Abstract
Common vetch (Vicia sativa L.) is a grain legume used in animal feeding, rich in protein content, fatty acid, and mineral composition that makes for a very adequate component to enrich feedstuff. In addition, relevant pharmacological properties have been reported in humans. The common vetch, similar to other legumes, can fix atmospheric nitrogen, a crucial feature for sustainable agricultural systems. These properties enhance the use of vetch as a cover crop and its sowing in intercropping systems. Moreover, several studies have recently pointed out the potential of vetch in the phytoremediation of contaminated soils. These characteristics make vetch a relevant crop, which different potential improvements target. Varieties with different yields, flowering times, shattering resistance, nutritional composition, rhizobacteria associations, drought tolerance, nitrogen fixation capacity, and other agronomic-relevant traits have been identified when different vetch accessions are compared. Recently, the analysis of genomic and transcriptomic data has allowed the development of different molecular markers to be used for assisted breeding purposes, promoting crop improvement. Here, we review the potential of using the variability of V. sativa genetic resources and new biotechnological and molecular tools for selecting varieties with improved traits to be used in sustainable agriculture systems.
1. Introduction
The common vetch (Vicia sativa L., Tribe Viciae, Family Fabaceae) is one of the world’s most economically important annual grain legumes, used as animal feed, as forage (grain, hay, and for silage production) or as grain legume as a cheap and rich source of protein and minerals of high digestibility and high energy content [1,2]. Additionally, vetch fixes atmospheric nitrogen through its symbiotic interactions with rhizobia soil bacteria, which could improve the soil fertility significantly, making its cultivation appropriate in sustainable agriculture, as a main crop, as an element of rainfed rotation, as a cover crop, or in rotation with cereals [3] by decreasing the use of fertilizers and reducing CO2 emissions and other pollutants [4]. The main bottleneck of this species is the presence of antinutritional factors in various parts of the plant, but the enormous dependence on proteins of vegetable origin for animal feed and the interest in the EU for the use of species of environmental value make the use of this crop a relevant agricultural option with an essential role in the implementation of environmental measures such as From Farm to Fork Strategy and the new Common Agricultural Policy (CAP) directive. In this work, we have tried to review the status and evolution of vetch cultivation and gene bank genetic resources throughout the world from a historical, economic, and environmental perspective. We have reviewed the main nutritional and pharmacological uses and environmental benefits of this crop as a nitrogen-fixer legume in cover crops, intercropping, or soil phytoremediators (Figure 1). The main abiotic and biotic threats to this crop have also been explored. Finally, we present the status of the main genomic and transcriptomic tools, from a biotechnological perspective and by means of the use of plant genetic resources collections to address the challenge of vetch crop improvement through combined strategies.
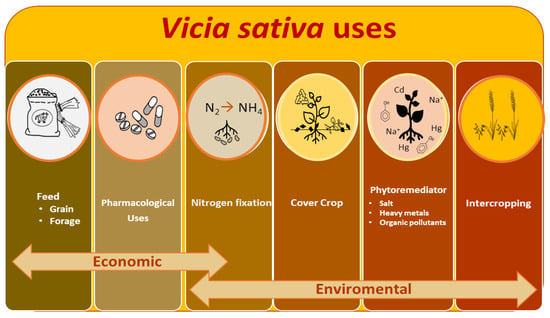
Figure 1.
V. sativa main uses. The scheme includes economically relevant nutritional and medical uses. Environmental applications for sustainable agriculture systems are also incorporated.
2. Taxonomy
The botanical tribe Viceae, from the subfamily Papilioboideae, of the family Fabaceae, includes some genus of agricultural interest such as Lens, Cicer, Pisum, Lathyrus, and Vicia [5]. The genus Vicia, whose center of origin and diversification has been placed in the Mediterranean and Irano-Turanian regions [6], includes a number of species ranged between 150 [7] and 210 [8], from which 34 are cultivated. The genus is, nowadays, distributed in temperate regions of the northern hemisphere in Asia, Europe, and North America, and also in the non-tropical region South America [9]. This genus has an enormous phenotypic variation [10]; in fact, there has been a big number of taxonomic revisions made over the genus, more than 20 since the original classification presented by Linneo (1735–1770) in the 18th century, following for those of Jaaska and other authors [9,11,12,13,14,15]. In a focus on V. sativa section Vicia, which includes the most important agricultural species, one of the last classifications was proposed by Van der Wouw et al. [16] after several studies focus on Vicia L. series Vicia, who presented a classification of this series in four species: V. babazitae Ten&Guss., V. incisa M.Bieb., V. pyrenaica Pourr., and V. sativa, which includes six subspecies: nigra (L.) Ehrh., segetalis (Thuill) Čelak., amphicarpa L.Batt, macrocarpa (Moris) Arcang., cordata (Wulfen ex Hoppe) Batt., and sativa. This group of forms is named the V. sativa aggregate.
Commercially vetch includes, in addition to V. sativa, V. villosa Roth. (winter vetch, hairy vetch) and other species of similar local importance such as V. pannonica L. in Turkey, V. pannonica, V. ervilia L. and V. articulata Hormen in Spain, and V. benghalensis L. in Australia; the same term, vetch, has been used for different species such as Lathyrus sativus L. and other Lathyrus species in Africa [17]. In this paper, we will use the name vetch to refer exclusively to cultivated species included in the Vicia sativa aggregate.
3. A Historical Crop
The common vetch, similar to other species of the Fabaceae family, has been cultivated together with cereals since the beginning of agriculture. Archaeological evidences indicate the Mediterranean Basis as the center of origin and primary diversification of this species [18,19]. We present below an historical revision of vetch use based on the Spanish Inventory of Traditional Knowledge about Agricultural Biodiversity [20], one of the most complete reviews of vetch uses.
Some authors indicate that the first archaeological references to vetch seeds date back to the Neolithic Periodic and the Bronze Age. This point is not clearly established because these seeds could also belong to wild species, associated with the crops. Additionally, others authors such as Zohary [21] disagree with this approach, dating the use of vetch into the agricultural systems in the Roman Empire, at a time when the use of vetch as a fodder species as already been reported, together with others species such as alfalfa and lupin or fenugreek, also associated with cereals and others grain legumes [22]. Columela, an ancient Roman scientist and writer who lived in the first century B.C. cited the use of vetch for poultry (hens and pigeons) feeding and as a fodder and green manure, together with other legumes such as alfalfa and fenugreek [23]. In the same time period, Plinius The Elder (First century B.C.) said that their use would improve soil fertility, giving indications about the sowing times in the function to the final use, including the use as fallow [24]. This author mentioned that vetch was the best feed for the bullocks. In the 4th century, Paladio described the use of a mixture of lupin and vetch as a soil improver when cutting in green, and they also made mention of the differences in the sowing date of the function of the final use. Isidore of Seville, who lived between the 6th–7th century, highlighted the scarce production of seed of vetch compared with other legumes [25]. In the Middle Age (11th and 12th century), vetch was a minority crop in Europe [26], even if the Andalusian author Abü l-Jayr indicated names such as Umda or Amank to identify different forms of vetches [27].
In the 16th century, Juan de Járava wrote that this species could be found among cereals and that it could be eaten as lentils, although it did not taste good [28]. In this century, vetch traveled to the New World, adapting perfectly to the local conditions of America, to the point that some escapees from cultivated vetches came to grow wild in the new environmental conditions [26]. Thus, in the 19th century, vetch was introduced to Argentina by Italian immigrants (settlers) establishing it as a well-known fodder [29]. To conclude this historical revision, a book from the 18th century used several names for cultivated and wild vetches and mentioned that they were a well-known crop in Europe, and that they could have reached Spain from the east by crossing France. Here, also, its uses as grain, green manure, and as a flour component to make bread in times of scarcity are mentioned [30].
4. Worldwide Vetch Cultivation
Due to its economic and ecological advantages (Figure 1), vetch is now widespread throughout many parts of the world. Figure 2A shows, based on data by FAOSTAT and the Spanish Ministry of Agriculture, Fisheries and Food [31], the surface and production of this crop from 1961 to 2021 are shown in Figure 2A. In the agricultural season of 2020–2021, the main producers were Ethiopia, the Russian Federation, Spain, Mexico, and Australia (Figure 2B,C).
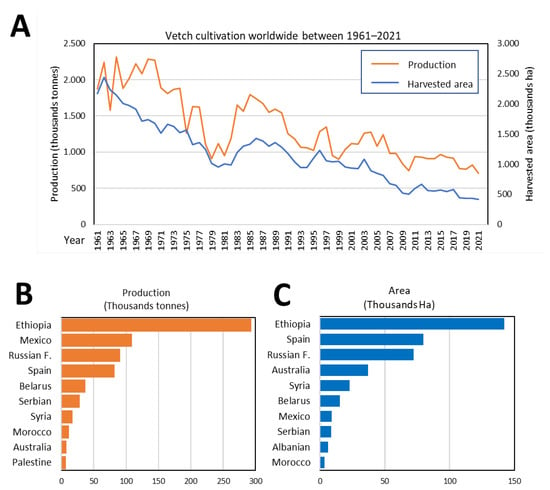
Figure 2.
Worldwide V. sativa cultivation. (A) Production data and cultivated area of vetch worldwide during the last 60 years. Main producers according to cultivated area (B) or production (C) during the year 2021.
According to FAO data from FAOSTAT, the economic value of the agricultural gross production of vetches worldwide was USD 139,237,000. This value was clearly well below the economic value of other legumes, partly due to its low production. Comparing its production worldwide with that of other legumes (average of last 5 years, 2017–2021), vetch production was 8 times less than lentil production or 18 times less than the production of chickpeas [31,32]. One of the reasons for this reduced production was the presence of antinutritional factors (ANFs) present in the grains. Therefore, as we discuss below, this is one of the main aims for common vetch improvement.
5. Nutritional and Pharmacological Properties
The nutritional value of the common vetch as a livestock feedstuff has been analyzed in different studies that have recently been reviewed [2,32]. The main conclusions of different works agree with the potential of common vetch grain, despite of the well-known deficit in sulfur amino acids (methionine and cysteine), as a rich source of proteins, minerals, and other nutrients, while being cheaper than other alternatives. The average crude protein values range from 21 to 39% (dry matter) and crude fat ranges from 9% to 38%, with high levels of palmitic and linoleic acids. The main essential and non-essential amino acids are leucine and glutamic acid, respectively. The seeds have high caloric content and are highly digestible [2]. These characteristics make vetch a potential nutrient-rich resource to be incorporated into animal diets and are very suitable to replace soy or a large proportion of cereals in certain feeds, maintaining their energy content. The nutritional content of the vetch seeds has been analyzed, and great differences in protein content, fatty acid composition, and mineral composition, including iron, were observed between accessions from different geographical origins. Although these studies have been carried out on a small scale, these data support the use of the variability of genetic resources from the gene banks of V. sativa for breeding purposes [33]. Remarkably, the large variation in crude protein and mineral content between different cultivars is much greater even than that due to climatic conditions [2,34,35]. This fact must be considered when selecting varieties with better nutritional conditions.
The medical uses of V. sativa have been also explored [36]. The seed flour and plant extract are traditionally used as an anti-poison and antiseptic [37,38], as an anti-asthmatic and respiratory stimulant in bronchitis [39], and as rheumatism treatment and an antipyretic [40]. Anti-acne [41] and antibacterial activity has been also validated [42]. However, most of the phytopharmacological mechanisms of action remain to be unraveled.
