Explicit Preston’s Equation Describes the Geometries of Egg-Shaped Tomato Cultivars and Its Potential for Estimating the Volume and Surface Area
Abstract
1. Introduction
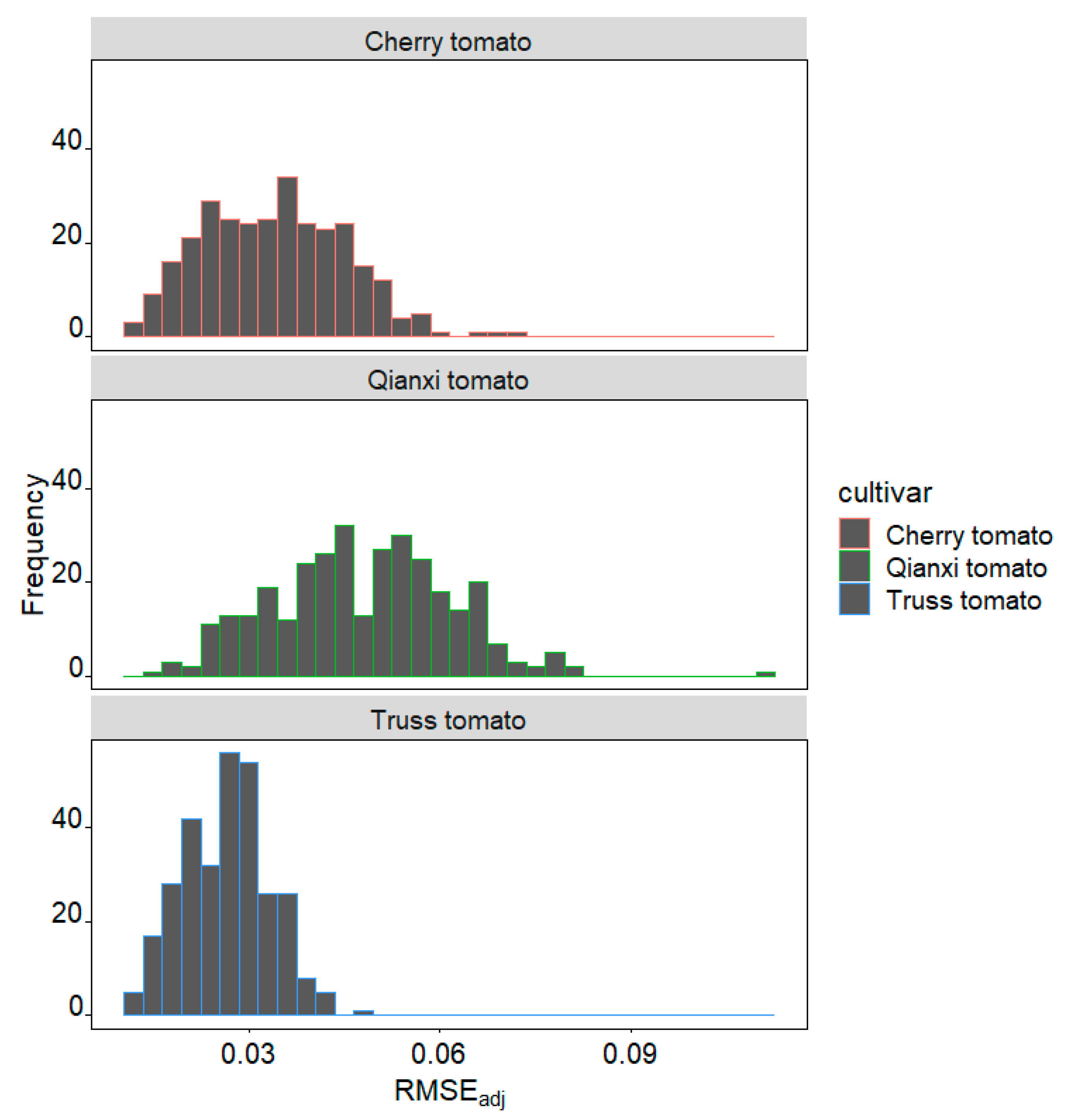
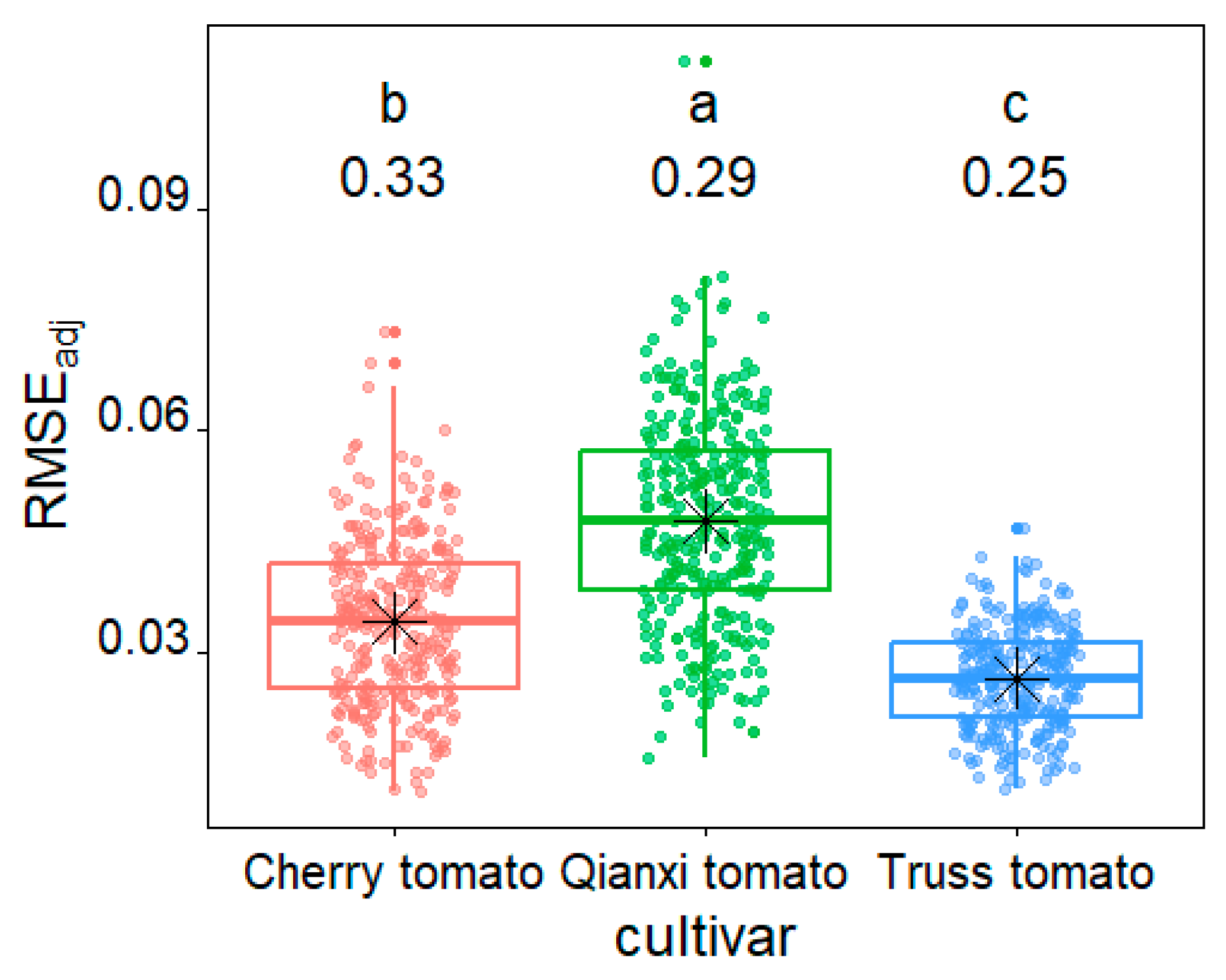
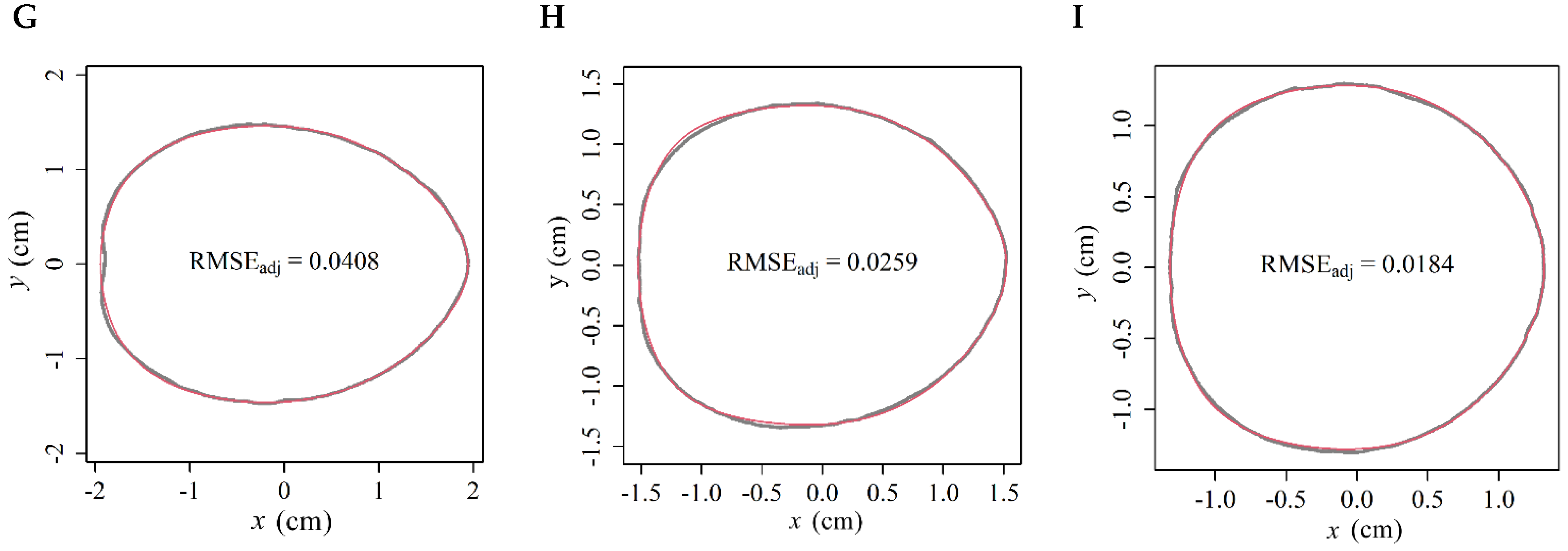
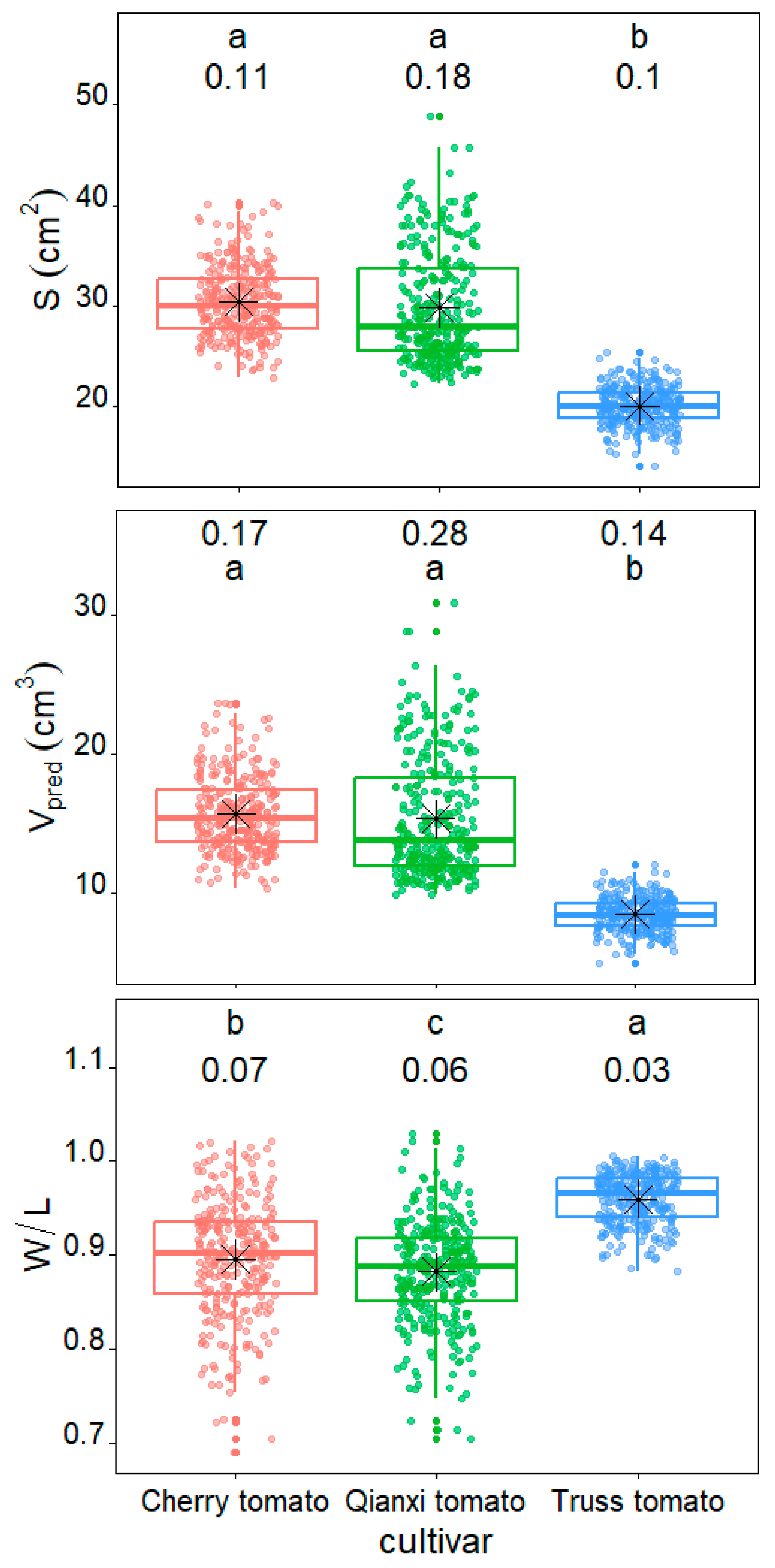
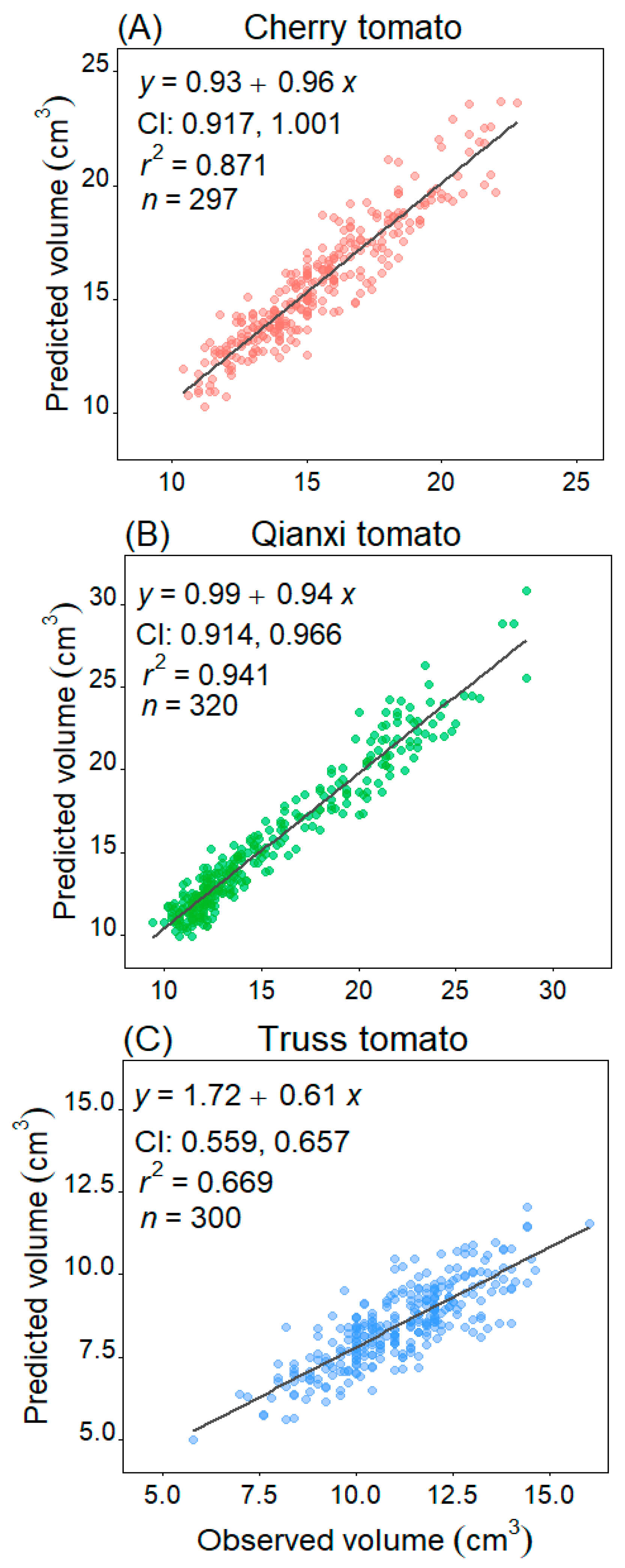
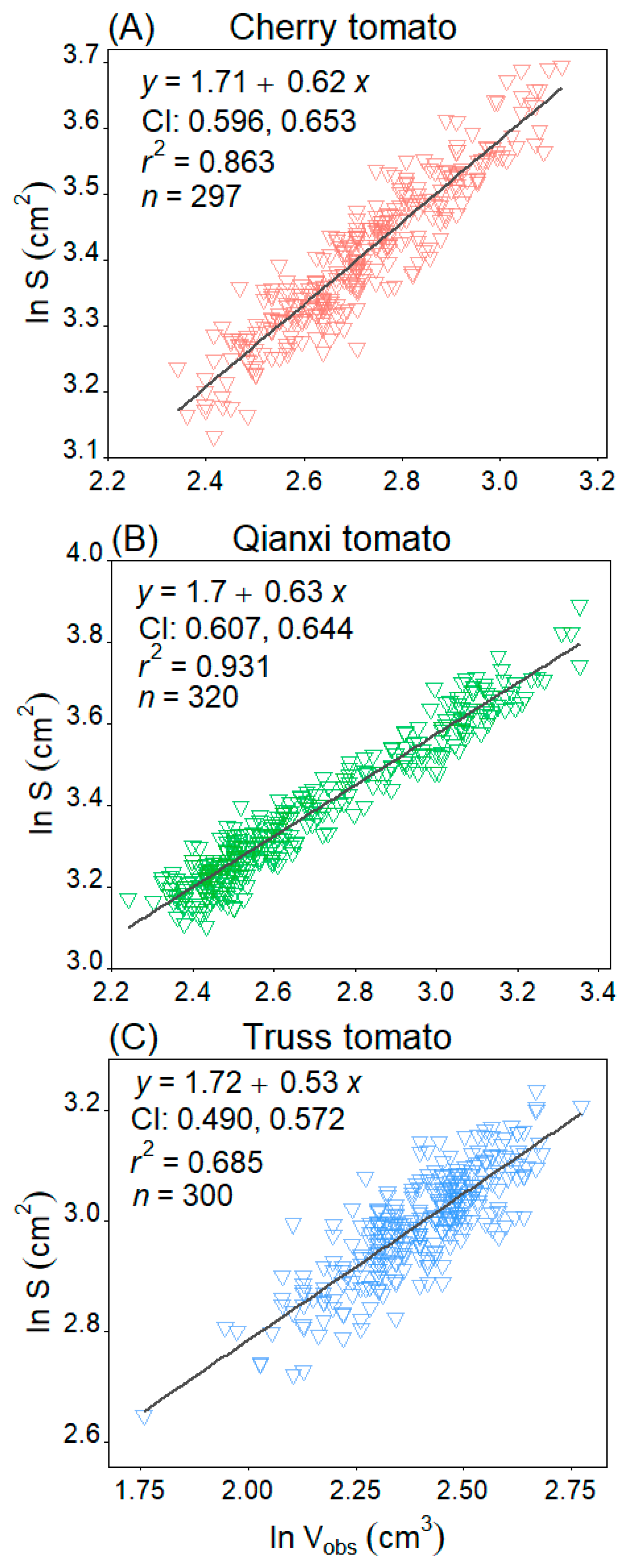
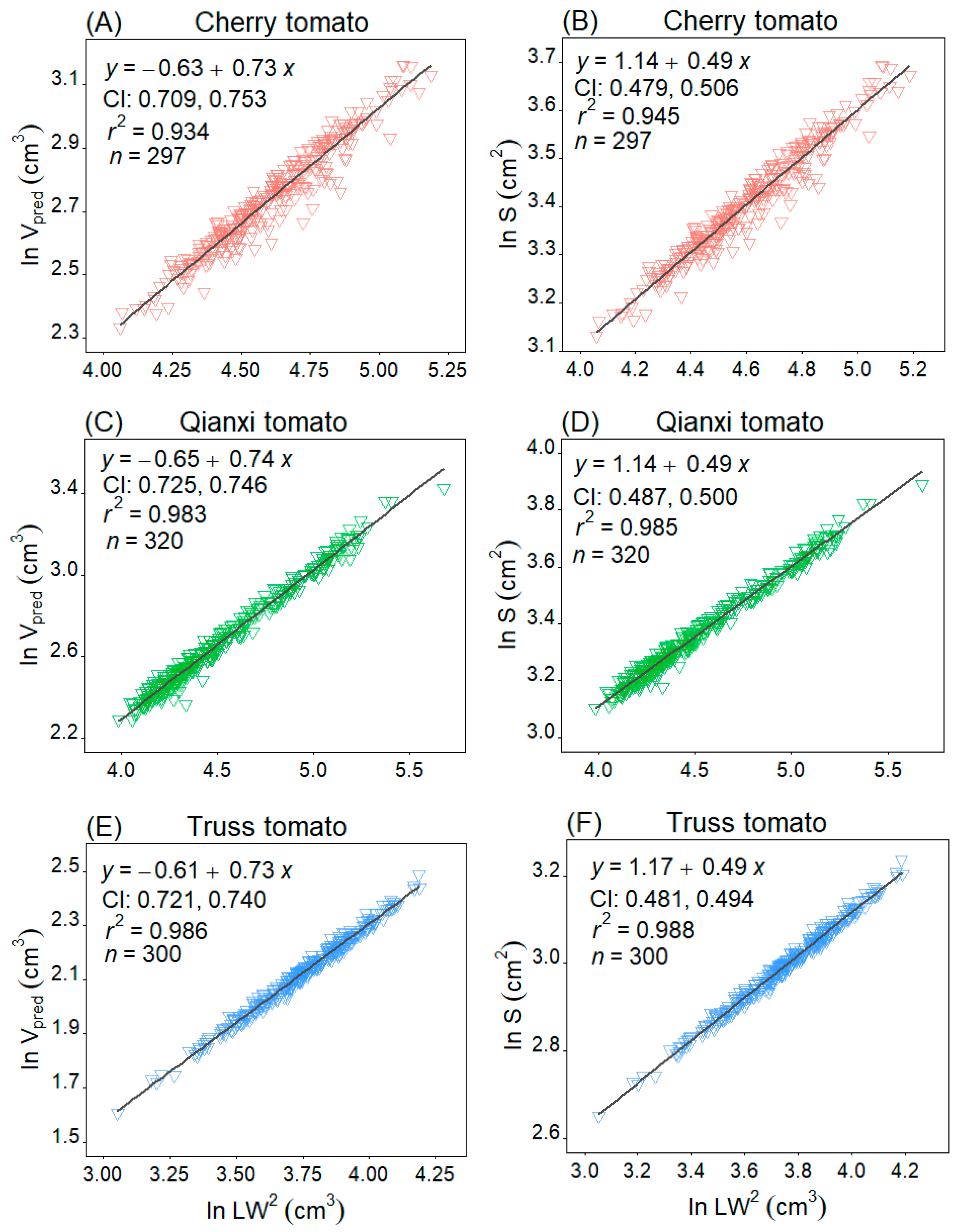
2. Results
3. Discussion
3.1. Validity of EPE in Modeling Three Tomato Cultivars
3.2. Are the Three Tomato Cultivars Studied Agrestis a Rotating Solid?
3.3. Can These Results Be Extended to Other Fruit Species and Used in Future Tomato Fruit Size Measurements?
4. Materials and Methods
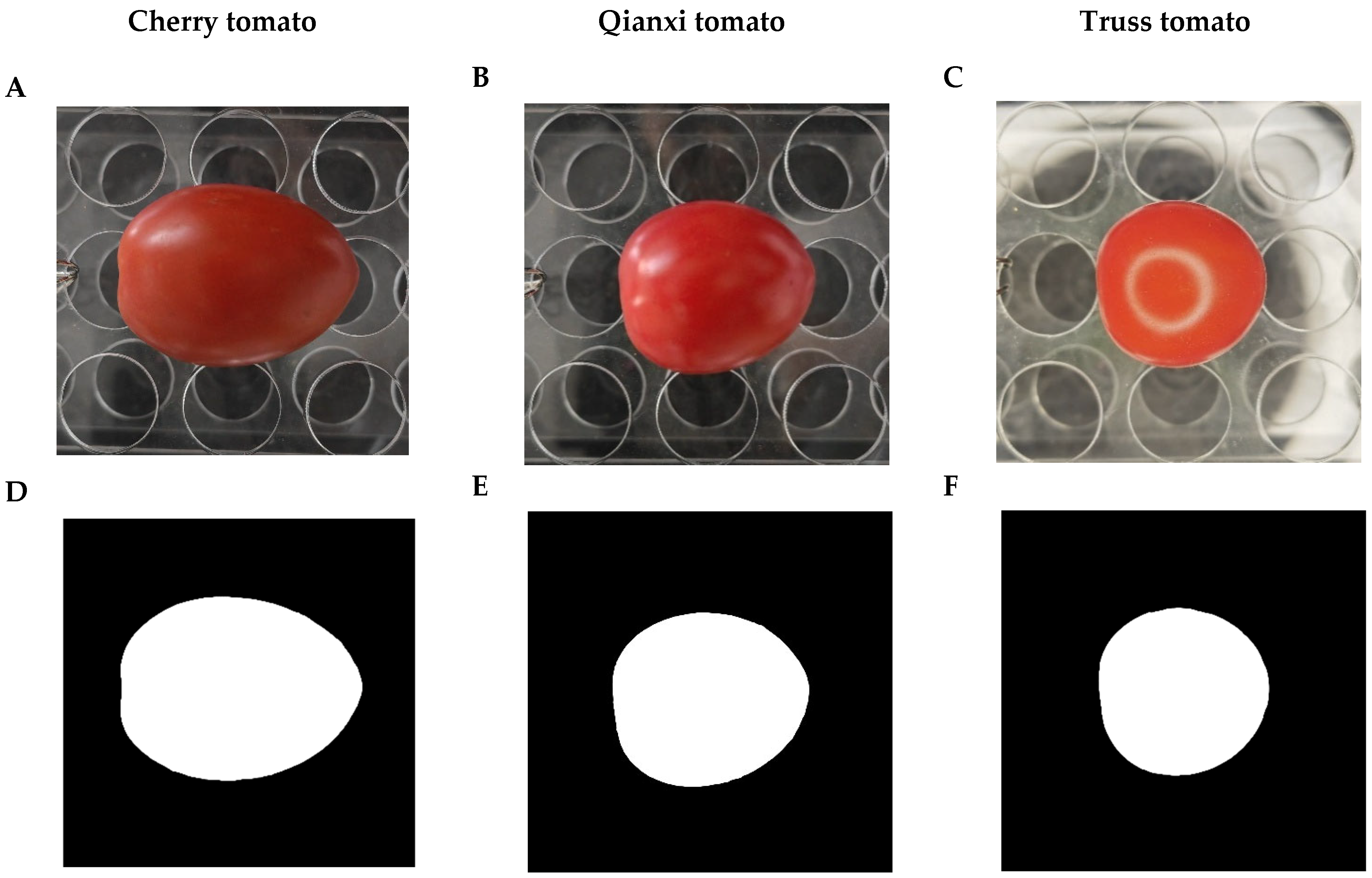
4.1. Sampling
4.2. Photographing and Data Acquisition
4.3. Data Fitting and Modelling
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| V | Volume |
| S | Surface area |
| Vpred | Predicted volume |
| Vobs | Observed volume |
| W | Maximum width |
| L | Length |
| RMSEadj | Adjusted root-mean-square errors |
| EPE | Explicit Preston equation |
References
- Lü, T.-F.; Gao, F.; Su, T.-B.; Dong, Y. Developmental genetics of fruit diversity in Brassicaceae. Curr. Opin. Plant Biol. 2025, 85, 102707. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Kourmpetli, S.; Drea, S. The fruit, the whole fruit, and everything about the fruit. J. Exp. Bot. 2014, 65, 4491–4503. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, T.; Zhao, Y.; Dong, H.; Chen, H.; Hu, Y.; Huang, C.