Abstract
Drought seriously affects wheat yield; it is therefore important to study the molecular mechanism of wheat resistance to drought stress to ensure national food security. Plants can remove harmful substances through autophagy, thus improving their drought resistance. The results of previous studies have shown that autophagy is involved in the drought stress response; however, the molecular mechanism of autophagy in response to drought stress has yet to be elucidated. In this study, molecular biological methods such as immunohistochemistry, Co-Immunoprecipitation (Co-IP), and pull-down were used to explain the molecular mechanism of autophagy in response to drought stress at the protein level. We found that a dehydrin protein called cold-regulated 410 (TaCOR410) interacts with autophagy-related 8 (TaATG8, a key protein of wheat autophagy). TaCOR410 interacted with TaATG8 through its ATG8-interacting motif (AIM), and interaction was inhibited after mutation of the AIM. Interference with TaCOR410 inhibited autophagy and reduced the drought resistance of wheat. In contrast, transient transfection of TaCOR410 promoted autophagy. In wheat, overexpression of TaATG8 improved the drought resistance of wheat. Following interference with TaATG5, TaATG7 inhibited autophagy and reduced the drought resistance of wheat. From the above results, it is evident that autophagy can improve the drought resistance of wheat and can respond to drought stress through the interaction of TaCOR410 with TaATG8.
1. Introduction
By 2025, it will be necessary for crop yields in drought-prone areas to be increased by 40% to feed the ever-increasing human population [1]. Global losses in crop production due to drought have amounted to approximately 30 billion US dollars over the past decade [2]. Bread wheat (Triticum aestivum L.) provides roughly 19% of global dietary energy [3]. Drought is a major environmental factor that limits wheat production worldwide [4]. In recent years, climate change has further exacerbated the problem and resulted in a higher frequency of extreme weather changes, leading to abiotic stress conditions [5]. To adapt to stress conditions, plant cells must recycle damaged or unwanted proteins and organelles by means of autophagy, which is a conserved eukaryotic mechanism employed for the degradation of cellular components in lytic organelles (vacuoles in yeast and plants and lysosomes in animals) [6]. Upon induction of autophagy, a double-membrane structure engulfs unwanted cellular components to form an autophagosome and transports them to the central vacuole for degradation and recycling [7]. A number of autophagy-related (ATG) proteins have been identified for autophagosome formation and delivery to vacuoles [8,9]. Autophagy can regulate cellular homeostasis by selectively degrading specific cargo materials [10]. The specificity of the cargo is determined by selective autophagy receptors, which function as sorting adaptors that recruit selected cargo into autophagosomes through their ability to interact with ATG8 proteins [11]. ATG8 is a ubiquitin-like protein modified by phosphatidylethanolamine (PE) for incorporation into the autophagosome membrane [8]. Specific interactions between selective autophagy receptors and ATG8 require the ATG8-interacting motif (AIM), a short motif within the selective autophagy receptor [12].
Autophagy plays an important role in plant drought resistance. In Arabidopsis thaliana ATG5 and ATG7 mutants, autophagy is blocked, and their drought resistance is reduced [13,14]. Tomato is hypersensitive to drought after interference with ATG8d and ATG18h [15]. Overexpression of MdATG8i or MdATG18a enhanced drought tolerance in transgenic apple [16,17]. In our previous study, drought induced the expression of wheat ATGs, and an increase in autophagy puncta and ATG8-PE; in addition, interference of ATG6 led to a reduction in drought tolerance [18]. However, the molecular mechanism of autophagy in response to drought stress in wheat requires further analysis.
Late embryogenesis abundant (LEA) proteins are an important group of functional proteins that reduce cell damage and protect cells under abiotic stress conditions [19]. The results of previous studies have suggested that dehydrins (DHNs), also known as LEA II proteins, exhibit various types of biochemical activities, such as ion isolation, membrane stabilization, and chaperoning [20]. DHNs accumulate during late seed developmental stages and in vegetative tissues subjected to dehydration, salt, and low-temperature stresses [21]. In general, DHNs contain three highly conserved K-, Y-, and S-segments: the K-segment is rich in lysine (EKKGIMDKIKEKLPG) and very conserved among different species; it is located near the C-terminal and can form an α-helix with both hydrophilic and hydrophobic characteristics, which is related to the combination of macromolecules [22]. The Y-segment (T/VDEYGNP) is partially similar to the nucleotide-binding site of the molecular chaperone, and it is usually located at the N-terminal of DHN [23]. The S-segment is composed of 4–10 serine residues, and it is the main site of phosphorylation [24]. Based on the number and arrangement characteristics of conserved sequences, DHNs are divided into five subgroups: YnSKn, YnKn, SKn, Kn, and KnS. Different subgroups of DHNs respond to different environmental stresses, and the YnSKn subgroup is mainly induced by dehydration stress and abscisic acid (ABA). The Kn and YnKn subgroups are mainly activated by low-temperature stress, the SKn subgroup responds to drought, low temperature, and salt stresses, and the KnS subgroup responds to drought and low-temperature stresses [25]. DHNs impart drought stress tolerance by enhancing water retention capacity, increasing chlorophyll content, maintaining photosynthetic machinery, activating reactive oxygen species (ROS) detoxification, and promoting the accumulation of compatible solutes [26]. The interaction of rice YSK2-type dehydrin (OsDhn-Rab16D) with a prolyl cis-transisomerase (OsFKBP, Os02g52290) improved drought resistance through ABA signaling [27]. The DHN MtCAS31 (cold acclimation-specific 31) promotes autophagic degradation under drought stress in Medicago truncatula [28]. Fifty-five DHN genes have been found in wheat [29]. Wheat TaCOR410 is a DHN that accumulates around the plasma membrane, is present in lesser amounts in the intercellular space [30], and is upregulated under drought stress [31].
The results of previous studies have shown that autophagy is involved in the drought stress response; however, the molecular mechanism of autophagy in response to drought stress has yet to be elucidated. In our study, TaCOR410 interacted with TaATG8 to promote wheat autophagy. Overexpression of TaATG8 improved the drought resistance of wheat, whereas inhibition of autophagy decreased its drought resistance.
2. Results
2.1. Drought Induces Autophagy in Wheat
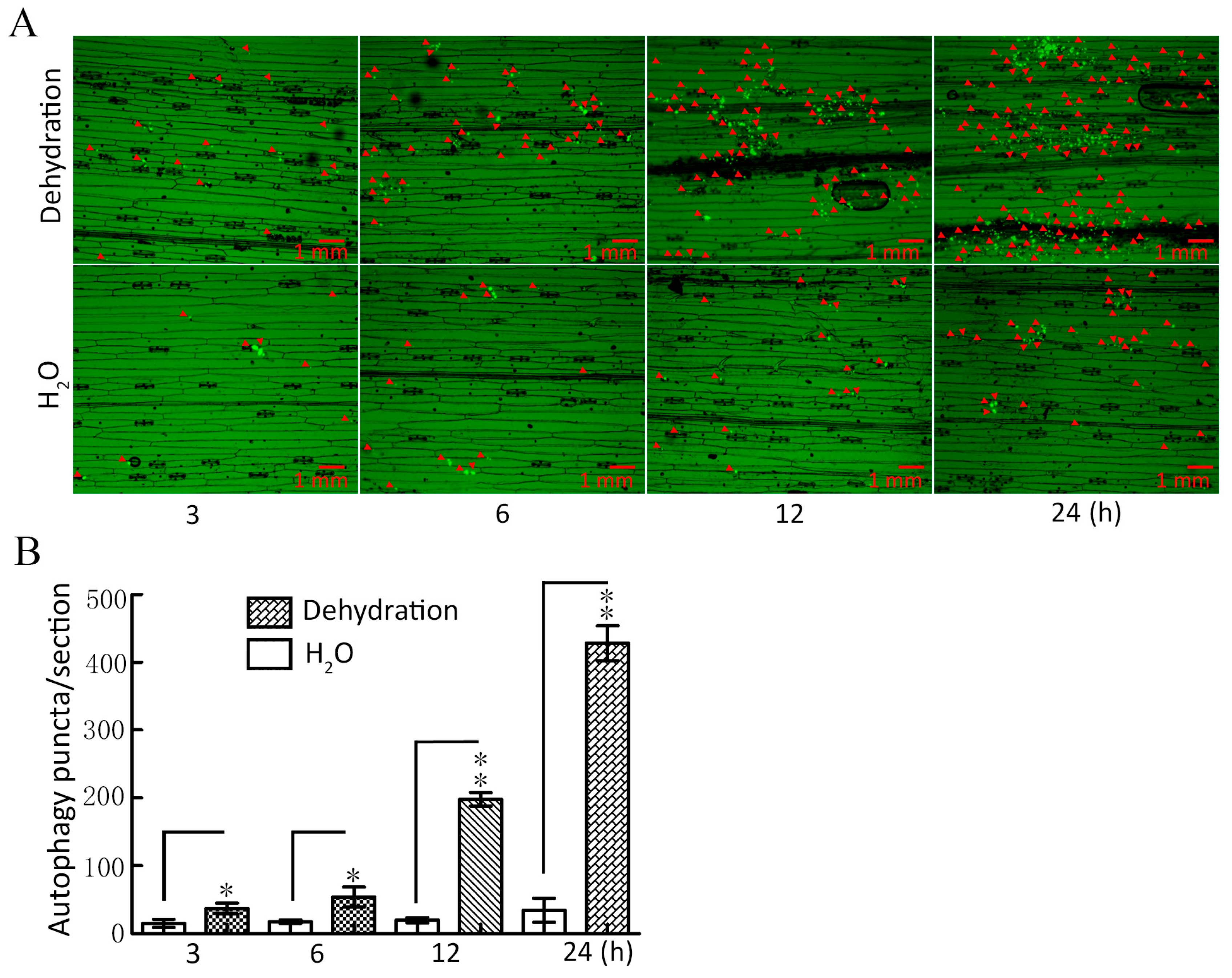
To investigate whether autophagy can respond to drought stress, ATG8-labeled autophagy puncta were used to detect plant tissue autophagy [32], and the expression of key autophagy genes was determined under dehydration conditions. The results showed that the abundance of autophagy puncta increased significantly under dehydration for 3–24 h when compared with the control (H2O); with the increase in dehydration time, the greater the abundance of autophagy puncta (Figure 1A,B). Additionally, our qRT-PCR results showed that the expression of key autophagy genes ATG3, ATG4, ATG5, ATG6, and ATG7 was significantly upregulated within 3–24 h (Figure S1). These findings provide conclusive evidence that drought induces autophagy in wheat.
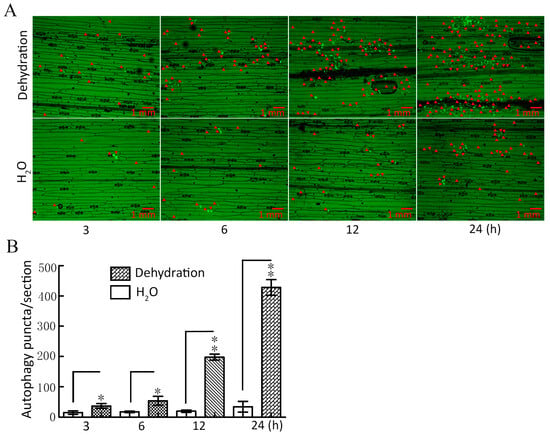
Figure 1.
Assay for autophagy puncta under dehydration conditions. (A) TaATG8-labeled autophagy puncta was detected under dehydration. Dehydration: 20% PEG-8000 was used to simulate drought stress at the indicated times. H2O was used as the control. The red arrows indicate autophagy puncta. Scale bars = 1 mm. (B) Significance analysis of the number of autophagy puncta per section in (A). Three sections were used for analysis. All experiments were conducted in triplicate. The error is the average SD of three experiments, and significant differences between the experimental and control groups were analyzed using the t-test (* p < 0.05; ** p < 0.01).
