Abstract
A planar solid-state ammonium-selective electrode, employing a composite mediator layer of graphite particles embedded in a polyvinyl butyral matrix on top of an inkjet-printed silver electrode, is presented in this paper. The effect of graphite powder mass fraction on the magnitude of the potentiometric response of the sensor was systematically verified using a batch-mode and a flow injection measurement setup. Under steady-state conditions, the paper electrode provided a Nernstian response of 57.30 mV/pNH4 over the concentration range of 10−5 M to 10−1 M with a detection limit of 4.8 × 10−6 M, while the analytical performance of the array in flow mode showed a narrower linear range (10−4 M to 10−1 M; 60.91 mV/pNH4 slope) with a LOD value of 5.6 × 10−5 M. The experimental results indicate that the prepared electrode exhibited high stability and fast response to different molar concentrations of ammonium chloride solutions. The pH-response of the paper NH4-ISE was also investigated, and the sensor remained stable in the pH range of 2.5–8.5. The potentiometric sensor presented here is simple, lightweight and inexpensive, with a potential application for in-situ analysis of environmental water samples.
1. Introduction
Desirable properties of instrumental analytical methods are high accuracy, selectivity for the analyte of interest, low-cost, reliability and ease of use. Ion-selective electrodes (ISEs) are characterised by such properties and are therefore an established tool for the direct determination of analytes in often complex matrices. One of the main advantages of ISEs is that they operate in a turbid solution without any sample pretreatment, providing a fast analytical response [1]. Potentiometry is a standard technique used in analytical laboratories around the world, and ion-selective electrodes for more than 50 different ions are commercially available. Among these sensors, ammonium ion-selective electrodes (NH4-ISEs) have proven to be an indispensable sensing tool in three different fields: (i) environmental water analysis [2,3], (ii) in the food industry for the detection of food freshness [4] and (iii) monitoring ammonium concentration in biologically important fluids for clinical diagnostics as well as for the purposes of sports practice [5].
The development of solid-contact ion-selective electrodes (SC-ISEs) has opened new possibilities in the fabrication of miniaturised portable devices of various sizes and shapes [6,7]. Historically, the first proposed concept of sensors without internal solution—coated wire electrodes—was extremely simple but, mainly because of the potential instability, not reliable [8]. To overcome this problem, solid transducer layers consisting of redox-polymers [9] or nanostructured materials [10] were introduced into the domain of SC-ISE sensor design. The transducer layer is usually built over the electrode material or over a noble metal contact. Generally, the conversion of ionic to electronic charge at the transducer-membrane interface occurs by a redox reaction (redox capacitance), or by charge generation (double-layer capacitance), both enabled by functional materials. For ammonium-selective electrodes, the redox capacitance process is enabled with the usage of conducting polymers such as poly(3-octylthiophene) [11], polypyrrole [12] or polyaniline [13]. Generation of the electric double-layer capacitance is achieved with the implementation of nanostructured material, mostly carbon nanotubes (CNTs) [5,14,15]. On the one hand, conjugated polymers suffer in terms of light and pH sensitivity, inadequate hydrophobicity and could be easily subjected to the redox reactions with interfering species. On the other hand, CNTs possess the advantages of superhydrophobicity and light insensitivity, but hardly form stable dispersions in many solvents, making the deposition of a homogeneous layer challenging. Thus, CNTs needs to be modified, but the presence of stabilisers may alter the membrane properties, leading to membrane detachment [16]. In addition to the solid contact used, the electrochemical performance of the ammonium-selective solid-state electrode is also highly dependent on the composition of the membrane and substrate employed in the sensor design. In terms of detection mechanism, ionophore-based potentiometric sensors with a polyvinyl chloride (PVC) membrane are the most commonly used ammonium sensor class [17].
In summary, diverse solid-state systems can be fabricated by varying the choice of ion-to-electron functional material, membrane solution composition and internal contact. In recent years, new directions in solid-state ISEs have focused on the fabrication of multicomponent ammonium-sensing architectures such as microfluidic chips [18], electronic tongues [19] and tattoo platforms [20]. Nowadays, all-solid-state ion-selective electrodes (ASS-ISEs), fabricated in a planar and flexible mode, are recognised as a new generation in the development of potentiometric sensors aiming at the development of integrated devices, especially wearable chemical sensors.
In this paper we present a low-cost, easy-to-fabricate, disposable ammonium-sensing platform made from conventional and commonly available materials. In the sensor design, a transducer consisting of a non-conductive polymer doped with microscopic graphite particles was “sandwiched“ between an inkjet printed inner silver electrode and an ammonium-sensitive membrane. Polyvinyl butyral (PVB) is a well-known thermoplastic, mostly used for applications that requires strong adhesion to many surfaces and flexibility [21]. In addition, PVB has been used in the construction of solid-state reference electrodes [22] but has never been used to formulate solid contacts in ion-selective electrodes. The potentiometric sensor is fabricated on paper, a low cost and widely available substrate [23], and can be easily integrated into more structured devices. Additionally, the choice of materials used for the design of the ammonium ISE presented in this study is explained in detail in each respective section.
2. Materials and Methods
2.1. Reagents
Ammonium chloride (NH4Cl) and potassium hexacyanoferrate [K3Fe(CN)6] were purchased from Kemika (Zagreb, Croatia). Polyvinyl chloride of high molar mass is obtained from Fluka (Buchs, Switzerland). Tetrahydrofuran (THF, 99.5% wt), polyvinyl butyral, bis(2-ethylhexyl) sebacate (DOS, 97.0% wt), ammonium ionophore I (Nonactin, 95.0% wt) and graphite powder (synthetic) were purchased from Sigma–Aldrich (St. Louis, MO, USA). For the preparation of Britton–Robinson (BR) buffer solutions, acetic acid (0.04 M), boric acid (0.04 M) and phosphoric acid (0.04 M) were used; all purchased from Kemika (Zagreb, Croatia). All chemicals were of analytical grade and used as received. Solutions were prepared by double destilled deionised water (conductivity 0.060 µS cm, MilliQ, Millipore, Burlington, MA, USA).
For ink-jet printing, the silver nanoparticle-based conductive ink JS-B25P was obtained from NovaCentrix (Austin, TX, USA), while the paper substrate (photo quality Premium satin RC glossy paper) was purchased from Orink (Zhuhai, China).
