Abstract
Plant communities may be co-invaded by invasive plants, sometimes even by congeneric invasive plants (CIPs). Despite the growing understanding of co-invasion in the environment, little is known about how CIP interactions and mechanisms regulate co-invasion. Darwin’s naturalisation conundrum predicts that the coexistence of closely related species is difficult due to their structural and behavioural similarities. Nevertheless, communities containing closely related species are more susceptible to being invaded because close relatives may favour similar environments; therefore, this hypothesis should be followed in the co-invasion of CIPs. To explore whether the phylogenetic relatedness and origins of invasive species to CIPs can promote or hinder co-invasion, we conducted a controlled interaction and soil-legacy greenhouse experiment to quantify the growth response of invasive plants and their congeners. We consistently found that CIPs of identical origin were more likely to co-invade compared to CIPs of distinct origins. CIPs of distinct origins exhibited an antagonistic effect on co-invasion by allelopathy. Invasive plant-conditioned soil was more conducive to the growth of CIPs of identical origin than CIPs of distinct origins. Our results revealed the different effects of invader–invader phylogenetic relatedness on co-invader success and impact, suggesting the operation of different mechanisms across co-invasion.
1. Introduction
Increasing anthropogenic activities, such as transportation, agriculture, and global trade, have significantly facilitated the invasion of species into suitable habitats [,]. The communities may be invaded by two or more invasive species, a phenomenon referred to as co-invasion []. By 2050, invasive species are projected to increase by 36%, implying an increased chance of co-invasion []. For instance, they may interact with one another or alter the rhizosphere microbial community, which influences the likelihood that more invasive plants will invade, ultimately impacting the ecosystem.
The presence of invasive species will unavoidably influence their invasive neighbours and subsequent invasive plants (either accelerating or decelerating). It has become an urgent ecological problem to predict which invasive plants will co-invade, the effect of invasive plants on subsequent invasive plants in the community, and how to effectively prevent, manage, and control co-invasion.
The emergence of phylogenetic biology has facilitated the use of phylogenetic relatedness and evolutionary history in investigating ecological problems [,,]. Darwin [] first proposed that phylogenetic relatedness plays a crucial role in plant competition. Alien species are less likely to invade communities containing their close relatives because of niche overlap and similar resource needs, resulting in strong competition, known as ‘Darwin’s naturalisation hypothesis’ (DNH) []. In addition, invasive plants occupy similar environments, leading to an early adaptation process for subsequently closely related plants. Environmental filtering further enhances the susceptibility of closely related species to invasion, referred to as the ‘Pre-adaptation hypothesis’ (PAH) []. The two hypotheses, collectively known as ‘Darwin’s naturalisation conundrum (DNC)’, describe the potential contrasting influences of phylogenetic relatedness on invasion [,,]. Studies have found that biotic similarity among invasive plants increases invasiveness [,]. Biologic interactions and environmental filtering have both been shown to be potential determinants of biological invasion []. However, the mechanisms underlying the advantages of co-invasion of closely related species over their respective invasion remain unclear.
Interactions between plants are usual and can have direct effect (space occupancy and competition for shared resources) [,] or indirect effect (through changes in plant surroundings, biodiversity, and ultimately ecosystem function) []. Resource competition can be divided into two dimensions: ‘competitive effect’ (the ability of species to take up high resources) [,,] and ‘competitive response’ (the ability of species to tolerate low resources) [,,,]. Allelopathy and soil microorganism regulation can have an indirect effect on the growth of other invasive species [,]. The allelopathy of invasive plants, an interference mechanism, is pervasive []. Co-invading species can synergistically affect the abundance of soil bacterial community [], and invasive plants can build an environment more conducive to competitive ability and thus the invasiveness of subsequent invasive plants (Invasional meltdown hypothesis) []. Noteworthy, invasive plant-conditioned soil was not likely to directly affect competition intensity from subsequent plants; instead, the similarity in soil microbial communities indirectly affected subsequent invasive plants []. As a result, we believe that the traditional phylogenetic method or the functional similarity approach to studying the effect of phylogenetic relatedness on co-invasion may overlook the contribution of some specific conditions (for example, differences in species origin or direct and indirect effects among plants).
Recent studies have revealed that invasive populations in invasive areas can evolve to have more significant ecophysiological advantages than native populations through altering biomass allocation, which is conducive to resource competition and vigorous growth [,,]. However, it is unclear whether co-invasion in invasive areas is affected by their origin (which could be attributed to allelopathic interference) []. In recent years, co-invasion research has relied primarily on observational data, with limited experimental data [,,,]. Simultaneously, these studies have not directly examined the intensity of interaction among species from the perspective of infrageneric relationships. Such data are crucial for understanding the consequences and mechanisms of co-invasion in a specific ecosystem. In this study, we identify the potential linkage between competition for shared resources and allelopathic interference with congeneric invasive plants (CIPs) that co-invade, and specifically test whether phylogenetic relatedness and the origin of species affect co-invasion of CIPs. Furthermore, we addressed potential mechanisms of co-invasion of CIPs through plant interaction and allelopathic legacy experiments. We investigated the following key questions: (1) Does significant interspecific competition exist among CIPs? If interspecific interaction is more significant than intraspecific interaction, we believe they will fail to co-invade or vice versa. Are their divergent outcomes of interactions depending on their origins? If so, (2) How do nutrition and allelopathy affect the co-invasion of CIPs, and (3) How do CIPs adjust the biomass allocation strategy when they co-invade?
2. Materials and Methods
2.1. Study Location and Species
The common garden experiment was carried out in a plastic canopy (8 m × 60 m) in the Teaching and Research Base of Shenyang Agricultural University (41°49′48.57″ N, 123°33′42.65″ E) from 2022 to 2023.
