Abstract
Yarlung Zangbo Grand Canyon National Nature Reserve has the most complete vertical vegetation belts in China. However, identification and distribution of vertical vegetation belts is still uncertain and in debate. To explore the above issues, 190 plots were surveyed within the reserve from 2019 to 2021. Based on the vegetation plot data, cluster analysis, ordination analysis, and biodiversity statistics were performed to reveal the structure of vertical vegetation belts–the driving factors of vegetation distribution–to describe the main biodiversity patterns. Five vertical vegetation belts were identified by clustering. NMDS ordination showed that the main factor of vegetation distribution is elevation. Based on the results of the analysis and previous literature, a new scheme of vertical vegetation belts in the south slope of the reserve was proposed. There was a lower montane seasonal rainforest belt (600–1100 m), a lower montane evergreen broadleaf forest belt (1100–1800 m), a middle montane semi-evergreen broadleaf forest belt (1800–2400 m), a subalpine evergreen needleleaf forest belt (2400–3800 m), a alpine shrubland and meadow belt (3800–4400 m), an alpine sparse vegetation belt (4400–4800 m), and a nival belt (4800–7782 m). Among them, the seasonal rainforest belts are the northernmost distribution of this type, and the semi-evergreen broadleaf forest belts exist only in the Eastern Himalayas. The study showed a unimodal pattern in plant species diversity, the peak of which is about 1900 m. The middle montane semi-evergreen broadleaf forest belt had the highest species diversity in the reserve. This study settled the issues regarding the vertical vegetation belts, the main drivers of vegetation and assessment of plant species diversity in the south slope of the Yarlung Zangbo Grand Canyon National Nature Reserve. It provides essential support for the management and conservation of these ecosystems in the reserve.
1. Introduction
Recognizing and using elevational subdivisions is at the core of biogeographical and ecological studies in mountain ecosystems [1]. One of their key research areas is the recognition and use of vertical vegetation belts. The study of Andean vertical vegetation belts by Humboldt and Bonpland is considered the first work [2]. According to indicator species and elevations, Andean vegetation was divided into seven vertical belts. After more than two hundred years, the record of vertical belts provides important evidence for the relationship between the rising of the vertical vegetation belt and global climate change [3]. The studies of vertical vegetation belts were carried out in the Rocky Mountains, Alps, Mount Kilimanjaro, and other mountain ranges [4,5,6,7,8]. Due to the limitation of latitude, climatic zone, and elevation span, their vertical vegetation belts are relatively simple. In the Alps, for example, the base belt is a deciduous broadleaf forest [6]. Such as with Mount Kilimanjaro, located near the equator, its base belt is savanna. Due to the extent of the elevational gradient, suitable latitude, and southwest monsoon, the Himalayas have abundant vertical vegetation belts and are an excellent place for testing macroecological and biogeographical hypotheses.
The Yarlung Zangbo Grand canyon, located in the Eastern Himalayas, has the most complete vertical vegetation belts in China, and is one of the most complete vertical vegetation belts in the world [9]. In addition, it is one of the deepest and longest canyons in the world, as well as the most important passageway of water vapor transported from the Indian Oceans to the Qinghai-Tibet Plateau [10,11,12]. The Mount Namjagbarwa above this canyon is 7782 m above sea level, which is the highest peak in the Eastern Himalayas. The abundant water vapor coupled with a great elevational gradient, together, create the diverse vertical vegetation belts. At the elevational gradient of more than 7000 m, the vertical vegetation belts gradually transit from the tropical seasonal rainforest to the alpine sparse vegetation and permanent snow [9,13]. The vertical vegetation belts are complete and unique. The tropical seasonal rainforest belt here is located at 29 degrees 37 s, north latitude, which is the northernmost tropical seasonal rainforest in the world [14,15]. In addition, there is a distinctive semi-evergreen broadleaf forest belt, which exists only in the Eastern Himalayas.
The Yarlung Zangbo Grand canyon is also one of the global biodiversity hotspots [16,17]. Complete vertical vegetation belts have diverse and complex ecosystems which provide diverse habitats for a large number of species. There are more than 3800 vascular plant species in an area of 9168 km2 [18]. In recent years, many new species of animals and plants are continuously discovered in the canyon [19,20,21,22,23,24,25,26,27]. For example, Medog County, located in the canyon, is the county with the largest number of new species discovered in China in 2020 [28]. In order to protect the water vapor channel, vertical vegetation belts and biodiversity, Yarlung Zangbo Grand Canyon is designated as a national nature reserve.
