Abstract
The rapid emergence of novel psychoactive substances (NPSs) after 2020 has created one of the most dynamic analytical challenges in modern forensic science. Hundreds of new synthetic cannabinoids, synthetic cathinones, synthetic opioids, hallucinogens, and dissociatives, appearing as hybrid or structurally modified analogues of conventional drugs, have entered the illicit market, frequently found in complex polydrug mixtures. This review summarizes recent advances in gas chromatography-mass spectrometry (GC-MS) for their detection, structural elucidation, and differentiation between 2020 and 2025 based on the ScienceDirect and Google Scholar databases. Due to its reproducible electron-ionization spectra, established reference libraries, and robustness toward complex matrices, GC-MS remains the primary tool for the separation and identification of emerging NPS. The current literature highlights significant improvements in extraction and pre-concentration procedures, derivatization strategies for thermally unstable analogues, and chromatographic optimization that enable discrimination between positional and stereoisomers. This review covers a wide range of matrices, including powders, herbal materials, vaping liquids, and infused papers, as well as biological specimens such as blood, urine, and hair. Chemometric interpretation of GC-MS data now supports automated classification and prediction of fragmentation pathways, while coupling with complementary spectroscopic techniques strengthens compound confirmation. The review emphasizes how continuous innovation in GC-MS methodology has paralleled the rapid evolution of the NPS landscape, ensuring its enduring role as a reliable, adaptable, and cost-effective platform for monitoring emerging psychoactive substances in seized materials.
1. Introduction
Recent decades have been marked by the appearance of new psychoactive substances (NPS) and illicit drugs. By definition, NPSs are synthetic compounds engineered to reproduce the effects of conventional illegal drugs, yet remain outside existing legal frameworks, thereby representing a significant international public health issue [1]. In the beginning, the term “new” or “novel” mainly signified their recent appearance on the illicit market, whereas the synthesis procedures had been available in the scientific literature for a longer time. Today, it is not uncommon to identify entirely new compounds among the hundreds present in seized samples [2]. A high number of substances, the lack of certified standards for the parent molecule and its metabolites, and limited data on the pharmacokinetics and pharmacodynamic profiles in biological fluids make the detection and identification of NPSs a very challenging task for forensic toxicologists [3].
In the years following the COVID-19 pandemic, the dynamics of drug distribution, synthesis, and consumption changed markedly, with online marketplaces, local synthesis routes, and unconventional formulations becoming dominant [4,5,6]. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) issued a warning in April 2020 focusing on the potential effects of the pandemic on the growing drug market [7]. At the same time, traditional plant-based (cannabis, cocaine), synthetic (amphetamine, MDMA) substances and pharmaceuticals (antihypertensives, antidepressants, psychiatric drugs, and ibuprofen) have shown increasing trends and remained the most-consumed [8,9]. NPSs can also be found as adulterants in mixtures with other psychoactive compounds [1,10]. Therefore, it is of utmost interest to monitor these compounds as there is limited knowledge of the health harms, interactions with other drugs, and specific effects.
The United Nations Office on Drugs and Crime (UNODC) is conducting an Early Warning Advisory (EWA) program that lists NPSs identified by international drug enforcement, intelligence, health, and toxicology agencies. In the last update, more than 1396 NPS were reported in 153 countries and territories. Within this framework, the classification includes stimulants, synthetic cannabinoids, classic hallucinogens, synthetic opioids, sedatives/hypnotics, dissociatives, and unassigned compounds [11]. In the post-COVID-19 era, the highest number of NPS was recorded in 2024 (688) and the lowest in 2023 (540). It should be emphasized that these numbers present the unique compounds that were identified each year, but the number of newly described NPS was 101 (2024), 44 (2023), 44 (2022), 87 (2021), and 46 (2020) [12]. Out of the total number of compounds in 2024, the most common were stimulants (33%), synthetic cannabinoids (24%), and classic hallucinogens (14%). The percentage of appearance is similar over the time period from 2020 to 2025.
In this review, a simplified classification was adopted, with NPSs separated into four groups: synthetic and semi-synthetic cannabinoids; synthetic cathinones; synthetic and semi-synthetic opioids; and hallucinogens, dissociatives, entactogens, and empathogens (Figure 1). These four groups represent the most frequently identified NPS families between 2020 and 2025 and form the structural basis for the organization of the subsequent sections. Synthetic and semi-synthetic cannabinoids constitute one of the largest and most chemically diverse groups, comprising indole-, indazole-, and cyclohexyl-derived receptor agonists, as well as emerging semi-synthetic products such as hexahydrocannabinol (HHC) and tetrahydrocannabiphorol (THCP). Synthetic cathinones form the second major class and include β-keto analogues of amphetamine with extensive structural variation through aromatic substitution, alkyl-chain extension, and N-functionalization. Synthetic and semi-synthetic opioids, ranging from fentanyl analogues to benzimidazole derivatives and modified pharmaceutical opioids, represent a smaller but disproportionately high-risk group due to their potency and frequent implication in fatal intoxications. Finally, a heterogeneous category of hallucinogens, dissociatives, entactogens, and empathogens encompasses novel tryptamines, phenethylamines, arylcyclohexylamines, and MDMA-like substances, many of which appear only sporadically yet require analytical workflows capable of distinguishing closely related positional or stereochemical isomers.
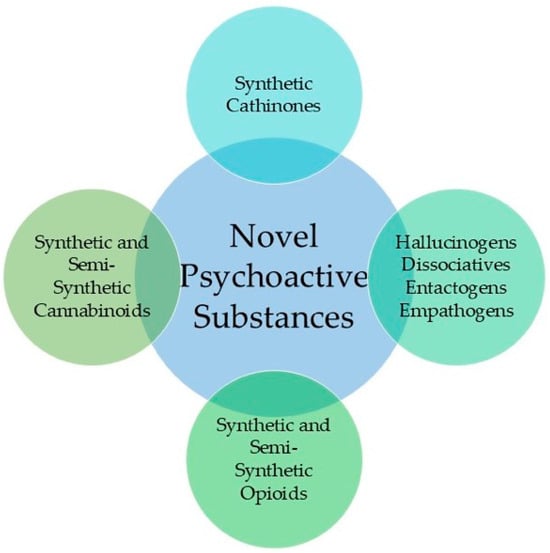
Figure 1.
The classes of Novel Psychoactive Substances covered in this review are for the period between 2020 and 2025.
Several factors further complicate the work of law enforcement and analytical laboratories. Among the most critical are: (i) the chemical and structural diversity of NPSs and their often ambiguous or non-uniform legal status; (ii) the rapid turnover and transient availability of new compounds on the illicit market; (iii) the frequent presence of NPSs in complex mixtures or disguised formulations; and (iv) their distribution in small, inconspicuous quantities via online trade and postal systems. These characteristics make timely recognition and forensic identification increasingly difficult. Moreover, significant heterogeneity exists in national and regional legal responses [6]. While some countries adopt generic or analogue-based legislative frameworks that simultaneously prohibit entire structural families, others continue to rely on substance-specific scheduling, leaving room for rapid structural modification and legal circumvention [13].
An innovative way for identifying NPSs is through social media, which presents platforms for discussion regarding purity, dosage, pharmacological and toxicological effects, ways of administration, and comparison with common drugs [14]. This so-called “social media listening” proved effective for different public health concerns [15]. NPSfinder® is a web crawler that helps with the identification and categorization of NPSs through discussion, websites/fora, and comparison with registry databases by EMCDDA and UNODC [16]. Catalani and coworkers identified 18 NPSs (six cathinones, six opioids, two synthetic cannabinoid receptor agonists, two phenylcyclohexylpiperidine (PCP)-like molecules, and two psychedelics) for the period between January and August 2020, 10 of which were not previously enlisted by law enforcement agencies [14].
Studies have shown that people of different socio-economic characteristics and motivations abuse these substances for both recreational and therapeutic reasons. Popular festivals, bars, nightclubs, and special events are prevalent occasions in which the use of psychoactive substances is exceptionally high [1,17] and associated with high alcohol consumption [18]. Compounds now appear in vaping liquids, herbal mixtures, edibles, and impregnated papers, often labeled as harmless alternatives to controlled drugs (“legal highs”, “bath salts”, “research chemicals”, “plant food”, “plant fertilizer”, “smokable herbal mixtures”, etc.) [19,20,21,22]. Vincenti and coworkers reported that NPSs were detected in 92.6% of the samples collected by the Italian police between May and October 2020 in postal parcels [2]. Examined samples were not reacting with the standard field kits for common drugs; therefore, it was suspected that NPSs were present. More than 200 cases of intoxication by NPSs were reported between January 2020 and March 2022, with more than 30% of cases involving the combined consumption of several substances [23]. This expansion in chemical and matrix diversity has made the reliable identification and quantification of NPSs one of the most demanding analytical tasks in contemporary forensic science.
Various analytical techniques are used to identify compounds in seized materials, such as nuclear magnetic resonance spectroscopy (NMR), gas chromatography-mass spectrometry (GC-MS), thin-layer chromatography, liquid chromatography with high-resolution mass spectrometry, infrared, and Raman spectroscopy, etc. [24,25,26,27,28,29]. Among the available instrumental techniques, GC-MS has maintained its role as the principal method, or the gold standard, for detection and confirmation [30,31,32]. Its strength lies in reproducible electron-ionization spectra and extensive reference libraries that facilitate inter-laboratory comparison. It should be noted that due to rapid market changes, many of the seized compounds are absent from spectral libraries, although comparisons with spectra of known compounds can be helpful [33,34]. Despite the development of liquid chromatography-high-resolution and tandem mass spectrometric systems, GC-MS remains indispensable because of its robustness, cost efficiency, and ability to provide both targeted and untargeted profiles from complex mixtures [35]. Compared to LC, GC is less susceptible to matrix effect and contamination, especially when highly concentrated biological samples are examined [36]. Also, lower maintenance and analysis costs are advantages of GC that make it a technique of choice in developing countries [37,38]. Several references emphasize the use of GC-MS for the metabolism study of these compounds [39,40,41]. Nevertheless, the importance of orthogonal techniques in NPS examination was suggested by several authors [31,42,43,44]. GC-MS is also widely used as a benchmark for the development of on-site and hyphenated techniques [35].
The post-pandemic environment posed analytical challenges that required adapting GC-MS workflows. Restrictions in precursor availability and disruptions in global supply chains resulted in numerous low-purity and chemically unstable analogues, frequently mixed with adulterants intended to alter volatility or mimic pharmacological effects [45,46]. Simultaneously, biological, herbal, beverage, and environmental matrices diversified, requiring optimized extraction and pre-concentration steps [47,48,49,50,51,52]. Techniques such as solid-phase extraction (SPE), liquid–liquid extraction (LLE), dispersive liquid–liquid microextraction (DLLME), dummy molecularly imprinted polymers (DMIP), and magnetic solid-phase extraction (MSPE) have become integral to GC-MS protocols, providing higher enrichment factors and cleaner chromatograms even at trace levels [21,47,48,53]. These advances underline the method’s flexibility and its ability to evolve alongside the illicit drug market.
Recent literature demonstrates that GC-MS is not confined to classical volatile analytes but can accommodate semi-volatile, thermally labile, or derivatized species that are characterized by complex mass fragmentation patterns [54,55]. The refinement of stationary phases, temperature programs, and chemical ionization has improved the separation of structural and positional isomers, while chemometric evaluation of electron-ionization spectra supports differentiation among closely related analogues [56,57]. Such approaches are particularly valuable for rapidly expanding compound families whose legal status depends on minute structural differences. Fragmentation mechanisms of various NPS classes, as related to different MS techniques, are also crucial for predicting the structural features of newly seized compounds [31,58]. In addition, GC-MS data increasingly serve as training input for pattern-recognition algorithms and chemometric models designed to automate classification and source attribution of seized materials [30,59,60].
This review is intended primarily for forensic toxicologists, forensic chemists, and analytical chemists who rely on GC-MS for the detection and characterization of emerging NPSs. It is also designed to serve as a comprehensive, accessible resource for graduate students and junior researchers entering the field. Accordingly, the review summarizes the methodological developments in the GC-MS analysis of novel psychoactive substances reported between 2020 and 2025, emphasizing new sample types, extraction and derivatization strategies, and combined approaches with complementary spectroscopic techniques. The primary search engines were Google Scholar and ScienceDirect, with the publication dates set from 2020. Some older references were included in the review when it was necessary to provide a general overview of a specific class or to mention notable examples. For analytical procedures, only references published after 2020 were included. The search terms included “GC-MS” AND one of the following groups: “synthetic cannabinoids”, “synthetic cathinones”, “designer benzodiazepines”, “fentanyls”, “hallucinogens”, “synthetic opioids”, and “polydrug NPS”. The discussion highlights how innovations in microextraction, column technology, and spectral interpretation have enhanced the relevance of GC-MS in the post-COVID analytical testing of NPSs. A special part of the review is devoted to the examination of polydrug mixtures and the application of advanced statistical methods in GC-MS analysis. The compounds are mentioned by their common names in the text, while the appropriate nomenclature is presented in the Abbreviations.
2. Analytical Challenges in GC-MS Analysis of NPS
The rapid evolution of the NPS market during the 2020–2025 period has required forensic chemists to rethink the way GC-MS workflows are conceptualized. Although the following sections of this review present detailed examples for specific substance families, several methodological principles apply across all classes of cannabinoids, cathinones, opioids, and hallucinogens. A unified overview is therefore necessary to understand why GC-MS remains effective despite the chemical heterogeneity and instability of many emerging compounds.
A central analytical challenge lies in the diversity of matrices and formulations encountered after 2020. NPSs now appear not only in powders and herbal materials but also in low-THC cannabis, disposable vapes, e-liquids, impregnated papers, counterfeit pharmaceutical tablets, biological samples, and even environmental matrices [61,62,63,64]. These heterogeneous substrates often contain pigments, excipients, stabilizers, plant resins, cutting agents, or polymeric residues, which can complicate chromatographic behavior. As a result, GC-MS workflows increasingly rely on matrix-specific extraction strategies rather than on a single universal protocol. Solid-phase extraction, liquid–liquid extraction, and modern microextraction approaches (DLLME, MSPE, DMIP) are used not because of their novelty but because they provide different selectivity windows, allowing laboratories to tailor cleanup to the physicochemical properties of each NPS class [40,48,65]. Extractive strategies further serve an interpretive purpose: cleaner chromatograms allow the analyst to differentiate true molecular features from thermally induced degradation products or polymer fragments originating from the sample matrix.
The thermal and structural stability of emerging NPS represents another shared analytical concern. Many post-2020 cannabinoids, cathinones, and benzimidazole opioids degrade, rearrange, or undergo side reactions in the GC injector. Derivatization, therefore, plays a dual role. Beyond reducing volatility, derivatization can suppress unwanted decomposition pathways, stabilize labile functional groups, or enhance diagnostic fragments that aid structural differentiation [66]. In contrast, for certain compound families, such as indazole-3-carboxamides and α-pyrrolidinophenones, derivatization may obscure class-specific fragments, making non-derivatized analysis preferable. The enantiometric purity of compounds can also be determined using appropriate derivatization [67]. The analyst must therefore balance increased chromatographic performance against the risk of generating artificially simplified or misleading mass spectra. This trade-off is increasingly important as clandestine laboratories introduce analogues with minimal structural changes intended to circumvent generic scheduling.
A third cross-cutting issue concerns chromatographic separation of closely related analogues. Many NPS families now include positional, constitutional, and stereochemical isomers with nearly identical EI-MS fragmentation profiles [68,69]. The separation of these analogues is challenging, particularly when analytes differ only by halogen placement (e.g., chlorocathinones), alkyl chain branching, or heterocyclic substitution. Adjustments in stationary-phase polarity, oven-temperature programs, and carrier-gas linear velocity often have a greater effect on discrimination than changes in the mass spectrometer settings. Recent work has demonstrated that column selectivity, rather than mass-spectral uniqueness, is now the primary factor determining whether two isomers can be differentiated in routine casework. This shift explains why laboratories are increasingly evaluating mid-polar or tailored stationary phases for NPS screening, especially when working with emerging semi-synthetic cannabinoids and benzimidazole opioids.
Despite these improvements, EI mass spectra remain the interpretive backbone of GC-MS-based NPS identification. Although fragmentation pathways differ among chemical classes, certain recurring patterns, for example, α-cleavage in cathinones, loss of tert-butyl esters in synthetic cannabinoids, and piperidine-ring fragmentation in fentanyls, serve as anchors for preliminary structural hypotheses [70,71]. The interpretive challenge arises when multiple structural analogues share the same diagnostic fragments, producing spectra that are highly similar yet evidence different pharmacological and legal implications [72]. In such cases, analysts increasingly rely on relative ion abundances, fragment–intensity ratios, and retention-time ordering rather than on the presence of any specific ion. Pattern recognition based solely on library matching is becoming insufficient for newly emerging analogues, many of which are absent from official reference databases.
The identification of many compounds within one NPS group has encouraged the development of chemometric and statistical tools such as PCA, hierarchical clustering, canonical discriminant analysis, and automated spectral-similarity metrics [30,73]. These approaches do not replace human interpretation but rather translate subtle spectral features into reproducible classifications [60]. Importantly, chemometrics also provides an orthogonal layer of evidence when chromatographic separation is incomplete, but the analyst must still determine the most plausible structural class [66]. As more laboratories perform these analyses, data standardization is emerging as a methodological priority, especially for sharing cross-border early warning signals about newly detected analogues.
3. Synthetic and Semi-Synthetic Cannabinoids
Synthetic cannabinoids (SCs) remain the most prevalent group of new psychoactive substances and continue to dominate forensic seizures worldwide. The term “cannabinoids” was initially used for the typical terpenophenolic C21 compounds in the cannabis plant, but the extended classification includes a large number of endocannabinoids, phytocannabinoids, and synthetic cannabinoids [74,75]. SCs first appeared around 2000, but were first detected in herbal products in 2008 by forensic scientists in Austria and Germany. Since then, the number of SCs in the market has steadily increased [76]. Out of 950 NPSs recorded by the EMCDDA, 254 are classified as SCs [77]. These compounds exhibit greater activity at cannabinoid receptors (CB1 and/or CB2) than ∆9-tetrahydrocannabinol (∆9-THC), resulting in more intense and short-lived effects [78,79]. SCs are often sold as solutions in organic solvents, sprinkled onto plant-based materials, and marketed online under names like “Spice” and “K2”. An overview of the pharmacological and toxicological properties of SCs is discussed in detail in the review by Roque-Bravo et al. [78]. Currently, 14 chemical families of SCs are recognized, some of which are not officially included in the banning laws [80]. Due to the complex structure and the plethora of functional groups in the core, tail, linker, and linked groups, several nomenclature systems are in use [81]. Their widespread distribution in herbal mixtures, e-liquids, disposable vapes, and even impregnated papers has created significant analytical challenges. Because of the lack of quality control of samples marketed as “synthetic marijuana”, the toxicity of SCs is unknown, as the individual compounds differ in potency, efficacy, and modes of action [82]. GC-MS has consistently been the frontline method for their detection, providing both targeted compound identification and non-targeted fingerprinting of complex mixtures. GC-MS remains particularly valuable because EI fragmentation generates highly class-diagnostic ions that facilitate the differentiation of indole-, indazole-, and cyclohexyl-derived scaffolds, even when reference standards are unavailable. However, many modern semi-synthetic cannabinoids are thermally labile and may partially degrade in the GC injector, making LC-MS or LC-HRMS more suitable for confirmatory analysis. In practice, GC-MS is most effective for rapid screening of herbal products, infused papers, and vaping materials, whereas LC-HRMS is required when resolving structural isomers or low-abundance metabolites. The study by Hubner et al. showed that available GC-MS spectral libraries are often insufficient to identify all tested samples purchased online [83].
3.1. Synthetic Cannabinoids in Powder and Herbal Mixtures
Recent studies have emphasized the diversity of SC formulations and matrices, such as paper, fabrics, and herb materials [84]. Low-THC cannabis or “CBD weed” is an industrially grown hemp. Most EU countries have an upper limit of allowed THC in these products of 0.2%. Several alerts have been issued in EU countries for the presence of synthetic cannabinoid MDMB-4en-PINACA (Figure 2) in low-THC products [85]. Out of 1142 examined in the article by Oomen and coworkers, 270 were found to contain this compound by GC-MS, and further proven by UPLC-QTOF [86]. Al-Eitan et al. investigated seized herbal blends from the Jordanian market, successfully quantifying AB-FUBINACA, AB-CHMINACA, and XLR-11 (all of which are presented in Figure 2) using GC-MS following liquid–liquid extraction, and highlighting wide variability in concentrations across samples [87] (Table 1). Similar findings were reported in Europe, where Pasin and colleagues identified CH-PIACA, a newly emerged indole-3-acetamide cannabinoid, in seized powders using combined GC-MS and high-resolution MS [88].
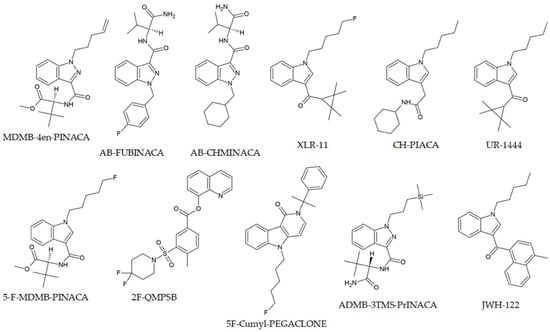
Figure 2.
Chemical structures of selected synthetic cannabinoids.
In 2021, a new NPS belonging to the synthetic cannabinoid class, 2F-QMPSB, depicted in Figure 2, was identified in three samples of herbal material by Tsochatzis et al. [89] during a screening by GC-MS. Along with 2F-QMPSB, the same study reported the spectral and chromatographic analysis of the acid precursor, 2F-MPSBA. The presence of the precursor was evident in the methanolic extract, suggesting that a transesterification reaction occurred, whereas the compound was absent from the chloroform extract. The choice of the stationary column (DB5-MS) used for the chloroform solution was not suitable for the analysis of acids [89].
Only the tentative identification of SCs by GC-MS was outlined by Al-Matrouk and Orabi, based on the investigation of samples seized in Kuwait [90]. Liu and coworkers reported the identification and quantification of 10 indole/indazole carboxamide synthetic cannabinoids in 36 herbal blends by GC-MS and NMR [91]. The concentration ranges of compounds determined by these methods were comparable (1.9–50.6 mg/g for GC-MS and 1.5–49.0 mg/g for NMR). The authors stated that GC-MS is more applicable for analyzing multicomponent mixtures when reference materials are available, as they enable quantification based on the molecular ion intensity. The linearity range was 0.05–2.5 mg/mL. The advantages of NMR were noted in the absence of reference materials. The presence of adulterants and preservatives masks the active compounds in herbal mixtures. Alves and coworkers reported the use of GC-MS, along with NMR, to characterize synthetic cannabinoids in nine different mixtures [92]. In addition to active compounds, the authors identified vitamin E, vitamin E acetate, and oleamide. Analysis of adulterants is important because these results can be used to link products to manufacturers [93,94]. These examples illustrate how clandestine laboratories continue to design novel scaffolds that challenge existing detection protocols.
3.2. Synthetic Cannabinoids in Infused Paper and Vapes
Alternative formulations of SCs have further complicated monitoring. Apirakkan et al. demonstrated that GC-MS, supported by complementary techniques, could identify AMB-FUBINACA and AB-CHMINACA not only in e-liquids but also in paper substrates intended for prison smuggling [62]. AMB-FUBINACA, a compound 85-fold more potent than THC, emerged in 2014 as a drug of abuse and is considered one of the most widely abused and seized [95,96]. In line with this, Abbott et al. confirmed the presence of multiple indazole-3-carboxamide SCs in impregnated letters intercepted in UK prisons, underlining the necessity of frequent spectral library updates [97]. Air monitoring approaches have also been explored; Paul et al. applied two-dimensional GC–TOFMS for airborne detection of SCs in prisons, though no direct confirmations were achieved, the study highlighted the potential of environmental monitoring as an early warning tool [98].
Beyond herbal and paper-based samples, vaping products have emerged as a key vector for SC delivery. Craft et al. recently reported that all seven illicit disposable vape pens submitted by a UK drug service contained only 5F-MDMB-PICA, at concentrations around 0.85 mg/mL, despite being sold as cannabis products [61]. Nguyen et al. described a validated GC-MS method for 5F-MDMB-PICA in the Vietnamese “American grass” market, achieving a detection limit as low as 0.06 µg/mL and quantifying an average content at 2.2% w/w in seized material [99]. These findings underscore the increasing shift toward vaping formulations and the importance of validated GC-MS methods for rapid, high-sensitivity detection.
A critical methodological development was presented by Sisco et al., who optimized a GC-MS protocol specifically for the discrimination of cannabinoids [100]. The method was validated on a panel of 50 compounds, encompassing both phytocannabinoids and a broad range of synthetic analogues. Using a DB-200 stationary phase and an isothermal oven program at 290 °C, the authors achieved reproducible retention times and improved separation between closely related analytes. Compound discrimination was further supported by automated spectral similarity testing, which allowed for differentiation even where the retention time overlap was minimal. Compared with a general confirmatory GC-MS method, the targeted protocol provided an order of magnitude higher sensitivity and markedly better retention time resolution. This study demonstrates that GC-MS can be adapted not only for screening but also for systematic classification of structurally related cannabinoids, a feature of considerable forensic value when large panels of emerging substances must be rapidly distinguished.
