Abstract
Cold shock proteins (CSPs) are DNA/RNA binding proteins with crucial regulatory roles in plant growth, development, and stress responses. In this study, we employed bioinformatics tools to identify and analyze the physicochemical properties, conserved domains, gene structure, phylogenetic relationships, cis-acting elements, subcellular localization, and expression patterns of the cotton CSP gene family. A total of 62 CSP proteins were identified across four cotton varieties (Gossypium arboreum, Gossypium raimondii, Gossypium barbadense, Gossypium hirsutum) and five plant varieties (Arabidopsis thaliana, Brassica chinensis, Camellia sinensis, Triticum aestivum, and Oryza sativa). Phylogenetic analysis categorized cotton CSP proteins into three evolutionary branches, revealing similar gene structures and motif distributions within each branch. Analysis of gene structural domains highlighted the conserved CSD and CCHC domains across all cotton CSP families. Subcellular localization predictions indicated predominant nuclear localization for CSPs. Examination of cis-elements in gene promoters revealed a variety of elements responsive to growth, development, light response, hormones, and abiotic stresses, suggesting the potential regulation of the cotton CSP family by different hormones and their involvement in diverse stress responses. RT-qPCR results suggested that GhCSP.A1, GhCSP.A2, GhCSP.A3, and GhCSP.A7 may play roles in cotton’s response to low-temperature stress. In conclusion, our findings underscore the significant role of the CSP gene family in cotton’s response to low-temperature stress, providing a foundational basis for further investigations into the functional aspects and molecular mechanisms of cotton’s response to low temperatures.
1. Introduction
Low temperatures are a significant environmental limitation on production and geographic distribution globally for plants, impacting growth and development [1]. Tropical and subtropical plants are more damaged by cold temperatures in general compared to overwintering plants with high cold tolerance. Low-temperature stress limits plant gene expression through direct inhibition of metabolic responses and indirect osmotic, oxidative, and other stresses [2]. To avert adverse effects, over the course of evolution, plants have devised sophisticated mechanisms to resist cold stress through the integration of transcription factors [3,4,5].
Contemporary research has demonstrated that freezing is tolerated by plants following cold exposure for an extended period, a process known as cold acclimation [6]. Cold domestication affects ion concentrations and metabolite transport, initiating downstream cold signaling [7]. The ICE-CBF-COR pathway is the most extensively studied regulatory pathway for low-temperature tolerance in plants today. The Inducer of CBF Expression (ICE) is a key factor in cold acclimation, and the regulatory gene C-repeat binding factors (CBF) plays a crucial role in cold acclimation. ICE1 induces the expression of CBF3 at low temperatures, while CBF can activate the expression of COR genes by binding to the CRT element (TGGCCCGAC) in the cold-responsive (COR) promoter [8]. Cold-inducible genes improve cold tolerance in plants by regulating the synthesis of compatible solutes (soluble sugars and proline), pigments (lutein and carotenoids), and cold-responsive proteins such as antifreeze proteins (AFPs), late embryogenesis abundance (LEAs) proteins, heat shock proteins (HSPs), and cold shock proteins (CSPs) [9,10,11,12].
Cold shock proteins (CSPs) act as DNA/RNA binding proteins that are conserved across bacterial and eukaryotic domains, including higher plants [13,14,15]. All plant CSPs are structurally similarly, with the cold shock domain (CSD) located at the N-terminus, and a large glycine-rich region at the C-terminus, which is randomly interspersed with 2–7 zinc finger domains (CCHC-type) [1,16]. CSP proteins have a role in cell proliferation, stress adaption, transcription and translation control, and cell division [17]. CSP proteins facilitate translation and destabilize RNA secondary structures to function as RNA chaperones, aiding the body in adapting to low-temperature stress [18].In eukaryotes, cold shock structural domain proteins are pleiotropic, and plant CSPs, in addition to being regulated by cold stress, are implicated in the regulation of embryo development, flowering time, fruit development, and stress responses, and this may be a function of the auxiliary structural domains in eukaryotic CSP proteins in addition to CSDs [16].
Up to now, the CSP family has only been systematically analyzed in Arabidopsis thaliana, Brassica chinensis, Camellia sinensis, Triticum aestivum, and Oryza sativa. In these species, four, six, three, two, and four CSP genes have been identified, respectively [18,19,20,21,22].The first CSP gene was identified in winter T. aestivum, and this WCSP1 gene was upregulated following low-temperature induction, possibly involved in regulating the cold tolerance of winter wheat. However, it was not regulated by other environmental stresses, suggesting that the function of WCSP1 was specific to cold adaptation [1]. Two cold shock structural domain proteins in O. sativa were cloned and functionally characterized, and it was determined that the expression of OsCSP1 and OsCSP2 was most elevated in rice spike tissues, as well as flowers and seeds. The expression of OsCSP1 and OsCSP2 was upregulated in rice root and stem tissues within 24 h of low-temperature stress treatment [23]. Overexpression of AtCSP3 enhances the tolerance and survival of transgenic plants exposed to multiple stresses, including salt, drought, and low temperatures [24,25]. Given that many CSP1-associated mRNAs are implicated in ribosome biogenesis, CSP1 may be essential for growth at low temperatures above freezing, with overexpression of AtCSP1 enhancing the translation of its targets under water limitation stress [26]. Overall, the CSP family proteins participates in the regulation of plant stress response and growth and development processes.
Cotton is a globally cultivated cash crop, with both diploid and allotetraploid varieties. Gossypium hirsutum and Gossypium barbadense are allotetraploids with the genetic composition AADD, which originated from the A genome of the diploid cotton Gossypium arboreum and the D genome of Gossypium raimondii [27]. Low-temperature cold damage is a common abiotic stress in cotton growing areas, where seed germination and seedling growth and development are inhibited, seriously impacting crop yield and quality. In cotton-producing areas of Xinjiang, the agrometeorological event with the greatest impact on cotton yield and quality is chilling, which occurs during the seedling stage in the Spring [28]. To improve responses to low-temperature cold damage, enhancing cotton genetic germplasm resources and identifying cold-tolerant genes are necessary approaches. The expression of GhDREB1 is positively regulated through the typical mechanisms of cold stress signaling, resulting in transgenic tobacco exhibiting higher cold tolerance compared to the wild type [29]. Overexpression of GhRaf19 positively regulates cold tolerance by modulating reactive oxygen species in cotton [30]. While some progress has been made in the mining of cold tolerance genes in cotton, there are a limited number of effective genes identified to respond to low-temperature stress and applied in the field [31]. Therefore, it is critical to locate cotton cold tolerance genes, characterize the cold tolerance mechanism, and develop cotton germplasm resources for resistance to low-temperature stress.
To date, functional examination of cotton CSP genes in hormone signaling, growth, development, and stress response has not been documented. In this study, we examined both the structure and evolutionary patterns of the CSPs gene family according to genome-wide data from the four cotton species. We used the expression of the CSP family under stress to characterize their responses. The findings elucidate the functions of the CSP gene family in cotton, and lay the foundation for the identification of genes for improving low-temperature tolerance in cotton.
2. Results
2.1. Identification of CSP Family Members across Four Cotton Species
To characterize members of CSPs across four cotton species (G. arboreum, G. raimondii, G. barbadense, and G. hirsutum), a search was performed using the Hidden Markov Model (HMM) and CSP protein sequences from Arabidopsis thaliana. A total of 43 genes containing CSP domains were identified, and 8 genes from G. boreum, 8 genes from G. aimondii, 13 genes from G. hirsutum, and 14 genes from G. barbadense were found. Seven genes were identified in the A genome of G. hirsutum tetraploid and the A and D genomes of G. borbadense, respectively. In comparison, six genes were identified in the D genome of G. hirsutum. Tetraploid cotton is an allotetraploid formed by hybridizing diploids with At and Dt genomes [32].The number of CSP genes in tetraploid cotton (G. hirsutum, G. barbadense) was nearly twice that of diploid cotton (G. arboreum, G. raimondii), indicating that the CSP family is conserved throughout evolution. These results indicate that the number of CSPs varies slightly across different cotton species, potentially due to the loss or duplication of genes over the course of cotton evolution.
Based on the position of these genes in the cotton chromosome, they were named GaCSP.1-8 (G. arboreum), GrCSP.1-8 (G. raimondii), GhCSP.A1-7 and GhCSP.D1-6 (G. hirsutum), and GbCSP.A1-7 and GbCSP.D1-7 (G. barbadense). Supplementary Table S1 indicates the gene ID number, coding region (CDS) length (bp), protein sequence length (aa), protein molecular weight (MW), isoelectric point (pI), and subcellular localization prediction results of the identified cotton CSP family proteins. The proteins encoded by the 43 genes had different physicochemical properties, varying in length from 90 to 300 amino acids, molecular weight from 8524.11 to 27,434.94 kDa, theoretical isoelectric points (pl) from 5.89 to 9.23, and subcellular localization predicted the localization of cotton CSP proteins primarily in the nucleus.
2.2. Analysis of Subcellular Localization of Cotton CSP Gene
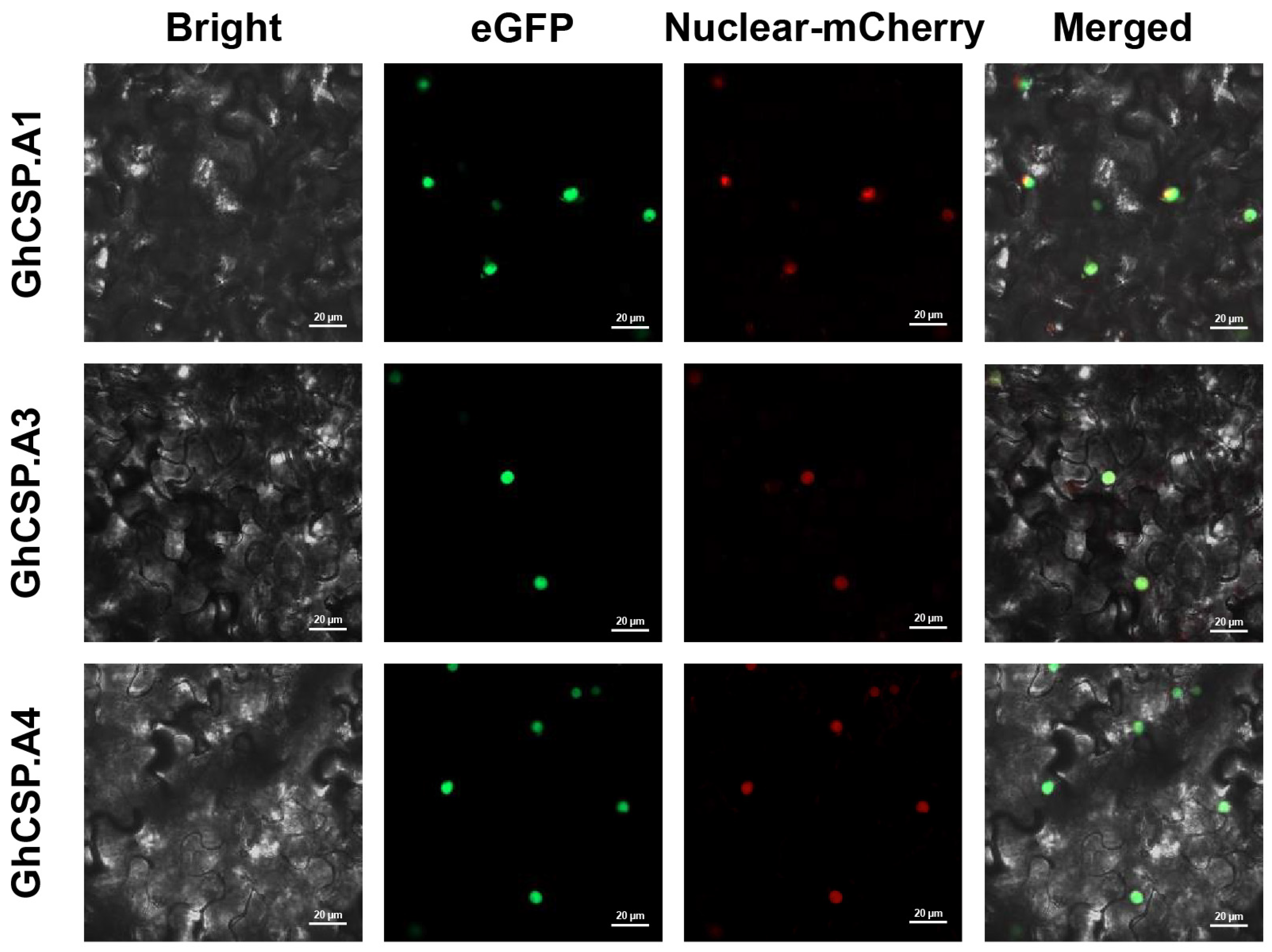
GhCSP.A1/A2/A3 was used as an example to validate subcellular localization. The vector was transiently expressed in tobacco leaves, and the GhCSP.A1/A2/A3 proteins were all localized to the nucleus as determined by the GFP fluorescence signals generated by laser confocal microscopy, in agreement with the prediction (Figure 1). This is similar to the subcellular localization results of A. thaliana AtCSP1~3 [25,26,33].