However, vegetation surveys and research in Yarlung Zangbo Grand Canyon National Nature Reserve are hampered by the complicated environments and inconvenient transportation. It was until 1980 that Li et al. systematically reported the vegetation here for the first time, based on fifteen months of fieldwork [9,13]. Vegetation on the south slope of the mountain is divided into eight vertical vegetation belts: one, a lower mountain evergreen monsoon rainforest belt (below 600 m); two, a lower mountain semi-evergreen monsoon rainforest belt (600–1100 m); three, a middle mountain evergreen broadleaf forest belt (1100–1800 m); four, a middle mountain semi-evergreen broadleaf forest belt (1800–2400 m); five, a subalpine hemlock forest belt (2400–2800 m); six, a subalpine fir forest belt (2800–4000 m); seven, an alpine shrubland and meadow belt (4000–4400 m); eight, an alpine subnival vegetation belt (4400–4800 m). Other researchers also report different classifications schemes of vertical vegetation belts in the region [29,30,31]. However, these studies are mainly based on descriptive materials and expert experiences and opinions. The vertical vegetation belts in Yarlung Zangbo Grand Canyon National Nature Reserve are still controversial due to the lack of quantitative analysis based on vegetation plots data, such as the validity of the semi-evergreen broadleaf forest and broadleaved mixed forest belts, which need to be verified. Different vertical vegetation belts were defined within the same elevation range [9,29,30,31]. In order to resolve these disputes, more quantitative vegetation studies are needed.
The purpose of this study is to identify main vertical vegetation belts, the main environmental drivers of vegetation distribution, as well as to assess composition of plant communities and plant species diversity in the south slope of Yarlung Zangbo Grand Canyon National Nature Reserve. There are notable conservation gaps for disturbance of future climate change on ecosystem functioning and services [32], which require vegetation data to provide the necessary guidance for ecosystem conservation [33,34,35,36]. Based on the results of statistical analysis of quantitative data, we hope that the study can improve the management of the reserve. Meanwhile, discovering its composition and biodiversity would contribute to protecting the habitats of endangered species and resolving the “Humboldt’s enigma”.
2. Results
2.1. Vertical Vegetation Belts
The vertical vegetation belts were identified by ward’s clustering and indicator species analysis. The clustering of all the 190 plots in the south slope of Yarlung Zangbo Grand Canyon National Nature Reserve produced five different groups by defining a K value = 5, based on fusion level value and silhouette width (Figure 1). The figures of fusion level value and silhouette width are provided in Appendix A. These groups correspond to five different vertical vegetation belts, respectively. There was a lower montane seasonal rainforest belt, a lower montane evergreen broadleaf forest belt, a middle montane semi-evergreen broadleaf forest belt, a subalpine evergreen needleleaf forest belt, and an alpine shrubland and meadow belt. In the five vertical vegetation belts, the top 20 indicator species of each belt are summarized in Appendix B. In addition, photos of typical alliances of each vertical belt are provided in Figure 1. The features of typical alliances in each vertical vegetation belt are also described in Figure 2.
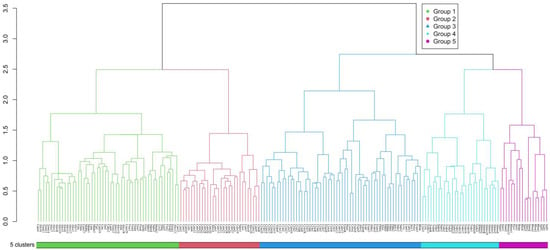
Figure 1.
Cluster dendrogram of the 190 plots in the south slope of Yarlung Zangbo Grand Canyon National Nature Reserve.

Figure 2.
Typical alliances of five vertical vegetation belts. (A–C), lower montane seasonal rainforest belt; (A), Terminalia myriocarpa forest alliance; (B), Altingia excels forest alliance; (C), the “slash-and-burn” farming method; (D–F), lower montane evergreen broadleaf forest belt; (D), Castanopsis indica forest alliance; (E), Castanopsis ceratacantha forest alliance; (F), Macaranga denticulate forest alliance; (G–J), middle montane semi-evergreen broadleaf forest belt; (G), Cyclobalanopsis kiukiangensis forest alliance; (H), Cyclobalanopsis lamellose forest alliance (April); (I), Exbucklandia populnea forest alliance; (J), Pinus bhutanica forest alliance; (K,L), subalpine evergreen needleleaf forest belt; (K), Tsuga dumosa forest alliance; (L), Abies delavayi var. motuoensis forest alliance; (M–O), alpine shrubland and meadow belt; (M), Salix annulifera deciduous broadleaf shrubland alliance; (N), Rhododendron chamaethomsonii evergreen broadleaf shrubland alliance; (O), Bergenia purpurascens alpine meadow grassland alliance.
