Seaweed Foliar Biostimulants Improve Growth and Phytochemicals of Thai Basil (Ocimum basilicum L.) in a Plant Factory
Abstract
1. Introduction
2. Results
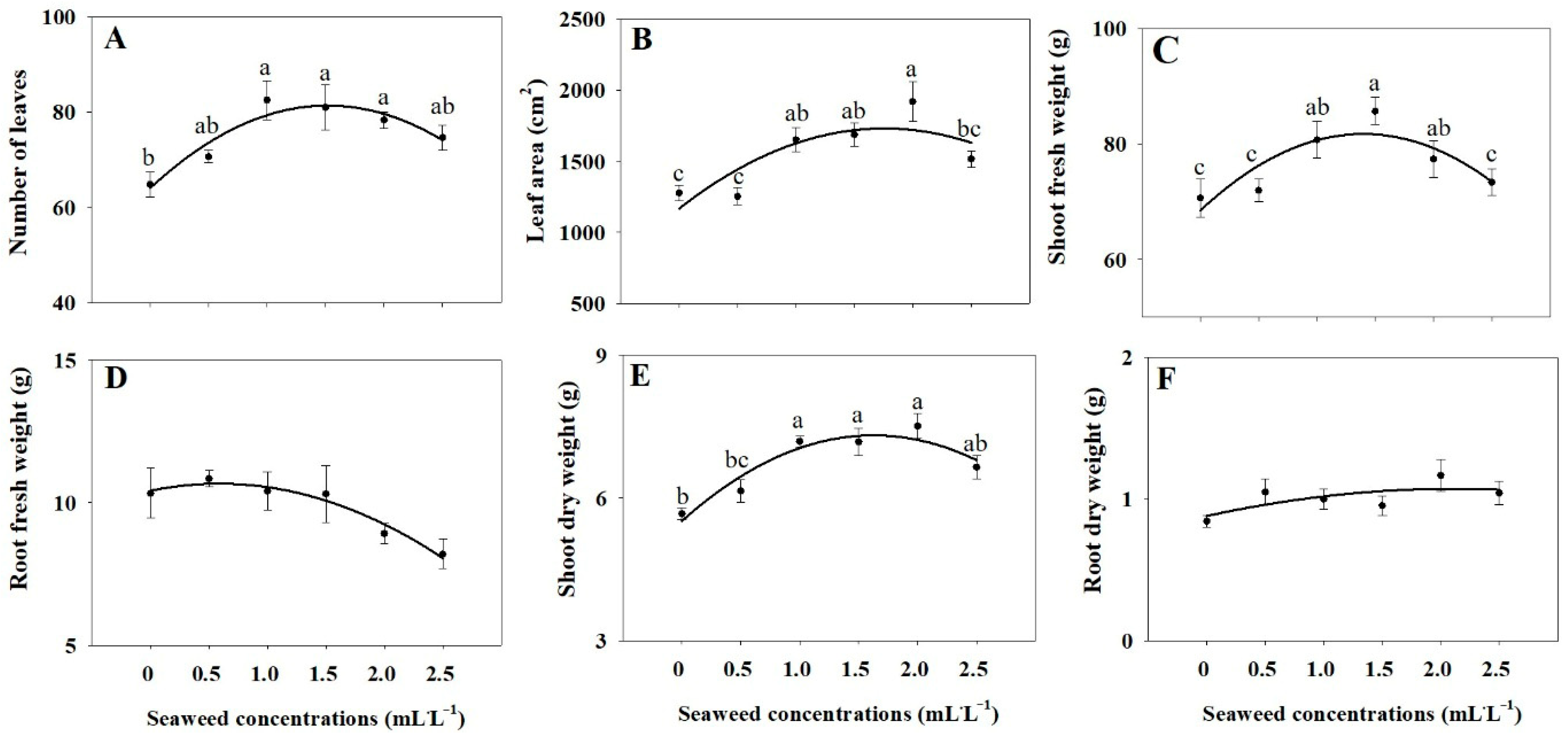
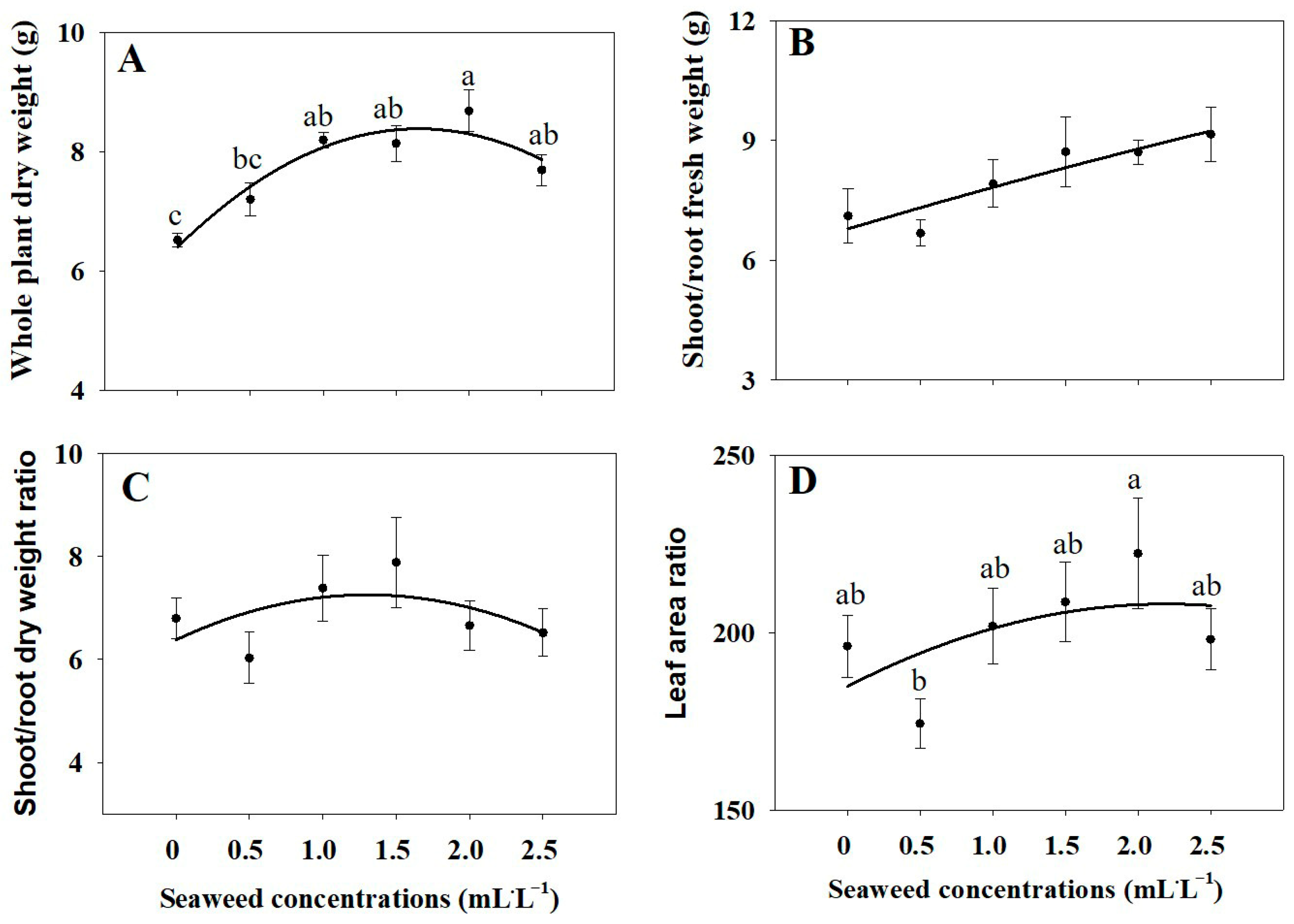
2.1. Growth Parameters
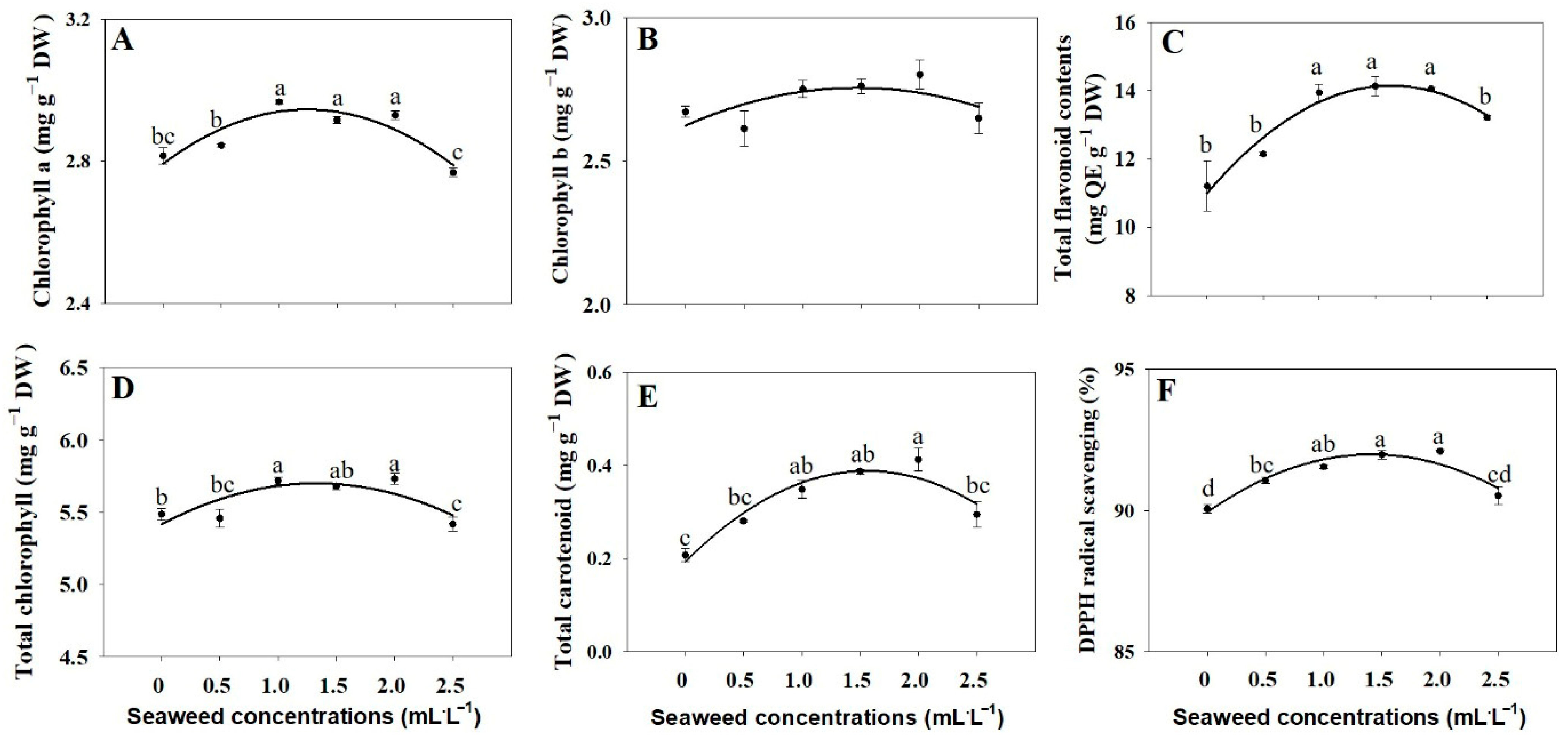
2.2. Chlorophyll, Carotenoids, and Total Flavonoid
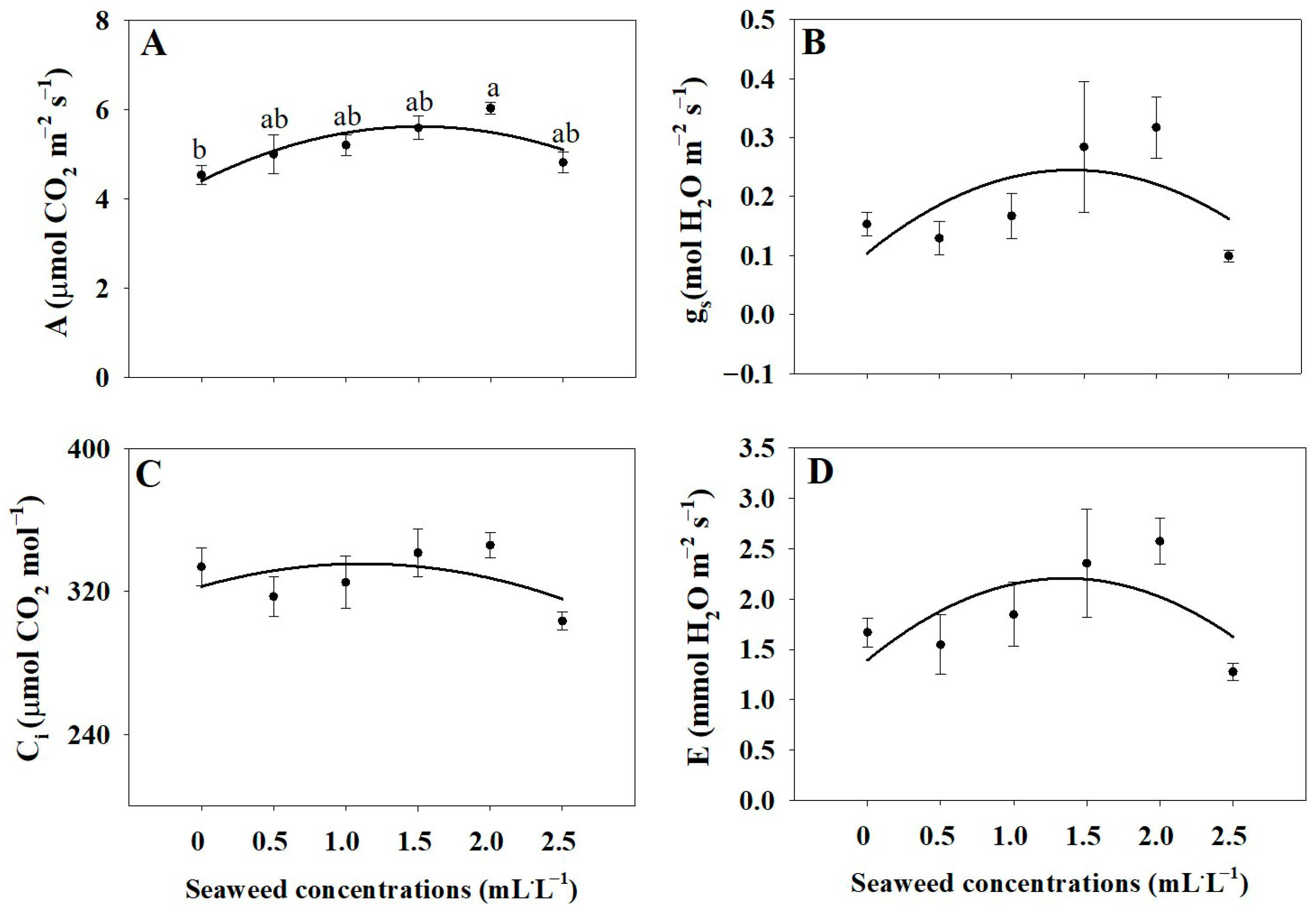
2.3. Photosynthetic Characteristics
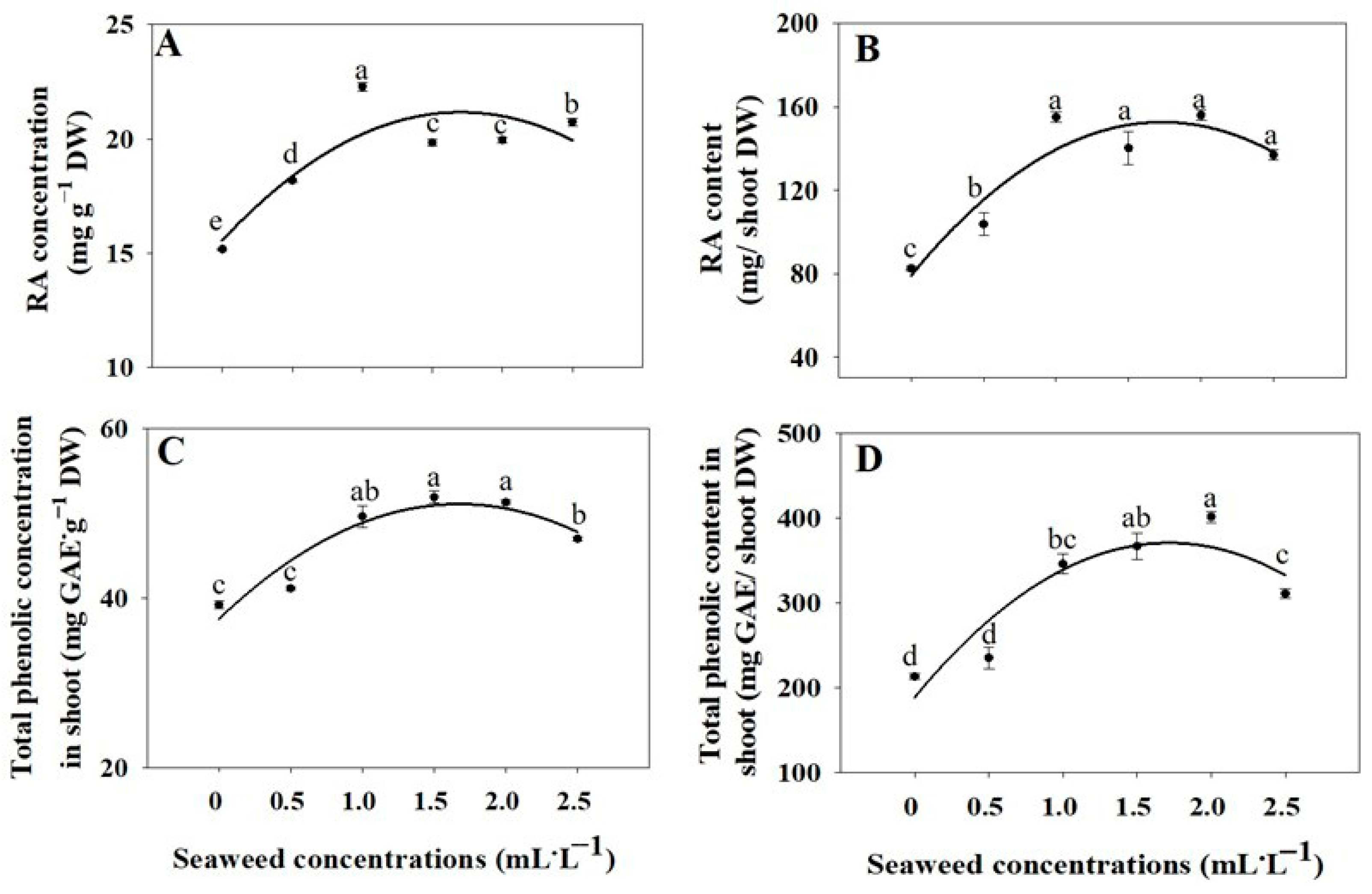
2.4. Rosmarinic Acid and Total Phenolic Concentrations and Contents
3. Discussion
3.1. Plant Growth Parameters
3.2. Chlorophyll and Total Carotenoid Levels
3.3. Photosynthetic Parameters
3.4. Rosmarinic Acid and Total Phenolic Concentrations and Contents
4. Materials and Methods
4.1. Plant Material and Seedling Conditions
4.2. Treatments and Experimental Design
4.3. Growth Parameter Measurements
4.4. Photosynthetic Characteristics
4.5. Photosynthetic Pigment Analysis
4.6. DPPH Radical Scavenging Activity Analysis
4.7. Total Flavonoid Content
4.8. Phenolic Compound Analysis by HPLC
4.9. Determination of Rosmarinic Acid
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimayu, G. Review on production and importance of basil (Ocimum basilicum L.) and roles of fertilizer on basil yield. J. Biol. Agric. Healthc. 2021, 11, 39–47. [Google Scholar]
