Dynamic Structural Changes in a Population of Rhododendron huadingense, a Rare and Endemic Species in Zhejiang, East China
Abstract
1. Introduction
2. Results
2.1. Stand-Scale Distribution Patterns and Diameter Class Structure of Rhododendron huadingense
2.2. Population Dynamics of Rhododendron huadingens
2.3. Static Life Table and Survival Rate Curve of Rhododendron huadingense
2.3.1. Static Life Table Analysis
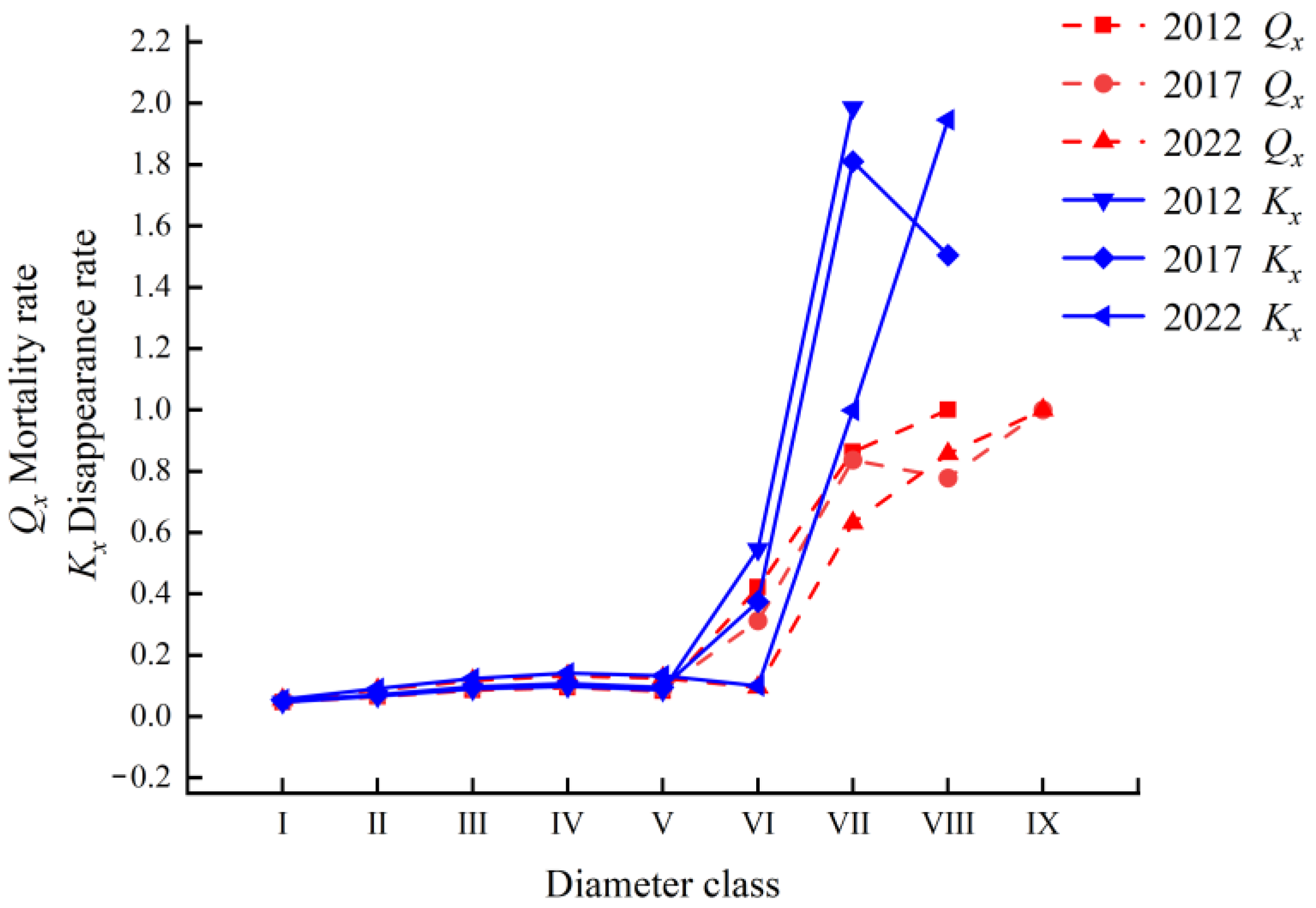
2.3.2. Mortality and Disappearance Rate Curve Analysis
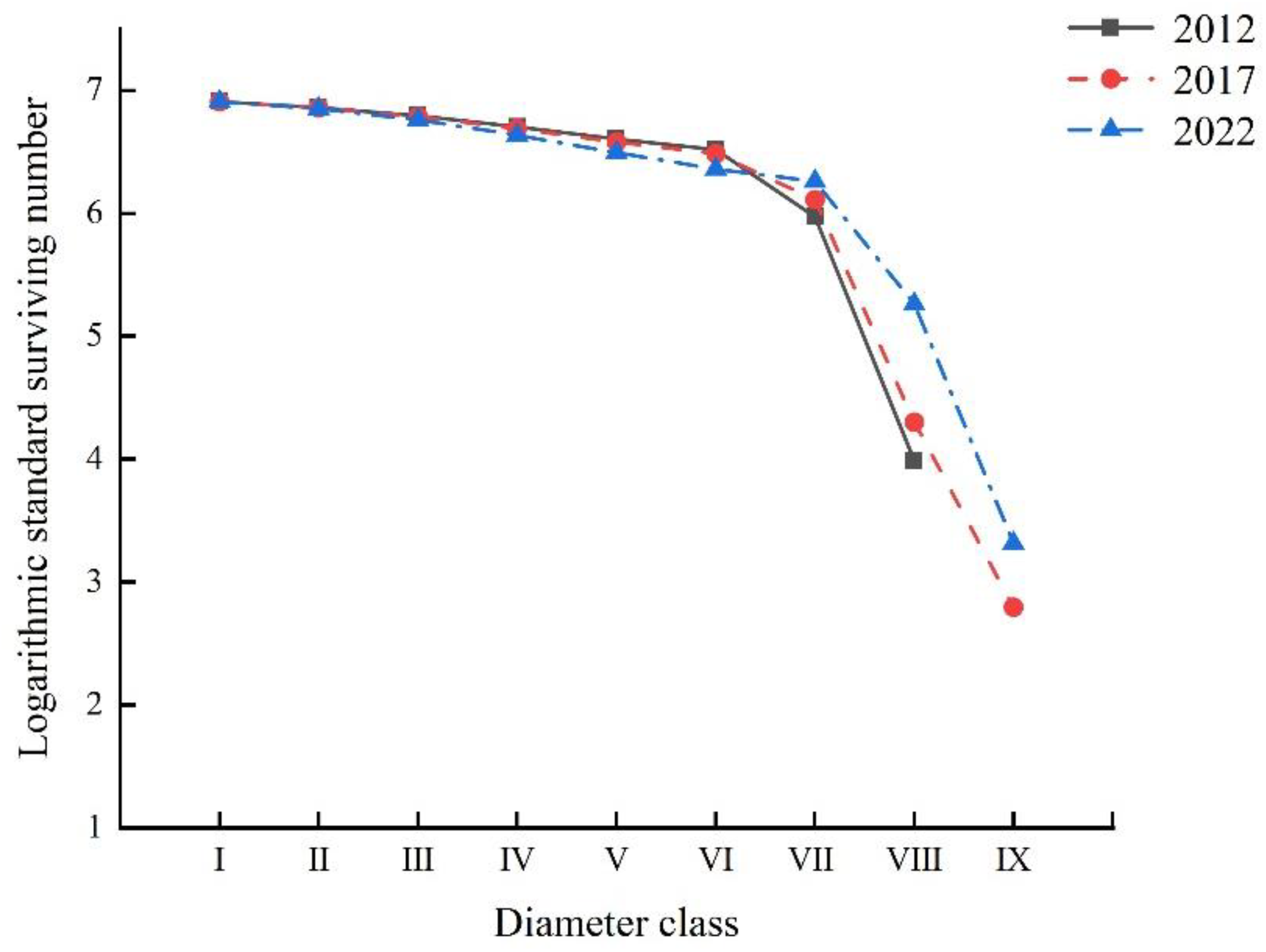
2.3.3. Survivorship Curve Analysis
2.4. Survival Analysis of Rhododendron huadingense
3. Discussion
3.1. Diameter-Class Structure of a Rhododendron huadingense Population
3.2. The Dynamic Trends of the Rhododendron huadingense Population
3.3. Conservation Implication for the Rhododendron huadingense
4. Materials and Methods
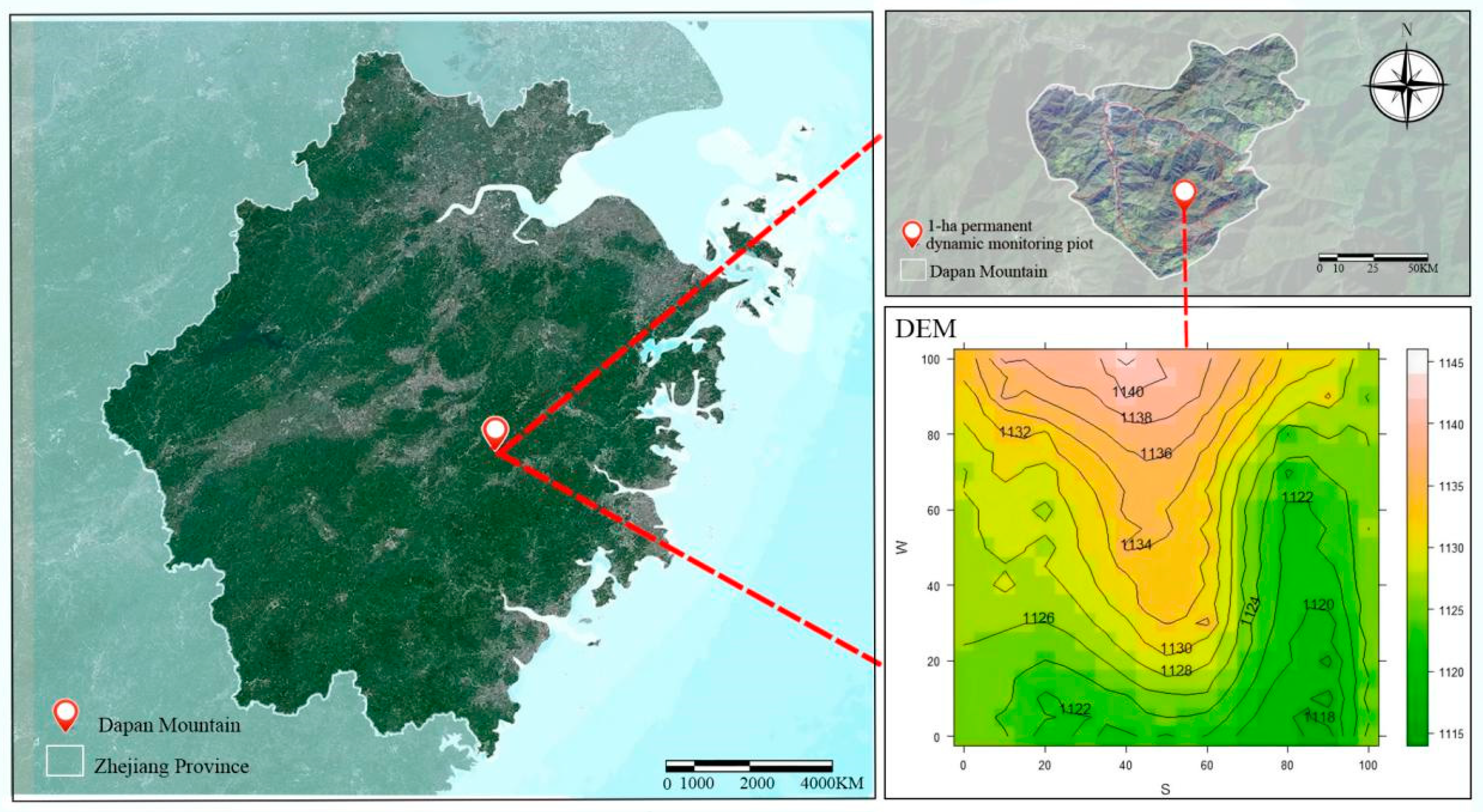
4.1. Study Area
4.2. Plot Establishment and Population Survey
4.3. Data Analysis
4.3.1. Diameter-Class Structure of Rhododendron huadingense Population
4.3.2. Quantification of Population Dynamics
4.3.3. Static Life Table and Survival Curve of Rhododendron huadingense
4.3.4. Survival Analysis
4.4. Data Processing and Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Yao, L.; Ai, X.; Zhu, J.; Liu, S. Structure and dynamic characteristics of Betula luminifera populations in different regions of Southwest Hubei Province, China. J. Appl. Ecol. 2020, 31, 357–365. [Google Scholar]
- Chhetri, P.K.; Bista, R.; Cairns, D.M. Population structure and dynamics of Abies spectabilis at Treeline Ecotone of Barun Valley, Makalu Barun National Park, Nepal. Acta Ecol. Sin. 2016, 36, 269–274. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Ren, H. Age structure of Picea schrenkiana forest along an altitudinal gradient in the central Tianshan Mountains, northwestern China. For. Ecol. Manag. 2004, 196, 267–274. [Google Scholar] [CrossRef]
- Hettt, J.M.; Loucks, O. Age Structure models of Balsam Fir and eastern Hemlock. J. Ecol. 1976, 64, 1029–1044. [Google Scholar] [CrossRef]
