Key Techniques in Tissue Culture of Scape Explants from Hemerocallis citrina
Abstract
1. Introduction
2. Results
2.1. Callus Induction and Adventitious Bud Differentiation from Floral Scape Explants
2.2. Multiplication Culture of Scape Explants
2.3. Rooting Culture of Tissue-Cultured Plantlets Derived from Floral Stalks
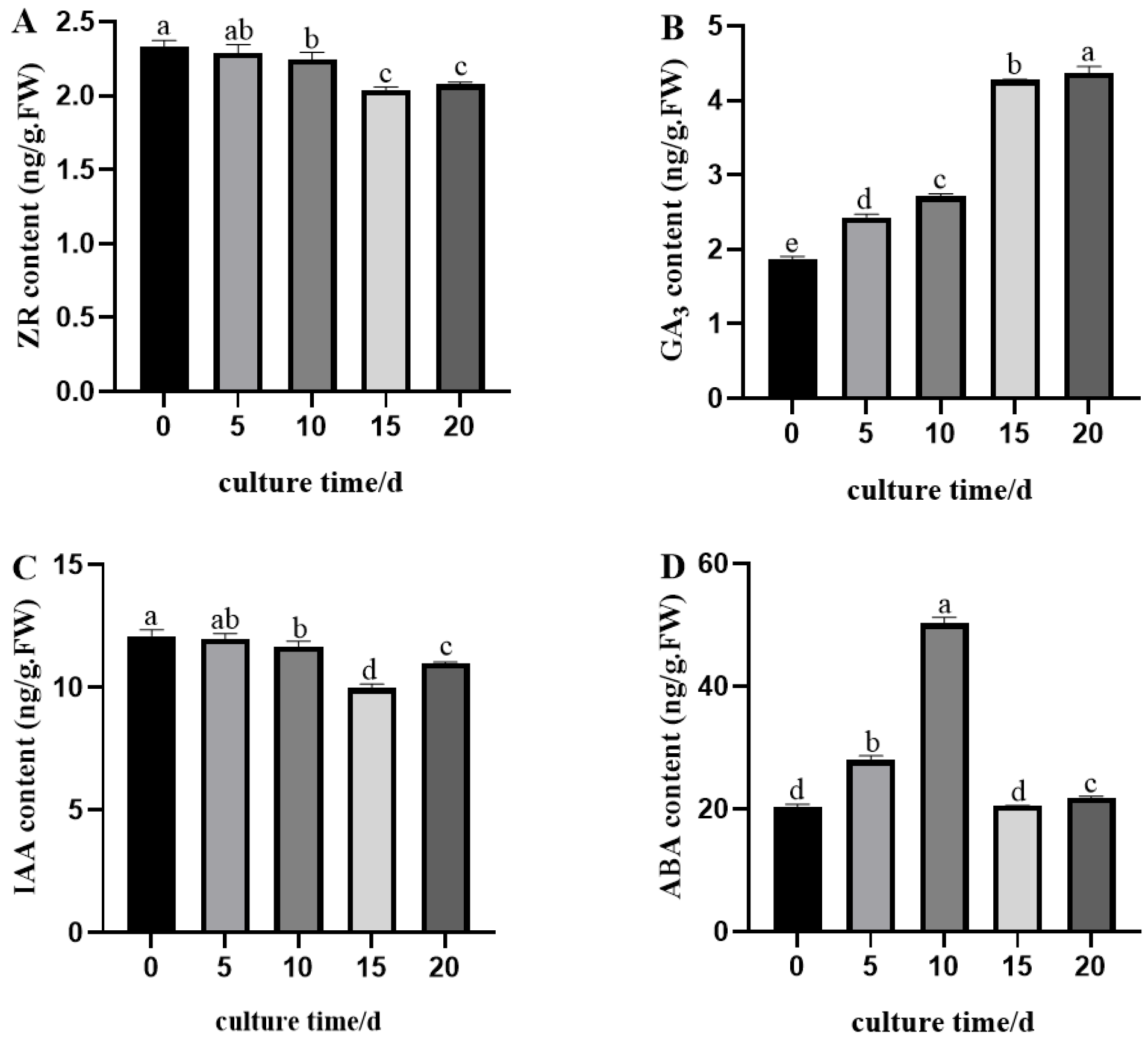
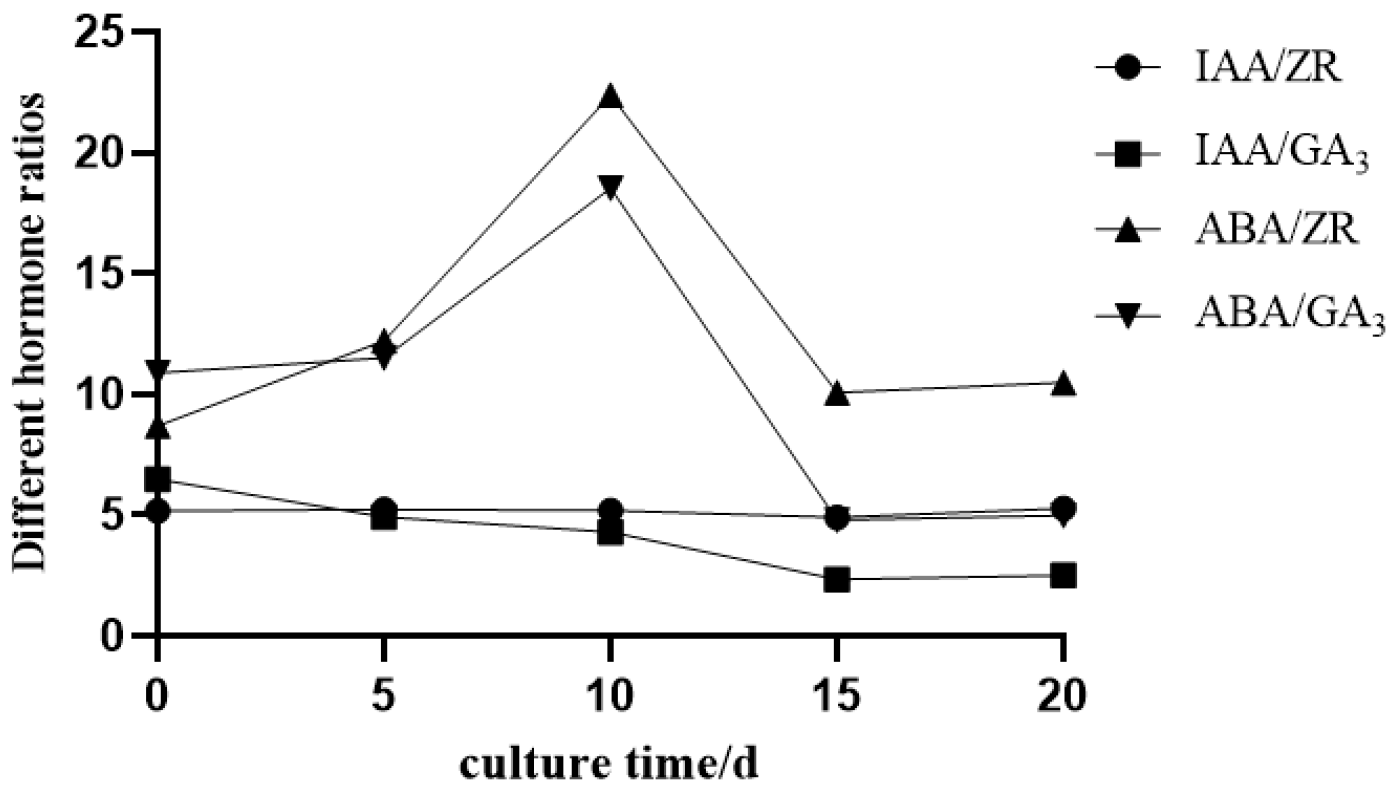
2.4. Dynamic Changes in Endogenous Phytohormone Levels During In Vitro Induction Culture of Floral Scape Explants
2.4.1. Changes in Zeatin Riboside (ZR), Gibberellic Acid (GA3), Indole-3-Acetic Acid (IAA), and Abscisic Acid (ABA) Contents
2.4.2. Changes in Endogenous Hormone Ratios
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Surface Disinfection of Explants
4.3. Treatment Protocols for Callus Induction and Adventitious Shoot Differentiation from Scape Explants Using Different Phytohormone Ratios
4.4. Adventitious Shoot Multiplication Culture from Scape Explants
