Abstract
Trichlorfon, an organophosphorus pesticide widely used in agriculture and other fields, poses a severe risk to both food safety and human health. We developed a photonic crystal film sensing platform for detecting trichlorfon, a hazardous organophosphorus pesticide. The method exploits trichlorfon’s inhibition of acetylcholinesterase (AChE). Normally, AChE catalyzes acetylcholine hydrolysis to produce acetic acid, which decomposes CaCO3 to release Ca2+. This triggers calcium alginate hydrogel formation, increasing solution viscosity and trapping water. When trichlorfon inhibits AChE, hydrogel formation fails, leaving the solution in a low-viscosity sol state with abundant free water. Immersing the film in trichlorfon-containing sodium alginate solutions causes water absorption and film swelling due to free water. Higher trichlorfon concentrations reduce hydrogel formation, increase free water, and amplify film swelling, resulting in proportionally higher reflectivity. The platform demonstrates a wide linear range (1–250 ng/mL) and a low detection limit (0.4 ng/mL) for trichlorfon. Successful analysis of real samples confirms its practicality for residue detection. This label-free thin-film sensor shows significant potential for monitoring trichlorfon and other organophosphorus pesticides.
1. Introduction
Organophosphorus pesticides (OPs) have been used continuously for over sixty years as agricultural and household pest control agents to ensure the normal growth and storage of crops [1,2,3,4,5]. Trichlorfon is one of the most commonly used OPs due to its excellent characteristics such as broad spectrum, high effectiveness, and low toxicity [4,6,7]. However, it is worth noting that the widespread use and misuse of trichlorfon in fruits, vegetables, and crops pose a serious threat to human health [8,9,10]. Against this backdrop, the maximum residue limits (MRLs) for trichlorfon have been established by different countries. For example, China has established MRLs of 0.1 to 1 mg/kg in vegetables and 0.02 mg/kg in fruits, while the United States has established an MRL of 0.1 mg/kg in vegetables [11].
At present, there are some traditional methods for the determination of trichlorfon residues [12,13,14,15,16,17] including chromatography [18], electrochemical method [19], capillary electrophoresis [20], and colorimetric method [21]. However, these methods suffer from a variety of drawbacks, such as high cost, professional operation, cumbersome sample processing, and special devices for signal output. These drawbacks hinder their suitability for rapid, on-site monitoring applications [22]. While innovative portable approaches like surface-enhanced Raman scattering (SERS) sensors [23] and smartphone-integrated syringe devices [24] have emerged, the demand persists for simpler, highly sensitive, specific, and truly portable techniques for routine pesticide residue analysis.
Using thin films as sensing materials has attracted much attention in recent years due to their exceptional sensing capabilities [25,26]. Examples include plasmonic-conducting metal–organic framework (MOF) films for detection [27] and photonic crystal (PC)-based sensors integrated into portable devices [28]. PC is a novel optical microstructure material, which is created through the periodic arrangement of material media with different refractive indices [29,30,31]. It possesses a photonic band-gap (PBG) in which light of a specific wavelength cannot propagate, but is reflected instead. When the lattice spacing and refractive index of the material are within a certain range, it can reflect light in the visible spectrum [32]. Due to the unique optical properties and responsiveness to the environment, PC shows great potential in the field of biosensing, including detection of metal ions, biomolecules, vapors, temperature, and electromagnetic fields, especially in the detection of OPs.
Common fabrication strategies for PC films encompass both bottom-up techniques, such as the self-assembly of colloidal particles or the deposition of zirconium dioxide and silicon dioxide films, and top-down approaches like advanced lithography [33,34,35]. To enable efficient detection of OPs, specific enzymes, such as methyl parathion hydrolase (MPH) [36], acetylcholinesterase (AChE) [28], and organophosphorus hydrolase (OPH) [37], are typically integrated into these PC films. Key enzyme integration methodologies include covalent binding, electrostatic adsorption, and immersion processes [36,38,39]. The binding of these enzymes to PC films can catalyze the degradation of OP or react specifically with it, which in turn causes changes in the optical properties of the PC films, such as structural color, reflectance spectra, and absorbance [40,41]. By monitoring these optical changes, quantitative detection of OPs can be realized. This inherent signal transduction mechanism—converting structural changes into measurable shifts in the Bragg diffraction peak wavelength or intensity—offers significant advantages for optical sensing [12,42,43].
Existing PC sensing platforms for OPs retain these advantages yet exhibit limitations in response kinetics, reusability, and long-term stability [44]. These platforms typically rely on internally confined signal transduction mechanisms, such as enzyme-embedded hydrogelation within the PC matrix. To overcome these constraints, we developed an externally-mediated gelation strategy. Hydrogels serve as stimuli-responsive matrices whose analyte-induced swelling/shrinking dynamically alters the PC’s lattice constant (d), thereby transducing molecular interactions into quantifiable optical signals through micrometer-scale structural changes.
