Drought-Driven Rhizosphere Microbiome and Metabolome Remodeling in Wild vs. Cultivated Saccharum arundinaceum
Abstract
1. Introduction
2. Results
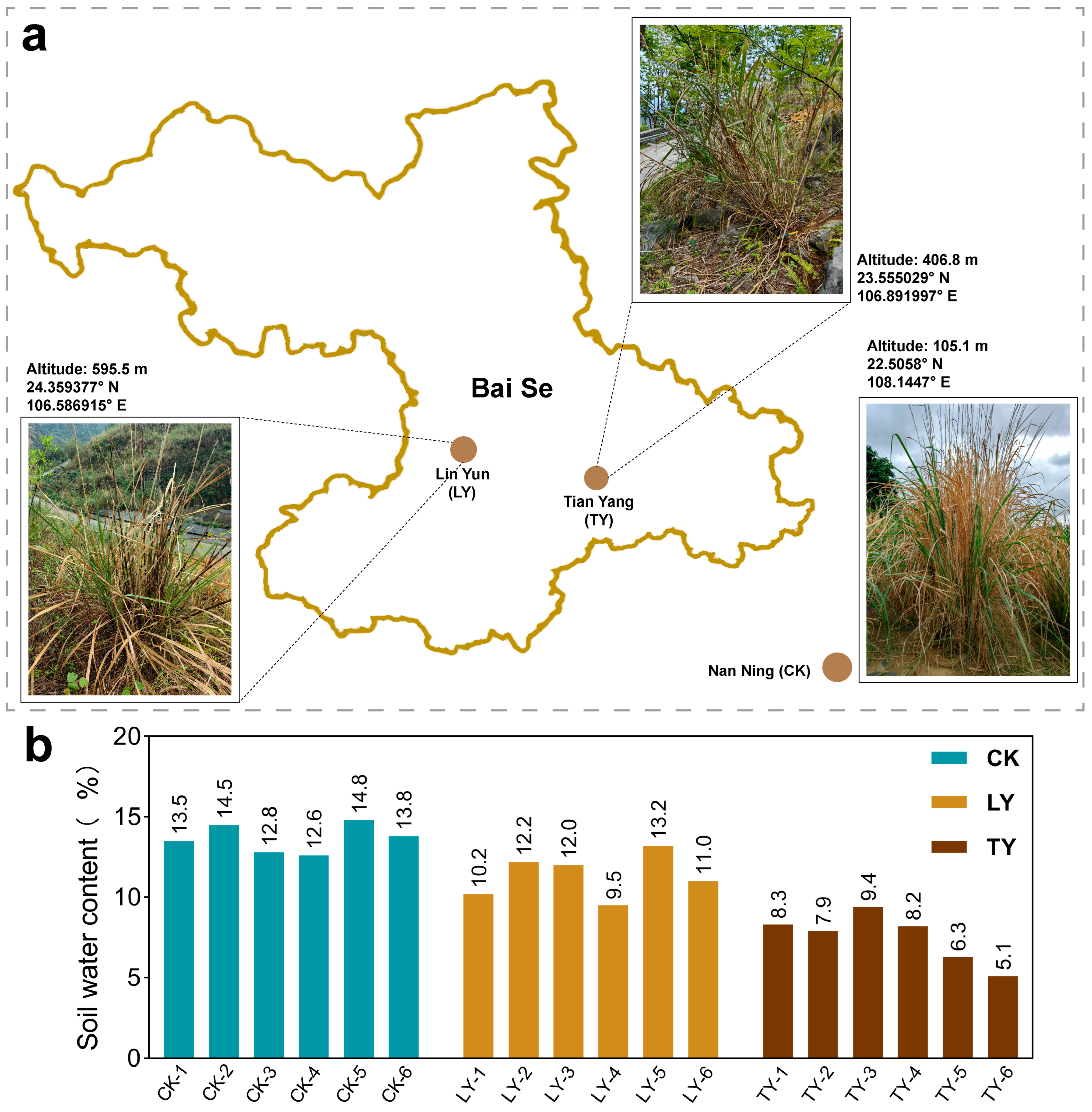
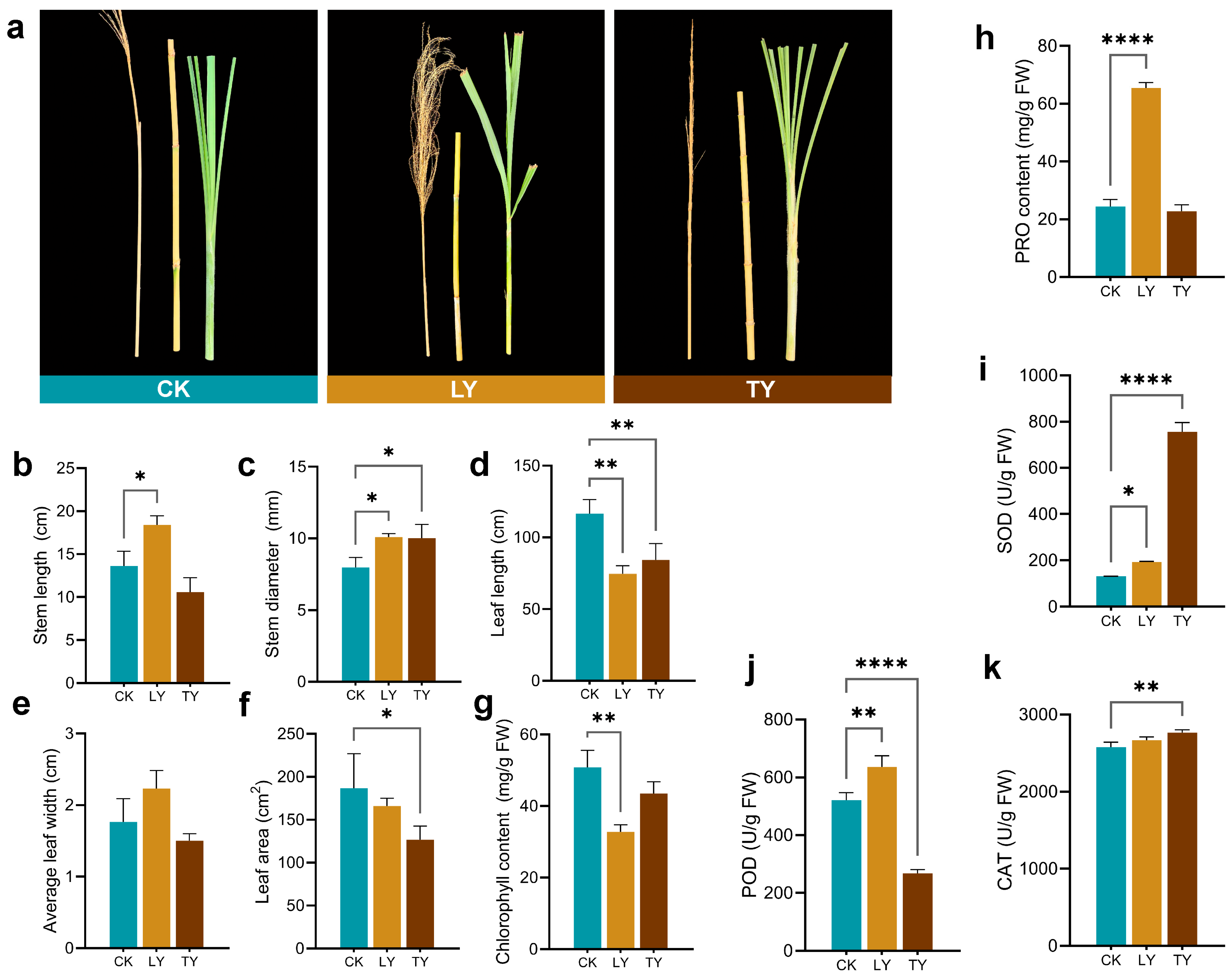
2.1. Environmental Variation, Morphological and Biochemical Responses in S. arundinaceum
2.2. Soil Physicochemical Properties
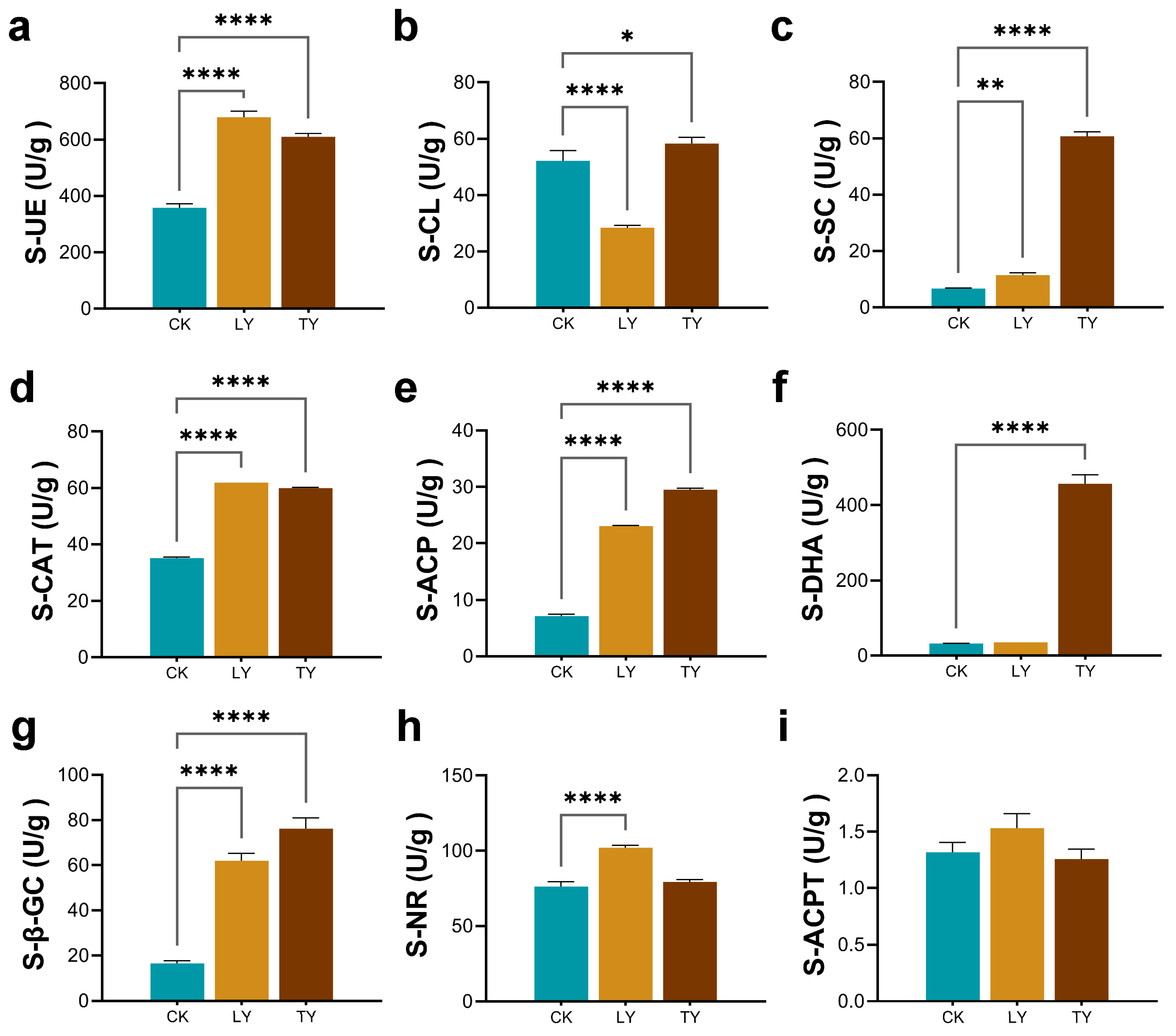
2.3. Soil Enzyme Activity
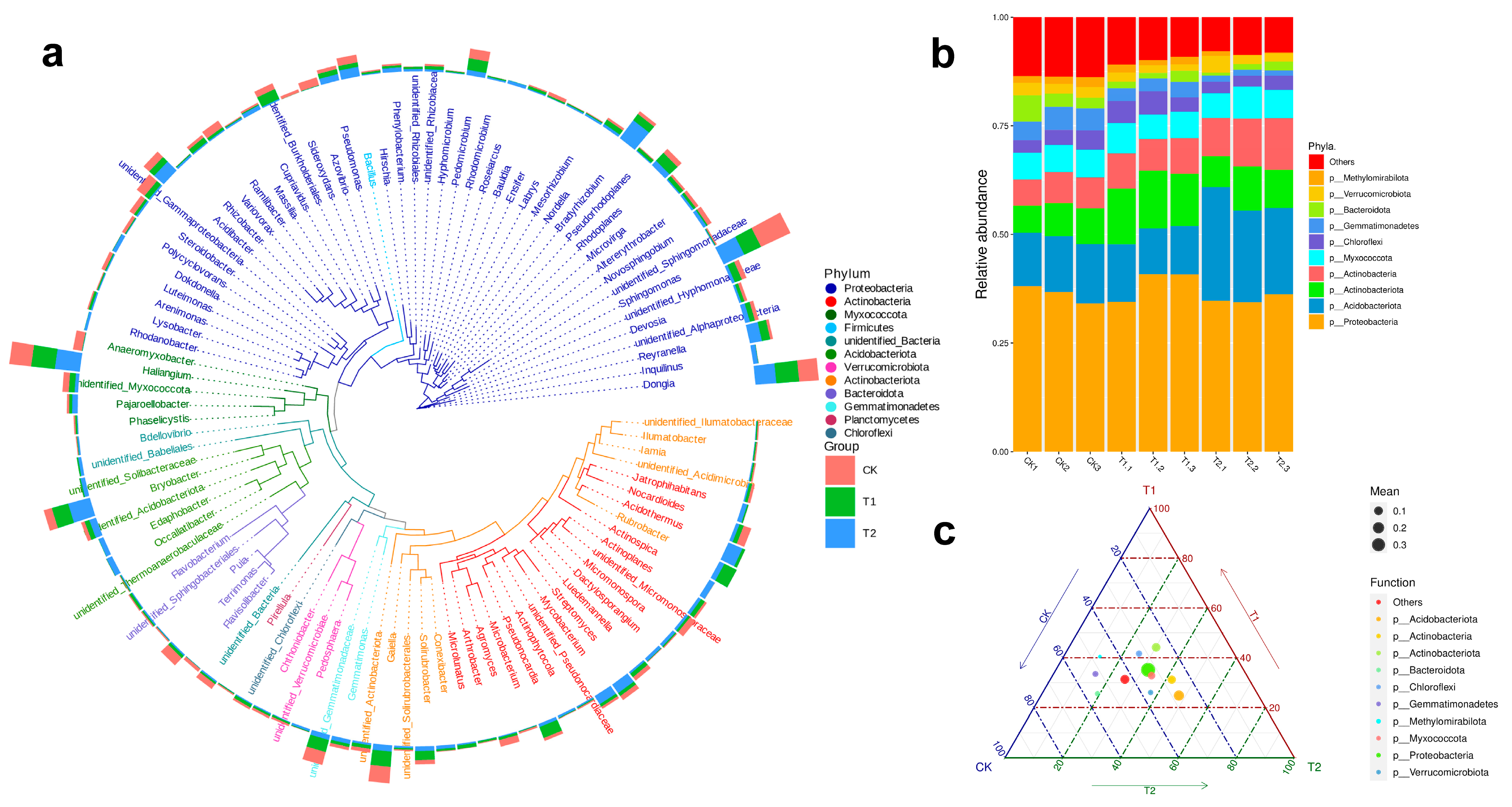
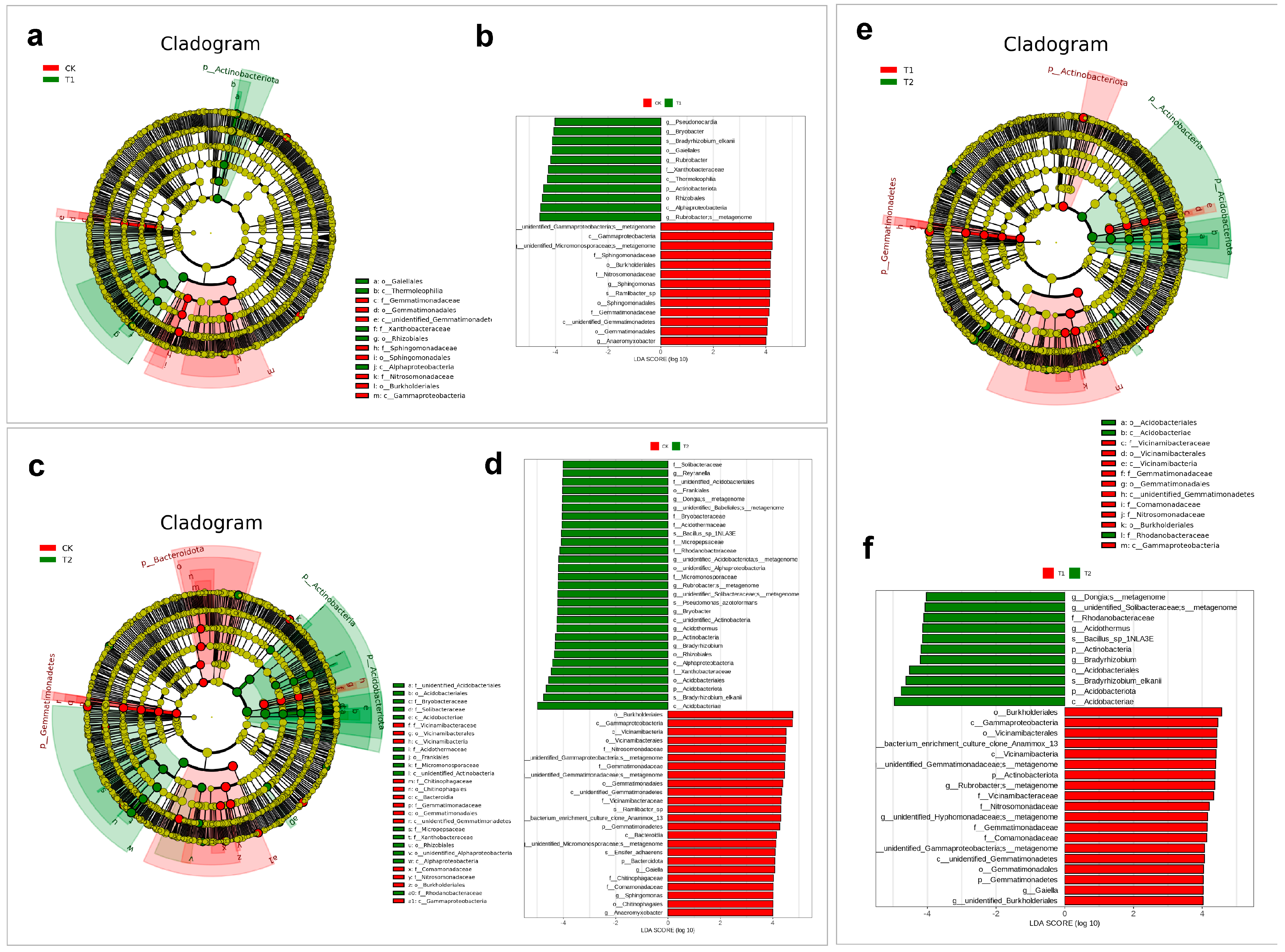
2.4. Soil Microbiota Composition Analysis of S. arundinaceum Rhizospheric Soil in Different Regions
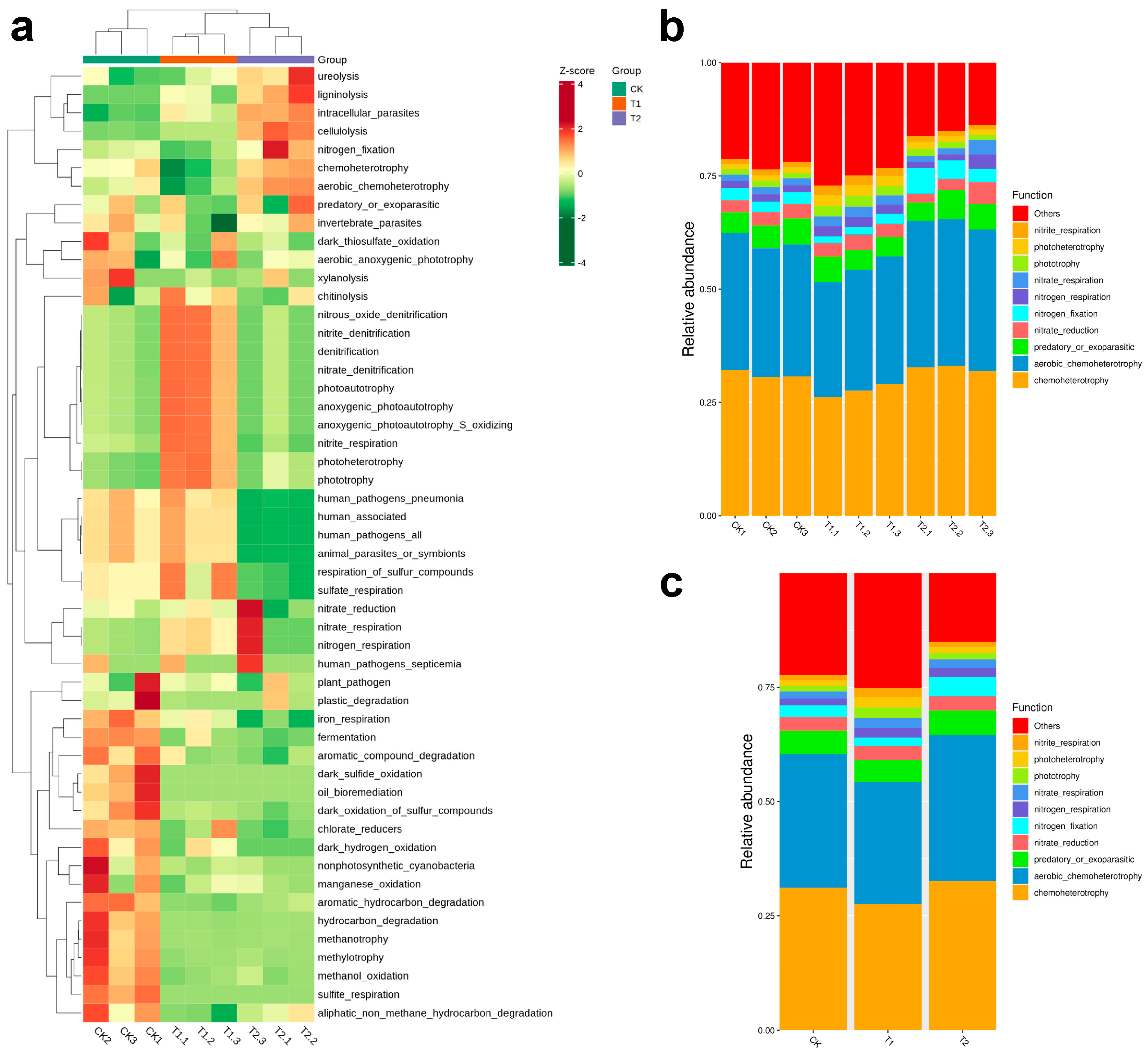
2.5. Shifts in Microbial Potential Under Drought and Cultivation
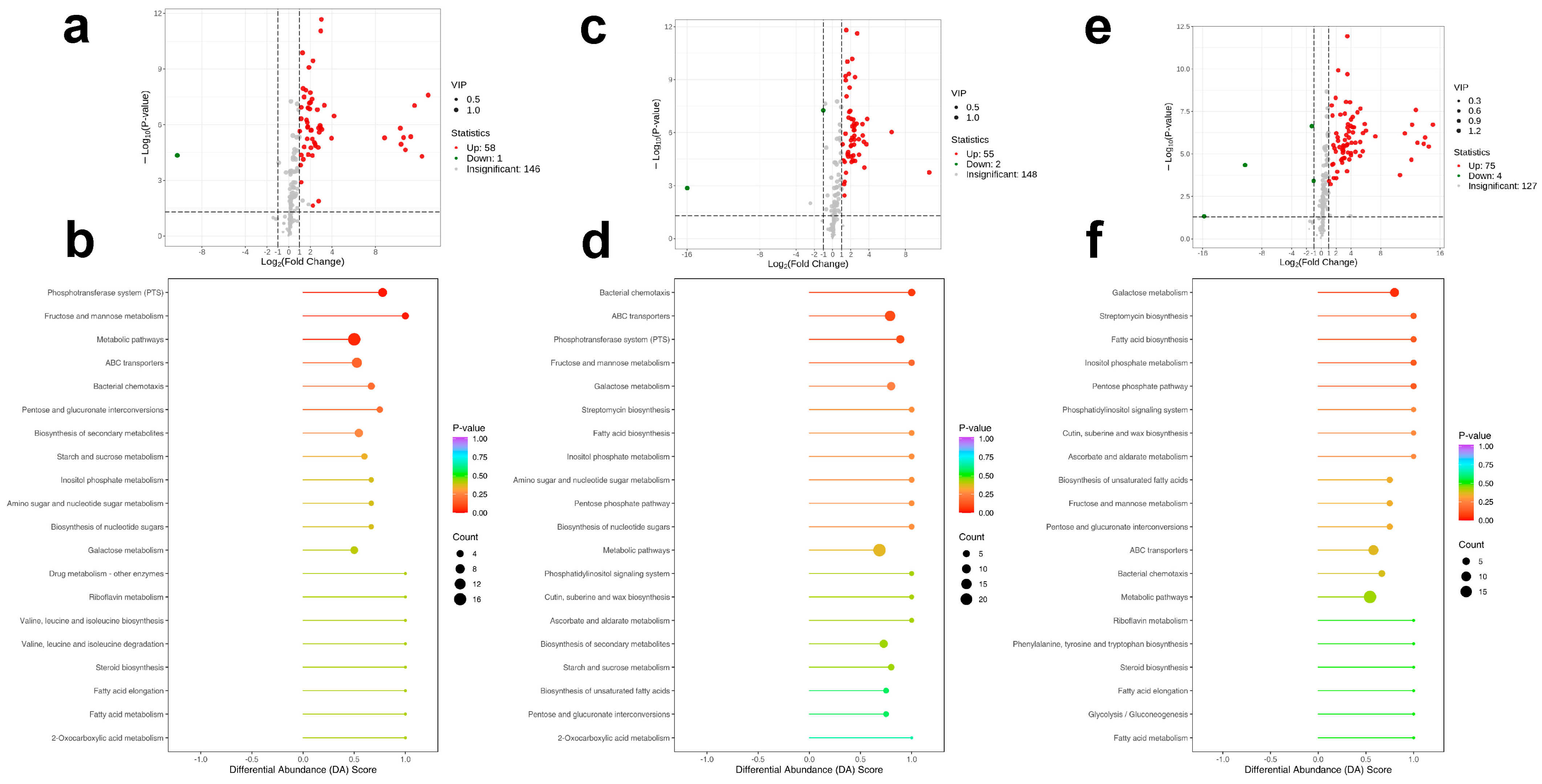
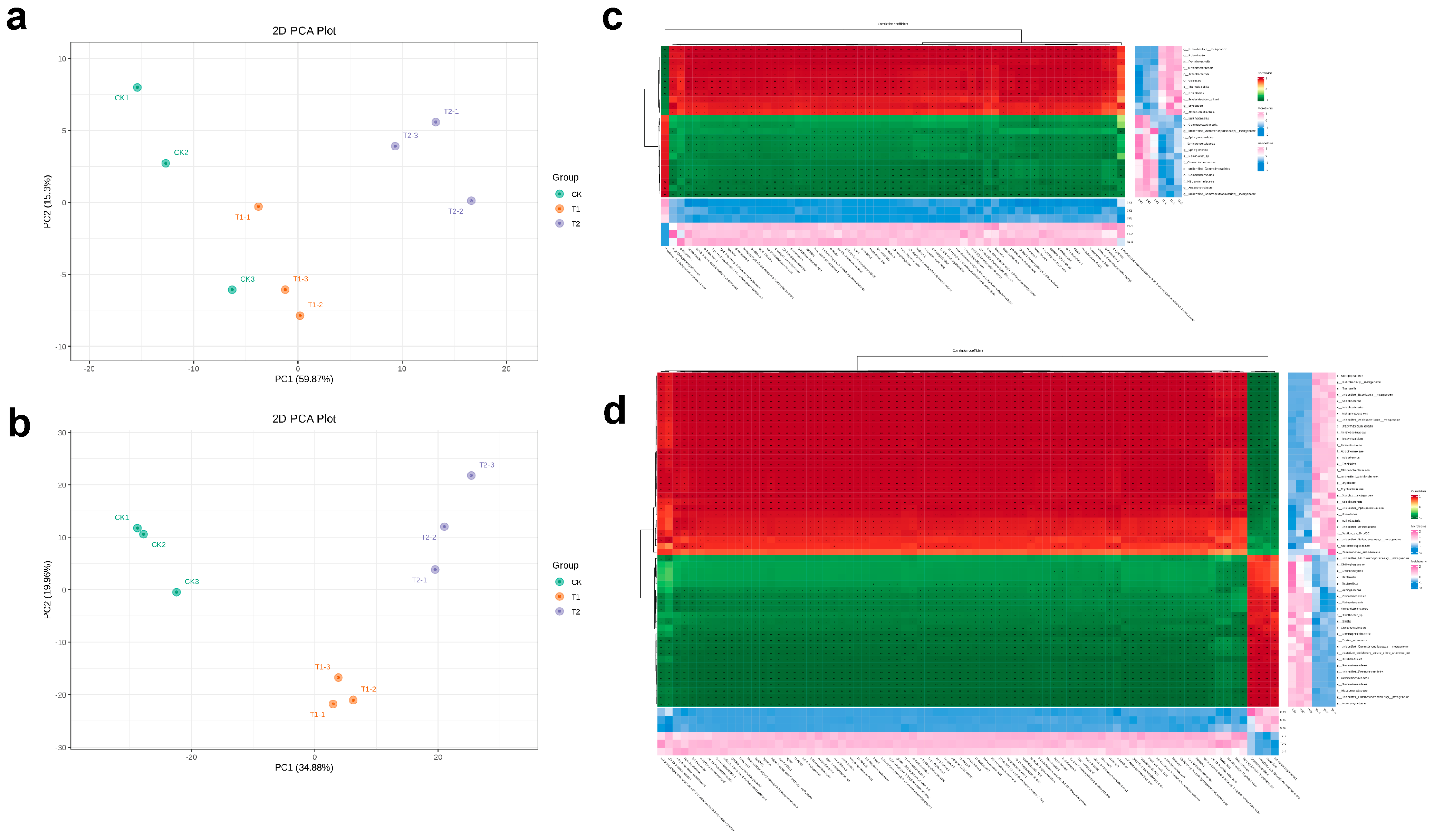
2.6. Non-Targeted Metabolomic Analysis of Rhizospheric Soil
2.7. Correlations Among Soil Metabolites and Soil Microbial Communities
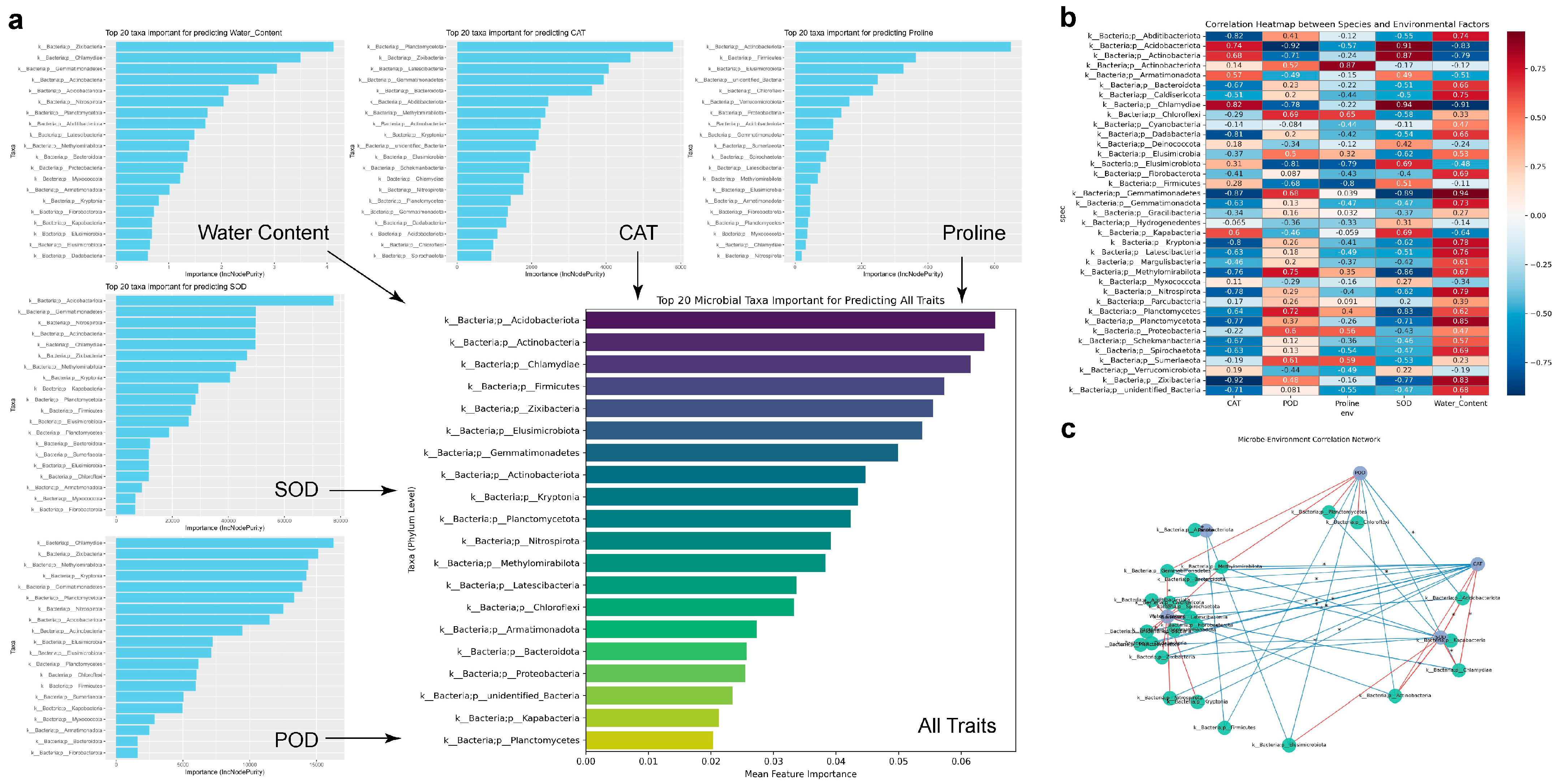
2.8. Correlation Analysis Between Drought Resistance-Related Indexes of S. arundinaceum and Differential Microbial Abundance in Rhizosphere Soil
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Characterization
4.2. Measurement of Plant Phenotypic Traits, Physiological Parameters, and Antioxidant Enzyme Activities
4.3. Soil Physicochemical Properties and Enzyme Activity Assays
4.4. Microbial Community Analysis
4.5. Soil Untargeted Metabolomics Analysis
