Nano-Elicitation Approaches for Boosting Secondary Metabolites in Medicinal Plant Cell Cultures
Abstract
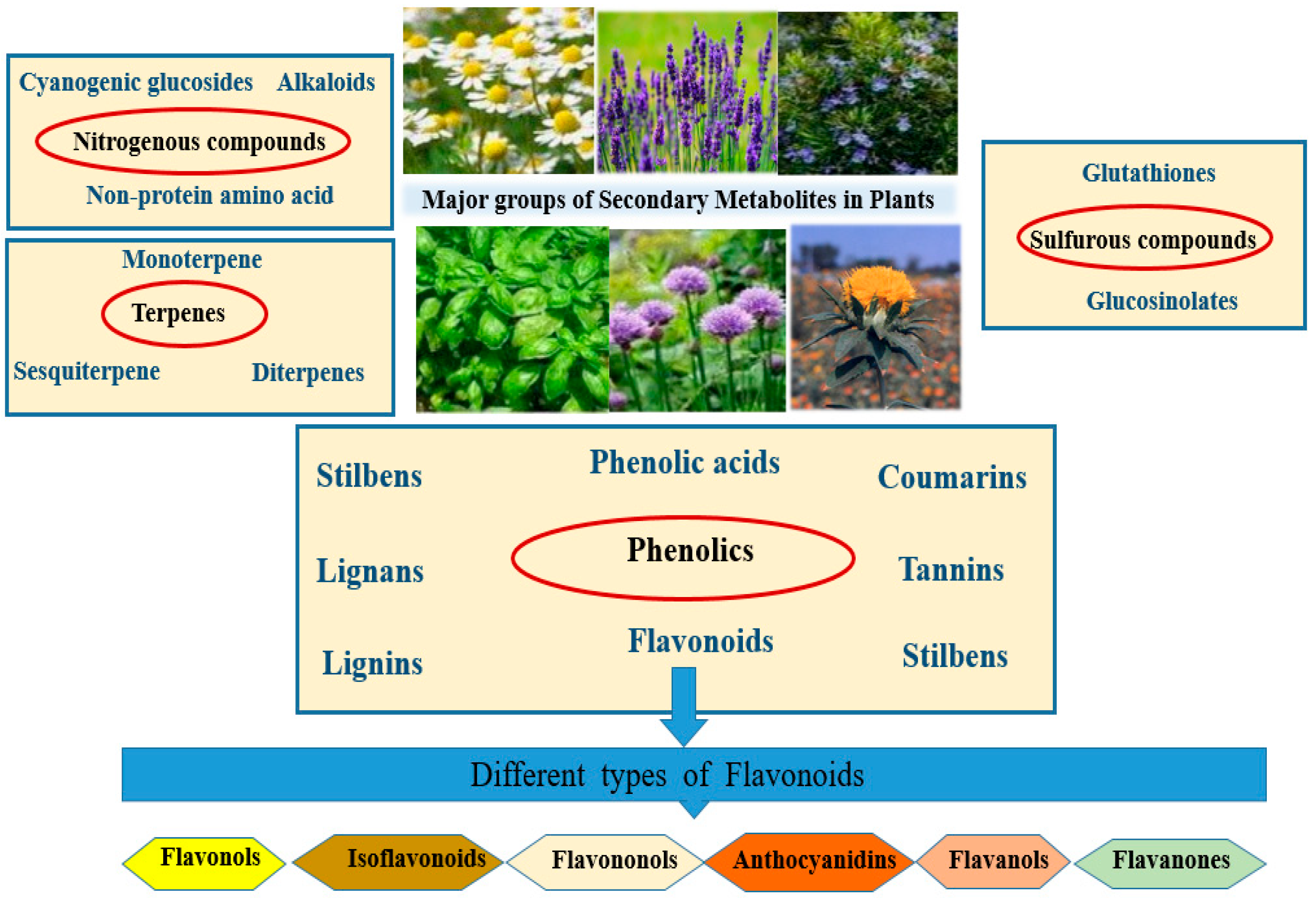
1. Introduction
2. Working Methodology
3. Plant Cell and Tissue Culture for SMs Production in Medicinal Plants
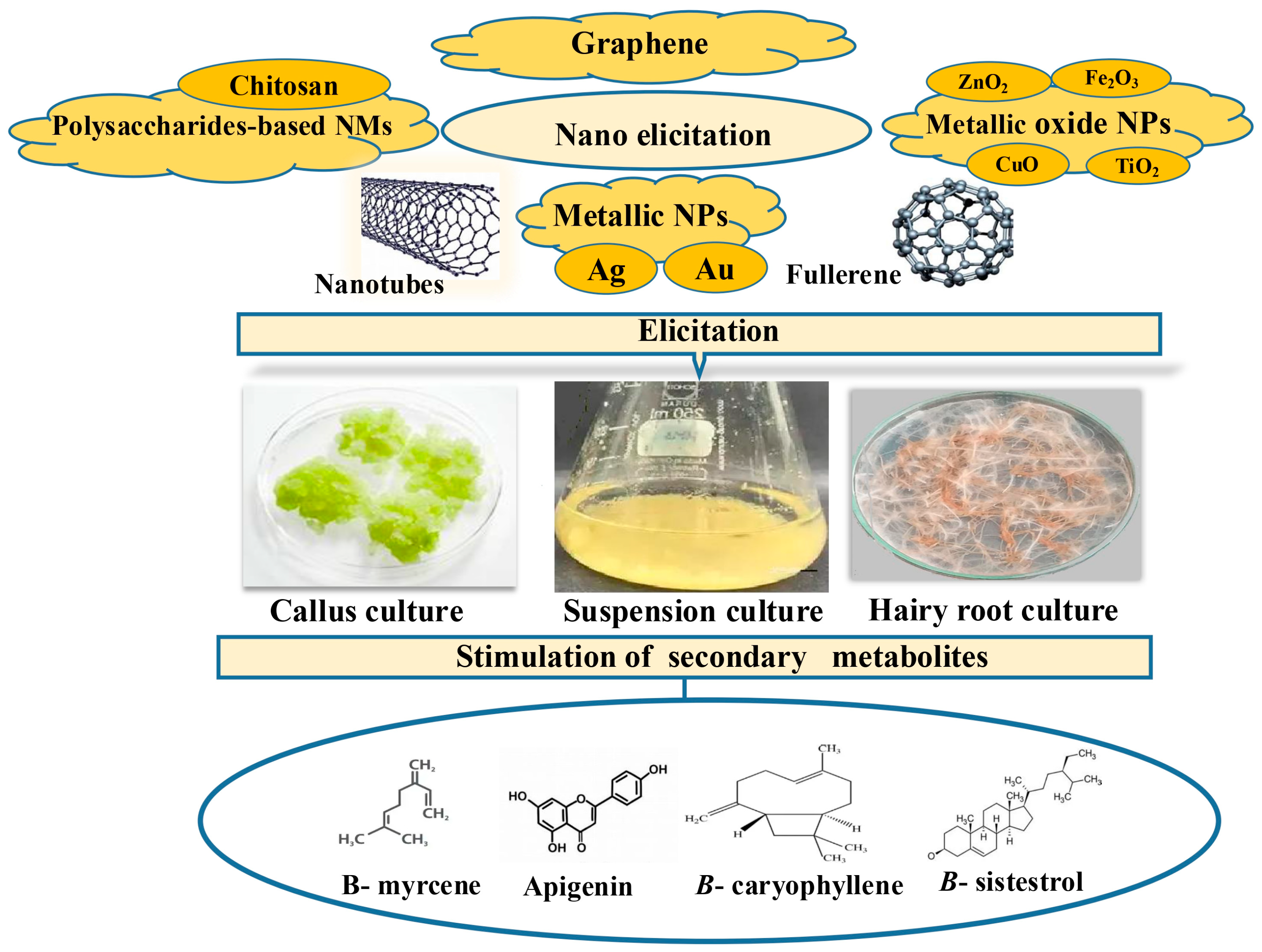
3.1. Callus Culture
3.2. Cell Suspension Culture
3.3. Hairy Root Cultures
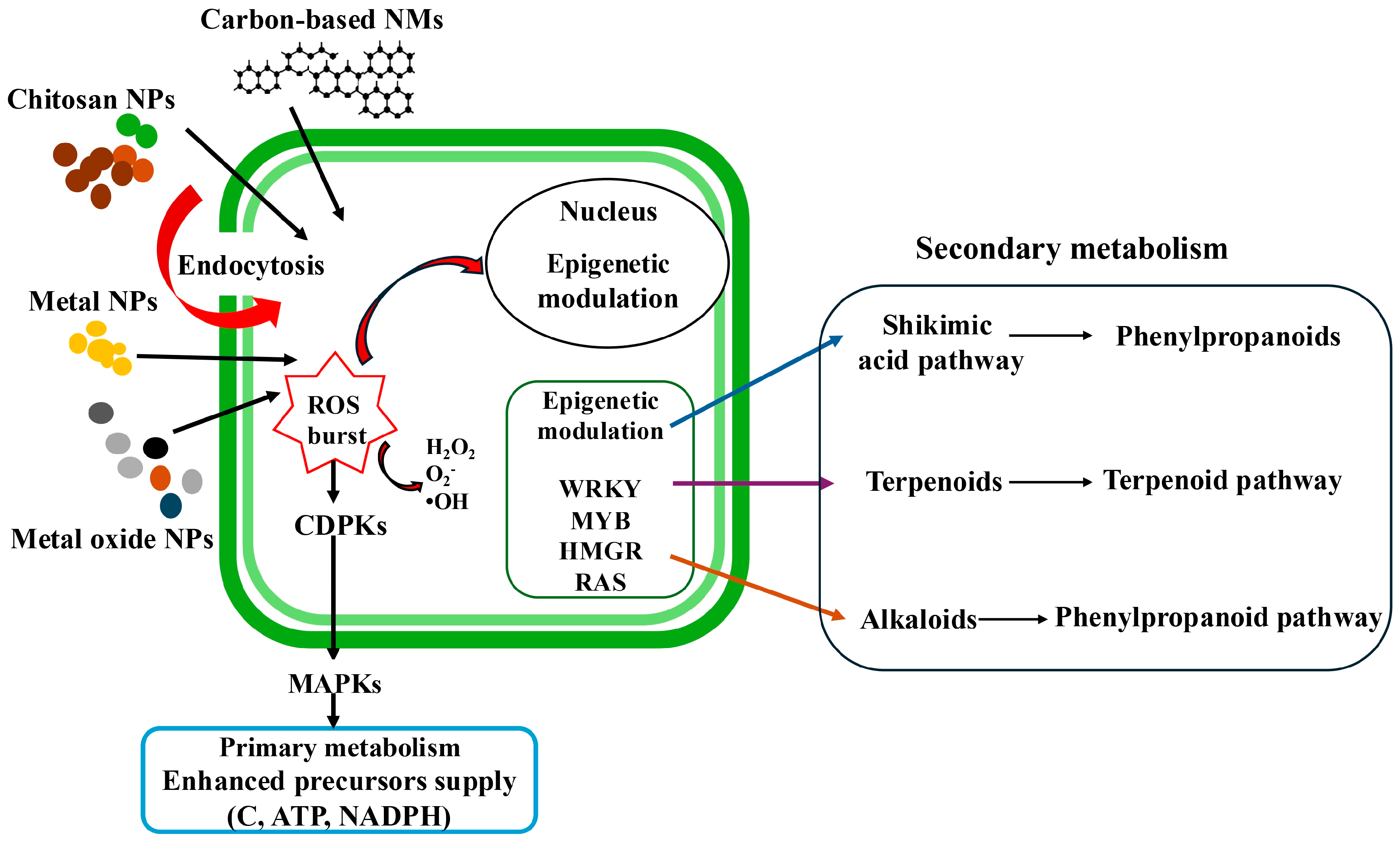
4. Mechanisms of NPs’ Action on SMs Production
5. Types of Nanoparticles as Elicitors in PCTC: Benefits, Mechanisms, and Applications
5.1. Metallic Nanoparticles (MNPs)
5.1.1. Silver Nanoparticles
5.1.2. Gold Nanoparticles
5.1.3. Selenium-Based Nanoparticles
5.2. Metal Oxide Nanoparticles
5.2.1. Iron Oxide NPs
5.2.2. Zinc Oxide NPs
5.2.3. Copper Oxide NPs
5.2.4. Cerium Oxide NPs
5.2.5. Silicon-Based NPs
5.3. Carbon-Based NMs
5.3.1. Multiwalled Carbon Nanotubes
5.3.2. Graphene
5.4. Polysaccharide-Based NMs
5.4.1. Chitosan
5.4.2. Chitin
5.5. Quantum Dots
5.6. Composite/ Hybrid NMs
5.7. Non-Metallic Elements
5.8. Integrative Considerations for Design and Scale-Up
6. Advantages and Challenges
6.1. Advantages
6.2. Research Gaps and Limitations
7. Conclusions and Future Recommendations
- (1)
- Elucidate the precise molecular mechanisms of nanoparticle–plant interactions, assessing long-term cytotoxic effects.
- (2)
- Establish standard guidelines for safe and efficient use of industrial-scale production of high-value SMs.
- (3)
- Evaluate the synergistic combinations of NPs with biotic elicitors for more potent effects.
- (4)
- Develop smart NPs designed for specific metabolic pathways.
- (5)
- Elucidate nanoparticle–cell interactions, redox regulation, and gene-level responses across different metal-based nano elicitors.
- (6)
- Integrate nanotechnology with omics-based approaches (genomics, transcriptomics, metabolomics) and metabolic engineering to reveal molecular mechanisms.
