Optimized SILAR Growth of Vertically Aligned ZnO Nanorods for Low-Temperature Acetone Detection
Abstract
1. Introduction
2. Experimental Section
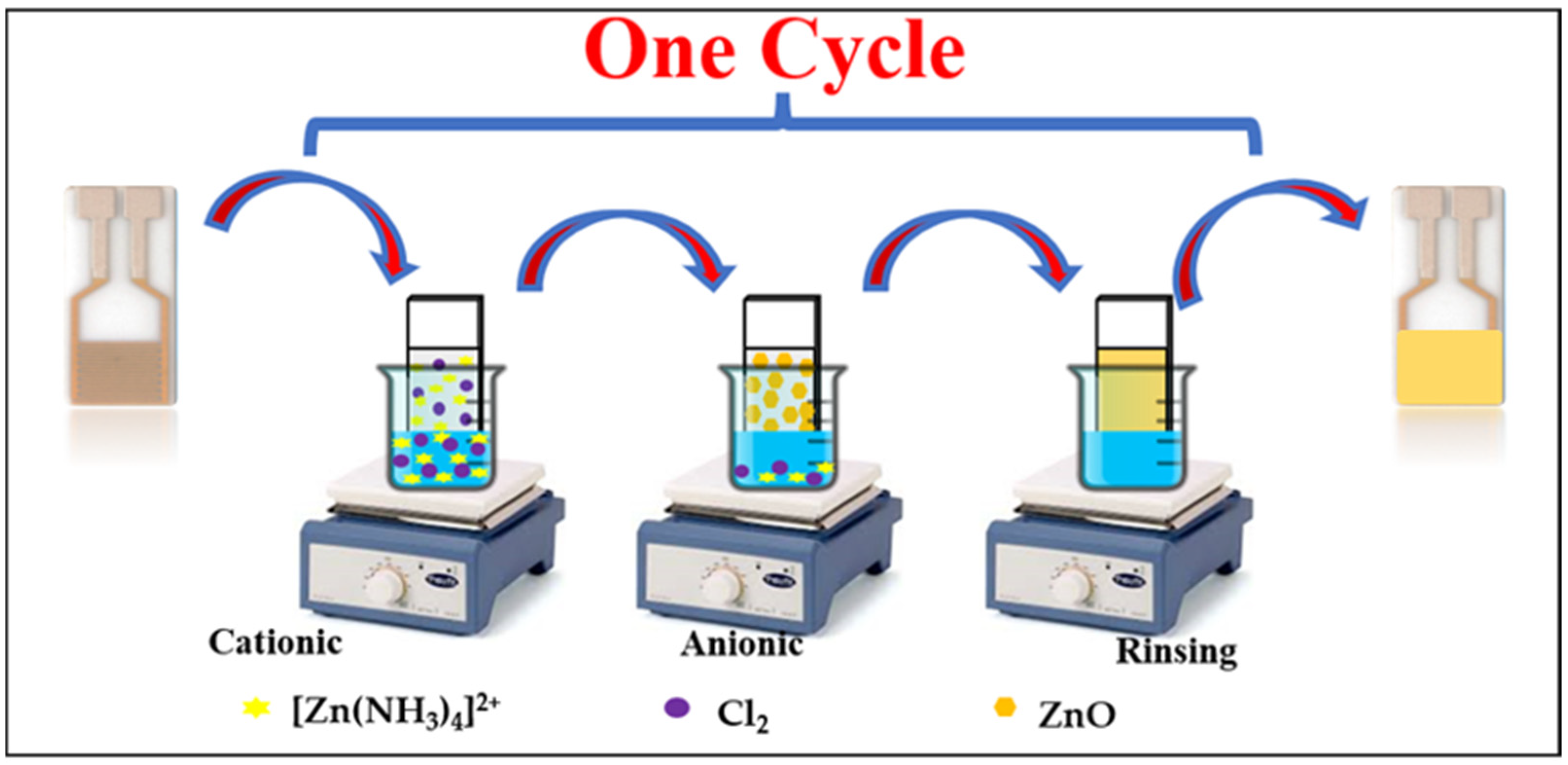
2.1. Synthesis of ZnO Nanorods
2.2. Characterization
2.3. Manufacture and Measurement of the Gas Sensor
3. Results and Discussion
3.1. Structural Study
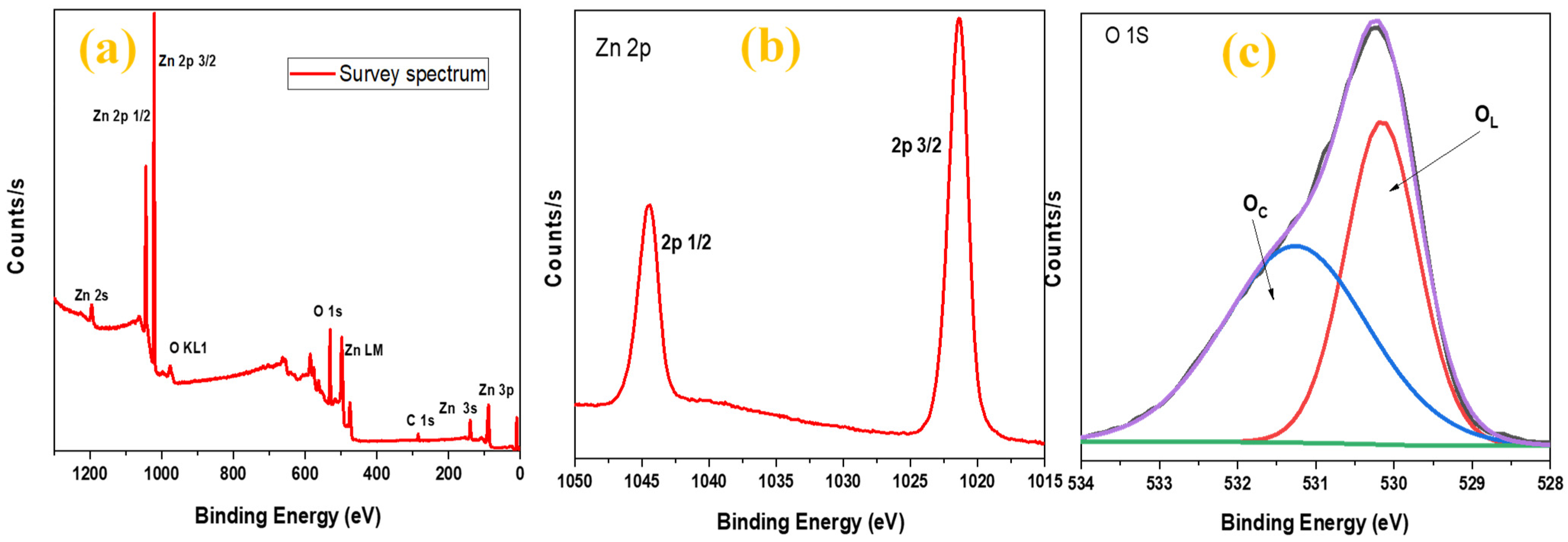
3.2. Compositional and Morphological Analysis
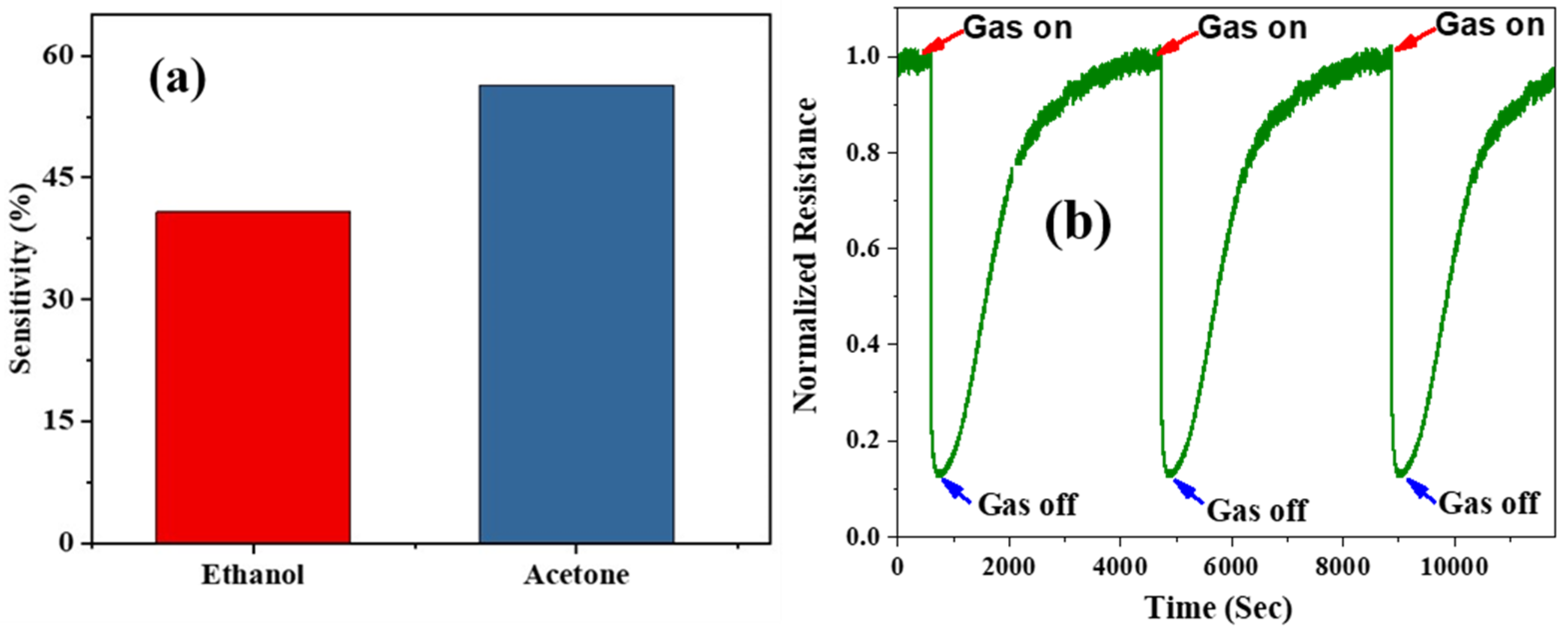
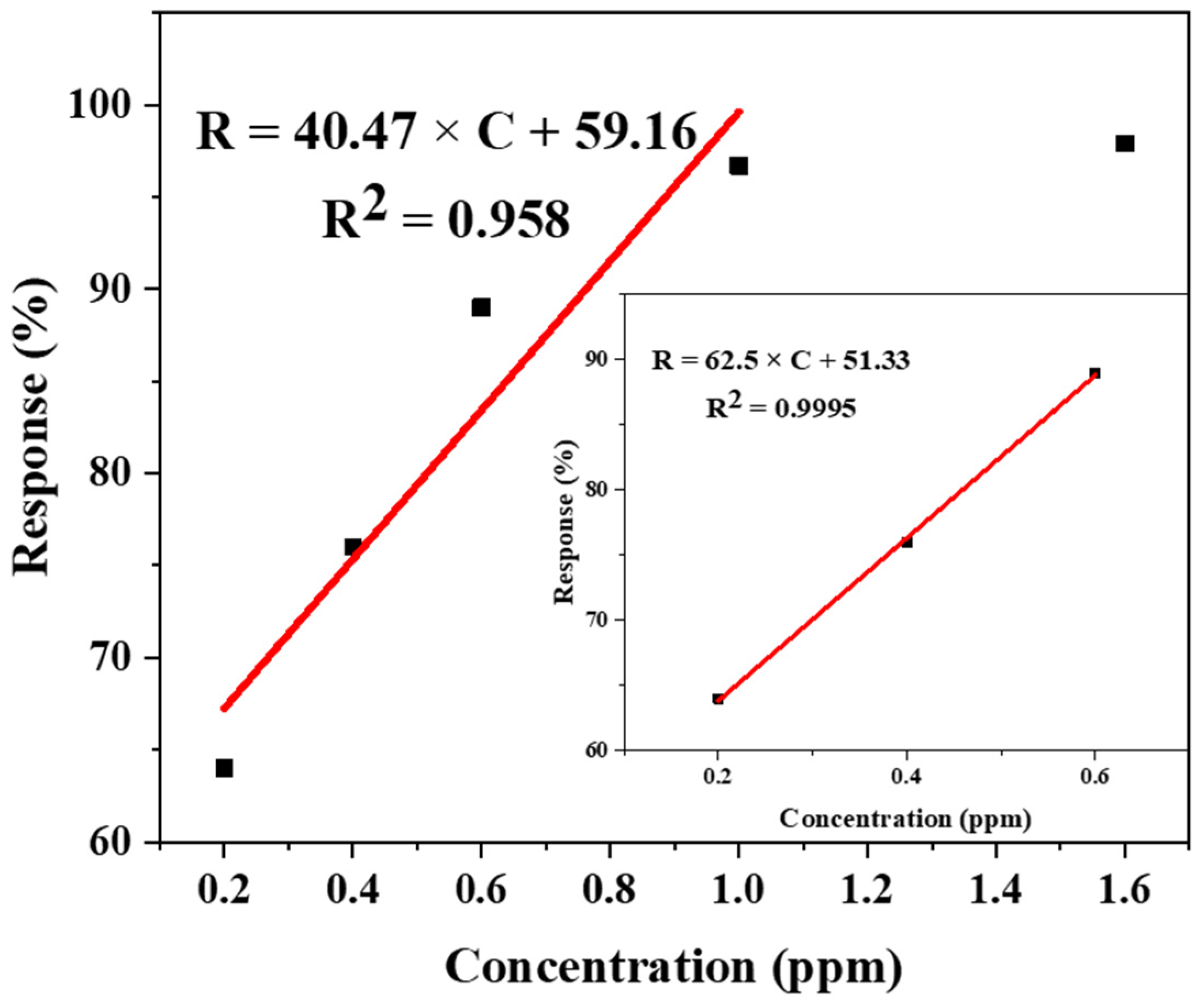
3.3. Gas Sensor Properties
| Material | T (°C) | Response (%) | Response Time (s) | Ref |
|---|---|---|---|---|
| ZnO | 260 | 8 | --- | [59] |
| ZnO nanorods | 219 | 12.9 | 13 | [28] |
| Ag/ZnO nanoneedles | 370 | 30.23 | 10 | [30] |
| ZnO pure | 400 | 2.3 | -- | [26] |
| ZnO indium | 200 | 19.3 | --- | [60] |
| ZnO nanorods | 320 | 50 | 60 | [61] |
| Al-ZnO nanorods | 250 | 50 | 12 | [62] |
| ZnO nanorods (aligned) | 280 | 150 | 9 | |
| ZnO nanoparticles (NPs) | 300 | 11.54 | 25 | [63] |
| 2% Ni-doped ZnO | RT | ~78 | 33 | [64] |
| 0.5% Fe-ZnO | 365 | 105.7 | 3 | [65] |
| Hierarchical ZnO + Au | 300 | 76 | 6 | [66] |
| 5 wt% Ni- ZnO NFs | 260 | 132.14 | 80 | [67] |
| Au-Pd ZnO nanorods | 225 | 14 | 7 | [31] |
| ZnO nanorods | 260 | 96 | 3 | This work |
3.4. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sens. Rev. 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagn. Rev. 2018, 8, 12. [Google Scholar] [CrossRef]
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications. Nanomaterials 2021, 11, 1927. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.K.; Chandu, B.; Puvvada, N. Recent Advances in Nanostructured Materials for Application as Gas Sensors. ACS Omega 2024, 9, 3092–3122. [Google Scholar] [CrossRef]
- Routoure, M.J. Elaboration and Characterization of 2% AZO Thin Films and Their Application to Gas Detection by Low-Frequency Noise Measurement; Abicha Agnaou, University of the Littoral Opal Coast, Dynamics Unit: Dunkirk, France, 2020. [Google Scholar]
- Fioravanti, A.; Carotta, M.C. Year 2020: A Snapshot of the Last Progress in Flexible Printed Gas Sensors. Appl. Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Abdulrahman, A.F.; Abd-Alghafour, N.M.; Almessiere, M.A. A High Responsivity, Fast Response Time of ZnO Nanorods UV Photodetector with Annealing Time Process. Opt. Mater. 2023, 141, 113869. [Google Scholar] [CrossRef]
