Abstract
Magnesia-dolomite refractories have emerged as sustainable alternatives to traditional carbon- or chromium-containing linings in steelmaking and cement industries. Their outstanding thermochemical stability, high refractoriness, and strong basic slag compatibility make them suitable for converters, electric arc furnaces (EAF), and argon–oxygen decarburization (AOD) units. However, their practical application has long been constrained by hydration and thermal shock sensitivity associated with free CaO and open porosity. Recent advances, including optimized raw material purity, fused co-clinker synthesis, nano-additive incorporation (TiO2, MgAl2O4 spinel, FeAl2O4), and improved sintering strategies, have significantly enhanced density, mechanical strength, and hydration resistance. Emerging technologies such as co-sintered magnesia–dolomite composites and additive-assisted microstructural tailoring have enabled superior corrosion resistance and extended service life. This review provides a comprehensive analysis of physicochemical mechanisms, processing routes, and industrial performance of magnesia–dolomite refractories, with special emphasis on their contribution to technological innovation, decarbonization, and circular economy strategies in high-temperature industries.
1. Introduction
Refractory materials have played a pivotal role in the progress of human civilization, supporting the technological development of ceramic, metallurgical, and glass industries since antiquity. They are inorganic, non-metallic materials designed to withstand extreme conditions of temperature, mechanical load, and chemical corrosion without undergoing structural or compositional degradation [1]. These materials are essential components in the operation of furnaces, converters, boilers, and other industrial equipment exposed to severe thermal and chemical environments. By maintaining structural integrity and thermal insulation under such conditions, refractories ensure the operational efficiency, safety, and longevity of high-temperature processes that sustain modern industry [2].
Refractory materials are broadly classified according to their chemical composition (acidic, basic, and neutral or special), manufacturing process (sintered or fused), form of implementation (shaped or unshaped), and porosity (dense or porous) [3,4]. Regardless of classification, their functional performance depends on their microstructure and phase assemblage, particularly the presence of highly refractory phases such as mullite, corundum, periclase, doloma, spinel, and alumina [5]. Structurally, refractories are composed of four main elements: coarse aggregates (1–2.5 mm), fine fillers (<150 µm), binders or cements, and a network of pores that influence mechanical and thermal behavior [6].
Among the various families, basic refractories mainly based on magnesia (MgO), dolomite (CaMg(CO3)2), forsterite, and spinel are indispensable due to their high melting temperatures (≥2000 °C), strong chemical stability in basic atmospheres, and excellent resistance to lime- and iron-rich slags [7,8]. They are extensively used for lining primary and secondary steelmaking furnaces, converters, ladles, and kilns, where extreme thermal and chemical stresses prevail [9]. Magnesia-based refractories have long been recognized for their exceptional thermal stability (melting point ≈ 2800 °C), resistance to basic slags, and mechanical integrity at elevated temperatures. The raw materials for magnesia production are derived from natural magnesite ores or synthesized from seawater and inland brines through precipitation and calcination processes [10,11]. Depending on the production route, magnesia may be sintered (dead-burned) or fused (electrocast), the latter conferring enhanced purity and microstructural homogeneity. These characteristics make magnesia refractories indispensable in steel, cement, and glass industries, where they serve as primary linings and thermal barriers [12].
Dolomite-based refractories derived from the calcination of dolomite into doloma (CaO + MgO) have attracted attention due to their thermodynamic compatibility with steelmaking slags, cost-effectiveness, and abundant availability. The CaO–MgO system exhibits an eutectic temperature near 2370 °C, endowing doloma with remarkable refractoriness [13,14]. However, the hydration susceptibility of doloma, particularly of its free lime content, remains a key limitation influencing handling, storage, and performance. Advances in sintering, impurity control, and microstructural stabilization have led to the development of improved doloma refractories with enhanced hydration resistance and densification behavior [15,16].
Within basic refractories, magnesia–dolomite (MgO-CaO) refractories have gained increasing relevance as high-performance and environmentally sustainable alternatives to conventional magnesia–chrome or magnesia–carbon systems. Their advantages include high refractoriness, strong resistance to basic slags, and the absence of chromium compounds, which eliminates toxic emissions associated with Cr6+ during service or disposal [17,18]. This aspect aligns closely with the global industrial transition toward cleaner production, circular economy strategies, and lower carbon footprints. However, the practical application of magnesia–dolomite refractories is not without challenges. Their main limitation lies in the susceptibility to hydration caused by the presence of free lime (CaO), leading to volumetric expansion, microcracking, and loss of mechanical integrity during storage or exposure to moisture and water vapor at service conditions [19,20]. In addition, these materials exhibit moderate thermal shock resistance, tendency to form low-melting phases in contact with Al2O3- or SiO2-rich slags, and difficulties in achieving a dense, homogeneous microstructure that guarantees long-term stability. Further constraints arise from the high cost of functional additives (TiO2, ZrO2, Al2O3, Fe2O3) and the limited industrial validation of laboratory-scale innovations, which still hinder large-scale deployment [18].
Historically, calcined dolomite refractories (doloma bricks) were first introduced in steelmaking furnaces and LD converters during the mid-20th century because of their ability to form protective lime-rich layers that enhance slag resistance and extend lining life. Despite these advantages, their widespread use was initially restricted by poor hydration resistance and short service life, prompting the development of hybrid magnesia–dolomite compositions with improved chemical and mechanical stability. The combination of MgO and CaO phases offers an optimal balance between refractoriness, slag compatibility, and cost-effectiveness, making MgO–CaO systems particularly attractive for modern steelmaking and cement industries [21,22].
Industrial validation of magnesia–dolomite refractory innovations faces several interconnected challenges that hinder the transition from laboratory-scale research to industrial implementation. Scale-up often introduces inconsistencies in sintering temperature control, raw material uniformity, and process atmosphere, affecting product reproducibility and quality [14]. Moreover, the high energy requirements of high-temperature processing increase production costs and carbon emissions, limiting rapid adoption [11]. The lack of pilot-scale facilities and the stringent standards for quality certification further constrain technology transfer. Overcoming these barriers demands stronger collaboration between academia and industry, focused investment in pilot-scale validation, and the development of scalable, energy-efficient production routes for advanced MgO–CaO refractories [11,14].
In recent decades, the design of magnesia–dolomite refractories has evolved from empirical formulation toward scientific material engineering, supported by phase diagram analysis, thermodynamic modeling, and advanced sintering technologies. The incorporation of functional additives, nanostructured oxides (nano-Al2O3), and in situ spinel formation mechanisms has markedly enhanced hydration resistance, densification kinetics, and thermal shock tolerance [23]. Emerging processing technologies—such as microwave sintering, spark plasma sintering, and additive manufacturing—offer new routes to control grain boundary chemistry and minimize energy consumption. These innovations have led to magnesia–dolomite refractories with higher chemical purity, finer microstructural control, and improved corrosion resistance, enabling their use in increasingly demanding thermal environments [18,24].
Beyond property optimization, sustainability considerations are reshaping refractory development. Novel approaches now emphasize recycling spent refractories, reducing CO2 emissions during MgO production, and reusing industrial by-products as raw materials, consistent with the broader principles of circular economy and resource efficiency. These strategies not only improve environmental performance but also contribute to lowering production costs and conserving critical mineral resources [25,26].
The present review aims to provide a comprehensive and critical analysis of advances in magnesia–dolomite refractories, encompassing their microstructural evolution, thermochemical behavior, mechanical and corrosion resistance, and industrial applications. Special emphasis is placed on recent technological developments and sustainability-driven innovations, including nanoparticle engineering, additive incorporation, sintering optimization, and recycling routes. The review also discusses current challenges and future prospects for magnesia–dolomite systems in next-generation high-temperature processes, particularly in steelmaking and cement manufacturing, where energy efficiency, emission reduction, and durability are strategic priorities.
2. Materials and Methods
2.1. Criteria for Selection and Exclusion of Research
To ensure transparency, rigor, and replicability in this systematic review, a clearly structured protocol was adopted. Systematic reviews require methodological precision and consistent criteria to evaluate the relevance and scientific quality of the included studies. In the absence of such rigor, many reviews risk producing incomplete or unreliable conclusions. Therefore, a strict set of inclusion and exclusion criteria was designed to preserve the thematic alignment and academic robustness of this research.
The review followed a structured methodology consistent with the PRISMA statement, ensuring transparency and reproducibility in the selection and synthesis of the scientific evidence. A comprehensive search and screening process was conducted across Scopus, ScienceDirect, SpringerLink, and MDPI databases, prioritizing recent advances published between 2010 and 2025. The structure and inclusion criteria were aligned with previously validated systematic approaches reported in related research (Retrieved from https://doi.org/10.17605/OSF.IO/DCHPW).
The study focuses on magnesia–dolomite refractories, with particular attention to their mechanical performance, thermal shock resistance, corrosion behavior, slag resistance, hydration resistance, and sustainability aspects. In this context, sustainability was understood to include energy efficiency during processing, the utilization of recycled or secondary raw materials, CO2 emission reduction strategies, life-cycle and circular economy approaches, and resource optimization within refractory production. Based on this research scope, the following criteria were established:
- Keyword Focus—Selected studies must explicitly address refractory systems based on magnesia, dolomite, or basic MgO–Dolomite compositions, combined with technical properties such as mechanical performance, thermal shock resistance, corrosion resistance, or recycling.
- Publication Date Range—Only publications between 2018 and 2025 were considered, ensuring a current perspective on recent advances.
- Subject Area Filtering—To maintain disciplinary focus, publications from non-engineering or non-materials domains such as dentistry, sociology, agriculture, mathematics, and business were excluded.
- Document Type—Only peer-reviewed journal articles in English were included. Non-article types such as patents, proceedings, book chapters, and technical reports were excluded to ensure consistency in the scientific rigor of the dataset.
- Thematic Relevance—Papers unrelated to refractory performance or focused on unrelated technologies (fuel cells, membranes, semiconductors, thin films, or medical applications) were excluded, as these do not align with the structural and thermomechanical objectives of this study.
- Final Search Code:
- The search string used was the following Listing 1:
| Listing 1. Scopus search string for magnesia—dolomite refractory materials |
| TITLE-ABS-KEY("magnesia refractory*" OR "dolomite refractory*" OR "basic refractory*" OR "MgO-CaO*") AND TITLE-ABS-KEY("mechanical properties" OR "thermal shock" OR "corrosion resistance" OR "slag resistance" OR "hydration resistance" OR "sustainability" OR "recycling") AND PUBYEAR > 2017 AND PUBYEAR < 2026 AND ( LIMIT-TO ( DOCTYPE,"ar" ) OR LIMIT-TO ( DOCTYPE,"English" ) OR EXCLUDE ( DOCTYPE,"p" ) OR EXCLUDE ( DOCTYPE,"k" ) OR EXCLUDE ( DOCTYPE,"d" ) OR EXCLUDE ( DOCTYPE,"Fuel Cells" ) OR EXCLUDE ( DOCTYPE,"Membranes" ) OR EXCLUDE ( DOCTYPE,"Semiconductors" ) OR EXCLUDE ( DOCTYPE,"Catalysts" ) OR EXCLUDE ( DOCTYPE,"Medical Applications" ) OR EXCLUDE ( DOCTYPE,"Thin Films" ) ) AND ( EXCLUDE ( SUBJAREA,"DENT" ) OR EXCLUDE ( SUBJAREA,"SOCI" ) OR EXCLUDE ( SUBJAREA,"AGRI" ) OR EXCLUDE ( SUBJAREA,"MATH" ) OR EXCLUDE ( SUBJAREA,"BUSI" ) ) |
2.2. Analysis Guide for Systemic Review of MgO–Dolomite Refractory Research
The analysis process also followed a three-phase framework adapted for the systematic review of basic refractories, specifically MgO–Dolomite systems. As illustrated in Figure 1, the initial search in Scopus retrieved 5215 articles. After refining the period of interest to 2017–2026 and restricting the scope to materials science and ceramic engineering, the dataset was reduced to 1567 articles. Finally, by applying strict inclusion and exclusion criteria focused on magnesia–dolomite refractory materials, 189 articles were selected for in-depth analysis.
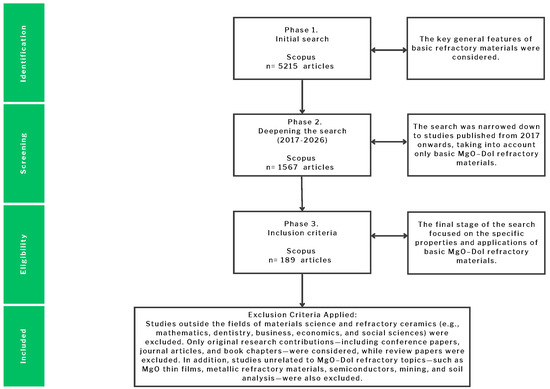
Figure 1.
Flow chart of the selection and exclusion process applied in the systematic review of magnesia–dolomite refractory materials.
This procedure ensured that the reviewed publications were aligned with the structural, thermomechanical, and corrosion-resistance aspects that directly influence the performance of basic refractories in high-temperature industrial applications. The progressive filtering process excluded studies outside of the core disciplinary scope, such as mathematics, dentistry, business, economics, and social sciences. Moreover, topics unrelated to MgO–Dolomite refractories (Thin films, metallic refractory alloys, semiconductors, mining, and soil analysis) were systematically removed to guarantee thematic relevance.
The final selection emphasizes research that investigates mechanical properties, hydration resistance, slag and corrosion resistance, thermal shock performance, and sustainability approaches such as recycling. Only original contributions—including journal articles, conference papers, and book chapters—were considered, while review papers were excluded to preserve methodological consistency and to focus on primary evidence.
2.3. Bibliometric Trends in Magnesia–Dolomite Refractory Research
Figure 2 illustrates the bibliometric network generated with VOSviewer (1.6.20), providing a comprehensive visualization of the co-occurrence of keywords associated with refractory materials. The color scale reflects the temporal distribution of research activity, thereby allowing a critical interpretation of how the scientific focus in this field has undergone significant evolution over the last decade.
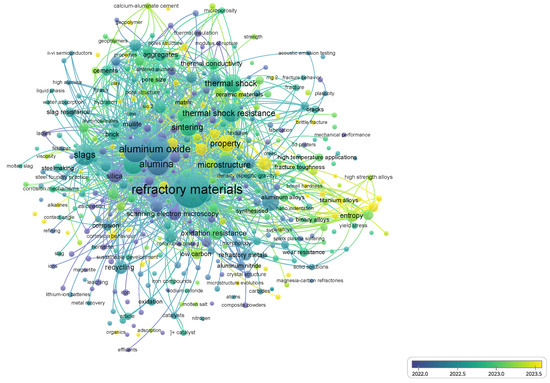
Figure 2.
Network visualization map of co-occurring keywords in refractory research, generated with VOSviewer. The color scale indicates the temporal evolution of research trends, highlighting the transition from early studies on microstructural characterization to sustainability-driven solutions and the incorporation of advanced processing technologies.
In the initial stage (before 2018), the dominant terms were strongly linked to fundamental aspects of refractory science, such as pore structure, hydration, calcination, and mechanical performance. These keywords indicate a period when research efforts were primarily focused on gaining a basic understanding of microstructural development, hydration resistance, and the mechanical characterization of bricks for conventional industrial use. This foundational stage was essential for establishing correlations between raw materials, processing routes, and intrinsic performance indicators.
From 2018 onward, the network shows a pronounced transition toward sustainability and environmental awareness. The increasing prominence of terms such as recycling, sustainable development, geopolymers, and effluents evidences the growing interest in minimizing the ecological footprint of refractory materials. This trend is consistent with global pressures for circular economy approaches, where the valorization of spent refractories, the substitution of carbon-intensive raw materials, and the integration of alternative binders (e.g., calcium aluminate cements or geopolymer matrices) are emerging as strategic research avenues. For magnesia–dolomite refractories in particular, such developments represent a paradigm shift from mere durability optimization to the design of systems that are both technically effective and environmentally responsible.
In the most recent period (2020–2025), the bibliometric map highlights the emergence of advanced processing technologies and performance-driven approaches. Notable keywords such as spark plasma sintering, 3D printing, nanoindentation, and acoustic emission testing illustrate a clear transition from conventional manufacturing to cutting-edge methods. These techniques enable a finer control over densification, microstructural tailoring, and in situ performance monitoring. Furthermore, the coupling of such innovations with computational modeling and real-time sensing aligns with the development of “smart refractories” that can withstand aggressive environments, improve thermal shock resistance, and enhance corrosion resistance under steelmaking conditions. This evolution demonstrates that the field has moved beyond classical material testing toward a more integrated framework that combines experimental, computational, and digital approaches.
Taken together, the observed keyword trends confirm the maturation of refractory research into a multidisciplinary domain that bridges traditional material science with sustainability imperatives and advanced technological solutions. For magnesia–dolomite refractories, this trajectory underscores their strategic relevance: they are no longer studied solely for their intrinsic properties, but increasingly as enablers of cleaner, more efficient, and digitally integrated industrial processes. This perspective is crucial for guiding future developments and aligning the laboratory innovations with industrial implementation. In contemporary industry, the study of materials has evolved from focusing solely on intrinsic properties to emphasizing their role as active enablers of cleaner, more efficient, and digitally integrated processes. This shift, driven by sustainability goals and Industry 4.0 technologies, highlights how refractories such as magnesia–dolomite composites contribute to energy efficiency, process optimization, and reduced environmental impact [27].
2.4. Comparative Analysis of Bibliometric Maps
Figure 2 and Figure 3 present two network visualizations obtained through VOSviewer, each based on different exclusion criteria in the Scopus query. The comparison between these maps provides valuable insights into how search strategies affect the thematic landscape of refractory research.
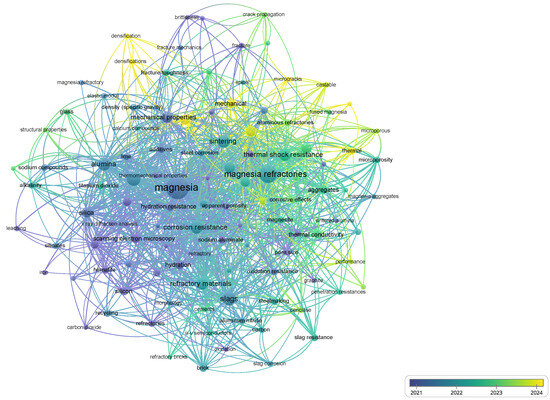
Figure 3.
Network visualization map of co-occurring keywords (strict exclusion criteria). Highlights the mechanical and chemical performance terms in refractories.
In the first network (Figure 2), which was generated with a broader set of results, the most recurrent keywords were associated with general materials science and sustainability. Terms such as geopolymers, sustainable development, effluents, and 3D printers were prominent, reflecting the expansion of refractory research into multidisciplinary areas that address environmental and technological innovation.
In contrast, the second network (Figure 3), which applied stricter exclusion filters to remove unrelated domains, revealed a more focused set of keywords directly linked to refractory performance. Concepts such as fracture mechanics, slag corrosion, hydration resistance, and oxidation resistance became central nodes, pointing to a technical and application-driven orientation. This suggests that narrowing the scope of the search reduces noise from peripheral disciplines and highlights the mechanical, chemical, and thermomechanical challenges that define the state-of-the-art in magnesia-based refractories.
The comparative results, summarized in Table 1, demonstrate that the exclusion strategy plays a critical role in shaping bibliometric analyses. While broader queries capture interdisciplinary trends and sustainability perspectives, refined queries emphasize the core scientific and engineering issues relevant for industrial applications.

Table 1.
Comparison of bibliometric maps obtained with different exclusion criteria.
2.5. Classification of Basic Refractories
Basic refractories are a crucial class of materials engineered to withstand high temperatures and corrosive environments dominated by basic oxides, such as those found in steelmaking, cement production, and specific non-ferrous metallurgical processes. Their primary constituents are magnesia (MgO) and doloma (CaO·MgO), which provide excellent resistance to chemical attack from slags rich in calcium and magnesium oxides, making them ideal for lining furnaces, ladles, and converters [7,13].
In refractory terminology, magnesia denotes magnesium oxide (MgO), typically obtained by calcining magnesite (MgCO3) or magnesium hydroxide (Mg(OH)2). Conversely, doloma refers both to the calcined form of dolomite (CaMg(CO3)2), consisting mainly of calcium oxide (CaO) and magnesium oxide (MgO), and to the family of refractory products such as bricks and monolithic concretes developed from this system. For this reason, doloma-based refractories are commonly designated as MgO-CaO materials in the literature and industry, reflecting their compositional basis rather than their raw mineral origin [22].
The performance of basic refractories depends on their chemical composition, microstructure, and manufacturing processes, with high-temperature firing and careful control of impurities, such as silica, being essential for optimizing strength and durability [28]. Advances in refractory technology have led to the development of castable and monolithic basic refractories that offer significant improvements over traditional shaped products. These innovations include low-cement and ultra-low-cement castables, cement-free systems, and advanced binder technologies, which result in higher densification, lower open porosity, and enhanced mechanical strength and durability [29]. The use of optimized particle packing, advanced plasticizers, and colloidal matrix additions (such as silica and alumina) further improves workability, flowability, and the final microstructure, contributing to superior thermal shock and slag corrosion resistance [30].
Installation flexibility has also increased with the introduction of self-flowing, shotcreting, and quick dry-out castables, as well as pre-cast shapes, allowing for more efficient and convenient application in complex industrial settings. These developments have blurred the line between shaped and unshaped refractories, with monolithic products now widely replacing bricks in many high-temperature processes due to their improved performance and ease of installation [31].
