Abstract
This study evaluated the effect of convective drying on the degradation of color and phenolic compounds of purple basil (Ocimum basilicum L.) leaves, and the hygroscopic behavior of dried leaves. The fresh leaves underwent drying at 40 °C, 50 °C, 60 °C, and 70 °C. Degradation of chlorophyll, flavonoids, and phenolic compounds were evaluated during drying and the hygroscopicity was evaluated through the moisture sorption isotherms. The drying mathematical modeling and the moisture sorption data were performed. The effective diffusivity for the drying increased from 4.93 × 10−10 m2/s at 40 °C to 18.96 × 10−10 m2/s at 70 °C, and the activation energy value (39.30 kJ/mol) showed that the leaves present temperature sensibility. The leaves dried at 40 °C had less degradation of phenolic compounds and color variation, but the drying process was too slow for practical purposes. Modified Page, Diffusion Approximation, and Verna models had excellent accuracy in drying kinetics. The isotherms showed that, in environments with relative humidity above 50%, the purple basil leaves are more susceptible to water gain, and at 8.83 g H2O/100 g db moisture, it guarantees the microbiological stability of the dried leaves. The Oswin model was the most suitable for estimating the moisture sorption isotherms of the dried leaves.
1. Introduction
The purple basil (Ocimum basilicum L.), also called ‘alfavaca-roxa’ and ‘alfavaca-comum’, is a plant species adapted to the Eastern Amazon, used in traditional medicine as a stimulant, in the treatment of hypertension and renal failure, against coughs, and in the form of baths to combat colds [1,2,3].
The purple basil presents in its composition fibers, carbohydrates, proteins, and minerals such as iron, calcium, and phosphorus [2]. Phenolic compounds are the secondary metabolites in this plant species, especially phenolic acids, flavonoids, tannins, and polyphenols, which act as reducing agents, metal chelators, and free radical scavengers [4]. The presence of these compounds gives Ocimum leaves antimicrobial, antioxidant, anti-inflammatory, and antihyperglycemic potential [2,5,6].
Due to medicinal properties, functional claims, and aromatic characteristics, basil has been gaining economic prominence due to the numerous possibilities of use in the pharmaceutical and food industries. Although basil occurs throughout the year, post-harvest treatments are necessary to control microbiological and biochemical changes and prolong its shelf life. Drying is a preservation method frequently used for the dehydration of leaves, and the literature shows that it has been used as a pre-treatment of basil leaves, to obtain essential oil [7] and for the infusion preparation [8].
Drying is a unit operation that removes water from cells and tissues. In this process, the transfer of heat and mass occurs simultaneously between the product and the air-drying. The increase in air-drying temperature causes the movement of water molecules and an increase in the partial pressure of water vapor in the product, which promotes a reduction in the water content and water activity (aw) [9]. The free water content is reduced to levels that can ensure a decrease in microbiological activity and enzymatic degradation, contributing to an increase in the shelf life of the product, in addition to favorably minimizing the packaging and transportation of the product, due to the reduction in volume [10,11].
On the other hand, the drying process can cause physical, chemical, and sensorial changes in the product; therefore, minimizing such effects is important to ensure the quality of the final product [10]. The degradation of compounds sensitive to heat, light, and oxygen, such as phenolic compounds, vitamins, and pigments, can be reduced or prevented by controlling the temperature, time, and drying method applied [12].
In the drying of aromatic plants, mathematical modeling predicts the drying behavior and can help in the definition of conditions for the process, capable of guaranteeing the preservation of the characteristics of the product, as well as a high efficiency for the drying [11,13]. Semi-theoretical models are the most used to describe the drying phenomenon. These models are based on the theory and data of drying kinetics, and they are derived from the simplification of Fick’s second law of diffusion or from the modification of simplified models. Such models consider that at a decreasing drying rate, the movement of liquid or vapor occurs in a porous solid in a given period, which is represented by the change in relative humidity [14].
To preserve the characteristics of an aromatic plant after drying, it is important to study the hygroscopic behavior of the product, through the moisture sorption isotherms. These isotherms define the relationship between the moisture content and the water activity (aw) of the product, and make it possible to determine the moisture content at which the product presents the greatest stability to degradative processes. Additionally, moisture sorption isotherms allow for the definition of drying conditions, as well as the packaging and storage, for the product [15,16].
There are several scientific works that apply different drying methods to the genus Ocimum and evaluate the post-drying behavior. Seczyk, Ozdemir, and Kolodziej [17] analyzed the effect of convective drying at 40 °C for 48 h, freezing at −20 °C, and lyophilization over the in vitro bioaccessibility of phytochemicals in basil, and found that convective drying achieved greater bioaccessibility of phytochemicals compared with those processed at low temperatures, despite having the highest amount of phenolics and antioxidant activity. Diaz-Maroto et al. [18] studied changes in basil aromas after convective drying at 45 °C, air-drying at room temperature, and lyophilization, and demonstrated that the temperature caused a decrease in volatile compounds and changes to the odor of the leaves.
Few works with Ocimum present data with moisture sorption isotherms. Lima-Corrêa et al. [19] evaluated the influence of drying basil leaves with fluidized beds and vibrofluidized on the volatile oil composition and color change, and applied the moisture sorption isotherm to evaluate the equilibrium moisture content as a function of temperature and relative humidity. Pääkkönen, Malmsten, and Hyvönen [20], in their work on basil and marjoram, concluded that the drying conditions, such as air-drying and lyophilization, modified the water adsorption in basil, and that hermetically closed packages can maintain the intensity of odor and flavor.
Based on the above, the objective of this work was to study the effect of convective drying, over a wider temperature range (40–70 °C), on the degradation of chlorophyll and phenolic compounds of the purple basil (O. basilicum), as well as to study the hygroscopic behavior to define the drying and storage conditions for the product. For the first time, this study presents practical data for the convective drying of purple basil leaves, as well as practical conditions for the storage of the dried leaves.
2. Results and Discussion
2.1. Characterization of Purple Basil Leaves
The results of the proximate composition and physical-chemical properties of the purple basil leaf are shown in Table 1. The average moisture content (83.56%) of the purple basil (O. basilicum) leaves was at the same order of magnitude as the value observed for basil (O. basilicum) by Reis et al. [21] (82.7%). On the other hand, Roberto et al. [22] observed higher values of moisture content (94.12%) for O. basilicum. Other plant species, typical from the Amazon region, had high moisture contents, such as jambu (Acmella oleracea) (92.99%) [23] and chicory (Eryngium foetidum) (87%) [24].

Table 1.