- Alfaro Cruz, M.R.; Garza-Hernández, R.; Horley, P.P.; Mata-Ramírez, J.; Martínez-G., E.; Aguirre-Tostado, F.S. Low Temperature ZnO Films Grown by Successive Ionic Layer Adsorption and Reaction Method. Thin Solid Films 2018, 663, 49–55. [Google Scholar] [CrossRef]
- Zhang, C.; Tu, Q.; Francis, L.F.; Kortshagen, U.R. Band Gap Tuning of Films of Undoped ZnO Nanocrystals by Removal of Surface Groups. Nanomaterials 2022, 12, 565. [Google Scholar] [CrossRef]
- Gaikwad, M.A.; Suryawanshi, M.P.; Maldar, P.S.; Dongale, T.D.; Moholkar, A.V. Nanostructured Zinc Oxide Photoelectrodes by Green Routes M-SILAR and Electrodeposition for Dye Sensitized Solar Cell. Opt. Mater. 2018, 78, 325–334. [Google Scholar] [CrossRef]
- Luh, N.; Septiani, W.; Saputro, A.G.; Kaneti, Y.V.; Maulana, A.L.; Fathurrahman, F.; Lim, H.; Yuliarto, B.; Dipojono, H.K.; Golberg, D.; et al. Hollow Zinc Oxide Microsphere-Multiwalled Carbon Nanotubes Composites for Selective Detection of Sulfur Dioxide. ACS Appl. Nano Mater. 2020, 3, 8982–8996. [Google Scholar] [CrossRef]
- Singh, A.; Sikarwar, S.; Verma, A.; Chandra Yadav, B. The Recent Development of Metal Oxide Heterostructures Based Gas Sensor, Their Future Opportunities and Challenges: A Review. Sens. Actuators A Phys. 2021, 332, 113127. [Google Scholar] [CrossRef]
- Ivanishcheva, A.P.; Sysoev, V.V.; Abdullin, K.A.; Nesterenko, A.V.; Khubezhov, S.A.; Petrov, V.V. The Application of Combined Visible and Ultraviolet Irradiation to Improve the Functional Characteristics of Gas Sensors Based on ZnO/SnO2 and ZnO/Au Nanorods. Chemosensors 2023, 11, 200. [Google Scholar] [CrossRef]
- Sarf, F. Metal Oxide Gas Sensors by Nanostructures. Gas Sens. 2020, 1–17. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent Progress in Hybrid Conducting Polymers and Metal Oxide Nanocomposite for Room-Temperature Gas Sensor Applications: A Review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Ydir, B.; Nidlhaj, A.; Bouaaliouat, O.; Ait-Wahmane, Y.; Bouabid, K.; El Fanaoui, A.; Ihlal, A.; Bousseta, M.; Lahlou, H. Towards the Development of a Gas Micro-Sensor Based on Nano-Structured Zinc Oxide Thin Film for Ethanol Gas Detection. Mater. Today Proc. 2021, 52, 89–94. [Google Scholar] [CrossRef]