4.5. Rooting Culture of Tissue-Cultured Plantlets Derived from Scape Explants
4.6. Endogenous Phytohormone Quantification
4.7. Culture Conditions
4.8. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, P.Z.; Li, K.X.; Zhang, C.F.; Zhao, Y.M.; Cao, J.K. Advances in bioactive compounds and functional properties of Daylilies. Food Ferment. Ind. 2022, 48, 330–336. [Google Scholar]
- Li, S.M. Cultivation management and processing technologies of Daylily (Hemerocallis citrina). For. By-Prod. Spec. China 2011, 5, 86–88. [Google Scholar] [CrossRef]
- Ke, J.J. Postharvest processing technologies for preventing deterioration of Daylily (Hemerocallis citrina). Northw. Hortic. 2024, 93–94. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RPbSoBw3VsEAmcL02lYkQVNPFIUqmhTe80NlkW_1R1GUgUqBxFPZkNkl18ZW4qZ0MMx3kS5mIjyrwNXif2CdNpNkuJuRmqTSeQEJPppkdTFQfHjUQuSMp6Sqzc71jnOS9A8lI1wXJo7d0djWaM6uBL9KQE09I1hECSYZ1mwxWfdKKU7eHjb96Q==&uniplatform=NZKPT&language=CHS (accessed on 10 June 2025).