Proximate composition and physical–chemical properties of the purple basil leaf (Ocimum basilicum L.).
The average contents of ash (1.63%), total proteins (0.57%), and total lipids (0.15%) observed in purple basil leaves were lower than the values reported by Oluwole et al. [25] for Ocimum gratissimum L. (7.12%, 3.71%, and 3.59%, respectively). The total carbohydrates in purple basil (3.14%) were also lower than that reported for O. basilicum leaves (5.03%) by Siti Mahirah et al. [26].
Total fibers make up approximately 11% of the composition of purple basil leaves. This content was similar to that observed in the leaves of O. gratissimum L. (10.34%) by Almeida et al. [27] and higher than that reported (7.80%) by Borges et al. [24]. The total energetic value of the purple basil leaves was 16.11 kcal/100 g, which was lower than that found by Borges et al. [28] (278.34 kcal/100 g) for O. gratissimum leaves.
The purple basil leaf had a 6.75 pH, an acidity of 0.72 mEq NaOH/100 g, and a total soluble solids content of 5.20 °Brix. According to Chitarra and Chitarra [29], organic acids are dissolved in cellular vacuoles that give the plant tissue acidity and cause the plant’s pH tissue to vary from 5 to 7. Henrique, Ferreira, and Nunes [30] observed an average pH value of 6.43, an acidity of 0.195 mEq NaOH/100 g, and soluble solids of 3.0 °Brix, for organic basil leaves (O. basilicum L.). The high value of aw (0.99) indicates that the purple basil leaves are very susceptible to the action of microorganisms, enzymes, and degradative processes in general [31]. Thus, drying is a process capable of reducing aw, at levels that ensures the degradative stability and thus increases the useful life of this leaf.
The different values observed for the composition and physical–chemical properties of the purple basil leaves when compared with the leaves of other plant species in the same genus and with plant species of other genera can be attributed to the place of cultivation, the soil and climate conditions, and the species or genus [32,33].
2.2. Drying of Purple Basil Leaves
2.2.1. Drying Kinetics
Figure 1 shows the drying curves of the basil leaves at temperatures of 40 °C, 50 °C, 60 °C, and 70 °C. In the initial stage of drying, a linear behavior was observed between moisture ratio (MR) and time, indicating that the process occurred at a constant drying rate, which increased with increasing temperature (increase in the straight slope) and it was much lower for drying at 40 °C, when compared with drying at temperatures from 50 °C to 70 °C. In the next drying stage, an exponential behavior of MR occurred with time, indicating that the process occurred with decreasing drying rates, for all conditions studied.
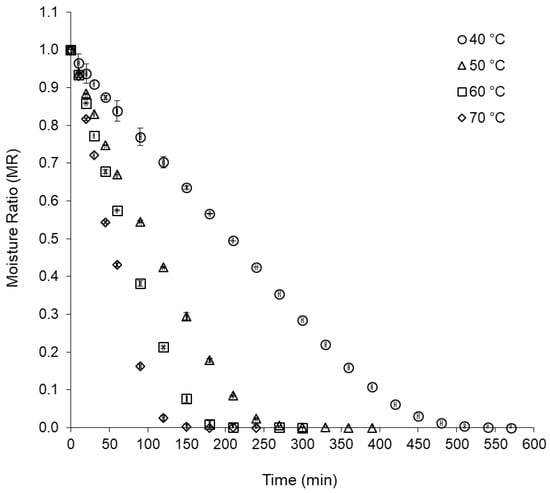
Figure 1.
Drying curves of purple basil leaves at temperatures of 40 °C, 50 °C, 60 °C, and 70 °C.
Similar drying behaviors were observed for rosemary leaves (Rosmarinus officinalis L.) dried by hybrid convective and microwave drying processes, under a vacuum [34]; for sage leaves (Salvia officinalis) dried in a cabin dryer [35]; and for basil leaves (O. basilicum L.) dried by microwave [36]. Mbegbu, Nwajunka, and Amaefule [37] reported that diffusion was the predominant mechanism for moisture transport during the thin layer drying of O. gratissimum and Ocimum africanum leaves.
Concerning the total drying time, a significant reduction was observed with the increase in the drying temperature, requiring a drying time of 2 h and 30 min at 70 °C; 3 h and 30 min at 60 °C; 4 h and 30 min at 50 °C; and 8 h and 30 min at 40 °C. Thus, there was a 40% increase in drying time at 60 °C compared with 70 °C, 29% at 50 °C compared with 60 °C, and 89% at 40 °C compared with 50 °C. These results indicate that the convective drying of purple basil leaves at 40 °C is a slow process, not suitable for practical purposes, except when higher temperatures degrade chemical compounds of interest. According to Babu et al. [31], air temperature and drying time can cause physical and structural changes and chemical losses due to the evaporation of volatile compounds, as they affect quality attributes, flavor, and the nutritional value of leaves. In this context, Rocha, Melo, and Radünz [38] reported that at 50 °C, the losses of chemical constituents were minimal during the drying of medicinal plants.
2.2.2. Effective Diffusivity and Activation Energy
Effective diffusivity (Deff) of water, for drying purple basil leaves, was 4.93 × 10−10 m2/s at 40 °C, 9.94 × 10−10 m2/s at 50 °C, 13.87 × 10−10 m2/s at 60 °C, and 18.96 × 10−10 m2/s at 70 °C. The behavior observed for Deff indicated that the increase in drying temperature favored the elimination of water from the leaves, confirming the behavior of the drying curves (Figure 1). According to Fernandez et al. [39], the Deff can vary depending on the internal conditions and the product structure, with the moisture content of the material, and with the drying temperature.
Seyedabadi [40] studied the effect of microwave power on the drying of basil leaves (O. basilicum L.) and observed that increasing power (from 90 W to 900 W) caused an increase in Deff (from 1.62 × 10−10 m2/s to 7.65 × 10−10 m2/s). Mbegbu, Nwajunka, and Amaefule [37] observed Deff values between 4.76 × 10−13 and 1.47 × 10−12 m2/s for the drying of sweet basil leaves (O. gratissimum), and between 4.83 × 10−13 and 2.06 × 10−12 m2/s for lemon basil leaves (O. africanum), both carried out in a vacuum oven, with temperatures between 30 °C and 70 °C. These Deff values were lower than the values observed in the drying of purple basil leaves, while the Deff values observed by Seyedabadi [40] were of the same magnitude order.
The activation energy (Ea), estimated for the drying process of purple basil leaves, was 39.30 kJ/mol. This value was higher than those reported for the drying of scent leaves (O. gratissimum) (Ea = 25.01 kJ/mol) and lemon basil leaves (O. africanum) (Ea = 32.35 kJ/mol) [37]. The highest Ea value indicates that the purple basil leaves are more sensitive to the drying air temperature.