2.2. Screening and Identification of TaATG8 Interaction Protein TaCOR410
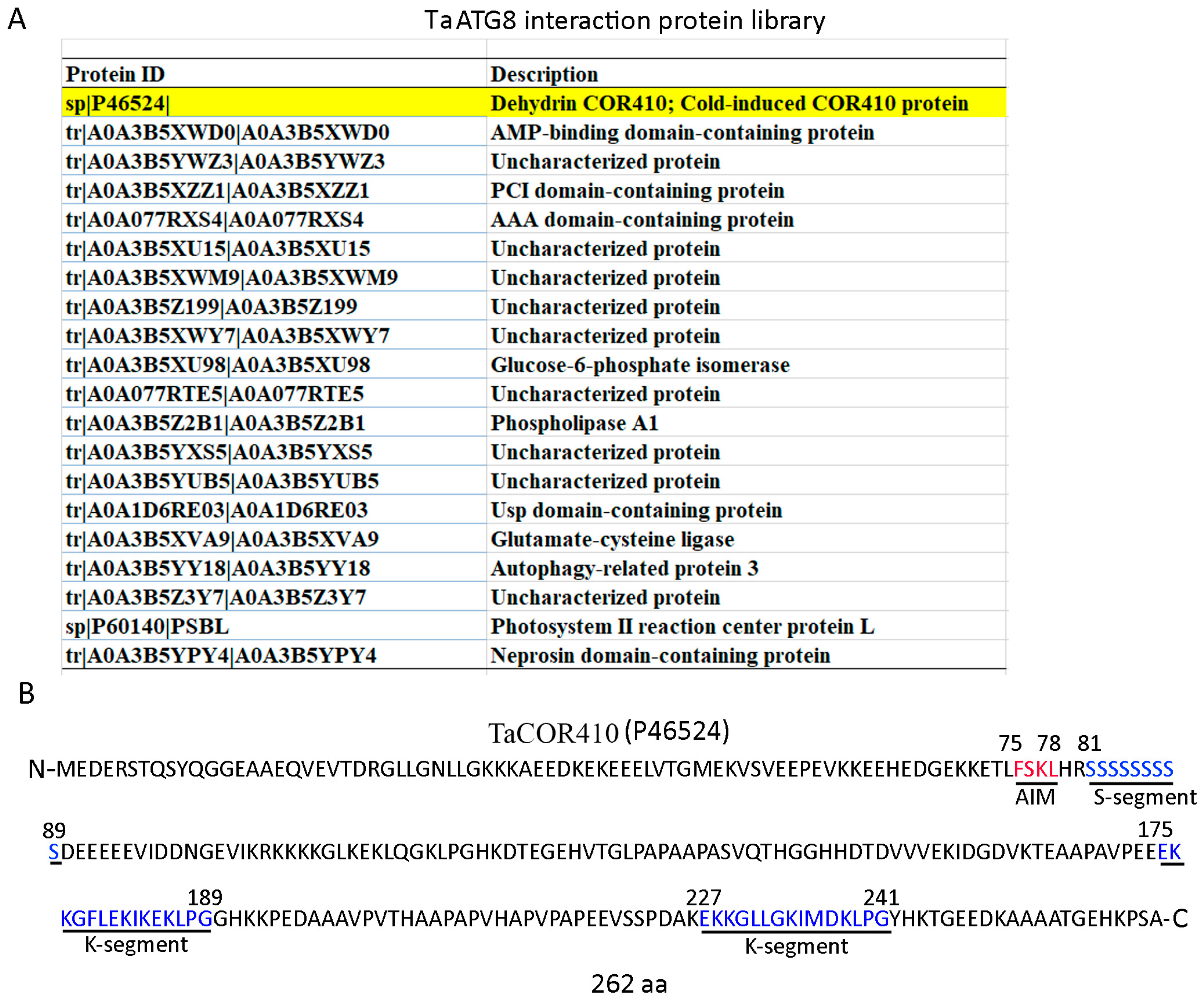
To investigate the molecular mechanism underlying the autophagy response to drought stress, a library of ATG8 interaction proteins was established by immunoprecipitating the total proteins of wheat leaves with a wheat ATG8 antibody and performing a mass spectrometry analysis. In this library, a wheat DHN protein, TaCOR410, was identified (Figure 2A). Due to DHNs being intrinsically disordered proteins and showing a tendency to interact with other biomolecules, we investigated whether TaCOR410 functions as a cargo receptor in the autophagy pathway. In general, ATG8 can bind to its interactors through the ATG8 interaction motif (AIM; W/F/YX1X2L/I/V) [33,34]. Hence, we analyzed the sequence of TaCOR410 and the predicted 1 AIM (75-FSKL-78). In addition, the protein contains 262 amino acids, an S-segment (81–89 amino acids), and two K-segments (175–189 and 227–241 amino acids) (Figure 2B), with it belonging to SK2 DHN.
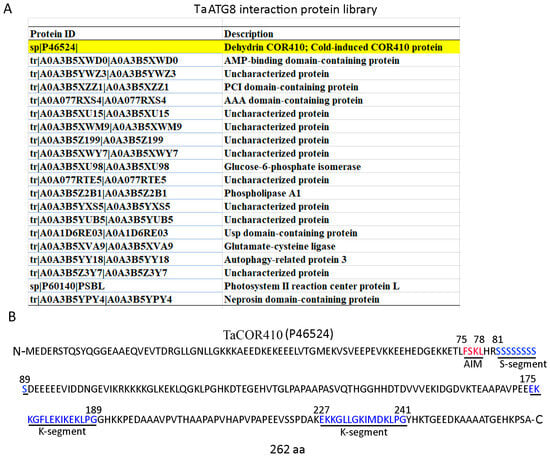
Figure 2.
Screening and identification of TaCOR410. (A) The TaATG8 interactive protein library; the screened TaCOR410 protein is highlighted in yellow. (B) TaCOR410 protein sequence analysis. The red letters represent the AIM, and the blue letters represent the S-segment and two K-segments. The numbers in the sequence represent the positions of amino acids.
2.3. TaCOR410 Specifically Interacts with TaATG8
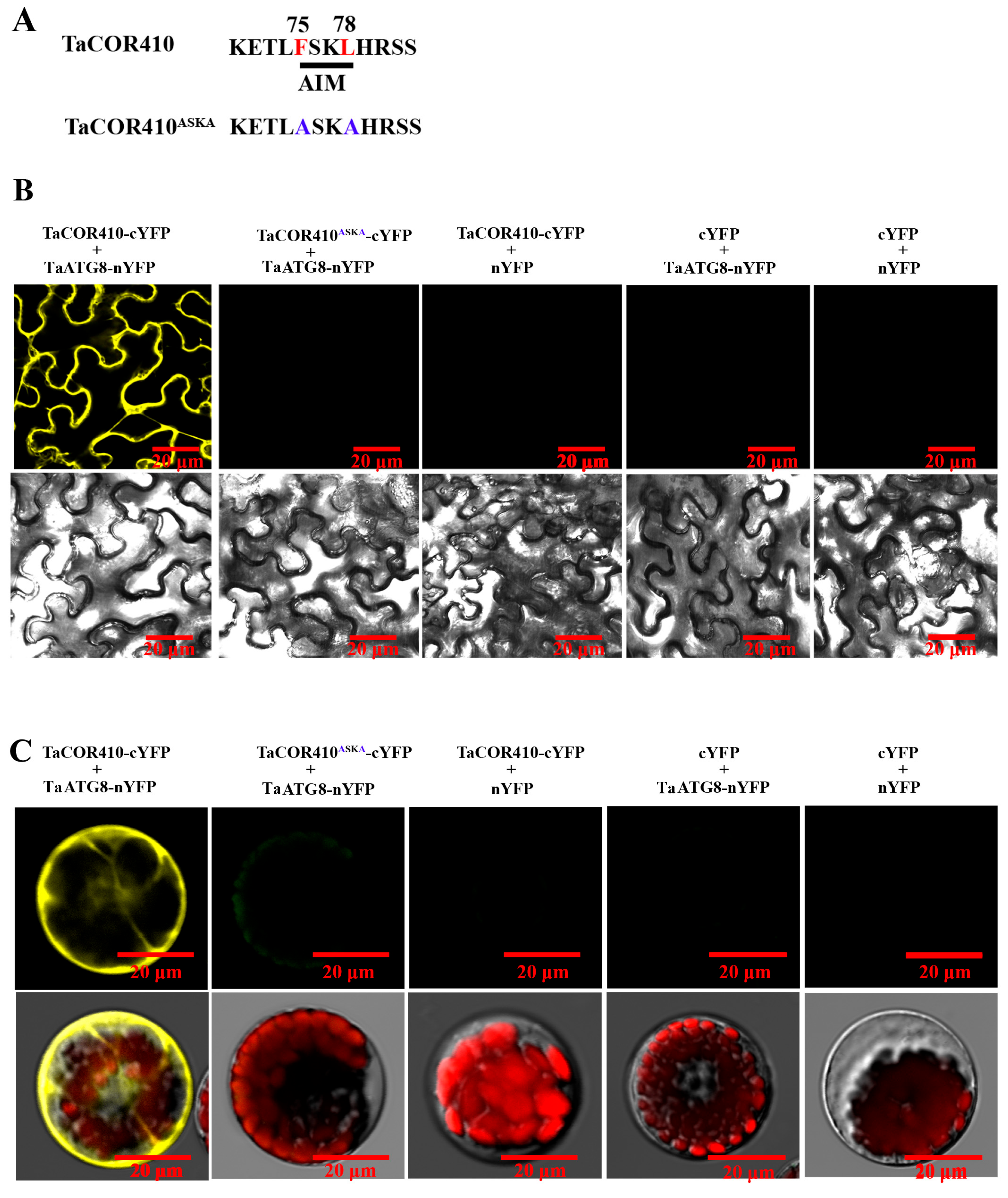
To further confirm the interaction of TaCOR410 with TaATG8, bimolecular fluorescence complementation (BiFC), co-immunoprecipitation (Co-IP), and pull-down experiments were conducted. The BiFC results showed that the yellow fluorescence signal was observed only when TaATG8-nYFP and TaCOR410-cYFP were co-transfected into the tobacco leaves and wheat protoplasts; in comparison, no yellow fluorescence was detected in the negative controls. However, the yellow fluorescence signal disappeared when the AIM FSKL (TaCOR410-cYFP) was mutated into ASKA (TaCOR410ASKA-cYFP) (Figure 3A–C).
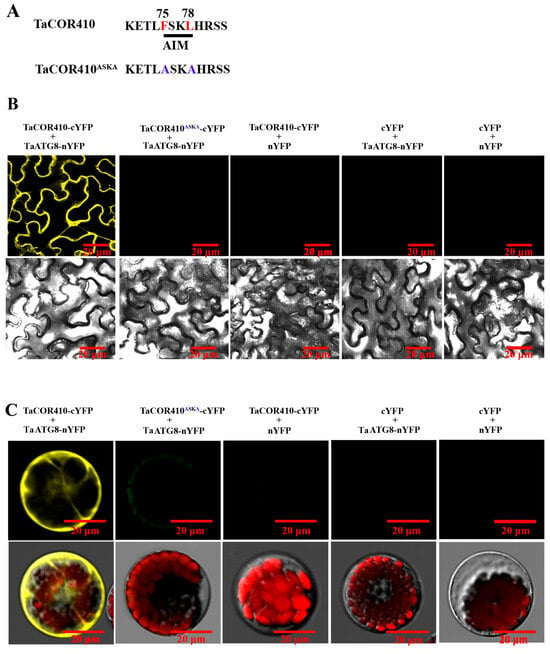
Figure 3.
BiFC assay confirming the interaction of TaCOR410 with TaATG8 in vivo. (A) TaCOR410 was mutated into TaCOR410ASKA. The 75–78th sequence is the AIM. The red letters indicate wild-type sites. The blue letters indicate mutant sites. (B) The BiFC assay was conducted using tobacco. TaATG8-nYFP and TaCOR410-cYFP are experimental groups, and the other groups are negative controls. (C) The BiFC assay was performed using the wheat protoplast. Images of all cells were obtained using a confocal microscope. Scale bars = 20 μm. All experiments were conducted in triplicate.