- Spence, C. Sweet basil: An increasingly popular culinary herb. Int. J. Gastron. Food Sci. 2024, 36, 100927. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) leaves as a source of bioactive compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Bhatti, M.; Kaur, D.; Thakur, M. A research article: Comparison on phytochemical analysis and antioxidant profile of two species of Ocimum (Ocimum basilicum and Ocimum sanctum). Der Pharma Chem. 2020, 12, 15–20. [Google Scholar]
- Shiam, M.A.H.; Alam, A.; Biswas, M.; Alam, M.; Zahid, M.A.; Alam, S.M.S.; Akhtaruzzaman, M.; Ahmed, S. A comprehensive review on basil seeds as a source of nutrients and functional ingredients with health benefit properties. Appl. Food Res. 2025, 5, 100859. [Google Scholar] [CrossRef]
- Eghbal, E.; Aliniaeifard, S.; Mehrjerdi, M.Z.; Abdi, S.; Hassani, S.B.; Rassaie, T.; Gruda, N.S. Growth, phytochemical, and phytohormonal responses of basil to different light durations and intensities under constant daily light integral. BMC Plant Biol. 2024, 24, 935. [Google Scholar] [CrossRef]
- Eskandarzade, P.; Mehrjerdi, M.Z.; Gruda, N.S.; Aliniaeifard, S. Phytochemical compositions and antioxidant activity of green and purple basils altered by light intensity and harvesting time. Heliyon 2024, 10, e30931. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellanos, L.F.; Navas-Gracia, L.M.; Lozano-Castellanos, I.C.; Correa-Guimaraes, A. Technologies applied to artificial lighting in indoor agriculture: A review. Sustainability 2025, 17, 3196. [Google Scholar] [CrossRef]