-H.; Xiang, J.; Ma, H. Angiosperm-wide analysis of fruit and ovary evolution aided by a new nuclear phylogeny supports association of the same ovary type with both dry and fleshy fruits. J. Integr. Plant Biol. 2024, 66, 228–251. [Google Scholar] [CrossRef]
- Ahn, J.; Gao, F.; Dong, Y. Developmental mechanisms of fruit diversification in angiosperms and the evolutionary implications. Plant Cell Environ. 2025, 48, 4585–4598. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; McGregor, C.; Liu, S.; Luan, F.; Gao, M.; Weng, Y. Genetic architecture of fruit size and shape variation in cucurbits: A comparative perspective. Theor. Appl. Genet. 2020, 133, 1–21. [Google Scholar] [CrossRef]
- Lomáscolo, S.B.; Levey, D.J.; Kimball, R.T.; Bolker, B.M.; Alborn, H.T. Dispersers shape fruit diversity in Ficus (Moraceae). Proc. Natl. Acad. Sci. USA 2010, 107, 14668–14672. [Google Scholar] [CrossRef]
- Bisht, I.S.; Bhat, K.V.; Tanwar, S.P.S.; Bhandari, D.C.; Joshi, K.; Sharma, A.K. Distribution and genetic diversity of Cucumis sativus var. hardwickii (Royle) Alef in India. J. Hortic. Sci. Biotechnol. 2004, 79, 783–791. [Google Scholar] [CrossRef]
- Yang, L.M.; Koo, D.H.; Li, Y.H.; Zhang, X.J.; Luan, F.S.; Havey, M.J.; Jiang, J.M.; Weng, Y.Q. Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 2012, 71, 895–906. [Google Scholar] [CrossRef]
