Abstract
A comparative metabolomic study of three varieties of wild Rosa (Rosa acicularis, Rosa amblyotis, and Rosa rugosa) from a Kamchatka expedition (2024) was conducted via extraction with supercritical carbon dioxide modified with ethanol (EtOH), and detection of bioactive compounds was realized via tandem mass spectrometry. Several experimental conditions were investigated in the pressure range 50–350 bar, with the used volume of co-solvent ethanol in the amount of 2% in the liquid phase at a temperature in the range of 31–70 °C. The most effective extraction conditions are the following: pressure 200 Bar and temperature 55 °C for Rosa acicularis; pressure 250 Bar and temperature 60 °C for Rosa amblyotis; pressure 200 Bar and temperature 60 °C for Rosa rugosa. Three varieties of wild Rosa contain various phenolic compounds and compounds of other chemical groups with valuable biological activity. Tandem mass spectrometry (HPLC-ESI–ion trap) was applied to detect the target analytes. A total of 283 bioactive compounds (two hundred seventeen compounds from the polyphenol group and sixty-six compounds from other chemical groups) were tentatively identified in extracts from berries of wild Rosa. For the first time, forty-eight chemical constituents from the polyphenol group (15 flavones, 14 flavonols, 4 flavan-3-ols, 3 flavanones, 1 phenylpropanoid, 2 gallotannins, 1 ellagitannin, 4 phenolic acids, 1 dihydrochalcone, and 3 coumarins) were identified in supercritical extracts of R. acicularis, R. amblyotis, and R. rugosa.
1. Introduction
The genus Rosa (family Rosaceae) is represented on the territory of Kamchatka by three species: Rosa rugosa Thumb., Rosa amblyotis C.A. Mey., and Rosa acicularis Lindl. Fresh fruits and leaves contain up to 900 mg% ascorbic acid per dry pulp weight. Fresh petals contain 0.25–0.38% essential oil. Its neutral volatile fraction contains 86.3% phenylethyl alcohol and some linalool, citronellol, geraniol, nerol, etc. Eugenol was found in the phenolic fraction, phenylacetic, benzoic, and other acids in the acid fraction. R. rugosa is a medicinal plant widely used in traditional and folk medicine. Extracts of R. rugosa have been valued for Asian culinary, cosmetic, and aromatherapy purposes and used in herbal medicines for diabetes mellitus and osteoarthritis [1]. In Korea, R. rugosa has been used as a traditional medicine for its anti-inflammatory, antioxidant, hypoglycemic, and hypocholesterolemic properties. These properties may be primarily associated with both the complex of polyphenolic compounds, including tannins, flavonoids, etc., and the terpene chemical group [2]. The medicinal effects seem to be involved in the presence of many phytochemicals in R. rugosa extracts, for example flavonoids, phenylpropanoid, tannins, fatty acids, and terpenoids [3].
Phenolic compounds are secondary plant metabolites whose presence and composition are important indicators of the nutraceutical and nutritional quality of fruits, vegetables, and other plants [4,5]. These compounds have an aromatic ring bearing one or more hydroxyl groups, and phenolic structures can range from a simple phenolic molecule to complex high-molecular polymers [6]. These compounds, as one of the most widespread groups of phytochemicals, directly affect plant physiology and morphology. Phenolic compounds can act as phytoalexins [7], pollinator attractants, antifeedants, plant pigmentation factors, and antioxidants and have a protective function against ultraviolet light [8]. A group of polyphenolic compounds directly contributes to the color and flavor characteristics of fruits and vegetables [9]. Natural polyphenolic compounds have been reported to have excellent properties as food preservatives and play a major role in protecting against several pathological disorders in the human body, such as atherosclerosis, brain dysfunction, and cancer (Gordon, 1996). Moreover, polyphenols also have many industrial applications; for example, polyphenols directly obtained from plant extracts can be used as natural colorants and preservatives for food products or in the production of paints, paper, and cosmetics [10].
Supercritical fluid extraction with the use of pressured CO2 (SC-CO2) has been used since the last 50 years in analytical methodologies to investigate the composition of food products, removal of undesirable substances, and isolation of the polyphenol group of compounds and other chemical constituents. The goal of this work was to identify and select polyphenolic compounds from three varieties of wild Rosa (R. acicularis, R. amblyotis, and R. rugosa) via extraction with SC-CO2. Also, a tandem mass spectrometry protocol was used for the detailed screening of phytochemicals present in three varieties of wild Rosa.
2. Materials and Methods
2.1. Materials
The subject of this study was the berries of wild Rosa (R. acicularis, R. amblyotis, and R. rugosa) collected and grown at the expedition work (Kamchatka) of N.I. Vavilov All-Russian Institute of Plant Genetic Resources, as depicted in Figure 1. The berries of R. acicularis, R. amblyotis, and R. rugosa were harvested at the end of July 2024. All plant tissues used in this work were conformed according to the standard established by the State Pharmacopoeia of the Russian Federation [11].

Figure 1.
Pictures of the studied three Rosa species.
2.2. Chemicals and Reagents
All reagents used in this study were of analytical grade. HPLC-grade acetonitrile was purchased from Fisher Scientific (Southborough, UK), and MS-grade formic acid and ethanol (EtOH) were purchased from Sigma-Aldrich (Steinheim, Germany). Ultrapure water was prepared from Siemens (SIEMENS water technologies, Munich, Germany).
2.3. Extraction
SC-CO2 extraction was performed using the SFE-500 supercritical pressure extraction system (Thar SCF Waters, Milford, CT, USA). System options include the following: a co-solvent pump (Thar Waters P-50 High Pressure Pump) for the extraction of polar samples; a CO2 flow meter (Siemens, Germany) to measure the amount of CO2 supplied to the system; and multiple extraction vessels to extract different sample sizes or to increase the throughput of the system. The flow rate was 10–25 mL/min for liquid CO2 and 1.00 mL/min for EtOH. Extraction samples of 200 g of berries (R. acicularis) were used. Extraction samples of 200 g of berries (R. amblyotis) were used. Extraction samples of 200 g of berries (R. rugosa) were used. Extraction time was counted after reaching working pressure and equilibrium flow and was 60–90 min for each sample. This method of SC-CO2 extraction of plant matrices was tested by the authors on numerous plant samples, including aboveground and underground parts of the plant [12,13].
2.4. Liquid Chromatography
HPLC was performed using a Shimadzu LC-20 Prominence HPLC (Shimadzu, Kyoto, Japan) equipped with a UV sensor and C18 silica reverse phase column (4.6 × 150 mm, particle size: 2.7 μm) to perform the separation of multicomponent mixtures. The mobile phase eluent A was deionized water containing 0.1% formic acid, and eluent B was acetonitrile containing 0.1% formic acid. The gradient elution was started at 0–2 min, with 0% eluent B for 2–50 min, and 0–100% B; control washing for 50–60 min with 100% B. The mobile phase flow rate and column temperature were maintained at 0.3 mL/min and 30 °C, respectively. The entire HPLC analysis was performed with a UV–vis detector SPD-20A (Shimadzu, Kyoto, Japan) at a wavelength of 230 nm for the identification of compounds. The injection volume was 10 μL. Additionally, liquid chromatography was combined with a mass spectrometric ion trap to identify compounds.
2.5. Mass Spectrometry
MS analysis was performed on an ion trap amaZon SL (Bruker Daltoniks, Leipzig, Germany) equipped with an ESI source in negative ion mode. MS analysis was carried out in electrospray ionization (ESI) mode using negative and positive polarity for all samples with data independent MSE acquisition. The optimized parameters were obtained as follows: ionization source temperature: 70 °C, gas flow: 4 L/min, nebulizer gas (atomizer): 7.3 psi, capillary voltage: 4500 V, end plate bend voltage: 1500 V, fragmentary voltage: 280 V, and collision energy: 60 eV. An ion trap was used in the scan range m/z 100–1700 for MS and MS/MS. The chemical constituents were identified by comparing their retention index, mass spectra, and mass spectrometry fragmentation with a home-library database built by the Group of Biotechnology, Bioengineering, and Food Systems at the Far-Eastern Federal University (Russia) based on data from other spectroscopic techniques, such as nuclear magnetic resonance, ultraviolet spectroscopy, and MS, as well as data from the literature that is continually updated and revised. The capture rate was one spectrum/s for MS and two spectrum/s for MS/MS. Data collection was controlled by Windows software for Bruker Daltoniks. All experiments were replicated thrice. A four-stage ion separation mode (MS/MS mode) was implemented.
2.6. Statistical Analysis
Statistix v 8.1 (https://statistix.informer.com/8.1/ (accessed on 15 February 2021)) was used to compare the global yield extract (mg/100) of the three Rosa varieties according to three-way ANOVA in a completely randomized design. To more clearly present the similarities and differences of bioactive substances identified in different variants of R. acicularis, R. amblyotis, and R. rugosa, the team of authors used the Jaccard index. The Jaccard index, also known as the Jaccard similarity coefficient, is a statistical measure used to evaluate the similarity and diversity of sets of samples. Nine replicate samples were analyzed. A mathematical application, available at https://molbiotools.com/listcompare.php (accessed on 12 September 2024), was used to calculate the Jaccard index in this article. Thee Venn diagram was prepared using an online tool InteractiVenn (https://www.interactivenn.net/ (accessed on 24 August 2023)).
3. Results
3.1. SC-CO2 Extraction of Berries (R. acicularis, R. amblyotis, and R. rugosa)
Three Rosa varieties, i.e., R. acicularis, R. amblyotis, and R. rugosa, were examined by SC-CO2 extraction under different extraction conditions. The supercritical pressures applied ranged from 100 to 400 bar, and the extraction temperature ranged from 31 to 70 °C. The co-solvent EtOH was used in an amount of 2% of the total solvent amount. Table 1 shows the global yield of bioactive compounds by SC-CO2 extraction in three Rosa species. Overall, we observed that temperature and pressure in different Rosa varieties can affect the global yield.

Table 1.
The global yield of extract (mg/100 mg) after SC-CO2 extraction of berries of three Rosa species.
The maximum global yield of bioactive substances from berries of R. acicularis was observed under the following extraction conditions:
- With a pressure of 200 Bar, extraction temperature of 55 °C, and extraction time of 1 h, the global yield of biologically active substances was 6.2 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
In the case of R. amblyotis, it was observed that the maximum global yield of bioactive substances was observed under the following extraction conditions:
- With a pressure of 250 Bar, extraction temperature of 60 °C, and extraction time of 1 h, the global yield of biologically active substances was 6.4 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
It was observed that the maximum global yield of bioactive substances from berries (R. rugosa) was observed under the following extraction conditions:
- With a pressure of 200 Bar, extraction temperature of 60 °C, and extraction time of 1 h, the global yield of biologically active substances was 6.7 mg/100 mg of plant sample; the share of the EtOH modifier was 2%.
3.2. Global Metabolome Profile of R. acicularis, R. amblyotis, and R. rugosa
The structural identification of each compound was carried out on the basis of its accurate mass and MS/MS fragmentation by HPLC-ESI-ion trap-MS/MS. We were able to identify 283 chemical compounds from the extracts of R. acicularis, R. amblyotis, and R. rugosa: 217 chemical compounds from the polyphenol group and 66 chemical compounds from other chemical groups. The other groups included amino acids, benzaldehydes, carboxylic acids, terpenoids (sesquiterpenoids and triterpenoids), sterols, iridoids, oxylipins, fatty acids, etc. (Figure 2A). Of the 283 compounds, 62 were commonly detected from all three Rosa species. Of the three species, R. acicularis was the richest in the diversity of the detected compounds with 84 uniquely detected compounds followed by R. amblyotis (29) and R. rugosa (24) (Figure 2B). All identified polyphenols and other compounds along with molecular formulae and MS/MS data for R. acicularis, R. amblyotis, and R. rugosa are summarized in Appendix A, Table A1. The polyphenols detected in our study were categorized as flavones, flavonols, flavan-3-ols, anthocyanidins, phenolic acids, chalcones, lignans, stilbenes, coumarins, etc. In total, the metabolites detected in our study belonged to 18 compound classes. The highest number of metabolites were flavonols (71), followed by flavones (38), flavan-3-ols (13), proanthocyanidins (5), anthocyanins (24), and phenolic acids (32). These numbers indicate that SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa are rich in flavonoids. The highest number of chemical compounds from other groups are oxylipins (6) and terpenoids (7).
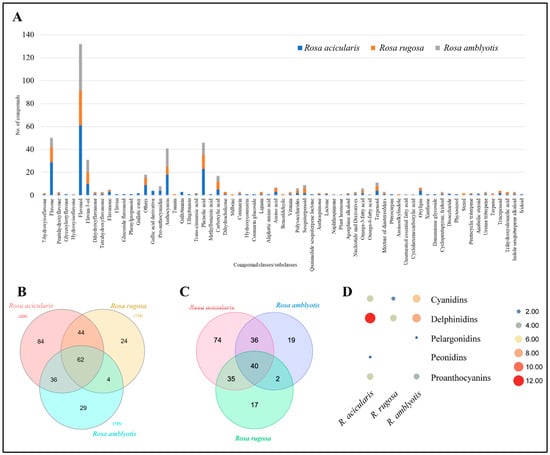
Figure 2.
Global metabolome profile of three Rosa species. (A) Bar plot showing compound classes (and subclasses) and respective number of compounds detected in each Rosa species. (B) Venn diagram showing number of common and specific compounds detected in three Rosa species. (C) Venn diagram showing number of common and specific polyphenolic compounds detected in three Rosa species. (D) Scatter plot showing number of anthocyanins detected in each Rosa species.
A Venn diagram showing the similarities and differences in the presence of various polyphenols in Rosa varieties (R. acicularis, R. amblyotis, and R. rugosa) is shown in Figure 2C. Forty compounds were commonly detected from the three Rosa varieties. These forty compounds belong to compound classes such as flavones, flavonols, anthocyanins, phenolic acids, etc., as major active compounds in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa. The applied methods were able to detect 188 (R. acicularis), 97 (R. amblyotis), and 95 (R. rugosa) polyphenolic compounds.
Moreover, to present the similarities and differences in bioactive substances in different variations of Rosa, we used the Jaccard index (Table 2). The Jaccard index, also known as the Jaccard similarity coefficient, is a statistic used to evaluate the similarity and diversity of sets of samples [14,15]. It showed that the highest degree of similarity is present between the varieties R. acicularis and R. amblyotis—0.3689.

Table 2.
Jaccard Index for three varieties of wild Rosa.
Twenty-nine compounds classified as anthocyanins (24) and pro-anthocyanins (five) were detected from the studied Rosa species. Generally, R. acicularis was rich in anthocyanins; except pelargonidins, other classes were detected. Next, R. amblyotis also exhibited cyanidins, delphinidins, and pelargonidins but not peonidin. Rosa rugosa had only seven anthocyanins belonging to cyanidins, delphinidins, and peonidin. However, it was deficient in pro-anthocyanidins (Figure 2D; Appendix A, Table A1).
To present the similarities and differences in bioactive substances in different variations of Rosa, we used the Jaccard index (Table 3). It showed that the highest degree of similarity is present between the varieties R. acicularis and R. amblyotis—0.4483.

Table 3.
Jaccard index for three varieties of wild Rosa.
