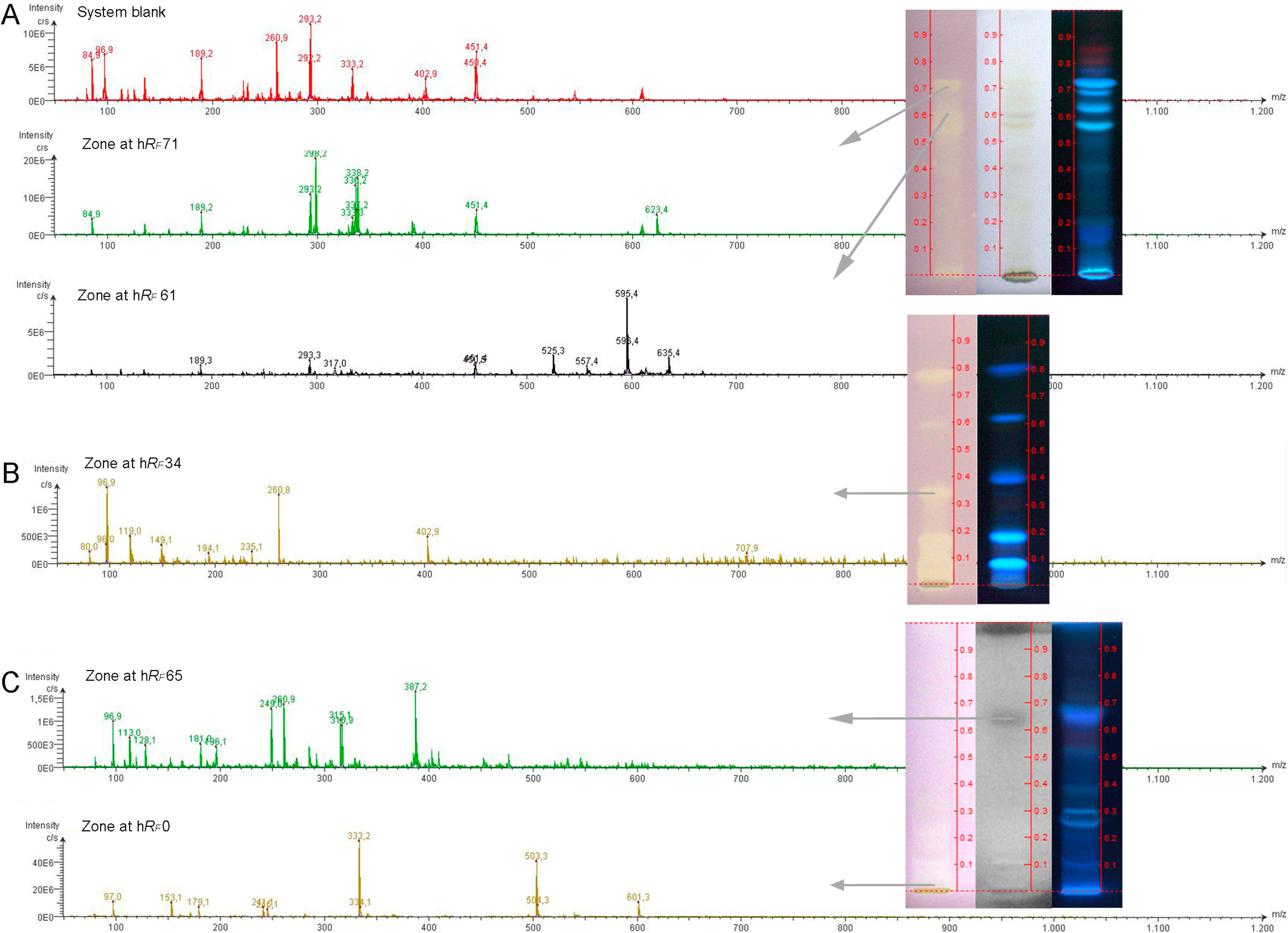
3.7. Characterization of Bioactive Compounds Using HPTLC-ESI-MS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA GAIN: India Oilseeds and Products Annual 2013. Available online: http://www.thecropsite.com/reports/?id=1882 (accessed on 6 October 2014).