4.6. Statistical Analysis
5. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinde, N.A.; Kawar, P.G.; Dalvi, S.G. Chitosan-based nanoconjugates: A promising solution for enhancing crops drought-stress resilience and sustainable yield in the face of climate change. Plant Nano Biol. 2024, 7, 100059. [Google Scholar] [CrossRef]
- Wang, L.; Ren, W. Drought in agriculture and climate-smart mitigation strategies. Cell Rep. Sustain. 2025, 2, 100386. [Google Scholar] [CrossRef]
- De Silva, S.; Kariyawasam Hetti Gamage, L.; Thapa, V.R. Impact of drought on soil microbial communities. Microorganisms 2025, 13. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Sharma, A.; Das, N.; Pandey, P.; Shukla, P. Plant-microbiome responses under drought stress and their metabolite-mediated interactions towards enhanced crop resilience. Curr. Plant Biol. 2025, 43, 100513. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Meddich, A.; Baslam, M. Plant-microbiome interactions under drought—Insights from the molecular machinist’s toolbox. Front. Sustain. Food Syst. 2023, 7. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Beyond correlation: Understanding the causal link between microbiome and plant health. Heliyon 2024, 10, e40517. [Google Scholar] [CrossRef]
- Taglialegna, A. Microbial allies in plant defence against drought. Nat. Rev. Microbiol. 2025, 23, 471. [Google Scholar] [CrossRef]
