Abstract
Trichoderma spp. are widely reported to regulate plant growth by improving nutrient uptake, photosynthesis, and abiotic stress tolerance. However, their possible application for bedding plants is little explored, especially when comparing different growing media. Considering that coconut coir dust is finding broader application in the ornamental plants sector as a peat substitute, this work was aimed to test the combination of Trichoderma atroviride AT10 and coconut coir dust on Impatiens walleriana plants. Four treatments were tested as a mix of: (i) two growing media (70:30), peat:perlite or coconut coir dust:perlite; and (ii) the absence or presence of a T. atroviride treatment. At the end of the production cycle, the biomass and ornamental parameters, leaf pigments, nutrient content of the plant tissues, and Trichoderma abundance were assessed. The results revealed that T. atroviride can readily colonize coir, and the same positive effects of inoculum were found in plants grown on both substrates. The biostimulant effect of T. atroviride was observed as an increase in the aboveground biomass, number and weight of flowers, pigments and nutrient concentration, thereby improving the commercial quality of I. walleriana. Thus, T. atroviride has shown its potential in making bedding plant cultivation more sustainable and improving the yield and aesthetic parameters of plants grown on peat and coconut coir dust substrates.
1. Introduction
Bedding and garden plants represent an important market share of the floriculture industry as they are considered space-saving products, especially in some countries, such as the United States [1,2], where they accounted for 47% of the wholesale in 2018, considering the overall market of floricultural products [3]. Looking at the European market, the wholesale was worth 2.2 billion in 2019, with the Netherlands, France, Italy, Germany, Spain, and Poland achieving a 27% quota of production destined for the Extra-EU export market [4]. Bedding plants are a typical soilless culture production that faces environmental and economic challenges, such as the use of more sustainable crop protection strategies and raw materials, including growing media. Indeed, soilless cultures are high-consuming systems that are increasingly asked to reduce inputs, including water, energy, agrochemicals, and the production of waste materials, such as plastics and spent growing media, although ensuring high product quality standards [5,6]. In this regard, molecules and microorganisms with a biostimulant function may play a crucial role in enhancing quality, together with reducing the use of phytochemicals, even if the spread of organic substrates based on other materials than peat requires improving the knowledge of possible interactions.
Coir has become one of the most commonly used peat alternative growing media constituents worldwide, at least in the last two decades [7,8,9], despite some emerging criticisms about its sustainability due to the environmental impact related to composting processes and shipping, as well as the social issues such as community infrastructure problems and human and labor rights respect [7,10]. It is a renewable material with optimal well-known physical–chemical properties [11,12], which allow the successful production of many soilless crops, such as bedding plants, including Impatiens spp., used both as a stand-alone substrate or in mixtures, starting from germination and seedling growth [13,14,15]. Coir, as organic matter, has higher microbiological activity compared to peat [16,17], and it has a recognized natural content of plant growth-promoting bacteria (PGPB), such as phosphate-solubilizing bacteria and indole-3-acetic acid-producing bacteria [18,19].
With the implementation of sustainable agronomic techniques, beneficial soil microorganisms are increasingly adopted to enhance the plant growth rate and quality [20,21]. Trichoderma spp. are ubiquitous free-living fungi (Hypocreaceae family), occurring widely in all soil types and representing major components of their mycoflora [22]. Trichoderma fungi can live in soil stress conditions such as salinity, alkalinity, nutrient deficiency, and drought [23]. Many Trichoderma species have been extensively studied in open fields and greenhouse cultivation due to their well-known biological control mechanism. Moreover, they are recognized to have a powerful capacity to improve plant growth, physiological traits, nutrient uptake, and yield [24,25,26,27]. Particularly, T. atroviride has been shown to increase the biomass of lettuce, zucchini, eggplants, tomato, and radiata pine seedlings [25,28,29,30,31], as well as to promote the root development of Arabidopsis seedlings [32,33]. The ability to stimulate plant growth has been positively correlated with the production of metabolites with hormone activities like indole-3-acetic acid or auxin analogues or the solubilization of nutrients like iron through siderophore secretion in the rhizosphere [34,35]. Consequently, the use of Trichoderma spp. as an active ingredient in biofertilizers, biopesticides, bioremediates, and natural resistance stimulants is becoming quite common. Despite this, to the best of our knowledge, there is no scientific information on the use of Trichoderma in bedding plant cultivation and little information on its application on ornamental plants in general, particularly when testing different growing media.