As described in other grain legumes, common vetch seeds contain a variety of antinutritional factors (ANFs), such as vicine, convicine, tannins, phenolic compounds, trypsin inhibitors, and cyano-alanines. Although some of these elements, such as polyphenols, have been studied as a source of antioxidants [43], these ANFs have partially limited the use of the seeds in food and/or feedstuffs, especially in the diets of monogastric animals [44]. However, the inclusion of a high proportion of common vetch seeds in the diet of ruminants does not produce relevant negative effects on their health [45,46,47,48,49,50]. The levels of anti-nutritional factors such as tannins, trypsin inhibitors, and hydrogen cyanide nutrients show huge variations between different accessions conserved in gene banks [33]. These variations have permitted the selection of low vicianine levels in common vetch accessions and have allowed the production of cultivars such as Blanchefluer without vicianine [51,52], extensively growing in Australia as a substitute for red lentils, although its consumption in humans is residual [17]. Last year, the molecular bases that regulate the hydrogen cyanide (HCN) synthesis from these cyanogenic glycosides have been unraveled in common vetch. Transcriptomic assays at different seed developmental stages enlighten important information about the regulatory network of this pathway. Eighteen key regulatory genes that are involved in HCN biosynthesis have been identified. These genes would be crucial as molecular markers for the selection and breeding of low HCN levelled vetch germplasm [53]. In any case, and especially for non-ruminant diets, it seems that these ANFs present in common vetch seeds need to be reduced or partially inactivated by adequate grain processing methods. A practical approach would be the selective breeding of varieties with a lower content of these antinutrients, but also the processing by soaking, chemical treatment, dehulling heat treatment, or germination. These treatments not only reduce the ANF content, but also improve the digestibility, palatability, and availability of the nutrients [35,54,55].
6. Environmental Benefits
The multiple benefits of common vetch for the farm as a versatile crop have been reviewed [2,32]. Plants need relatively large amounts of nitrogen for proper growth and development. The largest input of N into the terrestrial environment occurs through the process of biological nitrogen fixation (BNF). Therefore, BNF has great agricultural and ecological relevance, since N is often a limiting nutrient in many ecosystems [56]. The reduction of synthetic nitrogen fertilizers through the use of legumes not only has a decrease in environmental impact but also an economic one, due to the prices of these fertilizers, whose synthesis involves a large energy cost [57].
Rhizobia from legume-symbiotic systems make use of its nitrogenase enzyme to catalyze the conversion of atmospheric nitrogen (N2) to ammonia (NH3), which is a plant assimilable nitrogenous compound. This process utilizes energy produced by the legume photosynthesis and takes place in the symbiotic nodules of the legume roots. As other species of the Vicia genus, common vetch forms indeterminate-type root nodules through symbiosis with rhizobia to promote nitrogen fixation (Figure 3C). The interaction between the bacteria and host legume is so intricate that many rhizobial species nodulate in a host-specific manner despite the fact that the same symbiotic bacteria can infect different species, and even different genera, of legume. Rhizobium leguminosarum biovar viciae (Rlv) is the most common symbiont of V. sativa in which effective nitrogen fixation has been validated [56]. Furthermore, different strains of the Mesorhizobium and Bradyrhizobium genus have been isolated from V. sativa nodules, although there are no data about their ability to fix nitrogen [58]. Specific rhizobial nodule establishment in the plant host not only depends on the strain abundance in soil but also their nodulation competitiveness. R. leguminosarum biovar viciae establishes symbiosis with several legume genera, and genomics studies reveal plant preferences between specific rhizobial genotypes and the host V. sativa [59]. The complexity of these symbiotic associations and their specificity have been extensively addressed. These interactions present differences between V. sativa cultivars and wild relatives and are also affected by environmental conditions [58]. Moreover, the analysis of symbiotic genes of R. leguminosarum isolated from V. sativa from different geographical locations reveals a common phylogenetic origin, suggesting a close coevolution among symbiotic genes and legume host in this Rhizobium-Vicia symbiosis [60]. Symbiosis within V. sativa and Rlv has also been chosen as a model system to analyze different bacterial compounds, mainly oligosaccharides, and the plant-produced nod gene inducers (NodD protein activating compounds) involved in the establishment of the effective symbiosis with its host plant and the requirements for the host-plant specificity [61,62,63,64]. The bacterial nodulation genes (nod) are activated by flavonoids excreted by the common vetch roots [65], and, subsequently, the plant responds with the development of the root nodule [66]. Several physiological, biochemical, and transcriptomic analyses support an increase in drought tolerance in nodulated vetch plants compared to non-nodulated ones. Transcriptomic analysis has helped to discover specific drought pathways that are specifically activated in nodulated V. sativa plants, improving the understanding of the impact of the symbiosis-associated genetic pathways on the plant abiotic stress response [67].
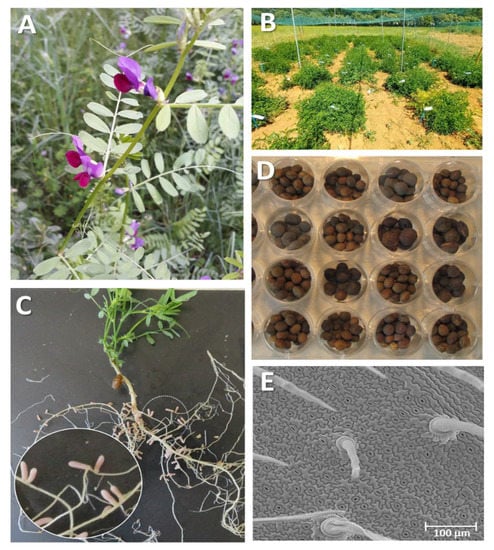
Figure 3.
V. sativa plants showing different tissues and growing stages. (A) Wild-growing common vetch at “Sierra Norte”-Madrid (Spain). (B) Field evaluation assay of different accessions (CRF-INIA/CSIC gene bank). (C) Indeterminate Rhizobium nodules of a common vetch root. (D) Diversity of size, shape, and color observed in seeds from different accessions from a CRF core collection. (E) Abaxial leaf surface, showing trichomes, stomas, and epidermal cells.
Crop systems in which legumes intercropped with cereals have traditionally been used in preference to legume or cereal monocultures, as it will result in higher forage yields and minimize synthetic fertilizers due to the nitrogen fixation ability of the legumes. The intercropping system of spring wheat (Triticum aestivum L.) with common vetch had a significant advantage on grain yield, beneficial effects on root development on both crops, and less N and P fertilizer requirements [68]. The use of vetch in the rotation of maize (Zea mays L.) and wheat helped to reduce the N deficiency, the increase in N concentration in the soil during next growing season, and the reduction in N losses by leaching [69]. Systems of oats (Avena sativa L.) or ryegrass (Lolium multiflorum Lam.) intercropped with common vetch have also proven to be especially profitable on dairy farms in central Mexico for silage cow feeding [70]. Finger millet (Eleusine coracana L.) is a widely grown cereal crop in some arid and semiarid areas in Africa, such as Ethiopia. Field assays in which ringer millet was intercropped with three vetch species, including V. sativa, concluded a general improving of the total dry matter yield and the quality of the intercrops [71,72,73]. Although the most well-characterized intercropped systems are those of legume cereal, other systems have been documented as intercropping with rapeseed (Brassica napus L.), which require higher levels of N fertilizers [73]. Additionally, the use of V. sativa in kiwifruit orchards increases the microbial community, moisture, and nutrients in the soil, activating plant growth [74].
Cover crops play an essential role in agroecosystems. They are unharvested plants grown in the gap between crops or integrated into rotations, which improve soil health, reduce erosion, enhance water availability, promote nutrient capture, are useful for controlling pests, weeds, and other diseases, and promote additional benefits for agriculture [75,76,77]. The use of legumes such as V. sativa as a cover crop allows the fixation of atmospheric nitrogen in the rhizobia symbiosis nodules, then the plant residue decomposes and remains available in the soil for the next harvest, acting as green manure by reducing the amount of inorganic fertilizer and reducing CO2 emissions [77,78]. It has recently been observed that V. sativa helps prevent water losses and soil erosion in vineyards (Vitis vinifera L.) [79]. In the USA, V. sativa is the most widely used legume cover crop [76]. In Argentina, V. sativa and V. villosa are the most important cultivated cover crop [80]. In Central Spain, the use of vetch as a cover crop in maize planted in the summer and autumn ensured the good production of principal crops and significant biomasses and N contents in the next following spring [81].
In recent years, environmental problems derived from soil and water contamination have begun to gain importance. In this context, the role that cover crops can have in phytoremediation is of great relevance [82]. V. sativa, together with V. faba, is the species of Vicia genus most frequently used in phytoremediation studies against inorganic and organic pollutants [83]. Different studies of phytoremediation, tolerance, and accumulation of inorganic and organic pollutants on V. sativa are summarized in Table 1. The relevance of V. sativa for the remediation of saline soils has recently been revealed. The phytodesalination process implies a high capacity of the plant to tolerate, absorb, and accumulate sodium in harvestable tissues [84]. Regarding the detoxification of organic compounds on V. sativa, the effect of the herbicide sulfosulfuron was evaluated without relevant effects on root or shoot growth parameters [85]. Similar studies assessed the effect of phenol and mepiquat chloride on seed yield and yield components in V. sativa plants, without drastic damages [86,87,88]. The growth, nodulation nitrogen fixation activity and V. sativa were less negatively affected by high concentrations of phenolics than in other tested legumes [89]. Wider studies on phytoremediation of diesel-fuel-contaminated soil were also developed in common vetch. The assays showed a greater tolerance of the vetch in diesel-contaminated soils and a greater capacity for decontamination of the soils compared to other crops [90]. Although V. sativa cannot be considered a hyperaccumulating plant capable of storing high concentrations of metals, copper tolerance has been described for germinative seeds [91,92]. Molecular mechanisms responsible for this tolerance remain to be explored, although it has been shown that vetch may prevent oxidative damage in the presence of some pollutants such as phenol by increasing the activity of lipid kinase and phosphatidic acid avoiding its toxicity [86,93]. V. sativa plants can also accumulate and concentrate different heavy metals. Curiously, these plants accumulate mercury (Hg) in the roots [94] but concentrate cadmium (Cd), lead (Pb), zinc (Zn), and nickel (Ni) in the aerial parts [95,96,97,98]. The tolerance of V. sativa to Cd seems to be related to antioxidant enzymes [99]. Phytochelatin synthases (PCS) and γ-Glutamylcysteine synthetase (γ-ECS) are directly involved in metal detoxification in plants. Ectopic overexpression of V. sativa PCS (VsPCS1) and γ-ECS (Vsγ-ECS) genes, which are Cd-inducible genes, are capable of increasing the tolerance to cadmium and the triggering of the detoxification pathway in Arabidopsis [100,101]. These results support the potential biotechnological use of these plants in phytoremediation processes against metal contamination.

Table 1.
Summary of studies of phytoremediation, tolerance, and accumulation of inorganic and organic pollutants on V. sativa.
Table 1.
