Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought
Abstract
1. Introduction
Drought Leads to Significant Yield Losses in Major Food Crops
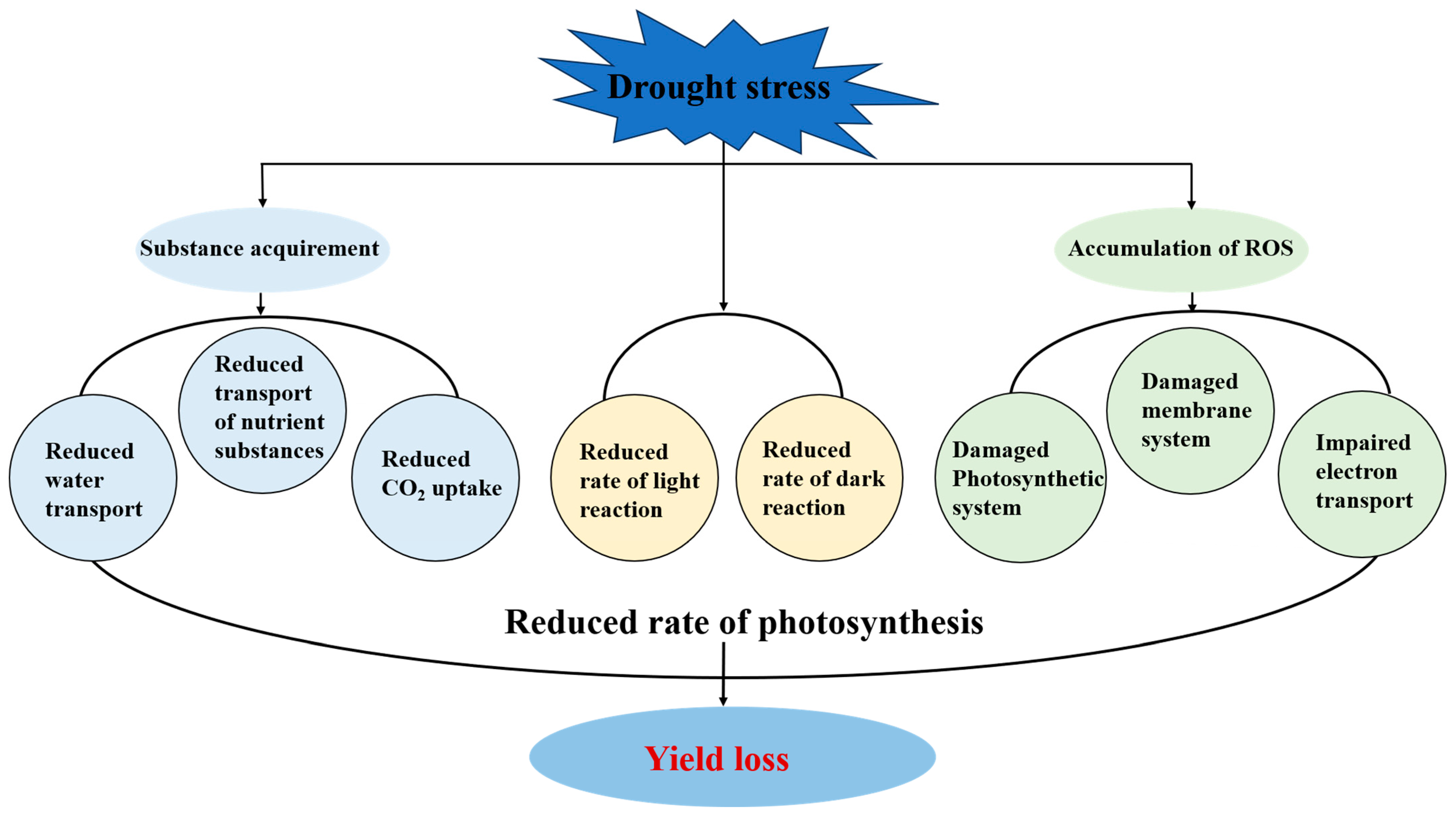
2. Impacts of Drought on Photosynthesis
2.1. Drought Affects Light-Dependent Reactions of Photosynthesis
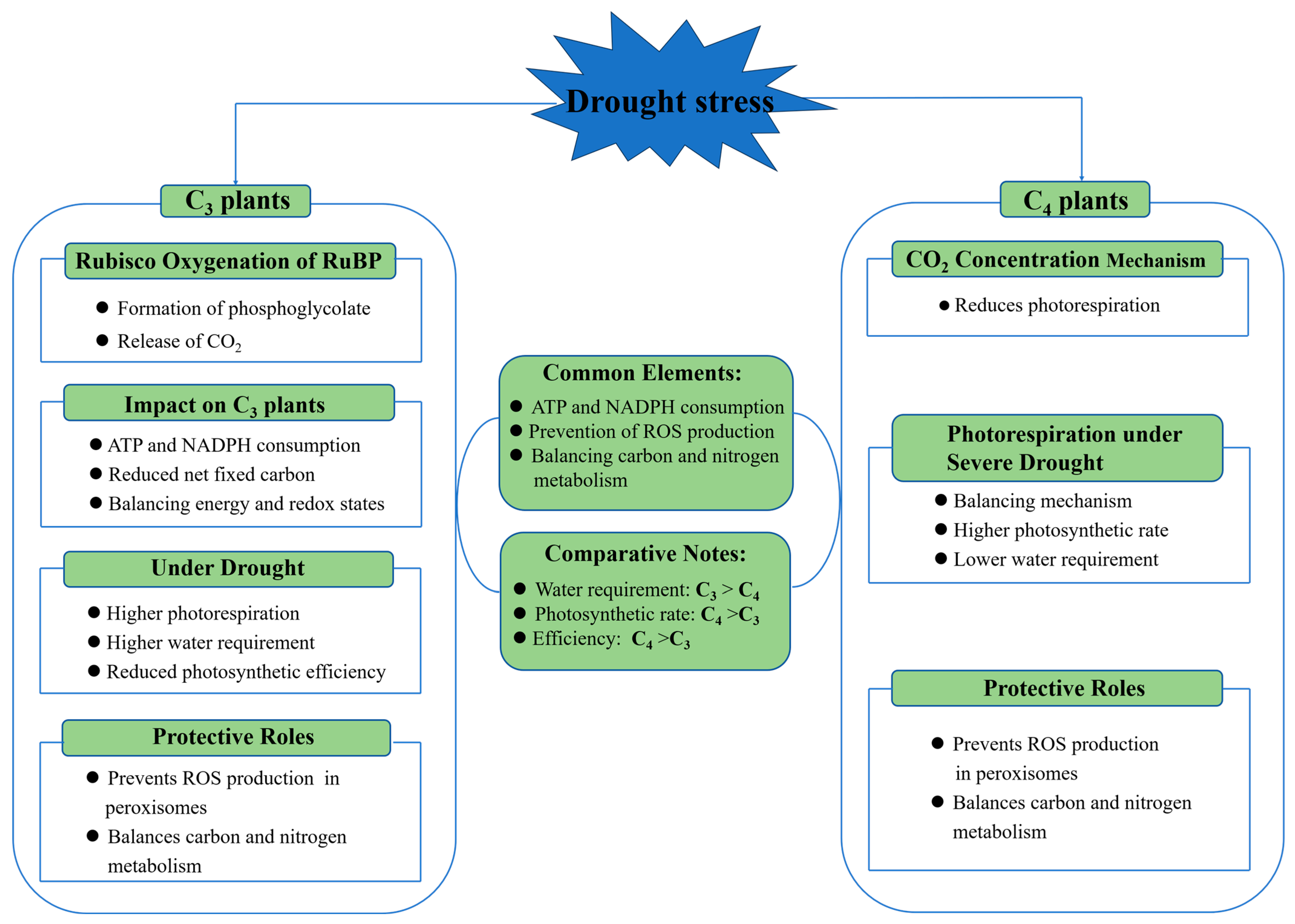
2.2. The Impact of Drought on Dark Reactions
3. Other Impacts of Drought on Major Food Crops
3.1. Reactive Oxygen Species (ROS) Production
3.2. Drought Affects the Channels for Substance Transport
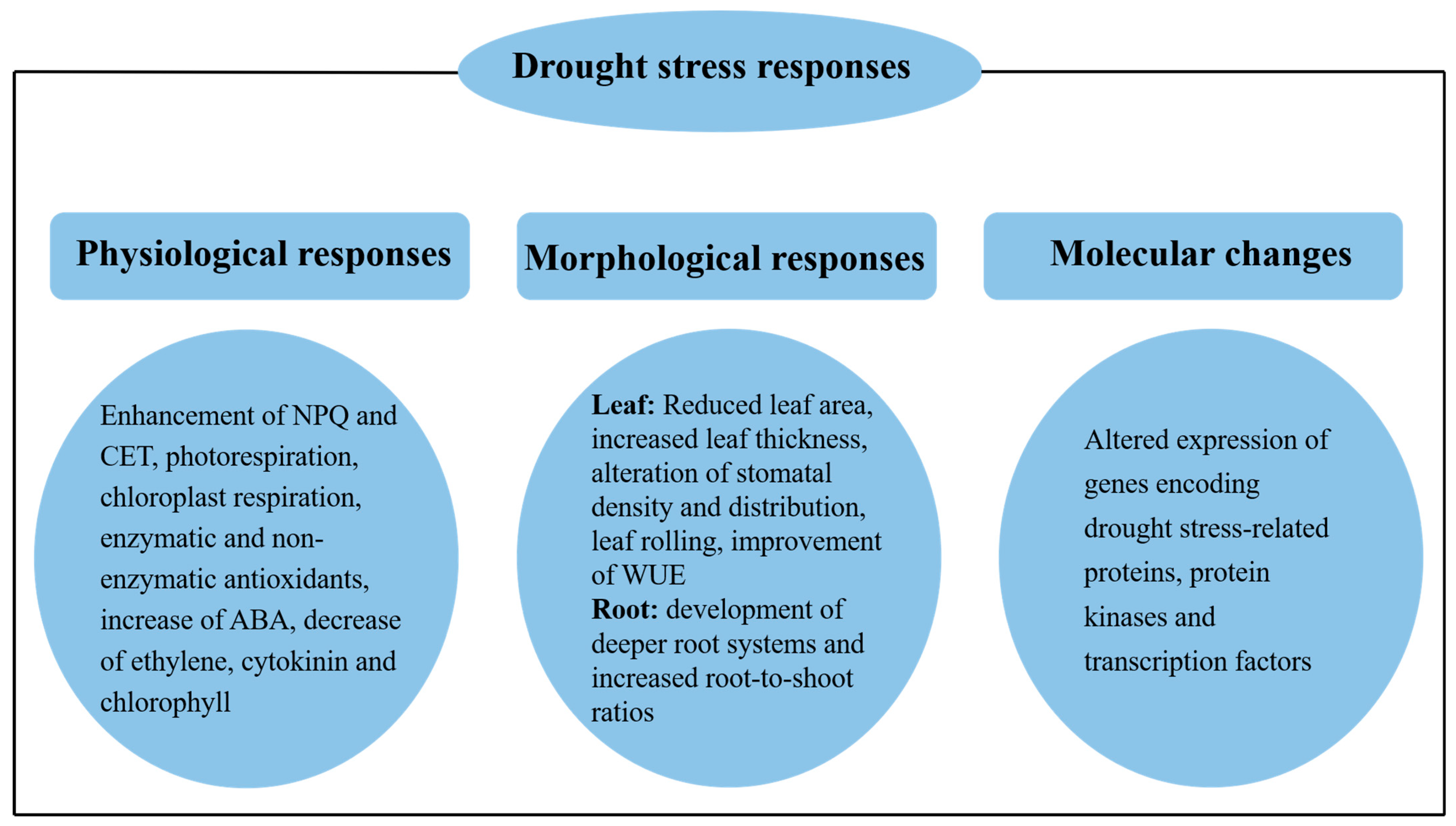
4. Mechanisms of Photosynthetic Response to Drought
4.1. Non-Photochemical Quenching Mechanisms
4.2. Enhancement of Alternative Electron Transfer
4.3. Photorespiration
4.4. Plant Hormones and Chlorophyll Content
4.5. Antioxidant Systems
4.6. Leaf Characteristics
4.7. Improvement of Water Use Efficiency
4.8. Root Structure Regulation
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, B.-M. Effects of Climate Change and Drought Tolerance on Maize Growth. Plants 2023, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.Y.; Lei, L.; Fan, X.W.; Li, Y.Z. Effects of high air temperature, drought, and both combinations on maize: A case study. Plant Sci. 2023, 327, 111543. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Reddy, A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Qi, M.; Liu, X.; Li, Y.; Song, H.; Yin, Z.; Zhang, F.; He, Q.; Xu, Z.; Zhou, G. Photosynthetic resistance and resilience under drought, flooding and rewatering in maize plants. Photosynth. Res. 2021, 148, 1–15. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Umezawa, T.; Fujita, M.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 2006, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Castorina, G.; Domergue, F.; Chiara, M.; Zilio, M.; Persico, M.; Ricciardi, V.; Horner, D.S.; Consonni, G. Drought-responsive ZmFDL1/MYB94 regulates cuticle biosynthesis and cuticle-dependent leaf permeability. Plant Physiol. 