Identification of Hypoglycemic Glycolipids from Ipomoea murucoides by Affinity-Directed Fractionation, In Vitro, In Silico and Dynamic Light Scattering Analysis
Abstract
1. Introduction
2. Results
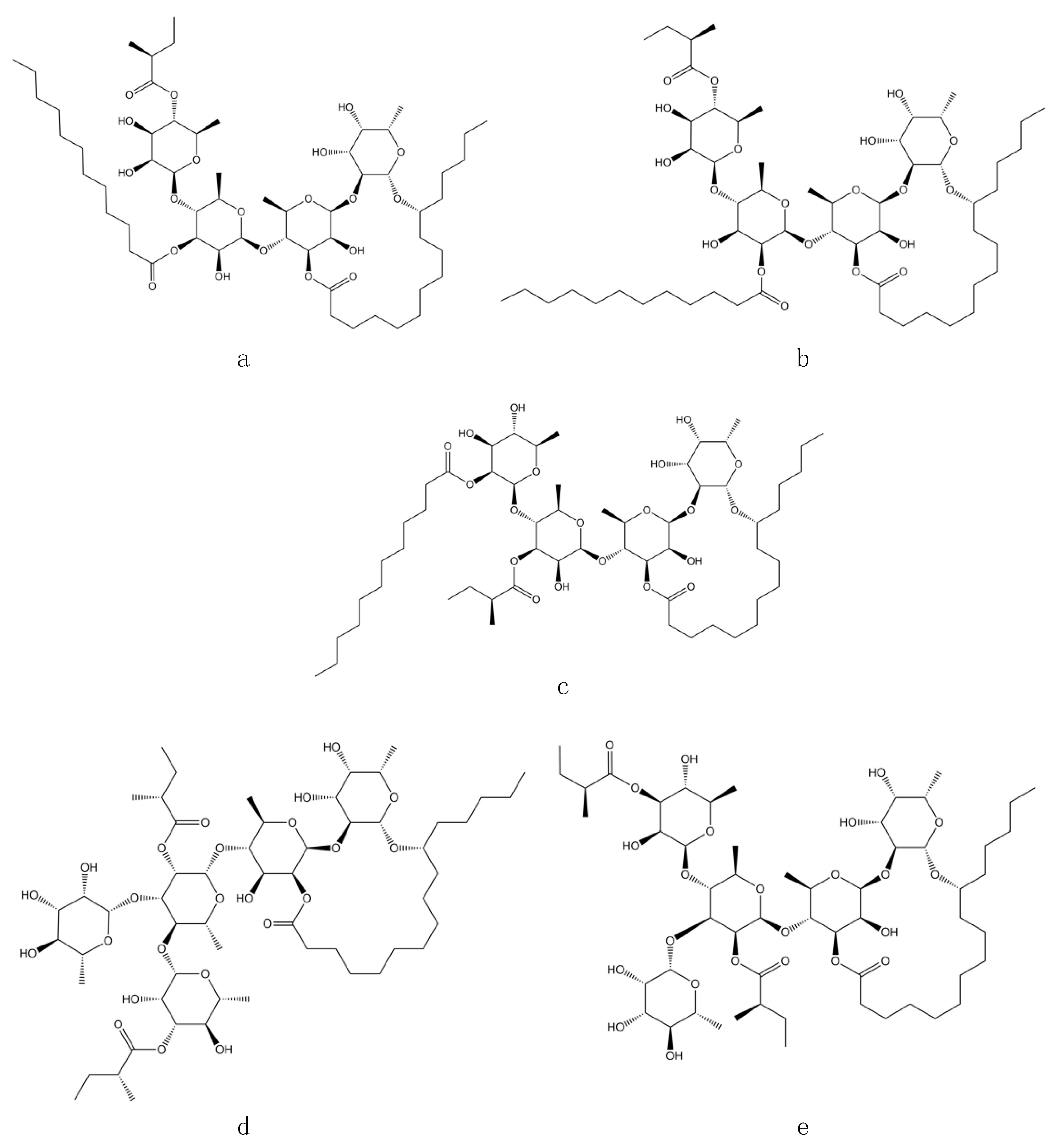
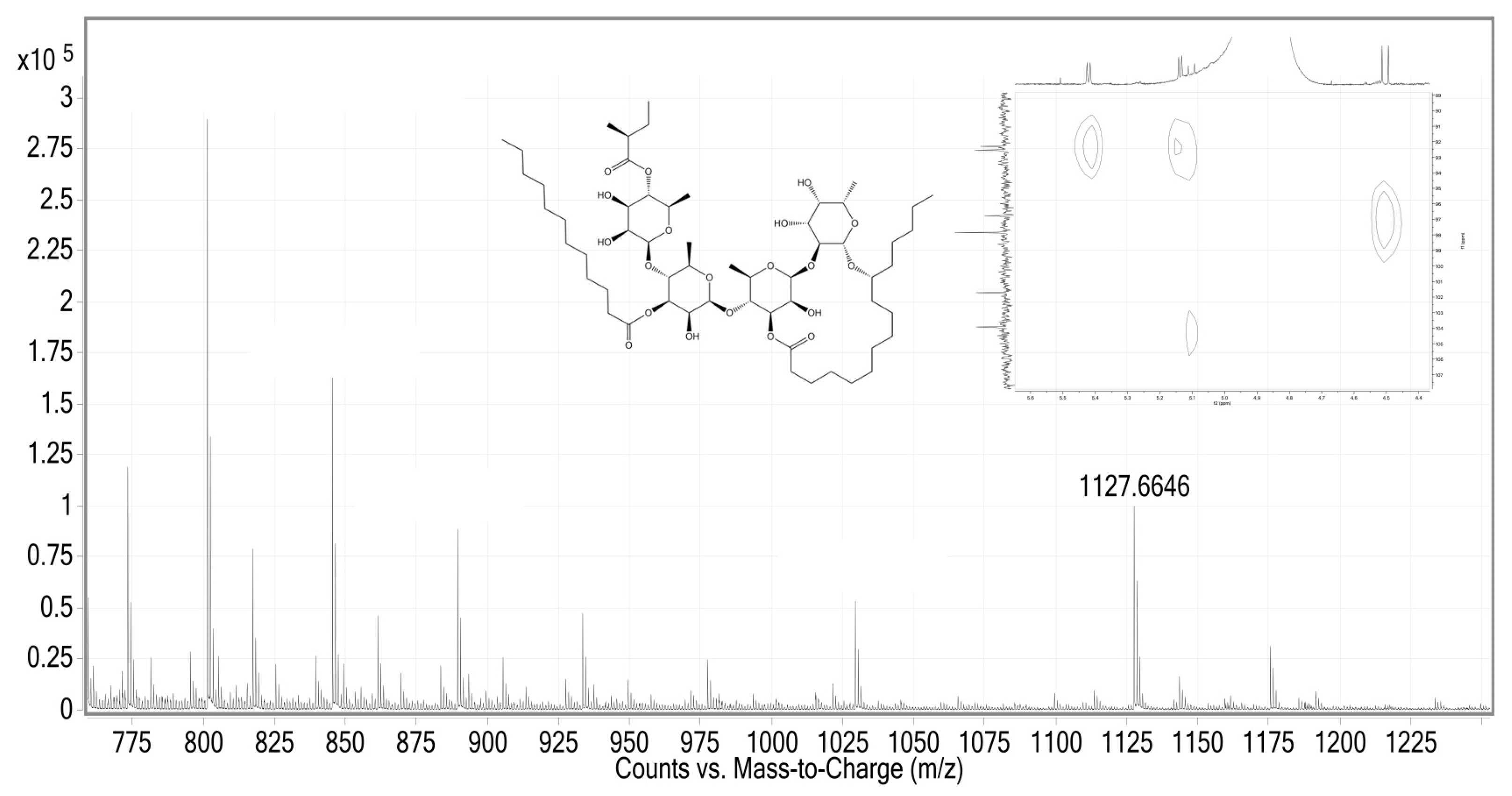
2.1. LRESI-MS Study of the Methanolic Extract from the Flowers of Cazahuate
2.2. Affinity-Directed Fractionation with α-Glucosidases and the Methanolic Extract from Flowers of Cazahuate
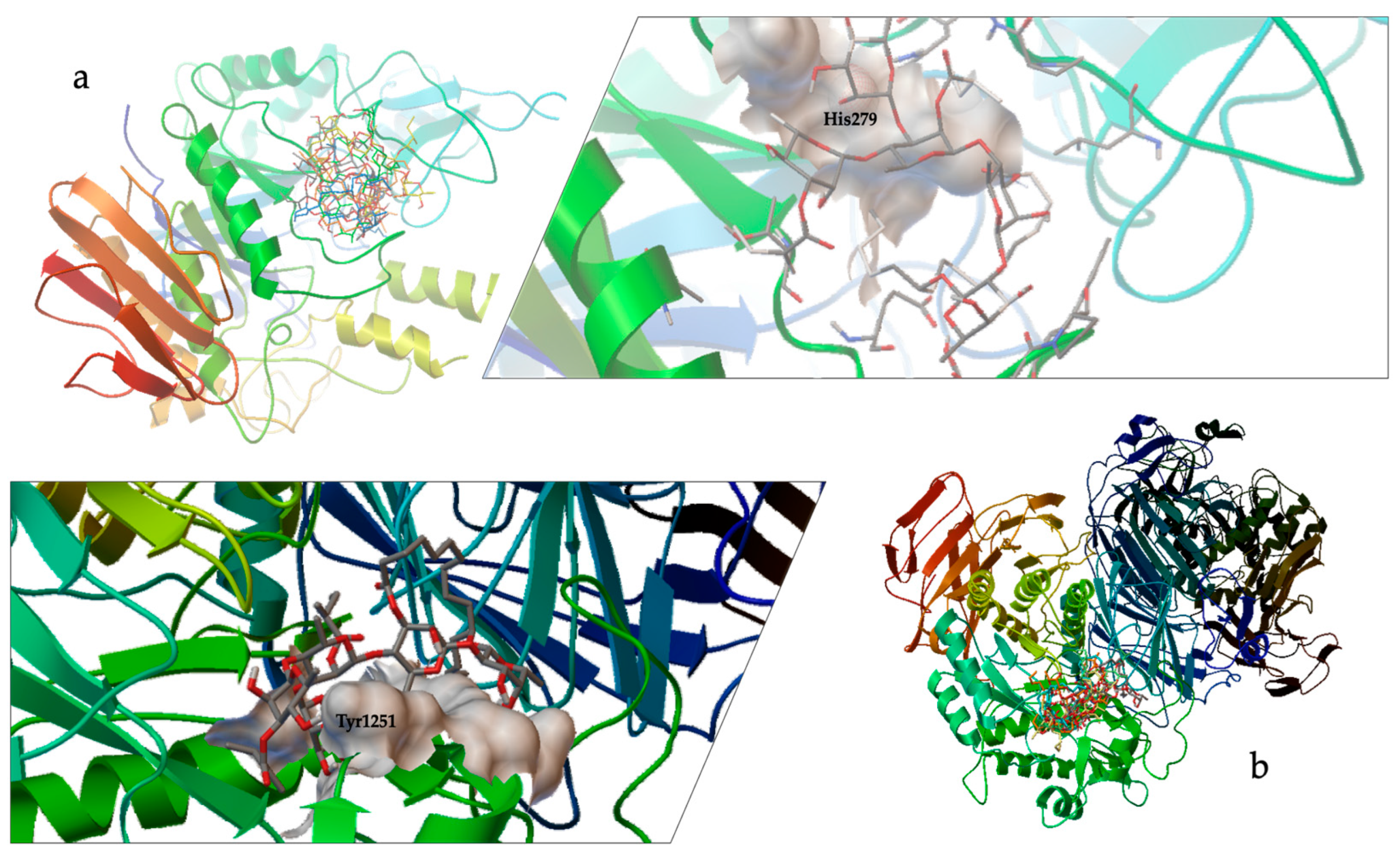
2.3. Molecular Docking
2.4. Affinity-Directed Fractionation with Glucose-6-Phosphatase (G6Pase) and the Methanolic Extract from Cazahuate Flowers
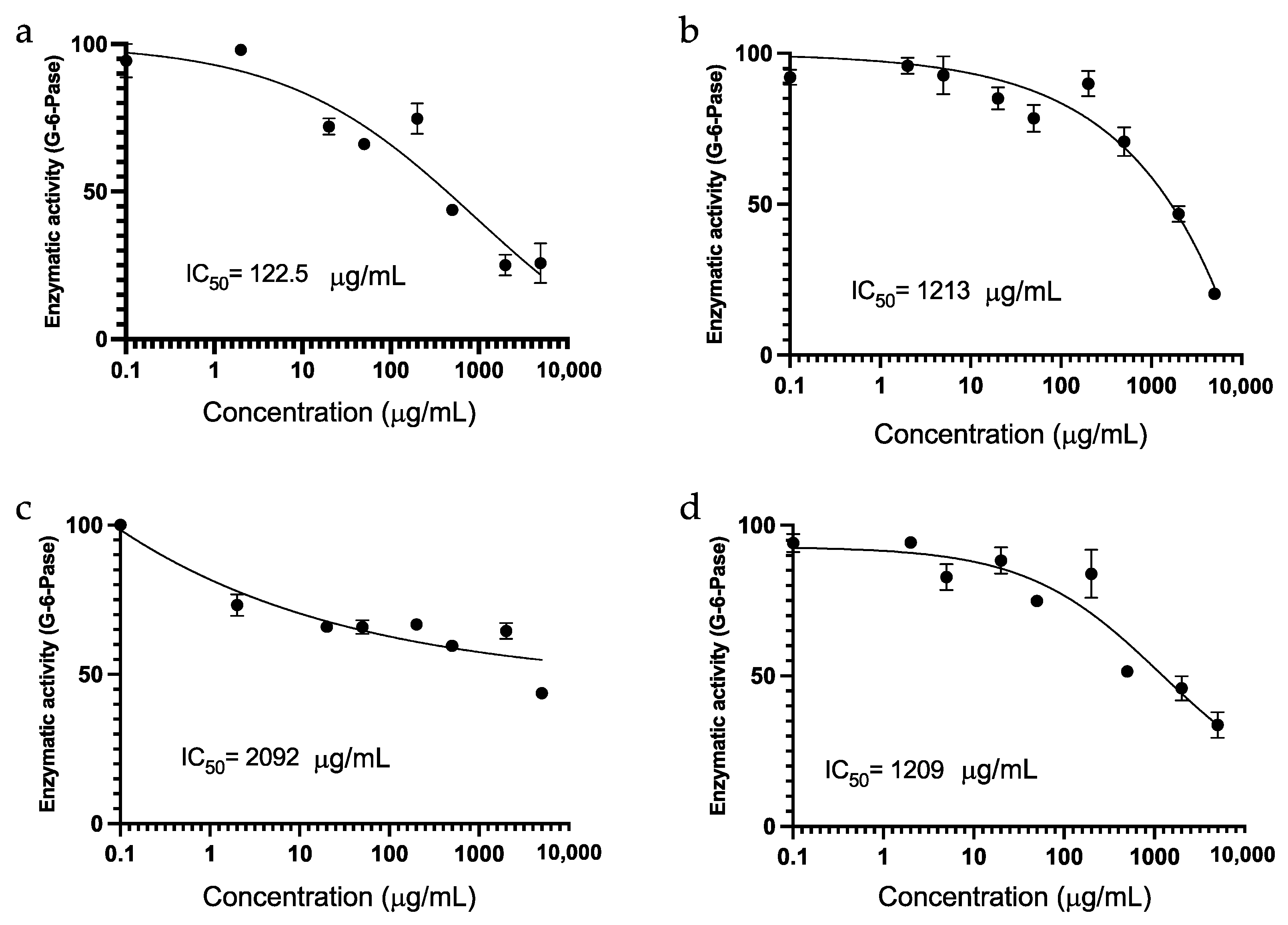
2.5. In Vitro Hepatic Glucose-6-Phosphatase (G6Pase) Activity
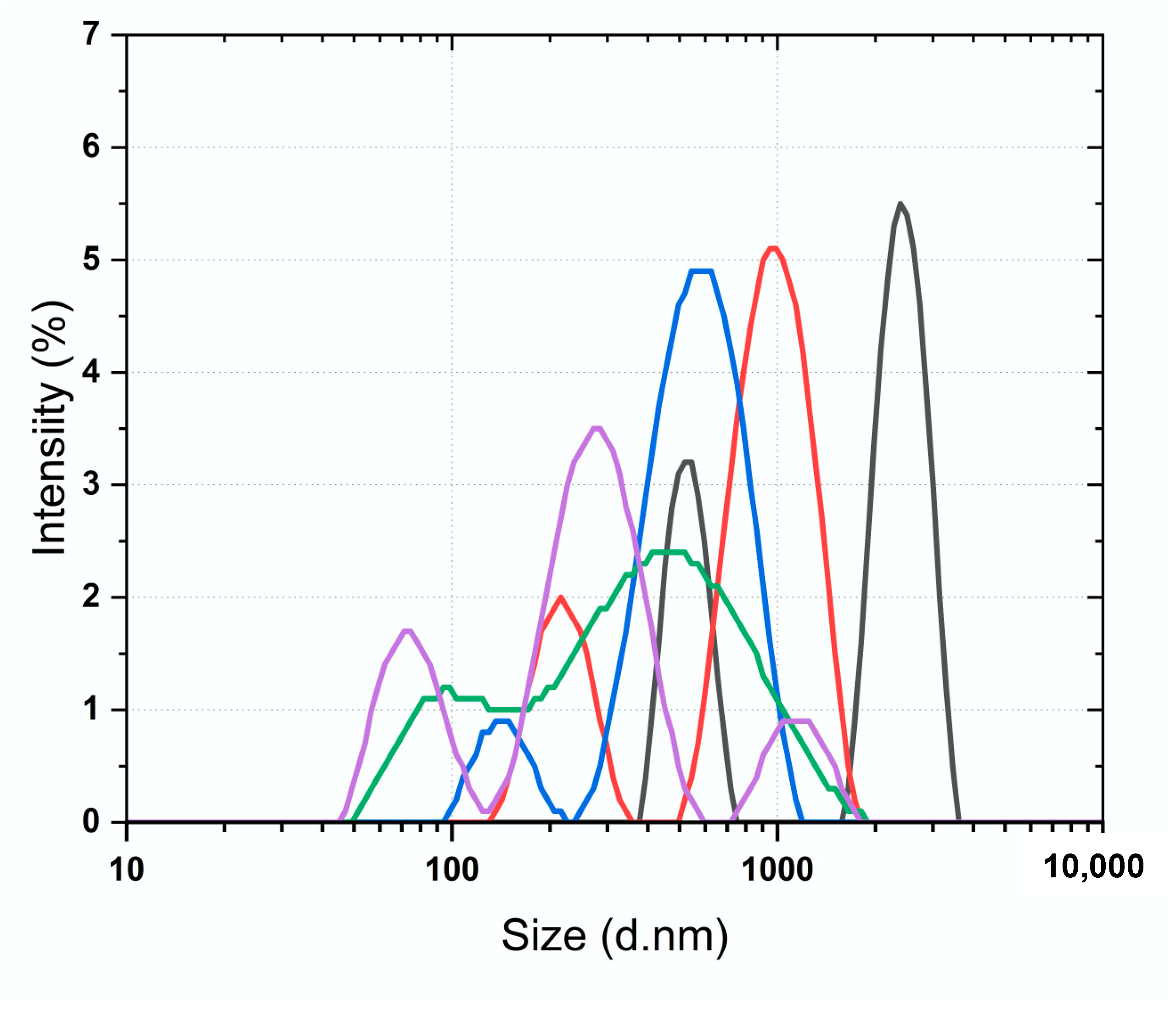
2.6. Dynamic Light Scattering (DLS) with Yeast α-D-Glucosidase and the SEC Protocol
3. Discussion
3.1. Affinity-Directed Fractionation with α-Glucosidases and the Methanolic Extract from Flowers of Cazahuate
3.2. Molecular Docking
3.3. Affinity-Directed Fractionation with Glucose-6-Phosphatase (G6Pase) and the Methanolic Extract from Cazahuate Flowers