2.2.3. Modeling of Drying Curves
The values of the statistics used to evaluate the quality of the mathematical fits and the parameters of the eight models fitted to the drying data of the purple basil leaves are presented in Table 2. Values of R2 > 0.95, χ2 < 0.007, and RMSE < 0.08 indicated that all models tested were capable of predicting, with good accuracy, the drying curves of purple basil leaves, in all conditions studied. However, the best fits for all drying conditions were observed for the modified Page models, with two parameters (R2 > 0.99, χ2 < 0.0014 and RMSE < 0.04), Diffusion Approximation (R2 > 0.99, χ2 < 0.0013, RMSE < 0.03), and Verna (R2 > 0.99, χ2 < 0.0013 and RMSE = 0.03), with three parameters. These models estimated with excellent precision the drying kinetics of the product, at all temperatures evaluated, as can be seen in Figure 2.

Table 2.
Statistics modeling and parameters of the mathematical models adjusted to the drying data of the purple basil leaves.
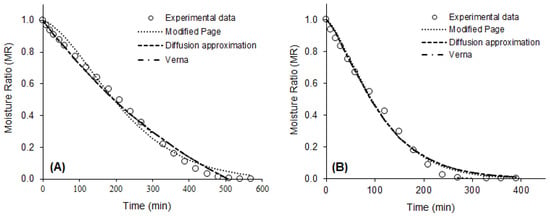
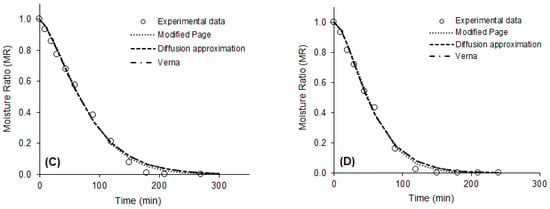
Figure 2.
Experimental drying data and drying curves predicted by the modified Page, Diffusion Approximation, and Verna models. (A) 40 °C; (B) 50 °C; (C) 60 °C; (D) 70 °C.
Altay, Hayaloglu, and Dirim [36] studied the drying of basil leaves (O. basilicum L.) by different methods. These authors observed that the Henderson and Pabis were the models that best described drying that occurred from the sun, the Logarithmic model presented the best fit for the freeze-drying data, and the modified Page was the model that more accurately predicted the drying in a convective dryer and in a microwave oven. Alibas et al. [41] observed that the modified Page model accurately described the natural drying curve of basil leaves.
2.3. Stability of Chlorophyll and Phenolic Compounds
Figure 3 shows the levels of chlorophyll a, chlorophyll b, total flavonoids (TF), and total phenolic compounds (TPC) in fresh purple basil leaves dried at 40 °C, 50 °C, 60 °C, and 70 °C. The content of chlorophyll a (4522.94 mg/g) (Figure 3A) in fresh basil leaves was higher than the content of chlorophyll b (2619.39 mg/g) (Figure 3B), and the value of TPC (105.1 mg GAE/g) (Figure 3C) was also higher than the total flavonoid content (TF) (31.19 mg RE/g) (Figure 3D).
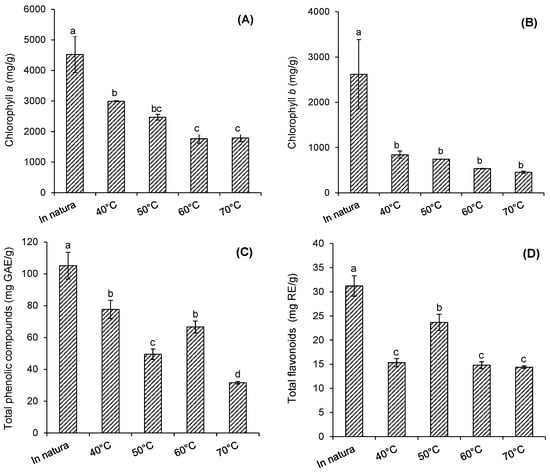
Figure 3.
Behavior of chlorophyll, phenolic compounds, and flavonoids in fresh purple basil leaves and those dried at 40 °C, 50 °C, 60 °C, and 70 °C. (A) chlorophyll a (mg/g), (B) chlorophyll b (mg/g), (C) total phenolic compounds (mg GAE/g), and (D) total flavonoids (mg/g). Means with the same letters in the same graph do not differ statistically (p ≤ 0.05) for Tukey’s test.
The chlorophyll a content of fresh purple basil leaves was significantly reduced (p ≤ 0.05) when the leaves were dried under different temperature conditions (Figure 3A). For the leaves dried at 40 °C, a degradation of 33.8% in chlorophyll a was observed, in relation to fresh leaves. When the drying temperature increased to 50 °C, a degradation of 45.4% was observed, and for dryings carried out at 60 °C and 70 °C, the observed losses corresponded to 60%, approximately (Figure 3A). For chlorophyll b, there was a degradation of 68% in the drying at 40 °C, in relation to fresh leaves (p ≤ 0.05), and no significant differences were observed among chlorophyll b values, for the leaves dried at the different temperatures (p > 0.05) (Figure 3B).
Chlorophylls are natural pigments responsible for the green color of leaves. When exposed to the action of temperatures, such as those practiced in drying processes, they can undergo degradation due to conversion into pheophytin, which has a green-brown color, or can release substrates for enzymatic browning reactions [42]. Alibas et al. [41] analyzed the chlorophyll degradation of O. basilicum L. leaves during natural, convective, and microwave drying. The authors observed that the chlorophyll values were significantly reduced by all drying methods.
As observed for chlorophyll, all drying conditions promoted a significant reduction (p ≤ 0.05) in the TPC (26.1% at 40 °C) (Figure 3C) and TF (50.9% at 40 °C) (Figure 3D). The increase in temperature, in turn, caused a reduction in the TPC (p ≤ 0.05) with a degradation of 70%, at 70 °C. On the other hand, there was no tendency to decrease the FT (p > 0.05) at the temperature range used.
Phenolic compounds and flavonoids are among the main chemical compounds found in basil leaves [4,43]. Sharma, Bhatia, and Kaur [44] reported the degradation of phenolic compounds and flavonoids during the drying of basil leaves by microwave, by exposure to the sun, and in a tray dryer, at temperatures of 45 °C, 50 °C, and 55 °C. They observed that the highest level of degradation occurred in tray drying at 55 °C. Other authors have also reported the loss of phenolic compounds and flavonoids during thermal processes [45,46].