Figure 2A–C, lower mountain seasonal rainforest belt (600–1100 m). Figure 2A, Terminalia myriocarpa forest alliance. The mean cover of the alliance is 70–80%. The mean height is 30–40 m. The community structure can be divided into tree layer, shrub layer and herb layer. In some primitive forests, the epiphytes and lianas are well developed. In tree layer, Terminalia myriocarpa is the dominant species and usually has plank buttresses root. Common species mainly are Garcinia pedunculata, Cordia dichotoma, Gynocardia odorata, Homalium ceylanicum, Syzygium balsameum, Turpinia pomifera and Talauma hodgsonii. In shrub layer, Dendrocnide sinuate, Boehmeria macrophylla var. rotundifolia, Glochidion hirsutum, Ficus heteropleura and Rhynchotechum ellipticum are common species. In herb layer, Phrynium placentarium, Curculigo capitulate, Nephrolepis cordifolia, Pteris wallichiana and Pronephrium medogensis are common species. Epiphytes and lianas mainly are Neottopteris nidus, Lysionotus serratus, Lemmaphyllum drymoglossoides, Tetrastigma hypoglaucum, Rhaphidophora luchunensis and Rhaphidophora decursiva. Figure 2B, Altingia excels forest alliance. The mean cover of the alliance is 80–90%. The mean height is 20–25 m. As the trunk of Altingia excels is white, this alliance was protected as fengshui forest near the village. But shrub layer and herb layer were more damaged. The community structure can be divided into tree layer, shrub layer and herb layer. In tree layer, Altingia excels is the dominant species. Common species mainly are Meliosma pinnata, Brassaiopsis hainla, Albizia sherriffii and Macaranga denticulate. In shrub layer, Oxyspora paniculata, Saurauia griffithii, Maesa montana, Glochidion hirsutum and Buddleja myriantha are common species. In herb layer, Nephrolepis cordifolia, Amischotolype hispida, Selaginella effuse, Impatiens namchabarwensis and Elatostema hookerianum are common species. Epiphytes and lianas mainly are Millettia pachycarpa, Pothos chinensis, Aeschynanthus stenosepalus, Poikilospermum suaveolens and Dalbergia mimosoides. Figure 2C,F, Macaranga denticulate forest alliance. The slash-and-burn farming method basically destroyed all the vegetation in the elevation range of 600–1900 m. After farmland was abandoned, secondary forest dominated by Macaranga denticulate was gradually formed. The forest has a simple structure and low species diversity. The mean cover of the alliance is 60–80%. The mean height is 22–28 m. Common species mainly are Castanopsis indica, Saurauia punduana, Ficus semicordata, Oxyspora paniculata, Phrynium placentarium and Piper thomsonii. Figure 2D–F, lower mountain evergreen broadleaf forest belt (1100–1800 m). Figure 2D, Castanopsis indica forest alliance. The mean cover of the alliance is 70–80%. The mean height is 22–28 m. The alliance is seriously disturbed by human activities. In tree layer, Castanopsis indica is the dominant species. Common species mainly are Macaranga denticulate, Musa balbisiana, Saurauia napaulensis, Engelhardtia spicata, Ficus semicordata, Meliosma pinnata, Radermachera sinica. In shrub layer, Saurauia griffithii, Ardisia crenata, Glochidion hirsutum, Luculia pinceana and Maesa montana are common species. In herb layer, Phrynium placentarium, Pronephrium medogensis, Piper thomsonii, Curculigo capitulate, Nephrolepis cordifolia, Dicranopteris ampla, Impatiens namchabarwensis, Colocasia esculentum var. antiquorum and Elatostema acuminatum are common species. Epiphytes and lianas mainly are Embelia floribunda, Embelia parviflora, Dalbergia mimosoides, Smilax aspericaulis, Hedyotis scandens, Loxostigma griffithii, Lysionotus serratus and Lemmaphyllum drymoglossoides. Figure 2E, Castanopsis ceratacantha forest alliance. The mean cover of the alliance is 80–90%. The mean height is 22–23 m. In tree layer, Castanopsis ceratacantha is the dominant species. Common species mainly are Macaranga denticulate, Meliosma pinnata, Saurauia punduana, Eurya trichocarpa, Cyclobalanopsis kiukiangensis and Pyrenaria tibetana. In shrub layer, Coriaria nepalensis, Oxyspora paniculata, Dendrocalamus tibeticus, Pyrenaria tibetana and Ardisia crenata are common species. In herb layer, Rubus metoensis, Oplismenus compositus, Nephrolepis cordifolia, Impatiens nyimana, Onychium siliculosum and Carpesium abrotanoides are common species. Epiphytes and lianas mainly are Helixanthera