3.4. In Vitro Hepatic Glucose-6-Phosphatase (G6Pase) Activity
3.5. Dynamic Light Scattering (DLS) with Yeast α-D-Glucosidase and the SEC Protocol
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Plant Material and Extracts
4.3. Affinity-Directed Fractionation
4.4. Glucose-6-Phosphatase Assay
4.5. Molecular Docking
4.6. Dynamic Light Scattering
4.7. Chromogenic Inhibition Assay of α-D-Glucosidase (EC 3.2.1.20) from S. cerevisiae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereda-Miranda, R.; Rosas-Ramírez, D.; Castañeda-Gómez, J. Resin Glycosides from the Morning Glory Family. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 92, pp. 77–153. [Google Scholar] [CrossRef]
- Chérigo, L.; Pereda-Miranda, R. Resin Glycosides from the Flowers of Ipomoea murucoides. J. Nat. Prod. 2006, 69, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Chérigo, L.; Pereda-Miranda, R.; Fragoso-Serrano, M.; Jacobo-Herrera, N.; Kaatz, G.W.; Gibbons, S. Inhibitors of Bacterial Multidrug Efflux Pumps from the Resin Glycosides of Ipomoea murucoides. J. Nat. Prod. 2008, 71, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Chérigo, L.; Pereda-Miranda, R.; Gibbons, S. Bacterial Resistance Modifying Tetrasaccharide Agents from Ipomoea murucoides. Phytochemistry 2009, 70, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Corona-Castañeda, B.; Chérigo, L.; Fragoso-Serrano, M.; Gibbons, S.; Pereda-Miranda, R. Modulators of antibiotic activity from Ipomoea murucoides. Phytochemistry 2013, 95, 277–283. [Google Scholar] [CrossRef] [PubMed]
- León, I.; Enríquez, R.G.; Nieto, D.A.; Alonso, D.; Reynolds, W.F.; Aranda, E.; Villa, J. Pentasaccharide Glycosides from the Roots of Ipomoea murucoides. J. Nat. Prod. 2005, 68, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- León, I.; Vera, L.G.; del Rio-Portilla, F.; Aranda, E.; Hernández-Velazquez, V.M.; Guevara Fefer, P.; Montiel, E.; Castillo, P.; Salinas Sanchez, D.O. Resin Glycosides from Ipomoea murucoides and their effects on growth of Spodoptera frugiperda. J. Entomolju. 2013, 10, 24–34. [Google Scholar] [CrossRef][Green Version]
