Abstract
One of the objectives of this study consists of the assessment of the antitumor activity of several extracts from three selected plant species: Xanthium spinosum L., Trifolium pratense L., and Coffea arabica L. and also a comparative study of this biological activity, with the aim of establishing a superior herbal extract for antitumor benefits. The phytochemical profile of the extracts was established by HPLC-MS analysis. Further, the selected extracts were screened in vitro for their antitumor activity and antioxidant potential on two cancer cell lines: A549—human lung adenocarcinoma and T47D-KBluc—human breast carcinoma and on normal cells. One extract per plant was selected for in vivo assessment of antitumor activity in an Ehrlich ascites mouse model. The extracts presented high content of antitumor compounds such as caffeoylquinic acids in the case of X. spinosum L. (7.22 µg/mL—xanthatin, 4.611 µg/mL—4-O-caffeoylquinic acid) and green coffee beans (10.008 µg/mL—cafestol, 265.507 µg/mL—4-O-caffeoylquinic acid), as well as isoflavones in the case of T. pratense L. (6806.60 ng/mL—ononin, 102.78 µg/mL—biochanin A). Concerning the in vitro results, the X. spinosum L. extracts presented the strongest anticancerous and antioxidant effects. In vivo, ascites cell viability decreased after T. pratense L. and green coffee bean extracts administration, whereas the oxidative stress reduction potential was important in tumor samples after T. pratense L. Cell viability was also decreased after administration of cyclophosphamide associated with X. spinosum L. and T. pratense L. extracts, respectively. These results suggested that T. pratense L. or X. spinosum L. extracts in combination with chemotherapy can induce lipid peroxidation in tumor cells and decrease the tumor viability especially, T. pratense L. extract.
1. Introduction
Cancer represents a disease in which certain pathophysiological alterations occur in the process of cell division. This pathogenesis consists of various types of tumors. Though many advances have been made concerning diagnosing and treating this dreadful disease, new information regarding phenotypic plasticity and disrupted differentiation have been recently established as new hallmarks of cancer [1]. According to recent statistics, more than 19.3 million cancer cases were diagnosed and reported worldwide [2]. Phytotherapy has been proposed as an adjuvant treatment or alternative to chemotherapy, radiation therapy, immunotherapy, surgery, and many other forms of approach to treating cancer. Unfortunately, these methods have been known to cause grave side effects as well as chemoresistance. Clinically tested and familiar plant-derived bioactive compounds are vinblastine, vincristine, podophyllotoxin, paclitaxel, and camptothecin [3].
The antiproliferative activity of polyphenolic compounds is widely known throughout the current scientific literature. It occurs as a result of their antioxidant properties, ROS scavenging, chelating transition elements, and regulating the activity and synthesis of antioxidant enzymes. Examples of bioactive compounds pertaining to this class with potent antitumor activity are epigallocatechin-3-gallate, trans-resveratrol, quercetin, and curcumin [4]. Other plant-derived bioactive compounds such as acetogenins and annomuricin from Annona muricata L. have demonstrated in vitro cytotoxicity or emodin, an anthraquinoid compound from Aloe vera L., also showed in vivo cytotoxicity [3].
The plant genus Xanthium L. is well known for its species richness in polyphenols, namely caffeoylquinic acids (CQA). Chlorogenic represent a class of esters formed between quinic and hydroxycinnamic acids, such as caffeic, ferulic, or p-coumaric acid. Principal classes of caffeoylquinic acids comprise CQA, dicaffeoylquinic acids (diCQA), and feruoylquinic acids. These compounds have been reported to exhibit numerous biological activities, some of which include antioxidant, antibacterial, anti-inflammatory, and neuroprotective activities as well as antitumor effects [5]. For practical reasons, this paper focuses on several isomers of the chemical class of CQA, such as 4-O-caffeoylquinic (4-CQA), 3,4-, 3,5-, and 4,5-dicaffeoylquinic acids (diCQA). The genus’ anti-cancer potential is also due to an innately secreted class of sesquiterpene lactones, the xanthanolides. Although phytotoxic in their primary nature, xanthanolides have been demonstrated to also possess anti-inflammatory, antimicrobial, and even anti-tumor activities [6,7,8]. Xanthatin is one of the main bioactive components of Xanthium extracts proven to inhibit tumor growth and cell proliferation by proposed mechanisms including inhibition of the mitotic spindle assembly, inducing G2-M arrest and impairment of anaphase entrance [9,10]. For practical reasons, this study focuses on xanthatin and its isomer, 8-epi-xanthatin. Xanthium spinosum L. was selected for the study due to the lack of research concerning it. Trifolium pratense L., or red clover by its common name, is widely known for its isoflavonic compounds such as formononetin, biochanin A, ononin, daidzein, and genistein. Not only being considered phytoestrogens with estrogenic, anti-inflammatory, cardioprotective, and neuroprotective activities, these compounds have also displayed antitumor effects as well [11,12,13,14,15,16,17]. Regarding their antitumor activity, the three isoflavonic compounds, biochanin A, formononetin, and ononin, have been reported to promote apoptosis, inhibit cell proliferation and differentiation, and other cell cycle phases, as well as inhibit cellular oxidative stress [18,19,20,21]. Coffee has garnered the attention of researchers as its consumption has been associated with a decreased risk of the development of various types of cancer [22,23,24]. The polyphenolic compounds of coffee beans, especially the unprocessed green coffee beans from Coffea arabica L. (also named Arabica), such as chlorogenic acids and CQA have been reported to inhibit cancer cell proliferation and apoptosis [25,26]. These compounds are found in larger amounts in unroasted, green coffee beans, as opposed to the roasted plant material [27,28,29]. In addition, the diterpenoid compounds, found in the lipidic fraction of green coffee beans, such as kahweol, for instance, have been reported to reduce cyclin D1 degradation, thus inhibiting the proliferation of human colorectal cancer cells [27,30].
The purpose of this study was to assess the antitumor activity of several extracts from the three plant species (mentioned above): Xanthium spinosum L., Trifolium pratense L., and Coffea arabica L. In addition, the authors have accomplished a comparative study of this biological activity for the three plant species, with the aim of establishing the superior herbal extract, in regard to the antitumor benefits.
Phytochemical characterization of the extracts was first performed in order to identify and quantify the antitumor bioactive compounds. Six extracts were studied in vitro for their anticancerous and antioxidant potentials. Based on the results of the phytochemical profiling and the results from the in vitro studies, only three extracts were further subjected to the in vivo assessment of the antitumor activity.
2. Results
2.1. HPLC-MS Analysis of the Extracts
The HPLC-MS results of the initially selected extracts are presented in the following sections. The nomenclature of the samples used in this section, only, can be found in previously published works on the selected plant species [31,32,33].
2.1.1. Analysis of Antitumor Compounds from the Xanthium Extracts
Xanthanolide Sesquiterpene Lactones
Two xanthanolides specific to the genus Xanthium, partially responsible for its antitumor activity, were quantified—xanthatin and its isomer—8-epi-xanthatin. Results are shown in Table 1. All extraction methods were able to yield the two compounds. For xanthatin, SE and UAE (30 min and 40 °C) yielded almost identical concentrations. UAE was the sole extraction method able to obtain high levels of the isomer 8-epi-xanthatin. The combination of TBE and UAE managed to attain the higher concentration levels for both compounds.

Table 1.
Antitumor compounds specific to the genus Xanthium, namely the species studied, X. spinosum L.
Caffeoylquinic Compounds
Four caffeoylquinic acids presenting antitumor activity, often found in the genus Xanthium, were also quantified in the selected extracts: 4-O-caffeoylquinic (4-CQA), 3,4-, 3,5-, and 4,5-dicaffeoylquinic acids (diCQA). Results are displayed in Table 1.
The highest concentration of 4-CQA was attained by SE. UAE values were comparable to those resulting from maceration, with TBE yielding the lowest levels of this compound. Results for the three diCQA were comparable to one another, as they are isomers, with few differences between the extraction methods. Respectively, UAE conditions of 30 min and 40 °C were the most favorable, closely followed by the UTE sample, and secondly by the conditions of 20 min and 50 °C. SE was the only classical method able to achieve results comparable to the lower values accomplished by UAE.
2.1.2. Evaluation of Antitumor Compounds from the Trifolium Extracts
Results for this section are shown in Table 2. Three antitumor compounds were investigated: formononetin, ononin, and biochanin A. The highest concentrations were found for formononetin, followed by ononin, and biochanin A. For formononetin and biochanin A, results of the UAE extracts at 30 min, 50 °C were comparable to those of the 60 min SE extracts. In the case of ononin, only SE at 60 min time reached the highest value.

Table 2.
Antitumor compounds specific to Trifolium pratense L., found in the selected extracts.
2.1.3. Analysis of Antitumor Compounds from the Green Coffee Bean Extracts
Caffeoylquinic Acids
The samples were screened for four caffeoylquinic compounds: 4-CQA, 3,4-, 3,5-, and 4,5-diCQA. Results are shown in Table 3. The levels of these compounds were similar to one another, due to their isomerism. High yields were observed especially in the 60 min SE samples and TBE samples, namely, four cycles of 5 min extraction time and 4000 rpm and 6000 rpm, respectively. Maceration, UAE, and UTE reached only medium to high levels of caffeoylquinic acids. A progressive increase in yields along with the increase in parameter values could be observed concerning all extraction methods, for all four compounds. This suggests an improvement in compound release due to the increase in temperature, time, and speed, respectively.

Table 3.
Antitumor compounds characteristic of green coffee beans.
Diterpenoid Compounds
The samples were also screened for two diterpenoid compounds of which present reports concerning their antitumor activity were found, that is, kahweol and cafestol. Results for these compounds can also be found in Table 3. Having chemical structures related to one another, these compounds exhibited similar behaviors in terms of extraction yields. Thus, the highest yields were attained for both kahweol and cafestol by UAE, specifically the 30 min and 40 °C sample, followed by other UAE samples with minor differences between each concentration value. Other extraction methods enabled only low to medium yields for these compounds. All extraction methods obtained higher diterpenoid yields as temperature and time values increased. For UAE as well as SE, a further increase in extraction time and temperature led to an abrupt decrease in diterpenoid yields due to possible degradation of the compounds as a consequence of prolonged exposure.
2.2. Evaluation of Biological Activities in Cell Culture for the X. spinosum L. Extracts
Once the phytochemical profile of the extracts was determined, two samples from each species were selected for further determination of in vitro biological activities. The selection was based on the number of identified antitumor compounds and the concentration levels of said compounds. Thus, for X. spinosum L., the extracts S60 (Soxhlet extraction, 60 min extraction time) and U34 (ultrasound-assisted extraction (UAE) at 30 °C extraction temperature, and 40 min extraction time) were selected. For T. pratense L., extracts S60 (Soxhlet extraction, 60 min extraction time) and U35 (UAE at 30 °C extraction temperature, 50 min extraction time). For green coffee beans, the extracts S60 (Soxhlet extraction, 60 min extraction time), and U35 (UAE at 30 °C extraction temperature, 50 min extraction time). Further, in order to promote the legibility of the study, the extracts were renamed in the sections discussing biological activities (see Table 4).

Table 4.
Nomenclature of the samples.
2.2.1. Evaluation of Cell Viability
The anticancerous effects of X. spinosum L. extracts were evaluated in parallel on cancerous and normal human cell lines (Figure 1). For the cancerous phenotype, human lung adenocarcinoma (A549) cells and human breast carcinoma (T47D-KBluc) were selected. As a normal cell line, human fibroblast cells (BJ) isolated from the foreskin were used. The evaluation was done after a 24 h and a 48 h incubation with the extracts at doses ranging from 50 to 1000 µg/mL.
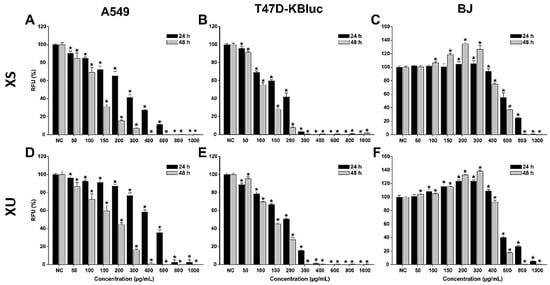
Figure 1.