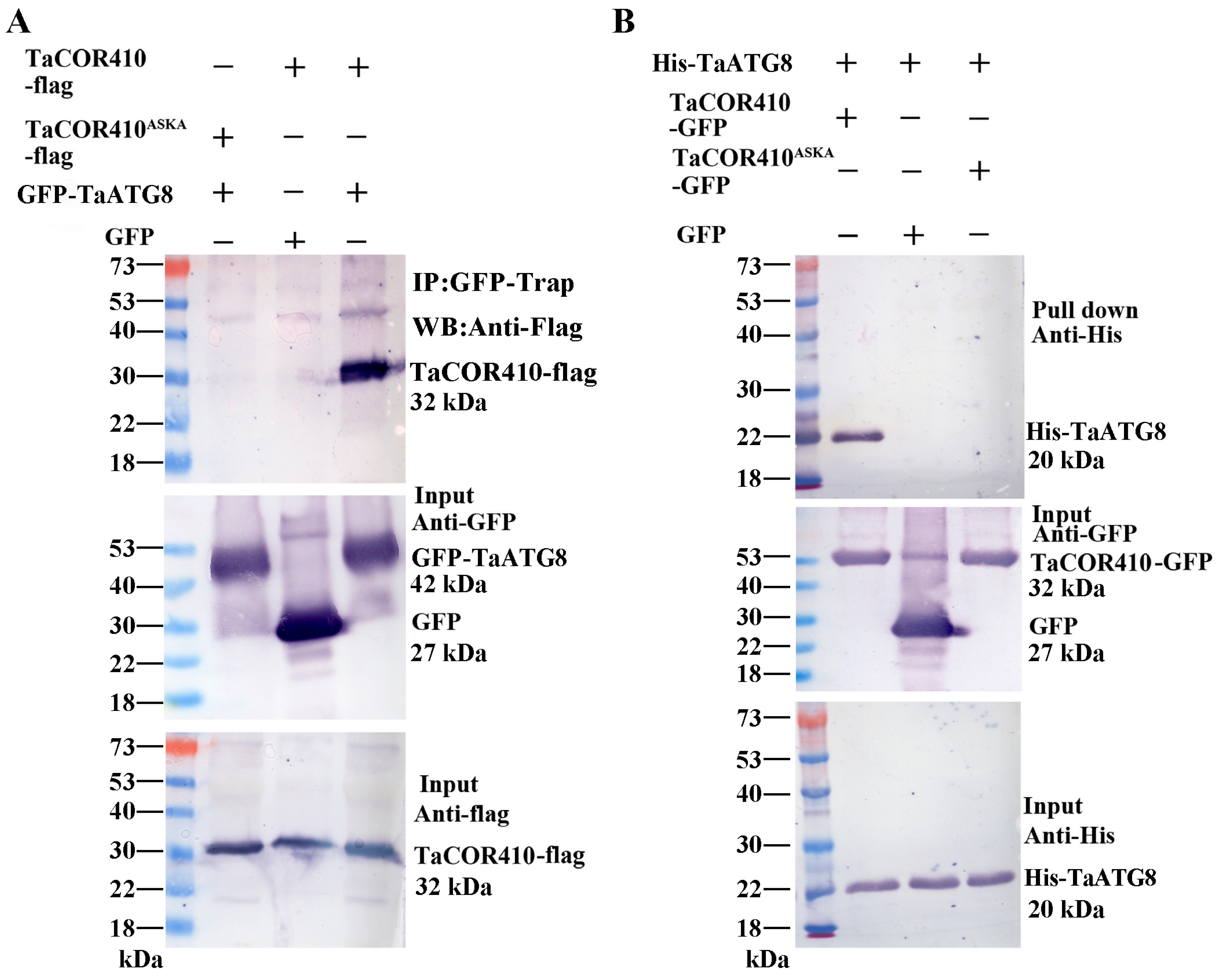
In addition, Co-IP was also performed to verify the results. TaCOR410-flag/GFP-TaATG8 or TaCOR410ASKA-flag/GFP-TaATG8 were co-expressed in tobacco leaves. Protein was immunoprecipitated with anti-GFP beads and detected with anti-flag. Only TaCOR410-flag and GFP-TaATG8 were able to bind specifically (Figure 4A). Lastly, we performed pull-down assays to confirm this interaction using GFP, TaCOR410-GFP, or TaCOR410ASKA-GFP protein as bait to precipitate the His-TaATG8 protein. As shown in Figure 4B, the TaCOR410-GFP protein bound specifically to His-TaATG8; however, neither GFP nor TaCOR410ASKA-GFP exhibited the same behavior. From the above results, it can be concluded that wheat DHN protein TaCOR410 interacts with TaATG8 in the AIM both in vitro and in vivo.
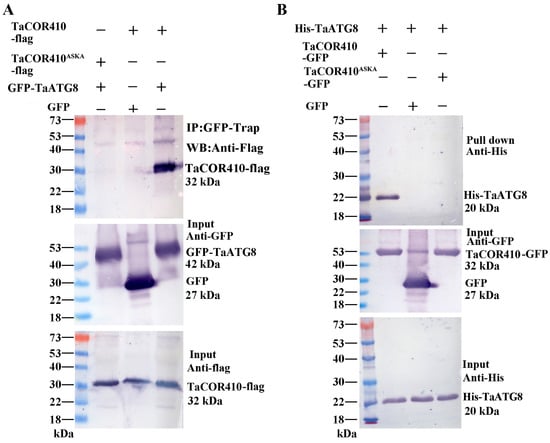
Figure 4.
Co-IP and pull-down assays to verify the interaction between TaATG8 and TaCOR410. (A) Detection of TaCOR410-TaATG8 interaction via Co-IP. TaCOR410 and TaCOR410ASKA were tagged with flag (TaCOR410-flag and TaCOR410ASKA-flag). GFP was tagged with TaATG8 (GFP-TaATG8). TaCOR410-flag/GFP and GFP-TaATG8/TaCOR410ASKA-flag were used as negative controls. Total proteins were extracted from tobacco leaves co-transformed with the indicated constructs and incubated with GFP beads to immunoprecipitate the target protein. Coprecipitated proteins were analyzed with Western blotting by using anti-flag and anti-GFP antibodies. (B) Detection of TaCOR410-TaATG8 interaction using the pull-down assay. His tag was fused with TaATG8 (His-TaATG8), and TaCOR410 and TaCOR410ASKA were fused with a GFP tag (TaCOR410-GFP and TaCOR410ASKA-GFP). TaCOR410-GFP, GFP, or TaCOR410ASKA-GFP alone was incubated with His-TaATG8 and precipitated with GFP beads. The precipitates were separated using SDS-PAGE and analyzed with Western blotting by using anti-His and anti-GFP antibodies. All experiments were conducted in triplicate.
2.4. Interference with TaCOR410 Reduces the Drought Resistance of Wheat
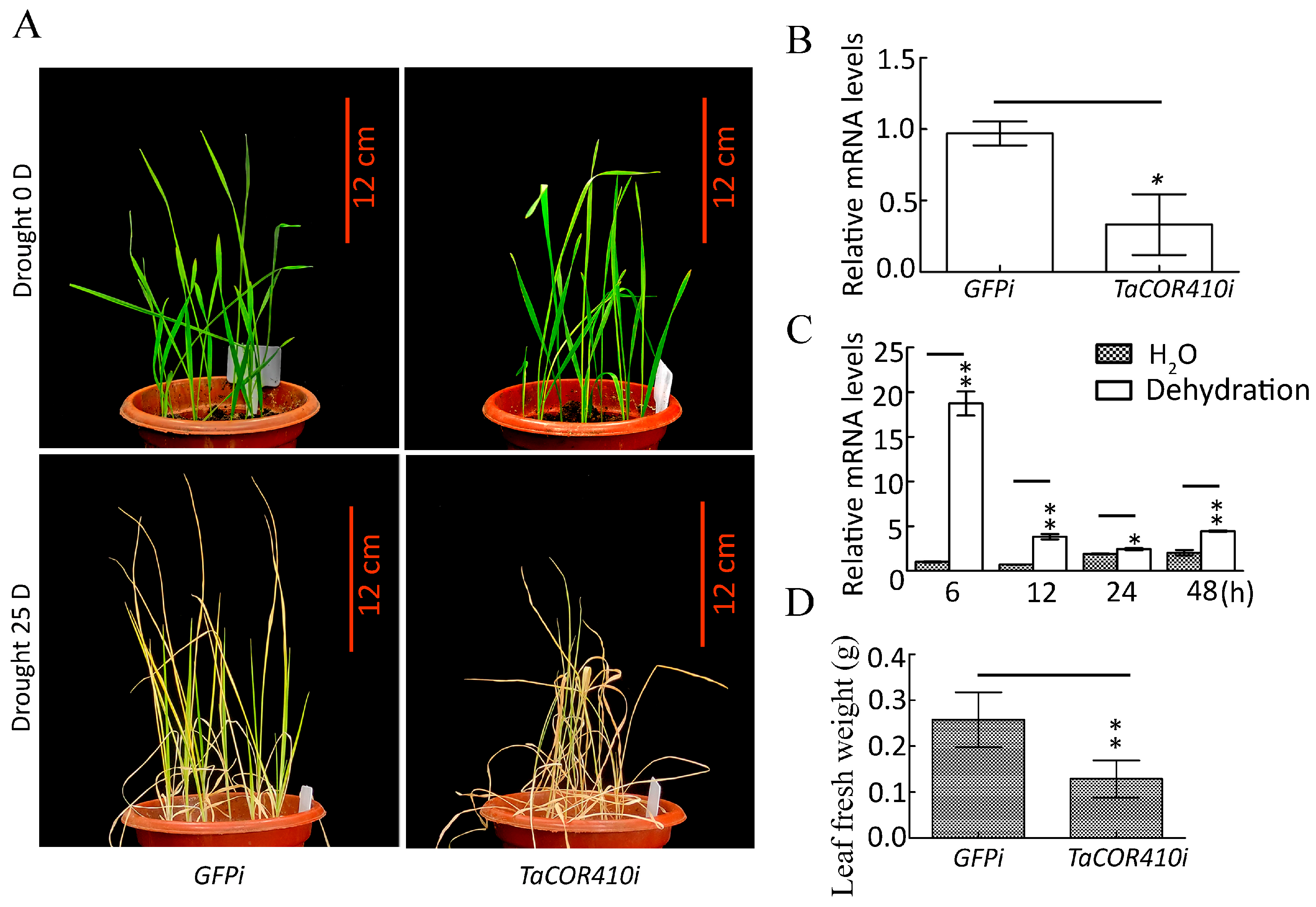
To investigate the role of TaCOR410 in wheat drought resistance, the drought resistance phenotype was identified after TaCOR410 was silenced by using the barley stripe mosaic virus (BSMV)-based virus-induced gene silencing (VIGS) method [35]. After TaCOR410 interference (TaCOR410i), the seedlings exhibited obvious dryness, and their fresh weight showed evident reduction when compared with the control (GFPi) after 25 days of drought stress (Figure 5A,B,D). The qRT-PCR results showed that TaCOR410 was significantly upregulated under drought stress from 6 to 48 h (Figure 5C). The results suggest that interference with TaCOR410 reduces the drought resistance of wheat, indicating that TaCOR410 responds to drought stress.
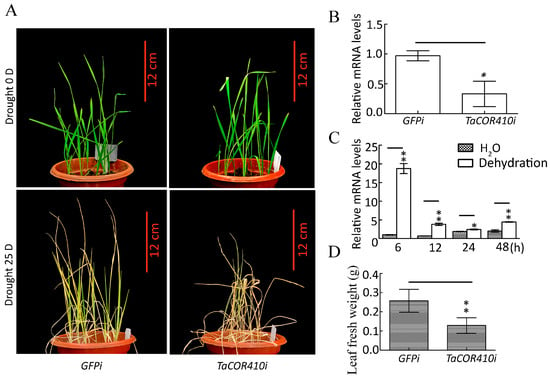
Figure 5.
Phenotype of wheat seedlings after TaCOR410 interference and expression of TaCOR410 under dehydration treatment. (A) Phenotype of wheat seedling after TaCOR410 interference. TaCOR410i represented the interference group. GFPi is the control of TaCOR410i. Scale bars = 12 cm. (B) Statistical analysis of the efficiency of TaCOR410 knockdown by qRT-PCR assay. β-actin was used as the internal reference and the relative expression was calculated using the 2−ΔΔCT method. (C) Expression of TaCOR410 under dehydration treatment. β-actin was used as the internal reference and the relative expression was calculated using the 2−ΔΔCT method. (D) The fresh weight was statistically analyzed after TaCOR410 interference. All experiments were conducted in triplicate. The error is the average SD of three experimental data, and significant differences between the experimental and control groups were analyzed using the t-test (* p < 0.05; ** p < 0.01).
