Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets
Abstract
1. Introduction
2. Results and Discussion
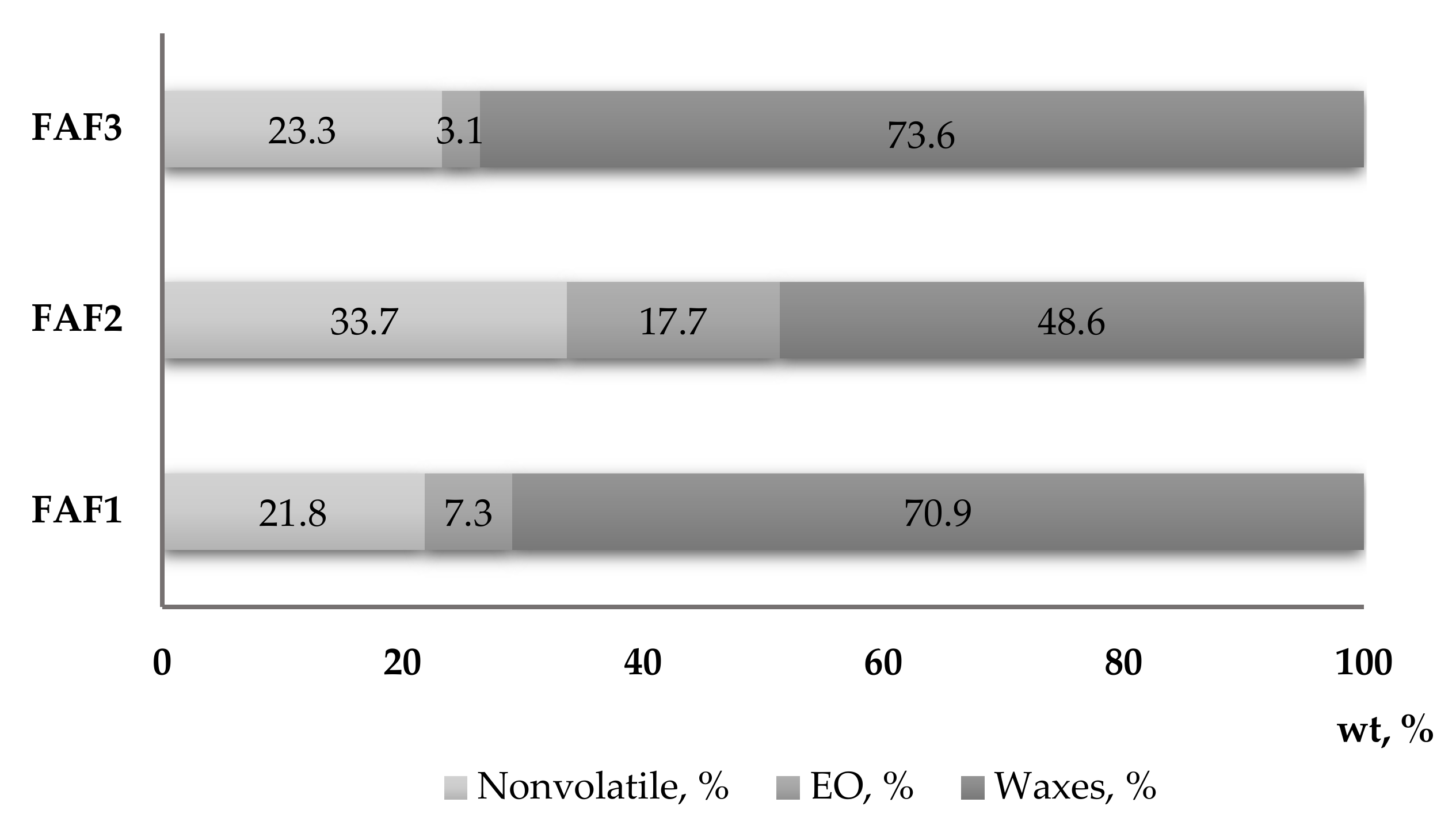
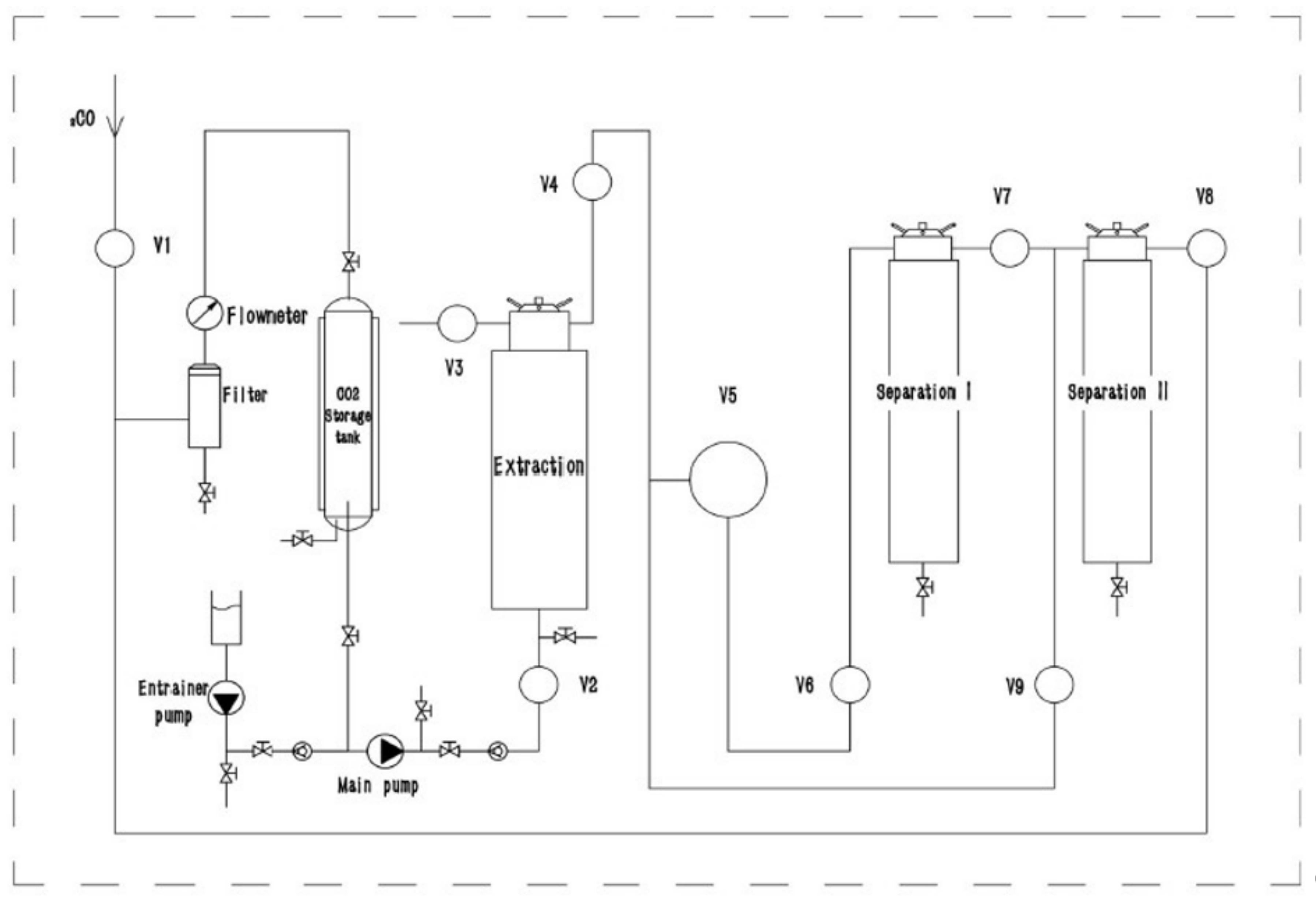
2.1. Supercritical Fluid Extraction
2.2. Phytochemical Screening of Matricaria recutita White Ray Ffloret Supercritical Fluid Extracts
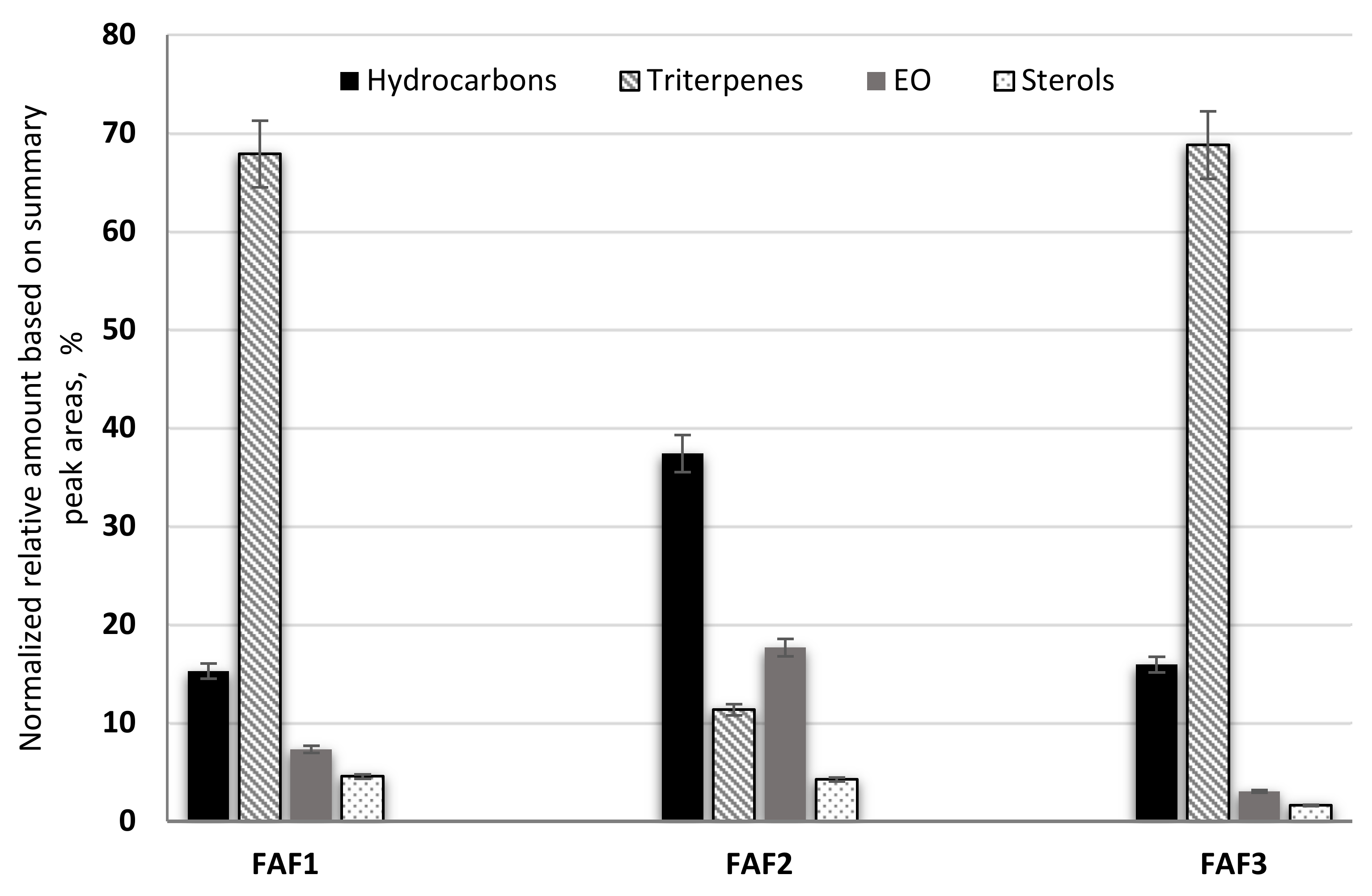
2.3. Qualitative Analysis of the Main Nonvolatile Plant Components in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
2.4. Quantitative Analysis of the Main Volatile Plant Components in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
2.5. Major and Minor Elements and Heavy Metal Screening in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
2.6. Antimicrobial Activity Screening of Ethanol Extracts from Matricaria recutita White Ray Floret Supercritical Fluid Extracts
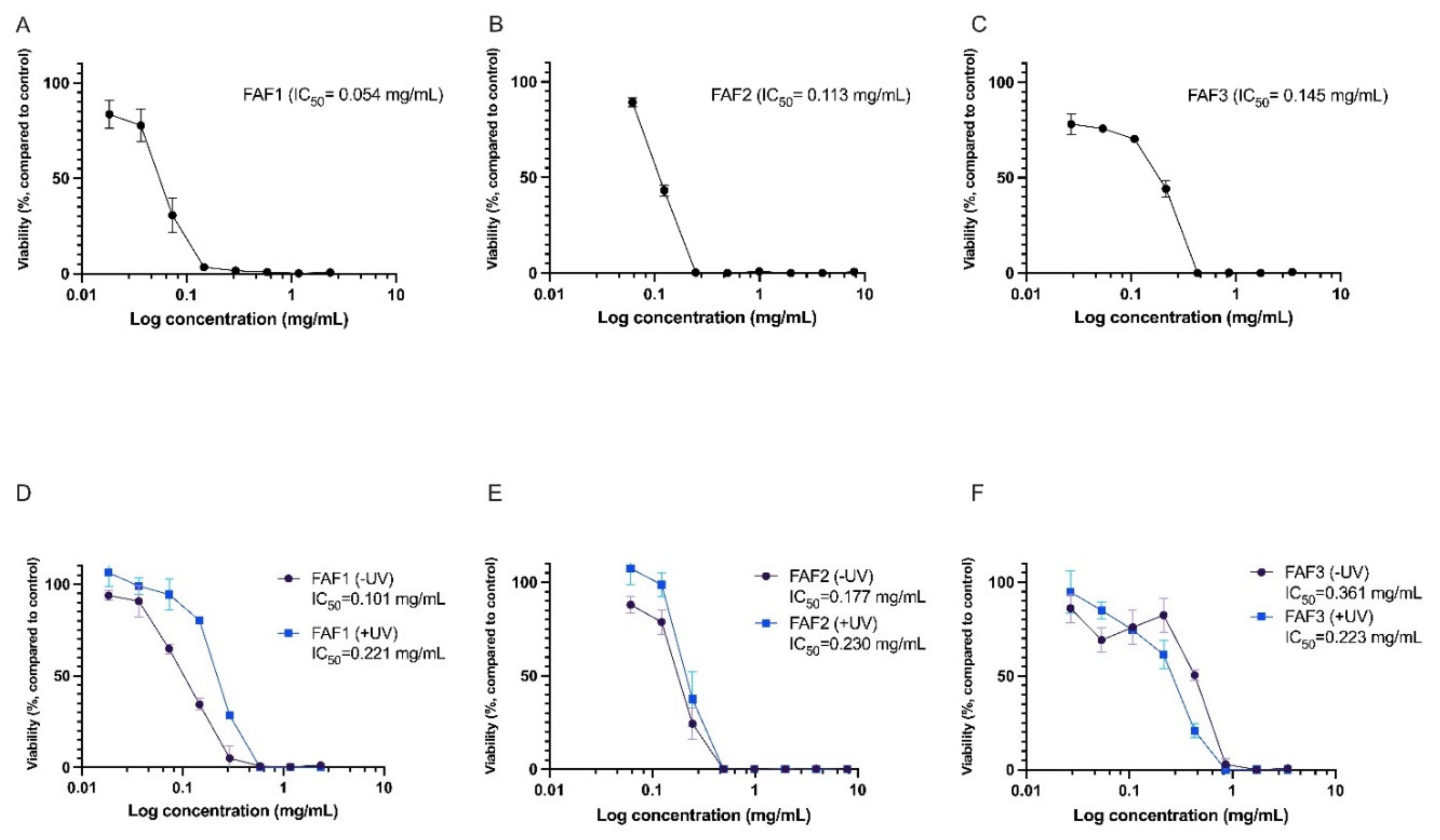
2.7. Cytotoxicity and Phototoxicity Assessment of Matricaria recutita White Ray Floret Supercritical Fluid Extracts
2.8. Statistical Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Supercritical Fluid Extraction
3.3. Chemicals and Reagents
3.4. Quantification of Essential Oils and Waxes
3.5. Determination of the Total Phenolic Content
3.6. Determination of the Total Flavonoid Content
3.7. Determination of the Total Tannin Content
3.8. Determination of the Total Sugar Content
3.9. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.10. Determination of Major and Minor Elements and Heavy Metals by MP-AES Analysis
3.11. UHPLC-HRMS Analysis
3.12. GC-MS Analysis
3.13. Antimicrobial Activity
3.14. Cytotoxicity
3.15. Phototoxicity
3.16. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.T.; Werner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E. Production of Essential Oils. In Handbook of Essential Oils: Science, Technology and Applications; Hüsnü, K., Baser, C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; p. 111. [Google Scholar]
- Torres-Leon, C.; Ramirez-Guzman, N.; Londono-Hernandez, L.; Martinez-Medina, G.A.; Diaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Perez, O.B.; Picazo, B.; Villarreal-Vazquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; De Almeida, L.F. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.P.; Werbitzky, O. Biocatalysis of Green Chemistry and Chemical Process Development. In How Green Can the Industry Become With Biotechnology; John and Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin protection by natural polyphenols. Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 5. [Google Scholar] [CrossRef] [PubMed]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Ultrasonic extraction of waste solid residues from the Salvia sp. essential oil hydrodistillation. Biochem. Eng. J. 2008, 42, 97–104. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Manzanera, M.C.A.S. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Papavassilopoulou, E.; Bardouki, H.; Vamvakias, M.; Bimpilas, A.; Oreopoulou, V. Antioxidant recovery from hydrodistillation residues of selected Lamiaceae species by alkaline extraction. J. Appl. Res. Med. Aromat. Plants 2018, 8, 83–89. [Google Scholar] [CrossRef]
