Defense and Adaptive Strategies of Crithmum maritimum L. Against Insect Herbivory: Evidence of Phenotypic Plasticity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material Sampling
2.2. Morphological Trait Measurements
2.3. Insect Attack Assessment
2.4. Sample Preparation for Biochemical and Mineral Analysis
2.5. Determination of Phenolic Content and In Vitro Antioxidant Activities
2.5.1. Total Polyphenol Content (TPC) and Total Condensed Tannins (TCT)
2.5.2. DPPH and ABTS Radical Scavenging Activities
2.6. Mineral Composition
2.7. Lipophilic Fraction Extraction and GC–MS Analysis
2.8. Statistical Analysis
3. Results
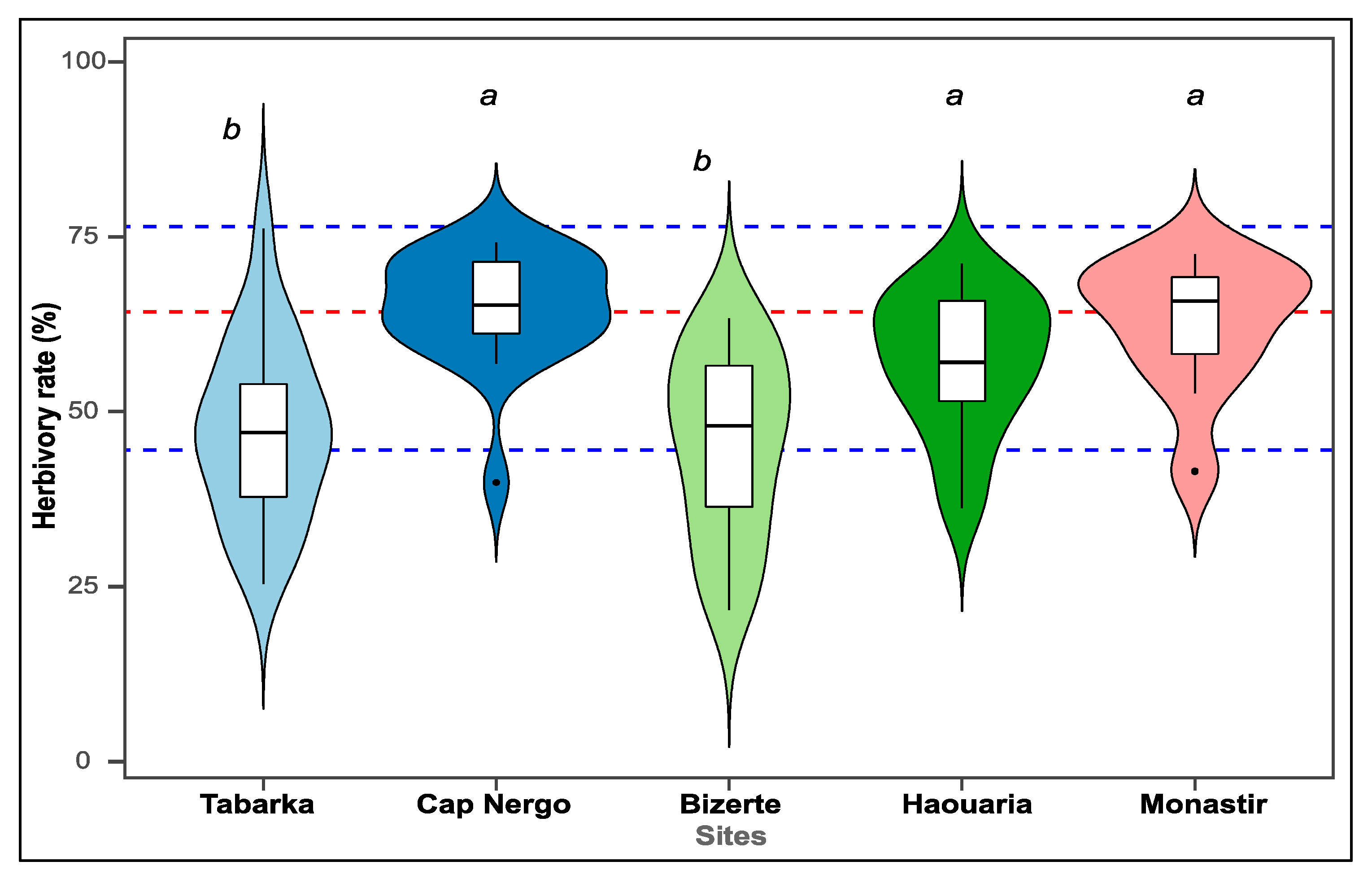
3.1. Herbivory Pressure Varies Significantly Across Sites
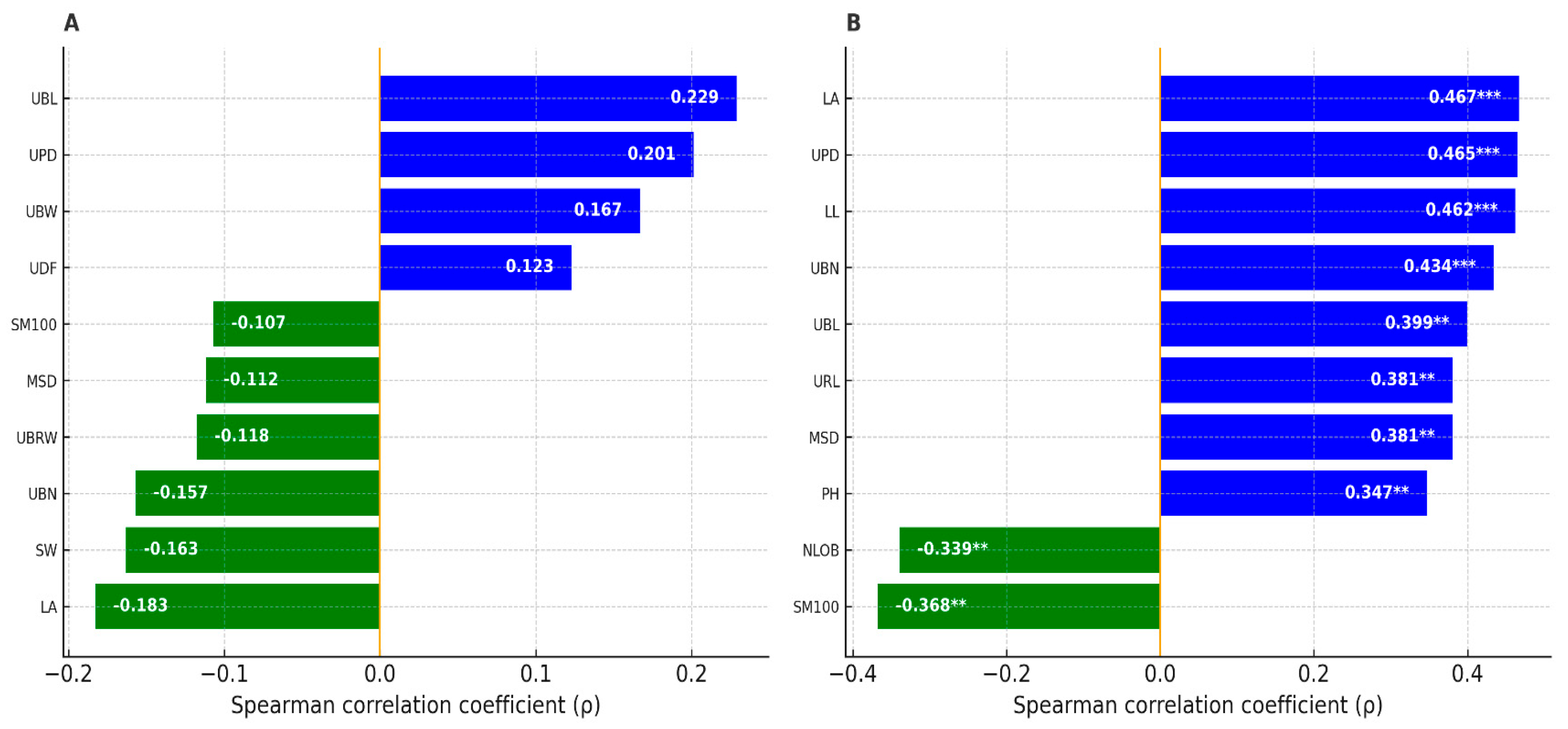
3.2. Effect of Herbivory on Morphological Traits
3.3. Effect of Herbivory on Biochemical Traits
3.4. Effect of Herbivory on Mineral Composition
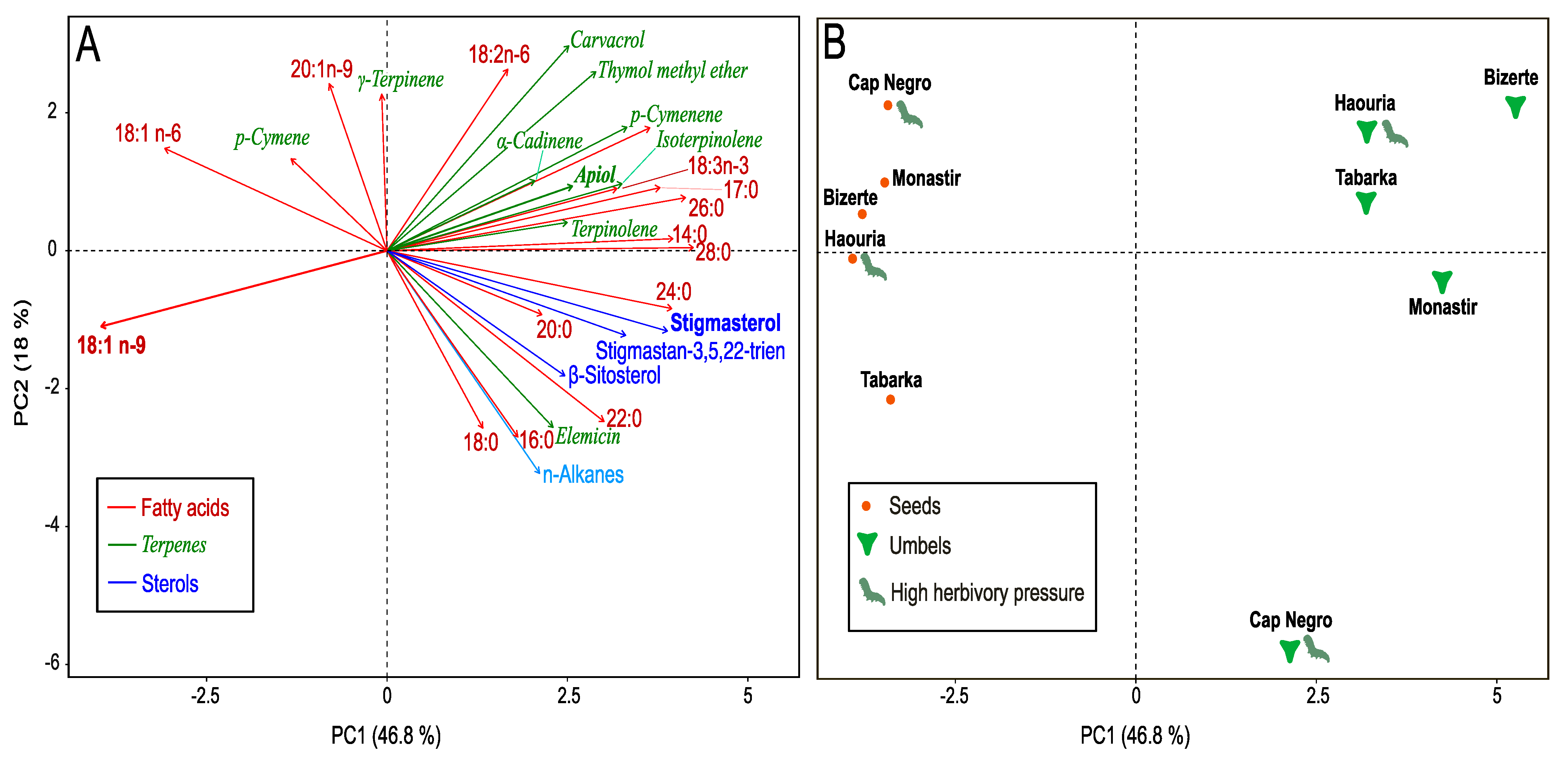
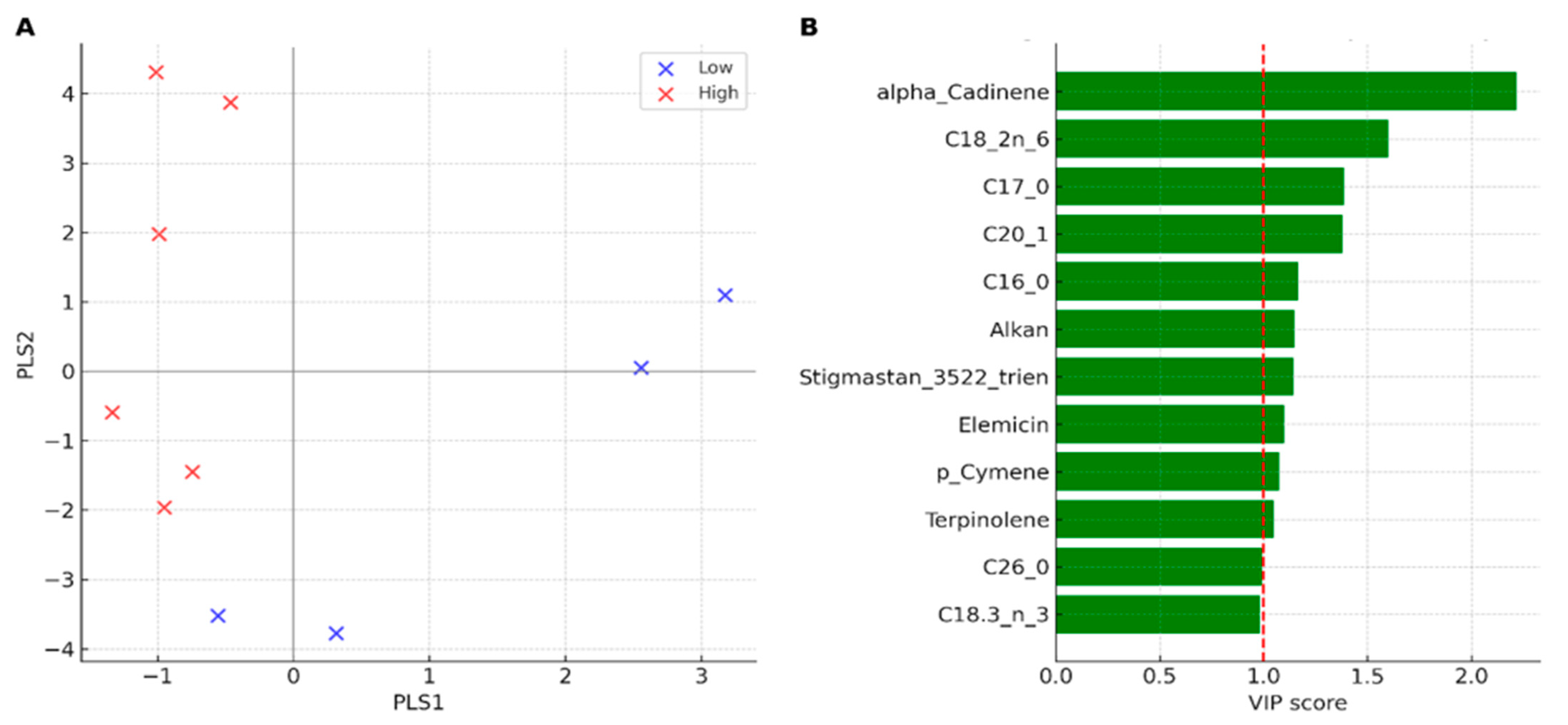
3.5. Effect of Herbivory on Lipophilic Fraction (GC–MS)
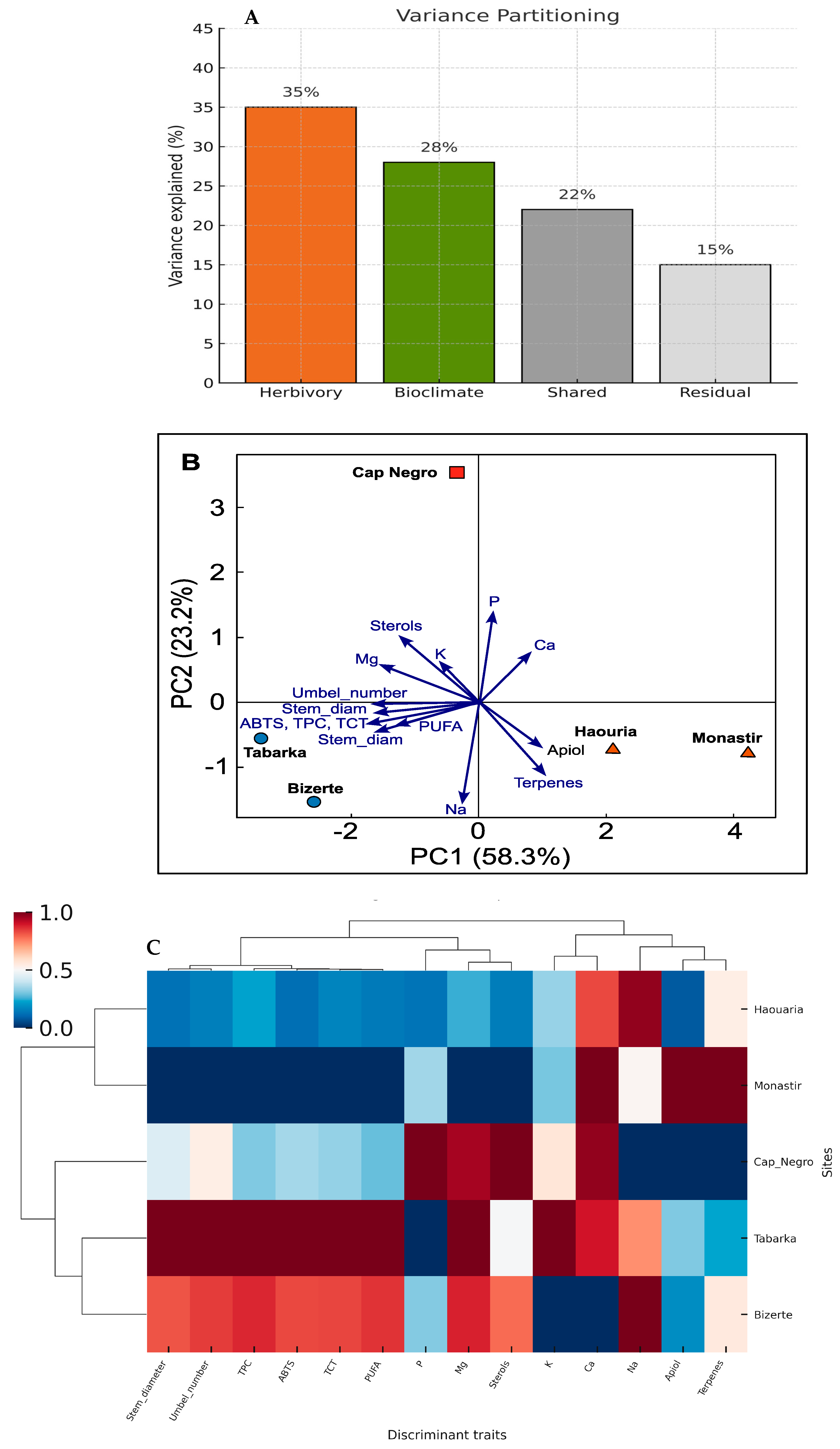
3.6. Integrative Analysis of Herbivory-Related Traits and Bioclimatic Context
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10397. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Đurić, M.; Jevremović, S.; Trifunović-Momčilov, M.; Milošević, S.; Subotić, A.; Jerinić-Prodanović, D. Physiological and oxidative stress response of carrot (Daucus carota L.) to jumping plant-louse Bactericera trigonica Hodkinson infestation. BMC Plant Biol. 2024, 24, 243. [Google Scholar] [CrossRef]
- Pastierovič, F.; Kalyniukova, A.; Hradecký, J.; Dvořák, O.; Vítámvás, J.; Mogilicherla, K.; Tomášková, I. Biochemical responses in Populus tremula: Defending against sucking and leaf-chewing insect herbivores. Plants 2024, 13, 1243. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Changes in phenolic compounds and antioxidant activity in horse chestnut leaves related to leaf miner infestation. Molecules 2014, 19, 14633–14649. [Google Scholar]