Herein, we prepared a sensing platform based on PC hydrogel film for detecting trichlorfon, which relied on the changes in reflectivity of the PC film during the drying and wetting processes. Our approach specifically leverages the simplicity of colloidal self-assembly using 200 nm silica nanoparticles to fabricate the initial PC structure. The principle of the platform can be described as follows. Acetylcholinesterase (AChE) can facilitate the breakdown of acetylcholine into choline and acetic acid, and the latter can trigger the decomposition of calcium carbonate (CaCO3), leading to the release of calcium ions (Ca2+). When alginate contacts Ca2+, a calcium alginate hydrogel is formed. In the context of this process, OPs such as trichlorfon function as inhibitors of AChE, impeding AChE-mediated hydrogelation of calcium alginate. The extent of hydrolysis of calcium alginate can influence the total amount of water that is absorbed by the PC film. The changes in water absorption, in turn, affect the thickness of the PC film, leading to changes in reflectivity. This modification generates measurable optical signals through Bragg diffraction shifts. Notably, minute molecular interactions are amplified into micrometer-scale deformations via the hydrogel matrix, ultimately converting to structural color changes. This amplification mechanism significantly boosts detection sensitivity, enabling efficient recognition of trace pesticides (Scheme 1).
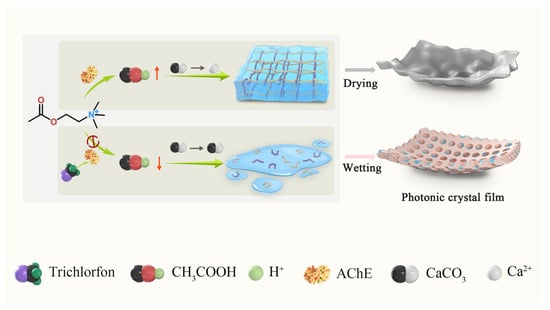
Scheme 1.
Diagram of film sensing platform for the detection of trichlorfon.
2. Materials and Methods
2.1. Reagents and Instruments
AChE and acetylcholine chloride were purchased from Sigma Aldrich Trading Co., Ltd., Shanghai, China. Sodium alginate, 2-hydroxy-2-methylpropiophenone (photoinitiator, 97%), and hydrofluoric acid solution (5%) were ordered from Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Poly (ethylene glycol) diacrylate (PEGDA), calcium carbonate, glucose, fructose, tryptophan, lysine, and boric acid buffer (0.5 M, pH = 8.0) were purchased from McLean Biochemical Technology Co., Ltd., Shanghai, China. Monodisperse silica microspheres were purchased from Nanjing Caina Bio-technology Co., Ltd., Nanjing, China. NaCl and KCl were purchased from Sinopharm Chemical Reagent Co., Ltd., Beijing, China. CdSO4·8H2O, CuSO4·5H2O, PbSO4, and ZnCl2 were purchased from Sinopharm Chemical Reagent Co., Ltd., Beijing, China. Ethanol was supplied by Tianjin Fuyu Technology Co., Ltd., Tianjin, China. All experiments were performed using ultrapure water with conductivity of 0.055 μS/cm.
Rheological properties were determined by the RheoStress 300 HAAKE rheometer (Thermo Fisher Scientific, Waltham, MA, USA). The Agilent Technologies Cary 5000 UV–Vis-NIR (Agilent Technologies, Mulgrave, VIC, Australia) spectrometer was utilized to measure UV–Vis spectra. Reflectance spectra were obtained using a fiber optic spectrometer model PG2000-PRO (Shanghai Fuxiang Optical Co., Ltd., China). Scanning electron microscopy (SEM) images were obtained using the SUPRATM55 field-emission microscope (Carl Zeiss, Oberkochen, Germany).
2.2. Inhibitory Effect of Trichlorfon on AChE
The impact of trichlorfon on the activity of AChE was assessed by measuring the viscosity of calcium alginate hydrogel. First, 0.1 g sodium alginate powder was dissolved in ultrapure water to prepare a 1% aqueous solution, which was then diluted 5-fold for subsequent use. Then, a 60 mM CaCO3 aqueous solution (the CaCO3 aqueous solution was supersaturated) and an acetylcholine solution (0.036 g acetylcholine was dissolved in 25 mM boric acid buffer solution) were prepared. The alginate solution, CaCO3 aqueous solution, acetylcholine solution, and AChE were mixed, and the viscosity of the mixture was analyzed using a Haake Rheometer (Thermo Fisher Scientific, MA, USA). The mixed solutions with and without trichlorfon were mixed with equal amounts of rhodamine B solution, and 100 μL of the upper solution was taken separately. The solutions were diluted with an equal volume of PBS and the UV–Vis absorption spectra of the mixed solutions were measured sequentially.
2.3. Preparation of PC Film
The SiO2 microsphere dispersion was centrifuged and washed multiple times with ethanol. Drying the resulting products in an oven at 70 °C for 12 h yielded SiO2 solids. A suspension containing 10% SiO2 microspheres dispersed in ethanol was made and sonicated for 30 min. Then, it was mixed with photoinitiator and PEGDA, and dried in an oven at 70 °C for 5 h to obtain the precursor solution. Next, 20 μL of the precursor solution was added at the center of the slide, and the upper and lower slides were separated by certified adhesive tapes (thicknesses: 0.06, 0.08, 0.10, 0.12 ± 0.002 mm) and clamped at both ends. The thicknesses of films were verified using a high-precision electronic thickness gauge. After irradiation under 365 nm UV light for 2 min and etching in a hydrofluoric acid solution for 8 h, the hollow photonic crystal film of 1 cm diameter was obtained.
