Uncovering Cyperus polystachyos in Europe: Nomenclatural Insights and New Historical Records
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Typification of the Names
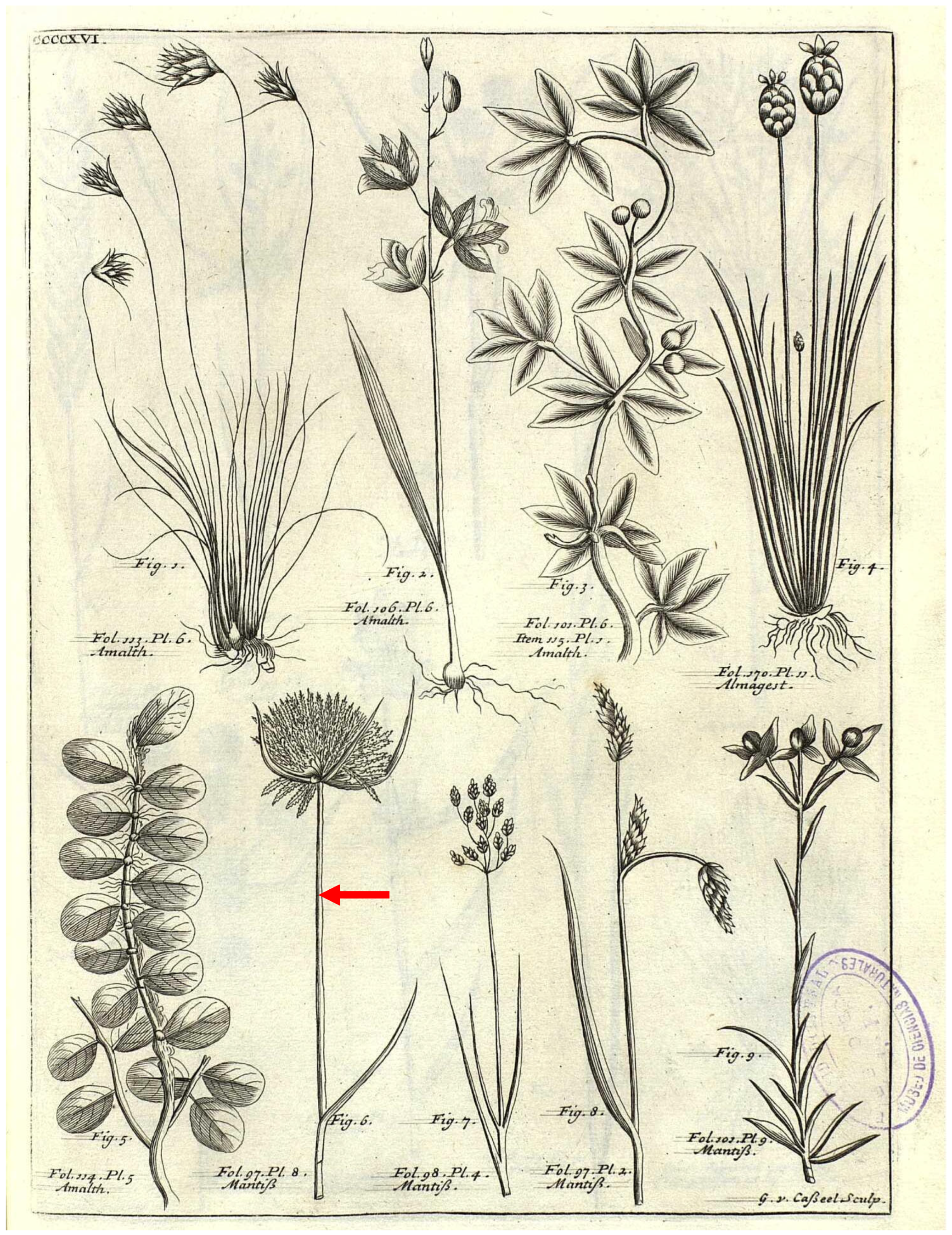
3.1.1. Cyperus polystachyos
3.1.2. Cyperus fascicularis
3.1.3. Cyperus scopellatus
3.1.4. Cyperus holosericeus
3.2. Taxonomic Notes
3.3. Taxonomic Treatment
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larridon, I. A linear classification of Cyperaceae. Kew Bull. 2022, 77, 309–315. [Google Scholar] [CrossRef]
- Larridon, I.; Zuntini, A.R.; Léveillé-Bourret, É.; Barrett, R.L.; Starr, J.R.; Muasya, M.; Villaverde, T.; Bauters, K.; Brewer, G.E.; Bruhl, J.J.; et al. A new classification of Cyperaceae (Poales) supportedby phylogenomic data. J. Syst. Evol. 2021, 59, 852–895. [Google Scholar] [CrossRef]
- POWO. Plant of the World Online. Available online: https://powo.science.kew.org/ (accessed on 30 May 2025).
