A pH-Responsive Liquid Crystal-Based Sensing Platform for the Detection of Biothiols
Abstract
1. Introduction
2. Materials and Methods
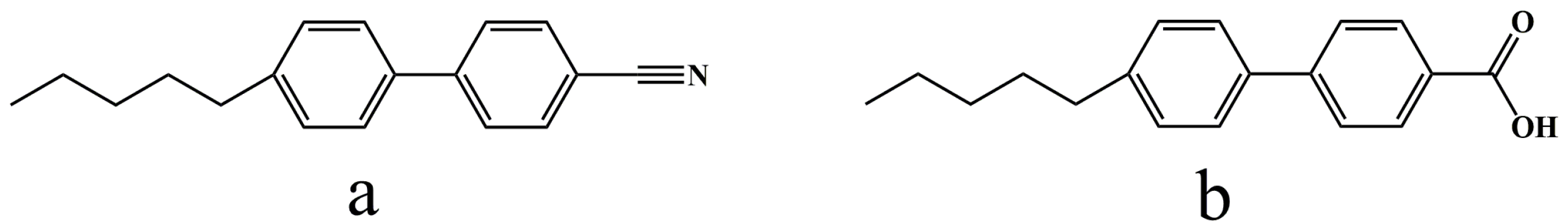
2.1. Reagents and Instruments
2.2. Cleaning of Glass Substrates
2.3. Silanization of Glass Substrates
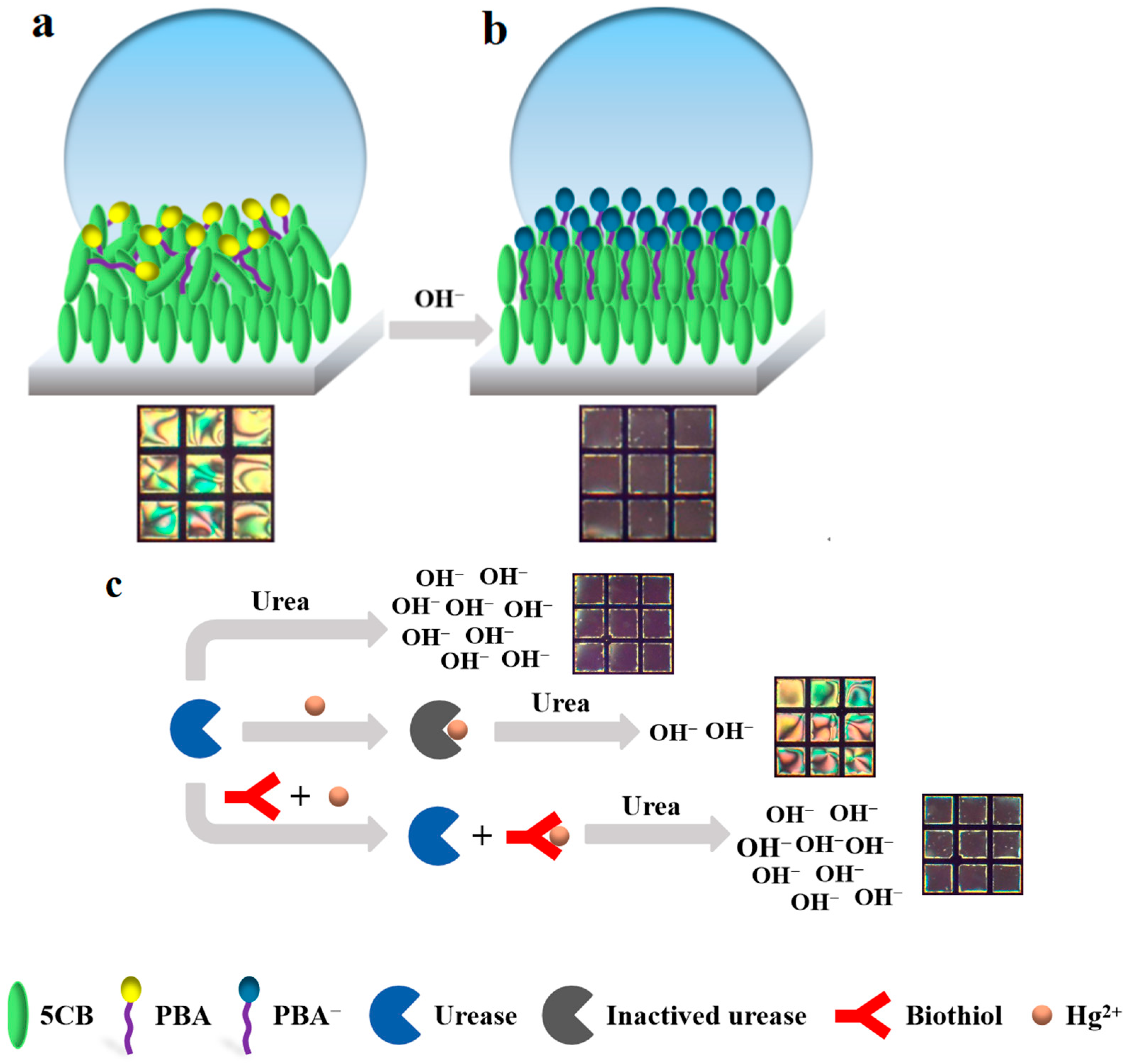
2.4. Preparation of LC Sensing Platform
2.5. Detection of Biothiols
2.6. Acquisition and Processing of LC Optical Images
3. Results and Discussion
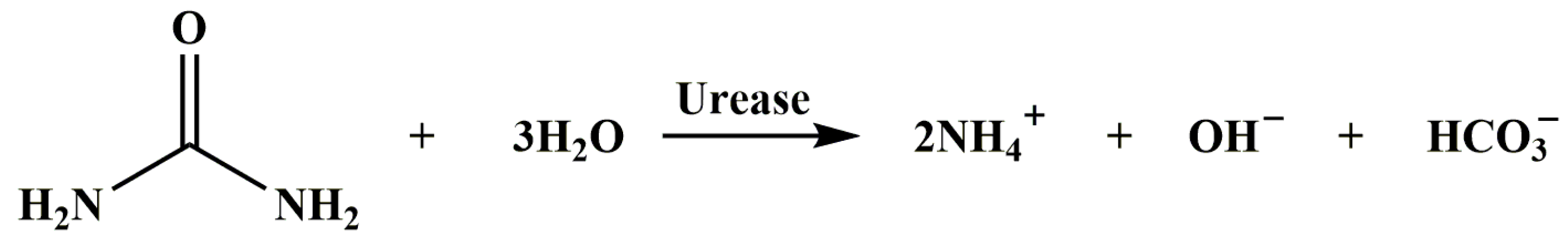
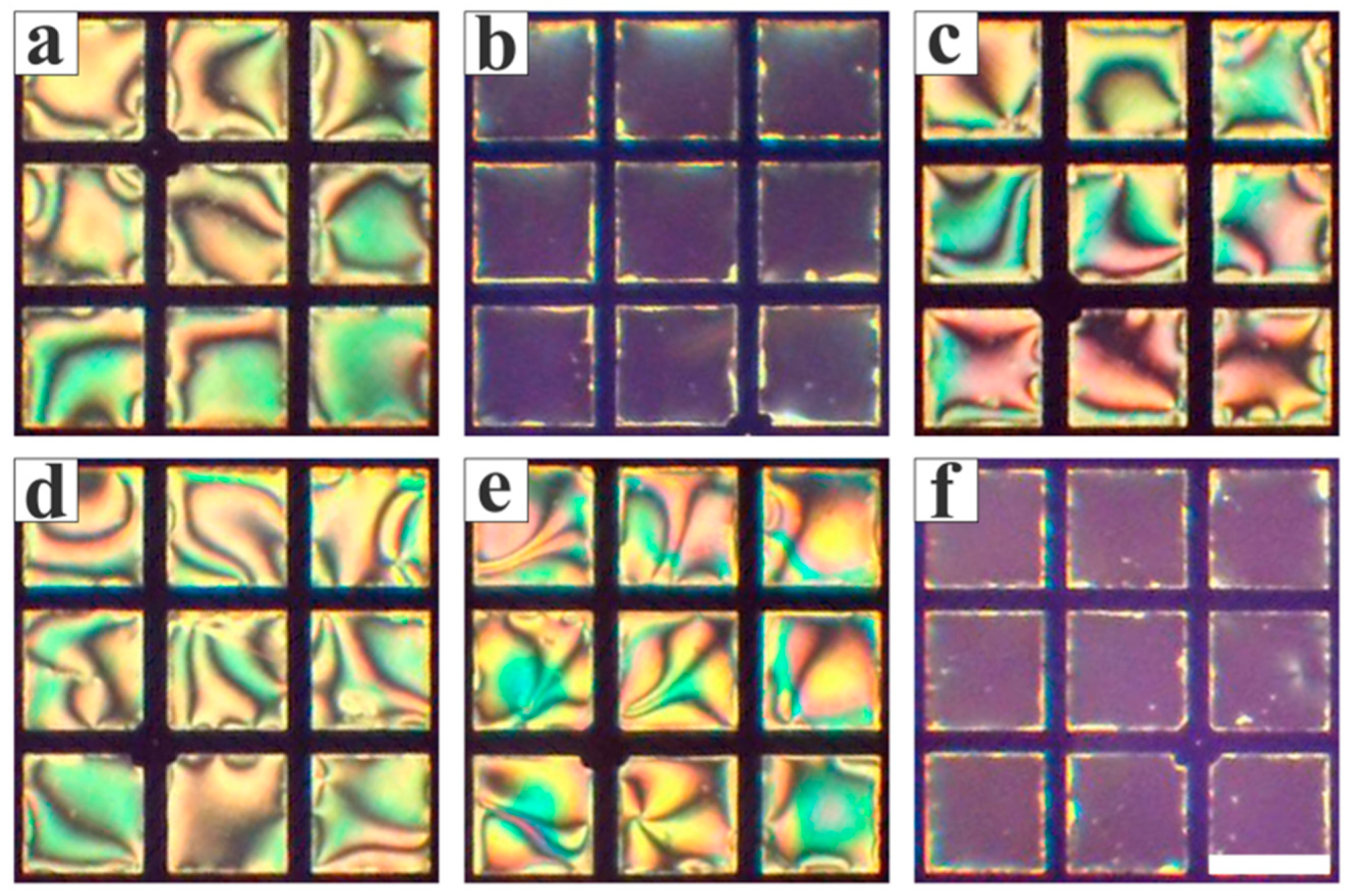
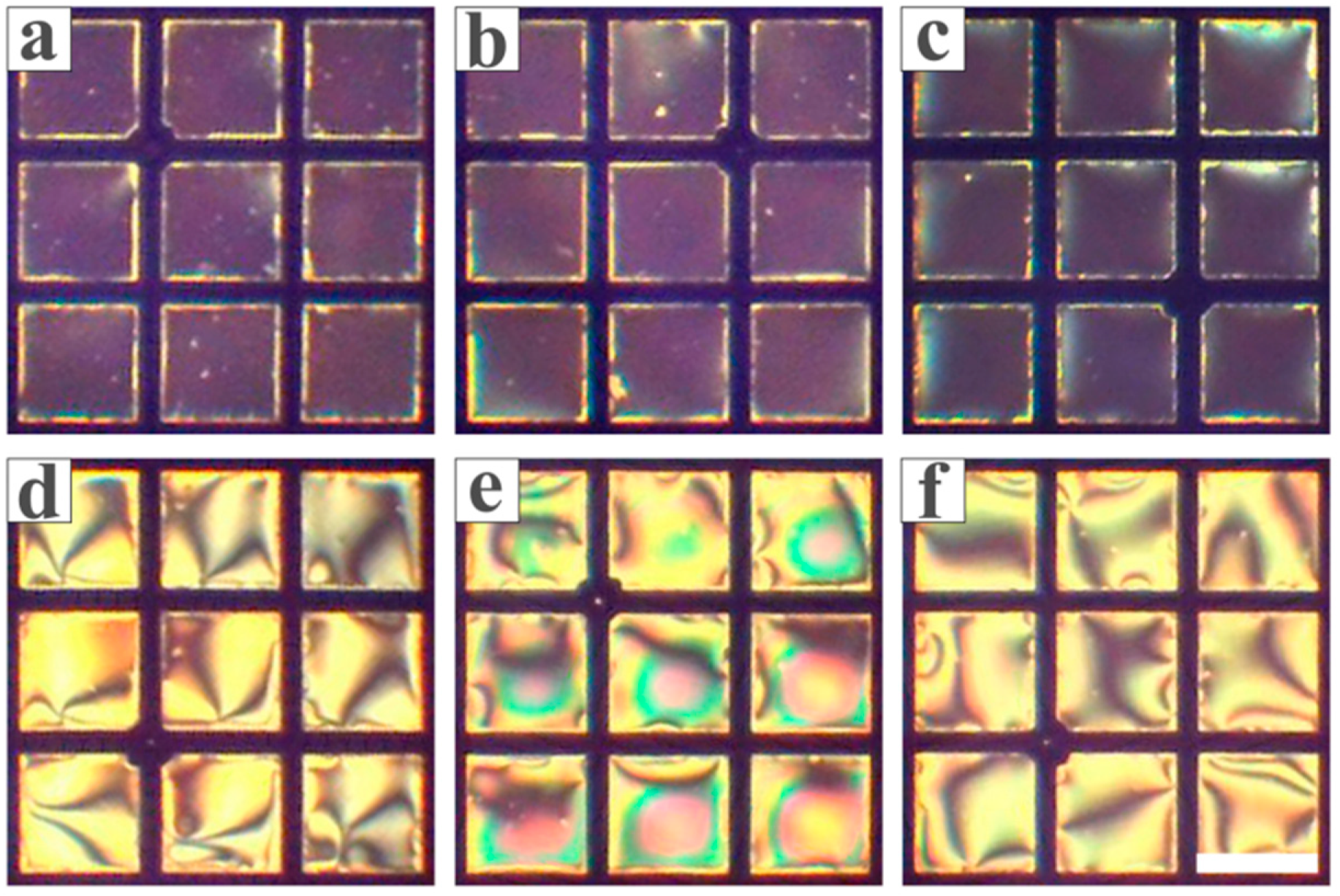
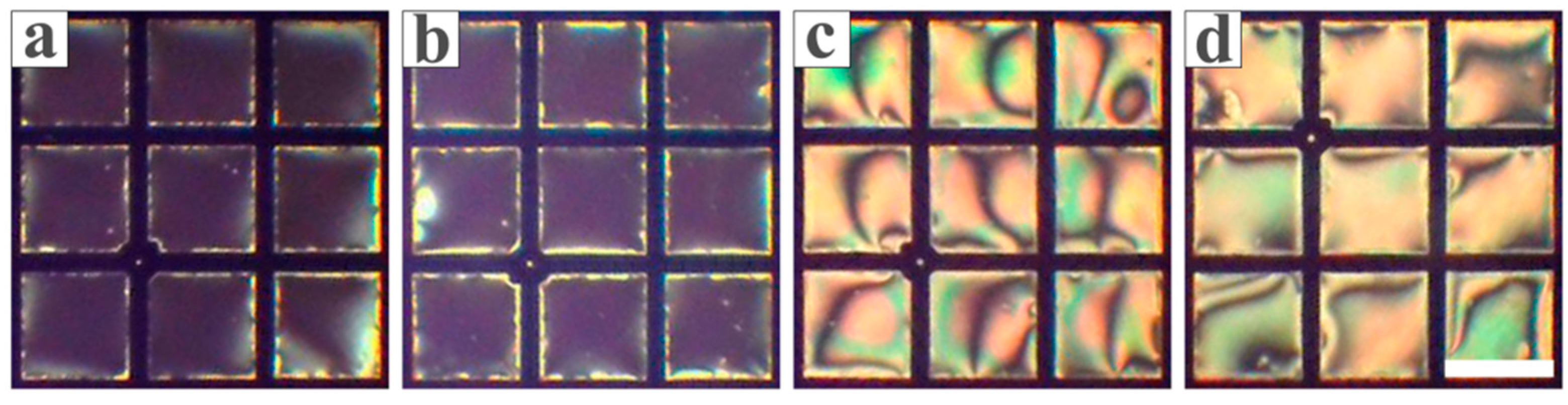
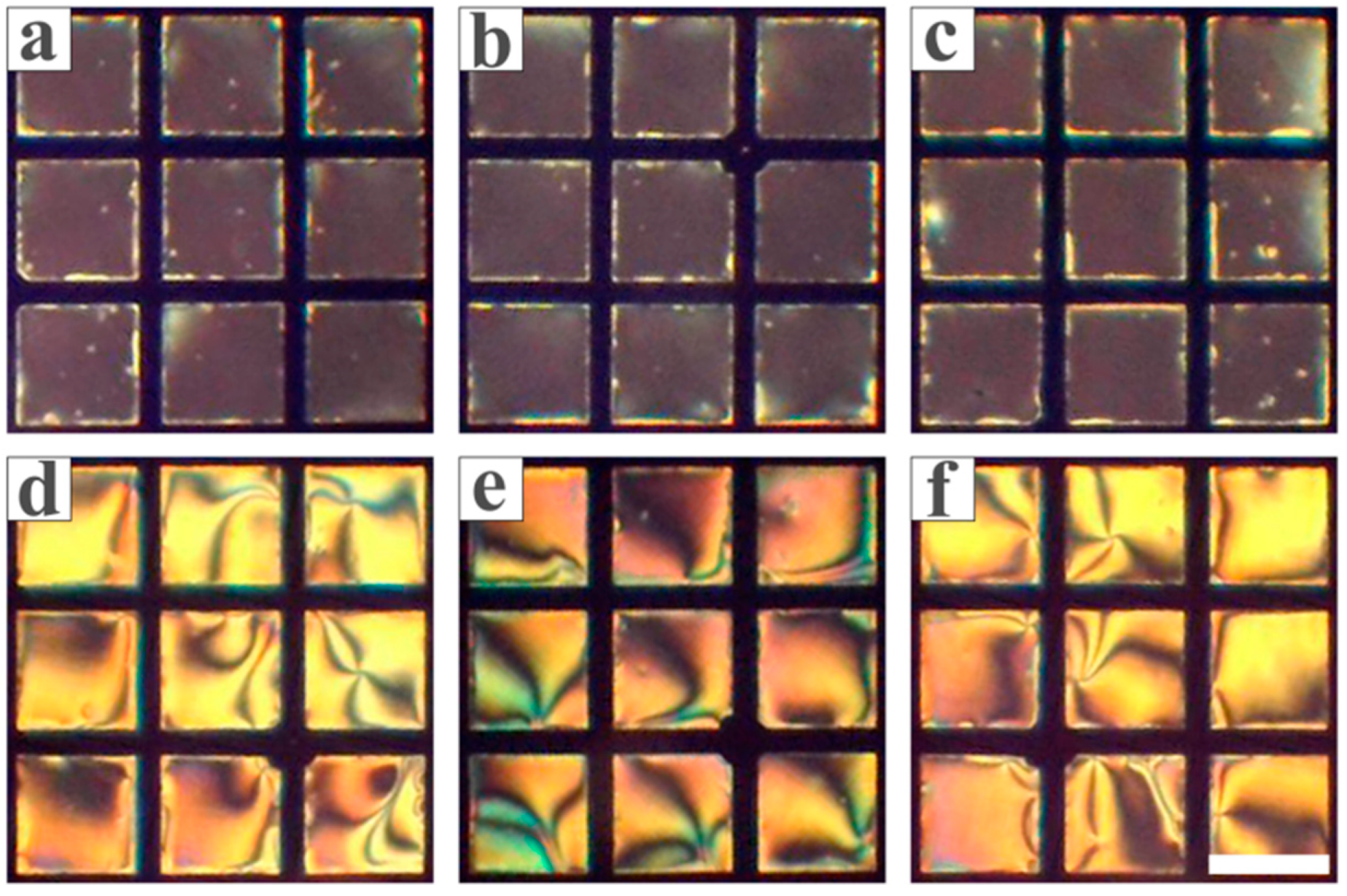
3.1. Feasibility Study
3.2. Optimization of the Conditions for the LC Sensing Platform
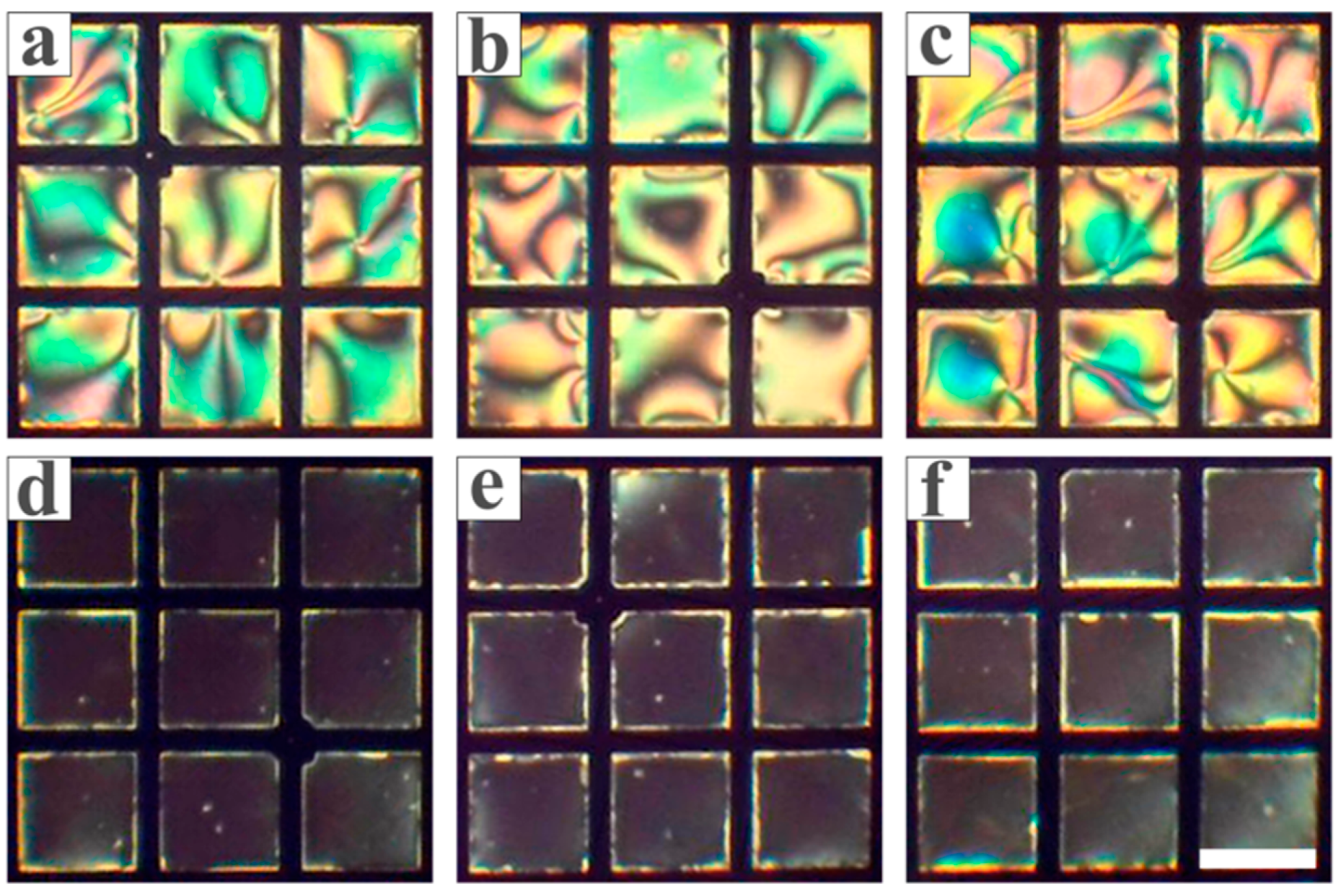
3.3. Inhibitory Effect of Heavy Metal Ions on Urease
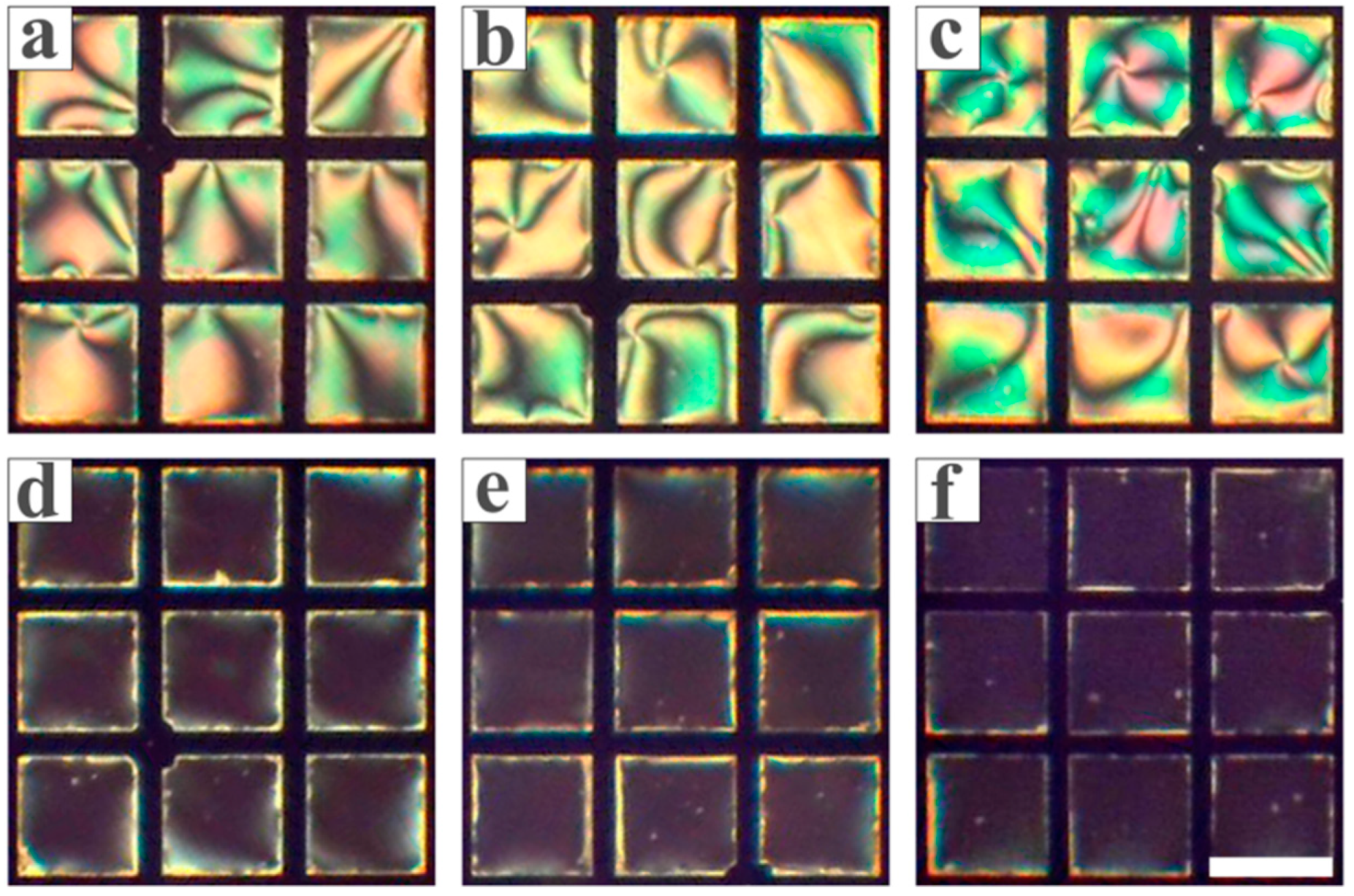
3.4. The Sensitivity and Specificity of the LC-Based Sensing Platform in Detecting Biothiols
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cys | Cysteine |
| Hcy | Homocysteine |
| GSH | Glutathione |
| LC | Liquid crystal |
| PBA | 4-n-pentylbiphenyl-4-carboxylic acid |
| 5CB | 4-n-pentyl-4-cyanobiphenyl |
| DMOAP | N, N-dimethyl-N-octadecyl-3-(trimethoxysilyl) propylammonium chloride |
| POM | Polarized optical microscope |
References
- Huang, W.; He, M.; Chen, S.; Yin, G.; Gan, Y.; Li, H.; Wu, C.; Yin, P. Dual-channel fluorescent detection of biothiols: A novel probe for distinguishing cysteine, homocysteine, glutathione, and N-Acetylcysteine in cellular environments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 1235221. [Google Scholar] [CrossRef] [PubMed]