2.4. Procedures of Trichlorfon Detection
For trichlorfon detection, 190 μL of alginate solution, 190 μL of CaCO3 solution, 10 μL of acetylcholine solution, and 5 μL AChE solution were incubated with 5 μL different concentrations of trichlorfon at 37 °C for 10 min. Subsequently, the prepared 1 cm diameter PC film was completely submerged in 0.4 mL of the mixture, and incubated 30 min in a constant-temperature incubator at 37 °C to achieve adsorption equilibrium prior, ensuring complete coverage and consistent environmental conditions throughout the incubation period. To eliminate optical interference from the liquid medium, specimens were carefully retrieved at 5 min intervals and placed it on a slide, gently dried under a stream of nitrogen gas to remove surface moisture, and subsequently subjected to reflectance spectroscopy in the dry state. Reflectance spectra were acquired using a fiber-optic spectrometer.
2.5. Detection of Trichlorfon in Real Samples
Trichlorfon-free apples were used for the method validation and recovery studies. To simulate a pesticide application scenario, 1.0 mL of trichlorfon standard solution (0.001, 0.01, 0.05, 0.1, 0.2 mg/kg) was sprayed uniformly at 20 cm from the apple surface using a calibrated sprayer (nozzle diameter 0.3 mm). To enhance the release efficiency, trichlorfon was extracted using the ultrasonic method described in the previous study for 5 min [45]. Finally, the extracted solution was collected and analyzed by the PC film sensing platform. All experiments were carried out at least three times.
2.6. Development of Portable Equipment and Software
To further enhance the practicality of the sensing platform for detecting trichlorfon, a simple portable organophosphorus pesticide detection device was designed. The device mainly consists of an LED torch (wavelength range: 620–625 nm), a black opaque 3D printing box (containing a sample stage and torch holder), and a smartphone. A calibration curve between trichlorfon concentration and illuminance was established based on three measurements. Entering the illuminance value enabled calculation of the target analyte concentration.
3. Results and Discussion
3.1. Feasibility Study
To investigate the potential impact of trichlorfon on the formation of calcium alginate hydrogel through the inhibition of AChE activity, a series of experiments were conducted. As shown in Figure 1a(i), the addition of 100 U/mL AChE to a mixture containing sodium alginate solution, CaCO3 solution, and acetylcholine solution successfully triggered the formation of calcium alginate hydrogel. In Figure 1a(ii), the addition of high concentration (1 mg/mL) of trichlorfon resulted in the inhibition of AChE activity, consequently impeding the formation of calcium alginate hydrogel. As illustrated in Figure 1b, in the presence of trichlorfon, water molecules released from the hydrogel could be effectively mixed with the rhodamine B solution due to the decrease in the viscosity of the system, resulting in a significant decrease in the absorbance of the solution, whereas in the control experiment without trichlorfon, the water molecules could not be released; accordingly, the system had a higher absorbance value. The inhibitory effect of trichlorfon on AChE was also demonstrated laterally by the ease of blending their mixed solution with the rhodamine B solution (Figure 1b).
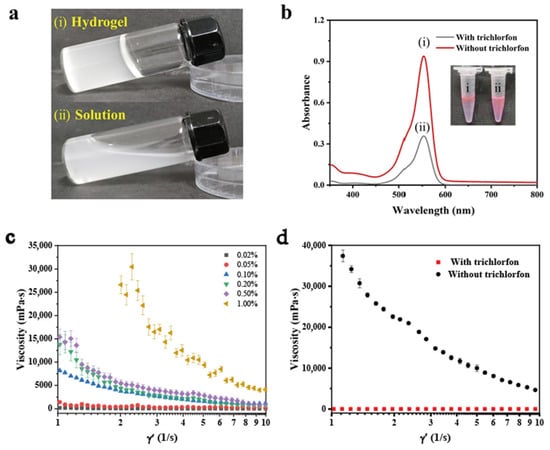
Figure 1.
Feasibility study. (a) (i) calcium alginate gel without trichlorfon, (ii) calcium alginate solution with 1 mg/mL trichlorfon. (b) UV–Vis spectra with rhodamine B solution under different experimental conditions: (i) without trichlorfon, (ii) with 1 mg/mL trichlorfon. (c) The graph of viscosity versus shear rate for the solutions of mixtures containing different concentrations of sodium alginate. (d) The pattern of viscosity as a function of shear rate for the mixed solutions without and with 1 mg/mL trichlorfon. Error bars represent standard deviations (n = 3).
Next, the concentration of sodium alginate was optimized, and this optimization is necessary to explore the impacts of different trichlorfon concentrations on the hydrogel formation based on the optimal results. As shown in Figure 1c, the viscosity of the calcium alginate mixture increased gradually with the increasing concentration of sodium alginate solution. When the concentration of sodium alginate solution reached 0.1%, its elastic modulus (G′) was obviously higher than the viscous modulus (G″), indicative of the formation of hydrogel (Figure 2). Figure 1d shows the rheological behavior of the mixed system with and without trichlorfon, respectively. The experimental results show that the addition of trichlorfon significantly reduces the viscosity and enhances the fluidity of the system.
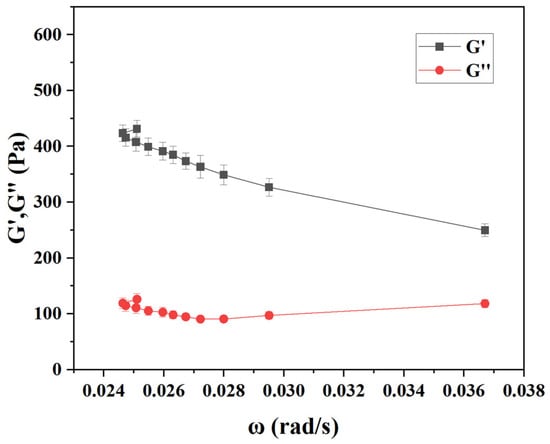
Figure 2.