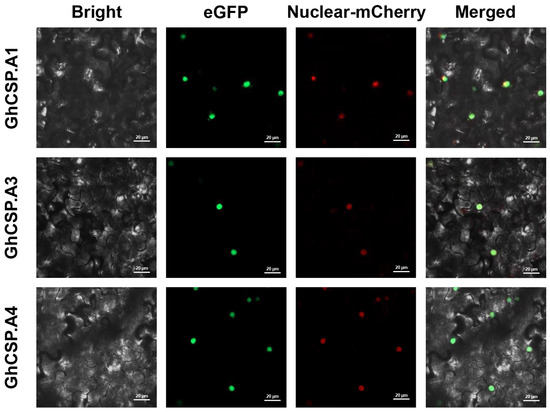
Figure 1.
Subcellular localization of GhCSP.A1-A3 proteins carried out in tobacco leaves. The Bright plot is the bright field. The green fluorescence in the eGFP plot represents the GFP fluorescence signal. The red fluorescence in the mCherry plot represents the organelle marker. The yellow fluorescence in the Merged plot represents the overlap of the green fluorescence from GFP with the red fluorescence from the marker.
2.3. Gene Structure, Conserved Domains, and Motif Analysis of Cotton CSP Genes
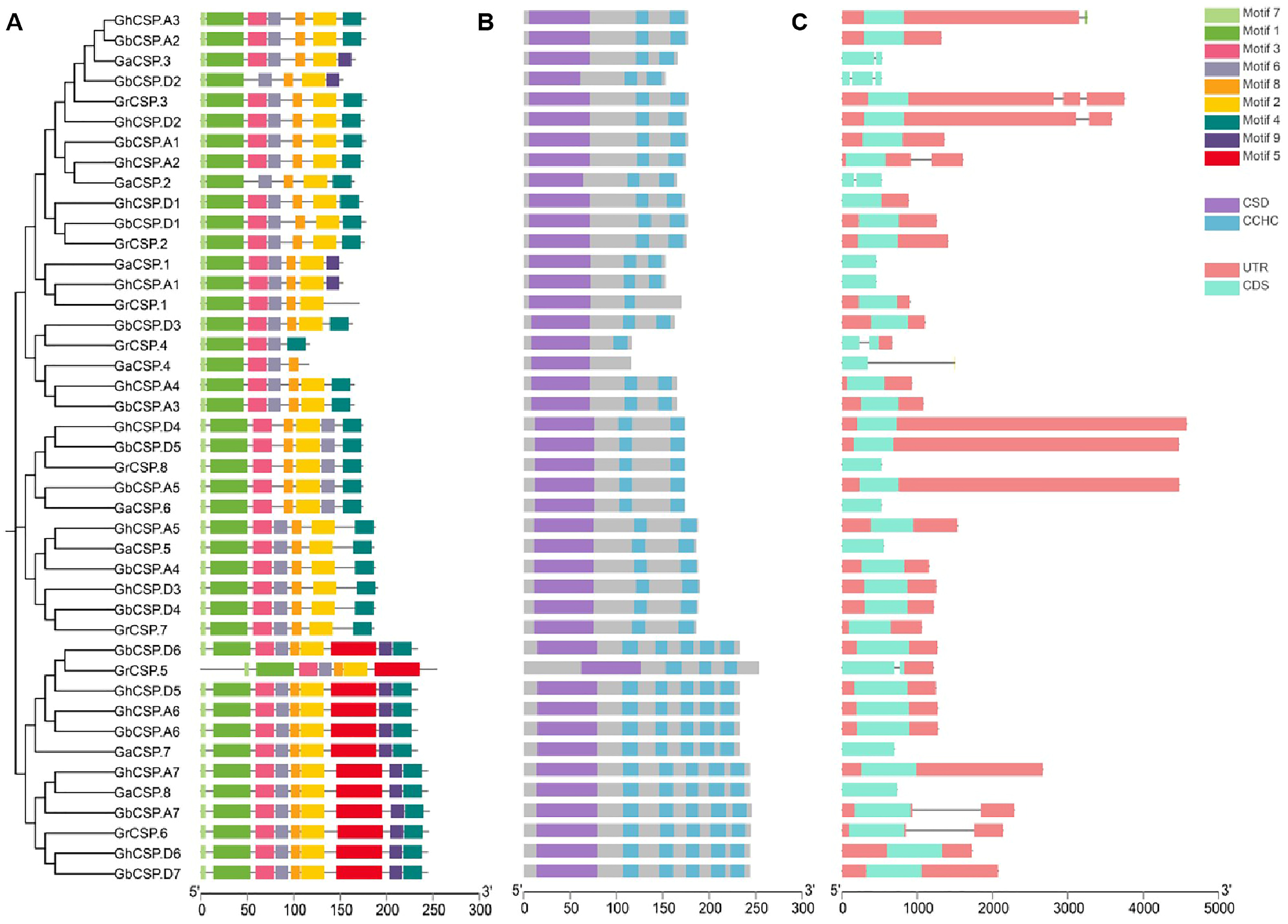
Gene structure is essential for determining the phylogenetic relationships among CSP genes [34]. To examine the evolutionary relationships throughout CSP genes in G. arboreum, G. raimondii, G. barbadense, and G. hirsutum, a phylogenetic tree was constructed using 43 characterized CSP protein sequences. Our findings showed three groups of CSP genes across the four cotton species closely distributed in each group.
Gene structure, conserved structural domains, and motifs were closely linked to gene function [35]. Consequently, cotton CSPs were analyzed according to phylogenetic trees. The coding sequences were mapped to the exon/intron sequences of the cotton genome to determine the gene structure of the cotton CSP family. The analysis demonstrated that the exon/intron structure of the cotton CSP genes was conserved across varieties. To further study the phylogenetic relationship of CSP genes, we investigated the arrangement of exons and introns in the CSP gene in cotton (Figure 2C). All group II genes had only one exon, while some members of groups I and III genes had three or five exon-intron structures. The sequence structure examination indicated that the majority of cotton CSP genes were composed of one exon, while a few of them consisted of two to five exons and introns, which was consistent with previous studies [16]. The exon and intron structures of cotton CSP genes are highly similar, supporting the reliability of the phylogenetic analysis. To further understand the structural characteristics of cotton CSP genes, we characterized nine conserved motifs using MEME. After analysis, it was determined that motifs 1, 2, 3, 4, 6, 7, and 8 appeared in nearly all CSP protein sequences from the three groups. Motif 5 was unique to Group I, and Motif 9 was absent in Group II (Figure 2A). Groups I was identical except for GrCSP.5, which lacks motifs 4 and 9. The group II CSP gene contains precisely the same motifs, but some of the motifs were arranged differently, lacking motifs 5 and 9 compared to group I. The motifs of group III were essentially the same as those of group II, but some of the motifs in group III were different, including GaCSP.4 and GrCSP.4, containing the lowest number of motifs. This finding demonstrates that members of the same group may differ in function. From the findings of conserved structural domains, similar to the A. thaliana CSP protein structural domains, all cotton CSP proteins contained both typical CSD and varied amounts of CCHC (Figure 2B).
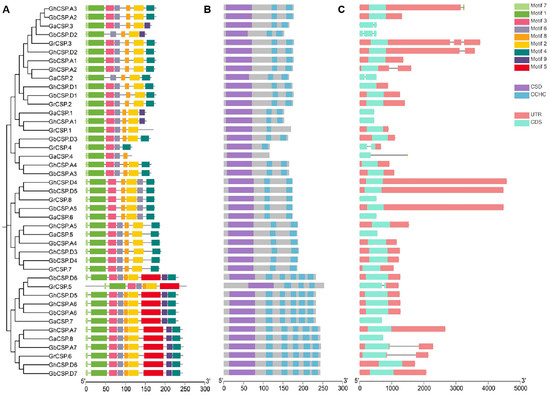
Figure 2.
Identification of conserved structural domains as well as gene structure analysis of cotton CSPs. (A) Evolutionary tree and conserved motifs throughout the cotton CSP family. (B) Conserved structural domains of the cotton CSP family. (C) Exon-intron structures of the cotton CSP family. Squares indicate exons. Lines indicate introns.