- Castorani, M.C.; Reed, D.C.; Raimondi, P.T. Fluctuations in population fecundity drive variation in demographic connectivity and metapopulation dynamics. Proc. R. Soc. B Biol. Sci. 2017, 284, 2086. [Google Scholar] [CrossRef]
- Thoen, R.D.; Hendricks, L.B.; Bailes, G.T.; Johnson, B.R. Spatiotemporal variation in population dynamics of a narrow endemic, Ranunculus austro-oreganus. Am. J. Bot. 2025, 112, e16446. [Google Scholar] [CrossRef]
- Chen, Y.K.; Yang, X.B.; Yang, Q.; Li, D.H.; Long, W.X.; Luo, W.Q. Factors affecting the distribution pattern of wild plants with extremely small populations in Hainan Island, China. PLoS ONE 2014, 9, e97751. [Google Scholar] [CrossRef]
- Reid, J.M.; Travis, J.M.; Daunt, F. Population and evolutionary dynamics in spatially structured seasonally varying environments. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1578–1603. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.K.; Ma, H.Y.; Wang, Y.H.; Wang, B.Y.; Shen, G.Z. The structure and dynamics of natural population of the endangered plant Euryodendron excelsum H. T. Chang. Acta Ecol. Sin. 2008, 28, 2404–2412. [Google Scholar] [CrossRef]
- Ma, K.; Li, G.Y.; Zhu, L.J.; Yan, C.X.; Xia, G.H. Population structure and distribution patterns of the rare and endangered Ardisia violacea (Myrsinaceae). Acta Ecol. Sin. 2013, 33, 72–79. [Google Scholar]
- Li, X.H.; Liu, J.; Li, D.D.; Zhang, Y.H. Population structure and dynamic characteristics of the endangered plant Rhododendron xiaoxidongense. Chin. J. Plant Ecol. 2025, 49, 1–14. [Google Scholar]
- Wambulwa, M.C.; Milne, R.; Wu, Z.Y. Spatiotemporal maintenance of flora in the Himalaya biodiversity hotspot: Current knowledge and future perspectives. Ecol. Evol. 2021, 11, 10794–10812. [Google Scholar] [CrossRef]
- Hu, S.Q.; Ding, B.Y.; Chen, Z.H. The critical regions for conservation of rare and endangered plant species diversity in Zheiiang Province. Biodiv. Sci. 2002, 01, 15–23. [Google Scholar]
- Chang, B.L.; Yu, S.; Chen, W.; He, X.Y.; Huang, Y.Q.; Zhang, Y. Population structure and dynamic characteristics of Taxus cuspidata in Baishilazi National Nature Reserve, China. Glob. Ecol. Conserv. 2024, 56, e03263. [Google Scholar] [CrossRef]