- Slavov, A.; Yantcheva, N.; Vasileva, I. Chamomile Wastes (Matricaria recutita): New Source of Polysaccharides. Waste Biomass Valoriz. 2018, 10, 2583–2594. [Google Scholar] [CrossRef]
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of various techniques for the extraction of umbelliferone and herniarin in Matricaria recutita processing fractions. Chem. Cent. J. 2017, 11, 78. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. J. Food 2018, 16, 1. [Google Scholar] [CrossRef]
- Hauthal, W.H. Advances with supercritical fluids Review. Chemosphere 2001, 43, 123–135. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale-up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Scalia, S.; Guiffreda, L.; Pallado, P. Analytical and preparative supercritical fluid extraction of Chamomile flowers and its comparison with conventional methods. J. Pharm. Biomed. Anal. 1999, 21, 549–558. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria recutita L.) collected in Molise (South-central Italy). Ind. Crops. Prod. 2014, 63, 256–263. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria recutita L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria recutita L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Mežaka, I.; Kronberga, A.; Nakurte, I.; Taškova, I.; Jakovels, D.; Primavera, A. Genetic, chemical and morphological variability of chamomile (Chamomilla recutita L.) populations of Latvia. Ind. Crops Prod. 2020, 154, 112614. [Google Scholar] [CrossRef]
- Reverchon, E.; Senatore, F. Supercritical carbon dioxide extraction of chamomile essential oil and its analysis by gas chromatography–mass spectrometry. J. Agric. Food Chem. 1994, 42, 154–158. [Google Scholar] [CrossRef]
- Povh, N.P.; Marques, M.O.M.; Meireles, M.A.A. Supercritical CO2 extraction of essential oil and oleoresin from chamomile (Recutita recutita [L.] Rauschert). J. Supercrit. Fluids 2001, 21, 245–256. [Google Scholar] [CrossRef]
- WHO, G. Who Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, Geneva; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Malik, J.; Szakova, J.; Drabek, O.; Balik, J.; Kokoska, L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008, 111, 520–525. [Google Scholar] [CrossRef]
