Dose-Related Pharmacokinetic and Pharmacodynamic Effects of Intramuscular Epinephrine in Healthy Neonatal Piglets
Abstract
Highlights
- Intramuscular epinephrine produced dose-dependent cardiovascular effects in newborn piglets.
- A dose of 0.1 mg/kg IM epinephrine achieved hemodynamic and pharmacokinetic effects comparable to standard 0.02 mg/kg IV epinephrine, while the lower IM dose (0.02 mg/kg) was largely ineffective.
- IM epinephrine at an adequate dose may represent a feasible alternative to IV administration during neonatal resuscitation when vascular access is delayed.
- Adoption of IM epinephrine could simplify vasopressor delivery, requiring less training, equipment, and time in emergency settings.
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Randomization
2.3. Blinding
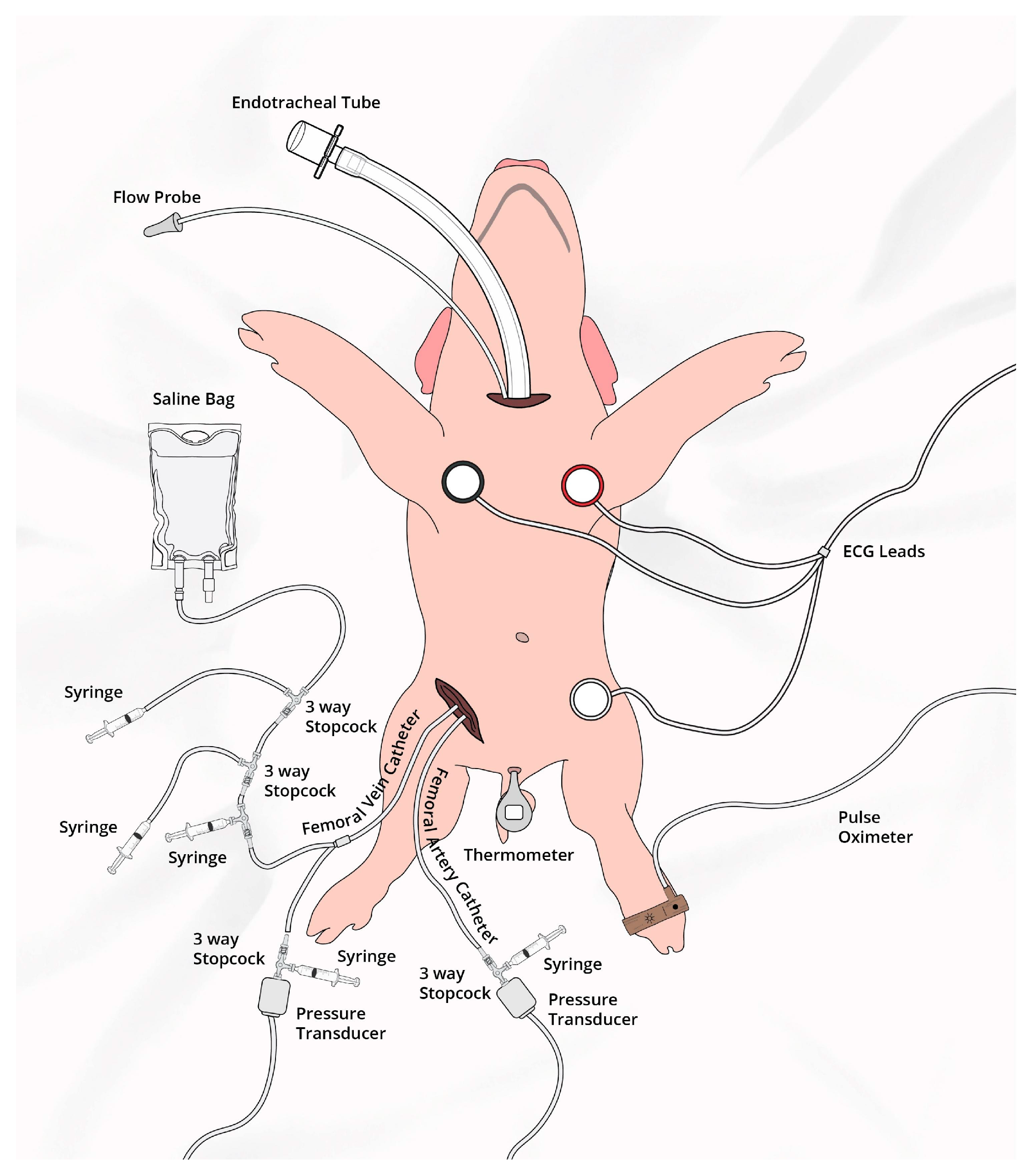
2.4. Animal Preparation
2.5. Hemodynamic Parameters
2.6. Cerebral Perfusion
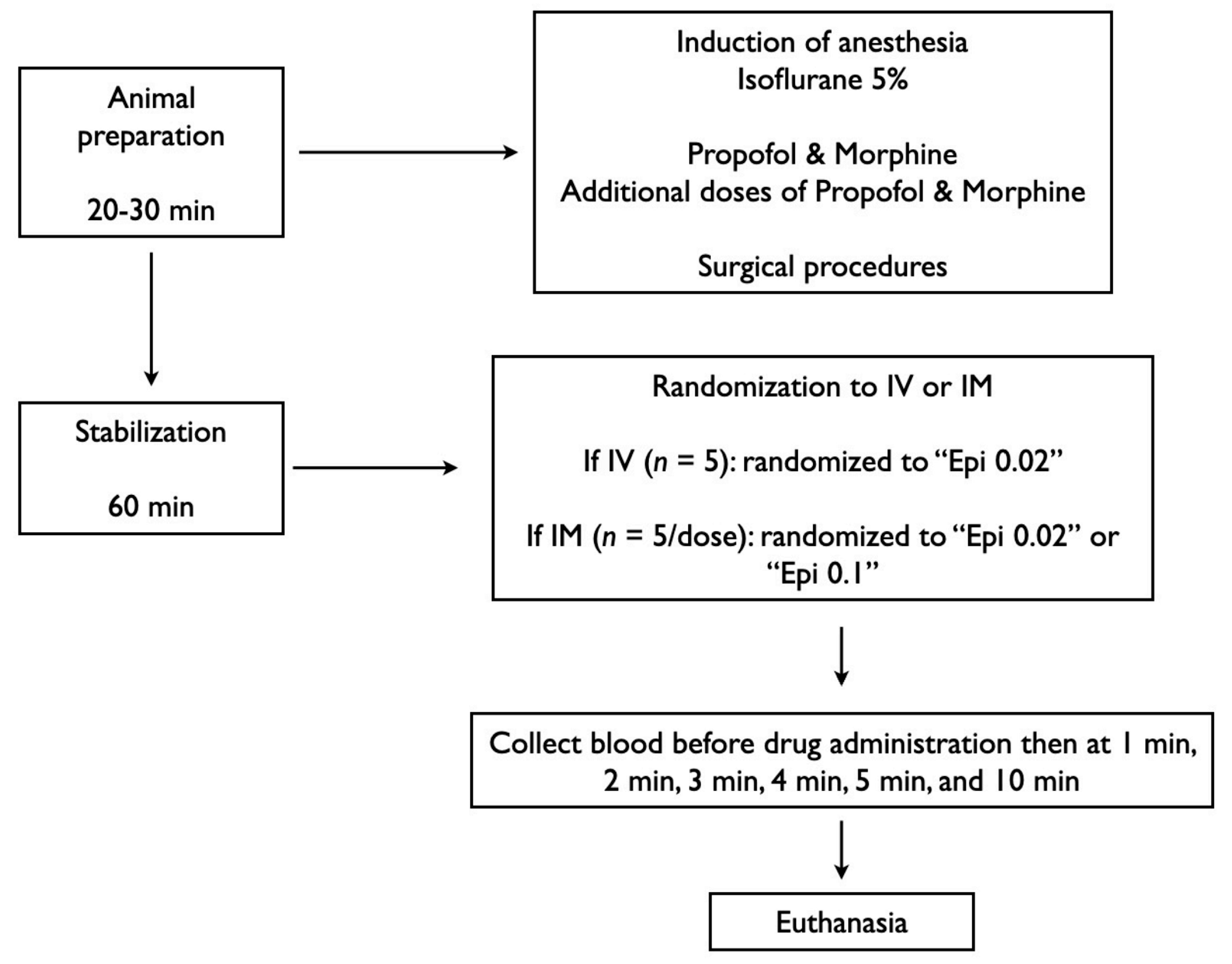
2.7. Experimental Protocol
2.8. Data Collection and Analysis
3. Results