- He, L.; Zhang, T.L. Biological characteristics and application values of Daylily (Hemerocallis citrina). Bull. Agric. Sci. Technol. 2012, 176–178. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RPbSoBw3VsFUQf1HKBuQnHCxhlP3EO9fjUC-EkkAetYGr8VpehO8d1Qv98-TlSoa7oIpYKpdfQKYjO5UjezWa92xbX1okxDueXiKDHcGbGl-AeuIfUAynarrhSKO3DH2OUBgCbU0tv9zr95f34KsGQPEsf_pNNf5BifSflzeVMN8OdERn5JUkw==&uniplatform=NZKPT&language=CHS (accessed on 10 June 2025).
- Wang, L.X.; Wang, Y.H.; Chen, C.; Liu, J.X.; Li, T.; Li, J.W.; Liu, P.Z.; Xu, D.B.; Shu, S.; Xiong, A.S. Advances in research on the main nutritional quality of daylily, an important flower vegetable of Liliaceae. PeerJ 2024, 12, e17802. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.L.; Cao, D.M.; Li, S.; Li, W.; Wu, Z.Q.; Xing, G.M. Research Progress on Germplasm Resources of Hemerocallis citrina Baroni. North. Hortic. 2024, 10, 119–125. Available online: https://link.cnki.net/urlid/23.1247.S.20240221.1308.004 (accessed on 23 June 2025).
- Gao, Z.H. Comparison on Nutritional Value of Hemerocallis citrine from Different Producing Areas. Heilongjiang Agric. Sci. 2019, 82–84. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RPbSoBw3VsFVs0Arb1QL9sZ1crZK97zlsSM2lcgG5-8zoPt5GeLG7PnuVYz95VYzB6TEv7Tm1SOjr_oT5d9_MLY8rbRZti8baETIVDithaHlj5hP7QL34D_LhXD0I0uiBzbt_COoP0tGxIbZDooTF_BgUcoyFdxX2_SrbBZ-aju01Ng8YJZK5A==&uniplatform=NZKPT&language=CHS (accessed on 10 June 2025).
- Jin, M.D.; Liu, J.; Jian, Y.W.; Zhang, P.H. Research progress on nutritional value, biological functions, and applications of Daylily (Hemerocallis citrina) straw in mutton sheep production. Chin. J. Anim. Sci. 2024, 60, 45–51. [Google Scholar]
- Wang, Y.; Zhang, H.L.; Xu, T.; Fan, B.; Liu, X.M.; Wang, F.Z. Study on the differences in nutritional and functional components among different varieties and parts of Daylily (Hemerocallis). Food Sci. Technol. 2017, 42, 68–71. [Google Scholar]
- Xie, X.M.; Li, R.X.; Li, C.Y.; Wei, X.C.; He, J.G.; Qiang, Z.Z.; Li, Y.; Yan, Y.T.; Fu, K.R. Research progress on chemical constituents and pharmacological effects of Daylily (Hemerocallis). Chin. Tradit. Pat. Med. 2024, 46, 3379–3387. [Google Scholar]
- Zhang, K.; Zhou, Y.; Shan, X.F.; Han, Z.P. Research advances in tissue culture of Daylily (Hemerocallis). North. Hortic. 2020, 130–137. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RPbSoBw3VsEvLnDtUSlVS5qZdnPPsH6lIa7enUHES_v2nWo10ImrKaf0rSV-wpEQ3QOO2dd_ilKf4yljjnGV43vVQa_nQhVpM_7TrXTg4qr_HDBW_FYpXr3qmDEbtm80wapL6t2hzddfHg9iiOU8wcg22DB_JsSLJ9yt13sVyopP_gnoOnVoGw==&uniplatform=NZKPT&language=CHS (accessed on 10 June 2025).