2.2. Preparation of a Conventional Internal Electrolyte Electrode
The ammonium-selective membrane mixture was prepared by dissolving 90.16 mg of PVC in 3.0 mL of THF, after which a neutral carrier (Nonactin, 187.04 mg) and a plasticiser (DOS, 250.0 µL) were added. This mixture was placed in a glass ring (d = 26 mm) resting on a clean glass plate. After evaporation of the solvent, the resulting membrane was peeled from the glass surface and disks of 6 mm were cut out. The ammonium ion-selective membrane was mounted in Philips IS 550 electrode body (The Netherlands) for batch-up potentiometric measurements. A 10 mM NH4Cl solution was used as the internal filling electrolyte.
2.3. Preparation of Solid-Contact Ammonium-Selective Electrodes
The ammonium-selective membrane consisted of ammonium ionophore I (1.0 wt %), PVC (32.2 wt %) and DOS (66.8 wt %); total 75.0 mg of the membrane components were dissolved in 3.0 mL THF. Aliquots of this mixture (25.0 µL) were applied to obtain PVC-based membrane layers on the surface of: (i) bare graphite disk electrode, (ii) solid contact-modified graphite disk electrode and (iii) solid-contact modified inkjet-printed planar silver electrode. The solid contact used to modify electrodes (ii) and (iii) was a composite made of 50–65 wt % graphite powder encapsulated within polyvinyl butyral resin. Deposition of the graphite-PVB transducer layer was conveniently achieved by dissolving in ethanol and simple drop-casting method (disk electrodes) and a dip-coating method (planar electrodes).
In order to fabricate an internal silver electrode, conductive nanosilver ink was loaded into the cartridge of a modified Epson Stylus D92 printer, and deposited onto paper substrate precoated with a polycation layer. The print settings were set as in our previous work [24,25]. Due to the presence of the polymer layer, the conversion of the inkjet-printed nanosilver suspension into its conductive form was performed by a room temperature sintering method. The obtained flexible paper silver electrode was further modified to obtain an ammonium-sensitive surface.
2.4. Electrochemical Measuring System
The cell assembly for the batch potentiometric measurements was as follows: Ag|AgCl|3 M KCl||2 M NaNO3||sample||selected working electrode. Conventional inner-filling electrode, disk graphite and silver inkjet-printed electrodes were tested for the development of ammonium-sensitive sensors.
The potentiometric flow cell (V = 0.2 mL) comprised of a flow Ag|AgCl|3 M KCl||2 M NaNO3 reference electrode, working ammonium-selective paper electrode, and inlet and outlet connections for the prepared solutions. To obtain the response of the prepared planar sensor, the solutions of increasing NH4+ activity, prepared in a 10 mM CaCl2, were pumped at 0.833 mL/min through the fluidics setup, while the potential was continuously recorded (Figure 1). Transport of the analyte solution to the ammonium sensor cell in a continuous flow mode was performed using a peristaltic pump. Sample injection into the carrier stream (10−6 M NH4+ in 10 mM CaCl2) for injection flow analysis was done with a syringe using an injector valve (Rheodyne model 7127) and the 100 µL sample loop.
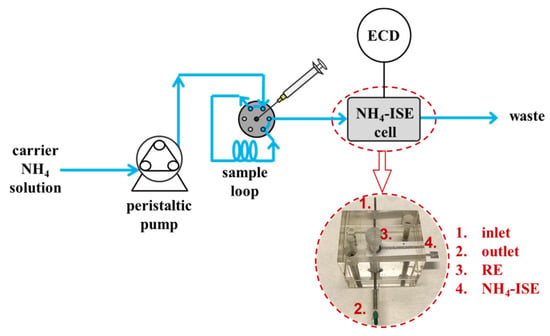
Figure 1.
Schematic presentation of the FIA device used for ammonium sensing with the potentiometric flow cell. RE—reference electrode; ECD—electrochemical detector.
Cyclic voltammetry (CV) measurements were made using EG&G PAR Polarographic Analyzer model 264A (Princeton, NJ, USA) connected to a PC for data analysis. A three-electrode electrochemical cell was used, including a graphite working electrode coated with graphite-PVB transducing layer, an Ag|AgCl|3 M KCl||2 M NaNO3 reference electrode, and a Pt auxiliary electrode. All electrochemical data were recorded at room temperature in a 10 mL electrochemical cell at a scan rate of 50 mV/s. Measurements were performed in a 5 mM [K3(Fe(CN)6] solution prepared in 0.1 M KCl primary electrolyte.
The electrochemical impedance spectroscopy (EIS) study was performed with an EG&G PAR Model 263A Polarographic Analyzer (Princeton, NJ, USA) connected to a frequency response detector applying single-sine technique. The same electrochemical cell, used in the CV study, was also used for the impedance spectra measurements. All experiments were carried out at DC potential of E = 310 mV; AC sine wave with 10 mV amplitude was applied. Impedance spectra were recorded in the frequency range between 1 mHz to 1 MHz.
2.5. Electron Microscopy Characterisation
The thickness of the printed bare silver traces on a paper substrate, the silver line coated with a layer of graphite-PVB solid contact as well as with an ion-selective membrane, were imaged using a scanning electron microscope (TESCAN Vega3 Easy Probe, Brno, Czech Republic) at an electron beam energy of 10.0 keV and a SE mode. Prior to imaging, all cross-sectional samples were coated with gold and palladium plasma for 60 s.
3. Results
For the purpose of developing a selective ammonium sensing platform, various electrode arrangements were tested, starting with the fabrication of the classical inner-filling solution electrode. In conventional glass-encapsulated ion-selective electrodes, the internal electrolyte plays a role in signal transduction from the selective membrane to the central metal conductor, resulting in a straightforward signal pathway across the interface [26]. The linear range of the prepared classical sensor extends from 10−6 to 10−1 M and shows an apparent Nernstian response of 58.87 mV/pNH4 within this range (Figure S1). Thus, it can be concluded that the prepared ion-selective membrane is suitable for further potentiometric sensor development.