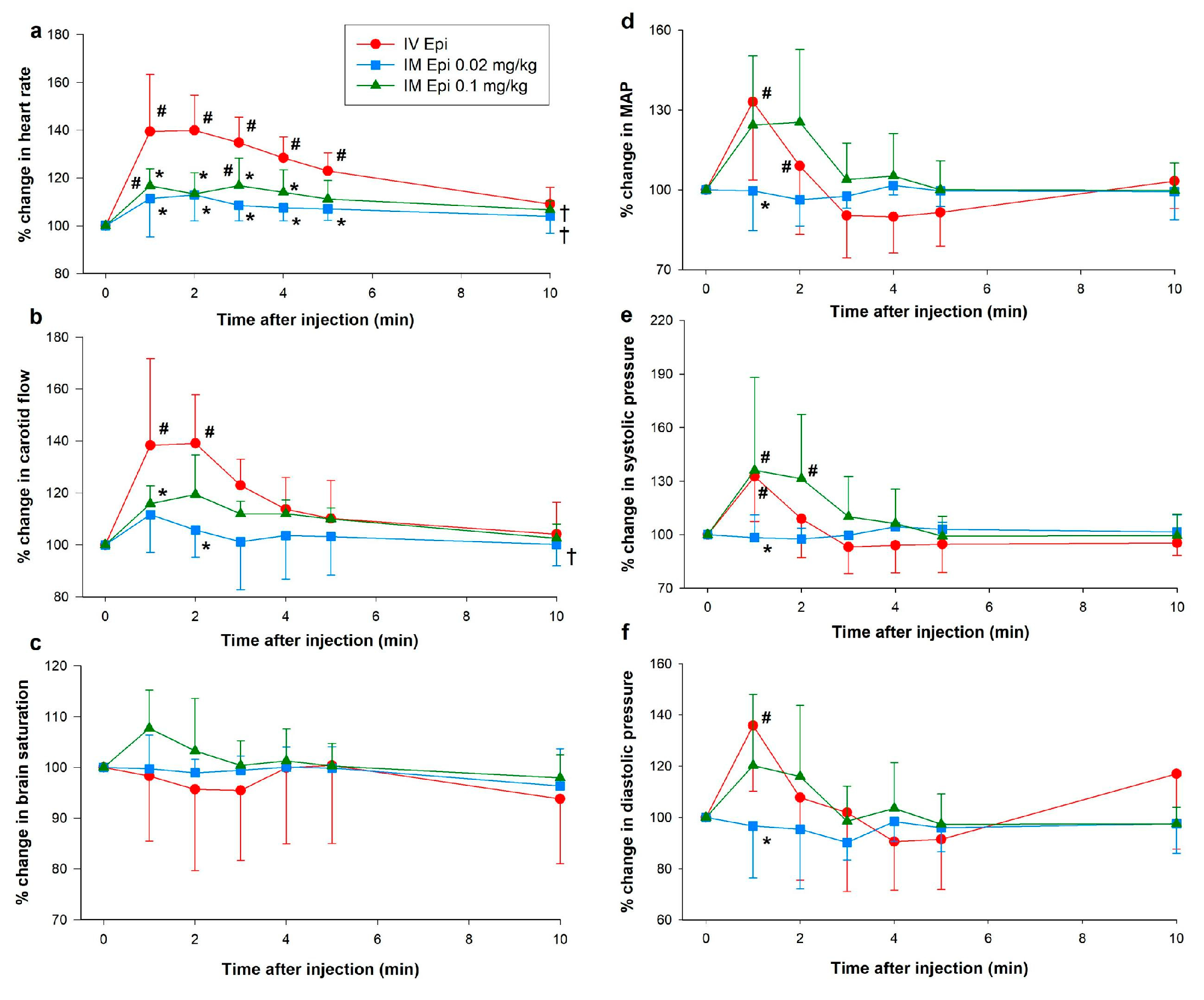
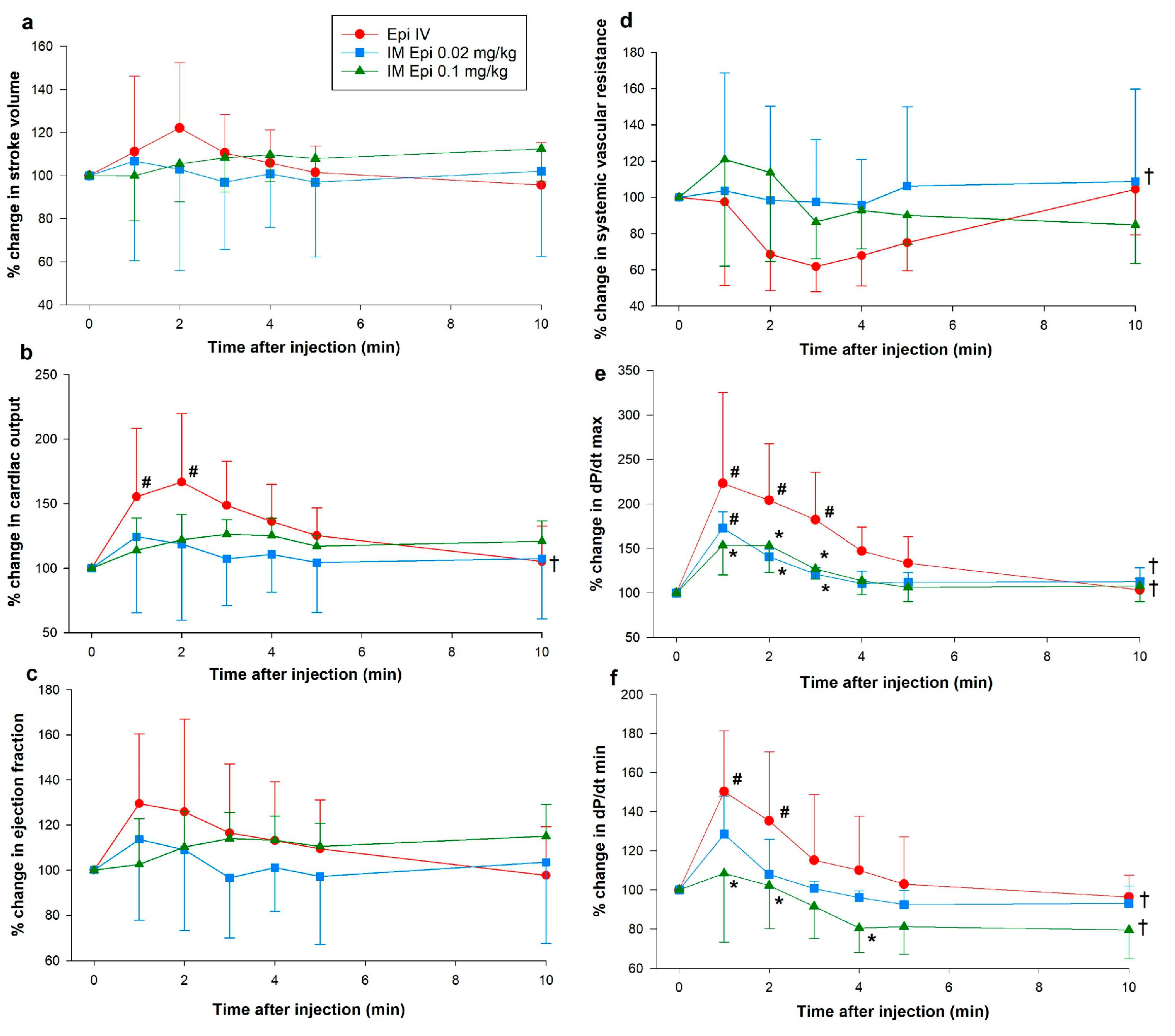
3.1. Hemodynamic Changes
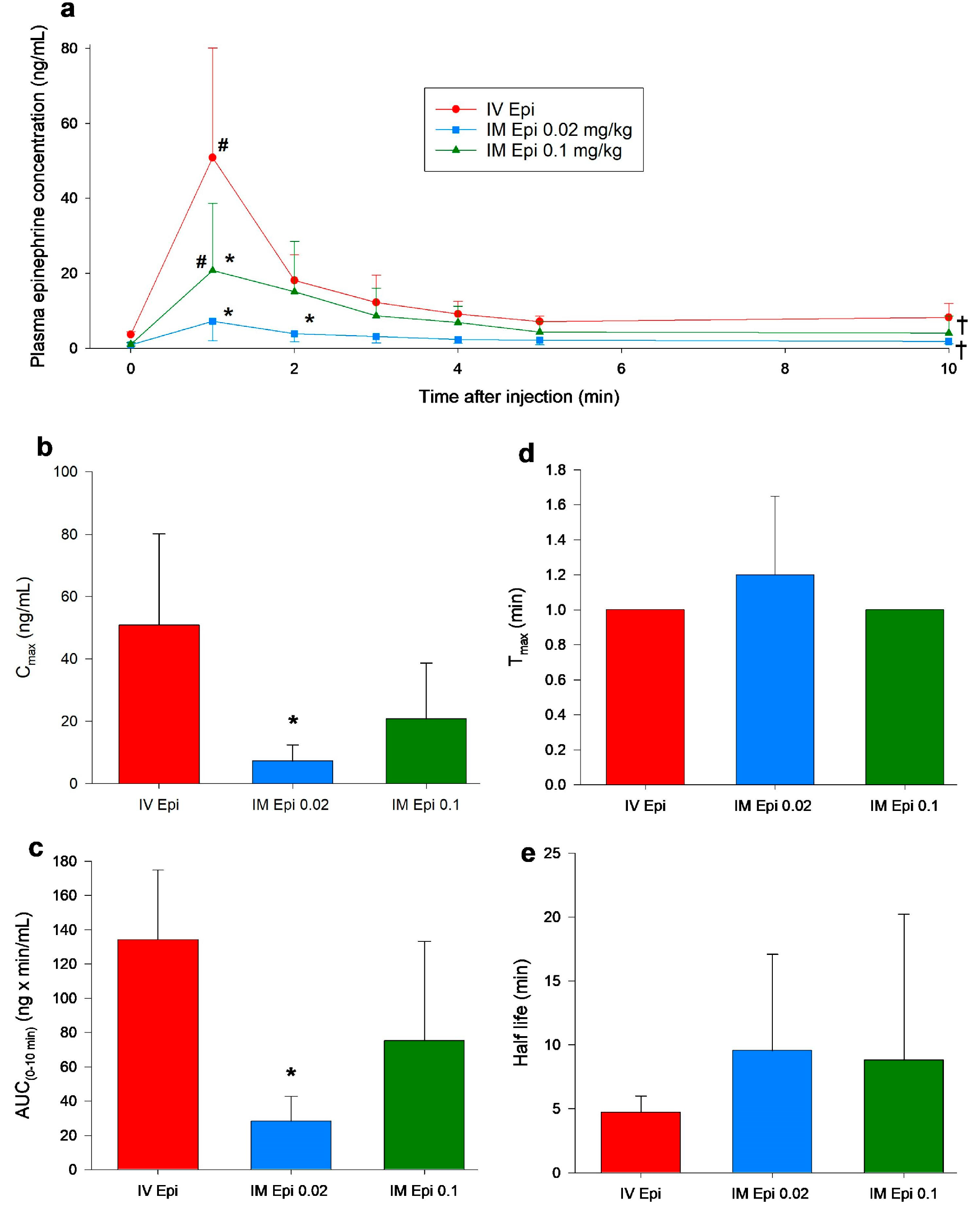
3.2. Changes in Plasma Epinephrine Concentrations
3.3. Pharmacokinetic Parameters
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vali, P.; Sankaran, D.; Rawat, M.; Berkelhamer, S.; Lakshminrusimha, S. Epinephrine in Neonatal Resuscitation. Children 2019, 6, 51. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. Endocrinol. 2021, 82, 193–197. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Aziz, K.; Escobedo, M.B.; Kapadia, V.S.; Kattwinkel, J.; Perlman, J.M.; Simon, W.M.; Weiner, G.M.; Zaichkin, J.G. Part 13: Neonatal Resuscitation. Circulation 2015, 132, S543–S560. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Schmicker, R.H.; Newgard, C.D.; Grunau, B.; Scheuermeyer, F.; Cheskes, S.; Vithalani, V.; Alnaji, F.; Rea, T.; Idris, A.H.; et al. Time to Epinephrine Administration and Survival From Nonshockable Out-of-Hospital Cardiac Arrest Among Children and Adults. Circulation 2018, 137, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol. 2024, 132, 124–176. [Google Scholar] [CrossRef]
