Application of a Low-Cost Electronic Nose to Differentiate Between Soils Polluted by Standard and Biodegradable Hydraulic Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- A
- Control (clean soil);
- B
- Soil with bio-oil 0.1 mL;
- C
- Soil with bio-oil 0.5 mL;
- D
- Soil with bio-oil 1.0 mL;
- E
- Soil with standard oil 0.1 mL;
- F
- Soil with standard oil 0.5 mL;
- G
- Soil with standard oil 1.0 mL.
2.2. Electronic Nose Device
2.3. Measurements by Electronic Nose
2.4. Electronic Nose Data Analysis
3. Results
3.1. Identification of Pollution Type
3.2. Differentiation Between Levels of Pollution Severity
Linear Discrimination Analysis of the Data
4. Discussion
4.1. Soil Contamination by Lubricating Oils in Forest Environments—Sources, Impacts, and Mitigation Strategies
4.2. Forest Machinery as a Source of Pollution
4.3. Tourism-Related Soil Pressure and Oil Leakage
4.4. Environmental Consequences and Food Safety Concerns
4.5. Preventive and Remediation Measures
- Mandatory use of biodegradable lubricants in forestry equipment operating on public or protected lands, consistent with EU Green Public Procurement (GPP) policies [39].
- Establishment of designated forest parking areas with impermeable surfaces and runoff retention systems to capture and contain potential leaks.
- Implementation of rapid soil monitoring tools, such as portable electronic noses or VOC detection systems based on GC-MS, to enable early diagnosis of contamination.
- Environmental education campaigns, including signage and outreach materials for recreational forest users, to raise awareness of the ecological impact of oil spills on soil and non-timber forest products.
5. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Kong, C.; Sun, L.; Li, X.; Yan, Y.; Chang, Z.; Li, M.; Gou, F.; Rong, B. Rapid and Simultaneous Detection of Petroleum Hydrocarbons and Organic Pesticides in Soil Based on Electronic Nose. Sensors 2025, 25, 380. [Google Scholar] [CrossRef]
- Wen, W.; Liang, X.; Han, B. Simulated Detection of VCHs in Soil by Using a Self-made Electronic Nose. Procedia Eng. 2012, 37, 202–207. [Google Scholar] [CrossRef]
- Lavanya, R.; Manimegalai, M.; Kumar, R. Indicative extent of humic and fulvic acids in soils determined by electronic nose. J. Environ. Chem. Eng. 2017, 5, 2378–2384. [Google Scholar] [CrossRef]
- Bieganowski, A.; Jaromin-Glen, K.; Guz, Ł.; Łagó’d, G.; Jozefaciuk, G.; Franus, W.; Suchorab, Z.; Sobczuk, H. Evaluating Soil Moisture Status Using an e-Nose. Sensors 2016, 16, 886. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, A.; Kempson, H.; Haseloff, J. Development of a Low-Cost Sensor System for Accurate Soil Assessment and Biological Activity Profiling. Micromachines 2024, 15, 1293. [Google Scholar] [CrossRef]
- Pineda, R.; Alvarez, M.; Ordoñez, J. SENose: An under $50 electronic nose for the monitoring of soil gas emissions. Comput. Electron. Agric. 2017, 142, 594–601. [Google Scholar] [CrossRef]
- Satybaldina, A.; Abdirakhman, A.; Tazhibayeva, S. Artificial Olfactory System for Distinguishing Oil-Contaminated Soils. Sensors 2023, 23, 5139. [Google Scholar] [CrossRef]