- He, S.R.; He, A.G.; Chen, Z.L.; Wang, P.; Xu, X.F.; Li, Q.G.; Lu, Y.F.; Jin, X.F. Community structure and dominant population dynamics of spatial distribution pattern of Rhododendron huadingense in Mt. Dapan. Acta Ecol. Sin. 2026. [Google Scholar] [CrossRef]
- Zhang, F.G. Flora of Zhejiang (New Edition); Zhejiang Science and Technology Press: Hangzhou, China, 2021; Volume 4. [Google Scholar]
- Ding, B.Y.; Fang, Y.Y. A new species of Rhododendron from Zhejiang. Bull. Bot. Res. 1990, 10, 31–33. [Google Scholar]
- Zhou, Y.Y.; Sun, L.; Chen, Z.L.; Zhu, W.K.; Yang, W.W.; Jin, X.F. Community characteristics of Rhododendron huadingense, a species endangered and endemic in China. J. Hangzhou Norm. Univ. Sci. Ed. 2012, 11, 211–216. [Google Scholar]
- Liu, P.; Jiao, J.; Wu, C.; Shao, W. Biodiversity patterns and community construction in subtropical forests driven by species phylogenetic environments. Plants 2025, 14, 2397. [Google Scholar] [CrossRef]
- Zhu, H.; Li, D.B.; Yue, C.L.; Li, H.P. Development of single nucleotide polymorphism and phylogenetic analysis of Rhododendron species in Zhejiang Province, China, using ddRAD-Seq technology. Plants 2025, 14, 1548. [Google Scholar] [CrossRef]
- Jin, S.H. Flora of Zhejiang (New Edition); Zhejiang Science and Technology Press: Hangzhou, China, 2021; Volume 1. [Google Scholar]
- Li, D.B.; He, L.P.; Li, X.P. Status quo and protection countermeasures of wild Rhododendron resources in Siming Mountain area. Chin. Wild Plant Res. 2021, 40, 77–81. [Google Scholar]
- Chu, W.K.; Zhou, Y.Y.; Chen, Z.L.; Qian, S.L.; Jin, X.F. On the community classification and species diversity of Rhododendron huadingense. J. Hangzhou Norm. Univ. Sci. Ed. 2013, 12, 240–244. [Google Scholar]
- Duan, Y.H.; Chen, F.; Zhang, H.W.; He, A.G.; Liu, J.L.; Liu, X.; Chen, X.R.; Ye, L.X.; Pang, C.M.; Yu, L.P.; et al. Resource status and priority for the protection of rare and endangered plants in Zhejiang Province, China. Guihaia 2024, 44, 2057–2066. [Google Scholar]