- Guo, M.; Jin, Z.; Ma, L.; Ou, S. Application of plant factory with artificial lighting in horticultural production: Current progress and future trends. Hortic. Plant J. 2025; in press. [Google Scholar] [CrossRef]
- Yoshida, H.; Shimada, K.; Hikosaka, S.; Goto, E. Effect of UV-B irradiation on bioactive compounds of red perilla (Perilla frutescens (L.) Britton) cultivated in a plant factory with artificial light. Horticulturae 2022, 8, 725. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; van der Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef]
- Mandal, S.; Anand, U.; López-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A review of seaweed extract’s potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Gatti, N.; Maghrebi, M.; Serio, G.; Gentile, C.; Bunea, V.V.; Vigliante, I.; Boitte, C.; Garabello, C.; Contartese, V.; Bertea, C.M.; et al. Seaweed and yeast extracts as sustainable phytostimulant to boost secondary metabolism of apricot fruits. Front. Plant Sci. 2025, 15, 1455156. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhou, W.L.; Yang, J.; Ao, J.H.; Huang, Y.; Shen, D.C.; Jiang, Y.; Huang, Z.R.; Shen, H. Effects of seaweed extracts on the growth, physiological activity, cane yield and sucrose content of sugarcane in China. Front. Plant Sci. 2021, 12, 659130. [Google Scholar] [CrossRef]