Cytotoxic effects of the XS (A–C) and XU (D–F) XU extracts observed on A549 (A,D), T47D-KBluc (B,E), and BJ (C,F) cells using the Alamar Blue Assay. The results are presented as relative means ± standard deviations of three biological replicates, where the negative control (NC) is 100%. Asterisks (*) indicate a statistically significant difference (p < 0.05) in comparison with the NC. RFU—relative fluorescence units.
Both extracts induced cytotoxic effects on all the cell lines used, with higher cytotoxicity being observed on the cancerous cell phenotypes. The cytotoxic effect was statistically significant on the cancerous cells starting from the lowest exposure dose of 50 µg/mL. Starting from this dose, a dose-dependent increase in cytotoxicity was observed for both extracts on the A549 and T47D-KBluc cells. Higher cytotoxicity was observed in the case of breast cancer cells, at concentrations above 300 (XS) and 400 (XU) µg/mL and an exposure of 24 h, the extracts reducing the cellular viability by almost 100%. This total cellular death was present only at the highest two concentrations of 800 and 1000 µg/mL in the case of A549 cells. Exposure of the cancerous cells to the extracts for 48 h induced a more pronounced cytotoxic effect.
Conversely, in the case of normal cells, an increase in the measured viability was recorded at low and intermediary doses. This hormetic response was more pronounced for the XU extract and after an exposure of 48 h. The cellular viability of BJ cells was increased up to 105 and 125% after a 24 h exposure to XS and XU, respectively. After a 48 h exposure to the XS and XU, the viability of BJ cells increased up to a value of 135 and 140%.
The hormetic effect observed could be related to the presence of CQA in both extracts, recent studies indicate that these phytocompounds increase intracellular adenosine triphosphate (ATP) levels in cells and thus the metabolic conversion of resazurin to resorufin [34]. Structure–activity relationship studies indicate that the activity is proportional to the number of caffeoyl moieties in the quinic acid, and explain the higher cellular viability measured in the case of XU which is more abundant in dicaffeoylquinic acids than the XS extract (Table 1) [35]. Even though low and intermediary doses increased the measured cellular viability, high doses of extracts (>600 µg/mL) induced strong cytotoxicity in the normal cells. At the highest tested dose of 1000 µg/mL, both extracts reduced the cellular viability to almost zero at both times of exposure.
For the potency evaluation of the X. spinosum L. extracts, 4-parameter logistic curves with the cellular viability as a function of the exposure doses were plotted, and the IC50 (half maximal inhibitory concentration) values were extrapolated (Table 5). The calculated IC50 values mirrored the previous observations, with lower values being obtained for the cancerous cells than for normal fibroblasts.

Table 5.
IC50 values (µg/mL) after exposure of A549, T47D–Kbluc, and BJ to the XS and XU extracts for 24 h.
The IC50 values for the T47D–Kbluc cells were approximately half of the values of A549 cells and a third of the values of BJ. Based on IC50 values, a more pronounced cytotoxic effect was observed for XS on the cancerous cells, whereas XU displayed higher cytotoxicity on the normal cells. Interestingly, the same XU extract that displays slightly higher cytotoxicity on the normal cells, induces a higher increase in cellular viability at intermediary doses. A three-way ANOVA with the cell type, extract type, and exposure dose as variables and the measured cellular viability as a response was performed to evaluate the differences between the conditions. The results of the statistical test indicated that all the variables significantly influenced the observed viability, the cell type, and the dose being the main contributors to the cellular viability observed.
2.2.2. Evaluation of Cell Viability
The antioxidant potentials of the X. spinosum L. extracts (XS and XU) were evaluated at three concentrations (100, 200, and 300 µg/mL) that did not induce cytotoxicity on the normal human fibroblast cells (Figure 2). The evaluation was done using the DCFH-DA assay, as previously described, in the presence or the absence of a pro-oxidant (H2O2) [36]. Exposure alone for 24 h with both extracts decreased in a dose-dependent manner the quantity of ROS. The antioxidant properties were more prominent for the XS extract, at the highest tested dose of 300 µg/mL decreasing ROS more than the positive control (NAC). For XU extract, in non-stimulated conditions, antioxidant effects similar to those observed for NAC were achieved at a dose of 300 µg/mL. Treatment with H2O2 increased by approximately 250% ROS quantities, an increase that was partially mitigated by NAC. Similar to NAC, both extracts partially decreased ROS in stimulated conditions, the statistical significance is observed from the lowest dose of 100 µg/mL for both extracts. Similar to the observations made in non-stimulated conditions, XS had a better antioxidant profile, with the highest tested dose decreasing ROS more than the positive control. XU extract had the same capacity as NAC in abrogating ROS at the maximum concentration.
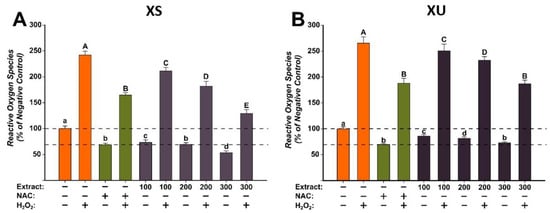
Figure 2.
The antioxidant effect of the X. spinosum L. extracts was evaluated using DCFH-DA assay on BJ cells: (A)—Xanthium spinosum L. Soxhlet extract (XS); (B)—Xanthium spinosum L. UAE extract (XU). Cells were pre-exposed to the extracts (100, 200, and 300 µg/mL) or NAC (20 mM) for 24 h, and further incubated with 50 µM DCFH-DA. The antioxidant effect of the XS extracts was evaluated after 2 h in non-stimulated (HBSS) and stimulated conditions (250 µM H2O2). The data are expressed as relative means ± standard deviations (six technical replicates for each of the three biological replicates) where the negative control is 100%. Different letters (a–d refers to comparisons on non-stimulated conditions, whereas A–E refers to comparisons in stimulated conditions) indicate significant differences (ANOVA + Holm-Sidak post hoc, p < 0.05).
2.3. Evaluation of Biological Activities in Cell Culture for the T. pratense L. Extracts
2.3.1. Evaluation of Cell Viability
In the case of both T. pratense L. extracts, higher cytotoxicity, which was dose-dependent, was observed in cancerous cells (Figure 3, Table 6). After a 24 h exposure to the extracts, the viability of A549 and T47D-KBluc cells statistically decreased from the doses of 300 and 150 µg/mL, respectively. The higher sensitivity of T47D-KBluc cells to the T. pratense L. extracts was also observed at higher doses, at the maximum concentration tested of 1000 µg/mL both extracts induced an almost 100% cytotoxicity.
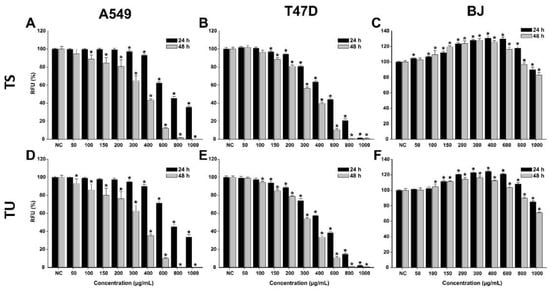
Figure 3.
Cytotoxic effect of the TS (A–C) and TU (D–F) observed using Alamar Blue assay on A549 (A,D), T47D-KBluc (B,E), and BJ (C,F) cells. The results are expressed as relative means ± standard deviations (six technical replicates for each of the three biological replicates), where the negative control (DMSO 0.2%) is 100%. Asterisks (*) indicate a significant difference (p < 0.05) in comparison with the negative control. RFU—relative fluorescence units.

Table 6.
IC50 values (µg/mL) after exposure of A549, T47D–Kbluc, and BJ to the TS and TU extracts for 24 h.
In the case of A549 cells, a 24 h exposure to both extracts at the highest dose tested decreased the cellular viability by approximately 65%. Interestingly, an increase in viability was observed for normal cells at low and intermediary doses (Figure 4). The obtained effect could be related to a hormetic response, non-toxic doses of T. pratense L. extracts increasing the metabolism of the cells and thus the analytical signal measured by the AB assay. At these doses, an increase in the cellular viability of up to 125% was observed. At the highest tested dose, both extracts induced a cytotoxic effect in BJ cells, decreasing the cellular viability by approximately 20% (Figure 4). In comparison with an exposure of 24 h, an exposure of 48 h to the T. pratense L. extracts was associated with higher cellular toxicity, especially in cancerous cells. Even though T. pratense L. extracts have been shown to possess anticancerous activities on different types of cancerous cells, isoflavonoid compounds abundant in T. pratense L. extracts, such as formononetin, show increased efficiency in estrogen receptor-positive cells, thus explaining the higher sensitivity of the T47D-KBluc cells [37].
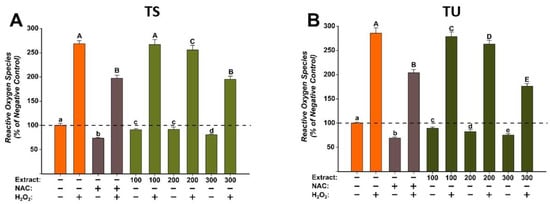
Figure 4.
The antioxidant effect of the T. pratense L.: (A)—Trifolium pratense L. Soxhlet extract (TS) and (B)—Trifolium pratense L. UAE extract (TU) was evaluated using DCFH-DA assay on BJ cells. Cells were pre-exposed to the extracts (100, 200, and 300 µg/mL) or NAC (20 mM) for 24 h, and further incubated with 50 µM DCFH-DA. The antioxidant effect of the T. pratense L. extracts was evaluated after 2 h in non-stimulated (HBSS) and stimulated conditions (250 µM H2O2). The data are expressed as relative means ± standard deviations (6 technical replicates for each of the three biological replicates) where the negative control is 100%. Different letters (a–e refers to comparisons on non-stimulated conditions, whereas A–E refers to comparisons in stimulated conditions) indicate significant differences (ANOVA + Holm-Sidak post hoc, p < 0.05).
For the anticancerous potency evaluation, IC50 values were calculated from four-parameter logistic curves (cellular viability as a function of exposure dose), see Table 6. IC50 values reflected the previous observations, T47D-KBluc cells being more sensitive to the cytotoxic effects of the T. pratense L. extracts.
2.3.2. Evaluation of the Antioxidant Potential
For the evaluation of the antioxidant effects of the T. pratense L. (TS and TU) extracts, three concentrations (100, 200, and 300 µg/mL) that did not induce significant cytotoxicity in BJ cells were selected. The antioxidant properties were evaluated using the DCFH-DA assay, as previously described [36]. The antioxidant potential was evaluated in stimulated/non-stimulated conditions, in the presence/absence of H2O2. Treatment alone with both extracts decreased in a dose-dependent manner to ROS (Figure 4). These effects were similar to NAC treatment, the positive control used. Exposure of cells to H2O2 induced a marked increase in ROS, the levels being approximately 250% of those observed for negative control. Pre-treatment with NAC for 24 h partially alleviated the increase in ROS induced by the H2O2. Similar to the antioxidant effects of NAC, both extracts decreased ROS at the exposure doses used (Figure 4). In the case of TS, a statistical decrease was observed starting from the dose of 200 µg/mL in non-stimulated/stimulated conditions, whereas in the case of TU, the decrease was present starting from the lowest dose evaluated of 100 µg/mL. Moreover, the antioxidant properties of TU were superior to the ones of the positive control, a statistically significant difference being observed at a dose of 300 µg/mL (Figure 4). The obtained results are congruent with the previously described antioxidant activity observed in the non-cellular assays [32].