- Hanaka, A.; Dresler, S.; Mułenko, W.; Wójciak, M.; Sowa, I.; Sawic, M.; Stanisławek, K.; Strzemski, M. Phenolic-based discrimination between non-symptomatic and symptomatic leaves of Aesculus hippocastanum infested by Cameraria ohridella and Erysiphe flexuosa. Int. J. Mol. Sci. 2023, 24, 14071. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef]
- Peng, C.; Brewer, G.J. Economic injury levels for the red sunflower seed weevil (Smicronyx fulvus) infesting oilseed sunflower. Can. Entomol. 1995, 127, 561–568. [Google Scholar] [CrossRef]
- Traw, M.B.; Dawson, T.E. Differential induction of trichomes by three herbivores of black mustard. Oecologia 2002, 131, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.R.; Waterhouse, G.I.N.; Smith, G.S.; Rogers, G. Silicon and sulfur alleviate herbivory via changes to leaf surface morphology. Plants 2020, 9, 643. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent sources of health-promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Kraouia, A.; Selmi, S.; Allagui, M.S.; Ben Haj Jilani, I.; Boukhchina, S. Variability of essential-oil composition of Crithmum maritimum L. growing wild in Tunisia. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K. Volatile constituents and antioxidant activity of leaves and stems of Greek Crithmum maritimum L. populations. Food Chem. 2016, 196, 104–111. [Google Scholar]
- Bendif, H.; Adouni, K.; Miara, M.D.; Kerrour, M.; Belhamra, M.; Laouer, H.; Boulekbache-Makhlouf, L.; Maggi, F. Essential-oil composition and antioxidant activity of wild Algerian Crithmum maritimum L. (Apiaceae). Nat. Prod. Res. 2018, 32, 1415–1420. [Google Scholar]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic effects of Mediterranean plant extracts on germination of weeds. Allelopath. J. 2009, 23, 479–492. [Google Scholar]
- Egea, T.; Candela, M.E.; Almela, L.; Romojaro, F. Antioxidant enzymes and peroxidase isoenzymes in leaves of Capsicum annuum as affected by insect herbivory. J. Plant Physiol. 2006, 163, 701–709. [Google Scholar]