- Petrović, S.M.; Savić, S.R.; Dimitrijević, M.L.; Petronijević, Ž.B. The determination of macro and microelements in chamomile teas (Matricaria chammomilla L.). Adv. Technol. 2015, 4, 37–42. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Mitteregge, M.S. Extractability of selected mineral and trace elements in infusions of chamomile. Int. J. Food Sci. Nutr. 2008, 59, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mihaljev, Ž.; Živkov-Baloš, M.; Ćupić, Ž.; Jakšić, S. Levels of some microelements and essential heavy metals in herbal teas in Serbia. Acta Pol. Pharm. 2014, 71, 385–391. [Google Scholar] [PubMed]
- Pohl, P.; Dzimitrowicz, A.; Jedryczko, D.; Szymczycha-Madeja, A.; Welna, M.; Jamroz, P. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 2016, 130, 326–335. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the Antibacterial Effects of a Variety of Essential Oils on Respiratory Tract Pathogens, Using a Modified Dilution Assay Method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Bawazeer, S.; Rauf, A. In Vitro Antibacterial and Antifungal Potential of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus. Bio. Med. Res. Int. 2021, 2021, 1543574. [Google Scholar] [CrossRef]
- Han, G.; Lee, D.G. Antibacterial Mode of Action of β-Amyrin Promotes Apoptosis-Like Death in Escherichia coli by Producing Reactive Oxygen Species. J. Microbiol. Biotechnol. 2022, 32, 1547–1552. [Google Scholar] [CrossRef]
- Shai, L.J.; McGaw, L.J.; Aderogba, M.A.; Mdee, L.K.; Eloff, J.N. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm. f) CA Sm. leaves. J. Ethnopharmacol. 2008, 119, 238–244. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Te Velde, A.A.; De Boer, L.; Vandenbroucke-Grauls, C.M.J.; Zaat, S.A.J. Two major medicinal honeys have different mechanisms of bactericidal activit. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef]
- Mizzi, L.; Maniscalco, D.; Gaspari, S.; Chatzitzika, C.; Gatt, R.; Valdramidis, V.P. Assessing the individual microbial inhibitory capacity of different sugars against pathogens commonly found in food systems. Lett. Appl. Microbiol. 2020, 71, 251–258. [Google Scholar] [CrossRef]
- Sak, K.; Nguyen, T.H.; Ho, V.D.; Do, T.T.; Raal, A. Cytotoxic effect of chamomile (Matricaria recutita) and marigold (Calendula officinalis) extracts on human melanoma SK-MEL-2 and epidermoid carcinoma KB cells. Cogent Med. 2017, 4, 1333218. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques. Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Pitchai, D.; Roy, A.; Ignatius, C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J. Adv. Pharm. Technol. Res. 2014, 5, 179–184. [Google Scholar] [CrossRef]
- Eldohaji, L.M.; Fayed, B.; Hamoda, A.M. Potential targeting of Hep3B liver cancer cells by lupeol isolated from Avicennia marina. Arch. Pharm. Weinh. 2021, 354, e2100120. [Google Scholar] [CrossRef]