- Fujizuka, K.; Nakamura, M.; Tamura, J.; Kawai-Kowase, K. Comparison of the efficacy of continuous intravenous infusion versus intramuscular injection of epinephrine for initial anaphylaxis treatment. Acute Med. Surg. 2022, 9, e790. [Google Scholar] [CrossRef] [PubMed]
- Sproat, T.; Hearn, R.; Harigopal, S. Outcome of babies with no detectable heart rate before 10 minutes of age, and the effect of gestation. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F262–F265. [Google Scholar] [CrossRef]
- Boddu, P.K.; Velumula, P.K.; Jani, S.; Fernandes, N.; Lua, J.; Natarajan, G.; Bajaj, M.; Thomas, R.; Chawla, S. Neonatal resuscitation program (NRP) guidelines and timing of major resuscitation events in delivery rooms at a level III NICU: Understanding deviations. Resusc. Plus 2024, 17, 100571. [Google Scholar] [CrossRef]
- Heathcote, A.C.; Jones, J.; Clarke, P. Timing and documentation of key events in neonatal resuscitation. Eur. J. Pediatr. 2018, 177, 1053–1056. [Google Scholar] [CrossRef]
- Mauch, J.; Ringer, S.K.; Spielmann, N.; Weiss, M. Intravenous versus intramuscular epinephrine administration during cardiopulmonary resuscitation—A pilot study in piglets. Pediatr. Anesth. 2013, 23, 906–912. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Vali, P.; Nair, J.; Gugino, S.; Helman, J.; Koenigsknecht, C.; Nielsen, L.; Lakshminrusimha, S. Inadequate Bioavailability of Intramuscular Epinephrine in a Neonatal Asphyxia Model. Front. Pediatr. 2022, 10, 828130. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; O’Reilly, M.; Labossiere, J.; Lee, T.-F.; Cowan, S.; Qin, S.; Bigam, D.L.; Cheung, P.-Y. Cardiopulmonary resuscitation with chest compressions during sustained inflations: A new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation 2013, 128, 2495–2503. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; O’Reilly, M.; Labossiere, J.; Lee, T.-F.; Cowan, S.; Nicoll, J.; Bigam, D.L.; Cheung, P.