Summary of studies of phytoremediation, tolerance, and accumulation of inorganic and organic pollutants on V. sativa.
| Pollutant | Developed Assay | References |
|---|---|---|
| Cd | Cd tolerance. Oxidative damage accumulation. | [96] |
| Cd and Zn | Zn and Cd accumulation in different tissues | [95] |
| Zn | Zn tolerance | [98] |
| Cu | Cu tolerance | [91,92] |
| Salt | Tolerance to salt. Na and K accumulation | [84] |
| Hg | Hg accumulation in different tissues | [94,100] |
| Ni | Ni accumulation. Oxidative damage accumulation. | [97] |
| Sulfosulfuron herbicide | Tolerance to sulfosulfuron | [85] |
| Diesel fuel | Tolerance to diesel | [90] |
| Phenol derivatives | Polychlorinated biphenyl (PCB) dissipation | [87] |
| Phenolics | Tolerance to phenolics. Effects on biomass, nodulation and nitrogen fixation activity | [89] |
| Mepiquat | Tolerance to mepiquat | [88] |
It has recently been shown that the rhizosphere microorganisms associated with the vetch roots can synergistically increase the decontamination potential by maximizing the efficiency of the process [102,103,104]. However, it is necessary to analyze the possible synergies or antagonisms derived from symbiosis to improve their efficiency in phytoremediation. On some occasions, bacterial strains tolerant to different pollutants do not show this activity when they are in symbiosis with V. sativa [83].
7. Pests and Diseases on Common Vetches
Diseases cause losses in quality and yield of common vetch crops and include viral, bacterial, and fungal infections, and, also, insect, spider, and nematode pests [105].
Many of the insects of forage legumes attack vetches, including beetle, flies, and aphids, promoting direct injuries or causing indirect damages by being vectors of virus transmission [106]. The herbivorous beetle vetch weevil (Bruchus rufipes Herbst.) is an important pest for legumes, including vetches. Their larvae feed on the grains, reducing the germination capacity of the seeds. The beetle Sitona lineatus L. is also a V. sativa common pest. Adults promote leaf damage, and the larvae produce symbiotic nodule destruction at a root level. Bemisia tabaci (Gennadius) flies cause damage to the crop, due to the suction of sap and the injecting of toxins through their saliva, which causes an overall weakening of the plant. Delia platura (Meigen), the seedcorn maggot, or the seed fly, is a polyphagous that significantly attacks vetch. Their larvae feed on seeds, young shoots, seedling stems, and roots, causing a general weakening or even vetch death. The pea moth (Laspeyresia nigricana Fabricius) is a lepidopteran that also attacks vetch. Their larvae cause damage to the pod and grain, promoting premature yellowing and the loss of its germinative power. Aphids also attack vetches. Thus, the black legume aphid (Aphis craccivora Koch) and the green legume aphid (Acyrthosiphon pisum Harris) attack young shoots, biting and sucking the sap, causing leaf deformation, shoots yellowing, and reducing photosynthesis. Other arthropods, such as the red spider mite (Tetranychus urticae Koch.), also promote severe damage to V. sativa. The affected leaves decrease their photosynthetic and transpiration capacity, causing defoliation, especially at the first phases of the crop that are the most sensitive period to the attack of this spider [107].
Fungal infections are the diseases most likely to cause the greatest losses in vetch. An extensive review has been carried out on fungal diseases affecting vetch, such as anthracnose, powdery mildew, rust, and botrytis [105]. At least 14 fungal diseases on V. sativa had been reported from 28 countries [108]. Over 43 fungal pathogenic species infect common vetch. These pathogens belong to the Deuteromycotina (58%), Ascomycotina (16%), Basidiomyotina (14%), and Mastigomycotina (12%) groups. Anthracnose is the main fungal disease in common vetch and is mainly caused by six pathogenic fungi: Colletotrichum vicia Dearness, C. villosum Weimer, C. sativum Horn, C. vicia-sativa Sawada, C. lentis Damm, and C. spinaciae Ellis & Halst. [108].
In vetch, there are no known severe diseases caused by bacterial pathogens. Bacterial blight was one of the first and more relevant infections identified in V. sativa. The disease, recorded in Australia for the first time, was caused by Pseudomonas stizolobii Wolf [109] that promotes necrotic lesions on stems, leaves, and flowers, which, in many cases, causes the complete wilting of the plant [110].
No severe economic losses associated with viral infections in common vetch crops have been reported. However, different viruses capable of infecting V. sativa and promoting effects on the quality and quantity of production have been identified [111]. Artichoke Yellow Ringspot Virus (AYRSV) infection in V. sativa was reported from Greece and Italy. This virus (Nepovirus genus; Secoviridiae Family) promotes severe stunting and leaf mottling in the infected vetch plants [112]. Additionally, Bean Yellow Mosaic Virus (BYMV) infections have been identified in Germany a long time ago. BYMV (Potyvirus genus; Potyviridae Family) promotes several symptoms, including leaf and stalk necrosis, distortion of plants, and dark to yellow green stripes along the veins on the lower surface. The virus is transmitted by aphid vectors in a non-persistent manner [113]. Broad Bean Stain Virus (BBSV) infection was reported from Slovakia in V. sativa crops. BBSV (Comovirus genus; Secoviridae Family) is transmitted by weevils [114]. Chickpea Chlorotic Stunt Virus (CpCSV) infection has been identified in V. sativa crops from Syria. Symptoms in virus-infected vetches include exhibit yellowing, reddening, and stunting. This virus (Polerovirus Genus; Luteoviridae Family) is transmitted by aphid vectors in a circulative, non-propagative manner [115]. For the Faba Bean Necrotic Yellows Virus (FBNYV), V. sativa-infection was identified from Azerbaijan. The virus-infected vetch plants exhibit leaf rolling, yellowing, and stunting symptoms. FBNYV (Nanovirus Genus; Nanoviridae Family) is transmitted by aphid vectors in a persistent but non-propagative manner [116].
Annual and parasitic weeds are the most important constraints for legume production, including vetches, because of their slow initial growth that causes poor competition to weeds. In the case of parasitic weeds, broomrapes (Orobanche and Phelipanche spp.) are obligate parasites that infect roots of dicotyledoneous plants, including Vicia genus. Broomrapes represent severe weed problems causing relevant yield losses on crops. Orobanche spp. are particularly important in Southern and Eastern Europe, the Middle East, and North Africa. Orobanche foetida Viv. is considered important as an agricultural parasite of common vetch crops in the western Mediterranean area, including Portugal, Spain, Morocco, Algeria, and Tunisia [117]. Recently, O. crenata Forssk. has been identified as a parasite on vetch crops in Spain [118]. Different components isolated from V. sativa root exudates were reported as stimulants of Orobanche or Phelipanche sp. seed germination [119]. The basis of host resistance to broomrapes is almost unknown, but vetch resistance based on inducing necrosis of broomrape tubercles by the formation of mucilage and the occlusion of host xylem has been reported [120]. The dodders (Cuscuta spp.) are also damaging parasitic plants of vetch crops that are highly susceptible to C. campestris. However, recently, some resistant genotypes have been identified in V. sativa germplasm collections [121].
The use of pre-emergence herbicides is effective, but the use in post-emergence is scarce due to their lack of safety [122] and the shortage of available active substances [123,124]. An additional constraint in some regions is the strict limitation on the use of plant protection for weed control (Council Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 that establishes a framework for Community action to achieve the sustainable use of pesticides). Strengthening research is the most adequate way to improve this situation, both in the search of environmentally friendly active ingredients as well as in the design of varieties with the genetic capacity to compete with weeds.
8. Germplasm Gene Banks and Common Vetch Genetic Diversity
Gene banks are relevant resources for the conservation of natural genetic diversity and provide a source of novel features for fluctuating circumstances associated with climate change and new disease outbreaks and are crucial for sustained crop improvement [125,126,127].
The total number of varieties and accessions of common vetch ex situ conserved is quite difficult to estimate due to the taxonomic complexity of this species. GENESYS (https://www.genesys-pgr.org/ accessed on 15 February 2023) is an online platform that includes information on Plant Genetic Resources (PGR) for food and agriculture conserved in gene banks worldwide, and it was accessed in February 2023. This data base includes a total of 54,743 accessions that belong to the genus Vicia, 13,694 of which belong to the species sativa, including the next taxons: V. sativa, V. sativa subsp sativa, V. sativa subsp nigra, V. sativa subsp cordata, V. sativa subsp amphicarpa, V. sativa subsp macrocarpa, V, sativa subsp incisa, V. cordata, V angustifolia, and V. macrocarpa, most of them landraces and wild forms. Regarding the provenance, these accessions has been mainly collected in Russia, Turkey, Spain, Italy, Syria, and Bulgaria. Figure 4 shows the number of accessions by holding institutions.
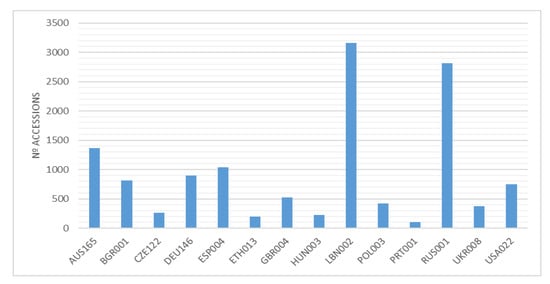
Figure 4.
Main collection of Vicia sativa accessions, by holding institution, reported at GENESYS. The holding institutions are identified by their WIEWS code, available at https://www.fao.org/wiews/data/organizations/en; (accessed on 15 February 2023).
Regarding Europe, EURISCO, the European catalog of plant genetic resources (https://eurisco.ipk-gatersleben.de/ accessed on 15 February 2023), includes a total of 7943 accessions named V. sativa, including also the species and some subspecies. The most important collections are those kept at the gene banks of the Russian Federation, Germany, Spain, and Bulgaria.
Despite the abundance of genetic resources, the situation regarding the availability of commercial varieties of vetch, at least in Europe, is limited. The European Common Catalogue (https://ec.europa.eu/food/plant-variety-portal/ accessed on 15 February 2023), includes 110 commercial varieties and 2 conservation varieties of this crop, registered by 14 countries. Italy, France, and Spain are the countries with the highest number of registered varieties.
One of the limiting factors for the use of these germplasm collections is the lack of characterization data; another limitation is the possible environmental influence on the expression of the agro-morphological traits. The solution to increase the value of these resources is the genotyping of these collections and the use of molecular marker-assisted selection.
9. Generating Genomic and Transcriptomic Tools
Recent years have witnessed the development of different types of increasingly efficient molecular markers that have allowed the characterization of the diversity of the accessions present in collections around the world (Summarized in Table 2). The first works were based on the use of retrotransposon-derived Sequence-Specific Amplified Polymorphism (SSAP) markers and allowed a preliminary characterization of the genetic diversity of the genus Vicia [128]. The use of Amplified Fragment Length Polymorphism (AFLP) derived markers allowed the diversity analysis of the genetic singularity coefficients in Russian V. sativa varieties [129]. Seed reserve protein patterns were also used to characterize the diversity present in the germplasm on a collection of Spanish vetches [130]. Start Codon Targeted (SCoT) markers have been used for analyzing the intra-population diversity of several common vetch varieties and optimizing the minimal sample size to assess their genetic diversity [131]. Inter Simple Sequence Repeats (ISSR) markers have been also used for characterizing more than 25 accessions from the Spanish V. sativa germplasm collection [132]. During the last few years, the rapid emergence and efficient development of next-generation sequencing (NGS) technologies have allowed the transcriptomic analysis of many species in a high-throughput manner with reasonable economic costs and in a time-efficient approach. This methodology has made the in-depth analysis of gene expression and the annotation of a large number of genes possible, but it has also been very useful for analyzing the presence of polymorphisms, managing to design molecular markers as simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs) associated with functional transcribed genes and associated traits [133,134]. Thus, the analysis of V. sativa transcripts from RNA-seq data has allowed the identification of cDNA-SSR molecular markers with a high degree of polymorphisms [135,136,137]. These markers have shown a high amplification potential in other species of the Vicia genus [138], supporting the possibility of using heterologous markers for diversity analysis of related species. These SSRs are mapped in coding regions of the genome, less polymorphic but potentially more conserved. This fact could favor the high rate of transferability across closely related species of the same genus. Recent works have developed a minimum set of 14 SSR reference alleles that have allowed the genotyping of over 545 common vetch worldwide accessions, including landraces, cultivars, and wild relatives. This analysis has allowed an exhaustive analysis of the diversity present in this collection based on which a Spanish vetch core collection (Figure 3B,D) has been created with a minimum loss of genetic diversity in comparison with the total collection [132]. Genome-derived polymorphisms have also been identified. Over 24,000 single nucleotide polymorphisms (SNP) have been identified from 1243 plants of the 12 natural V. sativa Japanese populations, and double-digest restriction-site associated DNA sequencing (ddRAD-Seq) was used to evaluate heterogeneity of these accessions [139]. A total of 76,810 different SSR have been also identified, tri- (36%) and tetra-nucleotide (13%) repetitions being the most abundant. Some of these SSR have been used to analyze the genetic diversity of Chinese common vetches [140].