3.3. Synthetic Cannabinoids in Biological Samples
Clinical and toxicological monitoring also benefit from GC-MS. La Maida et al. developed a dual approach combining GC-MS screening and UHPLC-HRMS confirmation for oral fluid analysis after the controlled administration of JWH-122, JWH-210, and UR-144. They demonstrated sub-ng/mL detection, providing the first disposition data for these compounds in human oral fluid [101]. In the study by Pellegrini et al., the hydroxy metabolites of JWH-122, JWH-210, and UR-144 were identified in urine samples, along with the parent molecules, at sub-ng/mL concentrations using GC-MS. These were further confirmed by UHPLC-HRMS [41]. Urine is the most common biological fluid used in forensic examination of drug abuse, but due to the fast metabolism of SCs, parent molecules are rarely detected [102]. Due to the similar metabolic pathways, misidentification of compounds may occur [103]. The hydroxy metabolites are the primary metabolites of SCs, as determined in the other studies [104,105,106]. Other chemical modifications include hydroxylation and carboxylation on the alkyl site, hydroxylation on the indole group, defluorination, and glucuronidation in the second methanolic phase, where metabolites are present [107,108]. The mentioned study emphasized the need for other metabolites, such as glucuronidated metabolites, for the proper examination of compounds.
The presence of thermally unstable compounds and the need for derivatization limit the use of GC-MS compared to LC-MS [109,110]. Recently, Wang and coworkers identified ADB-4en-PINACA, MDMB-4en-PINACA, and ADB-BUTINACA in human hair for the first time using GC-MS/MS [111,112]. Hair samples offer many advantages over other samples, such as target stability, a long detection window, and a long-term history of abuse of different compounds, as hair grows around 1 cm per month [113,114,115]. The sample requires complex preparation, including washing in 0.1% sodium dodecyl sulfate, 0.1% detergent, distilled water, and acetone to remove exogenous contaminants. The samples are then cut into 2 cm sections, and methanol extraction is performed. The linear concentration range for ADB-4en-PINACA was 20–1000 pg/mg, while LLOQ and LOD were 20 and 10 pg/mg, respectively. Due to the low concentration and background noise, the single-stage GC-MS analysis was not sufficient. This study emphasizes that the derivatization of compounds was not needed.
Post-mortem GC-MS analysis of samples is also used to assess SC concentrations. In the article by Giorgetti et al., four cases of death involving 5F-Cumyl-PEGACLONE (Figure 2), a fluorinated analogue of Cumyl-PEGACLONE, the first cannabinoid with a γ-carbolinone core structure, are reported [116]. The concentration range was 0.09–0.45 ng/mL in femoral blood. The cases of death related to SCs are common in the literature, although the significance of toxicological results is often questionable [117]. For this purpose, the Toxicological Significance Score (TSS) is used, with values ranging from 1 (Low, alternative cause of death) to 3 (High, NPS is probably the cause of death, although other drugs may be present). In the study by Giorgetti, the cases were assigned TSSs of 1, 2, and 3, as in only one case, a contributory role of 5F-Cumyl-PEGACLONE was suggested, along with an acute heroin intoxication.
The extraction procedure for the GC-MS analysis of complex biological samples is another parameter to consider. Dispersive liquid–liquid microextraction (DLLME) involves the use of small amounts of immiscible organic solvents in water, which renders the technique very cheap and eco-friendly [118]. Mercieca et al. applied ultrasound-assisted DLLME for the rapid GC-MS detection of 29 selected cannabinoids and their metabolites in blood and urine samples [40]. The observed limits of detection (1–5 ng/mL), limits of quantification (5 ng/mL), and linearity were satisfactory.
Another improvement to the extraction method was described by Shokoohi Rad and coworkers, who applied magnetic solid-phase extraction (MSPE) with ortho-aminophenol-coated Fe3O4 nanoparticles coupled with DLLME for the trace analysis of 5F-ADB-PINACA and AB-FUNBINACA in human plasma samples (Figure 2) [65]. The linear ranges were much higher, ranging from 0.2 to 50 ng/mL, with enrichment factors of 213 and 437. MSPEs are used for other extraction procedures because they are fast and straightforward to synthesize, and their surfaces are easily modified [119,120,121]. Microextraction by packed sorbent (MEPS) is a simple, fast, and miniaturized extraction method that has shown promise for the analysis of NPS in liquid samples [122]. Sorribes-Soriano et al. concluded that semi-automated MEPS extraction on a C18-packed sorbent for third-generation cannabinoids yielded quantitative recovery and high sensitivity in subsequent GC-MS analysis, with LODs of 10–20 mg/mL in saliva samples [103].
Two interesting SC compounds containing silicon were examined by Zschiesche et al. [123] These compounds, ADMB-3TMS-PrINACA (Figure 2) and Cumyl-3TMS-PrINACA, have a trimethylsilyl propyl moiety connected to the tertiary indazole nitrogen. GC-MS was used to identify 34 and 38 metabolites of these compounds. The specificity of their biotransformation is the side-chain monohydroxylation (specific marker) and TMS-group cleavage, initiated by oxidative Si-demethylation. These biomarkers were later discovered in biological samples (blood, urine, and stomach content) of a deceased polydrug user. The carbon-silicon switch or “sila-substitution” in these compounds is performed to avoid legal restrictions and affect pharmacodynamics and metabolic stability [124,125]. Other silicone analogues of psychoactive compounds are described in the literature, such as 1S-LSD [126].
3.4. Semi-Synthetic Cannabinoids
A special group of compounds, semi-synthetic cannabinoids (SSCs), is obtained by chemical modification of phytocannabinoids. The first SSC was (-)-trans-∆8-THC, discovered in the U.S. market in 2019 [127], produced from CBD through a ring closure reaction. Hexahydrocannabinol (HHC) is another SSC that was first reported to the EMCDDA in May 2022. By December 2022, it was found in almost all European countries [128]. These hexahydro derivatives of cannabinol are not scheduled under international drug conventions, which paves the way for their distribution in vape products and edibles [127]. HHC and cannabinol are the products of the thermal reduction and oxidation of tetrahydrocannabiphorol (Δ9-THCP) in e-cigarettes, as observed in the controlled conditions by GC-MS [64]. In the post-COVID-19 period, several other compounds have been determined by generic extraction in methanol and subsequent GC-MS analysis of the seized samples, including tetrahydrocannabidiol (H4-CBD), hexahydrocannabinol acetate (HHC-O-Acetate), hexahydrocannabiphorol (HHCP), and Δ9-THCP [129]. The structures of these compounds are shown in Figure 3. These were found in e-cigarette products (56%), hashish (20%), concentrates (17%), edibles (10%), and plant material (10%). ∆9 -THCP is a heptyl homolog of ∆9-THC, recently found in Cannabis sativa L. [130]. The low amounts in natural sources make it unprofitable for isolation, so it is usually produced by the chemical modification of CBD [131]. The manufacturers of this SSC claim it is derived from natural sources, but its legal status varies from country to country.
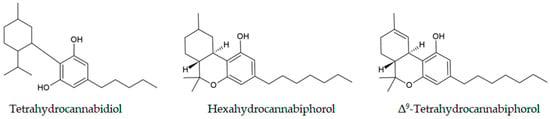
Figure 3.
Chemical structures of the cannabinoids identified in seized samples.
Schirmer et al. presented results of the GC-MS examination of a THCP vape liquid pen seized by Swiss customs [67]. Although the vape was labeled as having 90% THCP and 10% terpenes, the analysis showed the presence of enantiopure (6aR, 10aR)-∆9-THCP, along with isomeric compounds, derivatives, unreacted reactants, side products, and other chemical impurities. The results enabled the determination of the synthetic route, concluding that the compound was not isolated from natural sources. This provided authorities with the opportunity to control illegal production. Other studies in Japan and Germany also led to similar conclusions, as some of the samples did not contain THCP at all [67,132].

Table 1.
Selected GC-MS studies on synthetic cannabinoids (2020–2025).
Table 1.
Selected GC-MS studies on synthetic cannabinoids (2020–2025).
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| AB-FUBINACA, AB-CHMINACA, XLR-11 | Seized herbal blends | First profiling of the Jordanian market; wide concentration variability. | Al-Eitan et al., 2020 [87] |
| AMB-FUBINACA, MMB-CHMICA, others | E-liquids, powders, blotting papers, and prison letters | Highlighted new formulations (e-liquids, impregnated papers) used for prison smuggling. | Apirakkan et al., 2020 [62] |
| JWH-122, JWH-210, UR-144 | Oral fluid of consumers | Validated oral fluid method; first disposition data after controlled administration. | La Maida et al., 2020 [101] |
| Δ8-THC, THC-O acetates, CBD-di-O-A | Commercial edibles & herbal products | First report of THC-O acetates and CBD-di-O A in consumer products. | Holt et al., 2022 [133] |
| CH-PIACA (indole-3-acetamide SC) | Seized powders | First identification of CH-PIACA in Europe, linked to class-wide legislative bans. | Pasin et al., 2022 [88] |
| 5F-MDMB-PICA | “American grass” is a herbal drug | Avg. content 2.2% w/w in seized samples; method fully ICH-validated. | Nguyen et al., 2023 [99] |
| 5F-MDMB-PICA, MMB-4en-PICA, 4F-MDMB-BUTINACA, MDMB-4en-PINACA | Prison mail (impregnated paper) | Library updates required for prison screening systems. | Abbott et al., 2023 [97] |
| SCs (not confirmed in samples) | Prison air (fixed + personal samplers) | First attempt to detect airborne SCs; no direct confirmation, but showed feasibility. | Paul et al., 2021 [98] |
| 5F-MDMB-PICA | Seven disposable illicit vapes | All vapes mis-sold as cannabis contained only SCs, highlighting high user risk. | Craft et al., 2024 [61] |
| Semi-synthetic cannabinoids (Δ8-THC, HHC, H4-CBD, HHC-O-Ac, HHCP, THCP) | Seized cannabis products | Reprocessed historical GC-MS data to quantify emergence: 15% (n = 216) of products positive for SSCs; HHC 10% most frequent. | Jørgensen et al., 2024 [129] |
| 21 synthetic cannabinoids (reference set) | NPS bought online (powders) and laced plant material | Built an NMR + GC-MS reference dataset; shows why GC-MS alone can mis-ID some SCs and how pairing with NMR resolves ambiguities. | Hübner et al., 2025 [83] |
| JWH-122, JWH 210, UR-144 and their hydroxylated metabolites | Urine samples of consumers | The hydroxylated metabolites and parent molecules were determined in sub-ng/mL concentrations. The samples underwent initial hydrolysis with β-glucuronidase. | Pellegrini et al., 2020 [41] |
| 2F-QMPSB and precursor 2F-MPSBA | Herbal samples | The new compounds, along with their precursors, were determined in the samples. | Tsochatzis et al., 2021 [89] |
| 50 cannabinoids (synthetic and phytocannabinoids) | Reference standard panel | Targeted GC-MS method differentiated 50 cannabinoids by retention time and spectral dissimilarity; supports systematic classification. | Sisco et al., 2021 [100] |
| 5F-Cumyl-PEGACLONE | Femoral blood | The femoral blood concentrations of 5F-Cumyl-PEGACLONE were used to determine the cause of death. | Giorgetti et al., 2020 [116] |
| 10 indole/indazole carboxamide SCs | Herbal blends | For quantifying multi-component mixtures, GC-MS was preferred when reference materials were unavailable; when they were available, NMR proved more useful. | Liu et al., 2021 [91] |
| 29 cannabinoids and their metabolites | Blood and urine samples | Ultrasound-assisted DLLME was a highly efficient microextraction technique for the quantification of 29 compounds in blood and urine samples. | Mercieca et al., 2020 [40] |
| 5F-ADB-PINACA and AB-FUBINACA | Human plasma samples | Magnetic solid-phase microextraction coupled with DLLME showed enrichment factors of 213 and 437. | Rad et al., 2020 [65] |
| ∆9 -Tetrahydrocannabiphorol | THCP vape pen | GC-MS analysis enabled discrimination between natural and synthetic THCP sources by identifying chemical impurities. | Schirmer et al., 2024 [67] |
| Synthetic cannabinoids | Herbal mixtures | GC-MS and NMR were employed to determine the active compounds together with adulterants and preservatives (vitamin E, vitamin E acetate, oleamide). | Alves et al., 2021 [92] |
| ADB-4en-PINACA | Human hair sample | The human hair sample underwent extraction, but derivatization was not required. | Wang et al., 2023 [111] |
4. Synthetic Cathinones
Synthetic cathinones are the second most common type of NPSs and the largest subclass of synthetic stimulants [134,135]. Their structural basis is cathinone, a natural alkaloid obtained from the Catha edulis plant [136]. The structure consists of a benzyl group, a keto group on the β-carbon, and an amine terminal group. These structural moieties led to the development of various derivatives, such as aromatic ring substitution, N-functionalization, and a change in α-chain length [137,138]. In the review paper by Nadal-Gratacós and coworkers, the effects of the structural changes on the activity were examined in great detail [139].
Cathinones are usually produced in clandestine laboratories using procedures described in the scientific literature or patents. Most synthetic cathinones act as stimulants by producing effects similar to cocaine and amphetamine (euphoria, tachycardia, aggressive behavior, etc.) [140]. The origin of their activity lies in their inhibitory effects on dopamine and noradrenaline transporters. They are usually found in crystal or powder form, which allows their oral or nasal administration [31]. These NPSs are usually sold on the internet as “bath salts”, “research chemicals”, and “plant food” [141]. The regulation of synthetic cathinones lags behind the substances sold on the illicit market, as their scheduling is carried out on a case-by-case basis [134]. Therefore, only slight structural modifications of existing compounds are needed to avoid legislative restrictions [134].
4.1. Separation of Structural Isomers
The standard method for the identification of synthetic cathinones is GC-MS, although due to their thermal lability and degradation, the interpretation of mass spectra can be complicated [54]. Tseng and coworkers concluded that the slower thermal gradient heat speed with prolonged analysis time improved the peak resolutions [142]. The choice of GC column and thermal gradients was the main factor affecting compound separation.
The enamine formation of cathinones is commonly observed in MS spectra, with a -2 Da mass shift of the base peak due to oxidative degradation [54]. Depending on the jurisdiction, there may be requirements to identify specific isomers, such as when one is legally controlled while the other is not. To overcome this challenge, Liliedahl and Davison applied the canonical discriminant analysis (CDA) to differentiate cathinones by GC-MS [30]. The method is based on relative ion abundances in the MS spectrum. When 15 peaks were used, the classification rates were higher than 90% for chloroethcathinone, methoxymethcathinone positional isomers, and several constitutional isomers.
Other multivariate methods for the differentiation of NPS isomers using GC-MS data are given in references [59,143,144]. The fragmentation mechanism of N-alkylated cathinones depends strongly on the mass spectrometry technique used, as shown in reference [31]. ESI and DART ion sources produced protonated molecular ions with very similar spectra, and the dominant pathway involved the loss of H2O for secondary amines and the formation of alkylphenones for tertiary amines. When EI is used, the most abundant peaks include acylium and iminium ions as the radical-directed cleavages occur. These findings are important for the structural elucidation of novel compounds and for predicting spectra based on available instrumentation.
Dhabbah presented a derivatization technique with menthyl chloroformate that could distinguish between S and R cathinone isomers in different parts of Catha edulis [57]. S-isomer, as the most psychoactive stereoisomer, had higher concentrations in stems, but lower in leaves. Another derivatization method involves N-methylimidazole-catalyzed acylation with acid anhydrides, which leads to the formation of highly stable compounds, with over six years of shelf life [66]. This methodology allows for the identification of positional isomers based on retention times, especially when reference samples are available. Derivatization also influenced the fragmentation routes, which are determined by the basicity of specific compounds.
An interesting study by Nuñez-Montero and coworkers involved controlled administration of 4-chloromethcathinone, N-ethylpentedrone, and N-ethylhexedrone to consumers and GC-MS investigation of oral fluid and sweat [145] (Table 2). These compounds are depicted in Figure 4 to show their structural similarity. The ethyl acetate liquid–liquid extraction and derivatization with pentafluoropropionic anhydride (PFPA) provided the best compromise for sample preparation. The compounds administered intranasally were absorbed much more quickly than those administered orally, as indicated by the oral fluid analysis. Only 0.3% of the administered dose was determined in sweat. Although rare, this type of study is important for the precise examination of NPS metabolomic changes. Oral fluid is becoming a very popular alternative to other biological samples due to the relative ease of noninvasive collection and the potential for detecting parent molecules, although results are influenced by lipophilicity, matrix pH, and acid–base properties of compounds [146]. Other experimental studies with controlled administration of synthetic cathinones conducted in humans in the post-COVID-19 period are described in references [147,148,149,150,151,152,153].
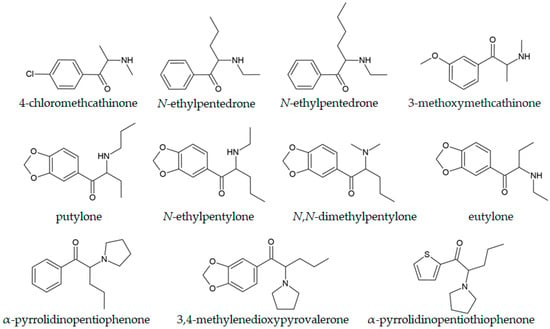
Figure 4.
Chemical structures of selected cathinones.
4.2. Synthetic Cathinones with a 3,4-Methylenedioxy Moiety
The addition of the 3,4-methylenedioxy moiety to the cathinone core is the primary step in the preparation of 3,4-methylenedioxy-N-alkylcathinones, which are analogues of MDMA [154,155]. Depending on the number of carbon atoms in the aliphatic chain attached to the amino group, there are several notable compounds in this sub-family, which constitutes around 18% of synthetic cathinones. These include methylone, butylone, ethylone, eutylone, pentylone, and N-ethylpentylone with 1–6 carbon atoms.
Dixon et al. examined a new compound in this subfamily, putylone (Figure 4), an N-propyl-substituted synthetic cathinone analogue of butylone [156]. The routine quantification was achieved by GC-MS, with an LOD and LOQ of 0.09 and 0.26 µg/mL, respectively, and was successfully applied to the determination of the compound in seized tablets. An interesting case was described in reference [157], in which forensic experts suspected that N-ethylpentylone was present in a seized sample. Only after the examination of EI mass spectra and assignment of the peak at 71 m/z to (CH3)2N+=CH-CH2• was it possible to conclude that the compound was dipentylone. Liliedhal and coworkers proved that N-butyl pentylone isomers (N-isobutyl pentylone, two diastereoisomers of N-sec-butyl pentylone, and N-tert-butyl pentylone) could be differentiated by employing the GC-EI-MS technique, as verified by the NMR experiments [158]. The ratio between the abundance of fragment ions at 128 and 72 m/z was sufficient for this purpose. This ratio is a consequence of the methyl substituent’s position, which influences the fragmentation pattern, and is essential for predicting the MS spectra of structurally similar compounds. In the case of N-isopropylbutylone, the GC-MS technique did not give a definitive answer because of mass spectral similarities with N,N-dimethylpentylone, N-ethylpentylone, and putylone [159]. All of these compounds are shown in Figure 4. Only with the use of advanced NMR techniques (correlation spectroscopy (COSY), distortionless enhancement by polarization transfer (DEPT), heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond correlation (HMBC)) was the structure univocally determined.
Armenta et al. presented results of the examination of a sample that was sold on the Internet as ketamine, although it was proven to be 3′,4′-methylenedioxy-2,2-dibromobutyrophenone, a precursor in the synthesis of other cathinones (pentylone and methylenedioxy pyrovalerone) [34,160]. The GC-MS analysis yielded two peaks with the same chemical composition, probably due to the decomposition in the injector. The peak at 65 m/z corresponded to the fragment found in other brominated 3,4-methylendioxy derivatives. Mata-Pesquera and coworkers performed characterization of N-cyclohexyl butylone, a recently reported cathinone [161]. The retention time of this compound was 8.465 min, with the major peaks in the mass spectrum at 58.1, 41.1, 121.0, 831, and 232.1 m/z, which did not allow for the identification of the compound after comparison with the spectral libraries. The structure was determined based on the fragments, and it contained a 3,4-methylenedioxy moiety, present in MDMA. The experimental research was followed by the in silico predictions of the activity of the compound, and they found that the predicted effects were similar to MDMA, just without the hallucinogenic effect. These in silico approaches are a good starting point for predicting the biological activity of newly obtained compounds by comparing them with structurally similar substances. Other theoretical methods, in the first place, Density Functional Theory (DFT) proved important for the determination of the absolute configuration of newly obtained compounds [162]. Mayer, Black, and Copp identified and characterized a new substance, N-cyclohexylpentylone, in a seized sample in New Zealand. The major peak at 154.2 m/z, and minor peaks at 72.1, 55.1, 149.0, and 121.0 m/z could not be matched to any compound in the databases. Through NMR analysis and the presence of impurities, the structure of the unknown compound was determined. Other new synthetic cathinones were described with spectral and chromatographic data ready to be updated in respective databases in references [72].
4.3. α-Pyrrolidinophenone Derivatives
α-Pyrrolidinophenone derivatives are some of the most abused designer drugs [135]. Their most notable structural feature is the addition of a pyrrolidine ring to the generic cathinone structure [163]. Notable examples include α-pyrrolidinopentiophenone (α-PVP, “Flakka”, Figure 4), α-pyrrolidinobutiophenone (α-PBP), 3,4-methylenedioxypyrovalerone (3,4-MDPV), α-pyrrolidinoheptanophenone (α-PV8), α-pyrrolidinopentiothiophenone (α-PVT, Figure 4), and α-pyrrolidinopropiophenone (α-PPP) [20,164]. The activity of the mentioned compounds is higher due to their more potent inhibition of monoamine transporters, and they more easily pass the blood–brain barrier because of the lipophilicity of the pyrrolidine substituent [165,166].
Davidson and coworkers examined the fragmentation pattern of 22 α-pyrrolidinophenone derivatives by GC-EI-MS, LS-ESI-MS/MS, and DART-MS [167] (Table 2). ESI and DART mass spectra were almost indistinguishable with eleven protonated ions. Meanwhile, GC-EI-MS spectra were dominated by iminium and acylium ions because of high-energy deposition from 70 eV electron fragmentation, which reduced the likelihood of rearrangements [168], as previously discussed for N-alkylated cathinones. The fragmentation mechanism was unaffected by the substitutions, enabling rapid identification of compounds via shifts in the mass axis. The authors have noted that EI generally produces odd-electron ions, whereas ESI produces even-electron ions [58]. The chromatographic separation and electron ionization-quadrupole mass spectrometry identification of 5-PPDI, an indanyl analogue of α-PBP, and three of its regioisomers were reported by Ishii and coworkers [169]. The order of elution (1, 4, 2, 5-positional isomer) was the same on three columns, namely a nonpolar 100% dimethylpolysiloxane, a micropolar phenyl arylene polymer column, and a mid-polar column virtually equivalent to a (50%-phenyl)-methylpolysiloxane.
Segurado and coworkers proposed a novel method of extraction, a bar adsorptive microextraction followed by microliquid desorption, of α-PVT, α-PVP, and MDPV from oral fluids, followed by GC-MS analysis (BAµE-µLD/GC-MS) [170]. The N-vinylpyrrolidone sorbent phase used as a coating material for the mentioned compounds demonstrated good performance due to hydrogen bonding, π–π interactions, and hydrophobic interactions. The average recovery rate ranged from 43.1 to 52.3%, and good selectivity was also observed. Blood concentration determination of α-PVT was reported in the paper by Machado and coworkers, which included the SPE with GC-MS analysis in the single-ion monitoring mode [171]. The peak at 140 m/z was selected for quantification based on selectivity and abundance. The linearity correlation was observed over the concentration range of 10–1000 ng/mL.
4.4. Samples with Several Cathinones
The separation of polydrug samples containing several cathinones is a tedious task due to their structural similarity, the absence of a molecular ion under EI ionization, and the statistical parameters used to describe spectral similarity, as outlined in references [71,172]. Woźniak et al. developed a simple procedure for the simultaneous determination of 45 amphetamines and synthetic cathinones in whole blood samples with high recovery (83.2–106%), accuracy (89.0–108%), and precision [173]. The procedure included extraction and pentafluoropropionyl derivatization. Another methodology was proposed by Antunes and coworkers, which included SPE and derivatization with N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) and trimethylchlorosilane (TMCS) for the accurate determination of four cathinones in whole blood samples [174]. The linearity range was 25–800 ng/mL, and efficiency was greater than 85%. Margalho et al. performed fast microwave derivatization of several cathinones with N-methyl-bis(trifluoroacetamide) (MBTFA) following SPE, a method that was successfully applied to 100 authentic blood samples [175]. The linearity range was similar to the previously mentioned derivatization procedure. The choice of derivatization agent depends on the structural features of the compounds, as concluded in reference [176]. For example, trimethylsilylation of chloromethcathinone isomers was needed for their complete separation [177]. Another standard biological sample containing synthetic cathinones is hair, which presents several effects that should be taken into account when discussing results, as reviewed by Bolcato and coworkers [52].
4.5. Synthetic Cathinones in Biological and Environmental Samples
GC-MS can also be used to quantify cathinones and their derivatives in plasma and brain tissue, as described by Bin Jardan and coworkers for methcathinone and its metabolite, ephedrine [47]. In complex matrices, the main advantage of GC-MS for synthetic cathinones lies in the consistent EI fragmentation pathways, as previously explained for α-cleavage and pyrrolidinyl-ring fragmentation, which support class confirmation and the identification of novel analogues. However, many halogenated or extended-chain cathinones are prone to thermal degradation or in-source rearrangement, reducing the reliability of GC-MS for trace-level quantification in biological matrices, where LC-MS/LC-HRMS provides superior sensitivity and stability. For this reason, GC-MS remains the method of choice for seized-drug screening and impurity profiling, while LC-MS is essential for metabolite analysis, stereoisomer resolution, and low-dose polydrug mixtures.