- Cai, X.; Chen, B.; Chen, F.; Chen, W.J.; Chen, Z.L.; Wang, P.; Chen, H.Z.; Jin, X.F. Population structure and interspecific association of Rhododendron huadingense, a rare and endemic species in China. J. Zhejiang Univ. Sci. Ed. 2019, 46, 354–363. [Google Scholar]
- Zeng, H.Y.; Ding, B.Y.; Fang, T. A Study on the community ecology of Rhododendron huadingense population in the Tiantaishan Mountains in Zhejiang Province. J. Zhejiang Univ. Sci. Ed. 2001, 06, 686–691. [Google Scholar]
- Chen, Y.; Yang, J.; Zhang, P.J. Population structure and spatial point pattern of Helianthemum soongoricum in West Ordos, Inner Mongolia, China. J. Desert Res. 2014, 34, 75–82. [Google Scholar]
- Gao, W.Q.; Ni, Y.Y.; Xue, Z.M.; Wang, X.F.; Kang, F.F.; Hu, J.; Gao, Z.H.; Jiang, Z.P.; Liu, J.F. Population structure and regeneration dynamics of Quercus variabilis along latitudinal and longitudinal gradients. Ecosphere 2017, 8, e01737. [Google Scholar] [CrossRef]
- Zhang, J.F.; Ge, S.S.; Liang, J.H.; Li, J.Q. Population age structure and dynamics of Pinus koraiensis in a broadleaved Korean pine forest in Changbai Mountain, China. Chin. J. Plant Ecol. 2022, 46, 667–677. [Google Scholar] [CrossRef]
- Wang, F.; Huo, H.C.; Zhao, Y. Population structure and dynamics of original Abies faxoniana Rehd.—Rhododendron simsii Planch. in high-mountain timberline of southern Gansu Province. Bull. Bot. Res. 2019, 39, 664–672. [Google Scholar]
- Jiang, S.B.; Yuan, C.J.; Yu, D.H. Population structure and dynamics of Rhododendron feddei. Zhejiang For. Sci. Technol. 2020, 40, 1–9. [Google Scholar]
- Li, K.J.; Liu, X.F.; Zhang, J.H.; Zhou, X.L.; Yang, L.; Shen, S.K. Complexity responses of Rhododendron species to climate change in China reveal their urgent need for protection. For. Ecosyst. 2023, 10, 100124. [Google Scholar] [CrossRef]
- Xu, Y.; Zang, R.G. Theoretical and practical research on conservation of wild plants with extremely small populations in China. Biodiv. Sci. 2022, 30, 84–105. [Google Scholar] [CrossRef]
