Abstract
Medicinal plants are a rich source of diverse secondary metabolites (SMs) with significant industrial and medicinal applications. However, the natural content of these compounds is often low and influenced by various environmental and biological factors, making large-scale extraction from conventionally cultivated plants challenging. This review comprehensively examines the efficacy and benefits of plant in vitro culture techniques, specifically, callus, cell suspension, and hairy root cultures, for enhanced SMs production. A primary focus is placed on the elicitation effects of various nanomaterials and their mechanisms of action in boosting SMs synthesis. We present successful case studies utilizing different classes of nanomaterials, including metal oxides, non-metal oxides, carbon-based materials, polysaccharides, and quantum dots, as nano-elicitors. Furthermore, the review discusses the advantages and current challenges of nanomaterial-based elicitation, as well as its future applications and prospects. The insights consolidated in this review underscore the potential of nanoparticle-mediated elicitation as a robust strategy for the efficient production of valuable SMs in plant cell cultures. Finally, we emphasize the broad utility of diverse nanomaterials and highlight critical areas requiring further investigation in this field.
1. Introduction
Plants are a source of bioactive metabolites that are used in various fields, including food, flavors, agrochemicals, and therapeutic proteins [1]. Plant metabolites, divided into primary and secondary, play crucial roles in the growth and survival of plant species [1,2]. Primary metabolites (such as amino acids, carbohydrates, lipids, and proteins) directly support essential cellular processes, including respiration, photosynthesis, growth, and development [2]. Secondary metabolites (SMs) or specialized metabolites are produced from primary metabolites through different pathways [3].
Plant SMs have various functions, including signaling, stimulatory and inhibitory effects on enzymes, catalytic and defensive activities, and interactions with other organisms [1,2]. These metabolites also have different functions in plant growth, such as adjusting to environmental stresses (e.g., elevated O3, ionizing radiation, and wounding) and shielding plants from pathogen infections [3,4]. The production of SMs is internally mediated by plant phytohormones, such as jasmonate, salicylic acid, and their derivatives, as signal transducers [3,5].
SMs are derived from primary metabolites by biosynthetic modifications, such as hydroxylation, glycosylation, and methylation [6]. SMs are considered key elements for ecosystem engagement [7,8]. Plant SMs have key applications across various industries, including pharmaceuticals, agriculture, cosmetics, dietary supplements, textiles, fragrances, flavors, dyes, and biostimulants [9].
Plant SMs are classified based on their chemical structures, functional groups, and methods of synthesis [1]. The four main categories are as follows: 1—Nitrogen-containing compounds, with main subgroups of alkaloids (e.g., morphine, quinine, nicotine, caffeine), cyanogenic glucosides (e.g., Dhurrin), and non-protein amino acids (e.g., azetidine). 2—Terpenes, which are derived from isoprene (C5) units and comprise several subgroups with distinct compounds within each group. The groups and examples are monoterpenes (C10, menthol), sesquiterpenes (C15, artemisinin), diterpenes (C20, taxol (paclitaxel)), triterpenes (C30, saponins), and tetraterpenes (C40, carotenoids). 3—Phenolics, which have aromatic ring compounds with one or more hydroxyl groups. This group is divided into different subgroups: simple phenols (e.g., hydroquinone), flavonoids (e.g., flavonols, flavanons, anthocyanidins), tannins (e.g., ellagitannins), lignins and lignans, stilbenes, and coumarins. 4—Sulfur-containing compounds derived from amino acids containing sulfur, e.g., glucosinolates, glutathione, and phytoalexines (Figure 1).
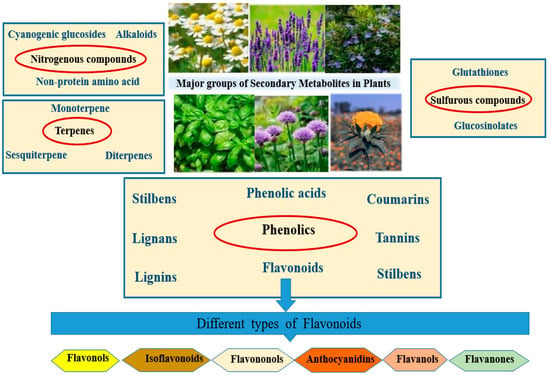
Figure 1.
The four major classes of plant secondary metabolites in medicinal plants are sulfur compounds, nitrogenous compounds, terpenes, and phenolic compounds.
Medicinal plants produce a vast array of over 100,000 known low-molecular-weight SMs [10] with beneficial therapeutic and pharmacological effects [6]. The biosynthesis, distribution, and accumulation of SMs are highly species-dependent and reflect the unique physiological and biochemical characteristics of each medicinal plant [9]. For example, in dragon’s head (Lallemantia iberica), anthocyanins and essential oils are accumulated in the leaves. In Zingiberaceae plants like ginger (Zingiber officinale) and turmeric (Curcuma longa) concentrate active ingredients—gingerol and curcumin, respectively—in their roots. In contrast, other plants, such as coffee (Coffea arabica), concentrate the active compound, caffeine, in the seeds. The biosynthesis of SMs starts through fundamental metabolic pathways, including glycolysis, the malonic acid pathway, the shikimic acid pathway, and the mevalonic acid pathway, before diverging into a variety of compounds.
The diversification in SMs is heavily influenced by cell type, the plant’s developmental stage, and environmental factors (e.g., temperature, humidity, and light intensity) [11,12]. Also, the SMs pathways and their regulation are highly susceptible to environmental variations because different stresses alter the expression of genes involved in their synthesis [9]. Additionally, reductions in plant populations, cultivation difficulties, low concentrations of essential metabolites in some species, and climate change have impacted the production of some SMs in medicinal plants [12,13].
Plant cell and tissue culture (PCTC) has emerged as a commercially viable solution for the pharmaceutical industry, offering a sustainable alternative to extracting these compounds from wild plants. In vitro elicitation is a key strategy that can activate stress-related pathways, alter cellular redox balance, and upregulate key biosynthetic genes involved in SMs production [11]. Among modern elicitation techniques, nano-elicitors have gained prominence as a practical and eco-friendly approach. Their unique physicochemical properties, such as nanoscale size and high reactivity, enable enhanced interaction with plant cellular machinery, thereby stimulating the biosynthesis of high-value SMs [5]. While nanomaterials (NMs), including metal-based nanoparticles (NPs), nanotubes, nanofibers, nanolayers, and nanosheets, have been widely developed for applications, such as food safety and plant growth, they also show great promise in PCTC.
NMs can modulate defense signaling pathways and redirect metabolic flux, leading to a significant increase in the accumulation of pharmaceutically important compounds, such as alkaloids, phenolics, terpenoids, and saponins [5]. However, despite a growing body of research, the precise mechanisms of nanoparticle-mediated elicitation in plant cell cultures remain poorly understood, and their effects are highly variable, depending on particle type, concentration, and exposure duration [5]. The core of this review is that NPs, beyond their role as simple abiotic stressors, can function as precise regulators of metabolic flux and SMs biosynthesis when optimally applied in plant in vitro systems.
This review seeks to evaluate the potential of nano-elicitors as powerful tools for enhancing SMs production and boosting the yield of valuable bioactive compounds in medicinal plants through PCTC. Furthermore, it aims to identify critical research gaps and outline future perspectives for the controlled and sustainable application of nano-elicitors in plant cell culture.
2. Working Methodology
This review was conducted through a comprehensive search of scientific literature related to the use of NMs as elicitors in plant cell, tissue, and hairy root culture systems. Scientific databases including Scopus, Web of Science, PubMed, Research Gate, and Google Scholar were searched using keywords including “cell culture,” “callus,” “hairy roots,” “nanomaterials,” elicitors,” and “metabolites.” Keywords were used in combination with Boolean operators to ensure precise and reproducible results. Specifically, we applied AND to combine different keywords, OR to include synonyms, and NOT to exclude irrelevant terms. All retrieved articles were analyzed and categorized by nanoparticle type, plant culture system, exposure concentration, and SMs type. A comparative evaluation across studies was performed to highlight the effective concentrations, response mechanisms, and potential advantages of nanoparticle-based elicitation for enhancing SMs production.
3. Plant Cell and Tissue Culture for SMs Production in Medicinal Plants
In vitro culture techniques present a viable alternative strategy for the efficient production of SMs, which, in natural conditions, is highly dependent on environmental fluctuations [14,15]. This approach enables the generation of substantial quantities within a reduced timeframe under strictly controlled, optimized environmental conditions [16,17]. The quality and quantity of SMs produced in vitro depend on various factors, including the composition of the culture media, pH, and culture environment (temperature, light, agitation, and aeration) [11,15]. Based on the nature of the explant and the nutrients provided in the culture medium, different in vitro culture systems can be established. in vitro culture has evolved into an effective tool for producing industrial and pharmaceutical compounds from plant cells under controlled, aseptic conditions [17,18,19,20]. The most relevant techniques for SM production in cell and tissue cultures include cell suspension culture, callus culture, and hairy root culture [17]. The methods based on PCTC have evolved into a scalable approach that provides precise control over SMs biosynthesis [20,21]. Here, we focused on the effects of different NMs on three applicable methods for producing SMs: callus culture, cell suspension culture, and hairy root culture.
3.1. Callus Culture
Callus is a tissue formed primarily from an unorganized mass of meristematic or proembryonic cells, typically induced from different parts of the whole plant or from aseptically germinated seeds [20,22]. Callus culture (CC) is an excellent tissue culture model for studying cellular processes in a tightly controlled environment, with rapid growth and high reproducibility. Moreover, CC serves as a precursor for cell suspension systems, which are increasingly used in the scalable production of bioactive compounds [23]. Unique Advantages of callus culture for SMs production could be detailed as controlled homogeneity and scalability within a uniform microenvironment, seasonal and geographical independence, and the possibility of applying different elicitors as a cutting-edge strategy to produce a wide range of SMs compounds. It is a suitable tool for genetic manipulation and somaclonal variation, as well as a conservation-oriented method for sustainable production. Also, callus cultures bypass geographical and climatic constraints, ensuring year-round, stable metabolite production.
3.2. Cell Suspension Culture
Cell suspension culture (CSC) is a crucial technique in cell biology where single cells or small aggregates are cultivated in an agitated liquid medium in a controlled environment [21]. This methodology is particularly effective for unattached cells. It relies on continuous agitation via orbital shakers, spinner flasks, or stirred bioreactors to maintain cells in suspension [24]. The versatility of CSC makes it ideal for high-yield biomanufacturing and provides uniform cell populations [18].
Unique advantages of the CSC method for SMs production include a homogeneous, controlled growth environment over environmental parameters such as pH, temperature, illumination, elicitation protocols, precursor feeding, and bioreactor optimization; better nutrient and oxygen transfer; and scalability in bioreactors [18,20,25]. Plant CSCs are now being employed for in vitro germplasm preservation, recombinant protein expression, scaled-up using bioreactors, transgenic plant development, generation of novel mutants through treating the cells with mutagens, and biotransformation [24]. Cell suspension cultures are also a good source for the examination of metabolomic and proteomic changes that take place during the production of various bioactive compounds [24]. Although CSCs have been successfully used to produce high levels of SMs, these initiatives face challenges, including poor cell efficiency, slow growth, genetic instability of high-producing cell lines, inadequate regulation of cellular differentiation, high costs, and difficult-to-control contamination [25].