Hobbs and coworkers described the first case of fatal intoxication by 4-fluoro-3-methyl-α-PVP by analyzing body fluids [178]. The concentrations were calculated to be 26 ng/L in gray-top femoral blood and 20 ng/L in red-top vitreous humor using selected ion monitoring (SIM). Methcathinone is an analogue of methamphetamine, and it is already a controlled substance; therefore, it cannot be considered as NPS, but it is presented here as the representative of the synthetic cathinone class. This method was validated as sensitive and selective for the simultaneous quantification of both compounds, with a linearity range of 5–1000 ng/mg. SIM signals usually include the base peak and two qualifier ions chosen to achieve higher selectivity and sensitivity [176].
Weng and coworkers applied the previously mentioned MSPE procedure for the extraction of 16 cathinones from a urine sample [21]. When concentrations of these compounds in urine are concerned, an expected detection concentration should be less than 10 ng/mL. The sorbent consisted of Fe3O4 with amino-modified multi-walled carbon nanotubes (NH2-MWCNTs). The achieved linearity range was 0.005–5.00 µg/mL in complex matrices, with eco-friendly, fast detection that was also efficient and sensitive. Another novel designer drug, 3′,4′-methylenedioxy-α-pyrrolidinohexanophenone (MDPHP) and its metabolites were examined in urine samples of consumers, as GC-MS provided sufficient information for the prediction of metabolism in human samples [39]. The peak intensity of metabolites can be higher than that of a parent molecule, allowing their quantification in all biological samples, as shown for 4′-methyl-α-pyrrolidinohexanophenone (MPHP) and its 4′-carboxyl metabolite [179]. The prediction of the possible metabolites in urine can also be conducted using the online software (GLORYx), as shown in the reference [180].
The examination of the prevalence of NPSs in environmental samples is a common way to determine the scale of substance abuse. Wastewater-based epidemiology (WBE) has become a complementary tool for obtaining near-real-time information on drug consumption in a given area or during special occasions, such as music festivals, holidays, or sports events [181,182]. In this approach, both the aqueous fraction and the suspended particulate matter (SPM) can be examined. Langa and coworkers examined SPE in Portuguese wastewater for the presence of several amphetamine-like compounds, including several synthetic cathinones, by GC-MS [53]. They also performed enantiomeric profiling of compounds that were sorbed on SPM using the chiral agent (R)-(−)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride ((R)-MTPA-Cl). The highest concentrations were observed for (S)-amphetamine, while the concentrations of other substances were lower than in other European countries; however, the method proved reliable for the examination of WBE. Selective extraction of cathinone, methcathinone, mephedrone, methylone, and ethylone from wastewater was conducted by Han et al. on dummy molecularly imprinted polymers [48]. The extraction procedure took less than 5 min, and subsequent GC-MS analysis showed low LOD values (0.002–0.1 ng/mL), and the used sorbent demonstrated better cleanup ability.
GC-MS is also important as a confirmatory technique when new methodologies for detecting synthetic cathinones are developed. Yen et al. presented results of the detection of this class of compounds by hydrophobic carbon dot probes, and the results were confirmed by GC-MS [183]. Several cathinones, among which 4-chloromethcathinone (4-CMC), 4-chloroethcathinone (4-CEC), eutylone, and butylone were found mixed with instant coffee powder in a so-called “toxic caffe packets” that are popular in Taiwan [50].

Table 2.
Selected GC-MS studies on synthetic opioids (2020–2025).
Table 2.
Selected GC-MS studies on synthetic opioids (2020–2025).
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| N-cyclohexyl butylone | Pills and crystal samples | Newly detected compound with MS spectra that did not match the libraries. | Mata-Pesquera et al., 2023 [161] |
| 3′,4′-methylenedioxy-2,2-dibromobutyrophenone | Powder | This precursor was identified through MS fragmentation in a sample that was sold as ketamine on the Internet. | Armenta et al., 2020 [34] |
| R and S-isomers of cathinone | Different parts of the Catha edulis plant | Derivatization with menthyl chloroformate and subsequent GC-MS analysis was successful for the quantification of different isomers in various parts of the plant. | Dhabbah, 2020 [57] |
| Five isomers of N-butyl pentylone | Pure compounds | The MS fragmentation pattern (the ratio of ion abundances at 128 and 72 m/z) can be used to distinguish isomers. | Liliedahl et al., 2023 [158] |
| Several amphetamine-like stimulants, including synthetic cathinones | Suspended particulate matter in wastewater | GC-MS technique with the derivatization agent efficiently determined concentrations of amphetamine isomers and other stimulants. | Langa et al., 2024 [53] |
| N-cyclohexyl pentylone | Pale brown powder | GC-MS fragmentation and NMR analysis were used to solve the structure of the newly encountered NPS | Mayer et al., 2024 [29] |
| Methcathinone and ephedrine | Plasma and brain tissues | The GC-MS method was developed for the simultaneous determination of both compounds with a high linearity range (5 to 1000 ng/mg). | Bin Jardan et al., 2021 [47] |
| cathinone, methcathinone, mephedrone, methylone, and ethylone | Wastewater | The selective sorption was done on dummy molecularly imprinted polymers; GC-MS analysis had LOD values between 0.002 and 0.1 ng/mL. | Han et al., 2022 [48] |
| 22 α-pyrrolidinophenone | Pure compounds | Fragmentation pathways were examined using different instruments: GC-EI-MS, LS-ESI-MS/MS, and DART-MS. GC-EI-MS fragmentation mechanism was not dependent on the substituents present. | Davidson et al., 2020 [167] |
| 5-PPDI and three regioisomers | Solution | The order of elution was the same for the three columns used, and compounds were differentiated by the second-generation product ion spectra in ESI-LIT-MS. | Ishii et al., 2022 [169] |
| putylone | Seized tablets | The proposed GC-MS method successfully quantified putylone in seized tablets. | Dixon et al., 2023 [156] |
| 4-CEC, α-PVP, 4-Cl-PVP, and MDPV | Whole blood samples | SPE and derivatization with MSTFA and TMCS, linearity range 5–800 ng/mL, extraction efficiency > 85%. | Antunes et al., 2021 [174] |
| 4-Fluoro-3-methyl-α-PVP | Femoral and heart blood, vitreous humor | Post-mortem analysis of body fluids by GC-MS helped identify this compound. | Hobbs et al., 2022 [178] |
| α-PVT, α-PVP, and MDPV | Oral fluid | BAµE-µLD/GC-MS of three components, with average recoveries between 43.1 and 52.3%, and good selectivity. | Segurado et al., 2022 [170] |
5. Semi-Synthetic and Synthetic Opioids
Synthetic and semi-synthetic opioids are agonists of different subclasses of the opioid receptors, offering analgesic and sedative effects [184]. Their standard structural features include a basic nitrogen atom (tertiary amine), an aromatic (heteroaromatic) ring, and polar groups, such as amide or phenolic/carbonylic. Opioids are polar compounds, and their structural characteristics allow for their analysis in a single chromatographic run. The pH of the solution dictates the solubility of a compound in organic and aqueous media. This is due to the presence of basic nitrogen, which allows liquid–liquid extraction (LLE) for their separation in various samples. Diversification of their structures and the introduction of new compounds led to the opioid epidemic, making it one of the significant public health concerns in North America [185]. Although newly detected opioids are smaller in number, synthetic opioids have a higher risk of fatal intoxication [186].
5.1. Semi-Synthetic Opioids
Semi-synthetic opioids (SSOs) include compounds obtained by chemical modification of naturally occurring compounds. Some representatives of this class include hydromorphone (Figure 5), oxycodone, and oxymorphone. Another rising issue is the pills sold as oxycodone, but counterfeited and illicitly manufactured to look similar to prescription pharmaceuticals, which contain harmful additives or irregular doses [187]. In the study by Nguyen and coworkers, 567 pills, most of which carried a “30M” imprint as 30 mg oxycodone tablets, were examined by GC-MS and GS-FID [188]. Out of this number, fentanyl was identified in 75.4%, while others contained benzodiazepines, other opioids, and their precursors. Usman and coworkers concluded that nanomaterial-based techniques now offer innovative tools for sensitive monitoring of classical opiates and their synthetic analogues, with lower LODs, on-site detection, and specific preliminary analyses, which have to be confirmed by standard chromatographic analysis [189]. Methorphan (Figure 5), a semi-synthetic morphine derivative, is commonly encountered in seized heroin samples. Its higher concentrations lead to effects similar to those of ketamine and phencyclidine. This compound is of particular interest, as its d-isomer is used as an antitussive. At the same time, the l-isomer is a potent narcotic opioid, raising questions about the low restrictions on specific isomers. The GC technique can be applied for the quantification of a particular isomer if a chiral derivatization agent is used, such as (-)-menthyl chloroformate [190]. Salerno et al. also presented the GC-MS examination of 45 heroin samples containing methorphan, and 24 out of 26 compounds in these samples were identified with similarities in the 85–97% without the need for derivatization [191]. The methorphan isomers were quantified by enantioselective HPLC-MS/MS.
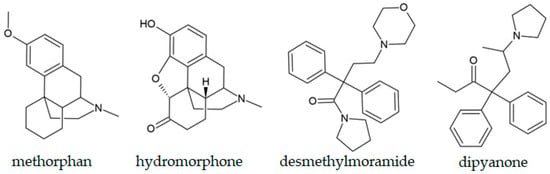
Figure 5.
Chemical structures of selected semi-synthetic opioids.
In 2021, two opioids related to methadone, a commonly prescribed analgesic, appeared on the drug market. The first one is dipyanone (Figure 5), a compound investigated for the analgesic activity comparable to methadone in the mid-20th century [192]. It was first identified in seized samples in Germany, followed by Slovenia and the USA. Desmethylmoramide (Figure 5), a second compound, was also discovered in Germany. This compound is structurally related to moramide, a controlled opioid [193]. As both compounds are related to the prescription opioids, their harm potential as NPS is poorly understood. Vandeputte and coworkers performed GC-MS analysis of dipyanone without derivatization, with the molecular ion located at 335 m/z and the pyrrolidino ring mass fragment at 98 m/z [194].
5.2. Fentanyl and Its Analogues
The increase in the consumption of fentanyl and its analogues created epidemic-level harm in some countries, as shown in references [195,196]. The constant increase in newly identified fentanyl-related opioids is evident, especially after 2009, when more than ten new compounds were reported each year [197]. Fentanyl, the most prominent synthetic opioid, has been synthesized and used since the 1960s. This compound is 50 to 100 times more potent than morphine. The structural features of fentanyl, based on the main chain of phenethyl piperidine, allow for different modifications, leading to a large group of compounds called “fentanyls” [197]. Unlike other groups of NPSs, fentanyl and some of its analogues are used in human and veterinary medicine for anesthesia, pain control in postoperative surgical procedures, and cancer, but there is also a long history of abuse [198,199]. Some of the examples of fentanyl derivatives are alfentanil, sufentanil, remifentanil, and carfentanil, and their structures are shown in Figure 6 [200]. So far, efforts by various government drug enforcement agencies to bring it under control have had limited success due to its availability and profitability [201]. The precursors for fentanyls are readily available, and the synthesis procedures are relatively straightforward [202]. Fentanyl is commonly encountered mixed with other natural and synthetic controlled substances, and even sold as “fake Norco” or MDMA [203,204].
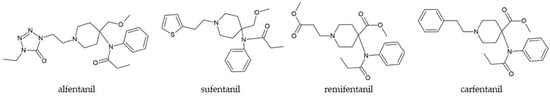
Figure 6.
Chemical structures of selected fentanyl derivatives.
Several different methodologies have been developed for the identification and quantification of fentanyl and its analogues. Commonly used methods include GC-MS and LC-MS/MS for the analysis of these compounds in biological samples. GC-MS is used routinely [205] for the identification of compounds, their precursors, and metabolites with great success, as shown in the review by Valdez [197]. The preprocessing of fentanyls for GC-MS analysis, including derivatization, makes the examination cumbersome and limits rapid detection. The detection limits are relatively high, increasing the risk of false negatives. Therefore, the importance of LC-MS/MS instrumentation is rising, as the method allows for high sensitivity and precision, high turnaround time, and a wide detection range [206,207]. The main drawback is the large volume of organic solvents required for analysis, especially when samples, such as hair, are hydrolyzed. Often, the lack of drug matrix references lowers the reliability and traceability of the obtained results [208].
When GC-MS is selected for fentanyl analysis, the critical step is sample preparation, as very low quantities are typically present in complex matrices. SPE is usually chosen for purification from the matrix, while LLE in phosphate buffer at pH 5–8.4 is the most widely used method for fentanyls, as outlined in the review paper by Carelli and coworkers [184]. Standard extraction techniques, such as SPE and LLE, are time-consuming and require large volumes of organic solvents. Therefore, microextraction techniques are constantly being developed for the analysis of biological samples. Ares-Fuentes and coworkers presented results of the fabric-phase sorptive microextraction (FPSE) and GC-MS analysis of nine fentanyl analogues in oral fluid [196]. The LOD and LOQs for these compounds were 1–15 ng/mL and 5–50 ng/mL, respectively, with recovery rates ranging from 94.5% to 109.1%.
Camedda et al. developed the first GC-MS procedure for the quantification of fentanyl and butyryl fentanyl with an LOQ of 1 ng/mL in oral fluid [209]. Sisco and coworkers obtained results on the application of fentanyl-targeted GC-MS separation of 11 analogues [210]. They concluded that the targeted analysis was up to 25 times more sensitive, and the retention time differences were increased by a factor of two. Gilbert et al. applied PCA and hierarchical clustering to EI-MS data for fentanyls, achieving 91% accuracy. The compounds were grouped based on the positions of modifications and chemical groups introduced into the structure, highlighting the importance of statistical tools in NPS identification [144]. Gilbert and coworkers proved that presumptive tests based on Eosin Y have the potential to detect a wide range of fentanyl derivatives [211]. Their results were confirmed by the GC-MS analysis, which was able to discriminate between 18 fentanyl-derived synthetic opioids after their dissolution in methanol within 20 min. The LOD and LOQ values were 0.007–0.822 µg/mL and 0.023–2.742 µg/mL.
Forensic coursework often involves the examination of non-standard samples. Vladez et al. described a method for fentanyl, acetyl fentanyl, and their metabolites in soil by the GC-MS technique [212]. Examination of these SO and metabolites in environmental samples can link the site to the illicit production of these compounds [213]. Active compounds were extracted from the soil using ethyl acetate, then reacted with 2,2,2-trichloroethoxycarbonyl chloride (Troc-Cl) to generate the modified compounds. The LOQ values obtained in this study were expressed in nanograms per milliliter of sample. Additionally, fentanyl and related compounds were examined using the same method from silt, a complex matrix due to its high water content and chemical impurities that shield analytes from derivatization agents [95], and from clay [214]. Valdez et al. used the same derivatization agent for urine and plasma samples spiked with fentanyl and acetyl fentanyl. They concluded that 2-phenylethyl chloride was detectable only by GC-MS, and not by LC-MS [215]. This compound is important as the second reaction product, which is crucial for determining the original opioid [216]. It is important to mention that fentanyls undergo similar fragmentation in mass spectra, including piperidine ring degradation, dissociation of phenethyl and piperidine rings, and cleavage of the piperidine ring and amide moiety. However, some differences were observed depending on the ionization mode [217]. Although similar, the fragmentation pathways depend on the substituent position and enable the differentiation of compounds, as observed in the mass spectra of fentanyl and acetyl fentanyl [70]. This allows for the structural elucidation of features when new compounds are encountered. In the paper by Wei and Su, nine fentanyl drugs were examined in hair samples, extracted by sonication and centrifugation, with the linear range between 0.5 and 5.0 ng/mg, with the detection limits between 0.02 and 0.05 ng/mg [208]. Another advancement in the analysis of fentanyls was described in a paper by Leary and coworkers, which presented the capabilities of a GC-MS instrument [218]. Out of 250 compounds (210 of which were fentanyl analogues), 90% were successfully detected onboard. These results might have a significant impact on the field analysis of illicit drugs.
5.3. Non-Fentanyl Compounds
A special class of synthetic opioids includes non-fentanyl compounds, belonging to piperazines (MT-45) and benzamides (U-50488, U-47700, U-69593, and AH-7941) [219,220]. The structures of representative compounds are shown in Figure 7. Most of these compounds were synthesized as analgesics that did not enter clinical trials. “U-compounds”, such as U-50488 and U-47700, were developed by the Upjohn Company in the 1970s and 1980s for their therapeutic properties [221]. These compounds possess desirable opioid effects, for example, euphoria and pain relief [222] with activity that is ten times higher than that of morphine in laboratory animals. A detailed review by Baumann and coworkers covers the pharmacological and structural features of these compounds [221]. The second group of non-fentanyl compounds is labeled as “AH-compounds”, and they include N-substituted cyclohexylmethylbenzamides [223]. These compounds were also discarded during the drug development because of unsatisfying results of drug tests, mainly due to severe side effects or increased potency [224]. MT-45 emerged in 2013. It is chemically similar to fentanyl and has a high analgesic effect [225,226]. Low cost of production and presence on the Internet as a “legal opioid” make MT-45 attractive to the illicit market. There are reports of this compound being mixed with methylone and sold as “wow” [226].
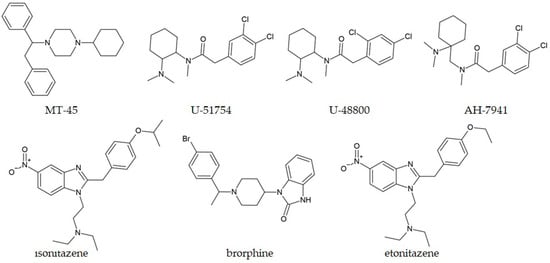
Figure 7.
Chemical structures of selected non-fentanyl compounds.
Pereira et al. discussed that the GC-MS technique alone was not sufficient for a definitive answer for the characterization of an unknown substance, as two matches were found in the mass spectra library (U-51754 and U-48800) [227]. NMR spectra and molecular dynamics simulations were also performed to elucidate the structural features of the unknown substance (U-48800) (Table 3). They concluded that bioinformatic tools are valuable in forensic research, especially when reference materials are unavailable. Uchiyama and coworkers identified MT-45 together with other NPSs in chemical and herbal products bought via the Internet by GC-MS, and further examined by other techniques [228].
Benzimidazole synthetic opioids are a special class of synthetic opioids, with activity that often exceeds that of fentanyl [229]. These compounds were developed by the Swiss chemical company in the late 1950s, but they have never been used in clinical practice. In 2020, Verougstraete et al. reported, for the first time, intoxication by brorphine (Figure 7), a piperidine benzimidazolone derivative, and characterized its structure from a powder sample obtained from a patient seeking medical help [230]. This compound is outside the scope of generic legislation, although the opioid activity of this class of compounds was reported in 1967 [230]. Another benzimidazole derivative, isotonitazene (Figure 7), was identified by GC-MS in 2019 by Blanckaert and coworkers [231]. The compound was sold online as white powder and marketed as etonitazene (Figure 7), a compound with a reported potency of a hundred to a thousand times that of morphine [232]. Biological studies involving isotonitazene showed its high potency and efficacy, labeling it as a potent opioid. The identification of isonitazene from nitazene, a structural isomer and a member of the benzimidazole opioid family, was difficult in the electron ionization mass spectra upon separation by GC, as concluded by Kanamori et al. [233]. The characteristic fragmentation in LC-MS allowed these compounds to be distinguished from one another.
5.4. Benzodiazepines
Benzodiazepines are allosteric modulators of γ-aminobutyric acid type A (GABAA) receptors, introduced in the 1960s and are used for their anxiolytic and sedative effects [234]. They are applied in the therapy of neurological disorders, insomnia, muscle spasms, epilepsy, and alcohol withdrawal. Benzodiazepine’s structure consists of fused benzene and heterocyclic diazepine rings. Some of these compounds are approved for veterinary use, although the number of compounds in the illicit market have increased in the following years. The most common prescription benzodiazepines present in Europe are diazepam (Valium) and alprazolam (Xanax). Griffin et al. commented that benzodiazepines are used in self-medication, in mixtures with other psychoactive substances, and can be found in drug-facilitated crimes [235].
A related group of compounds is “designer benzodiazepines” or “novel benzodiazepines” (NBs) that show similar pharmacological properties, but do not share the same structural features as benzodiazepines. These compounds can often be seized in illicit samples resembling original benzodiazepine tablets, making the consumers unaware of the specific drugs or dose that is consumed [236]. Unlike benzodiazepines, NBs are not registered as medications, and their side effects are less well-known, although their potency is considered to be greater than prescribed benzodiazepines [237]. It is assumed that NBs are partially used as “self-medication” combined with other psychoactive substances, or for the self-treatment of a sleep disorder. A review of the NBs up until 2019 is shown in the paper by Zawilska and Wojcieszak [236]. The number of intoxications with fatal outcomes involving NBs drastically increased. In Scotland, NBs were responsible for 842 out of 1330 drug-related deaths, as outlined by the National Records of Scotland [238]. The most common NBs are etizolam, diclazepam, flubromazolam, and phenazepam, which account for more than 80% of the seized tablets. The structures of diclazepam and flubromazepam are depicted in Figure 8. NBs are usually produced either by the derivatization of pharmaceutical drugs or structural modification, although there are cases in which active metabolites (desmethylflunitrazepam and 3-hydroxydesmethylflunitrazepam) of benzodiazepines are found [239].
Figure 8.
Chemical structures of selected designer benzodiazepines.
Desalkylgidazepam, or bromonordiazepam, is an active metabolite of gidazepam (Figure 8), recently discovered in forensic cases. This compound has a longer half-life than most benzodiazepines, and can be determined in urine and blood by GC-MS [240]. Bromazolam is a brominated analogue of alprazolam. Although it is not widely controlled, it is regulated in Germany and the UK. The first toxicological detection of this substance was reported in 2016, and since then, it has been found in various biological specimens [241,242]. Ballotari et al. presented results of the postmortem GC-MS analysis of blood and urine samples that were positive for fentanyl, desalkylgidazepam, and bromazolam [243]. Two designer benzodiazepines require different preparation: derivatization of desalkylgidazepam with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) for 45 min at 70 °C was needed, whereas bromozolam was analyzed directly. The concentrations of desalkylgidazepam and bromazolam in blood/urine were 1100/98 and 352/398 ng/mL, respectively (Table 3). Another fluorinated derivative of alprazolam, flualprazolam, was introduced in 2017 as tablets and blisters in several European countries. Giorgetti et al. described a case of fatal intoxication in 2022 involving this compound, with its concentration determined in body fluids and solid tissues [244]. This analysis involved GC-MS and LC-MS/MS, and several drugs (including GBH, amphetamine, bromazepam, THC-COOH) were detected in heart serum, peripheral blood, urine, brain, liver, and kidney. Based on the complex mixture of compounds, it was concluded that flualprazolam likely contributed to the death.
NBs were also found in 495 samples of paper, cards, blotters, tablets, powders, and clothing from Scottish prisons [245]. The most prevalent between 2020 and 2021 was etizolam, while flubromazepam (Figure 8) and bromazolam became more prevalent later. GC-MS analysis showed that concentrations were highest in tablets (0.34–2.33 mg per tablet), whereas benzodiazepine concentrations on cards varied across the surface (1.16–1.87 mg/cm2). These results highlight high risks of overdosing by individuals, as the amounts significantly differed among samples.

Table 3.
Selected GC-MS studies on synthetic opioids (2020–2025).
Table 3.
Selected GC-MS studies on synthetic opioids (2020–2025).
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Fentanyl and eight analogues | Oral liquids | FPSE microextraction followed by GC-MS provided good recovery, linearity, and selectivity in ng/mL concentrations. | Ares-Fuentes et al., 2022 [196] |
| Fentanyl and acetofentanyl | Soil | Extraction with ethyl acetate, followed by mixing with Troc-Cl and subsequent GC-MS analysis, allowed identification of metabolites. | Valdez et al., 2023 [212] |
| 11 fentanyl analogues | Methanolic solution | The targeted GC-MS technique developed for fentanyl analogues was 25 times more sensitive and had a two-fold increase in retention time difference, compared to standard methods. | Sisco et al., 2021 [210] |
| Fentanyl and butyryl fentanyl | Oral fluid | Developed GC-MS procedure with LOQ of 1 ng/mL. | Camedda et al., 2024 [209] |
| 18 fentanyl-derived opioids | Solution | Presumptive tests were used to detect compounds, which GC-MS further confirmed within 20 min. | Gilbert et al., 2020 [211] |
| U-48800 | White powder | GC-MS was unable to differentiate between closely related isomers; NMR and molecular dynamics provided definitive structural information. | Pereira et al., 2025 [227] |
| Dipyanone, a methadone-related compound | White powder | GC-MS identification was possible based on the peaks at 355 (molecular ion) and 98 (pyrrolidine ring). | Vandeputte et al., 2023 [194] |
| Nine fentanyl compounds | Hair sample | GC-MS analysis was done on the extracts after the sonication of the samples. | Wei and Su, 2022 [208] |
| Methyl-substituted fentanyl derivatives | Pure compounds | GC-MS retention times and fragmentation patterns depend on the position of substituents and allow their differentiation with high precision. | Pollard and Davison, 2023 [70] |
| Brorphine | Powder | Reported structural examination for the first time. | Verougstraete et al., 2020 [230] |
| Oxycodone pills and other prescription drugs | Pills | GC-MS and GC-FID were used to examine counterfeit pills with a physical appearance similar to genuine drugs. In most of them, fentanyl and other opioids were detected. | Nguyen et al., 2022 [188] |
| Fentanyl, desalkylgidazepam, and bromazolam | Blood and urine | GC-MS was applied for the quantification of three opioids; derivatization with MTBSTFA was needed for desalkylgidazepam. | Ballotari et al., 2024 [243] |
6. Hallucinogens, Dissociatives, and Entactogens/Empathogens
From the discovery of LSD in 1938 by Albert Hofmann, hallucinogens have always been present in the illicit drug market. In the 2000s, hundreds of new substances were sold as “legal” alternatives to those already listed. Among the most popular were those with hallucinogenic effects, including compounds classified as phenethylamines and indolalkylamines [246]. These compounds are known as serotonergic agonists.