- Chen, X.D. A study on the method of quantitative analysis for plant population and community structural dyanmics. Acta Ecol. Sin. 1998, 2, 104–107. [Google Scholar]
- Zhang, Y.; Wang, J.; Wang, X.; Wang, L.; Wang, Y.; Wei, J. Population structures and dynamics of Rhododendron communities with different stages of succession in northwest Guizhou, China. Plants 2024, 13, 946. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Wu, X.; Zhang, G.; Zong, D.; Li, D. Population dynamics and soil nutrient characteristics of the endangered Annamocarya sinensis in Yunnan, China. BMC Plant Biol. 2025, 25, 689. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.Q.; Mu, C.; Luo, K.; Li, Y.B.; Yang, R. Studies on community structure and population dynamics of Rhododendron xishuiense in Guizhou. J. Trop. Subtrop. Bot. 2023, 31, 766–778. [Google Scholar]
- Jiang, Z.M.; He, Z.S.; Shu, H.; Zhao, H.; Cai, J. Population structure and dynamic characteristics of endangered Syringa pinnatifolia Hemsl. Acta Ecol. Sin. 2018, 38, 2471–2480. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, Y.; Yin, H.; Qin, L.; Li, B.Y. Quantitative characteristics and dynamic analysis of the endangered species Rhododendron chrysanthum population in Changbai Mountain. Chin. J. Ecol. 2017, 36, 3123–3130. [Google Scholar]
- Jia, Y.Y.; Liu, H.Q.; Xie, X.R.; Wang, F.; Zhang, W.; Yang, Y.F. Age structure and population dynamics of rare and endangered Fraxinus sogdiana, China. Chin. J. Plant Ecol. 2015, 49, 760–772. [Google Scholar]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Liu, J.; Wei, M.; Lu, J.; Liu, S.; Li, X.; Wang, X. Ammopiptanthus nanus population dynamics: Bridging the gap between genetic variation and ecological distribution patterns. Biology 2025, 14, 105. [Google Scholar] [CrossRef]
- Chen, Z.H. Studies on Population Characteristics and Conservation Genetics of Rhododendron huadingense, a Rare Species Endemic to China. Master’s Thesis of Science, Hangzhou Normal University, Hangzhou, China, 2016. [Google Scholar]
- Huang, J.H.; Zang, R.G. Status and perspectives of plant diversity conservation in China. Terr. Ecosyst. Conserv. 2021, 1, 66–74. [Google Scholar]
