Abstract
Resurrection plants such as Ramonda serbica and Ramonda nathaliae are gaining scientific attention due to their exceptional ability to withstand extreme drought and cold. This study is the first to evaluate the changes in photosynthetic activity, antioxidant defense, and the role of protective proteins during the early hours of recovery of these species after freezing-induced desiccation. Specimens collected from natural habitats where temperatures dropped below −10 °C were rehydrated under controlled conditions, and measurements were taken at multiple time points from 1 h up to 7 days after recovery. Both species demonstrated a gradual increase in photosynthesis, with the CO2 assimilation rate significantly improving after 24 h and reaching full restoration by day 7. This recovery aligned with increases in relative water content and stomatal conductance. Photosystem II efficiency was fully restored within 72 h. Notably, R. nathaliae exhibited higher thermal dissipation during stress than R. serbica. Antioxidant activity peaked between 1 and 3 h of rehydration and returned to baseline by day 7. Additionally, early rehydration stages triggered the accumulation of stress-related proteins such as dehydrins, early light-inducible proteins, small heat shock proteins, and fatty acid amide hydrolase. These results provide valuable insights into the desiccation–rehydration mechanisms of Ramonda species, demonstrating that they fully recover physiological functions within seven days and highlighting species-specific stress responses during early rehydration.
1. Introduction
Extreme environmental conditions, including both low temperatures and drought, are among the most important factors that affect plant growth and development, and can also limit their distribution and productivity [1]. Climate change has extended dry periods during summer and extreme temperatures during winter in some areas [2]. Therefore, both drought and low positive and negative temperatures are stress factors for most non-tolerant plant species. Temperatures around 0 °C (cold stress) mainly affect basic physiological processes in plants, such as photosynthesis, sugar metabolism, electron transfer reactions, and protein synthesis [3,4]. However, subzero temperatures (freezing stress) can lead to cell dehydration or ice formation in the apoplast and can even cause damage to cellular compartments and tissue death [5]. The impact of these temperatures depends on plant species. Temperate species acclimatize during cold exposure to ensure freezing tolerance, whereas tropical and subtropical plants remain extremely sensitive even to mild cold [6,7]. Therefore, for the survival of perennials and resilient annuals, frost resistance is essential, relying on the ability of each plant to acclimate to low temperatures [8]. Consequently, the acclimatization period in fall is crucial for starting physiological changes that allow plants to survive freezing temperatures during winter.
Resurrection plants survive desiccation to an air-dried state and quickly restore normal physiological function upon rehydration [9]. The European resurrection angiosperm species, Haberlea rhodopensis Friv., Ramonda nathaliae Pančić & Petrović, Ramonda serbica Pančić and Ramonda myconi (L.) Rchb are capable of surviving both drought in summer as well as sub-zero temperatures in winter [10,11,12,13]. From these species, only R. myconi grows in the Iberian Peninsula, while all other species grow in the Balkan Peninsula [14,15,16,17]. They belong to the Gesneriaceae family and, unlike other tropical and subtropical gesneriads, these species have also developed mechanisms to tolerate subzero temperatures during winter. Furthermore, considering that R. serbica and R. nathaliae grow in their natural habitats at temperatures around 20–30 °C during summer [10], their ability to withstand frosts during winter and revive from these conditions becomes very important for research. Freezing tolerance in terms of photosynthetic adaptation was reported in H. rhodopensis [12,18], R. myconi [14], and in R. serbica and R. nathaliae [10,11]. R. serbica and R. nathaliae grow in different countries in the Balkans. The distribution of R. nathaliae populations mainly extends to North Macedonia, Kosovo, Serbia and Greece, while R. serbica mainly in Albania, Kosovo, Serbia, North Macedonia, Greece and Bulgaria [15,19,20]. These two species grow in natural environments with different conditions, R. serbica under cooler and more humid conditions, while R. nathaliae in harsher conditions during the summer, drier and with higher temperatures, and is considered more resistant to these conditions than R. serbica [21].
Tolerance to drought and extreme temperatures is a complex process that involves changes at the metabolic, molecular, biochemical, physiological, and morphological levels [22,23,24]. Furthermore, to survive these conditions, plants must overcome two main types of stress that prevail, oxidative and mechanical stress. In general, resurrection plants overcome the mechanical stress resulting from cell shrinkage through dehydration by cell wall folding and vacuole compartmentalization, which positively affect the maintenance of cellular integrity [25,26]. On the other hand, during dehydration, various metabolic disorders occur, that increase cellular oxidative stress, leading to the accumulation of reactive oxygen species (ROS). Resurrection plants have evolved two main mechanisms to balance the creation and elimination of ROS: poikilochlorophylly (chlorophyll degradation and dismantling photosynthetic apparatus) and homoiochlorophylly (preservation of most of the chlorophyll and photosynthetic apparatus) [24]. R. serbica and R. nathaliae belong to the homoiochlorophyllous group, as they retain their photosynthetic apparatus during dehydration both in summer and at sub-zero temperatures [10,11,21].
Photosynthesis is considered one of the most sensitive processes to water deficit resulting from both drought and frost [10,11,17]. During drying, the reduction in photosynthetic activity leads to a disruption in the balance between energy capture and energy utilization, thus leading to ROS production [27,28]. These excess molecules react with cellular macromolecules, causing oxidative damage and disruption of cellular functions. However, ROS also function as crucial signaling molecules, activating plant defense mechanisms [22,29]. These protective mechanisms for ROS scavenging are much more effective in resurrection plants by the activation of enzymatic (peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR)) and non-enzymatic (carotenoids, ascorbate, tocopherols, glutathione) antioxidant defenses [30,31,32,33,34]. Furthermore, an increase in antioxidant defense during low temperature stress under ex situ conditions has been previously reported in R. nathaliae, R. serbica and H. rhodopensis [10,30]. In resurrection plants, the oxidative stress at subcellular level is alleviated by the synthesis of numerous protective compounds such as sugars and various proteins [25]. Freezing-induced desiccation of R. nathaliae, R. serbica and H. rhodopensis was ensured by the accumulation of dehydrins and early-light inducible proteins (ELIPs) [11,12].
A characteristic feature of resurrection plants is downregulation of photosynthetic activity as a primary line of defense against freezing and drought stress, which is accompanied by increased thermal energy dissipation [24]. During cold acclimation and freezing-induced desiccation, resurrection plants such as H. rhodopensis, R. nathaliae, R. serbica, and R. myconi exhibit xanthophyll cycle de-epoxidation, modifications of photosynthetic proteins, and chlorophyll–protein rearrangements [10,11,12,13,35]. Homoiochlorophyllous species have also been shown to maintain high stability of thylakoid pigment–protein complexes under these conditions [10,36,37]. Cold stress reduces key photosynthetic proteins (D1, D2, PsaA/B, cytochrome b6f) in H. rhodopensis [12] and increases the abundance of some LHC proteins in R. nathaliae and R. serbica [10]. It has been found that frost stress significantly reduces CO2 assimilation rate in R. nathaliae, R. myconi, and R. serbica, primarily due to stomatal closure and decreased photochemical efficiency [10,14]. Following freezing-induced desiccation, full recovery of photosynthetic function occurs in H. rhodopensis and in both Ramonda species from the Balkan Peninsula after rehydration [10,35].
Although numerous studies have explored the physiological and biochemical defense strategies employed by resurrection plants under drought, low-temperature, and freezing conditions, the recovery mechanisms in resurrection plants following freezing-induced stress are less studied in general, and unexplored in R. nathaliae and R. serbica. Since these two species grow in different natural environments [15], we hypothesized that the ecological differences will affect their recovery processes after freezing, especially in the early stages, which are particularly vulnerable and important for their ability to restore their normal physiological function [35]. Therefore, the aim of this study was to investigate and compare for the first time the changes in photosynthetic activity, antioxidant defense and the role of protective proteins during the early hours of recovery of two resurrection plants R. nathaliae and R. serbica exposed to freezing stress under natural conditions. It should be noted that all previous studies on freezing stress in resurrection plants have been conducted under controlled or ex situ conditions. Additionally, by comparing data on recovery after drought [15,38,39,40,41,42] and freezing stress, we aimed to identify the common features that confer tolerance to R. nathaliae and R. serbica.
2. Results
2.1. Recovery of Relative Water Content
RWC dropped to around 13% in R. serbica leaves and approximately 11% in R. nathaliae leaves during the freezing period (0 h), resulting in freezing-induced desiccation (Figure 1). After the first hour of rehydration (1 h), the RWC increased to about 41% in R. serbica and 26% in R. nathaliae. This trend continued with significant increases at subsequent time points (3, 6, 9, and 12 h), and by 24 h, RWC values reached approximately 90%. Interestingly, R. serbica exhibited significantly higher RWC values after the first three hours of rehydration compared to R. nathaliae. However, by the sixth hour (6 h), the RWC values between the two species became approximately equal.