This work was aimed to evaluate the effect of T. atroviride addition on a typical bedding plant grown on a coir-based substrate as an alternative to peat growing media. Among the wide range of available herbaceous bedding plants, Impatiens walleriana has been chosen because it is much appreciated for its wide range of flower colors and long flower display, as well as for its attractive leaves.
2. Results
2.1. Biomass Measures
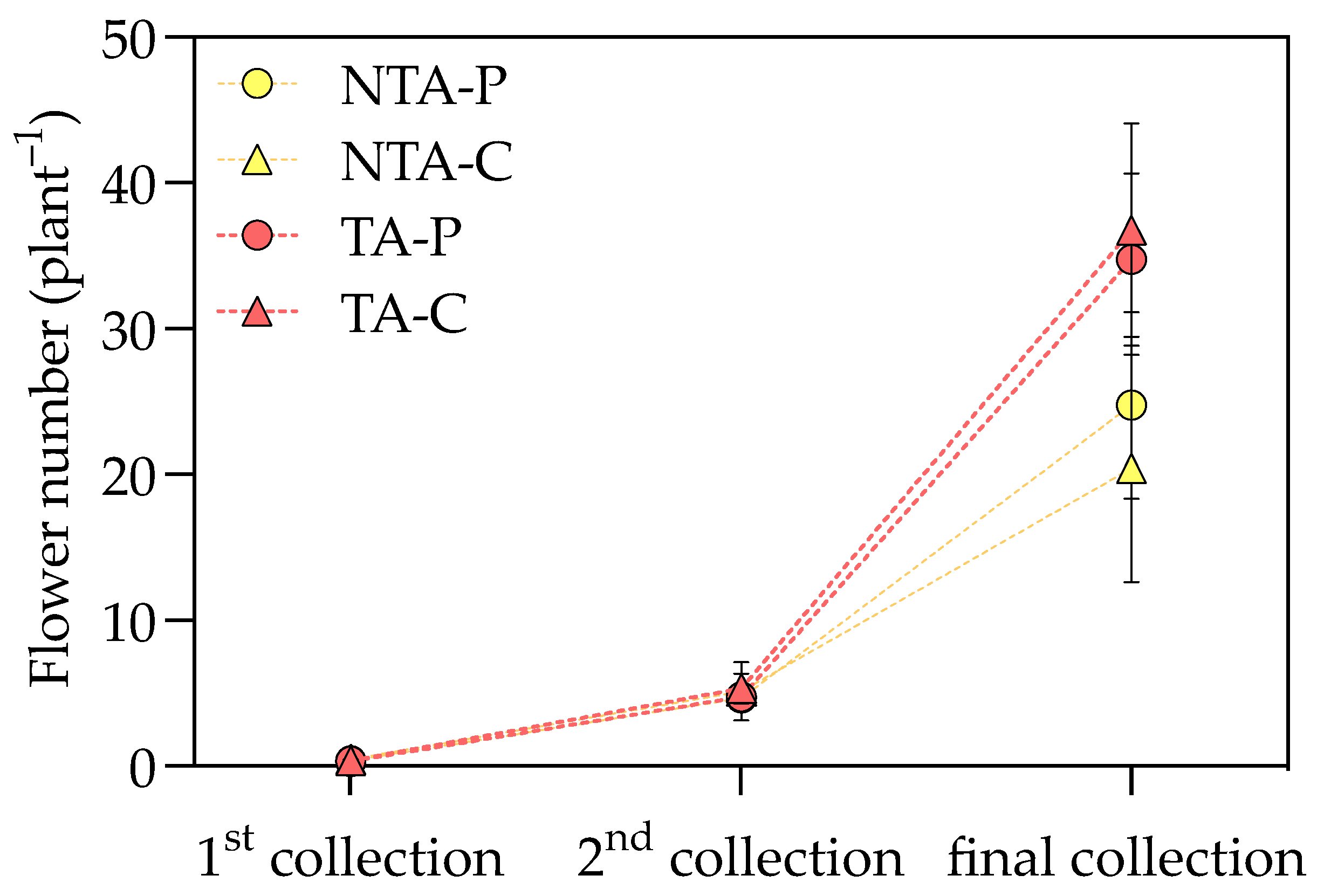
The application of T. atroviride statistically increased the aboveground biomass of I. walleriana plants, while the substrate type did not affect these parameters (Table 1). The plant shoot fresh (FW) and dry weight (DW) were positively influenced by T. atroviride treatment in both substrates (+26 g FW plant−1 and +0.8 g DW plant−1 in treated plants, a 24 and 16% increment, respectively, considering the average values on peat- and coir-based substrates). A positive effect was also found in flowers showing a higher FW and number, which resulted in 34 and 47% increases, respectively, considering the average values on peat- and coir-based substrates. Flower DW was unaffected by fungal treatment and substrate (Supplementary Table S1). By measuring the number of flowers produced during the growing cycle, the T. atroviride presence resulted in significant differences at the end of the trial but not at the first and second flower harvests (Figure 1). The positive effect on flowering is also displayed in Figure 2. The same trend was also observed in the leaf area, for which the treated plants had a 23% increase, considering the average values on peat- and coir-based substrates (Table 1). The specific leaf area (SLA) was not influenced by the treatment and substrate type (Supplementary Table S1).

Table 1.
Biometric parameters of I. walleriana plants at the end of the experiment.
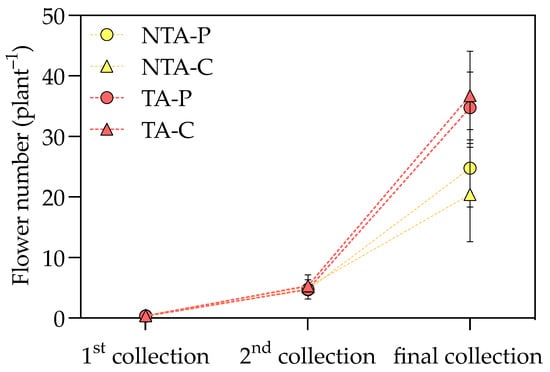
Figure 1.
The flower number measured at three time points during the experiment. Values represent the means (n = 4) ± SDs as Y-bars. NTA = no T. atroviride treatment, TA = T. atroviride treatment, P = peat, C = coir. Two-way ANOVA results = Treatment: p = ns at the first and second sampling points, p < 0.01 at the final sampling point. Substrate: p = ns at all the sampling points. Treatment × Substrate: p = ns at all the sampling points.

Figure 2.
Impatiens walleriana bedding plants at the end of the experiment, 56 days after transplant.
The substrate type influenced the root biomass, while the T. atroviride treatment did not improve this parameter (Table 1). Specifically, the I. walleriana plants had a statistically lower root biomass in coir than in peat (−15% considering the average values of not treated and treated plants).
2.2. Mineral Elements
T. atroviride treatment in the shoots influenced almost all the mineral elements, especially in the coir substrate (Table 2). The shoot total Kjeldahl nitrogen (TKN) was influenced by the interaction between the treatment and substrate, and the highest value was found in the not treated plants grown on the coir-based substrate. Even the shoot Ca was influenced by the interaction between the treatment and substrate, and specifically, it was higher in the treated plants than in the not treated ones in the coir-based substrate. Differently, P-PO4 and K increased with T. atroviride (+7 and 9%, considering the average values on peat- and coir-based substrates). In contrast, Mg was only influenced by the substrate type and was lower in coir than in peat (−9%, considering the average values of the not treated and treated plants). A lower number of statistically significant variations in the mineral elements were found in the roots (Supplementary Table S2). Specifically, K was higher in the treated plants (+18%, considering the average values on peat and coir), while Mg was higher in coir than in peat (+11%, considering the average values of the not treated and treated plants).

Table 2.
Nutrient concentrations (g kg−1 DW) measured in I. walleriana shoots at the end of the experiment.