- Corona-Castanñeda, B.; Pereda-Miranda, R. Morning glory resin glycosides as modulators of antibiotic activity in multidrug-resistant Gram-negative bacteria. Planta. Med. 2012, 78, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Liu, X.; Wang, M.; Xue, T.; Sun, B. Regulatory mechanism of the three-component system HptRSA in glucose-6-phosphate uptake in Staphylococcus aureus. Med. Microbiol. Immunol. 2016, 205, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Xu, X.; Gao, Z.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.T.; Sun, H. Silver’s multi-target mode of action against Staphylococcus aureus endows it with the capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ramírez, D.; Pereda-Miranda, R.; Escandón-Rivera, S.; Arreguín-Espinosa, R. Identification of α-Glucosidase Inhibitors from Ipomoea Alba by Affinity-Directed Fractionation-Mass Spectrometry. Rev. Bras. Farmacogn. 2020, 30, 336–345. [Google Scholar] [CrossRef]
- Lorber, B.; Fischer, F.; Bailly, M.; Roy, H.; Kern, D. Protein Analysis by Dynamic Light Scattering: Methods and Techniques for students. Biochem. Mol. Biol. Educ. 2012, 40, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mirasol, F. Stability Testing of Protein Therapeutics Using DLS. BioPharm. Int. 2021, 34, 41–43. [Google Scholar]