2.4. Chromosomal Location, Gene Duplication, and Evolutionary Relationships of Gossypium CSP Family Genes
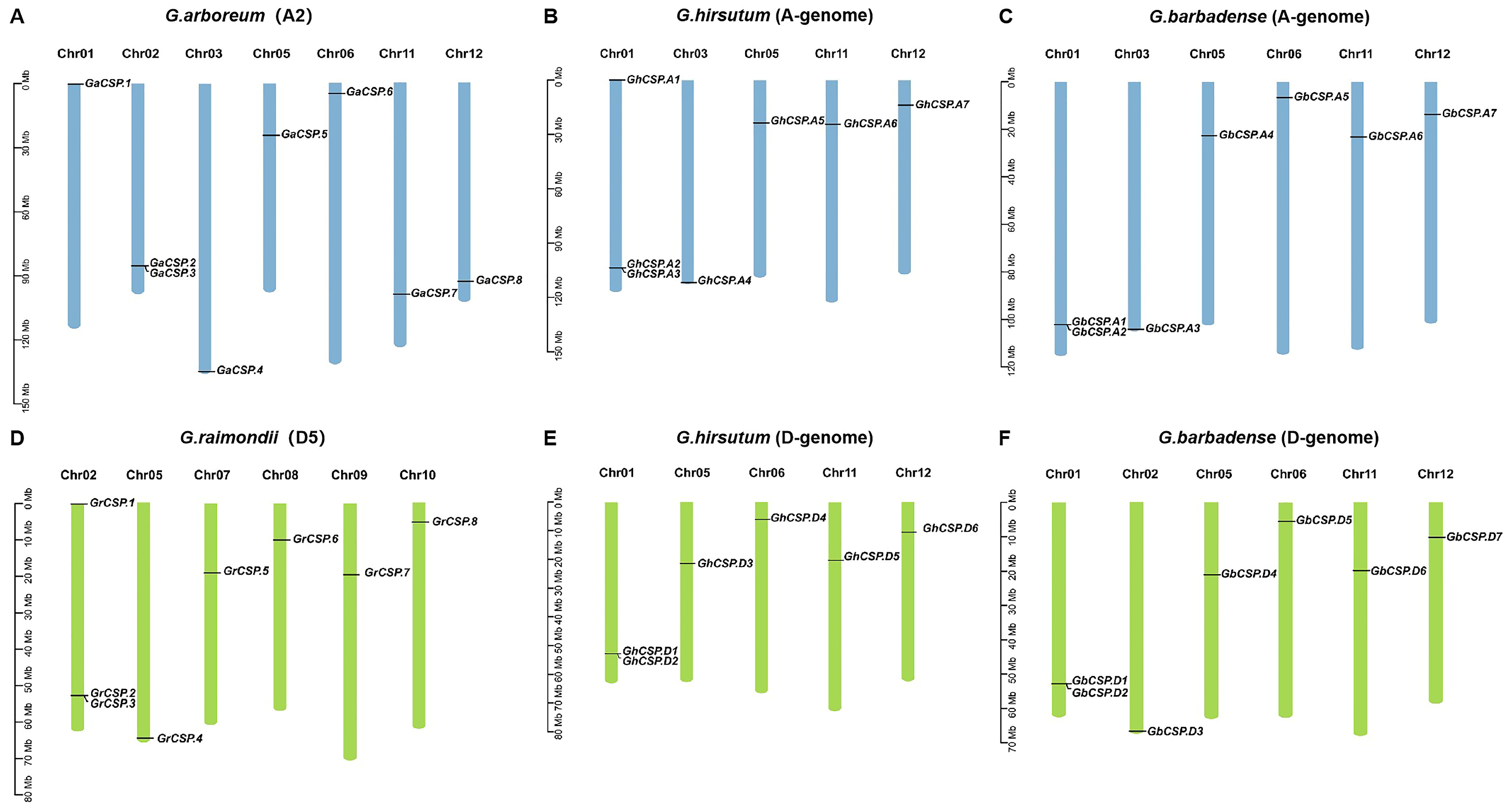
Cotton CSP genes are not distributed across all 13 chromosomes. It was found that 13 GhCSP genes were located on 10 chromosomes, of which seven were located on five chromosomes of the At genome, and the remaining six were located on five chromosomes of the Dt genome, according to the annotation information in the cotton genome (Figure 3). GhCSP.A1/D1, GhCSP.A2/D2, and GhCSP.A3 were located on chromosome A/D01, GhCSP.A5/D3, GhCSP.A6/D5, and GhCSP.A7/D6 were located on chromosome A05, 11, and 12, respectively, while GhCSP.A4 was located on chromosome A03 and GhCSP.D4 was located on chromosome A06. It was determined that 14 GbCSP genes were located on 12 chromosomes, with seven genes in each of the At and Dt genomes. The distribution of these two groups of genes on chromosomes was essentially identical. GbCSP.A1/D1 and GbCSP.A2/D2 were located on the A/D01 chromosomes, GbCSP.A4/D4, GbCSP.A5/D5, GbCSP.A6/D6, and GbCSP.A7/D7 were located on A/D05,6,11,12 chromosomes, respectively. Additionally, GbCSP.A3 was located on chromosome A03, while GbCSP.D3 was located on chromosome D02.
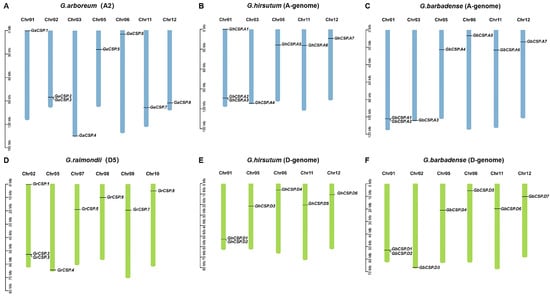
Figure 3.
Chromosome distribution and duplication of GhCSPs. (A) Eight GaCSPs were mapped onto seven chromosomes in G. arboreum. (B) Seven GhCSPs were mapped onto five chromosomes in G. barbadense (A-genome). (C) Seven GbCSPs were mapped onto six chromosomes in G. hirsutum (A-genome). (D) Eight GrCSPs were mapped onto six chromosomes in G. raimondii. (E) Six GhCSPs were mapped onto five chromosomes in G. barbadense (D-genome). (F) Seven GbCSPs were mapped onto six chromosomes in G. hirsutum (D-genome).
The GaCSP gene was localized on seven chromosomes, distributed across chromosomes 01 (GaCSP.1), 02 (GaCSP.2 and GaCSP.3), 03 (GaCSP.4), 05 (GaCSP.5), 06 (GaCSP.6), 11 (GaCSP.7), and 12 (GaCSP.8). The seven genes of GrCSP were situated on six chromosomes, including 02 (GrCSP.1, GrCSP.2, and GrCSP.3), 05 (GrCSP.4), 07 (GrCSP.5), 08 (GrCSP.6), 09 (GrCSP.7), and 10 (GrCSP.8). We determined that the A and D genomes of tetraploid cotton differed from diploid cotton in terms of the number of genes and chromosomal distribution, and these findings indicate that CSP genes may have undergone gene translocations, loss and duplication, and chromosomal deletions throughout the evolution of cotton.
To gain insight into the linkages between CSP genes and putative gene duplications in cotton genomes, we determined the non-synonymous (Ka) and synonymous (Ks) levels of 273 repeat gene pairs across 10 combinations of four cotton species. Selective pressure was predicted based on Ka/Ks values. Ka/Ks = 1 indicated neutral selection (pseudogenes), Ka/Ks < 1 indicated purification or negative selection, meaning that the gene evolution tends towards purification, and Ka/Ks > 1 indicated positive selection. The examination demonstrated that gene pairs with Ka/Ks > 1 appeared only in one pair throughout the Gb-Gb group, six gene pairs had Ka/Ks values between 0.5 and 1, while 233 gene pairs had Ka/Ks values below 0.5. Consequently, we hypothesized that the CSP gene family in cotton experienced strong purification selection (Supplementary Table S3).
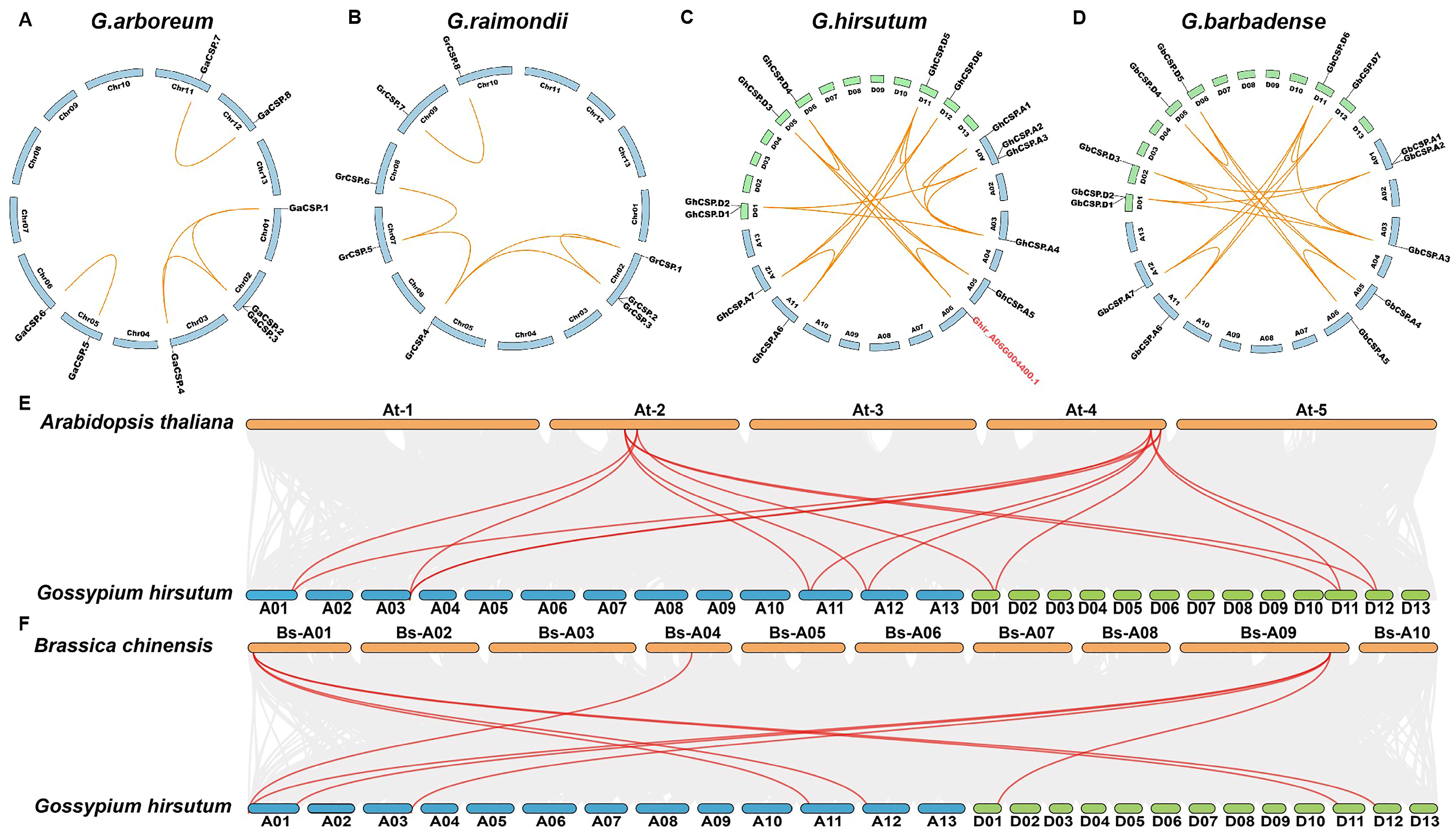
To investigate the genetic origin and evolutionary relationship of CSPs throughout cotton, we performed intraspecific and interspecific collinear analyses. Intraspecific collinear analysis of the four cotton varieties indicated that the collinearity of diploid cotton was effectively identical the same, with the exception of GrCSP.4 in G. raimondii being homologous to GrCSP.5 but divergent in G. arboreum. The genetic collinearity of tetraploid cotton was effectively equal, but alterations throughout cotton evolution and chromosome doubling were observed (Figure 4A–D). For instance, GhCSP.A1 does not have a homolog in the D genome, suggesting that it may have been ectopic or lost throughout evolution. Interestingly, Ghir_A06G004400.1 was determined to have collinearity with GhCSP.A5, GhCSP.D3, and GhCSP.D4, but it did not belong to the CSPs family, potentially due to an incomplete domain following gene replication in the evolutionary process. We conducted interspecific comparisons with A. thaliana and B. chinensis using G. hirsutum. It was identified that upland cotton possessed seven homologous gene pairs with A. thaliana and eight homologous gene pairs with B. chinensis, suggesting that these genes have elevated structural and functional similarity (Figure 4E,F).