- (7)
- Include membrane filters with a pore size of 0.1 µm (when possible) for removing bacteria, fungi, and mycoplasma to prevent tissue culture contamination.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Wiley: Hoboken, NJ, USA, 2023; Volume 12, pp. 1–18. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based on Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2023; pp. 153–167. [Google Scholar]
- Twaij, B.M.; Mohammed Aloubaidi, H.K.; Hasan, M.N. Nanoparticle-Mediated Enhancement of Alkaloid, Phenolic, and Flavonoid Production in Datura Callus Cultures. Plant Cell Tissue Organ Cult. 2025, 161, 72. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of Promising Secondary Metabolites to Confer Resistance against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar]
- Kumar, P.; Kumar, D.; Pal, S.; Singh, S. Plant Secondary Metabolites in Defense against Phytopathogens: Mechanisms, Biosynthesis, and Applications. Physiol. Mol. Plant Pathol. 2025, 138, 102639. [Google Scholar] [CrossRef]
- Jangpangi, D.; Patni, B.; Chandola, V.; Chandra, S. Medicinal Plants in a Changing Climate: Understanding the Links between Environmental Stress and Secondary Metabolite Synthesis. Front. Plant Sci. 2025, 16, 1587337. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, F. Secondary Metabolites as Plant Traits: Current Assessment and Future Perspectives. Crit. Rev. Plant Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Oliveira, M.E.B.S.; Cardoso, F.C.I. Advances and Challenges on the in vitro Production of Secondary Metabolites from Medicinal Plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Mmereke, K.M.; Venkataraman, S.; Moiketsi, B.N.; Khan, M.R.; Hassan, S.H.; Rantong, G.; Masisi, K.; Kwape, T.E.; Gaobotse, G.; Zulfiqar, F.; et al. Nanoparticle Elicitation: A Promising Strategy to Modulate the Production of Bioactive Compounds in Hairy Roots. Food Res. Int. 2024, 178, 113910. [Google Scholar] [CrossRef] [PubMed]
- Humbal, A.; Pathak, B. Harnessing Nanoparticle-Mediated Elicitation in Plant Tissue Culture: A Promising Approach for Secondary Metabolite Production. Plant Cell Tissue Organ Cult. 2023, 155, 385–402. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and in vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sanchez, R.; Lalaleo, L.; Bonfill, M.; Corchete, P.; Palazon, J. Biotechnological Production of Pharmaceuticals and Biopharmaceuticals in Plant Cell and Organ Cultures. Curr. Med. Chem. 2018, 25, 3577–3596. [Google Scholar] [CrossRef]
- Ibrahim, I.A.A.; Ramadan, W.M.; El-Nashar, H.A.S.; Elwan, M.W.M. Plant Tissue Culture: Current Status and Future Perspectives in Secondary Metabolite Production. Plants 2023, 12, 2895. [Google Scholar]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in vitro Technologies: Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Khalafalla, M.M. Plant Cell Suspension Culture for Plant Secondary Metabolite Production: Current Status, Constraints, and Future Solutions. Pol. J. Environ. Stud. 2025, 1, 1–14. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A. Hairy Roots: An Untapped Potential for Production of Plant Products. Front. Plant Sci. 2022, 13, 2808. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Rastegarnejad, F.; Mirjalili, M.H.; Bakhtiar, Z. Enhanced Production of Tanshinone and Phenolic Compounds in Hairy Root Culture of Salvia miltiorrhiza Bunge by Elicitation. Plant Cell Tissue Organ Cult. 2024, 156, 4. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy Root Culture: A Potent Method for Improved Secondary Metabolite Production of Solanaceous Plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Jampilek, J. Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Appl. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Khan, S.; Hossain, M.K. Classification and Properties of Nanoparticles. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–54. [Google Scholar]
- Pal, P.; Prakash, O.; Parveen, A.; Singh, A.K.; Gupta, R.; Sarangi, P.K.; Sahoo, U.K.; Rathore, S.S.; Singh, R.K. Nanoparticle-Driven Plant Signaling for Advancing Stress Resilience and Agricultural Productivity—A Review. J. Nanopart. Res. 2025, 27, 258. [Google Scholar] [CrossRef]
- Jan, S.; Jan, N.; Singh, S.; Shah, M.A.; Bhat, I.A. Nanotechnology in Plant Tissue Culture: A Review. Hortic. Plant J. 2025, in press. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Vafaie Moghadam, A.; Iranbakhsh, A.; Saadatmand, S.; Ebadi, M.; Oraghi Ardebili, Z. New Insights into the Transcriptional, Epigenetic, and Physiological Responses to Zinc Oxide Nanoparticles in Datura Stramonium; Potential Species for Phytoremediation. J. Plant Growth Regul. 2022, 41, 271–281. [Google Scholar] [CrossRef]
- Aseel, D.G.; Ibrahim, O.M.; Abdelkhalek, A. Biosynthesized Silver Nanoparticles Mediated by Ammi Visnaga Extract Enhanced Systemic Resistance and Triggered Multiple Defense-Related Genes, Including SbWRKY Transcription Factors, against Tobacco Mosaic Virus Infection. BMC Plant Biol. 2024, 24, 756. [Google Scholar] [CrossRef]
- Cui, Q.; Hu, S.; Chu, R.; Chen, Y.; Taheri, A.; Yang, F.; Li, X.; He, X.; Zheng, L.; Zhou, M. How DNA Methylation Regulates Plant Natural Product Biosynthesis: From Epigenetics to Secondary Metabolism. Plant Cell Environ. 2025. Online ahead of print. [CrossRef]
- Ayoobi, A.; Saboora, A.; Asgarani, E.; Efferth, T. Iron Oxide Nanoparticles (Fe3O4-NPs) Elicited Artemisia annua L. in vitro, toward Enhancing Artemisinin Production through Overexpression of Key Genes in the Terpenoids Biosynthetic Pathway and Induction of Oxidative Stress. Plant Cell Tissue Organ Cult. 2024, 156, 85. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de-Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon Nanotubes Induce Growth Enhancement of Tobacco Cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Oraghi Ardebili, Z. Multi-Walled Carbon Nanotubes Improved Growth, Anatomy, Physiology, Secondary Metabolism, and Callus Performance in Catharanthus roseus: An in vitro Study. 3 Biotech 2019, 9, 404. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F. Taxanes Content and Cytotoxicity of Hazel Cells Extract after Elicitation. Plant Physiol. Biochem. 2017, 110, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of Silver Nanoparticles Enhanced the Secondary Metabolites and Pharmacological Activities in Cell Suspension Cultures of Bitter Gourd. 3 Biotech 2018, 8, 412. [Google Scholar] [CrossRef]
