Abstract
Salicornia europaea is an extremely salt-tolerant annual halophyte. It occurs in coastal and inland saline habitats and is increasingly cultivated for food, nutraceuticals, and environmental remediation. This study examined whether inland populations from contrasting saline sites exhibit heritable differences in shoot and root morphology. Seeds from four isolated sites (Ciechocinek, Inowrocław, Salzgraben, and Soltauquelle) were grown at 0, 200, 400, and 1000 mM NaCl, and morphometric traits were quantified from digital images. Corresponding soil samples were also analyzed. A strong relationship was found between population origin and responses to salt stress. Optimal growth generally occurred at 200–400 mM NaCl. Shoot canopy area consistently best discriminated among populations. Inowrocław and Salzgraben performed best under extreme salinity (1000 mM), whereas Ciechocinek showed the weakest growth. Root analyses revealed a shift from radial expansion at moderate salinity to elongation at higher levels, with Salzgraben and Soltauquelle maintaining the longest roots. Soil analyses indicated substantial site differences: Ciechocinek had the highest salinity, resulting in the smallest trait development, Inowrocław was rich in Ca2+ and organic matter, while the German sites had lower salinity but higher pH and bicarbonates. These findings demonstrate population-specific adaptive strategies and soil-legacy effects, supporting targeted ecotype selection for saline agriculture and phytoremediation.
1. Introduction
Salicornia europaea L., commonly known as glasswort, sea asparagus, or marsh samphire, is a succulent annual halophyte of the Amaranthaceae family (subfamily Salicornioideae). As a euhalophyte, it completes its life cycle under saline conditions and is regarded as one of the most salt-tolerant terrestrial plants [1]. Salt tolerance in S. europaea is achieved through vacuolar sequestration of Na+ via NHX antiporters, selective ion uptake by HKT-type transporters, and accumulation of compatible osmolytes such as proline and glycine betaine [2,3,4]. Its simple highly succulent morphology with photosynthetic activity restricted to segmented green stems and vestigial leaves minimizes transpirational water loss and supports survival in hypersaline habitats [5]. Physiological and biochemical studies further demonstrate that salinity stimulates the accumulation of phenolic compounds, anthocyanins, and β-carotene, which act as antioxidants and enhance nutritional value [6,7,8]. The species develops shallow but extensive fibrous root systems that enable efficient water and ion uptake in waterlogged saline soils, contributing to productivity under brackish and hypersaline conditions [9,10,11].
The natural distribution of S. europaea spans European coasts, estuarine mudflats, and inland salt basins, but it has also colonized anthropogenically salinized landscapes, such as post-mining brine ponds, reflecting considerable ecological plasticity [12,13]. Cytogenetic and molecular analyses distinguish diploid S. europaea (2n = 18) from its polyploid relatives, consolidating its identity within the Eurasian diploid clade [14]. These features, combined with its simple architecture, short life cycle, and uniform growth habit, make it an excellent model species for studying halophyte adaptation. Beyond its ecological role, S. europaea has become increasingly relevant in saline agriculture, biotechnology, and remediation. Field trials in Europe and Asia confirm its potential for cultivation under brackish or seawater irrigation, with optimal biomass production at moderate salinity [8,15,16]. Commercial cultivation has been established in Mexico and the USA, where shoots are marketed as gourmet vegetables, while extracts are developed into functional foods, nutraceuticals, cosmetics, and forage products [17,18,19]. At the same time, phytoremediation studies highlight its ability to tolerate and accumulate heavy metals in contaminated saline soils, supporting its use in wastewater treatment and soil reclamation [20,21,22]. Collectively, S. europaea represents a multifunctional halophyte at the intersection of ecology, agriculture, and biotechnology.
Investigating this species at the population scale provides insights into local adaptation as distinct habitats exert strong selective pressures on functional traits. Populations are defined as groups of interbreeding individuals sharing a habitat, but their boundaries and divergence often depend on spatial scale and environmental heterogeneity [23]. In halophytes, variation across fine spatial scales may reflect short-term plasticity, while broader geographical separation can result in ecotypic differentiation and genetic divergence [24]. For S. europaea, exposure to fluctuating salinity regimes may promote trait plasticity, whereas populations from more stable saline environments often evolve specialized salt tolerance strategies [25]. Functional trait-based approaches are particularly effective for capturing these differences. Traits such as succulence, stem diameter, root length, and tissue dimensions directly reflect fitness under saline conditions, while anatomical modifications, such as lignification and cell wall remodeling, enhance osmotic stability [12,26,27]. Considerable intraspecific variability has been documented among natural populations, underlining the value of trait-based analyses for disentangling plastic versus genetic-based responses to salinity stress.
Based on this framework, the present study tests the hypothesis that inland populations of S. europaea from ecologically distinct saline habitats exhibit heritable differences in morphometric traits that reflect both adaptive genetic responses and maternal habitat legacies. We assumed that long-term exposure to divergent salinity regimes has shaped population-specific shoot and root growth strategies, resulting in distinct responses when grown under uniform controlled conditions. Specifically, we assess the shoot and root dimensions and functional traits across four populations subjected to graded NaCl treatments (0, 200, 400, and 1000 mM). By integrating population soil-characteristic origin with salinity effects, this study seeks to clarify the extent of local specialization and plasticity in this model halophyte.
2. Results
2.1. Shoot Trait Variability
The ANOVA-II results (Table 1) reveal significant effects of population for all the shoot traits (p < 0.001) and highly significant population × salinity interactions (p < 0.001), indicating that the magnitude and direction of responses differed among the populations. By contrast, the main effect of salinity was not significant for S-Projected area, S-Height, or S-Diameter, but S-Canopy (p = 0.022) and S-Shape perimeter (p < 0.001) were significantly influenced by NaCl concentrations.

Table 1.
Two-way ANOVA results for shoot morphometric traits of four S. europaea populations across salinity treatments. Values represent F-statistics with associated p-values; * indicates significance levels (p < 0.05) and ** (p < 0.01), while NS indicates not significant.
The morphometric analysis of shoots by one-way ANOVA (ANOVA-I; Table 2) confirmed distinct population-specific responses across the salinity treatments. At 0 mM NaCl, representing salt deprivation, growth was generally reduced compared with 200 and 400 mM, with smaller S-Projected areas, shorter S-Height, and restricted S-Canopy development, particularly in the Ciechocinek and Soltauquelle populations. Under non-saline conditions, the populations did not differ in S-Projected area or S-Shape perimeter (Table 2). The Salzgraben population had significantly greater S-Height and S-Diameter, and the Inowrocław population had the largest S-Canopy. Growth improved in all the populations at 200 and 400 mM NaCl, and the differences among them became more pronounced. The C-population consistently exhibited the lowest values across the traits. At 200 mM, the I-population developed the widest S-Canopy but the smallest S-Diameter. The So- and Sa-populations reached the greatest S-Height. At 400 mM, the Sa-population exhibited the widest S-Canopy, followed by the I-population. Moreover, the Sa-, I-, and So-populations showed significantly greater S-Projected area and S-Height compared with the C-population. At 1000 mM, strong population divergence was observed. The I- and Sa-populations performed best, showing significantly higher values across all the shoot traits, followed by the So- and finally C-populations.

Table 2.
Morphometric shoot traits of four S. europaea inland populations (C—Ciechocinek, I—Inowrocław, Sa—Salzgraben, and So—Soltauquelle) grown under different salinity levels (0, 200, 400, and 1000 mM NaCl). Values represent mean ± SE (n = 9). Different letters within each salinity treatment indicate significant differences between populations according to ANOVA followed by post hoc Tukey’s HSD test (p < 0.05).