- Chu, H.N.; Hou, F.F.; Jia, Y.; Li, F.F.; Li, X.L.; Li, S.; Kang, X.P.; Xing, G.M. Sterile sowing experiment of Daylily (Hemerocallis). J. Shanxi Agric. Sci. 2017, 45, 587–590. [Google Scholar]
- Wang, T.; Li, H.; Zhao, J.J.; Huang, J.L.; Zhong, Y.; Xu, Z.F.; He, F. Exploration of Suitable Conditions for Shoot Proliferation and Rooting of Quercus robur L. in Plant Tissue Culture Technology. Life 2025, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.P.; Zhang, H.X. Review on Propagation and Seedling Technique of Daylily. Hortic. Seed 2019, 25–28+34. [Google Scholar] [CrossRef]
- Wang, K. Study on Leaf Color Variation and Division Propagation Techniques in Cymbidium (Cymbidium spp.). Master’s Thesis, Northwest University of Agriculture and Forestry, Shaanxi, China, 2024. [Google Scholar]
- Prashant, S.P.; Bhawana, M. An update on biotechnological intervention mediated by plant tissue culture to boost secondary metabolite production in medicinal and aromatic plants. Physiol. Plant 2024, 176, e14400. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Salgueiro, L.; Canhoto, J. Plant Tissue Culture: Industrial Relevance and Future Directions. Adv. Biochem. Eng. Biotechnol. 2024, 188, 1–15. [Google Scholar]
- Yang, L.; Wu, K.L.; Qiu, S.X.; Cao, H.L.; Deng, S.L. A plant regeneration protocol from callus cultures of medicinal plant Rhodomyrtus tomentosa. Plant Cell Tissue Organ Cult. 2023, 153, 307–317. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X. Phytopesticides and plant cell and tissue culture. In Proceedings of the First National Academic Symposium on Botanical Pesticide Research and Development, Xian, China, 11–13 October 1999; pp. 151–154. [Google Scholar]
- Tomasiak, A.; Zhou, M.; Betekhtin, A. Buckwheat in tissue culture research: Current status and future perspectives. Int. J. Mol. Sci. 2022, 23, 2298. [Google Scholar] [CrossRef]
- Mahanta, M.; Gantait, S. Trends in plant tissue culture and genetic improvement of gerbera. Hortic. Plant J. 2025, 11, 974–988. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Ochatt, S.J. Less frequently used growth regulators in plant tissue culture. Methods Mol. Biol. 2024, 2827, 109–143. [Google Scholar] [PubMed]
- Alsaleh, M.M.; Natsheh, I.Y.; Shibli, R.A.; Alqadiri, H.M.; Darwish, M.M.; Alhrout, H.H.; Odat, O.S.; Albadawi, D.K. Impact of various combinations of phytohormones on the in vitro cultivation of Ammi visnaga (L.) Lam microshoots and calli. Plant Cell Tissue Organ Cult. 2025, 160, 56. [Google Scholar] [CrossRef]