- Cugnasca, B.S.; Santos, H.M.; Duarte, F.; Capelo-Martínez, J.L.; Dos Santos, A.A.; Lodeiro, C. Fluorescent discrimination of cysteine, homocysteine, and glutathione in urine samples using a novel seleno-BODIPY Probe. J. Mater. Chem. B 2024, 123, 123038–123049. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Wei, S.; Liu, X.; Li, J.; Wang, J. A pH-regulated colorimetric sensor array for discrimination of biothiols based on two different-sized β-cyclodextrin-functionalized AuNPs. J. Anal. Test. 2023, 7, 101–109. [Google Scholar] [CrossRef]
- Ding, X.; Yang, B.; Liu, Z.; Shen, M.; Fan, Z.; Wang, X.; Yu, W. A novel intramolecular charge transfer-based near-infrared fluorescent probe with large stokes shift for highly sensitive detection of cysteine in vivo. Anal. Chim. Acta 2023, 12380, 341873. [Google Scholar] [CrossRef]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Fontana, M.; Gunaydin Akyildiz, A.; D’Alonzo, C.; Giovannercole, F.; Zicchi, A.; Francioso, A.; Capuozzo, E.; De Biase, D. Synthesis and biological activity of homohypotaurine obtained by the enzyme-based conversion of homocysteine sulfinic acid using recombinant escherichia coli glutamate decarboxylase. Molecules 2024, 29, 3985. [Google Scholar] [CrossRef]
- Cotton, K.; Ayers, E.; Jin, Y.; Beauchet, O.; Derby, C.A.; Lipton, R.B.; Katz, M.; Galery, K.; Gaudreau, P.; Verghese, J. Elevated blood homocysteine increases the risk of incident motoric cognitive risk syndrome: A two-cohort study. J. Gerontol. Ser. A 2024, 79, 114. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, X.; Qiu, B. Genetically predicted circulating serum homocysteine levels on osteoporosis: A two-sample mendelian randomization study. Sci. Rep. 2023, 13, 9063. [Google Scholar] [CrossRef]
- Ruseva, S.; Popova, I.; Lozanov, V.; Mitev, V. Insight into the metabolite pattern of psoriasis: Correlation among homocysteine, methionine, and polyamines. Symmetry 2021, 13, 606. [Google Scholar] [CrossRef]