- Malekinejad, F.; Kheradmand, F.; Khadem-Ansari, M.H.; Malekinejad, H. Lupeol synergizes with doxorubicin to induce anti-proliferative and apoptotic effects on breast cancer cells. Daru 2022, 30, 103–115. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of b-sitosterol-3-o-glucoside and β -amyrin from Prunus africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef]
- Neto, S.F.; Prada, A.L.; Achod, L.D.R.; Torquato, H.F.V.; Lima, C.S.; Paredes-Gamero, E.J.; Silva de Moraes, M.O.; Lima, E.S.; Sosa, E.H.; De Souza, T.P.; et al. α-Amyrin-loaded nanocapsules produce selective cytotoxic activity in leukemic cells. Biomed. Pharmacother. 2021, 139, 111656. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package, version 1.0.8; R Foundation Statutes: Vienna, Austria, 2015. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package “factoextra” for R: Extract and Visualize the Results of Multivariate Data Analyses. R Package, version 1.0.3; R Foundation Statutes: Vienna, Austria, 2017. [Google Scholar]

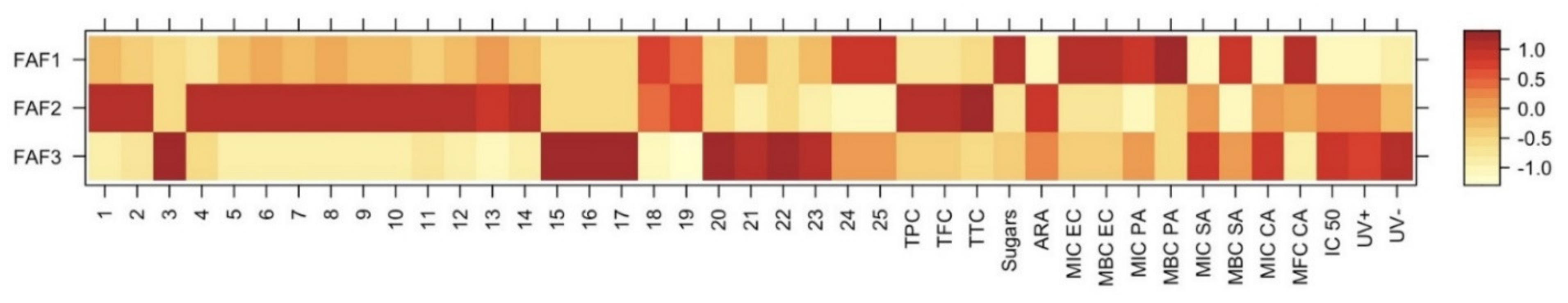
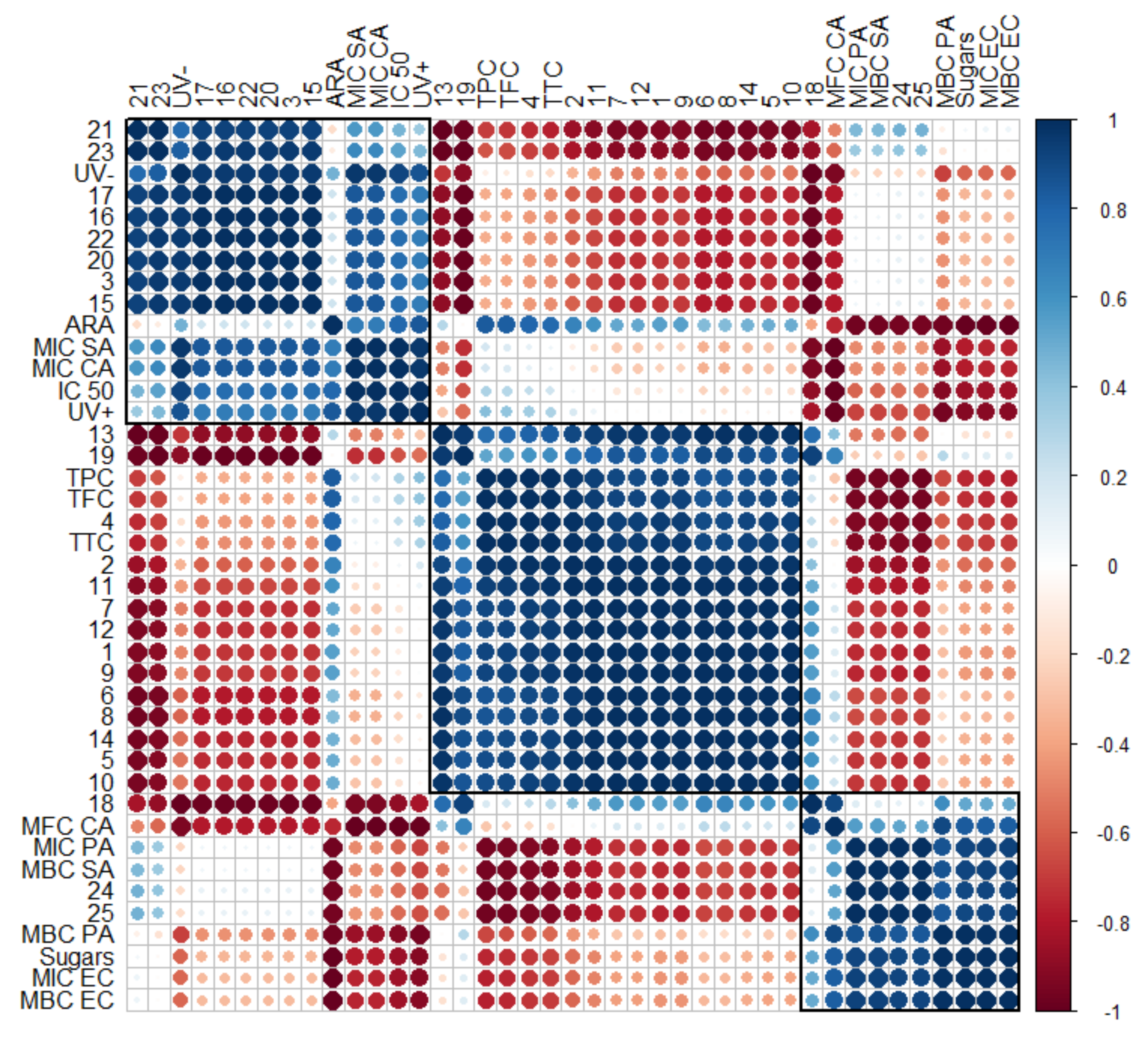

| Sample | FAF1 | FAF2 | FAF3 |
|---|---|---|---|
| Characterization | Yellow-green, slightly viscous | Bright green, slightly gelatinous | Bright yellow, runny |
| Yield, g 100 g−1 DW input | 13.51 ± 0.46 | 9.76 ± 0.76 | 18.21 ± 0.15 |
| Sample | TPC a, GAE mg/mL | TFC b, APE mg/mL | TTC c, TAE mg/mL | Sugars d, GLE mg/mL | ARA e, TE mg/mL | DPPH f Quenched, % |
|---|---|---|---|---|---|---|
| FAF1 | 98.1 ± 3.5 | 45.6 ± 2.7 | 46.4 ± 1.9 | 104.0 ± 3.6 | 3.87 ± 0.09 | 5.4% |
| FAF2 | 342.8 ± 17.3 | 201.8 ± 11.3 | 128.8 ± 12.4 | 53.0 ± 2.2 | 138.26 ± 0.07 | 66.5% |
| FAF3 | 138.1 ± 3.7 | 66.0 ± 5.1 | 49.8 ± 2.7 | 62.9 ± 3.1 | 95.06 ± 0.01 | 16.7% |
| No | Proposed Compounds | Tentative Molecular Formula | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | Sample FAF |
|---|---|---|---|---|---|---|
| 1 | Benzoic acid | C7H6O2 | 123.0441 | 123.0439 | −1.63 | 1,2 |
| 2 | Leucine/Isoleucine | C6H13NO2 | 132.1019 | 132.1018 | −0.76 | 1,2 |
| 3 | Furane | C6H8O3 | 129.0546 | 129.0549 | 2.32 | 1,2 |
| 4 | Norfuraneol | C5H6O3 | 115.0390 | 115.0394 | 3.48 | 1,2 |
| 5 | Phenylalanine | C9H11NO2 | 166.0863 | 166.0860 | −1.81 | 1,2 |
| 6 | N4-Acetylaminobutanal | C6H11NO2 | 130.0863 | 130.0862 | −0.77 | 1,2 |
| 7 | Pyrogallol | C6H6O3 | 127.0390 | 127.0391 | 0.79 | 1,2 |
| 8 | Tyrosine | C9H11NO3 | 182.0812 | 182.0812 | 0.00 | 1,2 |
| 9 | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone | C6H8O3 | 129.0546 | 129.0544 | −1.55 | 1,2 |
| 10 | Trans-3-Hexenyl acetate | C8H14O2 | 143.1067 | 143.1068 | 0.70 | 1,2 |
| 11 | Caprolactam | C6H11NO | 114.0913 | 114.0915 | 1.75 | 1,2 |
| 12 | Ethyl maltol | C7H8O3 | 141.0546 | 141.0544 | −1.42 | 1,2 |
| 14 | Cynaustine | C15H26ClNO4 | 320.1623 | 320.1622 | −0.31 | 1,2 |
