Managing Complexity in Rett Syndrome with a Focus on Respiratory Involvement: A Tertiary Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Sleep Study by Polygraphy
- -
- Total sleep time (TST): the total recording time (in minutes) excluding periods of artifacts and gross body movements.
- -
- Snoring time (%): Percentage of the TST spent snoring.
- -
- Apnea–hypopnea index (AHI): The number of apneas and hypopneas per hour of sleep.
- -
- Obstructive apnea–hypopnea index (oAHI): The number of obstructive and mixed apneas/hypopneas per hour of sleep.
- -
- Central apnea–hypopnea index (CAHI): The number of central apneas/hypopneas per hour of sleep.
- -
- Oxygen desaturation index (ODI): The number of ≥ 3% SpO2 desaturation events per hour of sleep.
- -
- Mean SpO2.
- -
- Percentage of the TST spent with an SpO2 < 90% (T90).
2.2. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Polygraphy Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RS | Rett syndrome |
| SDB | sleep-disordered breathing |
| oPG | overnight home polygraphy |
| LRTIs | lower respiratory tract infections |
| MECP2 | methyl-CoPG-binding protein 2 |
| CDKL5 | cyclin-dependent kinase-like 5 |
| URTIs | upper respiratory tract infections |
| TST | total sleep time |
| AHI | apnea–hypopnea index |
| oAHI | obstructive apnea–hypopnea index |
| CAHI | central apnea–hypopnea index |
| ODI | oxygen desaturation index |
| T90 | percentage of the TST spent with an SpO2 < 90% |
| OSA | obstructive sleep apnea |
| CSA | central sleep apnea |
| PtcCO2 | transcutaneous carbon dioxide |
| SD | standard deviation |
| ACT | airway clearance technique |
| ET-IV | endotracheal intubation |
| HFNC | high-flow nasal cannula oxygen therapy |
| PEG | percutaneous endoscopic gastrostomy |
| PSG | polysomnography |
References
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Asuncion, R.M.D.; Ramani, P.K. Rett Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Vilvarajan, S.; McDonald, M.; Douglas, L.; Newham, J.; Kirkland, R.; Tzannes, G.; Tay, D.; Christodoulou, J.; Thompson, S.; Ellaway, C. Multidisciplinary Management of Rett Syndrome: Twenty Years’ Experience. Genes 2023, 14, 1607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Writing Group For Practice Guidelines For Diagnosis And Treatment Of Genetic Diseases Medical Genetics Branch Of Chinese Medical Association; Guan, R.; Li, Q.; Fu, S. Clinical practice guidelines for Rett syndrome. Chin. J. Med. Genet. 