- Luan, X.; Kong, C.; Yao, Z.; Sun, Y.; Chang, Z. Hierarchical electronic nose detection and assessment technology for the reusage of land contaminated with petroleum hydrocarbons. Sens. Actuators B Chem. 2023, 390, 133940. [Google Scholar] [CrossRef]
- Managuli, V.; Patil, S.; Desai, S. Description and Identification of Soil Quality Measuring Development using UAV’s and E-Nose System. In Proceedings of the 2019 International Conference on Communication and Signal Processing (ICCSP), Chennai, India, 4–6 April 2019; pp. 607–611. [Google Scholar] [CrossRef]
- Bieganowski, A.; Król, A.; Krzesińska, D. Evaluation of Hydrocarbon Soil Pollution Using E-Nose. Sensors 2018, 18, 2463. [Google Scholar] [CrossRef]
- Zaytsev, D.; Frolov, Y.; Belyaev, A. Coding smell patterns of crude oil by the electronic nose: A soil pollution case. Ecol. Indic. 2024, 158, 111674. [Google Scholar] [CrossRef]
- Kong, X.; Ren, L.; Shi, X.; Chang, Z. Soil pesticides pollution detection and specific recognition using electronic nose. Sens. Actuators B Chem. 2024, 408, 135492. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Liu, G. A Novel Method for Soil Organic Matter Determination by Using an Artificial Olfactory System. Comput. Electron. Agric. 2019, 162, 3417. [Google Scholar] [CrossRef]
- Grassi, S.; Benedetti, S.; Opizzio, M.; di Nardo, E.; Buratti, S. Meat and Fish Freshness Assessment by a Portable and Simplified Electronic Nose System (Mastersense). Sensors 2019, 19, 3225. [Google Scholar] [CrossRef]
- Rusinek, R.; Dobrzański, B.; Gawrysiak-Witulska, M.; Siger, A.; Żytek, A.; Karami, H.; Umar, A.; Lipa, T.; Gancarz, M. Effect of the roasting level on the content of bioactive and aromatic compounds in Arabica coffee beans. Int. Agrophysics 2024, 38, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, 715763. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef]
- Rusinek, R.; Jeleń, H.; Malaga-Toboła, U.; Molenda, M.; Gancarz, M. Influence of Changes in the Level of Volatile Compounds Emitted during Rapeseed Quality Degradation on the Reaction of MOS Type Sensor-Array. Sensors 2020, 20, 3135. [Google Scholar] [CrossRef] [PubMed]
- Shell Tellus S2 VX46 Technical DataSheet. Available online: http://parsianlub.com/Technical%20Data%20/Tellus/Tellus%20S2%20VX%2046.pdf (accessed on 21 July 2025).
- Komatsu Product Data. Available online: https://www.komatsuforest.com/-/media/komatsu-forest/files/aftermarket-files/proselect/he-gen-ii-natura-en.pdf?la=en (accessed on 21 July 2025).
- Ministry of the Environment. Regulation of the Minister of Environment of September 1, 2016, on the Method of Conducting Soil Surface Contamination Assessmen. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20160001395 (accessed on 3 July 2025).
- Cheng, L.; Meng, Q.H.; Lilienthal, A.J.; Qi, P.F. Development of compact electronic noses: A review. Meas. Sci. Technol. 2021, 32, 062002. [Google Scholar] [CrossRef]
- Borowik, P.; Tkaczyk, M.; Pluta, P.; Okorski, A.; Stocki, M.; Tarakowski, R.; Oszako, T. Distinguishing between Wheat Grains Infested by Four Fusarium Species by Measuring with a Low-Cost Electronic Nose. Sensors 2024, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Figaro Engineering Inc. Figaro Gas Sensors & Modules. Available online: https://www.figarosensor.com/product/sensor/ (accessed on 30 June 2025).