- Escobedo-Martínez, C.; Pereda-Miranda, R. Resin Glycosides from Ipomoea pes-caprae. J. Nat. Prod. 2007, 70, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ramírez, D.; Escandón-Rivera, S.; Andrade-Cetto, A.; Arreguín-Espinosa, R. Glucose-6-Phosphatase and α-Glucosidase Inhibitors from Smilax moranensis Roots Identified by Affinity-Directed Fractionation. Rev. Bras. Farmacogn. 2020, 30, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Pereda-Miranda, R.; Bautista, E.; Martínez-Fructuoso, L.; Fragoso-Serrano, M. From relative to absolute stereochemistry of secondary metabolites: Applications in plant chemistry. Rev. Bras. Farmacogn. 2023, 33, 1–48. [Google Scholar] [CrossRef]
- Espinoza-Hernández, F.; Andrade-Cetto, A.; Escandón-Rivera, S.; Mata-Torres, G.; Mata, R. Contribution of fasting and postprandial glucose-lowering mechanisms to the acute hypoglycemic effect of traditionally used Eryngium cymosum F. Delaroche. J. Ethnopharmacol. 2021, 279, 114339. [Google Scholar] [CrossRef] [PubMed]
- Arion, W.J. Measurement of intactness of rat liver endoplasmic reticulum. Methods Enzymol. 1989, 174, 58–67. [Google Scholar] [CrossRef] [PubMed]
| Compound | Formula /MW | MAL12 | MGAM | ||
|---|---|---|---|---|---|
| Theoretical Ki | Hydrogen Bond | Theoretical Ki | Hydrogen Bond | ||
| Murucoidin XIV | C57H100O20/1104 | 2.86 mM | ASN241 HIS279 GLN322 | 12.1 mM | GLY1588 |
| Pescaprein V | C57H100O20/1104 | 1.23 mM | HIS279 | 346 mM | GLN1254 |
| Pescaprein VI | C57H100O20/1104 | 304 mM | HIS279 | 118 mM | GLN1254 TRP1369 GLN1372 ARG1377 |
| Murucoidin III | C56H96O24/1152 | 2.4 mM | HIS279 | 677 mM | ARG1377 THR1586 |
| Stoloniferin I | C56H96O24/1152 | 244 mM | HIS279 | 4.28 mM | SER1366 ASP1370 GLN1372 ARG1377 |
| Acarbose | C25H43NO18/645 | 51.4 μM | HIS279 GLN322 GLU304 ARG312 | 35.7 μM | TYR1251 GLN1372 ARG1377 GLN1561 GLY1588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Ramírez, D.; Arreguín-Espinosa, R.; Escandón-Rivera, S.; Andrade-Cetto, A.; Mata-Torres, G.; Pérez-Solís, R. Identification of Hypoglycemic Glycolipids from Ipomoea murucoides by Affinity-Directed Fractionation, In Vitro, In Silico and Dynamic Light Scattering Analysis. Plants 2024, 13, 644. https://doi.org/10.3390/plants13050644