2.5. TaCOR410 Promotes Autophagy in Wheat
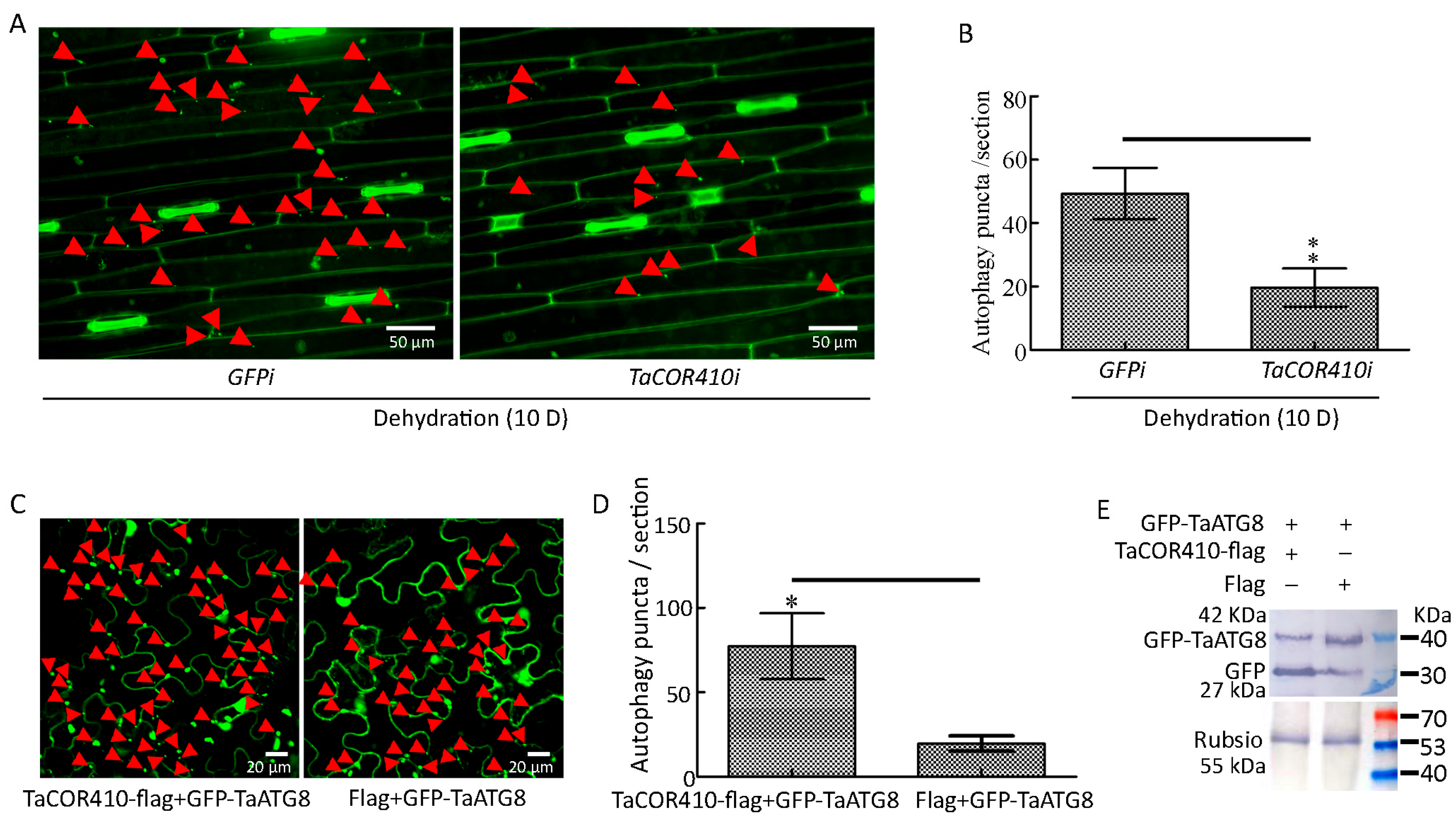
To examine whether TaCOR410 plays a role in drought-induced autophagy, we investigated the effects of TaCOR410 on autophagy under drought stress. The immunohistochemical results showed that interference with TaCOR410 led to a significant decrease in autophagy puncta under drought stress (Figure 6A,B). In addition, GFP-TaATG8 labeled autophagy puncta and cleavage of GFP-TaATG8 were measured to indicate autophagy after transfection of TaCOR410. In tobacco, co-transfection of TaCOR410-flag/GFP-TaATG8 produced more significant autophagy puncta than that of flag/GFP-TaATG8 (Figure 6C,D). Overexpression of TaCOR410-flag released more free GFP than flag (Figure 6E). These findings demonstrate that interference with TaCOR410 inhibits the formation of autophagy puncta and overexpression of TaCOR410 promotes the formation of autophagy puncta and cleavage of GFP-TaATG8. From the above results, it can be concluded that TaCOR410 promotes autophagy in wheat.
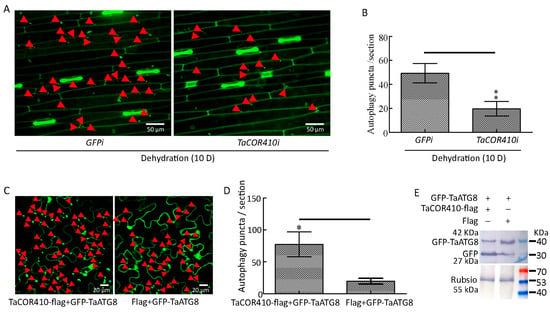
Figure 6.
TaCOR410 promotes autophagy in wheat. (A) TaATG8-labeled autophagy puncta was detected after TaCOR410 interference under drought stress. TaCOR410i represented the interference group, GFPi is the control of TaCOR410i. Dehydration: 20% PEG-8000 was used to simulate drought stress. The red arrows indicate autophagy puncta. Scale bars = 50 μm. (B) Significance analysis of the number of autophagy puncta per section in Figure 6A. Three sections were used for analysis. (C) Autophagy puncta were indicated by the transfection of the GFP-TaATG8 plasmid in tobacco leaves. Scale bars = 20 μm. Autophagy puncta were detected after co-transfection of TaCOR410-flag/GFP-TaATG8 and Flag/GFP-TaATG8 for 48 h. The red arrows indicate autophagy puncta. (D) Significance analysis of the number of GFP-TaATG8-labeled autophagy puncta per section in (C). Three sections were used for analysis. (E) Western blotting was used to detect the cleavage of GFP-TaATG8. Rubisco was used as the control. The SDS gel concentration was 12%. (* p < 0.05; ** p < 0.01).
2.6. Inhibition of Autophagy Reduces the Drought Resistance of Wheat
To elucidate the effects of autophagy on the drought resistance of wheat, the drought resistance phenotype of the seedlings was detected after autophagy was blocked by interference with TaATG5 or TaATG7. ATG5 and ATG7 play key roles in autophagy, and inhibition of ATG5 or ATG7 represses the autophagy pathway [36].
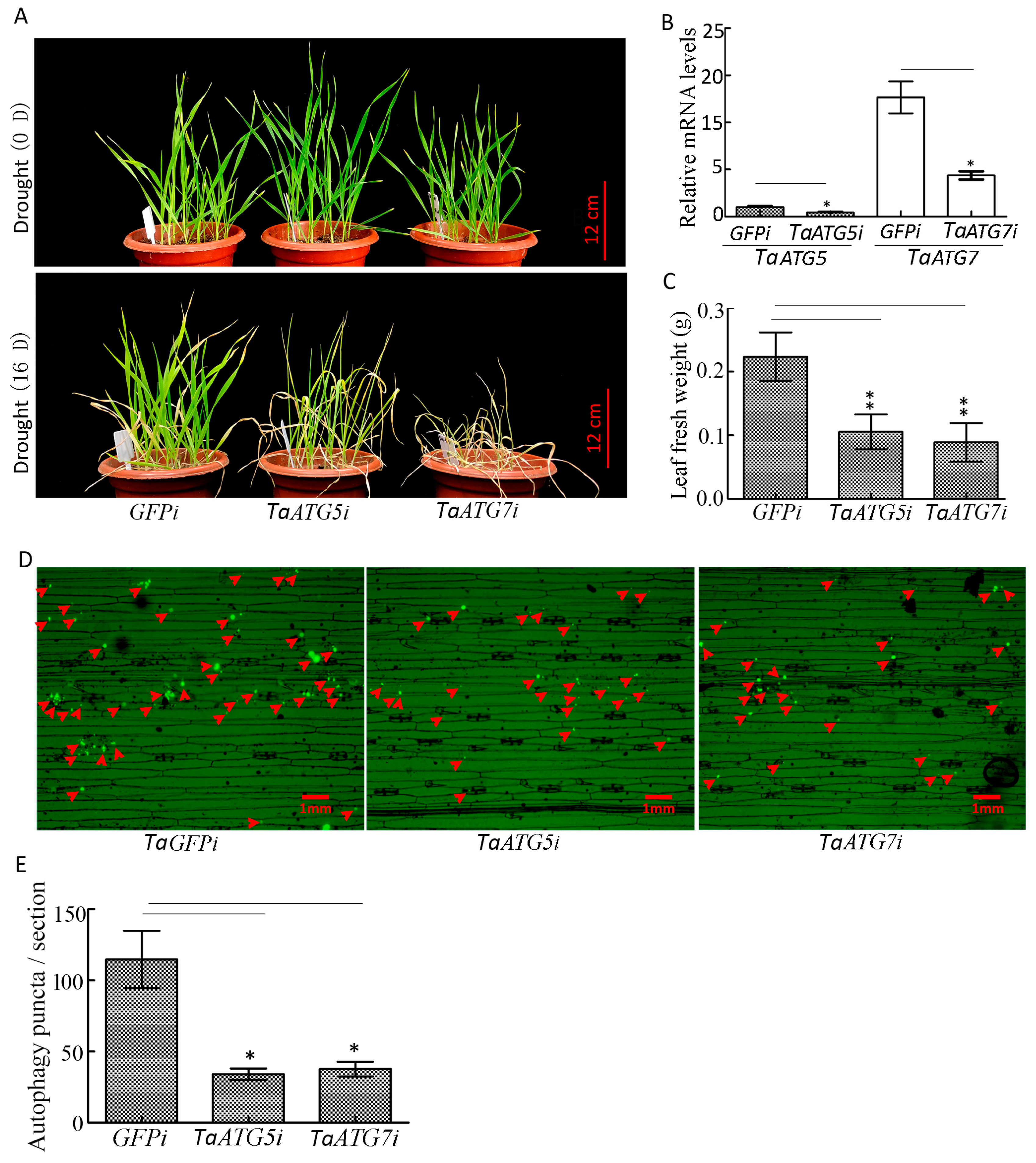
Our results demonstrated that interference with TaATG5 or TaATG7 led to a significant reduction in autophagy puncta, and seedlings wilted faster and the fresh weight of leaves decreased significantly under drought stress (Figure 7A–E), which indicates that inhibition of autophagy will accelerate the premature senescence of seedlings caused by drought.
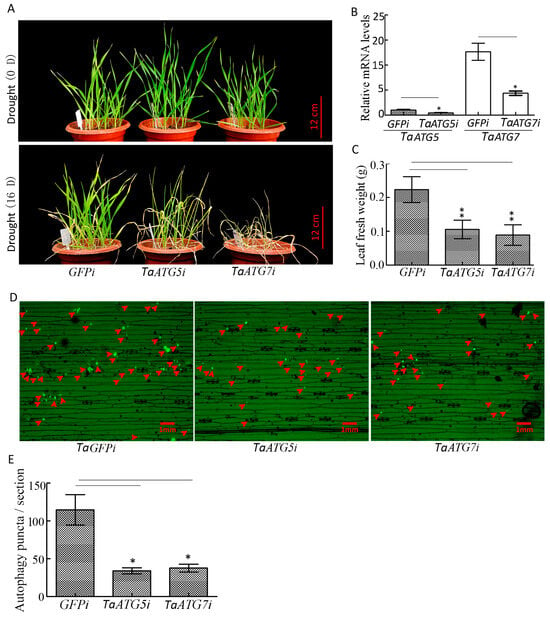
Figure 7.