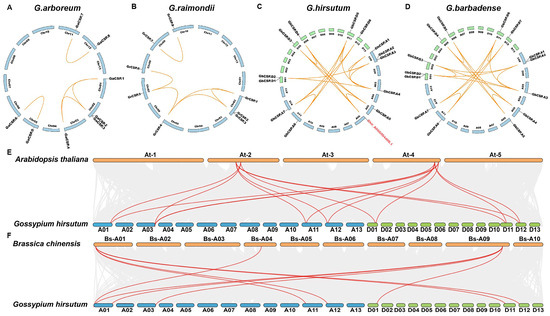
Figure 4.
Synteny examination of the GhCSP genes. (A) G. arboreum; (B) G. raimondii; (C) G. hirsutum; (D) G. barbadense; (E) A. thaliana and G. hirsutum; (F) B. chinensis and G. hirsutum.
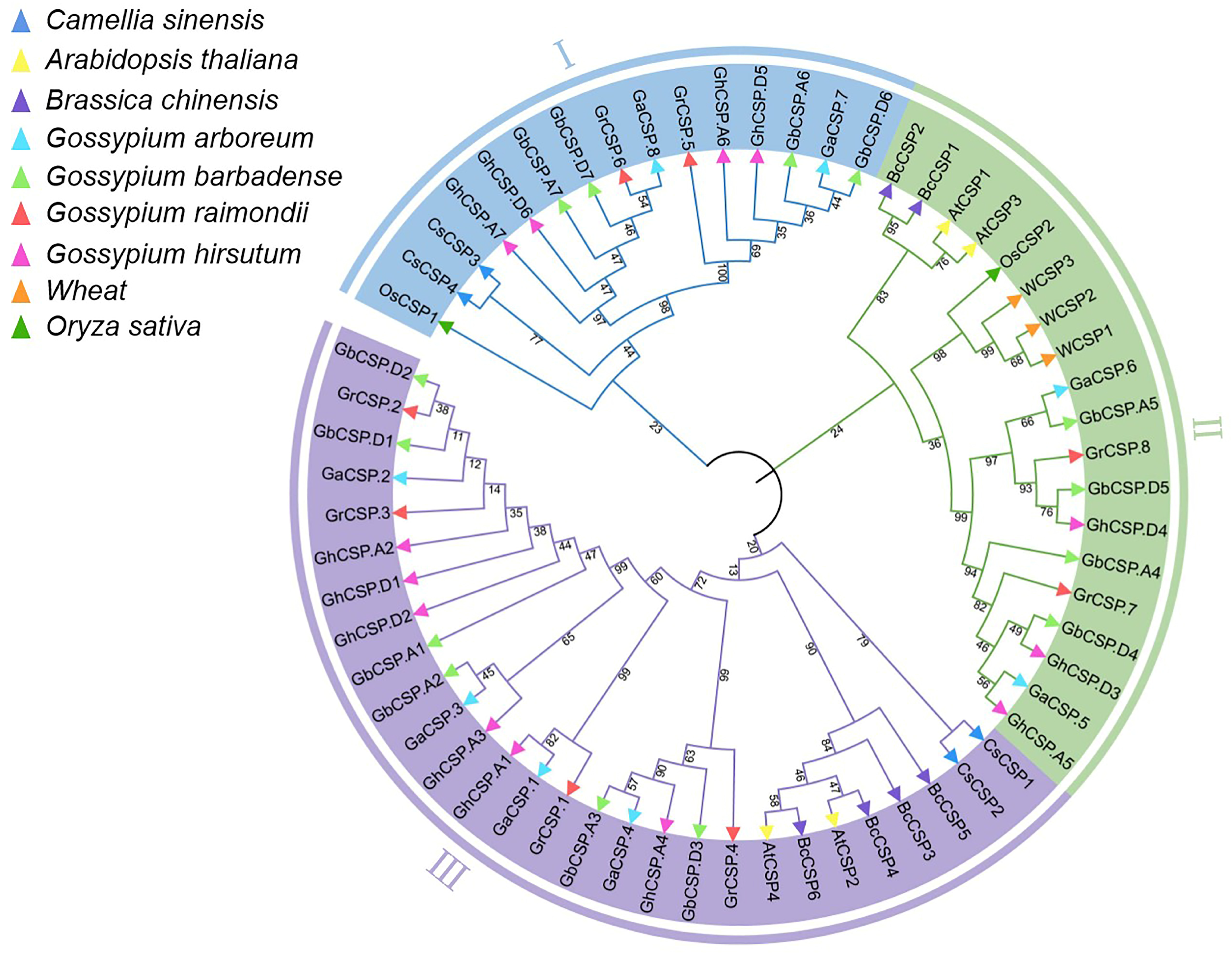
2.5. Phylogenetic Analysis
To investigate the evolutionary relationships of Cotton CSP proteins and those of five other plants, we created a neighbor-joining tree using multiple comparisons of CSP amino acid sequences. The phylogenetic tree indicated that CSPs had three distinct evolutionary branches, classified as CSP I–CSP III (Figure 5). CSP I contained 15 members, including two GrCSPs and GaCSPs, four GbCSPs and GhCSPs, two CsCSPs, and one OsCSP. CSP II possessed 19 members (three WCSPs, one OsCSP, two AtCSPs, two BcCSPs, three GhCSPs, four GbCSPs, two GrCSPs, and GaCSPs). CSP III was composed of the largest number of CSP members, including two CsCSPs, two AtCSPs, four BcCSPs, six GbCSPs and GhCSPs, and four GrCSPs and GaCSPs, for a total of 28. Prior reports have demonstrated that CsCSP4 is upregulated at low temperatures, and GhCSP.A7 is closely linked to CsCSP4, indicating that GhCSP.A7 may have comparable functions [22]. A close relationship between GhCSP.A4 and AtCSP2 was documented, while AtCSP2 has a negative regulatory role in low-temperature stress, GhCSP.A4 may also play a negative regulatory role [36]. Remarkably, each branch in the CSP gene family exhibits homologous genes across all four cotton species, suggesting a close relationship among their CSP genes. Notably, the proportion of CSPs in allotetraploid G. hirsutum and G. barbadense nearly maintains a 1:1 ratio, mirroring the similar ratio observed in diploid G. raimondii and G. arboreum. The proportion of CSPs in allotetraploid cotton and diploid cotton was below 2:1, potentially because of evolutionary selection during the hybridization of two diploid cotton lines to generate allotetraploid cotton.
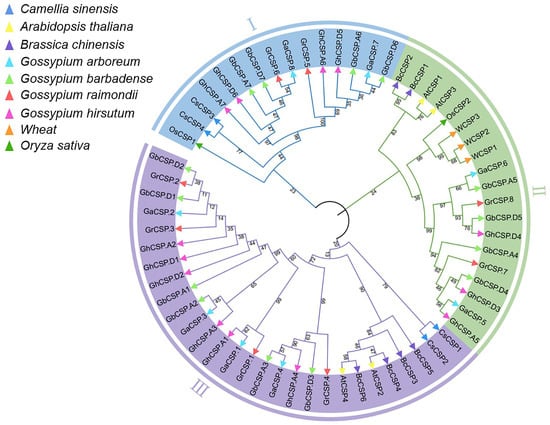
Figure 5.
Phylogenetic examination of CSP gene families. Phylogenetic analysis of the CSP gene families across cotton, A. thaliana, O. sativa, C. sinensis, B. chinensis, and T. aestivum. The inner circle is marked in blue, green, and purple, representing the CSPI, CSPII, and CSPIII clades, respectively. Diverse plants are indicated by small triangles of different colors. The number alongside the branch indicates the bootstrap value of each group derived from 1000 replicates.
2.6. Cis-Element Analysis in the Promoter Regions of Cotton CSP Genes
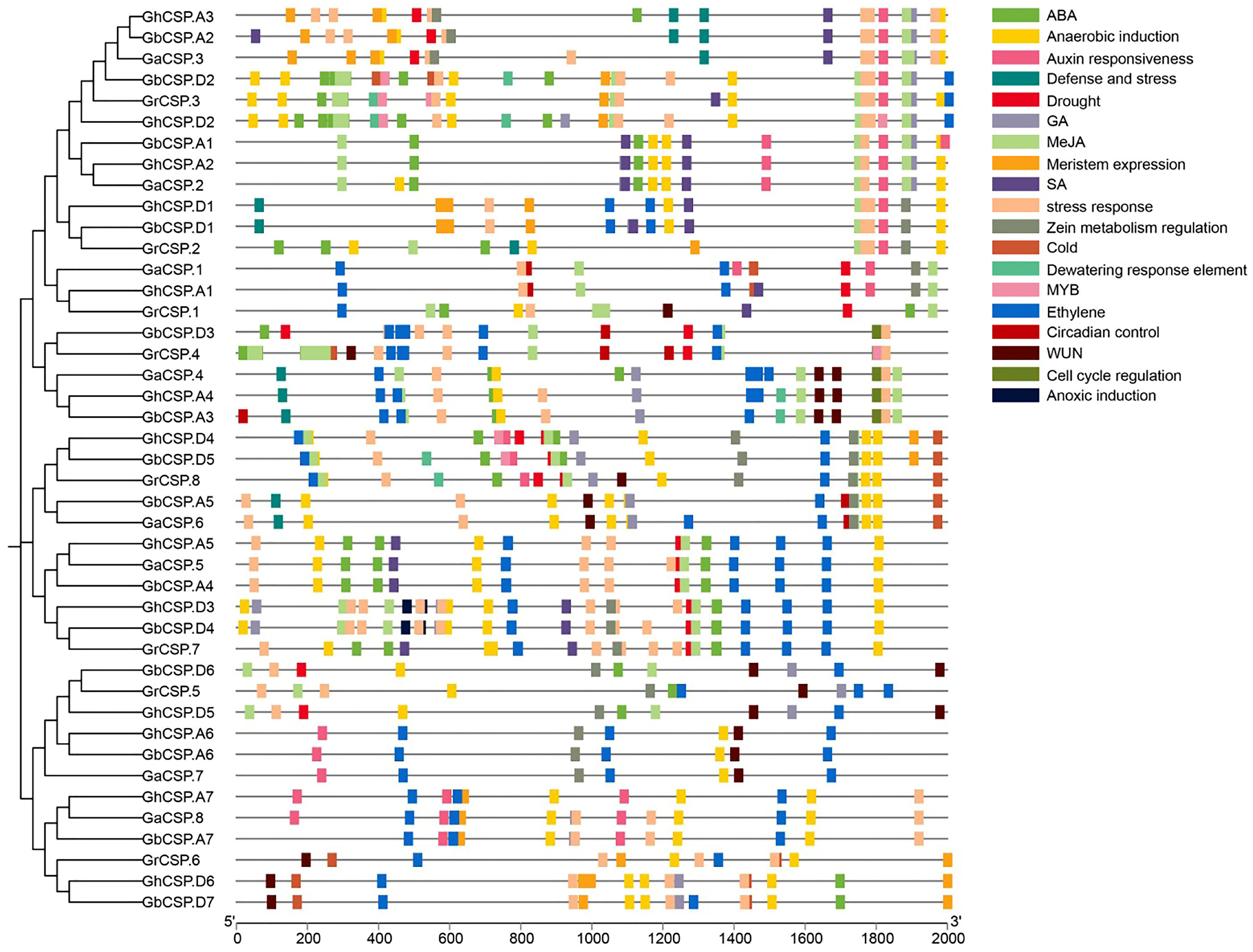
Gene expression is often regulated by cis-elements in the upstream promoter sequences [37]. These cis-elements in the non-coding DNA upstream of the transcription start site can modulate stress receptors or tissue-specific expression of the gene in diverse environments [35]. To comprehend the potential regulatory mechanisms of this family, we predicted the cis-acting elements of 43 cotton CSP proteins and examined their upstream 2000 bp promoter regions using PlantCARE. The findings indicated that in addition to common essential core elements (such as the A-box and TATA-box) and light-responsive elements (such as ACE), the promoter region of CSPs had numerous elements related to growth, development, and abiotic stress response (Figure 6).
