Assay of Two Antibacterial/Anticoccidial Drugs in Combination with Vitamin K3 for Oral Solutions: Stability Studies and Method Development Using HPLC-DAD: Appraisal of the Method’s Eco-Friendliness and Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatuses
2.2. Pure Standard Samples
2.3. Pharmaceutical Formulation
2.4. Solvents and Reagents
2.5. Stock and Working Solutions
2.6. Pharmaceutical Formulation Solutions
2.7. Analytical Procedures
2.7.1. HPLC Technique Development and Optimization
2.7.2. Evaluation of the Validation Standards of the HPLC-DAD Method
2.7.3. Guidelines for Achieving Stability Studies for MND, DMT, and SLF
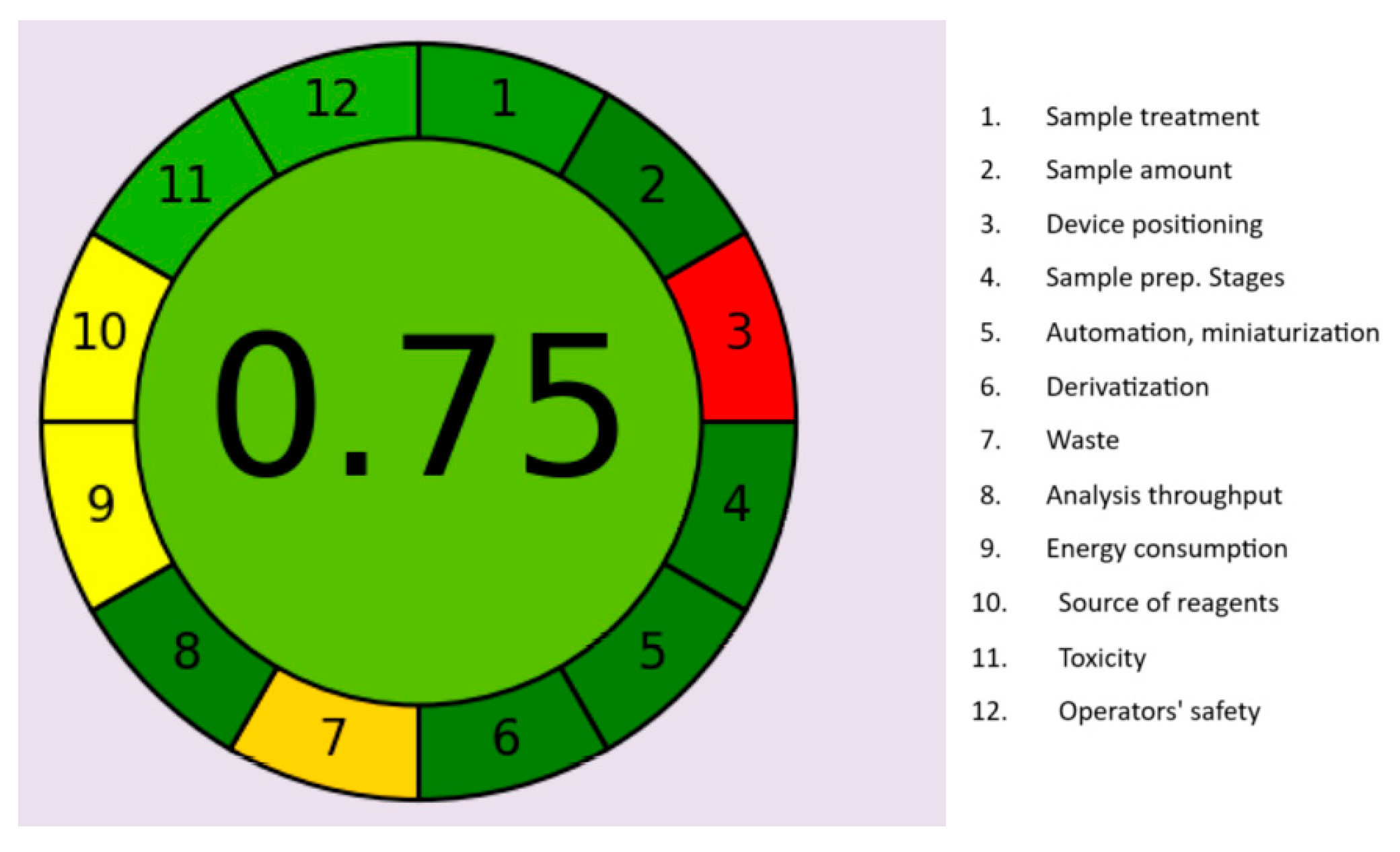
2.8. Evaluation of the Environmental Advantages of the HPLC Procedures Using AGREE and GAPI Tools
3. Results and Discussions
3.1. Assessing the Improvement of the HPLC Technique
3.2. Validation
3.3. Outcomes of the Detailed Degradation Studies
3.4. Evaluation of the Method’s Greenness and Comparisons with Previously Reported Chromatographic Approaches
3.5. The Advantages of the Novel HPLC Technique for the Industrial Pharmacy Field
3.6. Advice Concerning the Pharmaceutical Handling of MND, DMT, and SLF
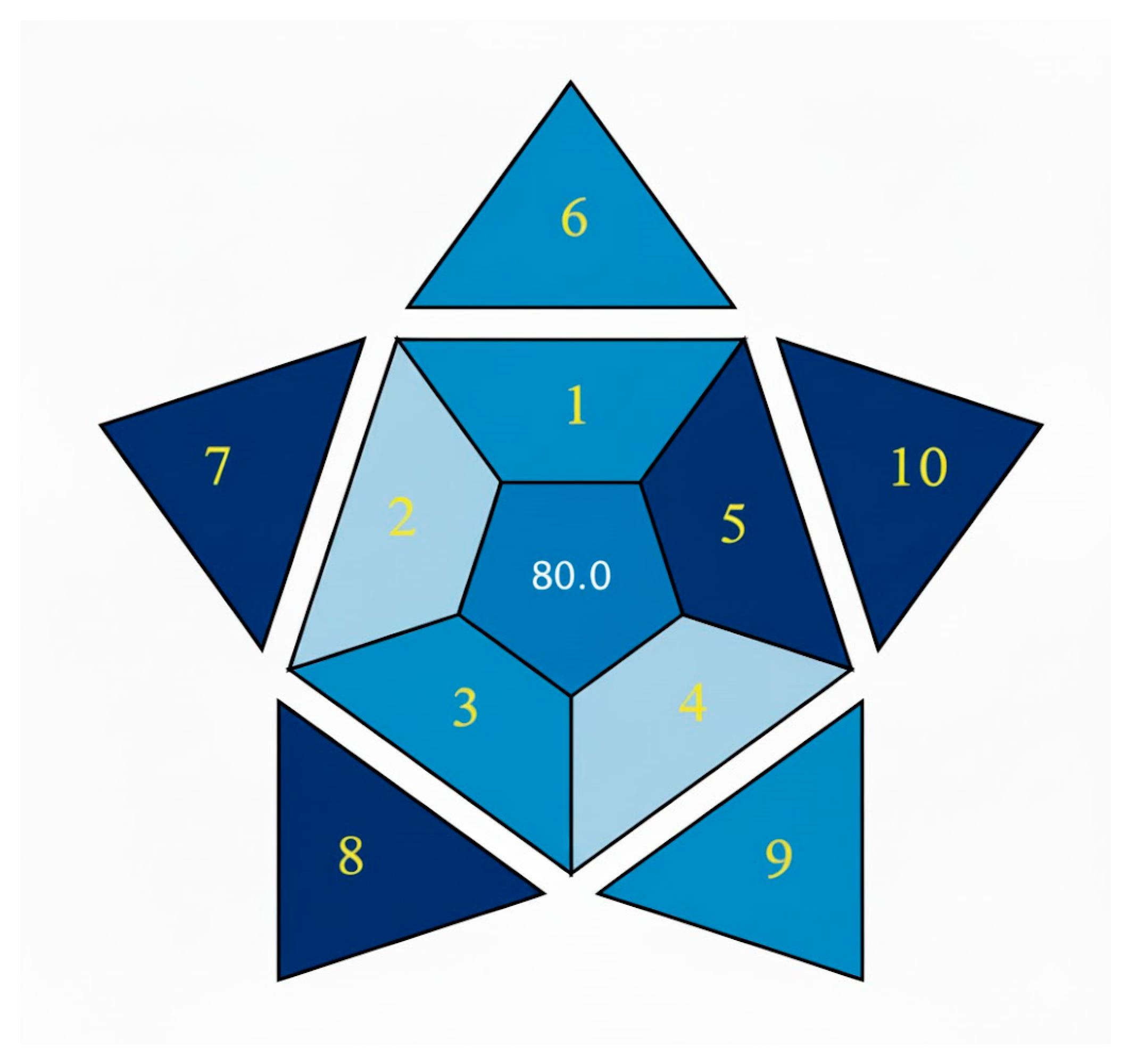
3.7. The Blue Applicability Grade Index (BAGI) Tool Is Used to Consider the Method’s Usefulness and Usability
3.8. The Estimated Constraints and Prospective Plans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehrawat, R.; Maithani, M.; Singh, R. Regulatory Aspects in Development of Stability-Indicating Methods: A Review. Chromatographia 2010, 72, 1–6. [Google Scholar] [CrossRef]
- Chew, Y.L.; Khor, M.A.; Lim, Y.Y. Choices of Chromatographic Methods as Stability Indicating Assays for Pharmaceutical Products: A Review. Heliyon 2021, 7, e06553. [Google Scholar] [CrossRef]
- Hegazy, A.M.; Batubara, A.S.; Abdelgawad, M.A.; El-Sherbiny, M.; Ghoneim, M.M.; Ahmed, A.M.; Gamal, M. Recommended and Verified Stability Indicating GC–MS Procedures for Green Separation of Quaternary Mixture of Naphazoline, Ephedrine, Methylparaben, and Naphazoline Impurity. Microchem. J. 2022, 183, 108058. [Google Scholar] [CrossRef]
- El-Maraghy, C.M. Implementation of Green Chemistry to Develop HPLC/UV and HPTLC Methods for the Quality Control of Fluconazole in Presence of Two Official Impurities in Drug Substance and Pharmaceutical Formulations. Sustain. Chem. Pharm. 2023, 33, 101124. [Google Scholar] [CrossRef]
- Bhukya, V.N.; Beda, D.P. Implementation of Green Analytical Principles to Develop and Validate the HPLC Method for the Separation and Identification of Degradation Products of Panobinostat, and Its Characterization by Using LC-QTOF-MS/MS and Its In-Silico Toxicity Prediction Using ADMET Software. Green Anal. Chem. 2024, 8, 100090. [Google Scholar] [CrossRef]
- Darji, H.; Dedania, Z. Simultaneous Estimation of Azelnidipine and Metoprolol Succinate with Greenness Assessment Using HPLC and UV-Spectrophotometric Methods. Green Anal. Chem. 2023, 7, 100079. [Google Scholar] [CrossRef]
- Kokilambigai, K.S.; Lakshmi, K.S. Analytical Quality by Design Assisted RP-HPLC Method for Quantifying Atorvastatin with Green Analytical Chemistry Perspective. J. Chromatogr. Open 2022, 2, 100052. [Google Scholar] [CrossRef]