Curves of elastic modulus (G′) and viscous modulus (G″) as a function of angular frequency for the gels formed at 0.1% sodium alginate. Error bars represent standard deviations (n = 3).
3.2. Characterization of PC Film
The PC film was prepared through a light curing process using a mixture of photoinitiator, SiO2 solution, and PEGDA [46]. The resulting PC film was characterized by scanning electron microscopy (SEM) (Figure 3a(i)) and atomic force microscopy (AFM) (Figure 4a). As depicted in Figure 3a(i), the size of the SiO2 microspheres in the PC gel film is about 200 nm. After hydrofluoric acid (HF) etching, some clear cavities appeared inside the film (Figure 3a(ii)). As illustrated in Figure 4, the surface structure of the film can be observed, and the HF-etched film shows significant wrinkles. Reflectivity of the film under dry and wet conditions was examined. As shown in Figure 3b, the dried film had no signal of reflectivity. However, when the film was immersed in water, a significant increase in reflectivity was observed. Motivated by these findings, the PC film sensing platform can be developed through modulating the water content of calcium alginate hydrogel via the addition of trichlorfon. Meanwhile, the stability of the PC film was explored, and it still maintained a good pore structure after being placed in dry and light-protected conditions for 30 days (Figure 3c), indicating that the film has good stability and is not prone to degradation.
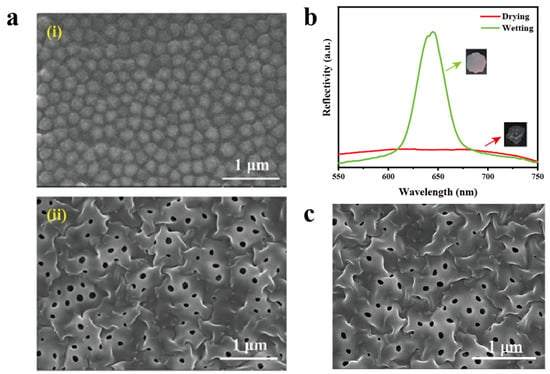
Figure 3.
Characterization of the as-prepared PC gel film. (a) SEM images of the PC gel film: (i) before HF etching, (ii) after HF etching. (b) Reflectivity spectra of the PC film under drying and wetting conditions (the insets are the corresponding optical photos of the film before and after detection). (c) SEM image of the PC film after 30-day storage in dark and dry conditions.
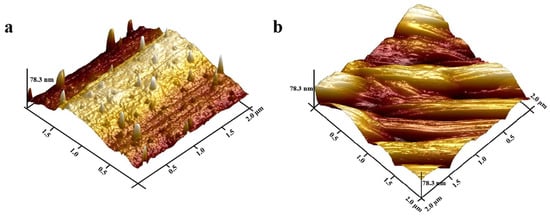
Figure 4.
AFM images of the as-prepared photonic crystal gel film. (a) Before HF etching; (b) after HF etching.
3.3. Optimization of the PC Film Sensing Platform
The efficacy of the PC hydrogel (an essential element of the sensing platform) is contingent upon the amount of enzyme employed. Thus, we firstly examined the effect of various concentrations of AChE (0, 0.1, 1, 10, 100 U/mL) on the gel-forming state of calcium alginate using a tube inversion method [47]. In Figure 5a, it can be found that only a concentration of 100 U/mL of AChE was able to trigger the hydrogel formation of calcium alginate, while lower concentrations failed. In addition, we optimized the thickness of the PC film. Films that are too thin are more prone to breakage. As shown in Figure 5b, when the thickness of film is less than 0.1 mm, it is fragile, and the film retains good integrity with the thickness more than 0.1 mm. Therefore, we chose the film with a thickness of 0.1 mm for the subsequent experiments.
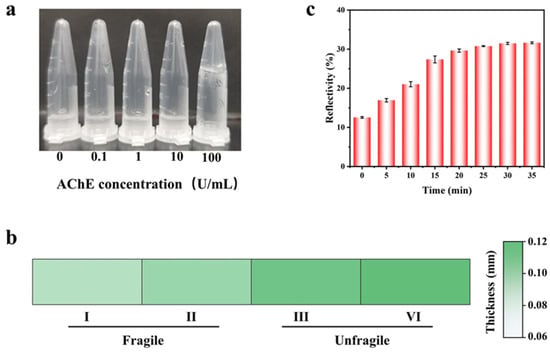
Figure 5.
Optimization of the experimental conditions for trichlorfon detection. (a) AChE concentration (0, 0.1, 1, 10, 100 U/mL gel-forming conditions). (b) The relationship between the thickness of the PC film and its fragility. (c) Histogram of reflectivity with incubation time for the mixture of AChE and trichlorfon. Error bars represent standard deviations (n = 3).
Furthermore, the optimal incubation time for the mixture of AChE and trichlorfon was determined. A high concentration of trichlorfon solution (1 mg/mL) was mixed with AChE (100 U/mL), and the prepared PC film was immersed into the solution for different incubation times, ranging from 0 to 35 min. As shown in Figure 5c, the film’s reflectivity increased over time and plateaued at 30 min. Thus, 30 min was chosen as the optimal incubation time.