We consider two species of the same genus as a group and then subgroup them according to geographical origin differences. If both species are native to the same continent, we consider them of an identical origin (IO). For example, Ambrosia trifida and Ambrosia artemisiifolia are native and widespread in North America. On the contrary, Solanum rostratum (North America) and Solanum sarrachoides (South America) are native to distinct continents. Therefore, we consider them as plants of distinct origins (DOs) (Figure 1, Table S1). All seeds were collected from the field. Additionally, to account for possible regional variations, invasive species belonging to the same genus were collected from distinct geographical regions (thus prioritizing plants originating from different provinces or cities when collecting CIPs). Based on the ‘Chinese Alien Plant List’ and the Chinese Biobank (https://species.sciencereading.cn, accessed on 22 June 2021), we identified the experimental species as alien species []. Among them, we selected 16 alien species (7 families), all successfully established in China.
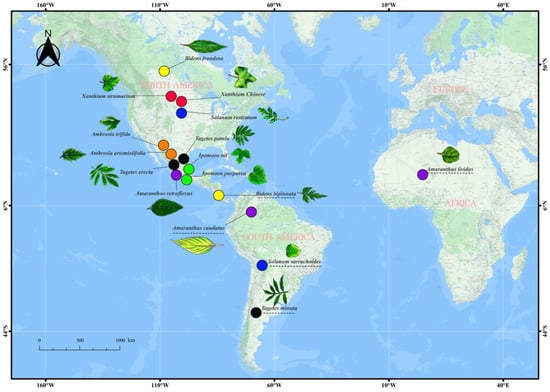
Figure 1.
The picture shows the origins of species in this experiment. The same colour represents the same genus. The underscore represents distinct origins of the same genus.
2.2. Experimental Set-Up
In April 2022, seeds for all plants were immersed in a 1% sodium hypochlorite solution for 20 min and then rinsed five times with distilled water. Subsequently, they were germinated in a light incubator or at room temperature under conditions of light/dark 25 °C/20 °C or light/dark 30 °C/25 °C, respectively. Once most of the seeds had germinated (with the radicle length equal to the seed length as the standard), we transferred them to hole plates for seedling cultivation. Seedlings that reached the ‘three-leaf and one-core stage’ and exhibited robust growth were transplanted into pots (27 cm × 17 cm). We sifted the soil in the field using a 1 cm screen to remove plant material and large stones while incorporating soil, substrate, and vermiculite into an experimental soil mixture in a 3:1:1 ratio.
Experiment 1 addressed the effects of origin differences, nutrition, and direct allelopathy on co-invasion of CIPs. We selected two plants per pot based on the combination requirements (Figure 2, Table S2). We planted 16 combinations of interactions: 11 combinations of interspecific interactions and 16 combinations of intraspecies interactions with a seedling spacing of 8 cm. Each group had five replicates of interspecific interactions, and one target species was taken from each pot to measure the experimental data. Intraspecific interaction was repeated three times in each group, and two target plants were taken from each pot to measure experimental data. We planted a total of 618 plants in 309 pots in the experiment. If seedlings died within two weeks of planting, they were replaced with new ones. After two weeks of planting, we conducted experimental treatments in three groups: high-nutrition group (+N), low-nutrition group (−N−AC), and allelopathic treatment group (+AC). We applied a water-soluble fertilizer (N:P:K:S = 24:12:14:4) to the soil in plants in the +N group. A 10 g solution dissolved in 4 L of water was administered to the roots monthly, totalling four applications. −N−AC group had no compound fertilizer and activated carbon. The +AC group contained additional activated carbon and a soil ratio of 20 mL/L. Pre-experiment and the literature proved that adding activated carbon had no significant effect on plant growth []. We irrigated the plants daily from 17:00 to 18:00 to maintain optimal soil moisture levels. Once planted, we sprayed all plants with pesticides to minimize pest and disease interference. We moved the pots randomly every two weeks throughout the experiment to prevent the influence of marginal effect on the experiment; 16 weeks after transplanting, we measured the plant phenotype (including height, coverage, and basal diameter). We used the LI-3000 leaf area meter (LI-Cor, Lincoln, NE, USA) to measure the collected leaves’ single leaf area (LA). Photosynthesis was measured using an LI-6400XT portable photosynthesis system (LI-Cor, Lincoln, NE, USA). We harvested plants at the ground level to obtain aboveground biomass and then separated the stems, leaves, and seeds for individual collection. Subsequently, we dried these plant parts in an oven at 80 °C until a constant weight was reached before weighing. Following this, we used high-pressure water to wash away the soil surrounding the roots of both species in each pot. The roots were carefully separated as much as possible in the water and dried in an oven until a constant weight was reached. We believe that losing a tiny part of fibrous root biomass during the separation process will not significantly affect the experimental results. As outlined by Poorter [], we derived several parameters from our measured data: leaf mass fraction (LMF), root mass fraction (RMF), stem mass fraction (support organ mass fraction, SOMF), seed mass fraction (SMF), and specific leaf area (SLA).
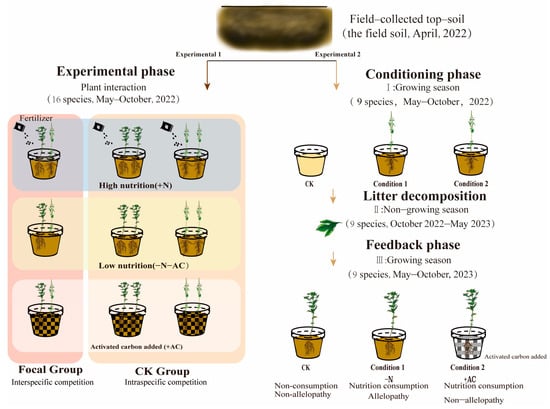
Figure 2.
The design of the two-phase experiment in this study.