- Calixto-Rodriguez, M.; Valdez Martínez, J.S.; Meneses-Arcos, M.A.; Ortega-Cruz, J.; Sarmiento-Bustos, E.; Reyes-Mayer, A.; González-Castañeda, M.; Domínguez García, R.O. Design and Development of Software for the Silar Control Process Using a Low-Cost Embedded System. Processes 2021, 9, 967. [Google Scholar] [CrossRef]
- Ydir, B.; Ajdour, A.; Antohe, I.; Socol, G.; Socol, M.; Toderascu, L.; Saadaoui, D.; Choulli, I.; Leghrib, R. Aluminum Doped Zinc Oxide Nanoplatelets Based Sensor with Enhanced Hydrogen Sulfide Detection. Sci. Rep. 2025, 15, 8633. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.G. Two-Dimensional Zinc Oxide Nanostructures for Gas Sensor Applications. Chemosensors 2018, 5, 17. [Google Scholar] [CrossRef]
- Bouaaliouat, O.; Ydir, B.; Ajdour, A.; Soumane, M.; Leghrib, R.; Lahlou, H. Inspecting Process-Diameter Relationships of Forcespun PVP Ultrafine Fibers via RSM and ANN-PCA Approaches. Fibers Polym. 2024, 25, 853–868. [Google Scholar] [CrossRef]
- Ydir, B.; Boualiouaat, O.; Lahlou, H.; Bousseta, M.; Akhouayri, E.; Fierro, V. Effect of UV-Light Illumination on Room-Temperature-ZnO Nanotubes for Ethanol Gas Sensing. J. At. Mol. Condens. Matter Nano Phys. 2020, 7, 155–165. [Google Scholar] [CrossRef]
- Ahmad, R.; Majhi, S.M.; Zhang, X.; Swager, T.M.; Salama, K.N. Recent Progress and Perspectives of Gas Sensors Based on Vertically Oriented ZnO Nanomaterials. Adv. Colloid Interface Sci. 2019, 270, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. ZnO Nanorods for Gas Sensors. In Nanorods and Nanocomposites; Ghamsari, M.S., Dhara, S., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Salehi, T.; Taherizadeh, A.; Bahrami, A.; Allafchian, A.; Ghafarinia, V.; Un, I. Towards a Highly Functional Hybrid ZnO Nanofiber–RGO Gas Sensor. Adv. Eng. Mater. 2020, 22, 2000005. [Google Scholar] [CrossRef]
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective Acetone-Gas Sensor Based ZnO Flowers Functionalized by Au Nanoparticle Loading on Certain Facet. Sens. Actuators B Chem. 2019, 288, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, S.; Qu, F.; Gong, S.; Wang, C.; Qiu, L.; Yang, M.; Cheng, W. High Performance Acetone Sensor Based on ZnO Nanorods Modified by Au Nanoparticles. J. Alloys Compd. 2019, 797, 246–252. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, Z.; Li, X.; Geng, Y.; Xiaoqing, T.; Du, Y. Enhanced Acetone-Sensing Properties to Ppb Detection Level Using Au/Pd-Doped ZnO Nanorod. Sens. Actuators B Chem. 2020, 310, 127129. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, Characterization and Acetone Gas Sensing Applications of Ag-Doped ZnO Nanoneedles. Ceram. Int. 2018, 43, 6765–6770. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Fan, C.; Wang, Q.; Li, M.; Yang, Z.; Gao, L. Enhanced Acetone Sensing Properties Based on Au-Pd Decorated ZnO Nanorod Gas Sensor. Sensors 2024, 24, 2110. [Google Scholar] [CrossRef]
- Meng, F.; Yang, J.; Wang, Y.; Zhang, R.; Yuan, Z. Acetone Gas Sensor Based on Spinel-Structured Hollow Sea Urchin-like Core-Shell ZnO/ZnFe2O4 Spheres Fanli. ACS Appl. Nano Mater. 2024, 7, 16066–16074. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Zhang, J.; Yu, J.; Tang, Z.; Yao, G.; Zhao, W.; Wu, G.; Jin, X. Enhanced Acetone-Sensing Performance of a Bilayer Structure Gas Sensor Composed of a ZnO Nanorod Top Layer and a ZnFe2O4 Nanoparticle Decorated ZnO Nanorod Bottom Layer. Sensors 2024, 24, 7851. [Google Scholar] [CrossRef]
- Peng, L.; Li, J.; Chen, L.; Gou, J.; Gao, D.; Wu, J.; Xie, Z. Pt-Decorated ZnFe2O4 Sensors Prepared via a Facile Impregnation Method Free of Reductants and Surfactants for Conductometric Acetone Sensing. Sens. Actuators B Chem. 2023, 380, 133386. [Google Scholar] [CrossRef]
- Hua, Z.; Qiu, Z.; Li, Y.; Zeng, Y.; Wu, Y.; Tian, X.; Wang, M.; Li, E. A Theoretical Investigation of the Power-Law Response of Metal Oxide Semiconductor Gas Sensors ΙI: Size and Shape Effects. Sens. Actuators B Chem. 2018, 255, 3541–3549. [Google Scholar] [CrossRef]
- Ydir, B.; Ben Hmamou, D.; Ait-Wahmane, Y.; Ihlal, A.; Bousseta, M.; Lahlou, H. Design, Implementation, and Characterization of an Automated SILAR System: Validation with ZnO Thin Film Deposition. Int. J. Adv. Manuf. Technol. 2022, 123, 1189–1201. [Google Scholar] [CrossRef]
- Ydir, B.; Ajdour, A.; Soumane, M.; Achouch, S.; Ben Hmamou, D.; Antohe, I.; Socol, G.; Toderaşcu, L.I.; Socol, M.; Luculescu, C.; et al. Assessing the combined effects of chemical and mechanical parameters on silar-grown nanostructured ZnO thin films. Rom. Rep. Phys. 2024, 76, 508. [Google Scholar] [CrossRef]
- Tien, C. Special Issue “Advanced Coating Technology by Physical Vapor Deposition and Applications”. Coatings 2023, 13, 467. [Google Scholar] [CrossRef]