3.4. Performance of the PC Film Sensing Platform
Based on the above optimization, the PC film sensing platform was utilized to test trichlorfon. As shown in Figure 6a, the reflectivity of the PC film gradually rises as the concentration of trichlorfon increases. When the film was immersed into the aqueous sodium alginate solutions containing different concentrations of trichlorfon, the amount of calcium alginate hydrogel formed in the system decreased as the concentration of trichlorfon increased. Subsequently, the number of free water molecules increased, leading to an increase in the swelling of the PC film, which exhibited a higher reflectivity. The standard curve between the reflectivity ratio (ΔR/R0) (R0 is the reflectivity value obtained after the film was immersed into a blank sample, Rn is the reflectivity value obtained after the film was immersed into a sample containing trichlorfon, and ΔR is the difference between Rn and R0) and the concentration of trichlorfon (0–250 ng/mL) was established and illustrated in Figure 6b. The detection limit was determined to be 0.4 ng/mL according to the 3σ/slope method (which is associated with σ, representing the standard deviation obtained from blank samples, and the slope of the standard curve). Compared to previous approaches for detection of trichlorfon, the PC film sensing platform exhibits a notable increase in sensitivity (Table 1).
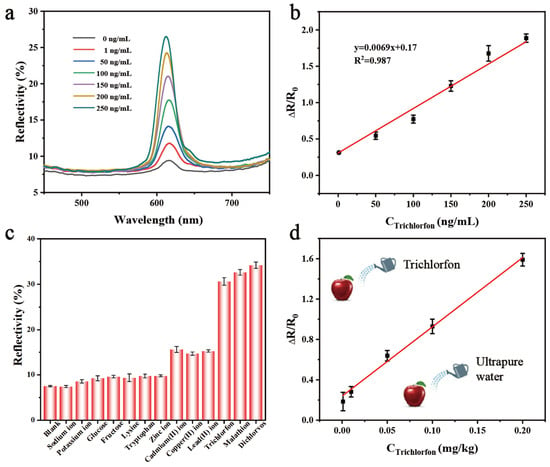
Figure 6.
Sensitivity and selectivity evaluations. (a) The reflectivity of the PC film with different concentrations of trichlorfon. (b) Relationship between ΔR/R0 values and trichlorfon concentrations. (c) The reflectivity of the proposed sensing platform in response to other chemicals, including OPs (dichlorvos and malathion), monosaccharides (glucose and fructose), amino acids (lysine, tryptophan), inorganic ions (Na+, K+), and heavy metal ions (Cd2+, Cu2+, Pb2+, Zn2+). (d) The calibration curve for trichlorfon detection in apple samples. Error bars represent standard deviations (n = 3).

Table 1.
Comparison of the detection limits of different methods for the detection of trichlorfon.
Subsequently, the selectivity of the proposed assay was further investigated against different potential interfering substances, including common OPs (200 ng/mL of dichlorvos and malathion), monosaccharides (200 μg/mL of glucose and fructose), amino acids (200 μg/mL of lysine and tryptophan), inorganic ions (200 μg/mL of Na+ and K+), and heavy metal ions (200 μg/mL of Cd2+, Cu2+, Pb2+, Zn2+). As shown in Figure 6c, the PC film sensing platform exhibits no cross-reaction with monosaccharides and inorganic ions; heavy metal ions induce reflectance enhancement; and the magnitude remains substantially lower than the signal variation elicited by trichlorfon at equivalent concentrations. This significant disparity confirms negligible interference from co-existing heavy metals in trichlorfon quantification. It demonstrates the ability to detect dichlorvos, malathion, and trichlorfon due to the property of OPs inhibiting the activity of AChE. Thus, this strategy can be used to detect OPs in complex environmental or food matrices.
3.5. Detection of Trichlorfon Residues in Practical Samples
Based on the excellent analytical performance, the present sensing platform was applied to detect trichlorfon in real samples [45]. Various concentrations of trichlorfon (0.001, 0.01, 0.05, 0.1, 0.2 mg/kg) were spiked on the apple samples using the standard addition method and measured by the PC film sensing platform. Due to the interference of sample matrix, the calibration curve for trichlorfon detection in apple samples was established (Figure 6d). The subsequent recovery experiments were carried out, and the found trichlorfon amounts were calculated through the utilization of the calibration curve. The detailed results in Table 2 demonstrate the analytical recoveries for trichlorfon ranging from 80.0% to 120.3% with the relative standard deviation (RSD) of 3.1~17.7%, which indicates the good practicability of the sensing platform for monitoring trichlorfon in real samples.

Table 2.
Detection of the trichlorfon in real samples by using the PC film sensing platform.
3.6. Use of Portable Device for the Detection of Trichlorfon
As illustrated in Figure 7a, this device comprises an LED flashlight (wavelength range: 620–625 nm), a black light-impermeable 3D-printed detection chamber, and a smartphone. After incubation and immersion of the PC films under optimal conditions, the film was placed on the slide within the chamber’s fixed sample stage, dried to remove moisture, and subjected to illuminance measurement under controlled illumination. The chamber’s design, featuring a pre-set flashlight holder and a dedicated smartphone slot, ensured precise alignment between the camera lens and the dried sample surface while maintaining parallel planes. We quantified the optical response of the PC film by measuring illuminance using smartphone-based Phyphox software (version 1.1.16). To enable direct concentration readouts, we developed a dedicated data analysis app implementing a least-squares linear regression algorithm (Figure 7b). Users simply input illuminance values acquired via Phyphox, and the app automatically calculates trichlorfon concentrations by interpolating inputs against a preloaded calibration curve. This integrated smartphone-device system significantly enhances portability and establishes a streamlined platform for simple, rapid OPs detection, demonstrating strong practical applicability. The effective monitoring of trichlorfon in the above actual samples justifies the design of the equipment.