Characterization of these materials involves assessing mechanical, thermal, and corrosion resistance properties to ensure reliability in demanding applications [5]. Environmental concerns and resource scarcity have also driven interest in recycling spent basic refractories, with closed-loop recycling gaining traction as a sustainable approach [32].
From a compositional standpoint, basic refractories can be classified according to their main oxide phase and the additives present, highlighting the following systems.
2.5.1. Magnesia-Based Basic Refractories
Magnesium oxide (MgO), or magnesia, is the primary component in basic refractories due to its exceptionally high melting point (2852 °C) and strong chemical stability in basic environments, making it ideal for lining furnaces in steel, iron, and cement industries [33,34,35]. High-purity MgO can be produced from sources such as seawater, dolomite, and salt-lake brine, with recent advances focusing on sustainable and cost-effective manufacturing processes that reduce energy consumption and CO2 emissions [36].
The addition of materials like calcium boride (CaB6) and yttrium oxide (Y2O3) to MgO-based refractories has been shown to significantly enhance properties such as bulk density, hot modulus of rupture, oxidation resistance, and resistance to molten slag, thereby improving performance in harsh service conditions [36,37].
However, MgO is susceptible to hydration, which leads to the formation of magnesium hydroxide (Mg(OH)2) and causes significant volume expansion, potentially limiting the reuse of spent refractories in construction applications. Research on the hydration behavior and swelling mechanisms of MgO granules has provided information on how to manage these effects and explore alternative uses, such as soil modification [38,39].
Furthermore, the interaction of MgO-based refractories with fuel ash at high temperatures can result in chemical wear, emphasizing the need for ongoing improvements in refractory formulations to enhance durability and reduce maintenance costs. Table 2 shows the main industrial grades.

Table 2.
Comparison of magnesia types used in basic refractories.
2.5.2. Calcium Oxide-Based Refractories
CaO-based refractories are valued for their exceptionally high refractoriness and strong basicity, making them suitable for specialized metallurgical applications such as steel refining where MgO contamination must be avoided. However, their practical use is limited by their tendency to rapidly hydrate and degrade upon exposure to moisture, necessitating careful storage and handling to maintain performance [46]. Research has focused on improving their hydration resistance and mechanical properties by incorporating additives such as microsilica, ZrO2, and calcium hexaluminate, which can reduce porosity, enhance strength, and improve thermal shock resistance [47,48,49,50]. For example, small additions of CaO in composite refractories can significantly increase hardness and strength, while the formation of protective layers like CA2 or CaZrO3 can enhance corrosion and hydration resistance [47,49,50]. In steelmaking, CaO refractories are particularly effective in desulfurization processes, as they react with sulfur in molten alloys to form CaS, thereby improving alloy purity [49,51].
2.5.3. Magnesia–Calcium Oxide Refractories
The combination of MgO and CaO in refractories creates materials with enhanced properties, leveraging the high refractoriness and thermal shock resistance of MgO alongside the basicity and slag resistance of CaO. These refractories typically form complex mineral phases such as periclase (MgO) and calcium silicates, which improve their performance in environments with variable or aggressive slags, such as those encountered in steelmaking and secondary refining furnaces [52]. However, a challenge is the tendency of free CaO to hydrate, which can compromise durability; see Figure 4.
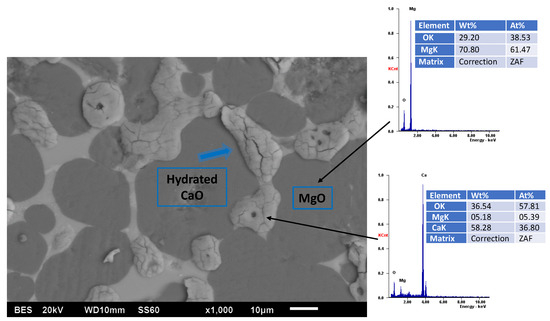
Figure 4.
Scanning electron microscopy image of a magnesia–dolomite refractory showing the expansive hydration process of lime.
This issue is being addressed through additives like TiO2 or CaZrO3 coatings that significantly improve hydration resistance and mechanical strength by forming protective layers at grain boundaries [19,53]. Advanced sintering techniques and the use of chemical binders further optimize the microstructure, densification, and thermal behavior of MgO–CaO refractories, resulting in better resistance to slag penetration and corrosion, even under demanding conditions such as electromagnetic fields or high-temperature cycling [54,55]. These improvements make MgO–CaO refractories suitable for use in high-temperature industrial processes, including steel refining and insulation applications, where both chemical stability and thermal efficiency are critical [48].
2.5.4. Magnesia–Carbon Refractories
The MgO–C (magnesia–carbon) refractory system is a cornerstone in the lining of electric arc furnaces and steel ladles due to its unique combination of high refractoriness, excellent thermal shock resistance, and strong resistance to slag and metal penetration. The addition of natural graphite enhances thermal shock resistance and reduces slag infiltration, but graphite is prone to oxidation at high temperatures, which can compromise the refractory’s integrity. To address this, antioxidant additives such as aluminum, silicon, or magnesium are incorporated to protect the carbon phase by preferentially reacting with oxygen, thereby extending the refractory’s service life and maintaining its mechanical strength [56]. Recent advances include the use of nano carbon to reduce overall carbon content without sacrificing performance, which not only improves oxidation resistance and mechanical properties but also addresses environmental concerns related to CO and CO2 emissions [57]. The microstructure and phase composition, including the formation of in situ ceramic phases like spinel, further influence the durability and corrosion resistance of these refractories [58]. Optimizing the balance between magnesia, carbon, and antioxidants is crucial for maximizing performance and longevity in the harsh environments of steelmaking [59].
2.5.5. Sintered Dolomite Refractories (Doloma)
Doloma refractories, produced by calcining dolomite (CaCO3·MgCO3) at temperatures above 1700 °C, yield materials predominantly composed of periclase (MgO) and calcium oxide (CaO) phases. These refractories are widely employed in steelmaking processes, such as LD (Linz–Donawitz) and AOD (Argon Oxygen Decarburization) converters, owing to their high refractoriness, strong resistance to basic slag attack, and excellent thermochemical stability under severe operating conditions [60]. Doloma refractories with a silica content below 1 wt% exhibit significantly higher thermodynamic stability compared with high-alumina and even magnesite-based refractories. Refractory-grade doloma generally contains less than 2.5 wt% of total impurities—mainly SiO2, Fe2O3, and Al2O3—and more than 97.5 wt% of combined CaO and MgO. However, most high-purity dolomite ores present considerable challenges during calcination and sintering, as achieving high density often demands specialized processing techniques. During production, the carbonate precursor (dolomite) is thermally decomposed to its oxide form (doloma) and subsequently sintered at temperatures above 1850 °C in either rotary or shaft kilns. Two principal manufacturing routes are employed for this purpose: the single-pass and the double-pass processes [13,61]. These refractories are particularly important in desulfurization, where in situ generated magnesium from decomposed dolomite effectively removes sulfur from molten iron, with the desulfurization rate primarily governed by reaction kinetics rather than diffusion processes, and showing optimal efficiency at elevated temperatures up to about 1623 K [62]. Doloma-based refractories are favored for their low cost and wide availability, though their service life is generally shorter than that of more advanced MgO–C refractories [63]. Studies indicate that the dissolution behavior and wear resistance of doloma refractories in contact with steelmaking slags depend on their microstructure, phase composition, and the basicity of the slag, with higher MgO content and optimized firing conditions improving performance [64]. Advances in processing, such as the development of off-fluxed dolomite compositions and improved sintering techniques, aim to enhance the mechanical and refractory properties of these materials, making them more competitive for industrial applications [65]. Despite some limitations in durability, doloma refractories remain a practical choice for many steelmaking operations due to their effectiveness in slag conditioning and sulfur removal, as well as their economic advantages [63].
2.6. Fundamental Physicochemical Properties
Basic refractories based on dolomite (CaCO3·MgCO3) and its calcined derivatives exhibit a distinctive set of physicochemical properties that determine their performance in high-temperature industrial applications, particularly in steelmaking processes. The most relevant properties include refractoriness, bulk density, chemical reactivity, slag resistance, and hydration resistance. Table 3 shows a summary of these properties.
The thermal decomposition of dolomite (CaMg(CO3)2) is a well-established two-step endothermic process, extensively characterized by Differential Thermal Analysis (DTA) and Thermogravimetric (TG) methods [66]. The first decomposition step occurs around 650–750 °C, where dolomite releases CO2 and forms a metastable mixture of MgO and CaCO3, often referred to as “half decomposition” [67]. The second, more intense endothermic event takes place between 800 and 950 °C, corresponding to the decomposition of the remaining CaCO3 into CaO and additional CO2 [68].
Above 1000 °C, solid-state reactions between CaO and MgO can occur, leading to limited sintering and the formation of periclase–lime aggregates, with the overall weight loss (typically 45–47%) reflecting the total CO2 released from the original carbonate structure [69]. The process is influenced by factors such as particle size, impurities, and the partial pressure of CO2, which can shift the decomposition temperatures. These transformations are crucial for understanding the calcination behavior and reactivity of doloma, especially in refractory and industrial applications where the properties of the resulting oxides are critical [70].

Table 3.
Physicochemical properties of dolomitic refractories and their relevance in steelmaking processes.
Table 3.
Physicochemical properties of dolomitic refractories and their relevance in steelmaking processes.
| Property | Description and Impact in Steelmaking | Ref. |
|---|---|---|
| Refractoriness | High melting point and thermal stability make dolomite suitable for lining furnaces and converters. | [71,72] |
| Bulk Density | Affects mechanical strength and resistance to slag penetration; influenced by calcination process. | [65,73] |
| Chemical Reactivity | Reactivity with slag and steel is determined by mineralogy, crystal size, and calcination degree. | [64,74] |
| Slag Resistance | Good resistance to basic slags (high CaO, MgO); calcined dolomite dissolves efficiently in slag. | [64,75] |
| Hydration Resistance | Calcined dolomite (Doloma) is prone to hydration; hydration resistance is lower than MgO bricks. | [72,76] |
The high refractoriness of the materials means that their ability to withstand extremely high temperatures without melting or deforming is crucial for applications such as steelmaking and high-temperature reactors. In dolomitic ceramic materials, their chemical composition, microstructure, and thermal stability are determined primarily. The presence of refractory phases such as periclase (MgO), calcium oxide (CaO), forsterite (Mg2SiO4), spinel, and corundum (Al2O3) contributes to high melting points, often above 1700 °C, ensuring structural integrity at elevated temperatures [77,78]. High-entropy ceramics also offer exceptional resistance to temperature due to their stable multielement microstructures [79,80]. A well-sintered, dense microstructure with minimal porosity enhances the resistance to thermal shock and mechanical strength [81], while additives like Cr2O3 promote the formation of high-temperature phases and improve densification [81]. Maintaining a high MgO/SiO2 ratio (>2.2) prevents the formation of low melting point compounds and ensures desirable phase assemblages [82]. In addition, chemical and mechanical stability at high temperatures further supports a long service life in aggressive steelmaking environments [83].
Calcined dolomitic refractory bricks are valued for their high bulk density, which typically ranges from 2.6 to greater 3.0 g/cm3, see Table 4. The higher bulk density in these bricks is closely related to a lower open porosity, resulting in improved mechanical strength and greater resistance to slag penetration. The performance of calcined dolomitic refractory bricks is strongly influenced by the relationship between bulk density and open porosity. Higher bulk density is generally associated with lower open porosity, which directly enhances compressive strength, resistance to chemical corrosion, and slag penetration [19,84]. The achievement of optimal density depends on several manufacturing parameters, including raw material quality, firing temperature, and binder selection. For example, the use of tar-based binders combined with high pressure compaction (500 kg/cm2) can consistently produce bricks with bulk densities exceeding 2.6 g/cm3 and low porosity [84]. Furthermore, higher sintering temperatures, such as 1400 °C, significantly improve densification and reduce porosity, as demonstrated in the fabrication of dolomite-based forsterite bricks [85].

Table 4.
Bulk density ranges of magnesia dolomite refractory materials by product.
Dolomitic refractories and dolomite-based materials are widely used for desulfurization in metallurgical and fuel processing industries due to their strong chemical reactivity with acidic slag components and sulfur compounds; see Figure 5. Their effectiveness in desulfurization is primarily due to the active participation of both calcium and magnesium oxides in sulfur capture reactions.
Figure 5.
Schematic representation of sulfur capture by calcined dolomite (CaO) in high-temperature gas environments. Modified from [62].
Dolomite, when decomposed and reduced such as in aluminothermic reactions, generates metallic magnesium, which dissolves in molten iron and reacts with sulfur to remove it efficiently. The desulfurization rate increases with higher temperatures and greater amounts of reactants, but tends to plateau above 1623 K. This process is primarily controlled by the chemical reaction kinetics rather than by mass diffusion, and it occurs predominantly in the homogeneous phase, rather than via magnesium vapor bubbles [62].
Dolomite and other calcium-based sorbents (such as limestone) are effective for capturing sulfur in coal gasification and fluidized-bed combustion systems. The active component, CaO (obtained from the calcination of dolomite), reacts with hydrogen sulfide (H2S) to form stable calcium sulfide (CaS), as shown in Equation (1):
Dolomite remains reactive at elevated temperatures (above 1023 K), making it suitable for high-temperature sulfur capture processes [89,90]. However, the presence of water vapor imposes thermodynamic limitations on the lowest achievable H2S concentrations due to equilibrium constraints. In contrast, the presence of CO2 does not significantly hinder the sulfur capture efficiency, though it may influence the initial calcination of the sorbent [90]. Dolomite is a promising raw material for SO2 sorbents in fluidized combustion systems, as both calcium oxide (CaO) and magnesium oxide (MgO) actively participate in sulfur dioxide binding. The resulting sulfation products, such as CaSO4 and MgSO4, are thermally stable up to 1100 °C, which supports the applicability of dolomite in high-temperature desulfurization processes [91].
Slag resistance in refractories is strongly influenced by the material’s microstructure, the MgO/CaO ratio, and the basicity of the contacting slag. A higher MgO content generally improves resistance to acidic slags, while the CaO content and the slag basicity affect the corrosion behavior and the formation of either protective or reactive interfacial layers.
MgO-CaO refractories are primarily governed by microstructural features, the MgO/CaO ratio, and the basicity of the contacting slag. Finer grain boundaries contribute to the formation of dense isolation layers that block slag penetration [92], while highly dispersed microstructures, especially those containing stabilizing phases like ZrO2, enhance chemical durability at the slag interface [93]. Chemically, increasing the MgO/CaO ratio enhances resistance to acidic slags and initially reduces slag viscosity, as shown in Figure 6. However, excessive MgO content may reverse this trend, leading to precipitation of MgO-rich phases and a subsequent increase in viscosity, reducing slag fluidity at high MgO/CaO ratios [44]. Higher slag basicity, typically expressed as CaO/SiO2, depolymerizes the slag structure and enhances flow, but overly basic compositions can lower corrosion resistance in MgO–CaO systems [94,95]. CaO content also promotes reactions with sulfur and other impurities, supporting steel refinement, but may increase corrosive interaction in low-basicity slags [92]. Optimal ranges—such as MgO/CaO ratios between 0.34 and 0.55 [93], and basicity ratios between 1.0 and 1.2 [94]—have been identified as the most effective balance between viscosity control and corrosion resistance.
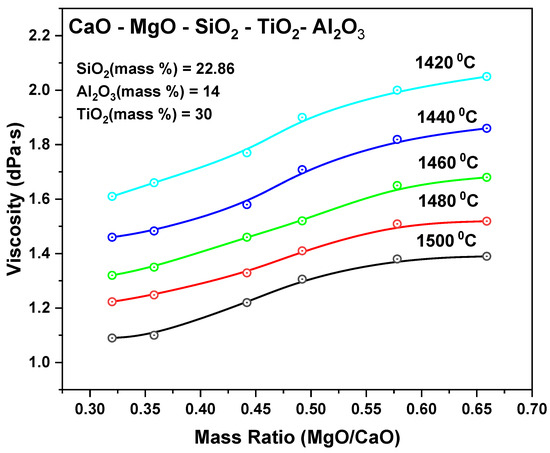
Figure 6.
Effect of MgO/CaO mass ratio on the viscosity of CaO–SiO2–Al2O3–MgO–TiO2 slags at various temperatures. Modified from [96].
Hydration resistance is a critical property for the presence of free lime in MgO–CaO refractories that makes these materials highly reactive when exposed to water in either liquid or vapor form. This reactivity originates from the weak Ca–O bond and the inherent structural instability of lime, which crystallizes in a face-centered cubic (FCC) structure [97]. Because the ionic radius of Ca2+ is relatively large, it cannot be stably accommodated within the sixfold coordination sites of the lattice. Consequently, CaO tends to react with H2O to form calcium hydroxide [Ca(OH)2] [98,99], as described by the following Equation (2):
The hydration of lime (CaO) to form calcium hydroxide [Ca(OH)2, also known as portlandite] is accompanied by a significant volume increase due to the lower density of portlandite (approximately 2.21 g/cm3) compared to lime (approximately 3.34 g/cm3). This expansion is well documented in cementitious systems and is a direct result of the crystallization and growth of Ca(OH)2 within the material matrix, which can lead to internal stresses and potential cracking if not properly managed [100]. The expansion is particularly pronounced when free lime is present in concrete or soil stabilization applications, as the hydration reaction causes the volume of the affected region to nearly double, especially for smaller CaO grains where a larger proportion of the grain hydrates [101].
Structurally, the hydration process involves an expansion in the direction perpendicular to the close-packed planes: from the (111) plane and [111] direction in FCC–CaO to the (001) plane and [001] direction in HCP–Ca(OH)2 [102]. The interplanar spacing changes significantly from approximately 2.78 Å to 4.91 Å, leading to nearly a twofold volumetric expansion.This anisotropic growth in the [001] direction generates internal stresses that can seriously damage the surrounding matrix and promote microcracking [103].
The hydration of CaO-based refractories proceeds through three consecutive stages [104]. In the first stage, water molecules come into direct contact with the exposed surface of the lime matrix, resulting in a rapid chemical reaction that forms an initial layer of hydrated compounds. As the reaction continues, the second stage begins, during which the growing hydration layer acts as a diffusion barrier that slows down the ingress of water and ionic species. Once this layer reaches a critical thickness, internal stresses generated by shrinkage exceed the mechanical strength of the material, leading to the development of microcracks. Finally, in the third stage, known as the “dusting stage,” the hydration rate rises abruptly, causing extensive structural damage and, in some cases, complete disintegration of the material due to the expansive formation of Ca(OH)2 [102].
The three-stage hydration process previously described can be visualized through the cylindrical pore model [102], which provides a clear geometrical interpretation of the reaction front propagation in porous CaO systems. Figure 7 schematically represents this mechanism, illustrating how H2O molecules diffuse through open cylindrical pores, forming a physically and chemically adsorbed water layer on the pore walls. During CaO hydration, H2O molecules diffuse through open cylindrical pores and form a physically and chemically adsorbed water layer on the pore walls. Within this adsorbed layer, the diffusion of OH− and Ca2+ ions facilitates the nucleation and growth of Ca(OH)2, causing the hydrated layer to progressively expand toward the unreacted CaO matrix. As the reaction front advances, the thickness of the Ca(OH)2 layer increases, which reduces the effective pore radius and leads to significant internal stresses due to the volumetric mismatch between the original CaO and the newly formed Ca(OH)2. This process can result in microcracking and, in extreme cases, dusting phenomena, as the weak tensile strength and poor crack resistance of the Ca(OH)2 layer make it prone to disintegration under stress. Pore blockage and the resulting internal stresses are key factors in the mechanical degradation of CaO-based materials during hydration, and the extent of these effects depends on factors such as pore structure, hydration rate, and the physical properties of the hydration products [105,106].
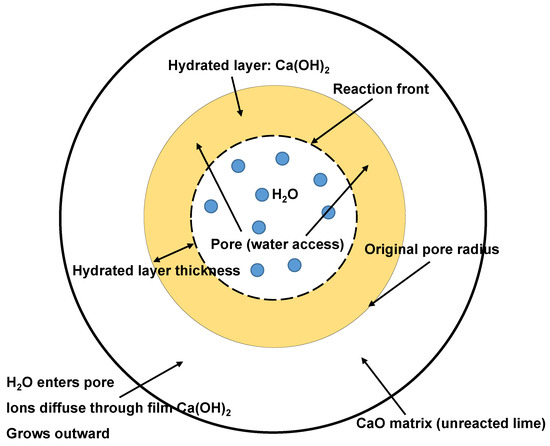
Figure 7.
Schematic representation of the hydration mechanism of CaO based on the cylindrical pore model. Modified from [102].
Several methods have been developed to improve the hydration resistance of calcium oxide (CaO), a critical issue in refractories and high-purity applications due to its high reactivity with moisture. One of the most effective strategies involves the incorporation of additives such as yttrium oxide, manganese oxide, chromium oxide, Fe2O3, and zirconia, which form solid solutions or secondary phases with CaO, thereby stabilizing the microstructure and impeding water ingress [107]. Complementary approaches like high-temperature sintering with additives and surface treatments lead to densification, reducing porosity and moisture penetration [108]. Microstructural control through advanced processing techniques such as cold isostatic pressing or plasma-assisted synthesis can further reduce grain boundary area and enhance hydration resistance [109]. Composite formation, particularly the incorporation of Ca2SiO4 nanoparticles, has shown promise in pinning CaO grains, mitigating agglomeration, and simultaneously improving hydration resistance and thermal behavior in energy storage contexts [110].
