Abstract
In this study, tannic acid-modified gold nanoparticles were found to have superior nanozyme activity and catalyze the oxidation reaction of 3,3′,5,5′-tetramethylbenzidine in the presence of hydrogen peroxide. Enhancing the catalytic activity of the nanozyme by Pb2+ ions caused by selectively binding metal ions by the tannic acid-capped surface of gold nanoparticles makes them an ideal colorimetric probe for Pb2+. The parameters of the reaction, including pH, incubation time, and concentration of components, were optimized to reach maximal sensitivity of Pb2+ detection. The absorption change is directly proportional to the Pb2+ concentration and allows the determination of Pb2+ ions within 10 min. The colorimetric sensor is characterized by a wide linear range from 25 to 500 ng×mL−1 with a low limit of detection of 11.3 ng×mL−1. The highly sensitive and selective Pb2+ detection in tap, drinking, and spring water revealed the feasibility and applicability of the developed colorimetric sensor.
1. Introduction
The widespread use of toxic metals in the manufacture of batteries, pigments, paints, ammunition, toys, and in other applications has led to environmental pollution and public health problems [1]. Pb2+ is one of the most dangerous pollutants as its high levels cause serious damage to the nervous system, renal, kidney, and brain. Moreover, prolonged exposure to low metal concentrations can lead to multiple adverse health effects [2]. In accordance with the World Health Organization guidelines, the level of Pb2+ in drinking water should not exceed 10 µg·L−1 [3]. Common methods for the determination of Pb2+ include flame atomic absorption spectroscopy [4], atomic emission spectroscopy [5], X-ray fluorescence [6], inductively coupled plasma mass spectrometry [7], and electrochemical methods [8,9]. However, implementation of these methods requires high-cost complex instrumentation and time-consuming procedures, which complicates on-site monitoring of target analyte.
The alternative approach is colorimetric methods that provide direct and simple detection of Pb2+ ions. The possibility to exclude sophisticated instruments staffed by highly trained personnel enables extensive use of colorimetric methods for the detection of Pb2+. The most widely applied analytic technique relies on the use of noble metal nanoparticles (most often, silver and gold nanoparticles) as carriers of various receptor molecules that specifically bind target metal ions. In this case, the surface modification of nanoparticles with various chelating ligands [10,11,12,13] and oligonucleotides [14,15,16,17] provides highly specific detection of Pb2+ [18]. In the presence of detectable heavy metal ions, aggregation of nanoparticles occurs, followed by a concentration-dependent color change, which is observed with the naked eye or recorded photometrically [19,20]. However, obtaining functionalized nanoparticles increases the complexity and cost of the analysis.
Recently, a great interest has been attracted by nanomaterials with enzyme-like catalytic properties, namely, nanozymes due to their ease of preparation, controlled activity, high stability, and low cost [21]. In the presence of metal ions, the catalytic activity of nanozymes may change, which enables the concentration-dependent colorimetric sensing of target ions [22,23]. Various metallic nanoparticles and nanohybrid materials were found to exhibit intrinsic peroxidase-mimicking activity and produce a colored product by catalyzing the oxidation of the enzyme substrate [21]. Thus, Tang et al. described the use of two-dimensional layered tungsten disulfide (WS2) nanosheets that possess peroxidase-like activity to design colorimetric sensor for Pb2+ detection in environmental and biological samples [24]. It was found that Pb2+ ions inhibit the catalytic activity of WS2 nanosheets, the proposed mechanism of which is blocking the electron transfer from the nanosheets to hydrogen peroxide and, as a result, the lack of (•OH) radicals for the oxidation of the enzyme substrate 3,3′,5,5′-tetramethylbenzidine (TMB). Among a variety of peroxidase-mimic nanomaterials, gold nanoparticles (AuNPs) are of particular interest because they are easily synthesized and functionalized by various receptor molecules. The ability of metal ions to affect the catalytic activity of AuNPs is used in a number of colorimetric sensors [25,26]. Thus, Lien et al. reported colorimetric analysis of Hg2+ and Pb2+ ions using peroxidase-mimic activity of AuNPs in the presence of Pt4+ and Bi3+ ions allowing to detect the target ions in nanomolar concentrations [27]. It should be noted that due to the mechanism of formation of metal-gold alloys, the colorimetric signal could be influenced by other interfering metal ions present in the sample. In this case, the surface modification of AuNPs makes it possible to improve the selectivity of the colorimetric sensor for detection of a target metal ion [28]. Citrate-stabilized gold nanoparticles were earlier proposed for aggregation analysis of different analytes, including heavy metal ions [29,30]. However, the use of unmodified gold nanoparticles for the determination of lead does not allow reaching a sensitivity higher than 18 μM [29]. It is explained by the absence of a specific recognizing molecule on the particle surface. Since the carboxyl groups of organic acids are capable to interact with many metal ions [31], a coating with functional groups is required to selectively recognize specific ions. Earlier, we developed a colorimetric technique for the determination of Pb2+ ions using tannic acid as a modifying agent [20]. The developed aggregation analysis made it possible to detect lead ions with a detection limit of 310 ng/mL. Changing sample/reactants volume ratio reduced the detection limit to 60 ng/mL. The use of catechin as an alternative tanning compound demonstrated the presence of a peroxidase-like activity in modified gold nanoparticles for the specific determination of lead in the H2O2-mediated oxidation of Amplex UltraRed [32]. The work of Yoosaf et al. explained the formation of the shell with necessary functional groups available for interaction with Pb2+ ions when synthesizing nanoparticles with the use of different tannins as reducing and stabilizing agents [33].