2.3. Leaf Pigments and Trichoderma Analysis
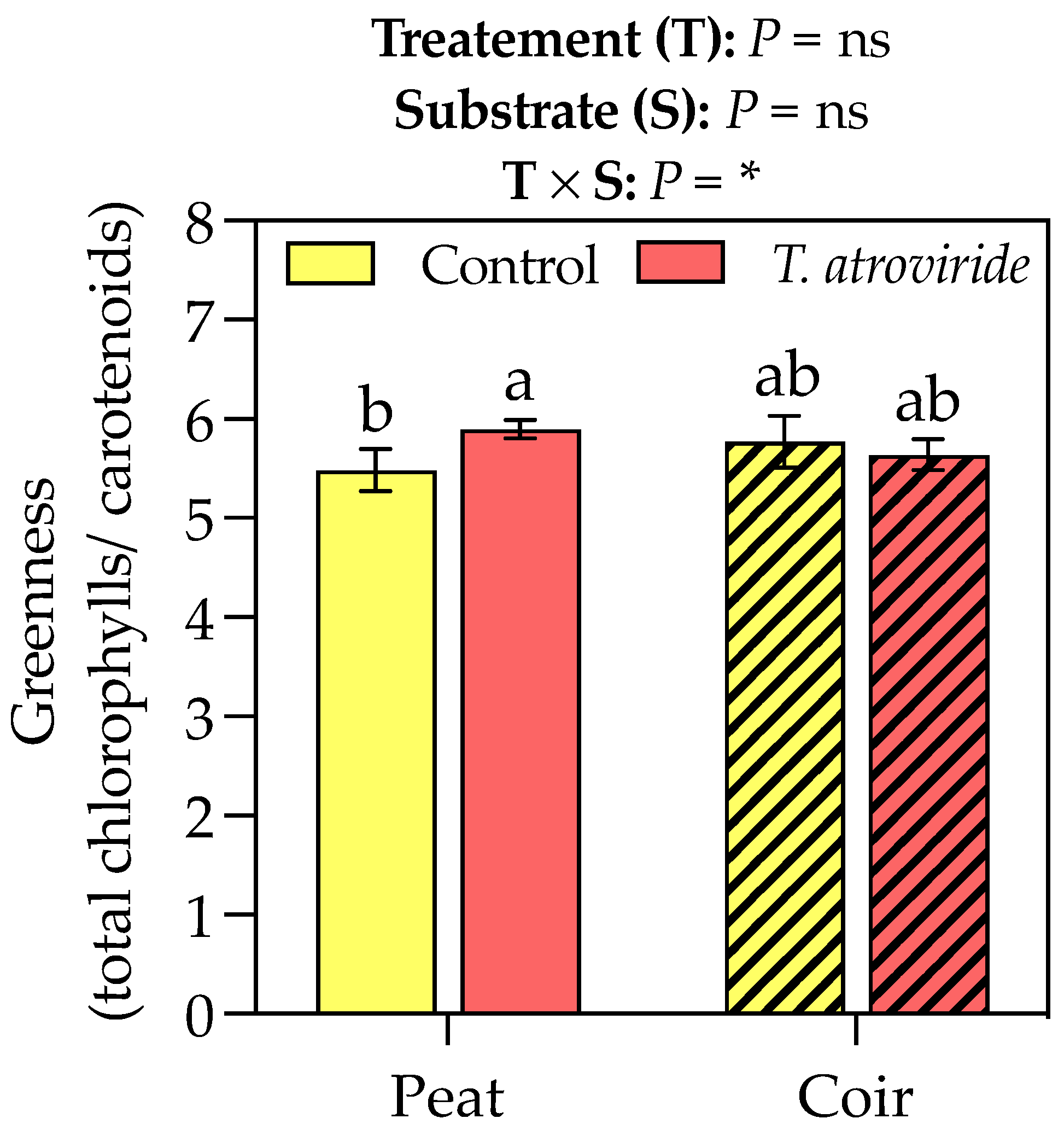
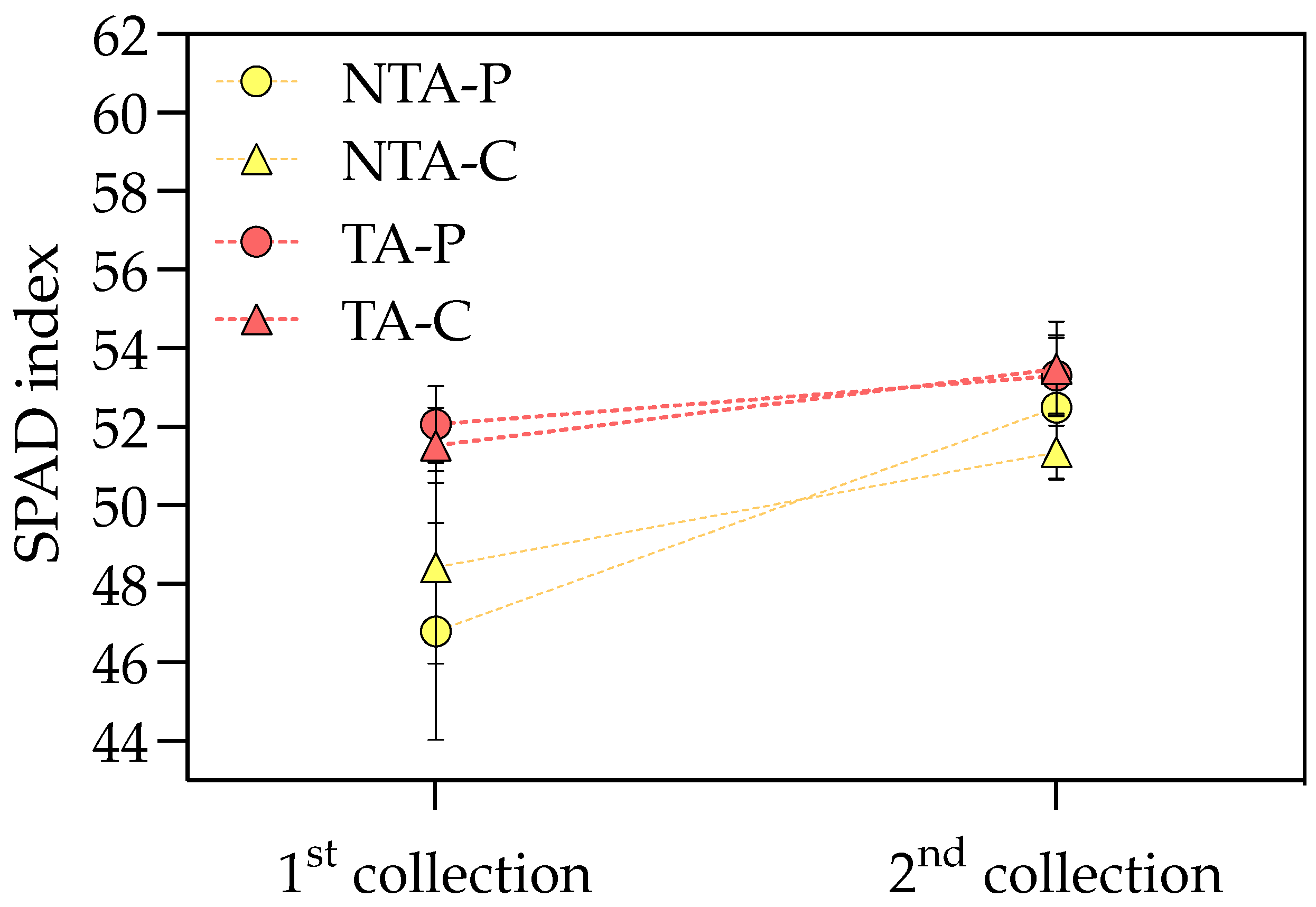
T. atroviride treatment promoted the chlorophyll a and carotenoid accumulation in the leaves, with values of +9 and 6% compared to the control, respectively, considering the average values on peat- and coir-based substrates (Table 3). Neither the treatment nor substrate showed any effect on chlorophyll b. The interaction between the treatment and substrate influenced the greenness index, which was higher for the plants grown on peat and treated with T. atroviride (Figure 3). On the coir-based substrate, the treatment with T. atroviride did not reveal any difference in the greenness index. The SPAD measures were also higher in the plants treated with T. atroviride at the intermediate sampling points (Figure 4).

Table 3.
Pigment concentrations (mg g−1 FW) measured in I. walleriana leaves at the end of the experiment.