- Mohamed, D.; Fouad, M.M. Application of NEMI, Analytical Eco-Scale and GAPI Tools for Greenness Assessment of Three Developed Chromatographic Methods for Quantification of Sulfadiazine and Trimethoprim in Bovine Meat and Chicken Muscles: Comparison to Greenness Profile of Reported HPLC Methods. Microchem. J. 2020, 157, 104873. [Google Scholar] [CrossRef]
- Elmansi, H.; Belal, F. Development of an Eco-Friendly HPLC Method for the Simultaneous Determination of Three Benzodiazepines Using Green Mobile Phase. Microchem. J. 2019, 145, 330–336. [Google Scholar] [CrossRef]
- Nugrahani, I.; Manosa, E.Y.; Chintya, L. FTIR-Derivative as a Green Method for Simultaneous Content Determination of Caffeine, Paracetamol, and Acetosal in a Tablet Compared to HPLC. Vibr. Spectrosc. 2019, 104, 102941. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Li, J.; Ma, J.; Chen, L. Hydrophilic Molecularly Imprinted Nanospheres for the Extraction of Rhodamine B Followed by HPLC Analysis: A Green Approach and Hazardous Waste Elimination. Talanta 2020, 215, 120933. [Google Scholar] [CrossRef]
- De Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Suresh, S.; Raghu, D.; Karunagaran, D. Menadione (Vitamin K3) induces apoptosis of human oral cancer cells and reduces their metastatic potential by modulating the expression of epithelial to mesenchymal transition markers and inhibiting migration. Asian Pac. J. Cancer Prev. 2013, 14, 5461–5465. [Google Scholar] [CrossRef]
- Zoughaib, M.; Pashirova, T.N.; Nikolaeva, V.; Kamalov, M.; Nakhmetova, F.; Salakhieva, D.V.; Abdullin, T.I. Anticancer and chemosensitizing effects of menadione-containing peptide-targeted solid lipid nanoparticles. J. Pharm. Sci. 2024, 113, 2258–2267. [Google Scholar] [CrossRef]
- Food Safety Commission of Japan. Risk Assessment Report: Dimetridazole (1,2-dimethyl-5-nitroimidazole) in Food-Producing Animals; Food Safety Commission Japan (FSCJ) Report; Food Safety Commission Japan: Tokyo, Japan, 2015. [Google Scholar]
- Mital, A. Synthetic Nitroimidazoles: Biological Activities and Mutagenicity Relationships. Sci. Pharm. 2009, 77, 497–520. [Google Scholar] [CrossRef]
- The public health issue of antibiotic residues in food and feed. Front. Microbiol. 2022, 13, 9047141.
- Meradji, S.; Basher, N.S.; Sassi, A.; Ibrahim, N.A.; Idres, T.; Touati, A. The Role of Water as a Reservoir for Antibiotic-Resistant Bacteria. Antibiotics 2025, 14, 763. [Google Scholar] [CrossRef]
- Sastry, C.S.P.; Rajendraprasad Singh, N.; Narayana Reddy, M.; Sankar, D.G. Spectrophotometric determination of menadione and menadione sodium bisulfite in pharmaceutical preparations. Int. J. Pharm. 1987, 39, 137–140. [Google Scholar] [CrossRef]
- Ruiz, T.P.; Lozano, C.M.; Tomas, V.; Martin, J. Flow-injection fluorimetric determination of menadione using on-line photo-reduction in micellar media. Anal. Chim. Acta 2004, 514, 259–264. [Google Scholar] [CrossRef]
- Gil Torró, I.; García Mateo, J.V.; Martínez Calatayud, J. Spectrofluorimetric determination of vitamin K3 by a solid-phase zinc reactor immobilized in a flow injection assembly. Analyst 1997, 122, 139–142. [Google Scholar] [CrossRef]