terrestris, Piper petiolatum, Tripterospermum volubile and Neottopteris simonsiana. Figure 2G–J, middle mountain semi-evergreen broadleaf forest belt (1800–2400 m). Figure 2G, Cyclobalanopsis kiukiangensis forest alliance. The mean cover of the alliance is 70–80%. The mean height is 35–45 m. The community structure can be divided into tree layer, shrub layer and herb layer. Primitive forests were well preserved, with well epiphytes and lianas. In tree layer, Cyclobalanopsis kiukiangensis is the dominant species and usually has plank buttresses root. In April and May, Cyclobalanopsis kiukiangensis shed all their leaves and quickly grow new ones in a dozen days. Common species mainly are Cyclobalanopsis kiukiangensis, Cinnamomum iners, Cerasus conadenia, Sorbus medogensis, Rhododendron arboretum, Acer pectinatum, Toxicodendron wallichii var. microcarpum, Machilus duthiei, Helicia tibetensis, Styrax grandifloras, Ilex longecaudata and Elaeocarpus varunua. In shrub layer, Chimonocalamus tortuosus, Smilax myrtillus, Damnacanthus indicus, Edgeworthia gardneri, Viburnum sympodiale, Ficus neriifolia, Skimmia melanocarpa and Lasianthus chinensis are common species. In herb layer, Campylandra aurantiaca, Arisaema concinnum, Elatostema hookerianum, Elatostema medogense, Rubus fockeanus, Pilea anisophylla, Ophiorrhiza rosea, Hydrocotyle salwinica, Laportea bulbifera, Sarcopyramis nepalensis, Fagopyrum dibotrys, Galium hoffmeisteri, Monotropastrum humile, Arisaema erubescens and Disporum longistylum are common species. Epiphytes and lianas mainly are Uncaria scandens, Embelia parviflora, Tetrastigma serrulatum, Trachelospermum jasminoides, Piper petiolatum, Rhaphidophora luchunensis, Rhaphidophora decursiva, Hedera nepalensis var. sinensis, Holboellia latifolia, Haplopteris doniana, Polypodiodes amoena, Hymenophyllum simonsianum, Pholidota articulate, Remusatia vivipara, Agapetes forrestii, Agapetes praeclara, Aeschynanthus angustissimus, Thladiantha cordifolia, Pleione hookeriana and Dendrobium salaccense. There are also more mosses and lichens in the forest. Its mean cover about is 20–30%. Figure 2H, Cyclobalanopsis lamellose forest alliance. This alliance is very similar to Cyclobalanopsis kiukiangensis forest alliance in community structure and species composition. Both of them share the same range of elevation and are the main type of middle mountain semi-evergreen broadleaf forest belt. Figure 2I, Exbucklandia populnea forest alliance. After forest of the belt was damaged, secondary forest dominated by Exbucklandia populnea was gradually formed. At the beginning of the succession, the alliance is small and dense with low species diversity. The mean cover of the alliance is 80–95%. The mean height is 15–25 m. In tree layer, Common species mainly are Schima parviflora, Symplocos lucida, Pinus bhutanica. Common shrubs and herbs mainly are Myrsine semiserrata, Ternstroemia biangulipes, Daphne bholua, Ardisia garrettii, Calanthe brevicornu, Tricholepidium normale, Campylandra aurantiaca, Ainsliaea latifolia. There are more litter on the ground, and its coverage about is 90%. Figure 2J, Pinus bhutanica forest alliance. On a degraded swamp, or bare ground after a landslide, secondary forest dominated by Pinus bhutanica was gradually formed. At the beginning of the succession, the alliance is small and sparse with low species diversity. But Pinus bhutanica grows very fast, it can grow more than 40 m high in about 60 years. The mean cover of the alliance is 50–80%. The mean height is 15–45 m. In tree layer, Common species mainly are Gaultheria leucocarpa var. cumingiana, Ilex denticulate, Houpoea rostrate, Diploknema butyracea, Cyclobalanopsis lamellose, Exbucklandia populnea, Cinnamomum tamala, Elaeocarpus varunua and Alsophila spinulosa. In shrub layer, Viburnum erubescens, Enkianthus deflexus, Litsea cubeba, Rosa sericea, Ilex nothofagifolia, Calamus acanthospathus, Mycetia nepalensis, Lasianthus biermannii and Psychotria calocarpa are common species. In herb layer, Beccarinda tonkinensis, Ophiorrhiza rosea, Elatostema hookerianum, Sarcopyramis nepalensis, Elatostema nasutum, Pilea anisophylla and Balanophora harlandii are common species. Epiphytes and lianas mainly are Melocalamus elevatissimus, Rhaphidophora luchunensis, Rhaphidophora decursiva, Trachelospermum jasminoides, Hymenophyllum simonsianum, Neottopteris