6.1. LSD and Its Analogues
With the undesired effects produced by the use of other hallucinogens, the synthesis of compounds similar to LSD gained attention. Some of the obtained compounds act as prodrugs and, during metabolism, change to LSD by N-deacetylation [247]. In 2020 and 2021, 1-cyclopropanoyl and 1-valeroyl-LSD derivatives were identified and characterized in references [248,249]. These two compounds are presented in Figure 9. In the electron ionization mass spectrum, both compounds show a molecular ion in high abundance, an iminium ion at 72 and 100 m/z, and clusters of ions originating from the lysergamide core at 151–156, 161–169, 178–182, 191–197, 205–208, and 219–221 m/z, which is consistent with other LSD-derived compounds [250,251]. It was concluded that some degradation products were detected only by GC-MS, rather than by LC-MS, due to the experimental conditions. Kavanagh and coworkers examined another LSD-derivative with a 12-carbon chain, namely 1-dodecanoyl-LSD (1DD-LSD, Figure 9) [252]. The authors proposed a complex fragmentation scheme, with the mass spectrum peaks specific for this compound at 462 (obtained by the loss of N-methylmethanimine), 403–406, and 222 m/z. During GC separation, six additional peaks were detected in full-scan mode, likely due to impurities from the synthesis.
Figure 9.
Chemical structures of selected LSD and tryptamine compounds.
The authors of a recent study published in 2022 identified by GC-MS a prodrug 1-acetyl-LSD (ALD-52) in nine seized blotter papers from São Paolo [253] (Table 4). It is important to note that Brazilian legislation did not control this compound at the time of publication. Tusiewicz and coworkers performed a GC-MS separation and identified 13 LSD analogues by varying experimental conditions [254]. They concluded that diethyl ether, tert-butyl methyl ether, dichloromethane, and acetone were solvents in which the best sensitivity and stability were obtained. On the other hand, methanol caused alcoholysis of the compounds, leading to false results. The major peaks for compound identification in the MS spectrum (221, 208, 207, 196, 194, 192, 181, 167, 154, 152, and 128 m/z) matched those reported in previous studies.
6.2. Tryptamines
Tryptamines are a diverse group of hallucinogenic substances, of which the most famous are psilocybin, dimethyltryptamine (DMT), and 5-methoxy DMT (5-MeOH-DMT) [255]. DMT, together with harmine and harmaline, is the main active component of ayahuasca, a hallucinogenic beverage that is used for ritualistic purposes in South America [256]. Although DMT is a highly controlled substance, its consumption is legitimized for religious purposes. Tavers et al. reported the results of the GC-MS analysis of sweat samples from participants in religious rituals [257,258]. A high linearity coefficient and concentrations ranging from 20 to 1500 ng/patch (cellulose patches were used for sweat collection) were obtained, indicating high selectivity and extraction efficiency exceeding 70%. This study emphasizes the use of sweat because of the cumulative record of drug exposure, painless collection, and detection period of up to two weeks following exposure.
Neukamm and coworkers examined a fatal case of aspiration due to the abuse of dipropyltryptamine (DPT, Figure 9) in reference [259]. This compound was used in psychotherapy for alcoholics in the 1960s and 1970s, but also as a hallucinogen due to its similar activity to DMT. Their examination of body fluids was conducted using LC-MS/MS. Still, they noted that GC-MS examination was not possible because DMP and its metabolites were not included in the spectral libraries. This raises an essential point: updating the relevant libraries when new compounds are encountered to facilitate their identification. Emen et al. presented the development of a chemometric method for the determination of 5-MeO-MiPT, a compound closely related to 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT, Figure 9), in urine samples [260]. 5-MeODiPT, shown in Figure 9, is a synthetic diisopropyl analogue of 5-MeOH-DMT. Based on the results, the proposed GC-MS was able to quantify 5-MeOMiPT, known as “moxy”, with LOD and LOQ values of 5 and 18 ng/mL, respectively. This result is also significant, as 5-MeOMiPT was the second most common NPS in the seized samples in Turkey [261].
6.3. Phenylalkylamines
Phenylalkylamine hallucinogens are a large group of molecules, usually separated into two classes. The first class resembles the structure of amphetamines, and incorporates naturally occurring compounds, such as mescaline, and 2,5-dimethoxy-4-bromoamphetamine [262]. The second class includes synthetic 4-substituted-2,5-dimethoxyphenethylamines (“2C-X”). The notation “2C” refers to the number of carbon atoms between the benzene ring and the amino group, while “X” represents different substituents at position 4 of the aromatic ring [263]. 2C-X compounds exhibit both stimulating and hallucinogenic effects, depending on the substituents present, as mentioned in the previous sections. N-benzylphenethylamine derivatives (NBOMes) are included in the second class, with an N-benzyl substituent, and they are particularly popular for the changes in sensory perception and mood; they were first described by Shulgin and Shulgin [264,265]. The best-known compounds of this class are N-(2-methoxybenzyl)-4-bromo-2,5-dimethoxyphenethylamine (25B-NBOMe) and N-(2-methoxybenzyl)-4-iodo-2,5-dimethoxyphenethylamine (25I-NBOMe) (Figure 10), secondary amines with high affinity for the 5HT2A receptor, one of the seven sub-families of serotonin receptors [266]. In the review paper by Katarzyna and coworkers, the adverse effects after the abuse of 25I-NBOMe include aggressiveness, psychotic behavior, panic attacks, and visual and auditory hallucinations [267]. A similar result was found for the chloro-derivative in the paper by Kamińska and coworkers [268]. The structural modifications of NBOMe, especially the N-(2-methoxybenzyl) substitution, led to substances with some of the highest affinities for the mentioned receptor at the microgram doses [269,270]. The concentrations of NBOMe compounds are most commonly determined by LC-MS, which allows a limit of quantification of pg/mL to ng/mL and separates compounds from their metabolites [267]. The applicability of GC-MS for their identification and quantification in blood and urine has also been demonstrated in the literature [271].
Figure 10.
Chemical structures of selected phenylalkylamine hallucinogens.
In the paper by Lukić and coworkers, a GC-MS method was described for the determination of 2,5-dimethoxy-4-bromophenethylamine (2C-B, Figure 10), a hallucinogenic phenethylamine, in urine samples [272]. The compound was derivatized using MBTFA and later separated on a DB-5MS capillary column. The quantification of the compound was performed using ions at 242, 229, and 149 m/z. Identification of 2X-NBO (X–halogen or alkyl substituent) is prone to thermal degradation, which leads to potentially incorrect identification [273]. Lopes et al. presented a thermal stability examination of 2X-NBO (X = Br, Cl, Et, I) from blotter papers using GC-MS, ATR-FTIR, SCXRD, and PXRD techniques, which can be used to develop new methodologies for the correct identification of these compounds [274]. The characteristic bands in ATR-FTIR and UV–Vis spectra were specific enough to allow differentiation, even after extraction, when the samples contained impurities.
Almalki and coworkers proactively prepared methylenedioxybenzyl analogues of 25X-NBO drugs with structures similar to those presented in Figure 10 [275]. This substituent was chosen as it is present in MDMA, one of the most famous party drugs for many years [276]. The enhancement of psychoactive effects was found in different drug classes upon the addition of this substituent [277]. The ten compounds obtained were separated on a 30 m column containing a silarylene film phase (Rxi®-17Sil MS) for 11 min [275]. During ionization, the iminium cation at 164 or 178 m/z was formed, along with halogenated dimethoxyphenyl fragments, as previously observed for similar compounds. Further GC-MS studies involved the separation and MS examination of compounds obtained by combining the structural features of amphetamines and NBOMe drugs [68,69]. The authors of this 2020 study examined the GC-MS characterization of nine regioisomers of 4-bromo-2,5-dimethoxybenzyl-substituted compounds (Figure 10), which are the direct inverse analogues of 25B-NBOMe [278]. Although these compounds were not encountered in the forensic coursework, they can be easily prepared and marketed. The authors have concluded that, based on the relative positions of the groups, the order of elucidation was: 2-methoxyphenethylamine, 3-methoxyphenethylamine, and 4-methoxyphenethylamine isomers, although their EI-MS spectra contained two bromine-containing and two non-brominated fragments. These results are important, as they allow for the prediction of elution times and fragmentation patterns for different isomers of NBO compounds.
6.4. Ketamine
Ketamine, although not considered an NPS, is commonly found in samples originating from young consumers and polyconsumption profiles. This substance was developed and used as an anesthetic and analgesic in veterinary medicine, but is classified as a dissociative drug [279]. Due to its importance, ketamine was not included in the list of NPSs, but as its illicit use has increased, different countries have introduced various measures for its control [280]. Ketamine is included in this review because several studies examined its presence in various biological samples. Matey and coworkers presented a GC-MS survey of hair samples from ketamine consumers [281]. The authors concluded that this technique was sufficient for the identification and quantification of ketamine and other NPSs in hair samples. However, the LOD and LOQ were lower when UHPLC-HRMS/MS methodology was used. On the other hand, GC-MS offered a much simpler procedure that did not require specialized training to interpret results.
Methoxetamine (MXE, Figure 11) is a psychoactive compound derived from ketamine, also able to produce hallucinogenic and dissociative effects [282]. After 2010, it became popular on the Internet, which resulted in its higher abuse rate and several fatal outcomes [283]. Alshamaileh et al. used human hepatocytes, a method highly popular for the in vitro prediction of the drugs’ metabolism, to study chemical conversions of MXE [284]. The metabolites of MXE were similar to those previously reported for ketamine, as expected given their structural resemblance. In this case, the metabolites were readily detected and identified by LC-MS, whereas the absence of signals in GC-MS after derivatization was attributed to their low volatility. The authors concluded that demethylation metabolites were more likely to be observed by GC-MS than hydroxylated metabolites, which are less volatile and more polar.
Figure 11.
Chemical structures of methoxetamine and ketamine.
6.5. Nitrous Oxide
Nitrous oxide (N2O) is a colorless and slightly sweet gas used as an analgesic and an anesthetic in medicine. Previous studies have shown subjective effects (euphoria, relaxation, and hallucinations) similar to those of other psychedelic drugs [285]. Due to its availability in small containers and cylinders as a food-grade compound, it is readily accessible to the younger population [286]. The abuse rate of the substance almost doubled between 2020 (9.7%) and 2021 (22.5%), as found by global studies. As the inhaled amount increases, the risk of tissue hypoxia and displacement of oxygen increases, leading to fatal accidents [287]. The most commonly used technique for N2O analysis is headspace gas chromatography-mass spectrometry (HS-GC-MS). However, the main difficulty in its quantification arises from the fact that its molar mass is the same as that of carbon dioxide. Li et al. showed that the use of n-pentane as an internal standard and sodium hydroxide solution to remove CO2 is promising for the precise quantification of N2O in blood samples in two cases of fatal overdose [288]. The obtained linearity range was 0.02–0.5 mL/mL, and the LOD was 0.005 mL/mL. Lindholm and coworkers used GC-MS to determine N2O concentrations in blood samples from traffic cases involving suspects accused of driving under the influence of this substance [289] (Table 4). Their methodology allowed for the determination of 0.1 to 48 mL N2O/L blood in 52 cases.
6.6. Hallucinogenic Compounds from Natural Sources
The hallucinogenic compounds from natural sources are not strictly considered as NPSs. However, since many of them have recently been examined by GC-MS in biological samples during the post-COVID-19 period, this review included some examples from the literature. Three active components of nutmeg (safrole, myristicin, and elemicin) are responsible for the intoxication due to overdose, which results in hallucinogenic effects, nausea, vomiting, vertigo, tachycardia, and agitation [290,291]. Usui et al. developed an efficient method for their extraction and quantification in human serum using the MonoSpin® extraction kit and GC-MS [292]. The detectable concentrations ranged from 0.5 to 300 ng/mL, which corresponded well to those reported in cases of nutmeg poisoning. Willard and coworkers conducted a study of the mass spectra of salvinorin A, a compound considered the most potent hallucinogen of natural origin (S. divinorum) [293]. This study is important because the authors developed a procedure for comparing the mass spectra of similar compounds based on ion abundances across the full scan range [294]. By applying the procedure, the authors were able to distinguish between salvinorin A, B, C, and D, which were extracted and chromatographically separated.

Table 4.
Selected GC-MS studies on novel hallucinogens/dissociatives (2020–2025).
Table 4.
Selected GC-MS studies on novel hallucinogens/dissociatives (2020–2025).
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Ketamine | Hair samples | GC-MS provided untargeted analysis, whereas UHPLC-HRMS/MS yielded lower LODs and LOQs. | Matey et al., 2021 [281] |
| 1-Acetyl-LSD | Blotter paper | Identified by GC-MS for the first time in Brazil. | Neves Junior et al., 2022 [253] |
| N2O | Blood | The GC-MS method allowed the determination of blood concentration between 0.1 and 48 mL N2O/L blood. | Lindholm et al., 2024 [289] |
| N2O | Blood | N2O was separated from CO2 using a sodium hydroxide solution before the GC-MS analysis, with n-pentane added as an internal standard. | Li et al., 2024 [288] |
| Safrole, myristicin, and elemicin | Human serum | MonoSpin® extraction kit and GC-MS were used for the extraction of three hallucinogenic compounds from nutmeg. | Usui et al., 2023 [292] |
| N-(bromodimethoxybenzyl)-2-, 3-, and 4-methoxyphenethylamines | Powder | The inverse analogues of the 25B-NBO drug were examined, and their elution times and fragmentation were determined based on the relative positions of substituents. | Almalki et al., 2020 [278] |
| Methylenedioxybenzyl analogues of 25X-NBOMe drugs | Powder | Methylenedioxybenzyl analogues were proactively synthesized and examined by GC-MS, with separation on a Rxi®-17Sil column, and the fragmentation pathways were explained. | Almalki et al., 2020 [275] |
| Methoxetamine (MXE) | Solution | The LC-MS technique was more appropriate for the examination of MXE’s metabolites from in vitro studies than GC-MS, due to their volatility. | Alshamaileh et al., 2020 [284] |
| 5-MeO-MiPT | Urine samples | The GC-MS methodology had LOD and LOQ values of 5 and 18 ng/mL, respectively, with linearity over 25–500 ng/mL. | Emen et al., 2023 [260] |
| 2C-B | Urine sample | Before GC-MS, derivatization was performed using MBTFA following SPE preparation. | Lukić et al., 2022 [272] |
| DTM, harmine, and harmaline | Sweat | GC-MS quantification of the constituents of Ayahuasca from sweat samples of the participants in the religious ritual. | Tavares et al., 2020 [257] |
7. Polydrug Mixtures from Different Classes
The presence of multiple psychoactive substances in seized materials and biological specimens represents a frequent and analytically demanding task in modern forensic practice. Polydrug mixtures are intentionally or unintentionally produced during synthesis, dilution, or distribution, and are often associated with elevated toxicity and unpredictable pharmacological effects and interactions. Analytical reports from the literature review indicated a sustained increase in the appearance of such mixtures, with the most common constituents being synthetic cathinones, amphetamine-type stimulants, dissociatives, and hallucinogens [3,22,173]. The combined intake of stimulants (methamphetamine, MDMA, and ketamine) and depressants (gamma hydroxybutyrate (GHB), benzodiazepines, and opioids) leads to complex pharmacodynamic profiles that are challenging for both clinical and postmortem interpretation. Although GC-MS instruments are available in most toxicology laboratories, methods for the simultaneous detection of a wide range of NPS are rare in the available literature [173,295]. Most GC-MS methods are developed for a single compound or category, as explained in previous sections.
In addition to their toxicological complexity, polydrug mixtures pose several analytical challenges beyond the simple coexistence of multiple compounds in a specimen. Structurally related stimulants, cathinones, fentanyl analogues, and semi-synthetic cannabinoids often share similar volatility and retention behavior, increasing the likelihood of partial or complete co-elution, even under optimized conditions. Under electron-impact ionization, abundant components may dominate fragmentation patterns and suppress diagnostic ions of low-level analytes, leading to the underrepresentation or misidentification of minor but toxicologically significant constituents. Thermal degradation can also be observed in mixtures, resulting in additional artifacts that complicate interpretation. For these reasons, the analysis of polydrug mixtures increasingly relies on the careful coordination of extraction, derivatization, and chromatographic parameters, as well as on deconvolution tools or chemometric classifiers when overlapping spectra cannot be separated visually. These considerations are rarely addressed explicitly in published studies but are essential for reliable GC-MS-based screening of contemporary NPS mixtures.
In the work by Ferreira et al., the SPE-GC-MS method was successfully applied for the detection and quantification of nineteen drugs of abuse, including cocaine, its metabolites, opiates, and NPSs, both in vitro and post-mortem whole blood samples [296]. The authors outlined the need for three working ranges to account for differences in the therapeutic, toxic, and lethal concentrations of compounds. The derivatization reagent included BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide) and 1% TMCS (trimethylchlorosilane), and identification was univocally conducted based on three diagnostic ions. The use of a single multi-drug method is beneficial as it reduces laboratory response time and reduces the amount of solvents and samples. Other notable examples of the polydrug mixtures found in fatal cases involving NPSs are presented in a review paper by Menéndez-Quintanal et al. [297].
Bouzoukas and coworkers reported a GC-MS method for the simultaneous detection of nine amphetamines, seven synthetic cathinones, and five phenethylamines in blood and urine samples [295]. The SPE was followed by the derivatization with pentafluoropropionic anhydride, and the separation was achieved in less than 11 min. The LODs were between 0.7 and 7.0 ng/mL, while LOQs ranged from 2.0 to 20 ng/mL. This method was successfully applied to post-mortem cases, and compounds were detected in 14 cases. Sim et al. examined methamphetamine, MDMA, ketamine, and their metabolites in urine using simple headspace solid-phase microextraction (HS-SPME) [36]. This solvent-free extraction technique is effective for extracting volatile and semi-volatile compounds from complex matrices. Their results proved the frequent occurrence of polydrug use, but also that the metabolites of ketamine were present in higher concentrations compared to those of other amphetamines, which suggested its faster metabolism.
In addition to applied multi-drug protocols, Nisbet et al. conducted a systematic comparison of six SPE and one supported liquid extraction (SLE) procedures and four derivatization reagents (two silylating and two acylation reagents) for the simultaneous GC-MS detection of 19 NPSs from multiple structural groups, including cathinones, arylcyclohexylamines, and benzofurans [298]. Extraction efficiencies ranged from 49 to 119%, and pentafluoropropionic anhydride in ethyl acetate yielded the highest sensitivity and most distinct spectra. This comprehensive optimization demonstrates that careful coordination of extraction and derivatization parameters enables the effective screening of structurally heterogeneous analytes within a single analytical workflow—an essential requirement in the analysis of polydrug mixtures.
The analysis of e-cigarettes from Scottish prisons seized between May 2022 and July 2023 showed the presence of NPSs combined with other restricted compounds, such as anabolic-androgenic steroids (AAS) [299]. Some e-cigarette cartridges contained visible residues from crushed tablets. The samples were subjected to examination by GC-MS without prior derivatization, and besides AAS (mestanolone and oxandrolone), they contained synthetic cannabinoids (ADB-BUTINACA and MDMB-4en-PINACA), cocaine, and Δ9-THC (Table 5). These results showed that the mode of administration changed from infused papers to powders and tablets, and subsequently to e-cigarettes, which were allowed on-site. One of the greatest concerns in this case is the unknown pyrolysis route of AAS and the effects of its products on vaping. Giorgetti and coworkers examined the paper infused with a novel opioid, AP-237, obtained from a German prisoner [300]. This compound was dissolved in an organic solvent, applied to a paper, estimated to be 1.2 µg/mL, and mixed with two other opioids, namely MDMB-4enPINACA and 5F-ADB. As previously described for synthetic opioids, NPSs circulating in prisons lead to increased risks of aggressive behavior, violence, debts, and other safety problems. The sample was soaked in methanol, and compounds were identified by GC-MS screening, while semi-quantitative analysis was performed by LC-MS. This was the first study to simultaneously determine both SA and SO in the infused paper.
Bloom, Sisco, and Lurie used a short column and a rapid (10’s °C/s) temperature program to separate psychoactive substances from several classes, adulterants, and diluents in less than 1 min, achieving other analytical parameters comparable to conventional GC-MS methods [301] (Table 5). Limited selectivity between positional isomers and structurally similar compounds was observed, but, as a preliminary method, it showed great potential. The same method for the Agilent QuickProbe gas chromatograph/mass spectrometer (QP-GCMS) instrument, requiring minimal sample preparation and fast separation, was later validated for other drugs seized by different research groups [302,303].
The GC-MS technique was successfully applied to identify and quantify 11 NPS in 67 seized chocolate samples [63]. These NPSs included NB (bromazolam), stimulants (3-FEA, 2-FEA, and 3-MMC), tryptamines (4-OH-MiPT), dissociatives (deoxymethoxetamine, 3-OH-PCP, 2-FDCK), synthetic cannabinoids (ADB-BUTINACA), and entactogens (6-APB and 4-APB) (Table 5). The extraction procedure used 1 mL of ethanol, sonication for 15 min, and centrifugation. The proposed methodology was not suitable for positional aromatic isomers because of their similar MS spectra; therefore, the results were further verified by NMR. The limits of quantification ranged from 0.01 to 0.03 mg/mL. The authors also stated that the GC-MS technique was superior to LC-MS, as LC-MS would require different sample preparation methods for different compound classes.
Smoking drugs of abuse leads to rapid effects and high concentrations, especially if consumed through a bucket bong. Fatal intoxications were reported for consumers of complex mixtures involving fentanyl and synthetic cannabinoids. The determination of these compounds involves GC-MS for screening of basic, neutral, and acidic drugs, and for quantification of THC and its metabolites by the same technique, while LC-MS/MS is necessary for other substances. Neukamm and coworkers reported fatal intoxication by the mixture of fentanyl, 5F-ADB, 5F-MDMB-P7AICA, and other synthetic cannabinoids (17 in total) [304]. These compounds were detected in the kidney, liver, urine, and hair. This study used both of the mentioned chromatography techniques to analyze biological samples.
As previously discussed, GC-MS results are used to validate less common techniques. Warner examined the mixture of substituted cathinones and fentanyl analogues by gas chromatography-infrared spectroscopy (GC-IRD), which allowed for the separation of 29 reference materials within 26 min [305]. Based on selectivity, repeatability, and reproducibility, GC-IRD proved complementary to GC-MS. On the other hand, GC-MS was used as a confirmatory technique for the examination of discarded drug paraphernalia (DDP), such as syringes, for the trace analysis of novel synthetic opioid β-U10 and other present psychoactive substances [306]. DDPs proved to be an important source of information about specific drug substances, adulterants, and polydrug mixtures that are present in the particular population of consumers. The compounds are usually present in microgram to nanogram quantities [307,308,309]. West and coworkers concluded that direct analysis in real-time (DART)-MS of DDP samples correctly identified the mentioned opioid, heroin, xylitol, degradation products of heroin (acetylcodeine, 6-monoacetylmorphine, and morphine), although those that were thermolabile (diphenhydramine) were not observed in the GC-MS workflow, and the amounts of compounds needed for the analysis were higher for the latter [306].

Table 5.
Selected GC-MS studies of polydrug mixtures (2020–2025).
Table 5.
Selected GC-MS studies of polydrug mixtures (2020–2025).
| Compounds | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Nine amphetamines, seven synthetic cathinones, five phenethylamines | Solution, blood, and urine | SPE followed by derivatization by pentafluoropropionic anhydride, separation in 11 min, with low LOQ and LOD. | Bouzoukas et al., 2025 [295] |
| Ketamine, methamphetamine, MDMA | Urine | HS-SPME-GC-MS analysis was suitable for the simultaneous detection of compounds and their metabolites in urine, allowing discussion of the pharmacokinetics. | Sim et al., 2025 [36] |
| Compounds from several groups | Powder | A short column and a rapid (10 s °C/s) temperature program allowed the separation of psychoactive compounds, diluents, and adulterants in less than 1 min. | Bloom et al., 2023 [301] |
| Anabolic-androgenic steroids + other NPS | e-cigarettes | GC-MS analysis without derivatization was an appropriate technique for verifying the presence of AAS and NPS. | Harries et al., 2024 [299] |
| AP-237, MDMB-4enPINACA, 5F-ADB | Infused paper | Screening by GC-MS and semi-quantitative analysis by LC-MS | Giorgetti et al., 2022 [300] |
| Eleven different NPS | Infused chocolate | Extraction by methanol, sonication, and centrifugation before GC-MS, with limits of detection between 0.05 and 0.1 mg/mL. | Song et al., 2023 [63] |
| β-U10 and heroin, xylitol, and degradation products of heroin | discarded drug paraphernalia | GC-MS confirmatory technique, although higher amounts were required, and not all compounds were detectable compared to DART-MS. | West et al., 2022 [306] |
| Nineteen psychoactive compounds | In vitro and post-mortem whole blood samples. | SPE-GC-MS method with derivatization mixture (BSTFA and TMCS), three working concentrations for therapeutic, toxic, and lethal concentrations. | Ferreira et al., 2021 [296] |
| Fentanyl, 5F-ADB, 5F-MDMB-P7AICA, and other SC | Bucket bong water, kidney, liver, urine, and hair | LLE followed by GC-MS for initial screening of compounds, quantification by GC-MS (THC and metabolites), and LC-MS/MS for other substances. | Neukamm et al., 2024 [304] |
8. Portable GC-MS Instruments and Machine Learning Advancements of GC-MS
Recent years have witnessed notable progress in miniaturized analytical instrumentation designed for rapid, on-site identification of controlled and emerging substances. These advances have been driven by the need to detect NPSs promptly in complex, dynamic environments such as music festivals, postal facilities, and supervised consumption sites. Portable instruments have gradually evolved from presumptive screening tools to sophisticated analytical systems capable of providing confirmatory data with accuracy comparable to that of conventional laboratory setups.