2020, 37, 308–312. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Cherchi, C.; Chiappini, E.; Amaddeo, A.; Chiarini Testa, M.B.; Banfi, P.; Veneselli, E.; Cutrera, R.; panel for the Problems in Patients with Rett Syndrome. Management of respiratory issues in patients with Rett syndrome: Italian experts’ consensus using a Delphi approach. Pediatr Pulmonol 2024, 59, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, N. Severe respiratory dysrhythmia in Rett syndrome treated with topiramate. J. Child Neurol. 2014, 29, NP118–NP121. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McLaren, A.T.; Bin-Hasan, S.; Narang, I. Diagnosis, management and pathophysiology of central sleep apnea in children. Paediatr. Respir. Rev. 2019, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Wong, K.; Jacoby, P.; Downs, J.; Leonard, H. Twenty years of surveillance in Rett syndrome: What does this tell us? Orphanet J. Rare Dis. 2014, 9, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Downs, J.; Torode, I.; Wong, K.; Ellaway, C.; Elliott, E.J.; Izatt, M.T.; Askin, G.N.; Mcphee, B.I.; Cundy, P.; Leonard, H.; et al. Surgical fusion of early onset severe scoliosis increases survival in Rett syndrome: A cohort study. Dev. Med. Child Neurol. 2016, 58, 632–638. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Bortolan, F.; Diliberto, D.; Villani, L. Pediatric interregional healthcare mobility in Italy. Ital. J. Pediatr. 2021, 47, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, C.; Armstrong, D.; Marsh, E.; Lieberman, D.; Motil, K.; Witt, R.; Standridge, S.; Nues, P.; Lane, J.; Dinkel, T.; et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr. Open 2020, 4, e000717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrelli, M.; Corcione, A.; Cimbalo, C.; Annunziata, A.; Basilicata, S.; Fiorentino, G.; Santamaria, F. Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography. Children 2023, 10, 1331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarber, K.M.; Howard, J.J.M.; Dye, T.J.; Pascoe, J.E.; Simakajornboon, N. Sleep-Disordered Breathing in Pediatric Patients With Rett Syndrome. J. Clin. Sleep Med. 2019, 15, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaddeo, A.; De Sanctis, L.; Arroyo, J.O.; Khirani, S.; Bahi-Buisson, N.; Fauroux, B. Polysomnographic findings in Rett syndrome. Eur. J. Paediatr. Neurol. 2019, 23, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hagebeuk, E.E.; Bijlmer, R.P.; Koelman, J.H.; Poll-The, B.T. Respiratory disturbances in rett syndrome: Don’t forget to evaluate upper airway obstruction. J. Child Neurol. 2012, 27, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Peri, F.; Cherchi, C.; Chiarini Testa, M.B.; Pavone, M.; Verrillo, E.; Cutrera, R. The Efficacy of Noninvasive Ventilation in Patients Affected by Rett Syndrome With Hypoventilation. Pediatr. Neurol. 2024, 158, 81–85. [Google Scholar] [CrossRef] [PubMed]
- MacKay, J.; Leonard, H.; Wong, K.; Wilson, A.; Downs, J. Respiratory morbidity in Rett syndrome: An observational study. Dev. Med. Child Neurol. 2018, 60, 951–957. [Google Scholar] [CrossRef] [PubMed]
- De Felice, C.; Guazzi, G.; Rossi, M.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Tonni, G.; Latini, G.; Valacchi, G.; Hayek, J. Unrecognized lung disease in classic Rett syndrome: A physiologic and high-resolution CT imaging study. Chest 2010, 138, 386–392. [Google Scholar] [CrossRef] [PubMed]

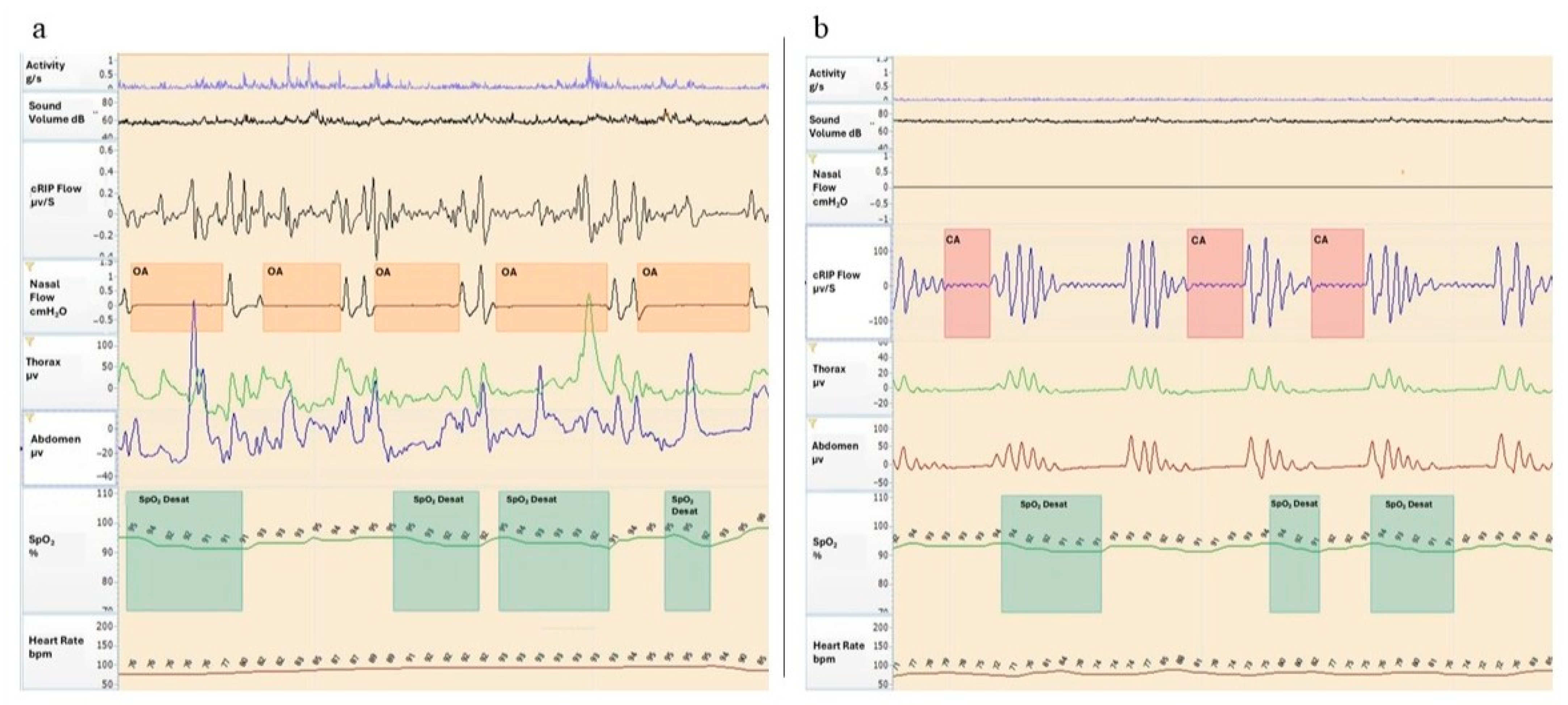

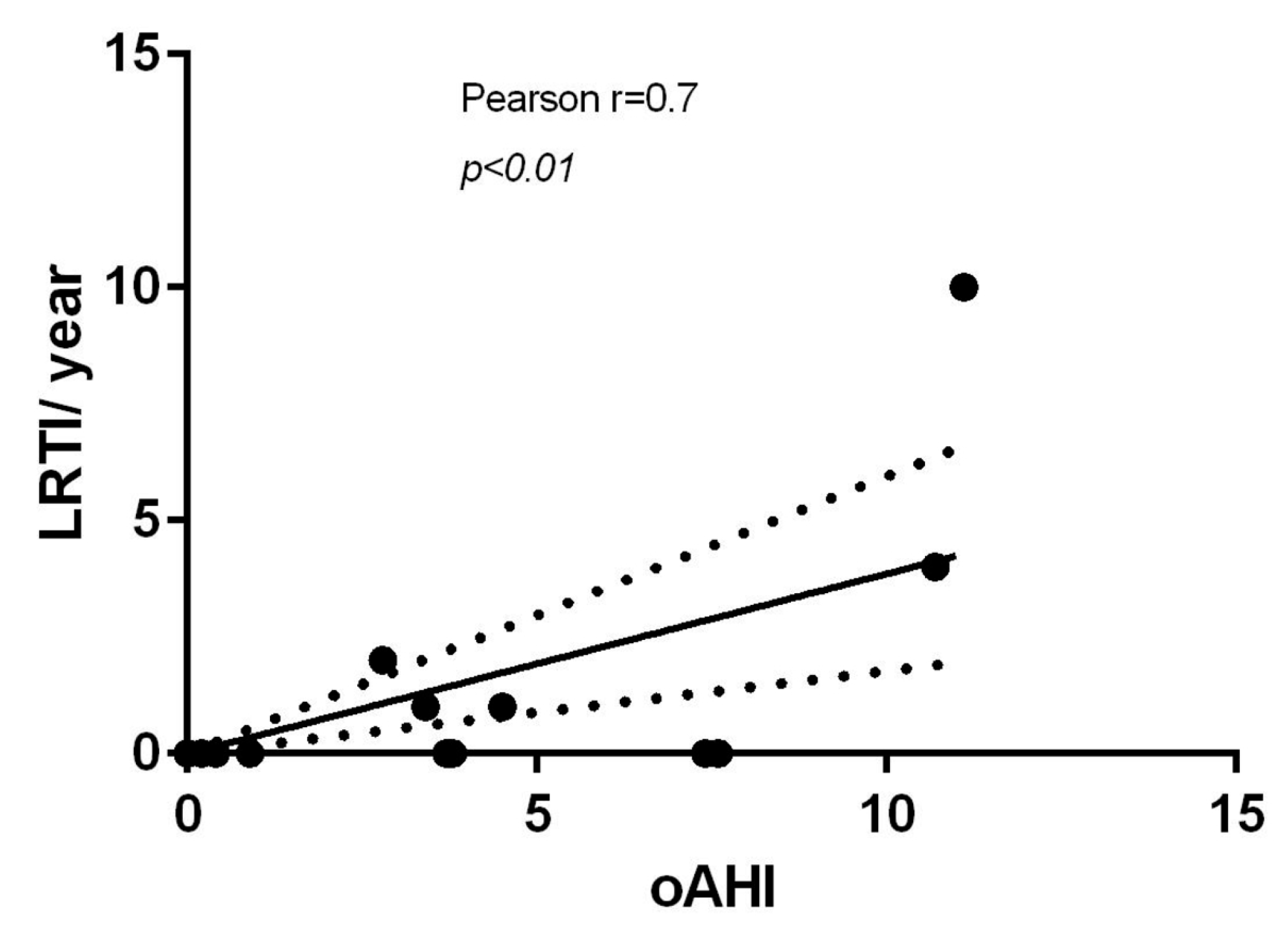
| CLINICAL FEATURES | |
|---|---|
| Epilepsy [n. (%)] | 15/23 (65) |
| Scoliosis [n. (%)] | 12/23 (52) |
| Oral solid feeding [n. (%)] | 16/23(70) |
| Oral semi-solid feeding [n. (%)] | 5/23(21) |
| URTIs [n. (%)] | 13/23 (56) |
| URTIs/last year [median (range)] | 1 (0–12) |
| LRTIs [n. (%)] | 9/23 (39) |
| LRTIs/last year [median (range)] | 0 (0–10) |
| n. hospitalizations due to respiratory issues/last year [median (range)] | 0 (0–3) |
| n. hospitalizations for non-respiratory-related issues/last year [median(range)] | 0 (0–1) |
| THERAPEUTIC MANAGEMENT | |