- Mikulics, M.; Mayer, J.; Hardtdegen, H.H. Cutting-Edge Nano-LED Technology. J. Appl. Phys. 2022, 131, 110903. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.S.; Castro-Lopes, S.; Cabrera-Baez, M.; Milani, R.; Padrón-Hernández, E.; Farias, B.V.; Soares, J.M.; Gusmão, S.S.; Viana, B.C.; Guerra, Y.; et al. The Role of PH on the Vibrational, Optical and Electronic Properties of the Zn1-XFexO Compound Synthesized via Sol Gel Method. Solid State Sci. 2022, 128, 106880. [Google Scholar] [CrossRef]
- Hossain, M.A.; Farhad, S.F.U.; Tanvir, N.I.; Chang, J.H.; Rahman, M.A.; Tanaka, T.; Guo, Q.; Uddin, J.; Patwary, M.A.M. Facile Synthesis of Cu2O Nanorods in the Presence of NaCl by Successive Ionic Layer Adsorption and Reaction Method and Its Characterizations. R. Soc. Open Sci. 2022, 9, 211899. [Google Scholar] [CrossRef]
- Fagadar-cosma, E.; Socol, G.; Mih, A.; Adam, L.; Sava, F.; Stefan, M. SnSe2-Zn-Porphyrin Nanocomposite Thin Films for Threshold Methane Concentration Detection at Room Temperature. Chemosensors 2020, 8, 134. [Google Scholar]
- Ydir, B.; Ajdour, A.; Saadaoui, D.; Choulli, I.; Ben Hmamou, D.; Lahlou, H. Conception and Realization of a Multiparametric Data Acquisition System Based on a Microcontroller for Gas Sensors. Rom. J. Phys. 2025, 70, 904. [Google Scholar] [CrossRef]
- Singh, S.; Sajana, S.; Varma, P.; Sreelekha, G.; Adak, C.; Shukla, R.P.; Kamble, V.B. Metal Oxide-Based Gas Sensor Array for VOCs Determination in Complex Mixtures Using Machine Learning. Microchim. Acta 2024, 191, 194. [Google Scholar] [CrossRef]
- Choi, K.; Koo, R.H.; Park, J.; Kim, D.; Kim, J.; Shin, H.; Jung, G.; Lee, J.H. Novel Gas Sensor Signal Acquisition Method: Amplifying Sensor Signals and Enabling Efficient Gas Identification. Adv. Sci. 2025, 12, 2415104. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Shi, X.; Zhu, J.; Xiong, H.; Wen, H. Recent Advances in Resistive Gas Sensors: Fundamentals, Material and Device Design, and Intelligent Applications. Chemosensors 2025, 13, 224. [Google Scholar] [CrossRef]
- Van Khai, T.; Van Thu, L.; Ha, L.T.T.; Thanh, V.M.; Lam, T.D. Structural, Optical and Gas Sensing Properties of Vertically Well-Aligned ZnO Nanowires Grown on Graphene/Si Substrate by Thermal Evaporation Method. Mater. Charact. 2018, 141, 296–317. [Google Scholar] [CrossRef]