Table 2.
Summary of molecular markers used for diversity characterization of V. sativa germplasm.
Table 2.
Summary of molecular markers used for diversity characterization of V. sativa germplasm.
| Type of Molecular Marker | Target | References |
|---|---|---|
| Retrotranspon-derived Sequence-Specific Amplified Polymorphism (SSAP) | Genomic sequences | [128] |
| Amplified Fragment Length Polymorphism (AFLP) | Genomic sequences | [129] |
| Seed reserve protein patterns | Protein | [130,132] |
| Start Codon Targeted (SCoT) marker | cDNA sequences | [131] |
| Inter Simple Sequence Repeats (ISSR) | Genomic sequences | [132] |
| cDNA-SSR | cDNA sequences | [132,135,136,138,140,141,142] |
| SNP | cDNA sequences | [132,139,142] |
| double-digest restriction-site associated DNA sequencing (ddRAD-Seq) | Genomic sequences | [139] |
| genomic-SSR | Genomic sequences | [140] |
9.1. Genomic Data and Transcriptomic Characterization of Some Traits and Developmental Stages
The lack of a high-quality and complete publicly available reference genome sequence of V. sativa has restricted the advances in molecular breeding and functional genomics efforts to improve this crop. Previous publications have reported controversial karyotypes with at least three different chromosome numbers (2n = 10, 12, and 14) inside the V. sativa aggregate. The four main taxa, sativa, macrocarpa, cordata, and angustifolia, suggest taxonomic ascription problems into the V. sativa aggregate or spontaneous amphidiploids or hybrid derivatives [143,144].
During this last year, two groups have sequenced the common vetch genome. Recently, a draft of common vetch chromosome-level genome has been published with a partial 85% assembly of over 1.5 Gb and 31,146 predicted genes [139], validating a ploidy of 2n = 14 for the sequenced cultivar (V. sativa subsp. sativa Cv. Studenica). Another lab has reported similar results with an estimated sequenced genome size of 1.6 Gb [140]. Further efforts will make it possible to have a complete and annotated genome, which will make it easier to have efficient genomic tools. Meanwhile, different transcriptional approaches by RNA-seq have been developed, making it possible to have gene expression data and sequences of transcripts from different tissues, developmental stages, and stress responses. These results have been very useful to understanding at genetic level processes such as flowering time, floral development, pod shattering, metabolic processes associated with HCN synthesis, or plant responses to different abiotic stress as drought, salt, or cold, and their interconnection between different plant tissues as root and leaves, summarized in Table 3, whose results are explained below.
Flowering time is an important determinant of harvesting time. In common vetch, early flowering promotes plant seed production, but it affects the yield of forage biomass. Therefore, understanding the flowering process is crucial for breeding purposes. To unravel the molecular mechanisms of flowering regulation, V. sativa accessions with different flowering times were analyzed at different developmental stages for integrative analyses of the transcriptomes and metabolomes. Among the differentially expressed genes (DEGs), synthesis and signal transduction of plant hormone pathways were the most enriched pathways. Moreover, the contents of three metabolites related to salicylic acid biosynthesis correlated with the observed differences in DEGs [145]. The development of the zygomorphic flower of V. sativa (Figure 3A) has also been analyzed at the transcriptional level by comparative expression analyses on six floral organs (sepals, dorsal petals, lateral petals, ventral petals, stamens, and carpels) in common vetch. Results show that these gene expression patterns of the vetch flower fitted a strict ABC model, similar to the core eudicots Arabidopsis [146].
The seed dispersion by pod shattering is the main form of propagation of many wild species and is one of the plant characters radically changed by crop domestication, already studied in legumes such as soya [147], chickpea [148], and common bean [149]. This behavior is frequent in cultivated V. sativa, and it is one of the most important defects that limits its utilization [150]. To better understand the pod shattering mechanism at a molecular level, comparative transcriptomic analysis of pod ventral sutures between shattering-susceptible and shattering-resistant vetch accessions has been performed. The most enriched pathways among the differentially expressed genes were processes related to cell wall modifications and hydrolases associated with pod shattering. The results helped to unravel the pod-shattering gene regulation networks in vetch. This information is relevant for the identification of pod-shattering-related genes and the design of future molecular markers assisted selection in breeding programs [142,151].
9.2. Genomic Data and Transcriptomic Characterization of Stress Responses
The physiological response of V. sativa germplasm collections has been explored under treatments with different concentrations of NaCl to select salt-tolerant accessions for breeding programs. Salt-tolerant vetches have a higher K+/Na+ ratio than salt-sensitive plants under these treatments. To unravel molecular mechanisms involved in salt tolerance, the expression of genes involved in ion homeostasis was evaluated. Salt-tolerant varieties had higher expression levels in the ion transporters NHX7, HKT1, AKT2, and HAK17, compared with the salt-sensitive ones. Proline levels, expression of the enzyme P5CS1 involved in proline biosynthesis, and antioxidant enzymes SOD, CAT, and APX were also higher in salt-tolerant varieties [152]. A comparative transcriptomic analysis of the leaves and roots of common vetch under salinity stress was also performed. This assay has allowed us to begin to unravel the complex molecular mechanisms associated with the salt stress response and the differences and interconnections that are established between the aerial and radicular parts of the plant and how these can help explain the behavior observed in some salt-tolerant varieties [153].
Drought is one of the main stresses that threatens current agriculture. It is estimated that its effects will be more dramatic in the coming years, as a direct consequence of climate change. Drought pressures vetch production, both in forage and grain. Therefore, it is important to understand the mechanisms of response and tolerance to drought in this legume. Physiological drought responses of V. sativa have been analyzed at a photosynthetic [154,155] and biochemical level [142]. Recent years have witnessed numerous studies addressing the drought response from a molecular perspective, trying to understand the genetic networks involved. Transcriptomes carried out on whole tolerant and sensitive vetch plants under different drought treatments reveal a functional enrichment of genes involved in relevant process as “oxidation reduction”, “lipid metabolism”, “oxidoreductase activity”, or “plant hormone signaling” [142]. Additionally, the aquaporin gene family seems to play an essential role during drought stress [156]. Moreover, miRNAs that are small noncoding RNAs that negatively regulate the expression of downstream target genes have been recently identified as regulators of the drought response in V. sativa. Potential targets of the identified drought-responsive miRNA include genes involved in various pathways, as cell wall biosynthesis, reactive oxygen removal, and protein transport, providing new insights into the miRNA-mediated regulatory networks of drought stress response in common vetch [155,157]. The response to drought stress is complex and involves different gene regulatory networks and physiological responses in the root and the aerial parts that are responsible for water uptake and stomatal evapotranspiration, respectively (Figure 3E). Comparative transcriptomic analyses have been performed in common vetch leaves and roots under drought stress. These studies shed light on the coordinated response of aerial and radicular tissues of common vetch to drought stress. Hormone signal transduction, starch and sucrose metabolism, and arginine and proline metabolism were extensively enriched in genes belonging to drought-responsive pathways, including the enzyme P5CS1, involved in the biosynthesis of proline. Various TFs’ (Transcription factors) family members (WRKY, bHLH, AREB/ABF, MYB, and AP2/ERF) seem to be crucial regulators in the crosstalk between leaves and roots during drought response. Previous studies support that the expression profile in the roots was more stable than that in the leaves. However, both the aerial and root parts coordinate the gene response to optimize the whole-plant adaption in drought stress by undergoing similar biological processes [155]. Similar approaches were performed combining drought and cold abiotic stresses, analyzing the crosstalk between aboveground and underground parts of common vetch. This study identifies specialized and unique responses to combined stresses in common vetch [158]. To understand the molecular networks involved not only in drought response, but also in adaptive mechanisms of drought tolerance, transcriptomic differences and specific polymorphic variants (mainly SNPs and SSRs) between tolerant and sensitive accessions under drought have been analyzed. This strategy has allowed the design of drought-associated markers to be used as new molecular breeding tools [142].

Table 3.
Developmental, metabolic, or stress processes with transcriptomic analysis in Vicia sativa. Analysis was performed at indicated plant tissue or developmental stage.
Table 3.
Developmental, metabolic, or stress processes with transcriptomic analysis in Vicia sativa. Analysis was performed at indicated plant tissue or developmental stage.
| Process | Analyzed Plant Tissue | References |
|---|---|---|
| Flower Development | Floral organs (dorsal, lateral and ventral petals, sepals, stamens, carpels) leaf, and roots | [146] |
| Flowering time | Aerial part at different stages | [145] |
| Pod Shattering | Pod ventral sutures | [151] |
| Drought Tolerance | Whole plant under different drought treatments | [137] |
| Drought Stress | Root, stem, and leaf tissue under PEG treatments | [156] |
| Drought Stress | Comparative leaf versus root | [155] |
| Drought response and tolerance | Aerial part of tolerant and sensitive varieties | [142] |
| Cold–drought combined stress | Comparative leaf versus root | [158] |
| Drought Stress | Aerial part under PEG treatments | [157] |
| Salinity Stress | Leaf versus root | [153] |
| Hydrogen Cyanide Synthesis | Seed development | [53] |
10. Perspectives for Future Breeding Strategies
Leguminous crops face several challenges, in the context of achieving sustainable agriculture. Among the main ones, it included the improvement of different agronomic and nutritional traits in common vetch by using several approaches. Traditional strategies have included the selection of cultivars with high forage and/or grain production. Additionally, the selection of varieties with flowering times or pod shattering adapted to different climatic conditions. Nowadays, to cope with increasing climate change in arid areas, breeding strategies for more tolerant and resilient crops, including common vetch, will be essential. The different tolerance mechanisms and management of drought stress and other associated abiotic stresses in grain legumes have been thoroughly reviewed and analyzed [159]. Legume crops face the challenge of managing water deficits by creating different ways for efficient water management at the same time as increasing crop yield [160]. Selecting drought-tolerant genotypes is strategically crucial to cope with water deficits. Breeding approaches as a result of the combination of traditional–classic techniques with novel tools of breeding techniques would generate yield improvements under drought. The use of classical breeding tools includes the exploitation of the diversity of plant genetic resources that is present in gene banks, including the use of crop wild relatives, for screening and selecting improved accessions. Novel strategies involve the use of biotechnological and molecular tools. V. sativa is particularly recalcitrant to transformation, thus obtaining transgenic plants transformed with Agrobacterium tumefaciens has been extremely difficult. Therefore, up to now the improvement in Vicia has been only reported by conventional methods. Hopeful results have only recently been achieved through the development of systems based on the use of R. rhizogenes for in vitro hairy root transformation by infecting different explants [161]. New approaches to cope with drought in legumes include the construction of transgenic legumes or Genome Editing (GE) tools, and also include the novel molecular and genomic techniques of Genome-Wide Association Studies (GWASs), Marker-Assisted Selection (MAS) with molecular markers, Genomic Selection (GS), and OMICs-based technologies by using transcriptome, genome, phenotype, proteome, and metabolome data as biotechnological tools. Within this strategy, several works have reported information on different drought-associated molecular markers (mainly SSRs and SNPs) in V. sativa to be used as new breeding tools for the molecularly assisted selection. Analysis of agronomic traits associated with drought have been carried out over a V. sativa core collection representative of the genetic diversity of a 545 whole collection (Figure 3B), selecting promising accessions.