- Tan, X.Y.; Ma, Y.H.; Li, C.; Wang, L.Y. Investigation on the incidence of daylily leaf rust and its control measures. China Cucurb. Veg. 2021, 34, 101–104. [Google Scholar]
- Zhang, X.; Tian, X.T.; Han, Z.P.; Zhang, S.W.; Li, X.Z.; Shi, Z.J. Se-enriched cultivation techniques for Datong Daylily (Hemerocallis). China Agric. Technol. Ext. 2025, 41, 62–65. [Google Scholar]
- Chen, P.R.; Lin, Y.; Zhang, Z.M.; Kong, J.; Fan, W.B.; Yang, P.R.; Zhang, C.J. Optimization of Enzymatic Hydrolysisand Fermentation Processes for DaylilyFlowers and Analysis of TheirAnti-Depressive Metabolic Pathways. Sci. Technol. Food Ind. 2025, 46, 1–22. [Google Scholar]
- Xu, C.X. Study on Browning and Contamination Control in Tissue Culture of Mongolian Oak (Quercus mongolica). Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2024. [Google Scholar]
- Sidky, R. Optimized Direct Organogenesis from Shoot Tip Explants of Date Palm. Methods Mol. Biol. 2017, 1637, 37–45. [Google Scholar] [PubMed]
- Dang, S.N.; Gao, R.M.; Zhang, Y.Q.; Feng, Y.M. In vitro regeneration and its histological characteristics of Dioscorea nipponica Makino. Sci. Rep. 2022, 12, 18436. [Google Scholar] [CrossRef]
- Mukherjee, E.; Sarkar, S.; Bhattacharyya, S.; Gantait, S. Ameliorated reserpine production via in vitro direct and indirect regeneration system in Rauvolfia serpentina (L.). Benth. ex Kurz. 3 Biotech 2020, 10, 294. [Google Scholar] [CrossRef]
- Romo Paz, F.D.J.; Orozco Flores, J.D.; Delgado Aceves, L.; Zamora Natera, J.F.; Salcedo Pérez, E.; Vargas Ponce, O.; Portillo, L. Micropropagation of Physalis angulata L. and P. chenopodifolia Lam. (Solanaceae) via indirect organogenesis. Vitr. Cell. Dev. Biol.-Plant 2023, 59, 497–506. [Google Scholar] [CrossRef]
- Xiao, X.J.; Luo, T.; Wang, F. Research progress on browning phenomenon and control measures in woody plant tissue culture. Jiangsu Agric. Sci. 2017, 45, 20–24. [Google Scholar]
- Liu, Y.; Zhou, N.F.; Luo, C.R.; Zhang, Q.; Sun, P.; Fu, J.m.; Li, S.Z.; Li, Z. Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng. Plants 2023, 12, 3507. [Google Scholar] [CrossRef]
- Vargas, R.; Vigo, C.N.; Juarez-Contreras, L.; Oliva-Cruz, M. Development and optimization of in vitro shoot regeneration in Physalis peruviana using cotyledon explants. J. Genet. Eng. Biotechnol. 2025, 23, 100463. [Google Scholar] [CrossRef]