Herein, AuNPs modified by tannic acid (TA) are proposed as a selective sensing probe for nanozyme-based colorimetric detection of Pb2+ in water. The implementation of TA as a capping agent showed a peroxidase-like activity of AuNPs for substrate containing H2O2 and TMB. The addition of Pb2+ ions further stimulated the catalytic activity of the nanoparticles, resulting in a faster formation of a colored product. The Pb2+-promoted nanozyme activity was investigated under different conditions (pH, concentrations of reactants). The practicability and reliability of nanozyme-based colorimetric sensor was validated through the analysis of Pb2+ in drinking, spring and tap water. The proposed approach allows for rapid, sensitive, and easy-to-use tool for monitoring of Pb2+ levels without sample pre-treatment.
2. Materials and Methods
2.1. Chemicals and Instruments
All of the heavy metal salts were purchased from the Center of Reference Materials and High-Purity Substances, LTD. (Saint-Petersburg, Russia) and used as stock solutions. Chloroauric acid (HAuCl4), sodium citrate, potassium carbonate, 3, 3, 5, 5-tetramethylbenzidine (TMB), dimethyl sulfoxide (DMSO) and tannic acid (TA) were obtained from Sigma Aldrich (St. Louis, MO, USA). 33% hydrogen peroxide was supplied by Fluka (St. Louis, MO, USA). All chemicals were of analytical grade. Ultrapure water (18.5 MΩ·cm at 22 °C; Millipore, Bedford, MA) was applied for the preparation of aqueous solutions. The colorimetric analysis was carried out in 96-well transparent Costar 9018 polystyrene microplates (Costar, Tewksbury, MA, USA).
Absorption spectra of gold nanoparticles were recorded using a UV-2450 spectrophotometer (Shimadzu Kyoto, Japan). The transmission electron microscopy (TEM) images were obtained by JEM CX-100 electron microscope (Jeol, Tokyo, Japan) with an accelerating voltage of 80 kV. The samples were placed onto 300-mesh grids (Pelco International, Redding, CA, USA) coated with formvar film. Subsequent image analysis was performed using Image Tool software (University of Texas Health Science Center, San Antonio, TX, USA).
2.2. Synthesis of TA-Capped AuNPs
The stock solution of TA-capped AuNPs was prepared according to our previous study [31]. Briefly, the solution 1 was prepared by adding 100 µL of 5% HAuCl4 to 39.5 mL of water. The solution 2 was prepared by mixing 2 mL of 1% sodium citrate, 250 µL of 1% TA, and 250 µL of 0.025 M potassium carbonate in 7.5 mL of ultrapure water. Both solutions were heated to 60 °C, followed by pouring solution 2 into solution 1, boiling for 4–5 min and cooling to room temperature. The concentration of prepared TA-capped AuNPs was 10−8 M. The solution was stored at 4–6 °C prior to use.
2.3. Comparison of Catalytic Activity of TA-Capped AuNPs in the Absence/Presence of Pb2+
The peroxidase-mimic activity of TA-capped AuNPs was investigated by assessing the ability of nanozyme to oxidize a colorless peroxidase substrate (TMB) into a colored product in the presence of H2O2. The assay was performed at a fixed concentration of TA-capped AuNPs using 500 μM freshly prepared TMB (1% TMB prepared in DMSO) and 500 mM H2O2 in sodium citrate buffer (pH 4.0). The kinetic analysis was performed by varying the concentration of H2O2 (0.1–0.6 M) at a fixed concentration of TA-capped AuNPs and TMB (500 μM). Similarly, the TMB-H2O2 catalytic reaction was carried out by varying the concentration of TMB (0.2–1 mM) with 500 mM H2O2. The reaction was started by adding an appropriate amount of H2O2, and the optical density at 650 nm corresponding to the maximum of TMB oxidation product was recorded using an EnSpire Multi-mode Plate Reader (PerkinElmer, USA). The initial reaction rates were determined from initial linear segment of “A650-time” curve, reflecting growing concentration of the TMB oxidation product. To calculate the initial reaction rates, the molar absorption coefficient ɛ (650 nm), equal to 39,000 M−1cm−1 was used. To determine Michaelis-Menten constant (Km) and maximum reaction velocity (Vmax), kinetic dependences were plotted in the Lineweaver-Burk coordinates and the following equation was used:
where —initial reaction velocity, —maximum reaction velocity, —initial substrate concentration, —Michaelis-Menten constant.