Mechanistically, hydration occurs rapidly upon exposure to water, releasing heat and forming Ca(OH)2; however, the addition of specific oxides and superplasticizers can delay this transformation and modify the morphology of the hydration products [98,111]. Improved resistance has been directly linked to the formation of secondary phases such as calcium ferrites and silicates, as well as to microstructural densification [107]. In high-purity applications such as CaO crucibles used in metal and alloy smelting, tailored additive strategies for improving magnesia–doloma refractories include the incorporation of metallic and ceramic nanoparticles such as ZrO2, TiO2, Al2O3, and Fe2O3, which promote sintering and form secondary phases that strengthen grain boundaries and enhance corrosion and thermal shock resistance [15]. Other approaches involve the use of spinel-forming oxides (MgAl2O4 or CaZrO3) to improve microstructural integrity, as well as hydrophobic additives and surface modifiers that reduce pore connectivity and prevent hydration of free CaO [16]. These strategies collectively enhance densification, mechanical strength, and durability while mitigating moisture-induced degradation in MgO-CaO systems to simultaneously maintain chemical purity and prevent premature hydration [112].
2.7. Raw Materials: Characteristics, Availability, and Geopolitics
The performance and economic viability of MgO-CaO refractories are critically dependent on the quality and characteristics of the raw materials used. Main parameters such as chemical purity, crystallinity, grain size, and the presence of minor oxides directly influence sintering behavior, hydration resistance, and mechanical integrity. High-purity dolomite and magnesite are preferred for ensuring optimal refractory behavior, while specific impurities (such as SiO2, Al2O3, or Fe2O3) can either degrade or, when carefully controlled, enhance certain properties by promoting the formation of stable secondary phases [19,20]. Well-crystallized, coarse-grained raw materials tend to improve sintering kinetics, lower porosity, and enhance hydration resistance and strength. In contrast, fine-grained or poorly crystalline sources exhibit higher reactivity and are more susceptible to moisture-induced degradation [19,113].
Geological origin also plays a pivotal role, as it determines the mineralogical structure and impurity profile of the raw materials, which in turn affects processing requirements and final refractory performance [114,115]. Sintering behavior benefits from high-purity inputs and optimal particle size distribution, facilitating densification and mechanical strength [19,20,113]. Hydration resistance can be further improved by minimizing free CaO and incorporating specific additives—such as Fe2O3, TiO2, ZnO, or hercynite—that encapsulate reactive grains and inhibit water diffusion [116,117]. Moreover, mechanical properties are significantly enhanced by combining compositional control with advanced processing techniques like high-temperature sintering or co-clinkering, which ensure microstructural uniformity and phase stability [114].
The global supply of high-grade dolomite and magnesite is unevenly distributed, with major producers such as China, Brazil, Turkey, the United States, and the European Union relying on distinct geological formations and industrial infrastructures; see Table 5. Geopolitical dynamics including export regulations, environmental policies, and supply chain vulnerabilities play a decisive role in determining market access and price stability for these critical refractory minerals. China holds a dominant position as the world’s leading supplier of magnesite, supported by substantial reserves and advanced refining capabilities. This strategic control allows China to influence global pricing and availability, often leading to volatility in markets dependent on magnesia-based refractories [118,119].

Table 5.
Major regions producing dolomite and magnesite and their industrial status.
Although Uzbekistan is not a major exporter, it possesses over 60 identified high-quality dolomite deposits. If further industrial infrastructure were developed, Uzbekistan could reduce regional dependence on imported magnesite and become a more significant player in the refractory raw material supply chain [14]. Other countries such as Brazil, Turkey, the United States, and various EU members also maintain notable dolomite and magnesite resources. However, their collective influence remains more limited in comparison to China’s export volume and market leverage [120,121].
Despite dolomite’s geological abundance in Europe, its classification as a critical raw material by the European Commission is primarily due to supply-chain vulnerabilities rather than scarcity. The production of high-purity calcined dolomite (doloma) for refractory and industrial applications is energy-intensive and subject to strict environmental regulations, which have led to a significant decline in domestic production capacity over recent decades [122]. As a result, Europe has become heavily reliant on imports especially from China for processed MgO-CaO materials, increasing exposure to market concentration and geopolitical risks [123].
This dependence is further exacerbated by the EUA broader vulnerability to supply disruptions in mineral raw materials, which can threaten economic activities and job security [123]. The dominance of external suppliers, particularly China, in critical mineral value chains raises concerns about potential coercive measures and supply restrictions, prompting the EU to consider strategies such as diversifying supply chains, reinvesting in domestic extraction and processing, and establishing partnerships with countries that uphold high socio-environmental standards [124]. These factors collectively explain why dolomite and magnesite are included in the EUA list of critical raw materials, despite their natural abundance within Europe [122].
Raw material selection must consider not only thermomechanical performance but also long term supply security and environmental impact. Strategies such as regional sourcing, beneficiation of lower grade ores, and the development of synthetic alternatives (seawater magnesia or bio-derived CaO) are being explored to ensure sustainable and resilient refractory manufacturing in a changing global context.
3. Processing and Synthesis Technologies
The production of magnesia–doloma refractory bricks traditionally relies on MgO-CaO systems, which are widely used in industries such as cement and steel due to their excellent high-temperature resistance and chemical stability.
3.1. Conventional Processes
Their production relies on the precise selection of raw materials, high-temperature calcination, and meticulous control of hydration and sintering processes. The most critical step in the manufacturing sequence is the high-temperature calcination of dolomite, which leads to the formation of calcined dolomite (doloma), typically performed at 1650–1800 °C, resulting in a composite of CaO and MgO phases [114]. This thermal treatment strongly influences the hydration resistance and mechanical properties of the final brick, making it essential for the long-term performance of the refractory in aggressive environments [125].
Sintering is then applied to densify the calcined powders, often at temperatures above 1700 °C, leading to the formation of interlocking grain structures and reduced porosity. During sintering, diffusion mechanisms promote bonding between adjacent particles, leading to densification, pore reduction, and the formation of grain boundaries [126], as shown in Figure 8.
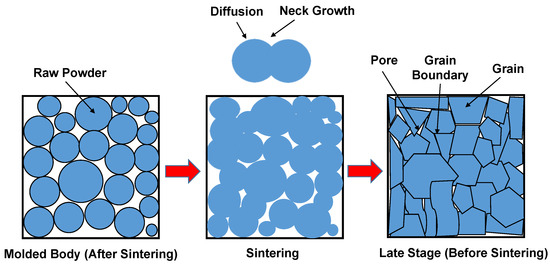
Figure 8.
Schematic representation of the sintering process showing powder consolidation, neck growth, and grain coalescence.
To further improve mechanical strength and corrosion resistance, additives such as graphite are introduced, which act as antioxidants and thermal shock absorbers in carbon-containing refractories [56,127,128,129]. Organic binders such as phenolic resins and pitch and thermosetting resins play a critical role in the processing of magnesia–doloma refractory bricks by providing the necessary green strength before firing, thereby facilitating compaction and handling [56]. These binders also influence the final density, porosity, and mechanical properties of the bricks; therefore, the optimal binder content must be carefully controlled, as excessive amounts can increase residual porosity and compromise the integrity of the material [130,131]. In advanced applications, particularly in additive manufacturing, clean burn-off binders such as polyvinylpyrrolidone vinyl acetate (PVP–VAc) are being developed to reduce carbon residues during firing and improve the microstructural quality of the final sintered product [132,133].
Dense brick fabrication is a process focused on achieving high packing density, low porosity, and strong intergranular bonding to ensure durability and performance in demanding applications. Optimization of particle size distribution and compaction methods is essential to produce bricks with these properties for high performance applications in steel converters, cement kilns, and non-ferrous metallurgy. This is shown in Table 6.

Table 6.
Main factors influencing dense brick fabrication for magnesia–dolomite refractories.
3.2. Innovative Processing Strategies
In response to the increasing demand for high performance and sustainable refractory materials, several innovative processing strategies have emerged to improve the structural, chemical, and environmental performance of MgO-CaO refractories.
One promising development is the production of fused magnesia–dolomite co-clinkers via melting of stoichiometric mixtures at temperatures exceeding 2000 °C. Figure 9 illustrates the industrial production flow of fused magnesia, including charging, arc melting, molten pool formation, and crystallization steps paralleling the co-clinker synthesis process where dolomite and magnesia are combined and densified through high-temperature fusion [139]. Fusion of magnesia–dolomite co-clinkers at temperatures above 2000 °C achieves superior densification and phase uniformity, which is critical for high-performance refractory materials [140]. However, this process is significantly more energy-intensive than conventional sintering (typically 1550–1700 °C), with specific energy demands for fusion routes exceeding 6 GJ t−1—almost double that of sintering (≈3–3.5 GJ t−1)—and results in 30–40% higher CO2 emissions [141]. The high energy consumption is primarily due to the elevated temperatures required for complete fusion and densification. Recent research highlights that energy-saving measures, such as improved furnace insulation, heat recovery from exhaust gases, and the gradual adoption of renewable electricity, can help mitigate these environmental impacts and align fused co-clinker production with circular economy strategies [11]. Additionally, alternative approaches like optimizing particle size for sintering have shown potential to achieve high densification at lower temperatures, further reducing energy use and emissions [141]. This process yields dense, homogeneous materials with improved grain bonding, reduced porosity, and enhanced thermal shock and hydration resistance. The fusion process eliminates residual carbonates and free CaO, resulting in microstructures that are more stable under aggressive service conditions [20,87].
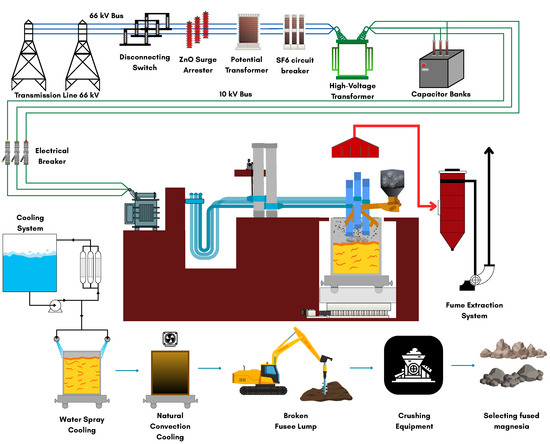
Figure 9.
Schematic overview of the fused magnesia industrial production process in electric arc furnaces. Modified from [139].
Another innovation involves the progressive addition of MgO to natural dolomite during calcination or sintering. This approach allows precise control of the MgO/CaO ratio, improving sinterability and hydration resistance while tailoring the final phase composition. By adjusting the additive proportions, it is possible to engineer multiphase microstructures that balance mechanical strength with corrosion and thermal resistance.
The controlled addition of MgO, or the use of dolomite with optimized Mg/Ca ratios, plays a central role in enhancing the performance of MgO-CaO refractories. From a processing standpoint, increasing MgO content improves sinterability by promoting the formation of dense and uniform microstructures. Homogeneous mixing at the atomic scale facilitates an even distribution of calcium and magnesium, which is essential for achieving consistent mechanical and thermal properties in the final product [142]. In terms of chemical stability, higher MgO content, particularly when combined with functional additives such as TiO2, leads to the development of protective phases like magnesium silicate hydrates, hydrotalcite, or calcium titanate (CaTiO3), which significantly enhance hydration resistance and long-term durability [19]. Furthermore, by carefully balancing the MgO/CaO ratio and additive content, it is possible to engineer multiphase microstructures that provide both early mechanical strength (primarily from CaO hydration) and extended service life (through the slower, stabilizing hydration of MgO) [143]. While the addition of MgO and functional oxides offers clear benefits, certain limitations must be considered to ensure optimal performance. Excessive MgO content may result in the presence of unreacted or poorly bonded particles within the microstructure, which can reduce mechanical strength and compromise reactivity [144]. Therefore, careful optimization of the MgO/CaO ratio is essential to balance densification, hydration resistance, and durability. Similarly, additives such as TiO2 are known to enhance hydration resistance and mechanical integrity by promoting the formation of protective ceramic phases; however, their effectiveness is highly dependent on proper dosage, as excess amounts may disrupt phase equilibrium or lead to undesirable microstructural effects [19].
Ensuring high purity dolomite and magnesite is crucial for industrial applications. Advanced flotation techniques, especially with selective depressants and collectors, are the primary methods for removing siliceous and ferrous impurities, resulting in consistent and high quality feedstock.
Recent advances in flotation-based purification techniques have significantly improved the selective separation of dolomite from magnesite, a critical step for obtaining high purity raw materials in refractory production. One major innovation involves the use of modern selective depressants such as EDTMPA, DTPMP, HEDP, Na2ATP, EGTA, sesbania gum, gellan gum, and tannin. These compounds preferentially bind to calcium ion sites (Ca2+) on the dolomite surface, increasing its hydrophilicity and thereby suppressing its floatability. This facilitates more effective separation from magnesite [121,145,146,147,148,149,150,151]. Many of these depressants are also biodegradable and environmentally friendly, aligning with green processing goals.
Advancements in collector agents such as -chloro-oleate acid and DBDP have enhanced the floatability of target minerals. Depending on the process design, these collectors can selectively increase either dolomite or magnesite recovery [152,153,154,155]. Additionally, physical enhancement methods like ultrasonic treatment have shown to improve flotation efficiency by increasing mineral surface roughness and reagent adsorption, resulting in better separation performance and cleaner product quality [156,157].
Beyond improvements in separation technologies, these advancements align with a broader shift in the refractory industry toward sustainability and resource efficiency. Current research and industrial practice emphasize energy efficient processing, the recycling of spent refractory materials, and the integration of circular economy principles. Key strategies include the valorization of industrial waste, the use of secondary raw materials from demolition or process residues, and the optimization of thermal treatments, all of which contribute to reducing the environmental footprint and improving long term resource security.
Several energy-saving measures have been identified to reduce the high energy consumption and CO2 emissions associated with MgO-CaO production, especially in fusion processes above 2000 °C. Upgrading to larger and more efficient electric arc furnaces—such as replacing 1600 kVA units with 3000 kVA models—can significantly lower unit power consumption in fused magnesia production [158]. Advanced process designs, such as transport bed flash calcination, utilize staged preheating and product cooling to recover heat, achieving energy efficiencies up to 66.8%, almost double that of conventional reverberatory furnaces [159].
Switching to alternative energy sources, such as integrating renewable electricity, nuclear power, or hydrogen, can further reduce the carbon footprint, with nuclear and hydrogen offering substantial reductions compared to coal or natural gas [160]. Partial substitution of fossil fuels with biomass in calcination can also cut CO2 emissions by up to 38% for dead-burned magnesia (DBM) [161]. Additional strategies include improved furnace insulation, heat recovery from exhaust gases, and the adoption of CO2 capture technologies, all of which contribute to making magnesia production more compatible with circular economy and sustainability goals [162]. While process optimization reduces the energy intensity of magnesia production, extending the material life cycle through recycling further enhances its overall environmental performance. In this context, the recycling of spent refractories is gaining momentum in the refractory industry as a response to growing environmental concerns, raw material supply limitations, and rising production costs. Despite this progress, recycled materials currently meet only about 7% of the total demand for refractory raw materials, largely due to quality variability and the lack of widespread high-value recycling applications [32,163]. Figure 10 illustrates examples of typical refractory waste materials from various industrial sectors. These spent materials form the basis for recycling strategies aimed at reducing environmental impact and raw material dependency. Nevertheless, closed loop recycling where used refractories are reintroduced into the production of new refractory products is emerging as a promising strategy to enhance resource efficiency and reduce dependence on virgin materials.

Figure 10.
Refractory waste materials from various applications: (a) Highly sintered chromite brick used in kiln lining; (b) corundum brick for combustion chamber; (c) MgO–C refractory brick after severe attack by molten slag in blast furnace conditions; (d) magnesia–zirconia brick for smelting furnaces.
Recent innovations such as electrodynamic fragmentation have shown potential in producing high-purity recycled fractions that retain comparable performance to conventional raw materials [164]. These methods enable effective separation and recovery of valuable phases, contributing to lower energy consumption and carbon emissions during processing. Supporting this, life cycle assessment (LCA) studies indicate that the reuse and recycling of refractory waste can lead to savings of approximately 0.54 tonnes of CO2-equivalent and 3 GJ of non-renewable energy per tonne of waste processed. Among the available options, direct reuse of materials offers the most significant environmental benefits [25]. Together, these developments position recycling and circular economy practices as central components of future sustainable refractory production systems.
4. Modification with Nanostructured Additives
The modification of MgO-CaO refractories with nanostructured additives has proven to be a highly effective approach for improving hydration resistance, structural integrity, and overall performance in aggressive environments.
4.1. Assessment of Additives
Commonly investigated additives include ZnO, ZrO2, TiO2, Fe2O3, and Al2O3, as well as spinel-forming compounds such as MgAl2O4 (spinel) and FeAl2O4 (hercynite) [113,165,166,167,168,169,170]. Beyond conventional additives, recent research has expanded the range of functional oxides to include MnO and SiO2, which offer additional advantages in phase stability, densification, and surface engineering [171,172,173,174]. From a thermodynamic perspective, MnO promotes the formation of MnCr2O4 spinel by shifting equilibrium away from Cr2O3, enabling selective chromium enrichment and improving slag interactions in steelmaking environments [175,176]. Simultaneously, MnO and SiO2 serve as effective network modifiers, depolymerizing silicate structures, lowering melt viscosity, and promoting densification during high-temperature processing [176,177,178]. These effects are crucial for optimizing the microstructure and minimizing porosity in advanced ceramics and refractories. Cr2O3 also demonstrates excellent thermodynamic stability in contact with Ga2O3 up to 600 °C, supporting its use in high temperature electronics and corrosion-resistant coatings [179,180]. Moreover, surface-engineered SiO2 and MnO2 core shell composites have shown excellent results in polishing systems due to their combined chemical and mechanical effects [181].
Additional studies have explored the use of B2O3 to form dense glassy phases that reduce moisture permeability; kaolin as a source of aluminosilicates promoting in situ spinel formation [182,183]; Si3N4 to enhance hydrophobicity and reduce wetting behavior; and MgSiO3 (enstatite) to generate intergranular protective barriers [184,185].
4.2. Impact of Nanostructured Additives on Performance Parameters
- Hydration Resistance
In conjunction with compositional advances, hydration resistance is primarily governed by the formation of protective phases at grain boundaries such as spinels, perovskites, and dense oxide layers which encapsulate reactive CaO and MgO, thereby reducing water exposure and limiting degradation. Nano-additives containing trivalent and tetravalent cations (Zr, Al, Ti) form solid solutions or low-melting compounds that densify grain boundaries and block moisture ingress. Sol-derived oxides like alumina sol enhance bonding and protective layer density, while hybrid systems such as Al–TiB2 suppress the formation of hydratable phases and improve thermal shock resistance. These microstructural modifications including grain encapsulation, densification, and reduced porosity—are essential to the durability and performance of advanced refractory systems [15,186,187,188]. In addition, Figure 11 provides direct SEM microstructural evidence supporting this mechanism. The image corresponds to an experimental formulation containing MgO-CaO with the addition of microparticles of hercynite (FeAl2O4), which incorporates trivalent Fe ions. During high-temperature sintering, this raw material reacts with CaO to form brownmillerite (), a low-melting phase that wets the grain boundaries between lime and magnesia. This phase behaves as a liquid-like collar surrounding the grains, promoting densification and reducing porosity and intergranular defects within the refractory matrix. Consequently, the formation of such continuous boundary films enhances the material’s resistance to hydration by limiting the penetration of moisture and blocking reactive sites associated with free CaO.
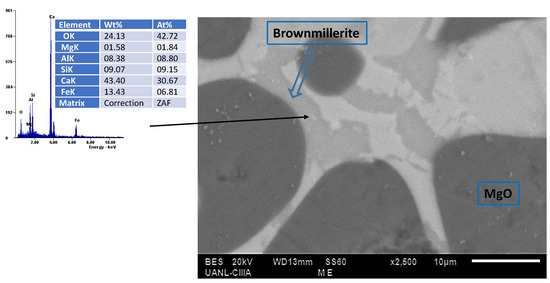
Figure 11.
SEM image of a MgO-CaO refractory showing the brownmillerite phase (), formed by the reaction of free lime () with the added hercynite (), encapsulating magnesia grains ().
- Mechanical Performance
Nanostructured additives significantly enhance mechanical performance. By refining grain size, increasing bulk density, and improving intergranular bonding, these additives contribute to tougher, more resilient microstructures. In both metallic and ceramic matrices, nanoparticles such as Y-Zr-O, Fe3O4, or graphene oxide disperse uniformly to form dense structures that resist crack propagation and improve compressive and flexural strength. These enhancements are attributed to grain boundary optimization, Orowan strengthening, and the formation of stable second phases. Combined with the densification and encapsulation mechanisms previously described, these effects establish nanostructured additives as powerful tools for engineering multifunctional materials with high durability, hydration resistance, and mechanical robustness [189,190,191,192,193,194,195].
- Thermal Behavior
Thermal performance is likewise improved through additive-induced phase engineering and microstructural control. The engineered formation of spinel and perovskite phases particularly when coupled with controlled porosity significantly enhances thermal shock resistance and reduces thermal conductivity. In magnesia-based refractories, in situ spinel formation creates fine, uniformly distributed pores that absorb thermal stress and inhibit crack propagation under cyclic heating. Similarly, perovskite structures, especially two-dimensional or high entropy variants, exhibit ultra-low thermal conductivity due to strong phonon scattering, lattice distortions, and defect engineering. These thermally insulating features, together with mechanical and hydration enhancements, position nanostructured additive systems as ideal candidates for high-performance applications exposed to extreme thermal cycling [196,197,198,199]. Overall, nanostructured additive engineering offers a promising route to tailor the microstructure and durability of magnesia–dolomite refractories, making them more suitable for demanding industrial applications where hydration and thermal stress are critical challenges.