3.2.1. Hydroxy(iso)flavones
The 7-hydroxyisoflavone formononetin (compound 1) has been already characterized as a component of Dracocephalum jacutense [16], Medicago varia [17], Maackia amurense [18], and Chinese herbal formula Jian-Pi-Yi-Shen pill [19]. Thus, our results indicate that the flavone formononetin is tentatively identified in the SC-CO2 extracts of both R. acicularis and R. rugosa (Appendix A, Table A1). The CID spectrum (collision-induced spectrum) in the positive ion mode for the flavone formononetin from the R. rugosa extract is shown in Figure 3. [M + H]+ ion produced three fragment ions with m/z 251.28, m/z 212.20, and m/z 179.93 (Figure 3). The fragment ion with m/z 251.28 produced three characteristic daughter ions with m/z 235.25, m/z 223.17, and m/z 179.08.
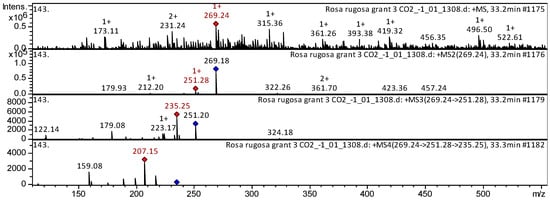
Figure 3.
CID spectrum of formononetin from SC-CO2 extracts of R. rugosa, m/z 269.24. Above is an MS scan in the range 100–1700 m/z and below is fragmentation spectra (top to bottom): MS2 of protonated formononetin ion (269.24 m/z, red diamond), MS3 fragment 269.24 → 251.28 m/z, and MS4 fragment 269.24 → 251.28 → 235.25 m/z.
3.2.2. Dihydroxyflavones
The flavones acacetin (compound 3), cirsimaritin (compound 10), cirsilineol (compound 17), dihydroxy-dimethoxy(iso)flavone (compound 11), and nevadensin (compound 12) (Appendix A, Table A1) have been already characterized as a component of Triticum aestivum [20], Mentha [21], Mexican lupine species [22], Artemisia annua [23], Rosmarinus officinalis [24], and Medicago varia [17]. We also tentatively identified these flavones in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa. The CID spectrum in positive ion modes for nevadensin from R. acicularis extracts is shown in Figure 4. The [M + H]+ ion produced three fragment ions with m/z 312.19, m/z 270.71, and m/z 179.05 (Figure 4). The fragment ion with m/z 312.19 produced two characteristic daughter ions with m/z 284.19 and m/z 269.13.
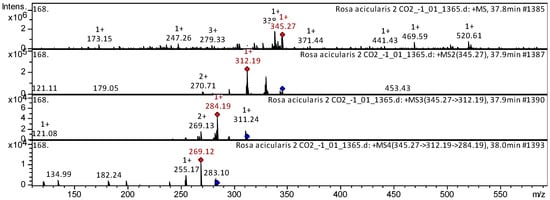
Figure 4.
CID spectrum of nevadensin from SC-CO2 extracts of R. acicularis, m/z 345.27. Above is an MS scan in the range 100–1700 m/z and below is fragmentation spectra (top to bottom): MS2 of protonated nevadensin ion (345.27 m/z, red diamond), MS3 fragment 345.27 → 312.19 m/z, and MS4 fragment 345.27 → 312.19 → 284.19 m/z.
3.2.3. Trihydroxyflavones
The flavones apigenin (compound 2), jaceosidin (compound 14), 5,6,4′-trihydroxy-7,8-dimethoxyflavone (compound 16), cirsiliol (compound 17), chrysoeriol [chrysoeriol] (compound 8), luteolin 7-O-glucoside (compound 25), kaempferide (compound 41), etc. (Appendix A, Table A1), have already been characterized as a component of Inula gaveolens [25], Lonicera henryi [26], Ribes meyeri [27], and Andean blueberry [28]. Trihydroxyflavones were tentatively identified in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa. The CID spectrum in positive ion modes for luteolin 7-O-glucoside from SC-CO2 extracts of R. amblyotis is shown in Figure 5. The [M + H]+ ion produced one fragment ion with m/z 287.19 (Figure 5). The luteolin 7-O-glucoside was identified in the bibliography in extracts from Vaccinium macrocarpon [29] and Lonicera henryi [26].
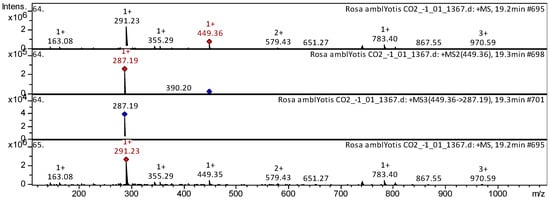
Figure 5.
CID spectrum of luteolin-7-O-glucoside from SC-CO2 extracts of R. amblyotis, m/z 449.36. Above is an MS scan in the range 100–1700 m/z and below is fragmentation spectra (top to bottom): MS2 of protonated luteolin-7-O-glucoside ion (449.36 m/z, red diamond), MS3 fragment 449.36 → 287.19 m/z, and MS4 fragment 449.36 → 287.19 → 287.19 m/z.
3.2.4. Anthocyanins
Delphinidin O-pentoside (compound 149), cyanidin-3-O-glucoside (compound 151), delphinidin 3-O-glucoside (compound 154), delphinidin 3-O-hexuronide (compound 157), cyanidin 3-O-coumaroyl hexoside (compound 161), etc. (Appendix A, Table A1), have already been characterized as a component of Ribes magellanicum; Berberis ilicifolia; Berberis empetrifolia; Ribes maellanicum; Ribes cucullatum; and Myrteola nummalaria. Their anthocyanins were tentatively identified in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa. The CID spectrum in positive ion modes for delphinidin O-pentoside from SC-CO2 extracts of R. rugosa is shown in Figure 6. The [M + H]+ ion produced one fragment ion with m/z 303.17 (Figure 6). The fragment ion with m/z 303.17 produced three characteristic daughter ions with m/z 285.17, m/z 229.17, and m/z 165.11. The anthocyanin delphinidin O-pentoside was tentatively identified in the bibliography in extracts from Myrtle [30], Gaultheria mucronate, Gaultheria antarctica [31], and Andean blueberry [28].
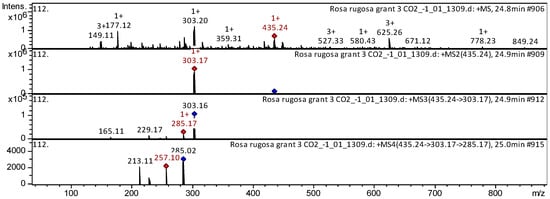
Figure 6.
CID spectrum of delphinidin O-pentoside from SC-CO2 extracts of R. rugosas, m/z 435.24. Above is an MS scan in the range 100–1700 m/z and below is fragmentation spectra (top to bottom): MS2 of protonated delphinidin O-pentoside ion (435.24 m/z, red diamond), MS3 fragment 435.24 → 303.17 m/z, and MS4 fragment 435.24 → 303.17 → 285.17 m/z.
3.3. Newly Detected Chemical Compounds in SC-CO2 Extracts of R. acicularis, R. amblyotis, and R. rugosa
Of the detected metabolites in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa, forty-eight chemical constituents from the polyphenol group (15 flavones, 14 flavonols, 4 flavan-3-ols, 3 flavanones, 1 phenylpropanoid, 2 gallotannins, 1 ellagitannin, 4 phenolic acids, 1 dihydrochalcone, and 3 coumarins) were identified for the first time. The newly identified polyphenols include flavones (acacetin, calycosin, diosmetin, odoratin, salvigenin, jaceosidin, nevadensin, cirsilineol, scutellarin, chrysoeriol 6-O-hexoside, chrysoeriol 6-O-glucoside, chrysoeriol 8-O-glucoside, chrysin 6-C-[2″-O-glucoside] -glucoside, and chrysin di-O-glucoside, lonicerin), flavonols (rhamnetin I, rhamnetin II, taxifolin 3-O-arabinofuranoside, isorhamnetin-3-O-arabinoside, isorhamnetin 3-O-pentoside, astragalin, dihydrokaempferol-O-hexoside, aromadendrin O-hexoside, taxifolin-3-O-glucoside, taxifolin-3-O-hexoside, quercetin 7-O-glucoside, quercetin-hexuronide, quercetin O-hexoside O-deoxyhexoside, and bioquercetin), flavan-3-ols (afzelechin, (epi)-afzelechin, gallocatechin, and (epi)-gallocatechin), flavanones (eriodictyol, hesperitin, and taxifolin-3-O-rhamnoside), phenylpropanoid vimalin, etc. (Appendix A, Table A1).
4. Discussion
Since the most biologically active substances in plants are usually polyphenolic compounds, extraction is the first and most important step in the stepwise separation of these compounds [32]. The most common extractions applicable to phenolic compounds are liquid/liquid and solid/liquid, and they are often used specifically for the separation of these components. The phenolic structure of polyphenols makes them relatively hydrophilic, so free phenolic compounds, including aglycones, glycosides, and oligomers are extracted using water, polar organic solvents such as ethyl acetate, methanol, ethanol, acetonitrile, acetone, and their mixtures with water or non-polar solvents (chloroform and diethyl ether) [4]. Nowadays, as a result of the negative impact of industrial extraction on the environment, the concept of green extraction has been introduced to protect both the environment and consumers. This also directly affects the increasing competition of industries to be more environmentally friendly (use of by-products and biodegradability), economical (lower energy and solvent consumption), and innovative [33]. In line with this approach to green extraction, non-traditional extraction methods such as microwave, ultrasonic extraction, and methods based on the use of compressed fluids as extracting agents such as subcritical water extraction (SWE), supercritical fluid extraction (SFE), pressurized fluid extraction (PFE), or accelerated solvent extraction (ASE) are used for the actual separation of phenolic compounds [34,35]. Generally, the most common extraction methods for phenolic compounds use acetone [36,37] or methanol [38] as phenolic compound extracting agents, but from the point of view of use in food and cosmetic industries, ethanol and water are definitely preferred [39].
As shown earlier, bioactive compounds of aboveground plant parts are efficiently extracted using organic solvents such as methanol and ethanol. However, the extraction products at the final stage require additional costs in terms of purification from trace amounts of the solvents used. SC-CO2 extraction, which is rightfully classified as a “green” extraction method, can be used as an alternative to traditional extraction methods: maceration or Soxhlet extraction [40,41]. SC-CO2 extraction has been used in the evaluation of food products, the isolation of bioactive substances, and the determination of lipid levels in food products and the levels of toxic substances. With SC-CO2 extraction, the products do not contain residues of organic solvents that are encountered in conventional extraction methods, and the solvents can be toxic, for example in the case of methanol and n-hexane. Easy removal of solvent from the final product, high selectivity, and moderate extraction temperatures are the main attractive features of SC-CO2 technology, which leads to a significant increase in research for applications in cosmetic, pharmaceutical, and food industries. When comparing possible supercritical solvents, carbon dioxide has the most attractive advantages: non-toxic, non-flammable, environmentally friendly, and, most importantly, a renewable resource [42]. Previously, our group of authors successfully used supercritical CO2 extraction in the extraction of the polyphenolic composition of the forage grass Medicago varia [17], the extraction of the aromatic component from Ribes fragrans leaves [43], the refinement of the polyphenolic composition of medicinal plants Ledum palustre [44] and Maackia amurense [18], the isolation of the sulfate compounds from Zostera marina [45], etc. Thus, the use of SC-CO2 extraction is an effective approach for the extraction of bioactive compounds.
High oxidation–reduction potential is the most important property of flavonoids, which allows them to act as singlet hydrogen quenchers, reducing agents, and hydrogen donors. Flavonoids have metal chelating potential [46]. Regular consumption of flavonoids has been directly associated with a reduced incidence of various cancers and heart disease [47]. There is currently great interest in flavonoid research due to the possibility of direct effects on health through diet, in which case preventive health care can be achieved through the consumption of fruits and vegetables. Flavonols are a class of flavonoids commonly found in many fruits and vegetables, and their content varies greatly depending on environmental factors such as growing conditions, climate, storage, and cooking conditions [48].
A total of 75 flavonols have been identified in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa, of which 14 compounds (rhamnetin I, rhamnetin II, taxifolin 3-O-arabinofuranoside, isorhamnetin-3-O-arabinoside, isorhamnetin 3-O-pentoside, astragalin, dihydrokaempferol-O-hexoside, aromadendrin O-hexoside, taxifolin-3-O-glucoside, taxifolin-3-O-hexoside, quercetin 7-O-glucoside, quercetin-hexuronide, quercetin O-hexoside O-deoxyhexoside, and bioquercetin) were identified for the first time in Rose SC-CO2 extracts, highlighting the great potential for applications in functional food technologies.
Flavanones can be characterized by the presence of a saturated three-carbon chain and an oxygen atom at the C4 position. They are usually glycosylated with a disaccharide at the C7 position. Flavanones are present in high concentrations only in citrus fruits but have also been identified in tomatoes and some aromatic plants (mint). The main aglycones are naringenin in grapefruit, hesperetin in oranges, and eriodictyol in lemons. A total of five flavanones have been identified in berry extracts, of which three compounds (hesperetin, quercetin O-hexoside O-deoxyhexoside, and taxifolin-3-O-rhamnoside) were identified for the first time in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa.
Anthocyanins are water-soluble pigments that turn red, purple, or blue depending on pH. They are also flavonoids but are synthesized via the phenylpropanoid pathway. Anthocyanins are found in all plant matrices, including leaves, stems, and roots, with particularly high concentrations in flowers and fruits. Anthocyanidins are the main structures of anthocyanins. Anthocyanidins consist of an aromatic ring A linked to a heterocyclic ring C containing oxygen, which is also linked via a carbon–carbon bond to a third aromatic ring B [49]. Many researchers have noted that glycoside derivatives of three unmethylated anthocyanidins (pelargonidin, cyanidin, and delphinidin) are the most common in nature, occurring in 69% of fruits, 50% of flowers, and 80% of pigmented leaves [50]. Six anthocyanidins are most commonly found in plants: delphinidin, pelargonidin, malvidin, cyanidin, peonidin, petunidin, and malvidin. Many anthocyanins have sugar residues acylated with aromatic or aliphatic acids [51]. In our studies, we were able to identify anthocyanins belonging to the following groups: delphinidin, pelargonidin, cyanidin, and peonidin—a total of 24 compounds from SC-CO2-extracts of R. acicularis, R. amblyotis, and R. rugosa.
5. Conclusions
Rosa species contain many polyphenolic components and components of other chemical groups that have valuable biological activities. SC-CO2 extraction of R. acicularis, R. amblyotis, and R. rugosa was successfully carried out by the team of authors; certain extraction conditions were selected. The extracts obtained showed both a high content of polyphenolic composition and a high content of the saponin group. Tandem mass spectrometry (HPLC-ESI—ion trap) was used to detect target analytes. Mass spectrometric data were recorded on an ion trap equipped with an ESI source in negative and positive ion mode. A four-stage ion separation mode is implemented. Two hundred and eighty-seven different biologically active compounds were found in SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa. For the first time, forty-eight chemical constituents from the polyphenol group (15 flavones, 14 flavonols, 4 flavan-3-ols, 3 flavanones, 1 phenylpropanoid, 2 gallotannins, 1 ellagitannin, 4 phenolic acids, 1 dihydrochalcone, and 3 coumarins) were identified in supercritical extracts of Rosa acicularis, Rosa amblyotis, and Rosa rugosa. Our research has shown for the first time the acceptability and effectiveness of the supercritical extraction method in extracting the largest number of chemical compounds of both the polyphenol group and compounds of other groups, which can be a start in improving applied food and pharmaceutical technologies.