- Onyilagha, J.C.; Gruber, M.Y.; Hallett, R.H.; Holowachuk, J.; Buckner, A.; Soroka, J.J.; Westcott, N.D. Constitutive and induced resistance to insect herbivory in Arabidopsis thaliana (Brassicaceae). J. Chem. Ecol. 2004, 30, 1937–1950. [Google Scholar]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Stamp, N. Plant interactions with arthropod herbivores: State of the field. Plant Physiol. 2003, 133, 895–903. [Google Scholar]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Schultz, J.C. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 1983, 221, 277–279. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect–Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Crawley, M.J. Plant Ecology, 2nd ed.; Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, R. Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. Am. Nat. 1998, 151, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Simms, E.L.; Triplett, J. Costs and benefits of plant responses to disease: Resistance and tolerance. Evolution 1994, 48, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Stowe, K.A.; Marquis, R.J.; Hochwender, C.G.; Simms, E.L. The evolution of tolerance to herbivory. Annu. Rev. Ecol. Syst. 2000, 31, 565–595. [Google Scholar] [CrossRef]
- Fineblum, W.L.; Rausher, M.D. Trade-off between resistance and tolerance to herbivore damage in a morning glory. Nature 1995, 377, 517–520. [Google Scholar] [CrossRef]
- Tiffin, P.; Inouye, B.D. Measuring tolerance to herbivory: Accuracy and precision of estimates made using two methods. Oecologia 2000, 123, 444–450. [Google Scholar]
- Rosenthal, J.P.; Kotanen, P.M. Terrestrial plant tolerance to herbivory. Trends Ecol. Evol. 1994, 9, 145–148. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Zangerl, A.R. Plant–insect interactions in terrestrial ecosystems. Ecology 2002, 83, 1093–1105. [Google Scholar]
- Ogran, A.; Ori, N.; Halpern, M.; Coll, N.S. Evolution of Phenotypic Plasticity: Genetic Differentiation and Additive Genetic Variation for Induced Plant Defence in Wild Arugula Eruca sativa. J. Evol. Biol. 2020, 33, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Raza, A.; Afzal, I.; Parveen, S. Plant Defense Responses to Biotic Stress and Its Interplay with Fluctuating Dark/Light Conditions. Front. Plant Sci. 2021, 12, 708635. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Schoettle, A.W.; Sparks, J.P. Coordinated Resource Allocation to Plant Growth–Defense Trade-Offs. New Phytol. 2021, 230, 2106–2120. [Google Scholar]