Therefore, it was observed that convective drying promoted a reduction in the contents of chlorophylls, TPC, and TF, when compared with fresh purple basil leaves; however, drying is a necessary operation for the post-harvest preservation of leaves, as well as a pre-treatment for other processes. Research carried out with medicinal and aromatic plants indicated that temperatures of 40 °C and 50 °C decreased the loss of chemical compounds and nutritional quality [38,47]. Thus, according to the results, drying purple basil leaves at 40 °C for 8 h and 30 min can be applied for less degradation of chlorophylls and TPC.
2.4. Color Measurements
The instrumental color parameters a*, b*, C*, L*, h°, and ΔE values, for fresh and dried purple basil leaves at 40 °C, 50 °C, 60 °C, and 70 °C, are presented in Table 3. The color pattern indicated that the fresh basil leaves have a light green color. However, the values of all the color parameters changed significantly (p ≤ 0.05) after the leaves were submitted to drying, even for drying at 40 °C. There was an increase in the value of a* and decreases in the values of the other parameters (b*, C*, L*, and h°). Regarding the drying temperature, there was no effect on a* (p > 0.05), but there was a tendency to decrease in the values of b* and L* (p ≤ 0.05), with increasing temperatures. Consequently, a tendency of decrease in the value of C* was also observed with the increase in the temperature; however, there was no significant variation in the parameter h°, in the range of drying temperatures studied. The values of ΔE, in turn, indicated that the increase in the drying temperature promoted a degradation in the color of the leaves. According to the color parameters, the purple basil leaves lost their greenish color characteristic (h° = 123.3) and adopted a light yellow color after drying (average value of h° = 88.8).

Table 3.
Instrumental color parameters for purple basil in natura and dried at different temperatures.
2.5. Hygroscopic Behavior of Purple Basil Leaves
2.5.1. Moisture Sorption Isotherms
The moisture adsorption and desorption isotherms of the basil leaves at 25 °C are shown in Figure 4. In the adsorption isotherm, the equilibrium moisture content (m) increased linearly with aw, between 0.1 and 0.5 aw (Δm = 5 g H2O/100 g db), when the behavior became exponential, up to 0.9 aw (Δm = 12.3 g H2O/100 g db). This behavior indicates that dried basil leaves will be more susceptible to moisture gain when exposed to an environment with relative humidity (RH) above 50%.
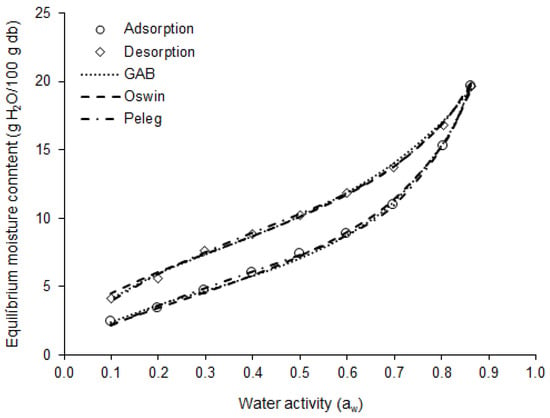
Figure 4.
Moisture sorption isotherm at 25 °C for purple basil leaves: experimental data and curves obtained by the GAB, Oswin, and Peleg models.
The moisture adsorption and desorption isotherms showed type II behavior (sigmoid shape), which is typical of biological materials [48]. Type II isotherms were observed by Lima-Corrêa et al. [19] for basil leaves (O. basilicum); by Canabarro et al. [49] for pitanga leaves (Eugenia uniflora L.); by Santos et al. [50] for jambu leaves (Acmella oleracea); and by Martins et al. [51] for guaco leaves (Mikania glomerata Sprengel).
The monolayer moisture content (mo) for adsorption indicated that 4.27 g H2O/100 g db is the moisture content level at which the dried purple basil leaves present the greatest stability under physical, chemical, and biological changes [52]. However, the adsorption isotherm indicates that the dried leaves will be microbiologically stable when they present a moisture content of less than 8.83 g H2O/100 g db (aw < 0.6), if stored at 25 °C [53]. In turn, the value of mo for desorption indicated that, when the purple basil leaves are subjected to drying, the moisture of the product should not reach moisture levels lower than 5.96 g H2O/100 g db, to avoid unnecessary power consumption, since below mo the water is strongly bound to the product.
2.5.2. Modeling of Moisture Sorption Isotherms
The results of the mathematical models’ fits to the experimental moisture adsorption and desorption data for dried purple basil leaves are presented in Table 4. The statistics used to assess the quality of the models’ fit indicated that the Oswin (R2 > 0.99, P < 3.1, RMSE < 0.25), GAB (R2 > 0.99, P < 2.4, RMSE < 0.23), and Peleg (R2 > 0.99, P < 2.7, RMSE < 0.15) models were able to describe with excellent accuracy the moisture adsorption and desorption isotherms of the dried basil leaves, as can be seen in Figure 4. The GAB and Peleg equations are more difficult to solve mathematically, as they are models with three and four parameters, respectively. In turn, as the Oswin is a model with two parameters, it is easy to solve it mathematically. Thus, for practical purposes, the Oswin model is the most suitable for estimating the moisture adsorption and desorption isotherms of the dried purple basil leaves.

Table 4.
Statistics and model’s coefficients for mathematical modeling of moisture sorption data of dried purple basil leaves at 25 °C.
Bensebia and Allia [54] observed that the GAB model was the one that best described the moisture adsorption and desorption isotherms of rosemary leaves, for a temperature range from 30 °C to 50 °C. Argyropoulos et al. [55] evaluated the fits of five moisture sorption models and observed that the modified Oswin model was the one that best fitted the moisture sorption data of lemon balm leaves, in the temperature range from 25 °C to 45 °C. Martins et al. [51], in turn, reported that the Oswin model was the one that best described the moisture sorption isotherms of guaco leaves, for a temperature range from 40 °C to 70 °C.
3. Materials and Methods
3.1. Plant Material
Purple basil (Ocimum basilicum L.) was collected in the municipality of Santa Izabel do Pará (1°23′00.0″ S 48°05′49.0″ W), located in the state of Pará, Brazil. Five plants were selected per production row, in a total of four rows. During the collection, part of the plant material was used for identification, exsiccate preparation, and deposit in the Marlene Freitas da Silva (MFS) herbarium of the State University of Pará—UEPA (Register MFS009423). Another part of the sample was immediately packed in a package that allowed air circulation and transported to the Federal University of Pará—UFPA (1°28′33.1″ S 48°27′26.2″ W). The samples (leaves with branches) were washed with running water to eliminate surface dirt. The leaves were then separated from the branches, and the torn, dark, and yellowed leaves were discarded. The selected leaves were submitted for sanitization in sodium hypochlorite solution with 100 mg/L of active chlorine for 15 min, followed by washing to eliminate residual chlorine. Part of these leaves was used for characterization, and another part was used for the drying experiments. The access to the species is registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge—SISGEN, under the following registration: A8627A9.