Spinel Phase Engineering for Thermal Shock Resistance
The enhancement of thermal shock resistance in ceramics through the engineered formation of spinel phases (notably MgAl2O4) is a well established phenomenon, supported by a robust body of research. Spinel phases, when introduced or formed in–situ within ceramic matrices, contribute to improved mechanical properties, reduced thermal conductivity, and increased resistance to crack propagation under cyclic heating and cooling. These improvements are attributed to mechanisms such as microcrack toughening, crack deflection, and the development of dense, well-bonded microstructures. Numerous studies have demonstrated that optimizing the content, distribution, and microstructure of spinel phases, often in combination with controlled porosity or secondary additives, leads to significant gains in thermal shock resistance across a variety of ceramic systems, including magnesia-based refractories, alumina spinel composites, and forsterite–spinel ceramics [200,201,202,203].
The primary mechanism by which spinel phases enhance thermal shock resistance is through microcrack toughening and crack deflection. The mismatch in thermal expansion coefficients between spinel and the matrix (magnesia or alumina) induces microcracks that dissipate thermal stress and hinder crack propagation [200,204,205], as shown in Figure 12. The formation of intergranular spinel phases also leads to a denser, more uniform microstructure, further improving resistance to thermal shock [169,200].
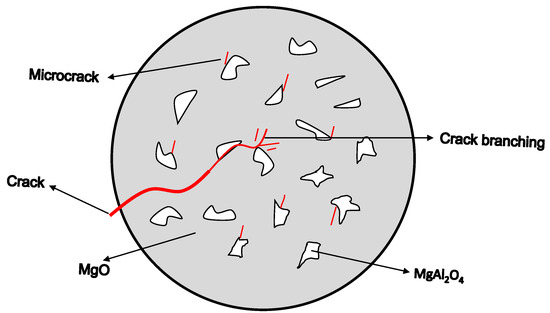
Figure 12.
Schematic of crack propagation. Modified from [206].
These mechanisms are foundational, but achieving optimal performance depends on further control over spinel content and distribution. Studies show that a well dispersed, interconnected spinel network maximizes crack deflection and energy absorption, while excessive or poorly distributed spinel can reduce mechanical strength [169,170,200]. The addition of secondary phases (rare earth oxides, aluminium titanate) or the use of nano-sized additives can further refine the microstructure and enhance performance [205].
Spinel phase engineering has been shown to improve thermal shock resistance in a wide range of ceramics, including magnesia–spinel refractories, alumina–spinel composites, forsterite–spinel ceramics, and zirconia-based systems [207,208,209]. The benefits are observed in both dense and porous ceramics, with microporous structures often providing additional thermal insulation and stress accommodation [210,211].
Controlled porosity, when combined with spinel formation, further enhances thermal shock resistance by accommodating thermal expansion and reducing thermal conductivity [196,203,210]. Additives such as rare earth oxides, aluminium titanate, and zirconia can synergistically improve both mechanical and thermal properties, as well as promote the formation of beneficial microstructures [169,211,212]. These synergies have been validated across a range of studies, as summarized in Table 7.

Table 7.
Selected studies on spinel engineering and its effect on thermal shock resistance in ceramic systems.
4.3. Industrial Applications and In-Service Performance
MgO-CaO refractory materials are widely used in industrial applications such as linings for furnaces, boilers, and cement kilns due to their high refractoriness, excellent resistance to basic slags, and suitability for clean steel production [13]. Their main advantages include stability in basic environments, high corrosion resistance, and relatively low production costs, making them attractive for industries seeking chrome-free alternatives with environmental and health benefits [214].
4.3.1. Steelmaking
MgO-CaO refractories are essential in the steel industry, particularly for lining Basic Oxygen Furnaces (BOFs), Electric Arc Furnaces (EAFs), and Argon Oxygen Decarburization (AOD) converters, due to their high resistance to basic slags, excellent thermal stability, and suitability for clean steel production [214]. Their use is driven by their ability to withstand chemically aggressive, high-temperature environments, offering advantages such as thermodynamic stability and cost-effectiveness compared to other basic refractories [215].
However, a main limitation has been their relatively low hydration resistance, which can lead to degradation during service. Recent advancements, such as the addition of nano- and micro-sized alumina, have significantly improved the physical and mechanical properties of MgO-CaO refractories, including bulk density, cold crushing strength, and thermal shock resistance, while also reducing their tendency to hydrate [216]. Innovative processing methods, like fused dolomite–magnesia co-clinkers, have further enhanced their chemical purity and resistance to corrosion, hydration, and thermal shocks, making them more reliable for demanding steelmaking applications [140,217]. Despite these improvements, the industry continues to seek ways to optimize their performance and extend their service life in steel production environments [65,218,219].
Refractories for Basic Oxygen Furnace (BOF) Units
Magnesia–doloma bricks are widely used in the slag lines and working linings of Basic Oxygen Furnaces (BOFs) because of their excellent resistance to basic slags rich in CaO and FeO. These bricks form a dense protective layer, such as Ca2SiO4, at the interface with the slag, which helps shield the refractory from direct corrosion and extends its service life, especially under static conditions [220]. The high basicity of the slag (high CaO/SiO2 ratio) further enhances the slag resistance of magnesia-based refractories, as it promotes the formation of stable, protective layers that inhibit slag penetration and chemical attack [92].
The presence of certain components like CaF2 or high iron oxide content in the slag can increase slag fluidity and aggressiveness, potentially reducing the thickness and effectiveness of the protective layer and accelerating refractory wear [221]. Increasing the magnesia content in dolomite–magnesite mixtures also improves resistance to slag attack and mechanical load, which is crucial for maintaining lining integrity in BOF operations [221,222].
Refractories for Electric Arc Furnace (EAF) Units
Current research does not directly address the use of magnesia–doloma bricks in Electric Arc Furnace (EAF) systems, but it is well established that these bricks are valued for being carbon-free and environmentally friendly alternatives to magnesia–carbon (MgO–C) refractories, especially in applications with basic slags and oxygen blowing. While MgO–C bricks dominate EAF slag zones and arc impact areas due to their superior thermal shock resistance, magnesia–dolomite bricks offer competitive performance in terms of slag resistance and thermal stability, particularly when improved with additives like hercynite spinel, which significantly enhance their mechanical strength and hydration resistance [223,224].
The environmental benefits of MgO-CaO refractories, such as being chrome- and carbon-free, make them attractive for steelmaking operations seeking to reduce emissions and health risks. However, their broader adoption in EAFs is still limited by their lower thermal shock resistance compared to MgO–C bricks, though ongoing material innovations are narrowing this gap [225].
Refractories for Argon Oxygen Decarburization (AOD) Converters
Magnesia–doloma bricks are valued in AOD converters for their high corrosion resistance against complex slags and their stability under variable gas atmospheres, which are common during stainless steel refining. Their oxidic stability and ability to bond sulfur from hot metal make them particularly effective in transition zones and around tuyères, where frequent redox cycles and aggressive chemical conditions impose significant mechanical and chemical stress on the refractory lining [226]. Recent advancements, such as the incorporation of sub-microscale TiO2 into doloma–carbon refractories, have further improved their mechanical, physical, and thermomechanical properties, allowing for reduced carbon content without sacrificing performance or durability [226]. This not only enhances the bricks’ resistance to wear and corrosion in demanding AOD environments but also supports environmental goals by lowering emissions. While some older studies suggest that MgO-CaO refractories can be a cost-effective and robust alternative to more expensive or less environmentally harmful materials, ongoing research continues to focus on optimizing their high-temperature strength and service life for large converter operations [227].
Comparison with Magnesia–Carbon and Chromium-Based Refractories
Compared to MgO–C (magnesia–carbon) refractories, magnesia–doloma bricks offer a more sustainable solution, as they are free from carbon-based emissions and do not require graphite, which is a non-renewable resource [228]. While MgO–C refractories are well known for their superior thermal shock resistance due to the graphite phase, recent advances in magnesia–dolomite materials—such as the incorporation of nano-sized additives like Al2O3, ZrO2, or TiO2—have significantly improved their mechanical strength, hydration resistance, and thermal shock performance, narrowing the performance gap with MgO–C bricks [24].
Life Cycle Assessment (LCA) studies indicate that carbonless magnesia-based bricks generally have lower environmental impacts across most categories compared to MgO–C refractories, except in certain areas such as mineral resource scarcity, where impacts may be higher due to the sourcing of high-purity raw materials [229]. The addition of nano-engineered phases not only enhances the durability and service life of magnesia–doloma bricks but also supports cleaner steel production by reducing refractory contamination and gaseous emissions [213].
Moreover, magnesia–doloma bricks offer a significant environmental and health advantage over chromium-based refractories (such as MgO–Cr2O3), as they eliminate concerns related to the formation of carcinogenic hexavalent chromium (Cr6+) during service [17]. While chromite-containing bricks are known for their high chemical and slag resistance, especially in stainless steelmaking, their use is declining due to these environmental risks, as shown in Figure 13, and regulatory pressures [24].
Figure 13.
Risks of Cr6+ exposure in refractory systems and its implications on human health.
Studies show that MgO-CaO refractories, particularly those modified with spinel-forming additives like hercynite or perovskite-forming additives such as TiO2, can achieve comparable or even superior mechanical strength, hydration resistance, and corrosion resistance in basic and oxidizing slag conditions [230,231]. For example, the addition of hercynite to magnesia–doloma bricks improves both mechanical properties and hydration resistance, while TiO2 has been identified as a promising additive to tailor thermal expansion and enhance refractory performance [24,232].
Overall, MgO-CaO refractories represent a viable and sustainable solution for several stages of the steelmaking process, particularly when thermal efficiency, basic slag resistance, and environmental compliance are prioritized; see Table 8.

Table 8.
Comparison of refractory types used in steelmaking applications.
4.3.2. Cement and Lime Industry
MgO-CaO refractories are indeed widely used in the cement and lime industries, especially for rotary kiln and preheater linings due to their excellent resistance to basic slags, high-temperature stability, and cost-effectiveness [239]. These refractories offer several advantages, including high corrosion resistance, thermodynamic stability in basic environments, and suitability for clean production processes, making them a preferred alternative to chrome-containing refractories from both environmental and occupational health perspectives [18].
However, a limitation of MgO-CaO refractories is their relatively low hydration resistance, which can lead to premature degradation during storage or operation. Recent studies have demonstrated that the incorporation of additives such as hercynite spinel or nano-sized alumina can substantially improve hydration resistance, mechanical strength, and thermal shock performance, thereby increasing the durability and reliability of these refractories in harsh kiln environments [18,24]. In addition, technological innovations like fused dolomite–magnesia co-clinkers and high-density magnesia–lime formulations have further enhanced refractory performance, delivering high chemical purity, increased resistance to corrosion and hydration, and improved thermal shock resistance [87].
Thermal Spalling in Rotary Kilns and Preheaters
Thermal spalling in refractory linings most frequently occurs during rapid temperature changes, such as kiln startups, shutdowns, or unexpected process interruptions, which induce significant mechanical stress due to thermal expansion mismatches between the hot face and the colder interior of the brick [240]; see Figure 14. If the refractory material lacks sufficient thermal shock resistance or the ability to relax these stresses, surface cracking, delamination, or even full layer detachment can result, compromising the integrity of the kiln and leading to costly maintenance [241].
Figure 14.
Illustration of brick spalling. Dashed line denotes brick after thermal spalling.
Research highlights that structural spalling is often initiated by thermal stress and can be exacerbated by factors such as slag or alkali penetration, phase transformations with high thermal expansion, and the presence of microstructural defects [242]. The use of proper insulating materials and the optimization of refractory composition—such as incorporating silicon carbide or designing low-porosity aggregates—can significantly improve thermal spalling resistance and reduce the intensity of thermal shock [243]. Additionally, operational practices like controlled heating and cooling cycles, as well as regular maintenance, are essential to minimize the risk of spalling and extend refractory service life [240].
To mitigate thermal spalling, several strategies are implemented:
- Material selection: Refractories with moderate-to-high thermal expansion and controlled porosity, such as magnesia–doloma bricks enhanced with spinel-forming additives (MgAl2O4, hercynite), offer better thermal shock resistance [169].
- Microstructural design: The presence of fine, uniformly distributed pores in the refractory structure helps absorb thermal stresses and reduces crack propagation during thermal cycling [196].
- Operational controls: Controlled heating and cooling rates, along with regular kiln shell scanning, help reduce the severity of thermal gradients during operation [244].
In preheater zones, refractory linings face not only rapid temperature fluctuations but also intense mechanical abrasion and chemical attack from alkalis and sulfur compounds. To address these challenges, low-porosity magnesia–doloma bricks with enhanced thermal conductivity and high mechanical strength are preferred, as they better resist spalling and wear. Research demonstrates that modifying MgO-CaO refractories with nano-oxides such as TiO2, ZrO2, and Al2O3 significantly improves their resistance to both thermal spalling and chemical corrosion [15].
The addition of these nano-oxides leads to denser microstructures, increased cold crushing strength, and reduced porosity, which collectively enhance thermal shock resistance and decrease the tendency for hydration and chemical degradation [113]. For example, nano-sized ZrO2 promotes the formation of closed pores and strong phase bonding, improving slag penetration resistance and mechanical durability [245]. Similarly, nano-Al2O3 and ZnO nanoparticles have been shown to boost both mechanical strength and hydration resistance, making these modified refractories especially suitable for harsh preheater environments [18].
4.3.3. Other Industrial Applications
In tandem steelmaking, MgO-CaO refractories have found increasing use in several high-temperature industrial sectors, including the glass industry, technical ceramics, and non-ferrous metallurgy, due to their basic slag resistance, cost-effectiveness, and low environmental impact.
Glass Industry
Magnesia–doloma bricks are increasingly considered as chrome-free alternatives in glass melting furnaces, particularly in forehearths and regenerators for soda-lime and container glass production. Their basic chemical nature makes them compatible with alkali-rich glass environments, and their low silica content helps minimize silica dissolution and the formation of unwanted secondary phases in the glass melt [13]. However, compared to traditional fused cast refractories, MgO-CaO bricks generally have lower corrosion resistance, which limits their use in direct-contact zones with aggressive glass melts [214].
Advancements, such as the development of fused MgO-CaO co-clinkers and the incorporation of nano-oxide additives like nano-Al2O3, have significantly improved the hydration resistance, mechanical strength, and thermal shock resistance of these refractories, expanding their applicability in selected furnace areas [140]. These improvements are especially valuable in regions where environmental or regulatory concerns restrict the use of Cr2O3-bearing refractories, allowing MgO-CaO bricks to serve as effective, more sustainable alternatives in specific glass furnace linings [18].
Technical Ceramics
MgO-CaO refractories are also utilized in kilns for firing technical ceramics such as alumina, zirconia, or silicon nitride components. These kilns often operate under cyclic thermal conditions and contain chemically aggressive atmospheres, making the thermal shock resistance and chemical stability of modified MgO–Doloma bricks highly desirable. Additives such as spinel-forming hercynite or nano-TiO2 have been shown to improve their thermal cycling durability and reduce the risk of structural spalling, thereby extending kiln lining life and reducing downtime [246].
Non-Ferrous Metallurgy
In the non-ferrous sector, particularly in copper and nickel smelting or refining, MgO-CaO bricks are used in furnaces and converter linings due to their resistance to basic slags and sulfur-rich atmospheres. Their lower silica content minimizes slag foaming and reaction with acidic oxides. Additionally, the absence of Cr6+ formation supports safer working environments and facilitates waste management. When modified with ZrO2 or Fe2O3 nano-additives, these refractories demonstrate enhanced structural integrity and improved performance under oxidizing conditions typical of non-ferrous metallurgy processes [220,247].
5. Technical and Competitive Strengths
MgO-CaO refractories offer a combination of high refractoriness and excellent dimensional stability, which makes them suitable for high-temperature industrial applications such as steelmaking, cement, and non-ferrous metallurgy. Their strong resistance to basic slags, combined with good thermal shock performance (especially when modified with nanostructured additives), ensures reliable performance under cyclic thermal and mechanical loads [17].
In terms of critical material substitution, MgO-CaO refractories offer a viable alternative to chromium-based and high-alumina materials. Their Cr-free composition eliminates the risks associated with hexavalent chromium (Cr6+) formation during service, while the addition of performance-enhancing phases (Ti-perovskites or Fe-spinel) partially compensates for the mechanical and thermal benefits typically associated with alumina-rich systems [24,230,248].
Moreover, MgO-CaO refractories demonstrate strong adaptability to circular economy practices due to their relatively simple chemistry and compatibility with recycling processes. Research shows that waste magnesia and dolomite refractories can be efficiently recovered and reintegrated into new refractory products without significant loss of performance, especially when using advanced recycling methods such as the incorporation of recycled aggregates or the use of waste lime from industrial processes [249,250]. For example, up to 30% recycled magnesia–carbon aggregate can be used in new bricks without degrading mechanical or thermal properties, and recycled magnesia from spent bricks can fully replace virgin magnesia in magnesium phosphate cement mortars while maintaining satisfactory strength and durability [125].
These recycling strategies reduce dependence on virgin raw materials, lower production costs, and support sustainability goals by minimizing waste and carbon footprint [251]. Additionally, the use of high-purity co-clinkers and the recovery of magnesia from spent refractories further enhance the environmental benefits and resource efficiency of MgO-CaO systems [125]. This adaptability makes MgO-CaO a promising choice for industries seeking to implement closed-loop recycling and circular economy principles.
6. Current Limitations and Technological Challenges
Despite the numerous advancements in MgO-CaO refractories, several limitations persist that hinder their widespread industrial adoption and long-term performance optimization.
6.1. Hydration Susceptibility Without Additives
One of the most persistent issues is the susceptibility of magnesia–doloma bricks to hydration, particularly in humid storage environments or during shutdown cycles. In the absence of proper additives such as nano-alumina, hercynite, or TiO2, reactive CaO within the matrix readily absorbs moisture, leading to expansion, cracking, and severe mechanical degradation [18,19,114], as shown in Figure 15. This limitation remains critical, especially in applications where bricks are exposed to fluctuating atmospheric conditions or water vapor.

Figure 15.
Laboratory specimens of an MgO-CaO formulation without additives. Stored without protection; complete hydration observed after 24 days.
6.2. Cost and Availability of Nanoparticles
While nanostructured additives such as nano-alumina, nano-TiO2, and ZrO2 nanoparticles have demonstrated significant improvements in hydration resistance, mechanical strength, and thermal performance of magnesia–doloma bricks, their widespread industrial adoption faces notable barriers. The high cost and limited commercial availability of these nanoparticles restrict their use in large-scale applications, making them less accessible for routine refractory manufacturing [18,252].
Additionally, challenges such as achieving uniform dispersion of nanoparticles within the brick matrix, preventing agglomeration, and ensuring safe handling during production further complicate their practical implementation [203]. These issues can lead to inconsistent material properties and may offset the performance benefits observed in controlled laboratory settings.
6.3. Industrial Scalability and Microstructural Uniformity
Scaling laboratory-developed magnesia–doloma brick formulations to industrial production presents significant challenges in achieving consistent microstructure, particularly regarding additive distribution, pore morphology, and phase homogeneity. Variations in raw material quality, pressing conditions, and sintering profiles can introduce heterogeneity, which undermines the performance benefits of advanced formulations and can lead to unpredictable in-service behavior.
Additionally, the lack of semi-industrial testing and advanced processing technologies for new raw material sources, such as dolomite deposits, further complicates the transition from laboratory to industrial production. Ensuring reliable performance requires not only technological validation of raw materials but also optimization of manufacturing processes to minimize variability. Without careful control and validation at each production stage, the advantages of laboratory-optimized formulations may not translate to consistent, high-quality industrial products [14].
Recent advances in pilot-scale sintering of MgO-CaO refractories confirm that precise control of temperature gradients and atmosphere composition is crucial for achieving homogeneous microstructures and consistent densification during scale-up. Studies show that using controlled-atmosphere furnaces with adjustable oxygen partial pressure can minimize over-burning and grain coarsening, leading to improved uniformity and mechanical properties in large refractory batches [214]. The integration of digital process monitoring systems, such as in-line porosity and temperature sensors, enables real-time quality control and early detection of microstructural deviations, which significantly enhances production consistency.
Additionally, the use of nano-sized additives (nano-Al2O3 or nano-MgO) and optimized sintering parameters such as particle size selection, rapid heating rates, and targeted temperature profiles can further improve densification, reduce porosity, and promote uniform grain growth [11]. Collaborative pilot trials combining these process optimizations with machine-learning-based data analysis have demonstrated up to 20% reduction in product porosity variability, supporting scalable manufacturing of high-performance MgO-CaO refractories [253]. These strategies collectively offer a feasible pathway toward more reliable and energy-efficient refractory production.
6.4. Recycling of Complex MgO–C and MgO–CaO–X Systems
Recycling used MgO-CaO refractories, including MgO–C and MgO-CaO-X materials, is challenging due to the presence of residual carbon, oxidation products, and complex multi-phase microstructures, which complicate separation and reprocessing. Current recycling methods often require high energy inputs or generate secondary waste streams, reducing both environmental and economic viability. For example, while waste magnesia refractory brick powder can be repurposed in magnesium phosphate cement mortar, the resulting material may have slightly inferior mechanical properties compared to those made with virgin magnesia, though it still meets engineering requirements and offers good water resistance and volume stability [125].