- Ashapkin, V.V.; Nurchatov, I.I.; Vanyushin, B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-Plant Metabolic Allocation under Water Stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Li, C. Phenotypic Plasticity and Integration Synergistically Enhance Plant Adaptability to Flooding and Nitrogen Stresses. Plant Soil 2025, 510, 129–147. [Google Scholar] [CrossRef]
- López-Goldar, X.; Zas, R.; Sampedro, L. Resource Availability Drives Microevolutionary Patterns of Plant Defences. Funct. Ecol. 2020, 34, 1452–1466. [Google Scholar] [CrossRef]
- Dar, F.A.; Mushtaq, N.U.; Saleem, S.; Rehman, R.U.; Dar, T.U.H.; Hakeem, K.R. Role of Epigenetics in Modulating Phenotypic Plasticity against Abiotic Stresses in Plants. Int. J. Genomics 2022, 2022, 1092894. [Google Scholar] [CrossRef]
- Xie, A.; Yang, J.; Wang, Y. Plasticity in Resource Allocation of the Invasive Phytolacca americana: Balancing Growth, Reproduction, and Defense along Urban–Rural Gradients. Sci. Total Environ. 2024, 919, 170402. [Google Scholar] [CrossRef]
- Hahn, P.G.; Agrawal, A.A.; Maron, J.L. Population Variation, Environmental Gradients, and the Evolutionary Ecology of Plant Defense against Herbivory. Am. Nat. 2019, 193, 40–56. [Google Scholar] [CrossRef]
- Du, B.; Liu, M.; Shen, Z.; Zhang, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 219–250. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Hastings, A.P. Trade-Offs Constrain the Evolution of an Inducible Defense within but Not between Plant Species. Ecology 2019, 100, e02757. [Google Scholar] [CrossRef] [PubMed]
- Dascaliuc, A.; Jelev, H.N.; Ralea, T. Phenotypic Plasticity and Plant Resistance to Abiotic Stress. In Proceedings of the Genetica, Fiziologia şi Ameliorarea Plantelor, Chişinău, Moldova, 7–8 October 2024. [Google Scholar]
- Yang, L.; Chen, Y.; Zhou, Y. WRKY Transcription Factors: Hubs for Regulating Plant Growth and Stress Responses. J. Integr. Plant Biol. 2025, 67, 1124–1142. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Shelp, B.J. Spermine Differentially Refines Plant Defense Responses against Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 117. [Google Scholar] [CrossRef]
