Applications of the Photoionization Detector (PID) in Occupational Hygiene. Estimation of Air Changes per Hour in Premises with Natural Ventilation
Abstract
1. Introduction
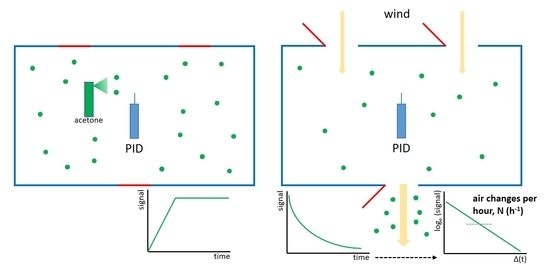
2. Materials and Methods
2.1. Selection of the Best Compound
2.2. Use of Acetone and a PID to Calculate the ACH in Different Ventilation Conditions
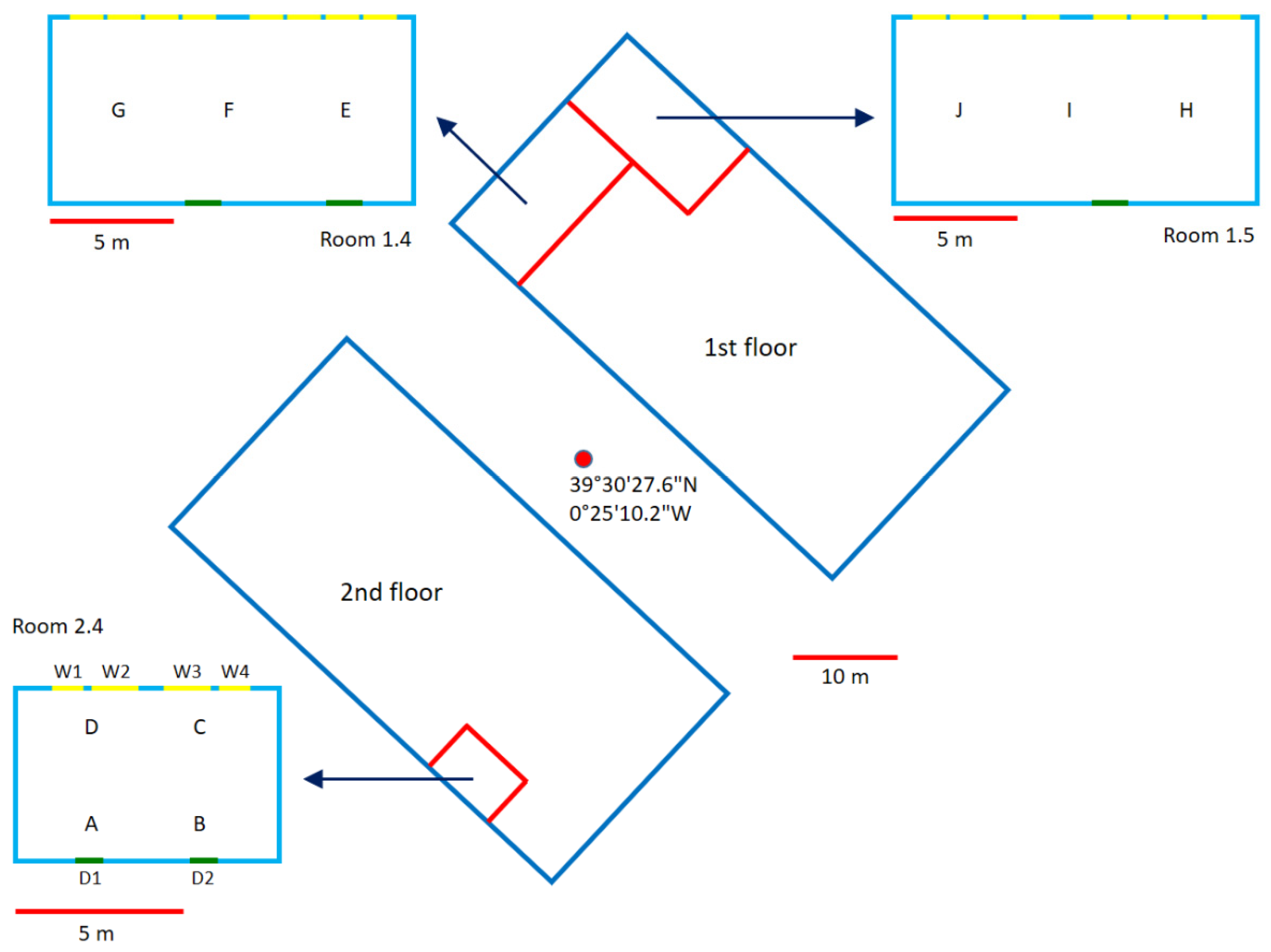
2.3. Use of Acetone and a PID to Calculate the ACH in Two Different Classrooms
3. Results
3.1. Selection of the Best Compound
3.2. Use of Acetone and a PID to Calculate the ACH
- (a)
- One window opened (W1, 0.80 m2) and the rest of the windows and doors closed. This study represents one of the worst possible situations for the ventilation of a room. The mass of evaporated acetone was 32.60 g (ambient concentration of 310.5 mg/m3). The results in the four corners are almost identical (Table 4) and provide an ACH value in this situation of about 2 h−1 (slopes of the regression lines, 1.98–2.05 h−1), with high precision (1.0–1.5%). The homogeneity of the results indicates that, in a small room like this, even small ventilation produces a high internal mixing. From a hygienic point of view, if 14 L/s/person is required, and eight persons work in this space, a minimum value of 4 ACH must be fulfilled. This value is then smaller and, if it represents the unique ventilation source, there exists a potential risk for the people staying inside. It was also the assay made with the lower external wind speed. After one hour, acetone concentration had only seen a sevenfold decrease. The wind speed through the window is estimated to be about 0.072 m/s, taking the sampling position D, the closest to the open window. At the moment of the measurement, the hourly mean external conditions were: wind speed = 0.2 m/s and wind direction = 167°.
- (b)
- Two windows opened (W1 and W4, 1.60 m2), and the rest of the windows and doors were closed. Therefore, the ventilation of the room should increase because this disposition causes an air gust inside the room. The mass of evaporated acetone was 38.20 g (ambient concentration of 364 mg/m3). The results on the four corners are similar, but minor differences can be identified (Table 4). The ACH values in this situation increase with regard to the previous assay (3.29–3.49 h−1). At the beginning of the assay, the concentrations of the measured points located near the closed doors remain almost unchanged, indicating a small air