Dopamine Spraying Protects Against Cadmium-Induced Oxidative Stress and Stimulates Photosynthesis in Soybean Plants
Abstract
1. Introduction
2. Results
2.1. DOP Minimized Cd Concentrations in Plants Exposed to Toxicity
2.2. Neurotransmitter Positively Regulates Nutrient Contents
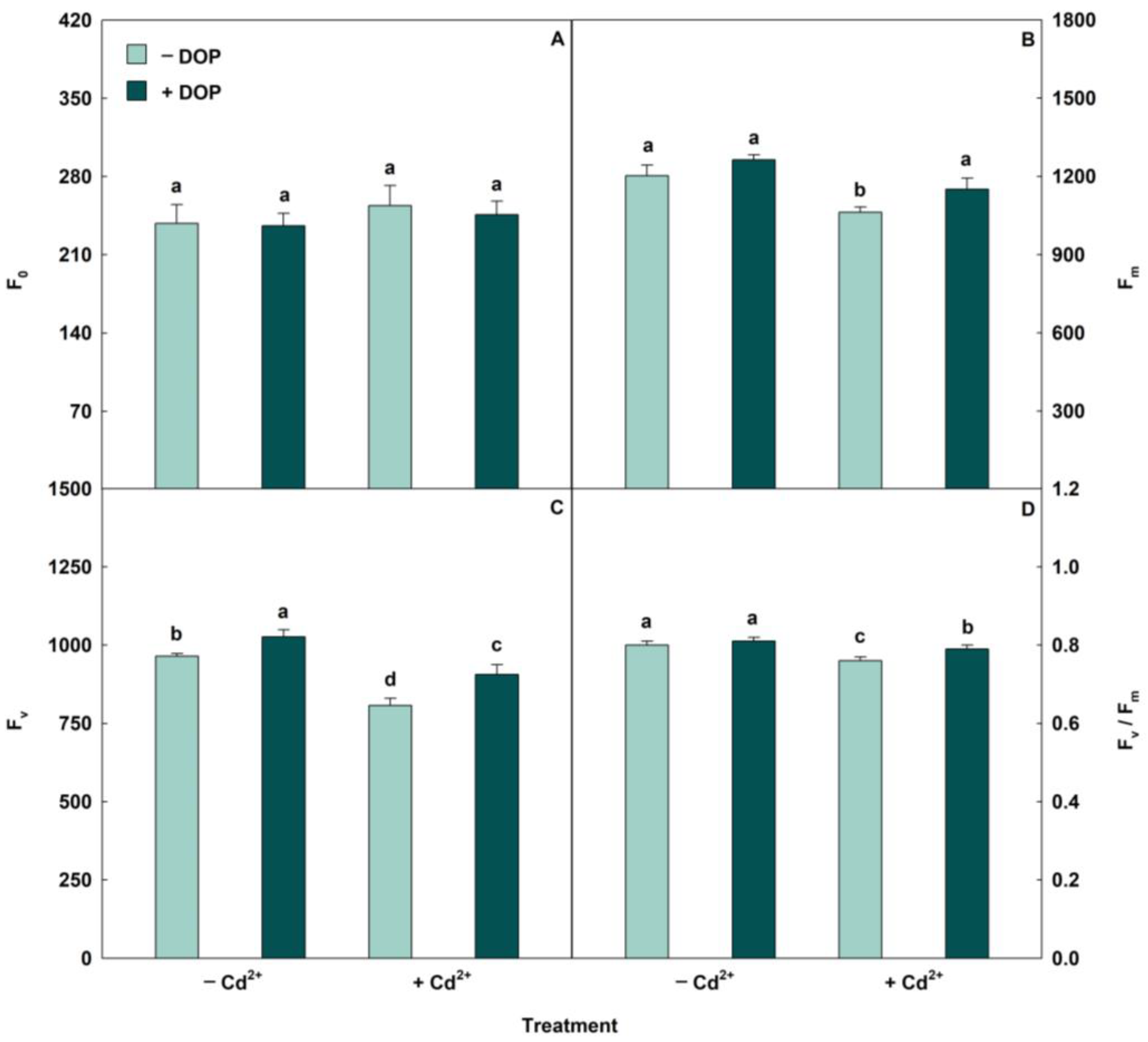
2.3. DOP Alleviated the Cd Impacts on Photosynthetic Apparatus
2.4. Benefits in Antioxidant Defense and Lower ROS Concentrations Induced by DOP
2.5. DOP Mitigated the Impacts Caused by Cd Excess on Biomass
3. Discussion
4. Materials and Methods
4.1. Geographical Location and Growth Parameters
4.2. Plants, Containers, and Acclimation
4.3. Experimental Design, Plant Nutrition and Cd Excess
4.4. Dopamine (DOP) Preparation and Application
4.5. Chlorophyll Fluorescence and Gaseous Exchange
4.6. Assessment of Antioxidant Enzymes, Soluble Proteins, and Stress Markers
4.7. Assessment of Photosynthetic Pigments, Nutritional Composition, and Biomass
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arias, A.C.R.; Chaparro-Giraldo, A.; López-Pazos, S.A. A Basic Scheme of Soybean Transformation for Glyphosate Tolerance Using Agrobacterium Tumefaciens through an Approximation of Patents: A Review. Agron. Colomb. 2021, 39, 145–155. [Google Scholar] [CrossRef]
- Tariq, A.; Jabeen, Z.; Farrakh, S.; Noreen, K.; Arshad, W.; Ahmed, H.; Haider, W. Exploring the Genetic Potential of Pakistani Soybean Cultivars through RNA-Seq Based Transcriptome Analysis. Mol. Biol. Rep. 2022, 49, 2889–2897. [Google Scholar] [CrossRef]
- Fao FAO Statistical Databases. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 15 May 2025).