- Dinesh Babu, K.S.; Janakiraman, V.; Palaniswamy, H.; Kasirajan, L.; Gomathi, R.; Ramkumar, T.R. A short review on sugarcane: Its domestication, molecular manipulations and future perspectives. Genet. Resour. Crop Evol. 2022, 69, 2623–2643. [Google Scholar] [CrossRef]
- Lu, G.; Liu, P.; Wu, Q.; Zhang, S.; Zhao, P.; Zhang, Y.; Que, Y. Sugarcane breeding: A fantastic past and promising future driven by technology and methods. Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef]
- Li, H.B.; Singh, R.K.; Singh, P.; Song, Q.Q.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Genetic diversity of nitrogen-fixing and plant growth promoting Pseudomonas species isolated from sugarcane rhizosphere. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pablo, C.H.D.; Mavrodi, O.V.; Weller, D.M.; Thomashow, L.S.; Mavrodi, D.V. Rhizosphere plant-microbe interactions under water stress. Adv. Appl. Microbiol. 2021, 115, 65–113. [Google Scholar] [CrossRef]
- Yusuf, A.; Li, M.; Zhang, S.; Luo, R.; Wu, Y.; Zhang, T.; Yunusa Ugya, A.; Zhang, Y.; Duan, S. Harnessing plant–microbe interactions: Strategies for enhancing resilience and nutrient acquisition for sustainable agriculture. Front. Plant Sci. 2025, 16. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin producing, plant growth promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Caddell, D.F.; Pettinga, D.; Louie, K.; Bowen, B.P.; Sievert, J.A.; Hollingsworth, J.; Rubanowitz, R.; Dahlberg, J.; Purdom, E.; Northen, T.; et al. Drought shifts sorghum root metabolite and microbiome profiles and enriches for pipecolic acid. Phytobiomes J. 2023, 7, 449–463. [Google Scholar] [CrossRef]