Experiment 2 addressed the effects of soil legacy (indirect allelopathy) and nutrition depletion on subsequent CIPs. During the soil treatment stage from May to October 2022, seedlings were carefully selected from each pot and cultivated throughout the growing season based on specific requirements (Figure 2, Table S3). We conducted experimental treatments under three conditions: the control group (no plants in the first year and no activated carbon added in the second year, designated as CK), condition 1 (plants in the first year and no activated carbon added in the second year, designated as −N), and condition 2 (plants in the first year and activated carbon added in the second year, designated as +AC). We planted the required number of each plant (9 species) and replicated each group eight times to give a total of 264 pots containing 176 plants. In order to replicate the natural growth conditions of plants in the field, we did not immediately remove plants; leaves were allowed to decompose naturally in the soil. We did this because of uncertainty about whether root exudate metabolites and leaf litter decomposition would have varying effects on the subsequent plants. Consequently, we removed the entire plant before conducting the soil feedback experiment in the second year. In the soil feedback stage, 264 pots and 264 plants (9 species) were planted under three conditions. Seedlings were selected per pot and cultivated throughout the growing season from May to October 2023. After 16 weeks of transplanting, we harvested the above-ground biomass by cutting the plants at ground level, and we harvested the below-ground biomass by washing the soil around the roots using high-ressure water. All biomass was dried until it reached a constant weight before being weighed.
2.3. Statistical Analyses
According to Goldberg’s definition, the competitive effect is the capacity to restrict the growth or reproduction of neighbouring species, and the competitive response is the ability to withstand the negative effects of neighbouring species [,]. To investigate the universality of phylogenetic relatedness between CIPs and avoid pseudoreplication, we conducted measurements of indices under consistent disturbance factors and environmental conditions. On the same day, we measured physiological indices of the same species, such as plant height, photosynthesis, and so on. The resulting data were logarithmically transformed for analysis purposes. We used a standard index and calculated the log response ratio (ln RR) []:
Xfocal represents the individual when the target species competes with neighbouring CIPs in interspecific interaction and XCK denotes the average total measurement of the target species in intraspecific interactions. The independent variables in this study were the experimental treatment conditions and the dependent variable was the response coefficient of the measurement index ln RR. This approach allowed for comparing competitive abilities among CIPs, regardless of plant size. For the convenience of analysis, we selected greater total biomass response coefficient values as predominant species (PS) in −N−AC and inferior species (IS) with lower values (Table S2). In the analysis, we examined the significance of plant origin on interaction and allelopathic legacy. We used a continuous index of allelopathy to test for the allelopathic legacy of CIPs in monocultures as follows []:
+AC represents the individual when the target species is in +AC, −N represents the individual when the target species is in −N, and CK denotes the average total measurement of the target species in CK. Negative and positive values indicate a negative and positive effect of allelopathic legacy of invasive plants, respectively.
Data were expressed as mean ± standard error (SE) and analysed using variance analysis followed by the Bonferroni test. All data were analysed using Prism 10.1.1 and pathway analysis was performed with R 4.3.0 software to evaluate the effect size and path of nutrition and allelopathy on plant competition.
3. Results
3.1. Interactive Effects of Species Origin Differences, Nutrition, and Direct Allelopathy
Under high-nutrition conditions (+N), the total biomass of PS was consistently higher than that of the low-nutrition group (−N−AC). This difference was significant for both identical origin (IO, mean difference (md) = 0.33, p = 0.003) and different origin (DO, md = 0.226, p = 0.03) species. At the same time, other biomass of PS of IO and DOs showed a positive increase, with significant increases in seed biomass (IO, md = 0.385, p = 0.002; DOs, md = 0.154, p = 0.53), stem biomass (IO, md = 0.4595, p = 0.007; DOs, md = 0.351, p = 0.001), and root biomass (IO, md = 0.359, p < 0.001; DOs, md = 0.098, p > 0.05). However, the total biomass of IS is not always higher than that of −N−AC. This difference was opposite for identical origin (IO, md = 0.217, p = 0.14) and different origin (DO, md = −0.22, p = 0.05) species (Figure 3). For the PS of IO, the leaf biomass was lower than that of the control group, while other biomass showed larger values (ln RR > 0). Only seed biomass (md = 0.376, p = 0.04) and SOMF (md = 7.817, p = 0.03) of IS of IO demonstrated significant increases (Figure 3 and Figure 4c). Under low-nutrition (−N−AC), the total biomass of all species was lower compared to the control group (ln RR < 0). Regardless of the origin of the species, there was a decrease in SOMF of PS and an increase in RMF with decreasing nutrition levels (Figure 4a,b). Under the condition without allelopathy (+AC), the total biomass of IS of DOs significantly increased (md = 0.22, p = 0.006), and the total biomass of other species was lower than that under the condition with allelopathy (−N−AC). Changes in the biomass of the root, stem, leaf, and seeds of PS and IS of DOs were the same as the total biomass (Figure 3b). In particular, after excluding allelochemicals from consideration, the effects of nutrition on all species were highly significant (PS of IO, md = 0.4, p < 0.001; IS of IO, md = 0.38, p = 0.003; PS of DOs, md = 0.251, p = 0.006; IS of DOs, md = 0.44, p < 0.001, Figure 3). Under the low-nutrition and without allelopathy conditions, the LMF of PS and IS was significantly higher than that of high-nutrition (−N−AC/+AC: PS of IO, md = 2.935/4.445, p = 0.01/0.002; IS of IO, md = 4.578/4.495, p = 0.01/0.006; PS of DOs, md = 3.916/5.757, p = 0.004/<0.001; IS of DOs, md = 3.842/3.571, p = 0.002/0.01, Figure 4). After allelochemicals were excluded, the change in seed biomass of PS and IS of IO was opposite to that of total biomass, and the seed biomass of PS showed a significant increase (md = 0.23, p = 0.009, Figure 3a).