| Antiepileptic drugs [n. (%)] | 16/23 * (70) |
| Arthrodesis [n. (%)] | 2/12 (16) |
| PEG [n. (%)] | 2/23 (9) |
| Antibiotic treatment/last year [median (range)] | 1 (0–12) |
| ACT [n. (%)] | 4/23 (17) |
| Nocturnal ventilation [n. (%)] | 3/23 (13) |
| Tracheostomy [n. (%)] | 1/3 (1) |
| Patient | Age (Years) | Epilepsy | Scoliosis | PEG | URTIs (n/Year) | LRTIs (n/Year) | Antibiotics (n/Year) | H (n/Year) | ACT | Nocturnal Ventilation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | N | Y | N | 0 | 0 | 0 | 0 | N | N |
| 2 | 2.6 | N | N | N | 4 | 1 | 3 | 1 | N | N |
| 3 | 16.8 | N | Y | N | 0 | 0 | 1 | 0 | N | N |
| 4 | 5.4 | Y | N | N | 0 | 0 | 1 | 0 | N | N |
| 5 | 5.4 | Y | N | N | 3 | 0 | 2 | 0 | N | N |
| 6 | 5 | N | N | N | 10 | 0 | 0 | 0 | N | N |
| 7 | 11.8 | Y | Y | N | 0 | 0 | 1 | 0 | N | N |
| 8 | 8.2 | N | Y | N | 2 | 1 | 1 | 0 | N | N |
| 9 | 16 | Y | Y | Y | 0 | 1 | 2 | 0 | Y | N |
| 10 | 6.9 | Y | N | N | 0 | 0 | 1 | 0 | N | N |
| 11 | 7.5 | Y | Y | N | 2 | 0 | 2 | 0 | N | N |
| 12 | 7.6 | Y | N | N | 4 | 1 | 12 | 0 | N | N |
| 13 | 5.9 | N | N | N | 1 | 0 | 1 | 0 | N | N |
| 14 | 15.9 | Y | Y | N | 0 | 0 | 0 | 0 | N | N |
| 15 | 6.9 | Y | N | Y | 4 | 4 | 5 | 0 | Y | Tracheostomy |
| 16 | 12.5 | Y | Y | N | 1 | 1 | 2 | 0 | N | N |
| 17 | 9.6 | Y | Y | N | 0 | 0 | 7 | 0 | N | N |
| 18 | 13.7 | Y | Y | N | 1 | 0 | 1 | 0 | N | N |
| 19 | 13.5 | Y | Y | N | 0 | 0 | 1 | 0 | N | N |
| 20 | 6.5 | Y | N | N | 2 | 1 | 2 | 1 | N | N |
| 21 | 7.6 | Y | N | N | 0 | 10 | 10 | 3 | Y | NIV |
| 22 | 5.6 | Y | Y | N | 12 | 2 | 2 | 0 | Y | NIV |
| 23 | 10.1 | N | N | N | 4 | 0 | 0 | 0 | N | N |
| Patient | TST (Minutes) | Snoring Time (%) | AHI (Events/h) | oAHI (Events/h) | CAHI (Events/h) | ODI (Events/h) | Mean SpO2 (%) | T90 (%) | PtcCO2 > 50 mmHg (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| NO OSA oAHI < 1 | 23 | 240 | 10.9 | 0.5 | 0 | 0 | 4.6 | 97 | 1.5 | - |
| 17 | 297 | 1.6 | 0.8 | 0.2 | 0.6 | 1.2 | 97 | 0.1 | - | |
| 14 | 423 | 4.1 | 1.4 | 0.4 | 0.1 | 1.3 | 97 | 0.2 | - | |
| 18 | 449 | 9.5 | 0.4 | 0.4 | 0 | 0.5 | 97 | 0 | - | |
| 10 | 336 | 9.7 | 1.4 | 0.9 | 0.4 | 1.1 | 96 | 0 | - | |
