Abstract
The long-term reliability of catalytic gas sensors is strongly influenced by changes in the chemical state and cleanliness of the catalyst surface. In this work, we investigate the surface composition and stability of the platinum (Pt) nanoparticle catalytic layer in Gas Metal-Oxide-Semiconductor (GMOS) sensors under varying environmental conditions. Using X-ray Photoelectron Spectroscopy (XPS) and High-Resolution (HR) XPS, we compared fresh, aged samples, thermally treated samples, and samples stored with or without a mechanical filter. The results show that prolonged ambient storage leads to the accumulation of adventitious carbon and nitrogen-containing species, as well as partial oxidation of platinum, which reduces the number of active metallic Pt sites. Thermal treatment at 300 °C for 30 min restores metallic Pt exposure by removing surface contaminants and narrowing the Pt 4f peaks. However, recontamination occurs during subsequent storage, with significant differences depending on surface protection. Sensors equipped with a mechanical filter exhibited obvious Pt metallic peaks in HR-XPS analysis, with lower carbon and nitrogen levels, compared to unprotected samples. These findings demonstrate that while heating refreshes catalytic activity, long-term stability requires complementary filtration to prevent re-adsorption of airborne species. The combined approach of heating and filtration is thus essential to ensure reliable performance of GMOS sensors for indoor and outdoor air quality monitoring.
1. Introduction
Air pollution remains a major threat to public health and environmental integrity, not only outdoors but in indoor spaces where people spend most of their time. Among the diverse pollutants, volatile organic compounds (VOCs) are particularly significant due to their ubiquity, health impacts, and atmospheric influence [1].
VOCs originate from a wide range of sources, such as solvents, paints, cleaning agents, building materials, consumer products, and combustion byproducts, and can be emitted both indoors and outdoors [2]. These compounds are known to cause sensory irritation, respiratory symptoms, neurological effects, and, in some cases, even carcinogenic and chronic health outcomes [3]. Therefore, monitoring air quality across urban, rural, and indoor environments is essential for identifying pollution sources, assessing exposure risks, guiding policy interventions, and safeguarding public health and ecosystems.
The Gas Metal-Oxide-Semiconductor (GMOS) sensor, which is a MOS-based gas sensor and is based on the Thermal Metal-Oxide-Semiconductor (TMOS) sensor, has been demonstrated to be efficient in detecting low concentrations of VOCs [4,5,6,7,8,9]. GMOS sensor, operating via a catalytic combustion reaction, offers notable advantages over conventional electrochemical and metal oxide semiconductor (MOX) gas sensors for air quality monitoring. GMOS detects gases by precisely measuring the heat release and ignition temperature during exothermic reactions on a catalytic layer. This approach enables highly selective and sensitive detection. Furthermore, the GMOS is compact, cost-effective, fast, energy-efficient, and capable of wireless data transmission, making it an excellent candidate for continuous monitoring of air quality, temperature, and humidity across diverse indoor environments, including laboratory spaces [4,5,6,7,8,9].
As reported in our earlier studies [4,5,6,7,8,9], the GMOS is a CMOS–Silicon-on-Insulator–Micro-Electro-Mechanical Systems (CMOS-SOI-MEMS) catalytic gas sensor that operates in a pellistor-like configuration. Its sensing element consists of a suspended transistor equipped with an integrated tungsten heater and coated with a nanoparticle catalyst, as depicted in Figure 1. When a target VOC, such as ethylene, is present, it undergoes an exothermic combustion reaction on the catalyst surface. The resulting heat changes the temperature of the sensing transistor, thereby altering its current-voltage characteristics. By measuring the differential response between sensor pixels with and without catalysts, the GMOS determines the presence and concentration of the analyte based on the magnitude of the temperature-induced electrical change.
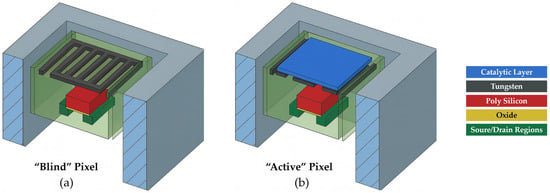
Figure 1.
Schematic cross-section of a GMOS sensor showing (a) a blind pixel and (b) an active pixel. Each pixel consists of a transistor, an embedded tungsten layer functioning as a heating resistor, and, in the active pixel, a catalytic layer. When a voltage is applied to the resistor, it heats both pixels to the ignition temperature of the analyte gas. In the active pixel, the catalytic layer initiates combustion of the analyte, producing an additional temperature rise, whereas the blind pixel, lacking a catalyst, remains at the baseline heater temperature. This temperature increase in the active pixel alters the current-voltage characteristics of its sensing transistor. During operation, the voltage difference between the blind and active transistors is measured, yielding a response proportional to the reaction-induced temperature change .
The catalyst temperature is precisely controlled by adjusting the voltage applied to the integrated heater. Each gas-catalyst pair exhibits a distinct relationship between the GMOS output signals at different heater voltages. By analyzing these relationships, optimal operating points for specific gases can be selected. Furthermore, multi-point measurements at different heater voltages enable selective discrimination among gases.
Sensor reliability is a critical factor for the practical deployment of GMOS devices. In our experience, sensors stored in ambient air for extended periods (several months) exhibited a marked reduction in sensitivity, whereas devices that were routinely operated at elevated pixel temperatures (250–350 °C) retained stable sensing performance. In addition, we observed that thermally treating aged sensors at 300–350 °C for ~30 min prior to VOC measurements significantly improved their response.
Since the pixel is fabricated using robust and well-established VLSI technology, the observed degradation is unlikely to originate from structural instability within the device itself. This suggested that surface-related processes were the likely source of performance variations.
Because the catalytic surface governs fundamental gas–solid interactions, its long-term stability is crucial for maintaining consistent sensing activity. Environmental exposure, storage conditions, and thermal treatment can all alter the chemical composition and oxidation state of the catalytic layer, thereby influencing sensor response.
The issue of sensor stability was reported in a number of papers and was commonly assigned to structural changes in a catalyst or sensor material. For example, the instability of catalytic microsensors for hydrogen detection was related to recrystallization and alloying of Pt nanoparticles [10]. The poor reliability of gas sensors using sputtered nanoporous Pt as well as alumina-supported Pt was caused by sintering and delamination of catalysts [11,12,13]. The stability of MOX SnO2 sensors with Pd catalyst was primarily dependent on the recrystallization of SnO2 [14].
The contamination and poisoning of catalysts is mainly studied for industrial applications where the feedstock influence is significant. The issue was reviewed in [15], where catalysts in electrochemical fuel cells were considered. One of the reasons for electrochemical catalyst degradation can be the partial oxidation of Pt alloys in the fuel cells [16]. The main drawbacks related to the lack of stability of gas sensors due to catalyst poisoning during methane detection are defined in [17].