- Xie, J.; Dawwam, G.E.; Sehim, A.E.; Li, X.; Wu, J.; Chen, S.; Zhang, D. Drought stress triggers shifts in the root microbial community and alters functional categories in the microbial gene pool. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Yue, H.; Yue, W.; Jiao, S.; Kim, H.; Lee, Y.H.; Wei, G.; Song, W.; Shu, D. Plant domestication shapes rhizosphere microbiome assembly and metabolic functions. Microbiome 2023, 11, 70. [Google Scholar] [CrossRef]
- Shi, S.; Chang, J.; Tian, L.; Nasir, F.; Ji, L.; Li, X.; Tian, C. Comparative analysis of the rhizomicrobiome of the wild versus cultivated crop: Insights from rice and soybean. Arch Microbiol. 2019, 201, 879–888. [Google Scholar] [CrossRef]
- Barnes, C.J.; Bahram, M.; Nicolaisen, M.; Gilbert, M.T.P.; Vestergård, M. Microbiome selection and evolution within wild and domesticated plants. Trends Microbiol. 2025, 33, 447–458. [Google Scholar] [CrossRef]
- Li, Y.R. In Sugarcane Germplasm Resources and Utilization; Liu, X.H., Zhang, G.M., Eds.; Modern Sugarcane Science. Agriculture Press: Beijing, China, 2010; pp. 103–115. [Google Scholar]