- Koffel, T.; Johnson, E.; Wright, I.J. Plant Strategies along Resource Gradients. Am. Nat. 2018, 191, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef]
- Sobral, M.; Sampedro, L.; Neylan, I.; Siemens, D.; Dirzo, R. Phenotypic Plasticity in Plant Defense across Life Stages. Proc. Natl. Acad. Sci. USA 2021, 118, e2005865118. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Roumet, C. Allocation, Morphology, Physiology, Architecture: Multiple Facets of Plant Responses to Resource Stress. New Phytol. 2018, 219, 67–84. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk between Responses to Biotic and Abiotic Stresses. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Patel, P.; Jha, B. Achieving Abiotic Stress Tolerance in Plants through Antioxidative Defense Mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Hasanuzzaman, M.; Nahar, K. Approaches to Enhancing Antioxidant Defense in Plants. Plants 2022, 11, 1448. [Google Scholar]
- Saini, N.; Sharma, P.; Singh, A. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants. Trends Plant Sci. 2024, 29, 812–826. [Google Scholar]
- Al-Zahrani, H.S.; Alzahrani, Y.M.; Alshareef, S. Antioxidative Defense System, Hormones, and Metabolite Crosstalk in Tolerant Plants under Heat Stress. Front. Plant Sci. 2022, 13, 911846. [Google Scholar] [CrossRef]
- Rao, M.J.; Khan, I.; Zhao, Y. Antioxidant Defense System in Plants: Management of ROS and RNS. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Marroquín, J.; Delgado, E.; Salinas, P. Redox Signaling and Oxidative Control of Plant Stress Responses. Plant Physiol. Biochem. 2023, 205, 107183. [Google Scholar]
- Li, S.; Zhang, X.; Zhao, Y. Reactive Oxygen Species Network and Its Regulation in Plant Abiotic Stress Tolerance. Plant Sci. 2021, 312, 111024. [Google Scholar]
- Santos, A.; Oliveira, C. Antioxidant Metabolism and Photoprotection Mechanisms under Combined Stresses. Physiol. Plant. 2022, 174, e13666. [Google Scholar]
- Mahanta, D.K.; Kundu, A.; Dey, S. Plant Defense Responses to Insect Herbivores through Signaling and Direct Defense. Front. Plant Sci. 2025, 16, 1569851. [Google Scholar]
- Turlings, T.C.J.; Erb, M. Trichomes, Glands, and Volatile Chemical Signals in Plant–Insect Interactions. Annu. Rev. Entomol. 2018, 63, 141–160. [Google Scholar]