- Conab Monitoring the Brazilian Harvest 2023/2024. 2024. Available online: https://www.gov.br/conab/pt-br/atuacao/informacoes-agropecuarias/safras/safra-de-graos/boletim-da-safra-de-graos/12o-levantamento-safra-2023-2024/12o-levantamento-safra-2023-2024 (accessed on 5 January 2025).
- Teixeira, W.F.; Soares, L.H.; Fagan, E.B.; da Costa Mello, S.; Reichardt, K.; Dourado-Neto, D. Amino Acids as Stress Reducers in Soybean Plant Growth under Different Water-Deficit Conditions. J. Plant Growth Regul. 2020, 39, 905–919. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Chang, W.; Li, K.; Zhang, M.; Pu, G.; Kurakov, A.V.; Ping, Y.; Song, F. Enhancing Soil Quality in Soybean Cultivation: Mycorrhizal Technology Combined with Intercropping under High Cadmium Stress. Ecotoxicol. Environ. Saf. 2025, 290, 117558. [Google Scholar] [CrossRef]
- Khan, K.; Khan, R.; Liu, Z.; Ali, S.; Naseer, M.A.; Shah, M.A.; Ahmad, H.; Zhou, X.B. Melatonin Mitigates Nickel Oxide Nanoparticles Induced Phytotoxicity in Soybean by Reducing Metal Accumulation, Enhancing Antioxidant Defense and Promoting Nitrogen Assimilation. J. Hazard. Mater. 2025, 485, 136861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S.U.; Qiu, Z.; Shahzad, S.M.; Nawaz, M.F.; Huang, J.; Naveed, S.; Li, L.; Wang, X.; Cheng, H. Toxic Effects of Cadmium on the Physiological and Biochemical Attributes of Plants, and Phytoremediation Strategies: A Review. Environ. Pollut. 2023, 325, 121433. [Google Scholar] [CrossRef] [PubMed]
- Abou Fayssal, S.; Kumar, P.; Popescu, S.M.; ud din Khanday, M.; Sardar, H.; Ahmad, R.; Gupta, D.; Kumar Gaur, S.; Alharby, H.F.; Al-Ghamdi, A.G. Health Risk Assessment of Heavy Metals in Saffron (Crocus Sativus L.) Cultivated in Domestic Wastewater and Lake Water Irrigated Soils. Heliyon 2024, 10, e27138. [Google Scholar] [CrossRef] [PubMed]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium Stress in Plants: A Critical Review of the Effects, Mechanisms, and Tolerance Strategies. Crit. Rev. Environ. Sci. Technol. 2020, 52, 675–726. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A.M. The Uptake, Transfer, and Detoxification of Cadmium in Plants and Its Exogenous Effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Tenuzzo, B.A.; Carata, E.; Panzarini, E.; Luvisi, A.; De Bellis, L.; Vergine, M. Effects of Cadmium on Root Morpho-Physiology of Durum Wheat. Front. Plant Sci. 2022, 13, 936020. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium Toxicity in Plants: Recent Progress on Morpho-Physiological Effects and Remediation Strategies; Springer International Publishing: Cham, Switzerland, 2022; Volume 22, ISBN 4272902100645. [Google Scholar]
- Ashraf, H.; Ghouri, F.; Ali, S.; Bukhari, S.A.H.; Haider, F.U.; Zhong, M.; Xia, W.; Fu, X.; Shahid, M.Q. The Protective Roles of Oryza Glumaepatula and Phytohormone in Enhancing Rice Tolerance to Cadmium Stress by Regulating Gene Expression, Morphological, Physiological, and Antioxidant Defense System. Environ. Pollut. 2025, 364, 125311. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Kamran, M.; Yasin, G.; Cheng, H.; Rehim, A.; Riaz, L.; Rizwan, M.; Ali, S.; Alsahli, A.A.; et al. Silicon Elevated Cadmium Tolerance in Wheat (Triticum aestivum L.) by Endorsing Nutrients Uptake and Antioxidative Defense Mechanisms in the Leaves. Plant Physiol. Biochem. 2021, 166, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Yasin, G.; Cheng, H.; Mehmood, F.; Zain, M.; Shehzad, M.; Ahmad, M.I.; Riaz, L.; Rahim, A.; et al. Role of Silicon on Root Morphological Characters of Wheat (Triticum aestivum L.) Plants Grown under Cd-Contaminated Nutrient Solution. Acta Physiol. Plant. 2021, 43, 1–13. [Google Scholar] [CrossRef]
- Muhammad, S.; Ulhassan, Z.; Munir, R.; Yasin, M.U.; Islam, F.; Zhang, K.; Chen, W.; Jan, M.; Afzal, M.; Muhammad, A.; et al. Nanosilica and Salicylic Acid Synergistically Regulate Cadmium Toxicity in Rice. Environ. Pollut. 2025, 364, 125331. [Google Scholar] [CrossRef]