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Tech. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Johnson, R. CRS Report for Congress: Hemp as an Agricultural Commodity (2013). Available online: http://fas.org/sgp/crs/misc/RL32725.pdf (accessed on 6 October 2014).
- FAO, Food Outlook Global Market Analysis Report (2012). Available online: http://www.fao.org/docrep/016/al993e/al993e00.pdf (accessed on 6 October 2014).
- Hessle, A.; Eriksson, M.; Nadeau, E.; Turner, T.; Johansson, B. Cold-pressed hempseed cake as a protein feed for growing cattle. Agr. Scand. A - Animal Sci. 2008, 58, 136–145. [Google Scholar]
- Lunger, A.N.; McLean, E.; Gaylord, T.G.; Kuhn, D.; Craig, S.R. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crop Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Zuk, M.; Kulma, A.; Bugajska-Prusak, A.; Gąsiorowski, K.; Kostyn, K.; Szopa, J. New dressing materials derived from transgenic flax products to treat long-standing venous ulcers—A pilot study. Wound Repair Regen. 2010, 18, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Żuk, M.; Dymińska, L.; Kulma, A.; Boba, A.; Prescha, A.; Szopa, J.; Mączka, M.; Zając, A.; Szołtysek, K.; Hanuza, J. IR and Raman studies of oil and seedcake extracts from natural and genetically modified flax seeds. Spec. Acta A–Mol. Biomol. Spec. 2011, 78, 1080–1089. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, T. TLC bioautography: High throughput technique for screening of bioactive natural products. Comb. Chem. High Throughput Screen. 2013, 16, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Urmann, O.; Morlock, G.E. Development of a planar chromatographic method for quantitation of anthocyanes in pomace, feed, juice and wine. J. Chromatogr. A 2013, 1289, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cretu, G.; Morlock, G.E. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014, 1, 104–112. [Google Scholar] [CrossRef]
- Das, B.; Paul, T.; Apte, K.G.; Chauhan, R.; Saxen, R.C. Evaluation of antioxidant potential & quantification of polyphenols of Diplazium esculentum Retz. with emphasis on its HPTLC chromatography. J. Pharm. Res. 2013, 6, 93–100. [Google Scholar] [CrossRef]
- Varghese, S.; Narmadha, R.; Gomathi, D.; Kalaiselvi, M.; Devaki, K. Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed. J. Acute Disease 2013, 2, 122–126. [Google Scholar] [CrossRef]
- Riffaulta, L.; Destandaua, E.; Pasquierb, L.; Andréb, P.; Elfakir, C. Phytochemical analysis of Rosa hybrida cv. ‘Jardin de Granville’ by HPTLC, HPLC-DAD and HPLC-ESI-HRMS: Polyphenolic fingerprints of six plant organs. Phytochemistry 2014, 99, 127–134. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Nawaz, Z.; Densmore, C.; Weigel, N.L.; Pham, T.A.; Clark, J.H.; O’Malley, B.W. High level expression of biologically active estrogen receptor in Saccharomyces cerevisiae. J. Steroid Biochem. Mol. Biol. 1991, 39, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, I.; Morlock, G.E. Sharp-bounded zones link to the effect in planar chromatography-bioassay-mass spectrometry. J. Chromatogr. A 2014, 1360, 288–295. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization, ISO 11348-1, Water quality –Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test) – Part 1: Method using freshly prepared bacteria. Geneva, Switzerland, 2007.