- De Clercq, P.; Pauwels, E.; Top, S.; Steppe, K.; Van Labeke, M.C. Effect of seaweed-based biostimulants on growth and development of Hydrangea paniculata under continuous or periodic drought stress. Horticulturae 2023, 9, 509. [Google Scholar] [CrossRef]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleraceae) following application of commercial seaweed extracts of the brown seaweed, (Ascophyllum nodosum). Agric. Food Sci. 2013, 22, 288–295. [Google Scholar] [CrossRef]
- Drygas, B.; Piechowiak, T.; Balawejder, M.; Matlok, N.; Kreczko, J.; Puchalski, C. The eliciting effect of aqueous extracts from Ascophyllum nodosum algae on the cultivation of arugula (Eruca sativa Mill.) microgreens. Sustainability 2024, 16, 7436. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Wang, X.Q.; Chen, B.C.; Zhang, M.; Ma, J.Z. Seaweed extract improved yields, leaf photosynthesis, ripening time, and net returns of tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plant 2021, 10, 1045. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Hussain, H.I.; Kasinadhuni, N.; Arioli, T. The effect of seaweed extract on tomato plant growth, productivity and soil. J. Appl. Phycol. 2021, 33, 1305–1314. [Google Scholar] [CrossRef]
- Laribi, B.; Annabi, H.A.; Bettaieb, T. Effects of ulva intestinalis linnaeus seaweed liquid extract on plant growth, photosynthetic performance and water status of two hydroponically grown lamiaceae species: Peppermint (Mentha × piperita L.) and purple basil (Ocimum Basilicum var. purpurascens Benth.). S. Afr. J. Bot. 2023, 158, 63–72. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Jamwal, S.; Kumari, A.; Veeragurunathan, V.; Prasad, K.; Ghosh, A.; Kumar, R. Enhancing growth, yield, essential oil content, and composition of holy basil (Ocimum tenuiflorum L.) using red algae-based bio-stimulant under acidic conditions of the Western Himalayas. BMC Plant Biol. 2025, 25, 84. [Google Scholar] [CrossRef]
- Pirani, H.; Ebadi, M.-T.; Rezaei, A. Effect of seaweed fertilizer foliar application on growth parameters, yield, essential oil content and composition of hyssop (Hyssopus officinalis L.). Iran. J. Med. Aromat. Plants Res. 2020, 36, 376–389. [Google Scholar] [CrossRef]
- Alibakhshi, M.; Asadi-Gharneh, H.A. Growth and biochemical properties of green basil (Ocimum basilicum L.) affected by foliar application of biostimulants. J. Crop Nutr. Sci. 2021, 7, 34–45. [Google Scholar]
- Sleight, N.J.; Volk, T.A.; Eisenbies, M. Belowground biomass and root:Shoot ratios of three willow cultivars at two sites. Forests 2023, 14, 525. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.D.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef] [PubMed]
- Arun, D.; Kothandaraman, G.; Chandran, M.; Dinakarkumar, Y. Studies on effect of seaweed extracts on crop plants and microbes. Int. J. ChemTech Res. 2014, 6, 4235–4240. [Google Scholar]