| 15 | N-(1-deoxy-D-fructos-1-yl)-L-Histidine | C12H19N3O7 | 318.1296 | 318.1298 | 0.63 | 1,2 |
| 16 | Tryptophyl-Proline | C16H19N3O3 | 302.1499 | 302.1500 | 0.33 | 1,2 |
| 18 | 1-Acetoxypinoresinol | C22H24O8 | 417.1544 | 417.1542 | −0.48 | 1,2,3 |
| 19 | Niacin | C6H5NO2 | 124.0393 | 124.0393 | 0.00 | 3 |
| 20 | Methyl hexadecanoate | C17H34O2 | 271.2632 | 271.2603 | −0.74 | 3 |
| 19 | 3-(1,1-dimethylallyl)herniarin | C15H16O3 | 245.1172 | 245.1173 | 0.41 | 1,2,3 |
| 20 | 7-O-Acetyllycopsamine-N-oxide A | C17H27NO7 | 358.1860 | 358.1862 | 0.56 | 1,2,3 |
| 21 | Austricin | C15H18O4 | 149.0597 | 149.0599 | 1.34 | 3 |
| 22 | Corchoinoside B | C19H28O9 | 263.1278 | 263.1277 | −0.38 | 3 |
| 23 | Cinnamic acid | C9H8O2 | 401.1806 | 401.1804 | −0.50 | 1,2,3 |
| 24 | Prolyl-Tryptophan | C16H19N3O3 | 255.1955 | 255.1958 | 1.18 | 1,2 |
| 25 | 4,4’’-bis(N-feruloyl)serotonin | C40H38N4O8 | 302.1499 | 302.1502 | 0.99 | 1,2 |
| 26 | Stilbene | C14H12 | 703.2762 | 703.2766 | 0.57 | 1,2,3 |
| 27 | Umbelliferone | C9H6O3 | 181.1012 | 181.1009 | −1.66 | 1,2,3 |
| 28 | Chrysanthetriol | C15H26O3 | 163.0390 | 163.0398 | 4.91 | 1,2,3 |
| 29 | Dihydrocaffeic acid | C9H10O4 | 183.0652 | 183.0650 | −1.09 | 1,2 |
| 30 | Loquatoside | C20H22O11 | 439.1235 | 439.1230 | −1.14 | 1,2 |
| 31 | p-Coumaric acid | C9H8O3 | 165.0546 | 165.0542 | −2.42 | 1,2,3 |
| 32 | Isoferulic acid | C10H10O4 | 195.0652 | 195.0650 | −1.03 | 1,2,3 |
| 33 | Epicatechin 3-gallate | C22H18O10 | 443.0973 | 443.0970 | −0.68 | 2,3 |
| 34 | Chrysoeriol 7-rutinoside | C28H32O15 | 609.1814 | 609.1819 | 0.82 | 3 |
| 35 | Hesperidin | C28H34O15 | 611.1970 | 611.1990 | 3.27 | 3 |
| 36 | Caffeic acid derivative | C21H30O13 | 491.1759 | 491.1763 | 0.81 | 3 |
| 37 | Coumaric acid pentoside hexoside | C20H26O12 | 459.1497 | 459.1499 | 0.44 | 3 |
| 38 | Catechin derivative | C15H14O6 | 291.0863 | 291.0867 | 1.37 | 3 |
| 39 | 4-Hydroxy-3,5,4’-trimethoxystilbene | C17H18O4 | 287.1278 | 287.1281 | 1.04 | 1,2 |
| 40 | Myricetin 3,3’-digalactoside | C27H30O18 | 643.1505 | 643.1511 | 0.93 | 1,2 |
| 41 | Apigenin 6-C-glucoside | C21H20O10 | 433.1129 | 433.1135 | 1.39 | 1,2 |
| 42 | Isomucronulatol | C17H18O5 | 303.1227 | 303.1234 | 2.31 | 1,2 |
| 43 | Epigallocatechin 3-cinnamate | C24H20O8 | 437.1231 | 437.1238 | 1.60 | 1,2 |
| 44 | (-)-Epigallocatechin 7-O-glucuronide | C21H22O13 | 483.1133 | 483.1128 | −1.03 | 2 |
| 45 | Glyphoside | C23H22O13 | 507.1133 | 507.1120 | −2.56 | 2 |
| 46 | Apigenin 7-O-malonylglucoside | C24H22O13 | 519.1133 | 519.1138 | 0.96 | 1,2,3 |
| 47 | Myricetin 3-glucoside | C21H20O13 | 481.0977 | 481.0982 | 1.04 | 2 |
| 48 | Oleacein | C17H20O6 | 321.1333 | 321.1339 | 1.87 | 2,3 |
| 49 | Herniarin | C10H8O3 | 177.0546 | 177.0551 | 2.82 | 1,2,3 |
| 50 | B3 (-)-gallocatechin-(4alpha-8)-(+)-catechin | C30H26O13 | 595.1446 | 595.1452 | 1.01 | 1, 3 |