- Nevado, J.J.; Pulgarin, J.A.; Laguna, M.A. Spectrofluorimetric study of the b-cyclodextrin: Vitamin K3 complex and determination of vitamin K3. Talanta 2001, 53, 951–959. [Google Scholar] [CrossRef]
- Vire, J.C.; El Maali, N.A.; Patriarche, G.J. Square-wave adsorptive stripping voltammetry of menadione (vitamin K(3)). Talanta 1988, 35, 997–1000. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Zhang, X.; Zhang, Z. Chemiluminescence analysis of menadione sodium bisülfite and analgin in pharmaceutical preparations and biological fluids. J. Pharm. Biomed. Anal. 1999, 21, 817–825. [Google Scholar] [CrossRef]
- Pérez-Ruíz, T.; Martínez-Lozano, C.; Tomás, V.; Martín, J. Flow injection determination of vitamin K3 by a photoinduced chemiluminescent reaction. Analyst 1999, 124, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.M.H. Potentiometric determination of Menadione (Vitamin K3). Microchim. Acta 2002, 138, 53–58. [Google Scholar] [CrossRef]
- Gaikwad, M.N.; Gaikwad, S.T.; Rajbhoj, A.S. Potentiometric study of vitamin K3 complexes with transition metals in methanol–water and acetonitrile–water medium. Int. J. ChemTech Res. 2012, 4, 1392–1395. [Google Scholar]
- Laffi, R.; Marchetti, S.; Marchetti, M. Normal-phase liquid chromatographic determination of menadione in animal feeds. J. Assoc. Off. Anal. Chem. 1988, 71, 826–828. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; García, M.A.; Martín, J. High-performance liquid chromatography: Photochemical reduction in aerobic conditions for determination of K vitamins using fluorescence detection. J. Chromatogr. A 2007, 1141, 67–72. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Li, J.; Wang, E. Detection of menadione sodium bisulfite (vitamin K3) by reversed-phase high performance liquid chromatography with series dual-electrode amperometric detector. Anal. Chim. Acta 1997, 338, 57–62. [Google Scholar] [CrossRef]
- Speek, A.J.; Schrijver, J.; Schreurs, W.H. Fluorimetric determination of menadione sodium bisulphite (vitamin K3) in animal feed and premixes by high-performance liquid chromatography with post-column derivatization. J. Chromatogr. 1984, 301, 441–447. [Google Scholar] [CrossRef]
- Saleh, E.M.; Chikako, S.; Naoya, K.; Kaname, O.; Mitsuhiro, W.; Naotaka, K. Development and Validation of the First Assay Method Coupling Liquid Chromatography with Chemiluminescence for the Simultaneous Determination of Menadione and Its Thioether Conjugates in Rat Plasma. Chem. Res. Toxicol. 2013, 26, 1409–1417. [Google Scholar] [CrossRef]
- Maya, K.; Yoshihisa, H.; Yoshitomo, S.; Naoko, T.; Kimie, N.; Toshio, O.; Hiroshi, H. Determination of Menadione by Liquid Chromatography–Tandem Mass Spectrometry Using Pseudo Multiple Reaction Monitoring. Anal. Sci. 2017, 33, 863–867. [Google Scholar]
- Castello, G.; Bruschi, E.; Ghelli, G. Gas chromatographic determination of the purity of vitamin K3 (menadione). J. Chromatogr. 1977, 139, 195–202. [Google Scholar] [CrossRef]
- Demirkaya-Miloglu, F.; Kadioglu, Y.; Senol, O. GC-FID and HPLC-DAD Methods for the Determination of Menadione Sodium Bisulphite Directly and by Converting Menadione Sodium Bisulphite to Menadione in Pharmaceutical Preparation. Iran J. Pharm. Res. 2014, 13, 353–364. [Google Scholar] [PubMed]