3.1. Effect of the Membrane Thickness
In the manufacturing process of all solid-state ISE, where the internal electrolyte solution is replaced by a rigid conductor, close contact between the selective membrane and the conductive material is crucial for ion-to-electron conduction to work correctly [27]. Graphite is an inexpensive and abundant material, with adequately low resistivity for the aforementioned purpose, and can be manufactured into a wide variety of forms, making it a popular choice as an electrode material [28]. The next step in the development of the ammonia sensing platform consisted of applying the ion-selective membrane mixture in volumes of 30 µL (electrode 1) and 50 µL (electrode 2) directly onto the surface of the graphite electrode [9]. Therefore, after drying at air conditions for 24 h, two different ammonium-selective membrane coated graphite electrodes were prepared and tested in the analyte solutions (Figure 2). The presented graphical plot clearly shows that both electrodes achieved an almost identical sub-Nernstian response of 55.39 mV/decade (electrode 1) and 55.91 mV/decade (electrode 2). Hence, the effect of membrane thickness is not significantly pronounced. However, since the electrode coated with 50 µL of the membrane mixture showed a slightly better response, it was selected for further investigation.
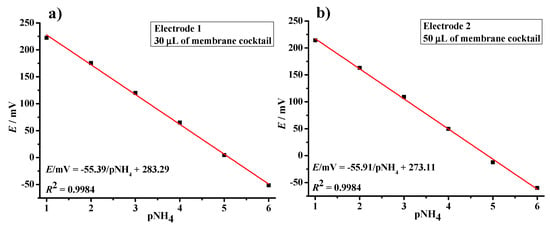
Figure 2.
Response of the graphite electrode coated with mixture of ammonium selective membrane; volumes of 30 µL (a) and 50 µL (b), respectively.
In solid-contact potentiometric sensors, it is also mandatory to avoid water ingress at the membrane-solid contact interface [17]. The formation of a thin film of water can lead to the spontaneous flow of ions between the membrane and the inner electrode, which alters the transboundary potential. Moreover, noticeable water uptake may disrupt the adhesion between the transducer-membrane interface, leading to delamination of the membrane. To investigate the phenomena of water uptake at the selective membrane-graphite electrode interface, the response of the prepared sensor was measured after soaking in deionised water for 24 h (Figure S2). It can be clearly seen that the direction of the slope did not change significantly. The electrode coated with 50 µL of the membrane mixture showed a linear response in the 10−1 to 10−6 M concentration range, with a sensitivity of 55.02 mV/decade. The result is slightly lower than the selectivity obtained with the sensor stored under dry conditions, indicating that a miniscule uptake of water occurred, although of limited significance for the stability of the selective membrane within the investigated timeframe. Thus, the response of the graphite electrode coated with layer of ammonium selective membrane is similar to those obtained using classical inner-filling electrode.
3.2. Cyclic Voltammetry and Impedance Studies
In order to improve the adhesion between the PVC layer and graphite electrode, as well as the analytical performance of the ammonium selective sensor, a layer of lipophilic material in combination with conductive material was introduced between the graphite electrode and the ammonium-selective membrane. In order to select the best formulation for the development of the potentiometric sensor, four different graphite-PVB formulations were prepared, layered over a graphite disk electrode, and tested as a transducer assembly. The bare graphite and graphite-PVB modified electrodes were electrochemically characterised using the CV and EIS techniques in a 5 mM [K3Fe(CN)6] with 0.1 M KCl as background electrolyte. Both CV and EIS measurements were performed in a three-electrode electrochemical cell (previously described in Section 2.4); results were plotted vs. reference electrode. The simple Randles cell with Warburg element was used to simulate the impedance spectra [29].
Cyclic voltammetry is a common and often the first electroanalytical technique of choice to obtain preliminary information about electrochemical reactions [30,31]. The reversible behaviour of the ferricyanide/ferrocyanide redox-couple was observed at the bare graphite electrode, with a pronounced anodic maximum at 302.7 mV, as well as a cathodic current trough at 143.4 mV (Figure 3a). The position of the peaks on the potential axis indicates a heterogeneous electrode surface consisting of edge and basal graphite planes [32], while the existence of only one distinct current wave indicates a one-electron transfer process, described as:
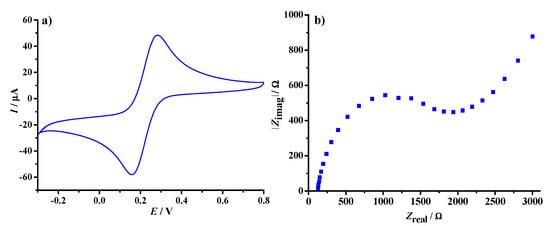
Figure 3.
Current response (a) and Nyquist plot (b) of the bare graphite electrode immersed in 5.0 mM K3[Fe(CN)6] with 0.1 M KCl solution. Cyclic voltammetric signature obtained at a scan rate of 50 mV/s; impedance spectra recorded within 1 mHz to 1 MHz and ΔE = ±10 mV.
The calculated standard electrode potential for the above process was E° = 0.223 V, which is in agreement with the value reported in the literature against Ag|AgCl|3 M KCl reference electrode [33]. The near-ideal behaviour of the hexacyanoferate(II/III) probe indicates that the graphite electrode provides fast electron-transfer rates, approaching those of bare metallic surfaces [34].
In addition to CV measurements, impedance spectroscopy is also a powerful tool for studying processes occurring at the electrode solution interface [35]. Detection methods based on impedance measurements have the potential to identify electron transfer at high frequency and detect low analyte concentrations. The Nyquist plot of the bare graphite electrode immersed in the redox probe solution is presented in Figure 3b. It contains a well-defined semicircular arc that intersects the real axis at high frequencies, followed by an extended arm in the low-frequency region. In the absence of the transducing layer, the entire surface of the bare graphite electrode is accessible to the reversible redox reaction. The sensor is thus characterised by a low-frequency capacitance and facilitates ion-to-electron transduction at the electrode/electrolyte interface.
The effect of the graphite-PVB coating, a key parameter in the development of the planar ammonium-selective sensor, is considered next and shown in Figure 4. In contrast to the bare-graphite electrode, the CV curves obtained for the graphite-PVB modified disk electrodes exhibited significantly lower current responses (Figure 4a). Coating the electrode with an asymmetric medium, in which the conductive graphite component is embedded in an insulating PVB layer, resulted in individual peaks that are reduced in size (significantly lower current responses compared to the bare graphite electrode) and widely separated. All voltammograms indicate difficult heterogeneous electron transfer, especially for the electrode layered with the conductor-insulator formulation with equal wt % (black CV curve). It can be seen that, as the modification of the bulk electrodes increases with the amount of graphite particles, the magnitude of the voltammetric peak heights also increases.
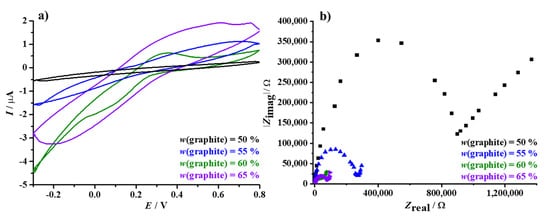
Figure 4.