The reported research was mainly concentrated on catalyst deactivation during the operation of sensors. In contrast, in our paper, we are focusing on Pt nanoparticle catalyst contamination during storage in the open air at room temperature because these are the conditions related to the air quality control sensors. We used a Pt nanoparticle catalyst deposited from Pt-NP ink as a promising material for catalyst printing in the mass production of cheap gas sensors.
In this study, we systematically examine how the surface composition of platinum nanoparticle catalysts evolves under different storage and operating conditions, with and without the use of a mechanical filter. By analyzing surface atomic concentrations and oxidation states, we provide insights into the mechanisms underlying sensor aging and outline strategies for improving the long-term reliability of GMOS devices for indoor and outdoor air quality monitoring.
2. Experiment Setup
2.1. Catalytic Layer Preparation
A platinum (Pt) catalytic layer was prepared using a 10% Pt nanoparticle ink (Fraunhofer Institute, Dresden, Germany) [18], consisting of Pt nanoparticles dispersed in a solvent. The ink was diluted with a proprietary thinner at a volumetric ratio of 1:2 (ink:thinner) to adjust viscosity for precise application. Deposition of the catalyst was performed manually with a fine-tipped brush to enable controlled, localized coating of the sensing area by Pt nanoparticle ink drops. Following deposition, the sensor was placed on a hot plate and thermally treated at approximately 200 °C for 30 min to promote solvent evaporation, ensure complete drying, and facilitate nanoparticle adhesion and catalyst layer solidification. Each sensor was also preheated prior to the first use to 300 °C. This prevented agglomeration and sintering during subsequent storage and sensing performance at lower temperatures.
2.2. Sample Conditioning
The samples used in this study were GMOS sensors that were fabricated using the same process. In order to study the impact of ambient exposure, thermal treatment, and mechanical filtration on surface contamination and the oxidation states of Pt, three aged GMOS devices that had been stored under ambient conditions for approximately three months were tested using X-ray Photoelectron Spectroscopy (XPS) measurements. These measurements were carried out for the samples under four distinct conditions: (i) one aged sample was analyzed in its aged state without any treatment; (ii) the same aged sample was subsequently heated on a hot plate at 300 °C for 30 min and re-analyzed; (iii) the second aged sample was heated under the same conditions and then stored in ambient air for 10 days before analysis; and (iv) the third aged sample underwent the same heating step but was immediately covered with a Polytetrafluoroethylene (PTFE) mechanical filter [19], and stored for 10 days before analysis.
The PTFE filter was not deposited directly on the Pt catalytic layer or on the sensing area. Instead, it was externally placed on the outer frame of the Dual In-line Package with 40 pins (DIP-40) package using a thin adhesive ring, as illustrated in Figure 2. This configuration ensured that the filter remained mechanically stable while preventing any physical contact with the pixels or the Pt surface. Consequently, no PTFE residues or signals are expected to be detected in the XPS spectra, as the filter does not interact with the analyzed surface.
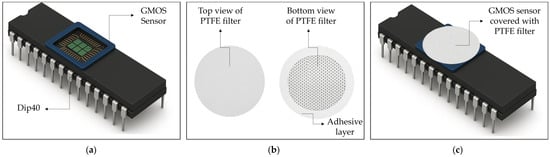
Figure 2.
Schematic illustration of (a) the GMOS sensor on a DIP-40 package, (b) the PTFE filter from top and bottom views showing the adhesive ring, and (c) the GMOS sensor covered with the PTFE filter.
In addition, Scanning Electron Microscopy (SEM) imaging was performed on a freshly prepared sample to evaluate its surface morphology and to assess the effect of heating on the structural features of the Pt layer. Energy-Dispersive X-ray Spectroscopy (EDX) measurements were carried out for both the freshly prepared and the aged (unheated) samples to determine their elemental composition and to enable a direct comparison between the two conditions.
These conditions were selected to investigate the roles of environmental exposure, thermal activation, and filtration in determining catalyst surface chemistry. The freshly prepared sample served as a reference, representing the state of the catalyst prior to any aging. Aging in ambient air was expected to promote the accumulation of adventitious carbon and other contaminants, leading to reduced catalytic activity in accordance with observed reduced sensitivity of GMOS sensors. Thermal treatment at 300 °C was anticipated to partially remove surface adsorbates and restore active Pt sites. Finally, the combination of heating with mechanical filtration was designed to evaluate whether limiting subsequent adsorption of airborne species could enhance surface stability during storage.
These considerations were based on our long-term experience with GMOS sensing of VOCs that showed a decrease in sensitivity to ethanol and ethylene by 30–50%.
2.3. Analytical Techniques
The surface of the platinum nanoparticle catalytic layers of the different samples was characterized using X-ray Photoelectron Spectroscopy (XPS) and High-Resolution XPS (HR-XPS). These techniques were employed to determine the surface atomic composition, chemical states, and spatial distribution of elements under the studied conditions.
XPS was performed using a VersaProbe III—PHI system (Physical Electronics, Chanhassen, MN, USA) operating under ultra-high vacuum (UHV, 2 × 10−10 Torr during analysis). Samples were irradiated with a focused, monochromatic Al Kα X-ray source (1486.6 eV) at 50 W and 15 kV, with a beam diameter of 200 μm. Emitted photoelectrons were collected by a Spherical Capacitor Analyzer (SCA), and surface charging was compensated using a Dual Beam Charge Neutralization system, which combines a conventional electron flood gun with a low-energy argon ion beam. Survey spectra were recorded to determine the surface elemental composition, with data acquired at a pass energy of 224 eV, a step size of 0.8 eV, and a dwell time of 20 ms, and the core level binding energies of the different peaks were normalized by setting the binding energy for the C1s at 284.8 eV. The accuracy of this peak position was estimated as ±(0.2–0.3) eV [20].
In XPS, the detected electrons originate from the top 5–10 nm of the sample surface. Peak assignments were performed with reference to established databases, listed in the reference section [20,21,22,23,24,25,26,27,28]. The detection limit of the technique is typically in the range of 0.1–1.0 atomic %.
The localization of platinum contact regions was achieved using the Secondary X-ray Imaging (SXI) mode, in which an electronically scanned X-ray beam generates secondary electron images. SXI was conducted using a 10 μm probe at 9 W and 15 kV.
High-resolution XPS (HR-XPS) spectra were acquired to investigate the chemical environment and oxidation states of platinum. These measurements were performed at a pass energy of 55 eV, with a step size of 0.1 eV and a dwell time of 20 ms. Table 1 summarizes the binding energies used for the assignments of the peaks.