- Shahzad, A.; Hameed, S.; Qin, M.; Li, H.; Zafar, S.; Siddiqui, S.; Sattar, S.; Mahmood, Z.; Mehwish, S. Cadmium (Cd) Detoxification and Activation of Plant Defense Enzymes in Wheat (Triticum aestivum) through the Use of Endophytic Bacillus Thuringiensis and Salix Alba Root Powder. Environ. Pollut. 2025, 364, 125147. [Google Scholar] [CrossRef]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium Caused Different Toxicity to Photosystem I and Photosystem II of Freshwater Unicellular Algae Chlorella Pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef]
- Song, J.; Sun, Z.; Saud, S.; Fahad, S.; Nawaz, T. Exploring the Deleterious Effects of Heavy Metal Cadmium on Antioxidant Defense and Photosynthetic Pathways in Higher Plants. Plant Stress 2025, 15, 100716. [Google Scholar] [CrossRef]
- Haider, F.U.; Zulfiqar, U.; Noor-ul-Ain; Mehmood, T.; Shahzad, B.; Liqun, C.; Yong, J.W.H.; Binobead, M.A. Effects of Titanium Oxide Nanoparticles and 24-Epibrassinosteroid to Mitigate the Toxicity of Cadmium (Cd) and Improve Physio-Morphological Traits of Soybean (Glycine max L.) Cultivated under Cd-Contaminated Soil. Environ. Technol. Innov. 2024, 36, 103811. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Wu, Q.; Chen, S.; Zou, Y.; Ma, F.; Li, C. Exogenous Dopamine Improves Apple Fruit Quality via Increasing Flavonoids and Soluble Sugar Contents. Sci. Hortic. 2021, 280, 109903. [Google Scholar] [CrossRef]
- Akula, R.; Mukherjee, S. New Insights on Neurotransmitters Signaling Mechanisms in Plants. Plant Signal. Behav. 2020, 15, 1737450. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Cao, H.; Tian, X.; Li, S.; Liu, C.; Zhang, Z.; Mao, K.; Ma, F.; Li, C. Dopamine Regulates Its Own Synthesis via MdORG2 to Improve Low-Nitrogen Tolerance in Apple Plants. Plant. Cell Environ. 2025, 48, 470–488. [Google Scholar] [CrossRef]
- Akcay, U.C.; Gencay, R.; Koc, F.Z. Effect of Dopamine and Progesterone on the Physiological and Molecular Responses of Tomato Seedlings to Drought and Salt Stress. Cogent Food Agric. 2024, 10, 2321308. [Google Scholar] [CrossRef]
- Abo-Shanab, W.A.; Diab, R.H. Dopamine Hydrochloride Alleviates the Salt-Induced Stress in Glycine max (L.) Merr. Plant. J. Soil Sci. Plant Nutr. 2024, 24, 3474–3490. [Google Scholar] [CrossRef]
- Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Cao, Y.; Han, R.; Shi, C.; Liang, B.; Xu, J. Melatonin and Dopamine Mediate the Regulation of Nitrogen Uptake and Metabolism at Low Ammonium Levels in Malus Hupehensis. Plant Physiol. Biochem. 2022, 171, 182–190. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.M.; Alharbi, B.M.; Alharby, H.F.; Abualresh, A.M.; Badawy, G.A.; Semida, W.M.; Rady, M. Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus Vulgaris. Plants 2022, 11, 3069. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J.; Liang, B. Melatonin and Dopamine Enhance Waterlogging Tolerance by Modulating ROS Scavenging, Nitrogen Uptake, and the Rhizosphere Microbial Community in Malus Hupehensis. Plant Soil 2023, 483, 475–493. [Google Scholar] [CrossRef]
- Ali, A.F.; Hatamnia, A.A.; Malekzadeh, P.; Sayyari, M.; Aghdam, M.S. Exogenous Dopamine Ameliorates Chilling Injury in Banana Fruits by Enhancing Endogenous Dopamine and Glycine Betaine Accumulation and Promoting ROS Scavenging System Activity. Postharvest Biol. Technol. 2023, 205, 112521. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine Alleviates Bisphenol A-Induced Phytotoxicity by Enhancing Antioxidant and Detoxification Potential in Cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, X.; Zhang, Z.; Wang, Y.; Ma, F.; Li, C. Dopamine Enhances the Resistance of Apple to Valsa Mali Infection. Phytopathology 2022, 112, 1141–1151. [Google Scholar] [CrossRef]