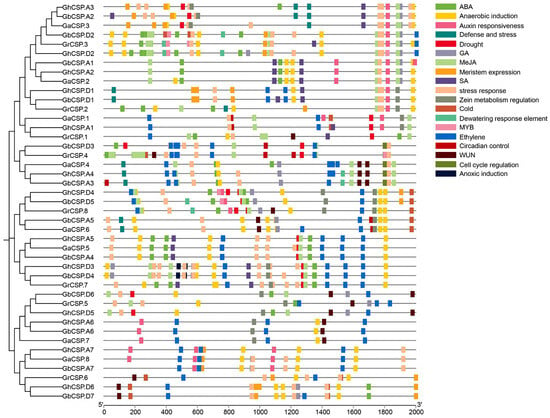
Figure 6.
Predicted hormone response, stress response, and growth-related cis-elements within the promoter regions of CSP genes.
Hormone response elements include those that react to auxin (AuxRE), ABA (ABRE), ethylene (ERE), methyl jasmonate (CGGTA motif and TGACG motif), gibberellin (GARE-motif, P-box, and TATC-box), and salicylic acid (SARE and TCA). The stress response elements include low-temperature response elements (LTR), dehydration response elements (DRE), drought response elements (MBS, Myb/Myc binding sites), defense and stress response elements (TC-rich repeats), anaerobic induction elements (ARE), and wound response elements (WUN and WRE3 motif). Growth and developmental elements include a meristematic tissue expression element (CAT-box), an AT-rich element (binding site of AT-rich DNA-binding protein), and an endosperm expression element (GCN4 motif). The findings indicate that CSPs may be regulated by diverse hormones involved in the growth of cotton and its response to environmental stresses. Various subfamilies have different gene promoter characteristics, including those of subfamilies II and III, typically containing a large number of hormone and stress-responsive elements, while the promoters of subfamilies I and III contain a large number of growth and developmental elements. This indicates that the expression of CSPs in different subfamilies is influenced by diverse regulatory factors.
2.7. Analysis of GhCSP Gene Expression under Various Stresses and Different Tissues
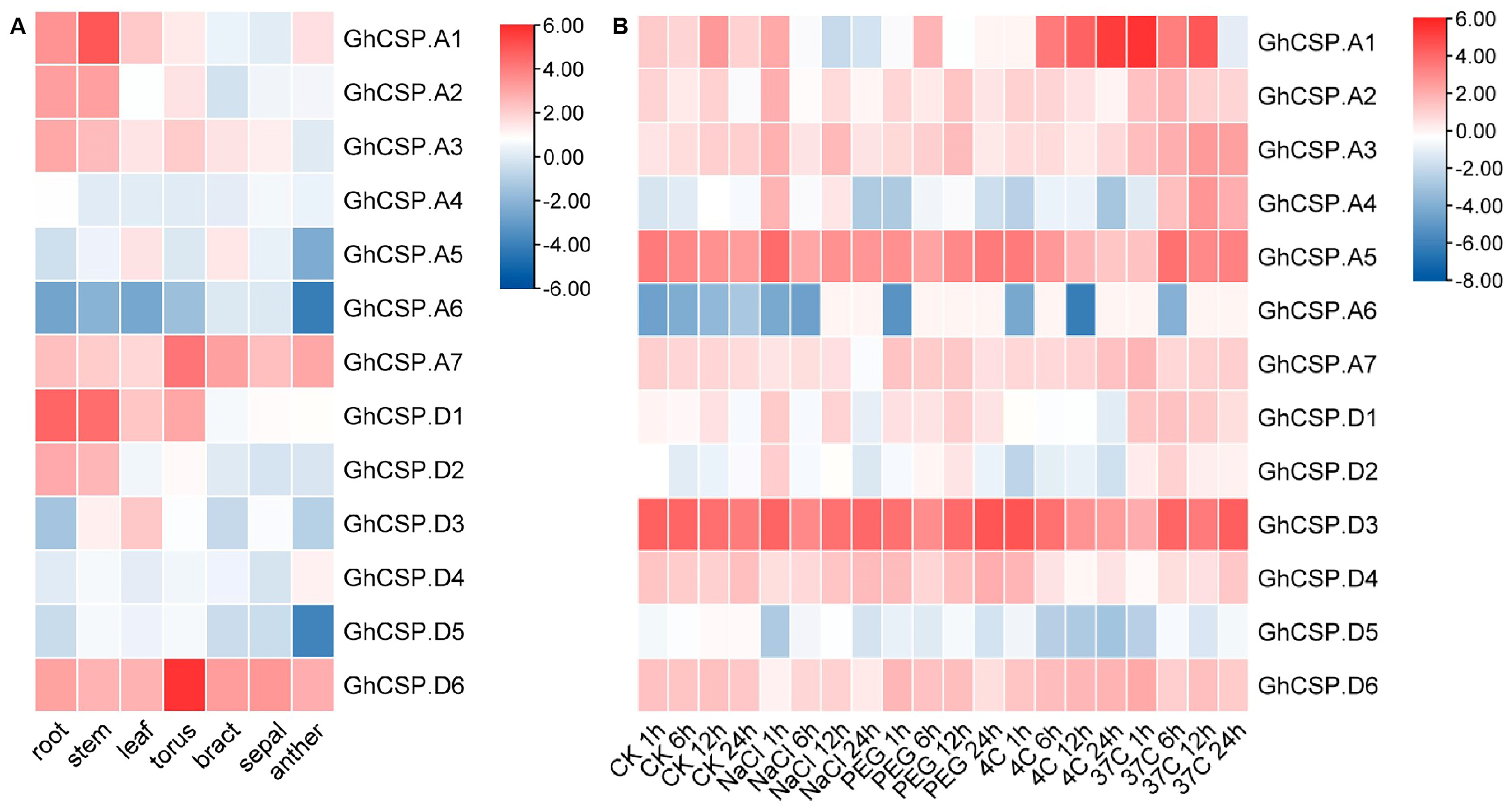
Given that gene expression is closely related to cis-acting elements, we examined abiotic stress and tissue-specific expression patterns for GhCSPs. As there is only information about upland cotton found in the database, we characterized upland cotton. The gene expression heatmap indicated that the same GhCSPs were expressed under different stress treatments, while the expression patterns of homologous genes were relatively similar across different tissues (roots, stems, leaves, and floral organs) and under various stress conditions (salt, drought, low temperature, and high temperature) (Figure 7). Moreover, while genes from the same subfamily possess similar motifs, they may differ functionally under different abiotic stresses related to stress response elements in the gene promoter region.
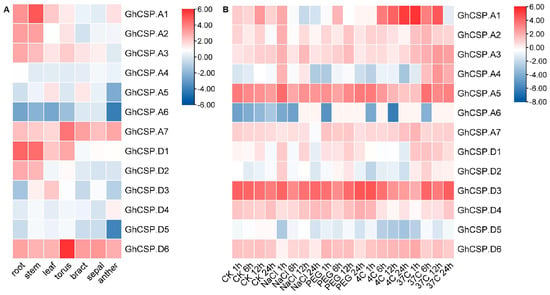
Figure 7.
Expression pattern examination of GhCSP. (A) Analysis of the expression patterns of GhCSP across different organs. (B) Expression pattern analysis of GhCSP under control, low temperature, high temperature, salt, and drought stress conditions, expressed as CK−control, NaCl−salt (400 mM NaCl), PEG−drought (20% PEG), 4C−low temperature (4°), and 37C−high temperature (37°), respectively.