Interference with TaATG5 or TaATG7 reduces the drought resistance of wheat. (A) Phenotype of wheat seedlings after interference with TaATG5 or TaATG7 under drought stress. TaATG5i and TaATG7i represented the interference group, while GFPi was used as the control group. Scale bars = 12 cm. (B) qRT-PCR analysis of the knockdown efficiency of TaATG5 and TaATG7. β-actin was used as the internal reference and the relative expression was calculated using the 2−ΔΔCT method. (C) The fresh weight was statistically analyzed after interference with TaATG5 or TaATG7. (D) Detection of autophagy puncta in wheat leaf after interference with TaATG5 or TaATG7 under drought stress. The red arrows indicate autophagy puncta. Scale bars = 1 mm. (E) Significance analysis of the number of autophagy puncta per section in (D). Three sections were used for analysis. The error is the average SD of three experiments, and significant differences between the experimental and control groups were analyzed using the t-test (* p < 0.05, ** p < 0.01).
2.7. Overexpression of TaATG8 Improves the Drought Resistance of Wheat
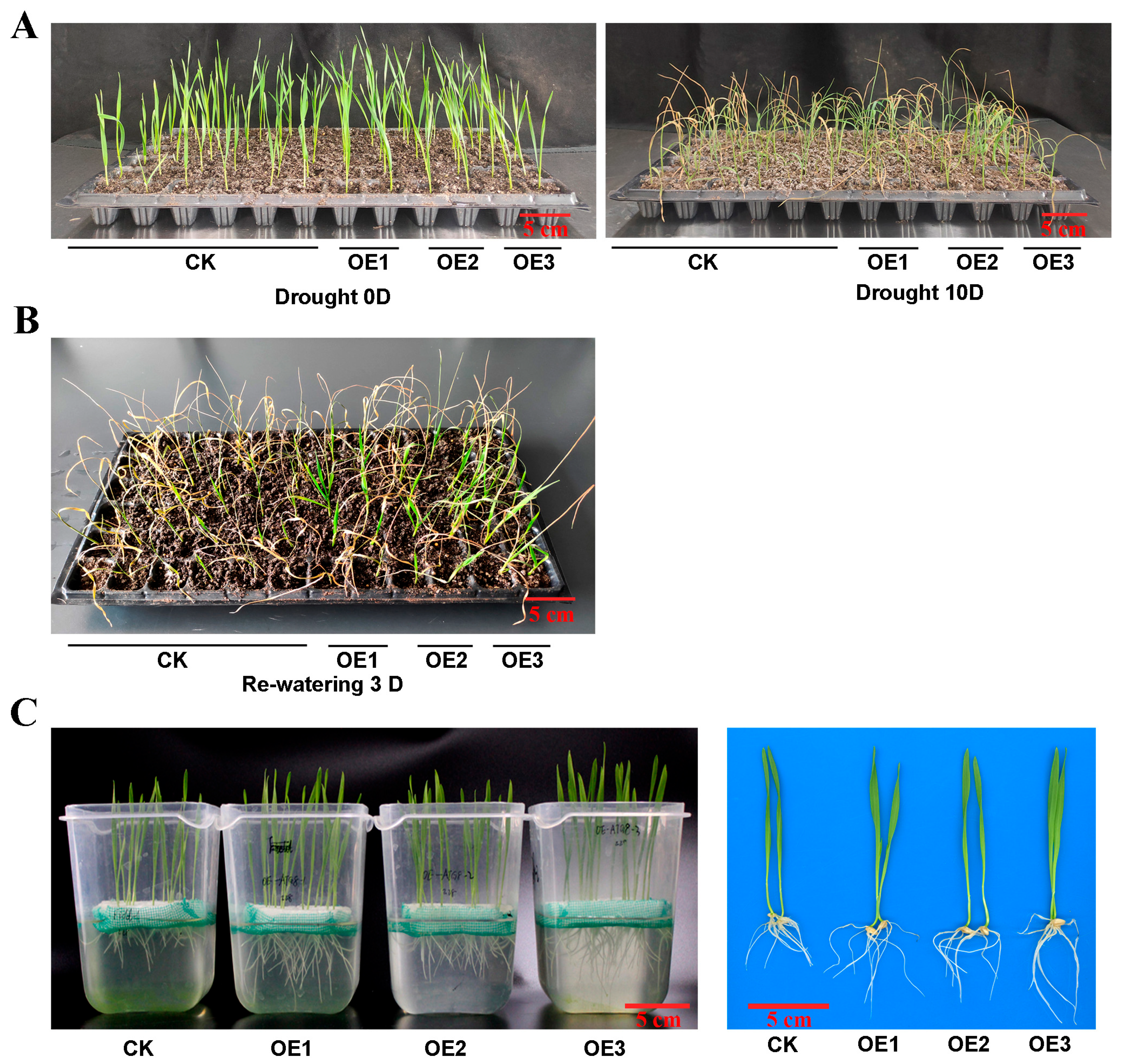
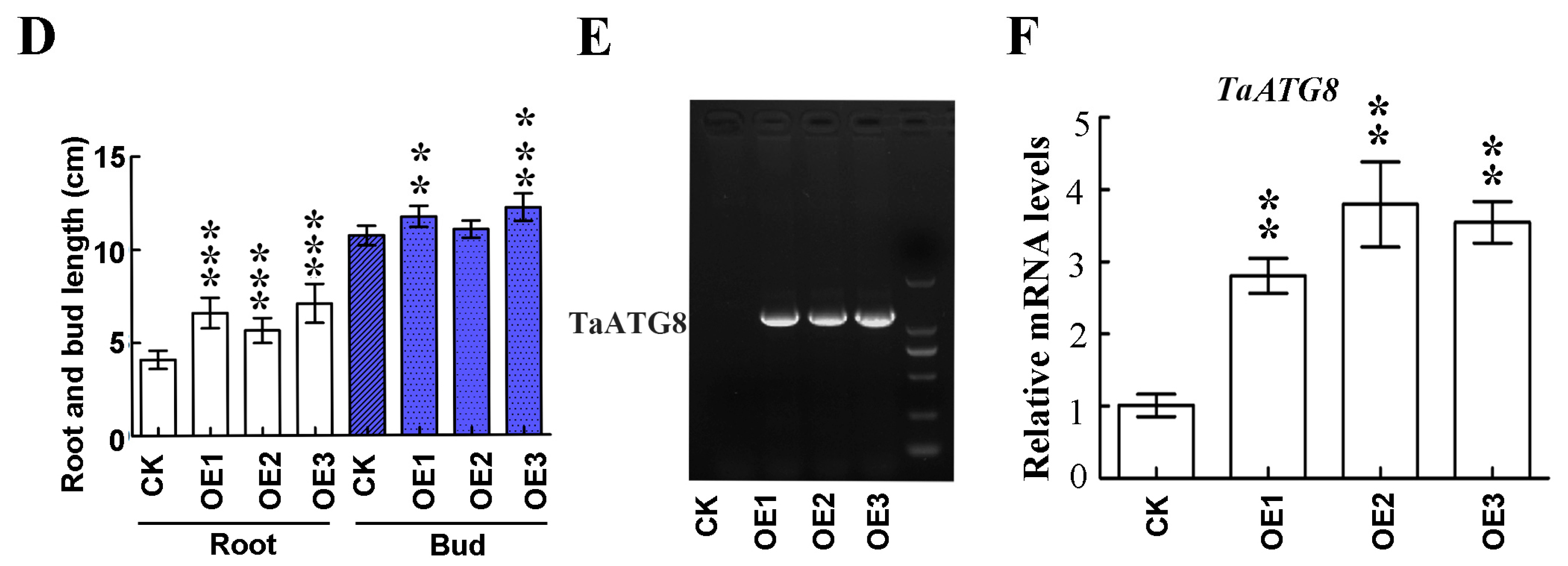
To investigate the role of TaATG8 under drought stress, we generated transgenic plants overexpressing TaATG8 (OE1, OE2, OE3), driven by the maize ubiquitin promoter, and Fielder was used as the receptor material. The transgenic lines wilted relatively slowly after 10 days of drought stress (Figure 8A). After 3 days of rehydration, the transgenic lines were reddish green; in comparison, the control Fielder were still dry (Figure 8B). In addition, the results of the drought simulation experiment showed that the root and bud lengths of the transgenic lines were significantly greater than those of recipient wheat Fielder (Figure 8C,D). All TaATG8 transgenic lines showed positive signals (Figure 8E), and the expression of TaATG8 was significantly higher than that of the receptor (Figure 8F). These results demonstrate that overexpression of TaATG8 can improve the drought resistance of wheat at the seedling stage.
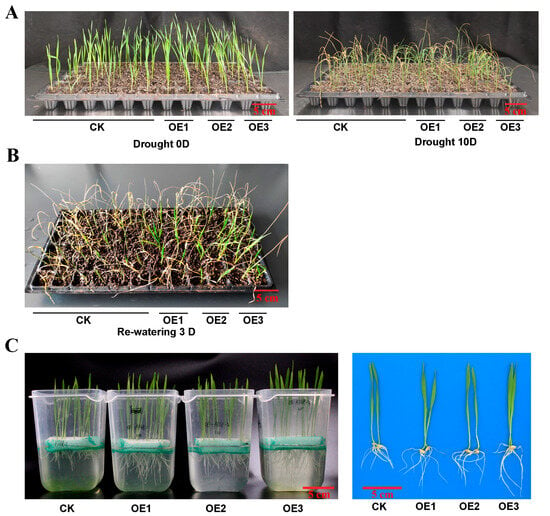
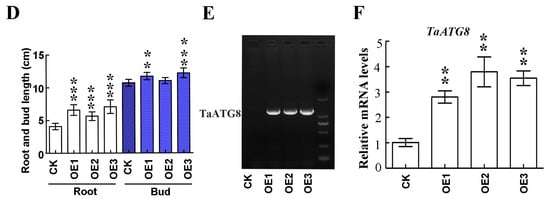
Figure 8.
Overexpression of TaATG8 improves the drought resistance of wheat. (A) Phenotype of TaATG8 transgenic lines at the seedling stage under drought stress. CK: Fielder wheat; OE1, OE2, and OE3, three independent TaATG8 transgenic lines. (B) Phenotype of seedlings after rehydration for 3 days. (C) Phenotypes of transgenic lines and controls were observed by treating the seedlings with 20% PEG-8000 to simulate drought. (D) The root and bud lengths were statistically compared between the transgenic lines and the control groups. (E) Transgenic detection of TaATG8 in 1% agarose gel. (F) qRT-PCR analysis of the expression of TaATG8 transgenic lines in wheat leaves. β-actin was used as the internal reference and the relative expression was calculated using the 2−ΔΔCT method. All experiments were conducted in triplicate. Scale bars = 12 cm. The error is the average SD of three experiments, and significant differences between the experimental and control groups were analyzed using the t-test (** p < 0.01; *** p < 0.001).
3. Discussion
Autophagy is essentially a process of digesting, degrading, and recycling harmful components in cells, and it can help plants survive under drought stress [37,38]. The results of a previous study demonstrated that drought promotes the expression of ATGs and the formation of ATG8-PE, thus activating autophagy in wheat [18]. However, the molecular mechanism of autophagy in response to drought has yet to be elucidated in wheat. In this study, we found that autophagy can respond to drought stress through the interaction of TaATG8 with TaCOR410. Interference with TaCOR410 inhibits autophagy and reduces the drought resistance of wheat, and transient transfection of TaCOR410 promotes autophagy. TaCOR410 interacts with TaATG8 through its AIM region, thus promoting autophagy. Overexpression of TaATG8 improves the drought resistance of wheat, and inhibition of autophagy reduces the drought resistance of wheat.
3.1. TaCOR410 Improves the Drought Resistance of Wheat Seedlings by Regulating Autophagy
DHNs are molecular protectors that protect membranes and macromolecules from denaturation under abiotic stress [39]. In wheat, TaCOR410 is a DHN that accumulates around the plasma membrane, and it is present in lesser amounts in the intercellular space [30]. In wheat, DHNs have been reported to accumulate in response to drought stress during anthesis [40]. The results of a previous study demonstrated that the number of mRNA transcripts of TaCOR410 significantly increased as water availability decreased in all wheat cultivars during the post-germination stage, presumably to enhance plant tolerance to drought stress through cell membrane protection, cryoprotection of enzymes, and prevention of ROS accumulation [41]. However, the molecular mechanism of TaCOR410 enhancing wheat drought stress requires further elucidation. Our findings demonstrated that interference with TaCOR410 inhibits drought-induced autophagy, resulting in decreased drought resistance in wheat. In contrast, overexpression of TaCOR410 promotes autophagy. Inhibition of autophagy leads to a decrease in drought resistance. From the above results, it can be concluded that TaCOR410 improves the drought resistance of wheat seedlings by regulating autophagy.