3.2. Characterization of the Leaves
3.2.1. Chemical Composition and Physical–Chemical Analysis
The fresh purple basil leaves were submitted for analysis of moisture content (method n° 920.151), ash (method n° 940.26), total proteins (method n° 920.152) (nitrogen-protein conversion factor of 5.75), pH (method n° 981.12), total titratable acidity (method n° 942.16), and total soluble solids (method no. 932.12), according to AOAC [56]. Total lipids were determined by the Bligh–Dyer method [57], total carbohydrates were estimated by difference, and total energy value was calculated according to the general Atwater conversion factors [58]. The water activity (aw) was determined by direct reading in a digital thermohygrometer (Decagon, Aqualab 4TEV, Pullman, EUA) at 25 °C.
3.2.2. Determination of Chlorophyll a and Chlorophyll b
The quantification of chlorophyll a and chlorophyll b was performed using the method proposed by Lichtenthaler and Buchmam [59]. The extraction of these pigments was carried out with 95% ethanol, followed by centrifugation (KASVI, K14-0815P). The reading of the supernatant was performed in a spectrophotometer (BEL, Photonics, BEL Engineering, Monza, Italy), at wavelengths of 652.2 nm and 665.2 nm. The equations 1 and 2 were used to calculate the two fractions.
where Ca = chlorophyll a (μg/mL); Cb = chlorophyll b (μg/mL); A665.2 = absorbance at 665.2 nm; A652.4 = absorbance 652.4 nm.
3.2.3. Determination of Total Phenolic Compounds
The determination of total phenolic compounds (TPC) was carried out according to the colorimetric method with the Folin−Ciocalteu reagent, proposed by Singleton, Orthofer, and Lamuela-Raventos [60]. An aliquot of 500 μL of 7% ethanolic extract of the leaves was added to 1250 μL of 10% Folin–Ciocalteu solution (v/v), and after two minutes of reaction in the dark, 1000 μL of the 7.5% sodium carbonate solution (w/v) were added to the mixture. Proceeding 30 min of reaction at room temperature and protected from light, the solution was read in the spectrophotometer at 760 nm. To quantify the TPC, an analytical curve of Gallic acid was used, obtained in the concentration range from 20 to 100 mg/L. TPCs were expressed in mg Gallic acid equivalent per g of sample (mg GAE/g of sample).
3.2.4. Determination of Total Flavonoids
The quantification of total flavonoids (TF) was carried out according to Pekal and Pyrzynska [61], with modifications. For this, 500 μL of 50% ethanolic extract of the leaves were added to 500 μL of 2% ethanolic aluminum chloride solution (w/v). After 10 min of reaction, at room temperature, and protected from light, the solution was read in a spectrophotometer at 425 nm. The analytical curve used to quantify TF was constructed in the concentration range from 5 to 200 mg/L, for rutin. Thus, the TF content was expressed in mg equivalent of rutin per gram of sample (mg ER/g of sample).
3.2.5. Color Measurements
The color was analyzed by tristimulus colorimetry in a digital colorimeter (Choma Mater CR-300, Konica Minolta, Osaka, Japan), by the CIELAB coordinate system (L*, a*, b*), using the 10° viewing angle and D65 illuminant standard. The L* coordinate refers to changes in luminosity, with a range from 0 = black to 100 = white; the chromatic coordinate a* represents the green–red dimension (−a = green and +a = red); and the chromatic coordinate b* is associated with the blue–yellow dimension (−b = blue and +b = yellow). The readings were performed in triplicate and, with the obtained values, the chroma (C*) (Equation (3)), the Hue angle (h°) (Equations (4) and (5)), and the total color difference (ΔE) (Equation (6)) were calculated [62].
where C* = chroma; h° = Hue angle; ΔE = total color difference; a* and b* = color coordinates; L* = luminosity.
3.3. Drying of the Leaves
The basil leaves were ground in a multiprocessor for 2 min and 10 g samples were spread on aluminum trays, with known mass and dimensions. The material was submitted to convective drying in a tray dryer (Quimis-Q316M5), at temperatures of 40 °C, 50 °C, 60 °C, and 70 °C, which were chosen according to the literature [47,51,63]. To monitor the process, the set (tray + sample) was weighed every 10 min, in the first 30 min; every 15 min, for an additional 45 min; and every 30 min until reaching constant weight (mass variation less than 1%). The dried leaves were ground in a knife mill, placed in hermetically closed glass jars, and stored at room temperature (≈25 °C). The dry matter of the sample was determined at 105 °C. The drying curves were obtained by correlating the moisture ratio (MR) (Equation (7)) with the drying time.
where MR = moisture ratio (dimensionless); X = moisture content at time t (g/100 g dry basis—db); Xi = initial moisture content (g/100 g db); Xe = equilibrium moisture content (g/100 g db).
In the dried leaves, analyses of chlorophyll a, chlorophyll b, total phenolic compounds, total flavonoids, and instrumental color were carried out according to the methodologies described in Section 3.2.
3.3.1. Calculation of Effective Diffusivity and Activation Energy
The value of effective diffusivity (Deff), a property that characterizes the mass transfer during the drying of a material, was determined from Fick’s second law of diffusion (Equation (8)) [64]. It is an important parameter that indicates the transfer of water within the product, and varies with drying conditions, so it is not intrinsic to the material [65]. The calculation was performed by linear regression, considering the temperature of the drying air and a bed thickness of 4 mm. Additionally, the activation energy (Ea) was calculated by the equation 9. The Ea value indicates the degree of water diffusivity in the material during drying [64].
where MR = moisture ratio (dimensionless); Deff = effective diffusivity coefficient (m2/s); L = bed thickness (m); t = drying time (s).
where Deff = effective diffusivity (m2/s); D0 = pre-exponential factor of the Arrhenius-type equation (m2/s); Ea = activation energy (J/mol); R = Universal gas constant (J/mol K); T = absolute temperature of the air-drying (K).
3.3.2. Mathematical Modeling of Drying
The experimental data on the drying of purple basil leaves were submitted to mathematical modeling. To this end, the mathematical fits of the semi-empirical models presented in Table 5 were evaluated. These models are used to describe the thin layer drying behavior [66].