- Erb, M.; Reymond, P. Molecular Mechanisms of Plant–Insect Interactions: Learning from Arabidopsis. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Hay, M.E.; Fenical, W. Chemical Mediation of Plant–Herbivore Interactions. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 379–403. [Google Scholar]
- Gu, X.; Sun, J.; Chen, X. Herbivore-Induced Terpene Biosynthesis and Volatile Signaling Networks in Higher Plants. Plant J. 2023, 116, 876–892. [Google Scholar]
- Yao, Y.; Li, H.; Xu, C. Regulation of Volatile Terpenoid Biosynthesis during Herbivore Attack. Plant Sci. 2024, 336, 111820. [Google Scholar]
- Mezzomo, R.; Oliveira, B.; Da Silva, C. Volatile-Mediated Defenses and Induced Resistance in Aromatic Plants. Ind. Crops Prod. 2023, 202, 117458. [Google Scholar]
- Benitez, P.; Martín-Peña, R.; Gómez, R. Sterol Composition and Membrane Dynamics in Stress-Resilient Plants. BBA-Biomembr. 2022, 1864, 183935. [Google Scholar]
- De Marcos, L.; Alvarez, R.; Hernández, M. Plant Sterols and Stress Tolerance: Role in Membrane Stability and Defense. Front. Plant Sci. 2021, 12, 662444. [Google Scholar]
- Alvarez, R.; Domínguez, P.; Gómez, R. Polyunsaturated Fatty Acids Modulate Oxidative Signaling in Stress Responses. Plant Physiol. 2020, 182, 2110–2125. [Google Scholar]
- Dufresne, M.; Boucher, J.; Garcia, A. PUFA-Derived Oxylipins and Jasmonate Crosstalk in Herbivory Responses. J. Exp. Bot. 2021, 72, 5014–5029. [Google Scholar]
- Wang, H.; Li, T.; Zhang, Y. The Role of Oxylipin Pathways in Balancing Growth and Defense. Plant Cell Rep. 2023, 42, 1157–1174. [Google Scholar]



| Site | Latitude (° N) | Longitude (° E) | Altitude (m, a.s.l.) | Annual Temperature (°C) | Annual Precipitation (mm) |
|---|---|---|---|---|---|
| Tabarka | 36.954 | 8.758 | 5 | 18.8 | 1010.4 |
| Cap Negro | 37.104 | 8.982 | 0 | 18.6 | 948 |
| Bizerte | 37.278 | 9.864 | 5 | 19 | 571 |
| Haouaria | 37.05 | 11.014 | 21 | 19.7 | 481.9 |
| Monastir | 35.769 | 10.819 | 10 | 20.6 | 383.7 |
| Trait Group | Recorded Traits (Abbreviations and Units) |
|---|---|
| Whole-Plant Traits | Height (PH, mm), length (PL, mm), and width (PW, mm). |