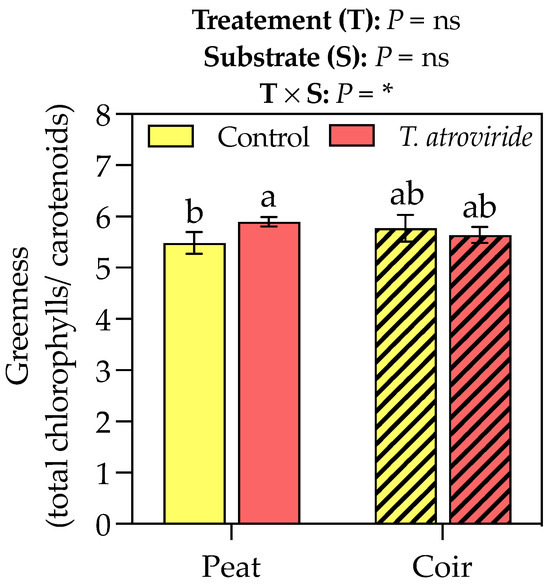
Figure 3.
Greenness index (chlorophyll a + b to total carotenoids ratio) at the end of the experiment. Bars represent the means (n = 4) ± SDs. Two-way ANOVA p-values and letters corresponding to Tukey’s post hoc results are reported in the figure (* p < 0.05; ns = not significant).
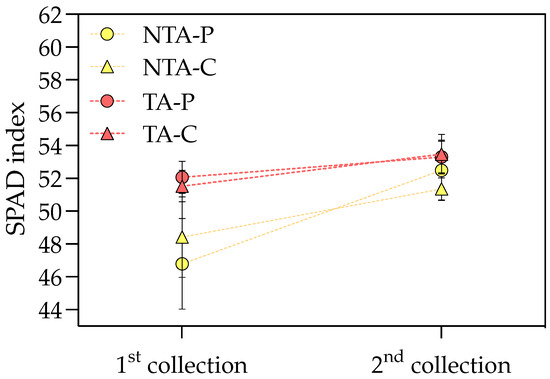
Figure 4.
SPAD index at the two intermediate sampling points during the experiment. Values represent the means (n = 4) ± SDs as Y-bars. NTA = no T. atroviride treatment, TA = T. atroviride treatment, P = peat, C = coir. Two-way ANOVA results = Treatment: p < 0.01 at the first sampling point, p < 0.05 at the second sampling point. Substrate: p = ns at both sampling points. Treatment × Substrate: p = ns at both sampling points.
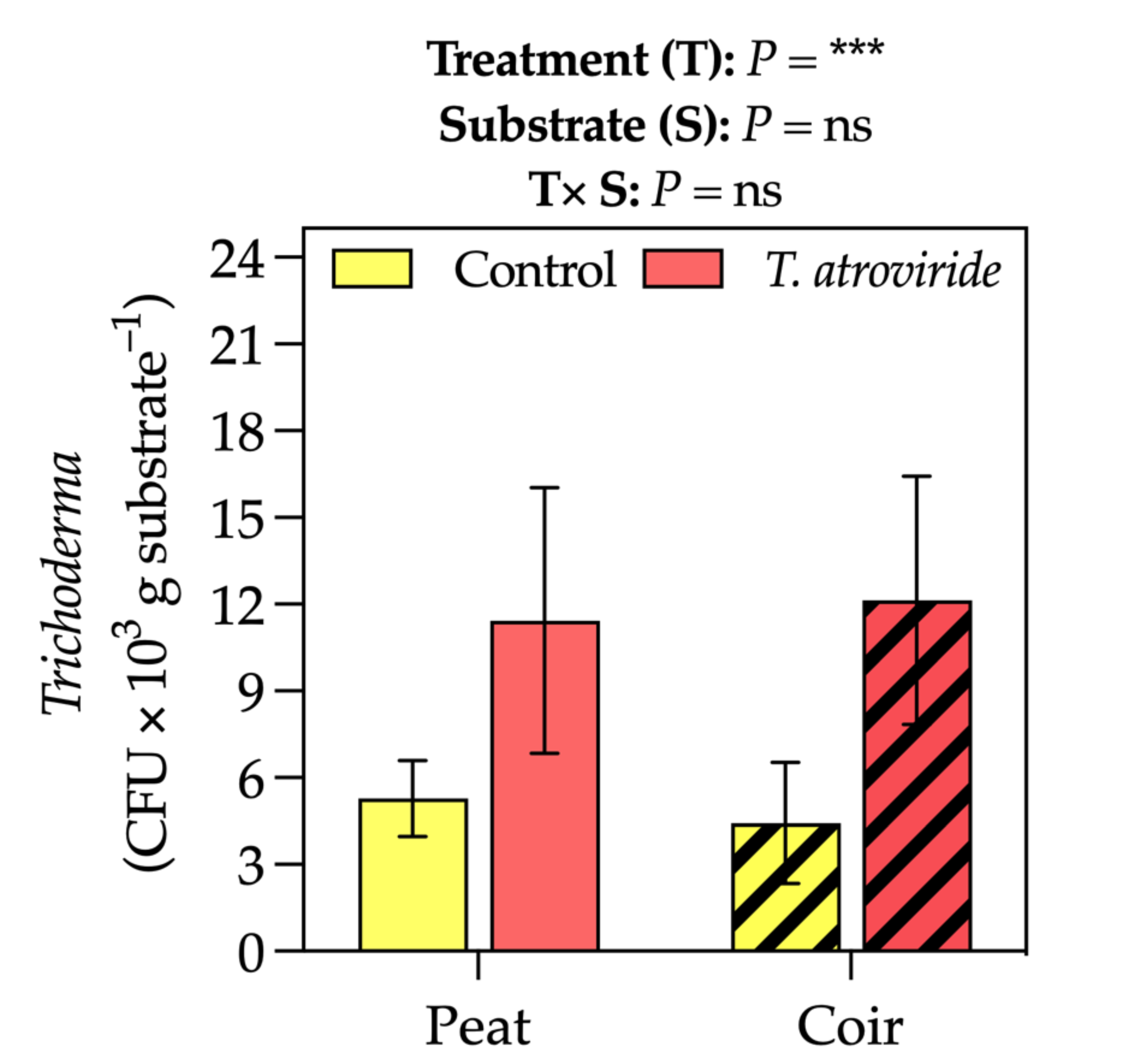
The fungal presence was higher in the pots of treated plants, resulting in an average of 11–12 Trichoderma colony forming unit (CFU) × 103 g substrate−1 (+59% of not treated plants, considering the average values on peat- and coir-based substrates, Figure 5).
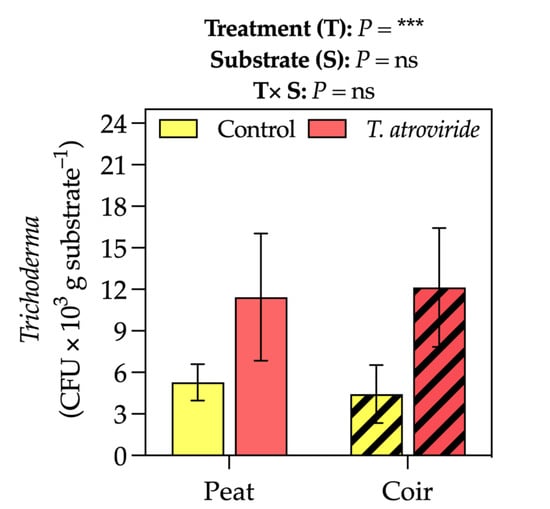
Figure 5.