2.4. Colorimetric Detection of Pb2+ in Aqueous Solutions
Briefly, 100 µL of different concentrations of Pb2+ solutions were added to 20 µL of TA-capped AuNPs. After incubation for 5 min at room temperature, 50 µL of TMB solution containing 6% H2O2 was added. Immediately, 50 µL of 1M H2SO4 was added to stop the reaction. To quantify the concentration of Pb2+ the absorbance at 450 nm (A450) was recorded. Each experiment was repeated three times. Dynamic range was determined by plotting A450 nm with increasing Pb2+ concentration. The limit of detection (LoD) was defined using the equation , where is the standard deviation of the colorimetric signal of the blank sample.
2.5. Specificity of the Analysis
The specificity of the proposed colorimetric sensor was evaluated by testing samples of 13 metal and metalloid ions, namely Hg2+, Zn2+, Cu2+, Co2+, Sr2+, Mn2+, Fe3+, Sn4+, Cr3+, Cd2+, As2+, Sb3+, and Ba2+. 100 μL of the listed ions at a concentration of 100 ng·mL−1 were added to 15 μL of TA-capped AuNPs. Furthermore, the experiment was carried out as described in Section 2.4.
2.6. Analysis of Real Water Samples
To verify the practical feasibility of the developed sensor, drinking, spring, and tap water samples were analyzed. The container was rinsed with water to be collected at least three times prior to sampling. The samples were purified using a membrane filter with a pore size of 0.22 μm, after which concentrated HNO3 was added to preserve the sample and adjust pH to 4.5–5.0. Water samples were stored in a freezer until analysis. A series of spiked water samples, containing Pb2+ at concentrations 25, 50, and 100 ng×mL−1, were prepared by diluting Pb2+ stock solution (0.1 mg·mL−1 in deionized water). The processing of obtained data was performed using Excel and OriginPro9 (Origin Lab, Northampton, MA, USA).
3. Results
3.1. Characterization of TA-Capped AuNPs
TA-capped AuNPs were prepared by reduction of chloroauric acid by citric and tannic acids, with the latter also providing a stabilizing effect. The as-prepared gold nanoparticles were characterized by wine-red color with an absorption peak at 515 nm (Figure 1a). The morphology of the nanoparticles was observed by TEM (Figure 1b) and revealed monodisperse nanoparticles with the particle size distribution of 6.8 ± 0.9 nm (the counted number of particles was 94, Figure 1c).
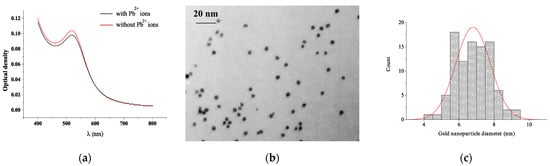
Figure 1.
(a) Absorbance spectra of TA-capped AuNPs with (black lines) and without (red lines) Pb2+ ions. (b) TEM image and (c) histogram of diameter distribution of the obtained TA-capped AuNPs.
The data on the reproducibility of the synthesis were evaluated in experiments with the determination of the optical density of nanoparticles. Thus, during the synthesis of nanoparticles, the optical density was estimated at 515 nm, which after five repeated syntheses was 0.98, 1.0, 0.98, 0.99, and 0.98, respectively. In addition, the value of the average error of detection was estimated when interacting with lead ions, which was no more than 5.8%.
3.2. Principle of the Nanozyme-Based Colorimetric Pb2+ Detection
The principle of proposed colorimetric sensor is illustrated in Figure 2 For Pb2+ sensing, TA-capped AuNPs were prepared and applied. Figure 2 clearly demonstrates the mechanism of reaction, absorbance peaks as well as visually observed changes occurred under interaction of nanoparticles with lead ions.
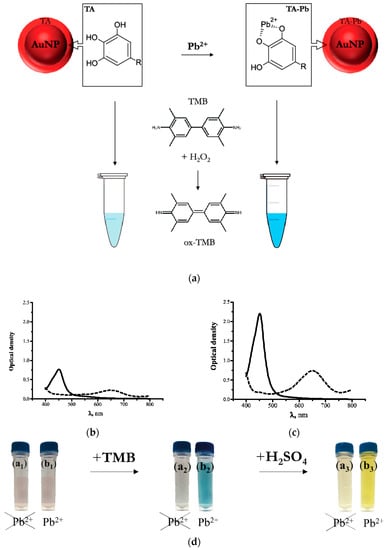
Figure 2.
Principle of the TA-capped AuNPs based colorimetric sensor for Pb2+ detection. (a) Scheme of catalytic oxidation of TMB in presence of AuNPs. (b) Absorbance spectra of TA-capped AuNPs without and with (c) Pb2+ ions after addition of TMB-H2O2 (dash lines) and stop reaction (solid lines) respectively. (d) Corresponding photographs of TA-capped AuNPs samples before/after catalytic reaction in the presence/absence Pb2+ ions.