3.3. Hairy Root Cultures
Hairy root cultures (HRCs) are generally classified as a type of organ culture, since they maintain the differentiated structure and physiological functions of plant roots under in vitro conditions [16].
This technique involves transforming plant explants with Rhizobium rhizogenes, which induces hairy roots (HRs) by inserting transfer DNA (T-DNA) [22]. However, this technique is often considered a bridge between organ and cell culture techniques in plant biotechnology [16]. HRCs systems are hormone-independent, genetically stable, and exhibit rapid growth rates due to their genetic transformation with T-DNA [13], which offers a cost-effective method to enhance the production of a wide variety of SMs [22]. HRs are a dependable source with high growth capacity that, when combined with strategies such as precursor feeding, immobilization, biotransformation, and elicitation, can be effective for SM production [26]. Additionally, HRCs tend to be more complex and costly than CSCs [27]. Although CC, CSC, and HRCs each possess their own advantages and limitations, as previously mentioned, all three approaches remain practically valuable and can be effectively employed depending on the specific objectives of the study. For instance, the growth rate and productivity of CC and CSC may be higher than those of HRCs in some cases; however, this depends strongly on plant species, genotype, and the type of SMs [16,18].
4. Mechanisms of NPs’ Action on SMs Production
Nanoparticles have transformed plant biotechnology by providing precise tools for modulating growth, defense, and metabolism. Their functional effects are derived from the physicochemical properties, such as size, shape, surface charge, and composition, which determine the uptake routes, cellular distribution, and biochemical reactivity [28,29]. When properly applied, NPs can amplify the biosynthesis of SMs, strengthen antioxidant systems, and enhance stress tolerance in medicinal plants. Mechanistically, these effects are orchestrated through finely tuned bursts of reactive oxygen species (ROS), calcium signaling, and activation of mitogen-activated protein kinase (MAPK) cascades that converge on the transcriptional reprogramming of biosynthetic genes [30]. The small size, high surface area, surface charge, and capacity for apoplastic or symplastic transport allow NPs to exhibit increased electrostatic interactions with cell membranes. These interactions can subsequently activate and regulate biosynthetic pathways, thereby enhancing SM synthesis in medicinal plant cells [31]. The production of SMs is initiated when nano-elicitors interact with specific receptors located on the plant cell’s plasma membrane (Figure 2). Following this interaction, NPs penetrate the membrane, leading to the generation of ROS, including hydrogen peroxide (H2O2) and superoxide (O2−). This ROS burst induces a calcium ion (Ca2+) influx into the cytosol through ion channels. This influx acidifies the cytosol, while a simultaneous efflux of chloride (Cl−) and potassium (K+) ions alkalinizes the extracellular environment. The rise in cytosolic Ca2+ is a pivotal elicitation event, as it regulates numerous cellular and physiological processes. Increased cytoplasmic Ca2 levels indirectly trigger defense responses via sensors such as Ca2-dependent protein kinases (CDPKs). This leads to the reversible phosphorylation and dephosphorylation of cytosolic and plasma membrane proteins, and the subsequent activation of MAPKs and other protein kinases (PKs). Furthermore, elevated intracellular Ca2+ enhances ROS production and activates NADPH oxidase. The resulting ROS act as secondary messengers, activating key signaling pathways involving jasmonate, ethylene, and salicylic acid. This cascade ultimately induces the expression of both the defense-related genes and key biosynthetic genes, such as PAL (phenylalanine ammonia-lyase), culminating in the biosynthesis of SMs [28]. It is important to note that the efficiency of SMs generation is highly dependent on several factors, including the plant species, types and concentrations of plant phytohormones, nutritional composition of the medium, the duration of elicitor exposure, and the physical characteristics and concentration of the elicitor itself [14,32].
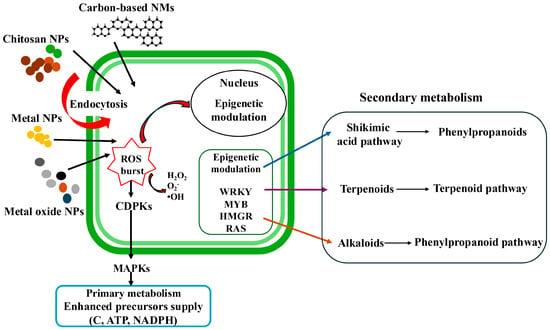
Figure 2.
Diagrammatic illustration describing possible mechanisms underlying nanoparticle-mediated elicitation of secondary metabolites in plant cell, callus, and hairy roots cultures.
Beyond these signaling cascades, nanoparticles can also trigger epigenetic modulation, which plays a role in the prolonged activation of secondary metabolism [33]. After NPs uptake and ROS signaling, certain factors and regulators in the nucleus change. These changes include how easily DNA can be accessed or how proteins called histones are modified. These adjustments help control gene activity. Key regulators affected by this process include WRKY transcription factors (named after their conserved WRKYGQK amino acid motif) and MYB transcription factors (originally identified from the myeloblastosis oncogene and which in plants regulate phenylpropanoid metabolism, flavonoid biosynthesis, and multiple stress-responsive pathways) [34,35]; HMGR (3-hydroxy-3-methylglutaryl-CoA reductase), a rate-limiting enzyme in the mevalonate pathway associated with terpenoid biosynthesis [36]; and RAS genes (rolB/rolC-associated signaling), which are linked to enhanced production of specialized metabolites [14]. Nano-elicitors help plant cells produce more phenylpropanoids, terpenoids, and alkaloids by altering signaling pathways and regulating gene expression.
5. Types of Nanoparticles as Elicitors in PCTC: Benefits, Mechanisms, and Applications
According to the literature, different kinds of NPs and NMs are gaining attention for their role as elicitors in plant biotechnology, especially in enhancing the production of valuable SMs in PCTC [37,38]. In this review, we focused on the eliciting effects of different nanoelicitation on SMs stimulation under CC, CSC, and HRCs (Figure 3). The following subsections examine the main classes of NPs, highlighting their biochemical functions, regulatory mechanisms, and their roles as elicitors under operational conditions.
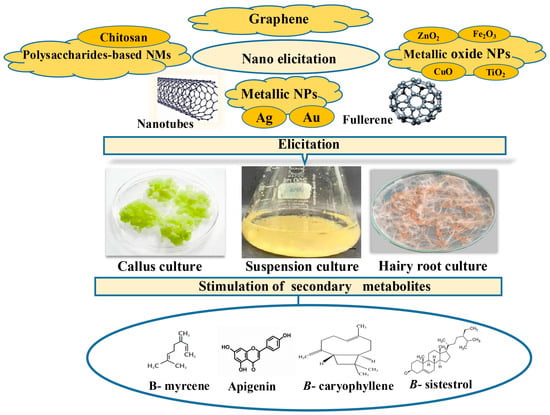
Figure 3.
Application of different elicitors on in vitro callus, cell suspension, and hairy roots culture towards the enhancement of secondary metabolites.
Table 1 summarizes the effects of various NPs used as elicitors across three in vitro culture systems: callus culture, cell suspension culture, and hairy root culture. For each nanoparticle type, the table reports biological responses, including changes in biomass, induction of different SMs, and biochemical alterations. Overall, Table 1 provides a comprehensive overview of nanoparticle-mediated elicitation across diverse medicinal plant culture systems and serves as a helpful basis for selecting suitable elicitors and culture platforms to optimize the production of valuable plant-derived compounds.

Table 1.
Effects of different nano-elicitation methods on the accumulation of secondary metabolites in various medicinal plants under callus, cell suspension, and hairy roots culture.
5.1. Metallic Nanoparticles (MNPs)
Metallic NPs have attracted particular attention as nano-elicitors due to their strong surface reactivity and controlled ion release, which create localized redox signals that reconfigure cellular metabolism. MNPs typically induce a transient ROS burst, upregulate antioxidant enzymes, such as superoxide dismutase and peroxidase, and trigger activation of the phenylpropanoid pathway via PAL [95]. These biochemical events, coupled with hormonal crosstalk, promote both protective and biosynthetic processes while sustaining growth if exposure remains within the hormetic range. In the following subsections, the specific metallic NPs employed in plant cell and tissue culture elicitation are detailed individually, highlighting their mechanisms of action, metabolite-specific responses, and species-dependent differences, thereby providing a comprehensive and nuanced understanding of their elicitation potential.
5.1.1. Silver Nanoparticles
Over the last decade, silver NPs (AgNPs) have emerged as potent elicitors in PCTC, enhancing the biosynthesis of diverse SMs by moderating oxidative effects, defense-related enzymes, and transcription factors, increasing PAL activity and phenylpropanoid flux [46,93,94,96]. In transgenic and in vitro systems, AgNPs also promote alkaloid biosynthesis by priming secondary metabolic pathways through redox-sensitive gene expression networks [45,49]. Ultimately, the balance between ROS generation and detoxification determines whether AgNPs act as stimulators or stressors, underscoring their role as efficient abiotic elicitors that enhance phenolics and alkaloids biosynthesis via redox-regulated, defense-associated pathways. For instance, the stimulatory effects of AgNPs have been reported to increase aloin in Aloe vera (L.) [97], hydroxybenzoic and flavanol acids in Bitter guard [40], hyoscine, scopolamine, and hyoscyamine in Hyoscyamus muticus [45]. In Stevia rebaudiana, two different concentrations of Ag NPs (45 and 60 mg L−1) increased the stevioside content of calli, i.e., 1.10 and 1.30-fold higher than the non-elicited treatments [43].
Notably, research indicates that Ag NPs function as effective signal transducers, eliciting an inductive response in Stevia cells. This suggests that Ag NPs could play a significant regulatory role in the glycosyltransferase pathway, thereby enhancing the biosynthesis of stevioside [98]. The concentration of 8 mg L−1 of Ag NPs resulted in the highest contents of carvacrol (1.06 mg L−1) and thymol (19.75 mg L−1) in Thymus kotschyanus and Thymus daenensis, under callus culture [42]. Elicitation by Ag NPs (10 mg L−1) in Corylus avellana resulted in the highest paclitaxel production through CSCs [44]. A 24 h post-elicitation period is optimal for maximizing the yield of various polyphenols, including rosmarinic acid, cinnamic acid, and rutin, in Hazel cells [39]. Treatment of Corylus avellana (L.) CSCs treated with 5 mg L-1 Ag NPs increased Taxol production [39]. In Echinacea purpurea, AgNPs (2-4 mg L−1) increased chicoric acid, chlorogenic acid, and caffeic acid levels, particularly in root-derived callus [49]. The treatment by AgNO3 elicitation, producing up to 9.5 mg g−1 DW chicoric acid within two days. In Perilla frutescens CSC, 100 mg L−1 AgNPs elevated caffeic acid (0.57 mg g−1 DW) and rutin (1.13 mg g−1 DW), coupled with high antioxidant activity and increased PAL, SOD (superoxide dismutase), and POD (peroxidase), indicating preferential activation of the phenylpropanoid branch via ROS-mediated signaling [91]. Similarly, in Hyoscyamus muticus HRs transformed with Agrobacterium rhizogenes (strain A4), AgNPs (100 mg L−1) markedly enhanced the accumulation of tropane alkaloids such as hyoscyamine and scopolamine [45]. Mechanistically, AgNPs operate through transient oxidative signaling that activates antioxidant and biosynthetic enzymes without inducing cytotoxicity.