Author Contributions
Conceptualization, M.P.R.; methodology, M.P.R. and M.A.N.; software, M.P.R.; validation, M.P.R., E.A.R. and K.S.G.; formal analysis, M.P.R. and M.A.N.; investigation, K.S.G. and M.P.R.; resources, K.S.G. and M.P.R.; data curation, E.A.R.; writing—original draft preparation—M.A.N. and M.P.R.; writing—review and editing M.A.N. and M.P.R.; visualization, M.P.R. and M.A.N.; supervision, M.A.N.; project administration, K.S.G. and M.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out at the N.I. Vavilov All-Russian Institute of Plant Genetic Resources at the expense of the Russian Science Foundation Grant No. 23-74-00044, https://rscf.ru/en/project/23-74-00044/ (accessed on 13 April 2023).
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors sincerely acknowledged the financial support by the Ministry of Science and Higher Education (Agreement No. 23-74-00044 dated 13 April 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Chemical compounds identified from the SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa in positive and negative ionization modes by HPLC-ion trap-MS/MS.
Table A1.
Chemical compounds identified from the SC-CO2 extracts of R. acicularis, R. amblyotis, and R. rugosa in positive and negative ionization modes by HPLC-ion trap-MS/MS.
| Class of Compounds | Identification | Formula | Calculated Mass | Observed Mass [M − H]− | Observed Mass [M + H]+ | MS/MS Stage 1 Fragmentation | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenolic Compounds | ||||||||||
| 1 | 7-hydroxyisoflavone | Formononetin [Biochanin B; Formononetol] | C16H12O4 | 268.2641 | 269 | 251; 137 | 233 | 150 | Dracocephalum jacutense [16]; Medicago varia [17]; Maackia amurense [18]; Chinese herbal formula Jian-Pi-Yi-Shen pill [19] | |
| 2 | Flavone | Apigenin [5,7-Dixydroxy-2-(40Hydroxyphenyl)-4H-Chromen-4-One] | C15H10O5 | 270.2369 | 271 | 253; 211; 136 | 225; 179; 134 | Inula gaveolens [25]; Lonicera henryi [26]; Ribes meyeri [27]; Andean blueberry [28] | ||
| 3 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] * | C16H12O5 | 284.2635 | 285 | 267; 211 | 187 | Triticum aestivum [20]; Mentha [21]; Mexican lupine species [22] | ||
| 4 | Flavone | Calycosin [3′-Hydroxyformononetin] * | C16H12O5 | 284.2635 | 285 | 253; 235; 217 | 217; 191; 155 | 133 | Chinese herbal formula Jian-Pi-Yi-Shen pill [19] | |
| 5 | Flavone | Prunetin [Padmakastein] | C16H12O5 | 284.2635 | 285 | 253; 235; 217 | 217; 191; 155 | 133 | Triticum aestivum [20]; Rosa rugosa [52] | |
| 6 | Flavone | Luteolin [Tetrahydroxyflavone] | C15H10O6 | 286.2363 | 287 | 269; 241; 227 | 153 | Inula gaveolens [25]; Lonicera henryi [26]; Ribes meyeri [27]; Rosa rugosa [52]; Jatropha [53] | ||
| 7 | Flavone | Diosmetin [Luteolin 4′-Methyl Ether; Salinigricoflavonol] * | C16H12O6 | 300.2629 | 301 | 286; 259 | 178; 136 | 150; 121 | Inula viscosa [54] | |
| 8 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | 300.2629 | 301 | 286; 259 | 178; 136 | 150; 121 | Mentha [21]; Mexican lupine species [22] | |
| 9 | Pentahydroxyflavone | Herbacetin [3,5,7,8-Tetrahydroxy-2-(4-hydro-xyphenyl)-4H-chromen-4-one] | C15H10O7 | 302.2357 | 303 | 203; 165 | 221; 175 | 202 | Triticum aestivum [20]; Lonicera caerulea [55] | |
| 10 | Flavone | Cirsimaritin [Scrophulein; 4′,5-Dihydroxy-6,7-Dimethoxyflavone; 7-Methylcapillarisin] | C17H14O6 | 314.2895 | 315 | 300 | 272; 168; 135 | 140 | Artemisia annua [23]; Rosmarinus officinalis [24]; Medicago varia [17] | |
| 11 | Flavone | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 314.2895 | 315 | 300 | 272; 168; 135 | 140 | Rosmarinus officinalis [24]; Medicago varia [17] | |
| 12 | Flavone | Odoratin * | C17H14O6 | 314.2895 | 315 | 300 | 255; 215; 136 | 168; 133 | Astragali radix [56,57] | |
| 13 | Flavone | Salvigenin * | C18H16O6 | 328.3160 | 327 | 312; 164 | 283; 149 | 268; 235; 150 | Dracocephalum palmatum [58]; Ocimum [59] | |
| 14 | Flavone | Jaceosidin [5,7,4′-trihydroxy-6′,5′-dimethoxyflavone] * | C17H14O7 | 330.2889 | 331 | 303; 203 | 203; 275 | 222 | Artemisia argyl [60]; Mentha [21] | |
| 15 | Flavone | Dimethoxy-trihydroxy(iso)flavone | C17H14O7 | 330.2889 | 331 | 303; 203 | 203; 275 | 222 | Jatropha [53] | |
| 16 | Flavone | 5,6,4′-Trihydroxy-7,8-dimethoxyflavone | C17H14O7 | 330.2889 | 331 | 313; 203 | 285 | 267; 241 | Mentha [21]; F. glaucescens; F. herrerae [61] | |
| 17 | Flavone | Cirsiliol | C17H14O7 | 330.2889 | 329 | 229; 311 | 209; 125 | 209 | Juglans mandshurica [62]; Ocimum [59]; Inula viscosa [54]; Medicago varia [17] | |
| 18 | Flavone | Nevadensin * | C18H16O7 | 344.3154 | 345 | 312; 270; 179 | 284; 269 | 269; 255; 182; 134 | Mentha [21]; Ocimum [59]; Medicago varia [17] | |
| 19 | Flavone | Cirsilineol [Eupatrin; Fastigenin; Cirsileneol] * | C18H16O7 | 344.3154 | 345 | 312; 270; 179 | 284; 269 | 269; 255; 182; 134 | Ocimum [59]; Medicago varia [17] | |
| 20 | Flavone | 5,6-Dihydroxy-7,3′,4′ -trimethoxyflavone | C18H16O7 | 344.3154 | 345 | 312; 270; 179 | 284; 269 | 269; 255; 182; 134 | Mentha [21]; A. cordifolia [61] | |
| 21 | Flavone | 5,6-Dihydroxy-7,8,3′,4′-tetramethoxyflavone | C19H18O8 | 374.3414 | 375 | 342; 317 | 314; 299 | 299; 284; 180; 151 | Mentha [21] | |
| 22 | Flavone | Tetrahydroxyflavanone pentoside | C20H20O10 | 420.3668 | 419 | 285 | 255 | 255; 227 | F. glaucescens [61] | |
| 23 | Flavone | Eriodictyol-O-pentoside | C20H20O10 | 420.3668 | 419 | 285 | 255 | 255; 227 | Andean blueberry [28] | |
| 24 | Flavone | Tetrahydroxy-methoxyflavone-O-pentoside | C20H16O12 | 448.3338 | 449 | 303 | 303; 257 | 229; 201 | Carpinus betulus [63] | |
| 25 | Flavone | Luteolin 7-O-glucoside [Cynaroside; Luteoloside] | C21H20O11 | 448.3769 | 449 | 287 | 287; 241; 213 | Vaccinium macrocarpon [29]; Lonicera henryi [26] | ||
| 26 | Flavone | Luteolin-O-hexoside | C21H20O11 | 448.3769 | 449 | 287 | 287; 241; 213 | Lepechinia [64]; Ribes meyeri [27]; Chilean currants [65] | ||
| 27 | Flavone | Luteolin-O-glucuronide | C21H18O12 | 462.3604 | 463 | 287 | 241; 165 | 241; 213; 157 | Andean blueberry [28] | |
| 28 | Glucosyloxyflavone | Scutellarin [Breviscapin; Scutellarin B; Scutellarin-7-glucuronide] * | C21H18O12 | 462.3604 | 463 | 287 | 241; 165 | 213; 157 | Perilla frutescens [66] | |
| 29 | Flavone | Chrysoeriol 6-O-hexoside * | C22H22O11 | 462.4036 | 463 | 445 | 427 | 409; 213 | Triticum aestivum L. [67] | |
| 30 | Flavone | Chrysoeriol 6-C-glucoside [Isoscoparin; Isoscoparine] * | C22H22O11 | 462.4035 | 463 | 445 | 427 | 409; 213 | Triticum aestivum L. [67] | |
| 31 | Flavone | Chrysoeriol 8-C-glucoside [Scoparin] * | C22H22O11 | 462.4035 | 463 | 445 | 427 | 409; 213 | Mexican lupine species [22]; Citrus sinensis [68] | |
| 32 | Flavone | Apigenin-7-O-rutinoside [Isorhoifolin] | C27H30O14 | 578.5187 | 579 | 417; 291 | 287 | 269; 255 | Dracocephalum palmatum [58]; Mentha [21] | |
| 33 | Flavone | Apigenin 8-C-deoxyhexoside-7-O-glucoside | C27H30O14 | 578.5187 | 579 | 417; 291; 163 | 287 | 269; 255 | Passiflora incarnata [69] | |
| 34 | Flavone | Chrysin 6-C-[2″-O-glucoside] -glucoside * | C27H30O14 | 578.5187 | 579 | 417; 291 | 287; 369 | 269; 255 | Passiflora incarnata [69] | |
| 35 | Flavone | Chrysin di-O-glucoside * | C27H30O14 | 578.5187 | 579 | 417; 291 | 287 | 269; 255 | Passiflora incarnata [69] | |
| 36 | Flavone | Lonicerin [Luteolin-7-O-Rhamnoside; Veronicastroside; Scolymoside; Luteolin-7-Rhamnoglucoside] * | C27H30O15 | 594.5181 | 595 | 449; 287 | 287 | 287; 153 | Lonicera caerulea [55] | |
| 37 | Flavone | Luteolin 7-O-(6-O-rhamnosyl-hexoside) | C27H30O15 | 594.5181 | 595 | 287 | 287; 241 | Lonicera henryi [26] | ||
| 38 | Hydroxy(iso)flavone | 2′-Hydroxygenistein C-diglucoside malonylated | C30H32O19 | 696.5637 | 695 | 487 | 469; 427; 353 | Mexican lupine species [22] | ||
| 39 | Flavonol | Kaempferol | C15H10O6 | 286.2363 | 287 | 227; 189; 153; 110 | 152 | Inula gaveolens [25]; Juglans mandshurica [62]; Ribes meyeri [27]; Andean blueberry [28] | ||
| 40 | Flavonol | Rhamnocitrin | C16H12O6 | 300.2629 | 301 | 283 | 199 | Astragali radix [56]; Lonicera caerulea [55] | ||
| 41 | Flavonol | Kaempferide [4′-O-Methylkaempferol] | C16H12O6 | 300.2629 | 301 | 286; 259 | 178; 136 | 150; 121 | Triticum aestivum [20]; Ocimum [59]; Ribes meyeri [27]; Juglans mandshurica [62] | |
| 42 | Flavonol | Isokaempferide [3-O-Methylkaempferol] | C16H12O6 | 300.2629 | 301 | 286; 259 | 178; 136 | 150; 121 | Rosa rugosa [52] | |
| 43 | Flavonol | Quercetin | C15H10O7 | 302.2357 | 303 | 303; 257; 165 | 229 | 201 | Ribes meyeri [27]; Capsicum annuum [70] | |
| 44 | Flavonol | Hesperitin [Hesperetin] | C15H10O7 | 302.2357 | 303 | 285; 165 | 257; 173 | 229; 201 | Andean blueberry [28]; Rosmarinus officinalis [24] | |
| 45 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | 304.2516 | 305 | 277 | 187 | Juglans mandshurica [62]; Camellia kucha [71]; Andean blueberry [28] | ||
| 46 | Flavonol | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether; 3-Methylquercetin] | C16H12O7 | 316.2623 | 317 | 271; 207; 179; 151 | 151 | Inula viscosa [54]; Vaccinium macrocarpon [72]; Andean blueberry [28]; Lonicera caerulea [55]; Ribes dikuscha [73]; Rosa rugosa [52]; Rosmarinus officinalis [24] | ||
| 47 | Flavonol | Rhamnetin I * | C16H12O7 | 316.2623 | 317 | 271; 207; 179; 151 | 151 | Inula viscosa [54]; Inula gaveolens [25]; Polygala sibirica [74]; Ribes dikuscha [73] | ||
| 48 | Flavonol | Rhamnetin II * | C16H12O7 | 316.2623 | 317 | 271; 207; 179; 151 | 151 | Inula gaveolens [25]; Polygala sibirica [74] | ||
| 49 | Flavonol | Myricetin | C15H10O8 | 318.2351 | 319 | 273; 219 | 191 | 209 | Juglans mandshurica [62]; Andean blueberry [28] | |
| 50 | Flavonol | Ampelopsin [Dihydromyricetin; Ampeloptin] | C15H12O8 | 320.251 | 321 | 273; 239; 219 | 191 | 173 | Juglans mandshurica [62] | |
| 51 | Flavonol | Kaempferol 3-O-pentoside | C20H18O10 | 418.3509 | 419 | 257 | 153 | Andean blueberry [28]; Cranberry [75]; Rosa canina [76]; Ribes dikuscha [73] | ||
| 52 | Flavonol | Avicularin (Quercetin 3-Alpha-L-Arabinofuranoside; Avicularoside) | C20H18O11 | 434.3503 | 433 | 301 | 179 | 151 | Rosa rugosa [77]; Ribes meyeri [27]; Cranberry [78]; Juglans mandshurica [62] | |