- Xiao, S.; Wu, Y.; Xu, S.; Jiang, H.; Hu, Q.; Yao, W.; Zhang, M. Field evaluation of TaDREB2B-ectopic expression sugarcane (Saccharum spp. hybrid) for drought tolerance. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, Y.; Yin, W.; Yang, Z.; Zhang, Y.; Lou, Y.; Pan, H.; Yang, Q.; Hu, G.; Zhuge, Y.; et al. Dose-dependent effects of Paecilomyces variotii extract on drought resistance in pear trees: Plant growth, soil enzyme activities, and root exudates. Agronomy 2025, 15. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8. [Google Scholar] [CrossRef]
- Ebrahimi-Zarandi, M.; Etesami, H.; Glick, B.R. Fostering plant resilience to drought with Actinobacteria: Unveiling perennial allies in drought stress tolerance. Plant Stress 2023, 10, 100242. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, L.; Qi, S.; Fang, J.; Li, P.; Xu, M.; Yan, J. Integrated analysis of 16 S endophytic bacteria and non-targeted metabolomics reveals the drought response mechanisms of Portulaca oleracea L. BMC Plant Biol. 2025, 25. [Google Scholar] [CrossRef]
- Aitken, K.; Li, J.; Piperidis, G.; Qing, C.; Yuanhong, F.; Jackson, P. Worldwide genetic diversity of the wild species Saccharum spontaneum and level of diversity captured within sugarcane breeding programs. Crop Sci. 2017, 58, 218–229. [Google Scholar] [CrossRef]
- Hernández-Álvarez, C.; García-Oliva, F.; Cruz-Ortega, R.; Romero, M.F.; Barajas, H.R.; Pinero, D.; Alcaraz, L.D. Squash root microbiome transplants and metagenomic inspection for in situ arid adaptations. Sci. Total Environ. 2022, 805, 150136. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, J.; Yu, B.; Liu, S.; Cao, X. Metagenomic and metabolomic perspectives on the drought tolerance of broomcorn millet (Panicum miliaceum L.). Microorganisms 2025, 13. [Google Scholar] [CrossRef]
- Ghorbanzadeh, Z.; Hamid, R.; Jacob, F.; Zeinalabedini, M.; Salekdeh, G.H.; Ghaffari, M.R. Comparative metabolomics of root-tips reveals distinct metabolic pathways conferring drought tolerance in contrasting genotypes of rice. BMC Genom. 2023, 24. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Singh, R.; Gong, Q.; Wu, Q.; Shi, J. Omics approaches in understanding the benefits of plant-microbe interactions. Front. Microbiol. 2024, 15. [Google Scholar] [CrossRef]
- Abou Jaoudé, R.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Soil microbiome transplantation to enhance the drought response of Salvia officinalis L. Front. Microbiol. 2025, 16. [Google Scholar] [CrossRef]
- Ishida, J.K.; Bini, A.P.; Creste, S.; Sluys, M.A.V. Towards defining the core Saccharum microbiome: Input from five genotypes. BMC Microbiol. 2022, 22. [Google Scholar] [CrossRef]
- Xing, Y.; Dao, J.; Chen, M.; Chen, C.; Li, B.; Wang, Z. Multi-omics reveals the sugarcane rhizosphere soil metabolism-microbiota interactions affected by drought stress. Appl. Soil Ecol. 2023, 190, 104994. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Park, S.; Mir, R.A.; Mushtaq, M.; Bhat, B.; Mahmoudi, H.; Bae, H. Deciphering the plant microbiome to improve drought tolerance: Mechanisms and perspectives. Environ. Exp. Bot. 2022, 201, 104933. [Google Scholar] [CrossRef]