In addition to these combined breeding strategies, new agronomy approaches are available. These tools include the exogenous application of plant Growth Regulators (PGRs), osmoprotectants, and bioinoculants of Plant-Growth-Promoting Rhizobacteria (PGPR). The improvement of biological nitrogen fixation by rhizobium symbiosis and the selection of new generation of highly effective rhizobia inoculants are some of the main challenges of sustainable agriculture research. The importance of the symbiotic interaction between legumes and rhizobium has promoted the production of commercial rhizobia inoculants. Inoculation of leguminous crops with rhizobia strains not only promotes sustainable farming systems by reducing fertilizer inputs, but also a considerable increase in crop yields from an economic and environmental point of view. In addition to selecting legume genotypes especially tolerant to abiotic stresses, another focus of the study is that the potential selection of rhizobia more tolerant to these types of stresses. This symbiotic systems with selected strains of Rhizobium could increase the legume production under drought conditions [67,162]. Selecting varieties with a high capacity for nitrogen fixation would be of interest to sustainable agriculture. In this sense, common vetch has shown novel applications and great potential for soil improvement in recent years linked with its ability as a phytoremediator, capable of reducing organic and inorganic pollutants in the soil, mediated by their roots’ bacterial communities. This capacity makes vetch a promising crop for improving soil health by decreasing contaminants.
Another potential target of improvement, as previously discussed, is associated with selecting varieties with increased nutritional properties. The large variations observed in protein and mineral content between different varieties open a promising gateway for selecting varieties with these characteristics. We must not forget that these selection strategies must correlate with the reduction in anti-nutritional factors, including tannins, trypsin inhibitors, and hydrogen cyanide, which also present variability between different varieties. Together with this, some authors have reported the use of V. sativa seeds as a source of functional components, mainly polyphenols, for the elaboration of functional foods [163]. The use of classical procedures to reduce the ANFs content (heating, washing, germination, fermentation, soaking…) and the new ones such as the Controlled Pressure Drop, ultrasound, or microwaves that could be used as an alternative to the cooking and germination to reduce the level of ANF [164] could be used to increase the use of legumes in general, and vetch, in particular, for animal and human consumption.
Author Contributions
L.D.l.R. and E.R.-P. are equally responsible for conceptualization, data curation, writing, reviewing, editing, approving the submitted version, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants RTI2018-094037-R-I00, PDI2021-122138OR-I00, from the Spanish Ministerio de Ciencia e Innovacion (MCIN/AEI/10.13039/501100011033); and by the “Severo Ochoa Program for Centres of Excellence in R&D” from the Agencia Estatal de Investigación of Spain, grant SEV-2016-0672 (2017–2021) to the CBGP; grant RF2007-00005 from the Spanish Ministry of Education and Science. CSIC partially supports open-access publication fees.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.fao.org/faostat/en/(accessed on 15 February 2023), https://www.genesys-pgr.org/ (accessed on 15 February 2023) and https://eurisco.ipk-gatersleben.de/apex/eurisco_ws/r/eurisco/home; (accessed on 15 February 2023).
Acknowledgments
We are grateful to Juan M. González (Universidad de Alcalá) for the critical proofreading of the article and to Roberto González for the English revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
AFLP: Amplified Fragment Length Polymorphism; ANF: Antinutritional Factors; AYRSV: Artichoke Yellow Ringspot Virus; BNF: Biological Nitrogen Fixation; BYMV: Bean Yellow Mosaic Virus; CAP: Common Agricultural Policy; CpCSV: Chickpea Chlorotic Stunt Virus; ddRAD-Seq: double-digest Restriction-site Associated DNA sequencing; DEG: differentially expressed genes; FAO: Food and Agriculture Organization; FBNYV: Faba Bean Necrotic Yellows Virus; GE: Genome Editing; GS: Genomic Selection; GWAS: Genome-Wide Association Studies; HCN: hydrogen cyanide; ISSR: Inter Simple Sequence Repeats; MAS: Marker-Assisted Selection; NGS: Next-Generation Sequencing; PCS: Phytochelatin synthases; PGPR: Plant-Growth-Promoting Rhizobacteria; PGR: Plant Genetic Resources; SCoT Start Codon Targeted; SNP: Single Nucleotide Polymorphisms; SSAP: Sequence-Specific Amplified Polymorphism; SSR: Simple Sequence Repeats; TF: Transcription Factors; γ-ECS: γ-Glutamylcysteine synthetase.
References
- Parissi, Z.; Irakli, M.; Tigka, E.; Papastylianou, P.; Dordas, C.; Tani, E.; Abraham, E.M.; Theodoropoulos, A.; Kargiotidou, A.; Kougiteas, L.; et al. Analysis of Genotypic and Environmental Effects on Biomass Yield, Nutritional and Antinutritional Factors in Common Vetch. Agronomy 2022, 12, 1678. [Google Scholar] [CrossRef]
- Huang, Y.F.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 807–823. [Google Scholar] [CrossRef]
- Lithourgidis, A.; Dordas, C.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: An alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Dalias, P.; Neocleous, D. Comparative Analysis of the Nitrogen Effect of Common Agricultural Practices and Rotation Systems in a Rainfed Mediterranean Environment. Plants 2017, 6, 61. [Google Scholar] [CrossRef]
- Maxted, N. An ecogeographical study of Vicia subgenus Vicia. In Systematic and Ecogeographic Studies on Crop Genepools; IPGRI: Rome, Italy, 1995; Volume 8, p. 184. [Google Scholar]
- Maxted, N. A phenetic investigation of Vicia L. subgenus Vicia (Leguminosae, Vicieae). Bot. J. Linn. Soc. 1993, 111, 155–182. [Google Scholar] [CrossRef]
- Hanelt, P.; Mettin, D. Biosystematics of the Genus Vicia L. (Leguminosae). Annu. Rev. Ecol. Syst. 1989, 20, 199–223. [Google Scholar] [CrossRef]
- Kupicha, F.K. The infrageneric structure of Vicia. Notes R. Bot. Gard. Edinb. 1976, 34, 287–326. [Google Scholar]
- Leht, M. Phylogenetics of Vicia (Fabaceae) based on morphological data. Feddes Repert. 2009, 120, 379–393. [Google Scholar] [CrossRef]
- Tate, M.; Ennerking, D. Vetches: From feed to food? Grain Legumes 2006, 47, 12–13. [Google Scholar]
- Potokina, E. Vicia sativa L. aggregate (Fabaceae) in the flora of former USSR. Genet. Resour. Crop Evol. 1997, 44, 199–209. [Google Scholar] [CrossRef]
- Jaaska, V. Isoenzyme diversity and phylogenetic affinities in Vicia subgenus Vicia (Fabaceae). Genet. Resour. Crop Evol. 1997, 44, 557–574. [Google Scholar] [CrossRef]
- Jaaska, V. Isozyme Variation and Phylogenetic Relationships in Vicia subgenus Cracca (Fabaceae). Ann. Bot. 2005, 96, 1085–1096. [Google Scholar] [CrossRef]
- van de Wouw, M.; Maxted, N.; Chabane, K.; Ford-Lloyd, B.V. Molecular taxonomy of Vicia ser. Vicia based on Amplified Fragment Length Polymorphisms. Plant Syst. Evol. 2001, 229, 91–105. [Google Scholar] [CrossRef]
- Yeater, K.M.; Bollero, G.; Bullock, D.; Rayburn, A.L. Flow cytometric analysis for ploidy level differentiation of 45 hairy vetch accessions. Ann. Appl. Biol. 2004, 145, 123–127. [Google Scholar] [CrossRef]
- van de Wouw, M.; Maxted, N.I.; Ford-Lloyd, B.V. A multivariate and cladistic study of Vicia L. ser. Vicia (Fabaceae) based on analysis of morphological characters. Plant Syst. Evol. 2003, 237, 19–39. [Google Scholar] [CrossRef]
- Ennerking, D.; Tate, M. Global vetch production. Grain Legumes 2006, 47, 14–15. [Google Scholar]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley (No. Ed. 3); Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Maxted, N.; Bennett, S. Plant Genetic Resources of Legumes in the Mediterranean; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 39. [Google Scholar]
- Tardío, J.; Pardo-de-Santayana, M.; Lazaro, A.; Aceituno, L.; Molina, M. Inventario Español de Conocimientos Tradicionales Relativos a la Biodiversidad Agrícola Volumen 2; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2022. [Google Scholar]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Varron, M. Rerum Rusticarum: Libri III; Cubero-Salmeron, J.I., Translator; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 2010; 1st century BC. [Google Scholar]
- Holgado-Redondo, A.; Columela, L.J.M. (1st Century)-Translation; “De re Rustica”: De los Trabajos de Campo; Holgado-Redondo, A., Translator; Ministerio de Agricultura: Madrid, Spain, 1988; 339p, ISBN 84-323-0622-3. [Google Scholar]
- Hernandez, L.; Huerta, J. Secundus Plinius The Elder (1st Century) Historia Natural; UNAM-Universidad Nacional Autónoma de Mexico: Mexico City, Mexico, 1976; Hernandez, L. (books 1–25), Huerta, J. (books 26–37). [Google Scholar]
- Oroz-Reta, J.; Marcos-Casquero., M.A. Isidore of Seville (6–7th Century)-Translation “Etymologiae” Etimologias; Oroz-Reta, J.; Marcos-Casquero, M.A., Translators; BAC: Madrid, Spain, 1982; Volume 15. [Google Scholar]
- Cubero, J.I. Historia General de la Agricultura; Guadalmazán: Córdoba, Spain, 2018; ISBN 9788494155239. p. 840. [Google Scholar]
- Carabaza-Bravo, J.M.; L-Jayr, A.; Al-Filāḥa, K. Tratado de Agricultura; (11st–12nd Century) Translation; Carabaza-Bravo, J.M., Ed.; Instituto de Cooperación con el Mundo Arabe: Madrid, Spain, 1991. [Google Scholar]
- Jarava, J. “Historia de las Yerbas y Plantas” (from Dioscoride Anazarbeo); Gorda, L.G., Ed.; Heirs of A. Byrcman: Antwerp, Belgium, 1557. [Google Scholar]
- Weber, L.H.; Schifino-Wittmann, M.T. The Vicia sativa L. aggregate (Fabaceae) in southern Brazil: Karyotypes, phenology and qualitative morphology. Genet. Resour. Crop Evol. 1999, 46, 207–211. [Google Scholar] [CrossRef]
- Gomez-Ortega, C. Continuacion de la Flora Española, ó Historia de las Plantas de España, Que Escribía Don Joseph Quer. Ibarra, J.: Madrid, Spain, 1784; Volume V–VI. [Google Scholar]
- Faostat. 2023. Available online: https://www.fao.org (accessed on 15 February 2023).