Some research has explored the use of waste lime and other industrial by-products to produce low-cost refractories, but these approaches are generally limited to specific applications and do not fully address the broader recycling challenges [250].
7. Future Perspectives and Research Directions
Many studies support a multi-faceted strategy for advancing MgO–CaO refractories, emphasizing materials innovation, real-world validation, environmental assessment, and intelligent monitoring. Hybrid systems incorporating CaO–ZrO2–MgO–SiO2 or ZrO2–MgO–CaO compositions have been developed to further refine microstructural homogeneity, reduce porosity, and tailor thermal expansion, resulting in improved resistance to chemical attack and enhanced mechanical properties at both ambient and elevated temperatures [254].
CaO–ZrO2–MgO–SiO2 refractories have attracted significant attention due to their high-temperature stability and corrosion resistance, which make them suitable for demanding industrial applications. Within the CaO–ZrO2–SiO2 system, ternary compounds such as Ca3ZrSi2O9 and Ca2ZrSi4O12 can form, although no quaternary compounds have been detected. Thermodynamic analyses reveal that stable refractory fields exist around 1600 °C, but these regions decrease sharply at 2000 °C owing to the detrimental influence of SiO2, which restricts suitable compositions to binary or low-percentage ternary combinations at higher temperatures [255]. Furthermore, the incorporation of ZrO2 into MgO-based refractories enhances cold strength but may reduce hot strength due to the formation of low-melting phases such as CaMgSiO4. Because ZrO2 preferentially reacts with CaO-containing phases, refractories with lower SiO2 or CaO contents exhibit better high-temperature performance, indicating that compositional optimization is essential for achieving high-performance MgO–ZrO2 materials [256].
At the microstructural level, the formation of CaZrO3 at grain boundaries—particularly when nano-sized ZrO2 is used—plays a vital role in improving intergranular bonding, enhancing slag penetration resistance, and strengthening the refractory matrix [165]. When these materials are exposed to slags containing CaO, SiO2, and other oxides, dense reaction layers composed mainly of CaZrO3 can form, reducing open porosity and thereby improving corrosion resistance [257]. However, under certain metallurgical conditions, such as contact with molten alloys, these refractories can participate in interfacial reactions that generate inclusions and modify alloy composition, which must be carefully considered for applications such as superalloy processing [258].
In conjunction, ZrO2-MgO-CaO refractories have been extensively studied for their enhanced densification, thermal shock resistance, and corrosion resistance, making them attractive for high-temperature service environments. The incorporation of nano-sized ZrO2 into MgO-CaO matrices promotes the in situ formation of CaZrO3 along grain boundaries, improving sintering behavior, increasing bulk density, and reducing porosity. These microstructural refinements contribute to better hydration and slaking resistance [259], and nano-sized ZrO2 proves to be more effective than its micro-sized counterpart owing to its higher surface reactivity and better dispersion, which allow smaller additions to achieve equivalent performance gains [260].
The presence of ZrO2 also enhances the viscosity of the liquid phase during slag exposure, thereby inhibiting slag infiltration and improving corrosion resistance. Nevertheless, excessive ZrO2 additions can adversely affect hydration resistance, emphasizing the need for optimal compositional balance [49]. In cement rotary kiln linings, MgO–CaO–ZrO2 refractories have demonstrated excellent thermal shock behavior, high softening temperature under load, and outstanding erosion resistance—properties attributed to their composite mineral structure rich in calcium silicate and zirconate phases [261].
Another critical research goal is validating the performance of optimized magnesia–doloma compositions under real operating conditions, which is a recognized research priority, but most available studies focus on laboratory-scale evaluations rather than full-scale industrial trials. Laboratory investigations have demonstrated that additives such as nano- and micro-alumina, hercynite spinel, and optimized dolomite blends can significantly improve hydration resistance, mechanical strength, and microstructural stability, as confirmed by X-ray diffraction and scanning electron microscopy analyses. These studies typically assess properties like compressive strength, cold crushing strength, and thermal shock resistance, and some have monitored microstructural evolution after exposure to simulated service conditions [262].
However, there is a lack of published pilot-scale or full-scale industrial trials that quantitatively assess long-term wear rates, spalling resistance, and post-service microstructural changes under fluctuating thermal, chemical, and mechanical loads. Most research calls for such real-world validation to provide the necessary feedback for further optimization and standardization of advanced MgO-CaO refractories. Bridging this gap will require coordinated efforts to conduct comprehensive field trials and systematic post-service analyses, ensuring that laboratory improvements translate into reliable in-service performance [18,24].
With these technical challenges, sustainability considerations are becoming increasingly relevant. Life Cycle Assessment (LCA) studies are now essential for guiding sustainable material selection and process improvements in the refractory industry. LCA enables quantitative comparison of environmental footprints, including CO2 emissions, energy consumption, and raw material depletion, which is crucial as environmental regulations tighten and circular economy goals become more prominent. An LCA of refractory waste management in a Spanish steelworks found that current practices—such as direct reuse and recycling of spent magnesia–carbon bricks—significantly reduce CO2 emissions, non-renewable energy use, land occupation, and water consumption compared to landfilling, with direct reuse offering the greatest benefits [25,263].
Other LCA studies on refractory brick production highlight that raw material extraction and the firing processes are major contributors to environmental impacts, including global warming potential and resource depletion [264]. Comparative LCA of different refractory brick systems (magnesia–carbon vs. magnesia–alumina) also shows that alternative formulations can substantially lower environmental impacts across multiple categories [237].
Figure 16 illustrates the life-cycle stages of MgO-CaO refractories, emphasizing reuse, formulation adjustment, and environmentally responsible disposal routes that contribute to the sustainability of refractory production systems.
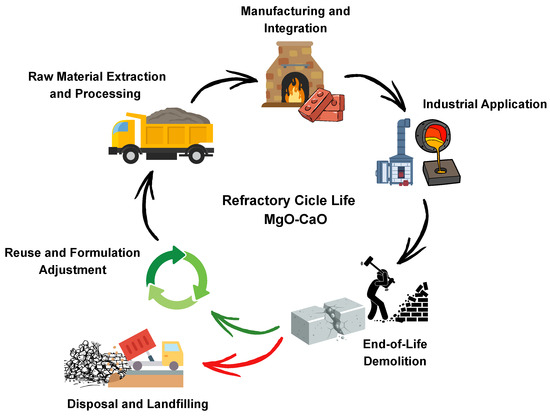
Figure 16.
Life Cycle Assessment (LCA) schematic for MgO–CaO refractories.
Finally, the integration of functional sensors and intelligent monitoring technologies into refractory bricks is rapidly advancing the concept of “smart refractories,” shown in Figure 17. Recent developments include embedding high-temperature electroceramic sensors—such as thermocouples, thermistors, and spallation/crack detectors—directly within refractory bricks, enabling real-time monitoring of temperature, strain, and corrosion or erosion processes without compromising the brick’s structural integrity [265,266]. These embedded sensors can withstand harsh environments (up to 1500 °C and high pressures), and their data can be wirelessly transmitted to central processing hubs for continuous health assessment and predictive maintenance [267].
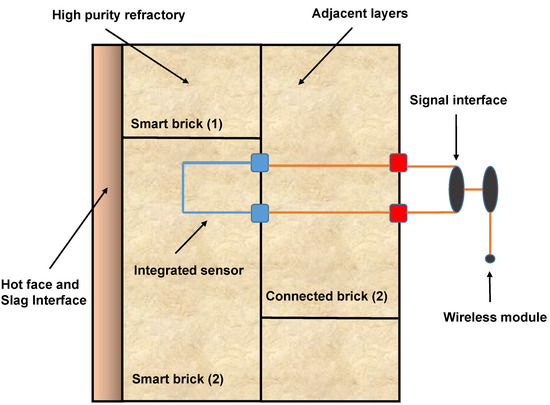
Figure 17.
Schematics of the refractory wall incorporating a smart refractory brick.
This approach allows for in–situ tracking of critical parameters, early detection of failure mechanisms, and the implementation of predictive maintenance strategies, significantly extending refractory service life and reducing unplanned downtime [268]. Advanced systems also incorporate models to estimate degradation and optimize sensor placement, while commercial solutions like radar-based Refractory Thickness Sensors and Furnace Tomography Sensors provide actionable, real-time data for asset management in industrial furnaces [269]. The fusion of materials engineering with Industry 4.0 concepts thus paves the way for next-generation smart refractories capable of self-diagnosis and enhanced operational reliability [270].
8. Discussion
8.1. Research Evolution and Thematic Shifts in Magnesia–Dolomite Refractory Materials
Figure 18 and Figure 19 illustrate the temporal distribution and thematic focus of scientific publications on refractory materials between 2018 and 2025. The data reveal a sustained increase in research activity, particularly from 2021 onward, driven by the growing demand for high-performance and environmentally sustainable refractories in metallurgical and cement industries.
Figure 18.
(A) Annual distribution of publications on refractory materials and aluminum oxide from 2018 to 2025. (B) Overall percentage of publications comparing refractory materials and aluminum oxide studies, showing a balanced research focus (51.86% and 48.14%, respectively). (C) Yearly percentage distribution highlighting the relative contribution of each year to the total scientific output. Together, the graphs illustrate the sustained growth and diversification of research interest in high-temperature ceramic materials.
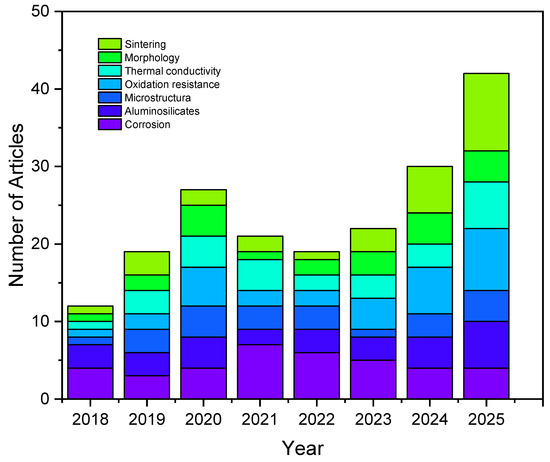
Figure 19.
Annual distribution of articles by research topic in refractory material studies (2018–2025).
As seen in Figure 18, studies on general refractory materials represent 51.86% of total publications, while aluminum oxide accounts for 48.14%. This near parity indicates that alumina-based refractories continue to dominate the research landscape, largely due to their high melting point, mechanical strength, and oxidation resistance [271]. However, a gradual shift toward magnesia–dolomite formulations is evident after 2023, aligned with the need for improved corrosion resistance against basic slags and lower environmental impact [272].
The thematic distribution in Figure 19 highlights that early studies (2018–2020) concentrated on sintering processes and morphology control, reflecting efforts to optimize density and porosity for improved mechanical performance. From 2021 onward, research diversified toward thermal conductivity, oxidation resistance, and microstructural design—key factors determining energy efficiency and durability in high-temperature applications [209]. In 2024–2025, there is a pronounced emergence of works focusing on corrosion and aluminosilicate integration, indicating a strategic trend toward multi-phase, self-healing, and thermochemically stable composites.
This evolution demonstrates the convergence of traditional ceramic processing with modern computational materials science and sustainable manufacturing. The integration of finite element (FE) modeling, life-cycle assessment (LCA), and AI-assisted design tools is expected to redefine refractory development in the coming decade [273,274]. These advancements aim to reduce the ecological footprint of refractory production while enhancing thermal performance, corrosion resistance, and structural integrity during service.
The bibliometric trends confirm a paradigm shift toward high-efficiency, environmentally conscious refractory systems. The growing number of publications on corrosion resistance and oxidation control underscores the importance of developing materials that ensure operational reliability under extreme conditions, reinforcing the vital role of MgO-CaO composites in the future of refractory technology.
8.2. Comparative Discussion with State-of-the-Art Reviews on Magnesia–Dolomite Refractories
Table 9 presents a detailed comparison between this study and main recent reviews concerning magnesia dolomite refractories. While previous works have explored specific aspects such as thermal behavior, microstructural design, or resource availability, few integrate these components holistically. This work stands out for incorporating thermochemical fundamentals, microstructural innovation, sustainability, and systematic review methodology under a unified framework.

Table 9.
Comparison of this work with recent reviews on magnesia–dolomite refractories. (✓: included; ✕: not addressed).
As evidenced in Table 9, earlier reviews provide fragmented insights: Resio (2024) [72] focuses on dolomite thermal decomposition and phase transformation, while Guler et al. (2024) [79] discuss high-entropy oxides with outstanding thermal stability but omit CaO–MgO systems. Kundu and Sarkar (2021) [56] establish reference benchmarks for MgO–C refractories, yet lack comparative evaluation with dolomitic counterparts. Ghasemi-Kahrizsangi et al. (2017) [22] emphasize nano-additive technology and microstructure refinement, whereas Alhaddad and Ahmed (2022) [275] address mineralogical resources without connecting to material performance.
By contrast, the present work (✓ in all categories) integrates these dimensions into a single framework, combining (1) thermochemical understanding of dolomite decomposition; (2) comparison across MgO–C and high-entropy analogs; (3) microstructural tailoring via nano-additives; (4) sustainability and raw material circularity; and (5) the PRISMA methodology for systematic transparency. This unified scope enables reproducible analysis and defines pathways for next-generation MgO–CaO refractories in industrial applications.
9. Conclusions
MgO-CaO refractories have emerged as sustainable alternatives to traditional carbon- and chromium-containing linings used in the steelmaking and cement industries. The reviewed studies demonstrate that advances in raw material purity, sintering control, and nano-engineering have led to remarkable improvements in densification, hydration resistance, and mechanical integrity. In particular, the incorporation of nano-additives such as TiO2, MgAl2O4, and FeAl2O4 has been shown to refine the microstructure, reduce open porosity, and enhance resistance to basic slag corrosion.
Despite these advances, several challenges remain unresolved. Long-term stability under cyclic thermal and chemical conditions, particularly in humid environments, continues to limit large-scale industrial deployment. Moreover, the variability in synthesis routes and testing methodologies makes it difficult to establish universal correlations between microstructure, composition, and performance. The lack of standardization in evaluating hydration kinetics and thermal spalling resistance also restricts reproducibility and comparability among studies.
Future research should focus on integrating advanced computational modeling, thermodynamic simulations, and machine learning techniques to predict phase evolution and optimize compositions in real time. Combining these digital approaches with experimental validation will enable the design of next-generation MgO-CaO refractories with improved service life and reduced environmental footprint. Furthermore, adopting circular economy strategies—such as recycling spent refractories and conducting life-cycle assessments (LCA)—will strengthen the role of these materials in achieving sustainable high-temperature industrial processes.
Author Contributions
Conceptualization, L.D.-T., J.F.L.-P. and E.A.R.-C.; Methodology, L.A.I.C. and M.A.; Software, L.A.I.C. and J.R.-R.; Validation, L.D.-T., J.F.L.-P. and J.R.-R.; Formal analysis, M.A. and E.A.R.-C.; Investigation, L.D.-T. and L.A.I.C.; Resources, J.F.L.-P. and J.R.-R.; Data curation, L.A.I.C. and M.A.; Writing—original draft preparation, L.D.-T. and L.A.I.C.; Writing—review and editing, E.A.R.-C. and J.R.-R.; Visualization, L.D.-T. and J.F.L.-P.; Supervision, E.A.R.-C. and J.R.-R.; Project administration, L.A.I.C. and J.R.-R.; Funding acquisition, J.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, S.; Xin, J.; Chen, R. Achieving high strength and low thermal conductivity: Additive manufacturing of mullite lightweight refractory. Ceram. Int. 2024, 50, 27880–27888. [Google Scholar] [CrossRef]
- Funch, C.V.; Proust, G. Laser-based additive manufacturing of refractory metals and their alloys: A review. Addit. Manuf. 2024, 94, 104464. [Google Scholar] [CrossRef]
- Ergashev, M. Exploring ceramic refractory materials: Classification and technological innovations. Int. J. Adv. Sci. Res. 2024, 4, 17–26. [Google Scholar] [CrossRef]
- Gilchrist, J.D. Classification of Refractories. In Fuels, Furnaces and Refractories; Elsevier: Amsterdam, The Netherlands, 1977; pp. 237–239. [Google Scholar] [CrossRef]
- Sengupta, P. Refractory: Characterization. In Refractories for the Chemical Industries; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Chandra, K.S.; Sarkar, D. Nanoscale reinforcement efficiency analysis in Al2O3–MgO–C refractory composites. Mater. Sci. Eng. A 2023, 865, 144613. [Google Scholar] [CrossRef]
- Sarkar, R. Refractory Technology: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Reitz, W. A Review of: “Refractories Handbook”. Mater. Manuf. Processes 2005, 20, 893–894. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Wojsa, J.; Psiuk, B.; Wala, T. Influence of zirconia addition on the properties of magnesia refractories. Ceram. Int. 2016, 42, 11373–11386. [Google Scholar] [CrossRef]
- Hou, Q.; Luo, X.; Xie, Z.; Li, Y.Z.; An, D.; Li, J. Preparation and characterization of microporous magnesia-based refractory. Int. J. Appl. Ceram. Technol. 2020, 17, 2629–2637. [Google Scholar] [CrossRef]
- Fu, L.; Yue, J.; Liu, W.; Han, Z.; Bai, D.; Xu, G. Analysis and experiment of sintering and densification of magnesia particles. Chem. Eng. Sci. 2022, 268, 118396. [Google Scholar] [CrossRef]
- Sado, S.; Jastrzębska, I.; Zelik, W.; Szczerba, J. Self-organizing maps as a tool to assess possible substitution of fused by sintered MgO aggregates in MgO–C refractories. Ceram. Int. 2024, 50, 14996–15012. [Google Scholar] [CrossRef]
- Sadik, C.; Moudden, O.; Bouari, A.E.; Amrani, I.E. Review on the elaboration and characterization of ceramics refractories based on magnesite and dolomite. J. Asian Ceram. Soc. 2016, 4, 219–233. [Google Scholar] [CrossRef]
- Asabaev, D.K.h.; Badalov, F.A.; Normurodov, A.A. Dolomites, their formation conditions and features of territorial distribution in western uzbekistan. J. Geogr. Reg. Plan. Dev. 2024, 1, 1–3. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S.; Karamian, E.; Shahmohammadian, F. Hydration resistance improvement of doloma particles using different nanoparticles. Ceram. Int. 2019, 45, 7390–7396. [Google Scholar] [CrossRef]
- Mohammadihooyeh, M.; Karamian, E.; Emadi, R. Effect of magnesium-aluminate spinel nano-particles on microstructure and properties behaviors of doloma-containing refractories. Ceram. Int. 2020, 46, 1662–1667. [Google Scholar] [CrossRef]
