Abstract
Food security and human health are directly related to the condition of agricultural soils. Soil contamination by heavy metals is a global environmental problem. Lead (Pb) is a toxic and non-biodegradable element posing a significant risk to ecosystems and human health. 24-Epibrassinolide (EBR) has multiple benefits in plant metabolism, including maximizing gas exchange. In plants, exogenous application of dopamine (DOP) confers tolerance to abiotic stresses, minimizing interferences on growth. This study aimed to investigate whether the exogenous application of EBR and DOP, administered independently or jointly, can contribute to mitigating the oxidative stress and impacts on photosystem II in Pb-stressed tomato, evaluating parameters related to nutritional status, photosystem II activity, gas exchange, antioxidant enzymes, and biomass. Better results were observed with the isolated EBR application, improving the photosynthetic efficiency, as evidenced by the increases in chlorophyll contents, effective quantum yield of PSII photochemistry, photochemical quenching coefficient, and electron transport rate, resulting in a higher net photosynthesis rate. Parallelly, treatment using both plant growth regulators (DOP and EBR) promoted significant increases of 14%, 18%, 13%, and 35% in the activities of superoxide dismutase, catalase, ascorbate peroxidase, and peroxidase, contributing to the reduction in oxidative stress in photosystem II of Pb-stressed plants. Therefore, this research proves that the exogenous application of DOP and EBR, alone or in combination, attenuates the toxic effects generated by Pb in tomato plants.
1. Introduction
Tomato (Solanum lycopersicum L.) belongs to the Solanaceae family and is a widely cultivated and consumed vegetable crop worldwide [1,2]. It is considered a model plant due to its complete genome sequence, which is widely used in basic and applied research [3,4]. At a global level, it contributes significantly to agricultural trade, representing more than 180 million metric tons produced in more than 161 countries [5]. In terms of production volume among vegetables, it ranks third, surpassed only by potatoes and sweet potatoes [6]. It stands out as the main horticultural product in terms of volume destined for industrial processing [7,8]. It is rich in mineral nutrients such as calcium, phosphorus, magnesium, as well as water-soluble vitamins (B complex and vitamin C), fat-soluble vitamins (A, E, and K), and bioactive compounds that directly contribute to promoting human health [9,10].
Food security and human health are directly related to agriculture conditions [11,12]. Soil contamination by heavy metals is a global environmental issue, posing significant risks, particularly for developing countries [13]. Several studies indicate that anthropogenic activities, such as industrial processes, mining, smelting, oil refineries, paint and pigment manufacturing, metal coatings, steelmaking, thermoelectric plants, and the transportation sector, are potential sources of heavy metal contamination in agricultural ecosystems [14,15,16].
Lead (Pb) is a toxic element that poses a significant risk to human health [17]. Its exposure is associated with damage to the nervous system, especially in children, and is also related to the development of cardiovascular and kidney diseases [18]. In plants, Pb negatively interferes with several physiological processes, such as germination [19], root system development, and nutrient absorption [20]. Additionally, it induces oxidative stress and compromises antioxidant defense mechanisms [21]. These effects culminate in reduced photosynthetic activity [22], impaired general plant metabolism, and, consequently, reduced biomass accumulation and a drop in crop production rates [23].
Plant growth regulators, including brassinosteroids (BRs), play multiple functions in plant metabolism, such as regulating cell division, photosynthesis, reproductive development, enzyme activation, and the synthesis and expression of proteins and genes [24]. These molecules also play a fundamental role in plant tolerance to various types of stress, including biotic and abiotic stress [25], contributing to the mitigation of phytotoxicity caused by heavy metals [26]. Among the BRs, 24-epibrassinolide (EBR) stands out as the most bioactive form naturally synthesized by plants [27,28].
Dopamine (DOP) is a natural catecholamine that acts as a neurotransmitter and neuromodulator in humans and animals, with significant activity also observed in higher plants [29]. In plants, the exogenous application of dopamine has been shown to confer greater tolerance to abiotic stress, promoting increased concentrations of photosynthetic pigments, stomatal conductance, CO2 assimilation, and maximum photochemical efficiency [30]. These effects lead to improved plant growth and increased biomass accumulation under adverse conditions [31].
The current literature presents gaps regarding the investigation of the combined action of EBR and DOP in tomato plants under Pb phytotoxicity conditions, evidencing a new technological and pioneering perspective that involves the use of hormonal regulators and plant neurotransmitters. We start from the hypothesis that EBR is capable of positively modulating plant growth and productivity through the regulation of morphological and physiological responses [32,33,34], while DOP contributes to the mitigation of oxidative stress [35,36]. This study aimed to investigate whether the exogenous application of EBR and DOP, administered independently or jointly, can contribute to mitigating the toxic effects of Pb in tomato plants, evaluating parameters related to gas exchange, antioxidant enzyme activity, photosynthetic efficiency and plant development.
2. Results
2.1. Pb Concentrations Were Minimized After Treatment with DOP and EBR
Treatment with 200 µM Pb caused a significant increase in the contents of this metal in the analyzed tissues (Table 1). However, plants exposed to Pb2+ stress and pretreated with DOP had significant reductions of 46% (leaf), 36% (stem) and 30% (root). The use of EBR in stressed plants promoted decreases in leaf (71%), stem (64%) and root (45%), compared to the same treatment in the absence of EBR. The simultaneous use of DOP + EBR promoted substantial attenuations (p < 0.05) of 58%, 45% and 42% in leaf, stem and root values, respectively, when compared to the treatment with plants exposed to Pb without pretreatment with DOP or EBR.

Table 1.
Pb contents in tomato plants treated with EBR and subjected to Pb toxicity.
2.2. DOP and EBR Stimulated the Nutritional Status in Pb-Stressed Plants
Exogenous DOP application increased nutrient levels in these tissues (Table 2), plants treated with DOP exposed to Pb (root) showed increases of 13%, 36%, 17%, 19%, 28% and 8% for Mg, K, Ca, Cu, Zn, and Mn, respectively, comparing to the same treatment without DOP. In the leaves, percentage increases of 15%, 14%, 8%, 27%, 36%, and 22% for Mg, K, Ca, Cu, Zn, and Mn were observed sequentially. Spraying with EBR promoted an increase in nutrient concentrations in these tissues, when compared to plants subjected to the same treatment without EBR. In roots, plants treated with EBR and exposed to Pb presented increases of 34%, 196%, 46%, 24%, 89%, and 29% in the levels of Mg, K, Ca, Cu, Zn, and Mn, respectively (Table 2). In the leaves, the corresponding increases were 41%, 51%, 22%, 48%, 80% and 26% for the same elements. The joint application of DOP and EBR molecules provided increases in nutritional status, being detected increases of 30% (Mg), 104% (K), 39% (Ca), 23% (Cu), 64% (Zn) and 24% (Mn) were observed in the roots (Table 2), when compared to plants subjected to the same treatment without DOP and EBR exposed to Pb. In the leaves, the percentage increases were 18% (Mg), 45% (K), 13% (Ca), 45% (Cu), 61% (Zn), and 25% (Mn) (Table 2).

Table 2.
Nutrient contents in tomato plants treated with EBR and subjected to Pb toxicity.
2.3. Plant Growth Regulators Provided Maintenance of Photosynthetic Pigments
Tomato plants exposed to Pb toxicity showed decreases in values of Chl a, Chl b, Total Chl and Car (Table 3). The application of DOP in plants with Pb excess promoted increases (p < 0.05) of 34%, 38%, 35%, and 55% in Chl a, Chl b, Total Chl and Car, when compared to the Pb2+ treatment without DOP. However, the combined Pb2+ and EBR treatment promoted increases in Chl a, Chl b, Total Chl and Car of 42%, 51%, 45% and 76%, respectively, compared to the Pb2+ treatment without EBR. The combination of DOP and EBR induced positive effects on the variables Chl a, Chl b, Chl Total and Car of 36%, 42%, 38% and 67% respectively, when compared to treatments with Pb2+ without DOP or EBR.

Table 3.
Photosynthetic pigments in tomato plants treated with EBR and subjected to Pb toxicity.