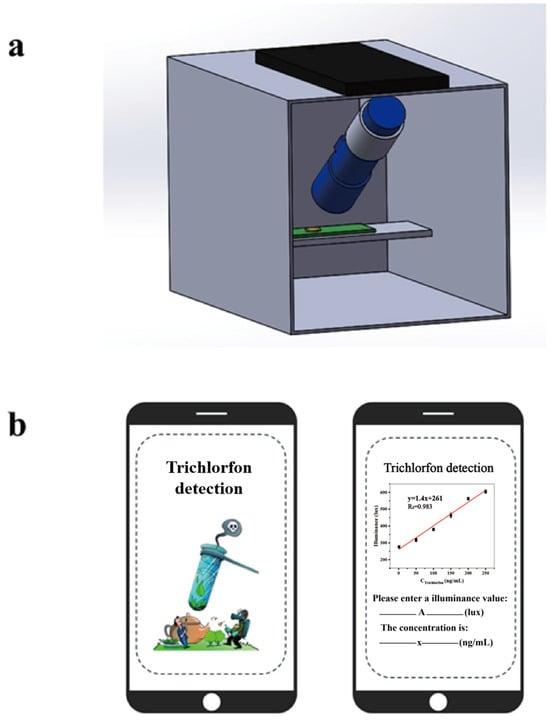
Figure 7.
Schematics for development of portable device. (a) The portable device for detecting trichlorfon. (b) The smartphone-based data analysis app.
4. Conclusions
This study developed a PC film sensing platform for detecting trichlorfon by taking advantage of the inhibitory effect of OPs on AChE. The proposed sensing platform exhibits a high level of selectivity for OPs, and also demonstrates an excellent sensitivity for trichlorfon detection with a detection limit of 0.4 ng/mL and a detection range of 1–250 ng/mL. Furthermore, the photonic materials exhibit promising anti-interference capabilities in real samples, indicating their potential for detecting OPs in food and other related fields.
Author Contributions
J.R.: Conceptualization, investigation, writing—original draft, data curation. X.L.: Methodology, formal analysis, writing—original draft, data curation. Z.W.: Conceptualization, supervision, funding acquisition, writing—review and editing. L.Y.: Resources, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Innovation Team Project of Jinan of China (202333024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no competing financial interests.
Abbreviations
The following abbreviations are used in this manuscript:
| PC | Photonic crystal |
| AChE | Acetylcholinesterase |
| OPs | Organophosphorus pesticides |
| MRLs | Maximum residue limits |
| SERS | Surface enhanced Raman scattering |
| MOF | Metal-organic framework |
| MPH | Methyl parathion hydrolase |
| OPH | Organophosphorus hydrolase |
| PBG | Photonic band-gap |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
| HF | Hydrofluoric acid |
| RSD | Relative standard deviation |
References
- Chen, Q.; Sun, Y.; Liu, S.; Zhang, J.; Zhang, C.; Jiang, H.; Han, X.; He, L.; Wang, S.; Zhang, K. Colorimetric and fluorescent sensors for detection of nerve agents and organophosphorus pesticides. Sens. Actuators B Chem. 2021, 344, 130278. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, H.; Mo, H.; Zhu, N. Organophosphorus Pesticide Photoelectrochemical/Electrochemical Dual-Mode Smartsensors Derived from Synergistic Co,N-TiO2@ZrO2/3DGH Platform. Chemosensors 2025, 13, 167. [Google Scholar] [CrossRef]
- Alhamami, M.A.M.; Algethami, J.S.; Rizk, M.A.; Abbas, A.M.; Khairy, G.M. A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples. Chemosensors 2022, 10, 391. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Xu, G.; Li, X.; Dong, J.; Luan, X.; Du, X. Smart controlled-release avermectin nanopesticides based on metal–organic frameworks with large pores for enhanced insecticidal efficacy. Chem. Eng. J. 2023, 475, 146312. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, W.; Ma, R.; Huang, L.; Yang, Y. A colorimetric analytical method based on a TCPP–CuCo2O4-like peroxidase for the detection of trichlorfon. Anal. Methods 2023, 15, 4331–4337. [Google Scholar] [CrossRef]
- Yuan, S.; Yu, H.; Guo, Y.; Xie, Y.; Cheng, Y.; Qian, H.; Yao, W. Removal of trichlorfon and phoxim from pak choi (Brassica campestris ssp. chinensis) by combined ultrasonic and ozone treatment and their effects on quality. J. Food Process Eng. 2023, 46, e14458. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, K.; Dao, F.; Li, P.; Huang, J. A recyclable AChE-nanoprobe based on nano-Fe3O4@CHO-β-CD for trichlorfon detection. Microchem. J. 2024, 197, 109860. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Chang, X.; Wang, X.; Feng, J.; Su, X.; Liang, J.; Li, H.; Zhang, J. Impact of chronic exposure to trichlorfon on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in common carp (Cyprinus carpio L.). Environ. Pollut. 2020, 259, 113846. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, R.; Liu, X.; Wu, X.; Chen, R.; Yang, M. Multiresidue Pesticide Analysis in Tea Using GC–MS/MS to Determine 12 Pesticide Residues (GB 2763-2021). Molecules 2022, 27, 8419. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Li, X.; Cai, Z.; Zhang, Y. Two-dimensional photonic crystal acetylcholinesterase hydrogel and organohydrogel sensors for efficient detection of organophosphorus compounds. Biosens. Bioelectron. 2025, 267, 116845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yan, Z.; Xiao, X. Peroxidase-mimetic carbon dot based nanozyme hydrogel colorimetric sensor for visual trichlorfon detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 336, 126027. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, L.; Jiang, X.