| MILD OSA 1 < oAHI ≤ 5 | 22 | 480 | N/A | 10.2 | 2.8 | 7.2 | 37 | 95 | N/A | 91 |
| 16 | 335 | 7.3 | 5.9 | 3.4 | 2.5 | 0.4 | 95 | 1.8 | - | |
| 11 | 467 | 50 | 5.1 | 3.7 | 1.3 | 8.7 | 94 | 0.6 | - | |
| 5 | 382 | 19.7 | 4.7 | 3.8 | 0.9 | 3.5 | 97 | 0.2 | - | |
| 19 | 240 | 3.1 | 6.8 | 3.8 | 2.7 | 9.2 | 95 | 0.1 | - | |
| 9 | 394 | 0.3 | 4.5 | 4.5 | 0 | 3.1 | 96 | 1.4 | - | |
| MODERATE OSA 5 < oAHI < 10 | 4 | 291 | N/A | 7.4 | 7.4 | 0 | 26.6 | 92 | 2.9 | - |
| 3 | 466 | 11.1 | 8.2 | 7.6 | 0.6 | 4.2 | 96 | 1.9 | - | |
| SEVERE OSA oAHI≥ 10 | 15 | 240 | N/A | 11.5 | 10.7 | 0.4 | 18 | 95 | N/A | 100 |
| 21 | 285 | 28.1 | 15.8 | 11.1 | 3.4 | 10.3 | 94 | 1.3 | 91 | |
| Mean ± DS | 355 ± 88.2 | |||||||||
| Median; range | 9.6; 0.3–50 | 4.9; 0.4–15.8 | 3.7; 0–11.1 | 0.4; 0–7.2 | 3.8; 0.4–37 | 96; 92–97 | 0.6; 0–2.9 | 91; 91–100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corcione, A.; Del Giudice, L.A.; Basilicata, S.; Maglio, M.; Aiello, S.; Cerchione, R.; Annunziata, A.; Amaddeo, A.; Borrelli, M. Managing Complexity in Rett Syndrome with a Focus on Respiratory Involvement: A Tertiary Center Experience. Children 2025, 12, 1181. https://doi.org/10.3390/children12091181
Corcione A, Del Giudice LA, Basilicata S, Maglio M, Aiello S, Cerchione R, Annunziata A, Amaddeo A, Borrelli M. Managing Complexity in Rett Syndrome with a Focus on Respiratory Involvement: A Tertiary Center Experience. Children. 2025; 12(9):1181. https://doi.org/10.3390/children12091181
Chicago/Turabian StyleCorcione, Adele, Luigi Antonio Del Giudice, Simona Basilicata, Mariantonia Maglio, Salvatore Aiello, Raffaele Cerchione, Anna Annunziata, Alessandro Amaddeo, and Melissa Borrelli. 2025. "Managing Complexity in Rett Syndrome with a Focus on Respiratory Involvement: A Tertiary Center Experience" Children 12, no. 9: 1181. https://doi.org/10.3390/children12091181
APA StyleCorcione, A., Del Giudice, L. A., Basilicata, S., Maglio, M., Aiello, S., Cerchione, R., Annunziata, A., Amaddeo, A., & Borrelli, M. (2025). Managing Complexity in Rett Syndrome with a Focus on Respiratory Involvement: A Tertiary Center Experience. Children, 12(9), 1181. https://doi.org/10.3390/children12091181