2020, 184, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cheng, J.; Lu, M.; Fang, T.; Zhu, Y.; Li, Z.; Wang, X.; Wang, Y.; Guo, Y.; Yang, S.; et al. Ca2+-independent ZmCPK2 is inhibited by Ca2+-dependent ZmCPK17 during drought response in maize. J. Integr. Plant Biol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Okumu, O.O.; Wendot, P.K.; Abraha, N.T.M. Constraints, interventions and prospects for improving Pearl Millet (Pennisetum glaucum L) production in the State of Eritrea—A review. Innov. Agric. 2023, 6, e32868. [Google Scholar] [CrossRef]
- Kumar, A.; Bernier, J.; Verulkar, S.; Lafitte, H.R.; Atlin, G.N. Breeding for drought tolerance: Direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crop. Res. 2008, 107, 221–231. [Google Scholar] [CrossRef]
- Melandri, G.; AbdElgawad, H.; Riewe, D.; Hageman, J.A.; Asard, H.; Beemster, G.T.S.; Kadam, N.; Jagadish, K.; Altmann, T.; Ruyter-Spira, C.; et al. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020, 71, 669–683. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.K.; Das, B.; Govaerts, B.; Vinayan, M.T. Maize production in a changing climate: Impacts, adaptation, and mitigation strategies. Adv. Agron. 2013, 114, 1–58. [Google Scholar]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Sharma, P.; Zheng, B. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar]
- Prasil, O.; Kolber, Z.; Berry, J.A.; Falkowski, P.G. Cyclic electron flow around Photosystem II in vivo. Photosynth. Res. 1996, 48, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, Y.Y.; Bai, Y.W.; Camberato, J.J.; Xue, J.Q.; Zhang, R.H. Effects of Drought Stress on the Photosynthesis in Maize. Russ. J. Plant Physiol. 2018, 65, 849–856. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Parry, M.A.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: Effects of drought stress. Ann. Bot. 2002, 89, 833–839. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, W.; Peng, W.; Jiang, M.; Chen, G.; Xiong, F. Sucrose transporter in rice. Plant Signal. Behav. 2021, 16, 1952373. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plan. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid in grapevines growing under drought conditions. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S. Phloem transport and drought. J. Exp. Bot. 2014, 65, 1751–1759. [Google Scholar] [CrossRef]