- Verloove, F. A conspectus of Cyperus s.l. (Cyperaceae) in Europe (incl. Azores, Madeira and Canary Islands), with emphasis on non-native naturalized species. Webbia 2014, 69, 179–223. [Google Scholar] [CrossRef]
- Larridon, I.; Huygh, W.; Reynders, M.; Muasya, A.M.; Govaerts, R.; Simpson, D.A.; Goetghebeur, P. Nomenclature and typification of names of genera and subdivisions of genera in Cypereae (Cyperaceae): 2. Names of subdivisions of Cyperus. Taxon 2011, 60, 868–884. [Google Scholar] [CrossRef]
- Reynders, M.; Huygh, W.; Larridon, I.; Muasya, A.M.; Govaerts, R.; Simpson, D.A.; Goetghebeur, P. Nomenclature and typification of names of genera and subdivisions of genera in the Cypereae (Cyperaceae): 3. Names in segregate genera of Cyperus. Taxon 2011, 60, 885–895. [Google Scholar] [CrossRef]
- Iamonico, D.; Nicolella, G. First record of the woody Melaleuca williamsii s.l. (Myrtaceae) out of its native range. Acta Bot. Croat. 2024, 83, 115–118. [Google Scholar] [CrossRef]
- Iamonico, D.; Nicolella, G. Carya illinoinensis (Juglandaceae), new to the Italian alien woody Flora and second record for continental Europe. Feddes Repert. 2023, 135, 156–159. [Google Scholar] [CrossRef]
- Iamonico, D. Biodiversity in urban areas: The extraordinary case of the Appia Antica Regional Park (Rome, Italy). Plants 2022, 11, 2122. [Google Scholar] [CrossRef]
- Iamonico, D.; Fortini, P.; Noor Hussain, A. On the occurrence and naturalization of Amaranthus hypochondriacus (Amaranthaceae) in some european countries, with notes on its climatic features. Hacquetia 2022, 21, 211–222. [Google Scholar] [CrossRef]
- Iamonico, D. First record of a naturalized population of the tropical Colocasia esculenta (Araceae) in Italy, and clarifications about its occurrence in southeastern Europe. Acta Bot. Croat. 2021, 80, 169–175. [Google Scholar] [CrossRef]
- Iamonico, D.; De Castro, O.; Di Iorio, E.; Nicolella, G.; Iberite, M. Taxonomy complexity of some Tyrrhenian endemic Limonium species belonging to L. multiforme group (Plumbaginaceae): New insights from molecular and morphometric analyses. Plants 2022, 11, 3163. [Google Scholar] [CrossRef]
- Del Guacchio, E.; Bureš, P.; Iamonico, D.; Carucci, F.; De Luca, D.; Zedek, F.; Caputo, P. Towards a monophyletic classification of Cardueae: Restoration of the genus Lophiolepis (= Cirsium p.p.) and new circumscription of Epitrachys. Plant Biosyst. 2022, 156, 1269–1290. [Google Scholar] [CrossRef]
- Iamonico, D. A nomenclature survey of the genus Amaranthus (Amaranthaceae). 7. Willdenow’s names. Willdenowia 2020, 50, 147–155. [Google Scholar] [CrossRef]
- Iamonico, D. Nomenclature survey of the genus Amaranthus (Amaranthaceae). 4. Detailed questions arising around the name Amaranthus gracilis. Bot. Serbica 2016, 40, 61–68. [Google Scholar]
- Iamonico, D. Studies on the genus Atriplex L. (Amaranthaceae) in Italy. II. Lectotypification of Atriplex elongata Guss. (Amaranthraceae). Candollea 2012, 67, 181–185. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2023; Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 30 May 2025).