Table 5.
Kinetic models used to describe the drying curves of purple basil leaves.
The coefficient of determination (Equation (18)), the reduced chi-square value (Equation (19)), and the relative mean square error (Equation (20)) were used to analyze the quality of the models’ fits.
where R2 = coefficient of determination; χ2 = reduced chi-square; RMSE = root mean square error; Yexp = experimental values; Ypre = values predicted by the model; N = number of observations; n = number of model parameters.
3.4. Hygroscopic Behavior of Dry Leaves
3.4.1. Obtaining Moisture Sorption Isotherms
To evaluate the hygroscopic behavior of the dried purple basil leaves, moisture adsorption, and desorption isotherms were obtained at 25 °C. Moisture sorption data were obtained by the DVS method (Dynamic vapor sorption), in the vapor sorption analyzer (VSA) equipment (Aqualab VSA, Decagon, Puma, WA, USA). A sample of the crushed dried leaves was weighed (≈650 mg), in a stainless steel capsule, on the analytical microbalance of the equipment, to obtain moisture adsorption and desorption data, in a range from 0.1 to 0.9 aW. Equilibrium data were obtained at different levels of relative humidity (RH) induced by changes in injections of dry steam and saturated steam. The equilibrium condition was set when three successive measures were below 0.05% (dm/dt%), where dm/dt is the ratio between mass variation and time variation, in percentage, between successive measurements. At the end of the process, the dry matter of the sample was determined at 105 °C [67].
3.4.2. Determination of Monolayer Moisture Content
The monolayer moisture content (mo) was determined by linear regression, from the linearized form of the BET equation (Equation (21)) [68].
where aw = water activity (dimensionless); m = equilibrium moisture content (g H2O/100 g db); mo = monolayer moisture content (g H2O/100 g db); c = constant related to the heat of sorption.
3.4.3. Mathematical Modeling of Moisture Sorption Isotherms
The moisture adsorption and desorption data were submitted to mathematical modeling, in which the fits of the six models shown in Table 6, were evaluated. The coefficient of determination (Equation (18)), the mean squared relative error (Equation (20)) and the mean absolute percentage error (Equation (22)) were used to assess the quality of the fits.
where P = mean absolute percent error; Yexp = experimental values; Ypre = values predicted by the model; N = number of observations.

Table 6.
Mathematical models used to describe the moisture sorption isotherms of purple basil leaves.
3.5. Statistical Analysis
The results of the properties evaluated under different conditions were submitted to analysis of variance (ANOVA) and to Tukey’s test for the means comparison, at a confidence of 95%. The fits of the drying and moisture sorption models to the experimental data were performed by non-linear regression, using the Levenberg–Marquardt estimation methodology, with a convergence criterion of 10−6. All statistical analyses were performed using the Statistica 7.0 program.
4. Conclusions
According to the results, convective drying is suitable for reducing aw, securing degradative stability, and prolonging the shelf life of purple basil leaves. The Deff values indicated that the increase in temperature (from 40 °C to 70 °C) favors the elimination of water from the leaves, but it causes losses of chlorophyll and phenolic compounds. Drying at 40 °C promoted the lowest losses of TPC (26.1%) and a change in the color of the leaves, but drying in this temperature is a slow process, not suitable for practical purposes. For thermosensitive conservation purposes, drying at 40 °C is recommended. Modified Page, Diffusion Approximation, and Verna models were efficient in predicting the drying kinetics of purple basil leaves. The moisture adsorption isotherm showed that dried purple basil leaves are more susceptible to water gain in an environment with relative humidity above 50%, and that the dried leaves present microbiological stability in moisture content less than 8.83 g H2O/100 g db, if stored at 25 °C. In addition, the moisture desorption isotherm indicated that the purple basil leaves should not be dried to moisture contents lower than 5.96 g H2O/100 g db. The Oswin mathematical model is indicated to describe the moisture sorption isotherms of purple basil leaves. Finally, this study can contribute to future research that is required for the drying of purple basil leaves, for applications in food preparation, essential oil extraction, and preparation of infusions, among others, as well as for the storage of these leaves.
Author Contributions
R.P.F.C. designed and performed the experiments, analyzed the data, and wrote the manuscript. A.L.d.A. contributed to the analysis of the results and to the writing of the manuscript. A.S.L. and R.d.S.P. designed the experiments and contributed to the analysis of the results and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for the scholarship of Rosane P. F. Chaves (88887.605381/2021-00), and Federal University of Pará (UFPA through PROPESP, Brazil).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guerra, M.E.C.; Medeiros Filho, S.; Costa, I.R.; Freitas, J.B.S.; Oliveira, A.B. Agronomic characterization of Ocimum gratissimum L (Rangpur alfavaca) and Ocimum sp (Purple alfavaca) cultivated in greenhouse and outdoor environment. Cult. Agron. 2014, 23, 123–134. Available online: http://www.repositorio.ufc.br/handle/riufc/63301 (accessed on 18 May 2022).