- Sams, M.J.; Strutt, P.R.; Barnes, K.A.; Damant, A.P.; Rose, M.D. Determination of dimetridazole, ronidazole and their common metabolite in poultry muscle and eggs by high performance liquid chromatography with UV detection and confirmatory analysis by atmospheric pressure chemical ionisation mass spectrometry. Analyst 1998, 123, 2545–2549. [Google Scholar] [CrossRef]
- Buizer, F.G.; Severijnen, M. Determination of dimetridazole in feedstuffs and pre-mixes by high-speed liquid chromatography. Analyst 1975, 100, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Liu, J.-Y.; Zhou, S.-P. Study on the determination of dimetrionidazole using a carbon paste electrode. Fenxi Shiyanshi 1998, 17, 40–43. [Google Scholar]
- Stubbings, G.; Bigwood, T. The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC-MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a Que Chers (Quick, Easy, Cheap, Effective, Rugged and Safe) approach. Anal. Chim. Acta 2009, 637, 68–78. [Google Scholar]
- Gentili, A.; Perret, D.; Marchese, S. Liquid chromatography-tandem mass spectrometry for performing confirmatory analysis of veterinary drugs in animal-food products. TrAC Trends Anal. Chem. 2005, 24, 704–733. [Google Scholar] [CrossRef]
- Cannavan, A.; Kennedy, D.G. Determination of dimetridazole in poultry tissues and eggs using liquid chromatography-thermospray mass spectrometry. Analyst 1997, 122, 963–966. [Google Scholar] [CrossRef]
- Miho, S.; Kazue, T.; Takeo, S.; Tomoko, K.; Hiroshi, H.; Setsuko, K.; Maki, K.; Toshihiro, N. Determination of Dimetridazole, Metronidazole and Ronidazole in Salmon and Honey by Liquid Chromatography Coupled with Tandem Mass Spectrometry. Food Hygiene Saf. Sci. 2011, 52, 51–58. [Google Scholar][Green Version]
- Petrovic, M.; Skrbic, B.; Zivancev, J.; Ferrando-Climent, L.; Barcelo, D. Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole linear ion trap in different types of water in Serbia. Sci. Total Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Sin, D.W.M.; Wong, K.M.; Tang, H.P.O. Determination of dimetridazole and metronidazole in poultry and porcine tissues by gas chromatography electron capture negative ionization mass spectrometry. Anal. Chim. Acta 2005, 530, 23–31. [Google Scholar] [CrossRef]
- Wang, J.H. Determination of three nitroimidazole residues in poultry meat by gas chromatography with nitrogen phosphorus detection. J. Chromatogr. A 2001, 918, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.D. Determination of Dimetridazole and Ipronidazole in Feeds at Cross-Contamination Levels. J. Assoc. Off. Anal. Chem. 1988, 71, 474–477. [Google Scholar] [CrossRef]
- Thompson, C.S.; Traynor, I.M.; Fodey, T.L.; Crooks, S.R. Improved screening method for the detection of a range of nitroimidazoles in various matrices by optical biosensor. Anal. Chim. Acta 2009, 637, 259–264. [Google Scholar] [CrossRef]
- Jallal, Z.; Yassine, E.; Chaimae, R.; Nadia, B.; Abderrahim, I.; Idriss, B.; Ali, A. Electrocatalytic detection of dimetridazole using an electrochemical sensor Ag. Anal. Appl. Milk, tomato juice and human urine. Sens. Int. 2021, 2, 100105. [Google Scholar]