Cyclic voltammograms (a) and impedance spectra (b) of the graphite disk electrode modified with a transducer layer of different wt % graphite, both recorded in the presence of [Fe(CN)6]4−/3− redox probe and 0.1 M KCl background electrolyte. CV and EIS parameters are also noted in the previous Figure.
Following the CV experiments, electrochemical impedance spectroscopy measurements were carried out using the same [Fe(CN)6]4−/3− redox probe to obtain additional information on the charge transfer processes. Nyquist plots were obtained for each step of the modification of the graphite surfaces, and are shown in Figure 4b. In contrast to the bare electrode (Figure 3b), coating the graphite electrode with the solid-contact layer resulted in an increase of the measured impedance value. The impedance spectra shown were similar in appearance, with pronounced differences in charge transfer resistances. The diameter of the semicircular arc decreased with increasing graphite content in the added transducer layer, followed by decrement in the measured value from initial 352.98 kΩ, through 84.96 kΩ and 18.78 kΩ, to the final value of 15.01 kΩ, respectively. This indicates decreasing charge transfer impedance, along with a low electrical double layer capacitance between the transducer film and the conductive substrate. The low electron transfer resistance (15.01 kΩ) of the electrode modified with graphite (65 wt %)-PVB pointed out that the fabricated sensor has the merits of high electron transfer ability, along with low resistance property. Equivalent circuit model used for simulating the impedance spectra, along with obtained modeling results, is provided in Supplementary Data (Figure S4). Therefore, the EIS result is in agreement with the CVs, and confirms good electrochemical behaviour (low impedance and high currents) of the graphite electrode coated with the PVB layer enriched with 65 wt % graphite particles and the [Fe(CN)6]4−/3− redox couple. Consequently, this formulation was chosen for further development of the planar potentiometric sensor.
3.3. Potentiometric Response of Solid Contact Electrodes
The next step in the development of the ammonium selective sensor was to apply an ion-selective membrane layer on top of the existing transducer layer, using two supporting electrode materials of different geometries: firstly a disk graphite electrode, and secondly a planar silver electrode.
3.3.1. Potentiometric Response under Steady Conditions
Graphite Disk Electrodes
This sensor is prepared using the layer-by-layer technique, where 2.5 µL of the solid contact formulation is applied first, followed by 10.0 µL of the ammonium selective membrane mixture. This particular amount of the transducer and ion-selective membrane suspensions were selected after optimizing the sensor, i.e., determining the best layer thickness parameters (Figure S3). 2.5 µL of a 50 wt % graphite-PVB mixture were selected as the basis for studying the effect of membrane thickness. A sensor fabricated on the surface of a graphite-PVB/graphite electrode coated with 10.0 µL of ammonium-selective membrane exhibited a sensitivity of 45.93 mV/pNH4.
The analytical performance of the sensor design with 10.0 µL of ammonium-selective membrane mixture applied on to the 2.5 µL volume of different transducer formulations was further tested under dry and wet (stored in water overnight) conditions (Figure 5). Notably, the sensitivity increased from 45.93 mV/pNH4, obtained for the PVB layer enriched with 50 wt % graphite particles, to the sub-Nernstian value of 54.98 mV/pNH4 for the sensor with 65 wt % conductive solid (Table 1). Compared to the response of the classical ammonium selective electrode, this sensor design exhibited a sub-Nernstian response, but the dynamic range of the sensor remained the same.
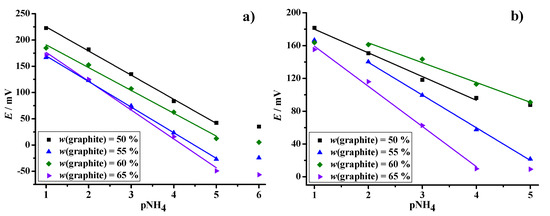
Figure 5.
Dynamic response diagrams of the graphite disk electrode modified with a transducer layer of different wt % graphite particles embedded in PVB and coated with a layer of ammonium-selective membrane. Measurements were performed in NH4Cl solution, c(NH4) = 10−1 M–10−6 M, after storage of the sensor under dry (a) and wet (b) conditions. Lines represent the linear fit of the data points, over the determined linear range.

Table 1.
Slopes of the calibration curves, after storage under dry and wet conditions, for the disk potentiometric sensors prepared with different graphite amounts.
The same trend in sensor response is seen after storing the electrode under wet conditions, only this time with significantly lower sensitivity values. More specifically, a decrease in sensitivity of 10 mV/decade was observed in all the electrodes tested, which can be attributed to the water uptake of the solid contact layer [36]. Compared to the negligible water uptake of the graphite disc electrode without solid contact (Figure S2), we see that the graphite-PVB layer is detrimental for the water uptake, which can in turn affect continuous measurements. However, we can note that increasing the graphite wt % generally improves hydrophobicity of the solid contact, thereby reducing water uptake. Overall, it can be stated that an increase in the mass fraction of graphite particles leads to an increase in sensor sensitivity under both dry and wet conditions. The ion-selective electrode stored under dry conditions showed good analytical performance and can be used for ammonium monitoring. The results also show that the response was linear for all the electrodes prepared over the investigated concentration range of 10−1 M–10−5 M. Thus, the dynamic range of the sensor does not depend on the mass fraction of graphite particles. On the other hand, storing the electrode under wet conditions leads to a significant loss of the linear range of the sensor. The ion-selective electrode, coated with 50 wt % graphite particle-PVB transducer formulation, exhibited a decrease in sensitivity in higher concentration ranges. Increasing the mass fraction of graphite particles embedded in the PVB matrix leads to a loss of sensitivity even in the lower concentration range. This study has shown that ion-to-electron solid contact (independent of the mass fraction of graphite particles) in combination with a PVC-based ion-selective membrane was not suitable for ammonium detection after storage under the wet conditions. As a water layer was formed between the solid contact and the ISM layer, the membrane structure was disturbed and the membrane became slightly delaminated.
Paper Electrode
The planar sensor was fabricated in two different ways: (i) by drop-casting (2.5 µL) the transducing graphite (65 wt %)-PVB mixture onto the inkjet-printed inner silver electrode or (ii) by dip-coating the inner contact in the above suspension. After this step, a membrane mixture of PVC, plasticiser and nonactin dispersed in THF (15.0 µL) was layered onto the decorated paper electrode using a pipette. The as-prepared planar ammonium ion-selective electrode was then integrated into an electrochemical cell for: (i) batch, and (ii) flow potentiometric analysis.