- Pitrat, M. Melon genetic resources: Phenotypic diversity and horticultural taxonomy. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: Berlin, Germany, 2017; pp. 25–60. [Google Scholar]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of environmental factors on growth and development of fruits. Trop. Plant Biol. 2021, 14, 226–238. [Google Scholar] [CrossRef]
- Génard, M.; Bertin, N.; Borel, C.; Bussières, P.; Gautier, H.; Habib, R.; Léchaudel, M.; Lecomte, A.; Lescourret, F.; Lobit, P.; et al. Towards a virtual fruit focusing on quality: Modelling features and potential uses. J. Exp. Bot. 2007, 58, 917–928. [Google Scholar] [CrossRef]
- Baldet, P.; Devaux, C.; Chevalier, C.; Brouquisse, R.; Just, D.; Raymond, P. Contrasted responses to carbohydrate limitation in tomato fruit at two stages of development. Plant Cell Environ. 2002, 25, 1639–1649. [Google Scholar] [CrossRef]
- Prudent, M.; Bertin, N.; Génard, M.; Muños, S.; Rolland, S.; Garcia, V.; Petit, J.; Baldet, P.; Rothan, C.; Causse, M. Genotype-dependent response to carbon availability in growing tomato fruit. Plant Cell Environ. 2010, 33, 1186–1204. [Google Scholar] [CrossRef] [PubMed]
- Okello, R.C.O.; de Visser, P.H.B.; Heuvelink, E.; Lammers, M.; de Maagd, R.A.; Struik, P.C.; Marcelis, L.F.M. A multilevel analysis of fruit growth of two tomato cultivars in response to fruit temperature. Physiol. Plant. 2015, 153, 403–418. [Google Scholar] [CrossRef]
- Guichard, S.; Gary, C.; Leonardi, C.; Bertin, N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. J. Plant Growth Regul. 2005, 24, 201–213. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Júnior, D.S.R.; Barbosa, A.C.O.; Batista, I.A.; Camillo, L.R.; Lopes, N.S.; Costa, M.G.C. Impact of moderate water deficit at the fruit development stage of tomato (Solanum lycopersicum L.): Effects on plant growth, physiology, fruit yield and quality and expression of carotenoid biosynthesis genes. Acta Physiol. Plant. 2023, 45, 65. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Yavuz, D.; Seymen, M.; Yavuz, N.; Çoklar, H.; Ercan, M. Effects of water stress applied at various phenological stages on yield, quality, and water use efficiency of melon. Agric. Water Manage. 2021, 246, 106673. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadakis, V.M.; Machado, M.; Psyllakis, E.; Nektarios, P.A. Non-invasive leaf hydration status determination through convolutional neural networks based on multispectral images in chrysanthemum. Plant Growth Regul. 2024, 102, 485–496. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Makraki, T.; Papadimitriou, D.; Nikoloudakis, N.; Taheri-Garavand, A.; Fanourakis, D. Non-Destructive Estimation of Area and Greenness in Leaf and Seedling Scales: A Case Study in Cucumber. Agronomy 2025, 15, 2294. [Google Scholar] [CrossRef]
- Kusumi, A.; Nishiyama, S.; Tao, R. Three-dimensional fruit growth analysis clarifies developmental mechanisms underlying complex shape diversity in persimmon fruit. J. Exp. Bot. 2024, 75, 1919–1933. [Google Scholar] [CrossRef]
- He, K.; Hui, C.; Yao, W.; Wang, J.; Wang, L.; Li, Q.; Shi, P. Evidence that field muskmelon (Cucumis melo L. var. agrestis Naud.) fruits are solids of revolution. Plants 2023, 12, 4186. [Google Scholar] [CrossRef]