The scheme of the reactants’ interaction and catalytic oxidation of TMB by TA-capped AuNPs is presented on Figure 2a. In the absence of Pb2+ ions AuNPs had weak catalytic activity toward TMB oxidation. Due to this, low absorbance at 650 nm was observed, and visually confirmed by the weak color development (Figure 2b). In the presence of lead, tannic acid residues chelated Pb2+ ions to form the complexes indicated at Figure 2a. This interaction promoted the catalytic activity of the AuNPs as nanozymes and resulted in increasing of absorbance peak at 650 nm related to oxidated product of TMB transformation (Figure 3c), the color of solution became bright blue. After adding the sulfuric acid stop solution, the blue color turned to yellow and thus absorbance peak at 450 nm increased (Figure 3d). The addition of a TMB solution to a solution of lead ions and tannic acid did not lead to any catalytic activity in the absence of AuNPs.
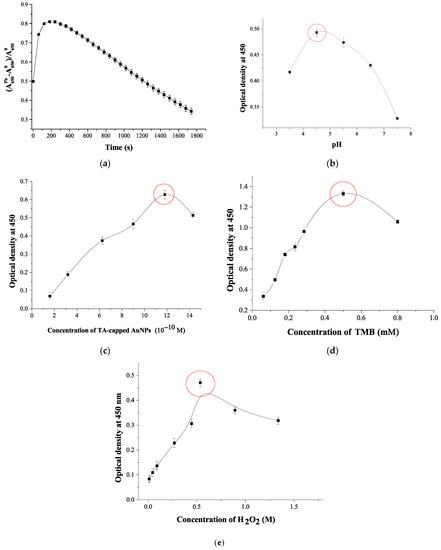
Figure 3.
Optimization of the analysis conditions: (a) Time dependence of the colorimetric response in the presence of Pb2+ relative to the background signal; (b) pH effect to analytical signal; (c) Concentration of TA-capped AuNPs; (d) Concentration of TMB; (e) Concentration of H2O2. Experimental conditions: 11.8 × 10−10 M TA-capped AuNPs, 0.5 mM TMB, 0.5 M H2O2, 100 ng·mL−1 Pb2+, pH 4.5. (N = 3).
It was previously shown that, in addition to the reducing properties of tannic compounds, they form complexes with metal ions [34,35,36]. The proposed mechanism of the analytical use of such tannic acid-containing nanoparticles is as follows. AuNPs synthesized with TA as a reducing and stabilizing agent have carbonyl groups on their surface and able to hydrolysis of phenolic compounds. They, in turn, specifically bind to Pb2+ ions, providing the formation of bridges that unite several nearby modified nanoparticles into clusters. This arrangement and the presence of free electron orbitals of Pb2+cations provide a free transition of an electron participating in the oxidation reaction of the substrate (TMB). The proposed principle of Pb2+ colorimetric detection corresponds to the previously described “turn-on” mechanism based on the enhancement of the catalytic activity of noble metal nanoparticles in the presence of metal ions [22].
The mechanism of oxidation of TMB catalyzed by AuNPs as a peroxidase-like nanozyme has been considered in earlier [28,37]. The catalytic process is similar to the Fenton reaction [38] and includes the following stages: (1) the formation of a hydroxyl radical (OH˙) from hydrogen peroxide in the presence of nanozyme, (2) the oxidation of TMB by the hydroxyl radical with the formation of the blue product TMB +. The high affinity of gold nanoparticles for TMB accelerates the excited electron transfer, which complicates the recombination of electron-hole pairs and, in turn, promotes the formation of OH˙. Thus, the peroxidase-like activity is increased. In addition, the presence of lead ions stimulates the formation of hydroxyl radicals [39].
3.3. Optimization of the Experimental Conditions for Colorimetric Pb2+ Detection
As shown in Figure 3a, the reaction of TMB oxidation by H2O2 catalyzed by TA-capped AuNPs reaches a plateau after 120 s. This incubation time was used as optimal in further experiments. To select the optimal pH, the catalytic activity of Pb2+-promoted TA-capped AuNPs was studied in a wide pH range from 3.5 to 7.5. As follows from Figure 3b, the highest signal after adding the stopping solution (A450) is recorded in the pH range 4.5–5. Furthermore, the ratios of the components in the colorimetric analysis were optimized by varying concentrations of TA-capped AuNPs, TMB, and H2O2. Figure 3c,d shows that the highest colorimetric signal was observed when 11.8×10−10 M TA-capped AuNPs and 0.5 mM TMB were applied. The peroxide-like activity of nanozymes also depends on the concentration of H2O2, since the latter forms hydroxyl radicals that oxidize TMB [40]. In this regard, the influence of the concentration of H2O2 on the catalytic activity of Pb2+-promoted TA-capped AuNPs was investigated (Figure 3e). The growth of the H2O2 concentration up to 0.53M gives rise to the signal enhancement.
3.4. Colorimetric Technique for Determining the Catalytic Activity of TA-Capped AuNPs
The peroxidase-mimic activity of bare TA-capped AuNPs was investigated using TMB. TMB is a common peroxidase substrate that is oxidized in the presence of H2O2 forming a blue product. The mixture of TMB with H2O2 is initially colorless; however, when TA-capped AuNPs are added, the mixture turns blue, which indicates the peroxidase-like activity. Nevertheless, the addition of Pb2+ to the mixture increases the catalytic activity and the solution instantly acquires a deep blue color (see Figure 2d).