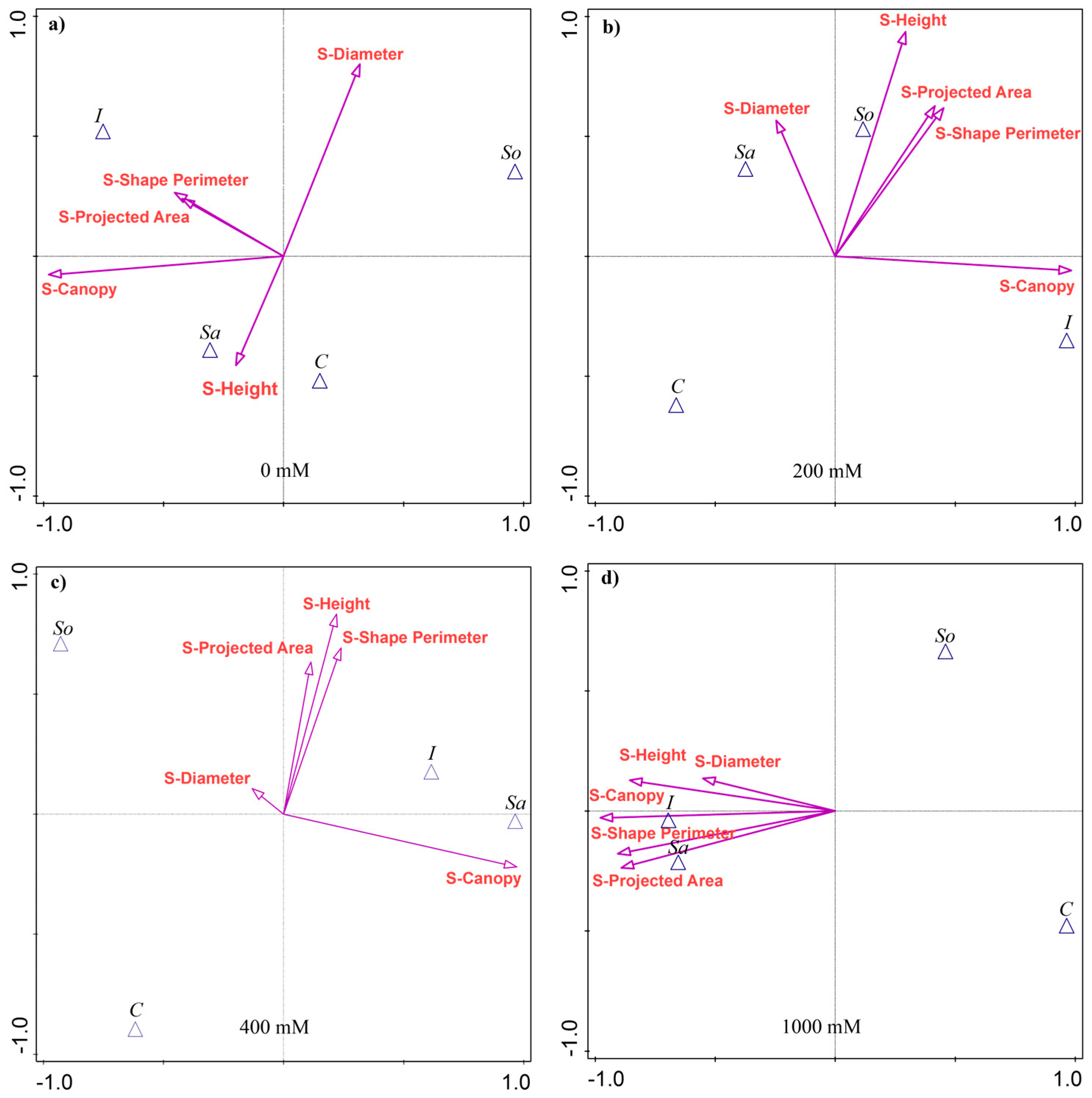
The results of the discriminant analysis showed that the shoot traits most separating the populations varied with treatment, although S-Canopy was consistently the strongest discriminator, explaining 22–30% of the variability among the populations (Appendix A Table A1). At 0 mM NaCl, in addition to S-Canopy, S-Diameter explained the most variability, followed by S-Height and S-Shape perimeter. The I- and Sa-populations were associated with larger S-Canopies, while the I- and So-populations were associated with greater S-Diameter (Figure 1a).
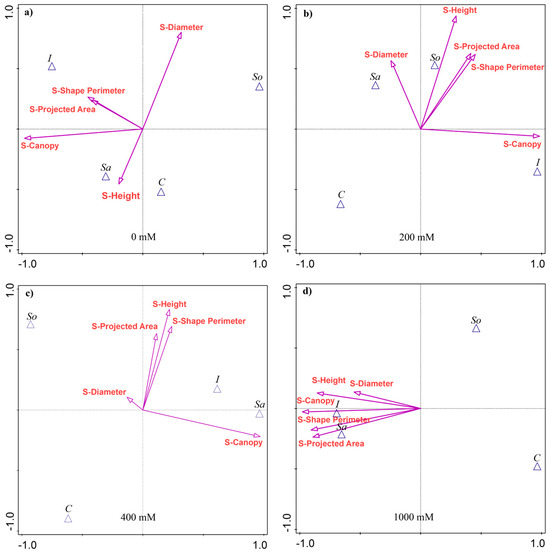
Figure 1.
Canonical variate analysis (CVA) of shoot morphological traits in four Salicornia europaea populations under different salinity treatments: (a) 0 mM NaCl, (b) 200 mM NaCl, (c) 400 mM NaCl, and (d) 1000 mM NaCl. Vectors indicate trait contributions to population discrimination. Population abbreviations: I—Inowrocław, C—Ciechocinek, Sa—Salzgraben, and So—Soltquelle.
At 200 mM, height was the second most discriminant trait (18% of variability), followed by S-Shape perimeter (13.3%) and S-Projected area (12.7%). The I-population was associated with the largest S-Canopy, while Sa- and So-populations were linked to other traits (Figure 1b). At 400 mM, the discriminant traits were the same as at 200 mM, although they explained a smaller share of the variability, except for S-Canopy (Table A1). The Sa- and I-populations were associated with larger S-Canopies, and, together with the So-population, with the remaining traits (Figure 1c). At 1000 mM, S-Canopy accounted for 29.7% of the variability, followed by S-Shape perimeter (26%), S-Projected area (25.3%), S-Height (22.9%), and S-Diameter (10.3%). The Sa- and I-populations were associated with high values across the traits, while the So- and C-populations performed poorly (Figure 1d).
2.2. Root Trait Variability
The two-way ANOVA (Table 3) revealed highly significant population effects for all the root traits (p < 0.001) and significant population × salinity interactions (p < 0.01). In contrast, the main effect of salinity was not significant for root projected area, shape perimeter, or length.

Table 3.
Two-way ANOVA results for root morphometric traits of four S. europaea populations under different salinity treatments. Values represent F-statistics with associated p-values; ** indicates significance levels (p < 0.01), while NS indicates not significant.
The one-way ANOVA (ANOVA-I; Table 4) confirmed distinct population-specific responses across the salinity treatments. At 0 mM, the I-population displayed significantly lower root values than the others, while the So-population had the longest roots. Similar results were observed at 200 mM (Table 4). At 400 mM, the Sa-, So-, and C-populations performed similarly, whereas I- again showed significantly reduced root traits. At 1000 mM, population divergence was more pronounced: the C-population had the lowest root growth, the I-population maintained reduced root length, and the Sa- and So-populations retained relatively high root development (Table 4).

Table 4.
Morphometric root traits of four Salicornia europaea inland populations (C—Ciechocinek, I—Inowrocław, Sa—Salzgraben, and So—Soltauquelle) grown under different salinity levels (0, 200, 400, and 1000 mM NaCl). Values represent mean ± SE (n = 3). Different letters within each salinity treatment indicate significant differences between populations according to ANOVA followed by post hoc Tukey’s HSD test (p < 0.05).