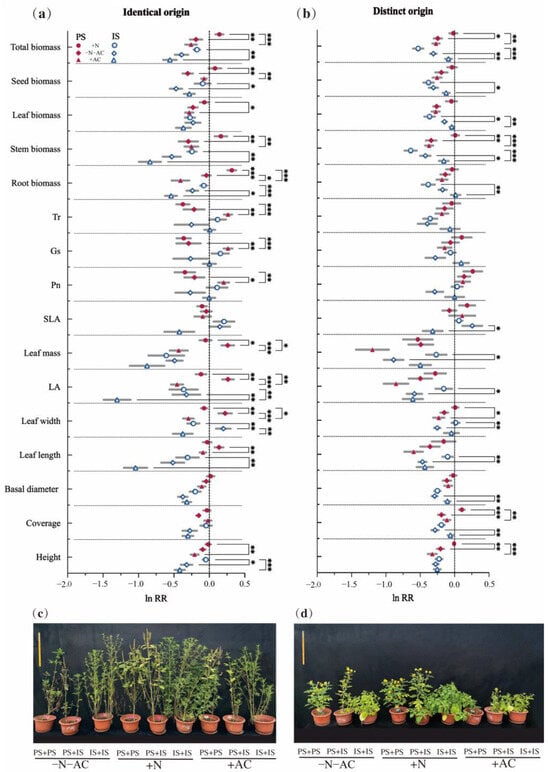
Figure 3.
The relative change in (a,c) identical origin (IO) and (b,d) distinct origin (DO) species in response to nutrition and allelopathy interactions. Grey lines represent the SE (standard error). Values are mean ± SE (relative changes). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Values of p indicate significant differences. Values of p > 0.05 are not presented for clarity. PS: predominant species, IS: inferior species, Tr: transpiration rate, Gs: stomatal conductance, Pn: net photosynthetic rate, SLA: specific leaf area, LA: single leaf area.
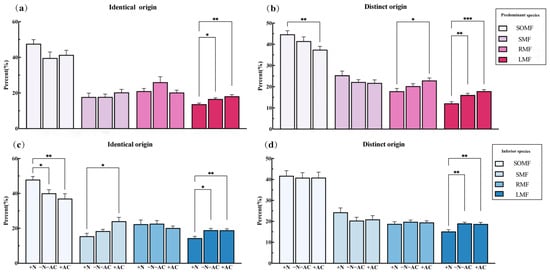
Figure 4.
The relative changes in the proportion of biomass in (a,c) identical origin and (b,d) distinct origin species. SOMF: support organ mass fraction, SMF: seed mass fraction, RMF: root mass fraction, LMF: leaf mass fraction. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Values of p indicate significant differences. Values of p > 0.05 are not presented for clarity.
3.2. Growth Traits Response to Nutrition and Allelopathy Changes
In general, nutrition had a positive effect on co-invasion by plant ‘Phenotypes’ (PS of IO = 0.2916; IS of IO = 0.315; PS of DOs = 0.328; IS of DOs = 0.2244, Figure 5). However, nutrition had significant and negative effects on the co-invasion of IS of DOs (direct effect: −0.46, p < 0.001), while they exhibited significant and positive effects on the co-invasion of PS of IO (direct effect: 0.53, p < 0.001). These findings were contrary to those observed for ‘Leaf size’ (PS of IO = −0.21, p > 0.05; IS of DOs = 0.45, p < 0.01) (Figure 5a,d). Allelopathy directly generated a negative influence on PS (direct effect: IO = −0.18, DO = −0.05) and a positive influence on IS (direct effect: IO = 0.20, DO = 0.15). Nutrition had no significant effects on ‘Photosynthesis’; however, allelopathy had a significant negative effect (direct effect: −0.56, p < 0.01) on ‘Photosynthesis’ of PS of IO and a positive effect (direct effect: 0.11, p > 0.01) on IS. Notably, the allelopathy’s effects on PS and IS of DOs were opposite (Figure 5c,d). Allelopathy positively influenced the ‘Phenotype’, ‘Leaf size’, and ‘Photosynthesis’ of IS of IO. In contrast, the opposite influenced IS of DOs and significantly and negatively influenced ‘Phenotype’ (direct effect: −0.55, p < 0.01) (Figure 5b,d).
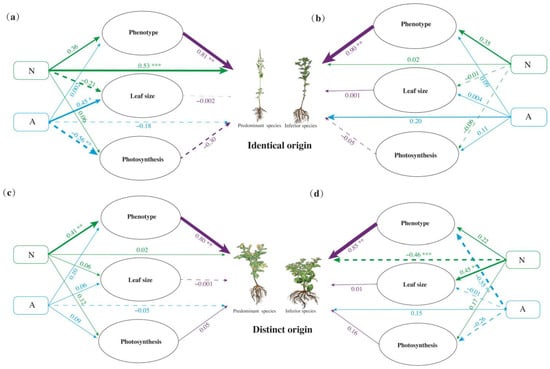
Figure 5.
Structural equation model (SEM) of the effects of nutrition (N) and allelopathy (A) interactions on the fitness of the predominant species (a,c) and inferior species (b,d). Solid and dotted lines indicate positive and negative correlations, respectively. Green, blue, and purple lines illustrate the nitrogen, allelopathy, and plant growth traits path diagram related to plant fitness, respectively. N: Nitrogen, A: Allelopathy. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Values of p indicate significant differences. Values of p > 0.05 are not presented for clarity.
3.3. Effects of Invasive Plants on Subsequent CIPs
Generally, the total biomass in IO and DOs varied because of the soil-egacy effect (Figure 6). The soil conditioned by invasive plants significantly and indirectly generated a negative influence on subsequent CIPs of DOs (Allelopathic legacy in monocultures: md = −0.151, p = 0.001) while promoting the invasion of IO (Allelopathic legacy in monocultures: md = 0.053, p > 0.05). Furthermore, the allelopathic legacy had a consistent impact on both above-and below-ground biomass.
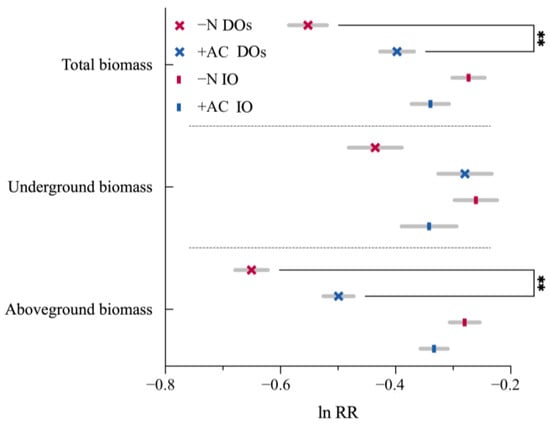
Figure 6.