- Lei, L.L. Effects of Different Plant Growth Regulators on the Growth of Tissue-cultured Seedlings of Actinidia arguta ‘Qiyimei No. 6’. Prat. Fruit. Grow. 2025, 32–36. Available online: https://kns.cnki.net/kcms2/article/abstract?v=RPbSoBw3VsE-8sEKQWAVI_eIORef0uOJSlBCBvUEDCoTpsN0mtTcuXvDPRpUj47WZiYsnrhFEsdgcrnu4Q2AydxozTI5CdIShE8B6bed9jT_n2_OObLCSeON0JYGCL4tcZsvnLQN7jRQYN1MuI1vJQHjdUd2rFr7eFu96kO_XcK16XfaKyJpNw==&uniplatform=NZKPT&langu (accessed on 10 June 2025).
- Li, Y.; Jiang, H.; Qin, M.; He, Y.; Zhang, J.; Gong, W.; Xiao, X.; Li, P.; Zhou, W. Establishment of in Vitro Efficient Regeneration System for Leaves and Hypocotyls of Toona sinensis, a Multifunctional Woody Plant. Ind. Crops Prod. 2025, 224, 120328. [Google Scholar] [CrossRef]
- Yin, X.Y.; Liang, H.L.; Zhang, J.W.; Chu, B.Y. Establishment of Tissue Culture and Regeneration System of New Germplasm ‘Red Doll’. J. Hebei For. Sci. Technol. 2025, 1, 13–18. [Google Scholar]
- Zuo, G.Y.; Ke, L.; Guo, Y.N.; Niu, X.R.; Yin, L.J.; Wu, Z.Q.; Zhang, X.M.; Cheng, X.J.; Yu, J.; Zheng, S.W.; et al. Development and Optimization of a Rapid in Vitro Micropropagation System for the Perennial Vegetable Day Lily, Hemerocallis citrina Baroni. Agronomy 2024, 14, 244. [Google Scholar] [CrossRef]
- Shen, R.M.; Lu, Y.; Li, X.Y.; Xing, B.L. Preliminary Study on Tissue Culture Regeneration from Young Leaves of Datong Daylily (Hemerocallis citrina). Bull. Agric. Sci. Technol. 2022, 7, 137–140. [Google Scholar]
- Zuo, G.Y. The Establishment and Application of Tissue Culture and Rapid Propagation System for Hemerocallis citrina cv. ‘Datong Huanghua’. Master’s Thesis, Shanxi Agricultural University, Taigu, China, 2022. [Google Scholar]
- Han, Z.P.; Wang, L.J.; Zhang, H.X.; Zhang, K. Study on Tissue Culture of Stem Segments of Daylily of Datong. Seed 2021, 40, 135–140+149. [Google Scholar]
- Cai, X.M.; Guo, W.J.; Zhang, J.; Fang, S.Z. Comparison of Primary Culture Using Different Explants of Hemerocallis citrina and Study of Rapid Propagation Technology. Southeast Hortic. 2020, 8, 21–25. [Google Scholar]
- Matand, K.; Shoemake, M.; Li, C.X. High Frequency in Vitro Regeneration of Adventitious Shoots in Daylilies (Hemerocallis sp.) Stem Tissue Using Thidiazuron. BMC Plant Biol. 2020, 20, 31. [Google Scholar] [CrossRef]
- Wang, J.; Hu, D.M.; Hou, J.; Bai, J. Study on Tissue Culture of Hemerocallis citrina Baroni ‘March Flower’. J. Sichuan Univ. (Nat. Sci. Ed.) 2019, 56, 167–172. [Google Scholar]
- Wang, R.M.; Chu, H.N.; Wang, J.Y.; Li, S.; Kang, X.P. Tissue Culture and Plant Regeneration with Dwarf Dtem of Hemerocallis Plants. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2019, 39, 23–28. [Google Scholar]