- Borowik, P.; Grzywacz, T.; Tarakowski, R.; Tkaczyk, M.; Ślusarski, S.; Dyshko, V.; Oszako, T. Development of a Low-Cost Electronic Nose with an Open Sensor Chamber: Application to Detection of Ciboria batschiana. Sensors 2023, 23, 627. [Google Scholar] [CrossRef]
- Borowik, P.; Tarakowski, R.; Tkaczyk, M.; Ślusarski, S.; Oszako, T. Application of a Low-Cost Electronic Nose to Detect of Forest Tree Pathogens: Fusarium oxysporum and Phytophthora plurivora. IEEE Access 2022, 10, 93475–93487. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning. Data Mining, Inference, and Prediction; Springer Series in Statistics; Springer: New York, NY, USA, 2009. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; 2010; pp. 92–96. Available online: https://proceedings.scipy.org/articles/proceedings-2010 (accessed on 30 July 2025).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Jones, G.M.; Spannuth, A.; Chongpinitchai, A.; Hurteau, M.D. Prescribed fire, managed burning, and previous wildfires reduce the severity of a southwestern US gigafire. For. Ecol. Manag. 2025, 580, 122540. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Malara, A.; Jośko, I.; Lesiuk, A. The Phytotoxicity Changes of Sewage Sludge-Amended Soils. Water Air Soil Pollut. 2012, 223, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Uppar, R.; Dinesha, P.; Kumar, S. Characterization of Bio-Lubricants with Nanoparticles Additives. Energy Sources Part A Recover. Util. Environ. Eff. 2024, 46, 3684–3706. [Google Scholar] [CrossRef]