| Stem Traits | Main stem diameter (MSD, mm). |
| Leaf Traits | Length (LL, mm), width (LW, mm), number of lobes (NLOB), projected leaf area (LA, mm2), Montgomery shape factor (ks) *, average lobe area (ALOB) ** |
| Umbel Traits | Peduncle length (UPL, mm), peduncle diameter (UPD, mm), number of rays (URN), length of rays (URL, mm), thickness of the rays (URT, mm), number of bracts forming the involucre (UBN), length of bracts (UBL, mm), width of individual bracts (UBW, mm). |
| Umbellet Traits | Number of pedicels for the secondary clusters (UNPED), number of bracteoles forming the involucel (UNBR), length of bracteole (UBRL, mm), width of bracteole (UBRW, mm) |
| Seed Traits | Seed length (SL, mm), seeds width (SW, mm), surface area (mm2), perimeter (SPER, mm), weight of 100 seeds (SM100, g). |
| Site | Herbivory Rate (%) * | Herbivory Group |
|---|---|---|
| Tabarka | 51.08 | Low |
| Bizerte | 54.92 | Low |
| Haouaria | 68.81 | High |
| Monastir | 70.64 | High |
| Cap Negro | 73.23 | High |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | Organ | K | Ca | Mg | P | Na | Fe | Zn | Mn | Cu |
| Tabarka | Flower | 619.74 | 509.29 | 97.11 | 15.08 | 616.61 | 4.68 | 0.42 | 0.23 | 0.09 |
| Leaf | 577.59 | 515.25 | 515.25 | 19.2 | 513.9 | 4.13 | 0.46 | 0.21 | 0.06 | |
| Stem | 784.99 | 227.32 | 68.55 | 21.72 | 579.6 | 2.2 | 0.48 | 0.07 | 0.08 | |
| Umbell | 890.63 | 392.22 | 99.83 | 41.08 | 623.06 | 3.21 | 0.46 | 0.17 | 0.13 | |
| Bizerte | Flower | 404.15 | 271.27 | 109.13 | 35.8 | 194.32 | 3.9 | 0.46 | 0.31 | 0.11 |
| Leaf | 503.45 | 413 | 413 | 14.66 | 857.63 | 8.79 | 0.46 | 0.36 | 0.06 | |
| Stem | 746.75 | 221.77 | 76.71 | 33.01 | 682.84 | 2.33 | 0.29 | 0.2 | 0.1 | |
| Umbell | 727.05 | 263.67 | 118.53 | 38.37 | 669.65 | 4.22 | 0.44 | 0.4 | 0.14 | |
| (b) | ||||||||||
| Site | Organ | K | Ca | Mg | P | Na | Fe | Zn | Mn | Cu |
| Cap Negro | Flower | 872.77 | 433.85 | 52.35 | 52.75 | 444.41 | 3.97 | 0.73 | 0.32 | 0.19 |
| Leaf | 887.93 | 542.37 | 542.37 | 64.69 | 541.61 | 9.84 | 0.62 | 0.42 | 0.15 | |
| Stem | 773.94 | 270.63 | 24.78 | 19.55 | 780.54 | 1.45 | 0.31 | 0.1 | 0.08 | |
| Umbell | 514.09 | 411.52 | 133.24 | 39.45 | 372.65 | 9.58 | 0.51 | 0.42 | 0.16 | |