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Recent advances in photonic crystal-based chemical sensors. Chem. Commun. 2024, 60, 9177–9193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.-Y.; Chen, J. A bimetallic nanozyme with high peroxidase-like activity for visual detection of organophosphorus pesticides. Talanta 2025, 295, 128309. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Wang, H.; Li, Z.; Huang, J.; Sun, X.; Guo, Y.; Du, F. Research progress on molecularly imprinted polymers (MIPs)-based sensors for the detection of organophosphorus pesticides. Food Chem. 2025, 490, 145137. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, K.; Duan, R.; An, C.; Dao, F.; Huang, J. Iron/manganese-zeolitic imidazolate framework (Fe/Mn-ZIF) nanozyme combined with acetylcholinesterase for colorimetric rapid detection of organophosphorus pesticides. Food Chem. 2025, 473, 143090. [Google Scholar] [CrossRef]
- Maguire, W.J.; Call, C.W.; Cerbu, C.; Jambor, K.L.; Benavides-Montes, V.E. Comprehensive Determination of Unregulated Pesticide Residues in Oregon Cannabis Flower by Liquid Chromatography Paired with Triple Quadrupole Mass Spectrometry and Gas Chromatography Paired with Triple Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 12670–12674. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, T.; Rong, S.; Zeng, D.; Yu, H.; Zhang, Z.; Chang, D.; Pan, H. A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH2 nanocomposite. Bioelectrochemistry 2019, 127, 163–170. [Google Scholar] [CrossRef]
- Wimmer, B.; Pattky, M.; Zada, L.G.; Meixner, M.; Haderlein, S.B.; Zimmermann, H.-P.; Huhn, C. Capillary electrophoresis-mass spectrometry for the direct analysis of glyphosate: Method development and application to beer beverages and environmental studies. Anal. Bioanal. Chem. 2020, 412, 4967–4983. [Google Scholar] [CrossRef]
- Deng, G.; Chen, H.; Shi, Q.; Ren, L.; Liang, K.; Long, W.; Lan, W.; Han, X.; She, Y.; Fu, H. Colorimetric assay based on peroxidase-like activity of dodecyl trimethylammonium bromide-tetramethyl zinc (4-pyridinyl) porphyrin for detection of organophosphorus pesticides. Microchim. Acta 2022, 189, 375. [Google Scholar] [CrossRef]
- Feng, D.; Wei, F.; Wu, Y.; Tan, X.; Li, F.; Lu, Y.; Fan, G.; Han, H. A novel signal amplified electrochemiluminescence biosensor based on MIL-53(Al)@CdS QDs and SiO2@AuNPs for trichlorfon detection. Analyst 2021, 146, 1295–1302. [Google Scholar] [CrossRef]
- Ye, C.; He, M.; Zhu, Z.; Shi, X.; Zhang, M.; Bao, Z.; Huang, Y.; Jiang, C.; Li, J.; Wu, Y. A portable SERS sensing platform for the multiplex identification and quantification of pesticide residues on plant leaves. J. Mater. Chem. C 2022, 10, 12966–12974. [Google Scholar] [CrossRef]
- Wei, D.; Wang, Y.; Zhu, N.; Xiao, J.; Li, X.; Xu, T.; Hu, X.; Zhang, Z.; Yin, D. A Lab-in-a-Syringe Device Integrated with a Smartphone Platform: Colorimetric and Fluorescent Dual-Mode Signals for on-Site Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2021, 13, 48643–48652. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Cao, Z.; Li, Y.; Li, Z.; Zhang, F.-L.; Gu, Y.; Han, C.; Yang, G.; Qu, L. Highly sensitive SERS substrates with multi-hot spots for on-site detection of pesticide residues. Food Chem. 2022, 381, 132208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, S.; Cai, R.; Tan, W. Rapid water-responsive shape memory films for smart resistive bending sensors. Nano Today 2021, 38, 101202. [Google Scholar] [CrossRef]
- Lin, F.-R.; Liu, Z.-Y.; Zhang, H.; Liu, M.; Luo, H.-B.; Zou, Y.; Ren, X.-M. Proton conductive thin films of metal-organic framework for impedance detection of formic acid. Microporous Mesoporous Mater. 2023, 360, 112722. [Google Scholar] [CrossRef]
- Qi, F.; Lan, Y.; Meng, Z.; Yan, C.; Li, S.; Xue, M.; Wang, Y.; Qiu, L.; He, X.; Liu, X. Acetylcholinesterase-functionalized two-dimensional photonic crystals for the detection of organophosphates. RSC Adv. 2018, 8, 29385–29391. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.; Kong, H.; Yang, G.; Wei, G.; Zhou, X. Recent advances in photonic crystal-based sensors. Coord. Chem. Rev. 2023, 475, 214909. [Google Scholar] [CrossRef]
- Wu, S.; Xia, H.; Xu, J.; Sun, X.; Liu, X. Manipulating Luminescence of Light Emitters by Photonic Crystals. Adv. Mater. 2018, 30, 1803362. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Wang, S.; Zhou, J.; Wu, Z. A responsive photonic crystal film sensor for the ultrasensitive detection of uranyl ions. Analyst 2020, 145, 5624–5630. [Google Scholar] [CrossRef]