Despite being the standard technique for identifying NPSs, GC-MS is a less common instrument for on-site testing due to its size, sample preparation, and derivatization, and the knowledge and experience requirements for the person performing the experiments [310]. The cost of instruments also varies widely, potentially limiting their on-site use [311]. Technological advancements and the miniaturization of portable GC-MS instruments enabled their use for more accurate, immediate testing of various compounds in forensic and environmental studies [312]. A critical line of development is to make these instruments accessible to individuals who may not have a scientific or analytical chemistry background, such as military personnel or law enforcement [313]. Several controlled substances have been successfully analyzed by these portable instruments, thus allowing fast and reliable results for workplace drug testing and border force checks [314,315,316]. Several studies have recently aimed to compare the performance of these instruments with that of standard laboratory-based analytical instruments.
A study by Gozdzialski et al. illustrated the utility of portable GC-MS in public health-oriented drug checking programs, where opioids adulterated with carfentanil and etizolam were analyzed directly at a supervised consumption site [309]. In that work, portable GC-MS identified over 60% of samples containing ultra-potent fentanyl analogues, outperforming Fourier transform infrared (FTIR) spectroscopy, which failed to detect carfentanil at trace levels. These results underscore the diagnostic advantage of mass-selective detection in harm-reduction contexts, where small quantities of active compounds may be masked within complex mixtures of excipients and diluents.
Frinculescu and co-workers assessed the Griffin G510 (Teledyne FLIR) and the Torion T-9 (PerkinElmer) portable GC-MS systems using seized “amnesty bin” samples containing MDMA, ketamine, 2C-B, and N-ethylpentylone [317]. The reported identification accuracy ranged from 74–86%, with detection limits down to 0.06 mg/mL and excellent ruggedness under variable environmental conditions. The results confirm that portable GC-MS can close the analytical gap between on-site screening and laboratory confirmation, particularly when supported by optimized method validation protocols (selectivity, carry-over, and intra-/inter-day precision).
Complementary work by the same group evaluated a single-quadrupole mass spectrometer coupled to an atmospheric solids analysis probe (ASAP-MS) for direct surface sampling of confiscated powders [318]. The method achieved more than 90% correct identifications when cross-checked against benchtop GC-MS results, demonstrating the potential of atmospheric pressure ionization interfaces for low-volume or heterogeneous samples. Such instruments have proven particularly valuable in festival and nightlife settings, where mixtures of stimulants, hallucinogens, and adulterants often appear in small quantities unsuitable for complete chromatographic workflows.
Broader overviews, such as the review by Alonzo et al., have emphasized that the future of field drug analysis lies in the convergence of miniaturized chromatographic, spectroscopic, and ambient ionization platforms [73]. Portable FTIR and Raman instruments remain indispensable for rapid, non-destructive screening; however, their limitations in mixture resolution and in the detection of low concentrations necessitate integration with more selective mass-based or electrochemical systems. Emerging platforms such as paper spray ionization (PSI-MS), direct analysis in real-time (DART-MS), and ion mobility spectrometry (IMS) have already demonstrated strong complementarity to portable GC-MS in differentiating structurally similar compounds, particularly cathinones and fentanyl analogues.
These studies demonstrate that portability no longer implies analytical compromise. Current portable GC-MS instruments can achieve mass accuracies within ±0.1 Da, rapid temperature cycling for sub-10 min analyses, and library-search reliability sufficient for judicial and harm-reduction reporting. While technical limitations persist, particularly in chromatographic resolution, spectral reproducibility, and reference-library completeness, the progress achieved between 2020 and 2025 confirms a clear trajectory toward decentralized, intelligent, and field-ready forensic analytics. Portable mass spectrometry and hybrid spectroscopic platforms thus represent the next logical stage in the evolution of NPS detection, complementing established GC-MS methodologies and ensuring a timely analytical response to the appearance of psychoactive substances.
In parallel, the field is moving toward data-driven and machine-learning-assisted analysis. Yang et al. developed supervised models trained on over 1200 GC-MS and LC–MS spectra of NPS and non-NPS compounds, achieving F1 scores up to 0.97 across four algorithms (k-nearest neighbors, support vector machines, random forest, and cascade forest) [60]. These models were later validated on real seizures containing synthetic cannabinoids, cathinones, and fentanyl derivatives, enabling early warning identification even in the absence of reference standards. The integration of spectral machine learning into portable devices is anticipated to redefine field detection workflows, reducing dependence on static libraries and allowing adaptive recognition of new analogues.
9. Conclusions and Outlook
Over the past five years, forensic analysis of novel psychoactive substances (NPSs) has been rapidly evolving due to the (re)appearance of hundreds of structurally diverse analogues, the expansion of online drug markets, and the persistent challenges of identifying complex mixtures and adulterated formulations. Gas chromatography coupled with mass spectrometry (GC-MS) has remained the principal analytical platform throughout this period because of its robustness, cost-efficiency, and compatibility with large spectral libraries. Numerous studies summarized in this review demonstrate that, despite the rapid turnover of the NPS, GC-MS continues to provide both targeted and non-targeted analytical capabilities applied for the screening, identification, and quantification of substances across a broad range of matrices (seized powders, herbal materials, vaping liquids, infused papers, blood, urine, and hair).
The post-COVID-19 period has revealed pronounced diversification of NPS formulations and the increasing complexity of polydrug combinations. Synthetic and semi-synthetic cannabinoids, synthetic cathinones, designer benzodiazepines, and opioids remain dominant, while a growing number of hybrid molecules and “sila-substituted” analogues illustrate the ongoing chemical innovation in clandestine synthesis. GC-MS applications have been successfully adapted to these changes through optimized extraction and microextraction protocols (SPE, LLE, DLLME, MSPE), the introduction of derivatization strategies for thermally labile compounds, and the refinement of chromatographic conditions, enabling the separation of structural and positional isomers. The inclusion of orthogonal spectroscopic techniques has further strengthened the reliability of identification and supported metabolite characterization in biological samples.
Another critical development concerns the coupling of GC-MS data with chemometric and multivariate statistical tools. Canonical discriminant analysis, principal component analysis, and cluster algorithms are now frequently employed to differentiate isomers, recognize fragment-ion patterns, and construct classification models linking samples to potential synthetic routes or distribution sources.
A persistent issue identified across the surveyed literature is the limited coverage of NPSs in commercial spectral libraries. Many newly discovered compounds lack reference spectra, complicating both routine casework and comparative analysis. The establishment of dynamic, open-access GC-MS spectral repositories—periodically updated through inter-laboratory collaborations and cross-validation with high-resolution MS—should therefore be considered a priority for the forensic community. Equally important is the need to standardize reporting formats for retention indices, derivatization reagents, and mass-fragmentation pathways, ensuring that newly published data can be seamlessly integrated into searchable libraries.
The most promising directions involve miniaturization, portability, and greener analytical workflows. Compact GC-MS and hybrid devices are already emerging as reliable tools for field deployment, particularly when combined with ambient ionization and direct-sampling techniques. Solvent-free and low-waste extraction protocols will continue to expand in alignment with green analytical chemistry principles, while microscale sorbents and coated fibers offer improved enrichment with minimal resource consumption. It can be expected that the forensic use of artificial-intelligence-driven chemometric models will likely mature, enabling the real-time spectral deconvolution, classification of unknowns, and predictive modeling of fragmentation patterns.
Funding
The author acknowledges the financial support of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Nos. 451-03-137/2025-03/200146 and 451-03-136/2025-03/200146).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-FDCK | 2-Fluorodeschloroketamine |
| 2-FEA | 2-Fluoroethamphetamine |
| 3-FEA | 3-Fluoroethamphetamine |
| 3-MMC | 3-Methylmethcathinone |
| 4-APB | 4-(2-Aminopropyl)benzofuran |
| 4-CEC | 4-Chloroethcathinone |
| 4-Cl-PVP | 4-Chloro-α-pyrrolidinovalerophenone |
| 4-HO-MiPT | 4-Hydroxy-N-methyl-N-isopropyltryptamine |
| 4-MeO-α-PVP | 4-Methoxy-α-pyrrolidinovalerophenone |
| 5-MeO-MiPT | 5-Methoxy-N-methyl-N-isopropyltryptamine |
| 5-PPDI | 1-(2,3-Dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)butan-1-one |
| 5F-ADB | Methyl 2-{[1-(5-fluoropentyl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate |
| 5F-MDMB-P7AICA | Methyl [2-(1-(5-fluoropentyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamido)-3,3-dimethylbutanoate] |
| 6-APB | 6-(2-Aminopropyl)benzofuran |
| ADB-4en-PINACA | N-[1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-(pent-4-en-1-yl)-1H-indazole-3-carboxamide |
| ADB-BUTINACA | N-[(2S)-1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-butyl-1H-indazole-3-carboxamide |
| ADMB-3TMS-PrINACA | N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(3-(trimethylsilyl)propyl)-1H-indazole-3-carboxamide |
| AH-7941 | N-(Cyclohexylmethyl)-N′-(3,4-dichlorobenzyl)benzamide |
| ALD-52 | 1-Acetyl-N,N-diethyllysergamide |
| AP-237 | 1-[4-(3-phenyl-2-propen-1-yl)-1-piperazinyl]-1-butanone |
| Brorphine | 3-{1-[1-(4-bromophenyl)ethyl]piperidin-4-yl}-1H-benzimidazol-2-one |
| CBD | Cannabidiol |
| CH-PIACA | Cyclohexyl indole-3-acetamide-type synthetic cannabinoid |
| Cumyl-3TMS-PrINACA | N-(2-phenylpropan-2-yl)-1-(3-(trimethylsilyl)propyl)-1H-indazole-3-carboxamide |
| Isotonitazene | N,N-Diethyl-2-[5-nitro-2-({4-[(propan-2-yl)oxy]phenyl}methyl)-1H-benzimidazol-1-yl]ethan-1-amine |
| LLE | Liquid-liquid Extraction |
| LOD | Limit of Detection |
| LOQ/(L)LOQ | Limit of Quantification/(Lower) Limit of Quantification |
| MDMB-4en-PINACA | 2-{[1-(pent-4-en-1-yl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate |
| MDPV | 3,4-Methylenedioxypyrovalerone |
| Methorphan | 3-Methoxy-N-methylmorphinan |
| MSPE | Magnetic Solid-Phase Extraction |
| MT-45 | 1-Cyclohexyl-4-(1,2-diphenylethyl)piperazine |
| MXE | Methoxetamine; 2-(ethylamino)-2-(3-methoxyphenyl)cyclohexanone |
| NB | Novel Benzodiazepines |
| NPS | Novel Psychoactive Substances |
| PCA | Principal Component Analysis |
| Putylone | 1-(1,3-Benzodioxol-5-yl)-2-(propylamino)butan-1-one |
| SC | Synthetic Cannabinoid |
| SO | Synthetic Opioids |
| SPE | Solid-Phase Extraction |
| SSC | Semi-synthetic Cannabinoid |
| THC | Δ9-Tetrahydrocannabinol |
| THCP | Δ9-Tetrahydrocannabiphorol |
| U-47700 | trans-3,4-Dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide |
| U-50488 | trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzamide |
| U-51754 | N-[2-(Dimethylamino)cyclohexyl]-N-methyl-4-methylbenzamide |
| U-69593 | trans-(±)-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzamide |
| α-PVP | α-Pyrrolidinovalerophenone |
| β-U10 | N-[2-(Dimethylamino)cyclohexyl]-N-methylnaphthalene-1-carboxamide |
References
- Bade, R.; Rousis, N.; Adhikari, S.; Baduel, C.; Bijlsma, L.; Bizani, E.; Boogaerts, T.; Burgard, D.A.; Castiglioni, S.; Chappell, A.; et al. Three Years of Wastewater Surveillance for New Psychoactive Substances from 16 Countries. Water Res. X 2023, 19, 100179. [Google Scholar] [CrossRef]
- Vincenti, F.; Gregori, A.; Flammini, M.; Di Rosa, F.; Salomone, A. Seizures of New Psychoactive Substances on the Italian Territory during the COVID-19 Pandemic. Forensic Sci. Int. 2021, 326, 110904. [Google Scholar] [CrossRef]
- Papa, P.; Valli, A.; Di Tuccio, M.; Buscaglia, E.; Brambilla, E.; Scaravaggi, G.; Gallo, M.; Locatelli, C.A. Prevalence of Stimulant, Hallucinogen, and Dissociative Substances Detected in Biological Samples of NPS-Intoxicated Patients in Italy. J. Psychoact. Drugs 2021, 53, 247–255. [Google Scholar] [CrossRef]
- Baumann, M.H.; Volkow, N.D. Abuse of New Psychoactive Substances: Threats and Solutions. Neuropsychopharmacology 2016, 41, 663–665. [Google Scholar] [CrossRef]
- Das, P.; Horton, R. The Global Drug Problem: Change but Not Progression. Lancet 2019, 394, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New Psychoactive Substances: Challenges for Drug Surveillance, Control, and Public Health Responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic: An Update from the EU Early Warning System; European Monitoring Centre for Drugs and Drug Addiction, Ed.; European Monitoring Centre for Drugs and Drug Addiction: Lisbon Portugal, 2020; ISBN 978-92-9497-557-7. [Google Scholar]
- Van Buskirk, J.; Griffiths, P.; Farrell, M.; Degenhardt, L. Trends in New Psychoactive Substances from Surface and “Dark” Net Monitoring. Lancet Psychiatry 2017, 4, 16–18. [Google Scholar] [CrossRef]
- Tomsone, L.E.; Neilands, R.; Kokina, K.; Bartkevics, V.; Pugajeva, I. Pharmaceutical and Recreational Drug Usage Patterns during and Post COVID-19 Determined by Wastewater-Based Epidemiology. Int. J. Environ. Res. Public Health 2024, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Browne, T.; Gold, M.S.; Martin, D.M. The Rapidly Changing Composition of the Global Street Drug Supply and Its Effects on High-Risk Groups for COVID-19. CPSP 2021, 10, 138–154. [Google Scholar] [CrossRef]
- Dei Cas, M.; Casagni, E.; Arnoldi, S.; Gambaro, V.; Roda, G. Screening of New Psychoactive Substances (NPS) by Gas-Chromatography/Time of Flight Mass Spectrometry (GC/MS-TOF) and Application to 63 Cases of Judicial Seizure. Forensic Sci. Int. Synerg. 2019, 1, 71–78. [Google Scholar] [CrossRef]
- UNODC (United Nations Office on Drugs and Crime) Data from the UNODC Early Warning Advisory on New Psychoactive Substances. Available online: https://www.unodc.org/LSS/Page/NPS/DataVisualisations (accessed on 18 October 2025).
- Salomone, A.; Palamar, J.J.; Vincenti, M. Should NPS Be Included in Workplace Drug Testing? Drug Test. Anal. 2020, 12, 191–194. [Google Scholar] [CrossRef]
- Catalani, V.; Arillotta, D.; Corkery, J.M.; Guirguis, A.; Vento, A.; Schifano, F. Identifying New/Emerging Psychoactive Substances at the Time of COVID-19; A Web-Based Approach. Front. Psychiatry 2021, 11, 632405. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, A.; Moosa, I.; Gittins, R.; Schifano, F. What About Drug Checking? Systematic Review and Netnographic Analysis of Social Media. CN 2020, 18, 906–917. [Google Scholar] [CrossRef]
- Arillotta, D.; Schifano, F.; Napoletano, F.; Zangani, C.; Gilgar, L.; Guirguis, A.; Corkery, J.M.; Aguglia, E.; Vento, A. Novel Opioids: Systematic Web Crawling Within the e-Psychonauts’ Scenario. Front. Neurosci. 2020, 14, 149. [Google Scholar] [CrossRef]
- Feltmann, K.; Elgán, T.H.; Böttcher, M.; Lierheimer, S.; Hermansson, S.; Beck, O.; Gripenberg, J. Feasibility of Using Breath Sampling of Non-Volatiles to Estimate the Prevalence of Illicit Drug Use among Nightlife Attendees. Sci. Rep. 2022, 12, 20283. [Google Scholar] [CrossRef]
- Gripenberg-Abdon, J.; Elgán, T.H.; Wallin, E.; Shaafati, M.; Beck, O.; Andréasson, S. Measuring Substance Use in the Club Setting: A Feasibility Study Using Biochemical Markers. Subst. Abus. Treat. Prev. Policy 2012, 7, 7. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Pérez-Acevedo, A.P.; Hladun, O.; Torres-Moreno, M.C.; Muga, R.; Torrens, M.; Farré, M. Cannabinoids: From Pot to Lab. Int. J. Med. Sci. 2018, 15, 1286–1295. [Google Scholar] [CrossRef]
- Patocka, J.; Zhao, B.; Wu, W.; Klimova, B.; Valis, M.; Nepovimova, E.; Kuca, K. Flakka: New Dangerous Synthetic Cathinone on the Drug Scene. Int. J. Mol. Sci. 2020, 21, 8185. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, X.; Ming, Z.; Jiang, L.; Zhou, Y. Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry. Separations 2021, 9, 3. [Google Scholar] [CrossRef]
- Cunha, R.L.; Oliveira, C.D.S.L.; De Oliveira, A.L.; Maldaner, A.O.; Do Desterro Cunha, S.; Pereira, P.A.P. An Overview of New Psychoactive Substances (NPS) in Northeast Brazil: NMR-Based Identification and Analysis of Ecstasy Tablets by GC-MS. Forensic Sci. Int. 2023, 344, 111597. [Google Scholar] [CrossRef]
- Lo Faro, A.; Berardinelli, D.; Cassano, T.; Dendramis, G.; Montanari, E.; Montana, A.; Berretta, P.; Zaami, S.; Busardò, F.; Huestis, M. New Psychoactive Substances Intoxications and Fatalities during the COVID-19 Epidemic. Biology 2023, 12, 273. [Google Scholar] [CrossRef]
- Yanini, A.; Esteve-Turrillas, F.A.; De La Guardia, M.; Armenta, S. Ion Mobility Spectrometry and High Resolution Mass-Spectrometry as Methodologies for Rapid Identification of the Last Generation of New Psychoactive Substances. J. Chromatogr. A 2018, 1574, 91–100. [Google Scholar] [CrossRef]
- Logan, B.K.; Reinhold, L.E.; Xu, A.; Diamond, F.X. Identification of Synthetic Cannabinoids in Herbal Incense Blends in the United States. J. Forensic Sci. 2012, 57, 1168–1180. [Google Scholar] [CrossRef]
- Pasin, D.; Cawley, A.; Bidny, S.; Fu, S. Current Applications of High-Resolution Mass Spectrometry for the Analysis of New Psychoactive Substances: A Critical Review. Anal. Bioanal. Chem. 2017, 409, 5821–5836. [Google Scholar] [CrossRef]
- Marginean, I.; Rowe, W.F.; Lurie, I.S. The Role of Ultra High Performance Liquid Chromatography with Time of Flight Detection for the Identification of Synthetic Cannabinoids in Seized Drugs. Forensic Sci. Int. 2015, 249, 83–91. [Google Scholar] [CrossRef]
- Muhamadali, H.; Watt, A.; Xu, Y.; Chisanga, M.; Subaihi, A.; Jones, C.; Ellis, D.I.; Sutcliffe, O.B.; Goodacre, R. Rapid Detection and Quantification of Novel Psychoactive Substances (NPS) Using Raman Spectroscopy and Surface-Enhanced Raman Scattering. Front. Chem. 2019, 7, 412. [Google Scholar] [CrossRef]
- Mayer, A.; Black, C.; Copp, B.R. Identification and Characterisation of the Recently Detected Cathinone N-Cyclohexyl Pentylone. Forensic Sci. Int. 2025, 367, 112387. [Google Scholar] [CrossRef]
- Liliedahl, R.E.; Davidson, J.T. The Differentiation of Synthetic Cathinone Isomers Using GC-EI-MS and Multivariate Analysis. Forensic Chem. 2021, 26, 100349. [Google Scholar] [CrossRef]
- Davidson, J.T.; Sasiene, Z.J.; Jackson, G.P. Fragmentation Pathways of Odd- and Even-Electron N-Alkylated Synthetic Cathinones. Int. J. Mass. Spectrom. 2020, 453, 116354. [Google Scholar] [CrossRef]
- Graziano, S.; Anzillotti, L.; Mannocchi, G.; Pichini, S.; Busardò, F.P. Screening Methods for Rapid Determination of New Psychoactive Substances (NPS) in Conventional and Non-Conventional Biological Matrices. J. Pharm. Biomed. Anal. 2019, 163, 170–179. [Google Scholar] [CrossRef]
- Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) MS Library, Version 3.14. Available online: https://www.swgdrug.org/ms.htm (accessed on 18 October 2025).