Soil treatments with congeneric invaders. DOs (×): CIPs native to distinct origins; IO (|): CIPs native to identical origins. **, p ≤ 0.01. Values of p indicate significant differences. Values of p > 0.05 are not presented for clarity.
4. Discussion
The origin of invasive species can have important ecological consequences for co-invasion of CIPs [,,]. At the same time, nutrition and allelopathy also play a crucial role in facilitating the co-invasion of CIPs of IO [,,,]. Specifically, under sufficient nutrition level, the total biomass of IO species has no significant difference between inter- and intra-specific interactions (Figure 3a). However, the total biomass of DO species shows a significant difference (Figure 3b). As a result, we believe CIPs of IO are more likely to co-invade a habitat than CIPs of DOs. In this experiment, CIPs co-invade at a high nutrition level, prioritising reducing stem biomass allocation while increasing the allocation to root and leaf biomass (Figure 4 and Figure 5). The co-occurring invasive–invasive plant strategy is not the same as the invasive–native plant strategy [,]. Invasive plants must adjust the allocation (higher or lower) of seed biomass according to their neighbouring collaborators or rivals for survival []. Following the exclusion of allelochemicals, PS and IS of IO will allocate a reduced amount of root biomass and invest more biomass into seed production, aiming to compete for additional nutrition and ensure stable reproduction (Figure 3 and Figure 4a,c). Notably, when CIPs of IO co-invade, PS of IO gain a competitive advantage, they exhibit significant reductions in single leaf mass, leaf area, leaf length, leaf width, and specific leaf area (SLA). In contrast, PS of DOs have the opposite strategy. The single-leaf growth pattern is in alignment with photosynthesis. These findings suggest that CIPs employ distinct nitrogen allocation strategies to regulate leaf construction costs during co-invasion []. The stems serve as the primary vegetative organs responsible for plant transportation and support, playing a crucial role in protecting against external environmental factors []. IS reduce biomass allocation to their stems, significantly reduce plant height, and adjust the cost of transport, thereby ensuring the stable reproduction of species. When CIPs of DOs co-invade, PS of DOs compete fiercely with IS. PS of DOs allocate more resources to support organ biomass and reduce the proportion of root and leaf biomass allocation to ensure greater height and coverage. Additionally, PS of DOs invest more resources into seed production to secure a reproductive advantage for their species. IS of DOs prefer allocating less leaf biomass while significantly increasing a single leaf’s area, mass, length, and width. Preferentially, IS of DOs allocate more biomass to seed biomass to ensure stable species reproduction. Notably, the strategy of IS of DOs is diametrically opposed to that of PS of DOs. The nitrogen allocation strategy of PS of IOs is active, while that of IS of DOs is passive. Thus, this study found that actively reducing leaf construction costs can promote species abundance and accelerate dispersal; passively reducing the construction cost can at least ensure survival until favourable conditions.
Plant neighbour allelobiosis and allelopathy have been widely reported among species [,,], but less is known about CIPs’ interactions. Various studies have demonstrated that the production of indirect defence traits is regulated by allelopathic interactions [,]. Allelopathy is broadly defined as a mechanism of inhibition, while allelobiosis is defined as a positive effect in chemical interactions [], although their primary functions in interactions between CIPs differ []. Competition becomes more apparent when allelochemicals are removed from CIPs of IO. We hypothesise that allelobiosis between CIPs may mediate co-invasion, allowing for adjustments in biomass allocation and avoiding excessive resource investment in competitive and defensive behaviours, ultimately benefiting both itself and the population. Additionally, the soil conditioned by invasive plants can enhance the establishment of subsequent CIPs of IO. We hypothesise that their potential shared adaptations to the corresponding regional environment with CIPs []. After the removal of allelochemicals, the total biomass of CIPs of DO was consistently decreased by direct allelopathy and allelopathic legacy []. Additionally, the total biomass of IS of DOs was significantly greater than that of PS and instead became the predominant species. All species allocated more resources to ensure the advantages of basal diameter and coverage, which were pivotal factors in competition and nutrition acquisition []. Our hypothesis is that there is considerable allelopathy between PS and IS of DOs, making co-invasion more challenging.
Alien species may also be attacked by natural enemies of closely related species, thereby hindering their invasion []. However, our field observations reveal that when A. trifida and A. artemisiifolia co-invade, Ophraella communa prefers A. artemisiifolia (Figure 7). This preference allows A. artemisiifolia to share the burden of natural enemies with CIPs (A. trifid) and increases the likelihood of successful invasion. We believe that this is one of the reasons that CIPs of IO are more likely to co-invade the field.

Figure 7.
(a) Co-invasion of Ambrosia trifida and Ambrosia artemisiifolia. (b) A. trifida is fed by Ophraella communa when it invades alone. (c) O. communa. (d) A. artemisiifolia is preferentially fed when A. trifida and A. artemisiifolia co-invade.
The process of co-adaptation characterizes the co-evolution of ecologically closely and distantly related species, and the outcomes of this coevolutionary relatedness are contingent upon it. Any evolutionary advancement in one species can exert competitive pressure on other species, even under constant environmental conditions []. CIPs of IO are capable of ‘resource sharing’ (it takes one to know one) and can coexist in a habitat while competing with other species for resources [,,]. CIPs of DO have apparent competition even under high nutrition levels. Allelopathic inhibition emerged as the dominant mechanism driving CIPs to compete when they co-invade, reflecting a strategic approach similar to ‘A bird in the hand is worth two in the bush’, thus preventing co-existence in a habitat. The co-invasion of CIPs varies due to differences in species origin, thus indicating that discussions on invasive plant dispersal should rely on more than just phylogeny [,].