5.1.2. Gold Nanoparticles
Gold NPs (AuNPs) represent a more recent and equally compelling class of elicitors [99]. They activate auxin-responsive transcription factors, such as ARF1 and ARF3, while upregulating 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme of the mevalonate pathway, demonstrating how nanogold bridges hormonal regulation with terpenoid biosynthesis [99]. Their actions illustrate how subtle redox cues and hormonal rebalancing can cooperatively drive secondary metabolism when the nanoparticle dosage is carefully optimized. AuNPs have also demonstrated strong elicitation potential in PCTC. Other metallic NPs, such as Fe and Zn, have been used as elicitors in PCTC. Overall, the collective evidence positions Fe and Zn nanostructures as highly promising, low-toxicity alternatives to noble-metal NPs (Ag and Au) in PCTC elicitation.
Different concentrations of FeNPs and ZnNPs (×, 2×, and 4× concentrations of B5 base medium) were used as elicitors on HRCs of fenugreek [54]. The results indicated that using Zn NPs can lead to higher levels of trigonelline (1.85 mg g−1 DW) under Zn (2×) treatment compared to the control group. The eliciting effects of other MNPs, such as Co [53] and Pd [55], have been reported (Table 1). The potential eliciting roles of other metallic or metal-based composites (e.g., Al and Co) remain poorly explored in PCTC. The eliciting effects of rare earth and lanthanide metallic NPs, such as cerium (Ce), lanthanum (La), neodymium (Nd), bimetallic and alloy nanostructures (e.g., Ag-Cu, Au-Ag, Co-Ni, among others), and metal–organic/biohybrid metallic composites, e.g., CuHARS (copper high-aspect ratio structures), have not been systematically evaluated.
5.1.3. Selenium-Based Nanoparticles
Selenium NPs (SeNPs) add a unique redox dimension to plant elicitation studies. Acting as both antioxidants and redox regulators, they improve biomass accumulation, enzymatic antioxidant defenses, and biosynthesis of multiple metabolite classes [100]. Their interaction with light signals further magnifies their effects. For instance, under blue LED illumination, SeNPs enhance PAL activity and phenolic biosynthesis in Santalum album, whereas selenium-doped ceria–magnetite composites double the chlorogenic acid content by transcriptionally activating key genes [101]. Thus, the efficiency of SeNPs is contingent not only on nanoparticle chemistry but also on the interplay between light quality and metabolic signaling.
5.2. Metal Oxide Nanoparticles
In contrast to metallic NPs, metal oxides offer greater chemical stability and lower toxicity. Their capacity to serve as both elicitors and micronutrient sources has positioned them as sustainable alternatives for enhancing SMs production. Metal oxide NPs often act through controlled oxidative priming, elevating antioxidant enzyme activity and triggering metabolic pathways without severely disrupting homeostasis [102].
5.2.1. Iron Oxide NPs
Iron oxide NPs (Fe3O4 and Fe2O3) have been used to enhance various SMs in cell cultures [56,57,61]. Their ability to induce significant metabolite gains makes them attractive for scalable biotechnological applications [56]. The significant potential of FeO NPs in photocatalysis arises from their unique magnetic properties and their ability to generate ROS upon light exposure [61,103,104]. According to the literature, iron oxide NPs upregulate PAL and rosmarinic acid synthase (RAS) genes, leading to markedly increased rosmarinic acid accumulation in Dracocephalum kotschy HRCs [56]. In addition, FeNPs stimulate secondary metabolism in other species, such as Hyoscyamus reticulatus, where they significantly enhance the production of tropane alkaloids, including hyoscyamine and scopolamine [60], through increased antioxidant enzyme activity and metabolic flux. Variations in light exposure can fine-tune these responses, revealing that iron NPs integrate redox control with photoresponsive regulation of cellular functions. In Artemisia scoparia, FeONPs (10–15 mg L−1) under variable light conditions promoted callus induction (92%), biomass accumulation (33 g L−1), and elevated total phenolics (37 mg GAE g−1 DW) and antioxidant activity (91%). These findings highlight Fe-based NPs as integrative elicitors that coordinate redox signaling, enzyme activation, and micronutrient balance to enhance metabolic flux toward antioxidant and bioactive compound biosynthesis. Such is the case of γ-Fe2O3 NPs, whose application in Bergenia ciliata CC enhanced biomass, phenolic and flavonoid contents, and antioxidant activity, accompanied by higher enzyme activity and volatile compound production [57]. This behavior suggests that Fe2O3 provides redox-based cues similar to those of Fe3O4, but, when combined with hormonal elicitors, it allows the jasmonate signaling pathway to converge with nanoparticle-derived oxidative signals, jointly boosting phenylpropanoid metabolism [66]. Mechanistically, FeONPs induced mild oxidative signaling, modulating SOD, POD, CAT, and APX activities, consistent with redox-mediated stimulation of secondary metabolism, which GC–MS profiling confirmed the presence of bioactive anti-leishmanial compounds under elicitation in Artemisia scoparia [61]. In another study, the xanthomicrol, cirsimaritin (as anticancer flavonoids), and isokaempferide content were increased by 11.87, 3.85, and 2.27-fold, respectively, under FeO NPS elicitation in Dracocephalum kotschyi HRCs [56].
5.2.2. Zinc Oxide NPs
Zinc is an essential micronutrient that functions as a catalytic cofactor for numerous plant enzymes, reinforcing biochemical activation cascades [105]. Among MONPs, zinc oxide NPs (ZnO NPs) have become one of the most extensively studied nano-elicitors due to their excellent biocompatibility, strong environmental sustainability potential, and demonstrated antioxidant, antifungal, and anticancer properties [105,106]. When applied at appropriate concentrations, engineered ZnO NPs stimulate a wide range of physiological and metabolic responses in plant cells. For example, in Stevia rebaudiana, exposure to 1 mg L−1 ZnO NPs doubled steviol glycoside content relative to the control and enhanced antioxidant activity, phenolics, and flavonoids. These responses were attributed to moderate ZnO NP–induced oxidative pressure and ROS generation, which activate antioxidant defenses and secondary metabolism, whereas higher concentrations caused metabolic suppression and phytotoxicity, underscoring a dose-dependent balance between stimulation and stress [107]. Optimal outcomes typically occur at mid-range concentrations and short elicitation periods, whereas overdosing reverses the stimulatory effect [107].
Consistent with this hormetic behavior, ZnO NPs have been shown to enhance diverse secondary metabolites (SMs) across multiple in vitro platforms, including callus cultures (CC), cell suspension cultures (CSC), and hairy root cultures (HRCs). In Linum usitatissimum CC and CSC, ZnO NP elicitation significantly increased phenolics and lignans [69,70]. In Thymus kotschyanus, the highest thymol content (22.83 mg L−1) occurred at 150 mg L−1 ZnO NPs, while in T. daenensis CC, the same concentration yielded maximal carvacrol levels (0.68 mg L−1) [108]. In Silybum marianum CC, 0.15 mg L−1 ZnO NPs produced the highest TPC (37 mg g−1 DW), TFC (8.9 mg g−1 DW), and silymarin (14.6 mg g−1 DW) [72]. Similarly, ZnO NP treatment of Delonix elata CC revealed peak phenolic (358.85 µg g−1 DW) and flavonoid (112.88 µg g−1 DW) accumulation at 30 mg L−1, accompanied by significant increases in gallic acid, quercetin, hesperidin, and rutin [109]. In Hyoscyamus reticulatus HRCs, ZnO NP elicitation (50–200 mg L−1) increased TPC by 3.2-fold and enhanced tropane alkaloids, scopolamine and hyoscyamine, by 1.2-fold [68]. Collectively, these findings demonstrate that ZnO NPs act as versatile and potent elicitors across plant tissue culture systems, with their efficacy strongly governed by concentration, exposure time, and species-specific metabolic capacity.
5.2.3. Copper Oxide NPs
Copper (Cu) is an essential micronutrient for plants and plays a critical role in regulating both primary and SMs in medicinal species. A growing body of research has explored the effects of copper oxide NPs (CuO NPs) on plant systems, highlighting their potential to influence biochemical pathways and SMs production [64]. CuO NPs combine trace-nutrient functionality with potent redox activity, resulting in notable increases in both phenolics and alkaloids [110]. CuO NPs activate WRKY, leading to the upregulation of pathway genes, such as tyrosine decarboxylase and several O- and N-demethylases in the alkaloid biosynthetic chain [70]. Various studies have demonstrated the effectiveness of CuO NPs under PCTC.
In Gymnema sylvestris, various concentrations of CuO NPs (1, 3, and 5 mg L−1) effectively increased gymnemic acid production by CSCs [64], resulting in a 2.3-fold increase compared to the control treatment. Callus elicitation of Ocimum basilicum showed that Murashige and Skoog media supplemented with 10 mg L−1 CuO NPs resulted in the highest contents for phenolic (27.5 mg g−1 DW), flavonoids (9.1 mg g−1 DW), rosmarinic acid (11.4 mg g−1 DW), chicoric acid (16.6 mg g−1 DW), and eugenol (0.21 mg g−1 DW) [74]. In Papaver orientale, a species valued for its benzylisoquinoline alkaloids, both chemically synthesized and green-derived CuO NPs (20–40 mg L−1) increased H2O2 accumulation and antioxidant enzyme activity, establishing oxidative stress as the initiating signal at 20 mg L−1 for 48 h [65]. The expression of key genes in the benzylisoquinoline alkaloid (BIA) pathway (PsWRKY, TYDC, SalSyn, SalAT, T6ODM, CODM) was upregulated, leading to higher levels of thebaine, codeine, and morphine [65]. This consistency between oxidative markers, transcriptional activation, and metabolite accumulation supports a ROS-to-transcription cascade as the central mechanism of CuO NP elicitation. Optimal elicitation typically occurs at moderate doses and longer exposure times, where oxidative signaling remains stimulatory rather than detrimental [111,112].
5.2.4. Cerium Oxide NPs
Cerium oxide NPs (CeO2 NPs) occupy a special niche among elicitors because of their reversible redox states (Ce3+/Ce4+) to enable ROS buffering and redox homeostasis [113]. When applied singly or in combination with iron oxides and selenium dopants, they markedly enhanced chlorogenic acid biosynthesis and broadened the phenolic profiles [114]. These effects extend beyond stress induction involving the transcriptional reprogramming of biosynthetic genes and metabolic intermediates; this performance illustrates a sophisticated biochemical leverage achievable through composite nanoformulations.