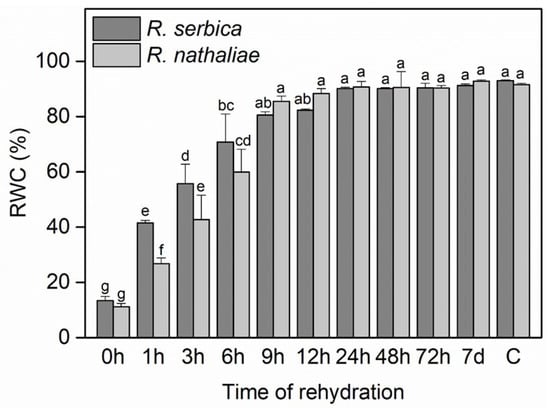
Figure 1.
Relative water content (RWC) in leaves of R. serbica and R. nathaliae after freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12, 24, 48, and 78 h, and 7 days), as well as in control plants (C). The values are presented as mean ± SE, n = 5. Identical letters in the graph indicate no significant differences among time points following rehydration after freezing-induced desiccation, compared with the control, for both species, as determined by Duncan’s multiple range test following one-way ANOVA at the 5% significance level (p ≤ 0.05).
2.2. Photosynthetic Activity During Stress and Recovery
The photosynthetic rate (A) of R. serbica and R. nathaliae after freezing stress, during early recovery, and in control plants showed significant differences between species, particularly after 24 h of rehydration (Figure 2A). During the freezing conditions that induced the severe desiccation (0 h), A showed a minimum value of 1.48 μmol CO2 m−2 s−1 in the leaves of R. serbica and 1.86 μmol CO2 m−2 s−1 in the leaves of R. nathaliae, compared to the control values (C), which were 6.29 and 7.07 CO2 m−2 s−1 respectively. The rate of CO2 assimilation did not change significantly in any of the species after the first hour (1 h) of rehydration (Figure 2A). It then slowly increased during rehydration up to 9 h, followed by a more significant increase after 24 h, 48 h, and 72 h of rehydration. Maximal and control-like values were fully restored by the seventh day (7 d) of rehydration, reaching 6.77 and 7.00 μmol CO2 m−2 s−1 in R. serbica and R. nathaliae, respectively. Overall, CO2 assimilation values during the first 12 h of recovery remained lower than those in the subsequent period.
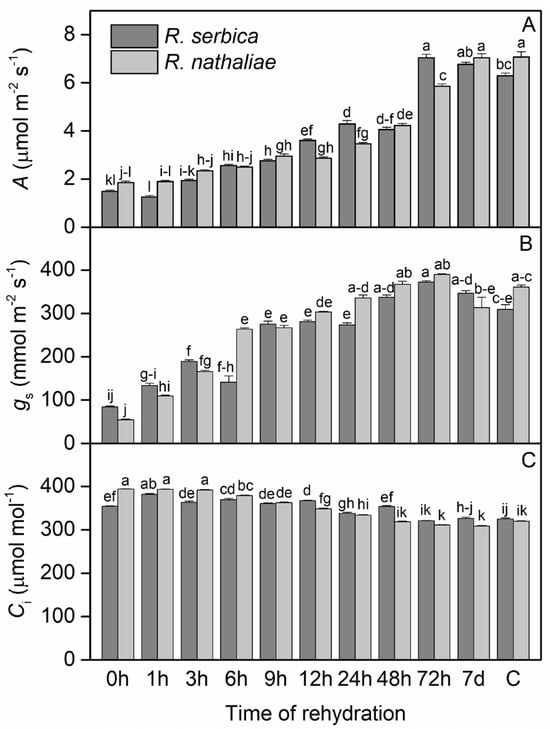
Figure 2.
Changes in CO2 assimilation rate (A; (A)), stomatal conductance (gs; (B)) and sub-stomatal CO2 (Ci; (C)) concentration in leaves of R. serbica and R. nathaliae after freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12, 24, 48, and 78 h, and 7 days), as well as in control plants (C). The values are presented as mean ± SE, n = 9. Identical letters in the graph indicate no significant differences among time points following rehydration after freezing-induced desiccation, compared with the control, for both species, as determined by Duncan’s multiple range test following one-way ANOVA at the 5% significance level (p ≤ 0.05).
Regarding stomatal conductance (gs), after the freezing period (0 h), its values dropped to a minimum in both species: 84.45 mmol m−2 s−1 in R. serbica and 54.82 mmol m−2 s−1 in R. nathaliae (Figure 2B). In contrast, sub-stomatal CO2 concentration (Ci) reached its maximum during this period, with values of 354 μmol mol−1 in R. serbica leaves and 394 μmol mol−1 in R. nathaliae (Figure 2C). Following rehydration, gs began to increase after the first hour (1 h) in both species, with a more pronounced rise by the third hour (3 h) of recovery (Figure 2B). This upward trend continued throughout the rehydration process, reaching control levels (C) after 24 h (24 h). As for Ci, our results indicate a gradual decline in both species over time during rehydration. This decrease becomes especially evident after 12 h (12 h, Figure 2C), with Ci values approaching control levels after 72 h of rehydration (72 h, Figure 2C).
2.3. Photochemical Efficiency and Energy Dispersion During Stress and Recovery
The maximum photochemical efficiency of PS II (Fv/Fm) and the photochemical efficiency of PSII in the light-adapted state (Fv′/Fm′) during the early stages of recovery from freezing stress, as well as in control plants of R. serbica and R. nathaliae, showed significant differences between species, particularly during the first 1–12 h of rehydration (Figure 3). After freezing-temperature stress, Fv/Fm values (Figure 3A) decreased significantly in R. nathaliae (0.18), while they showed a marked reduction in R. serbica, though to a lesser extent (0.49). The recovery of Fv/Fm was gradual and closely associated with the increase in RWC (Figure 1 and 3A, respectively), as RWC increased, Fv/Fm also improved.
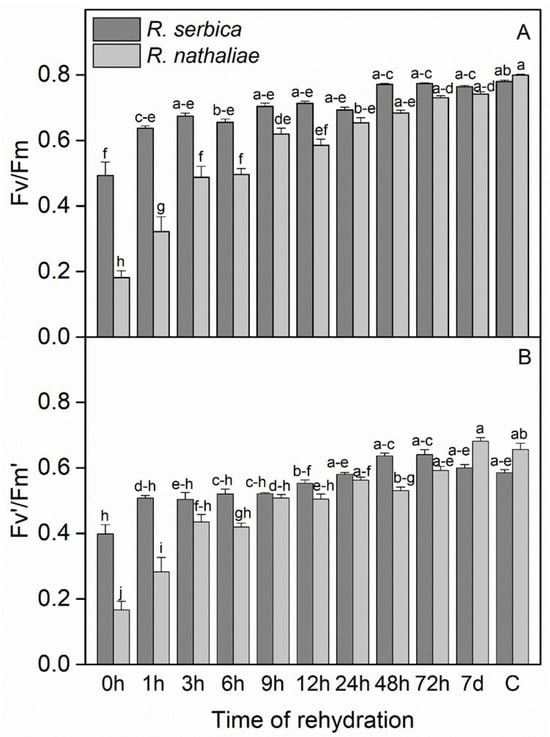
Figure 3.
Maximum photochemical efficiency of PSII (Fv/Fm, (A)) and photochemical efficiency of PSII in the light-adapted state (Fv′/Fm′, (B)) in leaves of R. serbica and R. nathaliae after freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12, 24, 48, and 78 h, and 7 days), as well as in control plants (C). The values are presented as mean ± SE, n = 9. Identical letters in the graph indicate no significant differences among time points following rehydration after freezing-induced desiccation, compared with the control, for both species, as determined by Duncan’s multiple range test following one-way ANOVA at the 5% significance level (p ≤ 0.05).
The initial increase in Fv/Fm occurred rapidly within the first hour (1 h) after rehydration, and this improvement continued progressively, becoming significantly more pronounced after 24 h (24 h) of rehydration (Figure 3A). Notably, the recovery was more marked in R. serbica, with a stronger statistical significance compared to R. nathaliae up to 6 h of rehydration. Both species reached control-like Fv/Fm values of approximately 0.8 after 72 h (72 h) of rehydration (Figure 3A), indicating near-complete recovery. Similar results were observed for Fv′/Fm′, where after freezing stress, the minimum values reached 0.17 in R. nathaliae and 0.40 in R. serbica (0 h, Figure 3B). However, the recovery of Fv′/Fm′ was also rapid. After nearly 24 h (24 h) of rehydration, both species exhibited significant recovery, reaching values similar to those of the control plants (C). Specifically, Fv′/Fm′ values were 0.59 for R. nathaliae and 0.66 for R. serbica, indicating a fast restoration of photochemical efficiency.
The efficiency of photochemical energy conversion in PSII under light (Y(II)) showed a significant decrease after freezing-induced desiccation, especially in R. nathaliae (Figure 4A and 4B, respectively). After rehydration, Y(II) did not show any significant increase during the first few hours. The efficiency of PSII gradually increased in the course of rehydration, reaching values close to the control after one week (7 d).