5. Conclusions
This study conducted a controlled experiment to investigate the differential growth responses of invasive species to interaction and allelopathic legacy on CIPs of origin differences. CIPs of IO co-invade through allelobiosis and share resources in a habitat. On the contrary, CIPs of DO could regulate investment in competition and defence by allelopathy, thereby monopolizing habitat nutrition. Hence, when implementing biological control measures, it is crucial to prioritize monitoring and managing co-invasion and re-invasion by CIPs of IO existing invaders in invasive habitats, as these habitats exhibit heightened vulnerability to invasion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13131807/s1, Table S1. Study species used in the experiment; Table S2. Species combinations used in Experiment 1; Table S3. Species combinations used in Experiment 2.
Author Contributions
Writing—original draft and Funding acquisition, Y.G. (Yujun Guo); Conceptualization, Y.G. (Yujun Guo) and B.Q.; Writing—review and editing, Formal analysis, Methodology, Y.G. (Yujun Guo), M.S. and P.G.; Investigation and Data curation, M.Y., L.G., Y.G. (Ying Gao) and L.M.; Validation and Supervision, B.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Key Research and Development Program of China (2023YFC2604500 and 2022YFF1301004). We greatly appreciate the anonymous reviewers for their insightful comments, which improved this manuscript greatly.
Data Availability Statement
Data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adams, C.R.; Hovick, S.M.; Anderson, N.O.; Kettenring, K.M. We Can Better Manage Ecosystems by Connecting Solutions to Constraints: Learning from Wetland Plant Invasions. Front. Environ. Sci. 2021, 9, 715350. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.D.; An, Y.; Song, T.J.; Tong, S.Z.; Wang, X. Effects of Land Use Changes on the Plant Community Characteristics in the Wetlands of the Semi-Arid Regions. Diversity 2022, 14, 1049. [Google Scholar] [CrossRef]
- Chen, D.; van Kleunen, M. Invasional Meltdown Mediated by Plant-Soil Feedbacks May Depend on Community Diversity. New Phytol. 2022, 235, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.; Kühn, I.; et al. Projecting the Continental Accumulation of Alien Species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.R.; Chen, B.J.W. Detect Thy Family: Mechanisms, Ecology and Agricultural Aspects of Kin Recognition in Plants. Plant Cell Environ. 2021, 44, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.A.J.; Briskie, J.V.; Burke, T.; Hatchwell, B.J. Genetic Analysis Reveals Diverse Kin-Directed Routes to Helping in the Rifleman Acanthisitta Chloris. Mol. Ecol. 2013, 22, 5027–5039. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. Plant Genomes: Markers of Evolutionary History and Drivers of Evolutionary Change. Plants People Planet 2021, 3, 74–82. [Google Scholar] [CrossRef]
- Darwin, C. The Evolution Debate 1813–1870, 1st ed.; Routledge: London, UK, 2003. [Google Scholar] [CrossRef]
- Daehler, C.C. Darwin’s Naturalization Hypothesis Revisited. Am. Nat. 2001, 158, 324–330. [Google Scholar] [CrossRef]
- Ricciardi, A.; Mottiar, M. Does Darwin’s Naturalization Hypothesis Explain Fish Invasions? Biol. Invasions 2006, 8, 1403–1407. [Google Scholar] [CrossRef]
- Diez, J.M.; Sullivan, J.J.; Hulme, P.E.; Edwards, G.; Duncan, R.P. Darwin’s Naturalization Conundrum: Dissecting Taxonomic Patterns of Species Invasions. Ecol. Lett. 2008, 11, 674–681. [Google Scholar] [CrossRef]
- Li, S.P.; Cadotte, M.W.; Meiners, S.J.; Hua, Z.S.; Shu, H.Y.; Li, J.T.; Shu, W.S. The Effects of Phylogenetic Relatedness on Invasion Success and Impact: Deconstructing Darwin’s Naturalisation Conundrum. Ecol. Lett. 2015, 18, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Gallien, L.; Boulangeat, I.; De Bello, F.; Münkemüller, T.; Roquet, C.; Lavergne, S. Resolving Darwin’s Naturalization Conundrum: A Quest for Evidence. Divers. Distrib. 2010, 16, 461–475. [Google Scholar] [CrossRef]
- Sheppard, C.S.; Carboni, M.; Essl, F.; Seebens, H.; Thuiller, W.; Pysek, P. It Takes One to Know One: Similarity to Resident Alien Species Increases Establishment Success of New Invaders. Divers. Distrib. 2018, 24, 680–691. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Xiao, H.G.; Wu, B.D.; Jiang, K.; Wang, C.Y. Co-Invasion of Daisy Fleabane and Canada Goldenrod Pose Synergistic Impacts on Soil Bacterial Richness. J. Cent. South Univ. 2020, 27, 1790–1801. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M.; Pyšek, P. Plant Invasions and Invasibility of Plant Communities. In Vegetation Ecology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 387–424. ISBN 978-1-118-45259-2. [Google Scholar]
- Gioria, M.; Osborne, B.A. Resource Competition in Plant Invasions: Emerging Patterns and Research Needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Z.; Cui, H.; Song, H.; Wang, J.; Gao, H.; Chen, S.; Liu, K.; Yang, Z.; Wang, Y.; et al. Direct and Indirect Effects of Dominant Plants on Ecosystem Multifunctionality. Front. Plant Sci. 2023, 14, 1117903. [Google Scholar] [CrossRef] [PubMed]
- Matzek, V. Trait Values, Not Trait Plasticity, Best Explain Invasive Species’ Performance in a Changing Environment. PLoS ONE 2012, 7, e48821. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wei, Z.; Friman, V.-P.; Xu, Y.; Shen, Q.; Kowalchuk, G.A.; Jousset, A. Resource Availability Modulates Biodiversity-Invasion Relationships by Altering Competitive Interactions. Environ. Microbiol. 2017, 19, 2984–2991. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Hardrath, A.; Jin, H.; van Kleunen, M. Increases in Multiple Resources Promote Competitive Ability of Naturalized Non-Native Plants. Commun. Biol. 2022, 5, 1150. [Google Scholar] [CrossRef]