| 53 | Flavonol | Quercetin-3-alpha-xylopyranoside [Quercetin 3-O-xylopyranoside] | C20H18O11 | 434.3503 | 433 | 301 | 179 | 151 | Vaccinium macrocarpon [72]; Cranberry [78] | |
| 54 | Flavonol | Quercetin 3-O-pentoside | C20H18O11 | 434.3503 | 433 | 301 | 179 | 151 | Rosa canina [76]; Ribes meyeri [27] | |
| 55 | Flavonol | Quercetin 3-D-xyloside [Reynoutrin] | C20H18O11 | 434.3503 | 435 | 303 | 257; 165 | 229; 201 | Juglans regia [79]; Embelia [80]; Cranberry [75] | |
| 56 | Flavonol | Quercetin-3-O-arabinoside [Guaijaverin; Guajaverin; Quercetin-3-Arabinopyranoside] | C20H18O11 | 434.3503 | 433 | 301 | 179 | 151 | Juglans regia [79]; Lonicera henryi [26]; Cranberry [78]; Cranberry [75]; Embelia [80] | |
| 57 | Flavonol | Taxifolin-O-pentoside [Dihydroquercetin pentoside] | C20H20O11 | 436.371 | 435 | 303 | 285; 125 | 241; 175 | A. cordifolia [61]; Rosa davurica [81]; Chilean currants [65] | |
| 58 | Flavonol | Taxifolin 3-O-arabinofuranoside [(+)-taxifolin 3-O-alpha-D-arabinopyranoside] * | C20H20O11 | 436.371 | 435 | 303 | 285; 125 | 241; 175 | Strawberry [82] | |
| 59 | Flavonol | Quercitrin [Quercetin 3-O-rhamnoside; Quercetrin] | C21H20O11 | 448.3769 | 449 | 303 | 227 | 229; 201 | Honey [83]; Carpinus betulus [63]; Inula viscosa [54]; Juglans mandshurica [62]; Vaccinium macrocarpon [29,72]; Cranberry [75,78] | |
| 60 | Flavonol | Quercetin 3-O-deoxyhexoside | C21H20O11 | 448.3770 | 449 | 303 | 227 | 229; 201 | Myrtle [30]; Stevia rebaudiana [84] | |
| 61 | Flavonol | Isorhamnetin-3-O-arabinoside * | C21H20O11 | 448.3769 | 447 | 315; 284; 235 | 300 | Vitis vinifera [85] | ||
| 62 | Flavonol | Isorhamnetin 3-O-pentoside * | C21H20O11 | 448.3769 | 447 | 315; 284; 235 | 300 | Andean blueberry [28]; Cranberry [75] | ||
| 63 | Flavonol | Astragalin [Kaempferol 3-O-glucoside; Kaempferol-3-Beta-Monoglucoside; Astragaline] * | C21H20O11 | 448.3769 | 449 | 287 | 287; 241; 213 | Juglans mandshurica [62]; Ribes meyeri [27] | ||
| 64 | Flavonol | Kaempferol-3-O-galactoside | C21H20O11 | 448.3769 | 447 | 285 | 287; 241; 213 | Triticum aestivum [20]; Vitis vinifera [85]; Ribes nigrum [76] | ||
| 65 | Flavonol | Kaempferol-3-O-hexoside | C21H20O11 | 448.3769 | 449 | 287 | 287; 241; 213 | Andean blueberry [28] | ||
| 66 | Flavonol | Kaempferol-C-hexoside | C21H20O11 | 448.3769 | 447 | 285; 255; 151 | 255 | 227 | Vaccinium macrocarpon [29]; Ribes dikuscha, Ribes pauciflorum [73] | |
| 67 | Flavonol | Methylquercetin-pentoside | C21H20O11 | 448.3769 | 449 | 317 | 285 | 257 | Vaccinium macrocarpon [72] | |
| 68 | Flavonol | Dihydrokaempferol 3-O-glucoside | C21H22O11 | 450.3928 | 451 | 289 | 271; 163; 127 | 229 | Punica granatum [86] | |
| 69 | Flavonol | Dihydrokaempferol-O-hexoside * | C21H22O11 | 450.3928 | 451 | 289; 273 | 271; 163; 127 | 229; 201 | Punica granatum [86] | |
| 70 | Flavonol | Aromadendrin O-hexoside * | C21H22O11 | 450.3928 | 451 | 289 | 271; 163; 127 | 229 | Andean blueberry [28] | |
| 71 | Flavonol | Kaempferol-3-O-glucuronide | C21H18O12 | 462.3604 | 463 | 287 | 241; 165 | 241; 213; 157 | Vitis vinifera [85]; Strawberry [87]; Ribes fragrans [43]; A. cordifolia; G. linguiforme [61]; Rosa canina [76] | |
| 72 | Flavonol | Kaempferol hexuronide | C21H18O12 | 462.3604 | 463 | 287 | 241; 165 | 241; 213; 157 | Hibiscus rosa sinensis [88] | |
| 73 | Flavonol | Quercetin-3-O-hexoside | C21H20O12 | 464.3763 | 465 | 303 | 257; 165 | 229; 201 | Cranberry [78]; Ribes dikuscha [73]; Stevia rebaudiana [84] | |
| 74 | Flavonol | Hyperoside (Quercetin 3-O-galactoside; Hyperin) | C21H20O12 | 464.3763 | 465 | 303 | 257; 165 | 229; 201 | Vaccinium macrocarpon [29,72]; Cranberry [75]; Andean blueberry [28]; Camelia kucha [71] | |
| 75 | Flavonol | Quercetin 3-glucoside [Isoquercetin; Isoquercitrin; Hirsutrin; Quercetin-3-O-Glucopyranoside; 3-Glucosylquercetin] | C21H20O12 | 464.3763 | 465 | 303 | 257; 165 | 229; 201 | Ribes meyeri [27]; Lonicera henryi [26]; Cranberry [75]; Andean blueberry [28]; Ribes fragrans [43] | |
| 76 | Flavonol | Quercetin 7-O-glucoside [Quercetin-7-O-Beta-Glucopyranoside] | C21H20O12 | 464.3763 | 465 | 303 | 257; 165 | 229; 201 | Passiflora incarnata [69]; Capsicum annuum [70] | |
| 77 | Flavonol | Taxifolin-3-O-glucoside * | C21H22O12 | 466.3922 | 465 | 285; 213 | 241; 217; 149 | 241; 197 | Vitis vinifera [85] | |
| 78 | Flavonol | Taxifolin-3-O-hexoside [Dihydroquercetin-3-O-hexoside] * | C21H22O12 | 466.3922 | 465 | 285; 213 | 241; 217; 149 | 241; 197 | Andean blueberry [28]; Chilean currants [65] | |
| 79 | Flavonol | Quercetin-3-O-glucuronide [Miquelianin] | C21H18O13 | 478.3598 | 479 | 303 | 257; 229 | 201 | Vitis vinifera [85]; Rosa canina [76]; Strawberry [87] | |
| 80 | Flavonol | Quercetin-hexuronide * | C21H18O13 | 478.3598 | 479 | 303 | 257; 165 | 229; 145 | Hibiscus rosa sinensis [88] | |
| 81 | Flavonol | Myricetin-3-O-galactoside | C21H20O13 | 480.3757 | 481 | 319 | 273; 245; 181; 137 | 245; 227; 157 | Juglans mandshurica [62]; Vitis vinifera [85]; Vaccinium macrocarpon [29,72]; Cranberry [75] | |
| 82 | Flavonol | Myricetin-3-O-glucoside | C21H20O13 | 480.3757 | 479 | 316; 179 | 271; 179 | 243 | Vitis vinifera [85]; Camellia kucha [71] | |
| 83 | Flavonol | Myricetin 3-O-hexoside | C21H20O13 | 480.3757 | 479 | 316; 179 | 271; 179 | 243 | Carpinus betulus [63]; Myrtle [30]; Andean blueberry [28]; Cranberry [78]; Chilean currants [65] | |
| 84 | Flavonol | Quercetin 3-O-malonylglucoside | C24H22O15 | 550.4225 | 551 | 303 | 257; 165 | 229; 201; 161 | Mexican lupine species [22]; A. cordifolia [61]; Strawberry [87] | |
| 85 | Flavonol | Quercetin 3-O-(6″-O-malonyl)hexoside | C24H22O15 | 550.4225 | 551 | 303 | 257; 165 | 229; 201; 161 | Bee-pollen [89] | |
| 86 | Flavonol | Kaempferol 3-O-rhamnosyl-galactoside | C27H30O15 | 594.5181 | 595 | 287; 165 | 287 | Pear [90] | ||
| 87 | Flavonol | Kaempferol 3-O-(6-O-rhamnosyl-glucoside) | C27H30O15 | 594.5181 | 595 | 287 | 241; 213; 165 | 213; 141 | Mexican lupine species [22]; Lonicera henryi [26] | |
| 88 | Flavonol | Kaempferol rhamnosyl glucoside | C27H30O15 | 594.5181 | 595 | 287; 219; 165 | 241; 165 | 213 | Tilla tomentosa [76] | |
| 89 | Flavonol | Kaempferol 3-O-deoxyhexoside-hexoside | C27H30O15 | 594.5181 | 595 | 287; 219; 165 | 241; 165 | 213 | Stevia rebaudiana [84]; Hibiscus rosa sinensis [88]; Rosa rugosa [91] | |
| 90 | Flavonol | Kaempferol 7-O-neohesperidoside | C27H30O15 | 594.5181 | 595 | 287; 219; 165 | 241; 165 | 213 | Honey [83] | |
| 91 | Flavonol | Kaempferol-3-O-coumaroylhexoside | C27H30O15 | 594.5181 | 595 | 287 | 213 | Strawberry [87] | ||
| 92 | Flavonol | Kaempferol 3-O-rutinoside | C27H30O15 | 594.5181 | 595 | 287 | 241; 213; 165 | 213; 141 | Ribes meyeri [27]; Spondias purpurea [92] | |
| 93 | Flavonol | Kaempferol 3-O-neohesperidoside | C27H30O15 | 594.5181 | 595 | 287 | 241; 213; 165 | 213; 141 | Rosa rugosa [1] | |
| 94 | Flavonol | Quercetin-3-hydroxyl-3-methylglutaroyl(HMG)-glucoside | C28H32O15 | 608.5447 | 609 | 303 | 285; 229; 165 | 201; 145 | Rubus ulmifolius [93] | |
| 95 | Flavonol | Rutin (Quercetin 3-O-rutinoside) | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Ribes meyeri [27]; Ribes magellanicum [64]; Lonicera henryi [26]; Spondias purpurea [92] | |
| 96 | Flavonol | Quercetin-O-rhamnoside-O-hexoside | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Spondias purpurea [92] | |
| 97 | Flavonol | Quercetin rhamnosyl glucoside | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | Mexican lupine species [22]; Tilla tomentosa [76]; Ribes dikuscha [73] | ||
| 98 | Flavonol | Quercetin-3-O-rhamnoside-7-O-glucoside | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Mexican lupine species [22]; Tilla tomentosa [76] | |
| 99 | Flavonol | Quercetin 7-O-rutinoside | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Pear [90]; Vitis vinifera [85] | |
| 100 | Flavonol | Quercetin O-hexoside O-deoxyhexoside * | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Andean blueberry [28] | |
| 101 | Flavonol | Bioquercetin [Quercetin-3-O-robinobioside] * | C27H30O16 | 610.5175 | 611 | 303 | 303; 257; 165 | 229 | Aspalathus linearis [94] | |
| 102 | Flavonol | Quercetin-O-(O-galloyl) hexoside | C28H24O16 | 616.4806 | 617 | 599; 303 | 257 | 229 | Juglans regia [79]; Myrtle [30] | |
| 103 | Flavonol | Quercetin galloyl-glucoside | C28H24O16 | 616.4806 | 617 | 303 | 257; 165 | 229; 158 | Rosa canina [76] | |
| 104 | Flavonol | Quercetin 3,4′-di-O-beta-glucopyranoside [Quercetin diglucoside] | C27H30O17 | 626.5169 | 627 | 465; 303 | 303; 257; 165 | 229 | Rosa rugosa [95]; Ribes nigrum [76]; Medicago varia [17] | |
| 105 | Flavonol | Quercetin-O-dihexoside | C27H30O17 | 626.5169 | 627 | 465; 304 | 303; 257; 166 | 230 | Inula viscosa [54]; Chilean currants [65]; Andean blueberry [28]; Medicago varia [17]; Hibiscus rosa sinensis [88] | |
| 106 | Flavonol | Quercetin-3,7-di-O-hexoside | C27H30O17 | 626.5169 | 627 | 465; 305 | 303; 257; 167 | 231 | Bee-pollen [89] | |
| 107 | Flavonol | Herbacetin-di-O-glucoside | C27H30O17 | 626.5169 | 627 | 465; 306 | 303; 257; 168 | 232 | Rhodiola rosea [96] | |
| 108 | Flavonol | Quercetin-3-O-xylosylrhamnosyl glucoside | C33H40O19 | 740.6593 | 739 | 587; 493; 269 | 285; 243 | Ribes meyeri [27] | ||
| 109 | Flavonol | Kaempferol-3-O-alpha-L-rhamnopyranosyl-(1->3)-(4-O-acetyl-alpha-L-rhamnopyranosyl)-(1->6)-beta-D-galactopyranoside | C34H22O22 | 782.5253 | 783 | 303; 463 | 285 | 257 | Actinidia valvata [97] | |
| 110 | Flavan-3-ol | Afzelechin * | C15H14O5 | 274.2687 | 275 | 245; 175 | 157 | Camellia kucha [71]; Pelargonium endlicherianum [98] | ||
| 111 | Flavan-3-ol | Epiafzelechin [(epi)Afzelechin] * | C15H14O5 | 274.2687 | 275 | 245; 219; 175 | 215; 193; 175; 157; 127 | 175; 157; 145 | A. cordifolia; F. glaucescens; F. herrerae [61] | |
| 112 | Flavan-3-ol | Catechin | C15H14O6 | 290.2681 | 291 | 261; 273; 231; 200 | 191; 173; 143 | 161; 143 | Ribes meyeri [27]; Ribes magellanicum [65] | |
| 113 | Flavan-3-ol | (Epi)-catechin | C15H14O6 | 290.2681 | 291 | 261; 273; 231; 200 | 191; 173; 143 | 161; 143 | Andean blueberry [28]; C. edulis [61]; Radix polygoni multiflori [99] | |