2.4. The Performance of Photosystem II Was Boosted Up After DOP and EBR Spray
Tomato plants subjected to Pb toxicity had an increase in F0 value, but reductions in Fv, Fm, and Fv/Fm values (Figure 1 and Table 4). However, the exogenous application of growth regulators (EBR and DOP) alleviated the damage on the photosynthetic machinery. In chlorophyll fluorescence (Figure 1 and Table 4), foliar spraying with EBR alone in plants under Pb excess induced a reduction in F0 (39%) and significant increases in Fm (22%), Fv (73%) and Fv/Fm (41%). While the single application of DOP in plants with Pb toxicity reduced F0 (35%) and increased the values of Fm (12%), Fv (50%) and Fv/Fm (35%). On the other hand, the joint application of EBR + DOP decreased F0 (39%) and maximized (p < 0.05) Fm (17%), Fv (63%) and Fv/Fm (39%), when compared with the treatment of tomato plants under Pb stress, but without the use of growth regulators. Pb caused decreases in the values of ΦPSII, qP, ETR, and ETR/PN, but increased the value of EXC (Table 4). However, the use of EBR promoted increases in ΦPSII, qP, ETR, and ETR/PN of 100%, 42%, 91%, and 31%, respectively, and reduced NPQ and EXC by 28% and 12%, in this order. Similarly, DOP increased the values of ΦPSII, qP, ETR and ETR/PN by 46%. 29%, 43%, and 20%, respectively, and induced reductions in NPQ (7%) and EXC (3%). Used together, EBR and DOP stimulated ΦPSII (77%), qP (31%), ETR (69%), and ETR/PN (24%), while decreasing NPQ (23%) and EXC (7%), when compared with the treatment without the use of EBR and DOP and subjected to heavy metal stress.
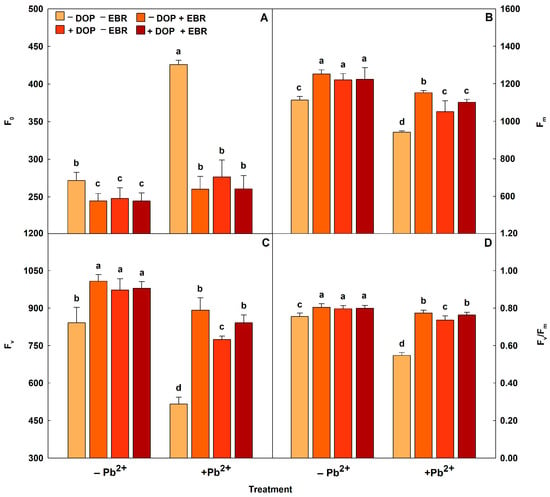
Figure 1.
Minimal fluorescence yield of the dark-adapted state (F0), maximal fluorescence yield of the dark-adapted state (Fm), variable fluorescence (Fv) and maximal quantum yield of PSII photochemistry (Fv/Fm) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott-Knott test (p < 0.05). Means ± SD, n = 5. (A) F0, (B) Fm, (C) Fv, (D) Fv/Fm.

Table 4.
Chlorophyll fluorescence in tomato plants treated with DOP and EBR and subjected to Pb toxicity.
2.5. Exogenous Spraying of DOP and EBR Maximized Gas Exchange
To gas exchange (Table 5), Pb caused deleterious effects. However, the combined treatment of DOP and EBR stimulated increases of 37%, 35%, 66% and 67%, respectively, in PN, gs, WUE and PN/Ci and a decrease in Ci (19%), when compared to plants exposed to Pb toxicity without DOP or EBR. The combination of Pb2+ with DOP positively altered the variables PN, gs, WUE, and PN/Ci by 19%, 24%, 35%, and 37%, respectively, compared with plants exposed to Pb2+ without DOP. EBR applied in plants with Pb2+ increased (p < 0.05) 46%, 47%, 80%, and 85% in PN, gs, WUE, and PN/Ci, when compared to the combination without EBR with Pb2+.

Table 5.
Gas exchange in tomato plants treated with EBR and subjected to Pb toxicity.
2.6. Neurotransmitter and Brassinosteroids Enhance Antioxidant Defense
Plants exposed to Pb2+ intoxication showed increases in the activities of antioxidant enzymes (SOD, CAT, APX and POX) (Figure 2). DOP application promoted significant increases in the enzymatic activities by 7% (SOD), 14% (CAT), 13% (APX) and 31% (POX). Regarding the use of EBR, increases (p < 0.05) of 23%, 25%, 31% and 52% were observed for the enzymes SOD, CAT, APX and POX, respectively. However, the treatment with DOP and EBR significantly maximized the enzymatic activities by 14% (SOD), 18% (CAT), 13% (APX) and 35% (POX), compared to the treatment with Pb and without DOP and EBR. Reactive oxygen species (ROS) (H2O2 and O2−) and membrane damage indicators (MDA and EL) increased in plants subjected to excess Pb2+ (Figure 3). However, plants exposed to Pb2+ toxicity and DOP application showed notable attenuations of 23%, 44%, 27% and 24% in H2O2 and O2−, MDA and EL, respectively. Plants treated with EBR showed reductions (p < 0.05) of 25%, 46%, 29% and 32% in H2O2, O2−, MDA and EL, respectively. While the combined use of DOP and EBR resulted in significant reductions of 25%, 45%, 28% and 27% in H2O2, O2−, MDA and EL, respectively, compared to the treatment exposed to excess Pb2+ and without DOP or EBR.
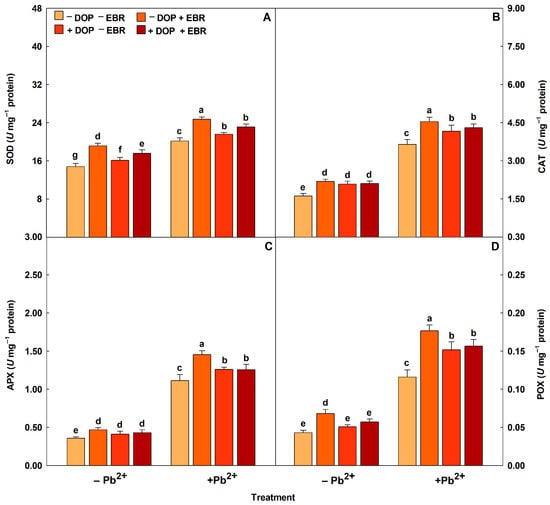
Figure 2.
Activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POX) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott-Knott test (p < 0.05). Means ± SD, n = 5. (A) SOD, (B) CAT, (C) APX, (D) POX.
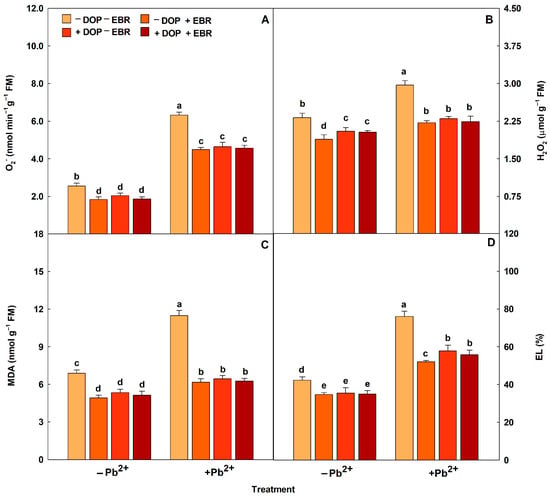
Figure 3.
Superoxide (O2−), hydrogen peroxide (H2O2), malondialdehyde (MDA) and electrolyte leakage (EL) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott-Knott test (p < 0.05). Means ± SD, n = 5. (A) O2−, (B) H2O2, (C) MDA, (D) EL.
2.7. DOP and EBR Promoted Higher Biomass Accumulation in Plants Exposed to Pb Excess
Stress caused by Pb caused a reduction in biomass (Table 6). Plants exposed to Pb2+ stress and pretreated with DOP had significant increases of 50% (LDM), 37% (RDM), 19% (SDM), and 39% (TDM). The use of EBR in stressed plants promoted increases in LDM (50%), RDM (57%), SDM (42%), and TDM (50%), comparing with same treatment in the absence of EBR. The simultaneous use of DOP + EBR promoted substantial increases (p < 0.05) of 48%, 48%, 36% and 45% in the values of LDM, RDM, SDM and TDM, respectively, when compared to the treatment with plants exposed to Pb without pretreatment with DOP or EBR.