- Stirk, W.A.; VanStaden, J. Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skaicka-Wozniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crops Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Shehata, A.M.; Walid, S.E.N. Response of sweet basil plants (Ocimum basilicum, L.) grown under salinity stress to spraying seaweed extract. Future J. Biol. 2019, 2, 16–28. [Google Scholar]
- EL Boukhari, M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. Using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Pacheco, A.C.; Sobral, L.A.; Gorni, P.H.; Carvalho, M.E.A. Ascophyllum nodosum extract improves phenolic compound content and antioxidant activity of medicinal and functional food plant Achillea millefolium L. Aust. J. Crop Sci. 2019, 13, 418–423. [Google Scholar] [CrossRef]
- Kisa, D.; Ceylan, Y.; Imamoglu, R. Accumulation of phenolic compounds and expression of phenylpropanoid biosynthesis-related genes in leaves of basil transformed with A. rhizogenes strains. Physiol. Mol. Biol. Plants 2023, 29, 629–640. [Google Scholar] [CrossRef]
- Azza, S.M.; Yousef, R.S. Response of basil plant (Ocimum sanctum L.) to foliar spray with amino acids or seaweed extract. J. Hortic. Sci. Ornam. Plants 2015, 7, 94–106. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids—Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lam, V.P.; Beomseon, L.; Anh, V.K.; Loi, D.N.; Kim, S.; Kwang-Ya, L.; Park, J. Effectiveness of silver nitrate application on plant growth and bioactive compounds in Agastache rugosa (Fisch. & C.A.Mey.) kuntze. Heliyon 2023, 9, e20205. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Yeo, H.J.; Baek, S.A.; Sathasivam, R.; Kim, J.K.; Park, S.U. Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa subsp. chinensis). Appl. Biol. Chem. 2021, 64, 3. [Google Scholar] [CrossRef]
- Meyer, P.; Förster, N.; Huyskens-Keil, S.; Ulrichs, C.; Geilfus, C.M. Phenolic compound abundance in Pak choi leaves is controlled by salinity and dependent on pH of the leaf apoplast. Plant Environ. Interact. 2021, 2, 36–44. [Google Scholar] [CrossRef]
- Lam, V.P.; Loi, D.N.; Shin, J.; Mi, L.K.; Park, J. Optimization of salicylic acid concentrations for increasing antioxidant enzymes and bioactive compounds of Agastache rugosa in a plant factory. PLoS ONE 2024, 19, e0306340. [Google Scholar] [CrossRef]

| w SE Concentration Treatments (mL·L−1) | Phenolic Compounds in Basil (mg·g−1 DW) | |||||
|---|---|---|---|---|---|---|
| Caffeic Acid (mg·g−1 DW) | Benzoic Acid (mg·g−1 DW) | Rutin (mg·g−1 DW) | Trans-Cinnamic Acid (mg·g−1 DW) | Quercetin (mg·g−1 DW) | Kaempferol (mg·g−1 DW) | |
| 0 | 0.42 ± 0.002 ab | 6.04 ± 0.475 d | 32.17 ± 0.266 d | 0.0159 ± 0.00019 a | 0.159 ± 0.0004 b | 0.395 ± 0.0040 c |
| 0.5 | 0.35 ± 0.005 c | 5.75 ± 0.208 d | 34.55 ± 0.020 c | 0.0067 ± 0.00011 b | 0.152 ± 0.00005 c | 0.335 ± 0.0066 d |
| 1.0 | 0.34 ± 0.008 c | 9.45 ± 1.228 c | 39.35 ± 0.531 a | 0.0039 ± 0.00026 b | 0.146 ± 0.0005 d | 0.383 ± 0.0044 c |