- Ali, A.; Mashwani, Z.-R.; Raja, N.I.; Mohammad, S.; Ahmad, M.S.; Luna-Arias, J.P. Exposure of Caralluma tuberculata to Biogenic Selenium Nanoparticles as In vitro Rooting Agent: Stimulates Morpho-Physiological and Antioxidant Defense System. PLoS ONE 2024, 19, e0297764. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, N.; Yousefifard, M.; Golkar, P.; Javed, R. Influence of Ag Nanoparticles on Physiological and Biochemical Aspects of Callus of Thymus Species and Zataria multiflora Boiss. Acta Agric. Slov. 2022, 118, 1–8. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of Stevia Glycosides Using Salicylic Acid and Silver Nanoparticles under Callus Culture. Sugar Tech 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Hazrati, R.; Zare, N.; Asghari-Zakaria, R.; Sheikhzadeh, P. Green Synthesized Ag Nanoparticles Stimulate Gene Expression and Paclitaxel Production in Corylus avellana Cells. Appl. Microbiol. Biotechnol. 2023, 107, 5963–5974. [Google Scholar] [CrossRef]
- Abdelkawy, A.M.; Alshammari, S.O.; Hussein, H.-A.A.; Abou El-Enain, I.M.; Abdelkhalek, E.S.; Radwan, A.M.; Kenawy, S.K.; Maaty, D.A.; Abed, N.N.; Sabry, S. Effect of Silver Nanoparticles on Tropane Alkaloid Production of Transgenic Hairy Root Cultures of Hyoscyamus muticus L. and Their Antimicrobial Activity. Sci. Rep. 2023, 13, 10397. [Google Scholar] [CrossRef]
- Alatar, A.A.; Qahtan, A.A.; Faisal, M. Eco-Friendly Synthesis of Silver Nanoparticles: Influence on Callus Proliferation, Secondary Metabolite Production, and Antioxidant Activities in Ruta chalepensis L. Plant Cell Tissue Organ Cult. 2024, 157, 55. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Iqbal, M.; Sabir, S.; Yasmeen, F. In vitro Seed Germination and Biochemical Profiling of Artemisia absinthium Exposed to Various Metallic Nanoparticles. 3 Biotech 2017, 7, 101. [Google Scholar] [CrossRef]
- Golinejad, S.; Mirjalili, M.H.; Rezadoost, H.; Ghorbanpour, M. Molecular, Biochemical, and Metabolic Changes Induced by Gold Nanoparticles in Taxus baccata L. Cell Culture. Ind. Crops Prod. 2023, 192, 115988. [Google Scholar] [CrossRef]
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced Production of Cichoric Acid in Cell Suspension Culture of Echinacea purpurea by Silver Nanoparticle Elicitation. Plant Cell Tissue Organ Cult. 2019, 139, 261–273. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H. Biochemical Changes and Enhanced Accumulation of Phenolic Compounds in Cell Culture of Perilla frutescens (L.) by Nano-Chemical Elicitation. Plant Physiol. Biochem. 2023, 204, 108151. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Shujait Ali, S.; Khan, A.; Wei, D.Q. Sustainable Production of Biomass and Industrially Important Secondary Metabolites in Cell Cultures of Selfheal (Prunella vulgaris L.) Elicited by Silver and Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Li, W.Y.; Wang, J.W. Stimulation of Artemisinin Production in Artemisia annua Hairy Roots by Ag–SiO2 Core–Shell Nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Ghasemi, B.; Hosseini, R.; Dehghan Nayeri, F.; Ghassemi, B.; Hosseini, R. The Effect of Nano Cobalt and Nano Chitosan on Artemisinin production and expression of SQS and DBR2 genes in Artemisia annua. Genet. Eng. Biosaf. J. 2015, 4, 25–39. [Google Scholar]
- Tariverdizadeh, N.; Mohebodini, M.; Chamani, E.; Ebadi, A. Iron and Zinc Oxide Nanoparticles: An Efficient Elicitor to Enhance Trigonelline Alkaloid Production in Hairy Roots of Fenugreek. Ind. Crops Prod. 2021, 162, 113240. [Google Scholar] [CrossRef]
- Kruszka, D.; Selvakesavan, R.K.; Kachlicki, P.; Franklin, G. Untargeted Metabolomics Reveals Elicitation of Important Secondary Metabolites upon Treatment with Various Metal and Metal Oxide Nanoparticles in Hypericum perforatum L. Cell Suspension Cultures. Ind. Crops Prod. 2022, 178, 114561. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Iron Oxide Nanoparticles: A Novel Elicitor to Enhance Anticancer Flavonoid Production and Gene Expression in Dracocephalum kotschyi Hairy-Root Cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef]
- Sardar, T.; Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Moussa, I.M.; Zaman, W.; Mahmoud, E.A. Methyl Jasmonate and Iron Oxide Nanoparticles Act as Elicitors to Stimulate Production of Bioactive Antioxidants and Metabolites in the In vitro Callus Cultures of Bergenia ciliata (Haw.) Sternb. S. Afr. J. Bot. 2023, 162, 201–210. [Google Scholar] [CrossRef]
- Sharafi, E.; Khayam Nekoei, S.M.; Fotokian, M.H.; Davoodi, D.; Mirzaei, H.H.; Hasanloo, T. Improvement of Hypericin and Hyperforin Production Using Zinc and Iron Nano-Oxides as Elicitors in Cell Suspension Culture of St John’s Wort (Hypericum perforatum L.). J. Med. Plants By-Prod. 2013, 2, 177–184. [Google Scholar]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of Secondary Metabolites and Antioxidant Activity in Dracocephalum Polychaetum Bornm. Cell Suspension Culture under Magnetite Nanoparticles and Static Magnetic Field Elicitation. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced Production of Hyoscyamine and Scopolamine from Genetically Transformed Root Culture of Hyoscyamus reticulatus L. Elicited by Iron Oxide Nanoparticles. In vitro Cell. Dev. Biol.-Plant 2017, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, R.; Khan, M.A.; Raza, A.; Ambreen; Ali, H.; Darwish, H.; Alharbi, K.; Kashgry, N.A.T.A.; Noureldeen, A. Iron Oxide Nanoparticles and Light Intensity Modulate Biomass, Antioxidant Capacity and Anti-Leishmanial Activity in Callus Cultures of Artemisia scoparia. Plant Cell Tissue Organ Cult. 2025, 160, 27. [Google Scholar] [CrossRef]
- Almagro, L.; Gea-Abellán, A.D.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A.; Pedreño, M.A. A Smart Strategy to Improve trans-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles. Polymers 2020, 12, 991. [Google Scholar] [CrossRef]
- Sobhanizadeh, A.; Giglou, M.T.; Behnamian, M.; Estaji, A.; Majdi, M.; Szumny, A. Effect of Plant Growth Regulators, Fe2O3–Chitosan Nanoparticles and LEDs on Growth and Biochemical Compounds of Black Seed (Nigella sativa L.) Callus In vitro. BMC Plant Biol. 2025, 25, 539. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Thiruvengadam, M. Impact of Copper Oxide Nanoparticles on Enhancement of Bioactive Compounds Using Cell Suspension Cultures of Gymnema sylvestre (Retz.) R. Br. Appl. Sci. 2019, 9, 2165. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M.R.; Motamedi, E.; Zargar, M. The Effects of Green and Chemically Synthesized Copper Oxide Nanoparticles on the Production and Gene Expression of Morphinan Alkaloids in Oriental Poppy. Sci. Rep. 2024, 14, 6000. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, T.; Khan, M.A.; Nadhman, A.; Aasim, M.; Khan, N.Z.; Ali, W.; Nazir, N.; Zahoor, M. Iron-Doped Zinc Oxide Nanoparticles-Triggered Elicitation of Important Phenolic Compounds in Cell Cultures of Fagonia indica. Plant Cell Tissue Organ Cult. 2021, 147, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of Secondary Metabolites in Callus Cultures of Stevia rebaudiana Bertoni Grown under ZnO and CuO Nanoparticles Stress. Sugar Tech 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of Nano-Zinc Oxide on Tropane Alkaloid Production, H6HGene Transcription and Antioxidant Enzyme Activity in Hyoscyamus reticulatus L. Hairy Roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C. Biogenic Zinc Oxide Nanoparticles-Enhanced Biosynthesis of Lignans and Neolignans in Cell Suspension Cultures of Linum usitatissimum L. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khurshid, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of Biogenic Zinc Oxide Nanoparticles on Growth and Oxidative Stress Response in Flax Seedlings vs. In vitro Cultures: A Comparative Analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef]