| Haouaria | Flower | 686.87 | 340.86 | 76.39 | 34.86 | 468.28 | 7.51 | 0.49 | 0.6 | 0.16 |
| Leaf | 607.9 | 574.42 | 133.02 | 28.5 | 937.28 | 17.76 | 0.54 | 0.91 | 0.17 | |
| Stem | 710.83 | 314.19 | 83.34 | 16.65 | 557.72 | 9.66 | 0.41 | 0.35 | 0.13 | |
| Umbell | 774.38 | 357.52 | 94.52 | 27.54 | 433.41 | 19.74 | 0.5 | 0.39 | 0.19 | |
| Monastir | Flower | 492.99 | 646.17 | 68.65 | 32.47 | 446.46 | 7.38 | 0.29 | 0.29 | 0.06 |
| Leaf | 835.06 | 335.05 | 74.99 | 28.58 | 941.51 | 3.66 | 0.5 | 0.22 | 0.11 | |
| Stem | 716.86 | 291.35 | 45.2 | 32.46 | 344.67 | 1.32 | 0.33 | 0.08 | 0.1 | |
| Umbell | 695.84 | 399.72 | 65.01 | 31.11 | 542.27 | 3.48 | 0.59 | 0.23 | 0.17 | |
| Site | Sterols | Terpenes | Apiol |
|---|---|---|---|
| Tabarka | 1.39 a ± 1.70 | 0.29 a ± 0.34 | 9.44 a ± 8.10 |
| Bizerte | 1.62 a ± 2.03 | 0.50 a ± 0.46 | 7.99 a ± 4.05 |
| Cap Negro | 1.79 a ± 2.28 | 0.14 a ± 0.12 | 5.85 a ± 2.21 |
| Haouaria | 1.12 a ± 1.33 | 0.49 a ± 0.62 | 6.78 a ± 8.04 |
| Monastir | 1.00 a ± 1.16 | 0.79 a ± 1.05 | 17.42 a ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naui, L.; M’rabet, Y.; Halouani, B.; Chaabene, N.; Mezni, F.; Khaldi, A.; Hosni, K. Defense and Adaptive Strategies of Crithmum maritimum L. Against Insect Herbivory: Evidence of Phenotypic Plasticity. Plants 2025, 14, 3403. https://doi.org/10.3390/plants14213403
Naui L, M’rabet Y, Halouani B, Chaabene N, Mezni F, Khaldi A, Hosni K. Defense and Adaptive Strategies of Crithmum maritimum L. Against Insect Herbivory: Evidence of Phenotypic Plasticity. Plants. 2025; 14(21):3403. https://doi.org/10.3390/plants14213403
Chicago/Turabian StyleNaui, Liliya, Yassine M’rabet, Bilel Halouani, Najet Chaabene, Faten Mezni, Abdelhamid Khaldi, and Karim Hosni. 2025. "Defense and Adaptive Strategies of Crithmum maritimum L. Against Insect Herbivory: Evidence of Phenotypic Plasticity" Plants 14, no. 21: 3403. https://doi.org/10.3390/plants14213403
APA StyleNaui, L., M’rabet, Y., Halouani, B., Chaabene, N., Mezni, F., Khaldi, A., & Hosni, K. (2025). Defense and Adaptive Strategies of Crithmum maritimum L. Against Insect Herbivory: Evidence of Phenotypic Plasticity. Plants, 14(21), 3403. https://doi.org/10.3390/plants14213403