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common Vetch: A Drought Tolerant, High Protein Neglected Leguminous Crop With Potential as a Sustainable Food Source. Front. Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolinska, W.; Rybinski, W.; Kiczorowska, B.; Kowalczuk-Vasilev, E.; Matras, J.; Wesolowska, S. Nutritional and Anti-Nutritional Factors in Vicia sativa L. Seeds and the Variability of Phenotypic and Morphological Characteristics of Some Vetch Accessions Cultivated in European Countries. Animals 2021, 11, 44. [Google Scholar] [CrossRef]
- Larbi, A.; El-Moneim, A.M.A.; Nakkoul, H.; Jammal, B.; Hassan, S. Intra-species variations in yield and quality determinants in Vicia species: 3. Common vetch (Vicia sativa ssp. sativa L.). Anim. Feed Sci. Technol. 2011, 164, 241–251. [Google Scholar] [CrossRef]
- Huang, Y.F.; Matthew, C.; Li, F.; Nan, Z.B. Common vetch varietal differences in hay nutritive value, ruminal fermentation, nutrient digestibility and performance of fattening lambs. Animal 2021, 15, 100244. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Reidah, I.M.; Sharopov, F.; Karazhan, N.; Sharifi-Rad, J.; Akram, M.; Daniyal, M.; Khan, F.S.; Abbaass, W.; Zainab, R.; et al. Vicia plants—A comprehensive review on chemical composition and phytopharmacology. Phytother. Res. 2021, 35, 790–809. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Shah, M.H.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015, 162, 333–345. [Google Scholar] [CrossRef]
- Shinwari, M.I.; Khan, M.A. Folk use of medicinal herbs of Margalla Hills National Park, Islamabad. J. Ethnopharmacol. 2000, 69, 45–56. [Google Scholar] [CrossRef]
- Prabhu, S.; Vijayakumar, S.; Yabesh, J.E.; Ravichandran, K.; Sakthivel, B. Documentation and quantitative analysis of the local knowledge on medicinal plants in Kalrayan hills of Villupuram district, Tamil Nadu, India. J. Ethnopharmacol. 2014, 157, 7–20. [Google Scholar] [CrossRef]
- Marc, E.; Nellya, A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [CrossRef]
- Nelson, K.; Lyles, J.T.; Li, T.; Saitta, A.; Addie-Noye, E.; Tyler, P.; Quave, C.L. Anti-Acne Activity of Italian Medicinal Plants Used for Skin Infection. Front. Pharmacol. 2016, 7, 425. [Google Scholar] [CrossRef]
- Saleem, M.; Karim, M.; Qadir, M.; Ahmed, B.; Rafiq, M.; Ahmad, B. In vitro antibacterial activity and phytochemical analysis of hexane extract of Vicia sativa. Bangladesh J. Pharmacol. 2014, 9, 189–193. [Google Scholar] [CrossRef]
- Megías, C.; Pastor-Cavada, E.; Torres-Fuentes, C.; Girón-Calle, J.; Barragán, M.A.; Juan, R.; Pastor, J.E.; Vioque, J. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur. Food Res. Technol. 2009, 230, 353–359. [Google Scholar] [CrossRef]
- Ford, R. Vetch pod rupture associated with unrelated streak-inducing viruses of peas. Phytopathology 1965, 55, 935. [Google Scholar]
- Mao, Z.; Fu, H.; Nan, Z.; Wan, C. Fatty acid, amino acid, and mineral composition of four common vetch seeds on Qinghai-Tibetan plateau. Food Chem. 2015, 171, 13–18. [Google Scholar] [CrossRef]
- Fırıncıoğlu, H.K.; Ünal, S.; Erbektaş, E.; Doğruyol, L. Relationships between seed yield and yield components in common vetch (Vicia sativa ssp. sativa) populations sown in spring and autumn in central Turkey. Field Crops Res. 2010, 116, 30–37. [Google Scholar] [CrossRef]
- Firincioğlu, H.K.; Tate, M.; Ünal, S.; Doğruyol, L.; Özcan, İ. A Selection Strategy for Low Toxin Vetches (Vicia sativa spp.). Turk. J. Agric. For. 2007, 31, 303–311. [Google Scholar]
- Matić, R.; Nagel, S.; Robertson, S.; Young, I.; Mihailović, V.; Mikić, A.; Kirby, G. Vetch (Vicia spp) expansion and use in Australia. Biotechnol. Anim. Husb. 2005, 21, 203–207. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global Synthesis of Drought Effects on Food Legume Production. PLoS ONE 2015, 10, e0127401. [Google Scholar] [CrossRef]
- Koumas, A.; Economides, S. Replacement of Soybean Meal by Broad Bean or Common Vetch Seed in Lamb and Kid Fattening Diets. Tech. Bull. 1987, 88, 1–5. [Google Scholar]
- Delaere, I. The Chemistry of Vivia sativa L. Selection; University of Adelaide, Department of Plant Science: Adelaide, Australia, 1996. [Google Scholar]
- Rathjen, J.M. The Potential for Vicia sativa L. as a Grain Legume for South Australia/Thesis Jane Mary Rathjen. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 1997. [Google Scholar]
- Li, M.; Zhao, L.; Zhou, Q.; Fang, L.; Luo, D.; Liu, W.; Searle, I.R.; Liu, Z. Transcriptome and Coexpression Network Analyses Provide In-Sights into the Molecular Mechanisms of Hydrogen Cyanide Synthesis during Seed Development in Common Vetch (Vicia sativa L.). Int. J. Mol. Sci. 2022, 23, 2275. [Google Scholar] [CrossRef]
- Akande, K.E.; Fabiyi, E.F. Effect of Processing Methods on Some Antinutritional Factors in Legume Seeds for Poultry Feeding. Int. J. Poult. Sci. 2010, 9, 996–1001. [Google Scholar] [CrossRef]
- Lambein, F.; Kuo, Y.H.; Ikegami, F.; Kusama-Eguchi, K.; Enneking, D. Grain legumes and human health. In Food Legumes for Nutritional Security and Sustainable Agriculture, Proceedings of the 4th International Food Legumes Research Conference, New Delhi, India, 18–22 October 2005; Indian Society of Genetics and Plant Breeding: New Delhi, India, 2009; pp. 422–432. [Google Scholar]
- Ampomah, O.; Huss-Danell, K. Genetic diversity of rhizobia nodulating native Vicia spp. in Sweden. Syst. Appl. Microbiol. 2016, 39, 203–210. [Google Scholar] [CrossRef]
- Daramola, D.A.; Hatzell, M.C. Energy Demand of Nitrogen and Phosphorus Based Fertilizers and Approaches to Circularity. ACS Energy Lett. 2003, 8, 1493–1501. [Google Scholar] [CrossRef]
- Lei, X.; Wang, E.T.; Chen, W.F.; Sui, X.H.; Chen, W.X. Diverse bacteria isolated from root nodules of wild Vicia species grown in temperate region of China. Arch. Microbiol. 2008, 190, 657–671. [Google Scholar] [CrossRef]
- Jorrin, B.; Imperial, J. Population Genomics Analysis of Legume Host Preference for Specific Rhizobial Genotypes in the Rhizobium leguminosarum bv. Viciae Symbioses. Mol. Plant Microbe Interact. 2015, 28, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martinez, E.R.; Valverde, A.; Ramirez-Bahena, M.H.; Garcia-Fraile, P.; Tejedor, C.; Mateos, P.F.; Santillana, N.; Zuniga, D.; Peix, A.; Velazquez, E. The analysis of core and symbiotic genes of rhizobia nodulating Vicia from different continents reveals their common phylogenetic origin and suggests the distribution of Rhizobium leguminosarum strains together with Vicia seeds. Arch. Microbiol. 2009, 191, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Laus, M.C.; van Brussel, A.A.; Kijne, J.W. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant Microbe Interact. 2005, 18, 1123–1129. [Google Scholar] [CrossRef]
- Laus, M.C.; van Brussel, A.A.; Kijne, J.W. Role of cellulose fibrils and exopolysaccharides of Rhizobium leguminosarum in attachment to and infection of Vicia sativa root hairs. Mol. Plant Microbe Interact. 2005, 18, 533–538. [Google Scholar] [CrossRef]
- Muszynski, A.; Laus, M.; Kijne, J.W.; Carlson, R.W. Structures of the lipopolysaccharides from Rhizobium leguminosarum RBL5523 and its UDP-glucose dehydrogenase mutant (exo5). Glycobiology 2011, 21, 55–68. [Google Scholar] [CrossRef]
- Tak, T.; van Spronsen, P.C.; Kijne, J.W.; van Brussel, A.A.; Boot, K.J. Accumulation of lipochitin oligosaccharides and NodD-activating compounds in an efficient plant--Rhizobium nodulation assay. Mol. Plant Microbe Interact. 2004, 17, 816–823. [Google Scholar] [CrossRef]
- Recourt, K.; Schripsema, J.; Kijne, J.W.; van Brussel, A.A.; Lugtenberg, B.J. Inoculation of Vicia sativa subsp. nigra roots with Rhizobium leguminosarum biovar Viciae results in release of nod gene activating flavanones and chalcones. Plant Mol. Biol. 1991, 16, 841–852. [Google Scholar] [CrossRef]
- Göttfert, M. Regulation and function of rhizobial nodulation genes. FEMS Microbiol. Rev. 1993, 10, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Aragón, R.; Manuel Palacios, J.M.; Ramirez-Parra, E. Rhizobial symbiosis promotes drought tolerance in Vicia sativa and Pisum sativum. Environ. Exp. Bot. 2023, 208, 105268. [Google Scholar] [CrossRef]
- Zhang, E.; Li, L.; Huang, G.; Huang, P.; Chai, Q. Regulation of fertilizer application on yield and root growth of spring wheat-faba bean intercropping system. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2002, 13, 939–942. [Google Scholar]