Morphological Characterization of the Planar Electrode
Paper is the most commonly used flexible substrate worldwide. From the perspective of inkjet technology, the high surface roughness of paper, together with its porous structure, can lead to absorption of the printed functional material. Moreover, the bonding between the functional material and the substrate is deficient due to its poor chemical and mechanical properties. For this reason, paper substrates are usually covered with a plastic film [37]. Therefore, for the development of a planar ammonium-selective potentiometric sensor, a paper substrate precoated with a polymer layer that allows sintering at room temperature is used. To gain insight into the sensor structure, SEM micrographs of the cross-section of the planar electrode were given in Figure 6. A paper structure with inkjet-printed silver line is shown in Figure 6a. The graphite-PVB layer is evenly distributed on the silver electrode fabricated by inkjet-printing and covers the entire inner contact area (Figure 6b). It can be seen that its structure is dense and uniform, which could allow a better bonding between the solid contact and the silver substrate. The ammonium-selective membrane forms a smooth, gel-like film over the deposited graphite-PBV transducer layer with randomly distributed pores in structure (Figure 6c). This homogeneous structure is the result of the addition of a plasticiser, which leads to the reduction of the brittleness of PVC [38].
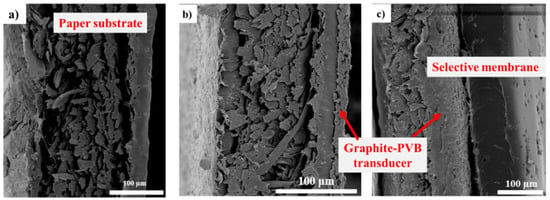
Figure 6.
SEM cross-section images: (a) paper substrate with inkjet-printed silver line (500× magnification); (b) internal silver electrode coated with graphite-PVB solid-contact (500× magnification); (c) ammonium-selective membrane/graphite-PVB/silver electrode (300× magnification).
Analytical Characterisation of the Planar Electrode
To investigate the analytical features of the potentiometric sensor, both electrodes (i.e., drop-casted and dip-coated) were tested in a dry and wet surrounding (Figure 7). To study the effects of wet conditions, the paper electrodes were immersed in a 10−3 M KCl solution for 24 h. The conditioning step is a crucial step to improve the sensor performance, since the membrane primarily does not contain ammonium ions. Thus, the lack of thermodynamic equilibrium in the electrode/solution interface can lead to a potential fluctuation when the prepared ammonium selective electrode comes into contact with the sample solution [39].
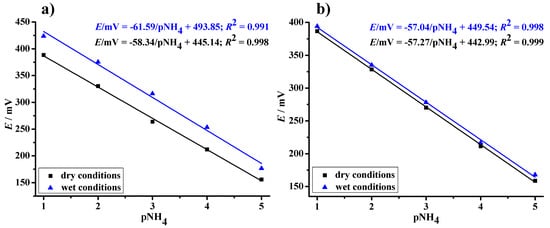
Figure 7.
Calibration curves of the planar sensor prepared by drop-casting (a) and dip-coating (b) the inkjet printed internal silver electrode into the transducing layer formulation.
The dynamic response curves of all prepared planar electrodes, obtained after measurements under both dry and wet conditions, showed linear concentration ranges from 10−1 to 10−5 M, resulting in a stable and reproducible response. It can be concluded that changing the sensor arrangement from a disk graphite substrate to a planar paper electrode does not diminish the performance of the solid-contact type sensor. It is known from the literature that PVB is a polymeric material, which is easily subjected to the incorporation of ionic salts that can further affect the polymer’s ionic conductivity and lead to an unexpected change in sensor performance. Since the sensitivity of the fabricated sensors is not impaired, we can also conclude that the pretreatment step causes a flux of ammonium ions from the solution into the PVC-based sensitive membrane and does not cause ion exchange between the transducing PVB layer and the selective membrane. Furthermore, the presence of graphite particles helps to avoid the water uptake due to their hydrophobic nature. The analytical parameters reported for the planar solid-state electrodes with a graphite-PVB layer placed between the ammonium-selective membrane and the supporting electrode compare well with those characterising classical ion-selective electrodes.
Effect of pH
The effect of buffer pH on the analytical signal of the planar ammonium sensor was investigated in Britton–Robinson buffer solution over a wide pH range (Figure 8). In buffer solutions where pH changes from 2.5 to 8.5, the response of the electrode varied slightly between 223.0 mV and 210.3 mV potential window. Since the potential change is not significantly pronounced in this range, it can be considered as the working pH range of the electrode [40]. The drift of the potential values at pH > 8.5, after which the measured potential drops abruptly, can be attributed to the formation of ammonia due to the pH change of the solution [41].
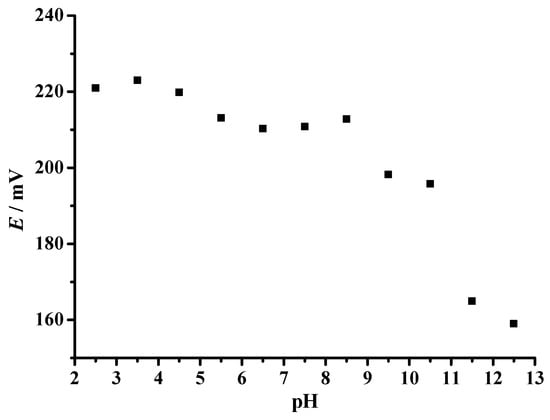
Figure 8.
Effect of pH on the response of the prepared planar ammonium selective electrode.
Effect of Interference–Selectivity
The potentiometric selectivity coefficient () indicates the ability of the electrode to respond only to the target ion i in the presence of other ions j [42]. One of the major challenges in the development of ammonium ISEs is to overcome problems associated with interfering ions in real matrices. The process to achieve a reliable sensor response via specific receptors involves extraction of the target ion from the sample into the ion-selective membrane, and unique binding between the analyte and the ionophore to form a complex. However, in real samples interfering ions compete with the primary ions, and can also be extracted into the selective membrane. Nonactin, the most commonly used ionophore in ammonium sensing devices, exhibits significant interference with potassium [43]. Therefore, the prepared ammonium electrodes were tested in view of their sensitivity towards sodium, potassium, lithium, magnesium and calcium ions. The selectivity coefficients were evaluated using both the separate solution method (SSM) and the fixed interference method (FIM). Both methods assume that the ISE response is in accordance with the Nicolsky–Eisenman [44] in Equation (2):
where Ei is the experimentally observed potential, is a constant, a represents the activity of the primary (ai) or interfering (aj) ion with the corresponding ionic charge number (zi/j). The selectivity coefficients are usually expressed as logarithmic value (), with negative values indicating an electrode preference for the primary ion over to the interfering one.