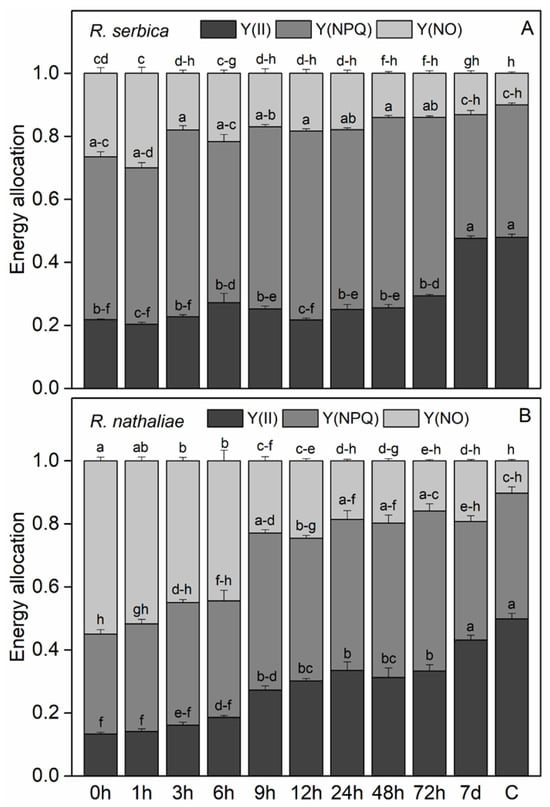
Figure 4.
Changes in the efficiency of photochemical energy conversion in PSII under light (Y(II)), non-regulated energy dissipation quantum yield (Y(NO)) and the quantum efficiency of regulated non-photochemical quenching (Y(NPQ)) in leaves of R. serbica (A) and R. nathaliae (B) after freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12, 24, 48, and 78 h, and 7 days), as well as in control plants (C). The values are presented as mean ± SE, n = 9. Identical letters in the graph indicate no significant differences among time points following rehydration after freezing-induced desiccation, compared with the control, for both species, as determined by Duncan’s multiple range test following one-way ANOVA at the 5% significance level (p ≤ 0.05).
The low quantum efficiency of PSII during the first hours of rehydration was accompanied by high quenching of excess excitation energy (Figure 4A and 4B, respectively).Regardless some enhancement in the quantum yield of non-regulated energy dissipation Y(NO) at the freezing stage and after 1 h of rehydration of R. serbica, the main part of excitation energy was dissipated as regulated non-photochemical quenching NPQ, indicating its important role in avoiding photoinhibition (Figure 4A). While the values of Y(NO) gradually decreased during rehydration, those of Y(NPQ) remained high up to 72 h of rehydration. In contrast, the main mechanism of dissipation of excess energy at the freezing stage and the first 6th h of rehydration of R. nathaliae was through Y(NO) (Figure 4B). With further rehydration of this plant species, Y(NO) decreased and Y(NPQ) increased, but both quenching parameters reached control level after 7d of rehydration.
2.4. Antioxidant Responses During Stress and Recovery
The results for total phenols (TP) and total flavonoids (TF) indicate that their levels in R. serbica and R. nathaliae were about 3 times higher after freezing stress compared to the control plants (C) (Table 1). Their content further increased after the first and third hours (1 and 3 h) of rehydration, but began to decline after the sixth hour (6 h), with a gradual decrease at each subsequent stage. By the seventh day (7 d), the levels were close to those observed in the control plants (C). Similar results were obtained for antioxidant capacity, determined by the phosphomolybdate (TAC) and ferric reducing antioxidant power (FRAP) assays, as well as free radical scavenging capacity measured by DPPH and ABTS•+ assays (Table 1). In this context, both species exhibited markedly higher antioxidant capacity, especially in the early phases of rehydration (1, 3 and 6 h) compared to the control plants, which gradually declined after further rehydration, reaching values similar to the control plants after the seventh day (7 d). This was particularly pronounced for FRAP and for the specie R. nthaliae in all phases of rehydration. Similar results were obtained for the changes in DPPH and ABTS•+, indicating a significantly higher free radical scavenging capacity after freezing stress (0 h) and following the first and third hours of rehydration (1 and 3 h) compared to the control plants (C) (Table 1). It is worth noting that DPPH had higher values after freezing stress and at the beginning of rehydration in R. serbica, while ABTS•+ was higher in R. nathaliae.

Table 1.
Secondary metabolites, antioxidant activity and scavenging capacity in leaves of R. serbica (RS) and R. nathaliae (RN) during freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12, 24, 48, and 78 h, and 7 days), as well as in control plants (C). The values are presented as mean ± SE, n = 9. Identical letters within a graph indicate no significant differences, as determined by Duncan’s Multiple Range Test at the 5% significance level (p ≤ 0.05) following ANOVA analysis.
2.5. Protein Changes During Stress and Recovery
The changes in the abundance of dehydrins, ELIPs, cytosolic sHsp Class I, cytosolic sHsp Class II, and fatty acid amide hydrolase (FAAH) during rehydration from freezing-induced desiccation of R. serbica and R. nathaliae were monitored by Western blot (Figure 5). Slight differences were observed in the patterns of dehydrin accumulation between R. serbica and R. nathaliae. In R. serbica leaves, dehydrin bands with apparent molecular weights of 50, 34, 20, 17–18, and 15 kDa were detected. In R. nathaliae, the bands appeared around 50, 40, 20, 17, and 15 kDa. In leaves of control R. serbica plants, faint dehydrin bands were detectable. Rehydration up to 12 h of dry R. serbica plants increased the abundance of dehydrins to some extent. After 24 h and full recovery of plants (7 d), their content decreased. In control R. nathaliae leaves, the dehydrin bands around 50 and 40 kDa were present only. The abundance of dehydrin proteins in R. nathaliae remained largely unchanged during the rehydration of desiccated plants. A slight decline in low-molecular weight dehydrins (20–15 kDa) was observed after 9 to 24 h of rehydration.
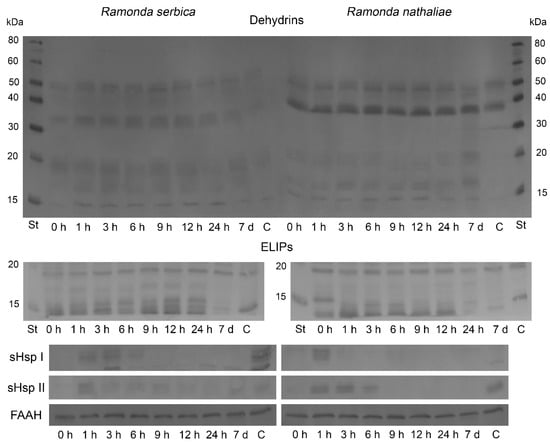
Figure 5.
Western blot of dehydrins, ELIPs, sHsp Class I, sHsp Class II and FAAH in leaves of R. serbica (left) and R. nathaliae (right) after freezing stress (0 h), and at various time points following rehydration from freezing-induced desiccation (1, 3, 6, 9, 12 and 24 h, and 7 days), as well as in control plants (C).
Several immunosignals of the Chl a/b binding proteins, ELIPs, within the 13–19 kDa molecular weight range were detected in both plants (Figure 5). ELIPs were detected in all analyzed samples, including control plants, but rehydration increased their content slightly up to 24 h and 12 h after recovery of plants, R. serbica and R. nathaliae, respectively. Subsequent rehydration led to a decline in the abundance of ELIPs as some isoforms of the proteins disappeared. In the controls of both Ramonda plants, their content was the lowest.
Western blot analysis showed the presence of the cytosolic sHsp 17.6 Class I during the first 6 h of rehydration of R. serbica (Figure 5). The protein was not detected in desiccated plants (0 h), but was present in controls (C). In R. nathaliae leaves, the sHsp Class I signal was only observed after 1 h of recovery. The protein was missing in desiccated leaves (0 h), and very faint signals could be detected in all other rehydrated samples.
Immunoblot analysis with cytosolic anti-sHsp 17.7 Class II antibody showed the presence of the protein only after 1 h of rehydration of R. serbica (Figure 5). sHsp Class II was not visible in desiccated plants, and very faint signals could be observed in all other rehydrated samples, including the control (C). In R. nathaliae plants, sHsp Class II was detected only after 1, 3, and 6 h after recovery, as well as in control plants. A faint band was present in air-dried samples (0 h).
The protein abundance of FAAH, an enzyme catabolizing N-acylethanolamines (NAEs), was enhanced during the early hours of rehydration of both Ramonda species (Figure 5). In R. serbica, after an initial slight decline in FAAH content, the protein accumulated after 3 h of recovery, and its levels remained higher compared to desiccated plants until the end of rehydration. On the other hand, FAAH content began to increase immediately after the start of rehydration in R. nathaliae leaves, and its abundance also remained high throughout the recovery process. At the end of rehydration (7 d), its content decreased by 25%.
3. Discussion
3.1. Dynamics of Relative Water Content
The early hours of rehydration of R. nathaliae and R. serbica following freezing-induced desiccation remained largely unexplored until now. Relative water content (RWC) is crucial for assessing recovery from dehydration and physiological restoration in resurrection plants [10,11,14,21,43]. In our study, winter temperatures in the natural habitats of both species were negative during January and February 2025, with several consecutive days below −10 °C from late January to early February. During the freezing phase (0 h), RWC was markedly reduced to approximately 10% in both R. serbica and R. nathaliae (Figure 1). These results aligned with previous findings on these two species regarding their adaptation during cold acclimation and freezing stress, where similar RWC values were observed [10]. During rehydration, R. serbica demonstrated significantly faster rehydration than R. nathaliae during the early hours (1–6 h), but differences became negligible after 9 h (Figure 1). This initial difference may be attributed to R. serbica greater frost resistance [10] and its larger biomass compared to R. nathaliae [21], factors that could contribute to a faster recovery. In contrast, our previous research during summer drought stress revealed that R. nathaliae demonstrated greater resistance and faster recovery than R. serbica [15], reflecting the different climatic adaptations of the two species. R. nathaliae populations are typically found in sub-Mediterranean climatic conditions, while R. serbica populations are adapted to continental climates with lower winter temperatures. Similarly to H. rhodopensis [32,35], full recovery of RWC in both Ramonda species was achieved by the seventh day (7 d), with RWC values comparable to those of the control samples (C) (Figure 1).