2.8. Validation of GhCSPs Expression Levels
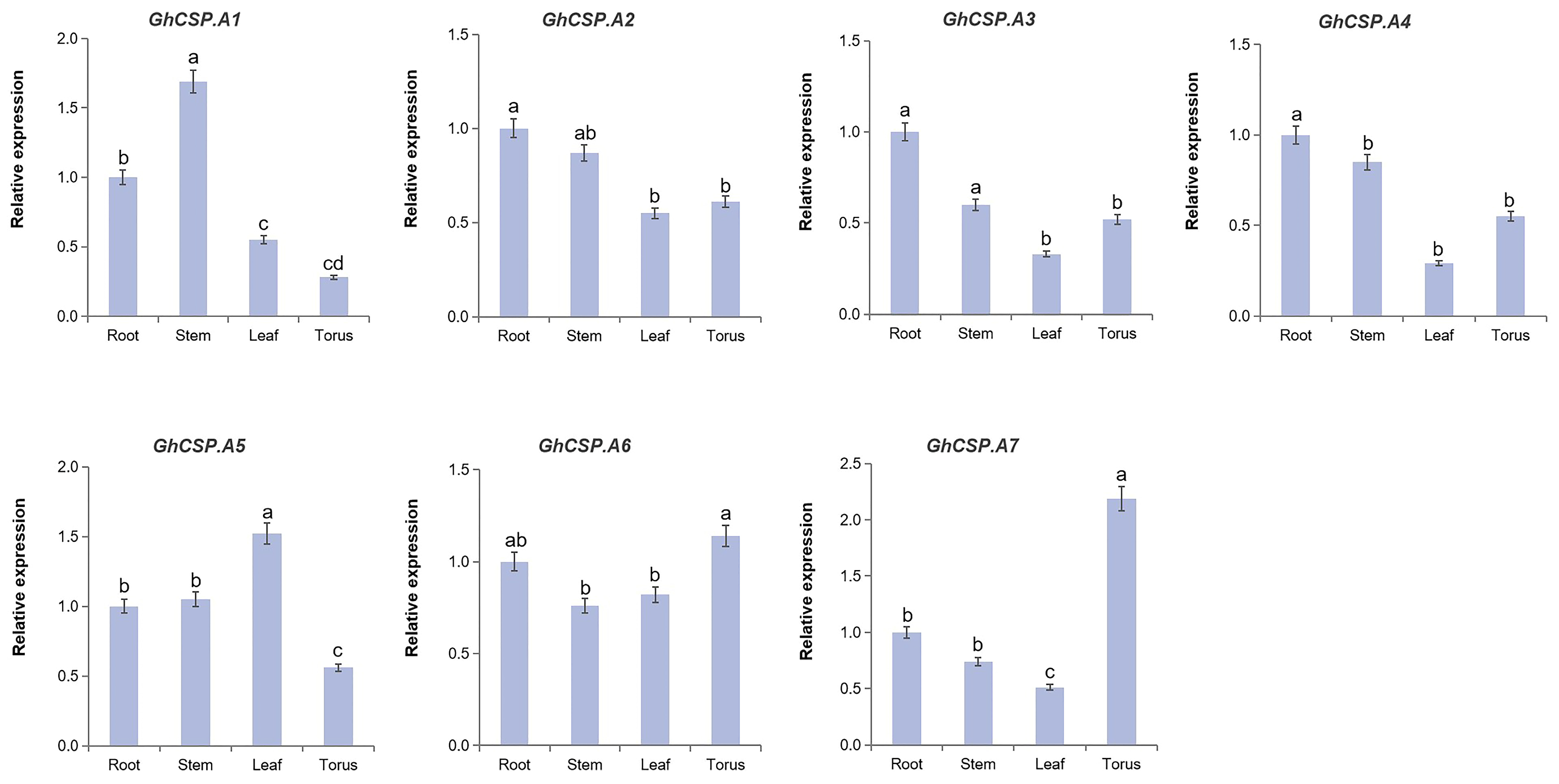
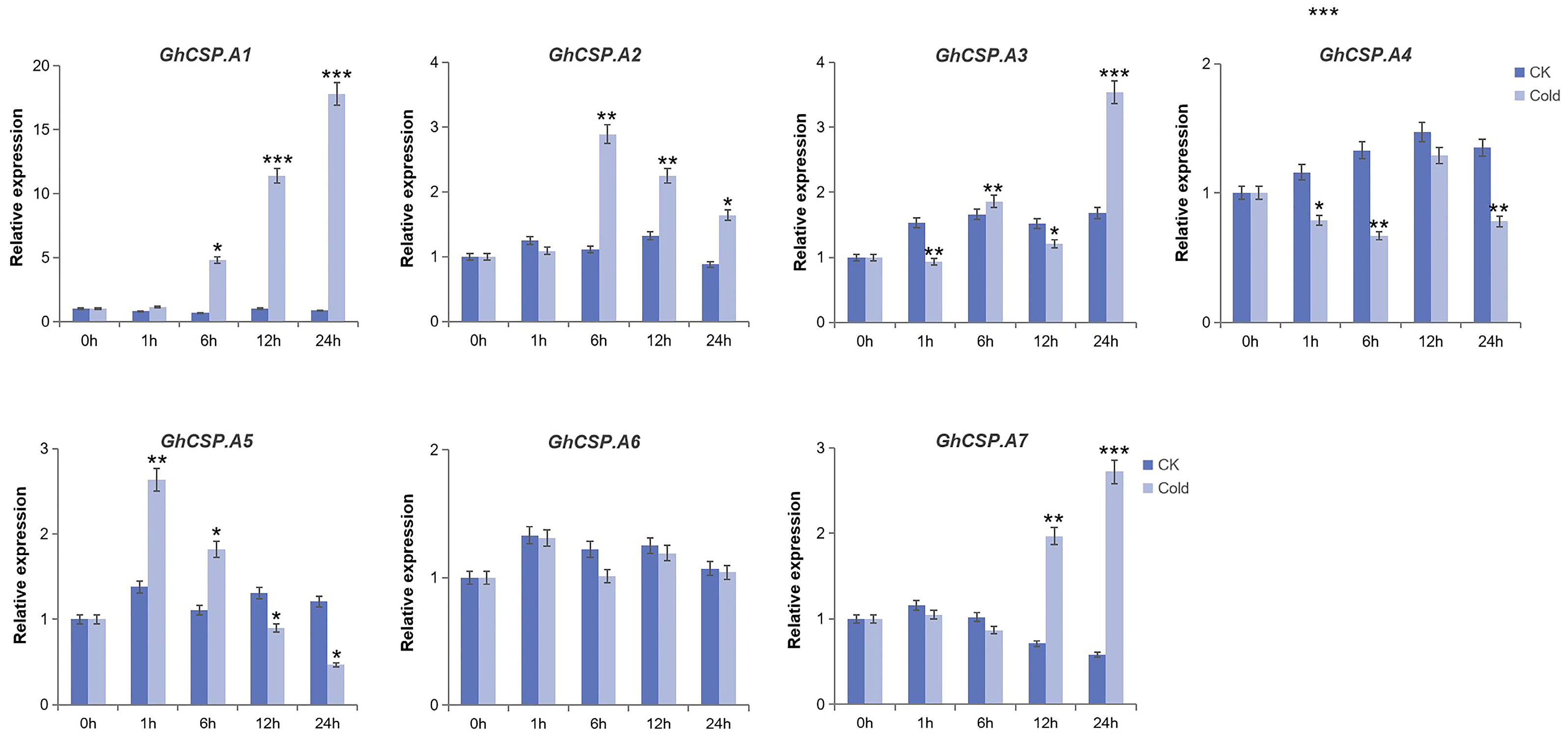
To investigate the tissue-specific expression of the GhCSPs gene family and its response to low-temperature stress, RT-qPCR verification and significance analysis were conducted on GhCSPs. Given the homology correlation, GhCSP.A1-A7 was selected. From the RT-qPCR results, the tissue-specific expression results were nearly consistent with the RNA-seq data, and most of the GhCSP genes were highly expressed in the roots or stems, while GhCSP.A5 and GhCSP.A7 were specifically highly expressed in the torus (Figure 8). We simulated low-temperature stress, and the results indicated that the expression levels of GhCSP.A1, GhCSP.A2, A3, and A7 were upregulated under low-temperature induction, except GhCSP.A6 which had no significant change (Figure 9). The expression of GhCSP.A1 was gradually upregulated following low-temperature treatment, reaching its highest level after 48 h, while the expression of GhCSP.A2 reached its highest level 6 h after low-temperature treatment and then gradually decreased. GhCSP.A3 expression was downregulated at 1 h and 6 h and significantly upregulated at 24 h, while GhCSP.A7 expression was significantly upregulated at 12 h and 24 h. The expression of GhCSP.A4 was downregulated, and GhCSP.A5 was upregulated at 1 h and 6 h. In contrast, both were downregulated at 12 h and 24 h, with the highest expression taking place at 1 h and gradually decreasing. Based on these results, most genes in the cotton CSP family can respond to low-temperature stress via expression regulation.
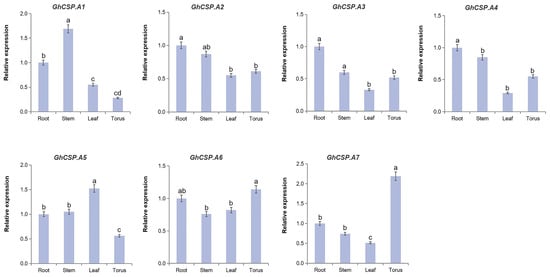
Figure 8.
The expression of GhCSP.A1–A7 across different organs. The qRT-PCR result of GhCSP.A1–A7 in the roots, stems, leaves, and flowers of G. hirsutum. Values are presented as the mean ± S.D (n = 3 replicates). Significant differences were assessed using one-way analysis of variance (ANOVA) with p < 0.05.
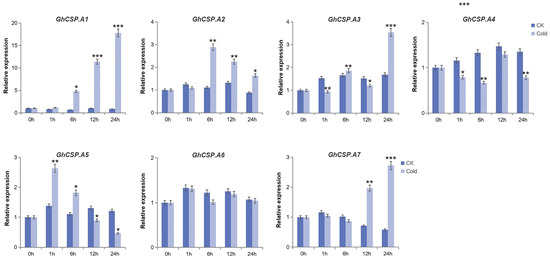
Figure 9.
Expression patterns of seven GhCSP genes responding to cryogenic treatment. Each sample was sampled at 0, 1, 6, 12, and 24 h following treatment. CK was the control group. Cold was the treatment group. The findings are presented as the mean of three replicates. Significant differences are indicated by * p < 0.05, ** p < 0.01, and *** p < 0.001, determined using a Student’s t-test.
3. Discussion
Low-temperature cold damage typically occurs throughout cotton growth and development, posing a significant threat and loss to agricultural production. Over the past two decades, efforts have been made to determine the pivotal elements of cold tolerance in plants, as well as analyze their regulatory mechanisms. Mining of low-temperature tolerance candidate genes has been important for this process [38]. A large number of studies have indicated that CSP proteins are ubiquitous in plants and play an essential role in adapting to various abiotic stresses (especially low-temperature stress) and regulating plant growth and development [16,23,39,40]. It was found that the number of CSPs in the four cotton varieties was nearly four times (G. barbadense and G. hirsutum) or twice (G. arboreum and G. raimondii) the number of CSPs identified in A. thaliana, C. sinensis, and T. aestivum. This difference may be attributed to the extensive gene amplification that cotton underwent to adapt to its environment during the evolutionary process.
The connection in the distribution of gene structures and conserved motifs in the same subfamily suggests that they have common roles in plant growth [41]. Upon studying the cotton CSP protein sequences, we discovered that the majority of CSP genes in cotton do not contain introns. Intronless genes are typically found in bacteria and primitive eukaryotes [35,42]. This suggests that CSP genes without introns are evolutionarily conserved and have been passed down from the past to the present. Furthermore, the CSD and CHCC zinc finger structural domains of cotton CSPs are highly conserved. These two domains can facilitate the binding of cold shock domain proteins to RNA, single-stranded DNA (ssDNA), and double-stranded DNA (dsDNA), demonstrating that cold shock domain proteins exhibit RNA chaperone activity [22].However, the motif distribution of cotton CSP proteins varies across subfamilies, and some motifs are specific to a particular subfamily. For instance, motif 5 is specific to subfamily I, which suggests that the functions of the genes may differ among subfamilies.
Chromosome mapping and collinearity analysis of cotton CSPs demonstrated that cotton had undergone gene deletion and chromosome translocation during evolution and chromosome doubling. Ghir_A06G004400.1 was found to be collinear with the CSP gene of upland cotton, but it was not identified as belonging to the CSP family. By examining the conserved domain, it lacks the CSD, the most critical cold shock domain in the CSP family, which may be lost during evolution. In addition, through interspecific comparison, we found that there were homologous gene pairs between cotton and dicotyledonous plants (A. thaliana, and B. chinensis), but not between cotton and monocotyledonous plants (O. sativa, and T. aestivum), related to species development.
Cis-acting elements on gene promoters can play an active role in plant stress responses, and the type of element dictates the response signal of the gene [43]. In the promoter sequences of the cotton CSP family, we identified various cis-acting elements in response to environmental stress (ARE, DRE, MBS, LTR, and WRE3, etc.), plant hormone response (ABRE, AuxRE, ERE, SARE, CGGTA motif, and P-box, etc.), and growth and development (GCN4 motif, and CAT-box, etc.). A large number of Myb and Myc transcription factor binding sites are present in the promoters of most CSP genes, and it has been demonstrated that these transcription factors are critical for plant stress tolerance [44,45].The distribution of these cis-acting elements on the promoter indicates that CSPs are likely to respond to adversity, stress, and growth and development.