- Wolf, S.; Deom, C.M.; Beachy, R.N.; Lucas, W.J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 1989, 246, 377–379. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Assoc. Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lee, S.K.; Yoo, Y.; Wei, J.; Kwon, S.Y.; Lee, S.W.; Jeon, J.S.; An, G. Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.M. Plant science. SWEET! The pathway is complete. Science 2012, 335, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, S.; Yunjuan, R.; Chen, S.; Liesche, J. Regulation of sucrose transporters and phloem loading in response to environmental cues. Plant Physiol. 2018, 176, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Wilson, S. The mechanism of Non-Photochemical Quenching in plants: Localization and driving forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Ruban, A.V. Photoprotective energy dissipation in higher plants involves alteration of the excited state energy of the emitting chlorophyll(s) in the light harvesting antenna II (LHCII). J. Biol. Chem. 2009, 284, 23592–23601. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.G.; Sane, P.V.; Hurry, V.; Oquist, G.; Huner, N.P. Photosystem II reaction centre quenching: Mechanisms and physiological role. Photosynth. Res. 2008, 98, 565–574. [Google Scholar] [CrossRef]
- Ilioaia, C.; Johnson, M.P.; Duffy, C.D.; Pascal, A.A.; van Grondelle, R.; Robert, B.; Ruban, A.V. Origin of absorption changes associated with photoprotective energy dissipation in the absence of zeaxanthin. J. Biol. Chem. 2011, 286, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2002, 403, 391–395. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Okoń, K.; Skorupka, M. Non-Photochemical Quenching under Drought and Fluctuating Light. Int. J. Mol. Sci. 2022, 23, 5182. [Google Scholar] [CrossRef]
- Krause, G.H.; Jahns, P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function. In Chlorophyll Fluoresc; Springer: Dordrecht, The Netherlands, 2004; pp. 463–495. [Google Scholar]