Table 6.
Biomass of tomato plants treated with EBR and subjected to Pb toxicity.
3. Discussion
Overall, the results of this research demonstrate that exposure to Pb caused significant disturbances in plant metabolism, affecting biochemical, nutritional, physiological, and morphological parameters. However, the exogenous application of DOP and EBR, as well as the joint administration of both molecules, attenuated the toxic effects of Pb in tomato plants, synergistically promoting improvements in leaf structure, photosynthetic activity, and plant growth.
DOP, EBR, and combined spray promoted reductions in Pb accumulation levels in the tissues (roots and leaves) of tomato plants. EBR plays a crucial role mitigating Pb toxicity, possibly by regulating ionic homeostasis and maintaining redox balance in plants [37]. DOP acts by increasing the activity of antioxidant enzymes, such as SOD, CAT, APX, and POX, minimizing the accumulation of reactive oxygen species (ROS), such as O2, H2O2, EL, and MDA, favoring PN [38,39], corroborated by the results obtained in this study. These effects are in agreement with previous studies that have demonstrated the ability of these phytohormones to modulate antioxidant responses and cellular defense mechanisms against heavy metal stress [40,41,42,43].
Pb transport in tomato plants occurs predominantly through root uptake [44]. In roots, Pb is mobilized into cells through transport proteins, and it is a non-essential ion; there are no specific transporters for Pb [45]. However, many transporters for divalent transition metals (Mg, K, Ca, Cu, Zn, Mn) can facilitate Pb entry through ionic similarity [46]. This interference can be explained due to Pb acting as a competitive analogue, thereby blocking the signal transduction of other nutrients and negatively affecting plant growth and development [47]. EBR treatment significantly reduced Cd accumulation in shoot of rice plants, decreasing the translocation of the metal from root to leaf [48]. Exogenous DOP application (DOP) suppressed the reduction in plant height, root length, chlorophyll levels, antioxidant enzyme activities, and photosynthetic performance in apple seedlings subjected to alkaline stress [38].
Pretreatment with DOP, EBR, and the joint application of both molecules in plants under Pb phytotoxicity conditions promoted positive effects on the levels of macronutrients (Mg, K, Ca) and micronutrients (Cu, Zn, Mn). EBR can mobilize essential minerals, such as manganese (Mn) and zinc (Zn), into the cells. These minerals, acting together, prevent the excessive accumulation of heavy metals, such as lead (Pb), by competing antagonistically for the various transporters and binding sites [49]. In parallel, DOP influences plant growth and development through its interaction with phytohormones, playing a relevant role in the intracellular regulation of ionic permeability and photophosphorylation in chloroplasts. This effect is associated with its high reducing power of this neurotransmitter, which contributes to the elimination of free radicals [50].
The nutrients, including magnesium (Mg), potassium (K) and calcium (Ca) are considered essential secondary macronutrients for the proper functioning of plant metabolism, contributing to increased plant resistance to different types of stress [51]. Calcium (Ca) plays a structural role in the cell wall and membranes, in addition to acting as a secondary messenger in signaling pathways associated with the perception of environmental stimuli, being fundamental in the adaptive responses of plants [52]. Nutritional imbalance, resulting from Mg and K deficiency, has negative repercussions on plant metabolism. K deficiency causes interveinal chlorosis, where the leaves become wrinkled and exhibit upward curling. In severe cases, the shoot tips die. Mg deficiency causes symptoms of leaf yellowing [53].
The micronutrients, such as copper (Cu), zinc (Zn) and manganese (Mn) are accumulated and translocated to different plant organs, such as leaves, roots and stem [54]. Cu acts to establish ideal conditions for plant development, being involved in several physiological processes, including photosynthesis, respiration, hormonal signaling and cellular metabolism [55]. Mn is essential for the functioning of the oxygen evolution complex in photosystem II, participating in water photolysis, a process responsible for releasing of oxygen during photosynthesis [56]. In addition, Mn contributes to the activity of the enzyme superoxide dismutase (SOD), which protects plant cells against oxidative stress by converting reactive oxygen species (ROS) into less toxic [57]. Corroborating our results, application of EBR significantly reduced the concentration of cadmium (Cd) in the roots and shoots of rice plants, attenuating the deleterious effects of Cd stress [58]. In addition, EBR treatment positively influenced the accumulation of iron (Fe) and manganese (Mn), indicating a possible role of these brassinosteroids in the modulation of mineral nutrition under conditions of heavy metal toxicity.
Lead (Pb2+) toxicity significantly decreased pigments and carotenoid levels in tomato plants. Pb toxicity impairs photosynthetic attributes by disrupting chloroplasts, causing ultrastructural changes in cells, and negatively affecting thylakoids and pigment synthesis [59]. Conversely, DOP treatment promoted an increase in Chl a, Chl b, total Chl, and Car levels, which is explained by the neurotransmitter to protect chloroplast structure and reduce damage. The damage caused by the heavy metal is related to the replacement of Mg2+, which is a component of the chlorophyll molecule, by Pb2+. This substitution was observed in studies with Cd [60,61], these authors showed a decrease in pigments associated with the replacement of Mg2+ by the heavy metal in the tetrapyrrole centers of chlorophyll, which causes the breakdown of the molecule and irreversible modifications of LHCII. The application of EBR improved the properties of leaf pigments by reducing chlorophyll degradation, corroborating the reduction in MDA and EL levels observed in this research. Carotenoids are responsible for protecting chlorophyll from oxidative damage by scavenging ROS, minimizing negative consequences on structure of the photosystems [62]. Research evaluating tomato plants, concluded that the application of EBR caused increases in carotenoid values, alleviating Cr toxicity [63]. A study demonstrated that apple plants under nutritional stress showed smaller declines in Chl a, Chl b, and total Chl levels in plants under DOP application [64].
Exogenous application of DOP and EBR increased ΦPSII, qP, ETR, and ETR/PN values, demonstrating that these growth regulators, used alone and in combination, reduced the damage caused by Pb toxicity by boosting electron transport and increasing the efficiency of photosynthetic activity. Plants under Pb stress exhibit decreased photosynthetic activity as a consequence of reduced CO2 assimilation, electron transport, inhibition of reaction centers, and photochemical limitation [65]. In contrast, EBR and DOP reduced NPQ and EXC values, confirming that these molecules acted to reduce the accumulation of excess energy and non-photochemical dissipation, favoring the energy availability for the photosynthetic machinery [66]. Improvements in photosynthesis and fluorescence parameters when analyzing cucumber plants pretreated with 150 μM DOP and stressed with 500 μmol L−1 nitrate, with increases in Fv/Fm, qP, and ETR [67]. Similarly, the study investigating the effects of EBR on Cr-intoxicated tomato plants, demonstrated positive results in chlorophyll content, specifically with increases of 53%, 29%, and 44% in Fv/Fm, ΦPSII, and qP, respectively [63].
Plants treated with DOP and EBR and subjected to 200 µM Pb2+ toxicity showed increases in CO2 fixation. Pb toxicity causes a reduction in gas exchange and induces stomatal closure, which limits CO2 entry into cells and water supply, as confirmed by the decrease in gs and E, negatively affecting PN [68,69]. Ref. [70] evaluating Pb-intoxicated cucumber plants, observed increases in Ci and decreases in PN and gs, corroborating this study. DOP clearly attenuated the negative effects of Pb, promoting improved water content and absorption, which can be explained by the increase in WUE [50]. Increases in PN and PN//Ci were identified in plants treated with EBR, proving that this molecule facilitates the absorption and flow of CO2 in the intercellular spaces, maximizing the activity of the RuBisCO enzyme, intrinsically linked to CO2 assimilation [71,72]. Effects associated with utilization of 100 μM DOP in apple plants under Cd stress promoted increases in E, gs, and PN, while Ci had a reduction, delaying the impact caused by this heavy metal on growth and development [35]. E. urophylla plants treated with EBR attenuated the toxic effects of Cd2+ on gas exchange, contributing to increases of 21% in WUE and 23% in PN//Ci [73].