nidus, Pothos chinensis, Epigeneium rotundatum, Aeschynanthus lasiocalyx, Pyrrosia lanceolate, Odontochilus lanceolatus, Eria tenuicaulis, Bulbophyllum reptans, Dendrobium hookerianum, Pholidota articulate and Pyrrosia sheareri. Figure 2K,L, subalpine evergreen coniferous forest belt (2400–3800 m). Figure 2K, Tsuga dumosa forest alliance. Tall and sparse primitive forests were well preserved. The mean cover of the alliance is 70–80%. The mean height is 35–45 m. The community structure can be divided into tree layer, shrub layer and herb layer. In tree layer, Tsuga dumosa is the dominant species. Common species mainly are Gamblea ciliate var. evodiifolia, Acer campbellii, Helwingia japonica, Lindera obtusiloba and Euonymus frigidus. In shrub layer, Daphne bholua, Berberis wilsoniae, Neillia thyrsiflora, Edgeworthia gardneri, Decaisnea insignis, Ribes glaciale, Leycesteria formosa, Euonymus sanguineus and Rhododendron delavayi are common species. In herb layer, Oxalis leucolepis, Circaea alpine, Ainsliaea latifolia, Plagiogyria glauca, Epipogium aphyllum, Pilea anisophylla, Anaphalis margaritacea, Botrychium robustum, Hydrocotyle salwinica, Maianthemum fuscum, Arisaema wattii and Impatiens tenuibracteata are common species. Epiphytes and lianas mainly are Lepisorus scolopendrium, Coelogyne corymbosa, Pleione bulbocodioides, Hymenophyllum simonsianum, Agapetes praeclara, Aristolochia griffithii, Actinidia venosa, Schisandra rubriflora and Vaccinium dendrocharis. There are also more mosses and lichens in the forest. Its mean cover about is 80–90%. Figure 2L, Abies delavayi var. motuoensis forest alliance. Tall and sparse primitive forests are well preserved. But near the forest line, the alliance becomes sparse and small. The mean cover of the alliance is 50–80%. The mean height is 15–45 m. The community structure can be divided into tree layer, shrub layer and herb layer. Lianas and epiphytes are almost absent. In tree layer, Abies delavayi var. motuoensis is the dominant species. Common species are Gamblea ciliate var. evodiifolia, Acer campbellii, Sorbus wilsoniana, Daphniphyllum himalense and Betula utilis. In shrub layer, Fargesia melanostachys, Ribes glaciale, Lonicera tangutica, Enkianthus quinqueflorus, Hydrangea aspera and Clethra delavayi are common species. In herb layer, Sinopodophyllum hexandrum, Aletris pauciflora, Impatiens nyimana, Circaea alpine, Maianthemum atropurpureum, Synotis longipes, Arisaema elephas, Arisaema decipiens and Dryopteris wallichiana are common species. Epiphytes and lianas mainly are Haplopteris linearifolia, Rhododendron dendrocharis and Agapetes praeclara. There are also more mosses and lichens in the forest. Its mean cover about is 80–90%. Figure 2M–O, alpine scrub and meadow belt (3800–4400 m). Figure 2M, Salix annulifera deciduous broadleaf shrubland alliance. The mean cover of the alliance is 70–90%. The mean height about is 0.2–0.4 m. In shrub layer, Salix annulifera is the dominant species. Common species are Gaultheria trichophylla, Berberis taronensis, Cassiope selaginoides, Diplarche multiflora, Rhododendron viridescens and Aster albescens var. levissimus. In herb layer, Polygonum viviparum, Cremanthodium phyllodineum, Bergenia purpurascens, Potentilla leuconota are common species. In winter, it will be covered by snow with a thickness of about 6 m. Figure 2N, Rhododendron chamaethomsonii evergreen broadleaf shrubland alliance. The mean cover of the alliance is 70–90%. The mean height about is 0.1–0.2 m. In shrub layer, Rhododendron chamaethomsonii which creeps and grows on the ground is the dominant species. Common species are Diplarche multiflora, Rhododendron mekongense, Salix anticecrenata, Lonicera myrtillus and Gaultheria trichophylla. In herb layer, Polygonum viviparum, Cremanthodium rhodocephalum, Cardamine macrophylla, Saxifraga wardii and Saxifraga melanocentra are common species. In winter, it will be covered by snow with a thickness of about 6 m. Figure 2O, Bergenia purpurascens alpine meadow grassland alliance. The mean cover of the alliance is 50–75%. The mean height about is 0.25 m. In herb layer, Bergenia purpurascens is the dominant species. Common species are Cardamine macrophylla, Senecio lingianus, Dryopteris lepidopoda, Saussurea nimborum. There are many large outcrops in the meadow. In winter, it will be covered by snow with a thickness of about 6 m.