In SSM the electrode potential is measured in a solution containing only one type of potential-generating ions—either the primary or the disturbing ones [45]. This method is simple to perform but is considered unreliable because it does not reflect the real analytical situation when primary and interfering ions are present in the solution and are in thermodynamic equilibrium with the ion-selective membrane. In FIM, measurements are performed in solutions of primary ions with increasing concentration and interfering ions with constant activity. The selectivity is determined by the intercept of the extrapolated linear response of E versus the logarithm of the activity of the primary ion. This method more accurately reflects experimental conditions and is recommended by IUPAC [43]. The potentiometric selectivity responses of the prepared planar sensor, measured with separate and mixed solutions, are shown in Figure 9. In addition, the selectivity coefficients calculated using SSM and FIM are provided in Table 2.
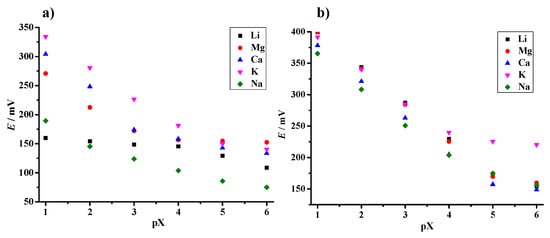
Figure 9.
Potentiometric selectivity of the planar ammonium selective electrode obtained by SSM (a) and FIM (b).

Table 2.
Potentiometric selectivity coefficients of the prepared planar NH4+-ISE based on graphite-PVB solid contact, calculated using methods of separate solutions and fixed interference, compared to the other values reported [15,41,46].
From the reported data, it is evident that the prepared planar sensor has high selectivity for ammonium ions over monovalent sodium and lithium and divalent calcium and magnesium. For these interfering ions, the selectivity coefficient values are on the order of 10−3 or less, indicating that they would not significantly disturb the detection of NH4. As expected, the lowest selectivity coefficients are those for potassium, as potassium and ammonium have similar ionic radii, thus causing greater interference. The calculated selectivity coefficients were in the range of previously reported values for nonactin-based ISEs (Table 2), which is significant taking into account the simple and inexpensive materials used to fabricate the ammonium-sensing platform.
3.3.2. Potentiometric Response under Flow Conditions
From their first appearance in the 1950s [47] to the present day, detection methods under flow conditions have introduced an entirely new concept into modern analytical chemistry [48]. These advantageous and feasible analytical techniques, characterised by a high degree of automation, satisfy the increasing demand in various applications for control and/or routine analysis. The easy miniaturisation of ion-selective electrodes of sufficient selectivity, and the design of a suitable flow-cell enable the potentiometric detection of the desired analyte. Thus, in order to obtain more accurate information on the performance of the prepared planar sensor, an analysis under flow conditions was performed in addition to the steady-state batch measurements.
Although the electrodes prepared by the drop-casting and dip-coating methods showed almost identical properties (as explained in Section 3.3.1), the dip-coating electrode was chosen for the flow measurements, due to smaller differences between the performance in dry and wet storage conditions. In this way, most of the paper electrode surface is covered with a highly hydrophobic layer, preventing possible damage of the inkjet printed internal contact and/or water ingress during successive measurements. The solid-contact ammonium ISE was incorporated into the simple flow-through system described in the Experimental Section, and the membrane selectivity was investigated using the mixed solution method. For this purpose, an aqueous CaCl2 solution was chosen because the potentiometric batch measurements showed that the interference by calcium was low (Figure 9; Table 2). Typical flow responses, obtained for a range of analyte/interference solutions in continuous and injection modes, are shown in Figure 10.
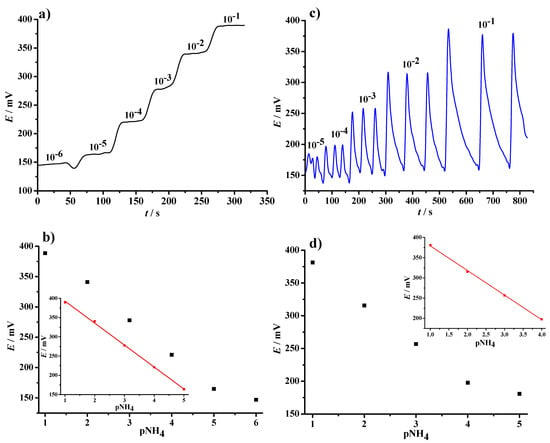
Figure 10.
Time-dependence response of NH4 selective paper sensor in a continuous flow (a) and injection flow conditions (c), obtained with a 0.833 mL/min flow rate of the carrier stream (10−6 M NH4Cl solution prepared in 10 mM CaCl2). Corresponding dynamic and linear response plots (b,d) were also given.
For continuous flow measurements, the analytical reading was carried out under conditions of chemical equilibrium, resulting in a step-like graphical plot with a well-defined plateau potential value (Figure 10a). The inset in the Figure 10b shows the five-point linear plot derived from the E vs. t curve, described mathematically by Equation (3):
The limit of detection (LOD) was calculated as three times the standard deviation of a low-concentration-level segment and the slope of the calibration curve [49], and was found to be 4.8 × 10−6 M.
In the case of flow injection analysis, the retention time of sample in the sensing cell is short, resulting in a typical peak-shaped signal [50]. Stable potentials were recorded (Figure 10c), with an obvious gradual increase in ISE response in the concentration range between 10−5 M and 10−1 M. The response of the planar sensor to the potential changes was rapid. This effect is attributed to the fast ion-exchange through the relatively thin PVC-based membrane (as shown in SEM micrographs in Figure 6) applied over the graphite-PVB transducing layer. Compared to the response observed in continuous measurements, the flow injection mode results in a slightly higher (i.e., super-Nernstian) slope, an order of magnitude lower detection limit, and a narrower linear range (10−4 M to 10−1 M). The four-point linear calibration curve (Figure 10d) is thus represented by the following Equation:
Table 3 Summarises the main analytical parameters of several ammonium ISEs based on the nonactin ionophore. The obtained results show similar analytical features of paper-based ammonium sensors, compared to the disk electrode substrates used (detection limit, sensitivity and linear range) within a concentration gradient relevant to many different field applications. Therefore, all-solid-state ammonium selective electrode fabricated on a paper substrate and presented in this work, could be used in place of conventional solid-state ISEs as a low-cost, bendable and disposable alternative for applications where classical electrodes are not effective.