Plants exposed to Pb2+ toxicity and treated with DOP and EBR showed increases in the activities of antioxidant enzymes (SOD, CAT, APX, and POX). These results can be justified by the action of DOP and EBR that influence the defense mechanisms in plants, boosting the enzymatic activities linked to redox metabolism, attenuating the imbalance caused by the high concentration of ROS in the tissues and damage to cell membranes [74]. The enzymes that perform antioxidant functions in metabolism are SOD (a key enzyme), which accelerates the reduction reaction of O2− to H2O2 and O2. CAT, APX, and POX reduce H2O2 to H2O or other molecules that are beneficial to plant cells and, consequently to metabolism [75]. The results obtained corroborate previous research, who investigated the contributions of DOP in cucumber plants under nitrate stress, stimulating the activities of antioxidant enzymes [76]. In general, ROS impair cellular components and disrupt the cytoplasmic balance responsible for signaling the antioxidant defense system and eliminating O2− and H2O2 [77]. However, our studies reveal that DOP and EBR mitigated the negative impacts caused by Pb2+ intoxication, possibly by regulating carbon utilization and hormonal signaling. Mechanisms of action of EBR aiming to alleviate the harmful effects caused by Cr, being detected increases in activities of SOD (27%), CAT (19%) and APX (107%), reducing oxidative stress, confirming the results of this research [63].
Reductions in ROS concentrations and membrane damage were found in plants treated with DOP and EBR, indicating that these molecules attenuated the toxic effects of ROS. This study demonstrated that the leaf tissues of plants subjected to Pb2+ poisoning suffered less oxidative damage (MDA and EL), these results being explained by the increases in antioxidant enzyme activities (POX, SOD, CAT and APX), previously described. Heavy metal toxicity can cause deleterious effects on plant cells, associated with the excess accumulation of O2− and H2O2, causing damage to cellular structures, such as membrane rupture, protein denaturation and reduced photochemical activity [78]. A study with Festuca arundinacea plants treated with EBR and under Pb stress, describing a 10% reduction in O2− concentrations [79]. Corroborating the results found in this research, tomato plants grown under chromium intoxication had an increase of 118% in EL. However, an exogenous application of DOP (0.1 mM) significantly reduced EL, preventing damage to cell membranes [29].
DOP and EBR positively regulate the biomass of Pb-stressed tomato plants through increases in LDM, SDM, RDM, and TDM, mitigating the toxic effects of this heavy metal. Exogenous DOP application mitigates Pb stress by increasing pigment concentrations, photochemical activity, and carbon fixation. These effects contribute to stimulating growth and favoring biomass accumulation [30]. Ref. [64] detected that DOP is capable of increasing stem height, promoting higher biomass accumulation in apple plants under nutritional stress conditions. EBR reduced the Pb uptake by altering cell membrane permeability and stimulating the production of enzymes linked to plant defense system [80]. Ref. [81] also observed that Zn-stressed soybean plants treated with EBR showed increased biomass. Ref. [73] observed that EBR application improved the biomass of Eucalyptus urophylla plants exposed to Cd toxicity. Pb excess within cells causes chloroplast disorganization and leaf damage, including ultrastructural lesions and even cell death. Furthermore, excess Pb impairs chlorophyll production and can interfere with nutrient transport and photosynthesis [82]. Studies such as that by [83] show that increased Pb application decreases biomass of bean plants.
4. Materials and Methods
4.1. Location and Growth Conditions
The experiment was performed at the Campus of Paragominas of the Universidade Federal Rural da Amazônia, Paragominas, Brazil (2°55′ S, 47°34′ W). The study was conducted in a greenhouse with controlled temperature and humidity. The minimum, maximum, and median temperatures were 23.6, 31.7 and 26.8 °C, respectively. The relative humidity during the experimental period varied between 60% and 80%, with photosynthetic photon flux density (PPFD) oscillating between 1150 and 550 µmol m−2 s−1 (measured at 12 am), and 12-h photoperiod.
4.2. Plants, Containers, and Acclimation
Seeds of Solanum lycopersicum L. cv Santa Clara were germinated using vegetable substrate and transplanted on the 15th day into 1.2-L pots filled with a mixed substrate of sand and vermiculite at a ratio of 3:1. Plants were cultivated under semi-hydroponic conditions containing 500 mL of nutritive solution. A nutritive solution was used as a source of nutrients [84]; the ionic strength started at 50% and was modified to 100% after two days.
4.3. Experimental Design
Experimental design had eight randomized treatments: Control − DOP − EBR (1), control − DOP + EBR (2), control + DOP − EBR (3), control + DOP + EBR (4), Pb+2 − DOP − EBR (5), Pb+2 − DOP + EBR (6), Pb+2 + DOP − EBR (7) and Pb+2 + DOP + EBR (8). Five replicates for each one of the eight treatments were conducted and used in the experiment, a total of 40 experimental units, with one plant in each unit.
4.4. Dopamine (DOP) and 24-Epibrassinolide (EBR) Preparations and Applications
Dopamine (DOP) solution with 100 µM (Sigma-Aldrich™, Saint Louis, MO, USA) was prepared [85]. A 100 nM 24-epibrassinolide (EBR) (Sigma-Aldrich™, Saint Louis, MO, USA) solution was used [86]. Fifteen-day-old plants were sprayed with single and combined applications of DOP and EBR (pre-treatment) at 7-d intervals until day 50.
4.5. Plant Nutrition and Pb Treatment
The plants received the following macro and micronutrients contained in the nutrient solution [87]: 8.75 mM KNO3, 7.5 mM Ca(NO3)2·4H2O, 3.25 mM NH4H2PO4, 1.5 mM MgSO4·7H2O, 62.50 µM KCl, 31.25 µM H3BO3, 2.50 µM MnSO4·H2O, 2.50 µM ZnSO4·7H2O, 0.63 µM CuSO4·5H2O, 0.63 µM NaMoO4·5H2O, and 250.0 µM NaEDTAFe·3H2O. To induce Pb stress, PbCl2 was used at concentrations of 0 and 200 µM Pb and was applied over 10 days (days 40–50 after the start of the experiment). During the study, the nutrient solutions were changed at 07:00 h at 3-day intervals, with the pH adjusted to 5.5 using HCl or NaOH. On day 50 of the experiment, physiological and morphological parameters were measured for all plants, and tissues were harvested for anatomical, biochemical and nutritional analyses.
4.6. Pb Determination
Milled samples (100 mg) of leaf tissue were pre-digested in conical tubes (50 mL) with 2 mL of sub-boiled HNO3. Subsequently, 8 mL of a solution containing 4 mL of H2O2 (30% v/v) and 4 mL of ultra-pure water were added and transferred to a Teflon digestion vessel [88]. Determination of Pb was performed using an inductively coupled plasma mass spectrometer (model ICP-MS 7900; Agilent, Santa Clara, CA, USA).
4.7. Measurement of Chlorophyll Fluorescence
The minimal fluorescence yield of the dark-adapted state (F0), maximal fluorescence yield of the dark-adapted state (Fm), variable fluorescence (Fv), maximal quantum yield of PSII photochemistry (Fv/Fm), effective quantum yield of PSII photochemistry (ΦPSII), photochemical quenching coefficient (qP), nonphotochemical quenching (NPQ), electron transport rate (ETR), relative energy excess at the PSII level (EXC), and ratio between the electron transport rate and net photosynthetic rate (ETR/PN) were determined using a modulated chlorophyll fluorometer (model OS5p; Opti-Sciences, Hudson, NH, USA). The chlorophyll fluorescence was measured in fully expanded leaves under light between 10:00 and 12:00 h. Preliminary tests determined that the acropetal third of leaves in the middle third of the plant and that adapted to the dark for 30 min yielded the greatest Fv/Fm ratio. Therefore, this part of the plant was used for measurements. The intensity and duration of the saturation light pulse were 7500 µmol m–2 s–1 and 0.7 s, respectively.
4.8. Evaluation of Gas Exchange
The net photosynthetic rate (PN), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were evaluated using an infrared gas analyser (model LCPro+; ADC BioScientific, Hoddesdon, UK). These parameters were measured at the adaxial surface of fully expanded leaves that were collected from the middle region of the plant. The water-use efficiency (WUE) was determined [89] and the instantaneous carboxylation efficiency (PN/Ci) was calculated [90]. Gas exchange was evaluated in all plants under a constant CO2 concentration (360 μmol mol−1 CO2), photosynthetically active radiation (800 μmol photons m−2 s−1), air-flow rate (300 µmol s−1), and temperature (28 °C) in the test chamber between 10:00 and 12:00 h.