- Khan, M.A.; Raza, A.; Yousaf, R.; Ali, H.; Darwish, H. Impact of Zinc Oxide Nanoparticles on Biosynthesis of Thymoquinone in Cell Cultures of Nigella sativa. Plant Nano Biol. 2024, 10, 100109. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Khan, M.A.; Ali, A.; Mohammad, S.; Noureldeen, A.; Darwish, H.; Ali, A.; Ahmad, A.; Khan, T.; Khan, R.S. Interactive Effects of Zinc Oxide Nanoparticles and Different Light Regimes on Growth and Silymarin Biosynthesis in Callus Cultures of Silybum marianum L. Artif. Cells Nanomed. Biotechnol. 2021, 49, 523–535. [Google Scholar]
- Fatima, K.; Abbas, S.R.; Zia, M.; Sabir, S.M.; Khan, R.T.; Khan, A.A.; Hassan, Z.; Zaman, R. Induction of Secondary Metabolites on Nanoparticles Stress in Callus Culture of Artemisia annua L. Braz. J. Biol. 2021, 81, 474–483. [Google Scholar]
- Nazir, S.; Jan, H.; Zaman, G.; Khan, T.; Ashraf, H.; Meer, B.; Zia, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Copper Oxide (CuO) and Manganese Oxide (MnO) Nanoparticles Induced Biomass Accumulation, Antioxidants Biosynthesis and Abiotic Elicitation of Bioactive Compounds in Callus Cultures of Ocimum basilicum (Thai Basil). Artif. Cells Nanomed. Biotechnol. 2021, 49, 625–633. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. Comparative Effects of Nano- and Bulk-Sized CuO and ZnO on Glycyrrhizin and Phenolics in Glycyrrhiza glabra L. Seedlings. Indian J. Plant Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Al-Oubaidi, H.K.M.; Al-Khafagi, M.F.J. In vitro Increasing Medical Compounds (Tannins and Phenols) of Punica granatum L. in Callus Using MgO NPs and CuO NPs. J. Pharm. Sci. Res. 2018, 10, 1085–1088. [Google Scholar]
- Shoja, A.A.; Çirak, C.; Ganjeali, A.; Cheniany, M. Phenolic Compounds Accumulation and Antioxidant Activity in In vitro Culture of Salvia tebesana Bunge in Response to Nano-TiO2 and Methyl Jasmonate Elicitors. Plant Cell Tissue Organ Cult. 2022, 149, 423–440. [Google Scholar] [CrossRef]
- Hedayati, A.; Naseri, F.; Nourozi, E.; Hosseini, B.; Honari, H.; Hemmaty, S. Response of Saponaria officinalis L. Hairy Roots to TiO2 Nanoparticles in Terms of Production of Valuable Polyphenolic Compounds and SO6 Protein. Plant Physiol. Biochem. 2022, 178, 80–92. [Google Scholar] [CrossRef]
- Tabarifard, M.; Cheniany, M.; Khalilian-Movahhed, M. Artificial Neural Network Prediction and Elicitation of Flavones and Rosmarinic Acid in Teucrium polium Calli by Methyl Jasmonate and Nano-Sized TiO2. Iran. J. Sci. 2025, 1, 1191–1208. [Google Scholar]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Inductive Effect of Titanium Dioxide Nanoparticles on Anticancer Compounds Production and Expression of Rosmarinic Acid Biosynthesis Genes in Dracocephalum kotschyi Transformed Roots. Plant Physiol. Biochem. 2021, 167, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Poborilova, Z.; Opatrilova, R.; Babula, P. Toxicity of Aluminium Oxide Nanoparticles Demonstrated Using a BY-2 Plant Cell Suspension Culture Model. Environ. Exp. Bot. 2013, 91, 1–11. [Google Scholar] [CrossRef]
- Hedayati, A.; Hosseini, B.; Palazon, J.; Maleki, R. Improved Tropane Alkaloid Production and Changes in Gene Expression in Hairy Root Cultures of Two Hyoscyamus Species Elicited by Silicon Dioxide Nanoparticles. Plant Physiol. Biochem. 2020, 155, 416–428. [Google Scholar] [CrossRef]
- AlOubaidi, H.K.M. Effect of SiO2 NPs on Increase of Active Compounds in Leaf Callus of Tagetes erecta L. (Marigold) In vitro. J. Pharm. Negat. Results 2022, 13, 86–92. [Google Scholar]
- Golkar, P.; Akbari, R.; Bazarganipour, M.; Javed, R. Biochemical and Phytochemical Responses of Ammi visnaga L. (Apiaceae) Callus Culture Elicited by SiO2 and Graphene Oxide–SiO2 Nanoparticles. Plant Physiol. Biochem. 2023, 200, 107741. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-Walled Carbon Nanotubes Stimulate Callus Induction, Secondary Metabolites Biosynthesis and Antioxidant Capacity in Medicinal Plant Satureja khuzestanica Grown In vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Farahani, A.H.K.; Hadian, J. Potential Toxicity of Nano-Graphene Oxide on Callus Cell of Plantago major L. under Polyethylene Glycol-Induced Dehydration. Ecotoxicol. Environ. Saf. 2018, 148, 910–922. [Google Scholar] [CrossRef]
- Golkar, P.; Bakhtiari, M.A.; Bazarganipour, M. The Effects of Nanographene Oxide on the Morpho-Biochemical Traits and Antioxidant Activity of Lepidium sativum L. under In vitro Salinity Stress. Sci. Hortic. 2021, 288, 110301. [Google Scholar] [CrossRef]
- Begum, S.; Khan, T.; Khan, M.A.; Zahoor, M.; Zaman, N.; Ali, W. Carbon Nanotubes-Mediated Production of Biomass and Phenolic Compounds in Callus Cultures of Fagonia indica. Ind. Crops Prod. 2023, 195, 116408. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Karim, S.; Zahoor, M.; Jan, T.; Khan, M.A.; Nadhman, A. Efficient Regeneration of Shoots and Roots in Graphene Oxide- and Carbon Nanotubes-Mediated Callus Cultures: A Qualitative and Quantitative Study. Ind. Crops Prod. 2023, 204, 117262. [Google Scholar] [CrossRef]
- Hassanen, S.A.; Hegazi, G.A.; Diab, M.I.; Mahdi, A.A.; Hendawey, M.H.; Farroh, K.Y. Effect of Coumarin and Phenylalanine Conjugated with Chitosan Nanoparticles on Secondary Metabolites Synthesis in Callus of Silybium marianum. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 112–130. [Google Scholar]
- Payamnoor, V.; Khodadadi, N.; Jafary Hajati, R. BioNanoparticles as Elicitors Increase Accumulation of Betulin and Betulinic Acid in Callus Cultures. S. Afr. J. Bot. 2021, 141, 431–439. [Google Scholar]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Chitosan Nanoparticles and Their Combination with Methyl Jasmonate for the Elicitation of Phenolics and Flavonoids in Plant Cell Suspension Cultures. Int. J. Biol. Macromol. 2022, 214, 632–641. [Google Scholar] [CrossRef]