| 114 | Flavan-3-ol | Gallocatechin [+(−)Gallocatechin] * | C15H14O7 | 306.2675 | 307 | 287; 153 | 259; 147 | 149 | Carpinus betulus [63]; G. linguiforme [61]; Ribes meyeri [27]; Embelia [80] | |
| 115 | Flavan-3-ol | (Epi)-Gallocatechin * | C15H14O7 | 306.2675 | 307 | 287; 153 | 259; 147 | 149 | Juglans regia [79]; Carpinus betulus [63]; Myrtle [30]; Ribes meyeri [27]; Ribes magellanicum [65]; G. linguiforme [61]; Camellia kucha [71] | |
| 116 | Flavan-3-ol | (Epi)-catechin derivative 1 | C19H22O8 | 378.3732 | 379 | 261; 275; 233 | 233; 207; 179 | 179; 165; 151 | Pubchem | |
| 117 | Flavan-3-ol | (Epi)-afzelechin derivative | C18H16O10 | 392.3136 | 393 | 275; 375 | 245; 175 | 157 | Zostera marina [45] | |
| 118 | Flavan-3-ol | (Epi)-catechin derivative 2 | C18H16O11 | 408.3130 | 409 | 291; 220; 155 | 272; 231; 187; 159 | 243; 213; 185 | Lonicera caerulea [55] | |
| 119 | Flavan-3-ol | (Epi)-catechin derivative 3 | 424 | 425 | 291 | 261 | 261; 173 | PubChem | ||
| 120 | Flavan-3-ol | Catechin glucoside | C21H24O11 | 452.4087 | 453 | 291; 273; 205 | 273; 165; 123 | 165; 123 | Glycine soja [100] | |
| 121 | Flavan-3-ol | (epi)-Catechin glucoside | C21H24O11 | 452.4087 | 453 | 291; 273; 205 | 273; 165; 123 | 165; 123 | Cassia abbreviata [101] | |
| 122 | Flavan-3-ol | (epi)-Catechin O-hexoside | C21H24O11 | 452.4087 | 453 | 291; 273; 205 | 273; 165; 123 | 165; 123 | Andean blueberry [28] | |
| 123 | Dihydroxyflavanone | Pinocembrin [(+)-Pinocembrin; Pihyrochrysin; (2S)-Pinocembrin] | C15H12O4 | 256.2534 | 257 | 259; 227; 197; 163 | 187; 155 | Ribes triste [73] | ||
| 124 | Tetrahydroxyflavanone | Dihydrokaempferol [Aromadendrin; Katuranin] | C15H12O6 | 288.2522 | 289 | 271; 177; 141 | 252; 193; 167 | 193 | Juglans mandshurica [62]; Andean blueberry [28]; F. glaucescens [61]; Camellia kucha [71] | |
| 125 | Flavanone | Quercetin O-hexoside O-deoxyhexoside [3′,4′,5,7-tetrahydroxy-flavanone] * | C15H12O6 | 288.2522 | 289 | 271; 242; 220; 127 | 253; 224; 193; 165 | 193 | Andean blueberry [28]; Embelia [80]; Rosa rugosa [52]; Rosmarinus officinalis [24]; Jatropha [53] | |
| 126 | Flavanone | Hesperitin [Hesperetin] * | C16H14O6 | 302.2788 | 303 | 303; 257; 165 | 229 | 201 | Andean blueberry [28]; Ribes triste [73]; Vitis vinifera [85]; Rosmarinus officinalis [24] | |
| 127 | Flavanone | Taxifolin-3-O-rhamnoside [Dihydroquercetin-3-O-rhamnoside; Astilbin; Isoastilbin; Neoastilbin] * | C21H22O11 | 450.3928 | 451 | 289; 147 | 243; 215; 153 | 215; 149 | Juglans mandshurica [62]; Vitis vinifera [85] | |
| 128 | Flavan | Dihydroxy-dimethoxy(iso)flavan | C16H16O5 | 288.2952 | 289 | 271; 177; 141 | 252; 193; 167 | 193 | Astragali radix [56] | |
| 129 | Glucoside flavonoid | Gossypitrin [Grossypitrin; Articularin; Equisporoside; Gossypetin-7-glucoside] | C21H20O13 | 480.3757 | 481 | 301; 241 | 241; 283; 199 | 199 | Triticum aestivum [20] | |
| 130 | Phenylpropanoid (cinnamic alcohol glycoside) | Vimalin * | C16H22O7 | 326.3417 | 327 | 309; 270; 249; 187 | 291; 235 | 250 | Medicago varia [17] | |
| 131 | Gallate ester | Beta-Glucogallin [1-O-Galloyl-Beta-D-Glucose; Galloyl glucose; Monogalloyl glucose] | C13H16O10 | 332.2601 | 333 | 314; 269; 214; 177; 141 | 269; 235; 197; 152; 136 | 134 | Ocimum [59]; Strawberry [82]; Eucalyptus [102]; Rosa canina [76]; Rosa acicularis [81]; Pelargonium [98] | |
| 132 | Gallate ester | Gallic acid hexoside | C13H16O10 | 332.2601 | 333 | 314; 269; 214; 177; 141 | 269; 235; 197; 152; 136 | 134 | Ribes meyeri [27] | |
| 133 | Methylgalloyl glucose | 346 | 345 | 183 | 168 | Rosa canina [76] | ||||
| 134 | Methylgalloyl hexose | 346 | 345 | 183 | 168 | Carpinus betulus [63] | ||||
| 135 | Galloyl-methylgalloyl hexose | 498 | 497 | 465; 345 | 183 | 168; 124 | Carpinus betulus [63] | |||
| 136 | Trigalloyllevoglucosan | C27H22O17 | 618.4534 | 619 | 303; 191 | 229; 165 | 201; 132 | Pubchem | ||
| 137 | Tri-galloyl hexoside | C27H24O18 | 636.4687 | 635 | 465 | 313; 295; 235; 168 | 295; 241; 193; 168 | Carpinus betulus [63]; Ribes dikuscha [73] | ||
| 138 | Tri-galloyl glucose | C27H24O18 | 636.4687 | 635 | 465 | 313; 295; 235; 168 | 295; 241; 193; 168 | Juglans regia [79]; Ribes dikuscha [73] | ||
| 139 | Gallic acid derivative | 1,2,3,6-Tetra-O-galloyl-β-D-glucopyranoside [1,2,3,6-Tetra galloyl glucose] | C34H28O22 | 788.5729 | 787 | 617; 573; 465; 295 | 465; 331; 277; 215 | 313; 221 | Eucalyptus [102] | |
| 140 | Gallic acid derivative | 1,2,3,4-Tetra-O-galloyl-β-D-glucopyranoside | C34H28O22 | 788.5729 | 787 | 617; 573; 465; 295 | 465; 331; 277; 215 | 313; 221 | Pubchem | |
| 141 | Gallic acid derivative | Tetra-O-galloylhexoside | C34H28O22 | 788.5729 | 787 | 617; 573; 465; 295 | 465; 331; 277; 215 | 313; 221 | Carpinus betulus [63] | |
| 142 | Gallic acid derivative | Tetra-O-galloyl glucose | C34H28O22 | 788.5729 | 787 | 617; 573; 465; 295 | 465; 331; 277; 215 | 313; 221 | Carpinus betulus [63] | |
| 143 | Pro-anthocyanidin | Procyanidin A-type dimer | C30H24O12 | 576.501 | 577 | 425; 245 | 407; 245; 217 | 217; 189 | Carpinus betulus [63]; Vitis vinifera [85]; Pear [90]; Vaccinium macrocarpon [29,72] | |
| 144 | Pro-anthocyanidin | Procyanidin B1; Procyanidin Dimer B1 [Epicatechin-(4beta->8)-ent-epicatechin] | C30H26O12 | 578.5202 | 579 | 409; 287 | 287; 257 | 245; 203 | Vaccinium macrocarpon [29]; Cranberry [78]; Andean blueberry [28]; Strawberry [87]; Cranberry [75] | |
| 145 | Pro-anthocyanidin | Procyanidin B2 [Epicatechin-(4beta->8)-epicatechin] | C30H26O12 | 578.5202 | 579 | 409; 287 | 287; 257 | 245; 203 | Punica granatum [86]; Glycine soja [100]; Vitis vinifera [85] | |
| 146 | Pro-anthocyanidin | Proanthocyanidin B3 [Proanthocyanidin; Catechin-(4 alpha->8)-catechin] | C30H26O12 | 578.5202 | 579 | 427; 291 | 409; 301 | 287; 257 | Punica granatum [86]; Vitis vinifera [85] | |
| 147 | Pro-anthocyanidin | Procyanidin B2 dimethyl gallate [Epicatechin-(4-8)-epicatechin-O-(3,4-dimethyl)-gallate] | C35H34O19 | 758.6331 | 759 | 617; 502; 280 | 443 | 160 | F. esculentum [103,104] | |
| 148 | Anthocyanin | Delphinidin | C15H11O7 | 303.2436 | 303 | 257; 165 | 229 | 201 | A. cordifolia [61] | |
| 149 | Anthocyanin | Delphinidin O-pentoside | C20H19O11 | 435.3583 | 433 | 301 | 179 | 151 | Myrtle [30]; Gaultheria mucronata; Gaultheria antarctica [31]; Andean blueberry [28] | |
| 150 | Anthocyanin | Delphinidin 3-O-xyloside | C20H19O11 | 435.3583 | 433 | 301 | 179 | 151 | Black currant [105] | |
| 151 | Anthocyanin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin; Chrysanthemin] | C21H21O11+ | 449.3848 | 450 | 287 | 287; 241; 213 | Black currant, Gooseberry, Chokeberry, Elderberry, Red currant [105]; Ribes magellanicum [65]; Berberis ilicifolia; Berberis empetrifolia; Ribes maellanicum; Ribes cucullatum; Myrteola nummalaria; Gaultheria mucronata; Gaultheria antarctica; Rubus geoides; Fuchsia magellanica [31] | ||
| 152 | Anthocyanin | Cyanidin-3-O-hexoside | C21H21O11+ | 449.3849 | 450 | 287 | 287; 241; 213 | Myrtle [30]; Andean blueberry [28] | ||
| 153 | Anthocyanin | Cyanidin-3-O-beta-galactoside | C21H21O11 | 449.3848 | 450 | 287 | 287; 139 | Black soybean [106]; Gaultheria mucronata [31] | ||
| 154 | Anthocyanin | Delphinidin 3-O-glucoside | C21H21O12+ | 465.3905 | 465 | 303 | 257; 165 | 229 | Ribes magellanicum [65]; Berberis ilicifolia; Berberis empetrifolia; Ribes maellanicum; Ribes cucullatum; Myrteola nummalaria [31]; Black currant [105] | |
| 155 | Anthocyanin | Delphinidin 3-O-hexoside | C21H21O12+ | 465.3905 | 465 | 303 | 257; 165 | 229; 201 | Myrtle [30]; Andean blueberry [28] | |
| 156 | Anthocyanin | Delphinidin 3-O-beta-galactoside | C21H21O12+ | 465.3905 | 465 | 303 | 257 | 229 | Vigna angularis [107]; Gautheria micronata; Gautheria antarctica [31] | |
| 157 | Anthocyanin | Delphinidin 3-O-hexuronide | C21H19O13+ | 479.3678 | 479 | 303 | 257; 229; 165 | 201 | Grape vine varieties [108] | |
| 158 | Anthocyanin | Delphinidin 3-O-glucuronide | C21H19O13+ | 479.3678 | 479 | 303 | 257; 229; 165 | 201 | Vine [109] | |
| 159 | Anthocyanin | Peonidin 3-O-glucoside-4-vinylphenol | C30H27O12 | 579.5282 | 579 | 417; 291 | 287 | 269; 255 | Vine [109] | |
| 160 | Anthocyanin | Cyanidin 3-O-(6-O-p-coumaroyl)glucoside | C30H27O13 | 595.533 | 595 | 287 | 287; 241 | 213 | Grape [110]; Vines [109]; Vitis vinifera [85]; Black currant, Gooseberry [105] | |
| 161 | Anthocyanin | Cyanidin 3-O-coumaroyl hexoside | C30H27O13 | 595.533 | 595 | 287 | 241; 213; 165 | 213; 141 | Grape vine varieties [108] | |
| 162 | Anthocyanin | Delphinidin 3-O-rutinoside [Tulipanin; Delphinidin 3-Rhamnosyl-Glucoside] | C27H31O16 | 611.5254 | 611 | 465; 303 | 303; 257; 165 | 229 | Black currant [105]; Berberis ilicifolia; Berberis empetrifolia; Ribes maellanicum; Ribes cucullatum [31] | |
| 163 | Anthocyanin | Delphinidin 3-O-(6-O-p-coumaroyl) glucoside | C27H31O17 | 611.5255 | 611 | 465; 303 | 303; 257; 165 | 229 | Vigna angularis [107]; Grape [110]; Vines [109]; Grape vine varieties [108] | |
| 164 | Anthocyanin | Delphinidin 3-(2″-galloylgalactoside) | C28H25O16 | 616.4885 | 617 | 303 | 257; 165 | 229; 201 | Aristotelia chinensis [111] | |
| 165 | Anthocyanin | Delphinidin-3,5-diglucoside | C27H31O17 | 627.5248 | 627 | 465; 304 | 303; 257; 166 | 230 | Vigna angularis [107]; Grape [110]; Aristotelia chilensis [111] | |
| 166 | Anthocyanin | Delphinidin 3,5-dihexoside | C27H31O17 | 627.5248 | 627 | 465; 305 | 303; 257; 167 | 231 | F. herrerae [61]; Andean blueberry [28]; Ribes dikuscha [73] | |
| 167 | Anthocyanin | Delphinidin-3,5-O-digalactoside | C27H31O17 | 627.5248 | 627 | 465; 306 | 303; 257; 168 | 232 | Vigna angularis [107] | |
| 168 | Anthocyanin | Pelargonidin-3-O-rutinoside-5-O-beta-D-glucoside | C33H41O19 | 741.6771 | 741 | 579; 553; 451; 355; 289 | 271 | 229; 187 | Gentiana lutea [112]; Iris dichotoma [113]; Camellia kucha [71] | |
| 169 | Anthocyanin | Delphinidin 3-O-(6-O-p-feruloyl)-5-O-diglucoside | 803.623 | 804 | 303 | 285 | 257 | Vitis vinifera [114] | ||
| 170 | Anthocyanin | Cyanidin 3-O-(caffeoyl)rutinoside-5-O-glucoside | C42H47O22 | 903.8219 | 901 | 449; 323; 269 | 269 | 225 | Iris dichotoma [113] | |