4.9. Extraction of Antioxidant Enzymes, Superoxide and Soluble Proteins
Antioxidant enzymes (SOD, CAT, APX, and POX), superoxide, and soluble proteins were extracted from leaf tissues [91]. The extraction mixture was prepared by homogenizing 500 mg of fresh plant material in 5 mL of extraction buffer, which consisted of 50 mM phosphate buffer (pH 7.6), 1.0 mM ascorbate, and 1.0 mM EDTA. Samples were centrifuged at 14,000× g for 4 min at 3 °C, and the supernatant was collected. Quantification of the total soluble proteins was performed [92]. Absorbance was measured at 595 nm, using bovine albumin as a standard.
4.10. Superoxide Dismutase Assay
For the SOD assay (EC 1.15.1.1), 2.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.6), 0.1 mM EDTA, 13 mM methionine (pH 7.6), 75 µM NBT, and 4 µM riboflavin was mixed with 0.2 mL of supernatant. The absorbance was then measured at 560 nm [93]. One SOD unit was defined as the amount of enzyme required to inhibit 50% of the NBT photoreduction. The SOD activity was expressed in unit mg–1 protein.
4.11. Catalase Assay
For the CAT assay (EC 1.11.1.6), 0.2 mL of supernatant and 1.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0) and 12.5 mM hydrogen peroxide were mixed, and the absorbance was measured at 240 nm [94].The CAT activity was expressed in μmol H2O2 mg–1 protein min–1.
4.12. Ascorbate Peroxidase Assay
For the APX assay (EC 1.11.1.11), 1.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA, and 1.0 mM hydrogen peroxide was mixed with 0.2 mL of supernatant and the absorbance was measured at 290 nm [95]. The APX activity was expressed in μmol AsA mg–1 protein min–1.
4.13. Peroxidase Assay
For the POX assay (EC 1.11.1.7), 1.78 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0) and 0.05% guaiacol was mixed with 0.2 mL of supernatant, followed by the addition of 20 µL of 10 mM hydrogen peroxide. The absorbance was then measured at 470 nm [96]. The POX activity was expressed in μmol tetraguaiacol mg–1 protein min–1.
4.14. Determination of Superoxide Concentration
For the determination of O2−, 1 mL of extract was incubated with 30 mM phosphate buffer [pH 7.6] and 0.51 mM hydroxylamine hydrochloride for 20 min at 25 °C. Seventeen mM sulphanilamide and 7 mM α-naphthylamine were then added to the incubation mixture for 20 min at 25 °C. After the reaction, ethyl ether was added in the identical volume and centrifuged at 3000× g for 5 min. The absorbance was measured at 530 nm [97].
4.15. Extraction of Nonenzymatic Compounds
Nonenzymatic compounds (H2O2 and MDA) were extracted [98]. Briefly, a mixture designed to extract H2O2 and MDA was prepared by homogenizing 500 mg of fresh leaf material in 5 mL of 5% (w/v) trichloroacetic acid. Samples were then centrifuged at 15,000× g for 15 min at 3 °C to collect the supernatant.
4.16. Determination of Hydrogen Peroxide Concentration
To measure H2O2, 200 µL of supernatant and 1800 µL of reaction mixture (2.5 mM potassium phosphate buffer [pH 7.0] and 500 mM potassium iodide) were mixed and the absorbance was measured at 390 nm [99].
4.17. Quantification of Malondialdehyde Concentration
MDA was determined by mixing 500 µL of supernatant with 1 mL of the reaction mixture, which contained 0.5% (w/v) thiobarbituric acid in 20% trichloroacetic acid. The mixture was incubated in boiling water at 95 °C for 20 min with the reaction terminated by placing the reaction container in an ice bath. The samples were centrifuged at 10,000× g for 10 min and the absorbance was measured at 532 nm. The nonspecific absorption at 600 nm was subtracted from the absorbance data. The amount of MDA–TBA complex (red pigment) was calculated [100], with minor modifications and using an extinction coefficient of 155 mM−1 cm−1.
4.18. Determination of Electrolyte Leakage
Electrolyte leakage was measured with minor modifications [101]. Fresh tissue (200 mg) was cut into pieces 1 cm in length and placed in containers with 8 mL of distilled deionized water. The containers were incubated in a water bath at 40 °C for 30 min and the initial electrical conductivity of the medium (EC1) was measured. The samples were then boiled at 95 °C for 20 min to release the electrolytes. After cooling, the final electrical conductivity (EC2). The percentage of electrolyte leakage was calculated using the formula EL (%) = (EC1/EC2) × 100.
4.19. Determination of Photosynthetic Pigments
Determinations of the chlorophyll and carotenoid levels were performed with 40 mg of leaf tissue. The samples were homogenized in the dark with 8 mL of 90% methanol (Sigma-Aldrich™, Saint Louis, MO, USA). The homogenate was centrifuged at 6000× g for 10 min at 5 °C. The supernatant was removed and the chlorophyll a (Chl a) and b (Chl b), carotenoid (Car), and total chlorophyll (total Chl) levels were quantified using a spectrophotometer (model UV-M51; Bel Photonics, Monza, Italy) [102].
4.20. Measurements of Biomass
The biomass of roots and leaves was measured based on constant dry weights (g) after drying in a forced-air ventilation oven at 65 °C.
4.21. Data Analysis
The data were subjected to an analysis of variance, and significant differences between the means were determined using the Scott-Knott test at a probability level of 5%, because this test better distinguishes the differences between averages [103]. Standard deviations were calculated for each treatment. Statistical analysis of the data was done using R™ software version 4.4.1 [104].
5. Conclusions
This study confirmed that the application of EBR (24-epibrassinolide) and DOP (dopamine), alone or in combination, mitigated the damage caused by Pb (lead) exposure in tomato plants. However, the most pronounced effects were observed with the isolated application of EBR. The exogenous application of EBR attenuated photoinhibition and contributed to the maintenance of photosynthetic efficiency, as evidenced by the increases in chlorophyll contents, effective quantum yield of PSII photochemistry, photochemical quenching coefficient, and electron transport rate, resulting in a higher net photosynthesis rate. Additionally, the defense system was enhanced by EBR and DOP treatments, indicating a key role in mitigating oxidative damage induced by Pb excess. These treatments clearly protected the photosynthetic machinery, upregulating the antioxidant system and improving gas exchange. Both treatments alleviated Pb-induced oxidative stress by stimulating enzymes linked to redox metabolism, detected with superoxide dismutase, catalase, ascorbate peroxidase, and peroxidase, which are involved in the detoxification of reactive oxygen species, including superoxide anion and hydrogen peroxide, in Pb-stressed plants. Simultaneously, the combination of DOP and EBR promoted synergistic effects, favoring the antioxidant system, the integrity of the photosynthetic machinery, and gas exchange, with positive repercussions on improved nutritional status and biomass accumulation, as verified in the results obtained. Therefore, this study demonstrated that EBR and DOP alleviated the negative interferences caused by Pb stress in tomato plants. However, it is recommended that future studies include molecular approaches to determine the modulated gene expression of DOP and EBR in plants under Pb toxicity.