The Pb2+-promoted catalytic activity was investigated comparing the nanoparticles with and without Pb2+ and plotting the time dependence of absorbance at 650 nm (stop solution was not added in these studies). As can be seen from the Figure 4, the presence of Pb2+ leads to a sharp increase of absorbance. A linear segment of the curve up to 120 s was used to calculate the initial velocity. Thus, the highest rate (3 × 10−3 s−1) was observed for the nanozyme in the presence of Pb2+, being three times the value of 1 × 10−3 s−1, observed in the absence of Pb2+. The estimated data confirm Pb2+-promoted catalytic activity of TA-capped AuNPs, which can be applied for detection of Pb2+ ions.
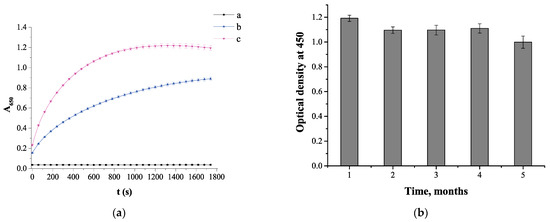
Figure 4.
(a) Peroxidase-like activity of TA-capped AuNPs: a—substrate mixture, 0.5 M H2O2 + 0.5 mM TMB; b—11.8 × 10−10 M TA-capped AuNPs + 0.5M H2O2 + 0.5 mM TMB; c—11.8 × 10−10 M TA-capped AuNPs + 100 ng×mL−1 Pb2+ + 0.5 M H2O2 + 0.5 mM TMB. (N = 3). (b) Pb2+-promoted catalytic activity of TA-capped AuNPs during long-term storage.
Furthermore, the stability of TA-capped AuNPs was evaluated. The TA-capped AuNPs were stored at 4 °C and Pb2+-promoted catalytic activity of the nanozyme was measured every month to investigate long-term stability. As shown in Figure 4b, the nanozyme displays stable high catalytic activity for 4 months, and after this period, it decreases slightly.
To investigate the catalytic properties of the TA-capped AuNPs the kinetic parameters were defined using by varying the concentration of both TMB and H2O2 and data processing by the Michaelis-Menten model as described in [41]. The Michaelis-Menten constants (Km) and maximum initial reaction rates (ʋmax) were calculated by fitting the Lineweaver-Burk plots (Figure 5b,d). As shown in Table 1, the TA-capped AuNPs exhibited a lower Km value when using TMB as a substrate compared to horseradish peroxidase (HRP) (0.2 mM versus 0.43 mM) indicating the stronger affinity of this nanozyme toward substrate. Pb2+ ions enhanced the catalytic activity of TA-capped AuNPs, which is reflected in a decrease of the Km to 0.09 mM. In contrast, the Km determination for H2O2 as a substrate revealed much higher values than that of HRP (190 mM versus 3.7 mM, see Table 1), highlighting the need for H2O2 concentrations to ensure maximum catalytic activity of the nanozyme. As previously shown [42], strong affinity of HRP for H2O2 is due to the presence of amino acids in the active centre of the natural enzyme. To sum up, these results demonstrated Pb2+-promoted nanozyme activity, and TA-capped AuNP is an appropriate sensing probe for Pb2+ detection.
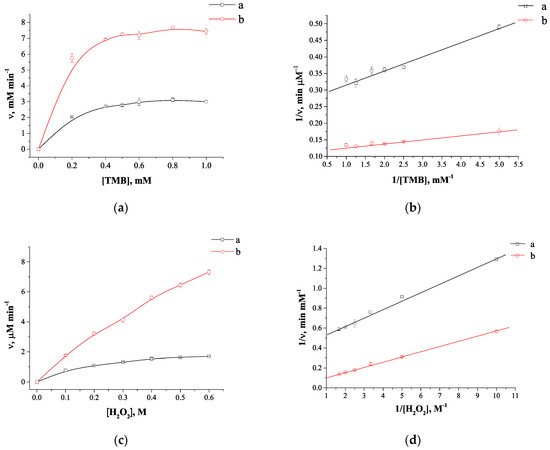
Figure 5.
Kinetic dependences of Michaelis-Menten and their linear transformations in double reciprocal plots (Lineweaver–Burk plots) for TA-capped AuNPs with (a, black lines) and without (b, red lines) Pb2+ ions varying the concentration of TMB from 0.2 to 1 mM with a fixed concentration of H2O2 at 0.5 M (a,b) and varying the concentration of H2O2 from 0.1 to 0.6 M with a fixed TMB at 0.5 mM (c,d). (N = 3). The values of deviations from the mean at the points for figures a–d are, respectively, 0.6–4.6%, 0.6–5.8%, 0.5–5.5%, 0.5–4.1%.

Table 1.
Comparison of kinetic parameters (Km and ʋmax) of the oxidation reaction catalyzed by HRP and the proposed colorimetric probe.