Table 1.
Binding Energies of Relevant Components Used for Identification of peaks in XPS Spectra [20,21,22,23,24,25,26,27,28].
3. Results and Discussion
3.1. Analysis of the Freshly Prepared Sample
To evaluate the structural stability of the Pt catalytic layer under thermal treatment and its morphology, a high-resolution SEM was performed. Imaging was performed using a Zeiss Ultra Plus field-emission SEM operated at a primary electron energy of 4 keV. Both Everhart–Thornley and in-lens secondary electron detectors were employed to enhance topographical contrast. Representative SEM images of the Pt layer are shown in Figure 3.
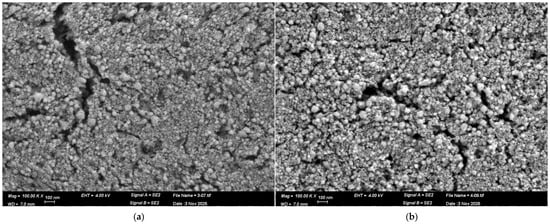
Figure 3.
SEM images of the Pt catalytic layer in the (a) freshly prepared sample, (b) freshly prepared sample that was heated at 300 °C, as captured at a magnification of 100,000×.
Figure 3a,b present HR-SEM images of the Pt catalytic layer under two conditions. Figure 3a corresponds to the freshly prepared device, which was initially calcined at 200 °C as part of the catalyst fabrication process to solidify the Pt layer. Figure 3b shows the same device after an additional heat treatment at 300 °C.
In both cases, the Pt film consists of densely packed nanoparticles forming a continuous and uniform layer with minimal voids. Such morphology provides a large specific surface area and abundant catalytic active sites, thereby facilitating efficient gas adsorption and oxidation processes. Importantly, no morphological changes or nanoparticle agglomeration are observed after heating to 300 °C. The nanoparticle distribution and surface roughness remain essentially unchanged, confirming that the Pt layer is thermally stable under these conditions.
Figure 4 and Figure 5 present the EDX analysis of the freshly prepared sample and the aged unheated sample, respectively. In both cases, the spectra show dominant peaks corresponding to Pt, confirming the presence of the catalytic layer. Signals associated with C, Si, and O are also detected. The appearance of Si and O is expected, as the Pt film is deposited on a SiO2 substrate, and the nanoparticle layer, although dense and nearly continuous, contains small voids through which the underlying surface is detected. The presence of carbon is consistent with adventitious surface contamination, which is commonly observed on samples exposed to ambient conditions and partly from residual organic components in the Pt ink used during deposition. Overall, the EDX results confirm the elemental composition of the catalytic layer and validate that both freshly prepared and aged samples maintain a Pt-rich surface.
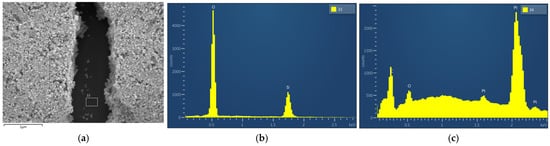
Figure 4.
SEM-EDX spectrum of Pt catalytic layer as captured from a freshly prepared sample; (a) SEM image; (b) EDX spectrum from position 33; (c) EDX spectrum from position 34.
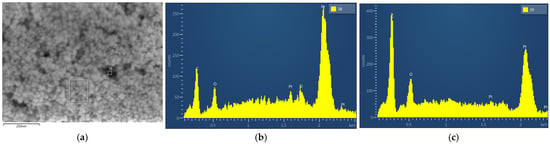
Figure 5.
SEM-EDX spectrum of Pt catalytic layer as captured from an aged sample; (a) SEM image; (b) EDX spectrum from position 38; (c) EDX spectrum from position 39.
Figure 6 presents the normalized EDX spectra of the freshly prepared sample (spectra 30) and the aged sample (spectra 39), where both spectra were normalized to the Pt peak to enable direct comparison of surface contaminants. A pronounced increase in the carbon signal is observed in the aged sample, indicating higher levels of surface contamination accumulated during storage in ambient conditions. This trend is consistent with the known adsorption of airborne hydrocarbons and organic species onto Pt surfaces over time. Additionally, the stronger small nitrogen peak that was detected in the aged sample compared to the freshly prepared sample further supports the presence of adsorbed environmental contaminants. These results demonstrate that while Pt remains the dominant surface element, the catalytic layer is susceptible to progressive accumulation of carbon- and nitrogen-containing species during storage in air.
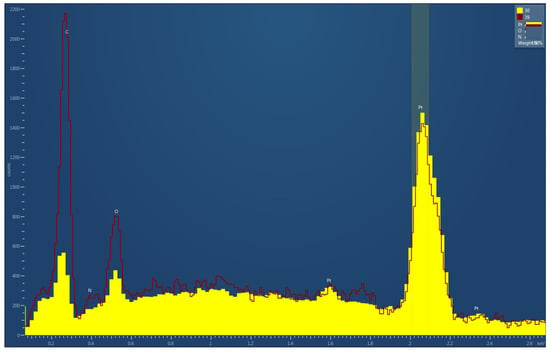
Figure 6.
Normalized EDX spectra of freshly prepared (30) and aged (39) GMOS sensor samples, both normalized to the Pt peak.
3.2. Aged Sample Without vs. with Thermal Treatment
XPS survey spectra of the aged sample, before and after heating for 30 min at 300 °C, are presented in Figure 7. For comparison, each spectrum was also normalized to the intensity of its respective Si 2s peak (153.18 eV for the aged sample and 153.94 eV for the heated sample), ensuring consistent scaling while preserving the original energy calibration. The normalized spectrum is presented in Figure 8.
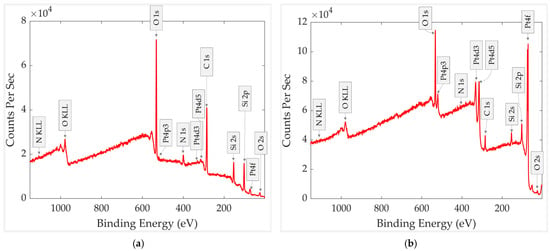
Figure 7.
XPS survey spectra of the aged GMOS sample (a) before heating and (b) after heating.
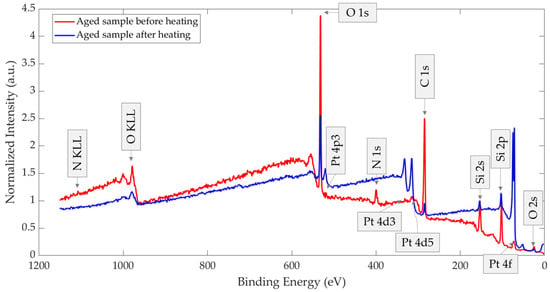
Figure 8.