-Y. 3:1 compression to ventilation ratio versus continuous chest compression with asynchronous ventilation in a porcine model of neonatal resuscitation. Resuscitation 2014, 85, 270–275. [Google Scholar] [CrossRef]
- Cheung, P.-Y.; Gill, R.S.; Bigam, D.L. A Swine Model of Neonatal Asphyxia. J. Vis. Exp. JoVE 2011, 56, e3166. [Google Scholar] [CrossRef]
- Wagner, M.; Cheung, P.-Y.; Li, E.S.; Lee, T.-F.; Lu, M.; O’Reilly, M.; Olischar, M.; Schmölzer, G.M. Effects of epinephrine on hemodynamic changes during cardiopulmonary resuscitation in a neonatal piglet model. Pediatr. Res. 2018, 83, 897–903. [Google Scholar] [CrossRef]
- Shen, W.; Xu, X.; Lee, T.-F.; Schmölzer, G.; Cheung, P.-Y. The Relationship Between Heart Rate and Left Ventricular Isovolumic Relaxation During Normoxia and Hypoxia-Asphyxia in Newborn Piglets. Front. Physiol. 2019, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Ramsie, M.; Cheung, P.-Y.; Lee, T.-F.; O’Reilly, M.; Schmölzer, G.M. Pharmacokinetics and pharmacodynamics of endotracheal versus supraglottic airway epinephrine in a healthy neonatal piglet model. Pediatr. Res. 2025, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Tijssen, J.A.; Lee, T.-F.; Ramsie, M.; Cheung, P.-Y.; Schmölzer, G.M. Intramuscular versus intravenous epinephrine administration in a pediatric porcine model of cardiopulmonary resuscitation. Resusc. Plus 2024, 20, 100769. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Gu, X.; Simons, K.J. Epinephrine absorption in adults: Intramuscular versus subcutaneous injection. J. Allergy Clin. Immunol. 2001, 108, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.; Buyssens, L.; Stroe, M.; Valenzuela, A.; Allegaert, K.; Smits, A.; Annaert, P.; Mulder, A.; Carpentier, S.; Van Ginneken, C.; et al. The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics 2021, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, J. Pediatric Out-of-Hospital Cardiac Arrest Resuscitation: Evaluation of IM Epinephrine (The PRIME Trial). Available online: https://theprimetrial.ca (accessed on 27 July 2025).