- Ding, H.; Liu, C.; Ye, B.; Fu, F.; Wang, H.; Zhao, Y.; Gu, Z. Free-Standing Photonic Crystal Films with Gradient Structural Colors. ACS Appl. Mater. Interfaces 2016, 8, 6796–6801. [Google Scholar] [CrossRef]
- Maity, A.; Mujumdar, S.; Polshettiwar, V. Self-Assembled Photonic Crystals of Monodisperse Dendritic Fibrous Nanosilica for Lasing: Role of Fiber Density. ACS Appl. Mater. Interfaces 2018, 10, 23392–23398. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Hong, Y.; Han, D.; Li, S.; Ning, B.; Li, Z.; Wang, J.; Bai, J.; Gao, Z.; Peng, Y. Aptamer-based photonic crystals enable ultra-trace detection of staphylococcal enterotoxin B without labels. Food Chem. 2022, 391, 133271. [Google Scholar] [CrossRef] [PubMed]
- Askar, K.; Leo, S.-Y.; Xu, C.; Liu, D.; Jiang, P. Rapid electrostatics-assisted layer-by-layer assembly of near-infrared-active colloidal photonic crystals. J. Colloid Interface Sci. 2016, 482, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Senbua, W.; Mearnchu, J.; Wichitwechkarn, J. Easy-to-use and reliable absorbance-based MPH-GST biosensor for the detection of methyl parathion pesticide. Biotechnol. Rep. 2020, 27, e00495. [Google Scholar] [CrossRef]
- Walker, J.P.; Kimble, K.W.; Asher, S.A. Photonic crystal sensor for organophosphate nerve agents utilizing the organophosphorus hydrolase enzyme. Anal. Bioanal. Chem. 2007, 389, 2115–2124. [Google Scholar] [CrossRef]
- Li, X.; Jia, M.; Yu, L.; Li, Y.; He, X.; Chen, L.; Zhang, Y. An ultrasensitive label-free biosensor based on aptamer functionalized two-dimensional photonic crystal for kanamycin detection in milk. Food Chem. 2023, 402, 134239. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Yang, X.; Zhao, T.; Jin, X.; Jiang, S.; Duan, P. Free-standing iridescent films: Crafting circularly polarized luminescence from blue to NIR-II for enhanced anti-counterfeiting performance. Nano Today 2024, 55, 102197. [Google Scholar] [CrossRef]
- Orosco, M.M.; Pacholski, C.; Miskelly, G.M.; Sailor, M.J. Protein-Coated Porous-Silicon Photonic Crystals for Amplified Optical Detection of Protease Activity. Adv. Mater. 2006, 18, 1393–1396. [Google Scholar] [CrossRef]
- Li, G.; Leng, M.; Wang, S.; Ke, Y.; Luo, W.; Ma, H.; Guan, J.; Long, Y. Printable structural colors and their emerging applications. Mater. Today 2023, 69, 133–159. [Google Scholar] [CrossRef]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Self-assembly of silica colloidal crystal thin films with tuneable structural colours over a wide visible spectrum. Appl. Surf. Sci. 2016, 380, 12–15. [Google Scholar] [CrossRef]
- Sun, H.; Zhong, H.; Chen, X.; Gan, Y.; Wang, W.; Zhou, C.; Lin, C. New modes of converting chemical information with colloidal photonic crystal sensing units. Talanta 2024, 267, 125154. [Google Scholar] [CrossRef]
- Li, B.; Wu, W.; Lin, J.-M.; Wang, T.; Hu, Q.; Yu, L. Water in liquid crystal emulsion-based sensing platform for colorimetric detection of organophosphorus pesticide. Food Chem. 2024, 436, 137732. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Huang, J.; Zhong, Y.; Wang, M.; Zhang, L.; Wang, D. Gelatinase-responsive photonic crystal membrane for pathogenic bacteria detection and application in vitro health diagnosis. Biosens. Bioelectron. 2022, 202, 114013. [Google Scholar] [CrossRef]
- Ping, J.; Wu, W.; Qi, L.; Liu, J.; Liu, J.; Zhao, B.; Wang, Q.; Yu, L.; Lin, J.-M.; Hu, Q. Hydrogel-assisted paper-based lateral flow sensor for the detection of trypsin in human serum. Biosens. Bioelectron. 2021, 192, 113548. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Zou, Y.; Jia, Q. Construction of CuNCs/AgNPs based fluorescent platform for specific enzyme-free detection of trichlorfon. Anal. Chim. Acta 2025, 1356, 344030. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Wu, S.; Chen, S.; Lin, Z.; Chen, Q. Highly sensitive colorimetric method for the detection of organophosphorus pesticides based on H2O2 etching of silver-coated gold nanostars. Sens. Actuators B Chem. 2025, 425, 137004. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, M.; Ju, Z.; Qiao, X.; Xu, Z. Development of direct competitive biomimetic immunosorbent assay based on quantum dot label for determination of trichlorfon residues in vegetables. Food Chem. 2018, 250, 134–139. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).