XPS survey spectra of the aged GMOS sample before heating and after heating, normalized to the Si 2s peak.
The XPS survey spectrum of the aged sample shows relatively high concentrations of carbon, oxygen, and nitrogen, suggesting the presence of a contamination surface layer. The negligible platinum signal indicates that Pt-containing regions are covered by this overlayer, effectively reducing the Pt 4f peak. The observed silicon peak is evidently related to the silicon dioxide layer of the pixel, serving as a substrate for the platinum nanoparticle catalyst.
The XPS survey spectrum of the thermally treated sample shows pronounced changes. The platinum signal increased markedly, the carbon concentration decreased, and nitrogen was almost no longer detected. Residual carbon was still observed, as expected, due to its presence in the ink constituents and the short contact with air before XPS measurements.
Surface atomic concentrations of the aged and thermally treated samples, calculated using elemental sensitivity factors and derived from the survey spectra, are summarized in Table 2, and the calculated atomic concentrations from HR Spectra for the same sample are presented in Table 3. The results clearly indicate that the thermal treatment cleans the catalyst surface, as evidenced by a pronounced increase in Pt concentration accompanied by a reduction in carbon and nitrogen. Accordingly, the silicon and oxygen concentrations are consistently growing and reaching almost a stoichiometric component ratio.

Table 2.
Surface atomic composition of the aged GMOS sample, before and after thermal treatment in air at 300 °C for 30 min.

Table 3.
Calculated Atomic Concentrations from HR Spectra for the GMOS sample, before and after thermal treatment in air at 300 °C for 30 min.
The comparison of the chemical states of Pt 4f for the aged sample before and after heating is presented in Figure 9. In both spectra, the Pt 4f doublets exhibit a spin–orbit splitting of ~3.3 eV, confirming correct peak assignment. Prior to heating (Figure 9a), the first component at 70.1 eV (Pt 4f7/2) with a corresponding 4f5/2 peak at 73.5 eV is attributed to metallic Pt0. A second doublet at 72.5 eV/75.7 eV indicates the presence of oxidized Pt, corresponding to Pt2+ species (PtO). After heating at 300 °C for 30 min (Figure 9b), the aged sample shows a shift of the metallic Pt0 component to 71.5 eV (4f7/2) and 74.8 eV (4f5/2), consistent with the restoration of a clean metallic Pt surface. The oxidized contribution also shifts to higher binding energy (74.1 eV/77.6 eV), characteristic of Pt4+ species (PtO2). However, this oxidized component becomes very small, indicating that most oxidized surface species were removed during thermal treatment, and the Pt surface is largely restored to a metallic state.
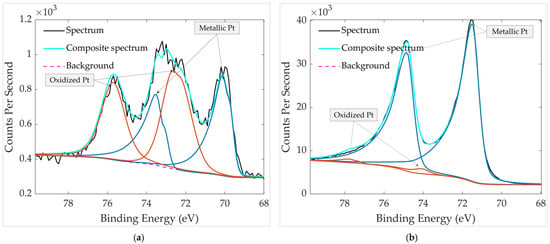
Figure 9.
HR XPS scan of Pt groups in the aged GMOS sample (a) before heating and (b) after heating. The spectra were recorded over a binding-energy range of 65–80 eV.
By comparing both chemical states in Figure 9, it is evident that thermal treatment significantly improves the surface condition of the aged sample. Heating increases the intensity of the metallic Pt0 component while greatly reducing the oxidized Pt fraction, indicating the removal of surface-bound oxygen species. The unheated aged sample shows a slight negative shift in the metallic Pt peak to 70.1 eV, suggesting an electronically enriched Pt surface in which increased electron density enhances final-state screening and lowers the observed binding energy [29]. After heating, this effect diminishes, and the Pt binding energy returns closer to the typical metallic Pt range. The metallic Pt peaks in the thermally treated sample are also narrower and exhibit greater asymmetry, a characteristic feature of conductive metallic systems in which electron–hole pair excitations near the Fermi level produce asymmetric line shapes [30,31,32]. Together, the reduced oxidation, peak narrowing, and enhanced asymmetry confirm that thermal treatment restores a predominantly metallic and cleaner Pt surface, re-exposing catalytically active sites.
The comparison of the chemical states of C 1s is presented in Figure 10. In the aged sample without thermal treatment (Figure 10a), high-resolution C 1s fitting reveals three main components at 284.3 eV, 285.7 eV, and 288.0 eV, corresponding to C–C, C–OH/C–N, and C=O/C=N species, respectively. The presence of multiple oxygen- and nitrogen-containing carbon bonds indicates surface contamination by organic residues and absorbed molecules. In contrast, after thermal treatment (Figure 10b), the spectrum contains only two detectable components at 284.8 eV (C–C) and 286.3 eV (C–OH/C–N). The second peak intensity decreases while the C=O/C=N peak at 288.0 eV nearly disappears, confirming the effective removal of oxidized carbon species during heating. The substantial reduction of oxygenated and nitrogen-containing carbon is consistent with the almost disappearance of N 1s in the spectrum of the heated sample (Figure 7b, Table 2), supporting thermal decomposition or desorption of heterocyclic contaminants such as pyridine- or quinoline-like molecules.
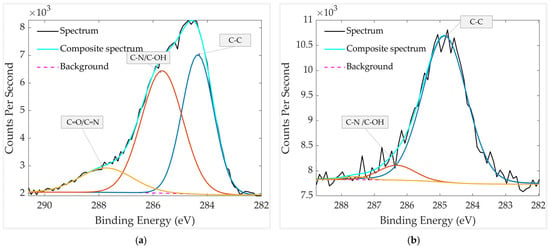
Figure 10.
HR XPS scan of carbon groups in the aged GMOS sample (a) before heating and (b) after heating. The spectra were recorded over a binding-energy range of 280–295 eV.
It should be noted that part of the detected C 1s signal originates from adventitious carbon, a ubiquitous hydrocarbon layer that forms on air-exposed surfaces due to adsorption of airborne organics [33]. This layer commonly produces C–C/C–H and oxygenated C–O or C=O components, explaining the complex carbon chemistry observed in the unheated sample. In addition, the Pt catalyst is deposited from an ink in which Pt nanoparticles are dispersed in a water-based organic formulation (exact composition undisclosed by the manufacturer). Therefore, residual C–C species are expected to remain even after heating, reflecting carbon-containing components inherently associated with the catalyst deposition process.