- Allende-Montalbán, R.; Martín-Lammerding, D.; del Mar Delgado, M.; Porcel, M.A.; Gabriel, J.L. Nitrate Leaching in Maize (Zea mays L.) and Wheat (Triticum aestivum L.) Irrigated Cropping Systems under Nitrification Inhibitor and/or Intercropping Effects. Agriculture 2022, 12, 478. [Google Scholar] [CrossRef]
- Garduno-Castro, Y.; Espinoza-Ortega, A.; Gonzalez-Esquivel, C.E.; Mateo-Salazar, B.; Arriaga-Jordan, C.M. Intercropped oats (Avena sativa)—common vetch (Vicia sativa) silage in the dry season for small-scale dairy systems in the highlands of central Mexico. Trop. Anim. Health Prod. 2009, 41, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Keba, W.; Tolemariam, T.; Mohammed, A. Straw dry matter yield and quality of finger millet intercropped with selected vetch species at different seeding ratios in western Oromia, Ethiopia. Heliyon 2022, 8, e10433. [Google Scholar] [CrossRef]
- Hontoria, C.; Garcia-Gonzalez, I.; Quemada, M.; Roldan, A.; Alguacil, M.M. The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci. Total Environ. 2019, 660, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Genard, T.; Etienne, P.; Laine, P.; Yvin, J.C.; Diquelou, S. Nitrogen transfer from Lupinus albus L., Trifolium incarnatum L. and Vicia sativa L. contribute differently to rapeseed (Brassica napus L.) nitrogen nutrition. Heliyon 2016, 2, e00150. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Li, J.; Wu, X.; Long, Y.; Su, Y. Intercropping Vicia sativa L. Improves the Moisture, Microbial Community, Enzyme Activity and Nutrient in Rhizosphere Soils of Young Kiwifruit Plants and Enhances Plant Growth. Horticulturae 2021, 7, 335. [Google Scholar] [CrossRef]
- Ogilvie, C.M.; Ashiq, W.; Vasava, H.B.; Biswas, A. Quantifying Root-Soil Interactions in Cover Crop Systems: A Review. Agriculture 2021, 11, 218. [Google Scholar] [CrossRef]
- Baldwin, K.; Creamer, N. Cover Crops for Organic Farms; Center for Enviromental Farming Systems, NCSU-NCA&TSU-NCDA&CS; North Carolina Cooperative Extension Service: Raleigh, NC, USA, 2006; pp. 1–22. [Google Scholar]
- Trenton, R.; Carrie, O.; Kelsey, H.; Hannah, W.; Tyler, D. Understanding cover crops. Agric. Nat. Resour. 2018, FS2156, 1–8. [Google Scholar]
- Wiesmeier, M.; Lungu, M.; Hübner, R.; Cerbari, V. Remediation of degraded arable steppe soils in Moldova using vetch as green manure. Solid Earth 2015, 6, 609–620. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J.; Terol, E.; Mora, G.; Giménez-Morera, A.; Cerdà, A. Vicia sativa Roth. Can Reduce Soil and Water Losses in Recently Planted Vineyards (Vitis vinifera L.). Earth Syst. Environ. 2020, 4, 827–842. [Google Scholar] [CrossRef]
- Renzi, J. Efecto de la estructura de cultivo y grado de madurez a cosecha sobre el rendimiento y la calidad de semillas de Vicia sativa L. y Vicia. villosa Roth., bajo riego. MSc. Thesis, Universidad nacional del Sur, Bahia Blanca, Argentina, 2009. [Google Scholar]
- Alonso-Ayuso, M.; Gabriel, J.L.; Pancorbo, J.L.; Quemada, M. Interseeding cover crops into maize: Characterization of species performance under Mediterranean conditions. Field Crops Res. 2020, 249, 107762. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Ibañez, S.; Medina, M.I.; Agostini, E. Vicia: A green bridge to clean up polluted environments. Appl. Microbiol. Biotechnol. 2020, 104, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lastiri-Hernández, M.A.; Alvarez-Bernal, D.; Bermúdez-Torres, K.; Cárdenas, G.C.; Ceja-Torres, L.F. Phytodesalination of a moderately saline soil combined with two inorganic amendments. Bragantia 2019, 78, 579–586. [Google Scholar] [CrossRef]
- Alonso-Prados, J.L.; Hernández-Sevillano, E.; Llanos, S.; Villarroya, M.; García-Baudín, J.M. Effects of sulfosulfuron soil residues on barley (Hordeum vulgare), sunflower (Helianthus annuus) and common vetch (Vicia sativa). Crop Prot. 2002, 21, 1061–1066. [Google Scholar] [CrossRef]
- Ibanez, S.G.; Sosa Alderete, L.G.; Medina, M.I.; Agostini, E. Phytoremediation of phenol using Vicia sativa L. plants and its antioxidative response. Environ. Sci. Pollut. Res. 2012, 19, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Halfadji, A.; Portet-Koltalo, F.; Touabet, A.; Le Derf, F.; Morin, C.; Merlet-Machour, N. Phytoremediation of PCB: Contaminated Algerian soils using native agronomics plants. Environ. Geochem. Health 2022, 44, 117–132. [Google Scholar] [CrossRef]
- Tan, M.; Temel, S. Effect of mepiquat chloride, a growth retardant, on seed yield and yield components in common vetch (Vicia sativa). Indian J. Agric. Sci. 2005, 75, 160–161. [Google Scholar]
- Machrafi, Y.; Prévost, D.; Beauchamp, C.J. Toxicity of phenolic compounds extracted from bark residues of different ages. J. Chem. Ecol. 2006, 32, 2595–2615. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. The effect of diesel fuel on common vetch (Vicia sativa L.) plants. Environ. Geochem. Health 2003, 25, 123–130. [Google Scholar] [CrossRef]
- Muccifora, S.; Bellani, L.M. Effects of copper on germination and reserve mobilization in Vicia sativa L. seeds. Environ. Pollut. 2013, 179, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.M.; Muccifora, S.; Giorgetti, L. Response to copper bromide exposure in Vicia sativa L. seeds: Analysis of genotoxicity, nucleolar activity and mineral profile. Ecotoxicol. Environ. Saf. 2014, 107, 245–250. [Google Scholar] [CrossRef]
- Ibañez, S.G.; Villasuso, A.L.; Racagni, G.E.; Agostini, E.; Medina, M.I. Phenol modulates lipid kinase activities in Vicia sativa plants. Environ. Exp. Bot. 2016, 122, 109–114. [Google Scholar] [CrossRef]
- Sierra, M.J.; Millán, R.; Esteban, E.; Cardona, A.I.; Schmid, T. Evaluation of mercury uptake and distribution in Vicia sativa L. applying two different study scales: Greenhouse conditions and lysimeter experiments. J. Geochem. Explor. 2008, 96, 203–209. [Google Scholar] [CrossRef]
- Bogatu, C.; Masu, S.; Lazarovici, M. Metals extraction from polluted soils by using of pillared zeolite and Vicia sativa. In Proceedings of the 14th Symposium on Analytical and Environmental Problems, Szeged, Hungary, 24 September 2007. [Google Scholar]
- Rui, H.; Zhang, X.; Shinwari, K.I.; Zheng, L.; Shen, Z. Comparative transcriptomic analysis of two Vicia sativa L. varieties with contrasting responses to cadmium stress reveals the important role of metal transporters in cadmium tolerance. Plant Soil 2018, 423, 241–255. [Google Scholar] [CrossRef]
- Ivanishchev, V.V.; Abramova, E.A. Accumulation of nickel ions in seedlings of Vicia sativa L. and manifestations of oxidative stress. Environ. Sci. Pollut. Res. 2015, 22, 7897–7905. [Google Scholar] [CrossRef]
- Masu, S.; Lixandru, B.; Bogatu, C. Zinc extraction from polluted soils by using zeolite and Vicia sativa plant. In Proceedings of the 3rd International Conference on Life Cycle Management, Zurich, Switzerland, 27–29 August 2007. [Google Scholar]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 2009, 168, 76–84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Chen, L.; Lu, Y.; An, Y. Ectopic expression gamma-glutamylcysteine synthetase of Vicia sativa increased cadmium tolerance in Arabidopsis. Gene 2022, 823, 146358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rui, H.; Zhang, F.; Hu, Z.; Xia, Y.; Shen, Z. Overexpression of a Functional Vicia sativa PCS1 Homolog Increases Cadmium Tolerance and Phytochelatins Synthesis in Arabidopsis. Front. Plant Sci. 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, S.G.; Merini, L.J.; Barros, G.G.; Medina, M.I.; Agostini, E. Vicia sativa-Rhizospheric bacteria interactions to improve phenol remediation. Int. J. Environ. Sci. Technol. 2014, 11, 1679–1690. [Google Scholar] [CrossRef]
- Anderson, T.A.; Guthrie, E.A.; Walton, B.T. Bioremediation in the rhizosphere. Environ. Sci. Technol. 1993, 27, 2630–2636. [Google Scholar] [CrossRef]
- Ibañéz, S.G.; Oller, A.L.W.; Paisio, C.E.; Alderete, L.G.S.; González, P.S.; Medina, M.I.; Agostini, E. The challenges of remediating metals using phytotechnologies. In Heavy Metals in the Environment: Microorganisms and Bioremediation; CRC Press: Boca Raton, FL, USA, 2018; pp. 173–191. [Google Scholar]
- Renzi, J.P.; Cantamutto, M.A. Vicias: Bases Agronómicas para el Manejo en la Región Pampeana; Ediciones INTA: Buenos Aires, Argentina, 2013; ISBN 978-987-521-470-5. [Google Scholar]
- InfoAgro. El Cultivo de la Veza (Vicia spp.). Available online: https://www.infoagro.com/documentos/el_cultivo_veza____em_Vicia__em__spp__.asp (accessed on 15 February 2023).