- Liang, B.; Gao, T.; Zhao, Q.; Ma, C.; Chen, Q.; Wei, Z.; Li, C.; Li, C.; Ma, F. Effects of Exogenous Dopamine on the Uptake, Transport, and Resorption of Apple Ionome under Moderate Drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of Dopamine on Growth, Carbon Metabolism, and Nitrogen Metabolism in Cucumber under Nitrate Stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Pontes, C.V.S.; Prestes, L.R.; Vieira Neto, A.F.; Lopes, L.K.C.; Barbosa, M.A.M.; Lobato, A.K.d.S. Single and Combined Applications of Dopamine and 24-Epibrassinolide in Soybean Seedlings under Water Deficiency: Tolerance Mechanisms Essential to Inhibit Deleterious Effects. Plant Biosyst. 2024, 158, 1347–1357. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Liu, Z.Y.; Shi, L.M.; Lu, X.Y.; Han, Y.Y.; Sun, Y. Mitigation Effect of Exogenous Dopamine Treatment on Downy Mildew-Infected Cucumber. J. Plant Growth Regul. 2024, 43, 2615–2631. [Google Scholar] [CrossRef]
- Sehrish, A.K.; Ahmad, S.; Ali, S.; Tabssam, R.; Ai, F.; Du, W.; Guo, H. Alleviation of Cadmium Toxicity by Improving Antioxidant Defense Mechanism and Nutrient Uptake in Wheat (Triticum aestivum L.) through Foliar Application of 24-Epibrassinolide under Elevated CO2. J. Hazard. Mater. 2024, 480, 136209. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, X.; Tang, Z.; Zhang, Z.; Gao, H.; Ma, F.; Li, C. Exogenous Dopamine and MdTyDC Overexpression Enhance Apple Resistance to Fusarium Solani. Phytopathology 2022, 112, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Chen, D.; Yang, M.; Huang, F.; Liu, J.; Yu, X.; Yu, L. Exploiting Synergy of Dopamine and Stressful Conditions in Enhancing Haematococcus Lacustris Biomass and Astaxanthin Yield. Bioresour. Technol. 2025, 417, 131848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of Dopamine in Plants: A Review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine Confers Cadmium Tolerance in Apples by Improving Growth, Reducing Reactive Oxygen Species, and Changing Secondary Metabolite Levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Zhang, J.; Ji, J.; Xu, J.; Liang, B. Dopamine Alleviates Cadmium Stress in Apple Trees by Recruiting Beneficial Microorganisms to Enhance the Physiological Resilience Revealed by High-Throughput Sequencing and Soil Metabolomics. Hortic. Res. 2023, 10, uhad112. [Google Scholar] [CrossRef]
- He, J.; Zhou, J.; Wan, H.; Zhuang, X.; Li, H.; Qin, S.; Lyu, D. Rootstock–Scion Interaction Affects Cadmium Accumulation and Tolerance of Malus. Front. Plant Sci. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, H.; Chen, G.; Wu, X.; Zeng, F. Cadmium Accumulation in Plants: Insights from Phylogenetic Variation into the Evolution and Functions of Membrane Transporters. Sustain. 2023, 15, 12158. [Google Scholar] [CrossRef]
- Cunha, L.F.S.d.; Oliveira, V.P.d.; Nascimento, A.W.S.d.; Silva, B.R.S.d.; Batista, B.L.; Alsahli, A.A.; Lobato, A.K.d.S. Leaf Application of 24-epibrassinolide Mitigates Cadmium Toxicity in Young Eucalyptus Urophylla Plants by Modulating Leaf Anatomy and Gas Exchange. Physiol. Plant. 2020, 173, 67–87. [Google Scholar] [CrossRef]
- Schäfer, E.D.; Owen, M.R.; Band, L.R.; Farcot, E.; Bennett, M.J.; Lynch, J.P. Modeling Root Loss Reveals Impacts on Nutrient Uptake and Crop Development. Plant Physiol. 2022, 190, 2260–2278. [Google Scholar] [CrossRef] [PubMed]
- Cochavi, A.; Cohen, I.H.; Rachmilevitch, S. The Role of Different Root Orders in Nutrient Uptake. Environ. Exp. Bot. 2020, 179, 104212. [Google Scholar] [CrossRef]
- Liu, J.; Ni, J.; Mo, A.; Fan, X.; Jiang, Y.; Xie, H.; Hu, J.; Zhu, Y.; Peng, C.; Yang, F. Cadmium Affects the Growth, Antioxidant Capacity, Chlorophyll Content, and Homeostasis of Essential Elements in Soybean Plants. South African J. Bot. 2023, 162, 604–610. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and Molecular Mechanisms of Medicinal Plants in Response to Cadmium Stress: Current Status and Future Perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure—Function Relations and Tissue-Specifica’c. Plants Rev. 2020, 9, 18. [Google Scholar]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine Alleviates Nutrient Deficiency-Induced Stress in Malus Hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-m.; Gao, T.