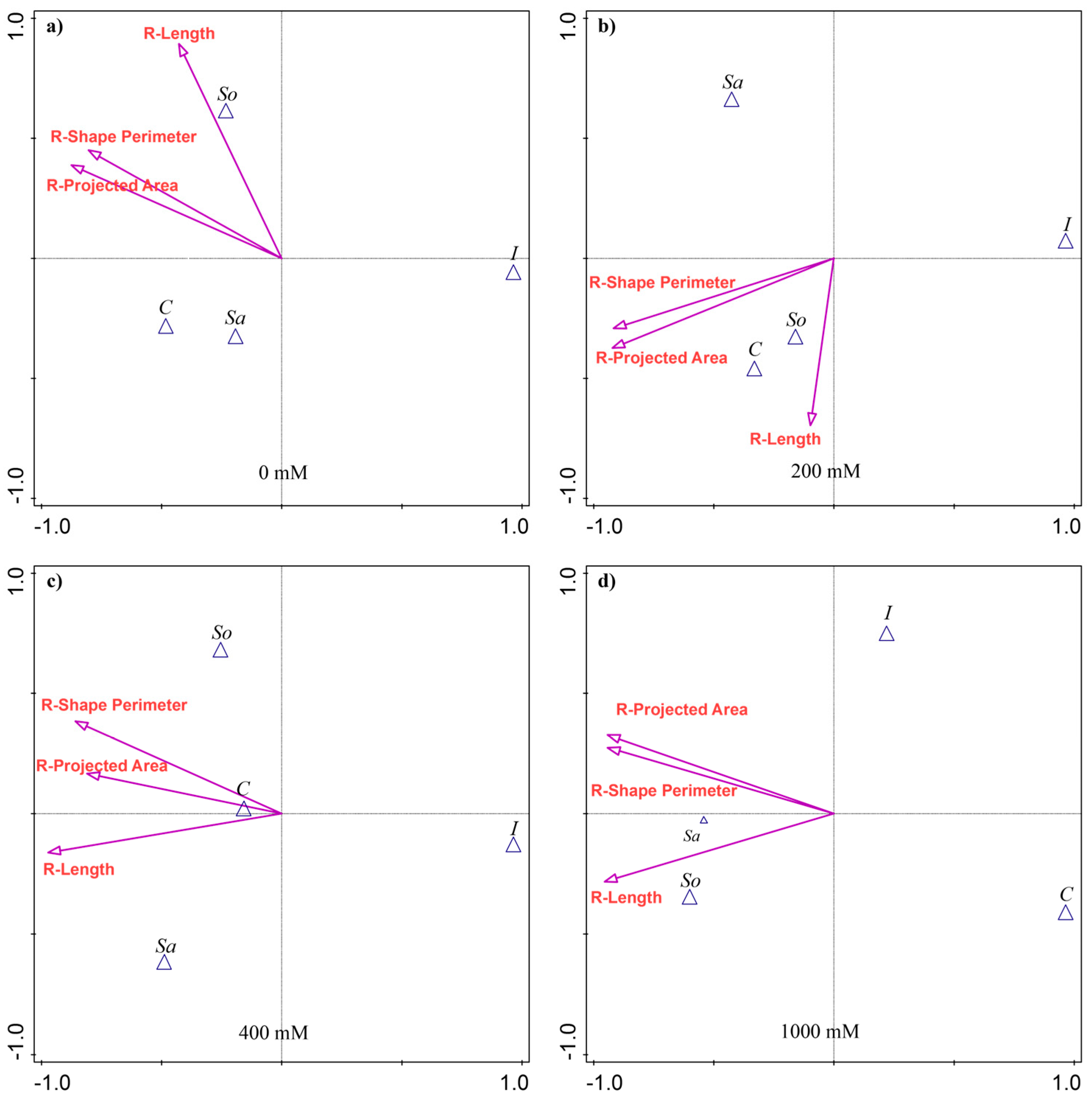
The discriminant analysis (CVA) showed that the root traits most separating the populations varied with treatment (Figure 2). At 0 mM, no traits contributed significantly to population variability. At 200 mM, the R-Projected area (25.7% of the explained variability) and the R-Shape perimeter (25.2%) accounted for most of the differences among the populations (Appendix A Table A2). The So- and C-populations were most associated with greater R-Length (Figure 2b). At 400 mM, R-Length explained the largest share of variation (26.1%), followed by R-Shape perimeter (22.7%), with the C-population relatively more aligned with R-Projected area (Figure 2c). At 1000 mM, all three traits contributed substantially, each explaining about 29% of the variability (Appendix A Table A2). Root R-Length dominated, followed by R-Projected area and R-Shape perimeter. The So-population was associated with greater R-Length, while the Sa-population corresponded more closely with R-Shape perimeter and R-Projected area. The C-population was positioned opposite, reflecting the lowest values for these traits (Figure 2d).
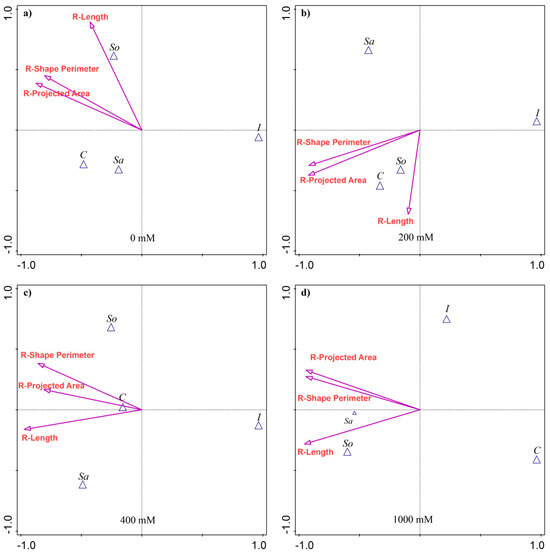
Figure 2.
Canonical variate analysis (CVA) of root morphological traits in four S. europaea populations under different salinity treatments: (a) 0 mM NaCl, (b) 200 mM NaCl, (c) 400 mM NaCl, and (d) 1000 mM NaCl. Vectors indicate trait contributions to population discrimination. Population abbreviations: I—Inowrocław, C—Ciechocinek, Sa—Salzgraben, and So—Soltquelle.
2.3. Comparison of Population Growth Performance
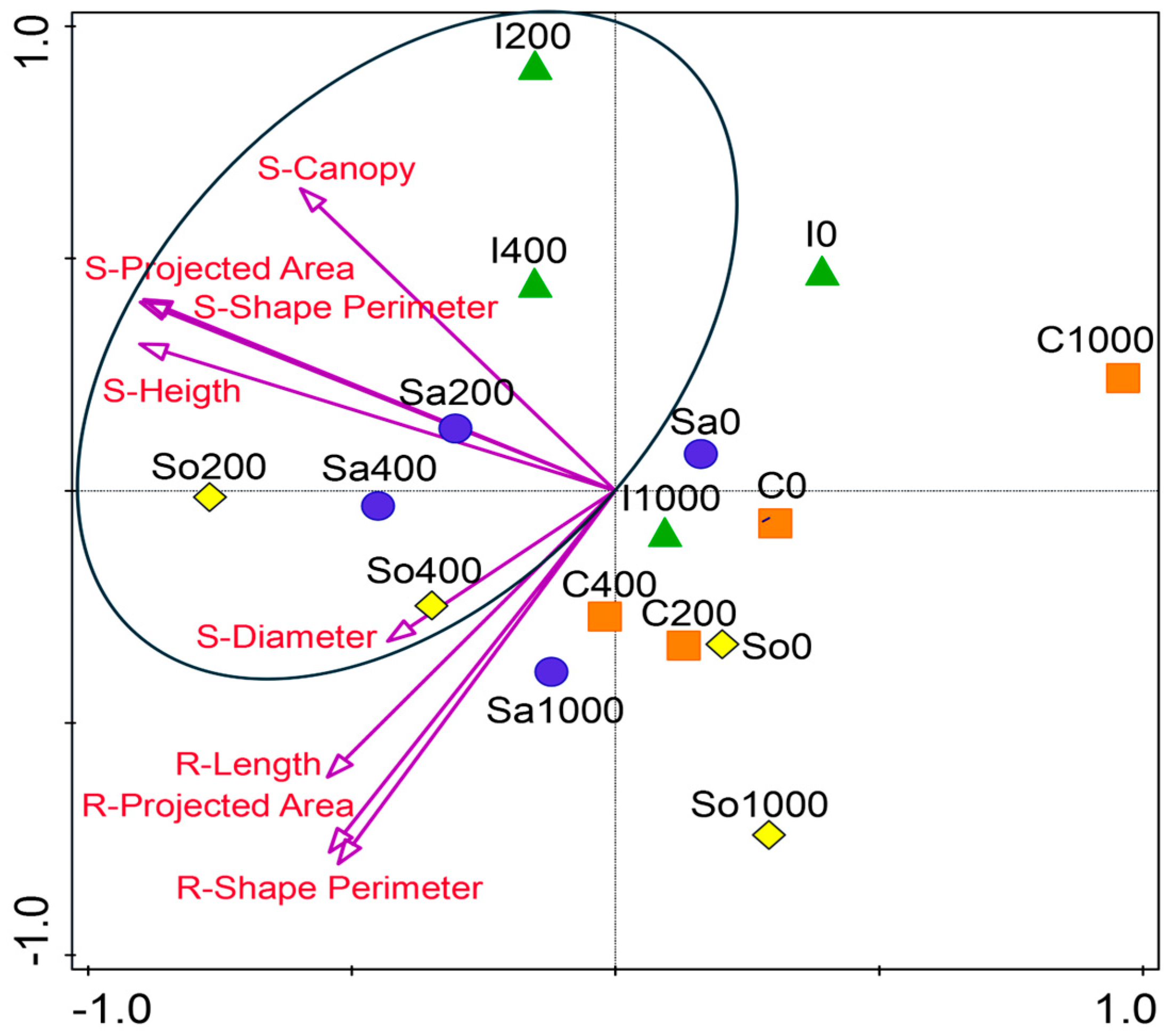
The NMDS ordination of standardized shoot and root traits revealed population-specific performance patterns across salinity levels (Figure 3). In most populations, optimum growth occurred at 200 or 400 mM NaCl, indicating that these levels supported the most favorable trait development in S. europaea. Populations I200, I400, Sa200, Sa400, So200, and So400 clustered along a shared axis in NMDS space, reflecting strong trait expression and alignment with the shoot and root vectors. In contrast, the C population remained clearly separated from the central cluster, showing the weakest alignment with the shoot traits across the treatments (Figure 3). However, within this population, growth at 200 and 400 mM was relatively improved compared with 0 and 100 mM NaCl.