- Shah, R.; Woydt, M.; Zhang, S. The Economic and Environmental Significance of Sustainable Lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Madanhire, I.; Mbohwa, C. Environmentally Adapted Lubricants. In Mitigating Environmental Impact of Petroleum Lubricants; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–178. [Google Scholar] [CrossRef]
- Falandysz, J.; Zhang, J.; Mędyk, M.; Zhang, X. Mercury in stir-fried and raw mushrooms from the Boletaceae family from the geochemically anomalous region in the Midu county, China. Food Control 2019, 102, 17–21. [Google Scholar] [CrossRef]
- Tuomi, I. The Impact of Artificial Intelligence on Learning, Teaching, and Education; Publications Office of the European Commission: Luxembourg, 2018. [Google Scholar] [CrossRef]

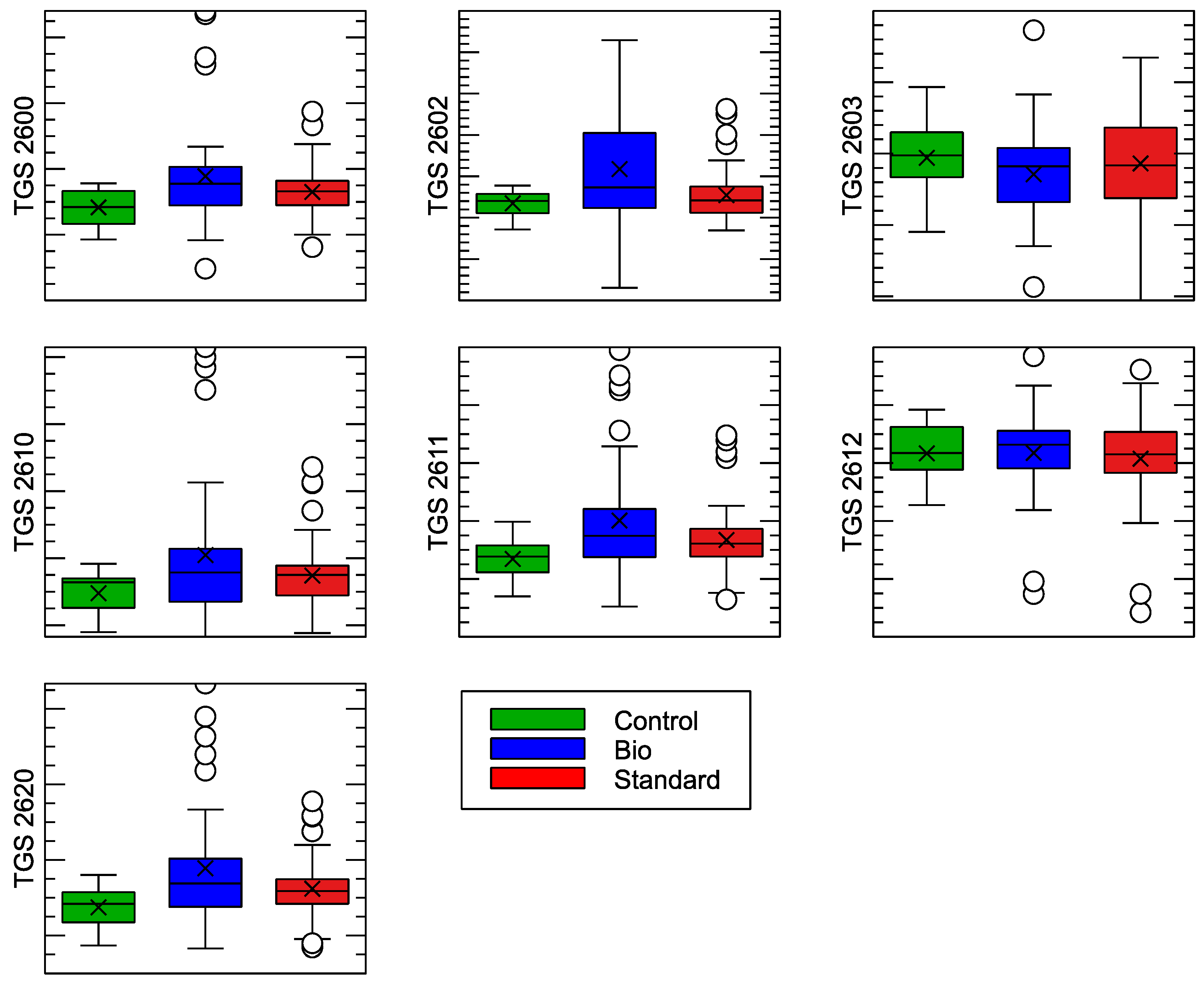

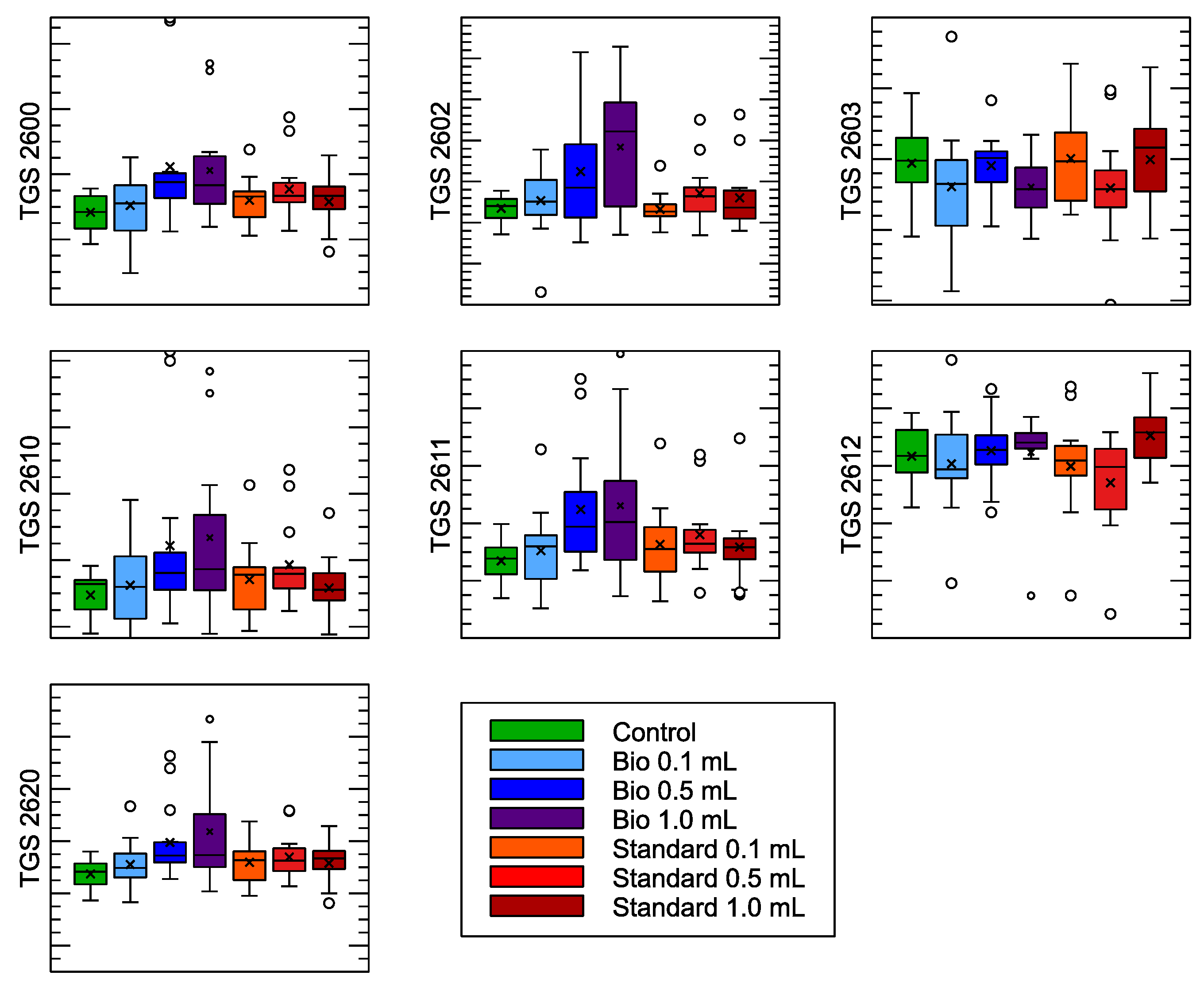
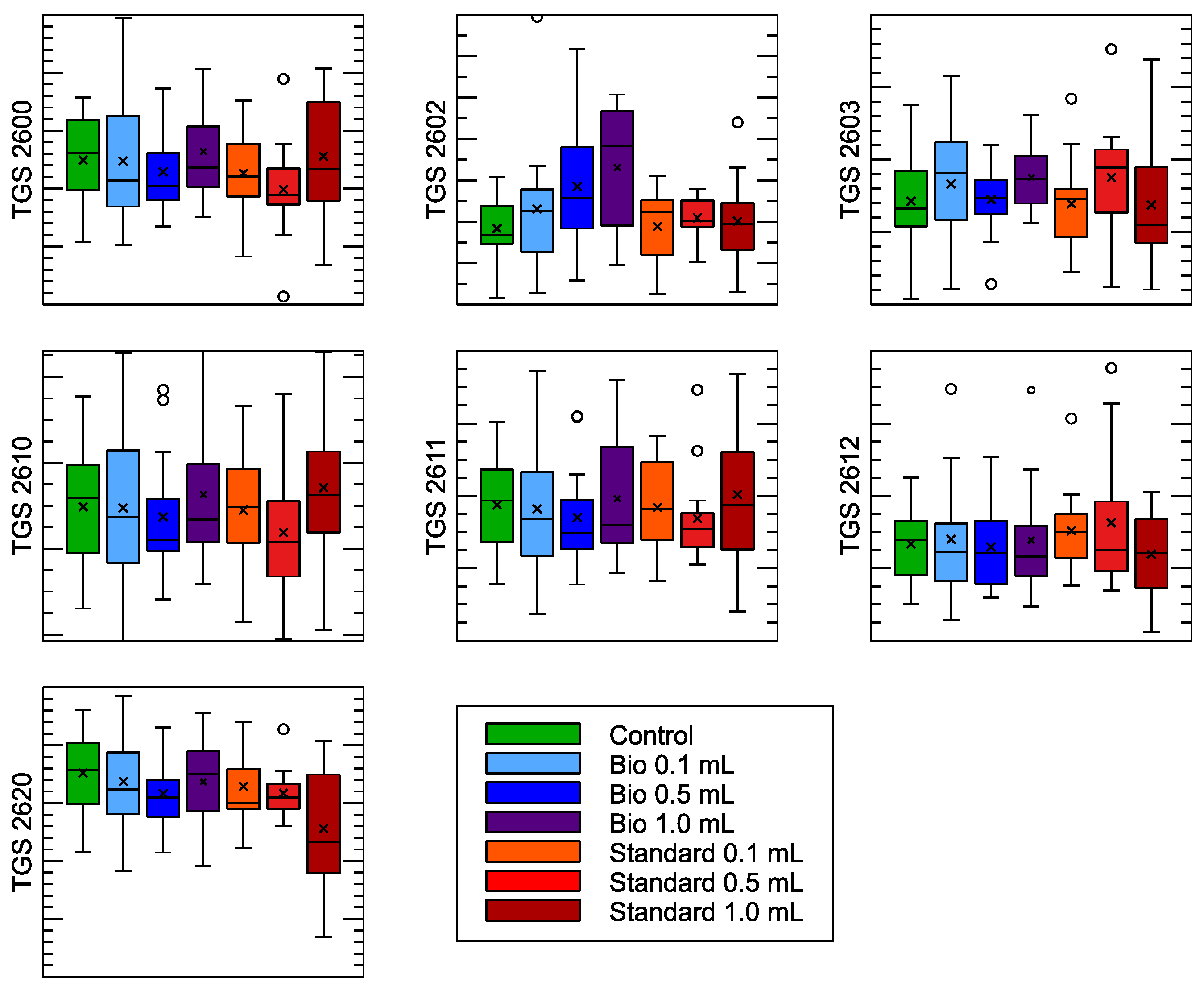

| Sensor Model | Target Gas Detection |
|---|---|
| TGS 2600 | Has a high sensitivity to low concentrations of gaseous air contaminants, such as hydrogen and carbon monoxide, which exist in cigarette smoke. The sensor can detect hydrogen at a level of several ppm. |
| TGS 2602 | Has high sensitivity to low concentrations of odorous gases, such as ammonia and H2S, generated from waste materials in office and home environments. The sensor also has a high sensitivity to low concentrations of VOCs, such as toluene, emitted from wood finishing and construction products. |
| TGS 2603 | Has high sensitivity to low concentrations of odorous gases, such as amine-series and sulfurous odors, generated from waste materials or spoiled foods such as fish. |