5.2.5. Silicon-Based NPs
Silicon dioxide NPs (SiO2 NPs) significantly modulate physiological mechanisms that enhance plant resilience against environmental stressors [115]. This efficacy is primarily attributed to the well-documented beneficial role of elemental silicon (Si) in promoting overall plant development and growth [115]. Nano elicitation with SiO2 NPs has increased the TPC and TFC in Ammi visnaga (15 mg L−1) [84] and Hyoscyamus reticulatus (100 and 200 mg L−1) [82]. Treatment of H. reticulatus HRs with 100 mg L−1 SiO2 NPs for 24 h resulted in the highest accumulation of the tropane alkaloids hyoscyamine and scopolamine, reaching levels of 140.15 μg/g FW and 67.71 μg g−1 FW, respectively [82]. This represented a dramatic increase of 1212% in hyoscyamine and a 272% increase in scopolamine production compared to the untreated control cultures. Other important MO NPs, such as TiO2 NPs, have been identified as significant elicitors [31,79] that promote key developmental processes in plants, such as cell division, cell expansion, and callus formation [78]. Furthermore, their physiological role extends to mimicking plant growth hormones [80]. The elicitation effects of TiO2 NPs on HRs of Saponaria officinalis L. showed that the highest rate of TPC (9.79 mg GAE g−1 FW) and total flavonoids content (TFC) (1.06 mg QE g−1 FW) were obtained in the treated HRs with 50 and 30 mg L−1 of TiO2 NPs under 24 and 48 h of treatments, respectively [78]. According to the literature, the eliciting effects of ZrO2 (zirconia), MgO, CaO, MnO, CeO2, NiO, CoO, and Cr2O3 oxides have not been studied under PCTC. These MO NPs lack mechanistic insights and a deficiency in dose-dependent toxicity. Most of the experiments have been limited to small-scale, so the industrial scalability of oxide-based elicitation remains untested.
5.3. Carbon-Based NMs
Carbon-based nanomaterials, including carbon nanotubes, graphene derivatives, and fullerenes, exert multifunctional effects by interacting with membranes and influencing electrical potential. This interaction reconfigures cellular redox balance and gene expression, enhancing phenylpropanoid and alkaloid pathways [113,116]. So, recent research suggests that carbon NMs may function as abiotic elicitors, enhancing the biosynthesis of SMs through various biochemical pathways [117]. The elicitation response observed in plant cell cultures is highly dependent on the physicochemical properties of these NMs, including their specific type, surface reactivity, functional groups, and tendency to agglomerate [86]. Hence, carbon nanotubes (CNTs) may be a suitable candidate for use as highly potent elicitors in PCTC, thereby modifying plant growth [38].
5.3.1. Multiwalled Carbon Nanotubes
Functionalized multiwalled carbon nanotubes (MWCNTs) have demonstrated pronounced elicitation effects even at low-to-moderate doses [118]. They promote morphogenesis and activate genes linked to alkaloid and phenylpropanoid biosynthesis (e.g., tyrosine aminotransferase, ornithine decarboxylase, putrescine N-methyltransferase) [119,120]. However, their hormetic nature is evident; high concentrations often result in cytotoxicity and excessive DNA methylation, emphasizing the narrow operational window for beneficial outcomes [121].
The stimulatory effects of CNTs [85,88] and MWCNTs [38] have been reported on the enhancement of SMs (Table 1). However, other researchers reported that MWCNTs at 5–500 µg mL−1 [37] enhanced cell growth compared to the control in tobacco callus culture. Thymoquinone (TQ), as the main active ingredient of the SMs, has been considered as an anti-liver cancer agent alone or in combination with other drugs [63]. According to the findings of [63], the highest value for TQ (295 mg L−1) was observed under treatment with FeO3-CTs NPs (100 mg L−1).
5.3.2. Graphene
Graphene oxide (GO) and reduced graphene oxide (rGO) have emerged as potent nano-elicitors for enhancing SM biosynthesis through stimulating antioxidant systems [122]. These NMs function as effective elicitors in plant cell cultures owing to their unique surface properties, such as high surface area, reactive functional groups, and tunable charge, as well as their strong interaction with cellular pathways. These interactions can enhance water transport, active stress-response signaling, and stimulate the biosynthesis os specialized metabolites, ultimately promoting regeneration and biomass accumulation [89]. The positive effects of GO NPs on the enhancement of TPC and TFC have been reported in P. major [86] and Lepidium sativum L. [87]. A comparison between rGO and CNTs showed that the callus treated with reduced GO showed increased TPC (107.4 µg GAE mL−1) and TFC (8.06 µg QE mL−1) as compared to TPC (32.3 µg GAE mL−1) and TFC (7.11 µg QE mL−1) observed in callus treated with CNTs. A recent study demonstrated that carbon-based NMs, particularly rGO, effectively promoted regeneration and SMs accumulation in Fagonia indica callus culture [89]. At low concentrations (1–2 mg L−1), rGO induced both shoot and root formation and achieved the highest biomass, TPC (107.4 µg GAE mL−1), and TFC (8.06 µg QE mL−1), outperforming CNTs. HPLC analyses confirmed enhanced biosynthesis of quercetin and gallic acid, indicating that rGO acts as a redox-modulating, biocompatible elicitor that reprograms secondary metabolism. The use of functionalized carbon-based NMs (e.g., carboxylated carbon nanotubes, aminated graphene) may enhance cellular uptake and signaling and, through PCTC, increase SMs. Investigating synergistic combinations of carbon-based NMs with metallic NPs or classical elicitors is needed. To the best of the authors’ knowledge, there are no studies on the elicitation effects of fullerenes (C60) and carbon quantum dots as elicitors in PCTC.
5.4. Polysaccharide-Based NMs
Polysaccharide-derived NMs, notably those based on chitosan and chitin, provide biocompatible and biodegradable elicitation pathways [123]. Unlike metallic NPs, plant cells perceive these NMs as pathogen or damage-associated molecular patterns, leading to innate immune activation and metabolic enhancement. Their structural similarity to natural polymers allows them to activate SMs biosynthesis with minimal cytotoxicity, positioning them as sustainable elicitors in plant biotechnological applications [124].
5.4.1. Chitosan
Chitosan NPs have unique properties, such as being nontoxic, biocompatible, and biodegradable, and exhibit stimulatory and antibacterial effects, which have been used for SMs production [90].
Chitosan NPs bind to negatively charged cell wall sites, initiating defense signaling and PAL activation [90,125]. When used as carriers for signaling molecules, such as methyl jasmonate, they enable controlled release, extending the duration of metabolic activation [92,126]. This dual functionality, both as an elicitor and a carrier, illustrates how biodegradable nanopolymers can enhance metabolite biosynthesis while ensuring environmental and biological compatibility.
The mixture of chitosan NPs and methyl jasmonate improved the production of phenols and flavonoids in SCS [127]. Under chitosan NPs elicitation in Silybium marianum, the highest record of taxifolin, silychristin, silybin B, and isosilybin A was obtained under 50 mg L−1 chitosan NPs that were coated with 40 mg L−1 phenylalanine [90]. The positive effect of chitosan NPs on artemisinin production in Artemisia annua CSC has also been reported [53]. Betulin and betulinic acid are two triterpenes with diverse pharmacological and physiological actions. An elicitation treatment with 0.5% cellulose nanofiber resulted in the highest betulin accumulation, yielding 0.7 mg g−1 in Betula pendula [91].
5.4.2. Chitin
Nanochitin operates through a closely related yet distinct mechanism, directly engaging plant immune receptors to induce robust defense metabolism by activating MAPK signaling cascades and subsequent triterpenoid pathways [91,128]. Short exposures to low concentrations favor both biomass and secondary metabolism, whereas prolonged treatment may shift the balance toward defense at the expense of growth. Compared to chitosan, nanochitin is a more potent activator of immune and phenolic pathways, making it particularly suitable for stimulating the production of high-value metabolites in controlled cultures [129]. According to the literature, no study has been done on the eliciting effect of chitin in PCTC.
5.5. Quantum Dots
Quantum dots (QDs) represent a new generation of elicitors effective at trace concentrations due to tunable redox properties [130,131]. Unlike polysaccharide or metallic NPs, they do not mimic biological ligands operating through redox-mediated signal transduction [132]. Carbon dots (CDs) are an example of this mode of action. For instance, when applied to Lactica sativa L. at low doses, they simultaneously promote biomass accumulation and redirect carbon flux toward triterpenoid and sterol biosynthesis [133]. Their elicitation mechanism involves the generation of superoxide radicals, which trigger antioxidant enzyme cascades and downstream Ca2+ and MAPK signaling [134]. Advanced modeling using artificial neural networks has captured these complex responses with high predictive accuracy, providing a robust computational framework for optimizing elicitor dose and timing to maximize productivity. Graphene-based quantum nanocomposites, such as rGO/Fe3O4 hybrids, illustrate how material engineering can balance elicitation intensity with cellular tolerance [135]. These systems enhance triterpenoid production while maintaining physiological stability, as their moderated redox activity induces metabolic shifts without causing excessive stress [136]. Such composites represent a promising path toward precision-controlled elicitation with a minimal ecological footprint.
5.6. Composite/ Hybrid NMs
Few studies have shown the effects of hybrid nanomaterials on SMs through PCTC [52]. In CC of Nigella sativa L., the highest levels of TPC, TFC, and amino acids were observed under Fe chitosan-coated nano iron (FeO3- CTs) (100 mg L−1) [63]. In comparison, FeO3- CTs at 100 and 200 mg L−1 exhibited significant effects on TPC, TFC, quercetin, gallic acid, and thymoquinone [63]. Under CC of Ammi visnaga L., a comparison between SiO2 NPs and GO-coated SiO2 NPs showed a superiority of GO-SiO2 NPs in stimulating TPC and TFC in comparison to SiO2 NPs [84]. Hybrid composites (GO–SiO2 and rGO/Fe3O4) stabilize redox states, preventing overoxidation and ensuring balanced metabolic activation [87,136]. These materials thus act as redox “priming agents,” improving plant readiness for stress and boosting secondary metabolite production through subtle oxidative and transcriptional cues under PCTC [137].
5.7. Non-Metallic Elements
According to the literature, few studies have examined the effects of non-metallic element NPs. HPLC quantitative analysis demonstrated a significant increase in phenolic and flavonoid compounds in Lotus arabicus CC treated with sulfur NPs (S NPs), particularly at 100 mg L−1, resulting in a 1.1-fold increase compared to the control [93]. The data further indicated a substantial increase in specific benzoic acid derivatives under the 100 mg L−1 S NP treatment. Notably, gallic acid increased by 1.37-fold, methyl gallate by 22.9-fold, and syringic acid by 2.4-fold. In contrast, the highest concentrations of ellagic acid and vanillin were observed at lower SNP doses of 25 mg L−1 and 50 mg L−1, respectively. To the authors’ knowledge, there are no studies involving nano-nitrogen compounds, selenium NPs, boron-based NPs, phosphorus-based NMs, or halogen-based NMs (Cl, Br, I) as elicitors in in vitro systems.