- Li, L.; Gu, W.; Li, J.; Li, C.; Xie, T.; Qu, D.; Meng, Y.; Li, C.; Wei, S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta 2011, 1807, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Carol, P.; Kuntz, M. A plastid terminal oxidase comes to light: Implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 2001, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N.; Stepien, P. Plastid Terminal Oxidase as a Route to Improving Plant Stress Tolerance: Known Knowns and Known Unknowns. Plant Cell Physiol. 2016, 57, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Horst, I.; Niessen, M.; Blume, C.; Kebeish, R.; Flugge, U.I.; Maurino, V.G. Photorespiration. Arab. Book. 2010, 8, e0130. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, J.; Kishimoto, N.; Nagata, Y.; Ishikawa, M.; Fujii, F.; Hashimoto, A.; Shimbo, K.; Shimatani, Z.; Kojima, K.; Suzuki, K.; et al. Genomics approach to abscisic acid- and gibberellin-responsive genes in rice. DNA Res. 2003, 10, 249–261. [Google Scholar] [CrossRef]
- Hu, X.; Wu, X.; Li, C.; Lu, M.; Liu, T.; Wang, Y.; Wang, W. Abscisic acid refines the synthesis of chloroplast proteins in maize (Zea mays) in response to drought and light. PLoS ONE 2012, 7, e49500. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Ren, Z.; Abou-Elwafa, S.F.; Pu, X.; Zhu, Y.; Dou, D.; Su, H.; Cheng, H.; Liu, Z.; et al. ZmERF21 directly regulates hormone signaling and stress-responsive gene expression to influence drought tolerance in maize seedlings. Plant Cell Environ. 2022, 45, 312–328. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, E.; Emam, Y. Effect of defoliation and drought stress on yield components and chlorophyll content of wheat. Pak. J. Biol. Sci. 2010, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vilalta, J.; Anderegg, W.R.L.; Sapes, G.; Sala, A. Greater focus on water pools may improve our ability to understand and anticipate drought-induced mortality in plants. New Phytol. 2019, 223, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of Wheat (Triticum aestivum L.) Genotypes for Drought Tolerance through Agronomic and Physiological Response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Goswami, A.; Banerjee, R.; Raha, S. Drought resistance in rice seedlings conferred by seed priming. Protoplasma 2013, 250, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Srivastava, G.C. Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop. Sci. 2001, 186, 49–55. [Google Scholar] [CrossRef]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss army knife: The diverse protective roles of anthocyanins in leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Shi, C.Q. Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Zhang, Q.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L. Maize Tolerance against Drought and Chilling Stresses Varied with Root Morphology and Antioxidative Defense System. Plants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. Int. 2016, 23, 17132–17141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2007, 58, 169–175. [Google Scholar] [CrossRef]
- Bahrun, A.; Jensen, C.R.; Asch, F.; Mogensen, V.O. Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J. Exp. Bot. 2002, 53, 251–263. [Google Scholar] [CrossRef]
- Kadioglu, A.; Terzi, R. A dehydration avoidance mechanism: Leaf rolling. Bot. Rev. 2007, 73, 290–302. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, G.; Li, G.; Wang, Q. Quantitative evaluation of the Trade-Off growth strategies of maize leaves under different drought severities. Water 2021, 13, 1852. [Google Scholar] [CrossRef]
- Chen, G.; Komatsuda, T.; Ma, J.F.; Li, C.; Yamaji, N.; Nevo, E. A functional cutin matrix is required for plant protection against water loss. Plant Signal. Behav. 2011, 6, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hao, J.; Lv, M.; Liu, P.; Ge, Q.; Zhang, S.; Yang, J.; Niu, H.; Wang, Y.; Xue, Y.; et al. A genome-wide association study identifies genes associated with cuticular wax metabolism in maize. Plant Physiol. 2024, 194, 2616–2630. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Kang, K.; Choi, Y.; Suh, M.C.; Paek, N.C. Mutation of OsMYB60 reduces rice resilience to drought stress by attenuating cuticular wax biosynthesis. Plant J. 2022, 112, 339–351. [Google Scholar] [CrossRef]
- Kränzlein, M.; Schmöckel, S.M.; Geilfus, C.M.; Schulze, W.X.; Altenbuchinger, M.; Hrenn, H.; Roessner, U.; Zörb, C. Lipid remodeling of contrasting maize (Zea mays L.) hybrids under repeated drought. Front. Plant Sci. 2023, 14, 1050079. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Figueiredo, J.; Laureano, G.; Machado, A.; Arrabaça, J.D.; Duarte, B.; Figueiredo, A.; Matos, A.R. Membrane remodelling and triacylglycerol accumulation in drought stress resistance: The case study of soybean phospholipases A. Plant Physiol. Biochem. 2021, 169, 9–21. [Google Scholar] [CrossRef]