2.4. Evaluation of Biological Activities in Cell Culture for the Green Coffee Bean Extracts
2.4.1. Evaluation of Cell Viability
The anticancerous potential of the green coffee bean extracts (S60 and U35) was assessed as previously described for the other two species. For both extracts, higher toxicity was observed in the case of cancerous cells, whereas in the case of normal cells, no toxicity was observed. After a 24 h exposure to both extracts, the viability recorded for A549 and T47D-KBluc cells decreased by increasing the exposure dose. At the highest tested dose, the viability of A549 at 24 h post-exposure was approximately 40 and 60% for CS and CU extract, respectively. A slightly higher cytotoxicity was observed in the case of T47D-KBluc cells, and at the highest tested dose, the recorded viabilities were approximately 20% for both extracts. A similar dose-dependent cytotoxicity profile on the cancerous cell lines was observed for both Coffea arabica L. extracts after incubation for 48 h. However, in this case, the cytotoxic effects were more pronounced, with both extracts decreasing the cellular viability below 50% at the exposure dose of 600 µg/mL. At the highest two doses of 800 and 1000 µg/mL, exposure to the extracts induced total cell death, with the recorded viabilities being approximately 0% (Figure 5).
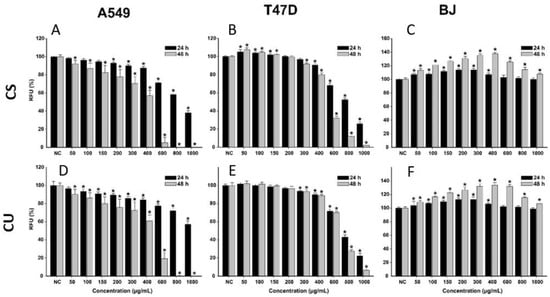
Figure 5.
Cytotoxic effect of the CS (A–C) and CU (D–F) on A549 (A,D), T47D-KBluc (B,E), and BJ (C,F) cells. Data are presented as relative means ± standard deviations (6 technical replicates for each of the three biological replicates), where the negative control (NC) is 100%. Asterisks (*) mark a significant difference (p < 0.05) in comparison with the NCRFU–relative fluorescence units.
Conversely, to the results obtained on the cancerous phenotypes, exposure of normal cells to low and intermediary (50–400 µg/mL) concentrations of the extracts for 24 h increased the cellular viability by 10–15%. The increase in viability can be explained by the presence of CQA in the extracts, as these phytocompounds have been shown to increase the metabolic reserve and metabolic capacity of cells [34]. Recent studies indicate that this benefic activity is proportional to the number of caffeoyl moieties in the quinic acid [35]. Regarding the content of CQA in the studied extracts, the extracts are almost alike in terms of qualitative and quantitative composition (Table 7). Due to this similarity, no difference in the cellular viability of the BJ cell line was observed between the two extracts. After a 48 h incubation with the extracts, the increase in viability on the normal cells was even higher, the viability reaching 140% of the value of negative control (Figure 5).

Table 7.
IC50 values (µg/mL) after exposure of A549, T47D–Kbluc, and BJ to the CS and CU extracts for 24 h.
Based on the experimental data, half maximal inhibitory concentration (IC50) values were extrapolated from four-parameter logistic curves of the cellular viability as a function of the exposure dose (Table 7). As to previous observations, the extracts displayed a similar potency, with slightly lower IC50 values being observed for the CS extract. Compared to a 24 h exposure, exposure for 48 h resulted in more pronounced toxicity in the A549 cells, the calculated IC50 at this time point being approximately half of the values obtained after a 24 h exposure. For T47D–Kbluc the differences between the IC50 after a 24 h and a 48 h exposure were not as prominent. The data were also analyzed using a Three-Way ANOVA with the measured cellular viability as a response, and the cell type, extract type, and exposure dose as variables at 24 h and 48 h exposures. In both cases, there was a statistical difference, with all variables influencing the measured viability. There was a clear difference between the normal and cancerous cell types, with the two phenotypes behaving differently. Moreover, the difference between the extracts increased at 48 h, with CS displaying higher potency.
2.4.2. Evaluation of the Antioxidant Potential
The antioxidant potentials of the green coffee bean extracts were evaluated at three concentrations (100, 200, and 300 µg/mL) that did not induce cytotoxicity on the normal human fibroblast cells. The evaluation was done using the DCFH-DA assay, as previously described, in the presence or the absence of a pro-oxidant H2O2 [36]. Exposure alone for 24 h with both extracts decreased in a dose-dependent manner the quantity of ROS. For CS, a statistical significance was observed starting from the intermediary dose of 200 µg/mL, whereas for CU, the difference was observed starting from the lowest dose of 100 µg/mL. Treatment with H2O2 increased by approximately 250% ROS quantities, an increase that was partially mitigated by NAC. Similar to NAC, both extracts partially decreased ROS in stimulated conditions, the statistical significance is observed from the dose of 200 µg/mL for both extracts (Figure 6).
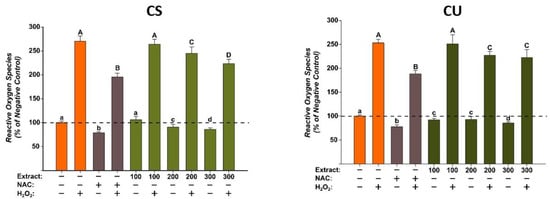
Figure 6.
The antioxidant effect of the CS and CU extracts was evaluated using DCFH-DA assay on normal fibroblast cells (BJ). Cells pre-exposed for 24 h to the extracts (100, 200, and 300 µg/mL) or NAC (20 mM), were further incubated with 50 µM DCFH-DA. The antioxidant properties of the CA extracts were measured after 2 h in non-stimulated (HBSS) and stimulated conditions (250 µM H2O2). The data are presented as relative means ± standard deviations of three biological replicates (six technical replicates/biological replicate) in comparison with the negative control (100%). Different letters (non-stimulated conditions (a–d) and stimulated conditions (A–D)) mark significant differences (ANOVA + Holm-Sidak post hoc, p < 0.05).
2.5. In Vivo Antitumor Activity Assessment
2.5.1. Tumor Cell Viability
Cell viability is an important assessment in the evaluation of the cytotoxicity of any bioactive compound. Results are presented in Figure 7. In addition to evaluating each extract separately, as stated above, a comparison between their effects was also attempted. Thus, CS, that is, the green coffee bean extract obtained by SE at 60 min extraction time presented the lowest cell viability (*** p < 0.001 vs. control group), compared to CYC. This was followed by TS, that is, the T. pratense L. extract obtained by SE, at 60 min extraction time (** p < 0.01 vs. control group), in comparison to CYC. XS, that is, the X. spinosum L. extract obtained by SE, at 60 min extraction time did not show any significant difference versus the CYC group or control, untreated group.
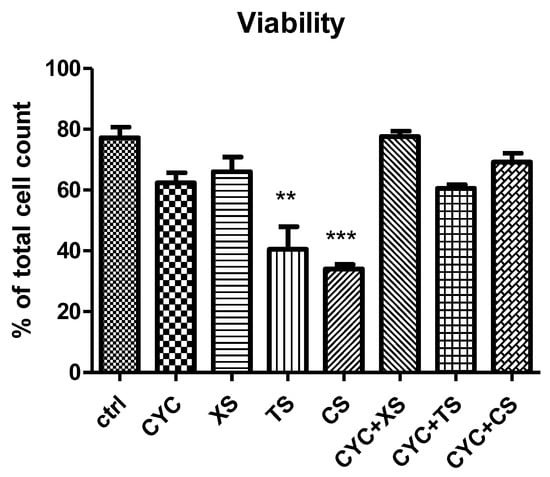
Figure 7.
Cell viability. Values are means ± SD. Statistical analysis was performed using a one-way ANOVA, with Tukey’s multiple comparisons post-test (** p < 0.01 and *** p < 0.001 vs. control group).
2.5.2. Evaluation of Oxidative Stress Markers from Ascites Samples
Redox imbalance markers determined in ascites samples can be seen in Figure 8. MDA levels increased after CYC treatment compared to controls, whereas the associations between CYC and XS or TS extracts caused a significant statistical increase in lipid peroxidation (p < 0.05). TS extract induced oxidative stress in ascites samples of mice close to the CYC group (p < 0.05), whereas the association with TS and CYC maintained the same values of MDA levels. Regarding the levels of non-endogenous antioxidants, for GSH, TS treatment and the association between CYC + CS showed comparable results, yet statistical significance was lacking. In the case of GSSG levels, there were no statistically significant differences between groups. Nevertheless, the GSH/GSSG ratio was slightly ameliorated after treatment with each extract individually. In the case of enzymatic antioxidants, CAT activity decreased after CYC treatment, whereas the extracts or extracts associated with CYC did not significantly modify the activity of the enzyme. GPx activity decreased after CYC administration, whereas the extracts elevated the enzyme activity, most notably XS and CS. Comparable results were reached by TS treatment and CYC + XS treatment. None of the data mentioned earlier presented statistical significance.
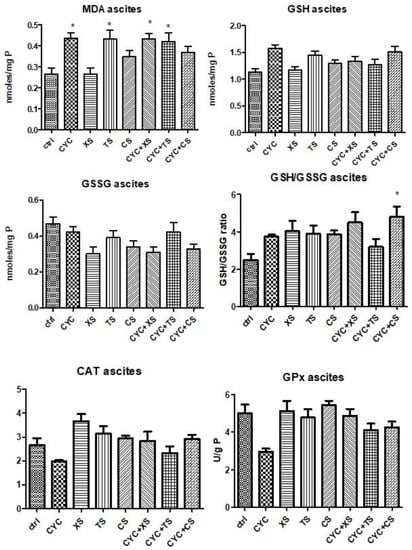
Figure 8.
Oxidative stress markers (MDA levels, GSG/GSSG ratio, CAT, and GPx activities) from the mice ascites samples after a 10-day treatment with CYC, XS, TS, CS, and associations of CYC and each of the separate extracts (Values are means ± SD. Statistical analysis was performed using a one-way ANOVA, with Tukey’s multiple comparisons post-test (* p < 0.05 vs. control group).
2.5.3. Evaluation of Oxidative Stress Markers from Liver Samples
Figure 9 presents the results of oxidative stress from the liver samples. MDA formation slightly increased after CYC administration but was statistically insignificant, whereas CYC + XS, that is, the X. spinosum L. SE extract, decreased MDA formation (p < 0.05), suggesting its antioxidant effect. Of the non-endogenous antioxidants, liver GSH levels were slightly increased for all individual extract treatments in comparison to the CYC group, as well as for the combination treatments. However, statistical significance was not present for these results. The liver GSSG levels increased after CYC administration but unfortunately decreased after treatment with extracts as well as the combination of therapies. Nonetheless, the GSH/GSSG ratio was positively impacted by the treatments containing extracts, with TS being moderately significant (** p < 0.01 vs. control group). Enzymatic antioxidant activities significantly increased compared to the control group and CYC group, namely TS (## p < 0.01 vs. control group), CS (** p < 0.01 vs. control group, and ### p < 0.001 vs. CYC group), for CAT. For GPx, the association CYC + XS was the only significant amelioration of enzyme activity (* p < 0.05 vs. the control group, and # p < 0.05 vs. the CYC group).
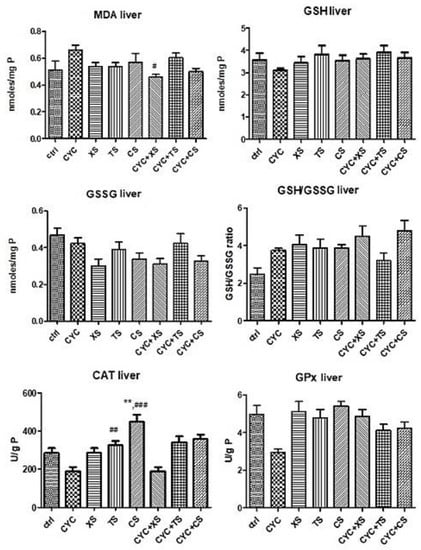
Figure 9.
Oxidative stress markers (MDA levels, GSG/GSSG ratio, CAT, and GPx activities) from the mice liver samples after a 10-day treatment with CYC, XS, TS, CS, and associations of CYC and each of the separate extracts (Values are means ± SD. Statistical analysis was performed using a one-way ANOVA, with Tukey’s multiple comparisons post-test (** p < 0.01 vs. control group, and # p < 0.05, ## p < 0.01, ### p < 0.001 vs. CYC group).