- Haseena, P.; Diwakar, L.; Ravindranath, V. Protein glutathionylation and glutaredoxin: Role in neurodegenerative diseases. Antioxidants 2022, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Erdos, T.; Masuda, M.; Venketaraman, V. Glutathione in HIV-associated neurocognitive disorders. Curr. Issues Mol. Biol. 2024, 46, 5530–5549. [Google Scholar] [CrossRef]
- Hu, Y.; Heo, C.H.; Kim, G.; Jun, E.J.; Yin, J.; Kim, H.M.; Yoon, J. One-photon and two-photon sensing of biothiols using a bis-pyrene-Cu(II) ensemble and its application to image GSH in the cells and tissues. Anal. Chem. 2015, 87, 3308–3313. [Google Scholar] [CrossRef]
- Yutani, R.; Venketaraman, V.; Sheren, N. Treatment of acute and long-COVID, diabetes, myocardial infarction, and alzheimer’s disease: The potential role of a novel nano-compound—The transdermal glutathione-cyclodextrin complex. Antioxidants 2024, 13, 1106. [Google Scholar] [CrossRef]
- Özyürek, M.; Baki, S.; Güngör, N.; Çelik, S.E.; Güçlü, K.; Apak, R. Determination of biothiols by a novel on-line HPLC-DTNB assay with post-column detection. Anal. Chim. Acta 2012, 750, 173–181. [Google Scholar] [CrossRef]
- Pudova, P.A.; Ivanov, A.V.; Popov, M.A.; Maslennikov, R.A.; Aleksandrin, V.V.; Galdobina, M.P.; Kubatiev, A. Determination of main aminothiols in blood plasma using capillary electrophoresis with UV detection. Biomed. Chromatogr. 2025, 39, e70017. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Takkar, P.; Gahlyana, P.; Kumar, R. A new pyranopyrazole based colorimetric chemosensor for the selective recognition of biothiols: Applications in real samples. Anal. Methods 2025, 17, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ni, P.; Sun, Y.; Wang, Y.; Wang, L.; Li, Z. Highly sensitive colorimetric determination of biothiols based on I−-H2O2-3,3′,5,5′-tetramethylbenzidine system. Sens. Actuators B 2018, 255, 3472–3478. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on carbon dot-based fluorescent detection of biothiols. Biosensors 2023, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zeng, Y.; Liu, M.; Yang, L.; Wang, J.; Song, X. Regulating sensing patterns in fluorescent probes for discriminative detection of biothiols. Anal. Chem. 2025, 97, 419–426. [Google Scholar] [CrossRef]
- Li, H.; An, Y.; Gao, J.; Yang, M.; Luo, J.; Li, X.; Lv, J.; Li, X.; Yuan, Z.; Ma, H. Recent advances of fluorescence probes for imaging of ferroptosis process. Chemosensors 2022, 10, 233. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Li, F.; Yu, Q.; Tan, S.; Chen, Y.; Yang, Z. Investigation of the Assembly Behavior of an Amphiphilic Lipopeptide at the Liquid Crystal-Aqueous Interface. Langmuir 2019, 35, 2490–2497. [Google Scholar] [CrossRef]
- Yang, C.; Chen, L.; Zhang, R.; Chen, D.; Laura, R.A.; David, A. Local high-density distributions of phospholipids induced by the nucleation and growth of smectic liquid crystals at the interface. Chin. Chem. Lett. 2022, 8, 3973–3976. [Google Scholar] [CrossRef]
- Satyabratt, P.; Madeeha, R.; Vishal, S.; Garima, S.; Chandan, B.; Rohit, V.; Dharm, D.; Ranjan, K.; Sachin, K. Real-time optical detection of mercury contamination in drinking water using an amphiphilic recognition probe at liquid crystal/aqueous interfaces. Anal. Methods 2024, 16, 8139–8147. [Google Scholar]
- Kinsinger, M.I.; Sun, B.; Abbott, N.L.; Lynn, D.M. Reversible control of ordering transitions at aqueous/liquid crystal interfaces using functional amphiphilic polymers. Adv. Mater. 2007, 19, 4208–4212. [Google Scholar] [CrossRef]
- Bi, X.; Hartono, D.; Yang, K.-L. Real-time liquid crystal pH sensor for monitoring enzymatic activities of penicillinase. Adv. Funct. Mater. 2009, 19, 3760–3765. [Google Scholar] [CrossRef]