- Wang, L.; Shi, P.; Chen, L.; Gielis, J.; Niklas, K.J. Evidence that Chinese white olive (Canarium album (Lour.) DC.) fruits are solids of revolution. Bot. Lett. 2024, 171, 109–115. [Google Scholar] [CrossRef]
- Eifert, J.D.; Sanglay, G.C.; Lee, D.-J.; Sumner, S.S.; Pierson, M.D. Prediction of raw produce surface area from weight measurement. J. Food Eng. 2006, 74, 552–556. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Pandiselvam, R.; Kothakota, A.; Ishwarya, S.P.; Chakraborty, S.K.; Kumar, M.; Cozzolino, D. Emerging non-destructive imaging techniques for fruit damage detection: Image processing and analysis. Trends Food Sci. Technol. 2022, 120, 418–438. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Hu, T.; He, Q.; Chen, Z.; Wang, J.; Liu, L.; Fang, C.; Luo, J.; Fu, L.; et al. Nondestructive 3D phenotyping method of passion fruit based on X-ray micro-computed tomography and deep learning. Front. Plant Sci. 2023, 13, 1087904. [Google Scholar] [CrossRef]
- Cano-Lara, M.; Rostro-Gonzalez, H. Tomato quality assessment and enhancement through Fuzzy Logic: A ripe perspective on precision agriculture. Postharvest Biol. Technol. 2024, 212, 112875. [Google Scholar] [CrossRef]
- Song, J.-Y.; Qin, Z.-S.; Xue, C.-W.; Bian, L.-F.; Yang, C. Fruit grading system by reconstructed 3D hyperspectral full-surface images. Postharvest Biol. Technol. 2024, 212, 112898. [Google Scholar] [CrossRef]