- Rami Reddy, Y.V.; Rajendra Kumar Reddy, P.; Suresh Reddy, C.; Jayarama Reddy, S. Polarographic determination of nitro group containing drugs in tablets. Indian J. Pharm. Sci. 1996, 58, 96–99. [Google Scholar]
- Zhang, J.; Chen, Z.P.; Tang, S.; Luo, X.G.; Xi, J.B.; He, Z.L.; Yu, J.X.; Wu, F.S. Fabrication of porphyrin-based magnetic covalent organic framework for effective extraction and enrichment of sulfonamides. Anal. Chim. Acta 2019, 1089, 66–77. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, W.; Zhang, Y.; Dai, Y.; Wang, W.; Wang, A. Determination of sulfadimethoxine in milk with aptamer-functionalized Fe3O4/graphene oxide as magnetic solid-phase extraction adsorbent prior to HPLC. J. Sep. Sci. 2020, 43, 3499–3508. [Google Scholar] [CrossRef]
- Mengelers, M.J.; Oorsprong, M.B.; Kuiper, H.A.; Aerts, M.M.; van Gogh, E.R.; van Miert, A.S. Determination of sulfadimethoxine, sulfamethoxazole, trimethoprim and their main metabolites in porcine plasma by column switching HPLC. J. Pharm. Biomed. Anal. 1989, 7, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Boison, J.O.; Keng, L.J. Determination of sulfadimethoxine and sulfamethazine residues in animal tissues by liquid chromatography and thermospray mass spectrometry. J. AOAC Int. 1995, 78, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Primus, T.M.; Jojola, S.M.; Robinson, S.J.; Johnston, J.J. Determination of Sulfadimethoxine Residues in Skunk Serum by HPLC. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2095–2102. [Google Scholar] [CrossRef]
- Dos Santos, L.F.; de Andrade, L.R.; Cardoso, T.F.; Caminha, K.B.; Pires, D.S.; Machado, E.P.; Severino, P.; do Amaral, M.S.; Souto, E.B.; Kassab, N.M. Innovative UV derivative spectrophotometric method for simultaneous quantification of sulfadimethoxine and metronidazole. J. Indian Chem. Soc. 2025, 102, 101655. [Google Scholar] [CrossRef]
- Ghanem, M.M.; Abu-Lafi, S.A. Validation of a stability-indicating RP-HPLC method for the simultaneous determination of trimethoprim and sulfadimethoxine sodium in oral liquid dosage form. Sci. Pharm. 2013, 81, 459. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Gamal, M. Critical analytical review: Rare and recent applications of refractive index detector in HPLC chromatographic drug analysis. Microchem. J. 2022, 178, 107339. [Google Scholar] [CrossRef]
- Ali, H.M.; Gamal, M.; Ghoneim, M.M.; Abd Elhalim, L.M. Quantitative Analysis of Abamectin, Albendazole, Levamisole HCl and Closantel in Q-DRENCH Oral Suspension Using a Stability-Indicating HPLC-DAD Method. Molecules 2022, 27, 764. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation (ICH). ICH Harmonised Guideline Q2(R2): Validation of Analytical Procedures; ICH: Geneva, Switzerland, 2023. [Google Scholar]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine: N -butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef]
- Abdelrahman, M.M. Green Analytical Chemistry Metrics and Life-Cycle Assessment Approach to Analytical Method Development. In Green Chemical Analysis and Sample Preparations; Springer: Cham, Switzerland, 2022; pp. 29–99. [Google Scholar] [CrossRef]