- Lal, R.K.; Gupta, P.; Chanotiya, C.S.; Sarkar, S. Traditional plant breeding in Ocimum. In The Ocimum genome, 2nd ed.; Shasany, A., Kole, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 89–98. [Google Scholar] [CrossRef]
- Sharifi-rad, J.; Adetunji, C.O.; Olaniyan, O.T.; Ojo, S.K.; Samuel, M.O.; Temitayo, B.T.; Roli, O.I.; Nimota, O.O.; Oluwabunmi, B.T.; Adetunji, J.B.; et al. Antimicrobial, antioxidant and other pharmacological activities of Ocimum Species: Potential to be used as food preservatives and functional ingredients. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight in composition of bioctive phenolic compounds in leaves and flowers of green and purple basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: And overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Gurav, T.P.; Dholakia, B.B.; Giri, A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2022, 21, 879–913. [Google Scholar] [CrossRef]
- Zotti-Sperotto, N.C.; Melo, E.C.; Souza, M.I.L.; Fonseca, M.C.M.; Gonzaga, D.A.; Ávila, M.B.R.; Demuner, A.J.; Ventrella, M.C.; Lelis, A.C.V. Effect of drying with ultrasonic pretreatment on the yield and quality of the essential oil of Varronia curassavica Jacq. and Ocimum gratissimum Linn. Ind. Crop. Prod. 2020, 147, 112211. [Google Scholar] [CrossRef]
- Kapepula, P.M.; Mungitshi, P.M.; Franck, T.; Mouithys-Mickalad, A.; Ngoyi, D.M.; Kalenda, P.D.T.; Ngombe, N.K.; Serteyn, D.; Tits, M.; Frederich, M.; et al. Antioxidant potentiality of three herbal teas consumed in Bandundu rural areas of Congo. Nat. Prod. Res. 2017, 31, 1940–1943. [Google Scholar] [CrossRef]
- Laws, N.; Parry, J.L. Mathematical modelling of heat and mass transfer in agricultural grain drying. Proc. R. Soc. A Math. Phys. Eng. Sci. 1983, 385, 169–187. [Google Scholar] [CrossRef]
- Boggia, R.; Zunin, P.; Hysenaj, V.; Bottino, A.; Comite, A. Dehydration of Brazil leaves and impact of processing composition. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: London, UK, 2015; pp. 645–653. [Google Scholar] [CrossRef]
- Dikmen, E.; Ayaz, M.; Kovaci, T.; Sahin, A.S. Mathematical modelling of drying characteristics of medical plants in vacum heat pump dryer. Int. J. Ambient Energy 2018, 40, 616–623. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Temple, S.J.; Van Boxtel, A.J.B. Thin layer drying of black tea. J. Agric. Eng. Res. 1999, 74, 167–176. [Google Scholar] [CrossRef]
- Lewis, L.W. The rate of drying of solid materials. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Rizvi, S.S.H. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods; Rao, M.A., Rizvi, S.S.H., Datta, A.K., Ahmed, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 359–436. [Google Scholar]
- Li, J.; Dong, L.; Xiao, M.; Qiao, D.; Wu, K.; Jiang, F.; Riffa, S.B.; Su, Y. A novel and accurate method for moisture adsorption isotherm determination of sultana raisins. Food Anal. Methods 2019, 12, 2491–2499. [Google Scholar] [CrossRef]
- Seczyk, L.; Ozdemir, F.A.O.; Kolodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest presenvation method-convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moroto, M.C.; Polomo, E.S.; Castro, L.; Viñas, M.A.G.; Perez-Coello, M.S. Changes produced in the aroma compounds and structural integrity of basil (Ocimum basilicum L.) during drying. J. Sci. Food Agric. 2004, 84, 2070–2076. [Google Scholar] [CrossRef]
- Lima-corrêa, R.A.B.; Andrade, M.S.; Silva, M.F.G.; Freire, J.T.; Ferreira, M.C. Thin-layer and vibrofluidized of basil leaves (Ocimum basilicum L.): Analysis of drying homogeneity and influence of drying conditions on the composition of essential oil and leaf colour. J. Appl. Res. Med. Aromat. Plants 2017, 7, 54–63. [Google Scholar] [CrossRef]
- Pääkkönen, K.; Malmsten, T.; Hyvönen, L. Drying, packaging, and storage effects on quality of basil, morjaram and wild marjoram. J. Food Sci. 1990, 5, 1373–1377. [Google Scholar] [CrossRef]
- Reis, R.C.D.; Devilla, I.A.; Ascheri, D.P.R.; Servulo, A.C.O.; Souza, A.B.M. Kinetics of drying of basil leaves (Ocimum basilicum L.) in the infrared. Rev. Bras. Eng. Agric. Ambient. 2012, 16, 1346–1352. [Google Scholar] [CrossRef][Green Version]
- Roberto, P.M.; Anunciação, P.C.; Lucia, C.M.D.; Pinheiro, S.S.; Souza, E.C.G.; Pinheiro-Sant’ana, H.M. Macronutrients, vitamins, minerals and bioactive compounds in fresh and dehydrated basil (Ocimum basilicum) and its hot and cold infusion. Acta Sci. 2020, 43, e55423. [Google Scholar] [CrossRef]
- Gomes, F.P.; Resende, O.; Sousa, E.P.; Damasceno, L.F. Comparison of powdered and fresh jambu (Acmella oleracea). Heliyon 2020, 6, E05349. [Google Scholar] [CrossRef]
- Leitão, D.S.T.C.; Siqueira, F.C.; Souza, S.H.B.; Mercadante, A.Z.; Chisté, R.C.; Lopes, A.S. Amazonian Erygium foetidum leaves exhibited very high contents of bioactive compounds and high singlet oxygen quenching capacity. Int. J. Food Prop. 2020, 23, 1452–1464. [Google Scholar] [CrossRef]
- Oluwole, S.O.; Fajana, O.O.; Ogun, M.L.; Ogbe, A.A.; Ademola, O.A. Proximate and mineral composition analysis of the leaves of Amaranthus cruentus and Ocimum gratissimum in some selected of Logos State, Nigeria. Int. J. Ecosyt. 2019, 9, 6–11. [Google Scholar] [CrossRef]
- Siti Mahirah, Y.; Rabeta, M.S.; Antora, R.A. Effects of diferent drying methods on the proximate composition and antioxidant activities of Ocimum basilicum leaves. Food Res. 2018, 2, 421–428. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; Lopes, M.F.G.; Sousa, P.H.M.; Nogueira, C.M.D.; Magalhães, C.E.C. Determination of moisture, fibers, lipids, ashes and silica in medicinal plants. Bol. Cent. Pesqui. Process. Aliment. 2003, 21, 343–350. [Google Scholar] [CrossRef]
- Borges, A.M.; Pereira, J.; Cardoso, M.G.; Alves, J.A.; Lucena, E.M.P. Determinação de óleos essenciais de alfavaca (Ocimum gratissimum L.), orégano (Origanum vulgares L.) e tomilho (Thymus vulgaris L.). Rev. Bras. Plantas Med. 2012, 14, 656–666. [Google Scholar] [CrossRef]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brazil, 2005. [Google Scholar]
- Henrique, V.A.; Ferreira, L.P.; Nunes, C.R. Análise físico-química e antioxidante de manjericão (Ocimum basilicum L.) orgânico. Rev. Interdiscip. Pensam. Cient. 2017, 2, 85–97. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Monga, S.; Dhanwal, P.; Kumar, R.; Kumar, A.; Chhokar, V. Pharmacological and physico-chemical properties of Tulsi (Ocimum gratissimum L.): An updated review. Pharma Innov. 2017, 6, 181–186. [Google Scholar]