Trichoderma colony-forming unit (CFU) in the substrate at the intermediate sampling point. Bars represent the means (n = 4) ± SDs. Two-way ANOVA p-values and Tukey’s post hoc results are reported in the figure (*** p < 0.001; ns = not significant).
2.4. Principal Component Analysis (PCA)
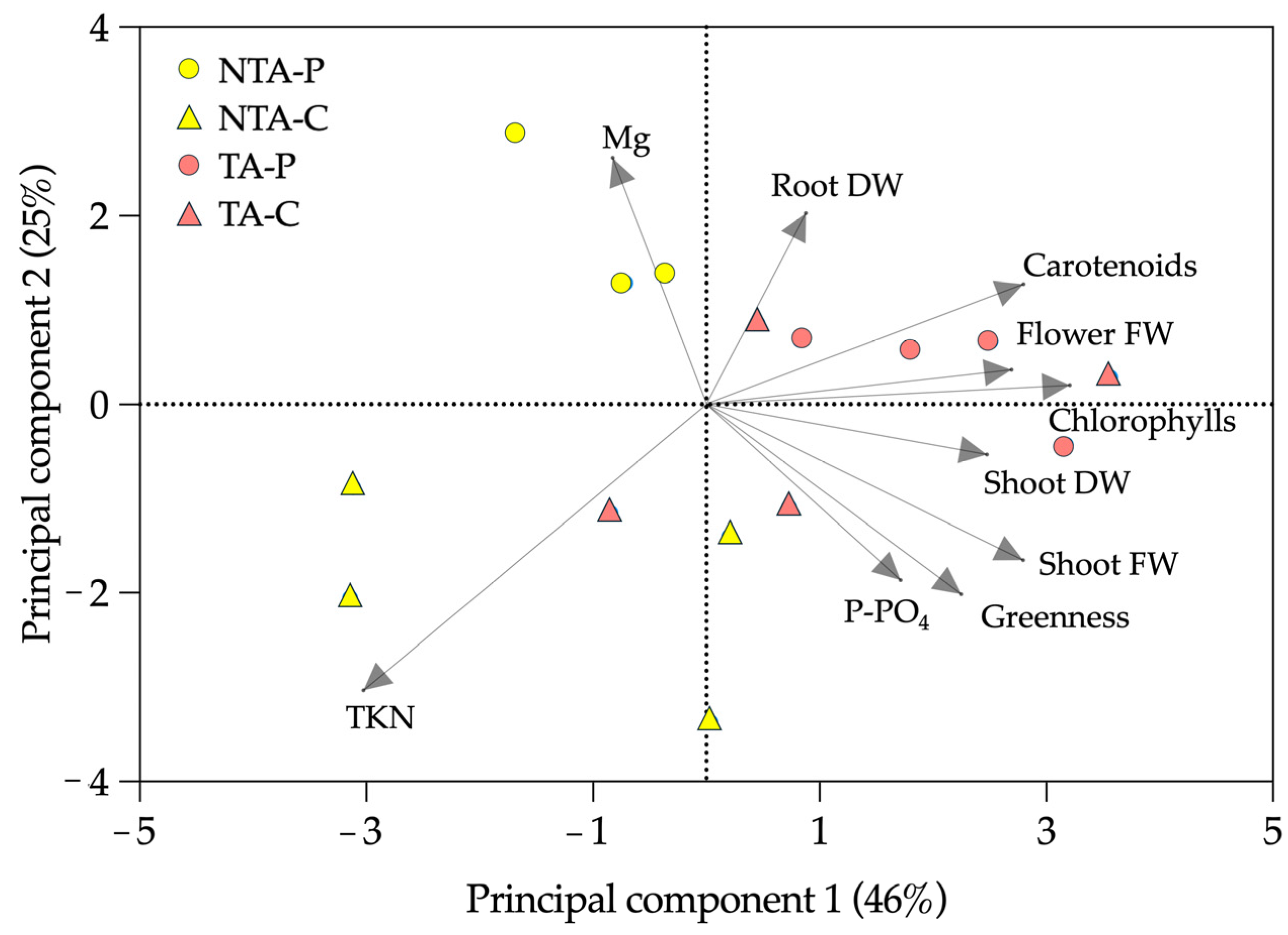
The PCA (Figure 6) highlighted that 46% of the variance could be explained by the differences between the treated and not treated plants, with the shoot TKN and Mg loading factors mostly identifying the not treated plants. On the contrary, 25% of the experimental variance could be explained mainly by the differences between coir and peat. The peat observations were mostly related to Mg, flower FW, chlorophylls, carotenoids, and root DW; the coir observations were mainly related to the shoot FW and DW, greenness index, and TKN.
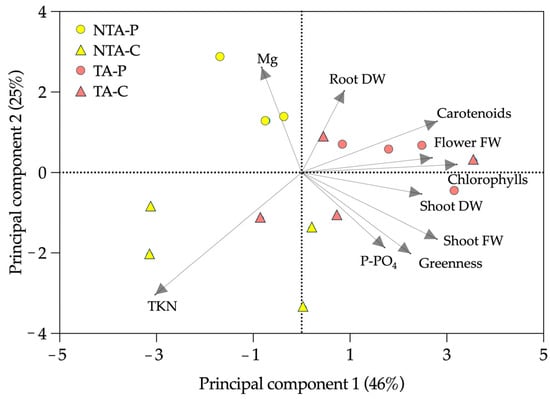
Figure 6.
Principal component analysis (PCA) of the experimental results. The loading factors (grey arrows) were selected using eigenvalues higher than 1.0. NTA = no T. atroviride, TA = T. atroviride, P = peat, C = coir, FW = fresh weight, DW = dry weight, TKN = total Kjeldahl nitrogen.
3. Discussion
The inoculation of the substrate with Trichoderma spp. is known to promote growth, flowering, quality, and nutritional status of ornamental plants regardless of the substrate type [36]. An increase in the biomass and leaf area was observed in I. walleriana plants in our experimental conditions, as already found in tulip and gladiolus [37,38], polianthes [39], Lantana camara [40], begonia [41], sea lavender, cypress, and camellia [42] inoculated with different Trichoderma strains. Moreover, a significant effect on flowering performance attributable to the elongation of inflorescences and the development of flowers has been also demonstrated in tulip, polianthes, and begonia [38,39,41]. Inoculating a peat-based growing media with T. harzianum, Ousley et al. [43] showed an improvement in the number of flowers in marigold and petunia, while in verbena, both the number and weight of the flowers increased; these results differed from the present work for the applied Trichoderma species (T. harzianum instead of T. atroviride) and its inoculum dose. Despite these promising results, the mechanisms supporting the beneficial effects of Trichoderma spp. on plant growth stimulation have not been fully explained. Contreras-Cornejo et al. [44] suggested that the plant growth promotion ability of T. virens is mediated by an auxin-dependent mechanism; through in vitro tests the authors confirmed the fungus’s ability to synthetize indole-3-acetic acid (IAA) and some derivatives, leading to greater root development. In the present experiment, however, the root biomass did not increase following the treatment with T. atroviride. Alternatively, the higher biomass accumulation can be attributed to improved nutrient availability [45]. Trichoderma spp. can in fact facilitate nutrient absorption from the substrate through mineral solubilization and increased element uptake [46]. Indeed, in our experimental conditions, T. atroviride increased the leaf macronutrient concentrations (P, K, and Ca), similarly as found by Andrzejak and Janowska [37] in gladiolus and Janowska et al. [47] in freesia, in both cases using a mixture of T. viride, T. harzianum, and T. hamatum.