- Toranjizadeh, H.; Shabani, P.; Ramezani, A. Ethanol Gas Sensing Improvement at High Humidity Levels and Optical Features Using Ba-Doped ZnO NPs. Mater. Res. Express 2019, 6, 095904. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Xu, L.; Zhou, Q.; Chen, X.; Zhu, S.; Dong, B.; Lu, G.; Song, H. Au@ZnO Functionalized Three–Dimensional Macroporous WO3: A Application of Selective H2S Gas Sensor for Exhaled Breath Biomarker Detection. Sens. Actuators B Chem. 2020, 324, 128725. [Google Scholar] [CrossRef]
- Mourya, A.K.; Singh, R.P.; Amin, M.; Barad, S.R.; Abedi, M.; Wankhade, A.V. Tuning Photocatalytic Performance via Oxygen Vacancies and in Situ ZnS Formation on ZnO for Hydrogen Evolution. Renew. Energy 2025, 249, 123166. [Google Scholar] [CrossRef]
- Dutta, T.; Noushin, T.; Tabassum, S.; Mishra, S.K. Road Map of Semiconductor Metal-Oxide-Based Sensors: A Review. Sensors 2023, 23, 6849. [Google Scholar] [CrossRef]
- Yuan, C.; Ma, J.; Zou, Y.; Li, G.; Xu, H.; Sysoev, V.V.; Cheng, X.; Deng, Y. Modeling Interfacial Interaction between Gas Molecules and Semiconductor Metal Oxides: A New View Angle on Gas Sensing. Adv. Sci. 2022, 9, 2203594. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview; Springer Nature: Singapore, 2023; Volume 15, ISBN 0123456789. [Google Scholar]
- Saxena, P.; Shukla, P. A Review on Recent Developments and Advances in Environmental Gas Sensors to Monitor Toxic Gas Pollutants. Environ. Prog. Sustain. Energy 2023, 42, e14126. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-Based Nanomaterials in Gas Sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Zhou, P.; Zhao, S.; Zhong, X.; Li, T.; Han, C.; Wei, D.; Meng, D. NO2 Sensing Properties of One-Pot-Synthesized ZnO Nanowires with Pd Functionalization. Sens. Actuators B Chem. 2019, 280, 151–161. [Google Scholar] [CrossRef]
- Fan, X.; Yang, S.; Huang, C.; Lu, Y.; Dai, P. Preparation and Enhanced Acetone-Sensing Properties of ZIF-8-Derived Co3O4@ZnO Microspheres. Chemosensors 2023, 11, 376. [Google Scholar] [CrossRef]
- Prabhu, N.N.; Shivamurthy, B.; Anandhan, S.; Rajendra, B.V.; Basanna, J.C.R.; Srivathsa, M. An Investigation on the Acetone and Ethanol Vapor-Sensing Behavior of Sol-Gel Electrospun ZnO Nanofibers Using an Indigenous Setup. ACS Omega 2023, 8, 49057–49066. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, M.; Ma, S.; Wang, P.; Zhang, G.; Chen, W.; Jiao, H.; Liu, L.; Xu, X. Enhanced Acetone Sensor Based on Au Functionalized In-Doped ZnSnO 3 Nanofibers Synthesized by Electrospinning Method. J. Colloid Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef]