| 51 | 9-Hexadecenoic acid | C16H30O2 | 255.2319 | 255.2325 | 2.35 | 1,2 |
| 52 | Isolariciresinol 3-glucoside | C26H34O11 | 523.2174 | 523.2179 | 0.96 | 1,2,3 |
| 53 | (2E,6E)-1-Hydroxy-2,6,10-farnesatrien-9-one | C15H24O2 | 237.1849 | 237.1843 | −2.53 | 1,2 |
| 54 | Muscomin | C18H18O7 | 347.1125 | 347.1120 | −1.44 | 2 |
| 55 | 2,3-Dihydroabscisic alcohol | C15H24O3 | 253.1798 | 253.1794 | −1.58 | 1,2 |
| 56 | Phenylethylbenzoate | C15H14O2 | 227.1067 | 227.1060 | −3.08 | 1,2 |
| 57 | Giberellin A | C19H24O5 | 333.1697 | 333.1695 | −0.60 | 1,2,3 |
| 58 | 2alpha-Hydroxyalantolactone | C15H20O3 | 249.1485 | 249.1480 | −2.01 | 1,2 |
| 59 | Methyl hexadecanoate | C17H34O2 | 271.2632 | 271.2627 | −1.84 | 1,2 |
| 60 | Arctiopicrin | C19H26O6 | 351.1802 | 351.1798 | −1.14 | 1,2,3 |
| 61 | Chrysosplenol | C18H16O8 | 361.0918 | 361.0922 | 1.11 | 3 |
| 62 | Isosyringinoside | C23H34O14 | 535.2021 | 535.2027 | 1.12 | 3 |
| 63 | Carnosic acid | C20H28O4 | 333.2060 | 333.2052 | −2.40 | 3 |
| 64 | Matricarin | C17H20O5 | 305.1384 | 305.1389 | 1.64 | 1,2,3 |
| 65 | β -D-Xylopyranosyl-(1->4)-a-L-rhamnopyranosyl-(1->2)-D-fucose | C17H30O13 | 443.1759 | 443.1762 | 0.68 | 1,2 |
| 66 | Cynaroside A | C21H32O10 | 445.2068 | 445.2069 | 0.22 | 1,2,3 |
| 67 | 9-Oxohexadecanoic acid | C16H30O3 | 271.2268 | 271.2264 | −1.47 | 1,2 |
| 68 | Retinol | C20H30O | 287.2369 | 287.2372 | 1.04 | 3 |
| 69 | Unidentified | C17H24O3 | 277.1798 | 277.1799 | 0.36 | 1,2 |
| 70 | 1-octen-3-ol-3-O-β-D-xylopyranosyl(1->6)- β -D-glucopyranoside | C19H34O10 | 423.2225 | 423.2230 | 1.18 | 1,2 |
| 71 | Pinoresinol | C20H22O7 | 375.1438 | 375.1443 | 1.33 | 1,2,3 |
| 72 | (E)-En-yn-dicycloether | C13H12O2 | 201.0910 | 201.0911 | 0.50 | 1,2 |
| 73 | Cubebininolide | C24H30O8 | 447.2013 | 447.2014 | 0.22 | 1,2 |
| 74 | Corchoionoside B | C19H28O9 | 401.1806 | 401.1807 | 0.25 | 1,2 |
| 75 | 9-Oxooctadecanoic acid | C18H34O3 | 299.2581 | 299.2583 | 0.67 | 1,2,3 |
| 76 | Spathulenol | C15H24O | 221.1900 | 221.1902 | 0.90 | 1,3 |
| 77 | Unidentified | C17H26O4 | 295.1904 | 295.1902 | −0.68 | 1 |
| 78 | Methyl dihydrojasmonate | C13H22O3 | 227.1642 | 227.1640 | −0.88 | 1,2 |
| 79 | Isospathulenol | C15H24O | 815.3332 | 815.3330 | −0.25 | 1,2,3 |
| 80 | Tricrocin | C38H54O19 | 221.1900 | 221.1901 | 0.45 | 3 |
| 81 | Camelledionol | C29H44O3 | 441.3363 | 441.3365 | 0.45 | 3 |
| 82 | Matricin | C17H22O5 | 307.1540 | 307.1544 | 1.30 | 3 |
| 83 | 13’-Hydroxy-alpha-tocotrienol | C29H44O3 | 441.3363 | 441.3363 | 0.00 | 3 |
| 84 | Oleanolic acid | C30H48O3 | 457.3676 | 457.3679 | 0.66 | 3 |
| 85 | Stigmasterol | C29H48O | 413.3778 | 413.3781 | 0.73 | 3 |
| 86 | 3-Hydroxy-1,10-bisaboladien-9-one | C15H24O2 | 237.1849 | 237.1842 | −2.95 | 1,2 |
