Variations in Flavonoid Metabolites Among Forsythia suspensa Populations in Response to Environmental Heterogeneity
Abstract
1. Introduction
2. Results
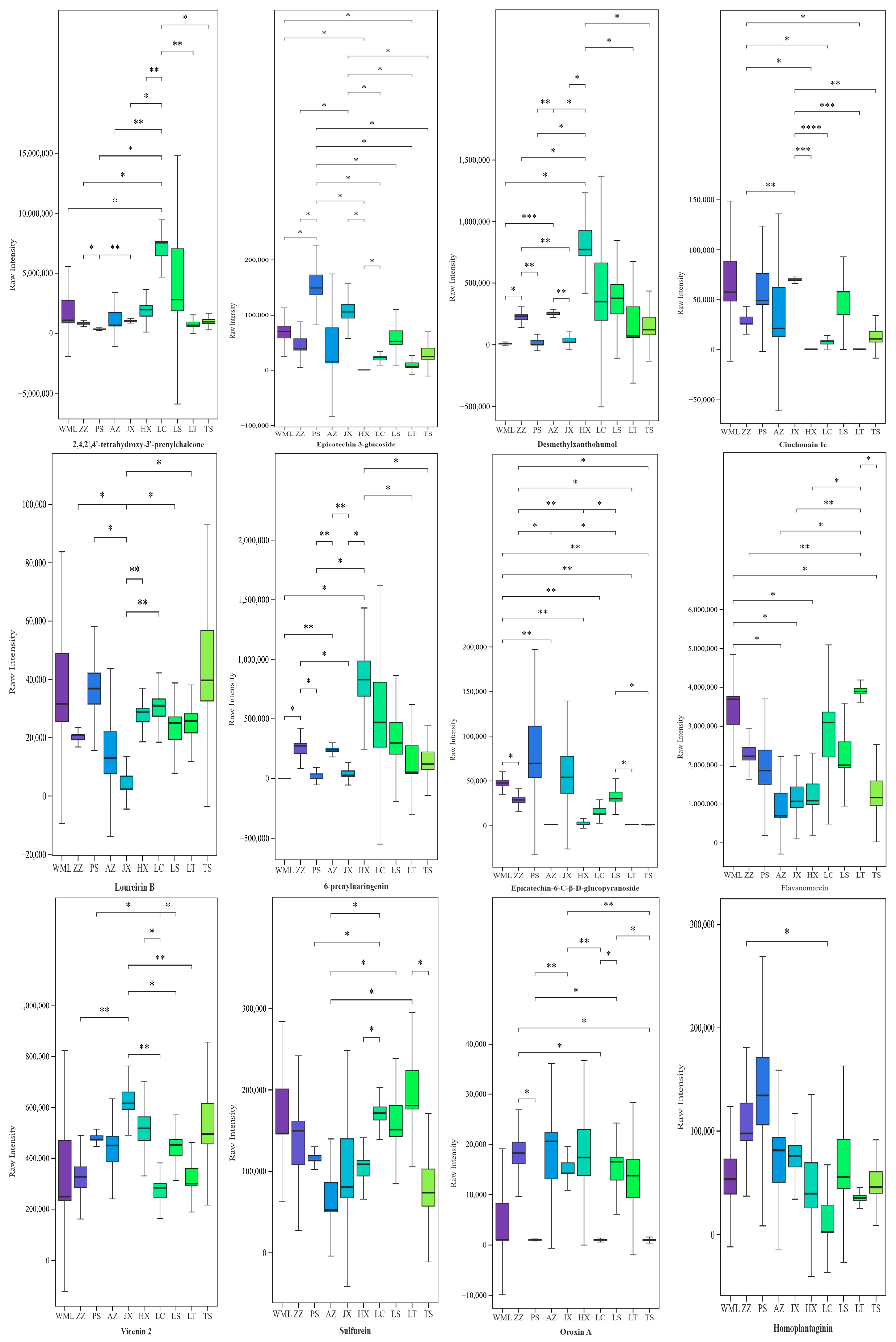
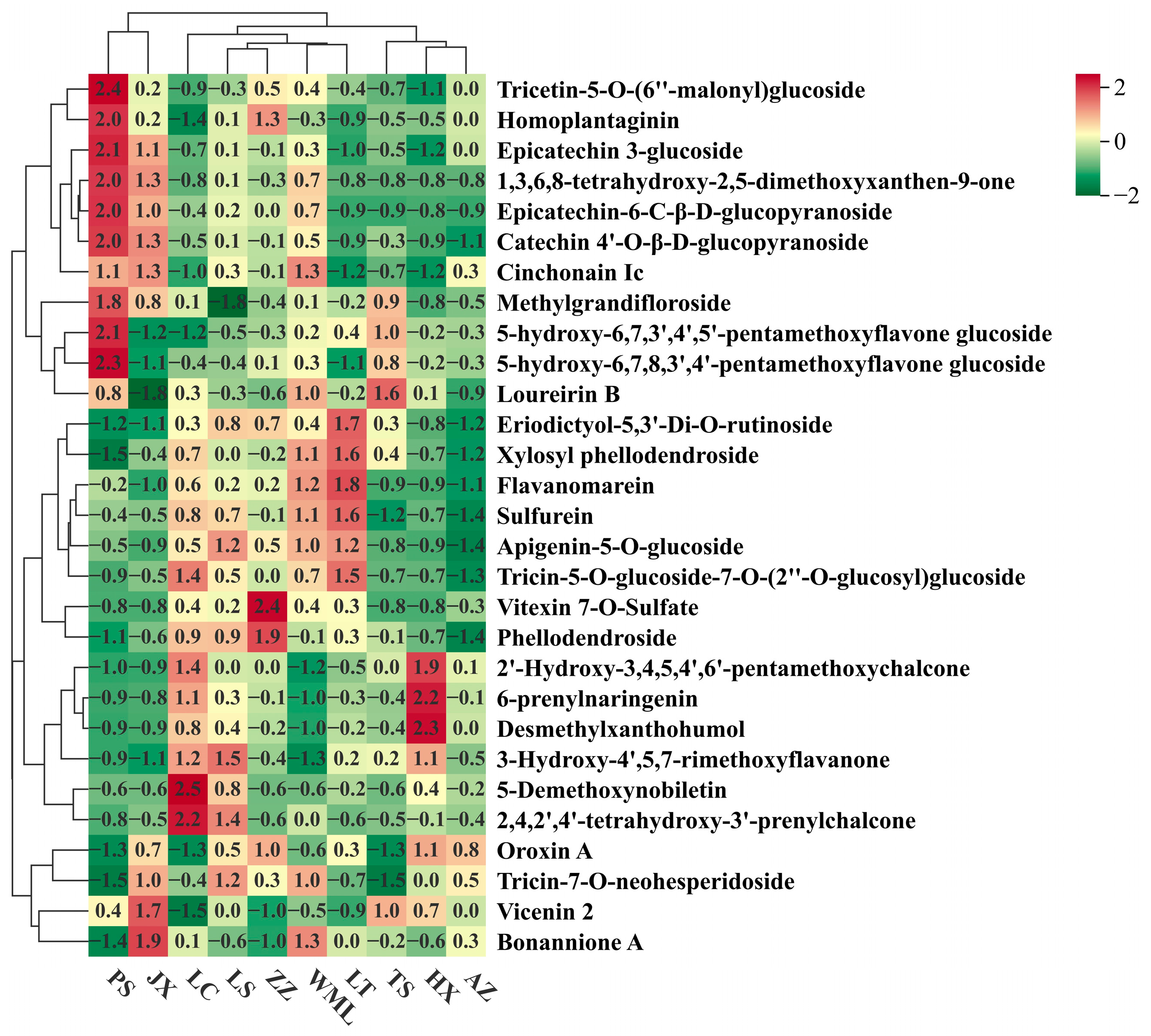
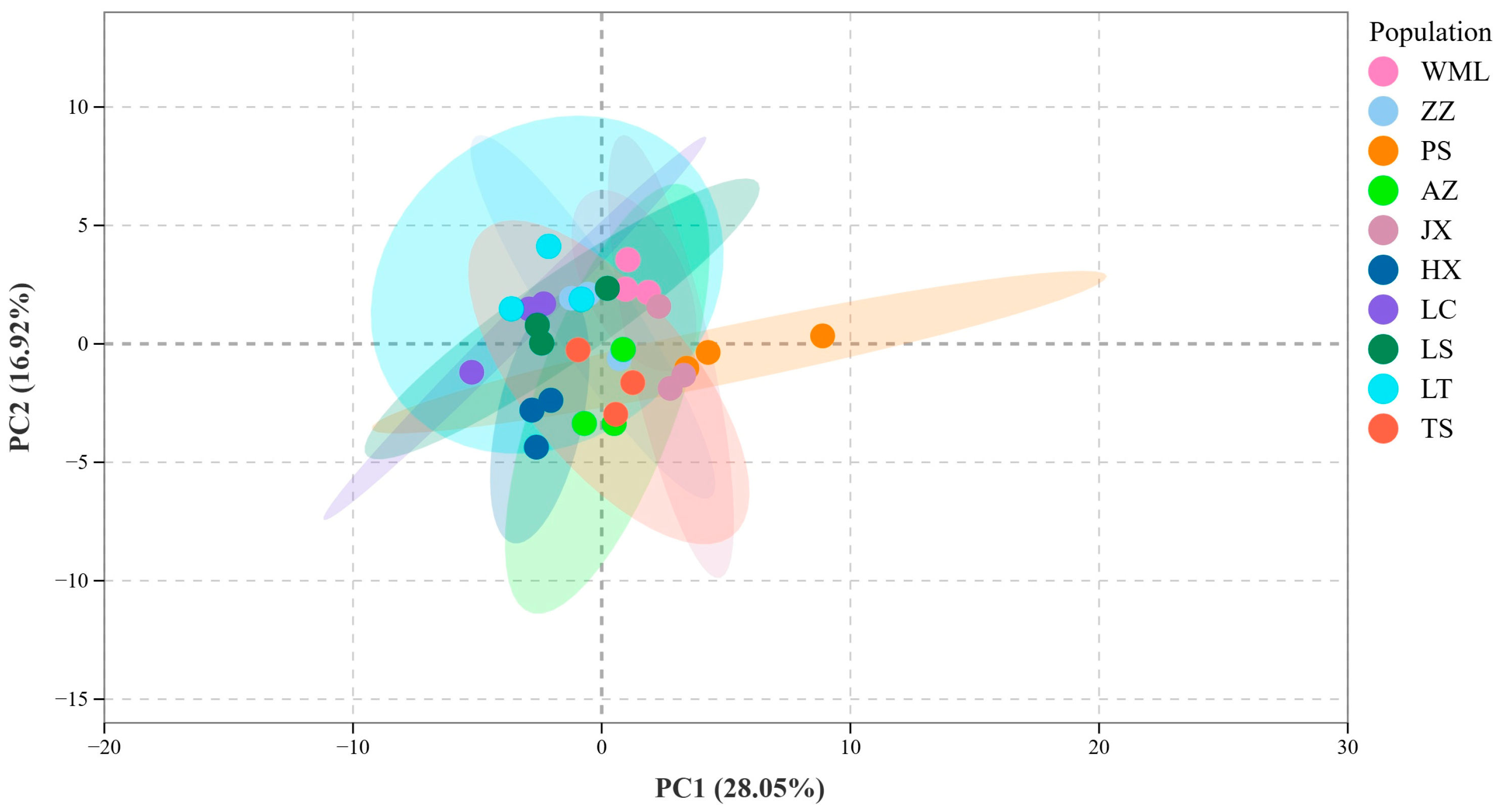
2.1. Variation in Flavonoid Metabolites Among F. suspensa Populations
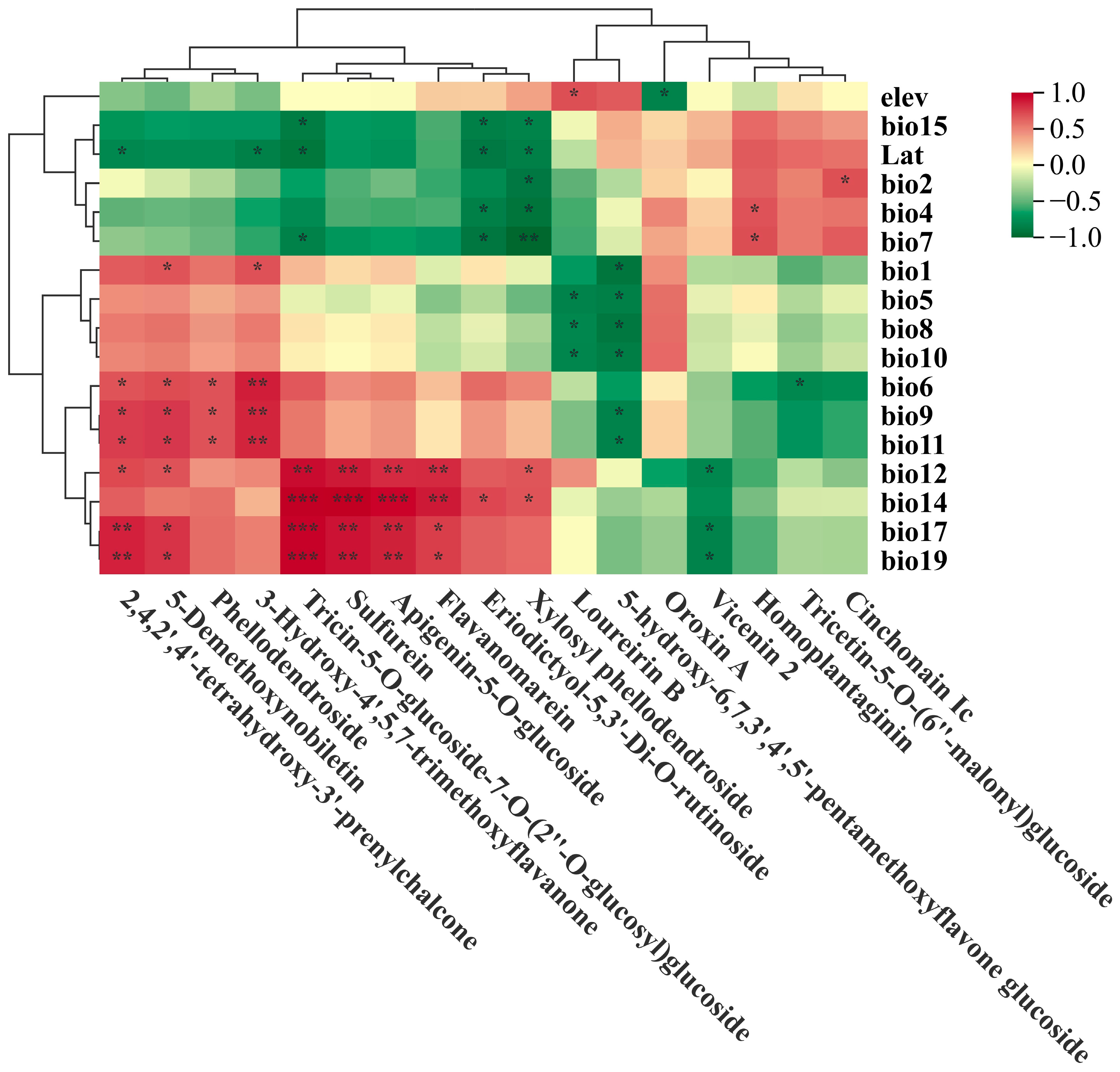
2.2. Relationship Between Environmental Variables and Flavonoid Metabolites in F. suspensa
2.2.1. Temperature-Correlated Metabolites
2.2.2. Precipitation-Correlated Metabolites
2.2.3. Latitude and Elevation Correlated Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Flavonoids Metabolic Profiling
4.3. Environmental Variables
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.; Lu, X.; Tong, X.; Dong, Y.; Tang, L.; Liu, M. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules 2017, 22, 1466. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. Japanese Pharmacopoeia, 17th ed.; The Stationery Office: Tokyo, Japan, 2016. [Google Scholar]
- The Korea Food and Drug Administration. South Korean Pharmacopoeia, Monografs Part II; Ministry of Health and Welfare: Se jong, Republic of Korea, 2015. [Google Scholar]
- Presbyterian Church in the United States. United States Pharmacopeia; The Pharmaceutial Association: Bethesda, MD, USA, 2017. [Google Scholar]
- Commission for Protection of Competition. Chinese Pharmacopoeia, 2020 Edition; China Medical Science Press: Beijing, China, 2020; Volume I. [Google Scholar]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, W.; Liu, Y.; He, D.; Li, Y. Adaptive genetic variation in the smoke tree (Cotinus coggygria Scop.) is driven by precipitation. Biochem. Syst. Ecol. 2015, 59, 63–69. [Google Scholar] [CrossRef]