| 171 | Anthocyanin | Cyanidin 3-(p-coumaroyl)-rutinoside-5-glucoside | C42H47O22 | 903.8219 | 901 | 449; 323; 269 | 269 | 225 | Solanum tuberosum [115] | |
| 172 | Tannin | HHDP-hexose | C20H18O14 | 482.3485 | 483 | 465; 433; 387; 337; 153 | 135 | Myrtle [30]; Carpinus betulus [63] | ||
| 173 | Gallotannin | Dehydro-galloyl-HHDP-hexoside * | C28H24O16 | 616.4806 | 617 | 303 | 257; 165 | 229; 158 | Terminalia arjuna [116] | |
| 174 | Ellagitannin | Pedunculagin [bis-HHDP [hexahydroxydiphenoyl]-O-hexoside; bis-HHDP hexose] * | C34H24O22 | 784.5412 | 785 | 599; 504; 284 | 501; 395; 319; 263 | Carpinus betulus [63]; Myrtle [30] | ||
| 175 | Gallotannin | Di-O-galloyl-HHDP-glucose [Heterophylliin A; Pedunculagin II] | C34H26O22 | 786.5570 | 785 | 483; 767; 633; 418; 301 | 313; 211 | Juglans regia [79]; Punica granatum [86]; Eucalyptus [102]; Rosa rugosa [117] | ||
| 176 | Gallotannin | Di-galloyl-HHDP-hexose * | C34H26O22 | 786.5570 | 785 | 483; 767; 633; 418; 301 | 313; 211 | Carpinus betulus [63] | ||
| 177 | Trans-cinnamic acid | Cinnamic acid (Trans-cinnamic acid; Phenylacrylic acid) | C9H8O2 | 148.1586 | 149 | 119 | Honey [83]; Potato [116]; Lonicera caerulea [55]; Ribes triste [73] | |||
| 178 | Hydroxybenzoic acid (Phenolic acid) | Protocatechuic acid | C7H6O4 | 154.1201 | 155 | 135 | Inula gaveolens [25]; Eucalyptus [102]; Ribes meyeri [27]; Vaccinium macrocarpon [29]; Ribes pauciflorum [73]; Camellia kucha [71]; Lepechinia [63]; Rosa rugosa [52]; Ocimum [59] | |||
| 179 | Hydroxybenzoic acid (Phenolic acid) | Gallic acid | C7H6O5 | 170.1195 | 171 | 139 | Carpinus betulus [63]; Inula gaveolens [25]; Ocimum [59]; Juglans mandshurica [62]; Eucalyptus [102]; Vaccinium macrocarpon [29]; Andean blueberry [28]; Ribes meyeri [27]; Camellia kucha [71]; Rosa rugosa [52]; Rosa rugosa [91] | |||
| 180 | Phenolic acid | Ethyl protocatechuate [3,4-Dihydroxybenzoic Acid Ethyl Ester] | C9H10O4 | 182.1733 | 183 | 153; 123 | 125 | Ocimum [59]; Medicago varia [17] | ||
| 181 | Phenolic acid | Veratric acid [3,4-Dimethoxybenzoic acid] | C9H10O4 | 182.1733 | 183 | 171; 153; 123 | 125; 134 | Rosa rugosa [52] | ||
| 182 | Phenolic acid | 3,5-Dimethoxybenzoic acid | C9H10O4 | 182.1733 | 183 | 171; 153; 123 | 125; 134 | Rosa rugosa [52] | ||
| 183 | Methylbenzoic acid | Methylgallic acid [Methyl gallate] * | C8H8O5 | 184.1461 | 185 | 153; 127 | 135; 125 | Andean blueberry [28]; Lonicera caerulea [55]; Grape [110] | ||
| 184 | Phenolic acid | 2,3,4,5-Tetrahydroxybenzoic acid [2-Hydroxygallussaure; 3,4,5-Trihydroxysalicylic acid] | C7H6O6 | 186.1189 | 187 | 145; 163; 127 | 127 | PubChem | ||
| 185 | Phenolic acid | Hydroxygallic acid * | C7H8O6 | 188.1348 | 188 | 170; 146 | 118; 143 | Embelia [80] | ||
| 186 | Hydroxycinnamic acid | 3,4-Dimethoxy cinnamic acid | C11H12O4 | 208.2106 | 209 | 177 | 145 | Embelia [80]; Rosa rugosa [52] | ||
| 187 | Hydroxycinnamic acid | Hydroxyferulic acid | C10H10O5 | 210.1834 | 211 | 193; 175; 147 | 175; 147; 129 | 129 | Andean blueberry [28]; Rosa davurica [81]; Lonicera caerulea [55]; Ribes dikuscha [73] | |
| 188 | Phenolic acid | Ibuprofen * | C13H18O2 | 206.2808 | 207 | 161 | 143 | 129 | Juglans mandshurica [62] | |
| 189 | Hydroxycinnamic acid | Sinapic acid [Sinapinic acid; 3,5-Dimethoxy-4-hydroxycinnamic acid; trans-Sinapic acid] | C11H12O5 | 224.21 | 225 | 207; 189; 161 | 189; 175; 161 | 171; 143 | Potato [115]; Vaccinium macrocarpon [29]; Cranberry [75]; Andean blueberry [28]; Ribes triste [73]; Vitis vinifera [85]; Rosa rugosa [52,77] | |
| 190 | Hydroxybenzoic acid (Phenolic acid) | Ellagic acid [Benzoaric acid; Elagostasine; Lagistase; Eleagic acid] * | C14H6O8 | 302.1926 | 301 | 257 | 229 | 201 | Ribes meyeri [27]; Strawberry [87]; Grape [110] | |
| 191 | Phenolic acid | Coumaroyl coumaric acid | C18H14O5 | 310.3008 | 311 | 163; 293 | 145 | 127 | Andean blueberry [28] | |
| 192 | Hydroxycinnamic acid | p-Coumaric acid-O-hexoside [Trans-p-Coumaric acid 4-glucoside] | C15H18O8 | 326.2986 | 327 | 309; 291; 276; 251; 230 | 261; 241; 175 | 227 | Carpinus betulus [63]; Inula viscosa [54]; Ribes meyeri [27]; Cranberry [78]; Ribes magellanicum [65]; Vaccinium macrocarpon [72]; Andean blueberry [28]; G. linguiforme [61]; Grape [110] | |
| 193 | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341; 281; 215 | 179; 161; 119 | 143 | Embelia [80] | ||
| 194 | Phenolic acid | Caffeoylquinic acid derivative | C17H18O10 | 382.3188 | 383 | 207; 249 | 179; 123 | 150; 123 | PubChem | |
| 195 | Hydroxycinnamic acid | Sinapic acid-O-hexoside | C17H22O10 | 386.3576 | 387 | 369; 207; 161 | 189; 149 | 159 | Andean blueberry [28]; Ribes magellanicum [65]; Cranberry [78] | |
| 196 | Hydroxycinnamic acid | 1-O-Sinapoyl-beta-D-glucose | C17H22O10 | 386.3576 | 387 | 369; 207; 161 | 189; 149 | 159 | Vitis vinifera [85]; Cranberry [75] | |
| 197 | Hydroxycinnamic acid | Hydroxycinnamic acid derivative | C18H24O12 | 432.3760 | 431 | 385; 223; 161 | 205; 153 | 187 | PubChem | |
| 198 | Phenolic acid | Ellagic acid pentoside [Ellagic acid 4-O-xylopyranoside] | C19H14O12 | 434.3073 | 435 | 303 | 257; 165 | 229; 201 | Juglans regia [79]; Carpinus betulus [63]; Strawberry [82]; Strawberry [87]; Ribes pauciflorum [73] | |
| 199 | Phenolic acid | Ellagic acid-O-arabinoside | C19H14O12 | 434.3073 | 433 | 301 | 179 | 151 | Phyllanthus [118] | |
| 200 | Phenolic acid | Methyl ellagic acid-O-pentose | C20H16O12 | 448.3338 | 449 | 317 | 285 | 257 | Pecan [119]; Strawberry [82] | |
| 201 | Phenolic acid | Ellagic acid-O-deoxyhexoside | C20H16O12 | 448.3338 | 449 | 303; 173 | 257 | Strawberry [87]; Punica granatum [86] | ||
| 202 | Phenolic acid | Ellagic acid-O-hexoside | C20H16O13 | 464.3332 | 465 | 303 | 257; 165 | 229; 201 | Carpinus betulus [63]; Myrtle [30]; Eucalyptus [102]; Strawberry [82]; Punica granatum [86]; Pelargonium endlicherianum [98] | |
| 203 | Phenolic acid | Ellagic acid-O-glucoside | C20H16O13 | 464.3332 | 465 | 303 | 257; 165 | 229; 201 | Punica granatum [86] | |
| 204 | Phenolic acid | Ellagic acid-O-glucuronide | C22H22O12 | 478.4029 | 479 | 303 | 257; 229 | 201 | Phyllanthus [118] | |
| 205 | Phenolic acid | Coumaroyl-methylgalloyl hexose | C23H24O12 | 492.4295 | 491 | 345; 183 | 183 | 167 | Carpinus betulus [63] | |
| 206 | Phenolic acid | Caffeoyl hexosyl trihydroxymethoxyphenyl propanoic acid | C25H28O14 | 552.4814 | 551 | 345; 183 | 183 | 168; 124 | Andean blueberry [28] | |
| 207 | Phenolic acid | Ellagic acid-galloyl-hexoside | C28H24O16 | 616.4806 | 617 | 303 | 303; 257; 165 | 229; 158 | Punica granatum [86] | |
| 208 | Phenolic acid | Ellagic acid-O-dihexoside | C26H26O18 | 626.4738 | 627 | 465; 303 | 257; 165 | 229 | Phyllanthus [118]; Carpinus betulus [63]; Punica granatum [86] | |
| 209 | Phenolic acid | 5-O-p-coumaroyl-4-O-caffeoyl-4-methylpentanoic acid-5-hydroxy-3-quinate | 694.25 | 695 | 353; 261 | 335; 261; 173; 123 | 243; 159 | Phoenix dactylifera [120] | ||
| 210 | Dihydrochalcone | Phloretin [Dihydronaringenin; Phloretol] | C15H14O5 | 274.2687 | 275 | 256 | 229 | 159 | Eucalyptus [102]; G. linguiforme [61]; Rosa rugosa [81]; Lonicera caerulea [55,120] | |
| 211 | Dihydrochalcone | Phlorizin [Phloridzin; Phlorizoside; Floridzin: phlorrhizin; Phloretin 2′-Glucoside; Phloretin-O-hexoside] * | C21H24O10 | 436.4093 | 435 | 273 | 167; 123 | 123 | Potato [115]; Eucalyptus [102]; Andean blueberry [28]; A. cordifolia [61] | |
| 212 | Stilbene | 3-Hydroxyresveratrol [Piceatannol] | C14H12O4 | 244.2427 | 245 | 218; 186; 145 | 158 | 141 | G. linguiforme [61]; Grape [110]; Rosa rugosa [81]; Ribes dikuscha [73] | |
| 213 | Coumarin | Umbelliferone hexoside * | C15H16O8 | 324.2827 | 325 | 271; 253; 223; 181 | 241; 181; 145 | 153 | G. linguiforme [61]; Lonicera caerulea [55] | |
| 214 | Hydroxycoumarin | Fraxidin [2H-1-Benzopyran-2-One, 8-Hydroxy-6,7-Dimethoxy-] * | C11H10O5 | 222.1941 | 224 | 207; 189 | 161; 143 | 143 | Rat plasma [121] | |
| 215 | Coumarin glucoside | Fraxin (Fraxetin-8-O-glucoside) * | C16H18O10 | 370.3081 | 371 | 352; 322; 213; 118 | 322; 292; 165 | 235; 168; 135 | Potato [116]; Actinidia deliciosa [122] | |
| 216 | Lignan | Pinoresinol | C20H22O6 | 358.3851 | 357 | 269; 225; 181 | 181 | Passiflora incarnata [69]; Rosa rugosa [81] | ||
| 217 | Lignan | Syringaresinol | C22H26O8 | 418.4436 | 419 | 255 | 239 | 211 | Annona montana [123] | |
| OTHERS | ||||||||||
| 218 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one [DDMP] | C6H8O4 | 144.1253 | 145 | 129 | 112 | Radix polygoni multiflori [99] | |||
| 219 | Aliphatic amino acid | L-Glutamic acid [L-Glutamate] | C5H7NO4 | 145.1134 | 145 | 127 | Medicago truncatula [124] | |||
| 220 | Glutamine family amino acid | Glutamine | C5H10N2O3 | 146.1445 | 147 | 129 | 111 | Triticum aestivum [125] | ||
| 221 | Benzaldehyde | Vanillin | C8H8O3 | 152.1473 | 153 | 133 | Potato [115] | |||
| 222 | Amino acid | L-Histidine | C6H9N3O2 | 155.1546 | 157 | 129 | 111 | Triticum aestivum [125]; Medicago truncatula [124]; Camellia kucha [71]; Actinidia deliciosa [122]; Lonicera caerulea [55]; Lonicera caerulea [119]; Ribes dikuscha [73] | ||
| 223 | Aromatic amino acid | Phenylalanine [L-Phenylalanine] | C9H11NO2 | 165.1891 | 166 | 120; 147 | 120 | Triticum aestivum [125]; Medicago truncatula [124]; Medicago varia [17]; Juglans mandshurica [62]; Passiflora incarnata [69]; G. linguiforme [61]; Camellia kucha [71] | ||
| 224 | Amino acid | L-theanine [Theanine; Theanin; N-Ethyl-L-glutamine] | C7H14N2O3 | 174.1977 | 175 | 158 | 140 | Camellia kucha [71]; Medicago varia [17]; Ribes aureum [73] | ||
| 225 | L-Ascorbic acid [Vitamin C] | C6H8O6 | 176.1241 | 177 | 145 | Phoenix dactylifera [120] | ||||
| 226 | Tricarboxylic acid | Citric acid [Anhydrous; Citrate] | C6H8O7 | 192.1235 | 191 | 111; 173 | strawberry [82]; Stevia rebaudiana [84] | |||
| 227 | Polyhydroxycarboxylic acid | Quinic acid | C7H12O6 | 192.1666 | 191 | 111; 173 | 111 | Inula graveolens [25]; Artemisia [23]; Ribes meyeri [27]; Andean blueberry [28]; Eucalyptus [102]; C. edulis [61]; pear [90]; Vitis vinifera [85]; Camellia kucha [71] | ||