- Shahrajabiana, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Ali, A.; Oon, C.C.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdylo, A.; Turkiewicz, I.P.; Szumny, A.; Lyczko, J. Volatile and polyphenol composition, anti-oxidant, anti-diabetic and anti-aging properties, and drying kinetics as affected by convective and hybrid vacuum microwave drying of Rosmarinus officinalis L. Ind. Crop. Prod. 2020, 151, 112463. [Google Scholar] [CrossRef]
- Doymaz, I.; Karasu, S. Effect of air temperature on drying kinetics, colour, changes and total phenolics content of sage leaves (Salvia officinalis). Qual. Assur. Saf. Crop. Foods 2018, 10, 269–276. [Google Scholar] [CrossRef]
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the drying kinetics and energy efficiency of purple basil (Ocimum basilicum L.) leaves using different drying methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Mbegbu, N.N.; Nwajunka, C.O.; Amaefule, D.O. Thin layer drying models and characteristics of scent leaves (Ocimum gratissimum) and lemon basil leaves (Ocimum africanum). Heliyon 2021, 7, E05945. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plant Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Fernandez, A.; Román, C.; Mazza, G.; Rodriguez, R. Determination of effective moisture diffusivity and thermodynamic properties variation of regional wastes under diferent atmosphere. Case Stud. Therm. Eng. 2018, 12, 248–257. [Google Scholar] [CrossRef]
- Seyedabadi, E. Drying kinetics modelling of basil in microwave dryer. Agric. Commun. 2015, 3, 37–44. [Google Scholar]
- Alibas, I.; Yilmaz, A.; Asik, B.B.; Erodogan, H. Influence of drying methods on the nutrients, proteins content and vitamin profile of basil leaves. J. Food Compos. Anal. 2021, 96, 103758. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.Q.; Thi, N.Q.N.; Thi, C.Q.N.; Truc, T.T.; Nghi, P.T.B. Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum L.). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012083. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Effect of drying methods on biochemical quality of basil leaf. Agric. Res. J. 2018, 55, 331–335. [Google Scholar] [CrossRef]
- Jyotshna; Srivastava, N.; Yadav, A.K.; Shanker, K.; Gupta, M.M.; Lal, R.K. Impact of postharvest processes on major phenolic constituents and antioxidant potentials of different Ocimum species. J. Appl. Res. Med. Aromat. Plants 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Le, N.L.; Le, T.T.H.; Ma, N.B. Effects of air temperature and blanching pre-treatment of phytochemical content, antioxidant activity and enzyme inhibition activities of the basil leaves (Ocimum basilicum var. thyrsiflorum). Food Res. 2021, 5, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Alakali, J.S.; Kucha, C.T.; Rabiu, I.A. Effect of drying temperature on the nutritional quality of Moringa oleifera leaves. Afri. J. Foood Sci. 2015, 9, 395–399. [Google Scholar] [CrossRef]
- Yanniotis, S.; Blahovec, J. Model analysis of sorption isotherms. LWT Food Sci. Technol. 2009, 42, 1688–1695. [Google Scholar] [CrossRef]
- Canabarro, N.I.; Ugalde, G.A.; Mazutti, M.A.; Ferreira, M.C. Conveyor-belt drying of Eugenia uniflora L. leaves: Influence of drying conditions on the yield, composition antioxidant activity and total phenolic content of supercritical CO2 extracts. Food Bioprod. Process. 2019, 116, 140–149. [Google Scholar] [CrossRef]
- Santos, O.V.; Soares, S.D.; Vieira, E.L.S.; Martins, M.G.; Nascimento, F.C.A.; Teixeira-Costa, B.E. Physicochemical properties and bioactive composition of the lyophilized Acmella oleracea powder. J. Food Process. Preserv. 2021, 45, e15354. [Google Scholar] [CrossRef]
- Martins, M.R.; Johann, G.; Palú, F.; Silva, E.A. Drying of guaco leaves: Experimental and modeling kinetic equilibrium isotherms and heat of desorption. J. Anal. Calorim. 2022, 147, 7411–7420. [Google Scholar] [CrossRef]
- Dalgıç, A.C.; Pekmez, H.; Belibağlı, K.B. Effect of drying methods on the moisture sorption isotherms and thermodynamic properties of mint leaves. J. Food Sci. Technol. 2012, 49, 439–449. [Google Scholar] [CrossRef]
- Araújo, A.L.; Pena, R.S. Moisture desorption behavior and thermodynamic properties of pulp and seed of jambolan (Syzygium cumini). Heliyon 2022, 8, e09443. [Google Scholar] [CrossRef]
- Bensebia, O.; Allia, K. Analysis of adsorption-desorption moisture isotherms of Rosemary leaves. J. Appl. Res. Med. Aromat. Plants 2016, 3, 79–86. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Alex, R.; Kohler, R.; Müller, J. Moisture sorption isotherms and isosteric heat of sorption of leaves and stems of lemon balm (Melissa officinalis L.) established by dynamics vapor sorption. LWT Food Sci. Technol. 2012, 47, 324–331. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Food and Agriculture Organization of the United Nations. Food Energy: Methods of Analysis and Conversion Factors Food Nutrition. 2002. Available online: http://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf (accessed on 22 April 2022).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2021, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.O. Analysis of total phenols and other oxidation substrates and antioxidants by means Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Brito, B.N.C.; Silva, R.S.; Lopes, A.S.; Chisté, R.C. Anthocyanins of jambolao (Syzygium cumini): Extraction and pH-dependent color changes. J. Food Sci. 2017, 82, 2286–2290. [Google Scholar] [CrossRef]
- Dorneles, L.N.S.; Goneli, A.L.D.; Cardoso, C.A.L.; Silva, C.B.; Hauth, M.R.; Oba, G.C.; Schoeninger, V. Effect of air temperature and velocity on drying kinetics and essential oil composition of Piper umbellatum L. leaves. Ind. Crops Prod. 2019, 142, 111846. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the thin-layer drying of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, G. Heat and mass transfer during low intensity convection drying. Chem. Eng. Sc. 1999, 54, 3899–3908. [Google Scholar] [CrossRef]
- Inyang, U.E.; Oboh, I.O.; Etuk, B.R. Kinetic models for drying techniques–Food materials. Adv. Chem. Eng. Sci. 2018, 8, 27–48. [Google Scholar] [CrossRef]
- Silva, D.A.; Pena, R.S. Thermodynamic properties of Buriti (Mauritia flexuosa) tree gum. Food Sci. Technol. 2018, 38, 390–398. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Chirife, J.; Iglesias, H.A. Equations for fitting water sorption isotherms of foods. Part 1–A review. J. Food Technol. 1978, 13, 159–174. [Google Scholar] [CrossRef]
- Maroulis, Z.B.; Tsami, E.; Arinos-Kouris, D.; Saravacos, G.D. Application of the GAB model to the sorption isotherms for dried fruits. J. Food Eng. 1988, 7, 63–70. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).