- Sobhy, S.E.; Khalifa, A.M.; Hafez, E.E.; Elsherif, D.E. Biosynthesized Sulfur Nanoparticles: A Novel Strategy to Enhance Antioxidant Secondary Metabolites in Lotus arabicus L. Callus Cultures. BMC Plant Biol. 2025, 25, 601. [Google Scholar] [CrossRef] [PubMed]
- Safary, R.; Golkar, P.; Bazarganipour, M. Effects of Graphene Oxide Incorporated with Silicon Dioxide on Some Secondary Metabolites of Levisticum officinale Koch. through Callus Culture. J. Plant Process Funct. 2023, 12, 321–336. [Google Scholar]
- Liu, W.; Zeb, A.; Lian, J.; Wu, J.; Xiong, H.; Tang, J.; Zheng, S. Interactions of Metal-Based and Metal-Oxide-Based Nanoparticles (MBNPs and MONPs) with Crop Plants: A Critical Review of Research Progress and Prospects. J. Hazard. Mater. 2020, 28, 294–310. [Google Scholar] [CrossRef]
- Chen, S.; Yan, X.; Peralta-Videa, J.R.; Su, Z.; Hong, J.; Zhao, L. Biological Effects of AgNPs on Crop Plants: Environmental Implications and Agricultural Applications. Environ. Sci. Nano 2022, 10, 62–71. [Google Scholar] [CrossRef]
- Raei, M.; Angaji, S.A.; Omidi, M. Effect of Abiotic Elicitors on Tissue Culture of Aloe vera. Int. J. Biosci. 2014, 5, 74–81. [Google Scholar]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered Silver Nanoparticles Are Sensed at the Plasma Membrane and Dramatically Modify the Physiology of Arabidopsis thaliana Plants. Plant J. 2016, 85, 245–257. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive Effect of AgNPs and AuNPs in In vitro Cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, K.; Geng, L.; Wang, Y.; Li, L.; Cheng, H. Selenium’s Role in Plant Secondary Metabolism: Regulation and Mechanistic Insights. Agronomy 2024, 15, 54. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Jafri, F.I.; Siddiqui, M.H.; Alamri, S.; Akhtar, M.S. Synergistic Effects of Selenium Nanoparticles and LED Light on Enhancement of Secondary Metabolites in Sandalwood (Santalum album) Plants through In vitro Callus Culturing Technique. PeerJ 2024, 12, e18106. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, P.; Hema, N.; Vijaya, P.P. In vitro Biocompatibility and Antimicrobial Activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. Bionanoscience 2020, 10, 112–121. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Galeas, S.; Guerrero, V.H. Phytosynthesis and Photocatalytic Activity of Magnetite (Fe3O4) Nanoparticles Using the Andean Blackberry Leaf. Mater. Chem. Phys. 2016, 179, 310–315. [Google Scholar] [CrossRef]
- Singh, R.P. Potential of Biogenic Plant-Mediated Iron and Iron Oxide Nanoparticles and Their Utility. In Plant Nanobionics: Volume 2, Approaches in Nanoparticles, Biosynthesis, and Toxicity; Springer: Singapore, 2019; pp. 77–113. [Google Scholar]
- Lilay, G.H.; Thiébaut, N.; du Mee, D.; Assunção, A.G.; Schjoerring, J.K.; Husted, S.; Persson, D.P. Linking the Key Physiological Functions of Essential Micronutrients to Their Deficiency Symptoms in Plants. New Phytol. 2024, 242, 881–902. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, Y.V.; Deryabin, A. Regulation of Pro-/Antioxidant Balance in Higher Plants by Nanoparticles of Metals and Metal Oxides. Russ. J. Plant Physiol. 2023, 70, 14. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of Zinc Oxide (ZnO) Nanoparticles on Physiology and Steviol Glycosides Production in Micropropagated Shoots of Stevia Rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of Callus Growth and Secondary Metabolites in Different Thymus Species and Zataria multiflora Micropropagated under ZnO Nanoparticles Stress. Biotechnol. Appl Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tarroum, M.; Alfarraj, N.S.; Al-Qurainy, F.; Al-Hashimi, A.; Khan, S.; Nadeem, M.; Salih, A.M.; Shaikhaldein, H.O. Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant. Metabolites 2023, 13, 905. [Google Scholar] [CrossRef]
- Darbahani, M.; Ghiyasi, M.R.; Rahaie, M. Nanoparticles as New Elicitors for the Production of Bioactive and Phytochemicals In vitro and In Vivo Plant Culture. Phytochem. Rev. 2025, 24, 3179–3203. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef]
- Trono, D. Elicitation as a Tool to Improve the Accumulation of Secondary Metabolites in Cannabis sativa. Phytochem. Rev. 2025, 24, 3119–3155. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as Novel Elicitors of Pharmacologically Active Plant Specialized Metabolites in Cell and Organ Cultures: Current Status and Future Outlooks. Plant Growth Regul. 2024, 104, 5–30. [Google Scholar] [CrossRef]
- Ashraf, K.; Liu, Z.; uz Zaman, Q.; Arshad, M.; Zaman, W.Q.; Shan, A.; Yu, J.; ur Rehman, T.; Zhuang, Y.; Guo, M. De Novo Synthesis of Selenium-Doped CeO2@Fe3O4 Nanoparticles for Improving Secondary Metabolite Biosynthesis in Carthamus tinctorius Cell Suspension Culture. Chem. Eng. J. 2025, 505, 159705. [Google Scholar] [CrossRef]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica Nanoparticles Protect Rice against Biotic and Abiotic Stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Abrishamchi, P.; Radjabian, T.; Saboora, A. Elicitation with Multi-Walled Carbon Nanotubes Improved Growth and Production of Polyphenolic Compounds in Melissa officinalis. Plant Biosyst. 2024, 158, 1125–1135. [Google Scholar] [CrossRef]
- Ghorbani, S.; Iranbakhsh, A.; Ebadi, M.; Oraghi Ardebili, Z. Carboxylic Acid-Functionalized Multi-Walled Carbon Nanotubes (COOH-MWCNTs) Elicit Concordant Variations in DNA Cytosine Methylation, Gene Expression, Growth, Morphogenesis, Metabolism, and Callogenesis in Salvia nemorosa: An In vitro Biological Assessment. J. Plant Growth Regul. 2023, 42, 4557–4569. [Google Scholar] [CrossRef]
- Tardast, Z.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Carboxylic Acid-Functionalized Multiwalled Carbon Nanotubes (COOH-MWCNTs) Improved Production of Atropine in Callus of Datura inoxia by Influencing Metabolism, Gene Regulation, and DNA Cytosine Methylation: An In vitro Biological Assessment. Plant Physiol. Biochem. 2023, 202, 107975. [Google Scholar] [CrossRef]
- Jabín, B.J.; Luis, S.J.; Eucario, M.-Á. Hormesis in Plant Tissue Culture. Plant Cell Tissue Organ Cult. 2024, 159, 16. [Google Scholar] [CrossRef]
- Soraki, R.K.; Gerami, M.; Ramezani, M. Effect of Graphene/Metal Nanocomposites on the Key Genes Involved in Rosmarinic Acid Biosynthesis Pathway and Its Accumulation in Melissa officinalis. BMC Plant Biol. 2021, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic Elicitors: A Boon for the In vitro Production of Plant Secondary Metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Tariq, A.; Bhawani, S.A.; Asaruddin, M.R.; Alotaibi, K.M. Introduction to Nanocomposites. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 15–37. [Google Scholar]