- Antonov, G.I.; Nedosvitii, V.P.; Kulik, A.S.; Semenenko, O. Stabilized Dolomite Refractories. Refract. Ind. Ceram. 2004, 45, 160–164. [Google Scholar] [CrossRef]
- Shahraki, A.; Ghasemi-Kahrizsangi, S.; Nemati, A. Performance improvement of MgO-CaO refractories by the addition of nano-sized Al2O3. Mater. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Li, Y.; Li, F.; Yan, W.; Shi, H. Effect of TiO2 addition on microstructures and properties of MgO–CaO refractory aggregates. J. Iron Steel Res. Int. 2024, 31, 1547–1554. [Google Scholar] [CrossRef]
- Kashaninia, F.; Sarpoolaky, H.; Naghizadeh, R.; Bagheri, A.; Zamanipour, M. Improving hydration resistance of magnesia-doloma refractories byiron oxide addition. Iran. J. Mater. Sci. Eng. 2011, 8, 34–40. [Google Scholar]
- Rabah, M.; Ewais, E. Multi-impregnating pitch-bonded Egyptian dolomite refractory brick for application in ladle furnaces. Ceram. Int. 2009, 35, 813–819. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Karamian, E.; Dehsheikh, H.G.; Ghasemi-Kahrizsangi, A. A Review on Recent Advances on Magnesia-Doloma Refractories by Nano-Technology. J. Water Environ. Nanotechnol. 2017, 2, 206–222. [Google Scholar] [CrossRef]
- Obregón, Á.; Rodríguez-Galicia, J.L.; López-Cuevas, J.; Pena, P.; Baudín, C. MgO–CaZrO3-based refractories for cement kilns. J. Eur. Ceram. Soc. 2011, 31, 61–74. [Google Scholar] [CrossRef]
- Díaz-Tato, L.; López-Perales, J.F.; Contreras, J.M.; Banda-Muñoz, F.; Suárez-Suárez, D.; González-Carranza, Y.; Gómez-Rodríguez, C.; Rodríguez, E. Hydration resistance and mechano-physical properties improvement of a magnesia-dolomite dense refractory by hercynite spinel. Mater. Chem. Phys. 2022, 287, 126314. [Google Scholar] [CrossRef]
- Muñoz, I.; Soto, A.; Maza, D.; Bayón, F. Life cycle assessment of refractory waste management in a Spanish steel works. Waste Manag. 2020, 111, 1–9. [Google Scholar] [CrossRef]
- Klitzsch, M.; Geith, M. Setting New Standards for Circular Economy in the Cement Industry. In REWAS 2022: Developing Tomorrow’s Technical Cycles; Springer: Cham, Switzerland, 2022; Volume I. [Google Scholar] [CrossRef]
- Kara, S.; Erdem, S.; Lezcano, R. MgO-Based Cementitious Composites for Sustainable and Energy Efficient Building Design. Sustainability 2021, 13, 9188. [Google Scholar] [CrossRef]
- Sengupta, P. Manufacturing and Properties of Refractories. In Refractories for the Cement Industry; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Lee, W.E.; Vieira, W.; Zhang, S.; Ahari, K.G.; Sarpoolaky, H.; Parr, C. Castable refractory concretes. Int. Mater. Rev. 2001, 46, 145–167. [Google Scholar] [CrossRef]
- Burgos-Montes, O.; Álvarez, M.; Aza, A.H.; Pena, P.; Baudín, C. The main role of silica—Based cement free binders on the microstructural evolution and mechanical behaviour of high alumina castables. J. Eur. Ceram. Soc. 2018, 38, 4137–4148. [Google Scholar] [CrossRef]
- Tomsu, F.; Palco, S. Refractory Monolithics versus Shaped Refractory Products. Interceram—Int. Ceram. Rev. 2017, 66, 20–23. [Google Scholar] [CrossRef]
- Horckmans, L.; Nielsen, P.; Dierckx, P.; Ducastel, A. Recycling of refractory bricks used in basic steelmaking: A review. Resour. Conserv. Recycl. 2019, 140, 297–304. [Google Scholar] [CrossRef]
- Jassim, A.; Salmtori, S.A.; Jassam, J. Sustainable manufacturing process applied to produce magnesium oxide from sea water. IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012021. [Google Scholar] [CrossRef]
- Wagri, N.K.; Carlborg, M.; Eriksson, M.; Ma, C.; Broström, M.; Andersson, B. High temperature interactions between coal ash and MgO-based refractories in lime kiln conditions. Fuel 2023, 342, 127711. [Google Scholar] [CrossRef]
- Chowdhury, A.; Panda, C.R. Preparation of high pure refractory grade magnesium oxide from east coast sea water. Indian J. Chem. Technol. 2023, 30, 614–622. [Google Scholar] [CrossRef]
- Hou, X.; Miao, Z.; Du, Y.; Chen, J.; Cao, Y.; Yan, W.; Xia, Y.; Wang, L.; Zhang, S.; Li, N. Fabrication Y2O3-doped MgO refractory raw materials based on magnesium hydroxide from salt-lake brine. Ceram. Inc. 2024, 50, 42729–42738. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Xu, Y.; Zhao, J.; Zhao, W.; Sun, Z.; Yang, D.; Ren, L.; Zhao, X.; Yan, H.; et al. Oxidation and corrosion resistance of resin-bonded magnesia-based refractories reinforced by CaB6 addition for secondary refining. Ceram. Inc. 2024, 50, 40700–40712. [Google Scholar] [CrossRef]
- Park, S.H.; Jeon, S.M.; Jang, J. Characteristic Behavior of Hydration of Magnesium Oxide. In Geotechnics for Sustainable Infrastructure Development; Springer: Singapore, 2019; pp. 1275–1279. [Google Scholar] [CrossRef]
- Park, S.H.; Ma, J.; Yun, T.; Jeon, S.M.; Byeun, Y.K.; Kang, D.; Jang, J. Pore-scale swelling mechanism of magnesium oxide granules during hydration. Constr. Build. Mater. 2020, 251, 119101. [Google Scholar] [CrossRef]
- Fedoročková, A.; Raschman, P.; Sučik, G.; Švandová, M.; Doráková, A. Reactive, Sparingly Soluble Calcined Magnesia, Tailor-Made as the Reactive Material for Heavy Metal Removal from Contaminated Groundwater Using Permeable Reactive Barrier. Minerals 2021, 11, 1153. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Rigaud, M. Sinterability of macrocrystalline and cryptocrystalline magnesite to refractory magnesia. Int. J. Ceram. Eng. Sci. 2020, 2, 303–309. [Google Scholar] [CrossRef]
- Huang, W.; Yu, S.; Wu, X.; Zhang, Q. Effect of firing temperature on the densification and properties of dead burned magnesia. J. Eur. Ceram. Soc. 2018, 38, 4140–4146. [Google Scholar] [CrossRef]
- Bouchekrit, C.; Kolli, M.; Altıner, M.; Doufnoune, R. Synthesis of high purity magnesia MgO from Algerian dolomite ore. J. Min. Metall. Sect. B Metall. 2023, 59, 53–64. [Google Scholar] [CrossRef]
- Zhao, X.; You, J.; Yue, J.; Luo, X.; Ma, B. Microstructure and Mechanistic analysis of high-calcium fused magnesia molten heap. J. Aust. Ceram. Soc. 2024, 60, 1529–1539. [Google Scholar] [CrossRef]
- Bilge, A.; Yaman, C.; Sarıoğlu, N. Turkey’s Magnesite for Production of Fused Magnesia, Properties and Uses in Refractory Applications. In Proceedings of the Processing Technology, 60th Inter Colloquium on Refractories EUROGRESS, Aachen, Germany, 18–19 October 2017. [Google Scholar]
- Chen, C.; Ma, S.; Sun, M.; Wang, Y.; Cao, H.; Zhang, M.; Li, Y.; Jiang, Z. Influence of refractories on cleanliness in high-purity C96V saw wire steel. Can. Metall. Q. 2024, 64, 1633–1648. [Google Scholar] [CrossRef]
- Cui, K.; Fu, T.; Zhang, Y.; Wang, J.; Mao, H.; Tan, T. Microstructure and mechanical properties of CaAl12O19 reinforced Al2O3-Cr2O3 composites. J. Eur. Ceram. Soc. 2021, 41, 7935–7945. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Wang, X.; Zhang, J.; You, D.; Wang, Y. Properties of CaO-MgO-SiO2 insulating refractories prepared from phosphorus tailings. Ceram. Inc. 2024, 50, 50867–50875. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Y.; Chen, J.; Li, N.; Han, B. Preparation of CaO-MgO-ZrO2 refractory and its desulfurization effect on Ni-based alloy in vacuum induction melting (VIM). J. Aust. Ceram. Soc. 2019, 56, 885–894. [Google Scholar] [CrossRef]
- Liu, N.; Gu, H.; Fu, L.; Huang, A.; Zhang, M. Improving corrosion resistance to CaO-Al2O3-SiO2 slag: Role of a novel dense calcium hexaluminate raw material. Constr. Build. Mater. 2025, 464, 140206. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, N.; Fan, S.; Sun, S.; Hou, G.; Li, J.; Zhou, Y.; Sun, X. Interfacial Desulfurization Reaction between Binary Ni-Al/Ti/Ta Alloy Melt and CaO Ceramic Refractory. Surf. Interfaces 2023, 44, 103718. [Google Scholar] [CrossRef]
- Cheng, Y.; Duan, S.; Zhang, L. Comparison Study of the Effect of MgO, MgO-CaO, MgO-Al2O3-C, and MgO-C Refractories on Cleanliness of a SiMn-Killed Steel. Steel Res. Int. 2025, 96, 535–555. [Google Scholar] [CrossRef]
- Xu, T.; Su, Y.; Shi, T.; Zhang, X. Improving hydration resistance of MgO–CaO ceramics by in situ synthesized CaZrO3 coatings prepared using a non-hydrolytic sol. Ceram. Inc. 2021, 47, 2165–2171. [Google Scholar] [CrossRef]
- Zan, W.; Ma, B.; Liu, K.; Yu, C.; Liu, H.; Wang, Z.; Deng, C.; Zhu, Q. Effects of YSZ and CA6 additives on densification and thermal shock resistance of Al2O3-MgO-CaO-Y2O3 refractories. Mater. Sci. Eng. A 2024, 901, 146564. [Google Scholar] [CrossRef]
- Ren, X.; Ma, B.; Li, S.; Li, H.; Liu, G.; Yang, W.; Qian, F.; Zhao, S.; Yu, J. Comparison study of slag corrosion resistance of MgO–MgAl2O4, MgO–CaO and MgO–C refractories under electromagnetic field. J. Iron Steel Res. Int. 2020, 28, 38–45. [Google Scholar] [CrossRef]
- Kundu, R.; Sarkar, R. MgO-C Refractories: A Detailed Review of These Irreplaceable Refractories in Steelmaking. Interceram—Int. Ceram. Rev. 2021, 70, 46–55. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on low carbon containing MgO-C refractory: Use of nano carbon. Ceram. Inc. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Bavand-vandchali, M.; Naghizadeh, R. Characterization and post-mortem analysis of Al2O3-MgO-C refractories used in steelmaking ladle furnaces. Eng. Fail. Anal. 2020, 116, 104697. [Google Scholar] [CrossRef]
- Behera, S.; Sarkar, R. Formation of in-situ Ceramic Phase in N220 Nano Carbon Containing Low Carbon Mgo-C Refractory. World Acad. Sci. Eng. Technol. Int. J. Mater. Metall. Eng. 2015, 2. Available online: https://dspace.nitrkl.ac.in/dspace/handle/2080/2367 (accessed on 10 November 2025).
- Filkoski, V.R.; Petrovski, J.; Gjurchinovski, Z. Energy optimisation of vertical shaft kiln operation in the process of dolomite calcination. Therm. Sci. 2018, 22, 2123–2135. [Google Scholar] [CrossRef]
- Stein, V.; Aneziris, C.; Gueguen, E.; Hill, K. A Prospective Way to Reduce Emissions in Secondary Steel Making Metallurgy by Application of Functionalized Doloma Carbon Refractories. Int. J. Appl. Ceram. Technol. 2011, 9, 615–624. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, Q.; Zhang, J.; Yao, X.; Yu, W. Numerical simulation of the dolomite in-situ desulfurization in molten iron. Mater. Res. Express 2023, 10, 016512. [Google Scholar] [CrossRef]
- Moorkah, H.I.; Abolarin, M.S. Investigation of the Properties of Locally Available Dolomite for Refractory Applications. Niger. J. Technol. 2005, 24, 79–86. [Google Scholar]
- Cheremisina, E.; Lesiak, S.; Rieger, J.; Schenk, J.; Firsbach, F.; Johnson, W.; Chopin, T.; Nispel, M. Assessment of the dissolution rate and behaviour of raw dolomite and limestone with different calcination degrees in primary steelmaking slags. Ironmak. Steelmak. 2022, 50, 379–391. [Google Scholar] [CrossRef]
- Dushevina, A. Study of the strength of caustic dolomite-based materials. Mech. Technol. 2024, 229–238. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar] [CrossRef]
- Mcintosh, R.M.; Sharp, J.; Wilburn, F. The thermal decomposition of dolomite. Thermochim. Acta 1990, 165, 281–296. [Google Scholar] [CrossRef]
- Subagjo; Wulandari, W.; Adinata, P.M.; Fajrin, A. Thermal decomposition of dolomite under CO2-air atmosphere. AIP Conf. Proc. 2017, 1805, 040006. [Google Scholar] [CrossRef]
- Sandu, V.C.; Selejan, A.D.; Cormos, C.; Pop, A.; Cormos, A. High-temperature dolomite decomposition: An integrated experimental and computational fluid dynamics analysis for calcium looping and industrial applications. Appl. Therm. Eng. 2024, 253, 123742. [Google Scholar] [CrossRef]
- Valverde, J.; Perejón, A.; Medina-Carrasco, S.; Pérez-Maqueda, L. Thermal decomposition of dolomite under CO2: Insights from TGA and in situ XRD analysis. Phys. Chem. Chem. Phys. 2015, 17, 30162–30176. [Google Scholar] [CrossRef] [PubMed]
- Sivrikaya, O. A study on the physicochemical and thermal characterisation of dolomite and limestone samples for use in ironmaking and steelmaking. Ironmak. Steelmak. 2018, 45, 764–772. [Google Scholar] [CrossRef]
- Resio, L.C. Dolomite thermal behaviour: A short review. Phys. Chem. Miner. 2024, 51, 19. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, Q.; Wu, G.; Yang, J.; Xu, M.; Ao, W.; Chen, Q. Recycling of Burned Dolomite Powder in Steelmaking. Metallurgist 2014, 57, 862–868. [Google Scholar] [CrossRef]
- Lesiak, S.; Cheremisina, E.; Rieger, J.; Schenk, J.; Firsbach, F.; Johnson, W.; Chopin, T.; Nispel, M. Calcination Condition of Dolomite-Based Materials Influencing Static Dissolution in Synthetic Electric Arc Furnace Slag. Steel Res. Int. 2022, 93, 2100675. [Google Scholar] [CrossRef]
- Feliciano, C.; Hernández, J.; Maldonado, J.; Cortes, K.; Schemmel, T.; Jansen, H. Use of MgO Briquettes in Electric Arc Furnaces: Principles and Industrial Experiences at Comsinac-Casimas. In Proceedings of the 6th Steel Industry Conference and Exposition (CONAC 2014), Monterrey, Mexico, 24 March 2014. [Google Scholar]
- Yu, Y.; Hwang, D.; Ahn, Y.; Cho, K.; Ahn, J.W.; Choi, J. A Comparative Study on the Calcination and Hydration of Dolomite Using Microwave and Electric Furnaces. J. Korean Soc. Miner. Energy Resour. Eng. 2021, 58, 107–118. [Google Scholar] [CrossRef]
- Sahu, N.; Biswas, A.; Kapure, G. Development of Refractory Material from Water Quenched Granulated Ferrochromium Slag. Miner. Process. Extr. Metall. Rev. 2016, 37, 255–263. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Tang, Y.; Xin, Y.; Yuan, H.; Ren, X.; Wan, Q. Synthesis mechanism and properties of lightweight mullite-corundum refractories obtained through high temperature liquid-assisted micrometer-scale Kirkendall effect. Ceram. Inc. 2020, 47, 9234–9244. [Google Scholar] [CrossRef]
- Guler, S.H.; Yakin, A.; Guler, O.; Chattopadhyay, A.K.; Şimşek, T. A critical review of the refractory high-entropy materials: RHEA Alloys, Composites, Ceramics, Additively Manufactured Alloys. Curr. Appl. Phys. 2024, 70, 87–124. [Google Scholar] [CrossRef]
- Miracle, D.; Tsai, M.; Senkov, O.; Soni, V.; Banerjee, R. Refractory high entropy superalloys (RSAs). Scr. Mater. 2020, 187, 445–452. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Gu, F.; Yang, L.; Tian, W.; Zhong, Q.; Rao, M.; Li, G.; Jiang, T. Chromium-promoted preparation of forsterite refractory materials from ferronickel slag by microwave sintering. Ceram. Inc. 2021, 47, 10809–10818. [Google Scholar] [CrossRef]
- Nanda, S.; Choudhury, A.; Chandra, K.; Sarkar, D. Raw materials, Microstructure, and Properties of MgO–C refractories: Directions for Refractory Recipe Development. J. Eur. Ceram. Soc. 2022, 43, 14–36. [Google Scholar] [CrossRef]
- Chandra, K.S.; Sarkar, D. Refractories and Failures. In Ceramic Processing; CRC Press: Boca Raton, FL, USA, 2019; pp. 167–213. [Google Scholar] [CrossRef]
- Kullatham, S.; Sirisoam, T.; Lawanwadeekul, S.; Thiansem, S. Forsterite refractory brick produced by talc and magnesite from Thailand. Ceram. Inc. 2022, 48, 30272–30281. [Google Scholar] [CrossRef]
- Kizinievič, O.; Gencel, O.; Kizinievič, V.; Sutcu, M.; Skamat, J. Recycling of dolomite powder in clay bricks: Effects on characteristics and gas release. Constr. Build. Mater. 2023, 404, 133217. [Google Scholar] [CrossRef]
- Yeprem, H.A.; Hübner, H. Effect of Fe2O3 Additions on Sinterability of Konya Dolomite of Turkey. Key Eng. Mater. 2004, 264–268, 1819–1822. [Google Scholar] [CrossRef]
- Suvorov, S.A.; Nazmiev, M.I.; Baranov, A.P.; Dmitrienko, A.A. A High-Density Water-Resistant Magnesia-Lime Material Based on Dolomite. Refract. Ind. Ceram. 2005, 46, 217–219. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Yan, W.; Schafföner, S.; Guiyuan, W.; Dai, Y.; Li, Y. A novel lightweight periclase-composite (Mg8−xFex+yAl16−yO32) spinel refractory material for cement rotary kilns. Ceram. Inc. 2021, 48, 615–623. [Google Scholar] [CrossRef]
- Yrjas, P.; Iisa, K.; Hupa, M. Limestone and dolomite as sulfur absorbents under pressurized gasification conditions. Fuel 1996, 75, 89–95. [Google Scholar] [CrossRef]
- Ay, Ş.; Atakül, H.; Sarioglan, A.; Akgün, F.; Isik-gulsac, I.; Çetin, Y.; Üresin, E.; Er, O.O.; Aksoy, P. Hot Gas Clean-Up with Dolomites: Effect of Gas Composition on Sulfur Removal Activity. Can. J. Chem. Eng. 2015, 93, 1643–1650. [Google Scholar] [CrossRef]
- Hycnar, E.; Ratajczak, T.; Sęk, M. Dolomites as SO2 Sorbents in Fluid Combustion Technology. Resources 2020, 9, 121. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, A.; Zou, Y.; Gu, H.; Fu, L.; Li, G. Corrosion modeling of magnesia aggregates in contact with CaO–MgO–SiO2 slags. J. Am. Ceram. Soc. 2020, 103, 2128–2136. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Dang, J.; Guo, J.; Zhou, H.; Lü, X. Viscosity and structure relationship with equimolar substitution of CaO with MgO in the CaO–MgO–Al2O3–SiO2 slag melts. Int. J. Miner. Metall. Mater. 2025, 32, 70–79. [Google Scholar] [CrossRef]
- Kong, W.; Liu, J.; Yu, Y.; Hou, X.; He, Z. Effect of w(MgO)/w(Al2O3) ratio and basicity on microstructure and metallurgical properties of blast furnace slag. J. Iron Steel Res. Int. 2021, 28, 1223–1232. [Google Scholar] [CrossRef]
- Li, P.; Ning, X. Effects of MgO/Al2O3 Ratio and Basicity on the Viscosities of CaO-MgO-SiO2-Al2O3 Slags: Experiments and Modeling. Metall. Mater. Trans. B 2016, 47, 446–457. [Google Scholar] [CrossRef]
- Pang, Z.; Lv, X.; Jiang, Y.; Ling, J.; Yan, Z. Blast furnace ironmaking process with super-high TiO2 in the slag: Viscosity and melting properties of the slag. Metall. Mater. Trans. B 2020, 29, 1170–1178. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, X.; Xing, Y.; Cui, X.; Wang, S.; Zhao, F.; Meng, W.; Ma, C.; Zhong, X. Improved hydration resistance of MgO–2CaO·SiO2–3CaO·SiO2 composite refractory using low-grade minerals. Mater. Res. Express 2020, 7, 085502. [Google Scholar] [CrossRef]
- Manzano, H.; Pellenq, R.; Ulm, F.; Buehler, M.; van Duin, A.V. Hydration of calcium oxide surface predicted by reactive force field molecular dynamics. Langmuir ACS J. Surf. Colloids 2012, 28, 4187–4197. [Google Scholar] [CrossRef]
- Fujimori, Y.; Zhao, X.; Shao, X.; Levchenko, S.; Nilius, N.; Sterrer, M.; Freund, H. Interaction of Water with the CaO(001) Surface. J. Phys. Chem. C 2016, 120, 5565–5576. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Cai, J.; Zhou, F.; Cheng, Z.; Zhang, J. Experimental Study on the Mechanical Properties and Microstructural Mechanisms of Coal Gangue-Based Cementitious Materials Synergistically Activated by Desulfurization Gypsum and Lime. Polymers 2025, 17, 932. [Google Scholar] [CrossRef]
- Barman, D.; Dash, S. Stabilization of expansive soils using chemical additives: A review. J. Rock Mech. Geotech. Eng. 2022, 14, 1319–1342. [Google Scholar] [CrossRef]
- Shahraki, A.; Keshavarz, M.; Malek Khachatourian, A.; Nemati, A.; Rezaei, B. Investigation of hydration, corrosion, and thermal shock resistance of MgO–CaO Ceramic Composites reinforced with electrospun carbon nanofibers. Mater. Res. Bull. 2025, 194, 113752. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Wu, C.; Chen, B.; Dai, M. Mechanism underlying effects of CaO-MgO combined expansive agent on hydration kinetics of cement-based systems. J. Sustain. Cem.-Based Mater. 2024, 13, 978–994. [Google Scholar] [CrossRef]
- Sun, Z.; Chi, H.; Fan, L. Physical and Chemical Mechanism for Increased Surface Area and Pore Volume of CaO in Water Hydration. Ind. Eng. Chem. Res. 2012, 51, 10793–10799. [Google Scholar] [CrossRef]