| TGS 2610 | Uses filter material in its housing, eliminating the influence of interference gases such as alcohol, resulting in a highly selective response to LP gas. |
| TGS 2611 | Uses filter material in its housing, which eliminates the influence of interfering gases such as alcohol, resulting in a highly selective response to methane gas. |
| TGS 2612 | Has high sensitivity to methane, propane, and butane, making it ideal for LNG and LPG monitoring. Due to its low sensitivity to alcohol vapors (a typical interference gas in the residential environment), the sensor is ideal for consumer market gas alarms. |
| TGS 2620 | Has high sensitivity to organic solvents and other volatile vapors, making it suitable for organic vapor detectors/alarms. |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Sensor | F-Statistics | p-Value | ||||
| TGS 2600 | 3.68 | 0.0290 | ||||
| TGS 2602 | 5.61 | 0.0050 | ||||
| TGS 2603 | 0.88 | 0.4171 | ||||
| TGS 2610 | 3.12 | 0.0488 | ||||
| TGS 2611 | 3.86 | 0.0245 | ||||
| TGS 2612 | 0.20 | 0.8227 | ||||
| TGS 2620 | 4.55 | 0.0131 | ||||
| (B) | ||||||
| TGS 2600 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0095 | 0.0317 | 0.0007 | 0.0183 | True |
| Control | Standard | 0.0047 | 0.4113 | −0.0041 | 0.0134 | False |
| Bio | Standard | −0.0048 | 0.1836 | −0.0112 | 0.0016 | False |
| TGS 2602 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0207 | 0.0212 | 0.0026 | 0.0388 | True |
| Control | Standard | 0.0049 | 0.7899 | −0.013 | 0.0229 | False |