3.2. Alterations in Photosynthesis and Chlorophyll Fluorescence Under Stress and During Recovery
Although the lowest assimilation rate of CO2 (A) (Figure 2A) was observed in both Ramonda species after freezing-induced desiccation, photosynthesis remained active. We found that the recovery of CO2 assimilation rate was slow at the beginning of rehydration and significant enhancement was observed 24–72 h after rehydration. The lower CO2 uptake during the first 12 h may be attributed to the limited stomatal conductance during the early phase of recovery, which directly influences CO2 uptake and photosynthetic rate and efficiency. Similar findings were reported in previous studies on R. serbica and R. myconi [10,14], and this could indicate a higher level of frost resistance, adaptive capacity and fast recovery upon rehydration. The reduction in photosynthetic activity during the desiccated state is crucial, as it minimizes the production of ROS and helps preserve the structure and function of the photosynthetic apparatus [44]. Furthermore, a strong association between the decline of A and RWC has been observed in R. myconi plants [14], H. rhodopensis [36] and Barbecina purpurea [45].
The lower values of stomatal conductance after freezing stress were accompanied with enhanced sub-stomatal CO2 concentration, showing an inverse relationship between these two physiological parameters in R. serbica and R. nathaliae (Figure 2B and Figure 2C, respectively). This inverse relationship occurs because under desiccation, the stomata close to prevent water loss, leading to a sharp decrease in gs. Consequently, the reduction in CO2 assimilation rate causes an accumulation of CO2 in the intercellular spaces, resulting in elevated Ci. Increased sub-stomatal CO2 concentrations under cold stress have been shown to activate photoprotective mechanisms in some resurrection plants, which helps minimize photooxidative damage and enhances their freezing tolerance [46]. Our results are also consistent with previous findings of a pronounced reduction in stomatal conductance in Ramonda species exposed to temperatures as low as −10 °C under ex situ conditions [11]. After rehydration, stomatal conductance slowly increased during the first 6 h in both species, reaching near-control levels after 24 h of recovery (Figure 2B). This pattern strongly correlates with the increase in RWC, suggesting that an RWC of 50% may represent a physiological threshold that allows recovery of CO2 assimilation. Previous studies on resurrection plants have shown that photosynthetic capacity is fully restored following rehydration. Upon rehydration, photosynthetic recovery is associated with increased gs and restored Rubisco activity [10,47,48].
Under freezing stress, the pronounced decrease in Fv/Fm values in both Ramonda species resulted in photoinhibition of PSII, more pronounced in R. nathaliae compared to R. serbica (Figure 3). These differences may be attributed to the distinct climatic conditions in which the two species naturally occur, as previously discussed. Therefore, the more pronounced decline in photochemical efficiency observed in R. nathaliae under freezing stress may represent a protective mechanism to prevent overexcitation of the photosynthetic apparatus, highlighting this species comparatively lower frost tolerance relative to R. serbica. This result is in agreement with our earlier research on the cold and freezing stress adaptations of these plants [10]. It has been reported that cold stress reduces Fv/Fm, with freezing temperatures causing a stronger decline in Ramonda species. Drought-induced dehydration also lowers Fv/Fm, particularly below 50% relative water content, while downregulating photosynthesis helps overwintering plants minimize winter damage [11,13,49,50]. Tissue freezing during winter caused a significantly reduction of Fv/Fm in R. myconi, which is linked to zeaxanthin formation; however, Fv/Fm rapidly recovered and was fully restored within six hours upon warming [13].
Regarding the fast restoration of Fv/Fm values of both Ramonda species (Figure 3A), similar results following freezing stress have been reported in other European Gesneriads. In H. rhodopensis, the recovery of Fv/Fm was observed after 24 h of rehydration [36], while in R. myconi, full restoration occurred within 6 h [13].
Freezing-induced desiccation led to a notable decline in PSII photochemical efficiency, particularly in R. nathaliae (Figure 4). Similarly to CO2 assimilation rate, Y(II) remained low during early rehydration, showing no immediate improvement. However, PSII efficiency gradually recovered over time, reaching near-control levels by day seven, indicating a successful restoration of photosynthetic function in both species. Slow recovery of photochemical activity of PSII during the early stages of rehydration has also been observed in H. rhodopensis. This correlates with lower RWC values and high excitation pressure, prompting plants to reduce antenna size or increase heat dissipation to restore energy balance [36].
The substantial differences between the two species in energy dissipation, especially with the higher thermal energy dissipation in R. nathaliae (Figure 4), may be due to the different climatic conditions in their natural habitats. As mentioned earlier, R. nathaliae, adapted to sub-Mediterranean habitats, dissipates excess energy as heat to protect itself against photoinhibition and oxidative stress during winter, as populations growing under high-radiation show especially efficient thermal energy dissipation [10,11].
3.3. Antioxidant Defense Mechanisms in Response to Stress and Recovery
One of the most important protective mechanisms during freezing stress, particularly in the early stages following rehydration, is the antioxidant defense system. Consequently, the activation of antioxidant responses during both stress and recovery phases plays a crucial role in maintaining cellular redox balance and minimizing oxidative damage in resurrection plants [10]. Our results show that total phenols (TP) and total flavonoids (TF) levels in R. serbica and R. nathaliae increased significantly during the early stages of rehydration (1 and 3 h), compared to both control and freezing-stressed plants. Similarly, antioxidant activity and flavonoid levels markedly increased upon severe desiccation of H. rhodopensis and remained elevated after rehydration, indicating sustained defense [31,51]. Elevated flavonoid levels during freezing stress have also been reported in H. rhodopensis [30] and both Ramonda species [10]. Although research on phenolic compounds in rehydrating plants remains limited, it is well established that their accumulation in resurrection plants is a key protective strategy, helping to prevent photoinhibition, particularly during early rehydration when water influx can damage cells. The enhanced synthesis of polyphenols at low temperatures in H. rhodopensis leaves has also been described as a protective strategy in this species [30]. Additionally, enhanced accumulation of phenolic acids in R. serbica has been shown to positively affect the preservation of membrane structure during dehydration [43]. The elevated accumulation of total phenolics under stress conditions plays a vital role in scavenging free radicals, thereby aiding plants in reducing oxidative damage [52].
It was found that both R. serbica and R. nathaliae exhibited significantly elevated antioxidant capacity during the early phases of rehydration (1, 3, and 6 h) compared to control plants (Table 1), indicating its crucial role in maintaining cellular redox balance and minimizing oxidative damage. The high values of antioxidant capacity during rehydration seem to have a direct relationship with the recovery of physiological functions, especially that of photosynthesis in these two species, which serves to mitigate ROS-induced damage. Furthermore, the high antioxidant activity of the resurrection plant Xerophyta viscosa during the early stages of rehydration is believed to play a crucial role in survival under water stress [53]. Similarly, DPPH and ABTS•+ assay results showed a progressive decrease in antiradical scavenging activity with the advancement of rehydration time. This may also be related to the production of ROS, which can be much higher during the early phases of rehydration and decreases with the restoration of RWC. It has been shown that high antioxidant activity is crucial for combating stress caused by frost, as well as for the revival of H. rhodopensis plants [18,30].
3.4. The Role of Protective Proteins During Stress and Recovery
Cumulation of proteins is a common response of resurrection plants to minimize the detrimental effect of ROS and protect cell macromolecules through the dehydration-rehydration cycle [18,25,35].
Dehydrins are hydrophilic proteins belonging to LEA2 group of proteins. They prevent aggregation and stabilize the proteins in plant cells during desiccation [54]. Dehydrins accumulated in response to drought and low temperatures in resurrection plant species [11,18,55,56,57]. Our results showed different expression profiles of dehydrins in R. serbica and R. nathaliae during recovery after freezing-induced desiccation, which reflected the differences in their natural conditions. We detected similar protein patterns during desiccation of both plants at low temperatures [10,11]. It had been shown that the expression of LEA genes depended on the climatic conditions during desiccation [58]. Dehydrins maintained high abundance during rehydration in both Ramonda species, especially the low molecular weight dehydrins (20–15 kDa), which indicated their important role in the recovery process [35]. In control plants, these proteins were not detected. Rehydration of H. rhodopensis from drought- and freezing-induced desiccation showed that the low molecular weight dehydrins (22–20 and 12 kDa) were localized in the chloroplast [35].