- Li, G.; Xiao, F.; Liao, S.; Chen, Q.; Zhou, J.; Wu, Z.; Yu, R. Label-free 2D colloidal photonic crystal hydrogel biosensor for urea and urease inhibitor. Sens. Actuators B 2018, 277, 591–597. [Google Scholar] [CrossRef]
- Liu, J.; Tai, W.; Wang, D.; Su, J.; Yu, L. Cholesteric liquid crystal photonic hydrogel films immobilized with urease used for the detection of Hg2+. Chemosensors 2022, 10, 140. [Google Scholar] [CrossRef]
- Recky, J.R.N.; Montero-Jimenez, M.; Scotto, J.; Azzaroni, O.; Marmisollé, W.A. Urea biosensing through integration of urease to the PEDOT-polyamine conducting channels of organic electrochemical transistors: pH-change-based mechanism and urine sensing. Chemosensors 2024, 123, 1234. [Google Scholar]
- Zhang, R.; Liao, W.; Sun, Y.; Heng, J.Y.Y.; Yang, Z. Investigating the role of glass and quartz substrates on the formation of interfacial droplets. J. Phys. Chem. C 2019, 123, 1151–1159. [Google Scholar] [CrossRef]
- Zhang, W.; Ang, W.T.; Xue, C.-Y.; Yang, K.-L. Minimizing nonspecific protein adsorption in liquid crystal immunoassays by using surfactants. ACS Appl. Mater. Interfaces 2011, 3, 3496–3500. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Zafiu, C.; Küpcü, S.; Pivetta, L.; Hollfelder, N.; Masutani, A.; Kilickiran, P.; Sinner, E.-K. Liquid crystal based sensors monitoring lipase activity: A new rapid and sensitive method for cytotoxicity assays. Biosens. Bioelectron. 2014, 56, 210–216. [Google Scholar] [CrossRef]
- Tsuei, M.; Shivrayan, M.; Kim, Y.-K.; Thayumanavan, S.; Abbott, N.L. Optical “Blinking” triggered by collisions of single supramolecular assemblies of amphiphilic molecules with interfaces of liquid crystals. J. Am. Chem. Soc. 2020, 142, 6139–6148. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Luo, L.; Liu, Q.; Chen, Z. Colorimetric sensor array for discrimination of heavy metal ions in aqueous solution based on three kinds of thiols as receptors. Anal. Chem. 2018, 90, 4770–4775. [Google Scholar] [CrossRef]