3. Discussion
Phytochemically-wise, Varga et al. have also reported the presence of xanthatin and its isomer, 8-epi-xanthatin, as well as CQA, such as 4-CQA and 3,5-diCQA in methanolic extracts of X. spinosum L. aerial parts, after extraction by UAE, followed by solid-phase extraction [37]. A notable observation was that of xanthatin being present in higher levels in the S60 sample, whereas its isomer, 8-epi-xanthatin, was present in higher concentrations in the UAE samples. A possible explanation could lie in the extraction parameters themselves, namely, UAE, with milder working conditions, lower temperatures (10–30 °C), and shorter extraction times (40–50 min), could have led to the loss of the isomer in the S60 sample (extraction conditions: boiling point of the hydroalcoholic solvent of approximately 79 °C, and 60 min extraction time), but its extraction by UAE. Another possibility that could be considered is the epimerization itself of xanthatin in the conditions of the UAE. As stated above, chlorogenic acids are a phytochemical class of esters formed between quinic and cis- or trans-cinnamic acids, such as caffeic, ferulic, or p-coumaric acid. The main classes of chlorogenic acids are CQA, diCQA, and feruoylquinic acids. These compounds have been reported to exhibit numerous biological activities, some of which include antioxidant, antibacterial, anti-inflammatory, and antitumor activities as well as neuroprotective effects [5]. Xanthatin has also been reported to inhibit both tumor growth and cell proliferation in various types of cancer cell lines [8,9,10,38,39].
Isoflavones were richly present in the T. pratense L., especially for UAE and SE extracts. The lower concentrations of ononin present for all extracts, with the exception of the S60 extract, as stated above, could be due to the chemical relation between ononin and formononetin. As ononin is the glycosylated derivative of formononetin. The loss of beta-D-glucopyranoside might be caused by the prolonged extraction times combined with the elevated extraction temperatures of the UAE. Or possibly due to the shear forces of the disperser, in the case of TBE.
CQA are specific to green coffee beans and have been reported to present antitumor activity [40,41]. Guglielmetti et al. have also reported a positive correlation between time and temperature for CQA yield in coffee silverskin, extracted by several extraction methods, including UAE [42]. Bianchin et al. have also reported a positive influence of longer extraction time over kahweol and cafestol yields for roasted coffee beans, subjected to UAE. UAE was also reported to have provided comparable results to a conventional liquid/liquid extraction; a finding not applicable to the present study [43]. Moeenfard et al. have also reported the UAE efficiency over a simple solvent extraction for cafestol esters yields in samples of green and roasted coffee beans, with the main reasons being a better mass transfer, swelling and enlargement of cellular wall pores, and improved intracellular penetration of the extraction solvent [44]. Two other studies have also reported the advantage of another innovative extraction method, microwave-assisted extraction (MAE), presented in terms of diterpene content, over conventional methods such as SE and conventional heating, for green coffee beans [45,46]. The degradation of the two diterpenoid compounds by long exposure to elevated extraction temperature and time values has also been reported by Chen et al. for green coffee oil, using a combination of UAE and MAE [47].
Similar to the current results, Ly et al. reported an IC50 value of 81.69 μg/mL for an ethanolic extract of X. strumarium L. leaves on HepG2 cells after an exposure of 48 h. Furthermore, the authors reported that treatment with the extracts induced cellular apoptosis marked by shrinkage of the cellular volume and the presence of apoptotic bodies [48]. The anticancerous activity of the X. strumarium L. extracts was also reported on other hepatic cancerous cells (Huh-7 and Hep3B). Kim et al. evaluated the cytotoxic potential of an ethanolic extract obtained from the fruits of X. strumarium L. and reported IC50 values of around 100 μg/mL on Huh-7 and Hep3B cells after a 72 h exposure. The authors reported that apoptosis was initiated in cancerous cells after the activation of caspase-3 and PARP. Moreover, in an ex vivo model, administration of the extracts inhibited tumoral growth and induced apoptosis by suppressing the PI3K/AKT/mTOR signaling pathways in the cancerous cells [49]. Comparable IC50 values were also reported for other cancerous cell types, including colorectal adenocarcinomas (HT-29 and C2BBe-1), breast adenocarcinomas (MDA-MB-231 and MCF-7), and ovarian carcinomas (SKOV-3 and ES-2) after treatment with an ethanolic extract of X. strumarium L. leaves [50]. Higher cytotoxicity was reported in Chinese hamster ovary cells after treatment with the whole extract of the aerial parts of X. strumarium L. The calculated IC50 in this latter case was 20.5 μg/mL [51]. Although not desirable due to the environmental impact, extraction with other non-polar solvents such as chloroform could result in extracts with higher concentrations of biologically active compounds. In a recent study, Alsabah et al. reported IC50 values between 2 and 3 μg/mL after a 72 h exposure to a chloroform-based extract from the leaves of the same species on three different breast cancer cell lines (AMN3, AMJ13, and MCF7) [52]. Same as in our study, the authors observed that their extract exerts more cytotoxicity on cancerous cells than on normal cells [52]. Similarly, higher cytotoxicity was observed in A549, HepG2, Jurkat, and L929 cells after treatment with an ethyl acetate fraction of a methanol extract of X. strumarium L. seed than with the whole methanol extract. In comparison with the crude extract, the ethyl acetate fraction was enriched in more lipophilic compounds thus explaining the observed differences. Interestingly, further purification of the ethyl acetate fraction yielded subfractions that lost their anticancerous properties, reiterating that the combination of different compounds is responsible for the inhibitory effect [38]. Slightly higher cytotoxicity than of the whole crude extract of X. strumarium L. leaves was also for a chloroform fraction by Piloto-Perrer et al. in CT26WT colon carcinoma cells [9]. Besides the evaluation of whole crude extracts, many scientific papers tried to fractionate crude extracts and isolate compounds with antitumoral activities from different parts of X. strumarium L. [10,49,53,54,55,56,57]. Xanthanolide sesquiterpene lactones, including xanthatin and 8-epi-xanthatin that were also quantified in the current study, are one of the most studied phytocompounds present in the Xanthium genus, being partially responsible for the anticancerous properties. Compared with crude extracts, these compounds present lower IC50 values. Ahn et al. reported antitumor activity for xanthin and 8-epi-xanthatin on several cancerous cells, with IC50 values varying between 0.1 and 1.7 µg/mL, depending on the cell line employed [58]. Similarly, Ramírez-Erosa et al. reported IC50 values of 13.9 and 4.8 µg/mL for these compounds in MDA-MB-231 cells and IC50 values of 6.15 and 2.65 µg/mL in WiDr (human colon cancer) cells [59]. Based on recent research, xanthatin alters the enzymatic activity of thioredoxin reductase by targeting a selenocysteine residue. The inhibition of the enzyme is followed by the induction of apoptosis due to increased oxidative stress [60]. Other effects related to an oxidative stress imbalance such as genotoxicity and cell cycle arrest were reported as mechanisms for the induction of apoptosis and the overall anticancerous effect [51,61].
Regarding the T. pratense L. extracts, both displayed similar cytotoxicity patterns, the calculated IC50 values being almost the same in both cancerous cell types (Table 6). Congruent with these results, Karakas et al. reported IC50 values higher than 200 µg/mL for T. pratense L. extracts obtained after extraction with hexane, dichloromethane, methanol, and water. The authors evaluated the cytotoxic effect on human breast cancer cells (MCF-7) and human hepatocellular carcinoma (HepG2) after an exposure of 24 h [62]. Similarly, Khazaei et al. reported that a hydroalcoholic extract of T. pratense L. presented a dose and time-dependent cytotoxicity against glioblastoma (U87MG) and mammary cancer (MCF-7 and MDA-MB-231) cells [63,64]. After a 24 h exposure to U87MG cells, the reported IC50 value was around 400 µg/mL, close to the values presented in our study [64]. In the case of the mammary cancerous cells, the calculated IC50s were higher than 1000 µg/mL for both cell types after an exposure of 24 h [63]. The anticancerous potential was also reported by Zakłos-Szyda et al. that observed higher cytotoxicity on cancerous cells than in normal human umbilical vein endothelial cells [36]. In addition to the polyphenolic and flavonoid compounds that have been shown to possess anticancerous activities, biochanin A, an isoflavone present in the TS and TU extracts, has been shown to possess anticancerous properties [19]. Biochanin A manifests antineoplastic properties by inhibiting cancerous cellular growth, activating apoptosis, and blocking angiogenesis. Its activities have been demonstrated in various cell types, including bladder, pancreatic, prostate, liver, and breast cancers [19]. Moreover, formononetin and ononin, another tw4o compounds present in T. pratense L. extracts, have been shown to possess anticancerous activity by inducing cell apoptosis and cell cycle arrest in cancerous cells [20].
Similar to the current in vitro results for the green coffee bean extracts, Amigo-Benavent et al. reported that an exposure to 1000 µg/mL methanolic extract of green coffee beans decreased the cellular viability of A549 cells by 40 and 45% after a 2 h and 24 h incubation, respectively [26]. These results are congruent with our results. After a 24 h exposure at a dose of 1000 µg/mL, the recorded viabilities decreased by 60% for CS and 40% for CU. Different from our study, the previously mentioned study reported relatively high toxicity on normal cells, exposure to the extract at a concentration of 1000 µg/mL for 24 h decreasing the cellular viability to 22% [26]. Even though fibroblasts were used as normal cells in both studies, the different origins could explain this discrepancy, as in our case we used normal dermal fibroblasts, and in their case, the fibroblasts were isolated from colon tissue. From the phytocompounds tested, 5-CQA and 3,5-diCQA were the most potent anti-proliferative phenols, their synergistic effect, together with the potential contribution of other bioactive compounds explaining the anticancerous effects [26]. Congruent results were also reported by Priftis et al. who observed a more than 20% decrease in viability at concentrations higher than 400 µg/mL of an aqueous extract of green coffee beans on the endothelial EA.hy926 cells [65]. Interestingly, they reported much higher cytotoxicity (viability < 80%) on the myoblastic C2C12 cells, cytotoxicity being present starting with an exposure dose of 20 µg/mL. C2C12 cells were less prone to toxicity when treated with extracts from roasted coffee beans, the exposure concentrations needed to decrease the cellular viability by more than 20% being at least 20 times higher than in the case of green coffee bean extract [65]. These results could be explained by the formation of melanoidins due to Maillard reactions between sugars and amino acids that present a high molecular mass and do not pass the cellular membranes. In addition to sugars and proteins, polyphenols can be sequestrated in these structures thus affecting the bioavailability of phytocompounds [66]. In a recent paper from 2022, the anticancerous potential of green, roasted, and spent coffee aqueous and ethanolic extracts was evaluated. Similar to the obtained results, the green coffee bean extract did not induce overt cytotoxicity up to a dose of 200 µg/mL and was less toxic than their roasted counterparts [67].
Cafestol and kahweol, two coffee-specific phytocompounds present in our extracts, have been shown to possess anti-carcinogenic, anti-angiogenic, and anti-inflammatory activities [68,69,70]. Several studies indicated that cafestol and kahweol display an inhibitory activity in multiple stages of cancer development by preventing tumorigenesis, metastasis, inhibiting cell proliferation, and inducing cellular apoptosis. One of the main antiproliferative mechanisms of action for cafestol is related to the downregulation in the expression of anti-apoptotic proteins such as Bcl-2. This effect has been reported in several studies on different cancer cell types, including human renal Caki cells, human glioma U251MG cells, and human breast carcinoma MDA-MD-231 cells [71,72,73]. Similar to cafestol, the structural-related kahweol displays anticancerous activities by down-regulating the expression of anti-apoptotic proteins and by inducing the caspase 3 activation and cytochrome release from the mitochondria [74,75,76,77]. In A549 cells, Kim et al. reported that kahweol exhibited antiproliferative and pro-apoptotic effects in a dose- and time-dependent manner. Moreover, cafestol and kahweol do not induce cytotoxicity on normal cell types, suggesting a cytotoxicity selectivity towards the cancerous phenotypes [71,75,78].