- Yang, J.; Miao, C.Y.; Mao, R.L.; Li, Y. Landscape Population Genomics of Forsythia (Forsythia suspensa) Reveal That Ecological Habitats Determine the Adaptive Evolution of Species. Front. Plant Sci. 2017, 8, 481. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, X.; Yang, J.; Qian, C.; Yin, X.; Fan, X.; Fang, T.; Gao, Y.; Chang, Y.; Liu, W.; et al. Variations in Flavonoid Metabolites Along Altitudinal Gradient in a Desert Medicinal Plant Agriophyllum squarrosum. Front. Plant Sci. 2021, 12, 683265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, J.; Qian, C.; Yin, X.; Yan, X.; Fan, X.; Fang, T.; Gao, Y.; Chang, Y.; Ma, X.-F. Organic acid metabolites involved in local adaptation to altitudinal gradient in Agriophyllum squarrosum, a desert medicinal plant. J. Plant Res. 2021, 134, 999–1011. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, S.; Qian, C.; Yan, X.; Yin, X.; Fan, X.; Zhao, P.; Liao, Y.; Shi, L.; Chang, Y.; et al. Corrigendum: Integrated metabolomics and transcriptomics insights on flavonoid biosynthesis of a medicinal functional forage, Agriophyllum squarrosum (L.), based on a common garden trial covering six ecotypes. Front. Plant Sci. 2022, 13, 1092707. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, C.; Xu, H.; Wang, Y.; Zhang, M.; Huo, C.; Shi, Q.; Yu, S. Chemical Constituents of Plants from the Genus forsythia. Mini-Rev. Org. Chem. 2012, 9, 303–318. [Google Scholar] [CrossRef]
- Liu, S.; Niu, X.; He, J.; Yue, C.; Jiang, Z.; Yu, Z.; Liu, Y. Chemical constituents from the fruits of Forsythia suspensa and their chemotaxonomic significance. Biochem. Syst. Ecol. 2025, 120, 104984. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Pu, Z.; Wang, J.; Zheng, Y.; Li, Y.; Wei, Y. Regulation, evolution, and functionality of flavonoids in cereal crops. Biotechnol. Lett. 2013, 35, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Clayton, W.A.; Deroles, S.C.; Schwinn, K.E.; Bowman, J.L.; Jordan, B.R.; Davies, K.M. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytol. 2018, 218, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Qi, X.; Lin, M.; Zhong, Y.; Sun, L.; Cui, W. Combined Analysis of the Fruit Metabolome and Transcriptome Reveals Candidate Genes Involved in Flavonoid Biosynthesis in Actinidia arguta. Int. J. Mol. Sci. 2018, 19, 1471. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef]
- Lei, Z.; Sumner, B.W.; Bhatia, A.; Sarma, S.J.; Sumner, L.W. UHPLC-MS Analyses of Plant Flavonoids. Curr. Protoc. Plant Biol. 2019, 4, e20085. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Fu, Z.; Li, Y.; Zhang, K.; Li, Y. Molecular data and ecological niche modeling reveal population dynamics of widespread shrub Forsythia suspensa (Oleaceae) in China’s warm-temperate zone in response to climate change during the Pleistocene. BMC Evol. Biol. 2014, 14, 114. [Google Scholar] [CrossRef]
- Fu, Z.; Lei, Y.; Peng, D.; Li, Y. Population genetics of the widespread shrub Forsythia suspensa (Oleaceae) in warm-temperate China using microsatellite loci: Implication for conservation. Plant Syst. Evol. 2016, 302, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Cushman, S.A.; He, Y.; Li, Y. Genome sequencing and population genomics modeling provide insights into the local adaptation of weeping forsythia. Hortic. Res. 2020, 7, 130. [Google Scholar] [CrossRef]