- Armenta, S.; Gil, C.; Ventura, M.; Esteve-Turrillas, F.A. Unexpected Identification and Characterization of a Cathinone Precursor in the New Psychoactive Substance Market: 3′,4′-Methylenedioxy-2,2-Dibromobutyrophenone. Forensic Sci. Int. 2020, 306, 110043. [Google Scholar] [CrossRef]
- Żubrycka, A.; Kwaśnica, A.; Haczkiewicz, M.; Sipa, K.; Rudnicki, K.; Skrzypek, S.; Poltorak, L. Illicit Drugs Street Samples and Their Cutting Agents. The Result of the GC-MS Based Profiling Define the Guidelines for Sensors Development. Talanta 2022, 237, 122904. [Google Scholar] [CrossRef]
- Sim, J.; Kyung, S.Y.; Jo, J.; Choi, H.; Jeong, S.; Lee, N.; Kim, S. Simultaneous Determination of Methamphetamine, MDMA, and Ketamine and Their Metabolites in Urine Using a Rapid and Simple HS-SPME-GC–MS Method: A Forensic Study on Drug Abuse Patterns in South Korea. J. Chromatogr. B 2025, 1264, 124720. [Google Scholar] [CrossRef]
- Peters, F.T.; Wissenbach, D.K.; Busardo, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2017, 23, 5455–5467. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, A.L.; Mohr, A.L.A.; Chan-Hosokawa, A.; Harper, C.; Huestis, M.A.; Limoges, J.F.; Miles, A.K.; Scarneo, C.E.; Kerrigan, S.; Liddicoat, L.J.; et al. Recommendations for Toxicological Investigation of Drug-Impaired Driving and Motor Vehicle Fatalities—2021 Update. J. Anal. Toxicol. 2021, 45, 529–536. [Google Scholar] [CrossRef]
- Grapp, M.; Kaufmann, C.; Schwelm, H.M.; Neukamm, M.A.; Blaschke, S.; Eidizadeh, A. Intoxication Cases Associated with the Novel Designer Drug 3′,4′-methylenedioxy-α-pyrrolidinohexanophenone and Studies on Its Human Metabolism Using High-resolution Mass Spectrometry. Drug Test. Anal. 2020, 12, 1320–1335. [Google Scholar] [CrossRef]
- Mercieca, G.; Odoardi, S.; Mestria, S.; Cassar, M.; Strano-Rossi, S. Application of Ultrasound-assisted Liquid–Liquid Microextraction Coupled with Gas Chromatography and Mass Spectrometry for the Rapid Determination of Synthetic Cannabinoids and Metabolites in Biological Samples. J. Sep. Sci. 2020, 43, 2858–2868. [Google Scholar] [CrossRef]
- Pellegrini, M.; Marchei, E.; Papaseit, E.; Farré, M.; Zaami, S. UHPLC-HRMS and GC-MS Screening of a Selection of Synthetic Cannabinoids and Metabolites in Urine of Consumers. Medicina 2020, 56, 408. [Google Scholar] [CrossRef]
- Verhoeven, M.; Bonetti, J.; Kranenburg, R.; Van Asten, A. Chemical Identification and Differentiation of Positional Isomers of Novel Psychoactive Substances–A Comprehensive Review. TrAC Trends Anal. Chem. 2023, 166, 117157. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Ng, J.Y.J.; Ong, M.C.; Lim, J.L.W.; Yap, T.W.A. Technical Note: Unequivocal Identification of 5-Methoxy-DiPT with NOESY NMR and GC-IRD. Forensic Sci. Int. 2020, 316, 110537. [Google Scholar] [CrossRef]
- Sim, S.B.D.; Lee, H.Z.S.; Ong, M.C.; Zhang, S.; Lim, K.A.; Lim, J.L.W.; Yap, T.W.A. Synthesis, Characterization and Differentiation of the Structural Isomers of Valine and Tert -leucine Derived Synthetic Cannabinoids. Drug Test. Anal. 2024, 16, 420–434. [Google Scholar] [CrossRef]
- Di Trana, A.; Carlier, J.; Berretta, P.; Zaami, S.; Ricci, G. Consequences of COVID-19 Lockdown on the Misuse and Marketing of Addictive Substances and New Psychoactive Substances. Front. Psychiatry 2020, 11, 584462. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, N.; Bonnet, U.; Hafermann, H.; Schifano, F.; Bender, S.; Grigoleit, T.; Kuhn, J.; Nyhuis, P.; Preuss, U.W.; Reymann, G.; et al. Availability of Illegal Drugs During the COVID-19 Pandemic in Western Germany. Front. Psychiatry 2021, 12, 648273. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Mohamed, K.; Abbas, N.; El-Gendy, M.; Alsaif, N.; Alanazi, M.; Mohammed, M.; Abounassif, M.; Hefnawy, M. Development and Validation of GC–MS Method for Determination of Methcathinone and Its Main Metabolite in Mice Plasma and Brain Tissue after SPE: Pharmacokinetic and Distribution Study. J. Pharm. Biomed. Anal. 2021, 194, 113798. [Google Scholar] [CrossRef]
- Han, C.; Tan, D.; Wang, Y.; Yu, Z.; Sun, X.; Wang, D. Selective Extraction of Synthetic Cathinones New Psychoactive Substances from Wastewater, Urine and Cocktail Using Dummy Molecularly Imprinted Polymers. J. Pharm. Biomed. Anal. 2022, 215, 114765. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Luan, T.; Jiang, R.; Ouyang, G. Sample Preparation and Instrumental Methods for Illicit Drugs in Environmental and Biological Samples: A Review. J. Chromatogr. A 2021, 1640, 461961. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-H.; Hsieh, C.-H.; Chaou, C.-H.; Chen, C.-K.; Yen, T.-H.; Liao, S.-C.; Seak, C.-J.; Chen, H.-Y. Synthetic Cathinone Poisoning from Ingestion of Drug-Laced “Instant Coffee Packets” in Taiwan. Hum. Exp. Toxicol. 2021, 40, 1403–1412. [Google Scholar] [CrossRef]
- Gould, O.; Nguyen, N.; Honeychurch, K.C. New Applications of Gas Chromatography and Gas Chromatography-Mass Spectrometry for Novel Sample Matrices in the Forensic Sciences: A Literature Review. Chemosensors 2023, 11, 527. [Google Scholar] [CrossRef]
- Bolcato, V.; Carelli, C.; Radogna, A.; Freni, F.; Moretti, M.; Morini, L. New Synthetic Cathinones and Phenylethylamine Derivatives Analysis in Hair: A Review. Molecules 2021, 26, 6143. [Google Scholar] [CrossRef]
- Langa, I.M.; Lado Ribeiro, A.R.; Ratola, N.; Gonçalves, V.M.F.; Tiritan, M.E.; Ribeiro, C. Amphetamine-like Substances and Synthetic Cathinones in Portuguese Wastewater Influents: Enantiomeric Profiling and Role of Suspended Particulate Matter. Forensic Sci. Int. 2024, 361, 112128. [Google Scholar] [CrossRef]
- Kerrigan, S.; Savage, M.; Cavazos, C.; Bella, P. Thermal Degradation of Synthetic Cathinones: Implications for Forensic Toxicology. J. Anal. Toxicol. 2016, 40, 1–11. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Kuwayama, K.; Kanamori, T.; Iwata, Y.T.; Inoue, H. Thermal Degradation of α-Pyrrolidinopentiophenone during Injection in Gas Chromatography/Mass Spectrometry. Forensic Sci. Int. 2013, 231, 296–299. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Wong, W.-C. Forensic Drug Analysis of Chloro-N,N-Dimethylcathinone (CDC) and Chloroethcathinone (CEC): Identification of 4-CDC and 4-CEC in Drug Seizures and Differentiation from Their Ring-Substituted Positional Isomers. Forensic Sci. Int. 2019, 298, 268–277. [Google Scholar] [CrossRef]
- Dhabbah, A.M. Determination of Chiral Cathinone in Fresh Samples of Catha Edulis. Forensic Sci. Int. 2020, 307, 110105. [Google Scholar] [CrossRef]
- Tyler Davidson, J.; Piacentino, E.L.; Sasiene, Z.J.; Abiedalla, Y.; DeRuiter, J.; Clark, C.R.; Berden, G.; Oomens, J.; Ryzhov, V.; Jackson, G.P. Identification of Novel Fragmentation Pathways and Fragment Ion Structures in the Tandem Mass Spectra of Protonated Synthetic Cathinones. Forensic Chem. 2020, 19, 100245. [Google Scholar] [CrossRef]
- Stuhmer, E.L.; McGuffin, V.L.; Waddell Smith, R. Discrimination of Seized Drug Positional Isomers Based on Statistical Comparison of Electron-Ionization Mass Spectra. Forensic Chem. 2020, 20, 100261. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Hua, Z.; Xu, P.; Wang, Y.; Di, B.; Liao, J.; Su, M. Machine Learning-Assisted Rapid Screening of Four Types of New Psychoactive Substances in Drug Seizures. J. Chem. Inf. Model. 2023, 63, 815–825. [Google Scholar] [CrossRef]
- Craft, S.; Sunderland, P.; Millea, M.F.; Pudney, C.R.; Sutcliffe, O.B.; Freeman, T.P. Detection and Quantification of Synthetic Cannabinoids in Seven Illicitly Sourced Disposable Vapes Submitted by an Individual Presenting to a UK Drug and Alcohol Service. Addiction 2025, 120, 549–554. [Google Scholar] [CrossRef]
- Apirakkan, O.; Frinculescu, A.; Denton, H.; Shine, T.; Cowan, D.; Abbate, V.; Frascione, N. Isolation, Detection and Identification of Synthetic Cannabinoids in Alternative Formulations or Dosage Forms. Forensic Chem. 2020, 18, 100227. [Google Scholar] [CrossRef]
- Song, C.; Jia, W.; Liu, C.; Hua, Z.; Meng, X.; Zhao, Y.; Li, T.; Cai, L.; Zhao, X. New Trends of New Psychoactive Substances (NPS)-Infused Chocolate: Identification and Quantification of Trace Level of NPS in Complex Matrix by GC-MS and NMR. Talanta 2023, 255, 124257. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kwon, E.; Oh, S.J.; Choi, M.R.; Lee, S.-R.; Jung, B.H.; Lee, W.; Hong, J. Thermal Transformation of CBD, CBDA, and Δ9-THC during e-Cigarette Vaping: Identification of Conversion Products by GC–MS. J. Chromatogr. A 2025, 1749, 465909. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi Rad, S.; Dalali, N.; Baheri, T. Combination of Magnetic Solid-phase Extraction with Dispersive Liquid–Liquid Microextraction Followed by GC-MS for Trace Analysis of Synthetic Cannabinoids in Plasma Samples. Micro Nano Lett. 2020, 15, 545–549. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Verduin, J.; Stuyver, L.I.; De Ridder, R.; Van Beek, A.; Colmsee, E.; Van Asten, A.C. Benefits of Derivatization in GC–MS-Based Identification of New Psychoactive Substances. Forensic Chem. 2020, 20, 100273. [Google Scholar] [CrossRef]
- Schirmer, W.; Schürch, S.; Weinmann, W. The Identification of Synthetic Impurities in a Vape Pen Containing Δ9-Tetrahydrocannabiphorol Using Gas Chromatography Coupled with Mass Spectrometry. Psychoactives 2024, 3, 491–500. [Google Scholar] [CrossRef]
- Abiedalla, Y.; Almalki, A.J.; DeRuiter, J.; Clark, C.R. GC–MS and GC–IR Analysis of Methylenedioxyphenylalkylamine Analogues of the Psychoactive 25X-NBOMe Drugs. Forensic Chem. 2021, 23, 100314. [Google Scholar] [CrossRef]
- Abiedalla, Y.; Almalki, A.J.; DeRuiter, J.; Clark, C.R. GC–MS and GC–IR Analysis of Substituted N-Benzyl 4-Bromo-2,5-Dimethoxyphenylisopropylamines. Forensic Chem. 2021, 24, 100326. [Google Scholar] [CrossRef]
- Pollard, A.; Davidson, J.T. Investigating the Effect of Substitution Location on Fentanyl Analog Identification for Methyl-Substituted Fentanyl Analogs Using GC-EI-MS. Forensic Chem. 2023, 36, 100534. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Caldeira, M.J.; Teixeira, H.M.; Câmara, J.S. Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques. Metabolites 2021, 11, 144. [Google Scholar] [CrossRef]
- Pulver, B.; Riedel, J.; Westphal, F.; Luhn, S.; Schönberger, T.; Schäper, J.; Auwärter, V.; Luf, A.; Pütz, M. A New Synthetic Cathinone: 3,4-EtPV or 3,4-Pr-PipVP? An Unsuccessful Attempt to Circumvent the German Legislation on New Psychoactive Substances. Drug Test. Anal. 2023, 15, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Alder, R.; Clancy, L.; Fu, S. Portable Testing Techniques for the Analysis of Drug Materials. WIREs Forensic Sci. 2022, 4, e1461. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, Cannabinoids, and Health. Dialogues Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [CrossRef]
- European Union Drugs Agency. European Drug Report 2024; European drug report ... (Online); Publications Office: Luxembourg, 2024. [Google Scholar]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; Da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef]
- Lobo Vicente, J.; Chassaigne, H.; Holland, M.V.; Reniero, F.; Kolář, K.; Tirendi, S.; Vandecasteele, I.; Vinckier, I.; Guillou, C. Systematic Analytical Characterization of New Psychoactive Substances: A Case Study. Forensic Sci. Int. 2016, 265, 107–115. [Google Scholar] [CrossRef]
- Mazzarino, M.; Torre, X.D.L.; Botrè, F. A Liquid Chromatography–Mass Spectrometry Method Based on Class Characteristic Fragmentation Pathways to Detect the Class of Indole-Derivative Synthetic Cannabinoids in Biological Samples. Anal. Chim. Acta 2014, 837, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Potts, A.J.; Cano, C.; Thomas, S.H.L.; Hill, S.L. Synthetic Cannabinoid Receptor Agonists: Classification and Nomenclature. Clin. Toxicol. 2020, 58, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr. Psychiatry Rep. 2016, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Hubner, E.; Schmid, M.G.; Manojlovic, V.; Gattringer, D.; Pferschy-Wenzig, E.; Kunert, O. NMR Spectroscopic Reference Data of Synthetic Cannabinoids Sold on the Internet. Magn. Reson. Chem. 2025, 63, 241–255. [Google Scholar] [CrossRef]
- Cozier, G.E.; Andrews, R.C.; Frinculescu, A.; Kumar, R.; May, B.; Tooth, T.; Collins, P.; Costello, A.; Haines, T.S.F.; Freeman, T.P.; et al. Instant Detection of Synthetic Cannabinoids on Physical Matrices, Implemented on a Low-Cost, Ultraportable Device. Anal. Chem. 2023, 95, 13829–13837. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. MDMB-4en-PINACA: EMCDDA Initial Report on the New Psychoactive Substance Methyl 3,3 Dimethyl 2 (1 (Pent 4 En 1 Yl) 1H Indazole 3 Carboxamido)Butanoate (MDMB 4en PINACA): In Accordance with Article 5b of Regulation (EC) No 1920/2006 (as Amended); Publications Office: Luxembourg, 2020. [Google Scholar]
- Oomen, P.E.; Schori, D.; Tögel-Lins, K.; Acreman, D.; Chenorhokian, S.; Luf, A.; Karden, A.; Paulos, C.; Fornero, E.; Gerace, E.; et al. Cannabis Adulterated with the Synthetic Cannabinoid Receptor Agonist MDMB-4en-PINACA and the Role of European Drug Checking Services. Int. J. Drug Policy 2022, 100, 103493. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Asa’ad, A.S.; Battah, A.H.; Aljamal, H.A. Application of Gas Chromatography–Mass Spectrometry for the Identification and Quantitation of Three Common Synthetic Cannabinoids in Seized Materials from the Jordanian Market. ACS Omega 2020, 5, 4172–4180. [Google Scholar] [CrossRef]
- Pasin, D.; Nedahl, M.; Mollerup, C.B.; Tortzen, C.; Reitzel, L.A.; Dalsgaard, P.W. Identification of the Synthetic Cannabinoid-type New Psychoactive Substance, CH-PIACA, in Seized Material. Drug Test. Anal. 2022, 14, 1645–1651. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Holland, M.V.; Reniero, F.; Palmieri, G.; Guillou, C. Identification and Analytical Characterization of a Novel Synthetic Cannabinoid-Type Substance in Herbal Material in Europe. Molecules 2021, 26, 793. [Google Scholar] [CrossRef]
- Al-Matrouk, A.; Orabi, K.Y. Identification and Chemical Structure Elucidation of Synthetic Cannabinoids Samples Seized in Kuwait during 2019–2023 Using GC–MS and NMR Spectroscopy. Forensic Sci. Res. 2025, 10, owae026. [Google Scholar] [CrossRef]
- Liu, C.; Jia, W.; Meng, X.; Hua, Z. Identification and Quantification of 10 Indole/Indazole Carboxamide Synthetic Cannabinoids in 36 Herbal Blends by Gas Chromatography-mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. J. Forensic Sci. 2021, 66, 2156–2166. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Caldeira, M.J.; Teixeira, H.M.; Câmara, J.S. Highly Sensitive Screening and Analytical Characterization of Synthetic Cannabinoids in Nine Different Herbal Mixtures. Anal. Bioanal. Chem. 2021, 413, 2257–2273. [Google Scholar] [CrossRef]
- Cole, C.; Jones, L.; McVeigh, J.; Kicman, A.; Syed, Q.; Bellis, M. Adulterants in Illicit Drugs: A Review of Empirical Evidence. Drug Test. Anal. 2011, 3, 89–96. [Google Scholar] [CrossRef]
- Grobério, T.S.; Zacca, J.J.; Botelho, É.D.; Talhavini, M.; Braga, J.W.B. Discrimination and Quantification of Cocaine and Adulterants in Seized Drug Samples by Infrared Spectroscopy and PLSR. Forensic Sci. Int. 2015, 257, 297–306. [Google Scholar] [CrossRef]
- Adamowicz, P.; Meissner, E.; Maślanka, M. Fatal Intoxication with New Synthetic Cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin. Toxicol. 2019, 57, 1103–1108. [Google Scholar] [CrossRef]
- Lobato-Freitas, C.; Brito-da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.J.; Dunnett, J.; Wheeler, J.; Davidson, A. The Identification of Synthetic Cannabinoids in English Prisons. Forensic Sci. Int. 2023, 348, 111613. [Google Scholar] [CrossRef]
- Paul, R.; Smith, S.; Gent, L.; Sutherill, R. Air Monitoring for Synthetic Cannabinoids in a UK Prison: Application of Personal Air Sampling and Fixed Sequential Sampling with Thermal Desorption Two-dimensional Gas Chromatography Coupled to Time-of-flight Mass Spectrometry. Drug Test. Anal. 2021, 13, 1678–1685. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Nguyen, H.C.; Nguyen, D.T.; Huynh, Q.C.; Le, M.H.; Huynh, H.T.; Pham, D.T. Into the Synthetic Cannabinoid 5-Fluoro-MDMB-PICA in “American Grass” Illicit Drug: Extraction, Isolation, Structure Elucidation, and GC/MS Determination. Talanta Open 2023, 8, 100246. [Google Scholar] [CrossRef]
- Sisco, E.; Burns, A.; Moorthy, A.S. A Framework for the Development of Targeted Gas Chromatography Mass Spectrometry (GC-MS) Methods: Synthetic Cannabinoids. J. Forensic Sci. 2021, 66, 1908–1918. [Google Scholar] [CrossRef]
- La Maida, N.; Pellegrini, M.; Papaseit, E.; Pérez-Mañá, C.; Poyatos, L.; Ventura, M.; Galindo, L.; Busardò, F.P.; Pichini, S.; Farré, M.; et al. Determination of the Synthetic Cannabinoids JWH-122, JWH-210, UR-144 in Oral Fluid of Consumers by GC-MS and Quantification of Parent Compounds and Metabolites by UHPLC-MS/MS. Int. J. Mol. Sci. 2020, 21, 9414. [Google Scholar] [CrossRef]
- Minakata, K.; Yamagishi, I.; Nozawa, H.; Hasegawa, K.; Suzuki, M.; Gonmori, K.; Suzuki, O.; Watanabe, K. Sensitive Identification and Quantitation of Parent Forms of Six Synthetic Cannabinoids in Urine Samples of Human Cadavers by Liquid Chromatography–Tandem Mass Spectrometry. Forensic Toxicol. 2017, 35, 275–283. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Verdeguer, J.; Pastor, A.; Armenta, S.; Esteve-Turrillas, F.A. Determination of Third-Generation Synthetic Cannabinoids in Oral Fluids. J. Anal. Toxicol. 2021, 45, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Scheidweiler, K.B.; Huestis, M.A. Simultaneous Quantification of 20 Synthetic Cannabinoids and 21 Metabolites, and Semi-Quantification of 12 Alkyl Hydroxy Metabolites in Human Urine by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1327, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.S.; Silva, I.; Ajenjo, A.C.; Dias, M.J. Validation and Application of an UPLC–MS/MS Method for the Quantification of Synthetic Cannabinoids in Urine Samples and Analysis of Seized Materials from the Portuguese Market. Forensic Sci. Int. 2014, 243, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Scheidweiler, K.B.; Jarvis, M.J.Y.; Huestis, M.A. Nontargeted SWATH Acquisition for Identifying 47 Synthetic Cannabinoid Metabolites in Human Urine by Liquid Chromatography-High-Resolution Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 883–897. [Google Scholar] [CrossRef]
- Kong, T.Y.; Kim, J.-H.; Kim, D.K.; Lee, H.S. Synthetic Cannabinoids Are Substrates and Inhibitors of Multiple Drug-Metabolizing Enzymes. Arch. Pharm. Res. 2018, 41, 691–710. [Google Scholar] [CrossRef]
- Abbate, V.; Schwenk, M.; Presley, B.C.; Uchiyama, N. The Ongoing Challenge of Novel Psychoactive Drugs of Abuse. Part I. Synthetic Cannabinoids (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1255–1282. [Google Scholar] [CrossRef]
- Rouxinol, D.; Dias Da Silva, D.; Silva, J.P.; Carvalho, F.; Bastos, M.D.L.; Carmo, H. Biodistribution and Metabolic Profile of 3,4-Dimethylmethcathinone (3,4-DMMC) in Wistar Rats through Gas Chromatography–Mass Spectrometry (GC–MS) Analysis. Toxicol. Lett. 2020, 320, 113–123. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Postmortem Distribution of AB-CHMINACA, 5-Fluoro-AMB, and Diphenidine in Body Fluids and Solid Tissues in a Fatal Poisoning Case: Usefulness of Adipose Tissue for Detection of the Drugs in Unchanged Forms. Forensic Toxicol. 2015, 33, 45–53. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Yi, L.; Liu, J.; Qiu, S.; Gu, J.; Bai, H.; Li, J.; Wurita, A.; Hasegawa, K. Newly Emerging Synthetic Cannabinoid ADB-4en-PINACA: Its Identification and Quantification in an Authentic Human Hair Sample by GC–MS/MS. Forensic Toxicol. 2023, 41, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.; Yang, H.; Liu, J.; Wurita, A.; Hasegawa, K. Quantification of MDMB-4en-PINACA and ADB-BUTINACA in Human Hair by Gas Chromatography–Tandem Mass Spectrometry. Forensic Toxicol. 2022, 40, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xiang, P.; Shen, M. Current Status of Hair Analysis in Forensic Toxicology in China. Forensic Sci. Res. 2021, 6, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P. Hair Analysis in Forensic Toxicology: An Updated Review with a Special Focus on Pitfalls. Curr. Pharm. Des. 2017, 23, 5480–5486. [Google Scholar] [CrossRef]
- Tsanaclis, L.; Andraus, M.; Wicks, J. Hair Analysis When External Contamination Is in Question: A Review of Practical Approach for the Interpretation of Results. Forensic Sci. Int. 2018, 285, 105–110. [Google Scholar] [CrossRef]
- Giorgetti, A.; Mogler, L.; Halter, S.; Haschimi, B.; Alt, A.; Rentsch, D.; Schmidt, B.; Thoma, V.; Vogt, S.; Auwärter, V. Four Cases of Death Involving the Novel Synthetic Cannabinoid 5F-Cumyl-PEGACLONE. Forensic Toxicol. 2020, 38, 314–326. [Google Scholar] [CrossRef]
- Elliott, S.; Sedefov, R.; Evans-Brown, M. Assessing the Toxicological Significance of New Psychoactive Substances in Fatalities. Drug Test. Anal. 2018, 10, 120–126. [Google Scholar] [CrossRef]
- Kokosa, J.M. Advances in Solvent-Microextraction Techniques. TrAC Trends Anal. Chem. 2013, 43, 2–13. [Google Scholar] [CrossRef]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically Assisted Matrix Solid Phase Dispersion for Extraction of Parabens from Breast Milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Latifeh, F.; Yamini, Y.; Seidi, S. Ionic Liquid-Modified Silica-Coated Magnetic Nanoparticles: Promising Adsorbents for Ultra-Fast Extraction of Paraquat from Aqueous Solution. Environ. Sci. Pollut. Res. 2016, 23, 4411–4421. [Google Scholar] [CrossRef]
- Dalali, N.; Habibizadeh, M.; Rostamizadeh, K.; Nakisa, S. Synthesis of Magnetite Multi-walled Carbon Nanotubes Composite and Its Application for Removal of Basic Dyes from Aqueous Solutions. Asia-Pac. J. Chem. Eng. 2014, 9, 552–561. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Gonçalves, J.; Porto-Figueira, P.; Figueira, J.A.; Alves, V.; Perestrelo, R.; Medina, S.; Câmara, J.S. Current Trends on Microextraction by Packed Sorbent–Fundamentals, Application Fields, Innovative Improvements and Future Applications. Analyst 2019, 144, 5048–5074. [Google Scholar] [CrossRef]
- Zschiesche, A.; Carlier, J.; Pietsch, J.; Scheu, M.; Seibt, J.; Busardò, F.P.; Auwärter, V.; Huppertz, L.M. Synthetic cannabinoid receptor agonists containing silicon: Exploring the metabolic pathways of ADMB- and Cumyl-3TMS-PrINACA in human urine specimens and post mortem material compared to In Vitro and in silico data. Arch. Toxicol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Sun, Y.; Wu, M.; Zhang, Z.; Zhang, T.; Wang, H.; Li, F.; Yang, L.; Xu, Y.; Liu, Z.-J.; et al. Carbon-Silicon Switch Led to the Discovery of Novel Synthetic Cannabinoids with Therapeutic Effects in a Mouse Model of Multiple Sclerosis. Eur. J. Med. Chem. 2021, 226, 113878. [Google Scholar] [CrossRef]
- Panayides, J.-L.; Riley, D.L.; Hasenmaile, F.; Van Otterlo, W.A.L. The Role of Silicon in Drug Discovery: A Review. RSC Med. Chem. 2024, 15, 3286–3344. [Google Scholar] [CrossRef]
- Tanaka, R.; Kawamura, M.; Ito, M.; Kikura-Hanajiri, R. Identification of Two Lysergic Acid Diethylamide Analogs, 1-(3-(Trimethylsilyl) Propionyl) Lysergic Acid Diethylamide (1S-LSD) and 1-(2-Thienoyl)-6-Allyl-nor-d-Lysergic Acid Diethylamide (1T-AL-LAD), in Paper Sheet Products Distributed on the Internet. Forensic Toxicol. 2025, 43, 370–376. [Google Scholar] [CrossRef]
- Ujváry, I. Hexahydrocannabinol and Closely Related Semi-synthetic Cannabinoids: A Comprehensive Review. Drug Test. Anal. 2024, 16, 127–161. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Hexahydrocannabinol (HHC) and Related Substances: Technical Report; Publications Office: Luxembourg, 2023. [Google Scholar]
- Jørgensen, C.F.; Rasmussen, B.S.; Linnet, K.; Thomsen, R. Emergence of Semi-synthetic Cannabinoids in Cannabis Products Seized in Eastern Denmark over a 6-year Period. J. Forensic Sci. 2024, 69, 2009–2017. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A Novel Phytocannabinoid Isolated from Cannabis Sativa L. with an in Vivo Cannabimimetic Activity Higher than Δ9-Tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Caprari, C.; Ferri, E.; Vandelli, M.A.; Citti, C.; Cannazza, G. An Emerging Trend in Novel Psychoactive Substances (NPSs): Designer THC. J. Cannabis Res. 2024, 6, 21. [Google Scholar] [CrossRef]
- Watanabe, S.; Murakami, T.; Muratsu, S.; Fujiwara, H.; Nakanishi, T.; Seto, Y. Discrepancies between the Stated Contents and Analytical Findings for Electronic Cigarette Liquid Products: Identification of the New Cannabinoid, Δ9 -tetrahydrocannabihexol Acetate. Drug Test. Anal. 2025, 17, 694–700. [Google Scholar] [CrossRef]
- Holt, A.K.; Poklis, J.L.; Peace, M.R. ∆8-THC, THC-O Acetates and CBD-Di-O Acetate: Emerging Synthetic Cannabinoids Found in Commercially Sold Plant Material and Gummy Edibles. J. Anal. Toxicol. 2022, 46, 940–948. [Google Scholar] [CrossRef]