- Qi, Y.; Yamauchi, Y.; Ling, J.; Kawano, N.; Li, D.; Tanaka, K. Cloning of a putative monogalactosyldiacylglycerol synthase gene from rice (Oryza sativa L.) plants and its expression in response to submergence and other stresses. Planta 2004, 219, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S.; Arikit, S.; et al. Induced genetic variations in stomatal density and size of rice strongly affects water use efficiency and responses to drought stresses. Front. Plant Sci. 2022, 13, 801706. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Henry, A.; Gowda, V.R.P.; Torres, R.O.; McNally, K.L.; Serraj, R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the Oryza SNP panel in rainfed lowland fields. Field Crop. Res. 2011, 120, 205–214. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Terzi, R.; Saruhan, N.; Saglam, A. Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Sci. 2012, 182, 42–48. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of squaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Lucob-Agustin, N.; Kawai, T.; Kano-Nakata, M.; Suralta, R.R.; Niones, J.M.; Hasegawa, T.; Inari-Ikeda, M.; Yamauchi, A.; Inukai, Y. Morpho-physiological and molecular mechanisms of phenotypic root plasticity for rice adaptation to water stress conditions. Breed. Sci. 2021, 71, 20–29. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Yamamoto, E. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef]
- Tuberosa, R.; Salvi, S.; Sanguineti, M.C.; Maccaferri, M.; Giuliani, S.; Landi, P. Searching for quantitative trait loci controlling root traits in maize: A critical appraisal. Plant Soil. 2003, 255, 35–54. [Google Scholar] [CrossRef]
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngara, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Babu, R.C.; Blum, A. Breeding for drought resistance in rice: Physiology and molecular genetics considerations. Crop Sci. 1997, 37, 1426–1434. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, R.; Sharma, R.; Kapoor, D.; Kohli, S.; Kumar, V.; Kaur, P. Hormonal regulation of drought stress responses in plants. Plant Physiol. 2013, 162, 209–217. [Google Scholar]