- Guo, Y.P.; Lin, L.G.; Wang, Y.T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chin. Med. 2015, 10, 16. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liu, X.; Li, C.; Xu, S.; Farooq, A. Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol. Biochem. Behav. 2013, 105, 134–141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Pei, N.; Li, Q.; Liu, H.; Yuan, W.; Zhang, H. The updated weeping forsythia genome reveals the genomic basis for the evolution and the forsythin and forsythoside A biosynthesis. Hortic. Plant J. 2023, 9, 1149–1161. [Google Scholar] [CrossRef]
- Wang, H. The Influence of Different Environmental Conditions on Growth Process of Forsythia suspense. Acta Chin. Med. 2014, 29, 1630–1631+1634. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Jin, J.; Cui, L.; Niu, S. Effects of Different Growth Time and Ecological Factors on Phillyrin Content in Forsythia suspensa Leaves. Mod. Chin. Med. 2025, 27, 1089–1093. [Google Scholar] [CrossRef]
- Suo, X.; Liu, C.; Zhao, Y.; Chenhui, D.; Ping, L.; Zhan, H.; He, R.; Shang, C.; Zhang, X.; Shi, T.; et al. Study on the Quality Regionalization of Forsythia suspensa (Thunb.) Vahl in Shanxi Province Based on MaxEnt Model and ArcGIS. Chin. J. Inf. TCM 2024, 31, 1–7. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Healing Effect of Vicenin-2 (VCN-2) on Human Dermal Fibroblast (HDF) and Development VCN-2 Hydrocolloid Film Based on Alginate as Potential Wound Dressing. Biomed. Res. Int. 2020, 2020, 4730858. [Google Scholar] [CrossRef]
- Le, L.; Fu, H.; Lv, Q.; Bai, X.; Zhao, Y.; Xiang, J.; Jiang, B.; Hu, K.; Chen, S. The protective effects of the native flavanone flavanomarein on neuronal cells damaged by 6-OHDA. Phytomedicine 2019, 53, 193–204. [Google Scholar] [CrossRef]