exchange at this period. However, after 3 min, a linear tendency is obtained, again showing a high internal mixing. In position D (close to one of the opened windows), some small deviations were detected, leading to the worst regression line. The ACH value is still small and does not meet the standard requirements. Acetone concentration decreased ten times after about 35 min. The wind speed through the open windows is estimated to be about 0.24–0.26 m/s, when taking the closest sampling positions C and D. At the moment of the measurement, the hourly mean external conditions were: wind speed = 1.8 m/s and wind direction = 90°.
- (c)
- One window (W4, 0.80 m2) and one door opened (facing, D2, 1.75 m2), the rest of the windows and the other door closed. With this distribution, the ventilation rate of the room should increase with respect to situation (b) because the air gust crosses the room diagonally. The mass of evaporated acetone was 42.73 g (ambient concentration of 407 mg/m3). The results on the four corners are again similar (Table 4). As expected, the ACH values in this situation increase, and are almost twice those of the previous assay (6.22–6.32 h−1). This ACH value meets the standard requirements. The acetone concentration decreased ten times after about 25 min. The wind speed through the open windows was estimated to be about 0.23 m/s, taking the closest sampling position C and, for the open door, about 0.105 m/s, taking the closest sampling position B. At the moment of the measurement, the hourly mean external conditions were: wind speed = 1.4 m/s and wind direction = 344°.
- (d)
- One window (W4, 0.80 m2) and one door opened (crossed, D1, 1.75 m2), the rest of the windows and the other door closed. The mass of evaporated acetone was 38.73 g (ambient concentration of 369 mg/m3). The differences between ACH values in this situation are now slightly large (5.45–6.25 h−1, Table 4), but all of them meet the standard requirements. The highest value for ACH was obtained near the open door, whereas the lower values were calculated near the closed door and the open window. Acetone concentration decreased by ten times after about 22 min. The wind speed through the open windows is estimated to be about 0.23 m/s, taking the closest sampling position C and, for the open door, about 0.09 m/s, taking the closest sampling position B. At the moment of the measurement, the hourly mean external conditions were: wind speed = 1.7 m/s and wind direction = 261°.