| 228 | Alpha,omega dicarboxylic acid | Sebacic acid [Decanedioic acid] * | C10H18O4 | 202.2475 | 203 | 175 | 143 | 111 | Lonicera caerulea [55]; Ribes dikuscha [73] | |
| 229 | Essential amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] * | C11H12N2O2 | 204.2252 | 205 | 145 | Triticum aestivum [125]; Medicago truncatula [124]; Strawberry [82]; Passiflora incarnata [69]; Camellia kucha [71] | |||
| 230 | Polysaccharides | Glucaric acid [D-Glucaric acid; Saccharic acid; D-Glutarate] * | C6H10O8 | 210.1388 | 210 | 193; 175; 147 | 175; 147 | 147; 129 | Soybean [126] | |
| 231 | Polysaccharides | Galactaric acid [Mucic acid; Saccharolactic acid; Galactarate] * | C6H10O8 | 210.1388 | 210 | 193; 175; 147 | 175; 147 | 147; 129 | Soybean [126]; Stevia rebaudiana [84] | |
| 232 | Alpha,omega dicarboxylic acid | Undecanedioic acid | C11H20O4 | 216.2741 | 217 | 199; 187; 171; 156; 137; 117 | 188; 160; 135 | G. linguiforme [61]; Jatropha [53] | ||
| 233 | Sesquiterpenoid | Aristolone | C15H22O | 218.3346 | 219 | 189 | 174 | 146 | Rumex hastatus [127] | |
| 234 | Sesquiterpenoid | Caryophyllene oxide [Caryophyllene-alpha-oxide] | C15H24O | 220.3505 | 221 | 219; 207; 189; 171; 161 | Rosa davurica [81] | |||
| 235 | Alpha,omega dicarboxylic acid | 3,4,5-Trimethoxyphenylacetic acid | C11H14O5 | 226.2259 | 225 | 178; 161; 143; 119 | 161; 143; 131 | Rosa rugosa [52] | ||
| 236 | Carboxylic acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227 | 209; 168 | 166; 139 | F. glaucescens [61]; Maackia amurensis [18] | ||
| 237 | Sesquiterpenoid | Atractylenolide I | C15H18O2 | 230.3022 | 231 | 203; 185; 159 | 157; 145 | Atractylodes macrocephalae rhizoma [128]; Chinese herbal formula Jian-Pi-Yi-Shen pill [19] | ||
| 238 | Quaianolide sesquiterpene lactone | Dehydrocostus Lactone | C15H18O2 | 230.3022 | 231 | 186; 158 | 168; 146 | 141 | Rosa davurica [81] | |
| 239 | Sesquiterpenoid | Atractylenolide II [Asterolide; 2-Atractylenolide] | C15H20O2 | 232.3181 | 233 | 186 | 168; 142 | Codonopsis Radix [128]; Chinese herbal formula Jian-Pi-Yi-Shen pill [19] | ||
| 240 | Anthraquinone | 1,8-Dihydroxy-anthraquinone [Chrysazin] | C14H8O4 | 240.2109 | 241 | 213 | 185 | 166 | Juglans mandshurica [62]; Ribes pauciflorum [73] | |
| 241 | Lactone | 1beta-Hydroxyalantolactone | C15H20O3 | 248.3175 | 249 | 231; 189; 149 | 185; 129 | 158; 128 | Inula viscosa [54] | |
| 242 | Naphthoquinone | 1,3,6,8-Tetrahydroxy-9(10H)-anthracenone | C14H10O5 | 258.2262 | 259 | 240; 213; 198; 184 | 191; 167; 139 | Juglans mandshurica [62]; Ribes pauciflorum [73] | ||
| 243 | Plant hormone | Abscisic acid [Dormin; Abscisin II; (S)-(+)-Abscisic acid] | C15H20O4 | 264.3169 | 265 | 204 | 174 | 146; 112 | Honey [83] | |
| 244 | Aporphine alkaloid | Anonaine | C17H15NO2 | 265.3065 | 266 | 249 | 203 | 157 | Rosa rugosa [81]; Dracocephalum jacutense [16]; Lonicera caerulea [55] | |
| 45 | Ribonucleoside composite of adenine (purine) | Adenosine | C10H13N5O4 | 267.2413 | 268 | 250; 232 | 232; 186 | 186; 168 | Medicago varia [17] | |
| 246 | Omega-3-fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid; Moroctic acid] | C18H28O2 | 276.4137 | 278 | 261; 173; 115 | 215; 115 | 129 | G. linguiforme [61]; Jatropha [53] | |
| 247 | Omega-3-fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.4296 | 279 | 261 | 219 | 163 | Jatropha [53]; Maackia amurensis [18] | |
| 248 | Linoleic acid amide | C18H33NO | 279.4607 | 280 | 262; 205; 149 | 149 | PubChem | |||
| 249 | Terpenoid | Rugosic acid A | C15H22O5 | 282.3322 | 283 | 215; 153 | Rosa rugosa [129] | |||
| 250 | Mixture of diastereomers | Fructose-leucine | C12H23NO7 | 293.3135 | 294 | 276; 235; 179 | 175; 189; 147 | 147 | Lonicera caerulea [55] | |
| 251 | Pterocarpan | 3-Hydroxy-9,10-dimethoxypterocarpan | C17H16O5 | 300.3059 | 301 | 283; 259; 195 | 268; 240; 171 | 240 | Radix astragali [56]; Chinese herbal formula Jian-Pi-Yi-Shen pill [19] | |
| 252 | Aminoalkylindole | Tryptamine * | C10H12N2 | 160.2157 | 161 | 143 | Hylocereus polyrhizus [130]; Ribes triste [73] | |||
| 253 | Unsaturated essential fatty acid | Oxo-eicosatetraenoic acid * | C20H30O3 | 318.4504 | 319 | 300 | 281; 149 | 249; 151 | F. potsii [61]; Ribes pauciflorum [73] | |
| 254 | Cyclohexenecarboxylic acid | Coumaroyl shiikimic acid | C16H16O7 | 320.2940 | 319 | 301; 173 | 127 | 142 | Andean blueberry [28]; Lonicera caerulea [55] | |
| 255 | Oxylipin | 9,10-Dihydroxy-8-oxooctadec-12-enoic acid [oxo-DHODE; oxo-Dihydroxy-octadecenoic acid] * | C18H32O5 | 328.4437 | 327 | 229; 291; 171 | 211; 155 | 209; 183 | Lonicera caerulea [55]; Dracocephalum jacutense [16] | |
| 256 | Oxylipin | Trihydroxyoctadecadienoic acid * | C18H32O5 | 328.4437 | 327 | 229; 291; 211; 171 | 211; 155; 125 | 209; 183; 125 | Artemisia absinthium [23] | |
| 257 | Oxylipin | Trihydroxyoctadecenoic acid * | C18H32O5 | 328.4437 | 327 | 229; 291; 211; 171 | 211; 155; 125 | 209; 183; 125 | Artemisia absinthium [23] | |
| 258 | Oxylipin | 13-Trihydroxy-Octadecenoic acid [THODE] * | C18H34O5 | 330.4596 | 329 | 229; 311; 171 | 211; 125 | 209; 183 | Jatropha [53]; Phoenix dactylifera [120] | |
| 259 | Dihydroxy eicosatrienoic acid | C20H34O4 | 338.4816 | 339 | 321; 303 | 275; 247; 235 | 207 | G. linguiforme; A. cordifolia; C. edulis [61] | ||
| 260 | Xanthone | Paxanthone | C19H16O6 | 340.3267 | 339 | 324 | 309 | 291; 163 | Inula viscosa [54] | |
| 261 | Dammarane glycoside | Blumenol C-glucoside [Byzantionoside B; 9-Epi-Blumenol C-glucoside] | C19H32O7 | 372.4532 | 373 | 211; 193 | 193 | 175 | Passiflora incarnata [69] | |
| 262 | Cyclopentapyran; Iridoid | Loganic acid * | C16H24O10 | 376.3558 | 377 | 346; 277 | 246 | 245; 166 | PubChem | |
| 263 | Disaccharide | Trehalose (+FA adduct) CH2O2 (46.0254) | C13H24O13 | 388.3219 | 387 | 341 | 179 | PubChem | ||
| 264 | Phytosterol | Ergosterol [Provitamin D2; Ergosterin] | C28H44O | 396.6484 | 397 | 379; 273 | 213 | F. glaucescens [61]; Lonicera caerulea [55] | ||
| 265 | Sterol | Beta-Sitosterin [Beta-Sitosterol] | C29H50O | 414.7067 | 413 | 381; 301; 263 | 301; 263; 235 | 283; 245; 214 | F. herrerae [61]; Atractylodes macrocephalae Rhizoma [128]; Carduus pycnocephalus [131] | |
| 266 | Pentacyclic triterpene | Lupeol [Fagarasterol; Clerodol; Monogynol B; Lupenol] * | C30H50O | 426.7174 | 427 | 409; 347; 251 | 347; 154 | 251 | Juglans mandshurica [62]; Carduus pycnocephalus [131] | |
| 267 | Sterol | Oxo-hydroxy sitosterol | C29H48O3 | 444.6896 | 445 | 427 | 335; 289; 261; 207 | 289; 261; 207; 133 | C. edulis; F. pottsii [61] | |
| 268 | Anabolic steroid | Vebonol * | C30H44O3 | 452.6686 | 453 | 435; 210 | 226; 336 | 210 | Hylosereus polyrhizus [130] | |
| 269 | Terpenoid | 1-Hydroxy-3-oxours-12-en-28-oic acid | C30H46O4 | 470.6838 | 471 | 407; 201 | 205 | 176 | Pear [90] | |
| 270 | Terpenoid | Triterpenoid | C30H46O4 | 470.6838 | 471 | 407; 205 | 205 | 176 | Brazilian propolis [132] | |
| 271 | Ursane triterpene | Annurcoic acid * | C30H46O5 | 486.6832 | 485 | 467; 370 | 423 | 393 | Annurca apple [133]; Pear [90] | |
| 272 | Terpenes | Triterpenoid | C30H48O5 | 488.6991 | 489 | 453; 407; 353; 262 | 407; 335; 249 | 389; 335; 267; 201 | PubChem | |
| 273 | Triterpenoid | Euscaphic acid * | C30H48O5 | 488.6991 | 487 | 469; 425 | 439 | Pear [90] | ||
| 274 | Triterpenoid | Tormentic acid [Jacarandic acid; Tomentic acid] * | C30H48O5 | 488.6991 | 487 | 469; 425 | 439 | Pear [90] | ||
| 275 | Trihydroxy oleanolic acid | Arjungenin [2alpha, 19 alpha, 23-Trihydroxyoleanolic acid] * | C30H48O6 | 504.6985 | 503 | 485 | 410; 307; 191 | PubChem | ||
| 276 | Trihydroxy oleanolic acid | Methyl arjunolate * | C31H50O5 | 502.7257 | 503 | 449; 259; 187 | 403; 335; 257 | 385; 321; 273 | G. linguiforme; C. edulis [61] | |
| 277 | Terpenoid | Triterpenoid | 505 | 506 | 471; 407; 355; 320 | 407; 351; 247 | 201; 133 | PubChem | ||
| 278 | Indole sesquiterpene alkaloid | Sespendole * | C33H45NO4 | 519.7147 | 520 | 184 | 125 | Hylosereus polyrhizus [130] | ||
| 279 | N′,N′,N‴-Tri-p-coumaroyl spermidine | C34H37N3O6 | 583.6741 | 584 | 339; 307; 216; 175 | 307; 275; 192 | 279 | Rosa rugosa [1] | ||
| 280 | N′,N′,N‴-Di-p-coumaroyl caffeoyl spermidine | C34H37N3O7 | 599.6735 | 598 | 503 | 485; 389 | Rosa rugosa [1] | |||
| 281 | Pheophorbide a | C35H34N4O6 | 606.6677 | 607 | 547; 461 | 461; 433 | 433 | [134] | ||
| 282 | Terpenoid | Triterpenoid | 667.234 | 668 | 453; 425; 351; 279 | 407; 323; 243; 201 | 389; 335; 277; 227 | PubChem | ||
| 283 | Iridoid | p-Coumaroyl monotropein hexoside | 698.8810 | 699 | 537; 365 | 375; 331; 259; 203 | Vaccinium myrtillus [135] |
* Compounds identified for the first time in R. acicularis, R. amblyotis, and R. rugosa.
References
- Yang, Y.; Zhang, J.-J.; Zhou, Q.; Wang, L.; Huang, W.; Wang, R. Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose (Rosa rugosa) bee pollen, and identification of bioactive components. J. Sci. Food Agric. 2019, 99, 5350–5357. [Google Scholar] [CrossRef]
- Seo, E.; You, Y.; Yoon, H.-G.; Kim, B.; Kim, K.; Lee, Y.-H.; Lee, J.; Chung, J.W.; Shim, S.; Jin, W. Rosa rugosa Aqueous Extract Alleviates Endurance Exercise-Induced Stress. J. Med. Food 2015, 18, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Ferreres, F.; Gil, M.I. Antioxidant phenolic metabolites from fruit and vegetables and changes during postharvest storage and processing. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 739–795. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Popa, V.I.; Dumitru, M.; Volf, I.; Anghel, N. Lignin and polyphenols as allelochemicals. Ind. Crops Prod. 2008, 27, 144–149. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Del Fabbro, P.; Manzocco, L.; Nunez, M.J.; Nicoli, M.C. Optimization of continuous phenol extraction from Vitis vinifera byproducts. Food Chem. 2005, 92, 109–117. [Google Scholar] [CrossRef]
- Pharmacopoeia of the Eurasian Economic Union, Approved by Decision of the Board of Eurasian Economic Commission No. 100 Dated 11 August 2020. Available online: https://eec.eaeunion.org/comission/department/deptexreg/formirovanie-obshchikh-rynkov/pharmacopoeia/ (accessed on 24 August 2023).