- Volis, S. Securing a future for China’s plant biodiversity through an integrated conservation approach. Plant Divers. 2018, 40, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Condit, R. Research in large, long-term tropical forest plots. Trends Eco. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Fei, S.M.; Jiang, J.M. Influences of substituting size variables for age on population survival analysis: A case study of Pinus tabulaeformis and P. armandii in Qingling Mountain China. J. Plant Ecol. 2008, 2, 448–455. [Google Scholar]
- Ding, B.Y.; Chen, D.L.; Luo, Z.R.; Chen, X.R.; Hu, R.Y.; Ye, Z.L. Zhejiang Baishanzu Forest Dynamics Plot: Tree Species and Their Distribution Patterns; China Forestry Publishing House: Beijing, China, 2013. [Google Scholar]
- Yang, X.P.; Zhou, L.; Jiang, L.L. Population statistics and survival analysis of Prunus divaricata. J. Arid Land Res. Environ. 2020, 34, 54–160. [Google Scholar]
- Wang, Y.T.; Huang, Z.H.; Wang, J.; Zhang, T.; Cui, G.F. The population structure and dynamic characteristics of Phellodendron amurense in Yanshan Mountains. Acta Ecol. Sin. 2021, 41, 2826–2834. [Google Scholar] [CrossRef]
- Wu, J.X.; Yang, X.B.; Li, D.H. Population structure and dynamics of Schefflera octophylla in Tongguling, Hainan Province. Guihaia 2020, 40, 1101–1110. [Google Scholar]
- Deevey, E.S. Life tables for natural populations of animals. Q. Rev. Biol. 1947, 22, 283–314. [Google Scholar] [CrossRef]
- Feng, S.Y. Survival analysis (III). J. Math. Pract. Theory 1983, 2, 70–76. [Google Scholar]


| Diameter Class | Year | Vn | Vpi | V′pi | Pmax | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | |||||
| Index value | 2012 | −0.53 | −0.7 | −0.41 | 0.24 | 0.44 | 0.4 | 0.86 | 1 | 0.177 | 0.003 | 0.016 |
| 2017 | −0.15 | −0.51 | −0.57 | 0.26 | 0.36 | 0.16 | 0.88 | 0.78 | 0.179 | 0.01 | 0.056 | |
| 2022 | 0.11 | 0.24 | −0.77 | 0.03 | 0.38 | −0.34 | 0.83 | 0.86 | 0.228 | 0.008 | 0.037 | |
| Year | Diameter Class | Ax (Plant) | ax (Plant) | lx (Plant) | Dx (Plant) | Qx | Lx (Plant) | Tx (Plant) | Ex (Plant) | Sx | Kx | lnlx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | I | 25.00 | 130.00 | 1000.00 | 46.15 | 0.05 | 976.92 | 5023.08 | 5.02 | 0.95 | 0.05 | 6.91 |