- (e)
- All windows (W1, W2, W3, W4, 3.96 m2) and doors (D1, D2, 3.49 m2) were opened. This distribution provided the highest possible ventilation. Because of this, the mass of evaporated acetone increased to 50.56 g (ambient concentration of 481.5 mg/m3). The results on the four corners were again similar (Table 4). The fast decrease in acetone concentration provided less precise data than those in the other cases. As was expected, the ACH values in this situation were the highest and almost triplicate the previous assay (16.2–16.7 h−1), but with those high ACH values, less precise results were obtained. This behavior is similar to that exposed in assay (d); door A and the window D were the zones with higher ACH values, and doors B and C were the zones with lower ACH values (the air moves in parallel, and this movement is preferred for the A-D direction more than in the B–C direction). This ACH value exceeds by far the minimum standard requirements. Acetone concentration decreased ten times after about 6–7 min. The wind speed through the open windows is estimated to be about 0.12 m/s, taking the closest sampling positions C and D, and for the open doors, about 0.12–0.13 m/s, taken the closest sampling positions A and B. These window speed values were smaller than before because ACH increased by more than 2.5 times, but the surface for windows increased even more (by five times, because central windows are bigger than extremity windows). At the moment of the measurement, the hourly mean external conditions were: wind speed = 1.4 m/s and wind direction = 95°.
3.3. Use of Acetone and a PID to Calculate the ACH in Two Different Classrooms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.-C. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. IJERPH 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed]
- Infection Prevention and Control during Health Care When Coronavirus Disease (COVID-19) Is Suspected or Confirmed. World Health Organization, 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2021.1 (accessed on 19 November 2021).
- Park, S.; Choi, Y.; Song, D.; Kim, E.K. Natural Ventilation Strategy and Related Issues to Prevent Coronavirus Disease 2019 (COVID-19) Airborne Transmission in a School Building. Sci. Total. Environ. 2021, 789, 147764. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 1027/2007, de 20 de Julio, Por el Que se Aprueba el Reglamento de Instalaciones Térmicas en los Edificios. Boletín Oficial del Estado (BOE), 2007. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2007-15820 (accessed on 19 November 2021).
- Minguillón, M.C.; Querol, X.; Felisi, J.M.; Garrido, T. Guía Para Ventilación de las Aulas CSIC; CSIC: Madrid, Spain, 2020. [Google Scholar] [CrossRef]
- Sherman, M.H. Tracer-Gas Techniques for Measuring Ventilation in a Single Zone. Build. Environ. 1990, 25, 365–374. [Google Scholar] [CrossRef]
- Almeida, R.M.S.F.; Barreira, E.; Moreira, P. A Discussion Regarding the Measurement of Ventilation Rates Using Tracer Gas and Decay Technique. Infrastructures 2020, 5, 85. [Google Scholar] [CrossRef]
- Laussmann, D.; Helm, D. Chapter 14. Air Change Measurements Using Tracer Gases. In Chemistry, Emission Control, Radioactive Pollution and Indoor Air Quality; Mazzeo, N.A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 365–406. ISBN 978-953-307-316-3. [Google Scholar] [CrossRef]
- Cui, S.; Cohen, M.; Stabat, P.; Marchio, D. CO2 Tracer Gas Concentration Decay Method for Measuring Air Change Rate. Build. Environ. 2015, 84, 162–169. [Google Scholar] [CrossRef]
- Remion, G.; Moujalled, B.; El Mankibi, M. Review of Tracer Gas-Based Methods for the Characterization of Natural Ventilation Performance: Comparative Analysis of Their Accuracy. Build. Environ. 2019, 160, 106180. [Google Scholar] [CrossRef]
- Paralovo, S.L.; Spruyt, M.; Lauwers, J.; Swinnen, R.; Lazarov, B.; Stranger, M.; Laverge, J. Developing a New Passive Tracer Gas Test for Air Change Rate Measurement. Int. J. Vent. 2020, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Fu, L.; Chen, C.; You, R.; Ren, W. Rapid Field Measurement of Ventilation Rate Using a Quartz-Enhanced Photoacoustic SF6 Gas Sensor. Meas. Sci. Technol. 2020, 31, 085105. [Google Scholar] [CrossRef]
- Coy, J.D.; Bigelow, P.L.; Buchan, R.M.; Tessari, J.D.; Parnell, J.O. Field Evaluation of a Portable Photoionization Detector for Assessing Exposure to Solvent Mixtures. AIHAJ–Am. Ind. Hyg. Assoc. 2000, 61, 268–274. [Google Scholar] [CrossRef]
- The PID Handbook. Theory and Applications of Direct-Reading Photoionization Detectors, 3rd ed.; RAE Systems Inc.: San Jose, CA, USA, 2013; ISBN 0-9768162-1-0. [Google Scholar]
- Instituto Nacional de Seguridad y Salud en el Trabajo, INSST. Límites de Exposición Profesional para Agentes Químicos. 2021. Available online: https://www.insst.es/el-instituto-al-dia/limites-exposicion-profesional-agentes-quimicos (accessed on 19 November 2021).