High-resolution XPS analysis of the O 1s region (Figure 11) reveals two main components in both the unheated and heated aged samples. The first peak at 530.6 eV is a small peak that corresponds to lattice oxygen (O2−) in Pt–O, and it is also observable after heating. The second component is centered at 532.2 eV in the unheated sample and shifts to 532.7 eV after heating. The O 1s BEs of several compounds and species fall within a narrow range of 532–533 eV (see Table 1). The 532.2 eV peak in the unheated sample can be attributed to the superposition of C=O, -OH and Si=O groups, while the higher binding-energy peak at 532.7 eV in the heated sample is attributed mainly to Si=O. The increase in binding energy after heating is consistent with the decreased contribution of C=O and C-OH bonds as well as the relative increase in Si=O composition and Si concentration in the heated sample, as shown in Table 2 and Table 3. This interpretation is further supported by the C 1s spectra, where the C=O component becomes negligible after heating, as well as the C-OH peak is significantly decreased (Figure 10b and Table 3). The presence of the small 530.6 eV peak in both spectra is also consistent with the trace amount of oxidized Pt observed in Figure 9b.
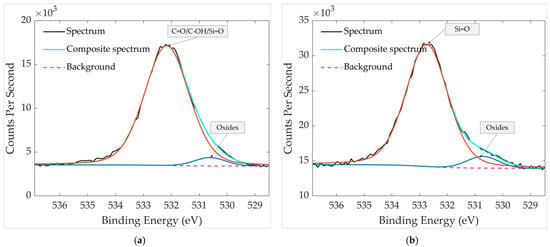
Figure 11.
HR XPS scan of oxygen groups in the aged GMOS sample (a) before heating and (b) after heating. The spectra were recorded over a binding-energy range of 525–540 eV.
3.3. Thermally Treated Samples: With vs. Without a Mechanical Filter
Figure 12 presents the XPS survey spectra of the two thermally treated GMOS samples, stored either without or with a mechanical filter. A normalized comparison of these spectra is provided in Figure 13, with each spectrum normalized to its respective Si 2s peak (154.02 and 154.06 eV, respectively). Relative to the Si 2s peak, the filtered sample shows a stronger Pt 4f signal together with a weaker C 1s signal, and the N 1s peak is nearly undetectable. Because both spectra are scaled to the substrate (Si 2s), these differences reflect true changes in the surface layer: the mechanical filter limits adsorption of airborne carbon- and nitrogen-containing species, thereby reducing overlayer contamination that would otherwise attenuate Pt, and leaving a larger fraction of Pt surface exposed after storage.
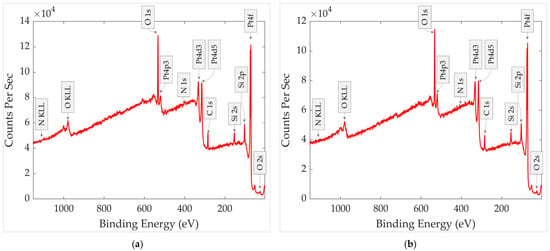
Figure 12.
XPS survey spectra of aged and thermally treated GMOS samples stored in air for 10 days: (a) without a mechanical filter and (b) with a mechanical filter.
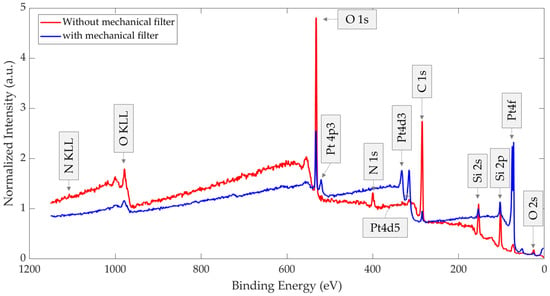
Figure 13.
XPS survey spectra of aged and thermally treated GMOS samples stored in air for 10 days, with and without a mechanical filter and, each normalized to its respective Si 2s peak.
Surface atomic concentrations for the two samples were calculated using elemental sensitivity factors derived from the survey spectra of each sample and are summarized in Table 4. Also, calculated atomic concentrations from HR Spectra for both samples are presented in Table 5. The results reveal that the sample protected by a mechanical filter contains a higher fraction of platinum, a lower fraction of carbon, and almost no nitrogen compared to the unprotected sample. These findings demonstrate that the use of a mechanical filter effectively reduces the adsorption and accumulation of airborne contaminants on the catalytic layer, thereby preserving its surface composition and minimizing potential deactivation of the catalyst.

Table 4.
Surface atomic composition of the aged and thermally treated GMOS sample, with and without a mechanical filter.

Table 5.
Calculated Atomic Concentrations from HR Spectra for the GMOS sample, with and without a mechanical filter.
High-resolution Pt 4f spectra of the thermally treated samples stored for 10 days, with and without a mechanical PTFE filter, are presented in Figure 14. In both cases, the Pt 4f doublets exhibit the expected spin–orbit splitting of ~3.33 eV and are dominated by metallic Pt. The unfiltered sample shows a metallic Pt0 component at 71.1 eV/74.4 eV, with no clearly resolved oxide peaks. In contrast, the filtered sample displays a metallic Pt0 peak at 71.3 eV/74.6 eV, together with a very weak oxidized Pt component at 73.9 eV/77.3 eV, attributed to Pt4+ (PtO2).
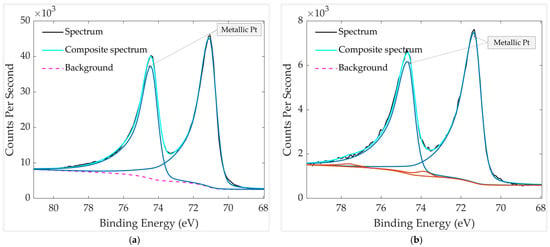
Figure 14.
HR XPS scan of Pt groups in aged and thermally treated GMOS samples: (a) without mechanical filter and (b) with mechanical filter. The spectra were recorded over a binding-energy range of 65–80 eV.
Importantly, the presence of this small, oxidized metal peak does not indicate greater oxidation in the filtered sample; rather, it reflects the cleaner surface produced by the PTFE filter. Reduced carbonaceous adsorption leaves the Pt surface more exposed, allowing even trace PtO2 to be detected, whereas airborne organics in the unfiltered sample form an overlayer that can attenuate or mask such weak features. This interpretation is further supported by surface atomic composition analysis and calculated atomic concentration for the two samples (Table 4 and Table 5), which show that the filtered sample contains a higher relative Pt 4f signal and a lower C 1s contribution compared to the unfiltered sample, as well as a higher Pt metal composition in the filtered sample. The stronger Pt signal and reduced carbon content confirm that the mechanical filter effectively suppresses adsorption of airborne contaminants, preserving a cleaner and more metallic Pt surface during storage.