ELIPs belong to the LHC superfamily and have photoprotective functions on the photosynthetic machinery, both under drought and freezing temperatures [59,60,61]. Western blot analysis showed the enhanced abundance of ELIPs in the process of rehydration of R. serbica and R. nathaliae after freezing-induced desiccation. This enhancement coincided with the increased levels of NPQ in both Ramonda species. It was reported that ELIP genes were among the most abundant transcripts during the dehydration-rehydration cycle of resurrection plants [35,58,62,63]. The restoration of PSII photochemistry was accompanied by a decrease in the ELIPs content after 7 d and 24 h rehydration of R. serbica and R. nathaliae plants, respectively. Similarly to dehydrins, we observed different patterns of accumulation of ELIPs during recovery of both Ramonda species and this is likely related to the different environmental conditions of their natural habitats. Previous studies showed that resurrection plants from Gesneriaceae family had different expression profiles of ELIPs during cold acclimation and freezing-induced desiccation [10,11].
Together with dehydrins and ELIPs, we investigated the content of the cytosolic sHsp Class I and II chaperones in the leaves of R. serbica and R. nathaliae during rehydration from freezing-induced desiccation. Plants possess a large number of over 30 different sHsp proteins [64]. Several studies report on the accumulation of sHsp in resurrection plants under drought and high temperature stresses and their importance for acquiring desiccation tolerance. Moreover, some of the sHsps are constitutively expressed in the leaves of resurrection plants, as well as in the roots [31,55,62,65,66,67,68]. Our results showed that the content of sHsp was elevated during the first hours of rehydration of R. serbica and R. nathaliae. We can suggest that sHsp Class I plays a more important role in the recovery process of R. serbica, being induced up to 6 h after the start of rehydration compared to R. natahaliae, in which this chaperone is only detected in the leaves after 1 h of rehydration. And vice versa, sHsp Class II plays a more important role during rehydration of R. nathaliae, being induced again up to 6 h after recovery and during the first hour after rehydration only in R. serbica.
FAAH is an enzyme that terminates the signaling pathway of N-acylethanolamines NAEs, which occur from polyunsaturated fatty acids [69] and is connected with ABA metabolism [70]. It was demonstrated that elevated levels of endogenous NAE 12:0 and ABA correlate with enhanced transcript content for ABA-responsive genes, coding proteins related to desiccation tolerance, including dehydrins, and lead to inhibition of seed growth in A. thaliana [70]. Our results demonstrated elevated protein abundance of FAAH after 3 h and 1 h of rehydration of R. serbica and R. nathaliae, respectively. We suggest that FAAH rapidly terminates the NAEs signal functions, leading to a shift in the metabolism towards recovery of both Ramonda species. It has been shown that similar genes are associated with desiccation tolerance of resurrection plants and seeds [71,72]. It is believed that since there is no difference in genomic organization in resurrection angiosperm species, they evolved their desiccation tolerance from the existing one in seeds [58,73].
4. Materials and Methods
4.1. Habitat Description, Sampling, and Experiment Setup
In this research, we used two species of European Gesneriaceae plants, R. nathaliae and R. serbica. The study was conducted on natural populations of R. serbica in Koprivnik (Bjeshkët e Nemuna; (42°37′47.3″ N, 20°16′01.0″ E)) and R. nathaliae in Glloboçicë (42°10′45.0″ N, 21°10′59.0″ E), both in Kosovo. As for R. nathaliae, it has a very limited distribution in Kosovo, occurring only in two populations in the Sharr Mountains (Glloboçicë and Gotovushë), growing at altitudes of about 900 m and in drier environments [19,74,75]. In contrast, R. serbica has a broader distribution, with several populations in the Sharr Mountains and the Bjeshkët e Nemuna, growing at altitudes ranging from 530 to 1700 m and in more humid habitats [13,19]. Regarding climatic conditions, Kosovo has a continental climate with sub-Mediterranean influences, characterized by cold winters and warm summers [76]. In this region, the lowest winter temperatures can reach as low as −20 °C in mountainous areas, while summer temperatures can rise up to 35 °C in the lowlands. In the area where the populations of R. nathaliae occur, there is a clear sub-Mediterranean climatic influence, while the populations of R. serbica experience stronger continental climatic conditions [77].
This research was carried out on plants and plant samples collected in the aforementioned locations during the first week of February 2025. During this period, minimum temperatures had reached below −10 °C for several consecutive days. During the expeditions, at least 15 leaves from different plant individuals of both species were collected and promptly frozen and preserved in liquid nitrogen to prevent degradation for subsequent analysis of secondary metabolites, antioxidant activity, and Western blot. Photosynthesis and chlorophyll fluorescence parameters (described below) were also measured during these field trips (0 h). Plants were later transferred to controlled growth conditions, at light intensity of 200 µmol m−2 s−1, temperatures ranging from 23 °C to 25 °C, and relative air humidity of 80%. In natural habitats, plants had entered a state of anabiosis due to freezing stress. After collection from their natural habitat, the plants were immediately transferred to controlled conditions (as described above), and recovery in both species was initiated by spraying and watering. Subsequently, Measurements for all the investigated parameters were taken at 1, 3, 6, 9, 12, 24, 48, 78 h, and on the 7th day after the recovery of both species in the vegetative room. For the control samples (C), all measurements were conducted in October, when meteorological conditions were considered optimal for the growth of these plant species.
4.2. Determination of Relative Water Content (RWC)
At each measurement stage, we assessed the relative water content (RWC) in leaves of both Ramonda species from control plants (C), during freezing stress (0 h), and throughout recovery (1, 3, 6, 9, 12, 24, 48, 72 h, and on the 7th day). To determine RWC, we first measured the fresh weight (FW), then the turgid weight (TW) was determined by soaking the leaves in distilled water for 24 h under laboratory conditions, and finally the dry weight (DW) (leaves were oven-dried for 24 h at 105 °C). The RWC was calculated using the formula: RWC (%) = (FW − DW)/(TW − DW) × 100 [21,78].
4.3. Quantification of Total Phenolic Content, Flavonoid Content, and Antioxidant Activity
The analysis of total phenolic and flavonoid contents, as well as antioxidant activity, was conducted on the leaves of both R. nathaliae and R. serbica, which were of approximately the same age and size. Leaves were collected from control plants (C) and those subjected to freezing stress (0 h), as well as at various time points during recovery: 1, 3, 6, 9, 12, 24, 48, and 72 h, and on day 7. The extraction followed [10], using 100 mg of dried leaves homogenized with 80% methanol. The resulting extract was then used for further analyses. For all the following spectrophotometric measurements, the Thermo Scientific BioMate 3S (Waltham, MA, USA), a compact UV-Vis spectrophotometer with a 190–1100 nm range, dual-beam optics, pre-configured assays, and temperature control, was used.
Total phenolic content (TP) was determined using the Folin–Ciocalteu reagent (Merck, Darmstadt, Germany) method with Na2CO3, as described [79], with minor modifications [10]. For each sample, 50 µL of methanolic extract was combined with 100 µL of Folin–Ciocalteu reagent (previously diluted with water 1:5 (v/v)) and allowed to react for 3 min, after which 100 µL 0.7 M L−1 of sodium carbonate (Merck, Darmstadt, Germany) was added. Absorbance was measured spectrophotometrically at 765 nm. TP concentrations were calculated based on a gallic acid standard (Merck, Hohenbrunn, Germany) curve (0–30 mg mL−1) and are reported as mg gallic acid equivalents per g dry weight (mg GAE/g DW).
Total flavonoid content (TF) measurement was conducted using a spectrophotometric method based on the protocol of [80], with minor adjustments as previously optimized for these plant species [10]. Specifically, 50 µL of the methanolic extract was mixed with 500 µL of water, followed by the addition of 100 µL of 5% NaNO2 (Merck, Darmstadt, Germany). The mixture was incubated at 50 °C for 5 min. Subsequently, 30 μL of 10% AlCl3 (Merck, Darmstadt, Germany) was added, and the mixture was allowed to stand for an additional 5 min. Finally, 200 μL of 1 M Na2CO3 and 240 μL of distilled water were added to the reaction mixture. Absorbance was measured at 510 nm against a blank prepared in the same manner, with the extract replaced by distilled water. TF concentrations were calculated from a catechin standard (Merck, Darmstadt, Germany) curve (0–20 mg mL−1) and expressed as mg catechin equivalents per g dry weight (mg CE/g DW).
Ferric reducing antioxidant power (FRAP) was conducted using the colorimetric method described [81]. A 0.1 mL methanolic extract was mixed with 1 mL sodium phosphate buffer and 1 mL of 1% K3[Fe(CN)6] (Merck, Darmstadt, Germany), then incubated at 50 °C for 20 min. Following a 10 min incubation at room temperature, the intensity of the blue-green color was measured spectrophotometrically at 700 nm. A standard curve was prepared using ascorbic acid (Sigma-Aldrich, St. Luis, MO, USA) at concentrations ranging from 1 to 2000 μM. FRAP of the samples was expressed as micromoles of ascorbic acid equivalents per gram of dry weight (μmol AAE/g DW).
DPPH radical scavenging activity was assessed using a modified version of the colorimetric method as described [82] with slight modification, 0.01 mL of the methanolic extract was adjusted to 3 mL with 80% methanol. The mixture was then combined with 2 mL of 0.25 mM DPPH solution (Sigma-Aldrich, St. Luis, MO, USA), thoroughly vortexed, and left to incubate in the dark for 30 min. Absorbance at 517 nm was used to quantify antioxidant activity, with a Trolox standard (Sigma-Aldrich, St. Luis, MO, USA) curve (1–2000 μM) for calibration. Results are expressed as μmol Trolox equivalents per g dry weight (μmol TE/g DW).