The antioxidant potential of the selected extracts from the three species observed on the BJ cell line is supported by the data obtained in the abiotic DPPH, FRAP, and ABTS+ antioxidant assays from previous works [31,32,33]. Main groups of active principles such as phenolics (chlorogenic acid, quercetin, caffeic acid) and CQA display antioxidant properties in vivo and in vitro. These compounds can directly neutralize ROS or indirectly can induce the nuclear translocation of Nrf2 and, as a consequence, increase the synthesis of cellular antioxidants (glutathione) and the activity of detoxifying enzymes (Heme-Oxygenase 1) alleviating the insults induced by ROS. One of the phenolic compounds with the highest concentration in the green coffee bean and X. spinosum L. extracts, namely chlorogenic acid, is recognized for its antioxidant and anti-inflammatory potential. Several studies showed that chlorogenic acid has a protective effect on LPS-induced inflammation in hepatic stellate cells and intestinal epithelial cells [79,80]. Treatment with chlorogenic acid inhibited the production of ROS in LPS-stimulated hepatic stellate cells and decreased the nuclear translocation of the pro-inflammatory NF-κB similar to the reference antioxidant NAC [79]. In a recent study, besides the direct free radical-scavenging activity, treatment with chlorogenic acid of H2O2-stimulated cells strikingly rescued cells from oxidative insults and cellular death. The authors reported that the effect is mediated by the upregulation of antioxidant cellular defense mechanisms such as heme oxygenase-1, glutathione, thioredoxin reductase 1, and thioredoxin 1 after the nuclear translocation of Nrf2 [81]. Besides activity in vitro, chlorogenic acid has been shown to act as an oxidant in ex vivo and in vivo models [82,83,84]. CQA that were present in the green coffee bean and X. spinosum L. extracts are a recognized class of antioxidants, the antioxidant capacity being proportional to the number of the caffeoyl groups bound to the quinic acid [5]. The antioxidant properties of this class have been shown in cell culture, an extract of Gymnaster koraiensis Kitam, and one of its major components, 3,5-di-CQA, exerts protective effects on hepatic cells (HepG2) treated with the pro-oxidant tert-butyl hydroperoxide (t-BHP) [85]. Moreover, the authors reported increases in the cellular glutathione level and a decrease in BHP-induced DNA damage in cells treated with the extracts. Similar to the increase in glutathione observed in the t-BHP model, a hexane fraction of the extract mitigated the acetaminophen-induced reductions in GSH levels, protecting the cells against cellular death [85]. Similarly, other research indicated the antioxidant properties of various caffeoylquinic acids in hepatic and neuronal cells [86,87,88,89,90,91]. The antioxidant mechanism of caffeoylquinic acid compounds is similar to the mechanism of chlorogenic acid, with both phytocompounds favoring the nuclear translocation of the Nrf2 with the consequent increase in the antioxidant cellular defense mechanisms [91].
The obtained results for the in vitro antioxidant effect of T. pratense L. extracts are congruent with the previously described antioxidant activity observed in the non-cellular assays (Table 6) and the data present in the literature [92,93,94]. Recently Lee et al. reported the anti-inflammatory and antioxidant effects of a T. pratense L. extract and an anthocyanin-enriched fraction from the T. pratense L. extract [95]. Both extracts alleviated the increase in ROS induced by the exposure of RAW-267.4 macrophages to lipopolysaccharides. The decrease in ROS was mediated by the inhibition of gene expression and phosphorylation of NADPH oxidase 1. Moreover, the extracts exerted anti-inflammatory potential by suppressing the nuclear translocation of the NF-kB [95]. Zakłos-Szyda et al. reported the antioxidant properties of the T. pratense L. extracts in non-stimulated conditions and in the presence of a pro-oxidant (t-BOOH). In both cases, treatment with T. pratense L. extracts decreased ROS in several cell types by approximately 20–40% [36]. In addition to the polyphenolic compounds with known antioxidant properties, the isoflavones formononetin and biochanin A have been shown to act as antioxidants [96,97]. In a recent study, Sugimoto et al. have shown that formononetin decreases ROS and mitigates the cytotoxicity of H2O2 in neuronal SH-SY5Y cells by enhancing the expression of antioxidant genes [97], whereas Wu et al. reported that biochanin A attenuates the production of ROS in a dose-dependent manner in LPS-stimulated microglial cells [96]. Other isoflavonoid compounds, such as genistein and daidzein, have been shown to elicit antioxidant properties, explaining the current results [98,99,100].
There is an incongruence (especially statistical) between the data obtained from the cell cultures vs. that obtained from the in vivo experiment. One possible explanation could be due to the wide evolutive difference between the species from which the samples were taken, for instance, the cell lines were of human origin (A549—human lung adenocarcinoma, T47D-KBluc—human breast cancer), and the tested animals were Swiss albino mice. Another possible explanation could be that, as seen in the in vitro assessments, large concentrations of extracts were necessary in order to obtain an antioxidant or antitumor effect. In this case, potentially, the doses of extracts administered to the subjects of the animal model were insufficient in order to induce an appropriate, statistically significant effect. Additionally, the tumor used was an aggressive one, a metastatic ovarian tumor with poor response to different treatments. The extracts used for additive effect with CYC have demonstrated efficacy on this type of tumor. As can be noted in the liver mouse samples, the major imbalance between the levels of GSH and GSSG might be due to the polyphenolic compounds’ induction of ROS which in turn led to the induction of cell apoptosis by mitochondrial enzyme activation. As is known, the antiproliferative properties of polyphenolic compounds not only lies in their antioxidant but also in their prooxidant properties [4].
Al Kury et al. managed to demonstrate the anticancer effects and immunomodulatory activities in a mouse model for an extract of X. spinosum L. obtained by maceration. The authors reported an enhancement of caspase-3 activity by 2.25 folds in treated mice compared to the negative control. The authors emphasized the pharmacological activities of the flavonoid compound eupatilin, present in higher levels in an ethanol fraction of the initial extract. They also noted that the aqueous fraction of the extract, containing xanthatin in higher levels, showed the highest fold increase in caspase-3 activity compared to other extracts and fractions [101].
In a review, Sarfraz et al. observed the apoptotic effects of biochanin A by blocking pro-inflammatory factors as well as caspase activity in rat chondrocytes [19]. Kim et al. studied the antitumor effects of formononetin an athymic NU/NU nude mouse model with multiple human myeloma xenografted tumors. The authors concluded that formononetin inhibited cell viability, induced apoptosis, and also suppressed constitutive STAT3 (tyrosine residue 705 and serine residue 727) and STAT5 (tyrosine residue 694/699) activation by suppressing the upstream kinases (JAK1, JAK2, and c-Src) via increased production of ROS due to a GSH/GSSG imbalance [102]. Gong et al. were able to demonstrate the antitumor effects of ononin on BALB/c nude mice injected with A549 cells, especially in combination with paclitaxel treatment. Respectively, the combined treatment inhibited the progression of non-small-cell lung cancer (NSLC) by blocking the PI3K/Akt/mTOR pathway, thus inducing cell apoptosis and limiting cell proliferation, migration, and metastasis [103].
Coffee has garnered the attention of researchers as its consumption has been associated with a decreased risk of the development of various types of cancer. Such studies varied from observing the effect of coffee bean consumption on the risks of brain tumors, including gliomas, to liver cancer (only a correlation was noticeable), and skin cancer (in this case, in a dose dependent-manner) in several Asian populations (Japanese and Chinese Singaporean patients) [24,25,26]. The polyphenolic compounds of coffee beans, especially the unprocessed green coffee beans, such as chlorogenic acids and CQA have been reported to inhibit cancer cell proliferation in a dose dependent-manner and possibly induce cell apoptosis by inhibiting lipoxygenase activity [26,30]. As stated above, these compounds are found in larger amounts in unroasted, green coffee beans, as opposed to the roasted plant material [27,28,29]. Despite this, several cohort studies have indicated that the consumption of green and/or tea did not ameliorate the risk of urinary cancer or biliary tract cancer in human patients, particularly for male smoker patients, concerning the formerly mentioned disease. For the first study, the authors also considered the lower rates of chronic disease in the population compared to the coffee nondrinker subjects, for instance, and other socio-economic factors, which sadly affected the lifestyles and quality of life of the studied patients [69,104]. Regarding the diterpenoid compounds, Park et al. have reported kahweol reduces cyclin D1 degradation in human colorectal cancer cells. The proposed mechanism consisted of threonine-286 phosphorylation of cyclin D1 dependent on ERK1/2, JNK, and GSK3β (proteins that are involved in cellular processes, including apoptosis) [30]. Ren et al. have proposed the detoxification effect of the combination of the two diterpenoids, cafestol and kahweol, to be the main origin of their antitumor effect. That is, the combination has been proven to down-regulate the CYP450s expression, which generally holds a pivotal role in carcinogen activation. The study also cited other previous research affirming the compounds’ capacity of inducing glutathione-S-transferase activation and increasing other phase II metabolizing enzymes. The two compounds were also reported to induce apoptosis by inhibiting certain anti-apoptotic genes and inhibit angiogenesis by modulating VEGFR2, a primary receptor of the vascular endothelial growth factor, holding great importance in the process of angiogenesis [69].
4. Materials and Methods
4.1. Chemicals, Reagents, and Equipment
All HPLC reagents and standards were of analytical grade and were procured from the companies Sigma–Aldrich (Taufkirchen, Germany) and Decorias (Rediu, Iași, Romania). A549 (human lung adenocarcinoma), T47D-KBluc (human breast cancer), and BJ (normal human fibroblasts) cells were purchased from ATCC (Manassas, VA, USA). Roswell Park Memorial Institute (RPMI) 1640 Medium, Dulbecco’s Modified Eagle Medium (DMEM), and fetal bovine serum (FBS) were bought from Antisel (Bucharest, Romania). The 2′,7′-dichlorofluorescin diacetate (DCFH-DA), dichlorodihydrofluorescein (DCFH), dichlorofluorescein (DCF), Hanks’ Balanced Salt Solution (HBSS), hydrogen peroxide (H2O2), and N-acetyl cysteine (NAC) were acquired from Sigma-Aldrich (Schnelldorf, Germany). Dimethyl sulfoxide (DMSO), phosphate-buffered saline solution (PBS), CMC, cyclophosphamide, xylazine, and ketamine hydrochloride were acquired from Sigma-Aldrich (Taufkirchen, Germany).
The HPLC system used was an 1100 series Agilent Technologies model (Darmstadt, Germany) consisting of a G1312A binary pump, an in-line G1379A degasser, a G1329A autosampler, a G1316A column thermostat, and an Agilent ion trap detector 1100 SL. Finally, a Biotek Synergy 2 multi-mode plate reader (The Lab World Group, Hudson, MA, USA) was employed for the in vitro assessments.
4.2. Plant Extracts
A total of 11 plant extracts were used. These were selected as a result of previous studies that aimed to identify the optimal extraction method and extraction parameters of bioactive compounds from the three plant species mentioned earlier: Xanthium spinosum L., Trifolium pratense L., and Coffea arabica L. The methods of extraction that were previously studied were maceration, Soxhlet extraction (SE), pertaining to the classical extraction methods, turboextraction (TBE), and ultrasound-assisted extraction (UAE), of the innovative extraction methods. The previous studies also aimed to establish a combined extraction method between TBE and UAE–UTE [31,32,33]. The used solvent was 70% ethanol and the sample-to-solvent ratio was 1:10 (w/v).
4.3. HPLC-MS Analysis of the Plant Extracts
The details regarding the methods and conditions used for the HPLC-MS analysis as well as the resulted chromatograms of the compounds from the resulted plant extracts can be found in the Supplementary Material accompanying the article [105,106,107,108,109].
4.4. In Vitro Assessment of Antioxidant and Anticancerous Activities
As stated before, two samples from each species were selected for further determination of in vitro biological activities, based on the determined phytochemical profile of the extracts.