-t.; Zhang, Z.; Tan, K.-x.; Jin, Y.-b.; Zhao, Y.-j.; Ma, F.-w.; Li, C. The Mitigation Effects of Exogenous Dopamine on Low Nitrogen Stress in Malus Hupehensis. J. Integr. Agric. 2020, 19, 2709–2724. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Z.; Liu, X.; Wu, Q.; Chen, Q.; Liu, Q.; van Nocker, S.; Ma, F.; Li, C. Physiological and Transcriptome Analyses of the Effects of Exogenous Dopamine on Drought Tolerance in Apple. Plant Physiol. Biochem. 2020, 148, 260–272. [Google Scholar] [CrossRef]
- Jiao, C.; Lan, G.; Sun, Y.; Wang, G.; Sun, Y. Dopamine Alleviates Chilling Stress in Watermelon Seedlings via Modulation of Proline Content, Antioxidant Enzyme Activity, and Polyamine Metabolism. J. Plant Growth Regul. 2021, 40, 277–292. [Google Scholar] [CrossRef]
- Hedges, D.M.; Yorgason, J.T.; Perez, A.W.; Schilaty, N.D.; Williams, B.M.; Watt, R.K.; Steffensen, S.C. Spontaneous Formation of Melanin from Dopamine in the Presence of Iron. Antioxidants 2020, 9, 1285. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, Y.; Guo, Z.; Han, M.; Yan, X. Zinc Oxide Nanoparticles Alleviate Cadmium Toxicity and Promote Tolerance by Modulating Programmed Cell Death in Alfalfa (Medicago Sativa L.). J. Hazard. Mater. 2024, 469, 133917. [Google Scholar] [CrossRef]
- Chen, J.; Qin, H.; Zhang, B.; Mao, W.; Lou, L.; Shen, C.; Mao, J.; Lin, Q. Development of Melatonin Nano-Delivery Systems to Reduce Cadmium Accumulation in Rice (Oryza Sativa L.) Seedlings: Insights from Photosynthetic Efficiency, Antioxidative Response and Gene Expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Farouk, S.; El-Hady, M.A.A.; El-Sherpiny, M.A.; Hassan, M.M.; Alamer, K.H.; Al-Robai, S.A.; Ali, E.F.; El-Bauome, H.A. Effect of Dopamine on Growth, Some Biochemical Attributes, and the Yield of Crisphead Lettuce under Nitrogen Deficiency. Horticulturae 2023, 9, 945. [Google Scholar] [CrossRef]
- Saeed, W.; Mubeen, S.; Pan, J.; Rehman, M.; Fang, W.; Luo, D.; Liu, P.; Li, Y.; Chen, P. Integrated Physiological and Metabolomic Responses Reveal Mechanisms of Cd Tolerance and Detoxification in Kenaf (Hibiscus Cannabinus L.) under Cd Stress. Front. Plant Sci. 2024, 15, 1332426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Z.; Feng, Y.; Qaharaduqin, S.; Liu, W.; Yan, Y. Impact of Cd and Pb on the Photosynthetic and Antioxidant Systems of Hemerocallis Citrina Baroni as Revealed by Physiological and Transcriptomic Analyses. Plant Cell Rep. 2024, 43, 226. [Google Scholar] [CrossRef] [PubMed]
- Roncel, M.; González-Rodríguez, A.A.; Naranjo, B.; Bernal-Bayard, P.; Lindahl, A.M.; Hervás, M.; Navarro, J.A.; Ortega, J.M. Iron Deficiency Induces a Partial Inhibition of the Photosynthetic Electron Transport and a High Sensitivity to Light in the Diatom Phaeodactylum Tricornutum. Front. Plant Sci. 2016, 7, 1050. [Google Scholar] [CrossRef]
- Santos, L.R.; Paula, L.S.; Pereira, Y.C.; Silva, B.R.S.d.; Batista, B.L.; Alsahli, A.A.; Lobato, A.K.d.S. Brassinosteroids-Mediated Amelioration of Iron Deficiency in Soybean Plants: Beneficial Effects on the Nutritional Status, Photosynthetic Pigments and Chlorophyll Fluorescence. J. Plant Growth Regul. 2021, 40, 1803–1823. [Google Scholar] [CrossRef]
- Assunção, N.S.; Ribeiro, N.P.; da Silva, R.M.; Soratto, R.P.; Fernandes, A.M. Tuber Yield and Allocation of Nutrients and Carbohydrates in Potato Plants as Affected by Limestone Type and Magnesium Supply. J. Plant Nutr. 2020, 43, 51–63. [Google Scholar] [CrossRef]