- Ji, H.; Wang, J.; Chen, F.; Fan, N.; Wang, X.; Xiao, Z.; Wang, Z. Meta-Analysis of Chitosan-Mediated Effects on Plant Defense against Oxidative Stress. Sci. Total Environ. 2022, 851, 158212. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; da Silva, F.F.A.; Bernardes, E.S.; Paulo Fabi, J. Plant-Derived Polyphenolic Compounds: Nanodelivery through Polysaccharide-Based Systems to Improve the Biological Properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 11894–11918. [Google Scholar] [CrossRef]
- Roy Chowdhury, M.; Mehmet, M.; Mukherjee, J.; Debnath, A.J.; Ražná, K. Chitosan as an Elicitor in Plant Tissue Cultures: Methodological Challenges. Molecules 2025, 30, 3476. [Google Scholar] [CrossRef]
- Nisar, N.; Tsuzuki, T.; Lowe, A.; Rossiter, J.T.; Javaid, A.; Powell, G.; Waseem, R.; Al-Mijalli, S.H.; Iqbal, M. Chitin Nanofibers Trigger Membrane-Bound Defense Signaling and Induce Elicitor Activity in Plants. Int. J. Biol. Macromol. 2021, 178, 253–262. [Google Scholar]
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2023, 35, 2203325. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, U.; Panigrahi, S.; Balyan, P.; Mehla, S.; Sihag, P.; Sagwal, V.; Singh, K.P.; White, J.C.; Dhankher, O.P. Nanoparticles as Novel Elicitors in Plant Tissue Culture Applications: Current Status and Future Outlook. Plant Physiol. Biochem. 2023, 203, 108004. [Google Scholar] [CrossRef]
- Kandoudi, W.; Németh-Zámboriné, É. Stimulating Secondary Compound Accumulation by Elicitation: Is It a Realistic Tool in Medicinal Plants In Vivo? Phytochem. Rev. 2022, 21, 2007–2025. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface Modification of Plant Fibers Using Environment Friendly Methods for Their Application in Polymer Composites, Textile Industry and Antimicrobial Activities: A Review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Cheng, B.; Ding, Z.; Yue, L.; Chen, F.; Cao, X.; Li, J.; Wang, C.; Wang, Z. Carbon Dots Enhanced Cold Tolerance of Lettuce (Lactuca sativa L.): Scavenging Reactive Oxygen Species, Modulating Hormones and Up-Regulating Gene Expression. Environ. Sci. Nano 2023, 10, 2849–2860. [Google Scholar] [CrossRef]
- Awere, C.O.; Sneha, A.; Rakkammal, K.; Muthui, M.M.; Govindan, S.; Çolak, A.B.; Bayrak, M.; Muthuramalingam, P.; Anadebe, V.C.; Archana, P. Carbon Dot Unravels Accumulation of Triterpenoid in Evolvulus alsinoides Hairy Roots Culture by Stimulating Growth, Redox Reactions and ANN Machine Learning Model Prediction of Metabolic Stress Response. Plant Physiol. Biochem. 2024, 216, 109142. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in Biologically Applicable Graphene-Based 2D Nanomaterials. Int. J. Mol. Sci. 2022, 23, 6253. [Google Scholar] [CrossRef]
- Darzian Rostami, A.; Yazdian, F.; Mirjani, R.; Soleimani, M. Effects of Different Graphene-Based Nanomaterials as Elicitors on Growth and Ganoderic Acid Production by Ganoderma lucidum. Biotechnol. Prog. 2020, 36, e3027. [Google Scholar] [CrossRef]
- Parthasarathy, S.P.; Archana, A.; Manickavasagam, M. Multi-Walled Carbon Nanotubes (MWCNTs) Elicitation Enhances L-Dopa Biosynthesis in Transgenic Hairy Root Cultures of Hybanthus enneaspermus (L.) F. Muell. Plant Cell Tissue Organ Cult. 2025, 163, 1–16. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis and Nanomaterials. From Biostimulation to Toxicity. In Plant Biostimulation with Nanomaterials; Springer: Cham, Switzerland, 2025; pp. 1–19. [Google Scholar]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and Toxicity: The Magnitude of the Impact of Nanomaterials in Microorganisms and Plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Komal, A.; Abbasi, B.H.; Hano, C. Nanoparticles as Elicitors of Biologically Active Ingredients in Plants. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Wiley: Hoboken, NJ, USA, 2021; pp. 170–202. [Google Scholar]
- Ugur, R. Development of In vitro Sterilization Protocol for DO-1 (Prunus domestica) Rootstock. Appl. Ecol. Environ. Res. 2020, 18, 2339–2349. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Alahakoon, A.M.P.D.; Polwaththa, K.P.G.D.M.; Galpaya, G.D.C.P.; Priyanjani, H.A.S.A.; Koswattage, K.R.; Senarath, W.T.P.S.K. Transforming Plant Tissue Culture with Nanoparticles: A Review of Current Applications. Plant Nano Biol. 2024, 10, 100102. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Hosseini-Mazinani, M.; Bagheri, S. Effects of Antimicrobial Activity of Silver Nanoparticles on In vitro Establishment of G × N15 (Hybrid of Almond × Peach) Rootstock. J. Genet. Eng. Biotechnol. 2014, 12, 103–110. [Google Scholar] [CrossRef]
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; The Vinh, B.; Khai, H.D.; et al. Silver Nanoparticles Improved Explant Disinfection, In vitro Growth, Runner Formation and Limited Ethylene Accumulation during Micropropagation of Strawberry (Fragaria × ananassa). Plant Cell Tissue Organ Cult. 2021, 145, 393–403. [Google Scholar] [CrossRef]
- Mohanlall, V. Plant Cell Culture Systems for the Production of Secondary Metabolites—A Review. J. Biotechnol. Biochem. 2020, 6, 35–47. [Google Scholar]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric Enhanced Stability of Functional Sub-10 nm Cerium and Iron Oxide Particles in Cell Culture Medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef]
- Vyavahare, G.D.; Patil, R.R.; Park, J.H. Nanoparticle-Assisted Elicitation of Therapeutically Important Secondary Metabolites in Plants. Plant Growth Regul. 2025, 1, 1–28. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as Elicitors and Harvesters of Economically Important Secondary Metabolites in Higher Plants: A Review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Asif, A.; Ahmed, Z.F.R. Nanoparticle-Enhanced Plant Defense Mechanisms Harnessed by Nanotechnology for Sustainable Crop Protection. In Nanoparticles in Plant Biotic Stress Management; Springer: Singapore, 2024; pp. 451–491. [Google Scholar]
| Nanomaterial | Type of Culture | Plant Name | Secondary Metabolite | Reference |
|---|---|---|---|---|
| Metallic NPs | ||||
| Ag | CSC | Aloe vera | Increase aloin | |
| Ag | CSC | Corylus avellana | Increase taxol and baccatin | [39] |
| Ag | CSC | Bitter guard | Increase TFC, TPC, hydroxycinnamic, hydroxybenzoic, and flavanol acids | [40] |
| Ag | CC | Caralluma tuberculata | Increase TPC and TFC | [41] |
| Ag | CC | T vulgaris, T. daenensis, T. Kotschyanus | Increase thymol and carvacrol | [42] |
| Ag | CC | Stevia rebaudiana | Increase steviol glycosides (stevioside and rebaudioside A | [43] |
| Ag | CC | Zataria multiflora | Increase thymol and carvacrol | [42] |