4.4.1. Cell Culture
For the in vitro biological activity evaluation, two malignant cell lines—A549 (human lung adenocarcinoma) and T47D-KBluc (human breast cancer)—and the normal human dermal BJ fibroblasts were used. The cells were bought from ATCC (Manassas, Virginia, USA), and grown in a humid incubator with 5% CO2 supplementation. T47D-KBluc cells were kept in Roswell Park Memorial Institute (RPMI) 1640 Medium, whereas BJ and A549 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) with low-glucose and high glucose. All media was supplemented with 10% Fetal Bovine Serum (FBS). The media was changed every two days, and the cells were subcultured when they had 70–80% confluence.
4.4.2. Preparation of Extracts Solutions
A 250 mg/mL stock solution in dimethyl sulfoxide (DMSO) was made from lyophilized extracts. The stock solution was further diluted in DMSO to obtain working solutions. The required concentrations in the cell culture medium, which ranged from 50 to 1000 µg/mL, were achieved using these working solutions.
4.4.3. Cytotoxicity—Alamar Blue Assay
The anticancerous potential of the extracts was assessed as previously described [110,111]. All three types of cells were seeded in 96-well plates and were left for 24 h to attach to the plastic substrate. The wells were washed with PBS (phosphate-buffered saline solution), and the remaining living cells were then exposed for an additional 24 or 48 h to the six selected extracts at concentrations ranging from 50 to 1000 µg/mL. The Alamar Blue (AB) assay was used to assess the ability of exposed cells to metabolically convert the non-fluorescent resazurin into the fluorescent product resorufin. Briefly, cells were washed with PBS and further incubated for 3 h with a 200 µM resazurin solution. Resorufin fluorescence was measured using a Synergy 2 multi-mode microplate reader at λexcitation = 530/25 and λemission = 590/35. The experiments were conducted on three biological replicates, each one including six technical replicates. The data are expressed as relative values in comparison to the negative control (NC) (100%).
4.4.4. Antioxidant Activities
The antioxidant properties of the extracts were evaluated in normal fibroblast cells using the reactive oxygen species (ROS) sensitive 2,7 dichloro-fluorescein diacetate (DCFH-DA) dye as previously described [112,113]. Following cellular internalization, intracellular esterases hydrolyze the non-fluorescent DCFH-DA dye to dichlorodihydrofluorescein (DCFH), which is contained inside the cellular compartment. In the presence of ROS, DCFH is further oxidized to dichlorofluorescein (DCF) which is proportional to the amount of intracellular ROS and can be quantified. Briefly, cells were pre-treated for 24 h with non-cytotoxic doses of the selected plant extracts and were incubated for 2 h with 100 µL of a 50 µM DCFH-DA solution prepared in Hanks’ Balanced Salt Solution (HBSS). After cell loading, non-internalized DCFH-DA was washed, and the cells were incubated for 2 h with 250 µM H2O2 in HBSS or solely HBSS to quantify ROS in stimulated and non-stimulated conditions. The fluorescence was measured using Synergy 2 multi-mode microplate reader at λexcitation = 485/20 and λemission = 528/20. The potency of the XS extracts was compared to N-acetyl cysteine (NAC) treatment (20 mM solution). The experiments were conducted on three biological replicates, each one including six technical replicates. The data are expressed as relative values in comparison to the negative control (NC) (100%).
4.5. In Vivo Assessment of the Antitumor Activity
4.5.1. Tumor Cell Viability
For this section of the study, only three extracts were used, one SE extract per plant. Sixty Swiss albino male mice with Ehrlich ascites carcinoma (EAC), 8 weeks old, and 30 g weight were used for the in vivo study of the antitumor activity of the extracts. The animals were acquired from the animal department of “Iuliu Hațieganu” University of Medicine and Pharmacy Cluj-Napoca. The animal subjects were left to acclimatize for 24 h in the vivarium of the Physiology Department.
Experiments were approved by the Ethics Committee Board of “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania (291/23.02.2022), on Animal Welfare in accordance with the Directive 2010/63/EU, regarding the protection of animals used for scientific purposes.
The mice were randomly divided into eight groups (n = 7). An aliquot of 1 mL of ascites liquid with 1 million cells from a donor animal was inoculated into the mice used in the experiment. After 24 h, the mice were enrolled in the experiment and treated by oral gavage for 10 days, as follows: group 1–0.5 mL 2% CMC (positive control group—CMC); group 2–25 mg/b.w. cyclophosphamide (CYC) administered intraperitoneally (negative control group); group 3–20 mg/b.w. XS in 0.2 mL CMC administered by gavage; group 4–20 mg/b.w. TS in 0.2 mL CMC administered by gavage; group 5–20 mg/b.w. CS in 0.2 mL CMC administered by gavage; group 6–20 mg/b.w. XS in 0.2 mL CMC administered by gavage and 25 mg/b.w. cyclophosphamide administered intraperitoneally; group 7–20 mg/b.w. TS in 0.2 mL CMC administered by gavage and 25 mg/b.w. cyclophosphamide administered intraperitoneally; and group 8–20 mg/b.w. CS in 0.2 mL CMC administered by gavage and 25 mg/b.w. cyclophosphamide administered intraperitoneally.
The animals were monitored for a period of 10 days. Under anesthesia induced by an intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine, at 24 h after the last treatment. Ascites volume, liver tissue, and blood samples were collected. Samples were stored at −80 °C until further analysis. The following biological parameters were evaluated: tumor cell viability and oxidative stress markers were also performed.
4.5.2. Evaluation of Stress Markers from Ascites and Liver Samples
The oxidative stress parameters were evaluated in ascites samples and in liver homogenates by quantification of malondialdehyde (MDA), a parameter of lipid peroxidation [114], and by measurement of glutathione-reduced (GSH) and oxidized (GSSG) levels and their ratio (GSH/GSSG) [115]. Additionally, catalase (CAT) and glutathione peroxidase (GPx) activities were evaluated from both tissues [116,117]. For oxidative stress assessment, the liver fragments were harvested and homogenized with a polytron homogenizer (Brinkman Kinematica, Switzerland) as previously published [118], and then a cytosolic fraction was prepared [118]. The protein level in liver tissue homogenates and in ascites samples were measured by a method described by Bradford [119].
4.6. Statistical Analysis of the In Vitro and In Vivo Assessments
Regarding the in vitro studies, all samples were analyzed in triplicate (n = 3) and the data were reported as the mean ± standard deviation (SD). The data were statistically analyzed by one-way analysis of variance (ANOVA) with a post hoc Holm-Sidak test using SigmaPlot 11.0 computer software (Systat, Software Inc., Chicago, IL, USA). Differences showing a p-level of 0.05 or lower were considered statistically significant.
Data analysis for the in vivo studies was also performed by one-way ANOVA and Tukey’s multiple comparisons post-test. GraphPad Prism 8 was used as software. A p-value < 0.05 was considered statistically significant. The results were expressed as mean ± standard deviation. The normality of distribution was tested with Kolmogorov–Smirnov test, a nonparametric test.
5. Conclusions
The antitumor activities of three separate plant species were analyzed: Xanthium spinosum L., Trifolium pratense L., and Coffea arabica L., namely green coffee beans. All extracts displayed higher cytotoxicity towards the tumor cell phenotypes than for the normal cell population. From the evaluated extracts, the Xanthium spinosum L. extracts showed the highest antitumoral effects, on both tumor cell lines and the highest antioxidant potential on the normal cells. All extracts induced in the normal cells a hormetic response at the intermediary doses effect associated with the presence of bioactive compounds that stimulate the cellular metabolism. In terms of cell viability, the most significant results were achieved by CS, that is, green coffee beans SE extract, followed by TS, that is, Trifolium pratense L. SE extract. In vivo study, ascites cell viability decreased after T. pratense L. and green coffee bean extract administration, whereas the oxidative stress was significant in tumor samples after T. pratense L. or CYC associated with X. spinosum L. and T. pratense L. extracts. Additionally, CYC associated with CS extract improved the activities of enzymatical antioxidants in ascites, whereas in liver samples, only CS and TS increased the CAT activities These results suggested that T. pratense L. or X. spinosum L. extracts in combination with chemotherapy can induce lipid peroxidation in tumor cells and decreased the tumor viability, especially T. pratense L. extract.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12091840/s1. Figure S1. Chromatogram of Xanthium spinosum L. S60 extract: upper spectra: UV chromatogram; lower spectra: MS extracted ion chromatogram: (1) 3,5-diCQA, (2) 4,5-diCQA, (3) 3,4-diCQA; Figure S2. MS chromatogram of green coffee beans S60 extract: upper spectra—cafestol extracted ion chromatogram; lower spectra—kahweol extracted ion chromatogram; Figure S3. MS chromatogram of Trifolium pratense L. S60 extract: upper spectra: total ion chromatogram; lower spectra: extracted ion chromatogram for Biochanin A; Figure S4. MS chromatogram of Xanthium spinosum L. S60 extract: (1) xanthatin, (2) 8-epi-xanthatin.
Author Contributions
O.G.: conceptualization, investigation, writing—original draft preparation, data interpretation; S.C.: supervision, review; R.M. and N.D.: methodology, investigation; A.-M.V.: methodology, investigation, formal analysis, resources, interpretation of data; G.A.F.: writing—review and editing, supervision; I.F. and A.P.: methodology, investigation, writing, data interpretation; P.V.: methodology and investigation; A.-M.V. and L.V.: project administration, funding acquisition; supervision, editing; G.C.: supervision, review. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been supported by the following research projects: UEFISCDI Romania, project PN-III-P1-1.1-PD-2019-0774, and “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, PhD grant PCD No. 1170/3/14.01.2021, respectively.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Dana Muntean for her aid regarding the assessment of the phytochemical profiling of the plant extracts.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
M—maceration; S40—Soxhlet extraction 40 min; S60—Soxhlet extraction 60 min; T24—turboextraction two cycles of 5 min, 4000 rpm; T26—turboextraction two cycles of 5 min, 6000 rpm; T44—four cycles of 5 min, 4000 rpm; T46—four cycles of 5 min, 6000 rpm; T48—four cycles of 5 min, 8000 rpm; U14—ultrasound-assisted extraction 10 min, 40 °C; U24—ultrasound-assisted extraction 20 min, 40 °C; U25—ultrasound-assisted extraction 20 min, 50 °C; U34—ultrasound-assisted extraction 30 min, 40 °C; U35—ultrasound-assisted extraction 30 min, 50 °C; UT—combination of ultrasound-assisted extraction and turboextraction.
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. Available online: https://pubs.thesciencein.org/cbl (accessed on 27 March 2023).