- Li, M.-C.; Liu, J.-J.; Liu, J.; Bai, H.-Y.; Zhao, M.-M.; Liu, J.-Y.; Xu, Y.-N.; Ren, X.-H. Sulfuretin: Unraveling its potent therapeutic potential in a holistic literature review. Fitoterapia 2025, 182, 106490. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Guo, Y.; Sun, H.; Zhang, G.; Kuang, M.; Yang, S.; Li, X.-M.; Díaz de la Garza, R.I.; Gou, J. Isolation of wheat mutants with higher grain phenolics to enhance anti-oxidant potential. Food Chem. 2020, 303, 125363. [Google Scholar] [CrossRef]
- Teng, Y.; Li, X.; Yang, K.; Li, X.; Zhang, Z.; Wang, L.; Deng, Z.; Song, B.; Yan, Z.; Zhang, Y.; et al. Synthesis and antioxidant evaluation of desmethylxanthohumol analogs and their dimers. Eur. J. Med. Chem. 2017, 125, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, P.; Yang, X.; Wang, X.; Cao, X.; Zhao, J. Homoplantaginin exerts therapeutic effects on intervertebral disc degeneration by alleviating TNF-α-induced nucleus pulposus cell senescence and inflammation. Front. Pharmacol. 2025, 16, 1526107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Cao, Z.; Luo, Y.; Liu, Y.; Cao, H.; Tang, X.; Fang, G. Loureirin B Reduces Insulin Resistance and Chronic Inflammation in a Rat Model of Polycystic Ovary Syndrome by Upregulating GPR120 and Activating the LKB1/AMPK Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 11146. [Google Scholar] [CrossRef]
- Ding, H.; You, Q.; Li, D.; Liu, Y. 5-Demethylnobiletin: Insights into its pharmacological activity, mechanisms, pharmacokinetics and toxicity. Phytomedicine 2022, 104, 154285. [Google Scholar] [CrossRef]
- Fahsi, N.; Mahdi, I.; Annaz, H.; Bitchagno, G.; Mahmoud, M.; Sobeh, M. Unlocking the therapeutic potential of cinchonains: A comprehensive review. Phytochem. Rev. 2024, 24, 197–233. [Google Scholar] [CrossRef]