Rosas-Ramírez D, Arreguín-Espinosa R, Escandón-Rivera S, Andrade-Cetto A, Mata-Torres G, Pérez-Solís R. Identification of Hypoglycemic Glycolipids from Ipomoea murucoides by Affinity-Directed Fractionation, In Vitro, In Silico and Dynamic Light Scattering Analysis. Plants. 2024; 13(5):644. https://doi.org/10.3390/plants13050644
Chicago/Turabian StyleRosas-Ramírez, Daniel, Roberto Arreguín-Espinosa, Sonia Escandón-Rivera, Adolfo Andrade-Cetto, Gerardo Mata-Torres, and Ricardo Pérez-Solís. 2024. "Identification of Hypoglycemic Glycolipids from Ipomoea murucoides by Affinity-Directed Fractionation, In Vitro, In Silico and Dynamic Light Scattering Analysis" Plants 13, no. 5: 644. https://doi.org/10.3390/plants13050644
APA StyleRosas-Ramírez, D., Arreguín-Espinosa, R., Escandón-Rivera, S., Andrade-Cetto, A., Mata-Torres, G., & Pérez-Solís, R. (2024). Identification of Hypoglycemic Glycolipids from Ipomoea murucoides by Affinity-Directed Fractionation, In Vitro, In Silico and Dynamic Light Scattering Analysis. Plants, 13(5), 644. https://doi.org/10.3390/plants13050644