| IV Epi 0.02 mg/kg (n = 5) | IM Epi 0.02 mg/kg (n = 5) | IM Epi 0.1 mg/kg (n = 5) | p-Value | |
|---|---|---|---|---|
| Age (days) | 2 (2–2) | 2 (2–2) | 3 (3–3) | 0.024 |
| Weight (kg) | 2 (1.9–2.2) | 1.9 (1.8–2) | 2 (1.8–2.2) | 0.423 |
| Sex (male/female) | 5/0 | 3/2 | 3/2 | 0.451 |
| pH | 7.45 (7.44–7.51) | 7.48 (7.48–7.49) | 7.48 (7.46–7.48) | 0.750 |
| PaCO2 (torr) | 35 (30–35) | 31 (31–33) | 30 (29–32) | 0.327 |
| PaO2 (torr) | 80 (71–81) | 69 (65–74) | 73 (72–74) | 0.867 |
| Base excess (mmol/L) | 0.8 (0.7~2) | −0.3 (−0.3~0.6) | −1.7 (−1.8~−0.5) | 0.119 |
| Lactate (mmol/L) | 4.73 (4.14–6.49) | 4.83 (4.63–5.37) | 6.27 (5.82–6.29) | 0.694 |
| Na+ (mmol/L) | 136 (135–137) | 138 (136–140) | 126 (130–137) | 0.134 |
| Hemoglobin (g/dL) | 8.3 (8.1–8.5) | 7.9 (7.5–8.1) | 7 (5.9–8.9) | 0.370 |
| Heart rate (bpm) | 186 (171–199) | 180 (163–192) | 205 (203–237) | 0.276 |
| Mean arterial pressure (mmHg) | 61 (61–67) | 64 (63–65) | 71 (70–74) | 0.088 |
| Systolic (mmHg) | 86 (85–89) | 89 (85–91) | 95 (87–96) | 0.943 |
| Diastolic (mmHg) | 45 (42–52) | 45 (44–48) | 60 (53–67) | 0.009 |
| Carotid blood flow (mL/kg/min) | 90 (72–93) | 81 (65–90) | 92 (74–104) | 0.530 |
| Cerebral oxygenation (%) | 48 (48–50) | 42 (39–46) | 41 (40–47) | 0.145 |
| Stroke volume (mL/kg/min) | 1.73 (1.54–2.97) | 1.44 (1.31–2.21) | 1.90 (1.42–2.41) | 0.512 |
| Cardiac output (mL/kg/min) | 322 (256–640) | 294 (220–429) | 338 (312–558) | 0.584 |
| Ejection fraction (%) | 53 (44–75) | 33 (23–49) | 49 (40–58) | 0.105 |
| Systemic vascular resistance | 0.21 (0.10–0.22) | 0.18 (0.13–0.28) | 0.19 (0.14–0.21) | 0.841 |
| dP/dt max (mmHg) | 2680 (2540–4392) | 2868 (2454–3230) | 4224 (3741–5403) | 0.033 |
| dP/dt min (mmHg) | −3959 (−3989~−2946) | −3030 (−3749~−2215) | −4799 (−6240~−3513) | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsie, M.; Cheung, P.-Y.; Hyderi, R.; Praveen, S.; Lee, T.-F.; O'Reilly, M.; Schmölzer, G.M. Dose-Related Pharmacokinetic and Pharmacodynamic Effects of Intramuscular Epinephrine in Healthy Neonatal Piglets. Children 2025, 12, 1180. https://doi.org/10.3390/children12091180
Ramsie M, Cheung P-Y, Hyderi R, Praveen S, Lee T-F, O'Reilly M, Schmölzer GM. Dose-Related Pharmacokinetic and Pharmacodynamic Effects of Intramuscular Epinephrine in Healthy Neonatal Piglets. Children. 2025; 12(9):1180. https://doi.org/10.3390/children12091180
Chicago/Turabian StyleRamsie, Marwa, Po-Yin Cheung, Raza Hyderi, Shrieya Praveen, Tze-Fun Lee, Megan O'Reilly, and Georg M. Schmölzer. 2025. "Dose-Related Pharmacokinetic and Pharmacodynamic Effects of Intramuscular Epinephrine in Healthy Neonatal Piglets" Children 12, no. 9: 1180. https://doi.org/10.3390/children12091180
APA StyleRamsie, M., Cheung, P.-Y., Hyderi, R., Praveen, S., Lee, T.-F., O'Reilly, M., & Schmölzer, G. M. (2025). Dose-Related Pharmacokinetic and Pharmacodynamic Effects of Intramuscular Epinephrine in Healthy Neonatal Piglets. Children, 12(9), 1180. https://doi.org/10.3390/children12091180