5.8. Integrative Considerations for Design and Scale-Up
Across NPs classes, a unifying principle emerges: elicitation efficacy follows dose–time hormesis, where short pulses at low-to-moderate concentrations maximize SMs while avoiding oxidative damage [138]. Surface functionalization, dopant chemistry, and composite formation often determine whether a nanoparticle acts as a stimulant or stressor [139]. Factors such as auxin balance, cell culture age, and light quality are vital for regulating nanoparticle signaling in both environmental and cell culture contexts [131]. To confirm mechanisms, it is crucial to combine ROS kinetics, antioxidant enzyme assays, and transcriptional profiling to establish causal links between nanoparticle properties and their metabolic impacts. For scaling up, emphasis should be placed on biocompatible formulations like ZnO, iron oxides, and polysaccharide carriers, as well as systems that are either magnetically recoverable or biodegradable [140]. By integrating experimental optimization with predictive modeling, it will be feasible to develop nano-elicitation strategies that enhance metabolite production in an efficient, sustainable, and reliable manner.
6. Advantages and Challenges
6.1. Advantages
As previously mentioned, PCTC supports agricultural activities by addressing issues such as mass propagation, germplasm conservation, genetic engineering, aseptic plant production, and increased SM output [117,141,142]. First, it was reported that NPs serve as efficient sterilizing agents at low concentrations, reducing microbial contamination during explant preparation and enhancing the overall success of the in vitro culture process [143]. Silver NPs are among the most widely used NPs in the disinfection of explants and culture media [31,144]. NPs, such as CuO, ZnO, TiO2, and graphite, have demonstrated decontamination power; however, there are no ionic counterparts to compare their effectiveness. Considering the eliciting effects of NMs under PCTC, the spectrum of used NPs as elicitors is significantly higher compared to traditional elicitors (such as ethylene, abscisic acid, gibberellic acid, and methyl jasmonate) [145]. Moreover, NPs can serve as carriers for genetic material, facilitating gene delivery and transformation processes in plant cells. The application of NPs also reduces the levels of plant phytohormones in culture media and promotes more natural growth patterns. Additionally, NPs can enhance nutrient and water uptake, thereby improving plant vigor and resilience under stress conditions. Overall, the use of NPs in plant tissue culture offers a multifaceted approach to enhancing plant growth, SMs production, and genetic transformation, thereby advancing the field of plant biotechnology. Nanomaterials showed greater stability compared with traditional elicitors. Their unique physicochemical properties, such as a high surface-area-to-volume ratio and the ability to be functionalized, enhance their dispersion and interaction with plant cells. For instance, studies have demonstrated that functionalized cerium and iron oxide NPs maintain colloidal stability in cell culture media, ensuring prolonged and consistent elicitation effects [146]. In contrast, conventional elicitors, including plant hormones and microbial extracts, often face challenges such as degradation, compositional variability, and batch-to-batch inconsistencies, leading to unpredictable responses in plant cultures [147]. The stability of NPs enables more controlled and reproducible elicitation of secondary metabolites, making them valuable tools for enhancing SM production in plant tissue cultures.
6.2. Research Gaps and Limitations
Despite the significant advantages shown by nanomaterials as elicitors in PCTC, the application of NPs as elicitors in plant tissue culture systems faces several substantial gaps and challenges that must be addressed to ensure reproducible and safe outcomes.
According to the literature, there is insufficient data available on optimal doses and toxic concentrations (dose-dependent responses) as threshold limits for many new types of NMs used as elicitors under PCTC. The magnitude and direction of metabolic responses to NPs are strongly concentration-and species-dependent. According to the literature, low to moderate NPs concentrations often increased SMs accumulation, whereas higher concentrations may lead to lipid peroxidation, membrane damage, DNA degradation, and inhibition of key antioxidant enzymes, ultimately reducing cell viability and growth under PCTC [6,117,148]. Long-term exposure to NPs also raises concerns about genomic instability, somaclonal variation, and changes in gene expression, which can compromise the regenerative capacity and genetic fidelity of plant cell culture [117,148]. Thus, one of the major concerns is about the potential toxicity of the used NPs, which depends on inappropriate concentrations, particle sizes of NPs, shapes, or chemical compositions of the used material [117,149], the exposure time, and the culture condition [149].
There is a lack of information on the scalability and biosafety of using NMs in large-scale methods, such as HRCs or CSCs. In addition, non-uniform dispersion and aggregation of NPs within the culture medium can decrease their bioavailability and efficacy. Additionally, the interactions with ions, organic compounds, and other components of the medium often lead to agglomeration or surface modifications that limit cellular contact [149]. The efficiency of cellular internalization of NMs depends on different factors such as nanoparticle size, coating, and their stability, which determine whether entry occurs via diffusion, endocytosis, or active transport [150]. According to the literature review, few studies have been reported regarding the mechanisms of NPs uptake into plant cells, translocation, and intracellular signaling under PCTC conditions. Also, the elicitation pathways for non-metallic NMs (ROS modulation, nutrient signaling, and membrane interactions) are poorly understood. Most studies have focused on MNP and MONPs in plant cells and HRCs, and non-metallic elicitors remain unexplored. Also, there is a lack of comparative studies for comparing metallic vs. non-metallic nanomaterials in the same culture system. Also, there is limited information on the combination of current elicitors (e.g., jasmonic acid, salicylic acid, biotic elicitors) with NMs.
Finally, environmental and biosafety implications must be considered, as residual NPs in culture biomass or effluents could accumulate in ecosystems or enter the food chain. Therefore, comprehensive risk assessment and life-cycle evaluation of NPs use in plant biotechnology are essential before large-scale application [147,148].
7. Conclusions and Future Recommendations
The present review highlighted the remarkable potential of different nanomaterials as novel and efficient elicitors in plant in vitro culture systems, including callus, suspension, and hairy root cultures. Nanoparticulate materials, including Ag, ZnO, CuO, TiO2, Fe3O4, and carbon-based NMs (graphene, carbon nanotubes), have been reported to modulate plant SMs through their unique physicochemical properties and ultimately enhance the biosynthesis of valuable SMs such as alkaloids, flavonoids, phenolic acids, terpenoids, and saponins. Overall, nanoparticle-mediated elicitation represents a promising, controllable, and sustainable strategy for enhancing the production of bioactive compounds in medicinal plants. However, to optimize nanomaterial application, further studies are required to:
- (1)
- Elucidate the precise molecular mechanisms of nanoparticle–plant interactions, assessing long-term cytotoxic effects.
- (2)
- Establish standard guidelines for safe and efficient use of industrial-scale production of high-value SMs.
- (3)
- Evaluate the synergistic combinations of NPs with biotic elicitors for more potent effects.
- (4)
- Develop smart NPs designed for specific metabolic pathways.
- (5)
- Elucidate nanoparticle–cell interactions, redox regulation, and gene-level responses across different metal-based nano elicitors.
- (6)
- Integrate nanotechnology with omics-based approaches (genomics, transcriptomics, metabolomics) and metabolic engineering to reveal molecular mechanisms.
- (7)
- Include membrane filters with a pore size of 0.1 µm (when possible) for removing bacteria, fungi, and mycoplasma to prevent tissue culture contamination.
Author Contributions
Conceptualization, P.G., E.V.-N., and J.R.P.-V.; methodology, P.G., E.V.-N., and J.R.P.-V.; investigation, P.G., E.V.-N., and J.R.P.-V.; writing—original draft preparation, P.G., E.V.-N., and J.R.P.-V.; writing—review and editing, P.G., E.V.-N., and J.R.P.-V.; supervision, J.R.P.-V. All authors have read and agreed to the published version of the manuscript.