- Mauxion, J.-P.; Chevalier, C.; Gonzalez, N. Complex cellular and molecular events determining fruit size. Trends Plant Sci. 2021, 26, 1023–1038. [Google Scholar] [CrossRef]
- Monforte, A.J.; Diaz, A.; Caño-Delgado, A.; van der Knaap, E. The genetic basis of fruit morphology in horticultural crops: Lessons from tomato and melon. J. Exp. Bot. 2014, 65, 4625–4637. [Google Scholar] [CrossRef]
- Preston, F.W. The shapes of birds’ eggs. Auk 1953, 70, 160–182. [Google Scholar] [CrossRef]
- Shi, P.; Chen, L.; Quinn, B.K.; Yu, K.; Miao, Q.; Guo, X.; Lian, M.; Gielis, J.; Niklas, K.J. A simple way to calculate the volume and surface area of avian eggs. Ann. N. Y. Acad. Sci. 2023, 1524, 118–131. [Google Scholar] [CrossRef]
- Todd, P.H.; Smart, I.H.M. The shape of birds’ eggs. J. Theor. Biol. 1984, 106, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, L.; Quinn, B.K.; Gielis, J. A new program to estimate the parameters of preston’s equation, a general formula for describing the egg shape of birds. Symmetry 2023, 15, 231. [Google Scholar] [CrossRef]
- Nyalala, I.; Okinda, C.; Nyalala, L.; Makange, N.; Chao, Q.; Chao, L.; Yousaf, K.; Chen, K. Tomato volume and mass estimation using computer vision and machine learning algorithms: Cherry tomato model. J. Food Eng. 2019, 263, 288–298. [Google Scholar] [CrossRef]
- Koc, A.B. Determination of watermelon volume using ellipsoid approximation and image processing. Postharvest Biol. Technol. 2007, 45, 366–371. [Google Scholar] [CrossRef]