- Lei, C.; Dai, H.; Fu, Y.; Ying, Y.; Li, Y. Colorimetric sensor array for thiols discrimination based on urease-metal ion pairs. Anal. Chem. 2016, 88, 8542–8547. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Z.; Wang, G. An overview: Metal-based inhibitors of urease. J. Enzym. Inhib. Med. Chem. 2023, 38, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; He, P.; Wang, D.; Liang, Y.; Gao, X.; Hou, X. Bimetallic Fe3O4@Co3O4/CN as a Nanozyme with Dual Enzyme-Mimic Activities for the Colorimetric Determination of Mercury(II). Chemosensors 2024, 123, 104. [Google Scholar] [CrossRef]
- Shaw, W.H.R. The inhibition of urease by various metal ions. J. Am. Chem. Soc. 1954, 76, 2160–2163. [Google Scholar] [CrossRef]
- Habala, L.; Devínsky, F.; Egger, A.E. Review: Metal complexes as urease inhibitors. J. Coord. Chem. 2018, 71, 907–940. [Google Scholar] [CrossRef]
- Huang, M.; Cui, P.; Zhou, J.; Liu, C.; Wang, Y. Theoretical study on the inhibition mechanisms of heavy metal ions on urease activity. Chemosphere 2023, 345, 140416. [Google Scholar] [CrossRef]
- Huang, Y.-K.; Qiu, X.-H. Development and application of the concept of Hard-Soft-Acid-Base (HSAB). Univ. Chem. 2016, 31, 45–50. [Google Scholar] [CrossRef]
- Qi, X.; Xie, Y.-L.; Niu, J.-Y.; Zhao, J.-W.; Li, Y.-M.; Fang, W.-H.; Zhang, J. Application of hard and soft acid-base theory to construct heterometallic materials with metal-oxo clusters. Angew. Chem. Int. Ed. 2025, 64, e202417548. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Q.; Tian, T.; Gao, Y.; Yu, L. A liquid crystal-based sensor for the simple and sensitive detection of cellulase and cysteine. Colloids Surf. B Biointerfaces 2016, 147, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kirtika, M.; Bachcha, S. Liquid crystal-based optical sensor for the detection of cysteine through competitive host-guest inclusion. Liq. Cryst. 2020, 48, 980–990. [Google Scholar]