| 87 | Farnesene | C15H24 | 205.1951 | 205.1955 | 1.95 | 1,2 |
| 88 | Acetyl tributyl citrate | C20H34O8 | 403.2326 | 403.2327 | 0.25 | 1,2 |
| 89 | Glycerophospate | C24H41O7P | 473.2663 | 473.2660 | −0.63 | 1,2,3 |
| 90 | Uzarigenin 3-[xylosyl-(1->2)-rhamnoside] | C34H52O12 | 653.3532 | 653.3530 | −0.31 | 2 |
| 91 | β-tocotrienol | C28H42O2 | 411.3258 | 411.3260 | 0.49 | 1,2 |
| 92 | ɣ-Tocotrienol | C28H42O2 | 411.3258 | 411.3259 | 0.24 | 1,2 |
| 93 | Camellioside D | C54H88O24 | 1121.5738 | 1121.5725 | −1.16 | 2 |
| 94 | 17-β-Hydroxy-2alpha-(methoxymethyl)17-methyl-5lapha-androstan-3-one | C22H36O3 | 349.2737 | 349.2730 | −2.00 | 1,2 |
| 95 | Zeaxanthin | C40H52 | 533.4142 | 533.4144 | 0.37 | 1,2 |
| 96 | Lutein | C40H52 | 533.4142 | 533.4148 | 1.12 | 1,2 |
| No | Compound b | RI a | FAF1 | FAF2 | FAF3 | Formula b | Class |
|---|---|---|---|---|---|---|---|
| 1 | (E)-β-Farnesene | 1444 | 2.10 | 5.90 | 0.67 | C15H24 | Sesquiterpenoids |
| 2 | Germacrene D | 1481 | 0.19 | 0.75 | 0.11 | C15H24 | Sesquiterpenoids |
| 3 | β-Selinene | 1486 | n.d. | n.d. | 0.06 | C15H24 | Sesquiterpenoids |
| 4 | Bicyclogermacrene | 1495 | n.d. | 0.37 | 0.03 | C15H24 | Sesquiterpenoids |
| 5 | Spathulenol | 1576 | 0.21 | 0.48 | 0.08 | C15H24O | Sesquiterpenoids |
| 6 | α-Bisabolol oxide B | 1655 | 1.10 | 2.28 | 0.34 | C15H26O2 | Tetrahydrofurans |
| 7 | α-Bisabolone oxide A | 1679 | 0.65 | 1.65 | 0.22 | C15H26O2 | Tetrahydrofurans |
| 8 | α-Bisabolol oxide A | 1744 | 1.38 | 2.53 | 0.65 | C15H26O2 | Tetrahydrofurans |
| 9 | cis-ene-yne-Dicycloether | 1849 | 1.39 | 3.11 | 0.75 | C13H12O2 | Spiroethers |
| 10 | (E)-Tonghaosu | 1902 | 0.29 | 0.60 | 0.14 | C13H12O2 | Spiroethers |
| 11 | Tetracosane | 2400 | 2.27 | 10.54 | n.d. | C24H50 | Hydrocarbons |
| 12 | Hexacosane | 2600 | 2.90 | 9.60 | n.d. | C26H54 | Hydrocarbons |
| 13 | Heptacosane | 2700 | 6.52 | 12.08 | 0.21 | C27H56 | Hydrocarbons |
| 14 | Octacosane | 2800 | 3.59 | 5.20 | 2.73 | C28H58 | Hydrocarbons |
| 15 | Nonacosane | 2900 | n.d. | n.d. | 3.11 | C29H60 | Hydrocarbons |
| 16 | Tritriacontane | 3300 | n.d. | n.d. | 6.23 | C33H68 | Hydrocarbons |
| 17 | Tetratriacontane | 3400 | n.d. | n.d. | 3.69 | C34H70 | Hydrocarbons |
| 18 | Stigmasterol | 3170 | 3.35 | 2.87 | 1.12 | C29H48O | Sterols |
| 19 | Sitosterol | 3173 | 1.22 | 1.39 | 0.51 | C29H50O | Sterols |
| 20 | Taraxerol | 2834 | n.d. | n.d. | 3.37 | C30H50O | Triterpenes |
| 21 | Lupeol | 3270 | 10.58 | 2.56 | 23.26 | C30H50O | Triterpenes |
| 22 | Ψ-Taraxasterol | 3290 | n.d. | n.d. | 0.7 | C30H50O | Triterpenes |
| 23 | Taraxasterol | 3293 | 2.31 | 0.69 | 5.9 | C30H50O | Triterpenes |
| 24 | α-Amyrin | 3328 | 24.04 | 2.97 | 15.32 | C30H50O | Triterpenes |
| 25 | β-Amyrin | 3337 | 30.97 | 5.13 | 20.24 | C30H50O | Triterpenes |