- Hadiyan, M. Sub-Ppm Acetone Gas Sensing Properties of Free-Standing ZnO Nanorods. J. Electroceram. 2019, 42, 147–155. [Google Scholar] [CrossRef]
- Benamara, M.; Rivero-Antúnez, P.; Dahman, H.; Essid, M.; Bouzidi, S.; Debliquy, M.; Lahem, D.; Morales-Flórez, V.; Esquivias, L.; Silva, J.P.B.; et al. Selective and Rapid Detection of Acetone Using Aluminum-Doped Zno-Based Sensors. J. Sol-Gel Sci. Technol. 2023, 108, 13–27. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Nassar, K.I.; Elkenany, E.B.; Al-Syadi, A.M. Zinc Oxide-Based Sensor Prepared by Modified Sol-Gel Route for Detection of Low Concentrations of Ethanol, Methanol, Acetone, and Formaldehyde. Semicond. Sci. Technol. 2024, 39, 115021. [Google Scholar] [CrossRef]
- Lokhande, S.D.; Awale, M.B.; Umadevi, G.; Mote, V.D. Effect of Ni Doping on Structural, Optical and Gas Sensing Properties of ZnO Films for the Development of Acetone Sensor Devices. Mater. Chem. Phys. 2023, 301, 127667. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Huang, D.; Wang, X.; Wang, Y.; Wang, W.; Yi, M.; Cheng, Q.; Song, Y.; Han, G. Highly Sensitive and Selective Acetone Gas Sensors Based on Modified ZnO Nanomaterials. Mater. Sci. Semicond. Process. 2022, 148, 106807. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.Y.; Ooi, P.C.; Hamzah, A.A.; Mohd Razip Wee, M.F.; Aniq Shazni Mohammad Haniff, M.; Dee, C.F.; Mahmoudi, E.; et al. Detection of Breath Acetone by Semiconductor Metal Oxide Nanostructures-Based Gas Sensors: A Review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Prabhu, N.N.; Shivamurthy, B.; Anandhan, S.; Rajendra, B.V.; Kulkarni, S.D. Effect of Ni Doping on the Acetone Vapor Sensing Performance of ZnO Nanofibers. Ceram. Int. 2024, 51, 730–740. [Google Scholar] [CrossRef]