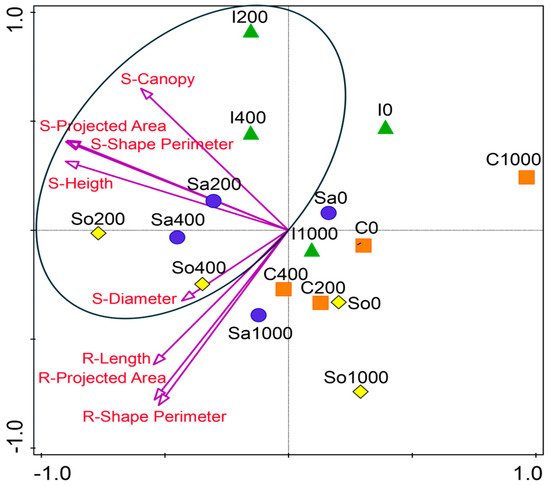
Figure 3.
Non-metric multidimensional scaling (NMDS) ordination of standardized shoot and root traits of Salicornia europaea populations under four salinity levels (0, 200, 400, and 1000 mM NaCl) based on Euclidean distance. Points represent populations (I—Inowrocław, C—Ciechocinek, Sa—Salzgraben, and So—Soltquelle); vectors indicate shoot (S) and root (R) traits.
2.4. Differences in Site Environmental Conditions
Soil variables were considered the main factors shaping population differences. The chemical analyses revealed pronounced variation in salinity and edaphic properties among the four inland sites where the S. europaea populations were collected (Table 5 and Table 6). For salinity (Table 5), the C-site showed the highest electrical conductivity (48.1 dS·m−1) and elevated Na+ (9427 mg·dm−3) and Cl− (16,990 mg·dm−3) concentrations. The I-site had the highest Ca2+ content (3578 mg·dm−3) and intermediate Na+ (3296 mg·dm−3). By contrast, the Sa- and So-sites had lower salinity, with mean ECe values of 12.7 and 11.0 dS·m−1, respectively, and comparatively lower ion concentrations.

Table 5.
Soil salinity characteristics of four inland sites (C—Ciechocinek, I—Inowrocław, Sa—Salzgraben, and So—Soltauquelle) where Salicornia europaea populations were collected. Values represent mean ± standard deviation (SD, n = 3). Different letters within each salinity treatment indicate significant differences between sites according to the Kruskal–Wallis test followed by post hoc Dunn’s test (p < 0.05).

Table 6.
Soil trophic characteristics of four inland sites (C—Ciechocinek, I—Inowrocław, Sa—Salzgraben, and So—Soltauquelle) where Salicornia europaea populations were collected. Values represent mean ± standard deviation (SD, n = 3) for total organic carbon (TOC), total nitrogen (TN), C/N ratio, soil pH (in H2O and KCl), calcium carbonate (CaCO3), and available phosphorus (Pca). Different letters within each salinity treatment indicate significant differences between sites according to the Kruskal–Wallis test followed by post hoc Dunn’s test (p < 0.05).
For trophic status (Table 6), the I-site had the highest total organic carbon (4.96%), total nitrogen (0.50%), and CaCO3 (29.5%), whereas the C-site had the lowest TOC (1.30%) and TN (0.15%). The Sa- and So-sites showed intermediate TOC and TN values, with So distinguished by the highest available phosphorus (Pca, 1628 mg·kg−1). The soil pH was slightly alkaline across all the sites, ranging from 7.3 to 8.5 in H2O and 7.7 to 8.3 in KCl.
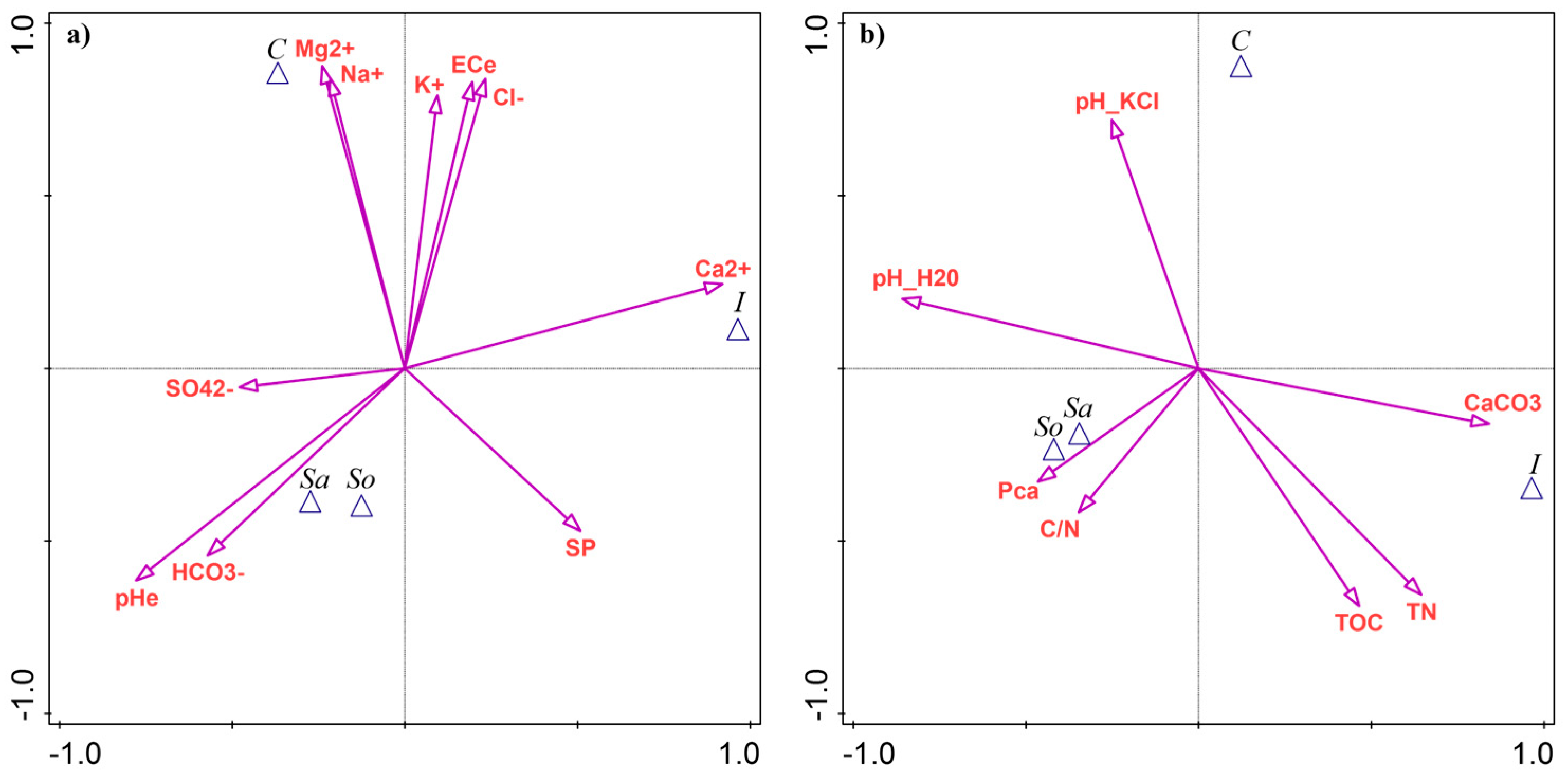
Based on CVA with forward selection and Monte Carlo permutation testing, soil pH (pHe) explained the largest proportion of variance between the sites (32.8%, Appendix A Table A3). The Sa- and So-sites had the highest pHe values (Figure 4a). Calcium (Ca2+) was the second most discriminating variable, contributing 30.6% of the explained variance, with elevated levels in the I-site (Figure 4a). The highest total salinity, expressed as ECe and ion contents (Mg2+, Na+, K+, and Cl−), was associated with the C-site, explaining 21–27% of the variability between sites (Table A3). Bicarbonates (HCO3−) explained 21.0% of the variance and were linked with the Sa- and So-sites (Figure 4a).