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as potential antiproliferative agents: Scientific trends. Curr. Opin. Food Sci. 2018, 24, 26–35. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef]
- Bosco, A.; Golsteyn, R.M. Emerging anti-mitotic activities and other bioactivities of sesquiterpene compounds upon human cells. Molecules 2017, 22, 459. [Google Scholar] [CrossRef] [PubMed]
- Piloto-Ferrer, J.; Sánchez-Lamar, Á.; Francisco, M.; González, M.L.; Merino, N.; Aparicio, G.; Pérez, C.; Rodeiro, I.; Lopes, M.T.P. Xanthium strumarium’s xanthatins induces mitotic arrest and apoptosis in CT26WT colon carcinoma cells. Phytomedicine 2019, 57, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lamar, A.; Piloto-Ferrer, J.; Fiore, M.; Stano, P.; Cozzi, R.; Tofani, D.; Cundari, E.; Francisco, M.; Romero, A.; González, M.L.; et al. Xanthium strumarium extract inhibits mammalian cell proliferation through mitotic spindle disruption mediated by xanthatin. J. Ethnopharmacol. 2016, 194, 781–788. [Google Scholar] [CrossRef]
- Atkinson, C.; Oosthuizen, W.; Scollen, S.; Loktionov, A.; Day, N.E.; Bingham, S.A. Modest protective effects of isoflavones from a red clover-derived dietary supplement on cardiovascular disease risk factors in perimenopausal women, and evidence of an interaction with ApoE genotype in 49–65 year-old women. J. Nutr. 2004, 134, 1759–1764. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, J.; Zhao, H.; Fan, M.; Gao, W. The anti-inflammatory effects of formononetin and ononin on lipopolysaccharide-induced zebrafish models based on lipidomics and targeted transcriptomics. Metabolomics 2019, 15, 153. [Google Scholar] [CrossRef]
- Kanadys, W.; Baranska, A.; Jedrych, M.; Religioni, U.; Janiszewska, M. Effects of red clover (Trifolium pratense) isoflavones on the lipid profile of perimenopausal and postmenopausal women—A systematic review and meta-analysis. Maturitas 2020, 132, 7–16. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wang, X.J.; Jin, Z.Y.; Xu, X.M.; Zhao, J.W.; Xie, Z.J. Protective effect of isoflavones from Trifolium pratense on dopaminergic neurons. Neurosci. Res. 2008, 62, 123–130. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Ahmed, M.; Surapaneni, K.M.; Veeraraghavan, V.P.; Arulselvan, P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J. Biol. Sci. 2021, 28, 4232–4239. [Google Scholar] [CrossRef]
- Gong, G.; Zheng, Y.; Kong, X.; Wen, Z. Anti-angiogenesis function of ononin via suppressing the MEK/Erk signaling pathway. J. Nat. Prod. 2021, 84, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ye, J.; Zhao, B.; Sun, J.; Gu, N.; Chen, X.; Ren, L.; Chen, J.; Cai, X.; Zhang, W.; et al. Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 2020, 36, 101677. [Google Scholar] [CrossRef] [PubMed]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total. Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Tay, K.C.; Tan, L.T.H.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Formononetin: A review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Lee, W.; Choo, S.; Sim, H.; Bae, J.S. Inhibitory activities of ononin on particulate matter-induced oxidative stress. Biotechnol. Bioprocess. Eng. 2021, 26, 208–215. [Google Scholar] [CrossRef]
- Ogawa, T.; Sawada, N.; Iwasaki, M.; Budhathoki, S.; Hidaka, A.; Yamaji, T.; Shimazu, T.; Sasazuki, S.; Narita, Y.; Tsugane, S. Coffee and green tea consumption in relation to brain tumor risk in a Japanese population. Int. J. Cancer 2016, 139, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. Available online: https://www.mdpi.com/1420-3049/24/7/1397 (accessed on 27 March 2023). [CrossRef]
- Oh, C.C.; Jin, A.; Yuan, J.M.; Koh, W.P. Coffee, tea, caffeine, and risk of nonmelanoma skin cancer in a Chinese population: The Singapore Chinese Health Study. J. Am. Acad. Dermatol. 2019, 81, 395–402. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Kowalska, I.; Pecio, Ł.; Durak, A.; Sęczyk, Ł. Lipoxygenase inhibitors and antioxidants from green coffee-mechanism of action in the light of potential bioaccessibility. Food Res. Int. 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; Wang, S.; Mateos, R.; Sarriá, B.; Bravo, L. Antiproliferative and cytotoxic effects of green coffee and yerba mate extracts, their main hydroxycinnamic acids, methylxanthine and metabolites in different human cell lines. Food Chem. Toxicol. 2017, 106, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Tanokura, M. Organic compounds in green coffee beans. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 149–162. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K. Unremitting problems with chlorogenic acid nomenclature: A review. Química Nova 2016, 39, 530–533. [Google Scholar] [CrossRef]
- Park, G.H.; Song, H.M.; Jeong, J.B. The coffee diterpene kahweol suppresses the cell proliferation by inducing cyclin D1 proteasomal degradation via ERK1/2, JNK and GKS3β-dependent threonine-286 phosphorylation in human colorectal cancer cells. Food Chem. Toxicol. 2016, 95, 142–148. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of different extraction techniques and their respective parameters on the phytochemical profile and biological activities of Xanthium spinosum L. extracts. Plants 2022, 12, 96. Available online: https://www.mdpi.com/2223-7747/12/1/96 (accessed on 27 March 2023). [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Novac, C.Ș.; Filip, G.A.; Vlase, L.; Crișan, G. Red clover and the importance of extraction processes—Ways in which extraction techniques and parameters affect Trifolium pratense L. extracts’ phytochemical profile and biological activities. Processes 2022, 10, 2581. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The effect of extraction methods on phytochemicals and biological activities of green coffee beans extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3, 5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Han, J.; Isoda, H.; Shigemori, H. Structure-activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 2011, 59, 502–507. Available online: https://pubmed.ncbi.nlm.nih.gov/21467684/ (accessed on 27 March 2023). [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Budryn, G. The effects of Trifolium pratense L. sprouts’ phenolic compounds on cell growth and migration of MDA-MB-231, MCF-7 and HUVEC cells. Nutrients 2020, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Domokos, E.; Kelemen, H.; Fulop, I.; Kursinszki, L. HPLC-ESI-MS/MS profiling of phenolic acids, flavonoids and sesquiterpene lactones from Xanthium spinosum. Rev. Chim. 2020, 71, 558–564. [Google Scholar] [CrossRef]
- Al-Mekhlafi, F.A.; Abutaha, N.; Mashaly, A.M.A.; Nasr, F.A.; Ibrahim, K.E.; Wadaan, M.A. Biological activity of Xanthium strumarium seed extracts on different cancer cell lines and Aedes caspius, Culex pipiens (Diptera: Culicidae). Saudi J. Biol. Sci. 2017, 24, 817–821. [Google Scholar] [CrossRef]
- Romero, M.; Zanuy, M.; Rosell, E.; Cascante, M.; Piulats, J.; Font-Bardia, M.; Balzarini, J.; De Clerq, E.; Pujol, M.D. Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties. Eur. J. Med. Chem. 2015, 90, 491–496. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Shyam Prasad, K. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Wellness 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Palmioli, A.; Ciaramelli, C.; Tisi, R.; Spinelli, M.; De Sanctis, G.; Sacco, E.; Airoldi, C. Natural compounds in cancer prevention: Effects of coffee extracts and their main polyphenolic component, 5-o-caffeoylquinic acid, on oncogenic Ras proteins. Chem. Asian J. 2017, 12, 2457–2466. [Google Scholar] [CrossRef]
- Guglielmetti, A.; D’Ignoti, V.; Ghirardello, D.; Belviso, S.; Zeppa, G. Optimisation of ultrasound and microwave-assisted extraction of caffeoylquinic acids and caffeine from coffee silverskin using response surface methodology. Ital. J. Food Sci. 2017, 29, 409–423. [Google Scholar]
- Bianchin, M.; Lima HHC de Monteiro, A.M.; de Benassi, M.T. Optimization of ultrasonic-assisted extraction of kahweol and cafestol from roasted coffee using response surface methodology. LWT 2020, 117, 108593. [Google Scholar] [CrossRef]
- Moeenfard, M.; Erny, G.L.; Alves, A. Determination of diterpene esters in green and roasted coffees using direct ultrasound assisted extraction and HPLC–DAD combined with spectral deconvolution. J. Food Meas. Charact. 2020, 14, 1451–1460. [Google Scholar] [CrossRef]
- Tsukui, A.; Santos Júnior, H.M.; Oigman, S.S.; De Souza, R.O.M.A.; Bizzo, H.R.; Rezende, C.M. Microwave-assisted extraction of green coffee oil and quantification of diterpenes by HPLC. Food Chem. 2014, 164, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Oigman, S.S.; De Souza, R.O.M.A.; Dos Santos Júnior, H.M.; Hovell, A.M.C.; Hamerski, L.; Rezende, C.M. Microwave-assisted methanolysis of green coffee oil. Food Chem. 2012, 134, 999–1004. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Ly, H.T.; Truong, T.M.; Nguyen, T.T.H.; Nguyen, H.D.; Zhao, Y.; Le, V.M. Phytochemical screening and anticancer activity of the aerial parts extract of Xanthium strumarium L. on HepG2 cancer cell line. Clin. Phytosci. 2021, 7, 14. [Google Scholar] [CrossRef]
- Kim, J.; Jung, K.H.; Ryu, H.W.; Kim, D.Y.; Oh, S.R.; Hong, S.S. Apoptotic effects of Xanthium strumarium via PI3K/AKT/mTOR pathway in hepatocellular carcinoma. Evid.-Based Complement. Altern. Med. 2019, 2019, 2176701. [Google Scholar] [CrossRef]
- Francisco Fernandez, M.; Charfi, C.; Piloto-Ferrer, J.; Lidia González, M.; Lamy, S.; Annabi, B. Targeting ovarian cancer cell cytotoxic drug resistance phenotype with Xanthium strumarium L. extract. Evid.-Based Complement. Altern. Med. 2019, 2019, 6073019. [Google Scholar] [CrossRef] [PubMed]
- Piloto Ferrer, J.; Cozzi, R.; Cornetta, T.; Stano, P.; Fiore, M.; Degrassi, F.; De Salvia, R.; Remigio, A.; Francisco, M.; Quiñones, O.; et al. Xanthium strumarium L. extracts produce DNA damage mediated by cytotoxicity in in vitro assays but does not induce micronucleus in mice. Biomed. Res. Int. 2014, 2014, 575197. [Google Scholar] [CrossRef]
- Alsabah, A.S.; Abd, A.H.; Al-Shammari, A.M. Cytotoxicity of Xanthium strumarium against breast cancer cell lines. J. Glob. Pharma Technol. 2018, 10, 767–776. [Google Scholar]
- Ferrer, J.P.; Zampini, I.C.; Cuello, A.S.; Francisco, M.; Romero, A.; Valdivia, D.; Gonzalez, M.; Salas, C.; Lamar, A.S.; Isla, M.I. Cytotoxic compounds from aerial organs of Xanthium strumarium. Nat. Prod. Commun. 2016, 11, 1934578X1601100313. [Google Scholar] [CrossRef]
- Karmakar, U.K.; Ishikawa, N.; Toume, K.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Sesquiterpenes with TRAIL-resistance overcoming activity from Xanthium strumarium. Bioorg. Med. Chem. 2015, 23, 4746–4754. [Google Scholar] [CrossRef]
- Xu, X.W.; Xi, Y.Y.; Chen, J.; Zhang, F.; Zheng, J.J.; Zhang, P.H. Phytochemical investigation of the fruits of Xanthium strumarium and their cytotoxic activity. J. Nat. Med. 2022, 76, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Chen, R.H.; Liu, D.C.; Zeng, D.S.; Liu, H. Chemical constituents from the fruits of Xanthium strumarium and their antitumor effects. Nat. Prod. Commun. 2020, 15, 1934578X20945541. [Google Scholar] [CrossRef]
- Wen, Y.L.; Li, M.J.; Ye, Z.J.; Liang, Y.M.; Wei, X.Q. Compounds isolated from the fruits of Xanthium strumarium, including a new neo-lignan, and their anticancer effects. Nat. Prod. Commun. 2020, 15, 1934578X20982782. [Google Scholar] [CrossRef]
- Ahn, J.W.; No, Z.S.; Ryu, S.Y.; Zee, O.P.; Kim, S.K. Isolation of Cytotoxic Compounds from the Leaves of Xanthium strumarium L. Nat. Prod. Sci. 1995, 1, 1–4. [Google Scholar]
- Ramírez-Erosa, I.; Huang, Y.; Hickie, R.A.; Sutherland, R.G.; Barl, B. Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can. J. Physiol. Pharmacol. 2007, 85, 1160–1172. [Google Scholar] [CrossRef]