- Cai, T.; Xu, X.; Dong, L.; Liang, S.; Xin, M.; Wang, T.; Li, T.; Wang, X.; Zheng, W.; Wang, C.; et al. Oroxin A from Oroxylum indicum improves disordered lipid metabolism by inhibiting SREBPs in oleic acid-induced HepG2 cells and high-fat diet-fed non-insulin-resistant rats. Heliyon 2024, 10, e29168. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, C.; Shen, L.; Yi, C.; Wu, R.; Sun, X.; Han, F.; Li, Y.; Liu, Y. The gap-free genome of Forsythia suspensa illuminates the intricate landscape of centromeres. Hortic. Res. 2024, 11, uhae185. [Google Scholar] [CrossRef]
- Cui, J.; Wu, R.; Sun, X.; Li, Y. Integrated morpho-physiological, transcriptomic and metabolomic data to reveal the differential chilling defense mechanisms of two ecologically diverged species of Forsythia. Hortic. Plant J. 2025, 11, 1291–1307. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.; Cushman, S.A. Transcriptomic responses and physiological changes to cold stress among natural populations provide insights into local adaptation of weeping forsythia. Plant Physiol. Biochem. 2021, 165, 94–103. [Google Scholar] [CrossRef]
- Aldred, E.M.; Buck, C.; Vall, K. Pharmacology: A Handbook for Complementary Healthcare Profes (Chapter 21—Phenols). In Pharmacology; Churchill Livingstone: Edinburgh, Scotland, 2009; pp. 149–166. [Google Scholar]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Lumingkewas, A.; Koesmaryono, Y.; Aziz, S.A.; Impron; Sugimoto, H. The influence of temperature to rutin concentration of buckwheat grains in humid tropic. Int. J. Sci. Basic Appl. Res. (IJSBAR) 2015, 20, 1–9. [Google Scholar]