| 1.5 | 0.40 ± 0.496 b | 13.78 ± 0.516 b | 37.14 ± 0.355 b | 0.0095 ± 0.00293 b | 0.174 ± 0.0004 a | 0.435 ± 0.0042 b |
| 2.0 | 0.42 ± 0.005 ab | 16.99 ± 0.100 a | 33.30 ± 0.147 cd | 0.0086 ± 0.00038 b | 0.147 ± 0.0016 d | 0.461 ± 0.0022 a |
| 2.5 | 0.43 ± 0.002 a | 6.09 ± 0.561 d | 39.88 ± 0.401 a | 0.0077 ± 0.00001 b | 0.144 ± 0.0004 d | 0.455 ± 0.0048 ab |
| Significance z | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, V.P.; Bok, G.; Loi, D.N.; Do, M.C.; Park, J. Seaweed Foliar Biostimulants Improve Growth and Phytochemicals of Thai Basil (Ocimum basilicum L.) in a Plant Factory. Plants 2025, 14, 3271. https://doi.org/10.3390/plants14213271
Lam VP, Bok G, Loi DN, Do MC, Park J. Seaweed Foliar Biostimulants Improve Growth and Phytochemicals of Thai Basil (Ocimum basilicum L.) in a Plant Factory. Plants. 2025; 14(21):3271. https://doi.org/10.3390/plants14213271
Chicago/Turabian StyleLam, Vu Phong, Gwonjeong Bok, Dao Nhan Loi, Manh Cuong Do, and Jongseok Park. 2025. "Seaweed Foliar Biostimulants Improve Growth and Phytochemicals of Thai Basil (Ocimum basilicum L.) in a Plant Factory" Plants 14, no. 21: 3271. https://doi.org/10.3390/plants14213271
APA StyleLam, V. P., Bok, G., Loi, D. N., Do, M. C., & Park, J. (2025). Seaweed Foliar Biostimulants Improve Growth and Phytochemicals of Thai Basil (Ocimum basilicum L.) in a Plant Factory. Plants, 14(21), 3271. https://doi.org/10.3390/plants14213271