- Santos, L.A.; Batista, B.L.; Lobato, A.K.d.S. 24-Epibrasinolide Delays Chlorophyll Degradation and Stimulates the Photosynthetic Machinery in Magnesium-Stressed Soybean Plants. J. Plant Growth Regul. 2023, 42, 183–198. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Yuce, M.; Ors, S.; Araz, O.; Torun, U.; Argin, S. Exogenous Dopamine Mitigates the Effects of Salinity Stress in Tomato Seedlings by Alleviating the Oxidative Stress and Regulating Phytohormones. Acta Physiol. Plant. 2024, 46, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Shen, J.; Zhang, L.; Wei, P.; Liu, A.; Song, H. The Alleviating Effect on the Growth, Chlorophyll Synthesis, and Biochemical Defense System in Sunflowers under Cadmium Stress Achieved through Foliar Application of Humic Acid. BMC Plant Biol. 2024, 24, 792. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Ma, X.; He, Y.; Ren, Q.; Huang, Y.; Wang, J.; Xue, Y.; Yang, R.; Guo, Y.; et al. New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna Turionifera 5511). Plants 2023, 12, 1996. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M.; Abed El-Mageed, T.A.; Howladar, S.M.; Abdelhamid, M.T. Alleviation of Cadmium Toxicity in Common Bean (Phaseolus Vulgaris L.) Plants by the Exogenous Application of Salicylic Acid. J. Hortic. Sci. Biotechnol. 2015, 90, 83–91. [Google Scholar] [CrossRef]
- Shahabivand, S.; Parvaneh, A.; Aliloo, A.A. Root Endophytic Fungus Piriformospora Indica Affected Growth, Cadmium Partitioning and Chlorophyll Fluorescence of Sunflower under Cadmium Toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, J.; Yang, C.; Ma, Y.; Gao, S.; Liu, Q.; Zhang, J.; Ma, F.; Li, C. Exogenous Dopamine Delays the Senescence of Detached Malus Hupehensis Leaves by Reducing Phytohormone Signalling and Sugar Degradation. Sci. Hortic. 2023, 319, 112151. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of Cadmium on Antioxidant Enzyme and Photosynthetic Activities in Leaves of Two Maize Cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Su, L.; Xie, Y.; He, Z.; Zhou, X.; Liu, Y.; Zhang, R.; Li, C. Selenium Mitigates Cd-Induced Oxidative Stress and Photosynthesis Inhibition in Two Cherry Tomato Cultivars. J. Soil Sci. Plant Nutr. 2022, 22, 3212–3227. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, Z.; Han, Y.; Sun, Y. Exogenous Dopamine Promotes Photosynthesis and Carbohydrate Metabolism of Downy Mildew-Infected Cucumber. Sci. Hortic. 2022, 295, 110842. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Zheng, J.; Zhang, Z.; Gao, T.; Li, C.; Ma, F. Overexpression of the Tyrosine Decarboxylase Gene MdTyDC in Apple Enhances Long-Term Moderate Drought Tolerance and WUE. Plant Sci. 2021, 313, 111064. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, J.; Li, L.; Yin, Y.; Xue, B.; Li, J.; Sun, F. Exploration of the Effects of Cadmium Stress on Photosynthesis in Oenanthe Javanica (Blume) DC. Toxics 2024, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, H.; Mehwish; Bukhat, S.; Rasul, S.; Rehmani, M.I.A.; Noreen, S.; Athar, H.U.R.; Zafar, Z.U.; Skalicky, M.; Soufan, W.; et al. Methyl Jasmonate Alleviated the Adverse Effects of Cadmium Stress in Pea (Pisum Sativum L.): A Nexus of Photosystem II Activity and Dynamics of Redox Balance. Front. Plant Sci. 2022, 13, 860664. [Google Scholar] [CrossRef]
- Arena, C.; Figlioli, F.; Sorrentino, M.C.; Izzo, L.G.; Capozzi, F.; Giordano, S.; Spagnuolo, V. Ultrastructural, Protein and Photosynthetic Alterations Induced by Pb and Cd in Cynara Cardunculus L., and Its Potential for Phytoremediation. Ecotoxicol. Environ. Saf. 2017, 145, 83–89. [Google Scholar] [CrossRef]
- Maia, C.F.; Pereira, Y.C.; Silva, B.R.S.d.; Batista, B.L.; Lobato, A.K.d.S. Exogenously Applied 24-Epibrassinolide Favours Stomatal Performance, ROS Detoxification and Nutritional Balance, Alleviating Oxidative Damage Against the Photosynthetic Apparatus in Tomato Leaves Under Nickel Stress. J. Plant Growth Regul. 2023, 42, 2196–2211. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Luo, D.; Hu, X.; Feng, P.; Mo, Y.; Li, H.; Gong, S. Integrated Effects of Soil Moisture on Wheat Hydraulic Properties and Stomatal Regulation. Plants 2024, 13, 2263. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.S.; Pereira, T.S.; Souza, C.L.F.d.C.; Lima, E.J.A.; Batista, B.L.; Lobato, A.K.d.S. Silicon Deposition in Roots Minimizes the Cadmium Accumulation and Oxidative Stress in Leaves of Cowpea Plants. Physiol. Mol. Biol. Plants 2018, 24, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Rizwan, M.; Zia ur Rehman, M.; Zubair, M.; Riaz, M.; Qayyum, M.F.; Alharby, H.F.; Bamagoos, A.A.; Ali, S. Application of Co-Composted Farm Manure and Biochar Increased the Wheat Growth and Decreased Cadmium Accumulation in Plants under Different Water Regimes. Chemosphere 2020, 246, 125809. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Qin, G.; Wang, H.; Zhu, Y.; Zhang, K.; Yang, H. Growth, Accumulation, and Antioxidative Responses of Two Salix Genotypes Exposed to Cadmium and Lead in Hydroponic Culture. Environ. Sci. Pollut. Res. 2019, 26, 19770–19784. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Lv, X.; Zhang, Y.; Wang, W. Effects of Biochar and Biofertilizer on Cadmium-Contaminated Cotton Growth and the Antioxidative Defense System. Sci. Rep. 2020, 10, 20112. [Google Scholar] [CrossRef]
- Santos, L.R.; Batista, B.L.; Lobato, A.K.d.S. Brassinosteroids Mitigate Cadmium Toxicity in Cowpea Plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Ali, A.; Rizwan, M.; Ali, S. Dopamine Alleviates Hydrocarbon Stress in Brassica Oleracea through Modulation of Physio-Biochemical Attributes and Antioxidant Defense Systems. Chemosphere 2021, 270, 128633. [Google Scholar] [CrossRef]
- Lima, J.V.; Lobato, A.K.d.S. Brassinosteroids Improve Photosystem II Efficiency, Gas Exchange, Antioxidant Enzymes and Growth of Cowpea Plants Exposed to Water Deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef]
- Pereira, Y.C.; Rodrigues, W.S.; Lima, E.J.A.; Santos, L.R.; Silva, M.H.L.; Lobato, A.K.d.S. Brassinosteroids Increase Electron Transport and Photosynthesis in Soybean Plants under Water Deficit. Photosynthetica 2019, 57, 181–191. [Google Scholar] [CrossRef]
- Maia, C.F.; Silva, B.R.S.d.; Lobato, A.K.d.S. Brassinosteroids Positively Modulate Growth: Physiological, Biochemical and Anatomical Evidence Using Two Tomato Genotypes Contrasting to Dwarfism. J. Plant Growth Regul. 2018, 37, 1099–1112. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced Tolerance to Salt Stress and Water Deficit by Overexpressing Superoxide Dismutase in Tobacco (Nicotiana Tabacu) Chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of Nitrite Formation from Hydroxylammoniumchloride: A Simple Assay for Superoxide Dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive Oxygen Metabolism in Mycorrhizal and Non-Mycorrhizal Citrus (Poncirus Trifoliata) Seedlings Subjected to Water Stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 431–438. ISBN 9780471709084. [Google Scholar]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective Procedures for the Determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and Rare Earth Elements in Plants and Foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Venables, W.N.; Smith, D.M.; R Core Team. An Introduction to R. Pr. Graph. Min. R 2024, 2, 27–52. [Google Scholar]




| Cd2+ | DOP | Cd in Root (µg g DM−1) | Cd in Stem (µg g DM−1) | Cd in Leaf (µg g DM−1) |
|---|---|---|---|---|
| − | − | 0.77 ± 0.05 c | 0.09 ± 0.01 c | 0.07 ± 0.01 c |
| − | + | 0.75 ± 0.01 c | 0.09 ± 0.01 c | 0.06 ± 0.01 c |
| + | − | 971.24 ± 24.31 a | 5.05 ± 0.10 a | 12.94 ± 0.20 a |
| + | + | 387.26 ± 16.56 b | 3.60 ± 0.07 b | 8.01 ± 0.23 b |
| Cd2+ | DOP | K (mg g DM−1) | Ca (mg g DM−1) | Mg (mg g DM−1) | Mn (µg g DM−1) | Fe (µg g DM−1) | Zn (µg g DM−1) |
|---|---|---|---|---|---|---|---|
| Contents in root | |||||||
| − | − | 34.52 ± 0.68 a | 13.20 ± 0.60 a | 14.05 ± 0.50 a | 444.66 ± 21.76 a | 4869.19 ± 156.89 a | 50.75 ± 0.72 b |
| − | + | 35.21 ± 0.35 a | 13.36 ± 0.05 a | 14.08 ± 0.20 a | 449.99 ± 13.20 a | 4907.15 ± 34.44 a | 52.74 ± 0.81 a |
| + | − | 23.54 ± 0.74 c | 8.61 ± 0.49 c | 10.61 ± 0.80 c | 222.51 ± 5.78 c | 2489.83 ± 106.04 c | 28.21 ± 1.15 d |