Author Contributions
A.K.d.S.L. was the advisor of this research, planned all phases of the research, and critically revised the manuscript. L.R.P., S.G.A.d.S., M.M.S.d.S., and M.A.F.G. conducted the experiment in the greenhouse, performing physiological, biochemical, and morphological determinations. They also wrote and edited the manuscript. Meanwhile, E.M.S.G.L., C.C.A., and B.L.B. conducted nutritional analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil): 080/2023.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
This research had financial supports from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Universidade Federal Rural da Amazônia (UFRA/Brazil) to A.K.d.S.L. In other hand, L.R.P. was supported with scholarship from Programa de Educação Tutorial (PET/Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mustafa, M.; Mwangi, R.W.; Szalai, Z.; Kappel, N.; Csambalik, L. Sustainable Responses to Open Field Tomato (Solanum lycopersicum L.) Stress Impacts. J. Agric. Food Res. 2025, 21, 101825. [Google Scholar] [CrossRef]
- Li, J.; Xian, J.; Zhang, S.; An, Y.; Li, N.; Zhou, D.; Sun, S.; Wang, J. Influence of Pigment Composition on Antioxidant Capacity of Different Tomato (Solanum lycopersicum L.). Lwt 2025, 224, 117871. [Google Scholar] [CrossRef]
- Cui, X.; Gu, J.; Liu, P.; Lu, R.; Ren, Z.; Zhang, Y.; Wang, F.; Qi, M.; Liu, Y.; Li, T. Genome-Wide Identification and Characterization of the Thioredoxin (TRX) Gene Family in Tomato (Solanum lycopersicum) and a Functional Analysis of SlTRX2 under Salt Stress. Plant Physiol. Biochem. 2025, 220, 109478. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Fao FAO—Food Agriculture Organization of the United Nations. Agricultural Production Statistics 2000–2022; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Bello, A.S.; Ahmed, T. Moringa Leaf Extract Alleviates Salt Stress in Tomato (Solanum lycopersicum L.) by Activating Antioxidant Defenses, Reducing Osmolyte Accumulation, Improving Water Status, and Enhancing Yield. Plant Stress 2024, 14, 100640. [Google Scholar] [CrossRef]
- Pizarro-Oteíza, S.; Salazar, F. Effect of UV-LED Irradiation Processing on Pectolytic Activity and Quality in Tomato (Solanum lycopersicum) Juice. Innov. Food Sci. Emerg. Technol. 2022, 80, 103097. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef]
- Kabir, M.S.; Roy, U.; Suvo, S.P.; Sobhan, A.; Kamal, M.M.; Akhter, M.J.; Akter, M.S.; Ahmed, M. Comparative Assessment of Fresh and Processed Tomato (Solanum lycopersicum) Pulps: Impact of Processing on Physicochemical, Antioxidant, and Enzymatic Behavior. Appl. Food Res. 2024, 4, 100550. [Google Scholar] [CrossRef]
- Jin, L.; Jin, N.; Wang, S.; Huang, S.; Yang, X.; Xu, Z.; Jiang, S.; Lyu, J.; Yu, J. Moderate Salt Stress Aids in the Enhancement of Nutritional and Flavor Quality in Tomato (Solanum lycopersicum L.) Fruits. Food Chem. X 2025, 26, 102330. [Google Scholar] [CrossRef] [PubMed]
- El-Sorogy, A.S.; Al-kahtany, K.; Alharbi, T.; Alarifi, S.S. Distribution Patterns, Health Hazards, and Multivariate Assessment of Contamination Sources of As, Pb, Ni, Zn, and Fe in Agricultural Soils. J. King Saud. Univ.—Sci. 2024, 36, 103489. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Li, H.Y.; Lü, L.Y.; Liang, G.J.; Wu, T.T.; Zhu, J.X. Distributions and Risk Assessment of Heavy Metals in Solid Waste in Lead-Zinc Mining Areas and Across the Soil, Water Body, Sediment and Agricultural Product Ecosystem in Their Surrounding Areas. China Geol. 2025, 8, 92–106. [Google Scholar] [CrossRef]
- Sanad, H.; Moussadek, R.; Mouhir, L.; Lhaj, M.O.; Zahidi, K.; Dakak, H.; Manhou, K.; Zouahri, A. Ecological and Human Health Hazards Evaluation of Toxic Metal Contamination in Agricultural Lands Using Multi-Index and Geostatistical Techniques across the Mnasra Area of Morocco’s Gharb Plain Region. J. Hazard. Mater. Adv. 2025, 18, 100724. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y. Effects of Atmospheric Deposition on Heavy Metals Accumulation in Agricultural Soils: Evidence from Field Monitoring and Pb Isotope Analysis. Environ. Pollut. 2023, 330, 121740. [Google Scholar] [CrossRef]
- Lima, F.A.; Bhattacharjee, S.; Jahangir Sarker, M.; Salam, M.A. Ecological Risk Assessment of Potentially Toxic Elements (PTEs) in Agricultural Soil, Vegetables and Fruits with Respect to Distance Gradient in Proximity to Lead-Acid Battery Industry. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100932. [Google Scholar] [CrossRef]
- Anjana, K.R.; Suresh, A.; Soman, V.; Rahman, K.H. Metal Contamination in the Ashtamudi Wetland Ecosystem: Source Identification, Toxicological Risk Assessment of Ni, Cd, Cr, and Pb and Remediation Strategies. Mar. Pollut. Bull. 2025, 212, 117534. [Google Scholar] [CrossRef]
- Yu, A.; Yang, Q.; Xu, B.; Yang, Y.; Ren, Z.; Li, K. Analysis of Lead Contamination Sources in Roadside Soil via the Isotope Tracing Method. J. Environ. Chem. Eng. 2024, 12, 114205. [Google Scholar] [CrossRef]
- Dejoie, E.; Lemay, M.A.; Fenton, N.J.; DesRochers, A.; Marion, J.; Savard, M.M.; Porter, T.J.; Proulx, D.; Gennaretti, F. Spatiotemporal Assessment of Lead (Pb) and Cadmium (Cd) Contamination in Urban Tree Rings near an Industrial Smelter: High Intraspecific Variability but Limited Spatial Differentiation. Atmos. Pollut. Res. 2025, 16, 102582. [Google Scholar] [CrossRef]
- Salmen, S.H.; Alharbi, S.A. Mitigating Pb-Induced Oxidative Stress in Rice Plants by Cerium Oxide and Iron Oxide Nanoparticles. S. Afr. J. Bot. 2024, 172, 544–555. [Google Scholar] [CrossRef]
- Feng, G.; Li, S.; Yang, X.; Hu, Y.; Zhang, X.; Chen, D.; Liu, W.; Yu, G.; Nie, G.; Huang, L.; et al. Integrative Multi-Omic Analyses Reveal the Molecular Mechanisms of Silicon Nanoparticles in Enhancing Hyperaccumulator under Pb Stress. Environ. Pollut. 2025, 368, 125677. [Google Scholar] [CrossRef]
- Noreen, S.; Malik, Z.; Luqman, M.; Fatima, I.; Tahir, U.A.; Dar, M.; Rizwan, M. Effect of Bacillus Strain and Fe-Modified Biochar on Lead (Pb) Bioaccumulation and Oxidative Stress in Wheat (Triticum aestivum L.) Grown in Pb Contaminated Soil. S. Afr. J. Bot. 2024, 172, 720–735. [Google Scholar] [CrossRef]
- Timori, Z.; Amirinejad, A.A.; Ghobadi, M. Biochar Improves Pb and Cd-Induced Stress in Mung Bean (Vigna radiata L. Wilczek). Environ. Chall. 2024, 16, 100992. [Google Scholar] [CrossRef]
- Maryam, H.; Rizwan, M.; Masood, N.; Waseem, M.; Ahmed, T.; Farooq, M.; Zia-ur-rehman, M.; Aziz, H. Mitigating Lead (Pb) Toxicity in Zea mays (L.) Plants Using Green Synthesized Iron Oxide Nanoparticles. S. Afr. J. Bot. 2024, 175, 657–668. [Google Scholar] [CrossRef]
- Torabi, S.; Rahmani, F. 24-Epibrassinolide Promotes Resilience against Arsenic Stress via Modulating Amino Acid Profiles and MRNA Abundance of CYP450 and MRP Genes in Zea mays L. Plant Physiol. Biochem. 2025, 221, 109631. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, G.; Pandey, S.; Singh, V.K.; Prasad, S.M. 24-Epibrassinolide Effectively Alleviates UV-B Stress-Induced Damage in the Cyanobacterium Anabaena Sp. PCC 7120 by Employing Nitric Oxide: Improved PS II Photochemistry, Antioxidant System, and Growth. Plant Physiol. Biochem. 2025, 221, 109667. [Google Scholar] [CrossRef]