- Khojastehnazhand, M.; Omid, M.; Tabatabaeefar, A. Determination of tangerine volume using image processing methods. Int. J. Food Prop. 2010, 13, 760–770. [Google Scholar] [CrossRef]
- Fellegari, R.; Navid, H. Determining the orange volume using image processing. In Proceedings of the International Conference on Food Engineering and Biotechnology (ICFEB 2011), Bangkok, Thailand, 7–9 May 2011; Citeseer: University Park, PA, USA, 2011; pp. 180–184. Available online: https://studylib.net/doc/25754476/34-b10018 (accessed on 6 August 2025).
- Omid, M.; Khojastehnazhand, M.; Tabatabaeefar, A. Estimating volume and mass of citrus fruits by image processing technique. J. Food Eng. 2010, 100, 315–321. [Google Scholar] [CrossRef]
- Visa, S.; Cao, C.; Gardener, B.M.; van der Knaap, E. Modeling of tomato fruits into nine shape categories using elliptic fourier shape modeling and Bayesian classification of contour morphometric data. Euphytica 2014, 200, 429–439. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. A general leaf area geometric formula exists for plants—Evidence from the simplified gielis equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef]
- Su, J.; Niklas, K.J.; Huang, W.; Yu, X.; Yang, Y.; Shi, P. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- Shi, P.; Gielis, J.; Quinn, B.K.; Niklas, K.J.; Ratkowsky, D.A.; Schrader, J.; Ruan, H.; Wang, L.; Niinemets, Ü. “Biogeom”: An R package for simulating and fitting natural shapes. Ann. N. Y. Acad. Sci. 2022, 1516, 123–134. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Huang, W.; Ma, K.; Gladish, D.K. Ellipse or superellipse for tree-ring geometries? evidence from six conifer species. Trees 2024, 38, 1403–1413. [Google Scholar] [CrossRef]