| Bio | Standard | −0.0157 | 0.0154 | −0.0289 | −0.0025 | True |
| TGS 2603 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | −0.0115 | 0.4839 | −0.0351 | 0.0122 | False |
| Control | Standard | −0.0039 | 0.9177 | −0.0274 | 0.0196 | False |
| Bio | Standard | 0.0076 | 0.5519 | −0.0097 | 0.0248 | False |
| TGS 2610 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0114 | 0.0568 | −0.0003 | 0.0231 | False |
| Control | Standard | 0.0053 | 0.5237 | −0.0063 | 0.0169 | False |
| Bio | Standard | −0.0061 | 0.2072 | −0.0146 | 0.0024 | False |
| TGS 2611 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0133 | 0.027 | 0.0012 | 0.0253 | True |
| Control | Standard | 0.0066 | 0.3956 | −0.0054 | 0.0185 | False |
| Bio | Standard | −0.0067 | 0.168 | −0.0155 | 0.0021 | False |
| TGS 2612 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0001 | 0.9996 | −0.0109 | 0.0112 | False |
| Control | Standard | −0.0019 | 0.9131 | −0.0128 | 0.0091 | False |
| Bio | Standard | −0.002 | 0.8261 | −0.01 | 0.0061 | False |
| TGS 2620 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0103 | 0.0158 | 0.0016 | 0.0191 | True |
| Control | Standard | 0.0049 | 0.3715 | −0.0038 | 0.0136 | False |
| Bio | Standard | −0.0054 | 0.1099 | −0.0118 | 0.0009 | False |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Sensor | F-Statistics | p-Value | ||||
| TGS 2600 | 0.53 | 0.5895 | ||||
| TGS 2602 | 5.70 | 0.0046 | ||||
| TGS 2630 | 0.50 | 0.6088 | ||||
| TGS 2610 | 0.03 | 0.9742 | ||||
| TGS 2611 | 0.03 | 0.9692 | ||||
| TGS 2612 | 0.21 | 0.8133 | ||||