Total antioxidant capacity (TAC) was measured via the phosphomolybdenum assay [83] and incorporating minor modifications [84]. In this method, 0.05 mL of the methanolic extract was combined with 5 mL of phosphomolybdate reagent and incubated at 95 °C for 60 min. Absorbance of the green PM complex was recorded at 695 nm using a blank for reference. Total antioxidant capacity was calculated from an ascorbic acid standard (Sigma-Aldrich, St. Luis, MO, USA) curve (0–50 mg mL−1) and expressed as mg AAE per g dry weight (mg AAE/g DW).
ABTS•+ radical scavenging activity was evaluated following the method [85]. The ABTS•+ radical cation was generated by mixing 7 mM ABTS•+ solution (Sigma-Aldrich, St. Luis, MO, USA) with 2.45 mM potassium persulfate (Merck, Darmstadt, Germany) and allowing the mixture to stand in the dark at room temperature for 12–16 h. Before use, the solution was diluted with methanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm. Then, 0.1 mL of plant extract was added to 3.9 mL of the diluted ABTS•+ solution. The decrease in absorbance was measured at 734 nm after 6 min of incubation. A Trolox solution (1–1000 μM) was used as a reference standard (Sigma-Aldrich, St. Luis, MO, USA). Sample absorbance was compared with the Trolox standards, and antioxidant activity was expressed as micromoles of Trolox equivalents per gram of dry weight (μmol TE/g DW).
4.4. Measurement of Photosynthesis and Chlorophyll Fluorescence
Gas exchange and chlorophyll fluorescence parameters were measured in R. serbica and R. nathaliae leaves using a CIRAS-4 infrared gas analyzer system (PP Systems, Amesbury, MA, USA), equipped with a broad-leaf cuvette and an integrated light source, enabling precise control of environmental conditions such as light intensity, CO2 concentration, temperature, and humidity during gas exchange analysis and also equipped with a Chlorophyll Fluorescence Module (CFM-4) (PP Systems, Amesbury, MA, USA), under standardized conditions and previously established protocols as described by Kastrati et al. [10].
Gas exchange parameters, including CO2 assimilation rate or net photosynthesis (A, μmol CO2 m−2 s−1), stomatal conductance (gs, mmol H2O m−2 s−1), and intercellular CO2 concentration or CO2 concentration in the sub-stomatal cavity (Ci, μmol mol−1), were measured on fully expanded leaves of similar age and size from both plant species at each phase of the investigation. The experimental conditions were standardized as follows: light intensity (PAR, Photosynthetically Active Radiation) was set to 200 µmol m−2 s−1; RGBW settings were 34% red, 33% green, 33% blue, and 0% white; cuvette temperature was maintained at 25 °C; relative air humidity in the leaf chamber was set to 80%; CO2 concentration in the leaf chamber was 400 µmol mol−1; cuvette flow rate was 300 cc min−1; and analyzer flow rate was 100 cc min−1.
Chlorophyll fluorescence parameters, including the maximal photochemical efficiency of PSII (Fv/Fm), photochemical efficiency in the light-adapted state (Fv′/Fm′), efficiency of photochemical energy conversion in PSII under light (Y(II)), non-regulated energy dissipation quantum yield (Y(NO)), and quantum efficiency of regulated non-photochemical quenching (Y(NPQ)), were measured on the same leaves used for gas exchange. Prior to fluorescence measurements, leaves were dark-adapted for 20 min using light-exclusion clips to allow full relaxation of the photosystems. Subsequently, a saturating light pulse (6000 µmol m−2 s−1, 1 s) was applied to determine maximal fluorescence in both dark- and light-adapted states. Actinic light was then provided at 200 µmol m−2 s−1 for steady-state fluorescence analysis. All fluorescence parameters were automatically calculated by the CFM-4 module, and nine leaves per species were measured at various time points following rehydration from the freezing-induced desiccation and from the control and used for statistical evaluation.
4.5. SDS-PAGE and Western Blot of Total Leaf Proteins
Total leaf proteins were isolated with the sample buffer as previously described [10]. Samples were separated by SDS-PAGE (SE260 Mighty Small II, Hoefer, Holliston, MA, USA) as described [86] with the only difference being that the gels contained 8.7% (v/v) glycerol. In total, 30 μg of protein was applied per lane. Semi-dry transfer unit TE70X (Hoefer, Holliston, MA, USA) was used to blot the proteins onto a nitrocellulose membrane. The conditions of SDS-PAGE and semi-dry transfer were described [10]. ROTI®Mark WESTERN PLUS (Carl Roth GmbH+Co. KG, Karlsruhe, Germany) prestained protein molecular weight marker for SDS-PAGE and detection on Western blot was used. For immunoblot analysis, the following primary antibodies were tested: anti-dehydrin (AS07 206A), anti-ELIP (AS06 147A), anti-Hsp 17.6 (Class I, AS07 254), anti-Hsp 17.7 (Class II, AS07 255) and anti-FAAH (AS16 3972) from Agrisera (Vännäs, Sweden). As secondary antibody, HRP-conjugated goat anti-rabbit antibody (AS09 602, Agrisera) was used. Enhanced chemiluminescence (ECL) was used to record the immunosignals on X-ray Blue films (Carestream Dental LLC, Atlanta, GA, USA). Films were scanned using an Epson Perfection V850 PRO scanner (Seiko Epson Corporation, Suwa, Japan), and band densitometry was performed with Gel-Pro Analyzer software (Media Cybernetics, Rockville, MD, USA).
4.6. Statistical Analyses
Data were presented as arithmetic mean and standard error (±SE). All statistical analyses were conducted using the SPSS software, version 25.0 (IBM Corp., Armonk, NY, USA), including Duncan’s Multiple Range Test at a 5% significance level (p ≤ 0.05). A one-way ANOVA was performed to assess significant differences among time points following rehydration (1, 3, 6, 9, 12, 24, 48, and 72 h, as well as on the 7th day) after freezing-induced desiccation, compared with the control (C).
5. Conclusions
This study demonstrates that both R. serbica and R. nathaliae rapidly restore their water status following freezing stress. Photosynthetic activity recovered slowly during the first hours but increased significantly after one day, becoming fully restored within a week, closely linked to rehydration and stomatal reopening. Despite exhibiting stronger initial damage, R. nathaliae recovered PSII efficiency faster than R. serbica, suggesting a robust repair capacity likely related to its ecological adaptation. Both species activated photoprotective mechanisms during early recovery, with species-specific differences in energy dissipation strategies. Increased antioxidant activity in both species during the early hours of rehydration protects plants from oxidative damage and promotes photosynthetic recovery, thus ensuring successful revival after freezing-induced desiccation. Rehydration from freezing-induced desiccation of R. nathaliae and R. serbica is accompanied by enhanced levels of protective proteins. Dehydrins and ELIPs play a role during the entire recovery process, while the cytosolic sHsp Class I and Class II proteins act at the onset of recovery (from 1 to 6 h).
Overall, these findings highlight distinct physiological and biochemical strategies employed by Ramonda species to cope with freezing stress, providing valuable insights into the protective mechanisms of recovery in resurrection plants, with potential applications for developing stress-resilient crops.
Author Contributions
Conceptualization, B.G., G.M. and K.G.; investigation, B.G., F.K., G.M., E.Ç., E.P., K.L.-R. and Q.R.; writing—original draft preparation, B.G., G.M. and K.G.; writing—review and editing, B.G., G.M. and K.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Science, Technology and Innovation of the Government of the Republic of Kosovo, under project number 2/2282-1.10.
Data Availability Statement
All datasets are contained within the article.
Acknowledgments
The authors would like to express their sincere gratitude to the Ministry for providing the funding and resources necessary for the successful implementation of this project. We also extend our appreciation to the staff of the University of Prishtina and the Bulgarian Academy of Sciences for their valuable support throughout the duration of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The State of Food Insecurity of the World: How Does International Price Volatility Affect Domestic Economies and Food Security? FAO; WFP; IFAD: Rome, Italy, 2011; Available online: https://reliefweb.int/attachments/76706889-dc9c-31d0-bfc7-1e42c0430aff/Full%20Report.pdf (accessed on 20 May 2022).