| Other compounds | 4.94 | 29.3 | 10.56 |
| Elements, mg/kg | FAF1 | FAF2 | FAF3 | Mean | Min | Max | CV, % |
|---|---|---|---|---|---|---|---|
| Ca | 510.0 | 503.1 | 507.2 | 506.8 | 503.1 | 510 | 0.7 |
| Fe | 1170.0 | 1189.3 | 1182.2 | 1180.5 | 1170 | 1189.3 | 0.8 |
| K | 0.17 | 0.18 | 0.18 | 0.2 | 0.17 | 0.18 | 3.3 |
| Mg | 50.0 | 52.2 | 55.9 | 52.7 | 50 | 55.9 | 5.7 |
| Na | 110.0 | 104.3 | 109.0 | 107.8 | 104.3 | 110 | 2.8 |
| Cu | 3.8 | 3.6 | 3.5 | 3.6 | 3.5 | 3.8 | 4.2 |
| Ni | 10.7 | 10.5 | 10.2 | 10.5 | 10.2 | 10.7 | 2.4 |
| Mn | 4.1 | 4.3 | 4.1 | 4.2 | 4.1 | 4.3 | 2.8 |
| Zn | 1.8 | 1.9 | 1.7 | 1.8 | 1.7 | 1.9 | 5.6 |
| Sample | E. coli | P. aeruginosa | S. aureus | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| FAF1 | 2.94 | 2.94 | 0.74 | 1.47 | 0.37 | 0.74 | 0.37 | 0.74 |
| FAF2 | 4.95 | 4.95 | 2.48 | 4.95 | 0.31 | 2.48 | 0.31 | 1.23 |
| FAF3 | 4.30 | 4.30 | 1.08 | 4.30 | 0.27 | 1.08 | 0.27 | 2.15 |
| Element | Flask Volume for Each Concentration, mL | Stock Solution, mg/L | Calibration Range |
|---|---|---|---|
| Ca, Fe, K, Mg, Na mix solution | 50 mL, 5% HNO3 | 500 mg/L | 0.25; 0.50; 1.0; 2.5; 4.0; 5.0; 7.5 mg/L (at least R2 = 0.990) |
| Cd, Co, As, Cr, Cu, Mn, Mo, Ni, Pb, Zn mix solution | 50 mL, 5% HNO3 | 5 mg/L | 0.1; 0.25; 0.5; 1.0; 2.5; 5 mg/L (at least R2 = 0.990) |
| Element | Wavelength, nm | Nebulizer Flow, L/min | Element | Wavelength, nm | Nebulizer Flow, L/min |
|---|---|---|---|---|---|
| Na | 588.995 | 0.95 | Cr | 425.433 | 0.90 |
| Mg | 285.213 | 0.90 | Cu | 324.754 | 0.70 |
| K | 766.491 | 0.75 | Mn | 403.076 | 0.90 |
| Ca | 393.366 | 0.60 | Mo | 379.825 | 0.85 |
| Fe | 371.993 | 0.65 | Ni | 361.939 | 0.70 |
| Cd | 226.502 | 0.50 | Pb | 283.305 | 0.75 |
| Co | 340.512 | 0.75 | Zn | 213.857 | 0.45 |
| As | 197.198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakurte, I.; Berga, M.; Pastare, L.; Kienkas, L.; Senkovs, M.; Boroduskis, M.; Ramata-Stunda, A. Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets. Plants 2023, 12, 396. https://doi.org/10.3390/plants12020396
Nakurte I, Berga M, Pastare L, Kienkas L, Senkovs M, Boroduskis M, Ramata-Stunda A. Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets. Plants. 2023; 12(2):396. https://doi.org/10.3390/plants12020396
Chicago/Turabian StyleNakurte, Ilva, Marta Berga, Laura Pastare, Liene Kienkas, Maris Senkovs, Martins Boroduskis, and Anna Ramata-Stunda. 2023. "Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets" Plants 12, no. 2: 396. https://doi.org/10.3390/plants12020396
APA StyleNakurte, I., Berga, M., Pastare, L., Kienkas, L., Senkovs, M., Boroduskis, M., & Ramata-Stunda, A. (2023). Valorization of Bioactive Compounds from By-Products of Matricaria recutita White Ray Florets. Plants, 12(2), 396. https://doi.org/10.3390/plants12020396