- Stark, S.; Julkunen-Tiitto, R.; Holappa, E.; Mikkola, K.; Nikula, A. Concentrations of foliar quercetin in natural populations of white birch (Betula pubescens) increase with latitude. J. Chem. Ecol. 2008, 34, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shang, X.; Ding, H.; Cao, Y.; Fang, S. Natural variations in flavonoids and triterpenoids of Cyclocarya paliurus leaves. J. For. Res. 2021, 32, 805–814. [Google Scholar] [CrossRef]
- Kalim, M.D.; Bhattacharyya, D.; Banerjee, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement. Altern. Med. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Müller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Schubert, B.; Stuppner, H. Altitudinal differences in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochem. Syst. Ecol. 2005, 33, 855–872. [Google Scholar] [CrossRef]
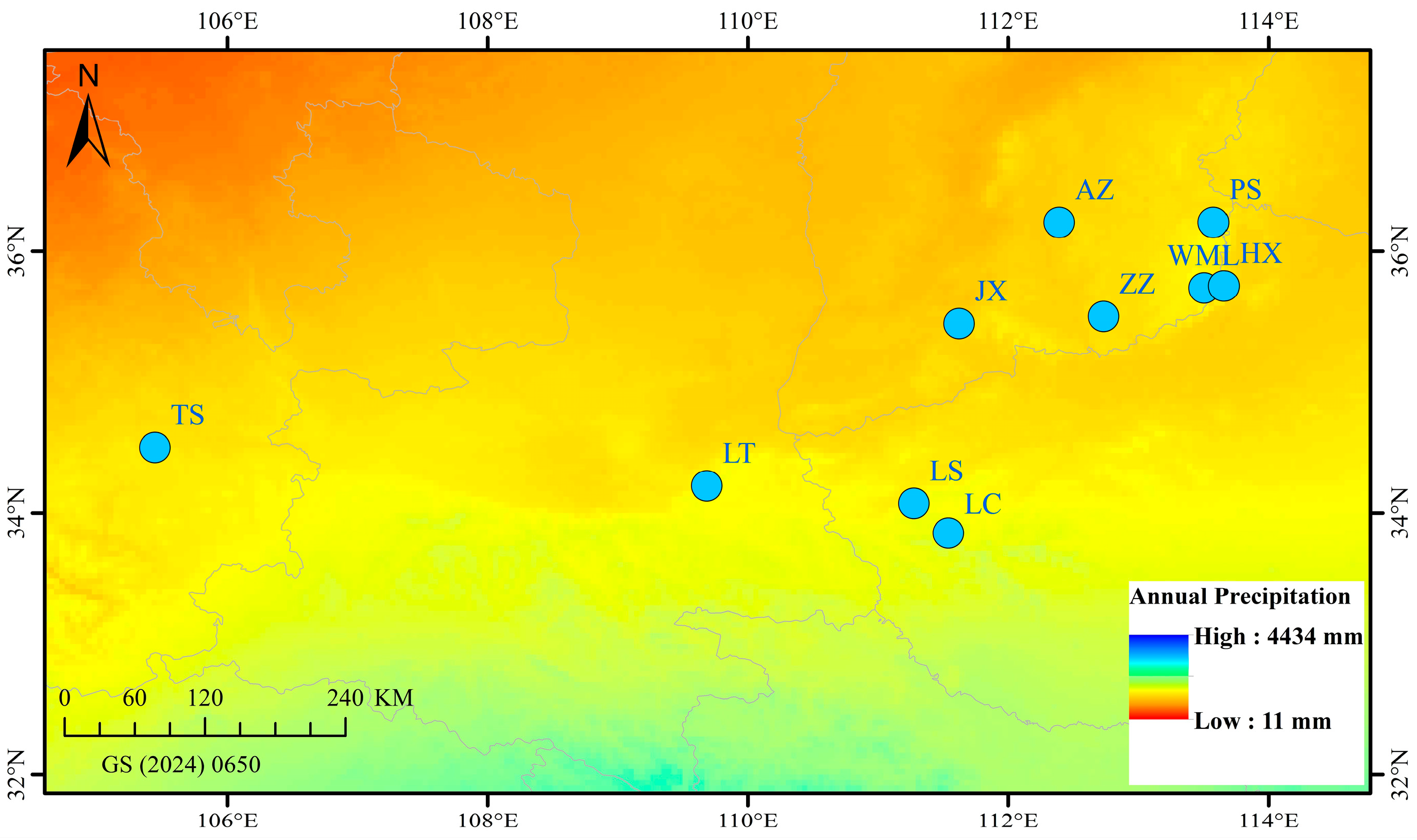
| Code | Location | Population Code | Longitude (N°) | Latitude (N°) |
|---|---|---|---|---|
| 1 | Wangmangling, Shanxi | WML | 113°30′20″ | 35°43′9″ |
| 2 | Zezhou, Shanxi | ZZ | 112°44′3″ | 35°30′7″ |
| 3 | Pingshun, Shanxi | PS | 113°34′36″ | 36°13′18″ |
| 4 | Anze, Shanxi | AZ | 112°23′33″ | 36°13′13″ |
| 5 | Jiangxian, Henan | JX | 111°37′25″ | 35°26′50″ |
| 6 | Huixian, Henan | HX | 113°39′26″ | 35°44′8″ |
| 7 | Luanchuan, Henan | LC | 111°32′30″ | 33°50′47″ |
| 8 | Lushi, Henan | LS | 111°16′32″ | 34°4′21″ |
| 9 | Lantian, Shaanxi | LT | 109°41′4″ | 34°12′27″ |
| 10 | Tianshui, Gansu | TS | 105°26′35″ | 34°29′59″ |
| Abbreviation | Environmental Variables | Unit |
|---|---|---|
| bio1 | Annual Mean Temperature | °C |
| bio2 | Mean Monthly Temperature Range | °C |
| bio3 | Isothermality (MMTR/TAR) (×100) | - |
| bio4 | Temperature Seasonality (standard deviation ×100) | - |
| bio5 | Max Temperature of Warmest Month | °C |
| bio6 | Min Temperature of Coldest Month | °C |
| bio7 | Temperature Annual Range | - |