- Su, C.G.; Zhang, X.G.; Zhang, S.L. Studies on Tissue Culture of Daylily Rhizomes. J. Southwest Agric. Univ. 1999, 21, 427–429. [Google Scholar]
- Zhao, C.; Zhang, L.; Zhang, X.; Xu, Y.; Wei, Z.; Sun, B.; Liang, M.; Li, H.; Hu, F.; Xu, L. Regulation of Endogenous Phytohormones Alters the Fluoranthene Content in Arabidopsis thaliana. Sci. Total Environ. 2019, 688, 935–943. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpoor, F.; Zare, N.; Asghari, R.; Sheikhzadeh, P. Sterilization Protocols and the Effect of Plant Growth Regulators on Callus Induction and Secondary Metabolites Production in in Vitro Cultures of Melia azedarach L. AMB Express 2022, 12, 3. [Google Scholar] [CrossRef]
- He, Z. Guidance to experiment on chemical control in crop plants. In Guidance to Experiment on Chemical Control in Crop Plants; He, Z.P., Ed.; Beijing Agricultural University Publishers: Beijing, China, 1993; pp. 60–68. [Google Scholar]
- Weiler, E.W.; Jourdan, P.S.; Conrad, W. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 1981, 153, 561–571. [Google Scholar] [CrossRef] [PubMed]

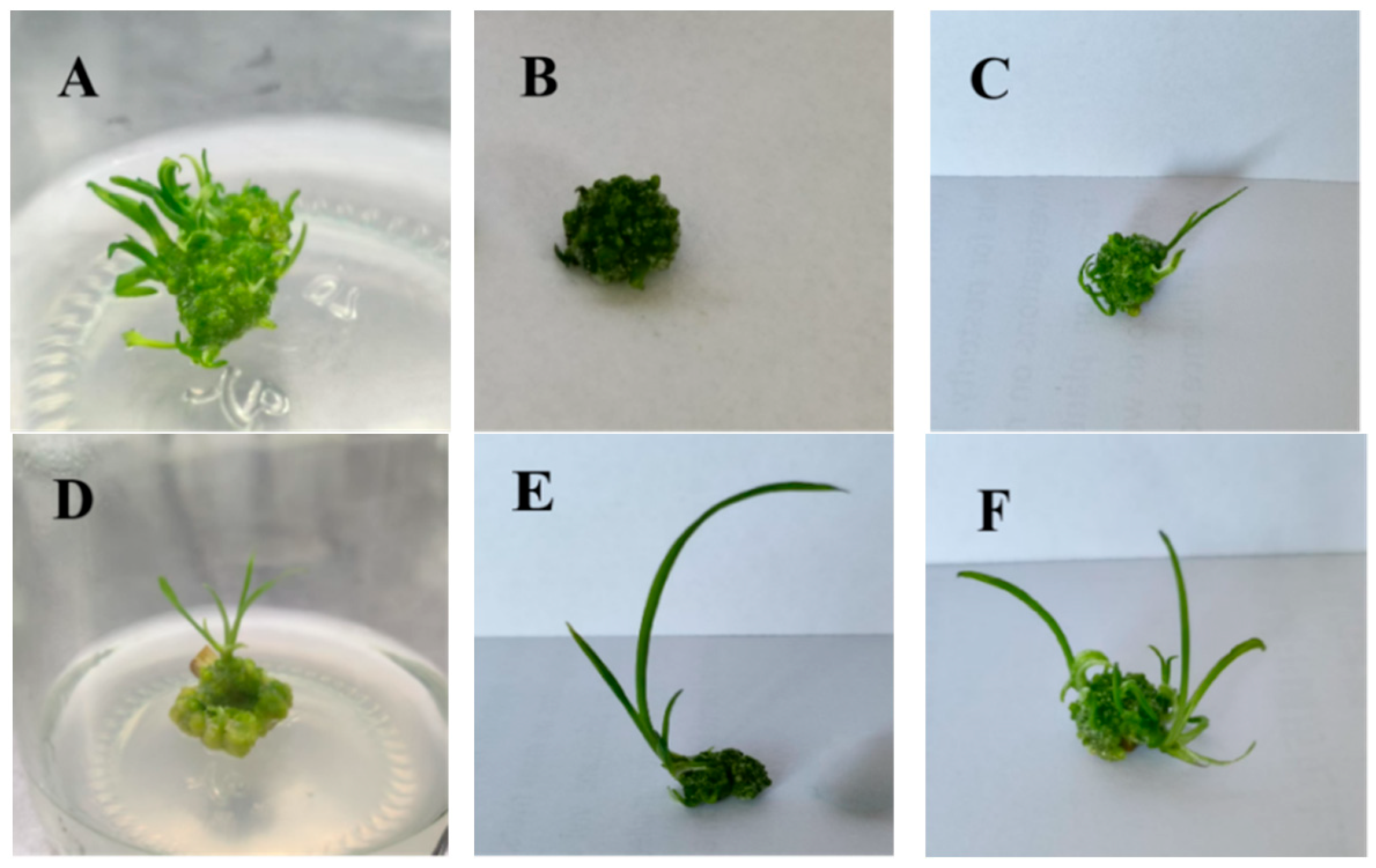


| Treatment | Plant Hormones | Number of Explants | Out of the Healing Number | Recovery Rate% | |
|---|---|---|---|---|---|
| ZT/(mg/L) | NAA/(mg/L) | ||||
| A1 | 1.0 | 0.0 | 60 | 9 | 12.05 ± 12.86 d |
| A2 | 1.0 | 0.1 | 60 | 11 | 20.83 ± 29.84 d |
| A3 | 1.0 | 0.3 | 60 | 15 | 25.00 ± 30.15 d |
| A4 | 1.0 | 0.5 | 60 | 17 | 29.17 ± 27.87 cd |
| A5 | 2.0 | 0.0 | 60 | 10 | 16.67 ± 30.77 d |
| A6 | 2.0 | 0.1 | 60 | 22 | 37.50 ± 40.59 cd |
| A7 | 2.0 | 0.3 | 60 | 25 | 41.67 ± 26.83 bcd |
| A8 | 2.0 | 0.5 | 60 | 20 | 33.33 ± 28.87 cd |
| A9 | 3.0 | 0.0 | 60 | 21 | 35.42 ± 36.08 cd |
| A10 | 3.0 | 0.1 | 60 | 35 | 58.33 ± 41.74 abc |
| A11 | 3.0 | 0.3 | 60 | 50 | 83.33 ± 32.57 a |
| A12 | 3.0 | 0.5 | 60 | 40 | 66.67 ± 38.92 ab |
| Treatment | Plant Hormones | Number of Explants | Induced Number | Adventitious Bud Induction Rate% | ||
|---|---|---|---|---|---|---|
| 6-BA/(mg/L) | NAA/(mg/L) | ZT/(mg/L) | ||||
| B1 | 3.0 | 0.1 | 0.0 | 30 | 20 | 66.80 ± 22.33 ab |
| B2 | 3.0 | 0.3 | 0.0 | 30 | 10 | 33.30 ± 35.21 c |
| B3 | 3.0 | 0.5 | 0.0 | 30 | 13 | 43.30 ± 31.75 bc |
| B4 | 0.0 | 0.1 | 3.0 | 30 | 11 | 36.60 ± 33.23 c |
| B5 | 0.0 | 0.3 | 3.0 | 30 | 25 | 83.40 ± 23.57 a |
| B6 | 0.0 | 0.5 | 3.0 | 30 | 15 | 50.10 ± 32.53 bc |
| Treatment | Plant Hormones | Number of Explants | Proliferation Coefficient | Growth Condition | ||