- Shabab, Z.; Sarada, D.V.L. 24-Epibrassinolide Mitigates Arsenate Stress in Seedlings of Oryza sativa (IR-20) via the Induction of Phenylpropanoid Pathway. Plant Physiol. Biochem. 2024, 215, 109023. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Pan, Y.; Wang, Y.; Xu, W. Treatment with 24-Epibrassinolide Protects ‘Youhou’ Sweet Persimmon Fruit against Chilling Injury During Cold Storage. Sci. Hortic. 2025, 343, 114087. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Xu, Z.; Li, Z.; Gao, X.; Yang, X.; Xie, Y.; Meng, X.; Jin, N.; Jin, L.; et al. Enhancing Industrial Value of Coriander (Coriandrum sativum L.): Effects of 2,4-Epibrassinolide on Aroma and Bioactive Compounds. Ind. Crops Prod. 2025, 230, 121139. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Sun, S.; Qu, K.; Chen, J.; Dong, Y.; Liu, A.; Chen, S. Hydrogen Peroxide Signaling Mediates Dopamine-Induced Chromium Stress Tolerance in Tomato. Environ. Pollut. 2025, 371, 125949. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X. Dopamine-Induced Abiotic Stress Tolerance in Horticultural Plants. Sci. Hortic. 2023, 307, 111506. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Shang, Y.; Ji, J.; Tan, L.; Zhang, X.; Xu, J.; Liang, B. Melatonin and Dopamine Alleviate Waterlogging Stress in Apples by Recruiting Beneficial Endophytes to Enhance Physiological Resilience. J. Integr. Agric. 2024, 23, 2270–2291. [Google Scholar] [CrossRef]
- Li, B.B.; Fu, Y.S.; Li, X.X.; Yin, H.N.; Xi, Z.M. Brassinosteroids Alleviate Cadmium Phytotoxicity by Minimizing Oxidative Stress in Grape Seedlings: Toward Regulating the Ascorbate-Glutathione Cycle. Sci. Hortic. 2022, 299, 111002. [Google Scholar] [CrossRef]
- Wen, J.; Tang, Y.; Li, J.; He, T.; Xiao, J.; Nangia, V.; Liu, Y. Effects of Exogenous Brassinosteroids on the Starch Structure, Physicochemical Properties and Digestibility of Wheat Under High-Temperature Stress at the Early Grain-Filling Stage. Int. J. Biol. Macromol. 2024, 283, 137690. [Google Scholar] [CrossRef]
- Barooti, S.; Edalat, M.; Oveisi, M.; Kazemeini, S.A.; Naderi, R. Does the Exogenous Application of Brassinosteroids Affect the Photosynthetic, Morphological Characteristics, and THC Concentrations of Cannabis sativa L. under Drought Stress? J. Appl. Res. Med. Aromat. Plants 2025, 46, 100635. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Zhang, J.; Ji, J.; Xu, J.; Liang, B. Dopamine Alleviates Cadmium Stress in Apple Trees by Recruiting Beneficial Microorganisms to Enhance the Physiological Resilience Revealed by High-Throughput Sequencing and Soil Metabolomics. Hortic. Res. 2023, 10, uhad112. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine Confers Cadmium Tolerance in Apples by Improving Growth, Reducing Reactive Oxygen Species, and Changing Secondary Metabolite Levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Niu, K.; Xiao, H.; Wang, Y.; Cui, T.; Zhao, C. A Meta-Analysis on Plant Growth and Heavy Metals Uptake with the Application of 2,4-Epibrassinolide in Contaminated Soils. Ecotoxicol. Environ. Saf. 2025, 289, 117439. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Liu, Z.Y.; Shi, L.M.; Lu, X.Y.; Han, Y.Y.; Sun, Y. Mitigation Effect of Exogenous Dopamine Treatment on Downy Mildew-Infected Cucumber. J. Plant Growth Regul. 2024, 43, 2615–2631. [Google Scholar] [CrossRef]
- Du, J.; Xu, H.; Zhang, D.X.; Feng, S. Chelation and Nanoparticle Delivery of Monomeric Dopamine to Increase Plant Salt Stress Resistance. Nat. Commun. 2025, 16, 4157. [Google Scholar] [CrossRef]
- Lv, M.; Li, J. Molecular Mechanisms of Brassinosteroid-Mediated Responses to Changing Environments in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2737. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Zhu, R.; Wang, Y.; Zhao, C.; Ma, H. 24-Epibrassinolide Improves Cadmium Tolerance and Lateral Root Growth Associated with Regulating Endogenous Auxin and Ethylene in Kentucky Bluegrass. Ecotoxicol. Environ. Saf. 2023, 249, 114460. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Lan, G.; Sun, Y.; Wang, G.; Sun, Y. Dopamine Alleviates Chilling Stress in Watermelon Seedlings via Modulation of Proline Content, Antioxidant Enzyme Activity, and Polyamine Metabolism. J. Plant Growth Regul. 2021, 40, 277–292. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, Z.; Han, Y.; Sun, Y. The Mitigation Effects of Exogenous Dopamine Treatment on Continuous Cropping Obstacles in Watermelon. J. Soil. Sci. Plant Nutr. 2023, 23, 4233–4249. [Google Scholar] [CrossRef]
- Sun, H.F.; Wang, X.N.; Li, Y.N.; Wang, L.L.; Li, Y.Y.; Ma, L.J.; Li, X.M. Long Non-Coding RNAs Modulate Glutathione Metabolism Gene Expression and Tolerance to Pb Stress in Root Tissue of Endophyte-Infected Rice Seedling. Ecotoxicol. Environ. Saf. 2025, 291, 117872. [Google Scholar] [CrossRef]
- Phuong, H.T.; Ba, V.N.; Thien, B.N.; Truong Thi Hong, L. Accumulation and Distribution of Nutrients, Radionuclides and Metals by Roots, Stems and Leaves of Plants. Nucl. Eng. Technol. 2023, 55, 2650–2655. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Govindasamy, C.; Sharma, A. Heavy Metal Accumulation in Root and Shoot Tapioca Plant Biomass Grown in Agriculture Land Situated around the Magnesite Mine Tailings. Environ. Res. 2024, 257, 119287. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Wu, X.; Duan, C.; Liu, C.; Yin, L.; Zhang, M.; Fu, D. Fertilization and Intercropping Reduce Pb Accumulation in Plants by Influencing Rhizosphere Soil Phosphorus Forms in Soil-Plant Systems. Ecotoxicol. Environ. Saf. 2025, 293, 118011. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Guo, R.; Jiang, Q.; Chen, C.Z.; Gao, Y.Q.; Jiang, M.M.; Shen, R.F.; Zhu, X.F.; Huang, J. Brassinosteroid Decreases Cadmium Accumulation via Regulating Gibberellic Acid Accumulation and Cd Fixation Capacity of Root Cell Wall in Rice (Oryza sativa). J. Hazard. Mater. 2024, 469, 133862. [Google Scholar] [CrossRef]
- Haider, F.U.; Zulfiqar, U.; Mehmood, T.; Shahzad, B.; Liqun, C.; Yong, J.W.H.; Binobead, M.A. Effects of Titanium Oxide Nanoparticles and 24-Epibrassinosteroid to Mitigate the Toxicity of Cadmium (Cd) and Improve Physio-Morphological Traits of Soybean (Glycine max L.) Cultivated Under Cd-Contaminated Soil. Environ. Technol. Innov. 2024, 36, 103811. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Yuce, M.; Ors, S.; Araz, O.; Torun, U.; Argin, S. Exogenous Dopamine Mitigates the Effects of Salinity Stress in Tomato Seedlings by Alleviating the Oxidative Stress and Regulating Phytohormones. Acta Physiol. Plant. 2024, 46, 59. [Google Scholar] [CrossRef]
- Niu, G.; Wang, R.; Zhou, H.; Yang, J.; Lu, X.; Han, X.; Huang, J. Nitrogen Addition and Mowing Had Only Weak Interactive Effects on Macronutrients in Plant-Soil Systems of a Typical Steppe in Inner Mongolia. J. Environ. Manag. 2023, 347, 119121. [Google Scholar] [CrossRef]
- Umar, A.W.; Naeem, M.; Hussain, H.; Ahmad, N.; Xu, M. Starvation from Within: How Heavy Metals Compete with Essential Nutrients, Disrupt Metabolism, and Impair Plant Growth. Plant Sci. 2025, 353, 112412. [Google Scholar] [CrossRef]
- Muneer, M.A.; Afridi, M.S.; Saddique, M.A.B.; Chen, X.; Zaib-Un-Nisa; Yan, X.; Farooq, I.; Munir, M.Z.; Yang, W.; Ji, B.; et al. Nutrient Stress Signals: Elucidating Morphological, Physiological, and Molecular Responses of Fruit Trees to Macronutrients Deficiency and Their Management Strategies. Sci. Hortic. 2024, 329, 112985. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Lin, G.; Shi, H.; Li, Z.; Shen, L. Bioaccessibility Assessment of Mn, Cu, Fe, and Cd in Henan Province Wheat Using Physiologically Based Extraction. Environ. Monit. Assess. 2025, 197, 600. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, T.A.; AlBlooshi, F.S.; Alharmoudi, A.; AlAlawi, M.S.; Alshkeili, A.M.; Albedwawi, A.; Masih, J.; Buty Alghfeli, M.M. Unveiling the Protective Role of Silicon Dioxide Nanoparticles against Copper-Induced Oxidative Damage in Soybean Plants through Altered Proline Metabolism and Antioxidants. Plant Nano Biol. 2025, 12, 100149. [Google Scholar] [CrossRef]