- Blamey, J.; Zhao, M.; Manović, V.; Anthony, E.; Dugwell, D.; Fennell, P. A shrinking core model for steam hydration of CaO-based sorbents cycled for CO2 capture. Chem. Eng. J. 2016, 291, 298–305. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, H.; Lee, S. Effect of Fe2O3 additions on the hydration resistance of CaO. J. Ceram. Process. Res. 2012, 13, 646–650. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, W.; Xu, J. Review of Research Development of Hydration Resistance of Calcium Oxide Refractories. Mater. Rev. 2005, 39, 364–367. [Google Scholar]
- Yi, N.; Ma, Y.; Wang, Z.; Liu, H.; Wang, X.; Dong, Y.; Xia, Z.; Zhu, Y.; Deng, C. Microstructural regulation and properties enhancement of MgO-CaO ceramics by doping Y2O3. J. Rare Earths 2022, 41, 1771–1779. [Google Scholar] [CrossRef]
- Guo, R.; Funayama, S.; Kim, S.T.; Harada, T.; Takasu, H.; Kato, Y. Hydration reactivity enhancement of calcium oxide–based media for thermochemical energy storage. Energy Storage 2021, 3, e232. [Google Scholar] [CrossRef]
- Kang, S.H.; Kwon, M.; Kwon, Y.H.; Moon, J. Effects of polycarboxylate ether (PCE)-based superplasticizer on the dissolution and subsequent hydration of calcium oxide (CaO). Cem. Concr. Res. 2021, 146, 106467. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; He, F. CO2 carbonation-induced improvement in strength and microstructure of reactive MgO-CaO-fly ash-solidified soils. Constr. Build. Mater. 2019, 229, 116914. [Google Scholar] [CrossRef]
- Chamhaydari, M.A.R.; Ahmadimoghadam, H.; Nilforoushan, M. Enhancing hydration resistance and mechanical properties of dolomite refractory through ZnO nanoparticle incorporation. Int. J. Appl. Ceram. Technol. 2024, 22, e14985. [Google Scholar] [CrossRef]
- Hadian, M.; Nazari, B. Influence of magnesia addition on hydration of iranian dolomite. Iran. J. Mater. Sci. Eng. 2010, 7, 51–56. [Google Scholar]
- Fan, Y. Production and Application Status of Magnesite-dolomite Refractories in China. Shandong Metall. 2006, 28, 28–30. [Google Scholar]
- Liu, J.; Chen, M.; Wang, N.; Sui, X. Effect of phase evolution and microstructure on thermal shock resistance and hydration resistance of low-carbon MgO-C refractories: Al-TiB2 hybrid addition. Constr. Build. Mater. 2025, 463, 140141. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Lee, W.E. Improving the hydration resistance of lime-based refractory materials. Int. Mater. Rev. 2008, 53, 1–20. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Liu, W.; Zhang, N.; Mao, Y.; Guo, Y. Impact and mechanism of bisphosphonate depressant 1-hydroxypropane-1,1-diphosphonic acid on flotation decalcification of dolomite-rich magnesite ore. Int. J. Min. Sci. Technol. 2024, 34, 1017–1032. [Google Scholar] [CrossRef]
- Yin, W.; Haoran, S.; Hong, J.; Hong, J.; Cao, S.; Bin, Y.; Won, C.; Song, M. Effect of Ca selective chelator BAPTA as depressant on flotation separation of magnesite from dolomite. Miner. Eng. 2019, 144, 106050. [Google Scholar] [CrossRef]
- Afify, A.M.; Sanz-Montero, M.; González-Acebrón, L. Dolomite–magnesite formation and polymetallic mineralization in a rift-sag basin on the western margin of the Red Sea: Paleoenvironmental, hydrothermal, and tectonic implications. J. Sediment. Res. 2022, 92, 144–165. [Google Scholar] [CrossRef]
- Yang, B.; Wang, D.; Cao, S.; Yin, W.; Xue, J.; Zhu, Z.; Fu, Y.; Yao, J. Selective adsorption of a high-performance depressant onto dolomite causing effective flotation separation of magnesite from dolomite. J. Colloid Interface Sci. 2020, 578, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.; Martins, L. Building a mineral-based value chain in Europe: The balance between social acceptance and secure supply. Miner. Econ. 2020, 34, 239–261. [Google Scholar] [CrossRef]
- Seaman, J. Critical Raw Materials, Economic Statecraft and Europe’s Dependence on China. Int. Spect. 2024, 60, 20–37. [Google Scholar] [CrossRef]
- Berthet, E.; Lavalley, J.; Anquetil-Deck, C.; Ballesteros, F.; Stadler, K.; Soytaş, U.; Hauschild, M.; Laurent, A. Assessing the social and environmental impacts of critical mineral supply chains for the energy transition in Europe. Glob. Environ. Change 2024, 86, 102841. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lian, L.; Jia, X.; Qian, J. Recycling of waste magnesia refractory brick powder in preparing magnesium phosphate cement mortar: Hydration activity, mechanical properties and long-term performance. Constr. Build. Mater. 2023, 402, 133019. [Google Scholar] [CrossRef]
- Baumgart, C.; Weigelt, C.; Krüger, L.; Aneziris, C.G. Investigations on the sintering response of steel-ceramic composites. IOP Conf. Ser. Mater. Sci. Eng. 2016, 139, 012013. [Google Scholar] [CrossRef]
- Vlad, E.; Buzduga, R.; Buzduga, M.; Caloian, V.; Plopeanu, E.; Pandelescu, C.; Dobrescu, C.; Constantin, N. Experimental research on the effect of additives on the sintering process of alumina-based refractory materials. J. Phys. Conf. Ser. 2021, 1781, 012066. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, Z.; Li, J. Sintering Strengthening Mechanism of MgO Sintering Additives Added in Fabricating Al2O3 Ceramics Using Binder Jetting. Adv. Eng. Mater. 2025, 27, 2402799. [Google Scholar] [CrossRef]
- Braulio, M.; Morbioli, G.G.; Medeiros, J.; Gallo, J.; Pandolfelli, V. Nano-Bonded Wide Temperature Range Designed Refractory Castables. J. Am. Ceram. Soc. 2012, 95, 1100–1104. [Google Scholar] [CrossRef]
- Tan, B.; Chen, K.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.W. Influence of the Binder Amount on the Properties of the Non-Sintering Ti(C,N)-Si3N4-SiC Composite Refractories. Adv. Mater. Res. 2013, 833, 221–224. [Google Scholar] [CrossRef]
- Apal’kova, G.D. The effect of nanodispersed iron oxide additives on the formation of the density of carbon refractories. Novye Ogneupor. 2018, 11, 50–52. [Google Scholar] [CrossRef]
- Gilmer, D.; Kim, S.; Goldsby, D.; Nandwana, P.; Elliott, A.; Saito, T. Predictive Binder Jet Additive Manufacturing enabled by Clean Burn-off Binder Design. Addit. Manuf. 2024, 80, 103955. [Google Scholar] [CrossRef]
- Bhandari, S.; Manière, C.; Sedona, F.; Bona, E.D.; Sglavo, V.; Colombo, P.; Fambri, L.; Biesuz, M.; Franchin, G. Ultra-rapid debinding and sintering of additively manufactured ceramics by ultrafast high-temperature sintering. J. Eur. Ceram. Soc. 2023, 44, 328–340. [Google Scholar] [CrossRef]
- Umar, A.M.; Wuritka, E.G.; Tokan, A.; Sadiq, Y.O. Production of dense zircon sand-alumina refractory brick from zircon sand of bakin ruwa for foundry application. Int. J. Appl. Adv. Eng. Res. 2025, 8, 44–54. [Google Scholar] [CrossRef]
- Reis, G.S.; Cazacliu, B.; Cothenet, A.; Poullain, P.; Wilhelm, M.; Sampaio, C.; Lima, E.; Ambrós, W.; Torrenti, J. Fabrication, microstructure, and properties of fired clay bricks using construction and demolition waste sludge as the main additive. J. Clean. Prod. 2020, 258, 120733. [Google Scholar] [CrossRef]
- Thuong, T.V.; Tashlykov, O.L.; Mahmoud, K. A unique Vietnam’s red clay-based brick reinforced with metallic wastes for γ-ray shielding purposes: Fabrication, characterization, and γ-ray attenuation properties. Nucl. Eng. Technol. 2024, 56, 1544–1551. [Google Scholar] [CrossRef]
- Odewale, I.O.; Aluma, C.C.; Idu, F.U.; Amaakaven, V.T.; Ogunkunle, D.; Olagunju, S. Consequence of Variations in Al2O3.2SiO2.2H2O and Grog Percentages on the Properties of Dense Refractory Bricks. Saudi J. Eng. Technol. 2021, 6, 451–462. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, Z.; Mao, H.; Han, H.; Yang, J. Recycling reuse of municipal sewage sludge in sustainable structural materials: Preparation, properties, crystallization and microstructure analyses. Constr. Build. Mater. 2023, 398, 132507. [Google Scholar] [CrossRef]
- Yang, J.; Lu, S.; Wang, L. Fused magnesia manufacturing process: A survey. J. Intell. Manuf. 2020, 31, 327–350. [Google Scholar] [CrossRef]
- Niesyt, M.; Psiuk, B. Fused dolomite-magnesia co-clinker for fired dolomite refractories. Ceram. Inc. 2017, 43, 51–59. [Google Scholar] [CrossRef]
- Korczak, K.; Kochański, M.; Skoczkowski, T. Mitigation options for decarbonization of the non-metallic minerals industry and their impacts on costs, energy consumption and GHG emissions in the EU—Systematic literature review. J. Clean. Prod. 2022, 358, 132006. [Google Scholar] [CrossRef]
- Suzuki, Y.; Morgan, P.; Ohji, T. Pressureless-Sintering of CaZrO3/MgO In Situ Composites Derived from Natural Dolomite with Various Additives. In Design Manufacturing Composites; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Qian, X.; Qin, Y.; Tao, Y.; Shen, P.; Hu, C.; Wang, F.; Hu, S. Development of highly reactive partially calcined dolomite precursor: Synergistic effect CaO and MgO. J. Am. Ceram. Soc. 2025, 108, e20530. [Google Scholar] [CrossRef]
- Kim, S.J.; Rocha, L.T.D.; Kim, S.; Jung, S. Effect of mill-scale and calcined dolomite on high Al2O3 sinter and its reduction behaviour. In Proceedings of the 12th International Conference of Molten Slags, Fluxes and Salts (MOLTEN 2024), Brisbane, Australia, 17–19 June 2024. [Google Scholar] [CrossRef]
- Liu, W.; Mao, Y.; Zheng, J.; Wang, Z.; Shang, C.; Liu, W.; Zhao, Q.; Zhao, S.; Shen, Y. Enhancing the flotation separation of magnesite and dolomite by introducing a phosphonic acid depressant during grinding. Sep. Purif. Technol. 2025, 361, 131412. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, C.; Chen, Q.; Li, Q.; He, D.; Li, Z.; Fu, Y. Effect of a novel environmental-friendly chelating depressant DTPMP on the flotation separation of magnesite and dolomite. Appl. Surf. Sci. 2025, 690, 162650. [Google Scholar] [CrossRef]
- Ban, X.; Yao, J.; Yin, W.; Xie, Y.; Zhang, T.; Du, W.; Wang, Y. Selective adsorption of eco-friendly inhibitor sesbania gum on dolomite for efficient flotation separation of magnesite and dolomite. Process Saf. Environ. Prot. 2024, 194, 630–640. [Google Scholar] [CrossRef]
- Luo, N.; Shi, J.; Yan, B.; Wang, X. Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals 2024, 14, 355. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Guo, J.; Yang, B.; Sun, H.; Yin, W.; Wang, Y.; Fu, Y. Role of tannin pretreatment in flotation separation of magnesite and dolomite. Int. J. Miner. Metall. Mater. 2024, 31, 452–461. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Huang, L.; Zhu, Y.; Li, F. Flotation separation of magnesite from dolomite with gellan gum as depressant and its depression mechanism. Miner. Eng. 2024, 212, 108718. [Google Scholar] [CrossRef]
- Wang, B.; Liu, C.; Fan, W.; Mao, Y.; Liu, W. An Environmentally Friendly Chelator for Improving the Flotation Separation of Magnesite and Dolomite: Flotation Behavior and Adsorption Mechanism. Minerals 2025, 15, 289. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Liu, W.; Li, P.; Shen, Y.; Dai, S. Utilization of a novel bisphosphonic acid surfactant for reverse froth flotation of magnesite and dolomite. Miner. Eng. 2022, 185, 107668. [Google Scholar] [CrossRef]
- Cheng, G.; Ma, Y.; López-Valdivieso, A.; Yin, W. Selective depression of phenoxyacetyl chloride on magnesite: Implications for effective flotation separation of magnesite from dolomite. Miner. Eng. 2024, 218, 109017. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Hu, K.; Cao, Y.; Chai, W. Insights Into Flotation Separation of Dolomite From Magnesite with SDS Collector: Experiments and DFT Calculation. Asia-Pac. J. Chem. Eng. 2025, 20, e70028. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Ma, Y.; Chen, K. Effects of ultrasonic treatment on the flotation behavior of magnesite and dolomite in a sodium oleate system. Green Smart Min. Eng. 2024, 1, 76–84. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Zhao, X.; Qi, Z.; Yang, B.; Yin, W.; Wang, Y. Effect of ultrasonic treatment on the surface roughness and floatability of magnesite and dolomite. J. Mol. Liq. 2024, 404, 125002. [Google Scholar] [CrossRef]
- Qi, G.; Shan, F.; Li, Q.; Yu, J. Energy Saving by Applying 3000kVA Electric Arc Furnace in Fused Magnesia Production. Mater. Sci. Forum 2013, 749, 299–302. [Google Scholar] [CrossRef]
- An, P.; Han, Z.; Wang, K.; Zhao, Z.; Situmorang, Y.A.; Rizkiana, J.; Abudula, A.; Guan, G. Energy-saving strategy for a transport bed flash calcination process applied to magnesite. Carbon Resour. Convers. 2021, 4, 122–131. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, J.; Dong, H. Analysis of carbon footprint and reduction approach of magnesia production in China. J. Clean. Prod. 2021, 334, 130194. [Google Scholar] [CrossRef]
- An, J.; Li, Y.; Middleton, R. Reducing energy consumption and carbon emissions of magnesia refractory products: A life-cycle perspective. J. Clean. Prod. 2018, 182, 363–371. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, W.; Liu, S.K. Performance Evaluation of a Full-Scale Fused Magnesia Furnace for MgO Production Based on Energy and Exergy Analysis. Energies 2021, 15, 214. [Google Scholar] [CrossRef]
- Spyridakos, A.; Alexakis, D.E.; Vryzidis, I.; Tsotsolas, N.; Varelidis, G.; Kagiaras, E. Waste Classification of Spent Refractory Materials to Achieve Sustainable Development Goals Exploiting Multiple Criteria Decision Aiding Approach. Appl. Sci. 2022, 12, 3016. [Google Scholar] [CrossRef]
- Seifert, S.; Dittrich, S.; Bach, J. Recovery of Raw Materials from Ceramic Waste Materials for the Refractory Industry. Processes 2021, 9, 228. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Fu, L. Formation Mechanism of In Situ Intergranular CaZrO2 Phases in Sintered Magnesia Refractories. Metall. Mater. Trans. A 2020, 51, 5328–5338. [Google Scholar] [CrossRef]
- Jin, E.; Zou, C.; Ding, D.; Xiao, G.; Duan, F.; Jiang, B.; Han, S.; Zheng, K. Enhanced mechanical properties and thermal shock resistance of magnesia refractories via in situ formation of tetragonal zirconia. Ceram. Inc. 2024, 50, 14968–14979. [Google Scholar] [CrossRef]
- Otroj, S. Synthesis of Hercynite under Air Atmosphere using MgAl2O4 Spinel. Mater. Sci. 2015, 21, 288–292. [Google Scholar] [CrossRef][Green Version]
- Jastrzębska, I.; Stępień, J.; Żukrowski, J. Stabilization of hercynite structure at elevated temperatures by Mg substitution. Mater. Des. 2023, 235, 112449. [Google Scholar] [CrossRef]
- Zan, W.; Ma, B.; Cao, Y.; Tian, J.; Zhou, Z.; Wang, L.; Jiang, Z. Preparation and performance optimization of MgAl2O4 spinel materials by single-step reaction sintering. Ceram. Inc. 2023, 49, 23567–23578. [Google Scholar] [CrossRef]
- Xinming, R.; Beiyue, M.; Zhang, G.; Gaofeng, F.; Yu, J.; Liu, G. Preparation and properties of MgAl2O4 spinel ceramics by double-doped Sm2O3–(Y2O3, Nb2O5 and La2O3). Mater. Chem. Phys. 2020, 252, 123309. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, W.; Wu, H.; Liu, Q.; Yao, L. Promoting effect of Si on MnOx catalysts for low-temperature NH2-SCR of NO: Enhanced N2 selectivity and SO2 resistance. Fuel 2023, 355, 129478. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, S.; Wu, Q.; Cao, R.; Cai, Z.; Liu, C.; Ma, Y.; Song, G.; Yang, W.; Wen, C. In-situ synthesis of Mn2SiO4 and MnxSi dual phases through solid-state reaction to improve the initial Coulombic efficiency of SiO anode for Lithium-Ion batteries. J. Electroanal. Chem. 2024, 977, 118845. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Chen, D.; Zhao, R. Hybrid MnO-SiOx@C Microspheres with a Hierarchical Mesoporous Structure for Advanced Lithium-Ion Battery Anodes. J. Alloys Compd. 2021, 899, 163251. [Google Scholar] [CrossRef]
- Woo, D.H.; Lee, H. Phase Equilibria of the MnO–SiO2–Al2O3–MnS System. J. Am. Ceram. Soc. 2010, 93, 2098–2106. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. Immobilizing chromium in stainless steel slags with MnO addition. J. Am. Ceram. Soc. 2021, 104, 697–705. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. Selective elemental concentration during the solidification of stainless steel slags for increased Cr recovery with MnO addition. J. Am. Ceram. Soc. 2020, 103, 6012–6024. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, J. Viscosity-structure relationship of alkaline earth silicate melts containing manganese oxide and calcium fluoride. J. Am. Ceram. Soc. 2019, 102, 4943–4955. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Z.; Zhang, Y.; Wang, C. Roles of MnO and MgO on structural and thermophysical properties of SiO2-MnO-MgO-B2O3 welding Fluxes: A molecular dynamics study. J. Mol. Liq. 2023, 386, 122501. [Google Scholar] [CrossRef]
- Ma, H.B.; Yan, J.; Zhao, Y.H.; Liu, T.; Ren, Q.; Liao, Y.; Zuo, J.; Liu, G.; Yao, M. Oxidation behavior of Cr-coated zirconium alloy cladding in high-temperature steam above 1200 °C. npj Mater. Degrad. 2021, 5, 7. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Tan, Y.; Feng, W.; Peng, S.; Zhang, H. A systematic study of the oxidation behavior of Cr coatings on Zry4 substrates in high temperature steam environment. Corros. Sci. 2020, 174, 108826. [Google Scholar] [CrossRef]
- Yang, R.; Lei, H.; Zhang, J. Preparation of SiO2@MnO2 composite abrasives and their performance in chemical-mechanical polishing of SiC substrates. Ceram. Inc. 2024, 50, 34796–34805. [Google Scholar] [CrossRef]
- Kaya, M.; Köksal, F.; Munir, M.; Kazmi, S.; Gencel, O.; Ozbakkaloglu, T. Effect of Natural and Artificial Silicon Additives on the Physicomechanical Performance of Dolomite-based Alkaline-Activated Mortar. Silicon 2023, 16, 215–230. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, D.; Liu, F.; Fan, B.; Wen, C.; Yu, X.; Xu, M. Scavenging of refractory elements (Ca, Mg, Fe) by kaolin during low rank coal combustion. Fuel 2018, 223, 198–210. [Google Scholar] [CrossRef]
- Vakalova, T.V.; Reshetova, A.A.; Revva, I.B.; Rusinov, P.G.; Balamygin, D.I. Effect of thermochemical activation of clay raw materials on phase formation, microstructure and properties of aluminosilicate proppants. Appl. Clay Sci. 2019, 183, 105335. [Google Scholar] [CrossRef]
- Gao, J.; Su, W.; Hou, J.; Song, X.; Bai, Y.; Wang, J.; Lv, P.; Yu, G. Inhibition effects of wetting and corrosion behavior of high-alkali coal using typical additives under gasification conditions. Fuel 2024, 380, 133158. [Google Scholar] [CrossRef]
- Kahrizsangi, S.G.; Nemati, A.; Shahraki, A.; Farooghi, M. The effect of nano-additives on the hydration resistance of materials synthesized from the MgO-CaO system (research note). Int. J. Eng. Trans. A Basics 2016, 29, 539–545. [Google Scholar] [CrossRef]
- Li, B.; Wei, Y.; Wang, J.; Chen, J.; Li, N. Improved hydration resistance of CaO granules via sol-processed metal oxide protective layers. J. Am. Ceram. Soc. 2021, 104, 4878–4890. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Bingrong, L.; Li, M.; Li, N. Core-shell structured CaO aggregate prepared by granulating with Al chelating compound and its hydration resistance. Ceram. Inc. 2020, 46, 10788–10796. [Google Scholar] [CrossRef]
- Yao, L.; Gao, Y.; Li, Y.; Huang, Y.; Wang, Y.; Peng, X.; Liu, Q. Microstructure, mechanical properties, and strengthening mechanisms of nanostructural Y-Zr-O oxide dispersion-strengthened (ODS) Mo alloys. J. Alloys Compd. 2022, 921, 166155. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Cai, X.; Ding, S.; Xin, S.; Sun, B.; Shen, T. Heterostructural nanolamellar oxide-dispersion-strengthened ferritic alloy with exceptional strength and ductility. Scr. Mater. 2024, 242, 115949. [Google Scholar] [CrossRef]