- Zhu, D.; Ren, A.; Xue, L. A mitochondria-targeted colorimetric and NIR ratiometric fluorescent probe for biothiols with large Stokes shift based on thiol–chromene click reaction. Org. Biomol. Chem. 2024, 22, 9113–9120. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Qiao, L.; Li, K.; Zhu, D.; Zhang, Y. Mitochondria-Targeted NIR ratiometric and colorimetric fluorescent probe for biothiols based on a thiol-chromene click reaction. Anal. Chem. 2024, 96, 17773–17780. [Google Scholar] [CrossRef]
- Li, X.; Yuan, R.; Ma, Y.; Li, G.; Ma, S. A highly selective and sensitive fluorescent probe with a large Stokes shift for Near-Infrared visualization of endogenous and exogenous biothiols in living cells. Front. Biosci.-Landmark. 2025, 30, 37240. [Google Scholar] [CrossRef]
- Yu, T.; Li, Y.; Li, J.; Gan, Y.; Long, Z.; Deng, Y.; Zhang, Y.; Li, H.; Yin, P.; Yao, S. Multifunctional fluorescent probe for simultaneous detection of ATP, Cys, Hcy, and GSH: Advancing insights into epilepsy and liver injury. Adv. Sci. 2025, 12, 2415882. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, J.; Yan, H.; Li, M.; Huang, C.; Wang, J. Label-free gold nanorods sensor array for colorimetric detection and discrimination of biothiols in human urine samples. Talanta 2019, 203, 220–226. [Google Scholar] [CrossRef]
- Hou, W.; Liu, X.; Lu, Q.; Liu, M.; Zhang, Y.; Yao, S. Etching and anti-etching strategy for sensitive colorimetric sensing of H2O2 and biothiols based on silver/carbon nanomaterial. Colloids Surf. B 2018, 162, 118–125. [Google Scholar] [CrossRef]
- Yuan, C.; Qin, X.; Xu, Y.; Li, X.; Chen, Y.; Shi, R.; Wang, Y. Carbon quantum dots originated from chicken blood as peroxidase mimics for colorimetric detection of biothiols. J. Photochem. Photobiol. A 2020, 396, 112529. [Google Scholar] [CrossRef]