- Uribe-Acosta, M.; Pascale, A.; Zhou, J.; Stringlis, I.A.; Pieterse, C.M. Roots: Metabolic architects of beneficial microbiome assembly. Plant Physiol. 2025, 199, kiaf349. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Lu, X.; Bai, Y.; Wang, G. Two diversities meet in the rhizosphere: Root specialized metabolites and microbiome. J. Genet. Genom. 2024, 51, 467–478. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.L.; Caddell, D.; et al. Ge-nome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat. Commun. 2021, 12, 3209. [Google Scholar] [CrossRef]
- Do, T.T.; Li, J.; Zhang, F.J.; Xing, Y.X.; Yang, L.T.; Li, Y.R.; Nguyen, T.H. Changes of antioxidant enzyme activities and contents of osmotic regulation substances in leaves of different sugarcane varieties under drought stress. Chin. J. Trop. Crops 2018, 39, 858–866. [Google Scholar]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and CAT enzyme activities in Rumex obtusifolius L. Ann. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar]
- Castrillo, G.; Teixeira, P.J.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Miec-zkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef]
- Xu, X.; Yu, Z.; Cheng, F.; He, L.; Cao, X.; Song, X. Molecular diversity and ecological characteristics of the eukaryotic phyto-plankton community in the coastal waters of the bohai sea, china. Harmful Algae 2017, 61, 13–22. [Google Scholar] [CrossRef]
- Ma, S.; Chen, X.; Su, H.; Xing, A.; Chen, G.; Zhu, J.; Zhu, B.; Fang, J. Phosphorus addition decreases soil fungal richness and alters fungal guilds in two tropical forests. Soil Biol. Biochem. 2022, 175, 108836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, H.; Wei, J.; Zhou, H.; Tang, Y.; Gui, Y.; Zhu, K. Drought-Driven Rhizosphere Microbiome and Metabolome Remodeling in Wild vs. Cultivated Saccharum arundinaceum. Plants 2025, 14, 3407. https://doi.org/10.3390/plants14223407
Huang S, Li H, Wei J, Zhou H, Tang Y, Gui Y, Zhu K. Drought-Driven Rhizosphere Microbiome and Metabolome Remodeling in Wild vs. Cultivated Saccharum arundinaceum. Plants. 2025; 14(22):3407. https://doi.org/10.3390/plants14223407
Chicago/Turabian StyleHuang, Sijie, Haibi Li, Jinju Wei, Hui Zhou, Yanhang Tang, Yiyun Gui, and Kai Zhu. 2025. "Drought-Driven Rhizosphere Microbiome and Metabolome Remodeling in Wild vs. Cultivated Saccharum arundinaceum" Plants 14, no. 22: 3407. https://doi.org/10.3390/plants14223407
APA StyleHuang, S., Li, H., Wei, J., Zhou, H., Tang, Y., Gui, Y., & Zhu, K. (2025). Drought-Driven Rhizosphere Microbiome and Metabolome Remodeling in Wild vs. Cultivated Saccharum arundinaceum. Plants, 14(22), 3407. https://doi.org/10.3390/plants14223407