- Adomako, M.O.; Yu, F.-H. Effects of Resource Availability on the Growth, Cd Accumulation, and Photosynthetic Efficiency of Three Hyperaccumulator Plant Species. J. Environ. Manag. 2023, 345, 118762. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Engelbrecht, B.M.; Lusk, C.H.; McDowell, N.G.; Poorter, H. Resource Limitation, Tolerance, and the Future of Ecological Plant Classification. Front. Plant Sci. 2012, 3, 246. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L. The Physiology of Invasive Plants in Low-Resource Environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef]
- Suding, K.N.; LeJeune, K.D.; Seastedt, T.R. Competitive Impacts and Responses of an Invasive Weed: Dependencies on Nitrogen and Phosphorus Availability. Oecologia 2004, 141, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Feng, Y.-L.; Chen, Y.-J.; Tian, Y.-H. Soil Microbes Alleviate Allelopathy of Invasive Plants. Sci. Bull. 2015, 60, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Brunel, C.; van Kleunen, M. Soil-Microorganism-Mediated Invasional Meltdown in Plants. Nat. Ecol. Evol. 2020, 4, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2020, 23, 367–371. [Google Scholar] [CrossRef]
- Fahey, C.; Koyama, A.; Antunes, P.M.; Dunfield, K.; Flory, S.L. Plant Communities Mediate the Interactive Effects of Invasion and Drought on Soil Microbial Communities. ISME J. 2020, 14, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Kama, R.; Javed, Q.; Liu, Y.; Li, Z.Y.; Iqbal, B.; Diatta, S.; Sun, J.F. Effect of Soil Type on Native Pterocypsela Laciniata Performance under Single Invasion and Co-Invasion. Life 2022, 12, 1898. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Brunel, C.; Van Kleunen, M. Evidence for Elton’s Diversity–Invasibility Hypothesis from Belowground. Ecology 2020, 101, e03187. [Google Scholar] [CrossRef]
- Lei, Y.B.; Feng, Y.L.; Zheng, Y.L.; Wang, R.F.; Gong, H.D.; Zhang, Y.P. Innate and Evolutionarily Increased Advantages of Invasive Eupatorium Adenophorum over Native E. Japonicum under Ambient and Doubled Atmospheric CO2 Concentrations. Biol. Invasions 2011, 13, 2703–2714. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Wang, C.; Cheng, J.; Qiang, S. A Comparative Study Reveals the Key Biological Traits Causing Bioinvasion Differences among Four Alien Species of Genus Veronica in China. J. Plant Ecol. 2023, 16, rtac068. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Eller, F.; Luo, Y.; Nong, Y.; Xing, L.; Xu, Z.; Li, H.; Lu, H.; Guo, X. Trait Value and Phenotypic Integration Contribute to the Response of Exotic Rhus Typhina to Heterogeneous Nitrogen Deposition: A Comparison with Native Rhus Chinensis. Sci. Total Environ. 2022, 844, 157199. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-L.; Fu, G.-L. Nitrogen Allocation, Partitioning and Use Efficiency in Three Invasive Plant Species in Comparison with Their Native Congeners. Biol. Invasions 2008, 10, 891–902. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, Z.; Zhou, L.; Yang, Q.; Zheng, S.; Li, S.P. Differential Roles of Species Richness versus Species Asynchrony in Regulating Community Stability along a Precipitation Gradient. Ecol. Evol. 2019, 9, 14244–14252. [Google Scholar] [CrossRef] [PubMed]
- Daehler, C.C. Performance Comparisons of Co-Occurring Native and Alien Invasive Plants: Implications for Conservation and Restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Ma, C.; Li, S.P.; Pu, Z.; Tan, J.; Liu, M.; Zhou, J.; Li, H.; Jiang, L. Different Effects of Invader-Native Phylogenetic Relatedness on Invasion Success and Impact: A Meta-Analysis of Darwin’s Naturalization Hypothesis. Proc. Biol. Sci. 2016, 283, 20160663. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Cheng, H.; Du, D. Which Factor Contributes Most to the Invasion Resistance of Native Plant Communities under the Co-Invasion of Two Invasive Plant Species? Sci. Total Environ. 2022, 813, 152628. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xiao, C.; Ma, J. A Dataset on Catalogue of Alien Plants in China. Biodivers. Sci. 2022, 30, 22127. [Google Scholar] [CrossRef]
- Dostal, P. Plant Competitive Interactions and Invasiveness: Searching for the Effects of Phylogenetic Relatedness and Origin on Competition Intensity. Am. Nat. 2011, 177, 655–667. [Google Scholar] [CrossRef]
- Poorter, L. Growth Responses of 15 Rain-forest Tree Species to a Light Gradient: The Relative Importance of Morphological and Physiological Traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Goldberg, D.E. Components of Resource Competition in Plant Communities. Perspect. Plant Compet. 1990, 27–49. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Fleetwood, L.M. Competitive Effect and Response in Four Annual Plants. J. Ecol. 1987, 75, 1131–1143. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Rajaniemi, T.; Gurevitch, J.; Stewart-Oaten, A. Empirical Approaches to Quantifying Interaction Intensity: Competition and Facilitation along Productivity Gradients. Ecology 1999, 80, 1118–1131. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Prati, D. The Relative Importance of Immediate Allelopathy and Allelopathic Legacy in Invasive Plant Species. Basic. Appl. Ecol. Basic. Appl. Ecol. 2015, 16, 28–35. [Google Scholar] [CrossRef]