Contemporary studies have indicated that CSP activity in plants is influenced by various abiotic stress factors, and the examination of gene expression patterns is often employed to characterize gene function [32]. Via analysis of expression patterns before and after stress, it was determined that the expression of four genes (GhCSP.A1, GhCSP.A2, GhCSP.A3, and GhCSP.A7) was upregulated under low temperatures. Similarly, the expressions of BcCSP1 and BcCSP3 in cabbage and CsCSP1 and CsCSP4 in Camellia were upregulated by low-temperature stress [20,22]. The expression of GhCSP.A1 was significantly upregulated by cold stress, but downregulated by salt and drought stress. The expression of GhCSP.A5 was upregulated under salt and drought stress, but downregulated after 6 h of low-temperature stress. Regrettably, no pleiotropic genes were identified in GhCSPs. Our laboratory acquired the DgCSP gene from Deinococcus gobiensis I-0, and subsequent overexpression in cotton confirmed its ability to increase yield and enhance drought and salt tolerance [46]. Studies have determined that following AtCSP2 deletion, the cold tolerance of the atcsp2 mutant did not exhibit any significant alterations. Still, overexpression of this gene reduced the cold resistance of transgenic A. thaliana. This negative regulatory mechanism is accomplished through inhibiting the expression of CBF (C-repeat binding factor) and its downstream genes [36]. Similar to AtCSP2, GhCSP.A4 is also inhibited by low-temperature treatment, suggesting that this gene may negatively regulate cotton cold resistance response, but its involvement in CBF regulation and downstream genes remains unclear. These results further validate the significant role of the GhCSP gene family in responding to stress. Under low-temperature conditions, the upregulated expression of GhCSP may enhance the activity of cotton CSP protein to improve RNA chaperone activity, promote correct folding of RNA, stimulate gene transcription and translation, and ultimately augment protein activity and cotton cold resistance [47].
Previous studies have indicated that AtCSPs are highly expressed in the early stages of shoot tip and floral tissues and may play a role in various stages of plant development [18]. Accumulation of CSPs was observed in winter T. aestivum and O. sativa during the development of embryonic roots, hypocotyls, and crown tissues (nutrient growth stage), flowers, and seeds under low-temperature stress conditions [21,23]. These findings suggest that CSPs can impact plant growth and development. Some cis-acting elements of CSP genes in cotton are linked to growth and development. Additionally, GhCSPs have expression specificity in different tissues. For example, GhCSP.A7 is highly expressed in floral organs, and GhCSP.A1, GhCSP.A2, and GhCSP.A3 are expressed in rhizomes. From this, we hypothesized that the CSPs are involved in cotton growth and development.Integrated with the examination of the expression patterns of GhCSP at various sites and under diverse stresses, we determined that the genes specifically highly expressed in rhizomes and floral organs were aligned with those upregulated under low-temperature stress. The accumulation of CSP proteins at these sites may be observed during stress and nutrient growth stages.
In this study, we conducted genome-wide identification and expression pattern analysis of CSP gene family members from across four cotton species. According to our findings, we concluded that CSP genes may play important regulatory roles in cotton growth and development alongside low-temperature stress response.
4. Materials and Methods
4.1. Identification of CSP Genes in Cotton
G. hirsutum and G. barbadense are allotetraploids, which evolved from two diploid cotton species, G.arboreum and G.raimondii. Therefore, these four cotton species were selected for gene family analysis. The whole genome sequence data and annotation information for four cotton species, G. hirsutum (HAU), G. barbadense (HAU), G. arboreum (CRI), and G. raimondii (JGI) (HAU, CRI, and JGI represent different assemblies of the genome), were obtained from the CottonFGD website (https://cottonfgd.net/ (accessed on 1 June 2023)) [48]. The CSP gene in A. thaliana was identified and downloaded from the TAIR database (https://www.arabidopsis.org/ (accessed on 3 June 2023)). A BUPP search was conducted in CottonFGD using the A. thaliana CSP protein sequence (AT4g36020, AT4g38680, AT2g17870, AT2g21060) with the e value set to 1e−10 to acquire the cotton CSP protein data. The Hidden Markov Models PF00313 (CSD) and PF00089 (zf-CCHC) corresponding to the CSP gene family were acquired from the PFAM database (http://pfam.xfam.org (accessed on 6 June 2023)) to characterize the CSP gene containing two domains in cotton [49]. The protein sequences from A. thaliana and cotton were aligned using HMM and Blastp, and domain information was identified using the CCD databases (http://www.NCBI.nlm.nih.gov/CDD/ (accessed on 9 June 2023)) of the SMART website (https://smart.embl.de/ (accessed on 9 June 2023)) and the NCBI website [50,51]. Following several screenings to remove ineligible proteins, the remaining non-redundant proteins were identified as cotton CSP proteins. The ExPAsy online tool (https://web.expasy.org/protparam/ (accessed on 15 June 2023)) was employed to determine the physicochemical parameters, including molecular weight, isoelectric point (pI), and protein length of CSP protein sequences across four cotton seeds [52]. WoLF PSORT tools (https://wolfpsort.hgc.jp/ (accessed on 21 June 2023)), CELLO (http://cello.life.nctu.edu.tw/ (accessed on 21 June 2023)), and YLoc (https://abi-services.cs.uni-tuebingen.de/yloc (accessed on 21 June 2023)) were utilized to predict subcellular localization of the proteins.
4.2. CSP Gene Subcellular Localization Analysis
The pCAMBIA1302 vector was digested with the restriction endonuclease NcoI to obtain a linear vector. Subsequently, the plasmids containing the target genes (GhCSP.A1, GhCSP.A2, GhCSP.A3) were ligated with the vector plasmid using a ClonExpress II One Step Cloning Kit (Vazyme) to create new vectors. The constructed vector was introduced into Agrobacterium rhizogenes GV3101 (pSoup) and then injected for transient expression on the underside of 4-week-old tobacco leaves. The infected area was subsequently excised for observation after 2 days of dark treatment. Bright-field fluorescence imaging of tobacco leaves was analyzed using a laser confocal super-resolution microscope (LSM980). All the primers used for this experiment are shown in Supplementary Table S2.
4.3. Phylogenetic Analysis
To investigate the evolutionary relationships between CSP proteins across different species, CSP protein sequences of A. thaliana, O. sativa, T. aestivum, B. chinensis, and C. sinensis were acquired from the Ensembl Plants database (https://plants.ensembl.org/index.html (accessed on 29 June 2023)) [53]. MEGA 11 was used for multiple sequence comparisons on the identified CSP sequences of different species, including cotton, and the phylogenetic tree was developed using the neighbor-joining method. The bootstrap value was established as 1000, and other parameters were set to default to obtain the NWK file. Finally, ITOL (https://itol.embl.de/ (accessed on 3 July 2020)) was applied to colorize this tree.
4.4. Conserved Motifs and Gene Structure Analysis of Cotton CSP Genes
TBtools software v2.031 was employed to trim and compare the protein sequences of cotton CSPs, and an evolutionary tree was developed using the IQtree function [54]. The online MEME tool (https://meme-suite.org/ (accessed on 12 July 2020)) was utilized to predict conserved motifs of CSP proteins. Employing the online tool GSDS (http://GSDS.cbi.pku.edu.cn/ (accessed on 22 July 2020)), we predicted the cotton CSPs’ genetic structure [55]. The NCBI conservative CDD structure domain database (https://www.ncbi.nlm.nih.gov/ (accessed on 26 July 2020)) was employed to predict the structure of the cotton CSP domain. Eventually, TBtools was employed to visualize the evolutionary tree, motif, domain, and gene structure.
4.5. Chromosomal Localization and Collinearity Analysis of Cotton CSP Genes
The chromosome distribution information of CSP genes was acquired from gene annotation files throughout the four cotton species (G. arboreum, G. raimondii, G. barbadense, and G. hirsutum) and visualized using TBtools. Repeat gene pair collinearity examination of the four cotton CSP protein sequences was conducted through the MCScanX program v2.031, and gene pairs meeting the criteria for tandem repeat genes were identified and visualized using TBtools. We calculated the ratio of the number of non-synonymous substitutions per non-synonymous site to the number of synonymous substitutions per synonymous site (Ka/Ks) for duplicated genes to investigate the selection pressure [55]. To examine the homology relationships of homologous CSP genes identified in cotton and other species, the multicollinearity scanning toolkit (MCScanX) was utilized to map the homology analysis [56].
4.6. Analysis of Cis-Acting Elements in CSP Genes from Cotton
The 2000 bp DNA sequence upstream of cotton CSPs was extracted based on the location of genes to analyze the promoter region of CSPs genes. Using PlantCare (http://bioinformatics.psb.ugent.be/webtools/PlantCare/html/ (accessed on 30 July 2020)) we identified the cis-acting regulatory elements in the promoter sequences, and used TBtools to visualize recognition elements.
4.7. RNA-Seq Analysis
The expression patterns of GhCSP genes across different parts and stresses were examined using original RNA-seq data. FPKM (fragments per kilobase of exon per million fragments mapped) indicated the amount of reads in each 1000 bases identified as a part of an exon across every million reads throughout the landscape. The FPKM approach was employed to examine gene expression. TBtools was used to construct a heatmap [35].
4.8. RT—qPCR Analysis
R15 (a conventional upland cotton cultivar) was planted in a blend of vermiculite and nutrient-rich soil, and cultivated in an incubator with a 16/8 h (light/dark) photoperiod and a temperature of 25 °C. Cotton seedlings aged three weeks were subjected to low-temperature stress (4 °C), while the control materials were left untreated. The leaves were collected at 0, 6, 12, and 24 h after treatment (3 repetitions), immediately frozen with liquid nitrogen, and stored at −80 °C. Total RNA was extracted from the samples using the FastPure Plant Total RNA Isolation Kit (RC401-01, Vazyme Biotech, Nanjing, China). The RNA was treated with DNase I to eliminate any DNA contamination. Reverse transcription was performed on 2 μg of total RNA using the HiScript III All-in-One RT SuperMix Perfect kit for qPCR, R333-01 (Vazyme). The ChamQ Universal SYBR qPCR Master Mix (Q711-02, Vazyme) and ABI 7500 Real-Time PCR system were employed for real-time quantitative PCR of the samples. Samples were analyzed using biological triplicates, and the internal reference gene was GhUBQ7 and GhHIS3. The 2−ΔΔCt method was employed to assess the relative expression levels of the target genes [57]. The data were analyzed for significant differences using one-way analysis of variance (ANOVA) and Student’s t-tests. Supplementary Table S2 indicates the primer sequences.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13050643/s1, Table S1: Characterization of 43 CSP genes in four cotton species. Table S2: The primers used for vector construction and RT-qPCR; Table S3: Statistical analysis of Ka/Ks values of four cotton species.
Author Contributions
Conceptualization: G.S.; Methodology: Y.Q.; Formal analysis and investigation: Y.Y. and Y.P.; Writing—original draft preparation: Y.Y.; Writing—review and editing: G.S.; Validation: Y.W., J.X. and T.Z.; Funding acquisition: G.S.; Resources: G.S.; Supervision: Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Winall Hi-tech Seed Co., Ltd. (Grant No GMLM2023). This research was funded by the project of Xinjiang Uygur Autonomous Region Key Research and Development Task and Special Project (Grant No 2022B02052-2), and the National Natural Science Foundation of China (Grant No 31771851).