| II | 39.00 | 124.00 | 953.85 | 61.54 | 0.06 | 923.08 | 4046.15 | 4.24 | 0.94 | 0.07 | 6.86 | |
| III | 118.00 | 116.00 | 892.31 | 76.92 | 0.09 | 853.85 | 3123.08 | 3.50 | 0.91 | 0.09 | 6.79 | |
| IV | 201.00 | 106.00 | 815.38 | 76.92 | 0.09 | 776.92 | 2269.23 | 2.78 | 0.91 | 0.10 | 6.70 | |
| V | 153.00 | 96.00 | 738.46 | 61.54 | 0.08 | 707.69 | 1492.31 | 2.02 | 0.92 | 0.09 | 6.60 | |
| VI | 85.00 | 88.00 | 676.92 | 284.62 | 0.42 | 534.62 | 784.62 | 1.16 | 0.58 | 0.55 | 6.52 | |
| VII | 51.00 | 51.00 | 392.31 | 338.46 | 0.86 | 223.08 | 250.00 | 0.64 | 0.14 | 1.99 | 5.97 | |
| VIII | 7.00 | 7.00 | 53.85 | 53.85 | 1.00 | 26.92 | 26.92 | 0.50 | 0.00 | - | 3.99 | |
| IX | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.00 | 0.00 | - | - | - | - | |
| 2017 | I | 35.00 | 122.00 | 1000.00 | 49.18 | 0.05 | 975.41 | 5057.38 | 5.06 | 0.95 | 0.05 | 6.91 |
| II | 41.00 | 116.00 | 950.82 | 65.57 | 0.07 | 918.03 | 4081.97 | 4.29 | 0.93 | 0.07 | 6.86 | |
| III | 83.00 | 108.00 | 885.25 | 81.97 | 0.09 | 844.26 | 3163.93 | 3.57 | 0.91 | 0.10 | 6.79 | |
| IV | 195.00 | 98.00 | 803.28 | 81.97 | 0.10 | 762.30 | 2319.67 | 2.89 | 0.90 | 0.11 | 6.69 | |
| V | 144.00 | 88.00 | 721.31 | 65.57 | 0.09 | 688.52 | 1557.38 | 2.16 | 0.91 | 0.10 | 6.58 | |
| VI | 92.00 | 80.00 | 655.74 | 204.92 | 0.31 | 553.28 | 868.85 | 1.33 | 0.69 | 0.37 | 6.49 | |
| VII | 77.00 | 55.00 | 450.82 | 377.05 | 0.84 | 262.30 | 315.57 | 0.70 | 0.16 | 1.81 | 6.11 | |
| VIII | 9.00 | 9.00 | 73.77 | 57.38 | 0.78 | 45.08 | 53.28 | 0.72 | 0.22 | 1.50 | 4.30 | |
| IX | 2.00 | 2.00 | 16.39 | 16.39 | 1.00 | 8.20 | 8.20 | 0.50 | 0.00 | - | 2.80 | |
| 2022 | I | 46.00 | 109.00 | 1000.00 | 55.05 | 0.06 | 972.48 | 5050.46 | 5.05 | 0.94 | 0.06 | 6.91 |
| II | 41.00 | 103.00 | 944.95 | 82.57 | 0.09 | 903.67 | 4077.98 | 4.32 | 0.91 | 0.09 | 6.85 | |
| III | 31.00 | 94.00 | 862.39 | 100.92 | 0.12 | 811.93 | 3174.31 | 3.68 | 0.88 | 0.12 | 6.76 | |