- Keil, C.B.; Ten Berge, W.F.; American Industrial Hygiene Association (Eds.) Mathematical Models for Estimating Occupational Exposure to Chemicals; AIHA Press: Fairfax, VA, USA, 2000; ISBN 978-0-932627-99-5. [Google Scholar]
- The National Institute for Occupational Safety and Health (NIOSH) Table of Immediately Dangerous to Life or Health Concentrations (IDLH) 1994. Available online: https://www.cdc.gov/niosh/idlh/intridl4.html (accessed on 19 November 2021).
- Lidwell, O.M. The Evaluation of Ventilation. J. Hyg. 1960, 58, 297–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Technical Committe ISO/TC 163. “Thermal Performance and Energy Use in the Built Environment” ISO 12569:2017. Thermal Performance of Buildings and Materials. Determination of Specific Airflow Rate in Buildings. Tracer Gas Dilution Method; CEN-CENELEC: Brussels, Belgium, 2017. [Google Scholar]
- Persily, A. What We Think We Know about Ventilation. Int. J. Vent. 2006, 5, 275–290. [Google Scholar] [CrossRef]
- Few, J.; Elwell, C. Measuring the Ventilation Rate in Occupied Buildings and Adapting the CO2 Tracer Gas Technique. In Proceedings of the 40th AIVC–8th TightVent–6th venticool Conference, Ghent, Belgium, 15–16 October 2019; Available online: https://www.aivc.org/resource/measuring-ventilation-rate-occupied-buildings-and-adapting-co2-tracer-gas-technique?volume=38539 (accessed on 19 November 2021).
- Mumovic, D.; Davies, M.; Ridley, I.; Altamirano-Medina, H.; Oreszczyn, T. A Methodology for Post-Occupancy Evaluation of Ventilation Rates in Schools. Build. Serv. Eng. Res. Technol. 2009, 30, 143–152. [Google Scholar] [CrossRef]
- Howard-Reed, C.; Wallace, L.A.; Ott, W.R. The Effect of Opening Windows on Air Change Rates in Two Homes. J. Air Waste Manag. Assoc. 2002, 52, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Song, D. How to Quantify Natural Ventilation Rate of Single-Sided Ventilation with Trickle Ventilator? Build. Environ. 2020, 181, 107119. [Google Scholar] [CrossRef]
- Saha, C.K.; Ammon, C.; Berg, W.; Loebsin, C.; Fiedler, M.; Brunsch, R.; von Bobrutzki, K. The Effect of External Wind Speed and Direction on Sampling Point Concentrations, Air Change Rate and Emissions from a Naturally Ventilated Dairy Building. Biosyst. Eng. 2013, 114, 267–278. [Google Scholar] [CrossRef]

| Substance | CAS Number | Vapor Pressure, 20 °C, kPa | Boiling Point, °C | Exposure Limit Values (mg/m3), TLV |
|---|---|---|---|---|
| Cyclohexane | 110-82-7 | 10.4 | 81 | 700 |
| Ethylbenzene | 100-41-4 | 0.95 | 136 | 884 |
| Ethanol | 64-17-5 | 5.8 | 78 | 1910 (STEL) |
| Isopropanol | 67-63-0 | 4.3 | 83 | 500 |
| Acetone | 67-64-1 | 24 | 56 | 1210 |
| Ethyl acetate | 141-78-6 | 9.69 | 77 | 734 |
| Tetrachloroethylene | 127-18-4 | 1.9 | 121 | 275 |
| Substance | n | Calibration Equation | Correlation Coefficient, r |
|---|---|---|---|
| Cyclohexane | 60 | S = (0.28 ± 0.04) + (0.0418 ± 0.0003) · C | 0.9989 |