Data Availability Statement
For privacy reasons, data can be provided via private mail.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Karlson, D.; Nakaminami, K.; Toyomasu, T.; Imai, R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J. Biol. Chem. 2002, 277, 35248–35256. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Magwanga, R.O.; Guo, X.; Kirungu, J.N.; Lu, H.; Cai, X.; Zhou, Z.; Wei, Y.; Wang, X.; Zhang, Z.; et al. Genome-Wide Analysis of Multidrug and Toxic Compound Extrusion (MATE) Family in Gossypium raimondii and Gossypium arboreum and Its Expression Analysis Under Salt, Cadmium, and Drought Stress. G3 2018, 8, 2483–2500. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Diouf, L.; Pan, Z.; He, S.P.; Gong, W.F.; Jia, Y.H.; Magwanga, R.O.; Romy, K.R.E.; Or Rashid, H.; Kirungu, J.N.; Du, X. High-Density Linkage Map Construction and Mapping of Salt-Tolerant QTLs at Seedling Stage in Upland Cotton Using Genotyping by Sequencing (GBS). Int. J. Mol. Sci. 2017, 18, 2622. [Google Scholar] [CrossRef]
- Graham, A.D.; Patterson, B.D. Responses of Plants to Low, Nonfreezing Temperatures: Proteins, Metabolism, and Acclimation. Annu. Rev. Plant Physiol. 1982, 33, 347–372. [Google Scholar] [CrossRef]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of Plant Metabolism during Cold Acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Lauersen, K.J.; Brown, A.; Middleton, A.; Davies, P.L.; Walker, V.K. Expression and characterization of an antifreeze protein from the perennial rye grass, Lolium perenne. Cryobiology 2011, 62, 194–201. [Google Scholar] [CrossRef]
- Yu, T.F.; Xu, Z.S.; Guo, J.K.; Wang, Y.X.; Abernathy, B.; Fu, J.D.; Chen, X.; Zhou, Y.B.; Chen, M.; Ye, X.G.; et al. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Sci. Rep. 2017, 7, 44050. [Google Scholar] [CrossRef]
- Griffith, M.; Ala, P.; Yang, D.S.; Hon, W.C.; Moffatt, B.A. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef]
- Makino, Y.; Ohga, T.; Toh, S.; Koike, K.; Okumura, K.; Wada, M.; Kuwano, M.; Kohno, K. Structural and functional analysis of the human Y-box binding protein (YB-1) gene promoter. Nucleic Acids Res. 1996, 24, 1873–1878. [Google Scholar] [CrossRef]
- Yamanaka, K.; Fang, L.; Inouye, M. The CspA family in Escherichia coli: Multiple gene duplication for stress adaptation. Mol. Microbiol. 1998, 27, 247–255. [Google Scholar] [CrossRef]
- Karlson, D.; Imai, R. Conservation of the cold shock domain protein family in plants. Plant Physiol. 2003, 131, 12–15. [Google Scholar] [CrossRef]
- Sasaki, K.; Imai, R. Pleiotropic roles of cold shock domain proteins in plants. Front. Plant Sci. 2011, 2, 116. [Google Scholar] [CrossRef]
- Kohno, K.; Izumi, H.; Uchiumi, T.; Ashizuka, M.; Kuwano, M. The pleiotropic functions of the Y-box-binding protein, YB-1. BioEssays 2003, 25, 691–698. [Google Scholar] [CrossRef]
- Nakaminami, K.; Hill, K.; Perry, S.E.; Sentoku, N.; Long, J.A.; Karlson, D.T. Arabidopsis cold shock domain proteins: Relationships to floral and silique development. J. Exp. Bot. 2009, 60, 1047–1062. [Google Scholar] [CrossRef]
- Liebthal, M.; Maynard, D.; Dietz, K.J. Peroxiredoxins and Redox Signaling in Plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Tang, J.; Hou, X. Identification, evolution and functional inference on the cold-shock domain protein family in Pak-choi (Brassica rapa ssp. chinensis) and Chinese cabbage (Brassica rapa ssp. pekinensis). J. Plant Interact. 2019, 14, 232–241. [Google Scholar] [CrossRef]
- Radkova, M.; Vítámvás, P.; Sasaki, K.; Imai, R. Development- and cold-regulated accumulation of cold shock domain proteins in wheat. Plant Physiol. Biochem. 2014, 77, 44–48. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Wang, Y.; Ding, Z.; Qian, W. Cloning and expression analysis of cold shock domain protein genes (CsCSPs) in Camellia sinensis. Plant Physiol. J. 2022, 58, 1105–1118. [Google Scholar] [CrossRef]
- Chaikam, V.; Karlson, D. Functional characterization of two cold shock domain proteins from Oryza sativa. Plant Cell Environ. 2008, 31, 995–1006. [Google Scholar] [CrossRef]
- Kim, M.H.; Sato, S.; Sasaki, K.; Saburi, W.; Matsui, H.; Imai, R. COLD SHOCK DOMAIN PROTEIN 3 is involved in salt and drought stress tolerance in Arabidopsis. FEBS Open Bio 2013, 3, 438–442. [Google Scholar] [CrossRef]
- Kim, M.H.; Sasaki, K.; Imai, R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 23454–23460. [Google Scholar] [CrossRef]
- Juntawong, P.; Sorenson, R.; Bailey-Serres, J. Cold shock protein 1 chaperones mRNAs during translation in Arabidopsis thaliana. Plant J. 2013, 74, 1016–1028. [Google Scholar] [CrossRef]
- He, P.; Yang, Y.; Wang, Z.; Zhao, P.; Yuan, Y.; Zhang, L.; Ma, Y.; Pang, C.; Yu, J.; Xiao, G. Comprehensive analyses of ZFP gene family and characterization of expression profiles during plant hormone response in cotton. BMC Plant Biol. 2019, 19, 329. [Google Scholar] [CrossRef]
- Xinjian, L.; Qing, H.; Yujiang, Y.; Fenglan, T. Cold disasters: The most serious meteorological disasters to the cotton production in Xinjiang, China. Proc. SPIE 2003, 4890, 406–411. [Google Scholar]
- Shan, D.-P.; Huang, J.-G.; Yang, Y.-T.; Guo, Y.-H.; Wu, C.-A.; Yang, G.-D.; Gao, Z.; Zheng, C.-C. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007, 176, 70–81. [Google Scholar] [CrossRef]
- Jia, H.; Hao, L.; Guo, X.; Liu, S.; Yan, Y.; Guo, X. A Raf-like MAPKKK gene, GhRaf19, negatively regulates tolerance to drought and salt and positively regulates resistance to cold stress by modulating reactive oxygen species in cotton. Plant Sci. 2016, 252, 267–281. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, C.; Meng, Z.; Li, Y.; Abid, M.A.; Askari, M.; Wang, P.; Wang, Y.; Sun, G.; Cai, Y.; et al. Leveraging Atriplex hortensis choline monooxygenase to improve chilling tolerance in cotton. Environ. Exp. Bot. 2019, 162, 364–373. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Xing, H.; Xin, M.; Saeed, M.; Wu, Q.; Wu, J.; Zhuang, T.; Zhang, X.; Mao, L.; et al. Genome-wide identification and expression analysis of the HVA22 gene family in cotton and functional analysis of GhHVA22E1D in drought and salt tolerance. Front. Plant Sci. 2023, 14, 1139526. [Google Scholar] [CrossRef]
- Sasaki, K.; Kim, M.H.; Imai, R. Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem. Biophys. Res. Commun. 2007, 364, 633–638. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, X.; Chen, X.; Zhao, L.; Han, M.; Wang, S.; Zhang, Y.; Fan, Y.; Ye, W. Genome-wide identification and function analysis of HMAD gene family in cotton (Gossypium spp.). BMC Plant Biol. 2021, 21, 386. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Chen, W.; Yao, J.; Li, Y.; Fang, S.; Lv, Y.; Li, X.; Pan, J.; Liu, C.; et al. Cotton DMP gene family: Characterization, evolution, and expression profiles during development and stress. Int. J. Biol. Macromol. 2021, 183, 1257–1269. [Google Scholar] [CrossRef]
- Sasaki, K.; Kim, M.H.; Imai, R. Arabidopsis COLD SHOCK DOMAIN PROTEIN 2 is a negative regulator of cold acclimation. New Phytol. 2013, 198, 95–102. [Google Scholar] [CrossRef]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Shim, K.-C.; Kim, S.; Le, A.Q.; Lee, H.-S.; Adeva, C.; Jeon, Y.-A.; Luong, N.; Kim, W.-J.; Akhtamov, M.; Ahn, S.-N. Fine Mapping of a Low-Temperature Germinability QTL qLTG1 Using Introgression Lines Derived from Oryza rufipogon. Plant Breed. Biotechnol. 2019, 7, 141–150. [Google Scholar] [CrossRef]
- Nakaminami, K.; Sasaki, K.; Kajita, S.; Takeda, H.; Karlson, D.; Ohgi, K.; Imai, R. Heat stable ssDNA/RNA-binding activity of a wheat cold shock domain protein. FEBS Lett. 2005, 579, 4887–4891. [Google Scholar] [CrossRef]
- Bhadauriya, P.; Yadav, B.S.; Singh, S.; Mani, A. Binding preferential of chickpea cold shock protein during nucleic acid interactions. J. Plant Biochem. Biotechnol. 2017, 26, 208–215. [Google Scholar] [CrossRef]
- Zafar, M.M.; Rehman, A.; Razzaq, A.; Parvaiz, A.; Mustafa, G.; Sharif, F.; Mo, H.; Youlu, Y.; Shakeel, A.; Ren, M. Genome-wide characterization and expression analysis of Erf gene family in cotton. BMC Plant Biol. 2022, 22, 134. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, P.; Tyagi, A.K.; Khurana, J.P. Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct. Integr. Genom. 2008, 8, 69–78. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.-W.; Zhou, J.-M.; Zhao, S.-P.; Zhang, X.-H.; Min, D.-H. Genome-wide analysis of the lectin receptor-like kinase family in foxtail millet (Setaria italica L.). Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 335–346. [Google Scholar] [CrossRef]
- Jan, R.; Aaqil Khan, M.; Asaf, S.; Lubna; Park, J.R.; Lee, I.J.; Kim, K.M. Flavonone 3-hydroxylase Relieves Bacterial Leaf Blight Stress in Rice via Overaccumulation of Antioxidant Flavonoids and Induction of Defense Genes and Hormones. Int. J. Mol. Sci. 2021, 22, 6152. [Google Scholar] [CrossRef]
- Wang, L.; Deng, R.; Bai, Y.; Wu, H.; Li, C.; Wu, Q.; Zhao, H. Tartary Buckwheat R2R3-MYB Gene FtMYB3 Negatively Regulates Anthocyanin and Proanthocyanin Biosynthesis. Int. J. Mol. Sci. 2022, 23, 2775. [Google Scholar] [CrossRef]
- Xia, W.; Zong, J.; Zheng, K.; Wang, Y.; Zhang, D.; Guo, S.; Sun, G. DgCspC gene overexpression improves cotton yield and tolerance to drought and salt stress comparison with wild-type plants. Front. Plant Sci. 2022, 13, 985900. [Google Scholar] [CrossRef]
- Sun, W.; Cao, G.; Yang, G.; Shao, N. Advances in RNA Chaperone. J. Med. Mol. Biol. 2004, 6, 23–26. [Google Scholar] [CrossRef]
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2014, 43, D222–D226. [Google Scholar] [CrossRef]
- Sharma, S.; Ciufo, S.; Starchenko, E.; Darji, D.; Chlumsky, L.; Karsch-Mizrachi, I.; Schoch, C.L. The NCBI BioCollections Database. Database 2019, 2019, baz057. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).