| IV | 132.00 | 83.00 | 761.47 | 100.92 | 0.13 | 711.01 | 2362.39 | 3.10 | 0.87 | 0.14 | 6.64 | |
| V | 128.00 | 72.00 | 660.55 | 82.57 | 0.13 | 619.27 | 1651.38 | 2.50 | 0.88 | 0.13 | 6.49 | |
| VI | 80.00 | 63.00 | 577.98 | 55.05 | 0.10 | 550.46 | 1032.11 | 1.79 | 0.90 | 0.10 | 6.36 | |
| VII | 122.00 | 57.00 | 522.94 | 330.28 | 0.63 | 357.80 | 481.65 | 0.92 | 0.37 | 1.00 | 6.26 | |
| VIII | 21.00 | 21.00 | 192.66 | 165.14 | 0.86 | 110.09 | 123.85 | 0.64 | 0.14 | 1.95 | 5.26 | |
| IX | 3.00 | 3.00 | 27.52 | 27.52 | 1.00 | 13.76 | 13.76 | 0.50 | 0.00 | - | 3.32 |
| Year | Fitting Equation | R2 | F | p |
|---|---|---|---|---|
| 2012 | y = 7.630x − 0.155 | 0.351 | 3.241 | 0.122 |
| y = 7.977e − 0.056x | 0.546 | 7.213 | 0.036 | |
| 2017 | y = 8.366x − 0.265 | 0.385 | 4.381 | 0.075 |
| y = 8.895e − 0.088x | 0.61 | 10.971 | 0.013 | |
| 2022 | y = 7.927x − 0.2 | 0.374 | 4.19 | 0.08 |
| y = 8.273e − 0.065x | 0.582 | 9.73 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, K.; He, A.; He, S.; Chen, W.; Chen, Z.; Cai, X.; Wang, P.; Chen, Y.; Lu, Y.; Jin, X. Dynamic Structural Changes in a Population of Rhododendron huadingense, a Rare and Endemic Species in Zhejiang, East China. Plants 2025, 14, 3406. https://doi.org/10.3390/plants14223406
Hao K, He A, He S, Chen W, Chen Z, Cai X, Wang P, Chen Y, Lu Y, Jin X. Dynamic Structural Changes in a Population of Rhododendron huadingense, a Rare and Endemic Species in Zhejiang, East China. Plants. 2025; 14(22):3406. https://doi.org/10.3390/plants14223406
Chicago/Turabian StyleHao, Ke, Anguo He, Shuran He, Weijie Chen, Zilin Chen, Xin Cai, Pan Wang, Yu Chen, Yifei Lu, and Xiaofeng Jin. 2025. "Dynamic Structural Changes in a Population of Rhododendron huadingense, a Rare and Endemic Species in Zhejiang, East China" Plants 14, no. 22: 3406. https://doi.org/10.3390/plants14223406
APA StyleHao, K., He, A., He, S., Chen, W., Chen, Z., Cai, X., Wang, P., Chen, Y., Lu, Y., & Jin, X. (2025). Dynamic Structural Changes in a Population of Rhododendron huadingense, a Rare and Endemic Species in Zhejiang, East China. Plants, 14(22), 3406. https://doi.org/10.3390/plants14223406