2.1.1. Lower Montane Seasonal Rainforest Belt (Group 1)
The elevation range of this belt was 600–1100 m. It lies at the base of the valley, which had experienced considerable slash-and-burn farming and longtime logging. Most of the primary vegetation had been destroyed, with some remnants in the valleys and steep slopes. There was a large area of secondary forests, but some saplings of dominant species from primary vegetation can be found in the underlayer. The top ten indicator species were Altingia excels, Impatiens stenantha, Phrynium placentarium, Sambucus adnata, Impatiens namchabarwensis, Blumea balsamifera, Mussaenda decipiens, Terminalia myriocarpa, Boehmeria macrophylla var. rotundifolia, and Lagerstroemia minuticarpa.
The Terminalia myriocarpa forest alliance, Altingia excels forest alliance, and Lagerstroemia minuticarpa forest alliance were the mainly primeval vegetation type. The secondary vegetation mainly included the Ficus semicordata forest alliance, Macaranga denticulate forest alliance, Saurauia polyneura var. paucinervis forest alliance, Albizia sherriffii forest alliance, Castanopsis indica forest alliance, Castanopsis hystrix forest alliance, Dendrocalamus tibeticus bamboo shrubland alliance, Ostodes paniculata forest alliance, Oxyspora paniculata evergreen broadleaf shrubland alliance, and Musa balbisiana shrubby grassland alliance.
2.1.2. Lower Montane Evergreen Broadleaf Forest Belt (Group 2)
The elevation range of this belt was 1100–1800 m. This vertical belt, which lies within the scope of human cultivation, had also experienced considerable destruction. The main reasons for primary forest destruction were slash-and-burn farming and longtime logging. The top ten indicator species were Castanopsis indica, Glochidion hirsutum, Oplismenus compositus, Triumfetta cana, Desmodium sequax, Polygonum capitatum, Pteris cretica, Impatiens arguta, Colocasia antiquorum, and Strobilanthes dimorphotricha.
The Castanopsis indica forest alliance, Castanopsis hystrix forest alliance, and Castanopsis ceratacantha forest alliance were the main primeval vegetation types. The secondary forest was similar to the lower montane seasonal rainforest belt.
2.1.3. Middle Montane Semi-Evergreen Broadleaf Forest Belt (Group 3)
The elevation range of this belt was 1800–2400 m. The top ten indicator species were Cyclobalanopsis lamellose, Exbucklandia populnea, Pholidota articulata, Ficus sarmentosa, Damnacanthus indicus, Disporum bodinieri, Cyclobalanopsis kiukiangensis, Arisaema concinum, Vaccinium kingdom-wardii, and Myrsine semiserrata.
The Cyclobalanopsis lamellose forest alliance and Cyclobalanopsis kiukiangensis forest alliance were the main primeval vegetation types. The Exbucklandia populnea forest alliance and Pinus bhutanica forest alliance were the main secondary forests. In addition, there were small contributions of the Alcimandra cathcartii forest alliance, Salix psilostigma forest alliance, Juglans sigillata forest alliance, and Populus wilsonii forest alliance. Usually, the Cyclobalanopsis lamellose forest alliance and Cyclobalanopsis kiukiangensis forest alliance were called the evergreen broadleaf forest. However, in the region, Cyclobalanopsis lamellose and Cyclobalanopsis kiukiangensis shed their leaves and grow new leaves within a month before the rainy season (April to May). Therefore, the alliances growing in Yarlung Zangbo Grand Canyon National Nature Reserve should be called a semi-evergreen broadleaf forest due to short time deciduous phenology.