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Gandhi, K.N.; Gravendyck, J.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Klopper, R.R.; Knapp, S.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Madrid Code); Regnum Vegetabile; University of Chicago Press: Chicago, IL, USA, 2025; Volume 162. [Google Scholar] [CrossRef]
- Rottböll, C.F. Descriptiones Plantarum Rariorum; N. Mölleri: Havnie, Denmark, 1772. [Google Scholar]
- Plukenet, C.F. Amaltheum Botanicum; Biodiversity Heritage Library: London, UK, 1705. [Google Scholar]
- HUH-Index of Botanists. Index of Botanists, Harvard University Herbaria & Libraries. Available online: https://kiki.huh.harvard.edu/databases/botanist_index.html (accessed on 30 May 2025).
- Pignatti, S.; Guarino, R.; La Rosa, M. (Eds.) Flora d’Italia, 2nd ed.; Edagricole: Milano, Italy, 2018; Volume 1. [Google Scholar]
- Poiret, J.L.M. Voyage en Barbarie: Ou Lettres Écrites de l’Ancienne Numidie Pendant les Années 1785 & 1786, sur la Religion, les Coutumes & les Moeurs des Maures & des Arabes-Bédouins: Avec un Essai sur l’Histoire Naturelle de ce Pays; J. B. F. Née de la Rochelle: Paris, France, 1789. [Google Scholar]
- Desfontaines, R. Flora Atlantica: Sive Historia Plantarum quae in Atlante, Agro Tunetano et Algeriensis Crescent; L.G. Desgranges: Paris, France, 1798; Volume 2. [Google Scholar]
- Richard, L.C.M. Catalogus Plantarum, ad Societatem, Ineunte Anno 1792, e Cayenna Missarum Domino le Blondre. Actes Soc. Hist. Nat. Paris 1792, 1, 105–114. [Google Scholar]
- Link, H.F. Hortus Regius Botanicus Berolinensis Descriptus; G. Reimer: Berolini, Germany, 1827; Volume 1. [Google Scholar]
- Eggli, U.; Leuenberger, B.E. Type specimens of Cactaceae names in the Berlin Herbarium (B) [De herbario berolinensi notulae 48]. Willdenowia 2008, 38, 213–280. [Google Scholar] [CrossRef]
- Hassemer, G.; Iamonico, D.; Rønsted, N.; Di Pietro, R. Typification of the Linnaean names Plantago serraria and P. subulata (Plantago subgenus Coronopus, Plantaginaceae). Taxon 2017, 66, 738–741. [Google Scholar] [CrossRef]
- Iamonico, D.; Hassemer, G.; Rønsted, N.; Di Pietro, R. The intricate nomenclatural questions around Plantago holosteum (Plantaginaceae). Phytotaxa 2017, 306, 75–84. [Google Scholar] [CrossRef][Green Version]
- Montesinos-Tubée, D.B.; Iamonico, D. Neotypification for five names linked to Arenaria (Caryophyllaceae) for the endemic flora of Peru and Bolivia. Phytokeys 2023, 230, 131–144. [Google Scholar] [CrossRef]
- Clarke, C.B. On the Indian species of Cyperus; with Remarks on some others that specially illustrate the subdivision of the Genus. J. Linn. Society. Bot. 1886, 21, 1–202. [Google Scholar] [CrossRef]
- Tucker, G.C.; Marcks, B.G.; Carter, J.R. Cyperus. In Flora of North America North of Mexico; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2002; Volume 23. [Google Scholar]
- Dai, L.-K.; Liang, S.-Y.; Zhang, S.; Tang, Y.; Koyama, T.; Tucker, G.C.; Simpson, D.A.; Noltie, H.J.; Strong, M.T.; Bruhl, J.J.; et al. Cyperaceae Juss. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; (Acoraceae through Cyperaceae); Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2010; Volume 23. [Google Scholar]
- Global Cyperaceae Database. Cyperus polystachyos var. holosericeus. Available online: https://www.cyperaceae.org/aphia.php?p=taxdetails&id=1702616 (accessed on 16 September 2025).