High-resolution XPS analysis of the C 1s region for the thermally treated samples, stored with and without a mechanical filter, is presented in Figure 15. In both cases, the spectra are dominated by the C–C component at 284.8 eV, accompanied by a smaller contribution from oxygen- or nitrogen-containing carbon species (C–OH/C–N) at 286.1 eV (unfiltered) and 286.4 eV (filtered). No peak at ~288 eV is observed in either sample, indicating the absence of detectable C=O or C=N groups and confirming that strongly oxidized surface carbon species are largely removed by thermal treatment. The filtered sample shows slightly lower overall carbon intensity, consistent with reduced adsorption of airborne hydrocarbons during storage. This agrees with the stronger Pt 4f signal observed in the filtered device, demonstrating that the PTFE filter effectively suppresses overlayer contamination, leaving a larger fraction of the Pt surface exposed.
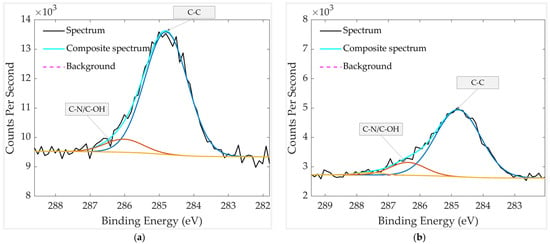
Figure 15.
HR XPS scan of carbon groups in aged and thermally treated GMOS samples: (a) without mechanical filter and (b) with mechanical filter. The spectra were recorded over a binding-energy range of 280–295 eV.
The O 1s spectra in Figure 16 reveal clear differences in the oxygen chemical environment depending on whether the sample was stored with or without a mechanical filter. The unfiltered sample exhibits two components at 530.8 eV and 532.7 eV. The peak at 530.8 eV corresponds to lattice oxygen in Pt–O, while the peak at 532.7 eV is attributed to Si=O. In contrast, the filtered sample shows only a single component at 532.7 eV, indicating the presence of Si=O and an absence of detectable oxide-related contributions. In addition to these compositional changes, the O 1s peak of the unfiltered sample is broader and less symmetric, consistent with the coexistence of more species. The filtered sample, by comparison, displays a narrower peak dominated solely by Si=O, reflecting a simpler and cleaner oxygen environment. These observations confirm that the mechanical filter effectively suppresses the adsorption of airborne oxidizing contaminants, thereby minimizing surface oxide formation during storage.
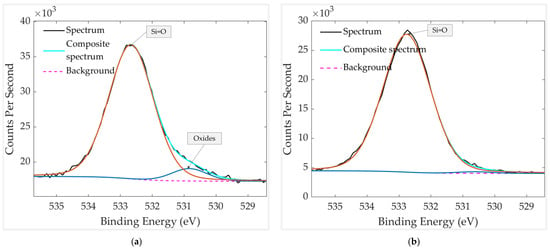
Figure 16.
HR XPS scan of oxygen groups in aged and thermally treated GMOS samples: (a) without mechanical filter and (b) with mechanical filter. The spectra were recorded over a binding-energy range of 525–540 eV.
Although the O 1s spectrum clearly shows a small oxide component at ~530.8 eV in the unfiltered sample, the corresponding Pt–O contribution is not well resolved in the Pt 4f region. This is expected for extremely low oxide coverage, where the metallic Pt signal dominates and the oxide-related Pt 4f features become too weak or too overlapped to be distinguished as a separate peak. The O 1s region, however, is highly sensitive to local oxygen chemistry and can detect subtle changes arising from adsorbed or chemisorbed oxygen-containing species. Frankcombe and Liu (2023) have shown that O 1s binding energies respond strongly to differences in surface oxygen environments, supporting the interpretation that the unfiltered sample contains surface oxide contamination acquired during storage, while the filtered sample remains cleaner and exhibits only the Si=O component [34].
3.4. Results Discussion
We examined aged GMOS sensors subjected to distinct conditioning: (i) aged, unheated; (ii) aged + thermal treatment (300 °C, 30 min); (iii) aged + thermal treatment, stored 10 days without a mechanical filter; and (iv) aged + thermal treatment, stored 10 days with a mechanical filter.
The initial assumption guiding this study was that the fresh, unaged device would exhibit the cleanest catalytic surface, consisting predominantly of exposed metallic Pt with minimal contamination. By contrast, aged samples stored under ambient conditions were expected to accumulate adventitious carbon and oxygen-containing species, reducing Pt visibility in XPS. Thermal treatment was anticipated to remove a significant portion of this surface contamination, whereas filtration during storage was expected to limit re-adsorption of airborne hydrocarbons and oxidizing species. The results obtained from SEM, EDX, and XPS confirm this expectation.
As expected, SEM and EDX analyses of the freshly prepared device versus the aged unheated device reveal a clear difference in surface cleanliness. The freshly prepared sample maintains a predominantly metallic Pt surface, with only minor carbon-related contributions originating from residual precursor ink and/or brief exposure to ambient air prior to SEM analysis, and almost no detectable nitrogen. In contrast, the aged unheated sample exhibits a higher carbon signal and the appearance of nitrogen-containing species, consistent with adsorption of airborne contaminants during prolonged storage. These results confirm that the surface of the catalyst becomes progressively contaminated over time during storage.
Regarding XPS analysis for the 3 samples under 4 different conditions, the aged sample stored under ambient conditions without heating shows substantial accumulation of carbon- and oxygen-containing functional groups (C–OH, C–N, C=O). The Pt 4f signal becomes weaker and less metallic, consistent with attenuation by carbonaceous overlayers and partial Pt oxidation. These effects limit the exposure of catalytically active Pt0 sites.
Thermal treatment at 300 °C for 30 min reverses much of this degradation. After heating, nitrogen-containing carbon species almost disappear, the carbon decreases, and the metallic Pt 4f peak intensifies, becoming narrower and more asymmetric. These changes confirm that heating removes surface-bound contaminants and restores metallic Pt0 as the dominant chemical state.
Storage experiments further demonstrate the protective role of the PTFE mechanical filter. After 10 days of ambient exposure, both unfiltered and filtered samples show some re-adsorption of carbon; however, the filtered sample exhibits lower carbon content, a stronger Pt 4f signal, and an almost absence of nitrogen. The Pt 4f spectra show only a very weak oxidized Pt component in the filtered sample, while the unfiltered one shows no resolved oxide due to attenuation by a thicker carbon overlayer. Similarly, the O 1s spectrum of the filtered sample displays a single Si=O component, whereas the unfiltered sample contains an additional oxide contribution. Together, these findings indicate that the filter suppresses adsorption of airborne carbon and nitrogen-containing molecules, which otherwise form an insulating overlayer and obscure surface Pt.