3.2. Autophagy Responds to Drought Stress Through the Interaction of TaCOR410 with TaATG8
Post-translational regulation is an important method for autophagy to cope with drought stress, and protein interaction is a form of post-translational regulation that exerts its effects in adaptation to various environmental stresses by promoting or inhibiting autophagy [42]. Cargo receptors play important roles in conferring autophagy selectivity by interacting with ATG8 via AIMs or the ubiquitin-interacting motifs [12,43]. To date, some cargo receptors related to plant drought resistance have been identified. In M. truncatula, the DHN MtCAS31 acts as a cargo receptor to form the plasma membrane intrinsic protein 2 (MtPIP2;7)-cold acclimation-specific 31 (MtCAS31)–MtATG8 complex, which facilitates MtPIP2;7 autophagic degradation and thus reduces water loss under drought stress and improves drought tolerance [28]. In Arabidopsis, the cargo receptor tryptophan-rich sensory protein/translocator (TSPO) interacts with ATG8 to promote the autophagic degradation of PIP2, thus improving drought resistance [44]. DOMINANT SUPPRESSOR OF KAR 2 (DSK2) is a ubiquitin-binding cargo receptor involved in the autophagic degradation of BRI1-EMSSUPPRESSOR 1 (BES1) to improve drought resistance mediated by the BR signaling pathway [45,46]. In this study, a cargo receptor, TaCOR410, was screened and identified from the interaction protein library of TaATG8; TaCOR410 can promote autophagy through the interaction of the AIM with TaATG8, thus improving the drought resistance of wheat. It cannot specifically bind to TaATG8 if the AIM is mutated. However, whether the interaction of TaCOR410 with TaATG8 is required to promote the autophagic degradation of PIP2 or any other substance in wheat requires further elucidation.
3.3. ATG8 Plays a Crucial Role in Wheat Drought Resistance
ATG8 plays an important role in maintaining the normal growth and development of plants and coping with various biotic and abiotic stresses. ATG8 is conjugated to the membrane lipid PE in a ubiquitin-like conjugation reaction that is essential for autophagosome formation [47]. In one study, Gossypium GhATG8f improved the salt tolerance of cotton by increasing superoxide dismutase, peroxidase, and catalase activities and proline accumulation [48]. Decreased ATG8F expression repressed the salt tolerance of transgenic rapeseed plants by significantly reducing root Na(+) retention under salt stress conditions and decreasing N uptake and translocation under N starvation [49]. ATG8b-mediated autophagy is involved in N recycling to grains and contributes to the grain quality of rice [50]. Silencing of ATG8c in the Chinese white pear (Pyrus bretschneideri Rehder) decreased its resistance to Botryosphaeria dothidea [51]. ATG8 is a positive regulator of the osmotic and drought stress response in wild emmer wheat [52]. In bread wheat, ATG8 is involved in grain development; interference with ATG8 will inhibit pericarp degradation, and the grain will become smaller and premature [53]. TaATG8j promotes resistance by regulating cell death in wheat affected by stripe rust [54]. In wheat, the function of TaATG8 has been investigated by means of only instantaneous interference. In this study, the role of TaATG8 in drought resistance was investigated using wheat transgenic materials with stable inheritance. In wheat, overexpression of TaATG8 can significantly increase the length of wheat seedling roots and buds and increase their resistance to drought stress.
4. Materials and Methods
4.1. Plant Material
The wheat cultivar Fielder was used as the receptor material in this study. Jimai 379, cultivated at the Crop Research Institute, Shandong Academy of Agricultural Sciences, is a water-saving and drought-resistant wheat variety.
4.2. Wheat Transformation
TaATG8 transgenic materials were obtained by Professor Li Genying’s group from the Crop Research Institute, Shandong Academy of Agricultural Sciences. Briefly, the open reading frame (ORF) of TaATG8 (TraesCS2A02G224000) was cloned into the binary vector pLGY02 at the SmaI and SpeI sites, forming a pLGY02-TaATG8 recombinant plasmid and maize Ubiquitin promoter-driven target gene expression. The construct pLGY02-TaATG8 was used to transform the drought-sensitive variety Fielder through Agrobacterium tumefaciens (Agrobacterium)-mediated transformation [55]. T1–T4 plants were tested for the presence of the transgene via PCR amplification, and Fielder was used as the negative control. Phenotypic identification of three group plants from each T4 line was performed at the seedling stage.
4.3. Wheat Seedlings Subjected to Drought Stress Treatment
Seeds of the wheat cultivar Jimai 379 were germinated at 22 °C. After 1 week, seedlings that showed consistent growth were selected for analysis. These seedlings were incubated in Hoagland nutrient solution for 3 days. Thereafter, some seedlings were transplanted into 20% PEG-8000 solution (P8260; Solarbio Science & Technology Co., Ltd., Beijing, China) to facilitate dehydration. Other seedlings were transplanted into flowerpots filled with peat soil soaked in Hoagland nutrient solution for drought stress experiments. The treatments were performed in a controlled environment chamber under a 16:8 h light–dark photoperiod and 22 °C temperature conditions.
4.4. Quantitative Real-Time Reverse Transcription (qRT)-PCR
Total RNA was extracted from wheat leaves by using a Rapid RNA extraction kit for plant tissues (DP452; TIANGEN, Beijing, China). After determining RNA quality via electrophoresis on a 1% agarose gel, 5 μg of RNA was reverse-transcribed into cDNA by using TransScript® First-Strand cDNA Synthesis SuperMix (AT301-02; TransGen Biotech, Beijing, China). The cDNAs were used as templates for qRT-PCR performed using TransStart® Top Green qPCR SuperMix (AQ131-01; TransGen Biotech), according to the manufacturer’s instructions; β-actin was amplified for internal standardization. The experiments were repeated three times, and the experimental data were analyzed using Student’s t-test. The relative expression data were analyzed using the 2−ΔΔCT method from the qRT-PCR experiments. The primers used are listed in Table S1.
4.5. Preparation of the TaATG8 Polyclonal Antibody
For the preparation of the TaATG8 polyclonal antibody, we followed the method outlined in a previous study [56]. Briefly, one Japanese white rabbit and one New Zealand white rabbit of experimental grade were raised. When the rabbits grew to 1–2 kg, 1 mL of well-mixed complete Freund’s adjuvant and 1 mL of TaATG8 polypeptide (N-KTEHPLERRQAESARIRE-C) at a concentration of 0.7 mg/mL were injected subcutaneously into each rabbit with a syringe. This stage was marked as day 1. On day 12, 1 mL of well-mixed incomplete Freund’s adjuvant and 1 mL of polypeptide at a concentration of 0.35 mg/mL were injected subcutaneously into the rabbits. On days 26, 40, and 54, 1 mL of well-mixed incomplete Freund’s adjuvant and 1 mL of polypeptide at a concentration of 0.35 mg/mL were injected intramuscularly into the rabbits. On day 66, the rabbit serum was taken for Western blot verification.
4.6. Establishment of the TaATG8 Interacting Protein Library
First, 1 mL of total protein of wheat leaf at a concentration of 2 mg/mL and 5 μL TaATG8 antibody at a concentration of 1.8 mg/mL were mixed and incubated on ice overnight; thereafter, 50 μL of Protein G Agarose (P2053, Beyotime, Shanghai, China) was added and incubated on ice for 3 h; it was then centrifuged at 400× g for 5 min and the supernatant was discarded. The gel-like substance at the bottom of the tube was retained, washed 5 times with PBS, and 40 μL of PBS was added and mixed after homogenization. Next, 20 μL of 5× protein loading buffer (G2075, Servicebio, Wuhan, China) was added, boiled for 10 min, and then proceeded by means of SDS-PAGE electrophoresis, with subsequent staining with SDS gel. The gel was subjected to mass spectrometry identification and analysis after decolorization.
The samples were processed using a standard in-gel digestion protocol, with trypsin used for in-gel digestion of the gel slices [57]. The specific experimental steps were as follows: the stained gel was incubated with trypsin (Promega) overnight at 37 °C. Peptides were extracted by means of incubation with 5% tallow fatty acid (TFA) for 1 h, followed by the addition of 2.5% TFA and 50% acetonitrile (ACN) at 37 °C for 1 h. The cleaved peptides were analyzed using a Nano LC-LTQ Orbitrap XL Liquid Chromatography–tandem mass spectrometry (LC-MS/MS) System (Thermo Scientific, Waltham, MA, USA). Data were analyzed using pFindStudio software (v.3.1.6) [58].
4.7. Western Blotting
Total proteins from wheat or tobacco leaves were extracted using the Plant Protein Extraction Kit (CW0885M; CoWin Biotech Co., Ltd., Taizhou, China). Equal amounts of protein (20 μg) from each sample were subjected to SDS-PAGE. The proteins were transferred electrophoretically onto a nitrocellulose membrane. The membrane was incubated with blocking buffer (2% skim milk powder dissolved in TBS) for 1 h at room temperature. Next, the membrane was incubated with a rabbit anti-ATG8 polyclonal antibody, rabbit anti GFP-Tag polyclonal antibody (AE011; ABclonal, Wuhan, China), or Anti-Rubisco mouse monoclonal antibody (Jining Bio, Shanghai, China) diluted 1:5000 in blocking buffer in TBS and incubated with the membrane for 2 h at room temperature. Thereafter, the membrane was washed with TBST (2% TWEEN-20 dissolved in TBS) 3 times and incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (A0239; Beyotime, Shanghai, China) diluted 1:10000 in the blocking buffer for 2–3 h at room temperature. The protein signal was visualized using the BCIP/NBT alkaline phosphatase chromogenic kit (C3266; Beyotime), according to the manufacturer’s instructions. The Prestained Protein Marker II (G2058, Servicebio, Wuhan, China) was used as a protein ruler.
4.8. Knock Down of TaATG5, TaATG7, and TaCOR410
A 206 bp fragment of TaATG5, a 216 bp fragment of TaATG7, and a 235 bp fragment of TaCOR410 from the conserved coding sequence were amplified and purified, and a 300 bp fragment of GFP was used as the control. The γ strand of BSMV was digested using ApaI and fused with the TaATG5, TaATG7, TaCOR410, and GFP fragments to form the vectors BSMVγ-TaATG5, BSMVγ-TaATG7, BSMVγ-TaCOR410, and BSMVγ-GFP, respectively. BSMVα, BSMVβ, BSMVγ-GFP, BSMVγ-TaATG5, BSMVγ-TaATG7, and BSMVγ-TaCOR410 were transformed into Agrobacterium GV3101. Agrobacterium GV3101 containing the target plasmid was cultured in YEB medium until OD = 0.6–0.8. The bacterial culture containing α, β, and γ components was mixed at a ratio of 1:1:1 and maintained at 28 °C for 4 h. Unfolded tobacco leaves at the 4–5 leaf stage were injected with the Agrobacterium solution. After 7–12 days, the injected leaves were collected and fully ground in buffer (1 g wheat leaf in 2–3 mL PBS buffer and 1% diatomite; pH = 7.2), and 15 µL of the juice was rubbed and inoculated onto the second leaf of wheat at the two-leaf and one-core stage. Six to eight days after inoculation, the target gene was detected using qRT-PCR.
4.9. Immunohistochemistry
Place the first leaf of the treated wheat seedling on the operating table, and gently make an incision in the middle part of the leaf with the tip of a dissecting knife. Then, using forceps, carefully peel off the lower epidermis from the leaf along the incision. The lower epidermis was treated with 4% paraformaldehyde at 4 °C overnight, placed in BSA and sealed at 37 °C for 1 h, incubated with a rabbit anti-ATG8 antibody diluted 1:200 in blocking buffer in TBS and incubated at 4 °C overnight, washed 3 times with PBS, incubated with the secondary antibody AF488-labeled Goat Anti-Rabbit IgG (A0423, Beyotime, Shanghai, China) diluted 1:1000 in BSA at 37 °C for 1 h, and washed 3 times with PBS. Thereafter, the lower epidermis tissue of the wheat leaves was placed on a glass slide for tableting, and a fluorescence microscope (HT7700; Hitachi, Tokyo, Japan) and a confocal microscope (FV3000; Olympus, Tokyo, Japan) were used to observe the samples and obtain images.