- Huang, W.; Ma, K.; Tan, J.; Wei, M.; Lu, Y. Superellipse equation describing the geometries of Abies alba tree rings. Plants 2024, 13, 3487. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.; Niklas, K.J.; Gielis, J.; Ding, Y.; Cao, L.; Shi, P. A superellipse with deformation and its application in describing the cross-sectional shapes of a square bamboo. Symmetry 2020, 12, 2073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Tan, J. Explicit Preston’s Equation Describes the Geometries of Egg-Shaped Tomato Cultivars and Its Potential for Estimating the Volume and Surface Area. Plants 2025, 14, 3398. https://doi.org/10.3390/plants14213398
Huang W, Tan J. Explicit Preston’s Equation Describes the Geometries of Egg-Shaped Tomato Cultivars and Its Potential for Estimating the Volume and Surface Area. Plants. 2025; 14(21):3398. https://doi.org/10.3390/plants14213398
Chicago/Turabian StyleHuang, Weiwei, and Jiaxin Tan. 2025. "Explicit Preston’s Equation Describes the Geometries of Egg-Shaped Tomato Cultivars and Its Potential for Estimating the Volume and Surface Area" Plants 14, no. 21: 3398. https://doi.org/10.3390/plants14213398
APA StyleHuang, W., & Tan, J. (2025). Explicit Preston’s Equation Describes the Geometries of Egg-Shaped Tomato Cultivars and Its Potential for Estimating the Volume and Surface Area. Plants, 14(21), 3398. https://doi.org/10.3390/plants14213398