Overall, Figure 17 summarizes these trends: aging introduces contamination that suppresses exposed Pt, thermal treatment restores metallic Pt and removes nitrogen- and oxygen-rich species, and filtration during storage significantly limits recontamination. These results demonstrate that thermal activation followed by filtration is necessary to preserve a chemically clean and catalytically active Pt surface in GMOS sensors.
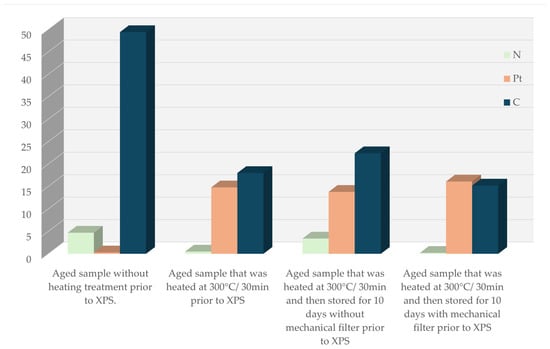
Figure 17.
A histogram that shows the calculated surface atomic concentrations of N, C, and Pt in the samples under different treatments.
The obvious reason for catalyst deactivation is a geometrical blocking of the catalyst by a shell of adventitious carbon, volatile compounds, as well as partial oxidation of Pt. In addition, it can also be catalyst poisoning due to the chemical interaction of Pt with nitrogen, whose content in the sample correlates with the aging time. It was reported that common poisons for Pt are nitrogen-heterocycles like pyridine and quinoline found in tobacco smell [35,36,37]. While the atomic concentration of nitrogen in aged samples seems relatively small (3.4–4.7%), it drags along a big heterocyclic organic molecule resulting in several times higher carbon concentration than that of nitrogen.
The binding energy of Pt to the nitrogen atom in similar compounds is reported as low as 36 kcal/mol, explaining the drop of nitrogen concentration at a heating temperature as low as 300 °C [38,39]. A proposed contamination model is illustrated in Figure 18.
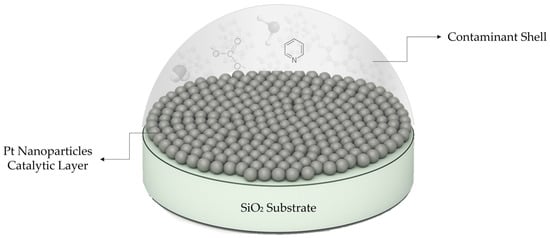
Figure 18.
Schematic representation of organic contaminant shell enclosing Pt catalysts.
4. Conclusions
In this study, we examined the origin of catalytic gas sensor degradation and regeneration and the impact of thermal treatment and mechanical filtration on the surface composition and stability of Pt-based GMOS sensors. The results obtained indicate that the combination of thermal treatment and mechanical filtration proved essential for maintaining catalyst cleanliness, providing stability of sensing. Heating alone was sufficient to restore Pt metallic exposure and reduce adventitious carbon, but without a filter, re-contamination with carbon species, VOCs, and nitrogen species occurred during storage. The results emphasize that thermal refresh must be complemented by mechanical filtration to achieve long-term surface stability, both of which are critical for reliable GMOS sensor performance.
Beyond surface contamination, other catalyst deactivation modes must also be considered. Catalyst layer delamination can be mitigated by employing porous or nanoparticle-based catalyst structures, as demonstrated in our earlier studies [4,5,6,7,8,9], while catalyst sintering is best avoided by pre-heating the sensor to a high temperature, around 350 °C, before its first use. Contamination remains a central challenge, where prevention can be achieved by using PTFE mechanical filters, and removal can be accomplished through periodic catalyst refreshment via short high-temperature heating cycles.
Taken together, these results highlight that long-term stability of GMOS sensors depends not only on effective surface treatments but also on preventive design strategies to minimize delamination, sintering, and contamination. Such robustness is vital for ensuring consistent and durable operation of nanomaterial-based chemo-sensors in air quality monitoring applications, where sensitivity, selectivity, and stability are indispensable for reliable detection of air pollutants and contaminants.
Author Contributions
Conceptualization, Y.N. and S.S.; methodology, S.S. and H.A.; software, H.A.; validation, T.B.; formal analysis, H.A. and S.S.; investigation, H.A. and S.S.; resources, Y.N.; data curation, T.B., H.A. and S.S.; writing—original draft preparation, H.A.; writing—review and editing, Y.N., S.S., H.A. and T.B.; visualization, H.A.; supervision, Y.N.; project administration, Y.N.; funding acquisition, Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
The generous funding of Todos Technologies Ltd. (https://www.todos-technologies.com).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Sara Stolyarova and Yael Nemirovsky were employed by the company Todos Technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Cincinelli, A.; Martellini, T. Indoor air quality and health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Jung, Y.S.; Chiang, S.; Athni, T.S.; Shah, J.; McCurdy, K.; Yu, S.E.; Kariveda, R.; Mitchell, M.; Ruan, M.; Zou, J.; et al. Characterizing everyday exposure to volatile organic compounds and upper respiratory health effects. Sci. Rep. 2025, 15, 23555. [Google Scholar] [CrossRef]
- Rumchev, K.; Brown, H.; Spickett, J. Volatile organic compounds: Do they present a risk to our health? Rev. Environ. Health 2007, 22, 39–55. [Google Scholar] [CrossRef]
- Ashkar, H.; Stolyarova, S.; Blank, T.; Nemirovsky, Y. The role of Pd–Pt bimetallic catalysts in ethylene detection by CMOS-MEMS gas sensor dubbed GMOS. Micromachines 2025, 16, 672. [Google Scholar] [CrossRef]
- Krayden, A.; Avraham, M.; Ashkar, H.; Blank, T.; Stolyarova, S.; Nemirovsky, Y. TinyML-based real-time drift compensation for gas sensors using spectral-temporal neural networks. Chemosensors 2025, 13, 223. [Google Scholar] [CrossRef]
- Nemirovsky, Y.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Zviagintsev, A.; Svetlitza, A.; Brouk, I. A new pellistor-like gas sensor based on micro machined CMOS transistor. IEEE Trans. Electron Devices 2018, 99, 5494–5498. [Google Scholar] [CrossRef]