- Valente, M.J.; Guedes De Pinho, P.; De Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and Synthetic Cathinones: A Review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P. Cathinone Derivatives: A Review of Their Chemistry, Pharmacology and Toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The Newest Cathinone Derivatives as Designer Drugs: An Analytical and Toxicological Review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Apirakkan, O.; Frinculescu, A.; Shine, T.; Parkin, M.C.; Cilibrizzi, A.; Frascione, N.; Abbate, V. Analytical Characterization of Three Cathinone Derivatives, 4-MPD, 4F–PHP and bk-EPDP, Purchased as Bulk Powder from Online Vendors. Drug Test. Anal. 2018, 10, 372–378. [Google Scholar] [CrossRef]
- Frański, R.; Gierczyk, B.; Zdrojewska, A.; Kasperkowiak, M. Comments on the Paper Entitled Rapid Tentative Identification of Synthetic Cathinones in Seized Products Taking Advantage of the Full Capabilities of Triple Quadrupole Analyzer. Forensic Toxicol. 2019, 37, 504–506. [Google Scholar] [CrossRef]
- Nadal-Gratacós, N.; Pazos, M.D.; Pubill, D.; Camarasa, J.; Escubedo, E.; Berzosa, X.; López-Arnau, R. Structure–Activity Relationship of Synthetic Cathinones: An Updated Review. ACS Pharmacol. Transl. Sci. 2024, 7, 2588–2603. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.; Barneo-Muñoz, M.; Carbón, X.; Hernández, F.; Martinez-Garcia, F.; Ventura, M.; Stove, C.P.; Sancho, J.V.; Ibáñez, M. Understanding the Pharmacokinetics of Synthetic Cathinones: Evaluation of the Blood–Brain Barrier Permeability of 13 Related Compounds in Rats. Addict. Biol. 2021, 26, e12979. [Google Scholar] [CrossRef] [PubMed]
- Barratt, M.J.; Ferris, J.A.; Winstock, A.R. Safer Scoring? Cryptomarkets, Social Supply and Drug Market Violence. Int. J. Drug Policy 2016, 35, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.-P.; Lan, Y.-S.; Lee, Y.-H.; Lee, Y.-C.; Chou, Y.-C.; Lee, H.-H.; Chang, M.-Y.; Liang, S.-S.; Lin, Y.-C. Optimizing Analytical Precision in the Identification of Synthetic Cathinones and Isomers: A Comparative Assessment of Diverse GC–MS Operating Parameters. Anal. Sci. 2024, 40, 1397–1407. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Peroni, D.; Affourtit, S.; Westerhuis, J.A.; Smilde, A.K.; Van Asten, A.C. Revealing Hidden Information in GC–MS Spectra from Isomeric Drugs: Chemometrics Based Identification from 15 eV and 70 eV EI Mass Spectra. Forensic Chem. 2020, 18, 100225. [Google Scholar] [CrossRef]
- Gilbert, N.; Mewis, R.E.; Sutcliffe, O.B. Classification of Fentanyl Analogues through Principal Component Analysis (PCA) and Hierarchical Clustering of GC–MS Data. Forensic Chem. 2020, 21, 100287. [Google Scholar] [CrossRef]
- Nuñez-Montero, M.; Lombroni, C.; Maida, N.; Rotolo, M.; Pichini, S.; Papaseit, E.; Hladun, O.; Ventura, M.; Poyatos, L.; Pérez-Mañá, C.; et al. GC–MS/MS Determination of Synthetic Cathinones: 4-Chloromethcathinone, N-Ethyl Pentedrone, and N-Ethyl Hexedrone in Oral Fluid and Sweat of Consumers under Controlled Administration: Pilot Study. Int. J. Mol. Sci. 2023, 24, 9387. [Google Scholar] [CrossRef]
- De Campos, E.G.; Da Costa, B.R.B.; Dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative Matrices in Forensic Toxicology: A Critical Review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Núñez-Montero, M.; Pérez-Mañá, C.; Hladun, O.; Poyatos, L.; Caicedo, D.A.; De La Rosa, G.; Argote, M.C.; Martín, S.; Ventura, M.; Maida, N.L.; et al. Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-Pentedrone. Pharmaceuticals 2025, 18, 721. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; De Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; De La Torre, R.; Farré, M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals 2021, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; De La Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Lo Faro, A.F.; Berardinelli, D.; Sprega, G.; Malaca, S.; Pichini, S.; Huestis, M.A.; Papaseit, E.; Pérez-Mañá, C.; Busardò, F.P.; et al. Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans. Int. J. Mol. Sci. 2022, 23, 14636. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; De La Rosa, G.; Martín, S.; Barriocanal, A.M.; Carabias, L.; Kelmendi, B.; Taoussi, O.; et al. Pharmacological Effects of Methylone and MDMA in Humans. Front. Pharmacol. 2023, 14, 1122861. [Google Scholar] [CrossRef] [PubMed]
- Di Trana, A.; La Maida, N.; De La Rosa, G.; Di Giorgi, A.; Graziano, S.; Aldhaehri, K.; Papaseit, E.; Hladun, O.; Farré, M.; Pérez, C.; et al. Early and Mid-Term Disposition of α-PVP and Its Unknown Metabolites in Urine and Oral Fluid Through a Multi-Analytical Hyphenated Approach Following a Single Non-Controlled Administration to Healthy Volunteers. AAPS J. 2025, 27, 25. [Google Scholar] [CrossRef]
- Zawilska, J.B. (Ed.) Synthetic Cathinones; Current Topics in Neurotoxicity; Springer International Publishing: Cham, Switzerland, 2018; Volume 12, ISBN 978-3-319-78706-0. [Google Scholar]
- Soares, J.; Costa, V.M.; Bastos, M.D.L.; Carvalho, F.; Capela, J.P. An Updated Review on Synthetic Cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Dixon, D.I.; Millea, M.F.; Wilcock, A.T.M.; Costello, A.; Ellison, J.R.; Lord, S.; O’Brian, K.A.; Mewis, R.E.; Sutcliffe, O.B. Synthesis, Characterisation and Quantification of the New Psychoactive Substance 1-(1,3-Benzodioxol-5-Yl)-2-(Propylamino)Butan-1-One (Bk-PBDB, Putylone). Forensic Chem. 2023, 35, 100523. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Li, Z.; Zhao, J.; Wang, X.; Wang, K.; Su, M.; Xiang, P. Separation and Identification of the Synthetic Cathinone Isomers Dipentylone and N-Ethylpentylone Using Chromatographic and Mass Spectral Characteristics. Forensic Chem. 2024, 37, 100551. [Google Scholar] [CrossRef]
- Liliedahl, R.E.; Hutzell, E.; Haley, M.; Predecki, D.P.; Davidson, J.T. The Differentiation of N-Butyl Pentylone Isomers Using GC-EI-MS and NMR. Forensic Sci. Int. 2023, 351, 111815. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Ong, M.C.; Lim, J.L.W.; Yap, T.W.A. Technical Note: N-Isopropylbutylone Unveiled–Differentiating the New Synthetic Cathinone in Ecstasy from Its Close Analogues with GC-MS and NMR Spectroscopy. Forensic Sci. Int. 2025, 370, 112448. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Freeman, S.; Sumnall, H.R.; Measham, F.; Cole, J. Analysis of NRG ‘Legal Highs’ in the UK: Identification and Formation of Novel Cathinones. Drug Test. Anal. 2011, 3, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pesquera, M.; Fabregat-Safont, D.; Gil, C.; Ventura, M.; Steinmetz, F.P.; Ibáñez, M. Characterization of the Recently Detected Cathinone N-Cyclohexyl Butylone: From Structure Elucidation to in Silico Supported Pharmacological/Toxicological Considerations. Microchem. J. 2023, 190, 108577. [Google Scholar] [CrossRef]
- Paškan, M.; Dobšíková, K.; Kuchař, M.; Setnička, V.; Kohout, M. Synthesis and Absolute Configuration of Cyclic Synthetic Cathinones Derived from A-tetralone. Chirality 2024, 36, e23646. [Google Scholar] [CrossRef]
- Zaitsu, K.; Katagi, M.; Tsuchihashi, H.; Ishii, A. Recently Abused Synthetic Cathinones, α-Pyrrolidinophenone Derivatives: A Review of Their Pharmacology, Acute Toxicity, and Metabolism. Forensic Toxicol. 2014, 32, 1–8. [Google Scholar] [CrossRef]
- Kavanagh, P.; Gofenberg, M.; Shevyrin, V.; Dvorskaya, O.; Dowling, G.; Grigoryev, A. Tentative Identification of the Phase I and II Metabolites of Two Synthetic Cathinones, MDPHP and α-PBP, in Human Urine. Drug Test. Anal. 2020, 12, 1442–1451. [Google Scholar] [CrossRef]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological Characterization of Designer Cathinones In Vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. 3,4-Methylenedioxypyrovalerone (MDPV): Chemistry, Pharmacology and Toxicology of a New Designer Drug of Abuse Marketed Online. Toxicol. Lett. 2012, 208, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.T.; Sasiene, Z.J.; Abiedalla, Y.; DeRuiter, J.; Clark, C.R.; Jackson, G.P. Fragmentation Pathways of α-Pyrrolidinophenone Synthetic Cathinones and Their Application to the Identification of Emerging Synthetic Cathinone Derivatives. Int. J. Mass. Spectrom. 2020, 453, 116343. [Google Scholar] [CrossRef]
- Namera, A.; Kawamura, M.; Nakamoto, A.; Saito, T.; Nagao, M. Comprehensive Review of the Detection Methods for Synthetic Cannabinoids and Cathinones. Forensic Toxicol. 2015, 33, 175–194. [Google Scholar] [CrossRef]
- Ishii, H.; Yokoyama, A.; Saito, K.; Kataoka, H. Synthesis and Analytical Differentiation of a Novel Synthetic Cathinone 1-(2,3-Dihydro-1H-Inden-5-Yl)-2-(Pyrrolidin-1-Yl)Butan-1-One (5-PPDI) and Its Regioisomers. Forensic Chem. 2022, 27, 100393. [Google Scholar] [CrossRef]
- Segurado, A.M.; Ahmad, S.M.; Neng, N.R.; Maniés-Sequeira, M.M.; Gaspar, H.; Nogueira, J.M.F. Simple Analytical Strategy for Screening Three Synthetic Cathinones (α-PVT, α-PVP, and MDPV) in Oral Fluids. Analytica 2022, 3, 14–23. [Google Scholar] [CrossRef]
- Machado, F.; Franco, J.; Vieira, D.N.; Margalho, C. Development and Validation of a GC–MS-EI Method to Determine α-PHP in Blood: Application to Samples Collected during Medico-Legal Autopsies. J. Anal. Toxicol. 2023, 47, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sisco, E.; Burns, A.; Moorthy, A.S. Development and Evaluation of a Synthetic Cathinone Targeted Gas Chromatography Mass Spectrometry (GC-MS) Method. J. Forensic Sci. 2021, 66, 1919–1928. [Google Scholar] [CrossRef]
- Woźniak, M.K.; Banaszkiewicz, L.; Wiergowski, M.; Tomczak, E.; Kata, M.; Szpiech, B.; Namieśnik, J.; Biziuk, M. Development and Validation of a GC–MS/MS Method for the Determination of 11 Amphetamines and 34 Synthetic Cathinones in Whole Blood. Forensic Toxicol. 2020, 38, 42–58. [Google Scholar] [CrossRef]
- Antunes, M.; Sequeira, M.; De Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC–MS. J. Anal. Toxicol. 2021, 45, 233–242. [Google Scholar] [CrossRef]
- Cláudia, M.; Pedro, A.; Tiago, R.; Francisco, C.R.; Eugenia, G. Determination of New Psychoactive Substances in Whole Blood Using Microwave Fast Derivatization and Gas Chromatography/Mass Spectrometry. J. Anal. Toxicol. 2020, 44, 92–102. [Google Scholar] [CrossRef]
- Júlio, S.; Ferro, R.A.; Santos, S.; Alexandre, A.; Caldeira, M.J.; Franco, J.; Barroso, M.; Gaspar, H. Synthesis of Emerging Cathinones and Validation of a SPE GC–MS Method for Their Simultaneous Quantification in Blood. Anal. Bioanal. Chem. 2023, 415, 571–589. [Google Scholar] [CrossRef]
- Synowiec, K.; Rojek, S.; Maciów-Głąb, M.; Kula, K.; Romańczuk, A.; Kłys, M. The Role of GC-EI-MS and Derivatization in the Detection of New Psychoactive Substances Exemplified by 49 Synthetic Cathinones. J. Anal. Chem. 2022, 77, 1315–1324. [Google Scholar] [CrossRef]
- Hobbs, J.M.; DeRienz, R.T.; Baker, D.D.; Shuttleworth, M.R.; Pandey, M. Fatal Intoxication by the Novel Cathinone 4-Fluoro-3-Methyl-α-PVP. J. Anal. Toxicol. 2022, 46, e101–e104. [Google Scholar] [CrossRef]
- Benedicte, L.; Camille, R.; Audrey, C.; Deborah, I.; Morgan, B.; Marie, D.; David, B.; Delphine, A.; Severine, F.; Guillaume, D.; et al. Case Report on Two-Cathinones Abuse: MPHP and N-Ethyl-4′methylnorpentedrone, with a Fatal Outcome. Forensic Toxicol. 2020, 38, 243–254. [Google Scholar] [CrossRef]
- Daziani, G.; Taoussi, O.; Berardinelli, D.; Bambagiotti, G.; Huestis, M.A.; Busardò, F.P.; Carlier, J. Comparison of Authentic Urine N-Ethylpentedrone Metabolites to Predicted in Silico and In Vitro Human Hepatocyte Metabolism. J. Pharm. Biomed. Anal. 2025, 267, 117170. [Google Scholar] [CrossRef]
- Langa, I.; Gonçalves, R.; Tiritan, M.E.; Ribeiro, C. Wastewater Analysis of Psychoactive Drugs: Non-Enantioselective vs Enantioselective Methods for Estimation of Consumption. Forensic Sci. Int. 2021, 325, 110873. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Castrignanò, E.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. Wastewater-Based Epidemiology and Enantiomeric Profiling for Drugs of Abuse in South African Wastewaters. Sci. Total Environ. 2018, 625, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-T.; Chang, H.-H.; Wang, Y.-T.; Chen, W.-T.; Lin, T.-C.; Chyueh, S.-C.; Chen, C.-F. Screening Synthetic Cathinones Mixed with Instant Coffee and Fruit Juice Powder Using Hydrophobic Carbon Dot Probes. Sens. Actuators B Chem. 2025, 442, 138127. [Google Scholar] [CrossRef]
- Carelli, C.; Radogna, A.; Bolcato, V.; Moretti, M.; Vignali, C.; Merli, D.; Morini, L. Old and New Synthetic and Semi-Synthetic Opioids Analysis in Hair: A Review. Talanta Open 2022, 5, 100108. [Google Scholar] [CrossRef]
- Garneau, B.; Desharnais, B.; Beauchamp-Doré, A.; Lavallée, C.; Mireault, P.; Lajeunesse, A. Challenges Related to Three Cases of Fatal Intoxication to Multiple Novel Synthetic Opioids. J. Anal. Toxicol. 2020, 44, 86–91. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments; Publications Office: Luxembourg, 2021. [Google Scholar]
- Bonner, L. DEA Issues Safety Alert on Counterfeit Meds. Pharm. Today 2021, 27, 21. [Google Scholar] [CrossRef]
- Nguyen, L.; Evans, A.; Frank, G.; Levitas, M.; Mennella, A.; Short, L.C. Genuine and Counterfeit Prescription Pill Surveillance in Washington, D.C. Forensic Sci. Int. 2022, 339, 111414. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Baig, Y.; Nardiello, D.; Quinto, M. How New Nanotechnologies Are Changing the Opioid Analysis Scenery? A Comparison with Classical Analytical Methods. Forensic Sci. Res. 2024, 9, owae001. [Google Scholar] [CrossRef]
- Koo, C.; Cox, M.; Klass, G.; Johnston, M. Stereochemical Analysis of Methorphan Using (−)-Menthyl Chloroformate. J. Forensic Sci. 2012, 57, 1549–1555. [Google Scholar] [CrossRef]
- Salerno, T.M.G.; Zamengo, L.; Coppolino, C.; Cucinotta, L.; Donato, P.; Trovato, E.; Vento, F.; Zancanaro, F.; Frison, G.; Mondello, L. Investigation of Adulterated Seized Heroin Samples and Direct Stereochemical Analysis of Methorphan by Chiral HPLC–MS/MS. Anal. Bioanal. Chem. 2025, 417, 5187–5198. [Google Scholar] [CrossRef]
- Janssen, P.A.J.; Jageneau, A.H. A New Series of Potent Analgesics. J. Pharm. Pharmacol. 1957, 9, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.C.; Sitkowski, K.; Heneghan, C.; Aronson, J.K. The Oxford Catalogue of Opioids: A Systematic Synthesis of Opioid Drug Names and Their Pharmacology. Brit J. Clin. Pharma 2021, 87, 3790–3812. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Walton, S.E.; Shuda, S.A.; Papsun, D.M.; Krotulski, A.J.; Stove, C.P. Detection, Chemical Analysis, and Pharmacological Characterization of Dipyanone and Other New Synthetic Opioids Related to Prescription Drugs. Anal. Bioanal. Chem. 2023, 415, 5165–5180. [Google Scholar] [CrossRef]
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.; Davis, N.L. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ares-Fuentes, A.M.; Lorenzo, R.A.; Fernández, P.; Fernández, A.M.; Furton, K.G.; Kabir, A.; Carro, A.M. Determination of Synthetic Opioids in Oral Fluid Samples Using Fabric Phase Sorptive Extraction and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1663, 462768. [Google Scholar] [CrossRef]
- Valdez, C.A. Gas Chromatography-Mass Spectrometry Analysis of Synthetic Opioids Belonging to the Fentanyl Class: A Review. Crit. Rev. Anal. Chem. 2022, 52, 1938–1968. [Google Scholar] [CrossRef]
- Payne, R.; Mathias, S.D.; Pasta, D.J.; Wanke, L.A.; Williams, R.; Mahmoud, R. Quality of Life and Cancer Pain: Satisfaction and Side Effects with Transdermal Fentanyl versus Oral Morphine. JCO 1998, 16, 1588–1593. [Google Scholar] [CrossRef]
- Donner, B.; Zenz, M.; Strumpf, M.; Raber, M. Long-Term Treatment of Cancer Pain With Transdermal Fentanyl. J. Pain. Symptom Manag. 1998, 15, 168–175. [Google Scholar] [CrossRef]
- Cannaert, A.; Ambach, L.; Blanckaert, P.; Stove, C.P. Activity-Based Detection and Bioanalytical Confirmation of a Fatal Carfentanil Intoxication. Front. Pharmacol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Saloner, B.; McGinty, E.E.; Beletsky, L.; Bluthenthal, R.; Beyrer, C.; Botticelli, M.; Sherman, S.G. A Public Health Strategy for the Opioid Crisis. Public Health Rep. 2018, 133, 24S–34S. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Mayer, B.P. An Efficient, Optimized Synthesis of Fentanyl and Related Analogs. PLoS ONE 2014, 9, e108250. [Google Scholar] [CrossRef]
- Stogner, J.M. The Potential Threat of Acetyl Fentanyl: Legal Issues, Contaminated Heroin, and Acetyl Fentanyl “Disguised” as Other Opioids. Ann. Emerg. Med. 2014, 64, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.G.; Pollack, H.A. Addressing the Fentanyl Threat to Public Health. N. Engl. J. Med. 2017, 376, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Dybowski, M.P.; Dawidowicz, A.L. Furanylfentanyl in Whole Blood Measured by GC–MS/MS after QuEChERS Extraction in a Fatal Case. Forensic Toxicol. 2020, 38, 496–504. [Google Scholar] [CrossRef]
- Qin, N.; Shen, M.; Xiang, P.; Wen, D.; Shen, B.; Deng, H.; Qiang, H.; Song, F.; Shi, Y. Determination of 37 Fentanyl Analogues and Novel Synthetic Opioids in Hair by UHPLC-MS/MS and Its Application to Authentic Cases. Sci. Rep. 2020, 10, 11569. [Google Scholar] [CrossRef] [PubMed]
- Strayer, K.E.; Antonides, H.M.; Juhascik, M.P.; Daniulaityte, R.; Sizemore, I.E. LC-MS/MS-Based Method for the Multiplex Detection of 24 Fentanyl Analogues and Metabolites in Whole Blood at Sub Ng mL−1 Concentrations. ACS Omega 2018, 3, 514–523. [Google Scholar] [CrossRef]
- Wei, Q.; Su, F.H. Determination of Nine Fentanyl Drugs in Hair Samples by GC-MS/MS and LC-MS/MS. ACS Omega 2022, 7, 19176–19182. [Google Scholar] [CrossRef]
- Camedda, N.; Dagoli, S.; Anzillotti, L.; Cecchi, R. Development and Validation of a Gas Chromatography-Mass Spectrometry Method for the Determination of Fentanyl and Butyryl Fentanyl in Oral Fluid. Anal. Sci. Adv. 2025, 6, e202400038. [Google Scholar] [CrossRef]
- Sisco, E.; Burns, A.; Moorthy, A.S. Development and Evaluation of a Synthetic Opioid Targeted Gas Chromatography Mass Spectrometry (GC-MS) Method. J. Forensic Sci. 2021, 66, 2369–2380. [Google Scholar] [CrossRef]
- Gilbert, N.; Antonides, L.H.; Schofield, C.J.; Costello, A.; Kilkelly, B.; Cain, A.R.; Dalziel, P.R.V.; Horner, K.; Mewis, R.E.; Sutcliffe, O.B. Hitting the Jackpot–Development of Gas Chromatography–Mass Spectrometry (GC–MS) and Other Rapid Screening Methods for the Analysis of 18 Fentanyl-derived Synthetic Opioids. Drug Test. Anal. 2020, 12, 798–811. [Google Scholar] [CrossRef]
- Valdez, C.A.; Rosales, J.A.; Leif, R.N. Determination of Fentanyl and Acetylfentanyl in Soil in Their Intact Form and Orthogonal Corroboration of Their Presence by EI-GC-MS Using Chloroformate Chemistry. Forensic Chem. 2023, 34, 100504. [Google Scholar] [CrossRef]
- Desage, M.; Guilluy, R.; Brazier, J.L.; Chaudron, H.; Girard, J.; Cherpin, H.; Jumeau, J. Gas Chromatography with Mass Spectrometry or Isotope-Ratio Mass Spectrometry in Studying the Geographical Origin of Heroin. Anal. Chim. Acta 1991, 247, 249–254. [Google Scholar] [CrossRef]
- Valdez, C.A.; Rosales, J.A.; Vu, A.K.; Leif, R.N. Detection and Confirmation of Fentanyls in High Clay-content Soil by Electron Ionization Gas Chromatography-mass Spectrometry. J. Forensic Sci. 2023, 68, 2138–2152. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.A.; Leif, R.N.; Corzett, T.H.; Dreyer, M.L. Analysis, Identification and Confirmation of Synthetic Opioids Using Chloroformate Chemistry: Retrospective Detection of Fentanyl and Acetylfentanyl in Urine and Plasma Samples by EI-GC-MS and HR-LC-MS. PLoS ONE 2022, 17, e0275931. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Sanner, R.D.; Corzett, T.H.; Dreyer, M.L.; Mason, K.E. Structural Modification of Fentanyls for Their Retrospective Identification by Gas Chromatographic Analysis Using Chloroformate Chemistry. Sci. Rep. 2021, 11, 22489. [Google Scholar] [CrossRef] [PubMed]
- Nan, Q.; Hejian, W.; Ping, X.; Baohua, S.; Junbo, Z.; Hongxiao, D.; Huosheng, Q.; Fenyun, S.; Yan, S. Investigation of Fragmentation Pathways of Fentanyl Analogues and Novel Synthetic Opioids by Electron Ionization High-Resolution Mass Spectrometry and Electrospray Ionization High-Resolution Tandem Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2020, 31, 277–291. [Google Scholar] [CrossRef]
- Leary, P.E.; Kizzire, K.L.; Chan Chao, R.; Niedziejko, M.; Martineau, N.; Kammrath, B.W. Evaluation of Portable Gas Chromatography–Mass Spectrometry (GC–MS) for the Analysis of Fentanyl, Fentanyl Analogs, and Other Synthetic Opioids. J. Forensic Sci. 2023, 68, 1601–1614. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. Synthetic Opioids: A Review and Clinical Update. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221139616. [Google Scholar] [CrossRef]
- Richeval, C.; Gaulier, J.-M.; Romeuf, L.; Allorge, D.; Gaillard, Y. Case Report: Relevance of Metabolite Identification to Detect New Synthetic Opioid Intoxications Illustrated by U-47700. Int. J. Leg. Med. 2019, 133, 133–142. [Google Scholar] [CrossRef]
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market. Brain Sci. 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Kyei-Baffour, K.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: U-47700. ACS Chem. Neurosci. 2020, 11, 3928–3936. [Google Scholar] [CrossRef]
- Solimini, R.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Giorgetti, R. Pharmacotoxicology of Non-Fentanyl Derived New Synthetic Opioids. Front. Pharmacol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Popławska, M.; Bednarek, E.; Naumczuk, B.; Kozerski, L.; Błażewicz, A. Identification and Structure Characterization of Five Synthetic Opioids: 3,4-Methylenedioxy-U-47700, o-Methyl-Acetylfentanyl, 2-Thiophenefentanyl, Benzoylfentanyl and Benzoylbenzylfentanyl. Forensic Toxicol. 2021, 39, 45–58. [Google Scholar] [CrossRef]
- Natsuka, K.; Nakamura, H.; Nishikawa, Y.; Negoro, T.; Uno, H.; Nishimura, H. Synthesis and Structure-Activity Relationships of 1-Substituted 4-(1,2-Diphenylethyl)Piperazine Derivatives Having Narcotic Agonist and Antagonist Activity. J. Med. Chem. 1987, 30, 1779–1787. [Google Scholar] [CrossRef]
- Kadem, S.N.; Arslan, Z.; Turkmen, Z. A New Synthetic Opioid Threat: A Comprehensive Review on MT-45. Forensic Sci. Int. 2025, 371, 112479. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.B.; Família, C.; Martins, D.; Cunha, M.; Dias, M.; Neng, N.R.; Gaspar, H.; Quintas, A. Drug-Checking and Monitoring New Psychoactive Substances: Identification of the U-48800 Synthetic Opioid Using Mass Spectrometry, Nuclear Magnetic Resonance Spectroscopy, and Bioinformatic Tools. Int. J. Mol. Sci. 2025, 26, 2219. [Google Scholar] [CrossRef]
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Identification of Two New-Type Designer Drugs, Piperazine Derivative MT-45 (I-C6) and Synthetic Peptide Noopept (GVS-111), with Synthetic Cannabinoid A-834735, Cathinone Derivative 4-Methoxy-α-PVP, and Phenethylamine Derivative 4-Methylbuphedrine from Illegal Products. Forensic Toxicol. 2014, 32, 9–18. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Papsun, D.M.; Walton, S.E.; Logan, B.K. Metonitazene in the United States—Forensic Toxicology Assessment of a Potent New Synthetic Opioid Using Liquid Chromatography Mass Spectrometry. Drug Test. Anal. 2021, 13, 1697–1711. [Google Scholar] [CrossRef]
- Verougstraete, N.; Vandeputte, M.M.; Lyphout, C.; Cannaert, A.; Hulpia, F.; Van Calenbergh, S.; Verstraete, A.G.; Stove, C. First Report on Brorphine: The Next Opioid on the Deadly New Psychoactive Substance Horizon? J. Anal. Toxicol. 2021, 44, 937–946. [Google Scholar] [CrossRef]
- Blanckaert, P.; Cannaert, A.; Van Uytfanghe, K.; Hulpia, F.; Deconinck, E.; Van Calenbergh, S.; Stove, C. Report on a Novel Emerging Class of Highly Potent Benzimidazole NPS Opioids: Chemical and In Vitro Functional Characterization of Isotonitazene. Drug Test. Anal. 2020, 12, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Bromig, G. Über neue starkwirkende Analgetika und ihre klinische Erprobung. Klin. Wochenschr. 1958, 36, 960–963. [Google Scholar] [CrossRef]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Iwata, Y.T. Analysis of Highly Potent Synthetic Opioid Nitazene Analogs and Their Positional Isomers. Drug Test. Anal. 2023, 15, 449–457. [Google Scholar] [CrossRef]
- Batlle, E.; Lizano, E.; Viñas, M.; Dolors Pujol, M. 1,4-Benzodiazepines and New Derivatives: Description, Analysis, and Organic Synthesis. In Medicinal Chemistry; Vašková, J., Vaško, L., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-173-1. [Google Scholar]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine Pharmacology and Central Nervous System–Mediated Effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- Zawilska, J.B.; Wojcieszak, J. An Expanding World of New Psychoactive Substances—Designer Benzodiazepines. NeuroToxicology 2019, 73, 8–16. [Google Scholar] [CrossRef]
- Hikin, L.J.; Coombes, G.; Rice-Davies, K.; Couchman, L.; Smith, P.; Morley, S. Post Mortem Blood Bromazolam Concentrations and Co-Findings in 96 Coronial Cases within England and Wales. Forensic Sci. Int. 2024, 354, 111891. [Google Scholar] [CrossRef] [PubMed]
- Drug-Related Deaths in Scotland in 2021; National Records of Scotland: Edinburgh, UK, 2022.