| bio8 | Mean Temperature of Wettest Quarter | °C |
| bio9 | Mean Temperature of Driest Quarter | °C |
| bio10 | Mean Temperature of Warmest Quarter | °C |
| bio11 | Mean Temperature of Coldest Quarter | °C |
| bio12 | Annual Precipitation | mm |
| bio13 | Precipitation of Wettest Month | mm |
| bio14 | Precipitation of Driest Month | mm |
| bio15 | Precipitation Seasonality (Coefficient of Variation) | - |
| bio16 | Precipitation of Wettest Quarter | mm |
| bio17 | Precipitation of Driest Quarter | mm |
| bio18 | Precipitation of Warmest Quarter | mm |
| bio19 | Precipitation of Coldest Quarter | mm |
| elev | Elevation | m |
| Lon | Longitude | - |
| Lat | Latitude | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Wu, L.; Zhang, Y.; Guo, Y.; Xi, J.; Li, D.; Ji, J. Variations in Flavonoid Metabolites Among Forsythia suspensa Populations in Response to Environmental Heterogeneity. Plants 2025, 14, 3329. https://doi.org/10.3390/plants14213329
Zhou S, Wu L, Zhang Y, Guo Y, Xi J, Li D, Ji J. Variations in Flavonoid Metabolites Among Forsythia suspensa Populations in Response to Environmental Heterogeneity. Plants. 2025; 14(21):3329. https://doi.org/10.3390/plants14213329
Chicago/Turabian StyleZhou, Shanshan, Longni Wu, Yahui Zhang, Yutong Guo, Jialan Xi, Danyang Li, and Jinlan Ji. 2025. "Variations in Flavonoid Metabolites Among Forsythia suspensa Populations in Response to Environmental Heterogeneity" Plants 14, no. 21: 3329. https://doi.org/10.3390/plants14213329
APA StyleZhou, S., Wu, L., Zhang, Y., Guo, Y., Xi, J., Li, D., & Ji, J. (2025). Variations in Flavonoid Metabolites Among Forsythia suspensa Populations in Response to Environmental Heterogeneity. Plants, 14(21), 3329. https://doi.org/10.3390/plants14213329