- Bennett, A.W. The Origin of Species by Means of Natural Selection; or the Preservation of Favoured Races in the Struggle for Life. Nature 1872, 5, 318–319. [Google Scholar] [CrossRef][Green Version]
- Khaleel, A.; Nasir, S.; Ismail, N.; Syazni, A. Origin of Invasive Fish Species, Peacock Bass Cichla Species in Lake Telabak Malaysia Revealed by Mitochondrial DNA Barcoding. Egypt. J. Aquat. Biol. Fish. 2020, 24, 311–322. [Google Scholar] [CrossRef]
- Sun, Y.; Müller-Schärer, H.; Maron, J.L.; Schaffner, U. Origin Matters: Diversity Affects the Performance of Alien Invasive Species but Not of Native Species. Am. Nat. 2015, 185, 725–736. [Google Scholar] [CrossRef][Green Version]
- Kato-Noguchi, H. Allelopathy and Allelochemicals of Imperata Cylindrica as an Invasive Plant Species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of Knotweeds as Invasive Plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef]
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.; Ciais, P.; Obersteiner, M.; Sigurdsson, B.; Penuelas, J. Plant Invasion Is Associated with Higher Plant-Soil Nutrient Concentrations in Nutrient Poor-Environments. Glob. Change Biol. 2016, 23, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-L.; Zhang, S.-Y.; Yuan, X.-F.; Sun, K.; Cai, J.-F.; Xue, J.-J.; Zhang, Y.; A, S.-H.; Yang, L.-J.; Cheng, R.; et al. Flat-Leaf Submerged Plants Are More Sensitive to Invasion Intensity and Water Nutrition Levels than Needle-Leaf Ones. Hydrobiologia 2023, 850, 3849–3863. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wei, M.; Wang, S.; Wu, B.; Du, D. Plant Height and Leaf Size: Which One Is More Important in Affecting the Successful Invasion of Solidago Canadensis and Conyza Canadensis in Urban Ecosystems? Urban For. Urban Green. 2021, 59, 127033. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Feng, Y.-L.; Liu, W.-X.; Liao, Z.-Y. Growth, Biomass Allocation, Morphology, and Photosynthesis of Invasive Eupatorium Adenophorum and Its Native Congeners Grown at Four Irradiances. Plant Ecol. 2009, 203, 263–271. [Google Scholar] [CrossRef]
- Martin, A.R. Crops and the Seed Mass-Seed Output Trade-Off in Plants. Int. J. Plant Sci. 2021, 182, 84–90. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Fu, G.-L.; Zheng, Y.-L. Specific Leaf Area Relates to the Differences in Leaf Construction Cost, Photosynthesis, Nitrogen Allocation, and Use Efficiencies between Invasive and Noninvasive Alien Congeners. Planta 2008, 228, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.J.; Liu, H.M.; Wu, Y.; Bing, J.; Zhang, G.F. New Advances in the Regulation of Stem Growth in Vascular Plants. Plant Growth Regul. 2023, 103, 65–80. [Google Scholar] [CrossRef]
- Wall, L.G.; Favelukes, G. Early Recognition in the Rhizobium Meliloti-Alfalfa Symbiosis: Root Exudate Factor Stimulates Root Adsorption of Homologous Rhizobia. J. Bacteriol. 1991, 173, 3492–3499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semchenko, M.; Saar, S.; Lepik, A. Plant Root Exudates Mediate Neighbour Recognition and Trigger Complex Behavioural Changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Bhatt, M.V.; Khandelwal, A.; Dudley, S.A. Kin Recognition, Not Competitive Interactions, Predicts Root Allocation in Young Cakile Edentula Seedling Pairs. New Phytol. 2011, 189, 1135–1142. [Google Scholar] [CrossRef]
- Vivanco, J.; Paschke, M.; Callaway, R. Allelochemical Control of Non-Indigenous Invasive Plant Species Affecting Military Testing and Training Activities. 2010. Available online: https://serdp-estcp.mil/projects/details/65a3b15a-c8db-4476-ae7b-2868bba8c10f/rc-1388-project-overview (accessed on 7 May 2024).
- Zhang, K.-M.; Shen, Y.; Zhou, X.-Q.; Fang, Y.-M.; Liu, Y.; Ma, L.Q. Photosynthetic Electron-Transfer Reactions in the Gametophyte of Pteris Multifida Reveal the Presence of Allelopathic Interference from the Invasive Plant Species Bidens Pilosa. J. Photochem. Photobiol. B Biol. 2016, 158, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, Z.; Kong, C. Allelobiosis in the Interference of Allelopathic Wheat with Weeds. Pest Manag. Sci. 2016, 72, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.B.; Peterson, D.A.; Ross, L.; Rosenheim, J.A. Does a Plant-Eating Insect’s Diet Govern the Evolution of Insecticide Resistance? Comparative Tests of the Pre-Adaptation Hypothesis. Evol. Appl. 2018, 11, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Ge, Y.; Wang, X.; Gao, S.; Chen, T.; Yu, F. Darwin’s Naturalization Conundrum Reconciled by Changes of Species Interactions. Ecology 2023, 104, e3850. [Google Scholar] [CrossRef] [PubMed]
- Berahim, Z.; Omar, M.H.; Zakaria, N.-I.; Ismail, M.R.; Rosle, R.; Roslin, N.A.; Che’Ya, N.N. Silicon Improves Yield Performance by Enhancement in Physiological Responses, Crop Imagery, and Leaf and Culm Sheath Morphology in New Rice Line, PadiU Putra. BioMed Res. Int. 2021, 2021, 6679787. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-Y.; Yang, Q.; Li, S.-P.; Fristoe, T.S.; Cadotte, M.W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; et al. A Latitudinal Gradient in Darwin’s Naturalization Conundrum at the Global Scale for Flowering Plants. Nat. Commun. 2023, 14, 6244. [Google Scholar] [CrossRef]
- Valen, L.V. A New Evolutionary Law (1973). In Foundations of Macroecology: Classic Papers with Commentaries; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar] [CrossRef]
- Kong, C.-H.; Zhang, S.-Z.; Li, Y.; Xia, Z.-C.; Yang, X.; Meiners, S.J.; Wang, P. Plant Neighbor Detection and Allelochemical Response Are Driven by Root-Secreted Signaling Chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).