4.10. Observing Autophagy Puncta in Tobacco
GFP was linked with the N-terminal ORF of TaATG8 to generate a GFP-TaATG8 fragment. The ligated fragment was cloned into a 35S-pBWA(V)HS-CCDB vector at BsaI and Eco31I sites, forming a 35S-GFP-TaATG8 reporter plasmid, which was constructed and transferred into Agrobacterium GV3101. When the OD of the bacterial culture reached 0.6–0.8, the culture was injected into tobacco with 4–6 leaves. Additionally, 100 μM E64D was injected into tobacco to inhibit enzyme activity in vacuoles. Autophagy puncta were observed using the aforementioned confocal microscope 48 h after injection.
4.11. GFP Cleavage Assay
The ORF of TaCOR410 (TraesCS6D02G234700) was cloned into the 35S-pBWA(V)KS-CCDB–flag vector at NdeI and EcoRI sites, forming a 35S-TaCOR410-Flag recombinant plasmid. GFP was linked with the N-terminal ORF of TaATG8 to generate the GFP-TaATG8 fragment; thereafter, the ligated fragment was cloned into the 35S-pBWA(V)HS-CCDB vector at the BsaI and Eco31I sites, forming a 35S-GFP-TaATG8 recombinant plasmid. The above plasmids, 35S-GFP-TaATG8, 35S-TaCOR410-flag, and 35S-flag, were transferred into Agrobacterium GV3101. The bacterial culture containing 35s-GFP-TaATG8 and the bacterial culture containing 35S-TaCOR410-flag or 35S-flag were evenly mixed at a ratio of 1:1 and centrifuged, and the supernatant was discarded; tobacco infection liquid was added to resuspend the bacteria and then injected into tobacco leaves, which were cultured for 48 h. The protein extract was analyzed with anti-GFP, and GFP-TaATG8 and free GFP were detected. In this assay, Rubisco was used as the reference.
4.12. BiFC Assay
The TaATG8 coding sequence was fused with the pCAMBIA1300-35S-C-YFPN vector at the BamHI and XbaI sites to generate the TaATG8-nYFP plasmid. In addition, TaCOR410 and TaCOR410ASKA were fused with the pCAMBIA1300-35S-C-YFPC vector at the BamHI and XbaI sites to generate 35S-TaCOR410-cYFP and 35S-TaCOR410ASKA-cYFP plasmids, which were separately transferred into Agrobacterium GV3101, and infiltration of tobacco was performed using a previously described method [59]. Infected tissues were analyzed at 48 h after infiltration. Fluorescence staining was visualized using a confocal microscope.
4.13. Pull-Down Assay
To investigate the interaction between TaCOR410 and TaATG8, the full-length coding sequence of TaATG8 was subcloned into the pET-30a vector and expressed in BL21 cells to produce a His-TaATG8 fusion protein. The 35S-TaCOR410-GFP, 35S-TaCOR410ASKA-GFP, and 35S-GFP plasmids were transferred into Agrobacterium GV3101 and injected into tobacco leaves. The total protein in tobacco was extracted after 48 h of culture. Different target proteins were incubated with 1 μg His-TaATG8 at 4 °C for 1 h with shaking. Thereafter, the GFP antibody was added, followed by shaking at 4 °C overnight. Next, protein A agarose was added to the samples for 2–3 h at 4 °C. The samples were centrifuged, and the precipitate was collected and washed with PBS 5 times. The precipitate was boiled in 2× SDS loading buffer, and the proteins were analyzed through immunoblotting using the anti-His tag (AE068; ABclonal) and anti-GFP tag.
4.14. Co-IP
Agrobacterium GV3101 harboring 35S-GFP/35S-TaCOR410-flag, 35S-GFP-TaATG8 /35S-TaCOR410-flag, or 35S-GFP-TaATG8/35S-TaCOR410ASKA-flag constructs was injected into tobacco leaves. The total protein in tobacco leaves was extracted after 48 h of culture; 5 μL anti-GFP tag antibody was added to 2 mL protein extract and incubated overnight at 4 °C. The extract was centrifuged, and the precipitate was collected and washed with PBS 5 times. The precipitate was boiled in 2× SDS loading buffer. For immunoblotting analysis, anti-GFP and anti-FLAG (AF0036, Beyotime) antibodies were used to detect the relevant fusion proteins.
4.15. Quantitative Statistics
Quantitative analysis of autophagy puncta using ImageJ1.8.0 software. The data are presented as the mean ± SD. Significant differences between the two groups were determined using Student’s t-test with GraphPad Prism 5 software. Asterisks indicate statistically significant differences (*, p < 0.05; **, p < 0.01).
5. Conclusions
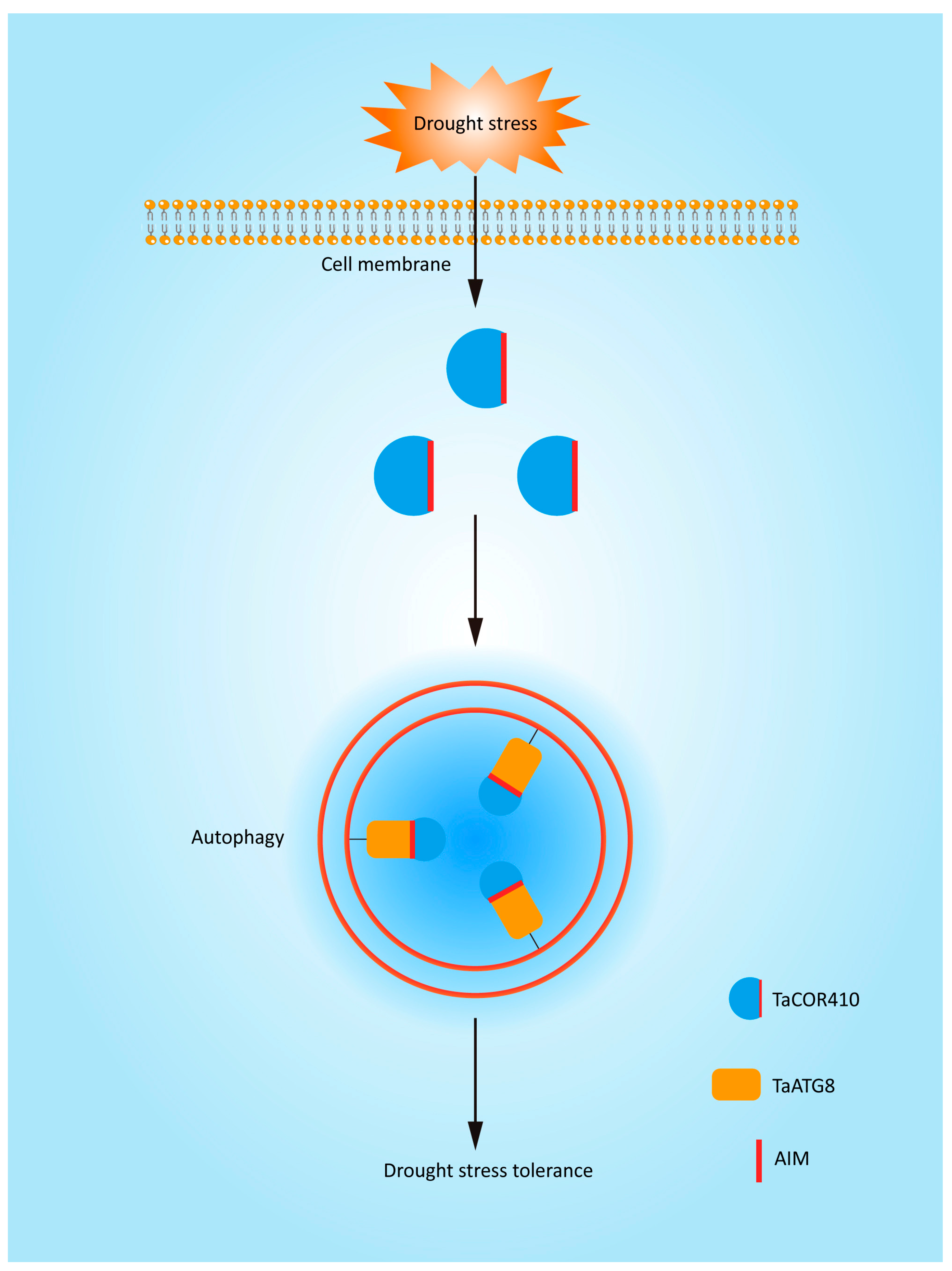
Based on the results of this study, we have produced a model to explain the molecular mechanism of autophagy in response to drought stress. Autophagy is involved in the drought stress response. TaCOR410 interacts with TaATG8 through its AIM to promote autophagy. Interference with TaCOR410 represses autophagy, and inhibition of autophagy reduces the drought resistance of wheat; in comparison, overexpression of TaATG8 improves the drought resistance of wheat. Overall, drought stress promotes autophagy through the interaction of TaCOR410 with TaATG8 (Figure 9).
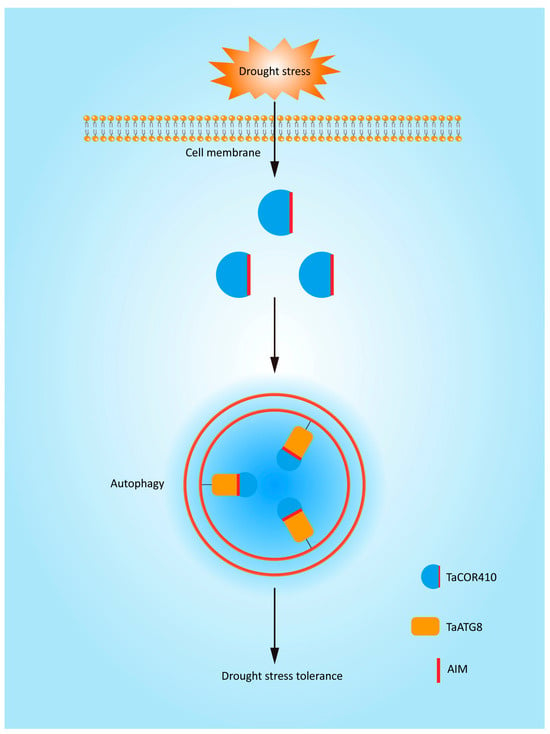
Figure 9.
A model to explain the molecular mechanism of autophagy in response to drought stress. TaCOR410 interacts with TaATG8 through its AIM to promote autophagy under drought stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14172726/s1, Figure S1: Effect of PEG-8000-induced stress simulating drought on autophagy related genes (ATGs)’ expression. Table S1: The primers used in this study.
Author Contributions
M.Y.: Writing—original draft; C.L.: Project administration; Y.-B.L.: Writing—review and editing, J.-L.W.: Funding acquisition; K.-Y.F.: Data curation; G.L.: Formal analysis; H.-D.S.: Validation. All co-authors have checked and confirmed their contribution statement. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Biological Breeding-National Science and Technology Major Project (No. 2023ZD04023), Shandong Key Research and Development Program, and the National Key Research and Development Program (No. 2023ZD04023; No. 2021LZGC009; No. 2022YFD1200205), the National Natural Science Foundation of China (32460455), the NGHJG project, the Xinjiang Tianshan Talent Project (2023TSYCCX0125), and the Xinjiang Production and Construction Corps Key Research and Development Program (2024AB009).