2.1.4. Subalpine Evergreen Needleleaf Forest Belt (Group 4)
The elevation range of this belt was 2400–3800 m. The top ten indicator species were Tsuga dumosa, Abies delavayi var. motuoensis, Acanthopanax evodiaefolius, Ribes glaciale, Acer campbellii, Pilea symmeria, Galium hoffmeisteri, Lindera obtusiloba var. heterophylla, Smilacina fusca, and Oxalis Leucolepis.
The main primeval vegetation types were the Tsuga dumosa forest alliance and Abies delavayi var. motuoensis forest alliance. The primeval vegetation in the range was subject to little human interference. In some plots, the average height of the dominant species was over 40 m and its average diameter at breast height was also over 1 m. However, above 3400 m, Abies delavayi var. motuoensis forests were often stunted by perennial avalanches. The thickness of the snow in the area in March can reach up to 6 m.
2.1.5. Alpine Shrubland and Meadow Belt (Group 5)
The elevation range of this belt was 3800–4400 m. It was covered by snow for 6 months each year. There were frequent avalanches here that cause habitat fragmentation. Therefore, meadows and shrublands were mixed in the same vertical belt. The top ten indicator species were Pleurospermum angelicoides, Dryopteris barbigera, Viola biflora, Athyrium attenuatum, Geranium polyanthes, Cardamine macrophylla, Polygonum polystachyum, Pedicularis lineata, Rosa taronensis, and Rhododendron viridescens.
The main primeval vegetation types were the Rhododendron chamaethomsonii evergreen broadleaf shrubland alliance, Rhododendron viridescens evergreen broadleaf shrubland alliance, Rhododendron pumilum evergreen broadleaf shrubland alliance, Salix annulifera deciduous broadleaf shrubland alliance, Salix flabellaris deciduous broadleaf shrubland alliance, Salix rehderiana deciduous broadleaf shrubland alliance, Bergenia purpurascens alpine meadow grassland alliance, Polygonum viviparum alpine meadow grassland alliance, and Polygonum macrophyllum alpine meadow grassland alliance.
2.2. Ordination of Vegetation
The joint ordination diagram was obtained through overlaying the classification results onto the NMDS diagram (Figure 3). The NMDS ordination showed significant differences of the five vertical vegetation belts and their relationship with environmental factors (Table 1). The plots representing the five vertical belts were clearly separated into distinct groups, except with partial overlaps between group 1 and group 2. The first axis was mainly related to elevation. From left to right of the diagram, the elevation gradually increased, and the vegetation gradually changed from the lower montane seasonal rainforest belt, the lower montane evergreen broadleaf forest belt, the middle montane semi-evergreen broadleaf forest belt, and the subalpine evergreen needleleaf forest belt to alpine shrubland and meadow belt. The elevation range of each vegetation belt was showed in the boxplot (Figure 4). The lower montane seasonal rainforest belt and lower montane evergreen broadleaf forest belt had a similar elevation range. The post-hoc Tukey test between five groups was showed in Table 2. It showed the similarity of elevation ranges between group 1 and group 2.
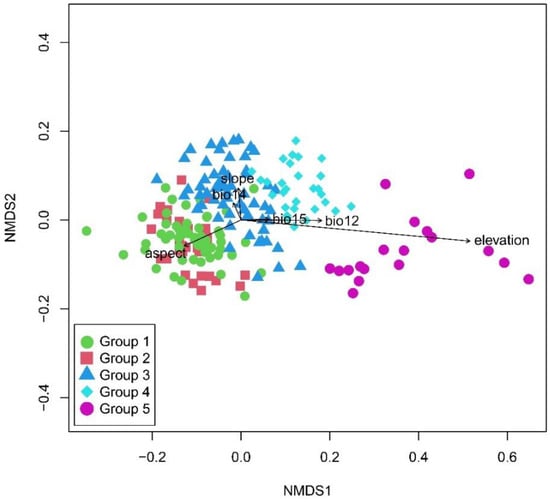
Figure 3.
NMDS ordination of 190 vegetation plots showing differences in species composition between five vegetation belts identified based on clustering analysis with passively fitted environmental variables presented as arrows. Abbreviations: Bio12 = annual precipitation, Bio14 = precipitation of driest month, and Bio15 = precipitation seasonality.

Table 1.
Relationships of environmental factors and the vertical vegetation belts.