Most research papers addressing the impact of Trichoderma spp. on the content of chloroplast pigments in leaves referred to edible species [48,49,50], while only a few studies investigated the stimulation of photosynthetic pigments on ornamental plants [46]. An increase in chlorophylls was found in Begonia × tuberhybrida [41] and Gladiolus hybridus ‘Advances Red’ [37] soaked in a mixture of spores of Trichoderma spp. and planted in peat substrate. According to Andrzejak et al. [41], the chlorophyll accumulation in Begonia × tuberhybrida was reflected by the greenness index. In our case, a significant accumulation of pigments, mainly chlorophyll a and carotenoids, and a higher greenness index, were found in leaves as a consequence of inoculum, therefore influencing the plant photosynthetic capability. In fact, even carotenoids are essential pigments in photosynthesis because they absorb in the blue–green region of the solar spectrum and transfer the absorbed energy to chlorophylls, so expanding the wavelength range of light, which can drive photosynthesis. Indeed, Harman et al. [49] stated that endophytic strains of Trichoderma determine an increase in the number of photosynthetic pigments or the expression of genes regulating the biosynthesis of chlorophylls, proteins in the light-harvesting complex, or components of the Calvin cycle.
Regarding the substrate suitability, no differences were observed in either the epigeal biomass production or flowering performance. The suitability of the coco-peat substrate for the application of Trichoderma spp. has already been highlighted by Sriram et al. [51], who monitored the T. harzianum fungal population density during the time, highlighting a stable population from 28 to 42 days after the inoculum. Our experimental results highlighted the high efficacy of inoculum also in coir alone.
4. Materials and Methods
4.1. Plant Material and Growing Condition
This experiment was carried out at the Research Centre for Vegetable and Ornamental Crops, Council for Agricultural Research and Economics, in Pescia (PT), Italy (lat. 43°54′ N, long. 10°42′ E, altitude 62 m). The trial was conducted in a greenhouse equipped with benches for soilless cultivation, a capillary fertigation system, and a basal heating system based on coaxial pipes circulating warm water powered by a compressor heat pump [52]. Basal heating was applied during the trial to maintain a temperature of 16 °C at the pot and root level. Cuttings of Impatiens walleriana Hook. f ‘Buddha F1 Carmine’ (Azienda Agricola Sentier, Mosnigo di Moriago della Battaglia, Treviso, Italy) were transplanted on 14 February 2019 in 1.2 L pots. Four treatments were applied as a combination of: (i) two growing media, i.e., peat:perlite and coconut coir dust (coir):perlite (both 70:30, v v−1); and (ii) amended growing media with or without 1 g L−1 of a Trichoderma atroviride-based inoculant (Tricoten, Trichoderma atroviride AT10 at 5 × 108 CFU g−1, Atens-Agrotecnologias Naturales SL, Tarragona, Spain). For each treatment, 4 replicates of 20 plants were set up for a total of 320 plants. The growing media pH and electrical conductivity (EC) were determined following the EN 13037/1999 [53] and EN 13038/1999 [54] methods, respectively, which consist of an electrometric determination on a substrate:water (1:5 v v−1) extract after 30 min shaking of a known volume of sieved wet samples. The physical characteristics (i.e., substrate bulk density, total porosity, available water content, water holding capacity, and air content) were determined as described by De Boodt and Verdonck [55]. The substrate characteristics are reported in Table 4. The trial started with the cutting transplant and ended on 11 April 2019, after 56 d of cultivation. The heating system was set to avoid an air temperature below 5 °C. Fertirrigation was managed according to the weather conditions by supplying a nutrient solution typically used for bedding plant production (i.e., 7.0 mM N-NO3, 0.7 mM N-NH4, 4.0 mM K, 2.5 mM Ca, 1.0 mM Mg, 1.8 mM S-SO4, 30.0 µM Fe, 25.0 µM B, 1.0 µM Cu, 5.0 µM Zn, 10.0 µM Mn, and 1.0 µM Mo) and maintaining a pH of roughly 6.0. During the experiment, the climate condition inside the greenhouse was monitored by a Testo data logger, mod. 175 (Testo SE & Co. KGaA, Titisee-Neustadt, Germany) and the recorded data were: average temperature 16.8 °C and air relative humidity 57.8%.

Table 4.
Main chemical–physical characteristics of the two used growing media.
4.2. Plant Biomass Measures
At the end of the experiment, the shoot (stems plus leaves) and flower FW and DW were determined in each biological replicate (i.e., in a bulk sample of 10 plants out of 20 plants constituting a replicate) by drying the samples in a ventilated oven at 60 °C until a constant weight. Before drying, the leaf area was measured in a significant portion of fresh leaves using a WinDIAS Image Analysis System (Delta-T Devices, Cambridge, UK) and used to determine the SLA (cm2 g DW−1). Flowers were periodically collected during the experiment from all the plants constituting every replicate (three times during the trial, i.e., 40, 49, and 56 d after transplanting, indicated as 1st collection, 2nd collection, and final collection) to obtain the total flower production, expressed as the number, FW, and DW. Roots were sampled at the end of the trial from the same plants used for the aboveground biomass measures (i.e., a bulk sample of 10 plants out of 20 plants constituting a replicate), washed in diluted acetic acid to remove the substrate, and used for the DW measures. The SPAD index was assessed in 4 leaves per plant, in 10 plants per replicate (the same plants used for biomass measures), by a SPAD-502 (Konica Minolta, Inc., Ishikawa-machi, Hachioji-shi, Tokyo, Japan) at the 1st and 2nd collections to monitor the leaf chlorophylls using a not-destructive technique.