- Cordo, H.A.; Logarzo, G.; Braun, K.; Di Iorio, O.R. Plagas y Enemigos naturales. In Catálogo de Insectos Fitófagos de la Argentina y sus Plantas Asociadas; Sociedad Entomológica Argentina Ediciones: Buenos Aires, Argentina, 2004; Volume 6, p. 734. [Google Scholar]
- Xu, S.; Li, Y.-Z. Research advances on fungal diseases of Vicia sativa. Acta Prataculturae Sin. 2016, 25, 203. [Google Scholar]
- Allen, R.; Hayward, A.C.; Halliday, W.G.; Fulcher, J.G. Bacterial Blight of Vicia sativa: Aetiology of the Disease and Identification of the Pathogen. Aust. J. Biol. Sci. 1970, 23, 597–606. [Google Scholar] [CrossRef]
- Martín-Sanz, A.; Palomo, J.L.; Caminero, C. First Report of Bacterial Blight Caused by Pseudomonas syringae pv. syringae on Common Vetch in Spain. Plant Dis. 2009, 93, 1348. [Google Scholar] [CrossRef]
- Sastry, K.S.; Mandal, B.; Hammond, J.; Scott, S.W.; Briddon, R.W. Vicia sativa (Vetch). In Encyclopedia of Plant Viruses and Viroids; Springer: New Delhi, India, 2019; pp. 2697–2699. [Google Scholar]
- Terzakis, M.; Avgelis, A.D.; Jones, A.T.; Katis, N.I. Artichoke yellow ringspot virus infecting Vetch (Vicia sativa) in Greece. Phytoparasitica 2002, 30, 195–197. [Google Scholar] [CrossRef]
- McCord, R.W.; Gudauskas, R.T. Properties of a strain of bean yellow mosaic virus isolated from vetch, Vicia sativa. Phytopathology 1968, 58, 1294–1297. [Google Scholar]
- Musil, M.; Leskova, O.; Kleja, S. The occurrence of broad bean stain virus on vetch plants in Slovakia. Ochr. Rostl. 1978, 14, 161–166. [Google Scholar]
- Asaad, N.Y.; Kumari, S.G.; Haj-Kassem, A.A.; Shalaby, A.B.A.; Al-Shaabi, S.; Malhotra, R.S. Detection and characterization of Chickpea Chlorotic Stunt Virus in Syria. J. Phytopathol. 2009, 157, 756–761. [Google Scholar] [CrossRef]
- Kumari, S.G.; Attar, N.; Mustafayev, E.; Akparov, Z. First Report of Faba bean necrotic yellows virus Affecting Legume Crops in Azerbaijan. Plant Dis. 2009, 93, 1220. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Sadiki, M.; Román, B. First Report of Orobanche foetida on Common Vetch (Vicia sativa) in Morocco. Plant Dis. 2005, 89, 528. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernandez-Aparicio, M.; Rodriguez, M.J. First Report of Crenate Broomrape (Orobanche crenata) on Lentil (Lens culinaris) and Common Vetch (Vicia sativa) in Salamanca Province, Spain. Plant Dis. 2008, 92, 1368. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Fernandez-Aparicio, M.; Rubiales, D.; Andolfi, A.; Melck, D. Soyasapogenol B and trans-22-dehydrocam- pesterol from common vetch (Vicia sativa L.) root exudates stimulate broomrape seed germination. Pest Manag. Sci. 2011, 67, 1015–1022. [Google Scholar] [CrossRef]
- Perez-de-Luque, A.; Lozano, M.D.; Cubero, J.I.; Gonzalez-Melendi, P.; Risueno, M.C.; Rubiales, D. Mucilage production during the incompatible interaction between Orobanche crenata and Vicia sativa. J. Exp. Bot. 2006, 57, 931–942. [Google Scholar] [CrossRef]
- Cordoba, E.M.; Fernandez-Aparicio, M.; Gonzalez-Verdejo, C.I.; Lopez-Grau, C.; Muñoz-Muñoz, M.V.; Nadal, S. Search for Resistant Genotypes to Cuscuta campestris Infection in Two Legume Species, Vicia sativa and Vicia ervilia. Plants 2021, 10, 738. [Google Scholar] [CrossRef]
- Maalouf, F.; Somanagouda, P.; Rajendran, K.; Hamwieh, A.; Goyal, A.; Agrawal, S.K. Breeding for post-emergence herbicide tolerance in cool-season food legumes. In Proceedings of the International Year Pulses ICARDA Conference, Marrakesh, Morocco, 18–20 April 2016. [Google Scholar]
- Kumar, N.; Nath, C.; Hazra, K. Efficient weed management in pulses for higher productivity and profitability. Indian J. Agron. 2016, 61, 5199–5213. [Google Scholar]
- Fraser, J.; McCartney, D.; Najda, H.; Mir, Z. Yield potential and forage quality of annual forage legumes in southern Alberta and northeast Saskatchewan. Can. J. Plant Sci. 2004, 84, 143–155. [Google Scholar] [CrossRef]
- Ramírez-Villegas, J.; Jarvis, A.; Fujisaka, S.; Hanson, J.; Leibing, C. Crop and forage genetic resources: International interdependence in the face of climate change. In Crop Genetic Resources as a Global Commons: Challenges in International Law and Governance; Halewood, M., López Noriega, I., Louafi, S., Eds.; Routledge: Abingdon, UK, 2013; pp. 78–98. [Google Scholar]
- Pellegrini, P.A.; Balatti, G.E. Noah’s arks in the XXI century. A typology of seed banks. Biodivers. Conserv. 2016, 25, 2753–2769. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Sanz, A.M.; Gonzalez, S.G.; Syed, N.H.; Suso, M.J.; Saldaña, C.C.; Flavell, A.J. Genetic diversity analysis in Vicia species using retrotransposon-based SSAP markers. Mol. Genet. Genom. 2007, 278, 433–441. [Google Scholar] [CrossRef]
- Potokina, E.K.; Aleksandrova, T.G. Genetic singularity coefficients of common vetch Vicia sativa L. accessions determined with molecular markers. Genetika 2008, 44, 1508–1516. [Google Scholar] [CrossRef]
- De la Rosa, L.; González, J.M. The genetic diversity associated with seed proteins in a collection of Spanish underground vetches (Vicia sativa L. subsp. amphicarpa (Dorthes) Asch. et Graebn.). Genet. Resour. Crop Evol. 2010, 57, 565–573. [Google Scholar] [CrossRef]
- Chai, X.; Dong, R.; Liu, W.; Wang, Y.; Liu, Z. Optimizing Sample Size to Assess the Genetic Diversity in Common Vetch (Vicia sativa L.) Populations Using Start Codon Targeted (SCoT) Markers. Molecules 2017, 22, 567. [Google Scholar] [CrossRef]
- De la Rosa, L.; López-Román, M.I.; González, J.M.; Zambrana, E.; Marcos-Prado, T.; Ramírez-Parra, E. Common Vetch, Valuable Germplasm for Resilient Agriculture: Genetic Characterization and Spanish Core Collection Development. Front. Plant Sci. 2021, 12, 617873. [Google Scholar] [CrossRef]
- Edwards, D.; Batley, J. Plant genome sequencing: Applications for crop improvement. Plant Biotechnol. J. 2010, 8, 2–9. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, D.S.; Gupta, S.; Dubey, S.; Gupta, P.; Kumar, S. Quantitative trait loci from identification to exploitation for crop improvement. Plant Cell Rep. 2017, 36, 1187–1213. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, T.S.; Suresh, S.; Lee, S.Y.; Cho, G.T. Development of 65 novel polymorphic cDNA-SSR markers in common vetch (Vicia sativa subsp. sativa) using next generation sequencing. Molecules 2013, 18, 8376–8392. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, P.; Luo, D.; Liu, W.; Wang, Y. Exploiting Illumina sequencing for the development of 95 novel polymorphic EST-SSR markers in common vetch (Vicia sativa subsp. sativa). Molecules 2014, 19, 5777–5789. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Xu, W.; Zhang, J.; Wang, X.; Nie, G.; Yao, L.; Wang, H.; Lin, C. De Novo Assembly and Discovery of Genes That Involved in Drought Tolerance in the Common Vetch. Int. J. Mol. Sci. 2019, 20, 328. [Google Scholar] [CrossRef]
- Raveendar, S.; Lee, G.A.; Jeon, Y.A.; Lee, Y.J.; Lee, J.R.; Cho, G.T.; Cho, J.H.; Park, J.H.; Ma, K.H.; Chung, J.W. Cross-amplification of Vicia sativa subsp. sativa microsatellites across 22 other Vicia species. Molecules 2015, 20, 1543–1550. [Google Scholar] [CrossRef]
- Shirasawa, K.; Kosugi, S.; Sasaki, K.; Ghelfi, A.; Okazaki, K.; Toyoda, A. Genome features of common vetch (Vicia sativa) in natural habitats. Plant Direct 2021, 5, e352. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Yan, M.; Liu, F.; Zhang, S.; Wang, X. Genome survey sequencing of common vetch (Vicia sativa L.) and genetic diversity analysis of Chinese germplasm with genomic SSR markers. Mol. Biol. Rep. 2022, 49, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Raveendar, S.; Suresh, S.; Lee, G.A.; Lee, J.R.; Cho, J.H.; Lee, S.Y.; Ma, K.H.; Cho, G.T.; Chung, J.W. Transcriptome Analysis of Two Vicia sativa Subspecies: Mining Molecular Markers to Enhance Genomic Resources for Vetch Improvement. Genes 2015, 6, 1164–1182. [Google Scholar] [CrossRef]
- De la Rosa, L.; Zambrana, E.; Ramirez-Parra, E. Molecular bases for drought tolerance in common vetch: Designing new molecular breeding tools. BMC Plant Biol. 2020, 20, 71. [Google Scholar] [CrossRef]
- Ladizinsky, G.; Shefer, Y. Polyploidy in the Vicia sativa aggregate. New Phytol. 1982, 91, 541–547. [Google Scholar] [CrossRef]
- Ladizinsky, G.; Temkin, R. The cytogenetic structure of Vicia sativa aggregate. Theor. Appl. Genet. 1978, 53, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cui, Y.; Dong, R.; Luo, D.; Fang, L.; Nan, Z.; Liu, Z. Integrative Analyses of Transcriptomes and Metabolomes Reveal Associated Genes and Metabolites with Flowering Regulation in Common Vetch (Vicia sativa L.). Int. J. Mol. Sci. 2022, 23, 6818. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Nan, Z.; Wang, Y. Comparative transcriptional profiling provides insights into the evolution and development of the zygomorphic flower of Vicia sativa (Papilionoideae). PLoS ONE 2013, 8, e57338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Kuang, H.; Hou, Z.; Gong, P.; Bai, M.; Zhou, S.; Yao, X.; Song, S.; Yan, L.; et al. Elimination of an unfavorable allele conferring pod shattering in an elite soybean cultivar by CRISPR/Cas9. Abiotech 2022, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Benitez, D.; Rubio, J.; Millán, T.; Gil, J.; Die, J.V.; Castro, P. Genetic analysis reveals PDH1 as a candidate gene for control of pod dehiscence in chickpea. Mol. Breed. 2020, 40, 40. [Google Scholar] [CrossRef]
- Parker, T.A.; Berny Mier y Teran, J.C.; Palkovic, A.; Jernstedt, J.; Gepts, P. Pod indehiscence is a domestication and aridity resilience trait in common bean. New Phytol. 2020, 225, 558–570. [Google Scholar] [CrossRef]
- Abd El-Moneim, A.M. Selection for Non-Shattering Common Vetch, Vicia sativa L. Plant Breed. 1993, 110, 168–171. [Google Scholar] [CrossRef]
- Dong, R.; Dong, D.; Luo, D.; Zhou, Q.; Chai, X.; Zhang, J.; Xie, W.; Liu, W.; Dong, Y.; Wang, Y.; et al. Transcriptome Analyses Reveal Candidate Pod Shattering-Associated Genes Involved in the Pod Ventral Sutures of Common Vetch (Vicia sativa L.). Front. Plant Sci. 2017, 8, 649. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xing, J.; Yu, X.; Lu, Y.; Xu, W.; Zhao, N.; Liu, Z.; Guo, Z. Evaluation of salt tolerance in common vetch (Vicia sativa L.) germplasms and the physiological responses to salt stress. J. Plant Physiol. 2022, 278, 153811. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Min, X.; Liu, W.; Liu, Z. Comparative Transcriptomic Analysis of Root and Leaf Transcript Profiles Reveals the Coordinated Mechanisms in Response to Salinity Stress in Common Vetch. Int. J. Mol. Sci. 2022, 23, 8477. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Lin, X.; Ndayambaza, B.; Wang, Y.; Liu, W. Coordinated mechanisms of leaves and roots in response to drought stress underlying full-length transcriptome profiling in Vicia sativa L. BMC Plant Biol. 2020, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Tenopala, J.; Gonzalez, F.; De la Barrera, E. Physiological responses of the green manure, Vicia sativa, to drought. Bot. Sci. 2012, 90, 305–311. [Google Scholar] [CrossRef]
- Wei, X.; Jin, X.; Ndayambaza, B.; Min, X.; Zhang, Z.; Wang, Y.; Liu, W. Transcriptome-Wide Characterization and Functional Identification of the Aquaporin Gene Family During Drought Stress in Common Vetch. DNA Cell Biol. 2019, 38, 374–384. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Xu, W.; Yao, L.; Wang, X.; Wang, H.; Xu, Y.; Li, L.; Duan, C.; Yi, Z.; et al. Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa). Open Life Sci. 2021, 16, 1111–1121. [Google Scholar] [CrossRef]
- Min, X.; Wang, Q.; Wei, Z.; Liu, Z.; Liu, W. Full-length transcriptional analysis reveals the complex relationship of leaves and roots in responses to cold-drought combined stress in common vetch. Front. Plant Sci. 2022, 13, 976094. [Google Scholar] [CrossRef]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Nguyen, V.; Searle, I.R. An Efficient Root Transformation System for Recalcitrant Vicia sativa. Front. Plant Sci. 2022, 12, 781014. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Drago, S.R.; González, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Physical and nutritional properties of extruded products based on whole grain with the addition of wild legumes (Vicia lutea subsp. lutea var. hirta and Vicia sativa subsp. sativa). Int. J. Food Sci. Technol. 2013, 48, 1949–1955. [Google Scholar] [CrossRef]
- Hernandez-Aguirre, A.I.; Téllez-Pérez, C.; San Martín-Azócar, A.; Cardador-Martínez, A. Effect of Instant Controlled Pressure-Drop (DIC), Cooking and Germination on Non-Nutritional Factors of Common Vetch (Vicia sativa spp.). Molecules 2020, 25, 151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).