3.5. Quantitative Pb2+ Detection Using Colorimetric Sensor
According to the TA-capped AuNPs-based colorimetric sensor design format, the amount of Pb2+ ions determine the peroxide-mimicking activity of the sensing probe. Consequently, the sensing responses of Pb2+ ions with different concentrations were investigated under optimal conditions. To assess the sensitivity and dynamic linear range of the developed sensor, concentrations of Pb2 + from 1 to 1000 ng×mL−1 were tested, and A450 was measured. As shown in Figure 6, with an increase in the Pb2+ concentration, A450 increases, accompanied by a color change from light blue to dark blue. The dynamic linear range for Pb2+ detection is 25–500 ng×mL−1 with the linear regression equation of y = 0.406 − 0.03x and correlation coefficient (R2) of 0.994 (Figure 6b). The detection limit is calculated to be 11.3 ng×mL−1 using the signal-to-noise ratio (S/N = 3), which is roughly in line with WHO drinking water guideline of 10 ng×mL−1. The linearity and Pb2+ detection limit of the developed sensor was mapped to previously reported colorimetric sensors and summarized in Table 2. The proposed colorimetric sensor allows the detection of Pb2+ ions in the nanogram range without additional sample pretreatment and demonstrates comparable or superior performance compared to other colorimetric analyses reported in the literature [13,41,43,44].
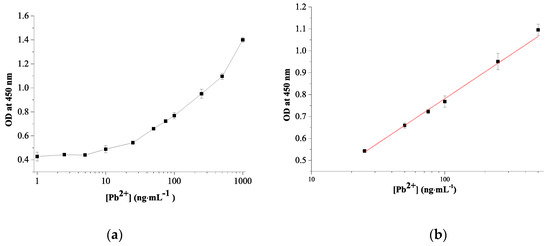
Figure 6.
Calibration curve for detection of Pb2+ ions using TA-capped AuNPs (a) and its linear range (b) (N = 3).

Table 2.
Comparison of the developed analysis for Pb2+ with previously reported colorimetric methods.
3.6. Selectivity
To confirm the specificity of the nanozyme-based colorimetric sensor, along with Pb2+, the contribution of other metals to the colorimetric response was studied. The concentration of all tested ions was 100 ng×mL−1. As shown in Figure 7, the reaction solution containing Pb2+ showed bright yellow while competing metal ions were almost colorless. All interfering metal ions demonstrated almost negligible changes in A450 after stopping the oxidation reaction (Figure 7). Thus, the results demonstrate excellent selectivity of TA-capped AuNPs toward Pb2+ ions among all interfering metal ions. The results obtained in this study are in accordance with previously reported data on strong adsorption capacity of tannin compounds toward Pb2+ ions in comparison to other metal [49,50].
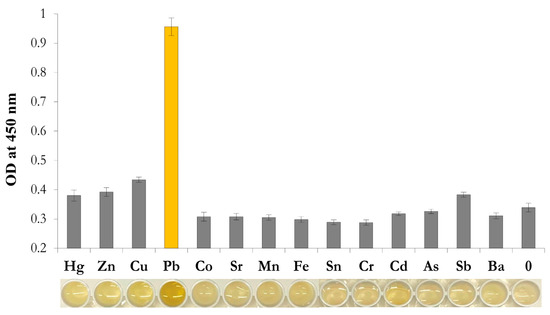
Figure 7.
Selectivity of the TA-capped AuNPs toward various metal ions with a concentration of 100 ng·mL−1. (N = 3). Insert: images of reaction media after testing the listed metal ions.
3.7. Evaluation of Nanozyme-Based Colorimetric Sensor
To evaluate the practicability of the developed approach, the detection of Pb2+ was performed in drinking, spring, and tap water. It was found that there were no Pb2+ ions in spring, bottled drinking and tap water samples, therefore known concentrations of Pb2+ (25, 50, and 100 ng×mL−1) were added to them. Table 3 shows the results of Pb2+ detection in spiked water samples. The obtained recoveries were from 106.6% to 133.4% with relative standard deviation less than 8%. These results indicate good precision and accuracy of the Pb2+ detection in real water samples.

Table 3.
Colorimetric detection of Pb2+ in spiked water samples.
4. Conclusions
To sum up, a novel colorimetric sensor for Pb2+ detection in water was developed based on peroxidase-like properties of small-sized TA-capped AuNPs. It was found that TA-capped AuNPs catalyze the oxidation of TMB by H2O2 under slightly acidic environment, thus providing a simple colorimetric detection of Pb2+ ions. The detection limit of Pb2+ ions was 11.3 ng×mL−1, which is close to the value established by the WHO guidelines of drinking water quality. The developed sensor was successfully applied to determine Pb2+ in real water samples from different sources with good recovery and RSD. Compared to conventional methods for the determination of Pb2+ in water, the proposed sensor has a number of tangible advantages, such as: (1) simplicity of the nanozyme preparation without an additional stage of gold nanoparticle modification; (2) excellent analytical performance for Pb2+ detection among other interfering metal ions; (3) analysis time takes 10 min. Taken together, these benefits enable to consider the developed colorimetric sensor as one of the approaches for the determination of Pb2+ ions in aqueous media, which can be further extended for practical use.