4.3. Tissue Analyses
At the conclusion of the experiment, fresh leaf disk samples were randomly collected from plants belonging to the same replicate (100 mg FW per replicate in two technical replicates) to assess the concentration of chlorophylls a and b and total carotenoids (mg g−1 FW). The samples underwent a methanol extraction (99%, v v−1) in darkness at −20 °C for 48 h and were analyzed using a spectrophotometer (Evolution™ 300 UV–Vis Spectrophotometer, Thermo Fisher Scientific Inc., Waltham, MA, USA) to measure the absorbances at 665.2, 652.4, and 470.0 nm, following the method described by Lichtenthaler and Buschmann [56]. The chlorophyll a, chlorophyll b, and carotenoid concentrations allowed the calculation of the greenness index [56] as follows:
Greenness Index = (Chlorophyll a + Chlorophyll b)/Carotenoids
The root and shoot (i.e., stems plus leaves) dry samples underwent a Kjeldahl nitrogen (TKN) analysis after a phospho-sulfuric acid digestion, as reported by Massa et al. [57]. In addition, the samples (250 mg DW) were digested in a mixture of nitric and perchloric acids (HNO3:HClO4 5:2 v v−1) at 230 °C for 1 h to determine the concentrations (mg g−1 DW) of potassium (K), calcium (Ca), magnesium (Mg), and phosphate (P-PO4). K, Ca, and Mg were quantified by atomic absorption spectrometry (AA-7000F Flame Atomic Absorption, Shimadzu, Japan), while the P-PO4 concentration was determined spectrophotometrically through the molybdenum blue method [58].
4.4. Trichoderma atroviride Analysis
Substrate samples were collected from the plant rhizosphere at the first floral sampling and the end of cultivation. Fungus quantification was performed by serial substrate dilutions in a Trichoderma-selective agar medium according to Fiorentino et al. [59], with some modifications. In detail, 10 g of root–substrate was suspended in sterile distilled water to provide serial dilutions (four replicates per dilution), and 100 μL aliquots of each sample were spread on the surface of 90 mm culture plates containing Rose Bengal Chloramphenicol agar (Merck KGaA, Darmstadt, Germany). The plates were incubated at 25 °C and examined daily for emerging fungal colonies. The results have been expressed as the CFU per g of dry substrate.
4.5. Statistical Analysis
The data were tested for a normal distribution through the Shapiro–Wilk test. Thus, the data were analyzed by a two-way ANOVA (T. atroviride treatment and substrate as variables), followed by a Tukey’s post hoc test (p < 0.05). The statistical analyses and graphs were processed using Prism 10 (GraphPad Software Inc., La Jolla, CA, USA). Principal component analysis (PCA) was performed using Prism 10, selecting eigenvalues higher than 1.0.
5. Conclusions
Coconut coir dust confirmed its suitability as an alternative substrate to peat for bedding plant production. Regarding fungal inoculum applications, T. atroviride improved both the quantitative and qualitative parameters of I. walleriana bedding plants. The inoculum positively affected the biomass parameters, nutrient uptake, and leaf pigment concentrations without particular differences between peat and coir. T. atroviride promoted the aesthetic parameters important for product marketability, such as the number and dimensions of the flowers and the greenness index. In light of our results, the application of coconut coir dust in combination with Trichoderma spp. is worthy to be explored in other bedding plants with the aim of supporting growers in the development of sustainable horticultural practices, including peat substrate replacement and use of beneficial microorganisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13050583/s1, Table S1: Biometric parameters of I. walleriana plants at the end of the experiment; Table S2: Nutrient concentrations (g kg−1 DW) measured in I. walleriana roots at the end of the experiment.
Author Contributions
S.T.: Formal analysis, data curation, visualization, validation, writing—original draft. M.C. (Mariateresa Cardarelli): Conceptualization, methodology, formal analysis, data curation, validation, writing—original draft. M.B.: Methodology, data curation, writing—review and editing. G.B.: Supervision, funding acquisition, writing—review and editing. M.C. (Maurizio Cutini): Methodology, data curation, writing—review and editing. M.F.: Methodology, supervision, project administration, funding acquisition, writing—review and editing. D.M.: Conceptualization, methodology, supervision, writing—review and editing. A.O.: Formal analysis, investigation, data curation, writing—review and editing. S.C.: Conceptualization, methodology, formal analysis, data curation, validation, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MASAF, Project “Energia dall’agricoltura: innovazioni sostenibili per la bioeconomia (AGROENER)”, Excutive Decree number 26329 del 01/04/2016.
Data Availability Statement
Data are contained within the article or supplementary material.
Acknowledgments
The authors would like to thank Angela Valentina Ceccarelli, Monica Michelotti, and Paolo Bini for their technical support during the trial.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Hand, F.P.; Garibaldi, A.; Gullino, M.L. Bedding plant production and the challenge of fungal diseases. Plant Dis. 2021, 105, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- USDA. Floriculture Crops: 2018 Summary. United States Department of Agriculture–National Agricultural Statistics Service. 2019. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/floran19.pdf (accessed on 16 February 2024).
- European Commission Working Document. Horticultural Products Flowers and Ornamental Plants Statistic 2010–2019. DGAGRI-G2 2020. Available online: https://agriculture.ec.europa.eu/system/files/2020-06/flowers-ornamental-plants-statistics_en_0.pdf (accessed on 16 February 2024).
- Pardossi, A.; Carmassi, G.; Diara, C.; Incrocci, L.; Maggini, R.; Massa, D. Fertigation and Substrate Management in Closed Soilless Culture; University of Pisa: Pisa, Italy, 2011. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Schmilewski, G. Growing medium constituents used in the EU. In Proceedings of the International Symposium on Growing Media 2007, Nottingham, UK, 2–8 September 2007; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; pp. 33–46. [Google Scholar] [CrossRef]
- Schmilewski, G. Growing media constituents used in the EU in 2013. In Proceedings of the International Symposium on Growing Media, Composting and Substrate Analysis—SusGro2015, Vienna, Austria, 7–11 September 2015; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2017; pp. 85–92. [Google Scholar] [CrossRef]
- Toboso-Chavero, S.; Madrid-López, C.; Villalba, G.; Gabarrell Durany, X.; Hückstädt, A.B.; Finkbeiner, M.; Lehmann, A. Environmental and social life cycle assessment of growing media for urban rooftop farming. Int. J. LCA 2021, 26, 2085–2102. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M. Efficacy of organic amendments used in containerized plant production: Part 2—Non-compost-based amendments. Sci. Hortic. 2020, 260, 108855. [Google Scholar] [CrossRef]
- Rhie, Y.-H.; Nam, S.; Kim, J. Seed germination and seedling growth of four bedding plants in substrate containing coal bottom ash mixed with coir dust. Agronomy 2021, 11, 1902. [Google Scholar] [CrossRef]
- Nelson, P.V.; Oh, Y.-M.; Cassel, D.K. Changes in physical properties of coir dust substrates during crop production. Acta Hortic. 2004, 644, 261–268. [Google Scholar] [CrossRef]