- Razgonova, M.; Zakharenko, A.; Kalenik, T.; Nosyrev, A.; Stratidakis, A.; Mezhuev, Y.; Burykina, T.; Nicolae, A.; Arsene, A.; Tsatsakis, A.; et al. Supercritical fluid technology and supercritical fluid chromatography for application in ginseng extracts. Farmacia 2019, 67, 2. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Ercisli, S.; Grudev, V.; Golokhvast, K. Comparative Analysis of Far East Sikhotinsky Rhododendron (Rh. sichotense) and East Siberian Rhododendron (Rh. adamsii) Using Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.C.; Miasojedow, B.; Startek, M.; Gambin, A. Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinform. 2019, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Lipkus, A.H. A proof of the triangle inequality for the Tanimoto distance. J. Math. Chem. 1999, 26, 263–265. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Rozhina, Z.G.; Egorova, P.S.; Golokhvast, K.S. Dracocephalum jacutense Peschkova from Yakutia: Extraction and Mass Spectrometric Characterization of 128 Chemical Compounds. Molecules 2023, 28, 4402. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Nawaz, M.A.; Ivanova, E.P.; Cherevach, E.I.; Golokhvast, K.S. Supercritical CO2-based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes 2024, 12, 1041. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K.S. Maackia amurensis Rupr. et Maxim.: Supercritical CO2-extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical characterisation and quantification of the major constituents in the Chinese herbal formula Jian-Pi-Yi-Shen pill by UPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int. 2020, 138, 109711. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, J.J.; Zhong, K.R.; Shang, Z.P.; Wang, F.; Wang, R.F.; Zhang, L.; Zhang, J.Y.; Liu, B. Analysis of non-volatile chemical constituents of Menthae haplocalycis herba by ultra-high performance liquid chromatography—High resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; Garcia-Lopez, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, L.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Wozniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Silinsin, M.; Bursal, E. UHPLC-MS/MS phenolic profiling and in vitro antioxidant activities of Inula graveolens (L.) Desf. Nat. Prod. Res. 2018, 31, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Muller, H.; Muller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF MS/MS and UHPLC-MS/MS. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.M.; Piovesana, S.; Lagana, A. Andean blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- D’Urso, G.; Sarais, G.; Lai, C.; Pizza, C.; Montoro, P. LC-MS based metabolomics study of different parts of Myrtle berry from Sardinia (Italy). J. Berry Res. 2017, 7, 217–229. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfild, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martinez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrncic, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, Y.S. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Jungfer, E.; Zimmermann, F.B.; Ruttkat, A.; Galensa, R. Comparing procyanidins in selected Vaccinium species by UHPLC-MS with regard to authenticity and health effects. J. Agric. Food Chem. 2012, 60, 9688–9696. [Google Scholar] [CrossRef] [PubMed]
- Jovancevic, M.; Balijagic, J.; Menkovic, N.; Šavikin, K.; Zdunic, G.; Jankovic, T.; Dekic-Ivankovic, M. Analysis of phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plant Res. 2011, 5, 910–914. [Google Scholar]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds frombilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. LWT Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Kupnik, K.; Leitgeb, M.; Primozic, M.; Postruznik, V.; Kotnik, P.; Kucuk, N.; Knez, Z.; Marevci, M.K. Supercritical Fluid and Conventional Extractions of High Value-Added Compounds from Pomegranate Peels Waste: Production, Quantification and Antimicrobial Activity of Bioactive Constituents. Plants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Reverchon, E. Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J. Supercrit. Fluids 2018, 134, 269–273. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Sabitov, A.S.; Senotrusova, T.A.; Lee, N.G.; Vitomskova, E.A.; Golokhvast, K.S. Ribes fragrans Pall.: Supercritical CO2-extraction and Complete Plant Metabolome. Bot. Pacifica 2024, 13, 61–72. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S. Investigation of the Supercritical CO2 Extracts of Wild Ledum palustre, L. (Rhododendron tomentosum Harmaja) and Identification of Its Metabolites by Tandem Mass Spectrometry. Russ. J. Bioorg. Chem. 2023, 49, 1645–1657. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K.S. Zostera marina L. Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A 2003, 1018, 29–40. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.; Shao, X. Determination of seven polyphenols in water by high performance liquid chromatography combined with preconcentration. Talanta 2008, 77, 679–683. [Google Scholar] [CrossRef]
- Caridi, D.; Trenerry, V.C.; Rochfort, S.; Duong, S.; Laugher, D.; Jones, R. Profiling and quantifying quercetin glucos ides in onion (Allium cepa L.) varieties using capillary zone electrophoresis and high performance liquid chromatography. Food Chem. 2007, 105, 691–699. [Google Scholar] [CrossRef]
- Konczak, I.; Zhang, W. Anthocyanins-more than nature colours. J. Biomed. Biotechnol. 2004, 5, 239–240. [Google Scholar]
- Dey, P.M.; Harborne, J.B. Plant Phenolics Methods in Plant Biochemistry, 2nd ed.; Academic Press Limited: London, UK, 1993; pp. 326–341. [Google Scholar]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; p. 384. [Google Scholar]
- Olech, M.; Pietrzak, W.; Nowak, R. Characterization of Free and Bound Phenolic Acids and Flavonoid Aglycones in Rosa rugosa Thunb. Leaves and Achenes Using LC–ESI–MS/MS–MRM Methods. Molecules 2020, 25, 1804. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sharmeen, J.B.; Brunetti, L.; Leone, S.; Di Simone, S.C.; Recinella, L.; et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Kheyar-Kraouche, N.; Bento da Silva, A.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Navaz, M.A.; Sabitov, A.S.; Zinchenko, Y.N.; Rusakova, E.A.; Petrusha, E.N.; Golokhvast, K.S.; Tikhonova, N.G. The Global Metabolome Profiles of Four Varieties of Lonicera caerulea, Established via Tandem Mass Spectrometry. Horticulturae 2023, 9, 1188. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.J.; Xu, W.; Huang, J.; Zhu, D.; Qiu, X.H. Rapid Characterization and Identification of Flavonoids in Radix astragali by Ultra-High-Pressure Liquid Chromatography Coupled with Linear Ion Trap Orbitrap Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 945–952. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Song, F.; Liu, Z.; Liu, S. Studies on principal components and antioxidant activity of different Radix astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 2009, 78, 1090–1101. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kim, E.; Kim, S.W.; Zulfugarov, I.S. New glycosides of eriodictyol from Dracocephalum palmatum. Chem. Nat. Compd. 2018, 54, 860–863. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Related Tech. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Chang, Y.; Zang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of Anti-Lipase Components of Artemisia argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharm. 2021, 12, 675396. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS—Bioassay Guided Approach. J. Chrom. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef]
- Huo, J.H.; Du, X.W.; Sun, G.D.; Dong, W.T.; Wang, W.M. Identification and characterization of major constituents in Juglans mandshurica using ultra performance liquid chromatography coupled with time-of flight mass spectrometry (UPLC-ESI-Q-TOF/MS). Chinese J. Nat. Med. 2018, 16, 0525–0545. [Google Scholar] [CrossRef] [PubMed]
- Felegyi-Toth, C.A.; Garadi, Z.; Darcsi, A.; Csernak, O.; Boldizsar, I.; Beni, S.; Alberti, A. Isolation and quantification of diarylheptanoids from European hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS characterization of its antioxidative phenolics. J. Pharm. Biomed. Anal. 2022, 210, 114554. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.A.; Villena, G.K.; Rodriguez, E.F. Phytochemical profile and rosmarinic acid purification from two Peruvian Lepechinia willd. species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Jimenez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Nakajima, J.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef]
- Ioset, J.-R.; Urbaniak, B.; Ndjoko-Ioset, K.; Wirth, J.; Martin, F.; Gruissem, W.; Hostettmann, K.; Sautter, C. Flavonoid profiling among wild type and related GM wheat varieties. Plant Mol. Biol. 2007, 65, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C-and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; de Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. A Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Braz. J. Pharmacol. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Pascale, R.; Acquuavia, M.A.; Cataldi, T.R.I.; Onzo, A.; Coviello, D.; Bufo, S.A.; Scrano, L.; Ciriello, R.; Guerrieri, A.; Bianco, G. Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI(-)-MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem. 2020, 412, 3005–3015. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef] [PubMed]
- Rafsanjany, N.; Senker, J.; Brandt, S.; Dobrindt, U.; Hensel, A. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-dominated uropathogenic Escherichia coli: A combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon. J. Agr. Food Chem. 2015, 63, 8804–8818. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Nawaz, M.A.; Sabitov, A.S.; Golokhvast, K.S. Genus Ribes: Ribes aureum, Ribes pauciflorum, Ribes triste, Ribes dikuscha, comparative mass spectrometric study of polyphenolic composition and other bioactive components. Int. J. Mol. Sci. 2024, 25, 10085. [Google Scholar] [CrossRef]
- Okhlopkova, Z.M.; Razgonova, M.P.; Kucharova, E.V.; Egorova, P.S.; Golokhvast, K.S. Rare Plant of Central Yakutia Polygala sibirica L.: Phytochemical Profile and In Vitro Morphogenic Culture. Russ. J. Plant Physiol. 2023, 70, 176. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; Harrington, P.; Chen, P. Nontargeted Metabolomic Study on Variation of Phenolics in Different Cranberry Cultivars Using UPLC-IM—HRMS. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J. Pharm. Biomed. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2013, 94, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Iswaldi, I.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; Uberos, J.; Lardon, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Characterization by high-performance liquid chromatography with diode-array detection coupled to time-of-flight mass spectrometry of the phenolic fraction in a cranberry syrup used to prevent urinary tract diseases, together with a study of its antibacterial activity. J. Pharm. Biomed. Anal. 2012, 58, 34–41. [Google Scholar]
- Medic, A.; Jakoric, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of the major phenolic constituents in Juglans regia L. peeled kernels and pellicles, using HPLC–MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.B.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Tree Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef]
- Chuah, W.C.; Lee, H.H.; Ng, D.H.J.; Ho, A.L.; Sulaiman, M.R.; Chye, F.Y. Antioxidants Discovery for Differentiation of Monofloral Stingless Bee Honeys Using Ambient Mass Spectrometry and Metabolomics Approaches. Foods 2023, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shaari, K. LC–MS metabolomics analysis of Stevia rebaudiana Bertoni leaves cultivated in Malaysia in relation to different developmental stages. Phytochem. Anal. 2021, 33, 249–261. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. Phytochemical A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamoclija, J.; Ciric, A.; Sokovic, M.; Heropoulos, G.; Proestos, C. Antiradical-antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Mejia, J.J.; Sierra, L.J.; Ceballos, J.G.; Martinez, J.R.; Stashenko, E.E. Color, Antioxidant Capacity and Flavonoid Composition in Hibiscus rosa-sinensis Cultivars. Molecules 2023, 28, 1779. [Google Scholar] [CrossRef] [PubMed]
- Mosic, M.; Trifkovic, J.; Vovk, I.; Gasic, U.; Tesic, Z.; Sikoparija, B.; Milojkovic-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef] [PubMed]
- Nijat, D.; Lu, C.-F.; Lu, J.-J.; Abdulla, R.; Hasan, A.; Aidarhan, N.; Aisa, H.A. Spectrum-effect relationship between UPLC fingerprints and antidiabetic and antioxidant activities of Rosa rugosa. J. Chromatogr. B 2021, 1179, 122843. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Grater, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra-high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Cendrowski, A.; Scibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of Polyphenolic Compounds of Liqueurs from Rose Petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef] [PubMed]
- Petsalo, A.; Jalonen, J.; Tolonen, A. Identification of flavonoids of Rhodiola rosea by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1112, 224–231. [Google Scholar] [CrossRef]
- Du, Q.-H.D.; Zhang, Q.-Y.Z.; Han, T.; Jiang, Y.-P.; Peng, C.; Xin, H.-L. Dynamic changes of flavonoids in Actinidia valvata leaves at different growing stages measured by HPLC-MS/MS. Chin. J. Nat. Med. 2016, 14, 66–72. [Google Scholar]
- Zengin, G.; Leyva-Jimenez, F.J.; Fernandez-Ochoa, A.; Bouyahya, A.; Yildiztugay, E.; Carrentero, A.S.; Mahomoodally, M.F.; de la Luz Cardiz-Gurrea, M. UHPLC-ESI-QTOF-MS metabolite profiles of different extracts from Pelargonium endlicherianum parts and their biological activities. Molecules 2023, 28, 2300728. [Google Scholar]
- Zhu, Z.W.; Li, J.; Gao, X.M.; Amponsem, E.; Kang, L.Y.; Hu, L.M.; Zhang, B.L.; Chang, Y.X. Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in Radix polygoni multiflori by LC–MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Wang, J.; Chen, G.; Tao, X.; Xu, S. Metabolomic Analysis Reveals Domestication-Driven Reshaping of Polyphenolic Antioxidants in Soybean Seeds. Antioxidants 2023, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.P.; Neto, C.P.; Silvestre, A.J.D. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from Eucalyptus wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Olschlager, C.; Rgos, I.; Zeller, F.J.; Treutter, D. Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum x F. homotropicum. Phytochemistry 2008, 69, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Kiprovski, B.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Xu, J.L.; Shin, J.S.; Park, S.K.; Kang, S.; Jeon, S.C.; Moon, J.K.; Choi, Y. Differences in the metabolic profiles and antioxidant activities of wild and cultivated black soybeans evaluated by correlation analysis. Food Res. Int. J. 2017, 100, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Park, J.E.; Lee, K.-S.; Seo, W.D.; Song, S.-B.; Lee, M.-H.; Kim, S.; Kim, J.-I.; Oh, E.; Pae, S.-B.; et al. Identification of anthocyanin compositions in black seed coated Korean adzuki bean (Vigna angularis) by NMR and UPLC-Q-Orbitrap-MS/MS and screening for their antioxidant properties using different solvent systems. Food Chem. 2021, 346, 128882. [Google Scholar] [CrossRef] [PubMed]
- Pantelic, M.M.; Dabic Zagorac, D.C.; Davidovic, S.M.; Todic, S.R.; Beslic, Z.S.; Gasic, U.M.; Tesic, Z.L.; Natic, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Fermo, P.; Comite, V.; Sredojevic, M.; Ciric, I.; Gasic, U.; Mutic, J.; Baosic, R.; Tesic, Z. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Flamini, R.H. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. Int. Sch. Res. Not. 2013, 1, 45. [Google Scholar] [CrossRef]
- Pineda, A.; Arenas, A.; Balmaceda, J.; Zuniga, G.E. Extracts of Fruits and Plants Cultivated In Vitro of Aristotelia chilensis (Mol.) Stuntz Show Inhibitory Activity of Aldose Reductase and Pancreatic Alpha-Amylase Enzymes. Plants 2022, 11, 2772. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Jin, X.; Capell, T.; Zhu, C.; Gomez-Gomez, L. Differential accumulation of pelargonidin glycosides in petals at three different developmental stages of the orange-flowered gentian (Gentiana lutea L. var. aurantiaca). PLoS ONE 2019, 14, e0212062. [Google Scholar] [CrossRef]
- Xu, W.; Luo, G.; Yu, F.; Jia, Q.; Zheng, Y.; Bi, X.; Lei, J. Characterization of anthocyanins in the hybrid progenies derived from Iris dichotoma and I. domestica by HPLC-DAD-ESI/MS analysis. Phytochemistry 2018, 150, 60–74. [Google Scholar] [CrossRef]
- Mazzuca, P.; Ferranti, P.; Picariello, G.; Chianese, L.; Addeo, F. Mass spectrometry in the study of anthocyanins and their derivatives: Differentiation of Vitis vinifera and hybrid grapes by liquid chromatography/electrospray ionization mass spectrometry and tandem mass spectrometry. J. Mass. Spectrom. 2005, 40, 83–90. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, S.; Rathi, B.; Bhrara, K.; Chhikara, B.S. Therapeutic analysis of Terminalia arjuna plant extracts in combinations with different metal nanoparticles. J. Mater. Nano Sci. 2015, 2, 1–7. [Google Scholar]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF–MS/MS Application to Investigate Chemical Compositions in a Fraction with Protein Tyrosine Phosphatase 1B Inhibitory Activity from Rosa Rugosa Flowers. Phytochem. Anal. 2013, 24, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Navaz, M.A.; Razgonova, M.P.; Rusakova, E.A.; Petrusha, E.N.; Sabitov, A.S.; Chunikhina, O.A.; Tikhonova, N.G.; Golokhvast, K.S. The Global metabolome profiles of four varieties of Lonicera caerulea, established via tandem mass spectrometry. Turk. J. Agric. For. 2024, 48, 10. [Google Scholar]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukui, M.; Nakazawa, T.; Hoshikawa, A.; Ohsawa, K. Metabolic Fate of Fraxin Administerd Orallyto Rats. J. Nat. Prod. 2006, 69, 755–757. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Boiko, A.P.; Zinchenko, Y.N.; Tikhonova, N.G.; Sabitov, A.S.; Zakharenko, A.M.; Golokhvast, K.S. Actinidia deliciosa: A high-resolution mass spectrometric approach for the comprehensive characterization of bioactive compounds. Turk. J. Agric. For. 2023, 47, 155–169. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Chang, F.-R.; Chen, S.-L.; Wu, C.-C.; Lee, K.-H.; Wu, Y.-C. Novel cytotoxic monotetrahydrofuranic Annonaceous acetogenins from Annona montana. Bioorg. Med. Chem. 2005, 13, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Dokwal, D.; Cocuron, J.-C.; Alonso, A.P.; Dickstein, R. Metabolite shift in Medicago truncatula occurs in phosphorus deprivation. J. Exp. Bot. 2022, 73, 2093–2111. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.; Papageorgiou, M.; Kalogiannis, S. Validation of a HILIC UHPLC-MS/MS Method for Amino Acid Profiling in Triticum Species Wheat Flours. Foods 2019, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, F.; Zeb, A.; Ayaz, M.; Ullah, F.; Sadiq, A. Evaluation of Rumex hastatus D. Don for cytotoxic potential against HeLa and NIH/3T3 cell lines: Chemical characterization of chloroform fraction and identification of bioactive compounds. BMC Complement. Altern. Med. 2016, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, P.; Leung, K.W.; Wang, H.; Kong, X.P.; Wang, L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. The Yin-Yang Property of Chinese Medicinal Herbs Relates to Chemical Composition but Not Anti-Oxidative Activity: An Illustration Using Spleen Meridian Herbs. Front. Pharm. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Kim, K.-H.; Park, Y.-J.; Jang, H.-J.; Lee, S.-J.; Lee, S.; Yun, B.-S.; Lee, S.W.; Rho, M.-C. Rugosic acid A, derived from Rosa rugosa Thunb., is novel inhibitory agent for NF-κB and IL-6/STAT3 axis in acute lung injury model. Phytother. Res. 2020, 34, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, L.A.; Hassan, W.H.B.; Al-Youssef, H.M. Phytochemical and biological studies of Carduus pycnocephalus L. J. Saudi Chem. Soc. 2012, 4, 1281–1287. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Oriano, P.; Pacifico, S. Annurcoic acid: A new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Canjura, F.L.; Schwartz, S.J. Identification of Chlorophyll Derivatives by Mass Spectrometry. J. Agric. Food Chem. 1991, 39, 1452–1456. [Google Scholar] [CrossRef]
- Bujor, O.-C. Extraction, Identification and Antioxidant Activity of the Phenolic Secondary Metabolites Isolated from the Leaves, Stems and Fruits of Two Shrubs of the Ericaceae Family. Ph.D. Thesis, Université d’Avignon, Avignon, France, 19 April 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).