- IPCC. Climate Change 2014 Synthesis Report; IPCC: Geneva, Szwitzerland, 2014; Volume 1059, p. 1072. [Google Scholar]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar] [CrossRef]
- Gołębiowska-Pikania, G.; Kopeć, P.; Surówka, E.; Krzewska, M.; Dubas, E.; Nowicka, A.; Rapacz, M.; Wójcik-Jagła, M.; Malaga, S.; Żur, I. Changes in protein abundance and activity involved in freezing tolerance acquisition in winter barley (Hordeum vulgare L.). J. Proteom. 2017, 169, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998, 118, 1–8. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Chen, M.; Thelen, J.J. Acyl-lipid desaturase 1 primes cold acclimation response in Arabidopsis. Physiol. Plant. 2016, 158, 11–22. [Google Scholar] [CrossRef]
- Farrant, J.M.; Hilhorst, H. Crops for dry environments. Curr. Opin. Biotechnol. 2022, 74, 84–91. [Google Scholar] [CrossRef]
- Kastrati, F.; Gashi, B.; Mihailova, G.; Georgieva, K.; Popova, E.; Çoçaj, E. Photosynthetic activity and antioxidative defense during cold and freezing stress of the resurrection plants Ramonda nathaliae and Ramonda serbica. Plant Stress 2025, 15, 100741. [Google Scholar] [CrossRef]
- Mihailova, G.; Gashi, B.; Krastev, N.; Georgieva, K. Acquisition of Freezing Tolerance of Resurrection Species from Gesneriaceae, a Comparative Study. Plants 2023, 12, 1893. [Google Scholar] [CrossRef]
- Mihailova, G.; Solti, Á.; Sárvári, É.; Keresztes, Á.; Rapparini, F.; Velitchkova, M.; Simova-Stoilova, L.; Aleksandrov, V.; Georgieva, K. Freezing tolerance of photosynthetic apparatus in the homoiochlorophyllous resurrection plant Haberlea rhodopensis. Environ. Exp. Bot. 2020, 178, 104157. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Neuner, G.; Kuprian, E.; Laza, J.M.; García-Plazaola, J.I.; Verhoeven, A. First evidence of freezing tolerance in a resurrection plant: Insights into molecular mobility and zeaxanthin synthesis in the dark. Physiol. Plant. 2018, 163, 472–489. [Google Scholar] [CrossRef]
- Fernández-Marin, B.; Nadal, M.; Gago, J.; Ferine, A.R.; Lopez-Pozo, M.; Artetxe, U.; Garcia-Plazaola, J.I.; Verhoeven, A. Born to revive: Molecular and physiological mechanisms of double tolerance in a paleotropical and resurrection plant. New Phytol. 2020, 226, 741–759. [Google Scholar] [CrossRef]
- Gashi, B.; Millaku, F.; Abdullai, K.; Daskalova, D.; Dontcheva, S.; Krasniqi, E.; Mata, V.; Kongjika, E. Ecological and morphological characteristics and in vitro conservation of Ramonda serbica Panc in Kosovo. Ekoloji 2013, 22, 19–28. [Google Scholar] [CrossRef]
- Daskalova, E.; Dontcheva, S.; Zekaj, Z.; Bacu, A.; Sota, V.; Abdullai, K.; Gashi, B.; Minkov, I.; Toneva, V.; Kongjika, E. Initial determination of polymorphism and in vitro conservation of some Ramonda serbica and Ramonda nathaliae populations from Albania, Macedonia and Bulgaria. Biotechnol. Biotechnol. Equip. 2012, 26, 16–25. [Google Scholar] [CrossRef]
- Farrant, J.M.; Moore, J.P. Programming desiccation-tolerance: From plants to seeds to resurrection plants. Curr. Opin. Plant Biol. 2011, 14, 340–345. [Google Scholar] [CrossRef]
- Georgieva, K.; Mihailova, G.; Fernández-Marín, B.; Bertazza, G.; Govoni, A.; Arzac, M.I.; Laza, J.M.; Vilas, J.L.; García-Plazaola, J.I.; Rapparini, F. Protective strategies of Haberlea rhodopensis for acquisition of freezing tolerance: Interaction between dehydration and low temperature. Int. J. Mol. Sci. 2022, 23, 15050. [Google Scholar] [CrossRef] [PubMed]
- Gashi, B.; Abdullai, K.; Sota, V.; Kongjika, E. Micropropagation and in vitro conservation of the rare and threatened plants Ramonda serbica and Ramonda nathaliae. Physiol. Mol. Biol. Plants 2015, 21, 123–136. [Google Scholar] [CrossRef]
- Rakić, T.; Lazarević, M.; Jovanović, Ž.S.; Radović, S.; Siljak-Yakovlev, S.; Stevanović, B.; Stevanović, V. Resurrection plants of the genus Ramonda: Prospective survival strategies–unlock further capacity of adaptation, or embark on the path of evolution? Front. Plant Sci. 2014, 4, 550. [Google Scholar] [CrossRef]
- Gashi, B.; Babani, F.; Kongjika, E. Chlorophyll fluorescence imaging of photosynthetic activity and pigment contents of the resurrection plants Ramonda serbica and Ramonda nathaliae during dehydration and rehydration. Physiol. Mol. Biol. Plants. 2013, 19, 333–341. [Google Scholar] [CrossRef]
- Colville, L.; Kranner, I. Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul. 2010, 62, 241–255. [Google Scholar] [CrossRef]
- Neeragunda Shivaraj, Y.; Barbara, P.; Gugi, B.; Vicré-Gibouin, M.; Driouich, A.; Ramasandra Govind, S.; Devaraja, A.; Kambalagere, Y. Perspectives on structural, physiological, cellular, and molecular responses to desiccation in resurrection plants. Scientifica 2018, 2018, 9464592. [Google Scholar] [CrossRef]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef]
- Morse, M.; Rafudeen, M.S.; Farrant, J.M. An overview of the current understanding of desiccation tolerance in the vegetative tissues of higher plants. Adv. Bot. Res. 2011, 57, 319–347. [Google Scholar] [CrossRef]
- Farrant, J.M. A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol. 2000, 151, 29–39. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, K.; Mihailova, G.; Gigova, L.; Dagnon, S.; Simova-Stoilova, L.; Velitchkova, M. The role of antioxidant defense in freezing tolerance of resurrection plant Haberlea rhodopensis. Physiol. Mol. Biol. Plants 2021, 27, 1119–1133. [Google Scholar] [CrossRef]
- Georgieva, K.; Mihailova, G.; Gigova, L.; Popova, A.V.; Velitchkova, M.; Simova-Stoilova, L.; Sági-Kazár, M.; Zelenyánszki, H.; Solymosi, K.; Solti, Á. Antioxidative defense, suppressed nitric oxide accumulation, and synthesis of protective proteins in roots and leaves contribute to the desiccation tolerance of the resurrection plant Haberlea rhodopensis. Plants 2023, 12, 2834. [Google Scholar] [CrossRef]
- Mihailova, G.; Vasileva, I.; Gigova, L.; Gesheva, E.; Simova-Stoilova, L.; Georgieva, K. Antioxidant defense during recovery of resurrection plant Haberlea rhodopensis from drought-and freezing-induced desiccation. Plants 2022, 11, 175. [Google Scholar] [CrossRef]
- Oliver, M.J.; Guo, L.; Alexander, D.C.; Ryals, J.A.; Wone, B.W.; Cushman, J.C. A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 2011, 23, 1231–1248. [Google Scholar] [CrossRef]
- Pandey, V.; Ranjan, S.; Deeba, F.; Pandey, A.K.; Singh, R.; Shirke, P.A.; Pathre, U.V. Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J. Plant Physiol. 2010, 167, 1351–1359. [Google Scholar] [CrossRef]
- Mihailova, G.; Christov, N.K.; Sárvári, É.; Solti, Á.; Hembrom, R.; Solymosi, K.; Keresztes, Á.; Velitchkova, M.; Popova, A.V.; Simova-Stoilova, L.; et al. Reactivation of the photosynthetic apparatus of resurrection plant Haberlea rhodopensis during the early phase of recovery from drought-and freezing-induced desiccation. Plants 2022, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, K.; Szigeti, Z.; Sarvari, E.; Gaspar, L.; Maslenkova, L.; Peeva, V.; Peli, E.; Tuba, Z. Photosynthetic activity of homoiochlorophyllous desiccation tolerant plant Haberlea rhodopensis during dehydration and rehydration. Planta 2007, 225, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hu, Z.A.; Wang, H.X.; Wen, X.G.; Kuang, T.Y. A comparison of photosynthetic apparatus of the detached leaves of resurrection plant Boea hygrometrica with its non-tolerant relative Chirita heterotrichia in response to dehydration and rehydration. Plant Sci. 2003, 165, 851–861. [Google Scholar] [CrossRef]
- Mihailova, G.; Büchel, C.; Dietzel, L.; Georgieva, K. Desiccation induced changes in photosynthesis related proteins of shade and sun Haberlea rhodopensis plants. C. R. Acad. Bulg. Sci. 2016, 69, 37–44. [Google Scholar]
- Rapparini, F.; Neri, L.; Mihailova, G.; Petkova, S.; Georgieva, K. Growth irradiance affects the photoprotective mechanisms of the resurrection angiosperm Haberlea rhodopensis Friv. in response to desiccation and rehydration at morphological, physiological and biochemical levels. Environ. Exp. Bot. 2015, 113, 67–79. [Google Scholar] [CrossRef]
- Sárvári, É.; Mihailova, G.; Solti, Á.; Keresztes, Á.; Velitchkova, M.; Georgieva, K. Comparison of thylakoid structure and organization in sun and shade Haberlea rhodopensis populations under desiccation and rehydration. J. Plant Physiol. 2014, 171, 1591–1600. [Google Scholar] [CrossRef]
- Mihailova, G.; Petkova, S.; Büchel, C.; Georgieva, K. Desiccation of the resurrection plant Haberlea rhodopensis at high temperature. Photosynth. Res. 2011, 108, 5–13. [Google Scholar] [CrossRef]