- Wells, M.; Dantus, M. Validation of chromatographic methods. In Ewing’s Analytical Instrumentation Handbook, 3rd ed.; Taylor and Fransis: Abingdon, UK, 2004; pp. 1015–1033. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A new tool for evaluating and/or selecting analytical methods: Summarizing the information in a hexagon. TrAC Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]

| Stationary phase | Supelcosil C18 (I.D 4.6 mm—length 25 cm—particle size 5 µ) | ||
| Mobile phase | 0.05 M KH2PO4: Acetonitrile (80:20 v/v) | ||
| Detector | At 260 nm—UV | ||
| Pumping sytem | Isocratic | ||
| Temperature | Ambient | ||
| The volume of Injection | 10 µL | ||
| Flow rate | 2.0 mL.min−1 | ||
| Full run time | 8 min | ||
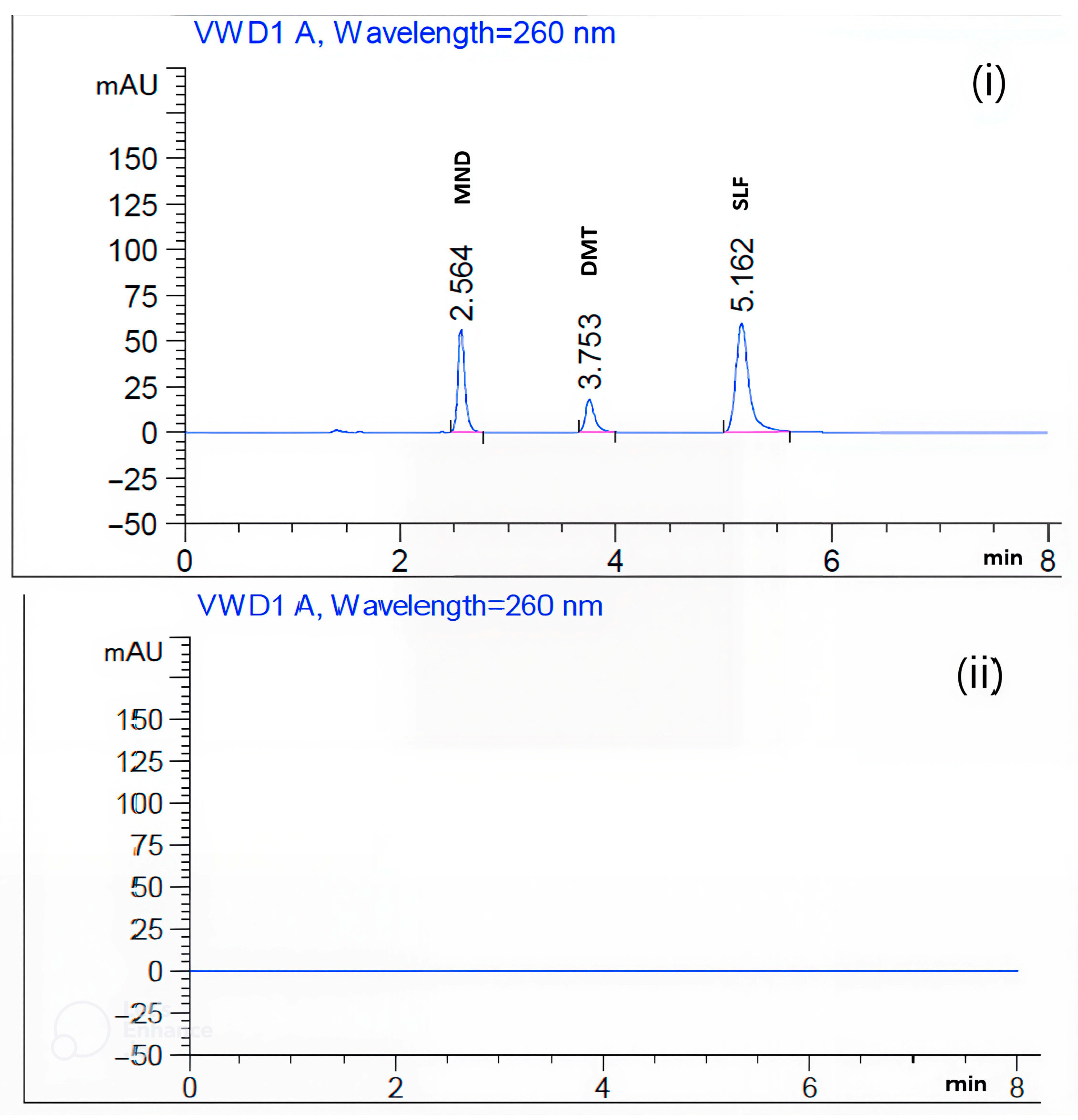
| Retention times | MND: 2.56 min | DMT: 3.75 min | SLF: 5.16 min |
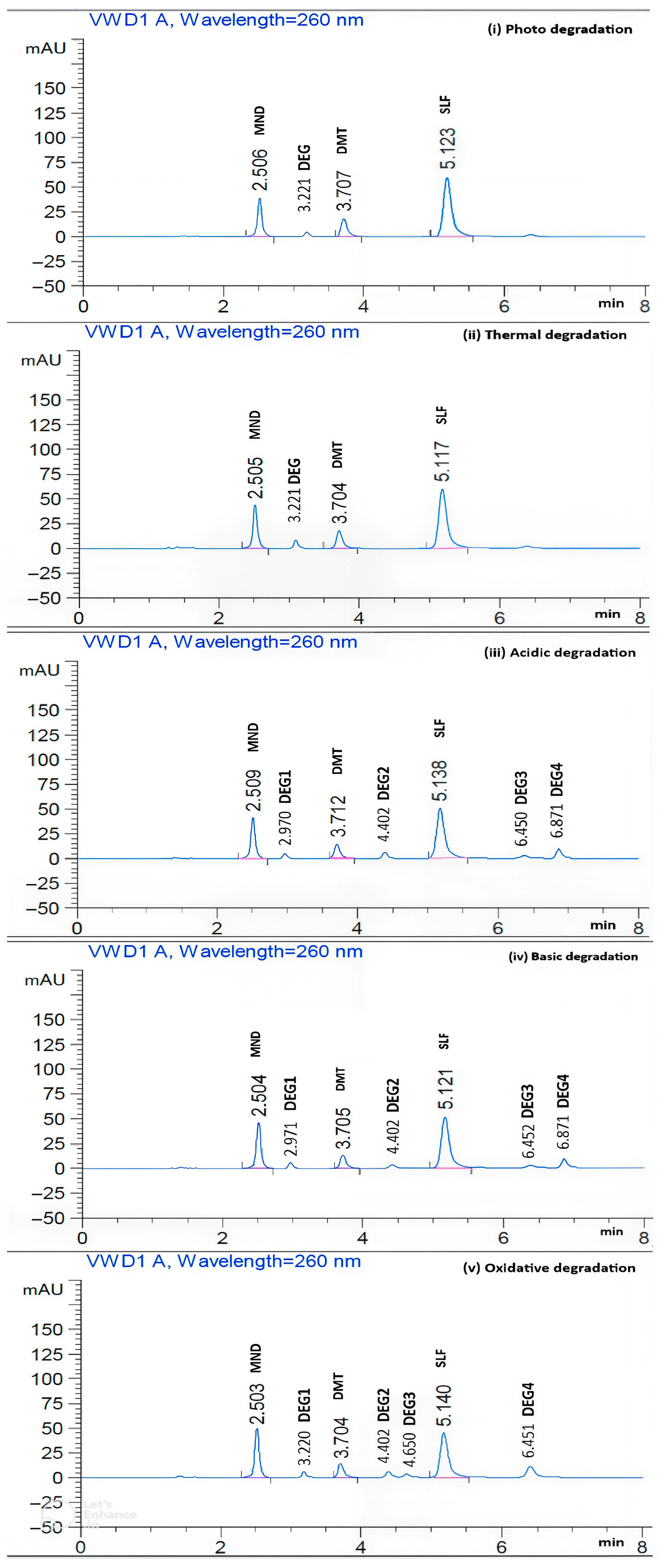
| Acidic and basic degradation products (2.97, 4.40, 6.45, and 6.87 min) Oxidative degradation products (3.22, 4.40, 4.65, and 6.45 min) Photo and thermal degradation products (3.22 min) | |||
| Validation Criteria | Values Measured | Approved Criteria That Follow the Guidelines of the ICH [59] | ||
|---|---|---|---|---|
| MND | DMT | SLF | ||
| Linearity and Range (Five concentration levels) | 10.0 to 30.0 µg.mL−1 r = 0.99936 | 20.0 to 60.0 µg.mL−1 r = 0.99998 | 20.0 to 60.0 µg.mL−1 r = 0.99999 | r ≥ 0.99 |
| Precision (for 6 replicates) | 0.19 | 0.71 | 0.47 | RSD ≤ 2% |
| Accuracy | 99.59% ±1.076 | 100.07% ±0.246 | 99.52% ±0.626 | 100 ± 2% |
| Specificity/Selectivity | Distinct peak for MND at a finer resolution than the other peaks for degradation products | Distinct peak for DMT at a finer resolution than the other peaks for degradation products | Distinct peak for SLF at a finer resolution than the other peaks for degradation products | There was no interference detected |
| Limit of Detection | 1.07 µg.mL−1 | 0.40 µg.mL−1 | 0.20 µg.mL−1 | Using the formula: 3.3 × SD/a (where SD is the response standard deviation and a is the calibration curve’s slope) |
| Limit of Quantitation | 3.26 µg.mL−1 | 1.22 µg.mL−1 | 0.61 µg.mL−1 | Using the formula: 10 × SD/a |
| Ruggedness | 2.41 (distinguished days) 0.66 (distinguished analysts) | 0.54 (distinguished days) 0.20 (distinguished analysts) | 1.92 (distinguished days) 0.64 (distinguished analysts) | Every alteration should have a pooled RSD of less than 3% |
| Robustness (modifies a few aspects of the mobile phase) | 0.64 | 0.06 | 0.39 | Every alteration should have a pooled RSD of less than 3% |
| Replicate | MND | DMT | SLF | Standards for Approval | |||
|---|---|---|---|---|---|---|---|
| Case One * | Case Two ** | Case One * | Case two ** | Case One * | Case Two ** | ||
| One | 241.47 | 239.09 | 107.69 | 107.79 | 512.68 | 515.01 | |
| Two | 240.47 | 237.94 | 107.62 | 107.71 | 512.80 | 516.32 | |
| Three | 241.04 | 238.05 | 107.63 | 107.74 | 513.03 | 517.40 | |
| Pooled average | 239.71 | 107.70 | 514.51 | ||||
| Pooled SD | 1.51 | 0.10 | 2.01 | ||||
| Pooled RSD | 0.64 | 0.06 | 0.39 | Equal or less than 3% | |||
| Parameters of System Suitability | Information Obtained from the Anticipated Technique | Reference Values [59] | |||
|---|---|---|---|---|---|
| MND | MT | SLF | |||
| Retention Time (Rt) ± SD | 2.56 ± 0.04 | 3.75 ± 0.02 | 5.16 ± 0.03 | More than 1 | |
| Capacity Factor (K) | 1.844 | 3.166 | 4.733 | 1–10 Good | |