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. Metabolites Replace the Parent Drug in the Drug Arena. The Cases of Fonazepam and Nifoxipam. Forensic Toxicol. 2017, 35, 1–10. [Google Scholar] [CrossRef]
- Maskell, P.D.; Wilson, G.; Manchester, K.R. Designer Benzodiazepines Gidazepam and Desalkygidazepam (Bromonordiazepam): What Do We Know? J. Anal. Toxicol. 2023, 47, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, P.F.; Deitche, A.; Wise, L.M.; Patrick, S.L.; Holloway-Beth, A.; Ellison, R.; Trecki, J.; Gerona, R.; Wahl, M.S. Notes from the Field: Seizures, Hyperthermia, and Myocardial Injury in Three Young Adults Who Consumed Bromazolam Disguised as Alprazolam—Chicago, Illinois, February 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 72, 1392–1393. [Google Scholar] [CrossRef]
- Wagmann, L.; Manier, S.K.; Felske, C.; Gampfer, T.M.; Richter, M.J.; Eckstein, N.; Meyer, M.R. Flubromazolam-Derived Designer Benzodiazepines: Toxicokinetics and Analytical Toxicology of Clobromazolam and Bromazolam. J. Anal. Toxicol. 2021, 45, 1014–1027. [Google Scholar] [CrossRef]
- Ballotari, M.; Truver, M.T.; Dhoble, L.R.; Kinsey, A.M.; Hoyer, J.L.; Chronister, C.W.; Goldberger, B.A. A Postmortem Case Report Involving Fentanyl, Desalkylgidazepam, and Bromazolam. J. Anal. Toxicol. 2024, 48, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Sommer, M.J.; Wilde, M.; Perdekamp, M.G.; Auwärter, V. A Case of Fatal Multidrug Intoxication Involving Flualprazolam: Distribution in Body Fluids and Solid Tissues. Forensic Toxicol. 2022, 40, 180–188. [Google Scholar] [CrossRef]
- Marland, V.; Reid, R.; Brandon, A.M.; Hill, K.; Cruickshanks, F.; McKenzie, C.; Norman, C.; Nic Daéid, N.; Menard, H. Changing Trends in Novel Benzodiazepine Use within Scottish Prisons: Detection, Quantitation, Prevalence, and Modes of Use. Drug Test. Anal. 2024, 16, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; McCorvy, J.D.; Meyer, M.R.; Wagmann, L.; Stratford, A.; Brandt, S.D. Pharmacological and Biotransformation Studies of 1-Acyl-Substituted Derivatives of -Lysergic Acid Diethylamide (LSD). Neuropharmacology 2020, 172, 107856. [Google Scholar] [CrossRef]
- Wagmann, L.; Richter, L.H.J.; Kehl, T.; Wack, F.; Bergstrand, M.P.; Brandt, S.D.; Stratford, A.; Maurer, H.H.; Meyer, M.R. In Vitro Metabolic Fate of Nine LSD-Based New Psychoactive Substances and Their Analytical Detectability in Different Urinary Screening Procedures. Anal. Bioanal. Chem. 2019, 411, 4751–4763. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Odland, A.U.; Klein, A.K.; Dowling, G.; Dempster, N.M.; Wallach, J.; Passie, T.; et al. Return of the Lysergamides. Part VI: Analytical and Behavioural Characterization of 1-cyclopropanoyl-d-lysergic Acid Diethylamide (1CP-LSD). Drug Test. Anal. 2020, 12, 812–826. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Pulver, B.; Morton, K.; Stratford, A.; Dowling, G.; Halberstadt, A.L. Return of the Lysergamides. Part VII: Analytical and Behavioural Characterization of 1-valeroyl-d-lysergic Acid Diethylamide (1V-LSD). Drug Test. Anal. 2022, 14, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Hoang, K.; Wallach, J.; Halberstadt, A.L. Return of the Lysergamides. Part I: Analytical and Behavioural Characterization of 1-propionyl-d-lysergic Acid Diethylamide (1P-LSD). Drug Test. Anal. 2016, 8, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Dowling, G.; Wallach, J.; Halberstadt, A.L. Return of the Lysergamides. Part V: Analytical and Behavioural Characterization of 1-butanoyl-d-lysergic Acid Diethylamide (1B-LSD). Drug Test. Anal. 2019, 11, 1122–1133. [Google Scholar] [CrossRef]
- Kavanagh, P.V.; Westphal, F.; Pulver, B.; Elliott, S.P.; Stratford, A.; Halberstadt, A.L.; Brandt, S.D. Analytical and Behavioral Characterization of 1-dodecanoyl-LSD (1DD-LSD). Drug Test. Anal. 2025, 17, 101–109. [Google Scholar] [CrossRef]
- Junior, L.F.N.; Fabris, A.L.; Barbosa, I.L.; De Carvalho Ponce, J.; Martins, A.F.; Costa, J.L.; Yonamine, M. Lucy Is Back in Brazil with a New Dress. Forensic Sci. Int. 2022, 341, 111497. [Google Scholar] [CrossRef]
- Tusiewicz, K.; Wachełko, O.; Zawadzki, M.; Szpot, P. Forensic Aspects of Designer LSD Analogs Identification by GC–MS (EI) and UV Spectroscopy. Molecules 2024, 29, 5717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiang, P.; Wen, D.; Shen, B.; Wang, X.; Li, L.; Deng, H.; Chen, H.; Yan, H.; Shen, M.; et al. Sensitive Quantitative Analysis of Psilocin and Psilocybin in Hair Samples from Suspected Users and Their Distribution in Seized Hallucinogenic Mushrooms. Forensic Toxicol. 2021, 39, 464–473. [Google Scholar] [CrossRef]
- Pires, A.P.S.; De Oliveira, C.D.R.; Moura, S.; Dörr, F.A.; Silva, W.A.E.; Yonamine, M. Gas Chromatographic Analysis of Dimethyltryptamine and β-carboline Alkaloids in Ayahuasca, an Amazonian Psychoactive Plant Beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef]
- Tavares, L.; Monedeiro, F.; Bordin, D.M.; De Martinis, B.S. Investigation of Ayahuasca β-Carboline Alkaloids and Tryptamine in Sweat Samples from Religious Community Participants by GC-MS. J. Anal. Toxicol. 2020, 44, 601–609. [Google Scholar] [CrossRef]
- De Martinis, B. Sweat as an Alternative Matrix for Amphetamines and MethylenEdioxy Derivatives Analysis. CPA 2008, 4, 274–278. [Google Scholar] [CrossRef]
- Neukamm, M.A.; Pollak, S.; Thoma, V.; Vogt, S.; Huppertz, L.M.; Auwärter, V. A Fatal Case of Aspiration Due to Consumption of the Hallucinogenic Tryptamine Derivative Dipropyltryptamine (DPT). J. Pharm. Biomed. Anal. 2024, 240, 115959. [Google Scholar] [CrossRef] [PubMed]
- Emen, E.; Aslan, R.; Aydoğdu, M.; Ertaş, H.; Annette Akgür, S. Development of a Chemometric Method in Urine Sample for Newly Designed Hallucinating Psychoactive Substances: 5-MeO-MiPT. Ege Tıp Derg. 2023, 62, 355–363. [Google Scholar] [CrossRef]
- Göl, E.; Çok, I. New Psychoactive Substances in Turkey: Narcotics Cases Assessed by the Council of Forensic Medicine between 2016 and 2017 in Ankara, Turkey. Forensic Sci. Int. 2019, 294, 113–123. [Google Scholar] [CrossRef]
- Nikolaou, P.; Papoutsis, I.; Stefanidou, M.; Spiliopoulou, C.; Athanaselis, S. 2C-I-NBOMe, an “N-Bomb” That Kills with “Smiles”. Toxicological and Legislative Aspects. Drug Chem. Toxicol. 2015, 38, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Effects of the Hallucinogen 2,5-Dimethoxy-4-Iodophenethylamine (2C-I) and Superpotent N-Benzyl Derivatives on the Head Twitch Response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Andrzejczak, D. Next Generation of Novel Psychoactive Substances on the Horizon–A Complex Problem to Face. Drug Alcohol. Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- Giulietti, M.; Vivenzio, V.; Piva, F.; Principato, G.; Bellantuono, C.; Nardi, B. How Much Do We Know about the Coupling of G-Proteins to Serotonin Receptors? Mol. Brain 2014, 7, 49. [Google Scholar] [CrossRef]
- Glennon, R.A.; Raghupathi, R.; Bartyzel, P.; Teitler, M.; Leonhardt, S. Binding of Phenylalkylamine Derivatives at 5-HT1C and 5-HT2 Serotonin Receptors: Evidence for a Lack of Selectivity. J. Med. Chem. 1992, 35, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Świt, P.; Malek, K. 2-(4-Iodo-2,5-Dimethoxyphenyl)- N -[(2-Methoxyphenyl)Methyl]Ethanamine (25I-NBOME): A Harmful Hallucinogen Review. J. Anal. Toxicol. 2021, 44, 947–956. [Google Scholar] [CrossRef]
- Kamińska, K.; Świt, P.; Malek, K. 25C-NBOMe Short Characterisation. Forensic Toxicol. 2020, 38, 490–495. [Google Scholar] [CrossRef]
- Kyriakou, C.; Marinelli, E.; Frati, P.; Santurro, A.; Afxentiou, M.; Zaami, S.; Busardo, F.P. NBOMe: New Potent Hallucinogens--Pharmacology, Analytical Methods, Toxicities, Fatalities: A Review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3270–3281. [Google Scholar]
- Hill, S.L.; Doris, T.; Gurung, S.; Katebe, S.; Lomas, A.; Dunn, M.; Blain, P.; Thomas, S.H.L. Severe Clinical Toxicity Associated with Analytically Confirmed Recreational Use of 25I–NBOMe: Case Series. Clin. Toxicol. 2013, 51, 487–492. [Google Scholar] [CrossRef]
- Poklis, J.L.; Dempsey, S.K.; Liu, K.; Ritter, J.K.; Wolf, C.; Zhang, S.; Poklis, A. Identification of Metabolite Biomarkers of the Designer Hallucinogen 25I-NBOMe in Mouse Hepatic Microsomal Preparations and Human Urine Samples Associated with Clinical Intoxication. J. Anal. Toxicol. 2015, 39, 607–616. [Google Scholar] [CrossRef]
- Lukić, V.; Micić, R.; Radosavljević, Ž. Veridentification and Determination of 2,5-Dimetoxy-4-Bromophenethylamine (2C-B) in Real Sample by GC-MS Methods and Derivatization after SPE Preparation. Bull. Nat. Sci. Res. 2022, 12, 1–4. [Google Scholar] [CrossRef]
- Coelho Neto, J.; Andrade, A.F.B.; Lordeiro, R.A.; Machado, Y.; Elie, M.; Ferrari Júnior, E.; Arantes, L.C. Preventing Misidentification of 25I-NBOH as 2C-I on Routine GC–MS Analyses. Forensic Toxicol. 2017, 35, 415–420. [Google Scholar] [CrossRef]
- Lopes, K.P.S.; Lucena, M.C.C.; Vidal, L.M.T.; Ayala, A.P.; Alencar, F.A.M.; Ricardo, N.M.P.S. Study of 25R-NBOHs (R = Br, Cl, Et, I): Thermal Stability Investigation and Presumptive Identification by SCXRD and PXRD Data. Forensic Chem. 2024, 40, 100588. [Google Scholar] [CrossRef]
- Almalki, A.J.; Clark, C.R.; Abiedalla, Y.; DeRuiter, J. GC–MS Analysis of Methylenedioxybenzyl Analogues of the Serotonin Receptor Agonists 25X-NBOMe Drugs. Forensic Chem. 2020, 21, 100284. [Google Scholar] [CrossRef]
- Cottler, L.B.; Womack, S.B.; Compton, W.M.; Ben-Abdallah, A. Ecstasy Abuse and Dependence among Adolescents and Young Adults: Applicability and Reliability of DSM-IV Criteria. Hum. Psychopharmacol. 2001, 16, 599–606. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- Almalki, A.J.; Clark, C.R.; Abiedalla, Y.; DeRuiter, J. GC–MS Analysis of N-(Bromodimethoxybenzyl)-2-, 3-, and 4-Methoxyphenethylamines: Inverse Analogues of the Psychoactive 25B-NBOMe Drug. Forensic Chem. 2020, 21, 100277. [Google Scholar] [CrossRef]
- Domino, E.F.; Chodoff, P.; Corssen, G. Pharmacologic Effects of CI-581, a New Dissociative Anesthetic, in Man. Clin. Pharma Ther. 1965, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Dalgarno, P.J.; Shewan, D. Illicit Use of Ketamine in Scotland. J. Psychoact. Drugs 1996, 28, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Matey, J.M.; Montalvo, G.; García-Ruiz, C.; Zapata, F.; López-Fernández, A.; Martínez, M.A. Prevalence Study of Drugs and New Psychoactive Substances in Hair of Ketamine Consumers Using a Methanolic Direct Extraction Prior to High-Resolution Mass Spectrometry. Forensic Sci. Int. 2021, 329, 111080. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B. Methoxetamine–a Novel Recreational Drug with Potent Hallucinogenic Properties. Toxicol. Lett. 2014, 230, 402–407. [Google Scholar] [CrossRef]
- Morris, H.; Wallach, J. From PCP to MXE: A Comprehensive Review of the Non-medical Use of Dissociative Drugs. Drug Test. Anal. 2014, 6, 614–632. [Google Scholar] [CrossRef]
- Alshamaileh, M.; Hussain, I.; Baron, M.; Croxton, R.; Vetter, M.; Gonzalez-Rodriguez, J. A Study of In Vitro Metabolism and Cytotoxicity of Mephedrone and Methoxetamine in Human and Pig Liver Models Using GC/MS and LC/MS Analyses. Open Chem. 2020, 18, 1507–1522. [Google Scholar] [CrossRef]
- Joncquel Chevalier-Curt, M.; Grzych, G.; Tard, C.; Lannoy, J.; Deheul, S.; Hanafi, R.; Douillard, C.; Vamecq, J. Nitrous Oxide Abuse in the Emergency Practice, and Review of Toxicity Mechanisms and Potential Markers. Food Chem. Toxicol. 2022, 162, 112894. [Google Scholar] [CrossRef]
- Brugnone, F.; Perbellini, L.; Cerpelloni, M.; Soave, C.; Cecco, A.; Giuliari, C. Nitrous Oxide in Blood and Urine of Operating Theatre Personnel and the General Population. Int. Arch. Occup. Environ. Heath 1996, 68, 22–26. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, L.; Ma, X.; Li, S.; Xue, Y.; Yan, P.; Chen, M.; Wu, J. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox. Res. 2021, 39, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Qiang, H.; Xie, W.; Su, M.; Xiang, P.; Shi, Y. Quantitative Determination of Nitrous Oxide in Human Blood by HS-GC–MS: Forensic Application of Two Fatal Poisoning Cases. Forensic Sci. Int. 2024, 360, 112067. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, A.Ø.; Nielsen, M.K.K.; Kristensen, M.; Rasmussen, B.S. Driving under the Influence of Nitrous Oxide–A Retrospective Study of HS-GC-MS Analysis in Whole Blood. Forensic Sci. Int. 2024, 354, 111904. [Google Scholar] [CrossRef]
- Atherton, R.R. The ‘Nutmeg Challenge’: A Dangerous Social Media Trend. Arch. Dis. Child. 2021, 106, 517–518. [Google Scholar] [CrossRef]
- Reynoard, J.; Torrents, R.; Domange, B.; Glaizal, M.; De Haro, L.; Simon, N. Nutmeg Poisoning: Ten Years (2008–2018) of Experience from the Marseille Poison Control Center. La Presse Médicale 2019, 48, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Kubota, E.; Kobayashi, H.; Fujita, Y.; Hatanaka, K.; Kamijo, Y.; Funayama, M.; Mimasaka, S. Detection of Major Psychoactive Compounds (Safrole, Myristicin, and Elemicin) of Nutmeg in Human Serum via GC–MS/MS Using MonoSpin® Extraction: Application in a Nutmeg Poisoning Case. J. Pharm. Biomed. Anal. 2023, 234, 115565. [Google Scholar] [CrossRef]
- Siebert, D.J. Localization of Salvinorin A and Related Compounds in Glandular Trichomes of the Psychoactive Sage, Salvia Divinorum. Ann. Bot. 2004, 93, 763–771. [Google Scholar] [CrossRef]
- Willard, M.A.B.; Hurd, J.E.; Smith, R.W.; McGuffin, V.L. Statistical Comparison of Mass Spectra of Salvinorins in Salvia Divinorum and Related Salvia Species. Forensic Chem. 2020, 17, 100192. [Google Scholar] [CrossRef]
- Bouzoukas, C.; Nikolaou, P.; Athanaselis, S.; Dona, A.; Spiliopoulou, C.; Papoutsis, I. Development, Validation and Applications of a GC/MS Method for the Simultaneous Determination of 9 Amphetamines and 12 NPS Analogues in Blood and Urine. Forensic Sci. Int. 2025, 370, 112469. [Google Scholar] [CrossRef]
- Ferreira, A.B.; Lobo Castro, A.; Tarelho, S.; Domingues, P.; Franco, J.M. GC-MS–Still Standing for Clinical and Forensic Analysis: Validation of a Multidrug Method to Detect and Quantify Illicit Drugs. Aust. J. Forensic Sci. 2023, 55, 107–128. [Google Scholar] [CrossRef]
- Menéndez-Quintanal, L.M.; Matey, J.M.; Del Fresno González, V.; Bravo Serrano, B.; Hernández-Díaz, F.J.; Zapata, F.; Montalvo, G.; García-Ruiz, C. The State of the Art in Post-Mortem Redistribution and Stability of New Psychoactive Substances in Fatal Cases: A Review of the Literature. Psychoactives 2024, 3, 525–610. [Google Scholar] [CrossRef]
- Nisbet, L.A.; Wylie, F.M.; Scott, K.S. Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances. Separations 2024, 11, 258. [Google Scholar] [CrossRef]
- Harries, R.L.; Norman, C.; Reid, R.; Nic Daéid, N.; Nisbet, L.A. Detection of Anabolic-Androgenic Steroids in e-Cigarettes Seized from Prisons: A Case Study. Forensic Sci. Int. 2024, 356, 111965. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Brunetti, P.; Pelotti, S.; Auwärter, V. Detection of AP-237 and Synthetic Cannabinoids on an Infused Letter Sent to a German Prisoner. Drug Test. Anal. 2022, 14, 1779–1784. [Google Scholar] [CrossRef]
- Bloom, M.B.; Sisco, E.; Lurie, I.S. Development and Validation of a Rapid GC–MS Method for Seized Drug Screening. Forensic Chem. 2023, 33, 100479. [Google Scholar] [CrossRef]
- Capistran, B.A.; Sisco, E. Validation of a Rapid GC–MS Method for Forensic Seized Drug Screening Applications. Forensic Chem. 2024, 41, 100609. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Russell, M.; Mayo, E.; Aitcheson, C. Rapid Analysis of Amphetamine-type Substances Using Agilent’s QuickProbe Gas Chromatograph/Mass Spectrometer Technology. J. Mass. Spectrom. 2023, 58, e4976. [Google Scholar] [CrossRef]
- Neukamm, M.A.; Halter, S.; Auwärter, V.; Schmitt, G.; Giorgetti, A.; Bartel, M. Death after Smoking of Fentanyl, 5F-ADB, 5F-MDMB-P7AICA and Other Synthetic Cannabinoids with a Bucket Bong. Forensic Toxicol. 2024, 42, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.L. Analysis of Substituted Cathinones and Fentanyl Analogs by Gas Chromatography-Infrared Spectroscopy (GC-IRD) Using Nitrogen Carrier Gas. Forensic Chem. 2025, 44, 100654. [Google Scholar] [CrossRef]
- West, H.; Fitzgerald, J.L.; Hopkins, K.L.; Leeming, M.G.; DiRago, M.; Gerostamoulos, D.; Clark, N.; Dietze, P.; White, J.M.; Ziogas, J.; et al. Trace Residue Identification, Characterization, and Longitudinal Monitoring of the Novel Synthetic Opioid β-U10, from Discarded Drug Paraphernalia. Drug Test. Anal. 2022, 14, 1576–1586. [Google Scholar] [CrossRef]
- Lefrancois, E.; Belackova, V.; Silins, E.; Latimer, J.; Jauncey, M.; Shimmon, R.; Mozaner Bordin, D.; Augsburger, M.; Esseiva, P.; Roux, C.; et al. Substances Injected at the Sydney Supervised Injecting Facility: A Chemical Analysis of Used Injecting Equipment and Comparison with Self-Reported Drug Type. Drug Alcohol. Depend. 2020, 209, 107909. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; Logan, B.K. Analytical Findings in Used Syringes from a Syringe Exchange Program. Int. J. Drug Policy 2020, 81, 102770. [Google Scholar] [CrossRef]
- Gozdzialski, L.; Aasen, J.; Larnder, A.; Ramsay, M.; Borden, S.A.; Saatchi, A.; Gill, C.G.; Wallace, B.; Hore, D.K. Portable Gas Chromatography–Mass Spectrometry in Drug Checking: Detection of Carfentanil and Etizolam in Expected Opioid Samples. Int. J. Drug Policy 2021, 97, 103409. [Google Scholar] [CrossRef]
- Harper, L.; Powell, J.; Pijl, E.M. An Overview of Forensic Drug Testing Methods and Their Suitability for Harm Reduction Point-of-Care Services. Harm Reduct. J. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Li, H.; Xing, Y.; Hou, K.; Li, H. Rapid On-Site Detection of Illegal Drugs in Complex Matrix by Thermal Desorption Acetone-Assisted Photoionization Miniature Ion Trap Mass Spectrometer. Anal. Chem. 2019, 91, 3845–3851. [Google Scholar] [CrossRef]
- Leary, P.E.; Kammrath, B.W.; Lattman, K.J.; Beals, G.L. Deploying Portable Gas Chromatography–Mass Spectrometry (GC-MS) to Military Users for the Identification of Toxic Chemical Agents in Theater. Appl. Spectrosc. 2019, 73, 841–858. [Google Scholar] [CrossRef]
- Leary, P.E.; Dobson, G.S.; Reffner, J.A. Development and Applications of Portable Gas Chromatography–Mass Spectrometry for Emergency Responders, the Military, and Law-Enforcement Organizations. Appl. Spectrosc. 2016, 70, 888–896. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, H.; Li, W.; Wang, C.; Cooks, R.G.; Ouyang, Z. Rapid Analysis of Synthetic Cannabinoids Using a Miniature Mass Spectrometer with Ambient Ionization Capability. Talanta 2015, 142, 190–196. [Google Scholar] [CrossRef]
- Mielczarek, P.; Silberring, J.; Smoluch, M. Miniaturzation in Mass Spectrometry. Mass. Spectrom. Rev. 2020, 39, 453–470. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; Krotulski, A.J.; Martin, D.M.; Browne, T.; Triplett, J.; Conti, T.; Logan, B.K. Detection of Cutting Agents in Drug-Positive Seized Exhibits within the United States. J. Forensic Sci. 2019, 64, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Frinculescu, A.; Shine, T.; Ramsey, J.; Couchman, L.; Frascione, N.; Abbate, V. Analysis of Drugs Seized from Amnesty Bins at Two Major United Kingdom Summer Music Festivals Using Two Portable Gas Chromatography-mass Spectrometry (GC–MS) Instruments. Drug Test. Anal. 2024, 16, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Frinculescu, A.; Mercer, B.; Shine, T.; Ramsey, J.; Couchman, L.; Douce, D.; Frascione, N.; Abbate, V. Assessment of a Single Quadrupole Mass Spectrometer Combined with an Atmospheric Solids Analysis Probe for the On-Site Identification of Amnesty Bin Drugs. J. Am. Soc. Mass. Spectrom. 2024, 35, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).