- Rottböll, C.F. Descriptionum et Iconum Rariores et Pro Maxima Parte Novas Plantas Illustrantium; Sumptibus Societatis Typograficae: Hafniae, Denmark, 1773. [Google Scholar]
- Anzalone, B.; Iberite, M.; Lattanzi, E. La Flora vascolare del Lazio. Inform. Bot. Ital. 2010, 42, 187–317. [Google Scholar]
- Sibilio, G.; Russo, A.; Vallariello, R.; Menale, B.; De Luca, P.; De Castro, O. The past, present and future of thermophilous Cyperus polystachyos Rottb. (Cyperaceae) on the island of Ischia (southern Italy). Plant Biosyst. 2015, 149, 933–942. [Google Scholar] [CrossRef]
- Sestili, M.L. Primo Contributo Allo Studio dell’Erbario Storico (1745–1889) Denominato “Antonio Orsini” Conservato ad Ascoli Piceno. Ph.D. Thesis, Polytechnic University of Marche, Ascoli Piceno, Italy, 2016. [Google Scholar]
- Conti, F.; Alessandrini, A.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bartolucci, F.; Bernardo, L.; Bonacquisti, S.; Bouvet, D.; Bovio, M.; et al. Integrazioni alla checklist della flora vascolare italiana. Nat. Vicentina 2007, 10, 5–74. [Google Scholar]
- Portal to the Flora of Italy. Available online: https://dryades.units.it/floritaly/index.php?procedure=taxon_page&tipo=all&id=8087 (accessed on 17 July 2025).
- Fabrini, G.; Crosti, R. Cyperus polystachyos Rottb. Inform. Bot. Ital. 2012, 44, 224–226. [Google Scholar]
- IPNI. International Plant Names Index. Available online: https://www.ipni.org/ (accessed on 30 May 2025).
- Merril, E.D. Studies on Philippines Rubiaceae, III. Philip. J. Sci. 1917, 12, 159–243. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iamonico, D.; Verloove, F.; De Mei, S. Uncovering Cyperus polystachyos in Europe: Nomenclatural Insights and New Historical Records. Plants 2025, 14, 3270. https://doi.org/10.3390/plants14213270
Iamonico D, Verloove F, De Mei S. Uncovering Cyperus polystachyos in Europe: Nomenclatural Insights and New Historical Records. Plants. 2025; 14(21):3270. https://doi.org/10.3390/plants14213270
Chicago/Turabian StyleIamonico, Duilio, Filip Verloove, and Sofia De Mei. 2025. "Uncovering Cyperus polystachyos in Europe: Nomenclatural Insights and New Historical Records" Plants 14, no. 21: 3270. https://doi.org/10.3390/plants14213270
APA StyleIamonico, D., Verloove, F., & De Mei, S. (2025). Uncovering Cyperus polystachyos in Europe: Nomenclatural Insights and New Historical Records. Plants, 14(21), 3270. https://doi.org/10.3390/plants14213270