| Theoretical Plate No. (N) | 8126 | 10,548 | 10,401 | As its worth rises, so does its efficacy | |
| HETP = height equivalent to theoretical plate (cm per plate) | 0.0031 | 0.0024 | 0.0024 | As the HETP score falls, column effectiveness rises | |
| Tailing Factor (T) | 0.77 | 0.69 | 0.71 | Less than or equal 2 | |
| Resolution factor (Rs) | 9.13 | 8.08 | More than or equal 1.5 | ||
| Selectivity factor (α) | 1.46 | 1.38 | More than 1 | ||
| Method of Degradations | A Description of the Circumstances | Area | Degradation % (Mean ± SD, n = 3) | ||||
|---|---|---|---|---|---|---|---|
| MND | DMT | SLF | MND | DMT | SLF | ||
| Light * | Light (48 h)/ UV (12 h) | 184.02 | 108.39 | 502.81 | 26.52 ± 1.04 | 0.00 ± 0.96 | 1.36 ± 1.08 |
| Heat | 80 °C (8 h) | 220.42 | 106.97 | 508.56 | 11.99 ± 1.64 | 0.79 ± 1.37 | 0.23 ± 1.18 |
| Acid | 1 N HCl/80 °C (1 h) | 213.60 | 91.07 | 441.52 | 14.71 ± 0.99 | 15.54 ± 1.10 | 13.39 ± 1.21 |
| Base | 1 N NaOH/80 °C (1 h) | 222.51 | 85.06 | 441.63 | 11.15 ± 0.89 | 21.12 ± 1.05 | 13.36 ± 0.94 |
| Oxidation | 0.50% H2O2/80 °C (1 h) | 226.46 | 85.63 | 371.33 | 9.58 ± 0.99 | 20.58 ± 1.12 | 27.16 ± 1.06 |
| Approaches | The Matrix and Merits | Quantification Constraints | Easy to Use and Reasonably Priced | Run Time | References |
|---|---|---|---|---|---|
| HPLC | Menadione assay in animal feed | 1.00 µg.g−1 | Pricey procedures for extraction are employed; assay MND only in the absence of DMT and SLF | 11 min | (Speek et al., 1984) [31] |
| HPLC | Menadione and Its Thioether Conjugates assay in Rat Plasma | 10.00 nMolar | Pricey procedures for extraction are employed; assay MND only in the absence of DMT and SLF | 9 min | (Saleh et al., 2013) [32] |
| LC-MS | Menadione assay using pseudo multiple reaction monitoring | 1.70 ng.mL−1 | Pricey procedures for extraction are employed; assay MND only in the absence of DMT and SLF | 14 min | (Maya et al., 2017) [33] |
| GC and HPLC | Menadione assay in Pharmaceutical Preparation | 0.50 µg.mL−1 | Pricey procedures for extraction are employed; assay MND only in the absence of DMT and SLF | 14 min for HPLC and GC | (Demirkaya et al., 2014) [35] |
| HPLC | Dimetridazole, ronidazole and their common metabolite assay in poultry muscle and eggs | 1.50 µg.kg−1 | Pricey procedures for extraction are employed; assay DMT only in the absence of MND and SLF | 12 min | (Sams et al., 1998) [36] |
| HPLC | Dimetridazole assay in feedstuffs | 150 mg.kg−1 | Pricey procedures for extraction are employed; assay DMT only in the absence of MND and SLF | 15 min | (Buizer et al., 1975) [37] |
| LC-MS | Dimetridazole assay in poultry tissues and eggs using | 5.00 ng.g−1 | Pricey procedures for extraction are employed; assay DMT only in the absence of MND and SLF | 15 min | (Cannavan et al., 1997) [41] |
| LC-MS | Dimetridazole, metronidazole and ronidazole assay in salmon and honey | 1.50 µg.kg−1 | Pricey procedures for extraction are employed; assay DMT only in the absence of MND and SLF | 20 min | (Miho et al., 2011) [42] |
| Polarography | Dimetridazole and nimesulide assay in tablets | 25.00 µg.mL−1 | Dropping mercury electrode is used which is poisonous, Surface area of a drop of mercury is never constant and applied voltage produces changes in surface tension and hence change in drop size. | 5 min | (RAMI REDDY et al., 1996) [49] |