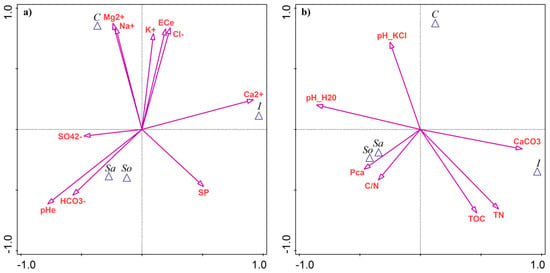
Figure 4.
Results of canonical variate analysis (CVA) as a discriminant analysis of soil parameters between four Salicornia europaea sites: (a) salinity-related variables; (b) edaphic parameters. Site abbreviations: C—Ciechocinek, I—Inowrocław, Sa—Salzgraben, and So—Soltauquelle. Soil parameters: pHe—pH of saturated paste extract, ECe—electrical conductivity of saturated paste extract, SP—saturation percentage, TN—total nitrogen, TOC—total organic carbon, and Pca—plant-available phosphorus.
Only four edaphic parameters contributed significantly to site separation, each explaining ~25% of the variance. Soil pH in water (pH-H2O) was followed by CaCO3, total nitrogen, and total carbon (Table A3). The Sa- and So-sites were associated with pH-H2O, while the I-site was linked with the remaining variables (Figure 4b).
3. Discussion
This study demonstrates that inland populations of S. europaea exhibit distinct above- and below-ground functional strategies when exposed to salinity gradients under controlled growth chamber conditions. The significant population and population × salinity effects revealed by ANOVA confirm that the shoot and root traits diverged consistently across the stress levels, indicating that intraspecific variability reflects phenotypic plasticity and population-specific differentiation. Such differentiation is increasingly recognized as a key driver of ecological stability in inland halophyte systems [5,27,28].
3.1. Aboveground Responses
The most responsive shoot trait was the S-Projected area, followed by S-Height, S-diameter, S-Shape perimeter, and S-Canopy. The populations differed in their strategies of canopy development under stress. Despite modest root investment, Inowrocław maintained strong canopy expansion (high S-Projected area and S-Shape perimeter), suggesting a strategy of prioritizing photosynthetic surface and carbon gain under salinity. Salzgraben sustained S-Projected area and R-Length, reflecting a balanced growth strategy across the compartments. Soltauquelle expressed high S-Height with long roots at moderate salinity, but this coordination declined at 1000 mM, indicating allocation trade-offs at extreme stress levels. In contrast, Ciechocinek consistently displayed low shoot trait values across the treatments, indicative of restricted tolerance thresholds and early growth limitations. These patterns align with previous observations in halophytes, where vertical elongation or canopy expansion serve as a key adaptive mechanism to sustain water uptake and photosynthetic efficiency under moderate salinity but are compromised under extreme ionic stress [1,29].
3.2. Belowground Responses
R-Projected area, R-Shape perimeter, and R-Length emerged as critical discriminants of tolerance strategies. The divergence across the populations reflected a shift from radial expansion (R-Projected area and R-Shape perimeter) under moderate salinity to axial elongation (R-Length) under higher stress. This is consistent with the findings regarding Suaeda salsa and Tamarix spp., where radial thickening initially enhances water and ion buffering, while elongation later supports deeper soil exploration under severe salt stress [30,31]. Salzgraben and Soltauquelle maintained higher R-Length under moderate and high salinity, whereas Inowrocław displayed consistently reduced root elongation, confirming compartment-specific trade-offs. These results reinforce the idea that belowground plasticity provides critical buffering capacity for aboveground growth but varies strongly with population origin.
3.3. Soil–Trait Linkages
Although the plants were grown under standardized conditions, the maternal-site soil analyses provided important ecological context. Ciechocinek, enriched in Na+ and Cl− with very high ECe, produced offspring with limited S- and R-traits across the treatments, reflecting inherited constraints under ionic toxicity. Inowrocław, originating from Ca2- and organic matter-rich soils, expressed strong S-Projected area and S-Shape perimeter, consistent with calcium’s role in maintaining membrane stability and Na+/K+ homeostasis [32]. Salzgraben displayed sustained R-Length and S-Projected area, and Soltauquelle showed high S-Height and R-Length at moderate salinity, highlighting the role of buffering capacity of carbonate soil and phosphorus availability in energy metabolism and osmotic adjustment [33]. The CVA ordinations confirmed these associations, indicating that morphometric responses under controlled salinity align with site-specific edaphic profiles. This suggests that populations retain an ecological “memory” of their soil environments, expressed through coordinated S–R trait combinations.
3.4. Applied Significance
From an applied perspective, population-specific strategies highlight the importance of selecting appropriate ecotypes for ecological restoration of inland salt marshes, breeding programs targeting biofunctional traits, or industrial uses such as biofuel and nutraceutical production. For example, populations investing in canopy expansion (Inowrocław, with high S-Projected area and S-Shape perimeter) may be more suitable for biomass applications, whereas those maintaining deeper root elongation (Salzgraben and Soltauquelle: high R-Length) may be better candidates for soil stabilization and remediation purposes. Furthermore, the link between soil ionic profiles and plant functional traits underscores the need to consider site edaphic history when designing restoration or cultivation programs.
3.5. Integration with Halophyte Ecology
These findings support the broader concept that halophyte populations adapt through general plasticity and site-specific selective pressures, resulting in ecotypic divergence [12,24]. In S. europaea, this divergence is expressed as distinct shoot–root trait combinations: canopy-dominant (Inowrocław: strong S-Projected area and S-Shape perimeter), balanced (Salzgraben: sustained S-Projected area and R-Length), vertically elongated (Soltauquelle: high S-Height and R-Length), and dwarf conservative (Ciechocinek: low S- and R-traits).
These patterns are summarized in Figure 3, which integrates the shoot, root, and soil parameters across the populations. The conceptual model illustrates that Ciechocinek is constrained by high-Na+/Cl− soils, leading to poor canopy and short roots; Inowrocław benefits from Ca2+-rich soils, sustaining canopy expansion despite modest root investment. Salzgraben and Soltauquelle, associated with carbonate-rich soils and enriched in available phosphorus (Pca), maintain balanced shoot–root elongation under moderate salinity. The figure underscores that populations retain ecological memory of their maternal soils, expressed as contrasting functional strategies under controlled salinity gradients. Such strategies mirror the patterns described in other halophytes (Atriplex, Tripolium, and Suaeda), where divergence across populations enhances community-level resilience under fluctuating saline conditions [5,29].