Funding
E.V.-N. gratefully acknowledges SECIHTI for the financial support provided through project PEE-2025-G-647.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Wiley: Hoboken, NJ, USA, 2023; Volume 12, pp. 1–18. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based on Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2023; pp. 153–167. [Google Scholar]
- Twaij, B.M.; Mohammed Aloubaidi, H.K.; Hasan, M.N. Nanoparticle-Mediated Enhancement of Alkaloid, Phenolic, and Flavonoid Production in Datura Callus Cultures. Plant Cell Tissue Organ Cult. 2025, 161, 72. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of Promising Secondary Metabolites to Confer Resistance against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar]
- Kumar, P.; Kumar, D.; Pal, S.; Singh, S. Plant Secondary Metabolites in Defense against Phytopathogens: Mechanisms, Biosynthesis, and Applications. Physiol. Mol. Plant Pathol. 2025, 138, 102639. [Google Scholar] [CrossRef]
- Jangpangi, D.; Patni, B.; Chandola, V.; Chandra, S. Medicinal Plants in a Changing Climate: Understanding the Links between Environmental Stress and Secondary Metabolite Synthesis. Front. Plant Sci. 2025, 16, 1587337. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, F. Secondary Metabolites as Plant Traits: Current Assessment and Future Perspectives. Crit. Rev. Plant Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Oliveira, M.E.B.S.; Cardoso, F.C.I. Advances and Challenges on the in vitro Production of Secondary Metabolites from Medicinal Plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Mmereke, K.M.; Venkataraman, S.; Moiketsi, B.N.; Khan, M.R.; Hassan, S.H.; Rantong, G.; Masisi, K.; Kwape, T.E.; Gaobotse, G.; Zulfiqar, F.; et al. Nanoparticle Elicitation: A Promising Strategy to Modulate the Production of Bioactive Compounds in Hairy Roots. Food Res. Int. 2024, 178, 113910. [Google Scholar] [CrossRef] [PubMed]
- Humbal, A.; Pathak, B. Harnessing Nanoparticle-Mediated Elicitation in Plant Tissue Culture: A Promising Approach for Secondary Metabolite Production. Plant Cell Tissue Organ Cult. 2023, 155, 385–402. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and in vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sanchez, R.; Lalaleo, L.; Bonfill, M.; Corchete, P.; Palazon, J. Biotechnological Production of Pharmaceuticals and Biopharmaceuticals in Plant Cell and Organ Cultures. Curr. Med. Chem. 2018, 25, 3577–3596. [Google Scholar] [CrossRef]
- Ibrahim, I.A.A.; Ramadan, W.M.; El-Nashar, H.A.S.; Elwan, M.W.M. Plant Tissue Culture: Current Status and Future Perspectives in Secondary Metabolite Production. Plants 2023, 12, 2895. [Google Scholar]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in vitro Technologies: Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Khalafalla, M.M. Plant Cell Suspension Culture for Plant Secondary Metabolite Production: Current Status, Constraints, and Future Solutions. Pol. J. Environ. Stud. 2025, 1, 1–14. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A. Hairy Roots: An Untapped Potential for Production of Plant Products. Front. Plant Sci. 2022, 13, 2808. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Rastegarnejad, F.; Mirjalili, M.H.; Bakhtiar, Z. Enhanced Production of Tanshinone and Phenolic Compounds in Hairy Root Culture of Salvia miltiorrhiza Bunge by Elicitation. Plant Cell Tissue Organ Cult. 2024, 156, 4. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy Root Culture: A Potent Method for Improved Secondary Metabolite Production of Solanaceous Plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Jampilek, J. Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Appl. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Khan, S.; Hossain, M.K. Classification and Properties of Nanoparticles. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–54. [Google Scholar]
- Pal, P.; Prakash, O.; Parveen, A.; Singh, A.K.; Gupta, R.; Sarangi, P.K.; Sahoo, U.K.; Rathore, S.S.; Singh, R.K. Nanoparticle-Driven Plant Signaling for Advancing Stress Resilience and Agricultural Productivity—A Review. J. Nanopart. Res. 2025, 27, 258. [Google Scholar] [CrossRef]
- Jan, S.; Jan, N.; Singh, S.; Shah, M.A.; Bhat, I.A. Nanotechnology in Plant Tissue Culture: A Review. Hortic. Plant J. 2025, in press. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Vafaie Moghadam, A.; Iranbakhsh, A.; Saadatmand, S.; Ebadi, M.; Oraghi Ardebili, Z. New Insights into the Transcriptional, Epigenetic, and Physiological Responses to Zinc Oxide Nanoparticles in Datura Stramonium; Potential Species for Phytoremediation. J. Plant Growth Regul. 2022, 41, 271–281. [Google Scholar] [CrossRef]
- Aseel, D.G.; Ibrahim, O.M.; Abdelkhalek, A. Biosynthesized Silver Nanoparticles Mediated by Ammi Visnaga Extract Enhanced Systemic Resistance and Triggered Multiple Defense-Related Genes, Including SbWRKY Transcription Factors, against Tobacco Mosaic Virus Infection. BMC Plant Biol. 2024, 24, 756. [Google Scholar] [CrossRef]
- Cui, Q.; Hu, S.; Chu, R.; Chen, Y.; Taheri, A.; Yang, F.; Li, X.; He, X.; Zheng, L.; Zhou, M. How DNA Methylation Regulates Plant Natural Product Biosynthesis: From Epigenetics to Secondary Metabolism. Plant Cell Environ. 2025. Online ahead of print. [CrossRef]
- Ayoobi, A.; Saboora, A.; Asgarani, E.; Efferth, T. Iron Oxide Nanoparticles (Fe3O4-NPs) Elicited Artemisia annua L. in vitro, toward Enhancing Artemisinin Production through Overexpression of Key Genes in the Terpenoids Biosynthetic Pathway and Induction of Oxidative Stress. Plant Cell Tissue Organ Cult. 2024, 156, 85. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de-Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon Nanotubes Induce Growth Enhancement of Tobacco Cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Oraghi Ardebili, Z. Multi-Walled Carbon Nanotubes Improved Growth, Anatomy, Physiology, Secondary Metabolism, and Callus Performance in Catharanthus roseus: An in vitro Study. 3 Biotech 2019, 9, 404. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F. Taxanes Content and Cytotoxicity of Hazel Cells Extract after Elicitation. Plant Physiol. Biochem. 2017, 110, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of Silver Nanoparticles Enhanced the Secondary Metabolites and Pharmacological Activities in Cell Suspension Cultures of Bitter Gourd. 3 Biotech 2018, 8, 412. [Google Scholar] [CrossRef]
- Ali, A.; Mashwani, Z.-R.; Raja, N.I.; Mohammad, S.; Ahmad, M.S.; Luna-Arias, J.P. Exposure of Caralluma tuberculata to Biogenic Selenium Nanoparticles as In vitro Rooting Agent: Stimulates Morpho-Physiological and Antioxidant Defense System. PLoS ONE 2024, 19, e0297764. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, N.; Yousefifard, M.; Golkar, P.; Javed, R. Influence of Ag Nanoparticles on Physiological and Biochemical Aspects of Callus of Thymus Species and Zataria multiflora Boiss. Acta Agric. Slov. 2022, 118, 1–8. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of Stevia Glycosides Using Salicylic Acid and Silver Nanoparticles under Callus Culture. Sugar Tech 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Hazrati, R.; Zare, N.; Asghari-Zakaria, R.; Sheikhzadeh, P. Green Synthesized Ag Nanoparticles Stimulate Gene Expression and Paclitaxel Production in Corylus avellana Cells. Appl. Microbiol. Biotechnol. 2023, 107, 5963–5974. [Google Scholar] [CrossRef]
- Abdelkawy, A.M.; Alshammari, S.O.; Hussein, H.-A.A.; Abou El-Enain, I.M.; Abdelkhalek, E.S.; Radwan, A.M.; Kenawy, S.K.; Maaty, D.A.; Abed, N.N.; Sabry, S. Effect of Silver Nanoparticles on Tropane Alkaloid Production of Transgenic Hairy Root Cultures of Hyoscyamus muticus L. and Their Antimicrobial Activity. Sci. Rep. 2023, 13, 10397. [Google Scholar] [CrossRef]
- Alatar, A.A.; Qahtan, A.A.; Faisal, M. Eco-Friendly Synthesis of Silver Nanoparticles: Influence on Callus Proliferation, Secondary Metabolite Production, and Antioxidant Activities in Ruta chalepensis L. Plant Cell Tissue Organ Cult. 2024, 157, 55. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Iqbal, M.; Sabir, S.; Yasmeen, F. In vitro Seed Germination and Biochemical Profiling of Artemisia absinthium Exposed to Various Metallic Nanoparticles. 3 Biotech 2017, 7, 101. [Google Scholar] [CrossRef]
- Golinejad, S.; Mirjalili, M.H.; Rezadoost, H.; Ghorbanpour, M. Molecular, Biochemical, and Metabolic Changes Induced by Gold Nanoparticles in Taxus baccata L. Cell Culture. Ind. Crops Prod. 2023, 192, 115988. [Google Scholar] [CrossRef]
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced Production of Cichoric Acid in Cell Suspension Culture of Echinacea purpurea by Silver Nanoparticle Elicitation. Plant Cell Tissue Organ Cult. 2019, 139, 261–273. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H. Biochemical Changes and Enhanced Accumulation of Phenolic Compounds in Cell Culture of Perilla frutescens (L.) by Nano-Chemical Elicitation. Plant Physiol. Biochem. 2023, 204, 108151. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Shujait Ali, S.; Khan, A.; Wei, D.Q. Sustainable Production of Biomass and Industrially Important Secondary Metabolites in Cell Cultures of Selfheal (Prunella vulgaris L.) Elicited by Silver and Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Li, W.Y.; Wang, J.W. Stimulation of Artemisinin Production in Artemisia annua Hairy Roots by Ag–SiO2 Core–Shell Nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Ghasemi, B.; Hosseini, R.; Dehghan Nayeri, F.; Ghassemi, B.; Hosseini, R. The Effect of Nano Cobalt and Nano Chitosan on Artemisinin production and expression of SQS and DBR2 genes in Artemisia annua. Genet. Eng. Biosaf. J. 2015, 4, 25–39. [Google Scholar]
- Tariverdizadeh, N.; Mohebodini, M.; Chamani, E.; Ebadi, A. Iron and Zinc Oxide Nanoparticles: An Efficient Elicitor to Enhance Trigonelline Alkaloid Production in Hairy Roots of Fenugreek. Ind. Crops Prod. 2021, 162, 113240. [Google Scholar] [CrossRef]
- Kruszka, D.; Selvakesavan, R.K.; Kachlicki, P.; Franklin, G. Untargeted Metabolomics Reveals Elicitation of Important Secondary Metabolites upon Treatment with Various Metal and Metal Oxide Nanoparticles in Hypericum perforatum L. Cell Suspension Cultures. Ind. Crops Prod. 2022, 178, 114561. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Iron Oxide Nanoparticles: A Novel Elicitor to Enhance Anticancer Flavonoid Production and Gene Expression in Dracocephalum kotschyi Hairy-Root Cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef]
- Sardar, T.; Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Moussa, I.M.; Zaman, W.; Mahmoud, E.A. Methyl Jasmonate and Iron Oxide Nanoparticles Act as Elicitors to Stimulate Production of Bioactive Antioxidants and Metabolites in the In vitro Callus Cultures of Bergenia ciliata (Haw.) Sternb. S. Afr. J. Bot. 2023, 162, 201–210. [Google Scholar] [CrossRef]