- Ovid’ko, I.; Valiev, R.; Zhu, Y. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Zhao, L.; Lee, T.; Zheng, S.; Zheng, W.; Ryu, S.; Zhang, D.; Guo, Q. Ultrastrong and Deformable Aluminum-Based Composite Nanolaminates with Transformable Binary Intergranular Films. Nano Lett. 2024, 24, 3843–3850. [Google Scholar] [CrossRef]
- Li, X.; Lu, L.; Li, J.; Zhang, X.; Gao, H. Mechanical properties and deformation mechanisms of gradient nanostructured metals and alloys. Nat. Rev. Mater. 2020, 5, 706–723. [Google Scholar] [CrossRef]
- Murashkin, M.; Sabirov, I.; Sauvage, X.; Valiev, R. Nanostructured Al and Cu alloys with superior strength and electrical conductivity. J. Mater. Sci. 2015, 51, 33–49. [Google Scholar] [CrossRef]
- Andrievski, R.; Glezer, A. Strength of nanostructures. Physics-Uspekhi 2009, 52, 315–334. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Huo, Y.; Fu, L.; Li, Y. Characterisation and properties of low-conductivity microporous magnesia based aggregates with in-situ intergranular spinel phases. Ceram. Inc. 2021, 47, 11063–11071. [Google Scholar] [CrossRef]
- Thakur, S.; Giri, A. Origin of Ultralow Thermal Conductivity in Metal Halide Perovskites. ACS Appl. Mater. Interfaces 2023, 15, 26755–26765. [Google Scholar] [CrossRef]
- Lou, Z.; Zhang, P.; Zhu, J.; Gong, L.; Xu, J.; Chen, Q.; Reece, M.; Yan, H.; Gao, F. A novel high-entropy perovskite ceramics Sr0.9La0.1(Zr0.25Sn0.25Ti0.25Hf0.25)O3 with low thermal conductivity and high Seebeck coefficient. J. Eur. Ceram. Soc. 2022, 42, 3480–3488. [Google Scholar] [CrossRef]
- Shi, Y.B.; Chen, Y.; Dong, H.; Wang, H.; Qian, P. Investigation of phase transition, mechanical behavior and lattice thermal conductivity of halogen perovskites using machine learning interatomic potentials. Phys. Chem. Chem. Phys. 2023, 25, 30644–30655. [Google Scholar] [CrossRef]
- Xuan, S.; Tian, Y.; Kong, X.; Hao, J.; Wang, X. Enhancement of thermal shock resistance of Al2O3–MgAl2O4 composites by controlling the content and distribution of spinel phase. Ceram. Inc. 2023, 49, 39908–39916. [Google Scholar] [CrossRef]
- Gruber, D.; Sistaninia, M.; Fasching, C.; Kolednik, O. Thermal shock resistance of magnesia spinel refractories—Investigation with the concept of configurational forces. J. Eur. Ceram. Soc. 2016, 36, 4301–4308. [Google Scholar] [CrossRef]
- Ma, S.H.; Shi, K.; Xia, Y.; Zhang, Y.; Han, X. Effect of modified MgO aggregates on mechanical properties of magnesium aluminate spinel refractories. Ironmak. Steelmak. 2020, 48, 292–298. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Yan, W.; Wang, J.; Ma, S.; Li, G. A comparative study on lightweight and dense periclase-magnesium aluminate spinel refractories from industrial preparation. J. Alloys Compd. 2023, 960, 170611. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, X.; Luo, X.; Zhao, J.; Wu, F. Effect of in-situ generated MgAl2O4 spinel on thermal shock resistance of magnesia-zirconia refractories. Ceram. Inc. 2024, 50, 35936–35945. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, W.; Yan, J.; Dai, Y.; Wang, X.; Wang, Q.; Li, N. Enhancement of strength and thermal shock resistance of MgO-MgAl2O4 ceramic filters with microporous MgO powder: Effect of α-Al2O3 micro-powder content. Int. J. Appl. Ceram. Technol. 2024, 22, e14921. [Google Scholar] [CrossRef]
- Gu, Q.; Zhao, F.; Liu, X.; Jia, Q. Preparation and thermal shock behavior of nanoscale MgAl2O4 spinel-toughened MgO-based refractory aggregates. Ceram. Inc. 2019, 45, 12093–12100. [Google Scholar] [CrossRef]
- Sokolov, A.; Deynega, G.I.; Kuzmina, N.; Kuzmina, I. Structural and mechanical properties of a composite material based on partially stabilized zirconium dioxide doped with magnesium aluminate spinel. Aviat. Mater. Technol. 2021, 66, 78–85. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolá, R. Formation and influence of magnesium-alumina spinel on the properties of refractory forsterite-spinel ceramics. Mater. Tehnol. 2020, 54, 135–141. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolář, R. Impact of Fly Ash as a Raw Material on the Properties of Refractory Forsterite–Spinel Ceramics. Minerals 2020, 10, 835. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, W.; Li, G.; Hong, S.; Li, N. Enhanced mechanical properties of novel Al2O3-based ceramic filter by using microporous corundum-spinel and nano-Al2O3 powders. J. Eur. Ceram. Soc. 2023, 44, 1070–1080. [Google Scholar] [CrossRef]
- Ceylantekin, R.; Aksel, C. Improvements on the mechanical properties and thermal shock behaviours of MgO–spinel composite refractories by ZrO2 incorporation. Ceram. Inc. 2012, 38, 995–1002. [Google Scholar] [CrossRef]
- Moritz, K.; Aneziris, C.; Hesky, D.; Gerlach, N. Magnesium Aluminate Spinel Ceramics Containing Aluminum Titanate for Refractory Applications. J. Ceram. Sci. Technol. 2014, 5, 125–130. [Google Scholar]
- Zhao, L.; Yao, S. Modification effect of Mg(OH)2–2Al(OH)3 composite gels on Y-PSZ ceramics. Ceram. Inc. 2024, 50, 30061–30067. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Liu, H.; Ma, Y.; Wang, X.; Yi, N.; Xia, Z.; Zhu, Y.; Deng, C.; Zhang, L. Selective sintering of magnesia–calcia materials by utilizing hot spots during induction sintering process. J. Iron Steel Res. Int. 2024, 31, 1914–1922. [Google Scholar] [CrossRef]
- Chandra, S.; Ramesh, K.V. Refractories in indian steel industry: Past and present scenario. Glob. J. Eng. Appl. Sci. 2011, 1, 50–52. [Google Scholar]
- Salomão, R.; Bittencourt, L.; Pandolfelli, V. A novel approach for magnesia hydration assessment in refractory castables. Ceram. Inc. 2007, 33, 803–810. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, D.; Luo, X.; Li, G.; Tian, L.; Zheng, Y. Enhanced thermal shock resistance in magnesia refractories through in situ formation of CaO·2La2O3·3SiO2. Int. J. Appl. Ceram. Technol. 2025, 22, e15075. [Google Scholar] [CrossRef]
- Shchekina, T.I.; Gramenitskii, E.N.; Batanova, A.M.; Kurbyko, T.A.; Likhodievskii, A.V.; Grigor’ev, B.N.; Pyrikov, A.N. Use of magnesian-dolomite mixtures in steel-melting furnace hearths and the mechanism of their wear in service. 1. Study of Ankerharth refractories. Refract. Ind. Ceram. 2006, 47, 317–325. [Google Scholar] [CrossRef]
- Badapalli, P.K.; Kottala, R.B.; Sree, P.; Rajasekhar, M. Occurrence and structures of dolomites in North Eastern part of Anantapur district, and their use in engineering materials. Mater. Today Proc. 2021, 50, 1005–1010. [Google Scholar] [CrossRef]
- Park, J.; Suk, M.; Jung, I.; Guo, M.; Blanpain, B. Interfacial Reaction between Refractory Materials and Metallurgical Slags containing Fluoride. Steel Res. Int. 2010, 81, 860–868. [Google Scholar] [CrossRef]
- Ewais, E.; Ahmed, A.; Kasem, A.A.; El-SKERIF, A.R. Attack under load of tempered tar/pitch-bonded Egyptian dolomite by BOF slag. J. Ceram. Soc. Jpn. 2002, 110, 931–936. [Google Scholar] [CrossRef]
- Preisker, T.; Gehre, P.; Schmidt, G.; Aneziris, C.; Wöhrmeyer, C.; Parr, C. Kinetics of the formation of protective slag layers on MgO–MgAl2O4–C ladle bricks determined in laboratory. Ceram. Inc. 2020, 46, 452–459. [Google Scholar] [CrossRef]
- Rahou, J.; Rezqi, H.; Ouahabi, M.E.; Fagel, N. Characterization of Moroccan steel slag waste: The potential green resource for ceramic production. Constr. Build. Mater. 2022, 314, 125663. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Cui, H.; Zhang, W.; Hu, L.; Mao, L. Effects of electric arc furnace slag on promoting quality and environmental safety of fired bricks incorporating municipal solid waste incineration fly ash. Constr. Build. Mater. 2022, 345, 128327. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Z.; Wang, Z.; Wang, N.; Wang, X. Splattering Suppression for a Three-Phase AC Electric Arc Furnace in Fused Magnesia Production Based on Acoustic Signal. IEEE Trans. Ind. Electron. 2017, 64, 4772–4780. [Google Scholar] [CrossRef]
- Stein, V.; Aneziris, C.; Gueguen, E. New Approach for the Application of Functional Ceramic Material in Carbon Bonded Doloma Refractories to Reduce Emissions. Adv. Eng. Mater. 2011, 13, 1135–1141. [Google Scholar] [CrossRef]
- Kudrina, A.; Krivchenko, Y.S.; Gul’ev, G.F. The influence of physicochemical factors of basic refractories on the life of linings of oxygen converters. Refractories 1963, 4, 434–439. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Antonio-Zárate, Y.; Revuelta-Acosta, J.; Verdeja, L.; Fernández-González, D.; López-Perales, J.F.; Rodríguez-Castellanos, E.A.; García-Quiñonez, L.; Castillo-Rodríguez, G.A. Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials 2021, 14, 2277. [Google Scholar] [CrossRef]
- Boenzi, F. Possible ecological advantages from use of carbonless magnesia refractory bricks in secondary steelmaking: A framework LCA perspective. Int. J. Environ. Sci. Technol. 2021, 19, 5877–5896. [Google Scholar] [CrossRef]
- Borges, O.; Aneziris, C.G.; Pandolfelli, V. Assessing and tailoring the dilatometric profile of novel chromium-free refractory raw materials. J. Am. Ceram. Soc. 2024, 108, e20271. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Huang, Z.; Zhao, J.; Zhao, W.; Yang, D.; Ren, L.; Zhao, X.; Yan, H.; Liu, L.; et al. Improved properties of in-situ MgAl2O4-TiO2 dense layer reinforced low-carbon MgO-based refractories. Ceram. Inc. 2023, 49, 33842–33850. [Google Scholar] [CrossRef]
- Kusiorowski, R. MgO-ZrO2 refractory ceramics based on recycled magnesia-carbon bricks. Constr. Build. Mater. 2020, 231, 117084. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, T.; Li, Y.; Sang, S. Microstructure and properties of MgO–C refractory with different carbon contents. Ceram. Inc. 2021, 47, 2538–2546. [Google Scholar] [CrossRef]
- Yu, C.; Dong, B.; Chen, Y.; Ma, B.; Ding, J.; Deng, C.; Zhu, H.; Di, J. Enhanced oxidation resistance of low-carbon MgO–C refractories with ternary carbides: A review. J. Iron Steel Res. Int. 2022, 29, 1052–1062. [Google Scholar] [CrossRef]
- Capelo-Avilés, S.; de Oliveira, R.T.; Stampino, I.I.G.; Gispert-Guirado, F.; Casals-Terré, A.; Giancola, S.; Galán-Mascarós, J. A thorough assessment of mineral carbonation of steel slag and refractory waste. J. CO2 Util. 2024, 82, 102770. [Google Scholar] [CrossRef]
- Mandal, S.; Hemrick, J.; Mahapatra, M. Chrome-free qandilite (Mg2TiO4) refractory aggregates: Role of titania source and evaluation of thermal expansion coefficient. J. Eur. Ceram. Soc. 2022, 42, 7343–7351. [Google Scholar] [CrossRef]
- Boenzi, F.; Meré, J.B.O.; Iavagnilio, R. Life Cycle Assessment Comparison of Two Refractory Brick Product Systems for Ladle Lining in Secondary Steelmaking. Sustainability 2019, 11, 1295. [Google Scholar] [CrossRef]
- Stoianov, O.; Niziaev, K.; Malii, K.; Kukhar, V. Application of refractory materials for steel ladles. Report. Priazovskyi State Tech. Univ. Sect. Tech. Sci. 2023, 46, 69–78. [Google Scholar] [CrossRef]
- Li, Z.; Hua, Y.; Chang, Z.; Yue, Y.; Qin, J.; Qian, J. Hydration, carbonation and strength development of reactive MgO cement blended with lime (CaO) under different curing conditions. J. Build. Eng. 2023, 76, 107082. [Google Scholar] [CrossRef]
- Burmistrova, E.; Abdrakhmanov, R.I.; Igonin, A. Refractory Materials for OAO MMK Degassers and main Areas for Improving their Operating Reliability1. Refract. Ind. Ceram. 2013, 54, 269–271. [Google Scholar] [CrossRef]
- Samanta, A.; Satpathy, S.; Tripathi, A.; Sengupta, S.; Tsuyuguchi, K.; Panda, P. Designing of low cement castables in Al2O3–SiO2–SiC system, having alkali and thermal spalling resistance and suitable for discharge zone of cement rotary kiln. Refract. Ind. Ceram. 2015, 43, 141–144. [Google Scholar]
- Blond, E.; Schmitt, N.; Arnould, O.; Hild, F.; Poirier, J.; Blumenfeld, P. Prevalent Material Parameters Governing Spalling of a Slag-Impregnated Refractory. Key Eng. Mater. 2004, 264–268, 1751–1754. [Google Scholar] [CrossRef]
- Jin, S.; Harmuth, H.; Gruber, D.; Buhr, A.; Sinnema, S.; Rebouillat, L. Thermomechanical modelling of a torpedo car by considering working lining spalling. Ironmak. Steelmak. 2018, 47, 145–149. [Google Scholar] [CrossRef]
- Gomez, R.S.; Porto, T.N.; Magalhães, H.L.F.; Moreira, G.; André, A.M.; Melo, R.B.F.; Lima, A.G.B. Natural Gas Intermittent Kiln for the Ceramic Industry: A Transient Thermal Analysis. Energies 2019, 12, 1568. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Fu, L.; Li, G. Fabrication and properties of in situ intergranular CaZrO3 modified microporous magnesia aggregates. Ceram. Int. 2020, 46, 16956–16965. [Google Scholar] [CrossRef]
- Booth, F.; Stábile, F.M.; Bruni, Y.; Gauna, M.; Rendtorff, N. Dolomite-zirconia reaction sintered bonded coarse magnesia ceramics: Effect of the bonding proportion. Cerâmica 2021, 67, 151–157. [Google Scholar] [CrossRef]
- Wei, C.; Ma, C.; Li, Y.; Bai, W.; Yang, J.; Cheng, Y.; Gao, L. Controllable preparation and slag corrosion resistance of novel MgO–MgAl2O4–ZrO2 refractory. Ceram. Int. 2024, 50, 21406–21416. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Ludwig, M.; Przystaś, J. Cr-free Refractories for Copper Metallurgy: Raw Materials and Factsage Thermodynamic Simulations. Ceram. Inc. 2024, 51, 3778–3791. [Google Scholar] [CrossRef]
- Ludwig, M.; Śnieżek, E.; Jastrzębska, I.; Prorok, R.; Sułkowski, M.; Goławski, C.; Fischer, C.; Wojteczko, K.; Szczerba, J. Recycled magnesia-carbon aggregate as the component of new type of MgO-C refractories. Constr. Build. Mater. 2021, 272, 121912. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Ikeda, K.; Ito, H. Manufacture of the Special Dolomite Refractories (Dolomitic, Magnesia-rich Refractories) by Utilizing Waste Lime from Acetylene Generator to Sea Water. J. Ceram. Assoc. Jpn. 1956, 64, 37–42. [Google Scholar] [CrossRef]
- Hao, C.; Yu, L.; Chen, X.; Chen, Y.; Li, C.; Qi, P.; Sun, J.; Zhou, H. Research on the development of recycling technology for magnesium refractory materials. Mater. Res. Express 2024, 11, 115504. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Fernández-González, D.; García-Quiñonez, L.; Castillo-Rodríguez, G.A.; Aguilar-Martínez, J.; Verdeja, L. MgO Refractory Doped with ZrO2 Nanoparticles: Influence of Cold Isostatic and Uniaxial Pressing and Sintering Temperature in the Physical and Chemical Properties. Metals 2019, 9, 1297. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Fu, L.; Li, G.; Wang, L.; Chen, D. Pore evolution of microporous magnesia aggregates with the introduction of nano-sized MgO. Ceram. Inc. 2022, 48, 18513–18521. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Rodríguez, M.A.; Aza, S.; Pena, P. Reaction sintering of zircon–dolomite mixtures. J. Eur. Ceram. Soc. 2001, 21, 343–354. [Google Scholar] [CrossRef]
- Berezhnoy, A.; Kordyuk, R.A. The CaO-MgO-ZrO2-SiO2 system and its significance in the production of refractories. Refractories 1962, 3, 69–73. [Google Scholar] [CrossRef]
- Song, Q.; Zha, X.; Gao, M.; Shi, J.; Ma, Y. Influence of ZrO2 on the phase composition and mechano-physical properties of MgO–ZrO2 refractories prepared by cold isostatic pressing. Ceram. Inc. 2024, 50, 30474–30482. [Google Scholar] [CrossRef]
- Park, J. Formation of CaZrO3 at the interface between CaO–SiO2–MgO–CaF2(–ZrO2) slags and magnesia refractories: Computational and experimental study. Calphad 2007, 31, 149–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Chen, C.; Li, J.; Li, X. Effect of a MgO–CaO–ZrO2-based refractory on the cleanliness of a K4169 Ni-based superalloy. Ceram. Inc. 2022, 49, 117–125. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO–CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Yu, J. Sintering and Performance of MgO-CaO Materials with Nano-Sized ZrO2 Addition. Mater. Sci. Forum 2007, 561–565, 623–626. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, M.B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia-doloma refractories. Ceram. Inc. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Zheng, W.; Xiao, X.; Chang, C.; Dong, J.; Wen, J.; Huang, Q.; Zhou, Y.; Li, Y. Characterizing properties of magnesium oxychloride cement concrete pavement. J. Cent. South Univ. 2019, 26, 3410–3419. [Google Scholar] [CrossRef]
- Haigh, R. A Decade Review of Research Trends Using Waste Materials in the Building and Construction Industry: A Pathway towards a Circular Economy. Waste 2023, 1, 935–959. [Google Scholar] [CrossRef]
- Özkan, A.; Günkaya, Z.; Tok, G.; Karacasulu, L.; Metesoy, M.; Banar, M.; Kara, A. Life Cycle Assessment and Life Cycle Cost Analysis of Magnesia Spinel Brick Production. Sustainability 2016, 8, 662. [Google Scholar] [CrossRef]
- Mena, J.; Sabolsky, E.; Sierros, K.A.; Sabolsky, K.; Jones, M.; Stepp, C.; Winch, N.; Raughley, M. Smart Refractory Sensors Development for Corrosion and Erosion Monitoring in High Temperature Systems. ECS Meet. Abstr. 2023, 1475. [Google Scholar] [CrossRef]
- Sabolsky, E.; Sabolsky, K.; Yakaboylu, G.A.; Buzzo, B. Fabrication and Testing of Smart Refractory for Energy System Monitoring in Harsh-Environments. In Proceedings of the 2018 XV International Scientific Conference on Optoelectronic and Electronic Sensors (COE), Warsaw, Poland, 17–20 June 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Ferreira, P.M.; Machado, M.; Carvalho, M.; Vidal, C. Embedded Sensors for Structural Health Monitoring: Methodologies and Applications Review. Sensors 2022, 22, 8320. [Google Scholar] [CrossRef]
- Huang, Q.; Bhattacharyya, D.; Pillai, R.C.; Sabolsky, K.; Sabolsky, E. Gasifier Health Monitoring using Smart Refractory Bricks. Struct. Health Monit. 2017, 16, 14160. [Google Scholar] [CrossRef]
- Bayram, Y.; Ruege, A.C.; Hagan, P.; Sperry, E.; Cetnar, D.P. Advanced Furnace Inspection and Monitoring Based on Radar Sensors. In Proceedings of the 76th Conference on Glass Problems, Columbus, OH, USA, 7–10 November 2016; pp. 117–132. [Google Scholar] [CrossRef]
- Bayram, Y.; Wechsel, J.; Sperry, E. New Industry Standard in Furnace Inspection. In 78th Conference on Glass Problems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Zanelli, C.; Dondi, M.; Raimondo, M.; Guarini, G. Phase composition of alumina–mullite–zirconia refractory materials. J. Eur. Ceram. Soc. 2010, 30, 29–35. [Google Scholar] [CrossRef]
- Betsis, K.; Kourtis, A.; Karalis, K.; Xenidis, A. Assessment of Magnesia Refractories Corrosion by Iron-Rich Slags. Mater. Proc. 2021, 5, 135. [Google Scholar] [CrossRef]
- Oliveira, R.; Rodrigues, J.; Pereira, J. Numerical simulations on refractory linings for steel casting vessels. Fire Saf. J. 2023, 138, 103794. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.S.; Faraji, G.; Baghani, M.; Hashemi, M.S.; Sheidaei, A.; Baniassadi, M. Design of Refractory Alloys for Desired Thermal Conductivity via AI-Assisted In-Silico Microstructure Realization. Materials 2023, 16, 1088. [Google Scholar] [CrossRef]
- Alhaddad, E.M.S.; Ahmed, H.; Alhaddad, M.S.; Ahmed, H.A. A review of magnesite mineral and its industrial application. Arab J. Sci. Publ. 2022, 2663, 5798. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).