3.6. Limitations and Perspectives
While the controlled chamber approach enabled direct testing of salinity gradients, it also restricted root exploration and microbial interactions, which are central in field conditions. Future studies should integrate physiological and biochemical measurements (ion accumulation, osmotic adjustment, and stress markers) and exploit non-destructive technologies, such as computer vision systems (CVSs) and hyperspectral imaging, for real-time monitoring of plant performance [34]. Combining morphometric, physiological, and omics approaches will enable a deeper mechanistic understanding of how S. europaea populations integrate soil legacy with functional trait responses.
4. Materials and Methods
4.1. Field Work
Seeds of S. europaea were collected in late autumn 2022 from four isolated populations (Figure 5): two in Poland—Ciechocinek (52°53′20.3″ N 18°47′06.4″ E; C-population) and Inowrocław (52°45′35.2″ N 18°13′39.9″ E; I population), sampled in November, and two in Germany—Salzgraben–Salzdahlum (52°11′56.8″ N 10°36′05.3″ E; Sa-population) and Soltauquelle (52°05′23.7″ N 10°49′17.9″ E; So-population), sampled in December. According to the Köppen–Geiger classification [35], the study areas in Poland and Germany have a warm-temperate fully humid climate with warm summers (Cfb). The mean annual air temperature and precipitation for the German sites are 9.9 °C and 614.8 mm, respectively (Braunschweig station, 1991–2020; http://sklima.de; accessed on 24 October 2025). For the Polish sites, the corresponding values are 8.9 °C and 548.8 mm (Toruń station, 1991–2020; https://klimat.imgw.pl; accessed on 26 October 2025). Sampling points were located in Salicornietum ramossissimae association [36]. In each site, 30 random individuals of S. europaea containing seeds were taken and then dried for seed collection. The C-population was located in the nature reserve protecting natural inland salt marshes, which was established in 1964 [37]. Due to the historical drainage of the Vistula Valley, halophytic vegetation is now restricted to a ditch system crossing the reserve. It is not regularly supported by brine from the graduation towers nearby, but the recorded soil salinity is very high, amounting to about 100 dS·m−1 [38]. The I-population originates from the Inowrocław–Mątwy industrial saline site located adjacent to the soda factory and affected by the saline waste of soda production, resulting in relatively stable and very high soil salinity around 55 dS·m−1 [28,39]. The Sa-population was collected from Salzgraben–Salzdahlum, a naturally inland saline wetland in northern Germany. This habitat is characterized by stable groundwater discharge with long-term consistent salinity exposure [12]. The So-population was gathered from Soltauquelle, a spring-fed inland wetland in northern Germany with shallow calcareous soils. This site experiences strong seasonal shifts in groundwater flow, leading to highly variable salinity conditions [12]. For soil characteristics, four soil samples were taken from the root zone (0–25 cm) for laboratory analyses at each site.
Figure 5.
Sampling locations in Germany and Poland.
4.2. Pot Experiment
Seeds of S. europaea were germinated in Petri dishes on filter paper moistened with distilled water. After germination, seedlings approximately 5 mm long were transplanted into individual plastic pots (5.3 cm height, 5.5 cm diameter, and 85.7 cm3 volume) filled with a 1:2 mixture of vermiculite and quartz sand. Before planting, nine pots per treatment and population were placed in cuvettes that lacked drainage and were watered to full substrate capacity with relevant solutions of 0, 200, 400, or 1000 mM NaCl. Initially, each pot contained 3–4 seedlings of the respective population, and, later, they were thinned to one plant per pot.
For the first 20 days after transplantation, pots were irrigated every three days with 300 cm3 of distilled water adjusted to the assigned NaCl concentration. Thereafter, irrigation continued every three days with 250 cm3 of modified Hoagland’s nutrient solution of the same parameters for each salt treatment. The stock solutions were prepared as follows: 236.1 g·dm−3 Ca(NO3)2·4H2O, 101.1 g·dm−3 KNO3, 136.1 g·dm−3 KH2PO4, and 246.5 g·dm−3 MgSO4·7H2O. Trace elements were prepared per 1 dm3 as 2.8 g H3BO3, 1.8 g MnCl2·4H2O, 0.2 g ZnSO4·7H2O, 0.025 g H2MoO4, and 0.1 g CuSO4·5H2O. Iron was supplied as Fe-EDTA, obtained by mixing 10.4 g EDTA·2Na and 7.8 g FeSO4·7H2O in 56.1 g KOH solution, with pH adjusted to 5.5 before mixing. The final Hoagland working solution per 1 dm3 contained 7 cm3 Ca(NO3)2, 5 cm3 KNO3, 2 cm3 KH2PO4, 2 cm3 MgSO4, 1 cm3 trace elements, and 1 cm3 Fe-EDTA solution. Plants were maintained under controlled growth chamber conditions: 25 °C day temperature, 50–60% relative humidity, and a 16 h light/8 h dark photoperiod.
4.3. Morphometric Plant Measurements
To quantify the morphological variation of S. europaea across salinity treatments and populations, all shoot and root traits were measured using digital image analysis. Each shoot was imaged in two orthogonal orientations, front (side-view) and top-view, against a uniform white background with a calibrated metric scale. Images were processed in Fiji (ImageJ v1.53c) using a standardized workflow to segment the shoot silhouette and extract morphometric traits [40,41]. The S-Projected area was defined as the two-dimensional area of the aboveground shoot silhouette captured in a standardized side-view image. S-Canopy referred to the planform area of the shoot obtained from a top-view image. S-Shape perimeter was the total length of the shoot silhouette contour measured on the segmented image. S-Height was the vertical distance from the stem base to the apical tip, and S-Diameter was the maximum width orthogonal to shoot height. For each population and NaCl treatment (0, 200, 400, and 1000 mM), nine shoots (n = 9 per population × treatment) were imaged under identical conditions, and all metrics were computed from the resulting binary masks in calibrated units.
Root traits comprised R-Projected area (cm2), R-Shape perimeter (cm), and R-Length (cm). Immediately after harvesting and gentle rinsing, roots were photographed in planar (front) view against a matte black background with an embedded scale bar. Images were analyzed in Fiji using the same segmentation workflow as for shoots. R-Projected area represents the total two-dimensional area of the root silhouette, R-Shape perimeter corresponds to the external contour of the whole root, and R-Length denotes the longest visible axis across the captured root system. A total of three root systems per population per salinity treatment (n = 3) were analyzed.
4.4. Soil Analyses
The soil samples were air-dried, passed through a 2 mm mesh sieve to remove large debris, and homogenized. The contents of total carbon (TC) and total nitrogen (TN) were determined using a Vario Macro Cube CHN/CHNS elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). The calcium carbonate (CaCO3) content was measured volumetrically using a Scheibler apparatus following the procedure described by Pansu and Gautheyrou [42]. Total inorganic carbon (TIC) was calculated from the CaCO3 content, and total organic carbon (TOC) was obtained as the difference between TC and TIC. Soil pH was measured potentiometrically with a CP-551 pH meter (Elmetron, Poland) in both a distilled water suspension (pH-H2O) and a 1 M KCl solution (pH-KCl) at a soil-to-solution ratio of 1:2.5. The plant-available phosphorus (Pca) content was determined colorimetrically using a UV-1601 spectrophotometer (Rayleigh, China) after extraction with ammonium citrate solution [43].