- Shlenkevich, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Nemirovsky, Y. A novel miniature and selective combustion on type CMOS gas sensor for gas mixture analysis—Part 1: Emphasis on chemical aspects. Micromachines 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Golan, G.; Nemirovsky, Y. A novel miniature and selective CMOS gas sensor for gas mixture analysis—Part 2: Emphasis on physical aspects. Micromachines 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Avraham, M.; Krayden, A.; Ashkar, H.; Aronin, D.; Stolyarova, S.; Blank, T.; Shlenkevitch, D.; Nemirovsky, Y. A novel miniature and selective CMOS gas sensor for gas mixture analysis—Part 4: The effect of humidity. Micromachines 2024, 15, 264. [Google Scholar] [CrossRef]
- Brauns, E.; Morsbach, E.; Kunz, S.; Bäumer, M.; Lang, W. A fast and sensitive catalytic gas sensor for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B Chem. 2014, 193, 895–903. [Google Scholar] [CrossRef]
- Sturm, H.; Brauns, E.; Seemann, T.; Zoellmer, V.; Lang, W. A highly sensitive catalytic gas sensor for hydrogen detection based on sputtered nanoporous platinum. Procedia Eng. 2010, 5, 123–126. [Google Scholar] [CrossRef]
- Shin, W.; Nishibori, M.; Houlet, L.F.; Itoh, T.; Izu, N.; Matsubara, I. Fabrication of thermoelectric gas sensors on micro-hotplates. Sens. Actuators B Chem. 2009, 139, 340–345. [Google Scholar] [CrossRef]
- Nishibori, M.; Shin, W.; Tajima, K.; Houlet, L.F.; Izu, N.; Itoh, T.; Matsubara, I. Long-term stability of Pt/alumina catalyst combustors for micro-gas sensor application. J. Eur. Ceram. Soc. 2008, 28, 2183–2190. [Google Scholar] [CrossRef]
- Pavelko, R.G.; Vasiliev, A.A.; Llobet, E.; Vilanova, X.; Barrabés, N.; Medina, F.; Sevastyanov, V.G. Comparative study of nanocrystalline SnO2 materials for gas sensor application: Thermal stability and catalytic activity. Sens. Actuators B Chem. 2009, 137, 637–643. [Google Scholar] [CrossRef]
- Choi, S.; Jang, I.; Lee, S. Advanced strategies for mitigating catalyst poisoning in low and high temperature proton exchange membrane fuel cells: Recent progress and perspectives. Crystals 2025, 15, 129. [Google Scholar] [CrossRef]
- Mehmood, A.; Scibioh, M.A.; Prabhuram, J.; An, M.-G.; Ha, H.Y. A review on durability issues and restoration techniques in long-term operations of direct methanol fuel cells. J. Power Sources 2015, 297, 224–241. [Google Scholar] [CrossRef]
- Karpova, E.; Mironov, S.; Suchkov, A.; Karelin, A.; Karpov, E.E.; Karpov, E.F. Increase of catalytic sensors stability. Sens. Actuators B Chem. 2014, 197, 358–363. [Google Scholar] [CrossRef]
- Fraunhofer Institute. Available online: https://www.dresden.fraunhofer.de/en.html (accessed on 14 August 2025).
- Cobetter. Membrane Filters. Cobetter Official Shop. Available online: https://shop.cobetter.com/collections/membrane-filters (accessed on 15 September 2025).
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights From a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, N.S. Studies of the oxidation of iron by water vapour using X-ray photoelectron spectroscopy and QUASES. Surf. Sci. 2004, 572, 217–227. [Google Scholar] [CrossRef]
- Available online: https://xpsdatabase.com/oxygen-o-z8/?v=4605f628f91d (accessed on 20 November 2025).
- Miller, D.J.; Öberg, H.; Kaya, S.; Sanchez Casalongue, H.; Friebel, D.; Anniyev, T.; Ogasawara, H.; Bluhm, H.; Pettersson, L.G.M.; Nilsson, A. Oxidation of Pt(111) under near-ambient conditions. Phys. Rev. Lett. 2011, 107, 195502. [Google Scholar] [CrossRef]
- John, F.; King, R.C., Jr. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- NIST X-Ray Photoelectron Spectroscopy (XPS) Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1990. Available online: https://srdata.nist.gov/xps/ (accessed on 16 August 2025).
- Thermo Scientific. XPS Simplified. Available online: http://www.xpssimplified.com/ (accessed on 16 August 2025).
- XPSFitting.com. XPS Peak Fitting Tutorial and Tools. Available online: https://www.xpsfitting.com/ (accessed on 20 September 2025).
- Thermo Fisher Scientific. Oxygen | XPS Periodic Table: Non-Metal Elements—Oxygen. Materials Science Learning Center. Available online: https://www.thermofisher.com/il/en/home/materials-science/learning-center/periodic-table/non-metal/oxygen.html (accessed on 20 September 2025).
- Bayindir, Z.; Duchesne, P.N.; Cook, S.C.; MacDonald, M.A.; Zhang, P. X-ray spectroscopy studies on the surface structural characteristics and electronic properties of platinum nanoparticles. J. Chem. Phys. 2009, 131, 244716. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Park, H.; Engelhard, M.H.; Li, D.; Sushko, P.V.; Du, Y. Reevaluation of XPS Pt 4f Peak Fitting: Ti 3s Plasmon Peak Interference and Pt Metallic Peak Asymmetry in Pt@TiO2 System. J. Vac. Sci. Technol. A 2024, 42, 063209. [Google Scholar] [CrossRef]
- Morgan, D.J. XPS Insights: Asymmetric Peak Shapes in XPS. Surf. Interface Anal. 2023, 55, 7215. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Baer, D.R.; Herrera-Gomez, A.; Sherwood, P.M.A. Introductory Guide to Backgrounds in XPS Spectra and Their Impact on Determining Peak Intensities. J. Vac. Sci. Technol. A 2020, 38, 063203. [Google Scholar] [CrossRef]
- XPSFitting.com. What Is Adventitious Carbon? January 2011. Available online: https://www.xpsfitting.com/2011/01/what-is-adventitious-carbon.html (accessed on 20 September 2025).
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Catalyst Poisoning. ChemEurope Encyclopedia. Available online: https://www.chemeurope.com/en/encyclopedia/Catalyst_poisoning.html (accessed on 31 August 2025).
- Maxted, E.V. The Poisoning of Metallic Catalysts. Adv. Catal. 1951, 3, 129–178. [Google Scholar]
- Schmeltz, I.; Hoffmann, D. Nitrogen-Containing Compounds in Tobacco and Tobacco Smoke. Chem. Rev. 1977, 77, 295–311. [Google Scholar] [CrossRef]
- Kutlu, E.; Kismali, G.; Emen, F.; Demindogen, R.E. Pyridine Derivative Platinum Complexes: Synthesis, Molecular Structure, DFT and Initial Anticancer Activity Studies. J. Mol. Struct. 2021, 1234, 130191. [Google Scholar] [CrossRef]
- Juhász, M.; Takahashi, S.; Arulmozhiraja, S.; Fujii, T. Bond Energies (Pt–NH3, Pt–Cl) and Proton Affinity of Cisplatin: A Density Functional Theory Approach. J. Struct. Chem. 2012, 53, 436–442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).