|---|---|---|---|---|---|---|
| 6-BA/(mg/L) | NAA/(mg/L) | ZT/(mg/L) | ||||
| 1 | 3.0 | 0.1 | 0.0 | 20 | 3.65 ± 1.00 a | +++ |
| 2 | 3.0 | 0.5 | 0.0 | 20 | 2.45 ± 0.69 b | ++ |
| 3 | 0.0 | 0.3 | 3.0 | 20 | 4.05 ± 1.07 a | ++++ |
| 4 | 0.0 | 0.5 | 3.0 | 20 | 2.25 ± 0.49 b | ++ |
| Treatment | Basic Medium | Plant Hormones | Rooting Rate% | Average Number of Roots | |
|---|---|---|---|---|---|
| IBA/(mg/L) | NAA/(mg/L) | ||||
| 1 | MS | 0.25 | 0.25 | 100% | 4.7 |
| 2 | MS | 0.50 | 0.50 | 98% | 3.1 |
| Research Materials | Type of Explant | Basic Culture Medium | Proliferation Coeffcient | Rooting Rate % | Source of Literature |
|---|---|---|---|---|---|
| Novel daylily cultivar ‘Red Baby’ | Shoot tip | MS + 0.2 mg/LIBA | 6.17 | 96% | [38] |
| ‘Datong Huanghua’ | Flowering stem | MS + 0.50 mg/LNAA | 90% | [39] | |
| ‘Datong Huanghua’ | Leaf blade | MS + 2 mg/L 6-BA + 0.5 mg/L IBA | 65% | [40] | |
| ‘Datong Huanghua’ | Scape | MS + 0.50 mg/LNAA | 90% | [41] | |
| ‘Datong Huanghua’ | Seed (stem of aseptic seedling) | 1/2 MS + 0.2 mg/LNAA | 45% | [42] | |
| Taiwanese daylily cultivar ‘Taichung No. 6’ | Shoot bud | Proliferation: MS + 6-BA 2.0 mg/L + NAA 0.1 mg/L Rooting: 1/2 MS + 0.05 mg/LNAA + 0.2 mg/L6-BA | 3.2 | 95% | [43] |
| ‘Alias Peter Parker’, ‘Sloam Gumdrop’, ‘Imprimatur’, along with 19 additional cultivars | Young inflorescence | MS | >95% | [44] | |
| ‘March Flower’ Daylily | Leaf blade | 1/2MS + 0.5 mg/L NAA | 93.33 | [45] | |
| Premium Xiamen Daylily | Rhizome | MS | 96% | [46] | |
| ‘Datong Huanghua’ | Shortened stem | MS + 1 mg/L6-BA + 0.1 mg/LNAA | 6.67 | [47] | |
| This study (on ‘Datong Huanghua’) | Scape | MS + 0.25 mg/L IBA + 0.25 mg/L NAA | 4.05 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, Q.; Zhang, Y.; Zheng, S. Key Techniques in Tissue Culture of Scape Explants from Hemerocallis citrina. Plants 2025, 14, 2761. https://doi.org/10.3390/plants14172761
Wang Y, Wei Q, Zhang Y, Zheng S. Key Techniques in Tissue Culture of Scape Explants from Hemerocallis citrina. Plants. 2025; 14(17):2761. https://doi.org/10.3390/plants14172761
Chicago/Turabian StyleWang, Ying, Qi Wei, Yamei Zhang, and Shaowen Zheng. 2025. "Key Techniques in Tissue Culture of Scape Explants from Hemerocallis citrina" Plants 14, no. 17: 2761. https://doi.org/10.3390/plants14172761
APA StyleWang, Y., Wei, Q., Zhang, Y., & Zheng, S. (2025). Key Techniques in Tissue Culture of Scape Explants from Hemerocallis citrina. Plants, 14(17), 2761. https://doi.org/10.3390/plants14172761