Analyses of the saturated paste extract were conducted using the methodology described by van Reeuwijk [43]. Portions of air-dried soil were placed into 250 cm3 beakers to about half their volume (≈200 g) and mixed with redistilled water added incrementally until a soil paste was obtained. The beakers were covered and left overnight. The paste was then vacuum-filtered on a Büchner funnel using a vacuum pump. The resulting saturated paste extract was used for further analysis. The extract pH (pHe) was measured potentiometrically using a CP-551 pH meter (Elmetron). Electrical conductivity at 25 °C (ECe) was determined by the conductometric method with a CPC-401 conductivity meter (Elmetron). Saturation percentage (SP) was determined gravimetrically by oven-drying. Magnesium (Mg2+) and calcium (Ca2+) were measured by atomic absorption spectrometry (AAS), whereas potassium (K+) and sodium (Na+) were determined by flame emission spectrometry (FES) using a SOLAAR Unicam 969 flame spectrometer (Unicam Ltd.,Cambridge, UK). Bicarbonate (HCO3−) was determined by titration following Pokojska [44]. Sulfate (SO42−) and chloride (Cl−) were measured by ion chromatography using a Thermo Scientific Dionex Aquion system (Thermo Scientific, Waltham, MA, USA).
4.5. Statistical Analysis
We evaluated morphological responses of S. europaea across four populations (Inowrocław, Ciechocinek, Saltzgrabe, and Soltquelle) subjected to four salinity levels (0, 200, 400, and 1000 mM NaCl) in a fully crossed two-factor design. Two-way ANOVA (ANOVA-II) tested the effects of population, salinity, and their interaction on morphological traits. Normality was checked with the Shapiro–Wilk test and homogeneity of variance by Levene’s test (p < 0.05). Normally distributed traits were further analyzed using one-way ANOVA (ANOVA-I) with Tukey’s HSD post hoc test, applied per trait and salinity level. The Kruskal–Wallis test with Dunn’s post hoc comparisons was used for skewed traits. Analyses were performed in PAST v.5.2.1 [45].
Canonical variate analysis (CVA) assessed trait contributions to population differentiation under each treatment, using forward selection and Monte Carlo permutation tests [46,47]. The simple effects model, focusing on independent trait contributions, was interpreted. All CVA analyses were run in Canoco v.5.
Morphometric differences across salinity treatments were summarized with non-metric multidimensional scaling (NMDS) using Euclidean distance [47]. Before analysis, shoot and root data were z-standardized (mean = 0, SD = 1). Analyses were based on population means per salinity level, with trait vectors overlaid to show direction and magnitude of influence.
Soil parameters were grouped into salinity-related (ECe, Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−) and edaphic variables (TOC, TN, C/N, CaCO3, P2O5, Pca, pH-H2O, and pH-KCl). Differences between sites were tested with the Kruskal–Wallis test and Dunn’s post hoc comparisons (PAST v.5.2.1). To evaluate variable contributions to site separation, CVA with forward selection and Monte Carlo permutation tests were applied to both soil groups in Canoco v.5.
5. Conclusions
We found a strong relationship between population origin and responses to salt stress. Shoot canopy area consistently best discriminated among populations, and the I- and Sa-populations performed best under extreme salinity (1000 mM), whereas the C-population showed the weakest growth. The root analyses revealed a shift from radial expansion at moderate salinity to elongation at higher levels, with the Sa- and So-populations maintaining the longest roots. The soil analyses indicated substantial site differences: the C-site had the highest salinity, resulting in the smallest plant trait development, the I-site was rich in Ca2+ and organic matter, while the Sa- and So-sites had lower salinity but higher pH and bicarbonates. These findings demonstrate population-specific adaptive strategies and soil-legacy effects, supporting targeted ecotype selection for saline agriculture and phytoremediation.
Author Contributions
A.P.: writing—original draft, data curation, formal analysis, methodology, review and editing; N.E.G.: formal analysis, methodology, review and editing; P.H.: formal analysis, methodology, data curation, review and editing; A.R.D.: formal analysis, data curation, review and editing; S.C.P.: writing—original draft, visualization, formal analysis, methodology, data curation, conceptualization, supervision, resources, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre in Poland, Project SONATA No. 2021/43/D/NZ8/01137.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Ricarda Pätsch from IBU Institute of Biology and Environmental Science, Carl Von Ossietzky Universität Oldenburg, for plant material and soil sample collection in Germany, and Adam Michalski, Adam Solarczyk, and Michał Dąbrowski from the Laboratory for Environmental Analysis, Faculty of Earth Sciences and Spatial Management, Nicolaus Copernicus University in Toruń, for their support in laboratory analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Supplementary data to the main text.

Table A1.
Results of canonical variate analysis (CVA) forward selection and Monte Carlo permutation test of shoot morphological trait effects in the discrimination between Salicornia europaea populations in different salt treatments (0, 200, 400, and 1000 mM NaCl). NS—not significant.
Table A1.
Results of canonical variate analysis (CVA) forward selection and Monte Carlo permutation test of shoot morphological trait effects in the discrimination between Salicornia europaea populations in different salt treatments (0, 200, 400, and 1000 mM NaCl). NS—not significant.
| Trait | % Variance Explained | p | % Variance Explained | p | % Variance Explained | p | % Variance Explained | p |
|---|---|---|---|---|---|---|---|---|
| 0 mM | 200 mM | 400 mM | 1000 mM | |||||
| S-Canopy | 22.8 | 0.002 | 28.9 | 0.002 | 30.7 | 0.002 | 29.7 | 0.002 |
| S-Diameter | 10.6 | 0.01 | 7.9 | 0.032 | 0.8 | NS | 10.3 | 0.008 |
| S-Height | 8.3 | 0.026 | 18.8 | 0.002 | 14 | 0.002 | 22.9 | 0.002 |
| S-Shape Perimeter | 7.5 | 0.036 | 13.3 | 0.002 | 9.9 | 0.008 | 26 | 0.002 |
| S-Projected Area | 6.7 | NS | 12.7 | 0.004 | 7.1 | 0.04 | 25.3 | 0.002 |

Table A2.
Results of canonical variate analysis (CVA) forward selection and Monte Carlo permutation test of shoot morphological trait effects in the discrimination between Salicornia europaea populations in different salt treatments (0, 200, 400, and 1000 mM NaCl).
Table A2.
Results of canonical variate analysis (CVA) forward selection and Monte Carlo permutation test of shoot morphological trait effects in the discrimination between Salicornia europaea populations in different salt treatments (0, 200, 400, and 1000 mM NaCl).
| Trait | % Variance Explained | p | % Variance Explained | p | % Variance Explained | p | % Variance Explained | p |
|---|---|---|---|---|---|---|---|---|
| 0 mM | 200 mM | 400 mM | 1000 mM | |||||
| R-Projected area | 15.9 | NS | 25.7 | 0.014 | 18.4 | NS | 29.4 | 0.004 |
| R-Length | 14.2 | NS | 13.8 | NS | 26.1 | 0.008 | 29.7 | 0.002 |
| R-Shape perimeter | 10.3 | NS | 25.2 | 0.014 | 22.7 | 0.016 | 29 | 0.004 |

Table A3.
Results of forward selection and Monte Carlo permutation test of canonical variate analysis (CVA)—simple effects of soil salinity parameters and soil edaphic conditions in the discrimination between S. europaea sites. pHe—pH of saturated paste extract, ECe—electrical conductivity of saturated paste extract, SP—saturation percentage, TN—total nitrogen, TOC—total organic carbon, and Pca—plant-available phosphorus.
Table A3.