- Tran, L.S.P.; Quach, T.N.; Guttikonda, S.K.; Aldrich, D.L.; Kumar, R.; Neelakandan, A.; Valliyodan, B.; Nguyen, H.T. Molecular characterization of stress-inducible GmNAC genes in soybean. Mol. Genet. Genom. 2009, 281, 647–664. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
| Country | Major Crops | Area (1000 Ha) | Yield (T/Ha) |
|---|---|---|---|
| China | Zea mays (maize) | 44,218 | 6.5 |
| Oryza sativa (rice) | 28,949 | 7.1 | |
| Triticum aestivum (wheat) | 23,627 | 5.8 | |
| Glycine max (soybean) | 10,470 | 2.0 | |
| Arachis hypogaea (peanut) | 4820 | 3.9 | |
| USA | Zea mays (maize) | 35,011 | 11.1 |
| Glycine max (soybean) | 33,328 | 3.4 | |
| Triticum aestivum (wheat) | 15,084 | 3.3 | |
| Sorghum bicolor (sorghum) | 2475 | 3.3 | |
| Oryza sativa (rice) | 1155 | 8.6 | |
| India | Oryza sativa (rice) | 48,000 | 4.2 |
| Triticum aestivum (wheat) | 31,401 | 3.5 | |
| Zea mays (maize) | 11,000 | 3.4 | |
| Panicum miliaceum (millet) | 9500 | 1.4 | |
| Glycine max (soybean) | 13,000 | 0.9 | |
| Brazil | Glycine max (soybean) | 45,900 | 3.4 |
| Zea mays (maize) | 21,500 | 5.7 | |
| Triticum aestivum (wheat) | 3470 | 2.3 | |
| Oryza sativa (rice) | 1545 | 6.9 | |
| Sorghum bicolor (sorghum) | 1541 | 3.1 | |
| EU | Triticum aestivum (wheat) | 24,200 | 5.5 |
| Zea mays (maize) | 8280 | 7.4 | |
| Hordeum vulgare (barley) | 10,300 | 4.6 | |
| Brassica napus (rapeseed) | 6220 | 3.2 | |
| Helianthus annuus (Sunflower seed) | 4801 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. https://doi.org/10.3390/plants13131808
Qiao M, Hong C, Jiao Y, Hou S, Gao H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants. 2024; 13(13):1808. https://doi.org/10.3390/plants13131808
Chicago/Turabian StyleQiao, Meiyu, Conghao Hong, Yongjuan Jiao, Sijia Hou, and Hongbo Gao. 2024. "Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought" Plants 13, no. 13: 1808. https://doi.org/10.3390/plants13131808
APA StyleQiao, M., Hong, C., Jiao, Y., Hou, S., & Gao, H. (2024). Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants, 13(13), 1808. https://doi.org/10.3390/plants13131808