Author Contributions
Conceptualization, K.V.S. and A.N.B.; methodology, N.S.K. and A.N.B.; software, N.S.K.; validation, N.S.K.; formal analysis, K.V.S. and N.S.K.; investigation, K.V.S. and N.S.K.; resources, A.V.Z.; data curation, A.V.Z.; writing—original draft preparation, K.V.S. and A.N.B.; writing—review and editing, K.V.S., A.V.Z. and B.B.D.; visualization, A.V.Z.; supervision, B.B.D.; project administration, B.B.D.; funding acquisition, A.N.B. and B.B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation (project # 19-44-02020, assay development) and the Ministry of Science and Higher Education of the Russian Federation (study of assay selectivity).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamed, M.; Siddiqui, M. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Daşbaşı, T.; Saçmacı, Ş.; Ülgen, A.; Kartal, Ş. A solid phase extraction procedure for the determination of Cd(II) and Pb(II) ions in food and water samples by flame atomic absorption spectrometry. Food Chem. 2015, 174, 591–596. [Google Scholar] [CrossRef]
- Sabarudin, A.; Lenghor, N.; Liping, Y.; Furusho, Y.; Motomizu, S. Automated Online Preconcentration System for the Determination of Trace Amounts of Lead Using Pb-Selective Resin and Inductively Coupled Plasma–Atomic Emission Spectrometry. Spectrosc. Lett. 2006, 39, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, X.-q.; Chen, B.; Luo, F.; Wu, X.; Jiang, D.; Luo, Z. Determination of heavy metals in soil by inductively coupled plasma mass spectrometry (ICP-MS) with internal standard method. Electron Sci Technol Appl. 2017, 4, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Dai, H.; Jin, Y. Electrochemical sensing of lead(II) by differential pulse voltammetry using conductive polypyrrole nanoparticles. Microchim. Acta 2019, 187, 23. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Berlina, A.N.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Rapid and selective electrochemical detection of pb2+ ions using aptamer-conjugated alloy nanoparticles. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric Detection of Pb2+ Using Glutathione Functionalized Gold Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Qi, L.; Shang, Y.; Wu, F. Colorimetric detection of lead (II) based on silver nanoparticles capped with iminodiacetic acid. Microchim. Acta 2012, 178, 221–227. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Dungchai, W. Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles. Talanta 2015, 132, 613–618. [Google Scholar] [CrossRef]
- Sengan, M.; Kamlekar, R.K.; Veerappan, A. Highly selective rapid colorimetric sensing of Pb2+ ion in water samples and paint based on metal induced aggregation of N-decanoyltromethamine capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118485. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Pridvorova, S.M.; Gaur, M.; Dzantiev, B.B. Rapid Visual Detection of Lead and Mercury via Enhanced Crosslinking Aggregation of Aptamer-Labeled Gold Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5489–5495. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, B.; Chen, Y. A colorimetric method for the determination of lead(II) ions using gold nanoparticles and a guanine-rich oligonucleotide. Microchim. Acta 2012, 177, 89–94. [Google Scholar] [CrossRef]
- Wang, F.; Dai, J.; Shi, H.; Luo, X.; Xiao, L.; Zhou, C.; Guo, Y.; Xiao, D. A rapid and colorimetric biosensor based on GR-5 DNAzyme and self-replicating catalyzed hairpin assembly for lead detection. Anal. Methods 2020, 12, 2215–2220. [Google Scholar] [CrossRef]
- Wang, G.; Chu, L.T.; Hartanto, H.; Utomo, W.B.; Pravasta, R.A.; Chen, T.-H. Microfluidic Particle Dam for Visual and Quantitative Detection of Lead Ions. ACS Sensors 2019, 5, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Jiang, X. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale 2011, 3, 1421–1433. [Google Scholar] [CrossRef]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Pridvorova, S.M.; Zherdev, A.V.; Dzantiev, B.B. Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions. Chemosensors 2021, 9, 290. [Google Scholar] [CrossRef]
- Berlina, A.; Sharma, A.K.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Colorimetric Determination of Lead Using Gold Nanoparticles. Anal. Lett. 2014, 48, 766–782. [Google Scholar] [CrossRef]
- Wong, E.L.S.; Vuong, K.Q.; Chow, E. Nanozymes for Environmental Pollutant Monitoring and Remediation. Sensors 2021, 21, 408. [Google Scholar] [CrossRef]