| Ag | CSC | Corylus avellana | Increase paclitaxel, taxol, baccatin | [44] |
| Ag | HRCs | Hyoscyamus muticus | Increase hyoscine, scopolamine, hyoscyamine | [45] |
| Ag | CC | Ruta chalepensis | Increase TPC, tannin, TFC, flavanols | [46] |
| Au | Artemisia absinthium | Increase TPC and TFC | [47] | |
| Au | CSC | Taxus baccato | Increase TPC and taxane | [48] |
| Ag | CC, CSC | Echinacea purpurea | Cichoric acid, chlorogenic acid, caffeic acid | [49] |
| Ag | HRCs | Hyoscyamus muticus | Tropane alkaloids (hyoscyamine, scopolamine) | [45] |
| Ag | CSC | Perilla frutescens | Caffeic acid, rutin; enhanced PAL, SOD, POD activity and antioxidant capacity via ROS-mediated phenylpropanoid activation | [50] |
| Ag and Au | CC | Prunella vulgaris | Callus proliferation, increase TPC, TFC | [51] |
| Ag-SiO2 | HRCs | Artemisia annua | Increase artemisinin | [52] |
| Co | CSC | Artemisia annua | Increase artemisinin | [53] |
| Zn | HRCs | Salvia miltiorrhiza Bunge | Increase tanshinone, rosmarinic acid, caffeic acid, and salvianolic acid | [26] |
| Zn and Fe | HRCs | Trigonella foenumgraecum | Increase TPC, TFC, and trigonelline | [54] |
| Ag, Au, Cu, Pd | CSC | Hypericum perforatum | Increase bisxanthone, gancaonin O, fusaroskyrin hyperxanthone C (Au), apigenin (Cu), emodin (Pd), | [55] |
| Metallic oxide NPs | ||||
| FeO | HRCs | Dracocephalum kotschyi | Increase TPC, TFC, rosmarinic acid, xanthomicrol, cirsimaritin, and isokaempferide | [56] |
| FeO | CC | Bergenia ciliata | Increase TPC, TFC, and volatile compounds | [57] |
| FeO and ZnO | CSC | Hypericum perforatum | Increase hypericin and hyperforin | [58] |
| Fe3O4 | CSC | Dracephalum polychaetum | Increase naringin, apigenin, rutin, rosmarinic acid, quercetin, thymol, and carvacrol | [59] |
| Fe3O4 | HRCs | Hyoscyamus reticulatus | Increase hyoscyamine and scopolamine | [60] |
| Fe3O4 | CC and CSC | Artemisia scoparia | Increase TPC, TFC, and volatile constituents | [61] |
| Fe3O4-β-cyclodextrin | CSC | Vitis vinifera | Increase resveratrol | [62] |
| FeO3-CTs | CC | Nigella sativa | Increase TPC, TFC, thymoquinone | [63] |
| CuO | CSC | Gymnema sylvestre | Increase TPC, TFC, and gymnemic acid II | [64] |
| CuO | CSC | Papaver orientale | Benzylisoquinoline alkaloids (thebaine, codeine, morphine) | [65] |
| Fe-ZnO | CSC | Fagonia indica | Increase TPC and epigallocatechin gallate | [66] |
| ZnO | CC | Stevia rebaudiana | Increase TPC, TFC, | [67] |
| ZnO | HRCs | Hyoscyamus reticulatus | Increase tropane alkaloids | [68] |
| ZnO | CSC | Linum usitatissimum | Increase lignans (secoisolariciresinol diglucoside, lariciresinol diglucoside) | [69] |
| ZnO | CC | T. vulgaris, T. daenensis, T. Kotschyanus | Increase thymol and carvacrol | [42] |
| ZnO | CC | Linum usitatissimum | Increase TPC, TFC, secoisolariciresinol diglucoside, lariciresinol diglucoside, dehydrodiconiferyl alcohol glucoside (25 mg L−1) | [70] |
| ZnO | CSC | Nigella sativa | Increase TPC, TFC, and thymoquinone | [71] |
| >ZnO | CC | Silybum marianum | Increase TPC, TFC, and silymarin | [72] |
| ZnO, CuO and CoO | CC | Artemisia annua | Increase TPC, TFC, rutin, gallic acid, and caffeic acid | [73] |
| CuO and MnO | CC | Ocimum basilicum | Increase TPC, TFC, rosmarinic acid, chicoric acid, eugenol | [74] |
| CuO and ZnO | Glycyrrhiza glabra | Increase glycyrrhizin | [75] | |
| MgO and CuO | CC | Punica granatum | Increase TPC, total tannins, gallic acid, ellagic acid, tannic acid | [76] |
| TiO2 | CC | Salvia tebesana | Increase TPC and TFC | [77] |
| TiO2 | HRCs | Saponaria officinalis | Increase TPC, total TFC, and SO6 anticancer protein | [78] |
| TiO2 | CC | Teucrium polium | Increase TFC, flavones, rosmarinic acid, | [79] |
| TiO2 | HRCs | Dracocephalum kotschyi | Increase TPC, TFC, rosmarinic acid, xanthomicrol, cirsimaritin, | [80] |
| Al2O3 and WO3 | CC | Datura spp. | Increase TPC, TFC, and alkaloids | [5] |
| Al2O3 | CSC | Tobacco | Increase total phenolics | [81] |
| SiO2 | HRCs | Hyoscyamus spp. | Increase TPC, TFC, tropane alkaloids (, scopolamine) | [82] |
| SiO2 | CC | Tagetes erecta | Increase phenolic compounds | [83] |
| SiO2 | CC | Ammi visnaga | Increase TPC and TFC | [84] |
| SiO2 | CC | Caralluma tuberculata | Increase TPC, TFC, coumarins, gallic acid, caffeic acid, ferulic acid, catechin, quercetin, and rutin | [41] |
| Carbon–based NMs | ||||
| CNTs | CC | Satureja khuzestanica | Increase TPC, TFC, rosmarinic acid, caffeic acid | [85] |
| CNTs | CC | Tobacco | Cell growth and division | [37] |
| MWCNTs | CC | Catharanthus roseus | Increase TFC | [38] |
| GO | CC | P. major | Increase TPC, TFC | [86] |
| GO | CC | Lepidium sativum | Increase TPC, TFC, and anthocyanin | [87] |
| CNTs and GO | CC | Fagonia indica | Increase TPC, TFC, caffeic acid, rutin, and benzoic acid | [88] |
| CNTs and reduced GO | Fagonia indica | Increase TPC, TFC, quercetin, and gallic acid | [89] | |
| Polymeric NMs | ||||
| Chitosan | CC | Silybium marianum | Increase silymarin isomers (taxifolin and silydianin) and some phenolics (P-OH-benzoic acid and protocatechuic acid | [90] |
| Cellulose nanofiber, chitosan nanofiber, chitin nanofiber | CC | Betula pendula | Increase betulin and betulinic acid | [91] |
| Chitosan | CSC | Artemisia annua | Increase artemisinin | [53] |
| Methyl jasmonate-loaded chitosan NPs (MJ-CNPs) | CC | Oriza sativa | Enhanced production of phenolics and flavonoids; prolonged PAL activity | [92] |
| Sulfur | CC | Lotus arabicus | Increase ellagic acid, vanillin, gallic acid (1.37-fold), methyl gallate (22.9-fold), and syringic acid (2.4-fold) under 100 mg L−1 | [93] |
| Composite/hybrid NMs | ||||
| GO-SiO2 | CC | Ammi visnaga | Increase TPC, TFC | [84] |
| GO-SiO2 | CC | Levisticum officinale Koch. | Increase TPC, TFC | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Golkar, P.; Vázquez-Núñez, E.; Peralta-Videa, J.R. Nano-Elicitation Approaches for Boosting Secondary Metabolites in Medicinal Plant Cell Cultures. Plants 2026, 15, 46. https://doi.org/10.3390/plants15010046
Golkar P, Vázquez-Núñez E, Peralta-Videa JR. Nano-Elicitation Approaches for Boosting Secondary Metabolites in Medicinal Plant Cell Cultures. Plants. 2026; 15(1):46. https://doi.org/10.3390/plants15010046
Chicago/Turabian StyleGolkar, Pooran, Edgar Vázquez-Núñez, and José R. Peralta-Videa. 2026. "Nano-Elicitation Approaches for Boosting Secondary Metabolites in Medicinal Plant Cell Cultures" Plants 15, no. 1: 46. https://doi.org/10.3390/plants15010046
APA StyleGolkar, P., Vázquez-Núñez, E., & Peralta-Videa, J. R. (2026). Nano-Elicitation Approaches for Boosting Secondary Metabolites in Medicinal Plant Cell Cultures. Plants, 15(1), 46. https://doi.org/10.3390/plants15010046