- Sharafi, E.; Khayam Nekoei, S.M.; Fotokian, M.H.; Davoodi, D.; Mirzaei, H.H.; Hasanloo, T. Improvement of Hypericin and Hyperforin Production Using Zinc and Iron Nano-Oxides as Elicitors in Cell Suspension Culture of St John’s Wort (Hypericum perforatum L.). J. Med. Plants By-Prod. 2013, 2, 177–184. [Google Scholar]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of Secondary Metabolites and Antioxidant Activity in Dracocephalum Polychaetum Bornm. Cell Suspension Culture under Magnetite Nanoparticles and Static Magnetic Field Elicitation. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced Production of Hyoscyamine and Scopolamine from Genetically Transformed Root Culture of Hyoscyamus reticulatus L. Elicited by Iron Oxide Nanoparticles. In vitro Cell. Dev. Biol.-Plant 2017, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, R.; Khan, M.A.; Raza, A.; Ambreen; Ali, H.; Darwish, H.; Alharbi, K.; Kashgry, N.A.T.A.; Noureldeen, A. Iron Oxide Nanoparticles and Light Intensity Modulate Biomass, Antioxidant Capacity and Anti-Leishmanial Activity in Callus Cultures of Artemisia scoparia. Plant Cell Tissue Organ Cult. 2025, 160, 27. [Google Scholar] [CrossRef]
- Almagro, L.; Gea-Abellán, A.D.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A.; Pedreño, M.A. A Smart Strategy to Improve trans-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles. Polymers 2020, 12, 991. [Google Scholar] [CrossRef]
- Sobhanizadeh, A.; Giglou, M.T.; Behnamian, M.; Estaji, A.; Majdi, M.; Szumny, A. Effect of Plant Growth Regulators, Fe2O3–Chitosan Nanoparticles and LEDs on Growth and Biochemical Compounds of Black Seed (Nigella sativa L.) Callus In vitro. BMC Plant Biol. 2025, 25, 539. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Thiruvengadam, M. Impact of Copper Oxide Nanoparticles on Enhancement of Bioactive Compounds Using Cell Suspension Cultures of Gymnema sylvestre (Retz.) R. Br. Appl. Sci. 2019, 9, 2165. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M.R.; Motamedi, E.; Zargar, M. The Effects of Green and Chemically Synthesized Copper Oxide Nanoparticles on the Production and Gene Expression of Morphinan Alkaloids in Oriental Poppy. Sci. Rep. 2024, 14, 6000. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, T.; Khan, M.A.; Nadhman, A.; Aasim, M.; Khan, N.Z.; Ali, W.; Nazir, N.; Zahoor, M. Iron-Doped Zinc Oxide Nanoparticles-Triggered Elicitation of Important Phenolic Compounds in Cell Cultures of Fagonia indica. Plant Cell Tissue Organ Cult. 2021, 147, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of Secondary Metabolites in Callus Cultures of Stevia rebaudiana Bertoni Grown under ZnO and CuO Nanoparticles Stress. Sugar Tech 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of Nano-Zinc Oxide on Tropane Alkaloid Production, H6HGene Transcription and Antioxidant Enzyme Activity in Hyoscyamus reticulatus L. Hairy Roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C. Biogenic Zinc Oxide Nanoparticles-Enhanced Biosynthesis of Lignans and Neolignans in Cell Suspension Cultures of Linum usitatissimum L. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khurshid, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of Biogenic Zinc Oxide Nanoparticles on Growth and Oxidative Stress Response in Flax Seedlings vs. In vitro Cultures: A Comparative Analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef]
- Khan, M.A.; Raza, A.; Yousaf, R.; Ali, H.; Darwish, H. Impact of Zinc Oxide Nanoparticles on Biosynthesis of Thymoquinone in Cell Cultures of Nigella sativa. Plant Nano Biol. 2024, 10, 100109. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Khan, M.A.; Ali, A.; Mohammad, S.; Noureldeen, A.; Darwish, H.; Ali, A.; Ahmad, A.; Khan, T.; Khan, R.S. Interactive Effects of Zinc Oxide Nanoparticles and Different Light Regimes on Growth and Silymarin Biosynthesis in Callus Cultures of Silybum marianum L. Artif. Cells Nanomed. Biotechnol. 2021, 49, 523–535. [Google Scholar]
- Fatima, K.; Abbas, S.R.; Zia, M.; Sabir, S.M.; Khan, R.T.; Khan, A.A.; Hassan, Z.; Zaman, R. Induction of Secondary Metabolites on Nanoparticles Stress in Callus Culture of Artemisia annua L. Braz. J. Biol. 2021, 81, 474–483. [Google Scholar]
- Nazir, S.; Jan, H.; Zaman, G.; Khan, T.; Ashraf, H.; Meer, B.; Zia, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Copper Oxide (CuO) and Manganese Oxide (MnO) Nanoparticles Induced Biomass Accumulation, Antioxidants Biosynthesis and Abiotic Elicitation of Bioactive Compounds in Callus Cultures of Ocimum basilicum (Thai Basil). Artif. Cells Nanomed. Biotechnol. 2021, 49, 625–633. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. Comparative Effects of Nano- and Bulk-Sized CuO and ZnO on Glycyrrhizin and Phenolics in Glycyrrhiza glabra L. Seedlings. Indian J. Plant Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Al-Oubaidi, H.K.M.; Al-Khafagi, M.F.J. In vitro Increasing Medical Compounds (Tannins and Phenols) of Punica granatum L. in Callus Using MgO NPs and CuO NPs. J. Pharm. Sci. Res. 2018, 10, 1085–1088. [Google Scholar]
- Shoja, A.A.; Çirak, C.; Ganjeali, A.; Cheniany, M. Phenolic Compounds Accumulation and Antioxidant Activity in In vitro Culture of Salvia tebesana Bunge in Response to Nano-TiO2 and Methyl Jasmonate Elicitors. Plant Cell Tissue Organ Cult. 2022, 149, 423–440. [Google Scholar] [CrossRef]
- Hedayati, A.; Naseri, F.; Nourozi, E.; Hosseini, B.; Honari, H.; Hemmaty, S. Response of Saponaria officinalis L. Hairy Roots to TiO2 Nanoparticles in Terms of Production of Valuable Polyphenolic Compounds and SO6 Protein. Plant Physiol. Biochem. 2022, 178, 80–92. [Google Scholar] [CrossRef]
- Tabarifard, M.; Cheniany, M.; Khalilian-Movahhed, M. Artificial Neural Network Prediction and Elicitation of Flavones and Rosmarinic Acid in Teucrium polium Calli by Methyl Jasmonate and Nano-Sized TiO2. Iran. J. Sci. 2025, 1, 1191–1208. [Google Scholar]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Inductive Effect of Titanium Dioxide Nanoparticles on Anticancer Compounds Production and Expression of Rosmarinic Acid Biosynthesis Genes in Dracocephalum kotschyi Transformed Roots. Plant Physiol. Biochem. 2021, 167, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Poborilova, Z.; Opatrilova, R.; Babula, P. Toxicity of Aluminium Oxide Nanoparticles Demonstrated Using a BY-2 Plant Cell Suspension Culture Model. Environ. Exp. Bot. 2013, 91, 1–11. [Google Scholar] [CrossRef]
- Hedayati, A.; Hosseini, B.; Palazon, J.; Maleki, R. Improved Tropane Alkaloid Production and Changes in Gene Expression in Hairy Root Cultures of Two Hyoscyamus Species Elicited by Silicon Dioxide Nanoparticles. Plant Physiol. Biochem. 2020, 155, 416–428. [Google Scholar] [CrossRef]
- AlOubaidi, H.K.M. Effect of SiO2 NPs on Increase of Active Compounds in Leaf Callus of Tagetes erecta L. (Marigold) In vitro. J. Pharm. Negat. Results 2022, 13, 86–92. [Google Scholar]
- Golkar, P.; Akbari, R.; Bazarganipour, M.; Javed, R. Biochemical and Phytochemical Responses of Ammi visnaga L. (Apiaceae) Callus Culture Elicited by SiO2 and Graphene Oxide–SiO2 Nanoparticles. Plant Physiol. Biochem. 2023, 200, 107741. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-Walled Carbon Nanotubes Stimulate Callus Induction, Secondary Metabolites Biosynthesis and Antioxidant Capacity in Medicinal Plant Satureja khuzestanica Grown In vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Farahani, A.H.K.; Hadian, J. Potential Toxicity of Nano-Graphene Oxide on Callus Cell of Plantago major L. under Polyethylene Glycol-Induced Dehydration. Ecotoxicol. Environ. Saf. 2018, 148, 910–922. [Google Scholar] [CrossRef]
- Golkar, P.; Bakhtiari, M.A.; Bazarganipour, M. The Effects of Nanographene Oxide on the Morpho-Biochemical Traits and Antioxidant Activity of Lepidium sativum L. under In vitro Salinity Stress. Sci. Hortic. 2021, 288, 110301. [Google Scholar] [CrossRef]
- Begum, S.; Khan, T.; Khan, M.A.; Zahoor, M.; Zaman, N.; Ali, W. Carbon Nanotubes-Mediated Production of Biomass and Phenolic Compounds in Callus Cultures of Fagonia indica. Ind. Crops Prod. 2023, 195, 116408. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Karim, S.; Zahoor, M.; Jan, T.; Khan, M.A.; Nadhman, A. Efficient Regeneration of Shoots and Roots in Graphene Oxide- and Carbon Nanotubes-Mediated Callus Cultures: A Qualitative and Quantitative Study. Ind. Crops Prod. 2023, 204, 117262. [Google Scholar] [CrossRef]
- Hassanen, S.A.; Hegazi, G.A.; Diab, M.I.; Mahdi, A.A.; Hendawey, M.H.; Farroh, K.Y. Effect of Coumarin and Phenylalanine Conjugated with Chitosan Nanoparticles on Secondary Metabolites Synthesis in Callus of Silybium marianum. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 112–130. [Google Scholar]
- Payamnoor, V.; Khodadadi, N.; Jafary Hajati, R. BioNanoparticles as Elicitors Increase Accumulation of Betulin and Betulinic Acid in Callus Cultures. S. Afr. J. Bot. 2021, 141, 431–439. [Google Scholar]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Chitosan Nanoparticles and Their Combination with Methyl Jasmonate for the Elicitation of Phenolics and Flavonoids in Plant Cell Suspension Cultures. Int. J. Biol. Macromol. 2022, 214, 632–641. [Google Scholar] [CrossRef]
- Sobhy, S.E.; Khalifa, A.M.; Hafez, E.E.; Elsherif, D.E. Biosynthesized Sulfur Nanoparticles: A Novel Strategy to Enhance Antioxidant Secondary Metabolites in Lotus arabicus L. Callus Cultures. BMC Plant Biol. 2025, 25, 601. [Google Scholar] [CrossRef] [PubMed]
- Safary, R.; Golkar, P.; Bazarganipour, M. Effects of Graphene Oxide Incorporated with Silicon Dioxide on Some Secondary Metabolites of Levisticum officinale Koch. through Callus Culture. J. Plant Process Funct. 2023, 12, 321–336. [Google Scholar]
- Liu, W.; Zeb, A.; Lian, J.; Wu, J.; Xiong, H.; Tang, J.; Zheng, S. Interactions of Metal-Based and Metal-Oxide-Based Nanoparticles (MBNPs and MONPs) with Crop Plants: A Critical Review of Research Progress and Prospects. J. Hazard. Mater. 2020, 28, 294–310. [Google Scholar] [CrossRef]
- Chen, S.; Yan, X.; Peralta-Videa, J.R.; Su, Z.; Hong, J.; Zhao, L. Biological Effects of AgNPs on Crop Plants: Environmental Implications and Agricultural Applications. Environ. Sci. Nano 2022, 10, 62–71. [Google Scholar] [CrossRef]