| Method | Detection Limit | Specificity | Cost | Response Time | Reference |
|---|---|---|---|---|---|
| Fluorescence | 97 nM (Cys) 94 nM (Hcy) 93 nM (GSH) | High | High | ≤10 min | [44] |
| Fluorescence | 29 nM (Cys) 16 nM (Hcy) 23 nM (GSH) | High | High | ≤30 min | [45] |
| Fluorescence | 0.129 μM (Cys) 0.143 μM (Hcy) 0.142 μM (GSH) | High | High | ≤7 min | [46] |
| Fluorescence | 12.5 nM (Cys) 1.0 nM (Hcy) 2.4 nM (GSH) | High | High | ≤13 min | [47] |
| Colorimetric | 31.12 μM (Cys) 13.24 μM (Hcy) 12.45 μM (GSH) | Medium | Medium | ≤30 min | [48] |
| Colorimetric | 0.03 μM (Cys) 0.05 μM (Hcy) 0.02 μM (GSH) | Medium | Medium | ≤30 min | [49] |
| Colorimetric | 0.4 μM (Cys) 0.6 μM (Hcy) 0.9 μM (GSH) | Medium | Medium | ≤25 min | [50] |
| Liquid Crystal | 0.1 μM (Cys) 0.1 μM (Hcy) 0.5 μM (GSH) | Medium | Low | ≤5 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, R.; Dong, X.; Wang, Z.; Yu, L. A pH-Responsive Liquid Crystal-Based Sensing Platform for the Detection of Biothiols. Chemosensors 2025, 13, 291. https://doi.org/10.3390/chemosensors13080291
Meng X, Zhang R, Dong X, Wang Z, Yu L. A pH-Responsive Liquid Crystal-Based Sensing Platform for the Detection of Biothiols. Chemosensors. 2025; 13(8):291. https://doi.org/10.3390/chemosensors13080291
Chicago/Turabian StyleMeng, Xianghao, Ronghua Zhang, Xinfeng Dong, Zhongxing Wang, and Li Yu. 2025. "A pH-Responsive Liquid Crystal-Based Sensing Platform for the Detection of Biothiols" Chemosensors 13, no. 8: 291. https://doi.org/10.3390/chemosensors13080291
APA StyleMeng, X., Zhang, R., Dong, X., Wang, Z., & Yu, L. (2025). A pH-Responsive Liquid Crystal-Based Sensing Platform for the Detection of Biothiols. Chemosensors, 13(8), 291. https://doi.org/10.3390/chemosensors13080291