- Aleksanyan, M.; Sayunts, A.; Aroutiounian, V.; Shahkhatuni, G.; Simonyan, Z.; Shahnazaryan, G. Gas Sensor Based on ZnO Nanostructured Film for the Detection of Ethanol Vapor. Chemosensors 2022, 10, 2445. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, C.; She, Y.; Wu, Z.; Zhao, C.; Pan, B.; Zhang, L. Heterostructures with Improved Gas Sensing Response. Nanoscale Res. Lett. 2020, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alenezy, E.; Sabri, Y.; Wang, T.; Motta, N.; Tesfamichael, T. Enhanced Amperometric Acetone Sensing Using Electrospun Non-Stoichiometric WO3−x Nanofibers. J. Mater. Chem. C 2021, 9, 671–678. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined Chemoresistive and in Situ FTIR Spectroscopy Study of Nanoporous NiO Films for Light-Activated Nitrogen Dioxide and Acetone Gas Sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]




| Scherrer Method | Williamson Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2θ (°) | hkl | d (Å) | FWHM (β) | Lattice Parameters (Å) | Crystallite Size D (Å) | Dislocation Density δ (×10+14) Lines m−2 | βcos(ϴ(rad)) | 4sin(ϴ(rad)) | Crystallite Size D (Å) |
| 31.82 | 1 0 0 | 0.281 | 0.29923 | a = 3.244 c = 5.18107 | 0.00599 | 1.0966 | |||
| 34.46 | 0 0 2 | 0.2601 | 0.29788 | 260.4 | 14.7 | 0.00434 | 1.1862 | 371.58 | |
| 36.29 | 1 0 1 | 0.2474 | 0.3561 | 0.00581 | 1.2467 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ydir, B.; Ajdour, A.; Soumane, M.; Antohe, I.; Socol, G.; Toderascu, L.-I.; Saadaoui, D.; Choulli, I.; Leghrib, R.; Lahlou, H. Optimized SILAR Growth of Vertically Aligned ZnO Nanorods for Low-Temperature Acetone Detection. Chemosensors 2025, 13, 289. https://doi.org/10.3390/chemosensors13080289
Ydir B, Ajdour A, Soumane M, Antohe I, Socol G, Toderascu L-I, Saadaoui D, Choulli I, Leghrib R, Lahlou H. Optimized SILAR Growth of Vertically Aligned ZnO Nanorods for Low-Temperature Acetone Detection. Chemosensors. 2025; 13(8):289. https://doi.org/10.3390/chemosensors13080289
Chicago/Turabian StyleYdir, Brahim, Amine Ajdour, Mouad Soumane, Iulia Antohe, Gabriel Socol, Luiza-Izabela Toderascu, Driss Saadaoui, Imade Choulli, Radouane Leghrib, and Houda Lahlou. 2025. "Optimized SILAR Growth of Vertically Aligned ZnO Nanorods for Low-Temperature Acetone Detection" Chemosensors 13, no. 8: 289. https://doi.org/10.3390/chemosensors13080289
APA StyleYdir, B., Ajdour, A., Soumane, M., Antohe, I., Socol, G., Toderascu, L.-I., Saadaoui, D., Choulli, I., Leghrib, R., & Lahlou, H. (2025). Optimized SILAR Growth of Vertically Aligned ZnO Nanorods for Low-Temperature Acetone Detection. Chemosensors, 13(8), 289. https://doi.org/10.3390/chemosensors13080289