- Hage, S.; Morlock, G.E. Book of Abstracts P-64 “HPTLC Hyphenated with Bioassays for the Screening of Bioactive Constituents of Serbian Salicaceae bud Extracts”; International Symposium for HPTLC 2014, Lyon, poster contribution; 2014. [Google Scholar]
- Akkad, R.; Schwack, W. Determination of organophosphorus and carbamate insecticides in fresh fruits and vegetables by high-performance thin-layer chromatography-multienzyme inhibition assay. J. AOAC Int. 2012, 95, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Aidij, M.; Morlock, G.E. Book of abstracts P-55 “Fast HPTLC‐direct bioautography using Bacillus subtilis for screening of antimicrobial components in plant extracts”; International Symposium for HPTLC 2014, Lyon, poster contribution; 2014. [Google Scholar]
- Morlock, G.E.; Klingelhöfer, I. Liquid Chromatography-Bioassay-Mass Spectrometry for Profiling of Physiologically Active Food. Anal. Chem. 2014, 86, 8289–8295. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.; Scholl, I.; Kunz, N.; Schroeder, A. Planar-chromatographic fingerprint of German propolis, CAMAG Bibliogr. Service 2013, 111, 13–15. [Google Scholar]
- Nair, V.P.; Hunter, J.M. Anticholinesterases and anticholinergic drugs. Contin. Educ. Anaesth. Crit. Care Pain. 2004, 4, 164–168. [Google Scholar] [CrossRef]
- Silva, D.; Chioua, M.; Samadi, A.; Agostinho, P.; Garção, P.; Lajarín-Cuesta, R.; de los Ríos, C.; Iriepa, I.; Moraleda, I.; Gonzalez-Lafuente, L.; et al. Synthesis, pharmacological assessment, and molecular modelling of acethycholinesterase/butyrylcholinesterase inhibitors: Effect against amyloid-β-induced neurotoxicity. ACS Chem. Neurosci. 2013, 4, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Gutierrez, R.; Diaz, G.; Gonzalez, C.; Perez, N.; Vega, S.; Noa, M. High-performance thin-layer chromatography -bioautography for multiple antibiotic residues in cow’s milk. J. Chromotogr. B 2003, 784, 315–322. [Google Scholar] [CrossRef]
- Knecht, B.G.; Strasser, A.; Dietrich, R.; Martlbauer, E.; Niessner, R.; Weller, M.G. Automated microarray system for the simultaneous detection of antibiotics in milk. Anal. Chem. 2004, 76, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; De Bruyne, T.; Apers, S.; Vanden Berghe, D.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Novak, J.T.; Grizzard, T.J.; Love, N.G. Estrogen receptor agonist fate during wastewater and biosolids treatment processes: A mass balance analysis. Environ. Sci. Technol. 2002, 36, 4533–4539. [Google Scholar] [CrossRef]
- Buchinger, S.; Spira, D.; Bröder, K.; Schlüsener, M.; Ternes, T.; Reifferscheid, G. Direct coupling of thin-layer chromatography with a bioassay for the detection of estrogenic compounds: Applications for effect-directed analysis. Anal. Chem. 2013, 85, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.A. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocrine Rev. 2001, 22, 319–341. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogen and western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Kurzer, M.S.; Hu, X. Dietary phytoestrogens. Ann. Rev. Nutri. 1997, 17, 353–381. [Google Scholar] [CrossRef]
- Bingham, S.A.; Atkinson, C.; Liggins, J.A. Phyto-oestrogens: Where are we now? Brit. J. Nutri. 1998, 79, 393–406. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012, 134, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G. Influence of the TLC/HPTLC plate and solvents on the background signals obtained in TLC/HPTLC-ESI-MS using the TLC-MS Interface. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2892–2914. [Google Scholar] [CrossRef]
- Matsuda, F.; Suzuki, F.; Sawada, Y. Database of RIKEN Plant Science Centre, Japan. 2009. Available online: http://www.massbank.jp/jsp/Result.jsp (accessed on 6 September 2014).
- Morlock, G.E.; Morlock, L.P.; Lemo, C. Streamlined analysis of lactose-free dairy products. J. Chromatogr. A 2014, 1324, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y.; Ogawa, H. Natural occurrence of cancer-preventive geranylgeranoic acid in medicinal herbs. J. Lipid Res. 2004, 45, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G. Chromatography combined with bioassays and other hyphenations—The direct link to the compound indicating the effect. ACS Syposium Series 2013, 1185, 101–121. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, S.-S.; Morlock, G.E. Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry. Chromatography 2015, 2, 125-140. https://doi.org/10.3390/chromatography2010125
Teh S-S, Morlock GE. Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry. Chromatography. 2015; 2(1):125-140. https://doi.org/10.3390/chromatography2010125
Chicago/Turabian StyleTeh, Sue-Siang, and Gertrud E. Morlock. 2015. "Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry" Chromatography 2, no. 1: 125-140. https://doi.org/10.3390/chromatography2010125
APA StyleTeh, S.-S., & Morlock, G. E. (2015). Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry. Chromatography, 2(1), 125-140. https://doi.org/10.3390/chromatography2010125