| TGS 2620 | 0.50 | 0.6092 | ||||
| (B) | ||||||
| TGS 2602 | ||||||
| group1 | group2 | meandiff | p-adj | lower | upper | reject |
| Control | Bio | 0.0096 | 0.0288 | 0.0008 | 0.0184 | True |
| Control | Standard | 0.0016 | 0.8993 | −0.0071 | 0.0104 | False |
| Bio | Standard | −0.008 | 0.0106 | −0.0144 | −0.0016 | True |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowik, P.; Pluta, P.; Tkaczyk, M.; Sztabkowski, K.; Tarakowski, R.; Oszako, T. Application of a Low-Cost Electronic Nose to Differentiate Between Soils Polluted by Standard and Biodegradable Hydraulic Oils. Chemosensors 2025, 13, 290. https://doi.org/10.3390/chemosensors13080290
Borowik P, Pluta P, Tkaczyk M, Sztabkowski K, Tarakowski R, Oszako T. Application of a Low-Cost Electronic Nose to Differentiate Between Soils Polluted by Standard and Biodegradable Hydraulic Oils. Chemosensors. 2025; 13(8):290. https://doi.org/10.3390/chemosensors13080290
Chicago/Turabian StyleBorowik, Piotr, Przemysław Pluta, Miłosz Tkaczyk, Krzysztof Sztabkowski, Rafał Tarakowski, and Tomasz Oszako. 2025. "Application of a Low-Cost Electronic Nose to Differentiate Between Soils Polluted by Standard and Biodegradable Hydraulic Oils" Chemosensors 13, no. 8: 290. https://doi.org/10.3390/chemosensors13080290
APA StyleBorowik, P., Pluta, P., Tkaczyk, M., Sztabkowski, K., Tarakowski, R., & Oszako, T. (2025). Application of a Low-Cost Electronic Nose to Differentiate Between Soils Polluted by Standard and Biodegradable Hydraulic Oils. Chemosensors, 13(8), 290. https://doi.org/10.3390/chemosensors13080290