- Raei, M.; Angaji, S.A.; Omidi, M. Effect of Abiotic Elicitors on Tissue Culture of Aloe vera. Int. J. Biosci. 2014, 5, 74–81. [Google Scholar]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered Silver Nanoparticles Are Sensed at the Plasma Membrane and Dramatically Modify the Physiology of Arabidopsis thaliana Plants. Plant J. 2016, 85, 245–257. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive Effect of AgNPs and AuNPs in In vitro Cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, K.; Geng, L.; Wang, Y.; Li, L.; Cheng, H. Selenium’s Role in Plant Secondary Metabolism: Regulation and Mechanistic Insights. Agronomy 2024, 15, 54. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Jafri, F.I.; Siddiqui, M.H.; Alamri, S.; Akhtar, M.S. Synergistic Effects of Selenium Nanoparticles and LED Light on Enhancement of Secondary Metabolites in Sandalwood (Santalum album) Plants through In vitro Callus Culturing Technique. PeerJ 2024, 12, e18106. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, P.; Hema, N.; Vijaya, P.P. In vitro Biocompatibility and Antimicrobial Activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. Bionanoscience 2020, 10, 112–121. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Galeas, S.; Guerrero, V.H. Phytosynthesis and Photocatalytic Activity of Magnetite (Fe3O4) Nanoparticles Using the Andean Blackberry Leaf. Mater. Chem. Phys. 2016, 179, 310–315. [Google Scholar] [CrossRef]
- Singh, R.P. Potential of Biogenic Plant-Mediated Iron and Iron Oxide Nanoparticles and Their Utility. In Plant Nanobionics: Volume 2, Approaches in Nanoparticles, Biosynthesis, and Toxicity; Springer: Singapore, 2019; pp. 77–113. [Google Scholar]
- Lilay, G.H.; Thiébaut, N.; du Mee, D.; Assunção, A.G.; Schjoerring, J.K.; Husted, S.; Persson, D.P. Linking the Key Physiological Functions of Essential Micronutrients to Their Deficiency Symptoms in Plants. New Phytol. 2024, 242, 881–902. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, Y.V.; Deryabin, A. Regulation of Pro-/Antioxidant Balance in Higher Plants by Nanoparticles of Metals and Metal Oxides. Russ. J. Plant Physiol. 2023, 70, 14. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of Zinc Oxide (ZnO) Nanoparticles on Physiology and Steviol Glycosides Production in Micropropagated Shoots of Stevia Rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of Callus Growth and Secondary Metabolites in Different Thymus Species and Zataria multiflora Micropropagated under ZnO Nanoparticles Stress. Biotechnol. Appl Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tarroum, M.; Alfarraj, N.S.; Al-Qurainy, F.; Al-Hashimi, A.; Khan, S.; Nadeem, M.; Salih, A.M.; Shaikhaldein, H.O. Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant. Metabolites 2023, 13, 905. [Google Scholar] [CrossRef]
- Darbahani, M.; Ghiyasi, M.R.; Rahaie, M. Nanoparticles as New Elicitors for the Production of Bioactive and Phytochemicals In vitro and In Vivo Plant Culture. Phytochem. Rev. 2025, 24, 3179–3203. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef]
- Trono, D. Elicitation as a Tool to Improve the Accumulation of Secondary Metabolites in Cannabis sativa. Phytochem. Rev. 2025, 24, 3119–3155. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as Novel Elicitors of Pharmacologically Active Plant Specialized Metabolites in Cell and Organ Cultures: Current Status and Future Outlooks. Plant Growth Regul. 2024, 104, 5–30. [Google Scholar] [CrossRef]
- Ashraf, K.; Liu, Z.; uz Zaman, Q.; Arshad, M.; Zaman, W.Q.; Shan, A.; Yu, J.; ur Rehman, T.; Zhuang, Y.; Guo, M. De Novo Synthesis of Selenium-Doped CeO2@Fe3O4 Nanoparticles for Improving Secondary Metabolite Biosynthesis in Carthamus tinctorius Cell Suspension Culture. Chem. Eng. J. 2025, 505, 159705. [Google Scholar] [CrossRef]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica Nanoparticles Protect Rice against Biotic and Abiotic Stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Abrishamchi, P.; Radjabian, T.; Saboora, A. Elicitation with Multi-Walled Carbon Nanotubes Improved Growth and Production of Polyphenolic Compounds in Melissa officinalis. Plant Biosyst. 2024, 158, 1125–1135. [Google Scholar] [CrossRef]
- Ghorbani, S.; Iranbakhsh, A.; Ebadi, M.; Oraghi Ardebili, Z. Carboxylic Acid-Functionalized Multi-Walled Carbon Nanotubes (COOH-MWCNTs) Elicit Concordant Variations in DNA Cytosine Methylation, Gene Expression, Growth, Morphogenesis, Metabolism, and Callogenesis in Salvia nemorosa: An In vitro Biological Assessment. J. Plant Growth Regul. 2023, 42, 4557–4569. [Google Scholar] [CrossRef]
- Tardast, Z.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Carboxylic Acid-Functionalized Multiwalled Carbon Nanotubes (COOH-MWCNTs) Improved Production of Atropine in Callus of Datura inoxia by Influencing Metabolism, Gene Regulation, and DNA Cytosine Methylation: An In vitro Biological Assessment. Plant Physiol. Biochem. 2023, 202, 107975. [Google Scholar] [CrossRef]
- Jabín, B.J.; Luis, S.J.; Eucario, M.-Á. Hormesis in Plant Tissue Culture. Plant Cell Tissue Organ Cult. 2024, 159, 16. [Google Scholar] [CrossRef]
- Soraki, R.K.; Gerami, M.; Ramezani, M. Effect of Graphene/Metal Nanocomposites on the Key Genes Involved in Rosmarinic Acid Biosynthesis Pathway and Its Accumulation in Melissa officinalis. BMC Plant Biol. 2021, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic Elicitors: A Boon for the In vitro Production of Plant Secondary Metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Tariq, A.; Bhawani, S.A.; Asaruddin, M.R.; Alotaibi, K.M. Introduction to Nanocomposites. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 15–37. [Google Scholar]
- Ji, H.; Wang, J.; Chen, F.; Fan, N.; Wang, X.; Xiao, Z.; Wang, Z. Meta-Analysis of Chitosan-Mediated Effects on Plant Defense against Oxidative Stress. Sci. Total Environ. 2022, 851, 158212. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; da Silva, F.F.A.; Bernardes, E.S.; Paulo Fabi, J. Plant-Derived Polyphenolic Compounds: Nanodelivery through Polysaccharide-Based Systems to Improve the Biological Properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 11894–11918. [Google Scholar] [CrossRef]
- Roy Chowdhury, M.; Mehmet, M.; Mukherjee, J.; Debnath, A.J.; Ražná, K. Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges. Molecules 2025, 30, 3476. [Google Scholar] [CrossRef]
- Nisar, N.; Tsuzuki, T.; Lowe, A.; Rossiter, J.T.; Javaid, A.; Powell, G.; Waseem, R.; Al-Mijalli, S.H.; Iqbal, M. Chitin Nanofibers Trigger Membrane-Bound Defense Signaling and Induce Elicitor Activity in Plants. Int. J. Biol. Macromol. 2021, 178, 253–262. [Google Scholar]
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2023, 35, 2203325. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, U.; Panigrahi, S.; Balyan, P.; Mehla, S.; Sihag, P.; Sagwal, V.; Singh, K.P.; White, J.C.; Dhankher, O.P. Nanoparticles as Novel Elicitors in Plant Tissue Culture Applications: Current Status and Future Outlook. Plant Physiol. Biochem. 2023, 203, 108004. [Google Scholar] [CrossRef]
- Kandoudi, W.; Németh-Zámboriné, É. Stimulating Secondary Compound Accumulation by Elicitation: Is It a Realistic Tool in Medicinal Plants In Vivo? Phytochem. Rev. 2022, 21, 2007–2025. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface Modification of Plant Fibers Using Environment Friendly Methods for Their Application in Polymer Composites, Textile Industry and Antimicrobial Activities: A Review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Cheng, B.; Ding, Z.; Yue, L.; Chen, F.; Cao, X.; Li, J.; Wang, C.; Wang, Z. Carbon Dots Enhanced Cold Tolerance of Lettuce (Lactuca sativa L.): Scavenging Reactive Oxygen Species, Modulating Hormones and Up-Regulating Gene Expression. Environ. Sci. Nano 2023, 10, 2849–2860. [Google Scholar] [CrossRef]
- Awere, C.O.; Sneha, A.; Rakkammal, K.; Muthui, M.M.; Govindan, S.; Çolak, A.B.; Bayrak, M.; Muthuramalingam, P.; Anadebe, V.C.; Archana, P. Carbon Dot Unravels Accumulation of Triterpenoid in Evolvulus alsinoides Hairy Roots Culture by Stimulating Growth, Redox Reactions and ANN Machine Learning Model Prediction of Metabolic Stress Response. Plant Physiol. Biochem. 2024, 216, 109142. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in Biologically Applicable Graphene-Based 2D Nanomaterials. Int. J. Mol. Sci. 2022, 23, 6253. [Google Scholar] [CrossRef]
- Darzian Rostami, A.; Yazdian, F.; Mirjani, R.; Soleimani, M. Effects of Different Graphene-Based Nanomaterials as Elicitors on Growth and Ganoderic Acid Production by Ganoderma lucidum. Biotechnol. Prog. 2020, 36, e3027. [Google Scholar] [CrossRef]
- Parthasarathy, S.P.; Archana, A.; Manickavasagam, M. Multi-Walled Carbon Nanotubes (MWCNTs) Elicitation Enhances L-Dopa Biosynthesis in Transgenic Hairy Root Cultures of Hybanthus enneaspermus (L.) F. Muell. Plant Cell Tissue Organ Cult. 2025, 163, 1–16. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis and Nanomaterials. From Biostimulation to Toxicity. In Plant Biostimulation with Nanomaterials; Springer: Cham, Switzerland, 2025; pp. 1–19. [Google Scholar]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and Toxicity: The Magnitude of the Impact of Nanomaterials in Microorganisms and Plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Komal, A.; Abbasi, B.H.; Hano, C. Nanoparticles as Elicitors of Biologically Active Ingredients in Plants. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Wiley: Hoboken, NJ, USA, 2021; pp. 170–202. [Google Scholar]
- Ugur, R. Development of In vitro Sterilization Protocol for DO-1 (Prunus domestica) Rootstock. Appl. Ecol. Environ. Res. 2020, 18, 2339–2349. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Alahakoon, A.M.P.D.; Polwaththa, K.P.G.D.M.; Galpaya, G.D.C.P.; Priyanjani, H.A.S.A.; Koswattage, K.R.; Senarath, W.T.P.S.K. Transforming Plant Tissue Culture with Nanoparticles: A Review of Current Applications. Plant Nano Biol. 2024, 10, 100102. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Hosseini-Mazinani, M.; Bagheri, S. Effects of Antimicrobial Activity of Silver Nanoparticles on In vitro Establishment of G × N15 (Hybrid of Almond × Peach) Rootstock. J. Genet. Eng. Biotechnol. 2014, 12, 103–110. [Google Scholar] [CrossRef]
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; The Vinh, B.; Khai, H.D.; et al. Silver Nanoparticles Improved Explant Disinfection, In vitro Growth, Runner Formation and Limited Ethylene Accumulation during Micropropagation of Strawberry (Fragaria × ananassa). Plant Cell Tissue Organ Cult. 2021, 145, 393–403. [Google Scholar] [CrossRef]
- Mohanlall, V. Plant Cell Culture Systems for the Production of Secondary Metabolites—A Review. J. Biotechnol. Biochem. 2020, 6, 35–47. [Google Scholar]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric Enhanced Stability of Functional Sub-10 nm Cerium and Iron Oxide Particles in Cell Culture Medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef]
- Vyavahare, G.D.; Patil, R.R.; Park, J.H. Nanoparticle-Assisted Elicitation of Therapeutically Important Secondary Metabolites in Plants. Plant Growth Regul. 2025, 1, 1–28. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as Elicitors and Harvesters of Economically Important Secondary Metabolites in Higher Plants: A Review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Asif, A.; Ahmed, Z.F.R. Nanoparticle-Enhanced Plant Defense Mechanisms Harnessed by Nanotechnology for Sustainable Crop Protection. In Nanoparticles in Plant Biotic Stress Management; Springer: Singapore, 2024; pp. 451–491. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.