| HPLC | Sulfadimethoxine assay in milk using Fe3O4/graphene oxide as adsorbent | 5.00 µg.L−1 | Pricey procedures for extraction are employed; assay SLF only in the absence of MND and DMT | 5 min | (Yinan et al., 2020) [51] |
| HPLC | Sulfadimethoxine, sulfamethoxazole, and trimethoprim assay in porcine plasma | 25.00 ng.mL−1 | Pricey procedures for extraction are employed; assay SLF only in the absence of MND and DMT | 15 min | (Mengelers et al., 1989) [52] |
| HPLC | Sulfadimethoxine and sulfamethazine residues assay in animal tissues | 6.00 ng.g−1 | Pricey procedures for extraction are employed; assay SLF only in the absence of MND and DMT | 10 min | (Boison et al., 1995) [53] |
| HPLC | Sulfadimethoxine residues assay in skunk serum | 0.10 µg.mL−1 | Pricey procedures for extraction are employed; assay SLF only in the absence of MND and DMT | 14 min | (Primus et al., 2007) [54] |
| Spectrophotometric | sulfadimethoxine and metronidazole in commercial veterinary tablets | 0.51 µg.L−1 | assay SLF only in the absence of MND and DMT | 15 min | (dos Santos et al., 2025) [55] |
| RP- HPLC | Trimethoprim and Sulfadimethoxine Sodium in Oral Liquid Dosage Form | 3.3 μg/mL | Pricey procedures for extraction are employed; assay SLF only in the absence of MND and DMT | 14 min | (Ghanem et al., 2013) [56] |
| HPLC-DAD | Considerably green technique for both genuine powder and powder oral solutions analysis and stability illustration | 10.00 µg.mL−1 for MND 20.00 µg.mL−1 for DMT and SLF | Affordable and does not need any special extraction techniques | 8 min | Current methodology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khateeb, L.A.; Elsayed, M.A.; Tony, R.M.; Gamal, M. Assay of Two Antibacterial/Anticoccidial Drugs in Combination with Vitamin K3 for Oral Solutions: Stability Studies and Method Development Using HPLC-DAD: Appraisal of the Method’s Eco-Friendliness and Functionality. Chemosensors 2025, 13, 406. https://doi.org/10.3390/chemosensors13120406
Al-Khateeb LA, Elsayed MA, Tony RM, Gamal M. Assay of Two Antibacterial/Anticoccidial Drugs in Combination with Vitamin K3 for Oral Solutions: Stability Studies and Method Development Using HPLC-DAD: Appraisal of the Method’s Eco-Friendliness and Functionality. Chemosensors. 2025; 13(12):406. https://doi.org/10.3390/chemosensors13120406
Chicago/Turabian StyleAl-Khateeb, Lateefa A., Mohamed Ahmed Elsayed, Rehab Moussa Tony, and Mohammed Gamal. 2025. "Assay of Two Antibacterial/Anticoccidial Drugs in Combination with Vitamin K3 for Oral Solutions: Stability Studies and Method Development Using HPLC-DAD: Appraisal of the Method’s Eco-Friendliness and Functionality" Chemosensors 13, no. 12: 406. https://doi.org/10.3390/chemosensors13120406
APA StyleAl-Khateeb, L. A., Elsayed, M. A., Tony, R. M., & Gamal, M. (2025). Assay of Two Antibacterial/Anticoccidial Drugs in Combination with Vitamin K3 for Oral Solutions: Stability Studies and Method Development Using HPLC-DAD: Appraisal of the Method’s Eco-Friendliness and Functionality. Chemosensors, 13(12), 406. https://doi.org/10.3390/chemosensors13120406