- BineshUnnikrishnana, C.-W.; Chu, H.-W.; Huang, C.-C. A review on metal nanozyme-based sensing of heavy metal ions: Challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123397. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, H.; Zhao, Q.; Xing, L.; Zheng, X.; Wang, W.; Zhao, Y.; Yu, Y.; Hu, L.; Yao, W. Recent developments of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst 2020, 145, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, Y.; Yang, Y.; Liu, B.; Wu, Y. A facile colorimetric sensor for ultrasensitive and selective detection of Lead(II) in environmental and biological samples based on intrinsic peroxidase-mimic activity of WS2 nanosheets. Anal. Chim. Acta 2020, 1106, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Choi, J.-S.; Kwon, J. Gold nanozyme-based paper chip for colorimetric detection of mercury ions. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-F.; Zhang, Y.-Y.; Wang, L.-Y.; Jin, Z.-Y.; Shao, G. Colorimetric assay for the simultaneous detection of Hg2+ and Ag+ based on inhibiting the peroxidase-like activity of core–shell Au@Pt nanoparticles. Anal. Methods 2017, 9, 4363–4370. [Google Scholar] [CrossRef]
- Lien, C.-W.; Tseng, Y.-T.; Huang, C.-C.; Chang, H.-T. Logic Control of Enzyme-Like Gold Nanoparticles for Selective Detection of Lead and Mercury Ions. Anal. Chem. 2014, 86, 2065–2072. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Park, S.H.; Kim, G.; Kim, D.-H.; Ahn, D.; Kim, Y.M.; Kwon, S.J.; Yoon, S.-Y.; Kang, H.J.; Chung, S.J. Metal-induced redshift of optical spectra of gold nanoparticles: An instant, sensitive, and selective visual detection of lead ions. Int. Biodeterior. Biodegrad. 2019, 144. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2019, 122, 115754. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Huang, F.-F.; Lin, Y.-W. Fluorescent Detection of Lead in Environmental Water and Urine Samples Using Enzyme Mimics of Catechin-Synthesized Au Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 1503–1509. [Google Scholar] [CrossRef]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In Situ Synthesis of Metal Nanoparticles and Selective Naked-Eye Detection of Lead Ions from Aqueous Media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Cruz, B.H.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Heavy Metal Binding by Tannic Acid: A Voltammetric Study. Electroanalysis 2000, 12, 1130–1137. [Google Scholar] [CrossRef]
- Zhan, K.; Li, Z.; Chen, J.; Hou, Y.; Zhang, J.; Sun, R.; Bu, Z.; Wang, L.; Wang, M.; Chen, X.; et al. Tannic acid modified single nanopore with multivalent metal ions recognition and ultra-trace level detection. Nano Today 2020, 33, 100868. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, M.; Wang, Y. Using gallic acid-modified gold nanoassemblies to detect the Pb2+ of tea. Anal. Methods 2012, 4, 3570–3574. [Google Scholar] [CrossRef]
- Long, Y.J.; Li, Y.F.; Liu, Y.; Zheng, J.J.; Tang, J.; Huang, C.Z. Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 2011, 47, 11939–11941. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Kannan, P.; Lin, Z.; Qiu, B.; Guo, L. Gold Nanorods as Colorful Chromogenic Substrates for Semiquantitative Detection of Nucleic Acids, Proteins, and Small Molecules with the Naked Eye. Anal. Chem. 2016, 88, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Held, K.D.; Sylvester, F.C.; Hopcia, K.L.; Biaglow, J.E. Role of Fenton Chemistry in Thiol-Induced Toxicity and Apoptosis. Radiat. Res. 1996, 145, 542–553. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wang, H.; Xi, J.; Liu, Q.; Meng, X.; Duan, D.; Gao, L.; Yan, X. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 2017, 53, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Josephy, P.D.; Eling, T.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [CrossRef]
- Noh, K.-C.; Nam, Y.-S.; Lee, H.-J.; Lee, K.-B. A colorimetric probe to determine Pb2+ using functionalized silver nanoparticles. Anal. 2015, 140, 8209–8216. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, R.; Jiang, Q.; Xiong, X.; Deng, S. Colorimetric Detection of Lead Ion Based on Gold Nanoparticles and Lead-Stabilized G-Quartet Formation. J. Biomed. Sci. Eng. 2015, 08, 7. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, E.; Pradhan, N. Metal-induced aggregation of valine capped gold nanoparticles: An efficient and rapid approach for colorimetric detection of Pb2+ ions. Sci. Rep. 2017, 7, 9278. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-J.; Shi, M.-R.; Wang, L.-Y.; Peng, C.-F.; Wei, X.-L. Colorimetric determination of Pb2+ ions based on surface leaching of Au@Pt nanoparticles as peroxidase mimic. Microchim. Acta 2020, 187, 255. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, Y.; Miao, L.; Xin, J.; Wu, A. The colorimetric detection of Pb2+ by using sodium thiosulfate and hexadecyl trimethyl ammonium bromide modified gold nanoparticles. Dalton Trans. 2013, 42, 5485–5490. [Google Scholar] [CrossRef]
- Okoli, B.J.; Shilowa, P.M.; Anyanwu, G.O.; Modise, J.S. Removal of Pb2+ from Water by Synthesized Tannin Resins from Invasive South African Trees. Water 2018, 10, 648. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Shao, P.; Zhang, K.; Yang, L.; You, D.; Shi, H.; Pavlostathis, S.G.; Lai, W.; Liang, D.; Luo, X. Tannic acid-based adsorbent with superior selectivity for lead(II) capture: Adsorption site and selective mechanism. Chem. Eng. J. 2019, 364, 160–166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).