Results of forward selection and Monte Carlo permutation test of canonical variate analysis (CVA)—simple effects of soil salinity parameters and soil edaphic conditions in the discrimination between S. europaea sites. pHe—pH of saturated paste extract, ECe—electrical conductivity of saturated paste extract, SP—saturation percentage, TN—total nitrogen, TOC—total organic carbon, and Pca—plant-available phosphorus.
| Soil Salinity | Soil Edaphic Condition | ||||
|---|---|---|---|---|---|
| Variable | Explains % | p | Variable | Explains % | p |
| pHe | 32.8 | 0.002 | pH-H2O | 25.3 | 0.002 |
| Ca2+ | 30.6 | 0.002 | CaCO3 | 24.7 | 0.01 |
| Mg2+ | 27.4 | 0.006 | TN | 24.6 | 0.006 |
| ECe | 25.3 | 0.006 | TOC | 19.3 | 0.032 |
| Na+ | 25.0 | 0.006 | pH-KCl | 15.5 | NS |
| Cl− | 24.2 | 0.004 | C/N | 10.5 | NS |
| K+ | 21.0 | 0.022 | Pca | 10.0 | NS |
| HCO3− | 21.0 | 0.008 | |||
| SP | 16.2 | NS | |||
| SO42− | 7.9 | NS | |||
References
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Xiao, X.; You, J.; Wang, J.; Wang, T.; Yao, Y.; Tian, C. Global transcriptome profiling of Salicornia europaea L. shoots under NaCl treatment. PLoS ONE 2013, 8, e65877. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of vacuolar membrane transport systems in plant salinity tolerance. J. Plant Growth Regul. 2023, 42, 1364–1401. [Google Scholar] [CrossRef]
- Rasouli, F.; Kiani-Pouya, A.; Shabala, L.; Li, L.; Tahir, A.; Yu, M.; Hedrich, R.; Chen, Z.; Wilson, R.; Zhang, H.; et al. Salinity effects on guard cell proteome in Chenopodium quinoa. Int. J. Mol. Sci. 2021, 22, 428. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes: Research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2012, 92, 83–91. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Piernik, A. Sabkha Ecosystems: Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Singh, D.; Buhmann, A.K.; Flowers, T.J.; Seal, C.E.; Papenbrock, J. Salicornia as a crop plant in temperate regions: Selection of genetically characterized ecotypes and optimization of their cultivation conditions. AoB Plants 2014, 6, plu071. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Orzoł, A.; Głowacka, K.; Pätsch, R.; Piernik, A.; Gallegos-Cerda, S.D.; Cárdenas-Pérez, S. The local environment influences salt tolerance differently in four Salicornia europaea L. inland populations. Sci. Rep. 2025, 15, 97394. [Google Scholar] [CrossRef]
- Piernik, A. Growth of three meadow species along a salinity gradient in an inland saline habitat: Transplant experiment. Pol. J. Ecol. 2006, 54, 117–125. [Google Scholar]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef] [PubMed]
- Katel, S.; Yadav, S.P.S.; Oli, S.; Adhikari, R.; Shreeya, S. Exploring the potential of Salicornia: A halophyte’s impact on agriculture, the environment, and the economy. Peruv. J. Agron. 2023, 7, 220–238. [Google Scholar] [CrossRef]
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Magné, C.; Foggi, G.; Conte, G.; Santin, M.; et al. Nutritional composition and bioactivity of Salicornia europaea L. plants grown in monoculture or intercropped with tomato plants in salt-affected soils. Horticulturae 2022, 8, 828. [Google Scholar] [CrossRef]
- Kamal, M.; Lele, W.; Shuzhen, T.; Jiandi, L.; Rongyan, Q.; Yanfeng, L.; Wenqi, W.; Xiangyu, C.; Cheng, Y. Influence of dietary Salicornia europaea L. extract supplementation on feed efficiency of Altay sheep by modifying their gastrointestinal bacteria communities. Front. Microbiol. 2024, 15, 1377314. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Piernik, A.; Łuczak, K.; Mendyk, Ł.; Hulisz, P. The behavior of potentially toxic elements in the technogenic soil–plant system: A study of Salicornia europaea L. from sites affected by the soda industry. Ecol. Modell. 2023, 486, 110517. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. The Salicornia europaea potential for phytoremediation of heavy metals in soils under different times of wastewater irrigation in northwestern Iran. Environ. Sci. Pollut. Res. 2021, 28, 47605–47618. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Parnian, A.; Moradi, A.; Piernik, A.; Garcia Caparros, P.; Hulisz, P. Salts phytoremediation by halophytes in soil and aquatic environments: New mechanisms, promising species, and challenges. Soil Sci. Annu. 2025, 76, 206904. [Google Scholar] [CrossRef]
- Rajhi, H.; Sanz, J.L.; Bardi, A.; Rojas, P. Marine sediments in the Gulf of Gabes (Tunisia) heavily polluted by phosphogypsum and human microbiota bacteria: Phytoremediation by Salicornia europaea as a nature-based solution. Environ. Sci. Pollut. Res. 2024, 31, 37652–37662. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Beierkuhnlein, C.; Thiel, D.; Jentsch, A.; Willner, E.; Kreyling, J. Ecotypes of European grass species respond differently to warming and extreme drought. J. Ecol. 2011, 99, 703–713. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Cárdenas Pérez, S.; Rajabi Dehnavi, A.; Leszczyński, K.; Lubińska-Mielińska, S.; Ludwiczak, A.; Piernik, A. Salicornia europaea L. functional traits indicate its optimum growth. Plants 2022, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas Pérez, S.; Strzelecki, J.; Piernik, A.; Rajabi Dehnavi, A.; Trzeciak, P.; Puchałka, R.; Mierek-Adamska, A.; Chanona Pérez, J.; Kačík, F.; Račko, V.; et al. Salinity-driven changes in Salicornia cell wall nanomechanics and lignin composition. Environ. Exp. Bot. 2024, 218, 105606. [Google Scholar] [CrossRef]
- Kalwasińska, A.; Hulisz, P.; Szabó, A.; Binod Kumar, S.; Michalski, A.; Solarczyk, A.; Wojciechowska, A.; Piernik, A. Technogenic soil salinisation, vegetation, and management shape microbial abundance, diversity, and activity. Sci. Total Environ. 2023, 905, 167380. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczak, A.; Kapusta, P.; Chapko, P.; Wojtasik, J.; Wojciechowska, A.; Piernik, A. Parental environment as a factor shaping salinity tolerance in halophyte Tripolium pannonicum L. Environ. Exp. Bot. 2024, 228, 106008. [Google Scholar] [CrossRef]
- Li, H.; Duijts, K.; Pasini, C.; van Santen, J.E.; Lamers, J.; de Zeeuw, T.; Verstappen, F.; Wang, N.; Zeeman, S.C.; Santelia, D.; et al. Effective root responses to salinity stress include maintained cell expansion and carbon allocation. New Phytol. 2023, 238, 1942–1956. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2018, 45, 9–27. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Cárdenas Pérez, S.; Grigore, M.-N.; Piernik, A. Prediction of Salicornia europaea L. biomass using a computer vision system to distinguish different salt-tolerant populations. BMC Plant Biol. 2024, 24, 1086. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Lubińska-Mielińska, S.; Kącki, Z.; Kamiński, D.; Pétillon, J.; Evers, C.; Piernik, A. Vegetation of temperate inland salt marshes reflects local environmental conditions. Sci. Total Environ. 2023, 856, 159015. [Google Scholar] [CrossRef] [PubMed]
- Wilkoń-Michalska, J. Zmiany sukcesyjne w rezerwacie halofitów Ciechocinek w latach 1954–1965. Ochr. Przyr. 1970, 35, 1–56. [Google Scholar]
- Lubińska-Mielińska, S.; Kamiński, D.; Hulisz, P.; Krawiec, A.; Walczak, M.; Lis, M.; Piernik, A. Inland salt marsh habitat restoration can be based on artificial flooding. Glob. Ecol. Conserv. 2022, 34, e02028. [Google Scholar] [CrossRef]
- Piernik, A.; Hulisz, P.; Rokicka, A. Micropattern of halophytic vegetation on technogenic soils affected by the soda industry. Soil. Sci. Plant Nutr. 2015, 61, 98–112. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Ludwiczak, A.; Duszyn, M.; Szmidt-Jaworska, A.; Chanona-Pérez, J.J. Image and fractal analysis as a tool for evaluating salinity growth response between two Salicornia europaea populations. BMC Plant Biol. 2020, 20, 233. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, T.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006; pp. 596–599. [Google Scholar]
- Van Reeuwijk, L.P. (Ed.) Procedures for Soil Analysis (Technical Paper 9); ISRIC—World Soil Information: Wageningen, The Netherlands, 2002. [Google Scholar]
- Pokojska, U. Przewodnik Metodyczny do Analizy Wód [Methodical Guide for Water Analysis]; Wydawnictwo UMK: Toruń, Poland, 1999. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Biometrics: Wageningen, The Netherlands; České Budějovice, Czech Republic, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).