| + | + | 31.49 ± 0.92 b | 11.80 ± 0.67 b | 12.92 ± 0.32 b | 352.31 ± 9.28 b | 3901.83 ± 107.38 b | 35.66 ± 1.21 c |
| Contents in stem | |||||||
| − | − | 61.06 ± 0.58 a | 12.02 ± 0.49 b | 2.55 ± 0.15 a | 29.24 ± 0.40 a | 44.07 ± 1.06 a | 12.31 ± 1.03 a |
| − | + | 62.00 ± 1.83 a | 12.88 ± 0.26 a | 2.65 ± 0.09 a | 30.07 ± 0.34 a | 45.41 ± 2.67 a | 12.75 ± 0.55 a |
| + | − | 41.64 ± 0.88 c | 7.98 ± 0.11 d | 1.26 ± 0.02 c | 14.77 ± 0.53 c | 24.23 ± 1.23 c | 8.30 ± 0.63 c |
| + | + | 49.75 ± 0.91 b | 9.86 ± 0.64 c | 1.90 ± 0.08 b | 20.19 ± 1.09 b | 32.59 ± 1.07 b | 9.77 ± 0.66 b |
| Contents in leaf | |||||||
| − | − | 39.38 ± 0.57 a | 14.02 ± 0.53 a | 4.46 ± 0.13 a | 74.17 ± 0.66 a | 141.72 ± 1.44 a | 25.87 ± 0.55 a |
| − | + | 40.55 ± 1.40 a | 14.51 ± 0.46 a | 4.51 ± 0.10 a | 75.35 ± 2.48 a | 147.74 ± 1.76 a | 26.75 ± 0.90 a |
| + | − | 29.50 ± 1.13 c | 10.51 ± 0.61 c | 3.27 ± 0.15 c | 56.46 ± 1.33 c | 105.14 ± 3.47 c | 20.48 ± 0.41 c |
| + | + | 35.37 ± 0.26 b | 12.22 ± 0.89 b | 4.08 ± 0.08 b | 69.18 ± 0.83 b | 128.56 ± 7.74 b | 22.96 ± 0.83 b |
| Cd2+ | DOP | Chl a (mg g−1 FM) | Chl b (mg g−1 FM) | Total Chl (mg g−1 FM) | Car (mg g−1 FM) | Ratio Chl a/Chl b | Ratio Total Chl/Car |
|---|---|---|---|---|---|---|---|
| − | − | 12.00 ± 0.35 a | 4.95 ± 0.29 a | 16.94 ± 0.41 b | 1.13 ± 0.06 a | 2.43 ± 0.18 a | 15.04 ± 0.98 a |
| − | + | 12.30 ± 0.21 a | 5.11 ± 0.14 a | 17.42 ± 0.10 a | 1.16 ± 0.03 a | 2.41 ± 0.11 a | 14.98 ± 0.51 a |
| + | − | 6.95 ± 0.45 c | 2.59 ± 0.19 c | 9.54 ± 0.53 d | 0.60 ± 0.03 c | 2.69 ± 0.22 a | 15.79 ± 0.60 a |
| + | + | 9.50 ± 0.26 b | 3.75 ± 0.26 b | 13.25 ± 0.15 c | 0.87 ± 0.02 b | 2.54 ± 0.23 a | 15.25 ± 0.45 a |
| Cd2+ | DOP | ΦPSII | qP | NPQ | ETR (µmol m−2 s−1) | EXC (µmol m−2 s−1) | ETR/PN |
| − | − | 0.381 ± 0.020 b | 0.559 ± 0.025 a | 0.90 ± 0.11 b | 55.98 ± 2.89 b | 0.525 ± 0.025 b | 2.96 ± 0.20 a |
| − | + | 0.405 ± 0.007 a | 0.572 ± 0.010 a | 0.80 ± 0.07 b | 59.46 ± 1.07 a | 0.502 ± 0.010 c | 3.12 ± 0.02 a |
| + | − | 0.285 ± 0.011 d | 0.472 ± 0.013 b | 1.10 ± 0.06 a | 41.85 ± 1.69 d | 0.626 ± 0.011 a | 3.13 ± 0.09 a |
| + | + | 0.358 ± 0.017 c | 0.556 ± 0.036 a | 1.03 ± 0.09 a | 52.62 ± 2.57 c | 0.545 ± 0.021 b | 3.06 ± 0.23 a |
| Cd2+ | DOP | PN (µmol m−2 s−1) | E (mmol m−2 s−1) | gs (mol m−2 s−1) | Ci (µmol mol−1) | WUE (µmol mmol−1) | PN/Ci (µmol m−2 s−1 Pa−1) |
| − | − | 18.91 ± 0.56 a | 1.86 ± 0.07 b | 0.228 ± 0.011 a | 232 ± 7 b | 10.15 ± 0.25 b | 0.082 ± 0.004 b |
| − | + | 19.05 ± 0.40 a | 1.70 ± 0.07 c | 0.246 ± 0.021 a | 201 ± 14 c | 11.24 ± 0.60 a | 0.095 ± 0.008 a |
| + | − | 13.38 ± 0.79 c | 2.03 ± 0.03 a | 0.132 ± 0.013 c | 262 ± 23 a | 6.60 ± 0.43 d | 0.051 ± 0.005 d |
| + | + | 17.31 ± 0.93 b | 1.90 ± 0.03 b | 0.178 ± 0.013 b | 245 ± 8 b | 9.12 ± 0.44 c | 0.071 ± 0.003 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmo, A.S.; Pontes, C.V.d.S.; Augusto, C.C.; Batista, B.L.; Lobato, A.K.d.S. Dopamine Spraying Protects Against Cadmium-Induced Oxidative Stress and Stimulates Photosynthesis in Soybean Plants. Plants 2025, 14, 3411. https://doi.org/10.3390/plants14223411
Carmo AS, Pontes CVdS, Augusto CC, Batista BL, Lobato AKdS. Dopamine Spraying Protects Against Cadmium-Induced Oxidative Stress and Stimulates Photosynthesis in Soybean Plants. Plants. 2025; 14(22):3411. https://doi.org/10.3390/plants14223411
Chicago/Turabian StyleCarmo, Andreza Sousa, Caio Victor da Silva Pontes, Caroline Cristine Augusto, Bruno Lemos Batista, and Allan Klynger da Silva Lobato. 2025. "Dopamine Spraying Protects Against Cadmium-Induced Oxidative Stress and Stimulates Photosynthesis in Soybean Plants" Plants 14, no. 22: 3411. https://doi.org/10.3390/plants14223411
APA StyleCarmo, A. S., Pontes, C. V. d. S., Augusto, C. C., Batista, B. L., & Lobato, A. K. d. S. (2025). Dopamine Spraying Protects Against Cadmium-Induced Oxidative Stress and Stimulates Photosynthesis in Soybean Plants. Plants, 14(22), 3411. https://doi.org/10.3390/plants14223411