Conflicts of Interest
Authors Mei Yan and Hua-Dong Song was employed by the company Shandong Luyan Seed Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pennisi, E. Plant genetics. The blue revolution, drop by drop, gene by gene. Science 2008, 320, 171–173. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.M.; Kunert, K.J.; Cullis, C.A. Cysteine proteases and wheat (Triticum aestivum L) under drought: A still greatly unexplored association. Plant Cell Environ. 2017, 40, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Li, S.; Chen, B.; Jian, C.; Mei, F.; Zhang, Y.; Li, F.; Chen, N.; Li, T.; Du, L.; et al. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant 2022, 15, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Bassham, D.C.; Laporte, M.; Marty, F.; Moriyasu, Y.; Ohsumi, Y.; Olsen, L.J.; Yoshimoto, K. Autophagy in development and stress responses of plants. Autophagy 2006, 2, 2–11. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Chen, Q.; Have, M. Regulation of nutrient recycling via autophagy. Curr. Opin. Plant Biol. 2017, 39, 8–17. [Google Scholar] [CrossRef]
- Michaeli, S.; Galili, G.; Genschik, P.; Fernie, A.R.; Avin-Wittenberg, T. Autophagy in Plants—What’s New on the Menu? Trends Plant Sci. 2016, 21, 134–144. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef]
- Yang, F.; Kimberlin, A.N.; Elowsky, C.G.; Liu, Y.; Gonzalez-Solis, A.; Cahoon, E.B.; Alfano, J.R. A Plant Immune Receptor Degraded by Selective Autophagy. Mol. Plant 2019, 12, 113–123. [Google Scholar] [CrossRef]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Marshall, R.S.; Hua, Z.; Mali, S.; McLoughlin, F.; Vierstra, R.D. ATG8-Binding UIM Proteins Define a New Class of Autophagy Adaptors and Receptors. Cell 2019, 177, 766–781.e24, Erratum in Cell 2022, 185, 1101–1102. https://doi.org/10.1016/j.cell.2022.03.002. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, Y.; Bassham, D.C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009, 5, 954–963. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Cheng, Y.; Chi, Y.J.; Fan, B.; Yu, J.Q.; Chen, Z. NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 2013, 9, e1003196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zou, L.; Li, Y.; Yao, X.; Xu, F.; Deng, X.; Zhang, D.; Lin, H. Mitochondrial alternative oxidase-dependent autophagy involved in ethylene-mediated drought tolerance in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gong, X.; Jia, X.; Li, X.; Wang, Y.; Wang, P.; Huo, L.; Sun, X.; Che, R.; Li, T.; et al. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. Int. J. Mol. Sci. 2021, 22, 5517. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef]
- Li, Y.B.; Cui, D.Z.; Sui, X.X.; Huang, C.; Huang, C.Y.; Fan, Q.Q.; Chu, X.S. Autophagic Survival Precedes Programmed Cell Death in Wheat Seedlings Exposed to Drought Stress. Int. J. Mol. Sci. 2019, 20, 5777. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. Cell Mol. Biol. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Hara, M.; Fujinaga, M.; Kuboi, T. Metal binding by citrus dehydrin with histidine-rich domains. J. Exp. Bot. 2005, 56, 2695–2703. [Google Scholar] [CrossRef]
- Koag, M.C.; Wilkens, S.; Fenton, R.D.; Resnik, J.; Vo, E.; Close, T.J. The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes. Plant Physiol. 2009, 150, 1503–1514. [Google Scholar] [CrossRef]
- Perdiguero, P.; Collada, C.; Soto, A. Novel dehydrins lacking complete K-segments in Pinaceae. The exception rather than the rule. Front. Plant Sci. 2014, 5, 682. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Lv, Q.; Zhu, D.; Qiu, T.; Xu, Y.; Bao, F.; He, Y.; Hu, Y. Physcomitrella Patens Dehydrins (PpDHNA and PpDHNC) Confer Salinity and Drought Tolerance to Transgenic Arabidopsis Plants. Front. Plant Sci. 2017, 8, 1316. [Google Scholar] [CrossRef]
- Hara, M. The multifunctionality of dehydrins: An overview. Plant Signal. Behav. 2010, 5, 503–508. [Google Scholar] [CrossRef]
- Rurek, M. Diverse accumulation of several dehydrin-like proteins in cauliflower (Brassica oleracea var. botrytis), Arabidopsis thaliana and yellow lupin (Lupinus luteus) mitochondria under cold and heat stress. BMC Plant Biol. 2010, 10, 181. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2022, 41, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Dhar, Y.V.; Srivastava, D.; Kidwai, M.; Chauhan, P.S.; Bag, S.K.; Asif, M.H.; Chakrabarty, D. Genome-wide analysis of rice dehydrin gene family: Its evolutionary conservedness and expression pattern in response to PEG induced dehydration stress. PLoS ONE 2017, 12, e0176399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Feng, H.; Deng, J.; Zhang, R.; Wen, J.; Dong, J.; Wang, T. Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 2020, 16, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, M.; Cui, Y.; Kong, L.; Wang, H. Genome-wide survey of the dehydrin genes in bread wheat (Triticum aestivum L.) and its relatives: Identification, evolution and expression profiling under various abiotic stresses. BMC Genom. 2022, 23, 73. [Google Scholar] [CrossRef]
- Rorat, T. Plant dehydrins—Tissue location, structure and function. Cell. Mol. Biol. Lett. 2006, 11, 536–556. [Google Scholar] [CrossRef]
- Hassan, N.M.; El-Bastawisy, Z.M.; El-Sayed, A.K.; Ebeed, H.T.; Nemat Alla, M.M. Roles of dehydrin genes in wheat tolerance to drought stress. J. Adv. Res. 2015, 6, 179–188. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Zhang, H.; Wang, M.; Li, P.; Fang, X.; Cai, X. Detection of autophagy processes during the development of nonarticulated laticifers in Euphorbia kansui Liou. Planta 2018, 247, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Avin-Wittenberg, T.; Michaeli, S.; Honig, A.; Galili, G. ATI1, a newly identified atg8-interacting protein, binds two different Atg8 homologs. Plant Signal. Behav. 2012, 7, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Honig, A.; Avin-Wittenberg, T.; Ufaz, S.; Galili, G. A new type of compartment, defined by plant-specific Atg8-interacting proteins, is induced upon exposure of Arabidopsis plants to carbon starvation. Plant Cell 2012, 24, 288–303. [Google Scholar] [CrossRef]
- Wang, F.; Lin, R.; Li, Y.; Wang, P.; Feng, J.; Chen, W.; Xu, S. TabZIP74 Acts as a Positive Regulator in Wheat Stripe Rust Resistance and Involves Root Development by mRNA Splicing. Front. Plant Sci. 2019, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Minina, E.A.; Moschou, P.N.; Vetukuri, R.R.; Sanchez-Vera, V.; Cardoso, C.; Liu, Q.; Elander, P.H.; Dalman, K.; Beganovic, M.; Lindberg Yilmaz, J.; et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018, 69, 1415–1432. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef]
- Su, T.; Li, X.; Yang, M.; Shao, Q.; Zhao, Y.; Ma, C.; Wang, P. Autophagy: An Intracellular Degradation Pathway Regulating Plant Survival and Stress Response. Front. Plant Sci. 2020, 11, 164. [Google Scholar] [CrossRef]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef]
- Lopez, C.G.; Banowetz, G.M.; Peterson, C.J.; Kronstad, W.E. Wheat dehydrin accumulation in response to drought stress during anthesis. Funct. Plant Biol. FPB 2002, 29, 1417–1425. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Tanash, A.A. Effect of drought stress on wheat (Triticum durum) growth and metabolism: Insight from GABA shunt, reactive oxygen species and dehydrin genes expression. Funct. Plant Biol. FPB 2024, 51, NULL. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Qi, G.; Cui, D.; Huang, C.; Sui, X.; Li, G.; Fan, Q. Mechanisms of autophagy function and regulation in plant growth, development, and response to abiotic stress. Crop J. 2023, 11, 1611–1625. [Google Scholar] [CrossRef]
- Xie, Q.; Tzfadia, O.; Levy, M.; Weithorn, E.; Peled-Zehavi, H.; Van Parys, T.; Van de Peer, Y.; Galili, G. hfAIM: A reliable bioinformatics approach for in silico genome-wide identification of autophagy-associated Atg8-interacting motifs in various organisms. Autophagy 2016, 12, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Veljanovski, V.; Reinhardt, H.; Guillaumot, D.; Vanhee, C.; Chaumont, F.; Batoko, H. The Arabidopsis abiotic stress-induced TSPO-related protein reduces cell-surface expression of the aquaporin PIP2;7 through protein-protein interactions and autophagic degradation. Plant Cell 2014, 26, 4974–4990. [Google Scholar] [CrossRef]
- Nolan, T.M.; Brennan, B.; Yang, M.; Chen, J.; Zhang, M.; Li, Z.; Wang, X.; Bassham, D.C.; Walley, J.; Yin, Y. Selective Autophagy of BES1 Mediated by DSK2 Balances Plant Growth and Survival. Dev. Cell 2017, 41, 33–46.E7. [Google Scholar] [CrossRef]
- Fatimababy, A.S.; Lin, Y.L.; Usharani, R.; Radjacommare, R.; Wang, H.T.; Tsai, H.L.; Lee, Y.; Fu, H. Cross-species divergence of the major recognition pathways of ubiquitylated substrates for ubiquitin/26S proteasome-mediated proteolysis. FEBS J. 2010, 277, 796–816. [Google Scholar] [CrossRef]
- Ryabovol, V.V.; Minibayeva, F.V. Molecular Mechanisms of Autophagy in Plants: Role of ATG8 Proteins in Formation and Functioning of Autophagosomes. Biochemistry 2016, 81, 348–363. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Wu, Z.; Lu, X.; Yin, Z.; Zhao, L.; Huang, H.; Meng, Y.; Fan, Y.; Guo, L.; et al. Systematic analysis and expression of Gossypium ATG8 family reveals the roles of GhATG8f responding to salt stress in cotton. Plant Cell Rep. 2024, 43, 58. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, T.; Zhang, Y.; Chen, J.; Song, H.; Wu, P.; Yue, C.; Huang, J.; Zhang, Z.; Hua, Y. Genome-Wide Identification and Functional Characterization Reveals the Pivotal Roles of BnaA8.ATG8F in Salt Stress Tolerance and Nitrogen Limitation Adaptation in Allotetraploid Rapeseed. Int. J. Mol. Sci. 2022, 23, 11318. [Google Scholar] [CrossRef]
- Fan, T.; Yang, W.; Zeng, X.; Xu, X.; Xu, Y.; Fan, X.; Luo, M.; Tian, C.; Xia, K.; Zhang, M. A Rice Autophagy Gene OsATG8b Is Involved in Nitrogen Remobilization and Control of Grain Quality. Front. Plant Sci. 2020, 11, 588. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.; Xu, W.; Chen, Q.; Wang, Y.; Ban, Q.; Xing, C.; Zhang, S. Genome-wide identification and expression analysis of the pear autophagy-related gene PbrATG8 and functional verification of PbrATG8c in Pyrus bretschneideri Rehd. Planta 2021, 253, 32. [Google Scholar] [CrossRef]
- Kuzuoglu-Ozturk, D.; Cebeci Yalcinkaya, O.; Akpinar, B.A.; Mitou, G.; Korkmaz, G.; Gozuacik, D.; Budak, H. Autophagy-related gene, TdAtg8, in wild emmer wheat plays a role in drought and osmotic stress response. Planta 2012, 236, 1081–1092. [Google Scholar] [CrossRef]
- Li, Y.B.; Yan, M.; Cui, D.Z.; Huang, C.; Sui, X.X.; Guo, F.Z.; Fan, Q.Q.; Chu, X.S. Programmed Degradation of Pericarp Cells in Wheat Grains Depends on Autophagy. Front. Genet. 2021, 12, 784545. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Tang, C.; Sun, Y.; Islam, M.N.; Liu, P.; Wang, X.; Kang, Z. Wheat Gene TaATG8j Contributes to Stripe Rust Resistance. Int. J. Mol. Sci. 2018, 19, 1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, R.; Song, G.; Gao, J.; Li, W.; Han, X.; Chen, M.; Li, Y.; Li, G. Targeted mutagenesis using the Agrobacterium tumefaciens-mediated CRISPR-Cas9 system in common wheat. BMC Plant Biol. 2018, 18, 302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, D.; Huang, C.; Sui, X.; Fan, Q.; Chu, X. Preparation of highly specific wheat ATG8 antibody and its application in the detection of autophagy. ACTA Agron. Sin. 2022, 48, 2390–2399. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Chi, H.; Liu, C.; Yang, H.; Zeng, W.F.; Wu, L.; Zhou, W.J.; Wang, R.M.; Niu, X.N.; Ding, Y.H.; Zhang, Y.; et al. Comprehensive identification of peptides in tandem mass spectra using an efficient open search engine. Nat. Biotechnol. 2018, 36, 1059–1061. [Google Scholar] [CrossRef]
- Cui, X.; Fan, B.; Scholz, J.; Chen, Z. Roles of Arabidopsis cyclin-dependent kinase C complexes in cauliflower mosaic virus infection, plant growth, and development. Plant Cell 2007, 19, 1388–1402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).