Table 3.
Comparison of various nonactin-based potentiometric sensors for selective determination of NH4.
4. Conclusions
In summary, this work has demonstrated that through the combination of easily accessible compounds, bendable paper substrates along with a simple preparation process, a type of simple and low-cost potentiometric sensor with good analytical performance can be built. In particular, an ion-selective electrode with graphite-PVB composite as ion-to-electron transducer, a PVC-based nonactin ammonium-sensitive membrane and an inkjet-printed silver inner electrode was successfully produced. Along with comparable sensitivity, selectivity and linear range, the electrode exhibited LOD in the micromolar range, which is comparable to previous articles regarding nonactin-based ammonium ISE. The exhibited LOD and linear range are suitable for detecting ammonium in environmental waters [51]. This type of sensor holds high promise for future development of flexible and wearable potentiometric devices.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/chemosensors9120333/s1, Figure S1: Calibration curve of the conventional ammonium ion-selective electrode immersed in 10−6–10−1 M NH4Cl solutions; Figure S2: Calibration curve of the graphite disc electrode coated with 50 µL of ammonium membrane mixture. Measurements were performed in 10−6–10−1 M NH4Cl solutions after immersion of the prepared sensor in water for 24 h; Figure S3: Optimization of the sensor parameters. Linear range of ammonium-selective ISE prepared with various amounts of graphite—PVB (50 wt %) transducer formulation layered over a graphite disc electrode and different volumes of membrane mixture. The measurements were performed in the same solutions as in the previous Figure; Figure S4: R(Q(RW)) circuit for simulation of impedance spectra (a) and results of circuit modeling (b) of graphite (65 wt %)—PVB coated graphite electrode using ZSimpWin simulation programme. Rs—solution resistance, Rct—charge transfer resistance, ZW—Warburg impedance, Q—constant phase element.
Author Contributions
Conceptualisation, I.I.; methodology, I.I., S.M. and P.K.; validation, I.I. and S.M.; formal analysis, I.I.; investigation, I.I. and A.R.; writing–original draft preparation, I.I.; writing–review and editing, I.I. and P.K.; visualisation, I.I. and P.K.; supervision, S.M.; project administration, S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was fully supported by the University of Zagreb, Grant No. 121079.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Y.; Chen, J.F.; Yuan, D.X.; Yang, Z.; Shi, X.L.; Li, H.L.; Jin, H.Y.; Ran, L.H. Development of analytical methods for ammonium determination in seawater over the last two decades. Trac-Trends Anal. Chem. 2019, 119, 14. [Google Scholar] [CrossRef]
- Pedersen, J.W.; Larsen, L.H.; Thirsing, C.; Vezzaro, L. Reconstruction of corrupted datasets from ammonium-ISE sensors at WRRFs through merging with daily composite samples. Water Res. 2020, 185, 12. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, F.; Reifsnyder, S.; Sobhani, R.; Cisquella-Serra, A.; Madou, M.; Rosso, D. Functional behaviour and microscopic analysis of ammonium sensors subject to fouling in activated sludge processes. Environ. Sci.-Wat. Res. Technol. 2020, 6, 2723–2733. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; van Boekel, M. A non-destructive ammonium detection method as indicator for freshness for packed fish: Application on cod. J. Food Eng. 2012, 110, 254–261. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Yamamoto, N.A.D.; Toor, A.; Payne, M.E.; Woods, C.; Pister, V.I.; Khan, Y.; Evans, J.W.; Arias, A.C. Optimization of printed sensors to monitor sodium, ammonium, and lactate in sweat. APL Mater. 2020, 8, 10. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Ying, Y.B.; Ping, J.F. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, R.; Bono, M.S.; Braganza, S.; Vaishnav, C.; Karnik, R.; Hart, A.J. In-field determination of soil ion content using a handheld device and screen-printed solid-state ion-selective electrodes. PLoS ONE 2018, 13, e0203862. [Google Scholar] [CrossRef] [Green Version]
- Alberti, G.; Zanoni, C.; Losi, V.; Magnaghi, L.R.; Biesuz, R. Current Trends in Polymer Based Sensors. Chemosensors 2021, 9, 108. [Google Scholar] [CrossRef]
- Yin, T.J.; Qin, W. Applications of nanomaterials in potentiometric sensors. Trac-Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Ding, L.; Ding, J.W.; Ding, B.J.; Qin, W. Solid-contact Potentiometric Sensor for the Determination of Total Ammonia Nitrogen in Seawater. Int. J. Electrochem. Sci. 2017, 12, 3296–3308. [Google Scholar] [CrossRef]
- Quan, D.P.; Quang, C.X.; Duan, L.T.; Viet, P.H. A conductive polypyrrole based ammonium ion selective electrode. Environ. Monit. Assess. 2001, 70, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Li, J.; Yin, T.Y.; Jia, J.J.; Ding, Q.; Zheng, H.; Chen, C.T.A.; Ye, Y. A novel all-solid-state ammonium electrode with polyaniline and copolymer of aniline/2,5-dimethoxyaniline as transducers. J. Electroanal. Chem. 2015, 741, 87–92. [Google Scholar] [CrossRef]
- Jang, C.W.; Byun, Y.T.; Jhon, Y.M. Detection of 10 nM Ammonium Ions in 35 parts per thousand NaCl Solution by Carbon Nanotube Based Sensors. J. Nanosci. Nanotechnol. 2012, 12, 1765–1769. [Google Scholar] [CrossRef]
- Athavale, R.; Kokorite, I.; Dinkel, C.; Bakker, E.; Wehrli, B.; Crespo, G.A.; Brand, A. In Situ Ammonium Profiling Using Solid-Contact Ion-Selective Electrodes in Eutrophic Lakes. Anal. Chem. 2015, 87, 11990–11997. [Google Scholar] [CrossRef]
- Maksymiuk, K.; Stelmach, E.; Michalska, A. Unintended Changes of Ion-Selective Membranes Composition-Origin and Effect on Analytical Performance. Membranes 2020, 10, 266. [Google Scholar] [CrossRef]