- Xian, Z.; Guo, F.; Chen, M.; Wang, Y.; Zhang, Z.; Wu, H.; Dai, J.; Zhang, X.; Chen, Y. Plant-Microbe Involvement: How Manganese Achieves Harmonious Nitrogen-Removal and Carbon-Reduction in Constructed Wetlands. Bioresour. Technol. 2024, 402, 130794. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Dubey, K.; Goswami, S.; Hasija, S.; Pandey, R.; Singh, P.K.; Singh, B.; Sareen, S.; Rai, G.K.; Singh, G.P.; et al. Heterologous Expression and Characterization of Novel Manganese Superoxide Dismutase (Mn-SOD)—A Potential Biochemical Marker for Heat Stress-Tolerance in Wheat (Triticum aestivum). Int. J. Biol. Macromol. 2020, 161, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Y.; Liu, S.; Wang, H.; Gao, F.; Shao, G. 24-Epibrassinolide Mitigates Cadmium Toxicity in Rice Plants by Activating the Plant Detoxification System and Regulating Genes Expression Involved in Cd/Fe Uptake and Translocation. Plant Stress 2024, 12, 100485. [Google Scholar] [CrossRef]
- Ferreyroa, G.V.; Lagorio, M.G.; Trinelli, M.A.; Lavado, R.S.; Molina, F.V. Lead Effects on Brassica Napus Photosynthetic Organs. Ecotoxicol. Environ. Saf. 2017, 140, 123–130. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P.; Nasim, S.A. Physiological and Antioxidant Responses of Salvinia Natans Exposed to Chromium-Rich Wastewater. Ecotoxicol. Environ. Saf. 2009, 72, 1790–1797. [Google Scholar] [CrossRef]
- Gillet, S.; Decottignies, P.; Chardonnet, S.; Le Maréchal, P. Cadmium Response and Redoxin Targets in Chlamydomonas Reinhardtii: A Proteomic Approach. Photosynth. Res. 2006, 89, 201–211. [Google Scholar] [CrossRef]
- Kaur, M.; Sandhu, K.S.; Singh, N. Comparative Study of the Functional, Thermal and Pasting Properties of Flours from Different Field Pea (Pisum sativum L.) and Pigeon Pea (Cajanus cajan L.) Cultivars. Food Chem. 2007, 104, 259–267. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine Alleviates Nutrient Deficiency-Induced Stress in Malus Hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Santos, C. Low Doses of Pb Affected Lactuca Sativa Photosynthetic Performance. Photosynthetica 2017, 55, 50–57. [Google Scholar] [CrossRef]
- Figlioli, F.; Sorrentino, M.C.; Memoli, V.; Arena, C.; Maisto, G.; Giordano, S.; Capozzi, F.; Spagnuolo, V. Overall Plant Responses to Cd and Pb Metal Stress in Maize: Growth Pattern, Ultrastructure, and Photosynthetic Activity. Environ. Sci. Pollut. Res. 2018, 26, 1781–1790. [Google Scholar] [CrossRef]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of Dopamine on Growth, Carbon Metabolism, and Nitrogen Metabolism in Cucumber under Nitrate Stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2019; Volume 249, pp. 153–197. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Ji, G. Responses of Biomass Allocation and Photosynthesis in Mulberry to Pb-Contaminated Soil. Acta Physiol. Plant. 2022, 44, 43. [Google Scholar] [CrossRef]
- Burzyński, M.; Kłobus, G. Changes of Photosynthetic Parameters in Cucumber Leaves Under Cu, Cd, and Pb Stress. Photosynthetica 2004, 42, 505–510. [Google Scholar] [CrossRef]
- Pereira-Matos, Y.C.; Lima, E.J.d.F.; Ribeiro, A.T.; Lange, C.N.; Batista, B.L.; El-Beltagi, H.S.; Bajguz, A.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide Reverses Disturbances in Zinc-Stressed Tomato by Synergistically Stimulating Leaf Structures, Photosynthesis and Growth. S. Afr. J. Bot. 2023, 159, 447–460. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The Role of 24-Epibrassinolide in the Regulation of Photosynthetic Characteristics and Nitrogen Metabolism of Tomato Seedlings Under a Combined Low Temperature and Weak Light Stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Cunha, L.F.S.; Oliveira, V.P.; Nascimento, A.W.S.; Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; da Silva Lobato, A.K. Leaf Application of 24-Epibrassinolide Mitigates Cadmium Toxicity in Young Eucalyptus Urophylla Plants by Modulating Leaf Anatomy and Gas Exchange. Physiol. Plant. 2020, 173, 67–87. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early Events in Plant Abiotic Stress Signaling: Interplay Between Calcium, Reactive Oxygen Species and Phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Springer: Singapore, 2017; pp. 1–329. [Google Scholar] [CrossRef]
- Lan, G.; Shi, L.; Lu, X.; Liu, Z.; Sun, Y. Effects of Dopamine on Antioxidation, Mineral Nutrients, and Fruit Quality in Cucumber Under Nitrate Stress. J. Plant Growth Regul. 2022, 41, 2918–2929. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Revisiting Carotenoids and Their Role in Plant Stress Responses: From Biosynthesis to Plant Signaling Mechanisms during Stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerlands, 2018; pp. 207–232. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-Epibrassinolide on Reactive Oxygen Species and Antioxidative Defense Systems in Tall Fescue Plants under Lead Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; Lobato, A.K.D.S. 24-Epibrassinolide Improves Root Anatomy and Antioxidant Enzymes in Soybean Plants Subjected to Zinc Stress. J. Soil. Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-Lead Interactions: Transport, Toxicity, Tolerance, and Detoxification Mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Yüce, M.; Ekinci, M.; Yildirim, E.; Turan, M.; Ors, S. Changes in Bean Plant Growth in Lead-Contaminated Soil. In Proceedings of the International Conference on Agriculture, Virtual, 11–12 August 2022; Volume 7, pp. 29–33. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; 58p. [Google Scholar]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Bajguz, A.; Lobato, A.K.D.S. 24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants Under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures. Agronomy 2022, 12, 1985. [Google Scholar] [CrossRef]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective Procedures for the Determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and Rare Earth Elements in Plants and Foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L. Photosynthesis, Transpiration, and Water Use Efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. Photosynthetica 2004, 42, 65–70. [Google Scholar] [CrossRef]
- Aragão, R.M.; Silva, E.N.; Vieira, C.F.; Silveira, J.A.G. High Supply of NO3−Mitigates Salinity Effects Through an Enhancement in the Efficiency of Photosystem II and CO2 Assimilation in Jatropha curcas Plants. Acta Physiol. Plant. 2012, 34, 2135–2143. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced Tolerance to Salt Stress and Water Deficit by Overexpressing Superoxide Dismutase in Tobacco (Nicotiana tabacu) Chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of Nitrite Formation from Hydroxylammoniumchloride: A Simple Assay for Superoxide Dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive Oxygen Metabolism in Mycorrhizal and Non-Mycorrhizal Citrus (Poncirus trifoliata) Seedlings Subjected to Water Stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV—VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Venables, W.N.; Smith, D.M.; R Core Team. An Introduction to R; R Core Team: Vienna, Austria, 2021; 105p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).