- Georgieva, K.; Röding, A.; Büchel, C. Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. J. Plant Physiol. 2009, 166, 1520–1528. [Google Scholar] [CrossRef]
- Sgherri, C.; Stevanovic, B.; Navari-Izzo, F. Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol. Plant. 2004, 122, 478–485. [Google Scholar] [CrossRef]
- Dinakar, C.; Djilianov, D.; Bartels, D. Photosynthesis in desiccation tolerant plants: Energy metabolism and antioxidative stress defense. Plant Sci. 2012, 182, 29–41. [Google Scholar] [CrossRef]
- Nadal, M.; Carriquí, M.; Badel, E.; Cochard, H.; Delzon, S.; King, A.; Lamarque, L.J.; Flexas, J.; Torres-Ruiz, J.M. Photosynthesis, leaf hydraulic conductance and embolism dynamics in the resurrection plant Barbacenia purpurea. Physiol. Plant. 2023, 175, e14035. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; Sack, L.; Farquhar, G.D. Optimal plant water economy. Plant Cell Environ. 2017, 40, 881–896. [Google Scholar] [CrossRef]
- Georgieva, K.; Maslenkova, L.; Peeva, V.; Markovska, Y.; Stefanov, D.; Tuba, Z. Comparative study on the changes in photosynthetic activity of the homoiochlorophyllous desiccation-tolerant Haberlea rhodopensis and desiccation-sensitive spinach leaves during desiccation and rehydration. Photosynth. Res. 2005, 85, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Guidi, L.; Stevanovic, B.; Navari, F. CO2 fixation and chlorophyll a fluorescence in leaves of Ramonda serbica during a dehydration-rehydration cycle. J. Plant Physiol. 2008, 165, 723–733. [Google Scholar] [CrossRef]
- Rakić, T.; Gajić, G.; Lazarević, M.; Stevanović, B. Effects of different light intensities, CO2 concentrations, temperatures and drought stress on photosynthetic activity in two paleoendemic resurrection plant species Ramonda serbica and R. nathaliae. Environ. Exp. Bot. 2015, 109, 63–72. [Google Scholar] [CrossRef]
- Míguez, F.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Activation of photoprotective winter photoinhibition in plants from different environments: A literature compilation and meta-analysis. Physiol. Plant. 2015, 155, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Moyankova, D.; Mladenov, P.; Berkov, S.; Peshev, D.; Georgieva, D.; Djilianov, D. Metabolic profiling of the resurrection plant Haberlea rhodopensis during desiccation and recovery. Physiol. Plant. 2014, 152, 675–687. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Farrant, J.M.; Cooper, K.; Hilgart, A.; Abdalla, K.O.; Bentley, J.; Thomson, J.A.; Dace, H.J.; Peton, N.; Mundree, S.G.; Rafudeen, M.S. A molecular physiological review of vegetative desiccation tolerance in the resurrection plant Xerophyta viscosa (Baker). Planta 2015, 242, 407–426. [Google Scholar] [CrossRef]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef]
- Mladenov, P.; Zasheva, D.; Planchon, S.; Leclercq, C.C.; Falconet, D.; Moyet, L.; Brugière, S.; Moyankova, D.; Tchorbadjieva, M.; Ferro, M.; et al. Proteomics evidence of a systemic response to desiccation in the resurrection plant Haberlea rhodopensis. Int. J. Mol. Sci. 2022, 23, 8520. [Google Scholar] [CrossRef] [PubMed]
- Giarola, V.; Challabathula, D.; Bartels, D. Quantification of expression of dehydrin isoforms in the desiccation tolerant plant Craterostigma plantagineum using specifically designed reference genes. Plant Sci. 2015, 236, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, A.; Stevanović, S.; Komić, S.M.; Kilibarda, N.; Vidović, M. In silico characterisation of the late embryogenesis abundant (LEA) protein families and their role in desiccation tolerance in Ramonda serbica Panc. Int. J. Mol. Sci. 2022, 23, 3547. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, G.; Zhang, L.; Yang, X.; Zhao, S.; Ji, Z.; Zhou, Q.; Hu, M.; Wang, Y.; Chen, M.; et al. The resurrection genome of Boea hygrometrica: A blueprint for survival of dehydration. Proc. Natl. Acad. Sci. USA 2015, 112, 5833–5837. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Bartels, D. Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci. 2001, 160, 1161–1170. [Google Scholar] [CrossRef]
- Hutin, C.; Nussaume, L.; Moise, N.; Moya, I.; Kloppstech, K.; Havaux, M. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. USA 2003, 100, 4921–4926. [Google Scholar] [CrossRef]
- Verhoeven, A. Sustained energy dissipation in winter evergreens. New Phytol. 2014, 201, 57–65. [Google Scholar] [CrossRef]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.R.; Marriott, A.S.; Bergström, E.; et al. Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef]
- VanBuren, R.; Pardo, J.; Man Wai, C.; Evans, S.; Bartels, D. Massive tandem proliferation of ELIPs supports convergent evolution of desiccation tolerance across land plants. Plant Physiol. 2019, 179, 1040–1049. [Google Scholar] [CrossRef]
- Sun, X.; Sun, C.; Li, Z.; Hu, Q.; Han, L.; Luo, H. AsHSP17, a creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signalling to attenuate plant response to abiotic stress. Plant Cell Environ. 2016, 39, 1320–1337. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins–evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, J.; Almoguera, C.; Bartels, D.; Jordano, J. Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum. Plant Mol. Biol. 1995, 29, 1093–1099. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Sun, D.; Deng, X. Molecular cloning and differential expression of sHSP gene family members from the resurrection plant Boea hygrometrica in response to abiotic stresses. Biologia 2013, 68, 651–661. [Google Scholar] [CrossRef]
- Mihailova, G.; Solti, Á.; Sárvári, É.; Hunyadi-Gulyás, É.; Georgieva, K. Protein changes in shade and sun Haberlea rhodopensis leaves during dehydration at optimal and low temperatures. Plants 2023, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.K.; Cravatt, B.F. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005, 74, 411–432. [Google Scholar] [CrossRef]
- Teaster, N.D.; Motes, C.M.; Tang, Y.; Wiant, W.C.; Cotter, M.Q.; Wang, Y.S.; Kilaru, A.; Venables, B.J.; Hasenstein, K.H.; Gonzalez, G.; et al. N-Acylethanolamine metabolism interacts with abscisic acid signaling in Arabidopsis thaliana seedlings. Plant Cell 2007, 19, 2454–2469. [Google Scholar] [CrossRef]
- Illing, N.; Denby, K.J.; Collett, H.; Shen, A.; Farrant, J.M. The signature of seeds in resurrection plants: A molecular and physiological comparison of desiccation tolerance in seeds and vegetative tissues. Integr. Comp. Biol. 2005, 45, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.D.; Farrant, J.M.; Oliver, M.J.; Ligterink, W.; Buitink, J.; Hilhorst, H.M. Key genes involved in desiccation tolerance and dormancy across life forms. Plant Sci. 2016, 251, 162–168. [Google Scholar] [CrossRef]
- Costa, M.C.D.; Artur, M.A.; Maia, J.; Jonkheer, E.; Derks, M.F.; Nijveen, H.; Williams, B.; Mundree, S.G.; Jiménez-Gómez, J.M.; Hesselink, T.; et al. A footprint of desiccation tolerance in the genome of Xerophyta viscosa. Nat. Plants. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Kurtto, A.; Lampinen, R.; Junikka, L. (Eds.) Atlas Florae Europaeae. Distribution of Vascular Plants in Europe. 13 Rosaceae; The Committee for Mapping the Flora of Europe and Societas Biologica Fennica: Helsinki, Finland, 2004; pp. 14–15. [Google Scholar]
- Berisha, N.; Millaku, F.; Gashi, B.; Matevski, V. Ramondo-Ostryetum carpinifoliae—A new association from the hop-hornbeam forests of the Sharri Mountains, Kosovo. Hacquetia 2019, 18, 323–336. [Google Scholar] [CrossRef]
- Gavrilov, M.B.; Marković, S.B.; Janc, N.; Nikolić, M.; Valjarević, A.; Komac, B.; Zorn, M.; Punišić, M.; Bačević, N. Assessing average annual air temperature trends using the Mann–Kendall test in Kosovo. Acta Geogr. Slov. 2018, 58, 7–25. [Google Scholar] [CrossRef]
- Berisha, N.; Krasniqi, E.; Bytyqi, V. Biogeographical regionalization of Kosovo: Integrating vegetation, climate and topography. Mediterr. Bot. 2025, 46, e99744. [Google Scholar] [CrossRef]
- Gashi, B.; Kongjika, E.; Osmani, M.; Luma, V. Activity of δ-aminolevulinic acid dehydratase at Ramonda nathaliae and Ramonda serbica plants during dehydration and rehydration. Biol. Futur. 2019, 70, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Gashi, B.; Buqaj, L.; Vataj, R.; Tuna, M. Chlorophyll biosynthesis suppression, oxidative level and cell cycle arrest caused by Ni, Cr and Pb stress in maize exposed to treated soil from the Ferronikel smelter in Drenas, Kosovo. Plant Stress 2024, 11, 100379. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).