- Cuartero, M.; Colozza, N.; Fernandez-Perez, B.M.; Crespo, G.A. Why ammonium detection is particularly challenging but insightful with ionophore-based potentiometric sensors—An overview of the progress in the last 20 years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef] [Green Version]
- Chiang, T.Y.; Lin, C.H. A microfluidic chip for ammonium sensing incorporating ion-selective membranes formed by surface tension forces. RSC Adv. 2014, 4, 379–385. [Google Scholar] [CrossRef]
- Nunez, L.; Ceto, X.; Pividori, M.I.; Zanoni, M.V.B.; del Valle, M. Development and application of an electronic tongue for detection and monitoring of nitrate, nitrite and ammonium levels in waters. Microchem. J. 2013, 110, 273–279. [Google Scholar] [CrossRef]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef]
- Brendgen, R.; Grassmann, C.; Grethe, T.; Mahltig, B.; Schwarz-Pfeiffer, A. Coatings with recycled polyvinyl butyral on polyester and polyamide mono- and multifilament yarns. J. Coat. Technol. Res. 2021, 18, 819–829. [Google Scholar] [CrossRef]
- Guinovart, T.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 2014, 821, 72–80. [Google Scholar] [CrossRef]
- Tobjörk, D.; Österbacka, R. Paper Electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Ivanisevic, I.; Kassal, P.; Milinkovic, A.; Rogina, A.; Milardovic, S. Combined Chemical and Thermal Sintering for High Conductivity Inkjet-printed Silver Nanoink on Flexible Substrates. Chem. Biochem. Eng. Q. 2019, 33, 377–384. [Google Scholar] [CrossRef]
- Milardovic, S.; Ivanisevic, I.; Rogina, A.; Kassal, P. Synthesis and Electrochemical Characterization of AgNP Ink Suitable for Inkjet Printing. Int. J. Electrochem. Sci. 2018, 13, 11136–11149. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Michalska, A. All-Solid-State Ion Selective and All-Solid-State Reference Electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Murphy, A.; O’Riordan, A.; O’Connell, I. Equivalent Impedance Models for Electrochemical Nanosensor-Based Integrated System Design. Sensors 2021, 21, 3259. [Google Scholar] [CrossRef]
- Ivanisevic, I.; Rukavina, V.; Kassal, P.; Milardovic, S. Impact of Weak Organic Acids on Precipitation of Poly (acrylic acid) Stabilized Silver Nanoparticles; an Electrochemical Approach. Croat. Chem. Acta 2018, 91, 491–499. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Kassal, P.; Zlatar, M. Electrochemical and spectroscopic characterization of AgNP suspension stability influenced by strong inorganic acids. Electrochim. Acta 2021, 377, 138126. [Google Scholar] [CrossRef]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005, 7, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Vyas, R.N.; Wang, B. Electrochemical Analysis of Conducting Polymer Thin Films. Int. J. Mol. Sci. 2010, 11, 1956–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, S.C.S.; Patel, A.N.; McKelvey, K.; Unwin, P.R. Definitive Evidence for Fast Electron Transfer at Pristine Basal Plane Graphite from High-Resolution Electrochemical Imaging. Angew. Chem. Int. Edit. 2012, 51, 5405–5408. [Google Scholar] [CrossRef]
- Jin, S.; Lee, J.S.; Kang, Y.; Heo, M.; Shin, J.H.; Cha, G.S.; Nam, H.; Lee, J.Y.; Helal, A.; Kim, H.S.; et al. Voltammetric ion-channel sensing of ammonium ion using self-assembled monolayers modified with ionophoric receptors. Sens. Actuator B Chem. 2015, 207, 1026–1034. [Google Scholar] [CrossRef]
- Abramova, N.; Bratov, A. Application of Photocured Polymer Ion Selective Membranes for Solid-State Chemical Sensors. Chemosensors 2015, 3, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.M.; Nassar, J.M.; Hussain, M.M. Paper as a Substrate and an Active Material in Paper Electronics. ACS Appl. Electron. Mater. 2021, 3, 30–52. [Google Scholar] [CrossRef]
- Casadella, A.; Schaetzle, O.; Loos, K. Ammonium across a Selective Polymer Inclusion Membrane: Characterization, Transport, and Selectivity. Macromol. Rapid Commun. 2016, 37, 858–864. [Google Scholar] [CrossRef]
- Michalska, A.; Wojciechowski, M.; Bulska, E.; Maksymiuk, K. Experimental study on stability of different solid contact arrangements of ion-selective electrodes. Talanta 2010, 82, 151–157. [Google Scholar] [CrossRef]
- Isildak, O.; Ozbek, O. Application of Potentiometric Sensors in Real Samples. Crit. Rev. Anal. Chem. 2021, 51, 218–231. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Thomas, J.D.R. Poly (vinyl chloride) type ammonium ion-selective electrodes based on nonactin: Solvent mediator effects. Anal. Commun. 1994, 31, 181–183. [Google Scholar] [CrossRef]
- Capella, J.V.; Bonastre, A.; Campelo, J.C.; Ors, R.; Peris, M. A New Ammonium Smart Sensor with Interference Rejection. Sensors 2020, 20, 7102. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes Part I. Inorganic cations—(Technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Ren, K. Selectivity problems of membrane ion-selective electrodes—A method alternative to the IUPAC recommendation and its application to the selectivity mechanism investigation. Fresenius J. Anal. Chem. 1999, 365, 389–397. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Buhlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Novell, M.; Parrilla, M.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. Paper-Based Ion-Selective Potentiometric Sensors. Anal. Chem. 2012, 84, 4695–4702. [Google Scholar] [CrossRef]
- Skeggs, L.T. An automatic method for colorimetric analysis. Am. J. Clin. Pathol. 1957, 28, 311–322. [Google Scholar] [CrossRef]
- Hansen, E.H.; Wang, J.H. The three generations of flow injection analysis. Anal. Lett. 2004, 37, 345–359. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2010; pp. 103–105. [Google Scholar]
- Trojanowicz, M.; Kolacinska, K. Recent advances in flow injection analysis. Analyst 2016, 141, 2085–2139. [Google Scholar] [CrossRef]
- Du, Y.; Ma, T.; Deng, Y.; Shen, S.; Lu, Z. Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China. Environ. Sci. Process. Impacts 2017, 19, 161–172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).