- Carlile, W.R.; Raviv, M.; Prasad, M. Organic Soilless Media Components. In Soilless Culture; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 303–378. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Dewi, T.K.; Mubarok, W.Z.; Antonius, S. Study of plant growth promoting bacteria from coconut coir dust. IOP Conf. Ser. Earth Environ. Sci. 2020, 439, 012037. [Google Scholar] [CrossRef]
- Chromkaew, Y.; Kaeomuangmoon, T.; Mawan, N.; Mukjang, N.; Khongdee, N. Is coconut coir dust an efficient biofertilizer carrier for promoting coffee seedling growth and nutrient uptake? PeerJ 2023, 11, e15530. [Google Scholar] [CrossRef] [PubMed]
- Bardin, M.; Pugliese, M. Biocontrol agents against diseases. In Integrated Pest and Disease Management in Greenhouse Crops. Plant Pathology in the 21st Century; Gullino, M., Albajes, R., Nicot, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 9, pp. 385–407. [Google Scholar] [CrossRef]
- Saini, I.; Aggarwal, A.; Kaushik, P. Inoculation with mycorrhizal fungi and other microbes to improve the morpho-physiological and floral traits of Gazania rigens (L.) Gaertn. Agriculture 2019, 9, 51. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bissett, J.; Druzhinina, I.; Kullnig-Gradinger, C.M.; Szakacs, G. Genetic and metabolic diversity of Trichoderma: A case study on South East Asian isolates. Fungal Genet. Biol. 2003, 38, 310–319. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Abdel Lateif, K.S.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.M.; El-Meihy, R. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; De Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.; Fadiji, A.E.; Hyder, S.; Babalol, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Cardarelli, M.; Ceccarelli, A.V.; Colla, G. Effectiveness of Trichoderma application through drip-irrigation to reduce Sclerotinia disease incidence and improve the growth performance of greenhouse lettuce. Acta Hortic. 2020, 1268, 199–204. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Ntatsi, G.; La Bella, S.; Baldassano, S.; Rouphael, Y. Stand-alone or combinatorial effects of grafting and microbial and non-microbial derived compounds on vigour, yield and nutritive and functional quality of greenhouse eggplant. Plants 2022, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Regliński, T.; Rodenburg, N.; Taylor, J.T.; Northcott, G.L.; Ah Chee, A.; Spiers, T.M.; Hill, R.A. Trichoderma atroviride promotes growth and enhances systemic resistance to Diplodia pinea in radiata pine (Pinus radiata) seedlings. For. Pathol. 2012, 42, 75–78. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Ruíz-Herrera, L.F.; Pelagio-Flores, R.; Macías-Rodríguez, L.I.; Martínez-Trujillo, M.; López-Coria, M.; Sánchez-Nieto, S.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma atroviride-emitted volatiles improve growth of Arabidopsis seedlings through modulation of sucrose transport and metabolism. Plant Cell Environ. 2021, 44, 1961–1976. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; López-Bucio, J.S.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ramos-Vega, M.; Guevara-García, Á.A.; López-Bucio, J. Mitogen-activated protein kinase 6 and ethylene and auxin signaling pathways are involved in Arabidopsis root-system architecture alterations by Trichoderma atroviride. Mol. Plant-Microbe Interact. 2015, 28, 7011–7710. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; Estrada-Rivera, M.; Olguin-Martínez, I.; Del Carmen Rocha-Medina, M.; Islas-Osuna, M.A.; Casas-Flores, S. TBRG-1 a Ras-like protein in Trichoderma virens involved in conidiation, development, secondary metabolism, mycoparasitism, and biocontrol unveils a new family of Ras-GTPases. Fungal Genet. Biol. 2020, 136, 103292. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Trichoderma spp. improves flowering, quality, and nutritional status of ornamental plants. Int. J. Mol. Sci. 2022, 23, 15662. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, R.; Janowska, B. Flowering, nutritional status, and content of chloroplast pigments in leaves of Gladiolus hybridus L. ‘Advances Red’ after application of Trichoderma spp. Sustainability 2022, 14, 4576. [Google Scholar] [CrossRef]
- Mazhabi, M.; Nemati, H.; Rouhani, H.; Tehranifar, A.; Mahdikhani-Moghadam, E.; Kaveh, H. Does Trichoderma harzianum really increase growth parameters in plants? Res. J. Biol. Sci. 2010, 5, 739–744. [Google Scholar] [CrossRef]
- Mazhabi, M.; Nemati, H.; Rouhani, H.; Tehranifar, A.; Moghadam, E.M.; Kaveh, H.; Rezaee, A. The effect of Trichoderma on polianthes qualitative and quantitative properties. J. Anim. Plant Sci. 2011, 21, 617–621. [Google Scholar]
- Yahya, A.B.; Al-Sawaf, M.D.; Al-Morad, N.Y. Effect of biofertilizer Trichoderma harzianum t-22 application, growing medium and training methods on some characteristics for Lantana camara plants. Mesop. J. Agric. 2021, 49, 95–103. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B.; Reńska, B.; Kosiada, T. Effect of Trichoderma spp. and fertilization on the flowering of Begonia × tuberhybrida Voss. ‘Picotee Sunburst’. Agronomy 2021, 11, 1278. [Google Scholar] [CrossRef]
- Prisa, D.; Sarrocco, S.; Forti, M.; Burchi, G.; Vannacci, G. Endophytic ability of Trichoderma spp. as inoculants for ornamental plants innovative substrates. J. Biocontrol Plant Pathog. Sustain. Agric. IOBC–WPRS Bull. 2013, 86, 169–174. [Google Scholar]
- Ousley, M.A.; Lynch, J.M.; Whipps, J.M. The effects of addition of Trichoderma inocula on flowering and shoot growth of bedding plants. Sci. Hortic. 1994, 59, 147–155. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ’omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Gupta, V.G., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T. The influence of fungi of the Trichoderma genus on the flowering of Freesia refracta Klatt ‘Argentea’ in winter. Hortic. Sci. 2020, 47, 203–210. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Shabana, Y.M.; Ismail, A.E.; Rashad, Y.M. Trichoderma harzianum: A biocontrol agent against Bipolaris oryzae. Mycopathologia 2007, 164, 81–89. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Metwally, R.A.; Al-Amri, S.M. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 2020, 70, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Savitha, M.J.; Ramanujam, B. Trichoderma-enriched coco-peat for the management of Phytophthora and Fusarium diseases of chilli and tomato in nurseries. J. Biol. Control 2010, 24, 311–316. [Google Scholar]
- Terrosi, C.; Cacini, S.; Burchi, G.; Cutini, M.; Brambilla, M.; Bisaglia, C.; Massa, D.; Fedrizzi, M. Evaluation of compressor heat pump for root zone heating as an alternative heating source for leafy vegetable cultivation. Energies 2020, 13, 745. [Google Scholar] [CrossRef]
- EN 13037/1999; Soil Improvers and Growing Media—Determination of pH. CEN, European Committee for Standardization: Brussels, Belgium, 1999.
- EN 13038/1999; Soil Improvers and Growing Media—Determination of Electrical Conductivity. CEN, European Committee for Standardization: Brussels, Belgium, 1999.
- De Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. In Proceedings of the III Symposium on Peat in Horticulture 26, Dublin, Ireland, 28 June–3 July 1971; pp. 37–44. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Massa, D.; Prisa, D.; Montoneri, E.; Battaglini, D.; Ginepro, M.; Negre, M.; Burchi, G. Application of municipal biowaste derived products in Hibiscus cultivation: Effect on leaf gaseous exchange activity and plant biomass accumulation and quality. Sci. Hortic. 2016, 205, 59–69. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. xi-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).