- Liu, R.; Shi, D.; Zhang, J.; Li, X.; Han, X.; Yao, X.; Fang, J. Xanthatin promotes apoptosis via inhibiting thioredoxin reductase and eliciting oxidative stress. Mol. Pharm. 2018, 15, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tao, L.; Ruan, J.; Li, W.; Wu, Y.; Yan, L.; Zhang, F.; Fan, F.; Zheng, S.; Wang, A.; et al. Xanthatin induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma MKN-45 cells. Planta Med. 2012, 78, 890–895. [Google Scholar] [CrossRef]
- Karakas, F.P.; Yildirim, A.B.; Bayram, R.; Yavuz, M.Z.; Gepdiremen, A.; Turker, A.U. Antiproliferative activity of some medicinal plants on human breast and hepatocellular carcinoma cell lines and their phenolic contents. Trop. J. Pharm. Res. 2015, 14, 1787–1795. [Google Scholar] [CrossRef]
- Khazaei, M.; Pazhouhi, M. Antiproliferative effect of Trifolium pratens L. extract in human breast cancer cells. Nutr. Cancer 2019, 71, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Pazhouhi, M.; Khazaei, S. Evaluation of hydro-alcoholic extract of Trifolium pratens L. for its anti-cancer potential on U87MG cell line. Cell J. 2018, 20, 412–421. [Google Scholar]
- Priftis, A.; Panagiotou, E.M.; Lakis, K.; Plika, C.; Halabalaki, M.; Ntasi, G.; Veskoukis, A.S.; Stagos, D.; Skaltsounis, L.A.; Kouretas, D. Roasted and green coffee extracts show antioxidant and cytotoxic activity in myoblast and endothelial cell lines in a cell specific manner. Food Chem. Toxicol. 2018, 114, 119–127. [Google Scholar] [CrossRef]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.S.; Domingues, M.R.M.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef]
- Díaz-Hernández, G.C.; Alvarez-Fitz, P.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Parra-Rojas, I.; Flores-Alfaro, E.; Salazar, R.; Ramírez, M. Antibacterial, antiradical and antiproliferative potential of green, roasted, and spent coffee extracts. Appl. Sci. 2022, 12, 1938. Available online: https://www.mdpi.com/2076-3417/12/4/1938 (accessed on 27 March 2023). [CrossRef]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W.W.; Schilter, B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Quesada, A.R.; Medina, M.A. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS ONE 2011, 6, e23407. [Google Scholar] [CrossRef]
- Woo, S.M.; Min, K.J.; Seo, B.R.; Nam, J.O.; Choi, K.S.; Yoo, Y.H.; Kwon, T.K. Cafestol overcomes ABT-737 resistance in Mcl-1-overexpressed renal carcinoma Caki cells through downregulation of Mcl-1 expression and upregulation of Bim expression. Cell Death Dis. 2014, 5, e1514. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Park, E.J.; Oh, J.H.; Min, K.J.; Yang, E.S.; Kim, Y.H.; Lee, T.J.; Kim, S.H.; Choi, Y.H.; Park, J.W.; et al. Cafestol, a coffee-specific diterpene, induces apoptosis in renal carcinoma Caki cells through down-regulation of anti-apoptotic proteins and Akt phosphorylation. Chem. Biol. Interact. 2011, 190, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Chae, J.I.; Shim, J.H. Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J. Biomed. Sci. 2012, 19, 60. [Google Scholar] [CrossRef]
- Cárdenas, C.; Quesada, A.R.; Medina, M.Á. Insights on the antitumor effects of kahweol on human breast cancer: Decreased survival and increased production of reactive oxygen species and cytotoxicity. Biochem. Biophys. Res. Commun. 2014, 447, 452–458. [Google Scholar] [CrossRef]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol. Ther. 2015, 23, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Hwang, Y.P.; Choi, J.H.; Jin, S.W.; Lee, G.H.; Han, E.H.; Chung, Y.H.; Chung, Y.C.; Jeong, H.G. Kahweol inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. Food Chem. Toxicol. 2018, 121, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.I.; Jeon, Y.J.; Shim, J.H. Anti-proliferative properties of kahweol in oral squamous cancer through the regulation specificity protein 1. Phytother. Res. 2014, 28, 1879–1886. [Google Scholar] [CrossRef]
- Min, K.J.; Um, H.J.; Kim, J.I.; Kwon, T.K. The coffee diterpene kahweol enhances sensitivity to sorafenib in human renal carcinoma Caki cells through down-regulation of Mcl-1 and c-FLIP expression. Oncotarget 2017, 8, 83195–83206. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Dang, X.; Liu, Y.; Jiang, J.; Wang, Y.; Lu, X.; Guo, X. Effect of chlorogenic acid on LPS-induced proinflammatory signaling in hepatic stellate cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Palócz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS-induced intestinal inflammation and oxidative stress in IPEC-J2 cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gul, Z.; Demircan, C.; Bagdas, D.; Buyukuysal, R.L. Protective effects of chlorogenic acid and its metabolites on hydrogen peroxide-induced alterations in rat brain slices: A comparative study with resveratrol. Neurochem. Res. 2016, 41, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Kang, K.; Oidovsambuu, S.; Lee, E.H.; Jung, S.H.; Shin, I.S.; Nho, C.W. Gymnaster koraiensis and its major components, 3, 5-di-O-caffeoylquinic acid and gymnasterkoreayne B, reduce oxidative damage induced by tert-butyl hydroperoxide or acetaminophen in HepG2 cells. BMB Rep. 2013, 46, 513–518. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.O. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J. Food Sci. 2011, 76, C250–C256. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, S.; Liu, B. Three common caffeoylquinic acids as potential hypoglycemic nutraceuticals: Evaluation of α-glucosidase inhibitory activity and glucose consumption in HepG2 cells. J. Food Biochem. 2020, 44, e13361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Bai, J.P.; Zhang, Q.; Hu, X.L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.H.; Zhao, Q.C. Caffeoylquinic acid derivatives protect SH-SY5Y neuroblastoma cells from hydrogen peroxide-induced injury through modulating oxidative status. Cell. Mol. Neurobiol. 2017, 37, 499–509. [Google Scholar] [CrossRef]
- Xiao, L.; Liang, S.; Ge, L.; Wan, H.; Wu, W.; Fei, J.; Wu, S.; Zhou, B.; Zeng, X. 4, 5-di-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. targets the Keap1/Nrf2 pathway to attenuate H2O2-induced liver oxidative damage in HepG2 cells. Phytomedicine 2020, 70, 153219. [Google Scholar] [CrossRef]
- Tian, X.; Gao, L.; An, L.; Jiang, X.; Bai, J.; Huang, J.; Meng, W.; Zhao, Q. Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells. Neurol. Res. 2016, 38, 1079–1087. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef]
- Tripathi, N.; Chaudhary, S. A review on chemical and biological activity of Trifolium pretense. PharmaTutor 2014, 2, 93–101. Available online: http://www.pharmatutor.org/magazines/articles/march-2014/review-chemical-biological-activity-trifolium-pratense (accessed on 27 March 2023).
- Vlaisavljevic, S.; Kaurinovic, B.; Popovic, M.; Djurendic-Brenesel, M.; Vasiljevic, B.; Cvetkovic, D.; Vasiljevic, S. Trifolium pratense L. as a potential natural antioxidant. Molecules 2014, 19, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Schwartsova, H.; Vojinovic-Miloradov, M. Antioxidant profile of Trifolium pratense L. Molecules 2012, 17, 11156–11172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Brownmiller, C.R.; Lee, S.O.; Kang, H.W. Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide-stimulated RAW-267.4 macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wu, Y.Y.; Huang, H.; He, C.; Li, W.Z.; Wang, H.L.; Chen, H.Q.; Yin, Y.Y. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015, 35, 391–398. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ko, R.; Goshima, H.; Koike, A.; Shibano, M.; Fujimori, K. Formononetin attenuates H2O2-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology 2021, 85, 186–200. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.H.; Han, N.S.; Kim, D.O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Rawat, S.; Pathak, S.; Gupta, G.; Singh, S.K.; Singh, H.; Mishra, A.; Gilhotra, R. Recent updates on daidzein against oxidative stress and cancer. EXCLI J. 2019, 18, 950–954. [Google Scholar] [PubMed]
- Al Kury, L.T.; Taha, Z.; Mahmod, A.I.; Talib, W.H. Xanthium spinosum L. extracts inhibit breast cancer in mice by apoptosis induction and immune system modulation. Pharmaceuticals 2022, 15, 1504. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Ganesan, K.; Xiong, Q.; Zheng, Y. Antitumor effects of ononin by modulation of apoptosis in non-small-cell lung cancer through inhibiting PI3K/Akt/mTOR pathway. Genet. Res. 2022, 2022, 5122448. [Google Scholar] [CrossRef]
- Hashemian, M.; Sinha, R.; Murphy, G.; Weinstein, S.J.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Albanes, D.; Loftfield, E. Coffee and tea drinking and risk of cancer of the urinary tract in male smokers. Ann. Epidemiol. 2019, 34, 33–39. [Google Scholar] [CrossRef]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a valuable source of bioactive polyphenols: HPLC profile, in vitro antioxidant and antimicrobial potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Vlase, L.; Olah, N. Phytochemical analysis of isoflavons from some Fabaceae species extracts. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 57–60. Available online: https://www.notulaebotanicae.ro.corten-garden.com/index.php/nbha/article/view/3608 (accessed on 27 March 2023).
- Benedec, D.; Vlase, L.; Oniga, I.; Toiu, A.; Tǎmaş, M.; Tiperciuc, B. Isoflavonoids from Glycyrrhiza sp. and Ononis spinosa. Farmacia 2012, 60, 615–620. Available online: https://farmaciajournal.com/wp-content/uploads/2012-05-art3.benedec.pdf (accessed on 27 March 2023).
- Benedec, D.; Hanganu, D.; Filip, L.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Gheldiu, A.-M.; Raita, O.; Vlase, L. Chemical, antioxidant and antibacterial studies of Romanian Heracleum sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Toiu, A.; Vlase, L.; Gheldiu, A.M.; Vodnar, D.; Oniga, I. Evaluation of the antioxidant and antibacterial potential of bioactive compounds from Ajuga reptans extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Pop, A.; Fizes, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.S. Enhanced recovery of phenolic and tocopherolic compounds from walnut (Juglans regia L.) male flowers based on process optimization of ultrasonic assisted extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In vitro evaluation of biological activities of canes and pomace extracts from several varieties of Vitis vinifera L. for inclusion in freeze-drying mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizesan, I.; Pop, A.; Mocan, A.; Gheldiu, A.M.; Babota, M.; Vodnar, D.C.; Jurj, A.; Berindan-Neagoe, I.; Vlase, L.; et al. Walnut (Juglans regia L.) Septum: Assessment of Bioactive Molecules and In Vitro Biological Effects. Molecules 2020, 25, 2187. [Google Scholar] [CrossRef] [PubMed]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P. Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater. Sci. Eng. C 2020, 25, 111117. [Google Scholar] [CrossRef] [PubMed]
- Bolfa, P.; Vidrighinescu, R.; Petruta, A.; Dezmirean, D.; Stan, L.; Vlase, L.; Damian, G.; Catoi, C.; Filip, A.; Clichici, S. Photoprotective effects of Romanian propolis on skin of mice exposed to UVB irradiation. Food Chem. Toxicol. 2013, 62, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar]
- Pippenger, C.E.; Browne, R.W.; Armstrong, D. Regulatory antioxidant enzymes. In Free Radical and Antioxidant Protocols. Methods in Molecular Biology; Armstrong, D., Ed.; Humana Press Inc.: Totowa, NJ, USA, 1998; Volume 108, pp. 299–313. [Google Scholar]
- Bidian, C.; Filip, G.A.; David, L.; Moldovan, B.; Baldea, O.; Olteanu, D.; Filip, M.; Bolfa, P.; Poatra, M.; Toader, A.M.; et al. Viburnum opulus fruit extract-capped gold nanoparticles attenuated oxidative stress and acute inflammation in carrageenan-induced paw edema model. Ggreen Chem. Rev. 2022, 15, 319–335. [Google Scholar] [CrossRef]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Bolfa, P.; Catoi, C.; Baldea, I.; Bolojan, L.; Olteanu, D.; Muresan, A.; Postescu, I.D. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B 2011, 105, 133–142. [Google Scholar] [CrossRef]
- Noble, J.E.; Bailey, M.J. Quantitation of protein. Methods Enzymol. 2009, 463, 73–95. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).