| Ethylbenzene | 25 | S = (−0.11 ± 0.05) + (0.0937 ± 0.0019) · C + (−3.76 ± 0.16) · 10−4 · C2 | 0.9989 |
| Ethanol | 51 | S = (0.220 ± 0.006) + (0.01250 ± 0.00011) · C + (−2.04 ± 0.04) · 10−5 · C2 | 0.984 |
| Isopropanol | 45 | S = (0.589 ± 0.014) + (0.0253 ± 0.0003) · C + (−6.73 ± 0.15) · 10−5 · C2 | 0.9990 |
| Acetone | 61 | S = (0.66 ± 0.09) + (0.0796 ± 0.0005) · C | 0.9987 |
| Ethyl acetate | 65 | S = (1.234 ± 0.016) + (0.0113 ± 0.0009) · C | 0.9981 |
| Tetrachloroethylene | 16 | S = (0.43 ± 0.04) + (0.0372 ± 0.0011) · C | 0.994 |
| Location A | n | Linear Equation | Correlation Coefficient, r |
|---|---|---|---|
| a | 30 | loge S(t) = 3.533 − 2.052 · (t − ti) (h) EN = 0.028 | −0.997 |
| b | 36 | loge S(t) = 3.205 − 3.382 · (t − ti) (h) EN = 0.099 | −0.986 |
| c | 33 | loge S(t) = 3.970 − 6.315 · (t − ti) (h) EN = 0.132 | −0.993 |
| d | 30 | loge S(t) = 3.458 − 5.986 · (t − ti) (h) EN = 0.211 | −0.983 |
| e | 15 | loge S(t) = 3.41 − 16.60 · (t − ti) (h) EN = 1.04 | −0.975 |
| A | B | C | D | |
|---|---|---|---|---|
| a | 2.05 ± 0.03 | 2.04 ± 0.03 | 1.99 ± 0.03 | 1.98 ± 0.02 |
| b | 3.38 ± 0.10 | 3.43 ± 0.10 | 3.32 ± 0.09 | 3.58 ± 0.13 |
| c | 6.32 ± 0.13 | 6.30 ± 0.09 | 6.20 ± 0.13 | 6.12 ± 0.08 |
| d | 5.99 ± 0.21 | 5.45 ± 0.25 | 5.45 ± 0.21 | 6.25 ± 0.33 |
| e | 16.6 ± 1.0 | 16.2 ± 1.3 | 16.2 ± 1.3 | 16.7 ± 0.9 |
| Classroom 1.4 | E | F | G |
| 1 | 19.5 ± 1.2 | 18.7 ± 1.5 | 19.8 ± 1.4 |
| 2a | 25.4 ± 1.2 | 23.4 ± 1.4 | 22.9 ± 1.0 |
| 2b | 27.0 ± 1.0 | 27.5 ± 1.1 | 31.1 ± 1.3 |
| Classroom 1.5 | H | I | J |
| 1 | 16.4 ± 1.2 | 15.9 ± 1.0 | 16.9 ± 0.9 |
| 2a | 35.2 ± 2.7 | 38.0 ± 2.6 | 43.8 ± 2.5 |
| 2b | 35.4 ± 2.5 | 33.6 ± 2.2 | 35.4 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeso-García, M.D.; Esteve-Turrillas, F.A.; Verdú-Andrés, J. Applications of the Photoionization Detector (PID) in Occupational Hygiene. Estimation of Air Changes per Hour in Premises with Natural Ventilation. Chemosensors 2021, 9, 331. https://doi.org/10.3390/chemosensors9120331
Maeso-García MD, Esteve-Turrillas FA, Verdú-Andrés J. Applications of the Photoionization Detector (PID) in Occupational Hygiene. Estimation of Air Changes per Hour in Premises with Natural Ventilation. Chemosensors. 2021; 9(12):331. https://doi.org/10.3390/chemosensors9120331
Chicago/Turabian StyleMaeso-García, María D., Francesc A. Esteve-Turrillas, and Jorge Verdú-Andrés. 2021. "Applications of the Photoionization Detector (PID) in Occupational Hygiene. Estimation of Air Changes per Hour in Premises with Natural Ventilation" Chemosensors 9, no. 12: 331. https://doi.org/10.3390/chemosensors9120331
APA StyleMaeso-García, M. D., Esteve-Turrillas, F. A., & Verdú-Andrés, J. (2021). Applications of the Photoionization Detector (PID) in Occupational Hygiene. Estimation of Air Changes per Hour in Premises with Natural Ventilation. Chemosensors, 9(12), 331. https://doi.org/10.3390/chemosensors9120331