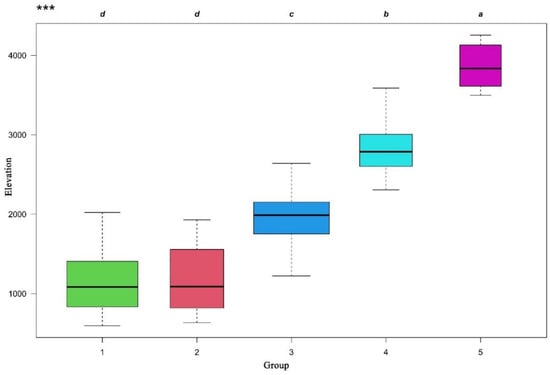
Figure 4.
Boxplots presenting differences in elevation between five groups of vegetation belts evaluated by ANOVA and post-hoc Tukey test. Groups with the same letter did not differ significantly at p = 0.05. *** p ≤ 0.001.

Table 2.
The post-hoc Tukey test for elevation between five groups.
2.3. Species Diversity
In the reserve, 1416 vascular plants from 190 plots were recorded, belonging to 165 families and 609 genera. Angiosperms included 136 families, 543 genera, and 1273 species; Gymnosperms included 3 families, 6 genera, and 10 species; Pteridophytes included 26 families, 60 genera, and 133 species. The family with the highest number of species was Orchidaceae, including 35 genera and 81 species.
The species richness, Shannon diversity index, Simpson diversity index, and Pielou diversity index were compared among five groups (Table 3). The maximum value of species richness was group 3. A unimodal pattern was showed in the scatter diagram between species richness and elevation (Figure 5), peaking at 1900–2000 m. Both of them showed that the middle montane semi-evergreen broadleaf forest belt had the highest biodiversity. There were 823 vascular plants recorded in the belt.

Table 3.
Differences in plant species diversity metrics between five vegetation belts, evaluated by post-hoc Kruskal–Wallis test. Groups with the same letter did not differ significantly at p = 0.05.
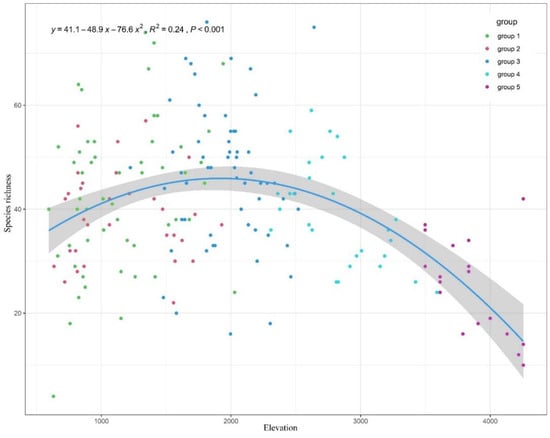
Figure 5.
The relationship between species richness and elevation in the south slope of Yarlung Zangbo Grand Canyon National Nature Reserve.
2.4. The New Division Scheme of Vertical Vegetation Belts
Based on these results and previous literature, we proposed a new scheme for vertical vegetation belts in Yarlung Zangbo Grand Canyon National Nature Reserve. There were seven vertical vegetation belts: the lower montane seasonal rainforest belt (600–1100 m), lower montane evergreen broadleaf forest belt (1100–1800 m), middle montane semi-evergreen broadleaf forest belt (1800–2400 m), subalpine evergreen needleleaf forest belt (2400–3800 m), alpine shrubland and meadow belt (3800–4400 m), alpine sparse vegetation belt (4400–4800 m), and nival belt (4800–7782 m). This scheme was similar to the previous schemes in the lower montane seasonal rainforest belt, alpine shrubland and meadow belt, alpine sparse vegetation belt, and nival belt, but different in the lower montane evergreen broadleaf forest belt, middle montane semi-evergreen broadleaf forest belt, and subalpine evergreen needleleaf forest belt (Figure 6).
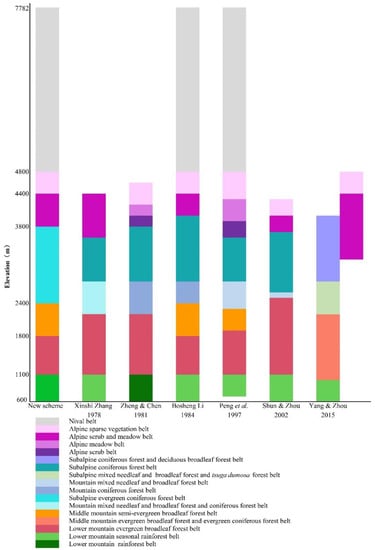
Figure 6.
Comparison of different distribution schemes of vegetation belts in the study site.
3. Discussion
3.1. Comparison of Vegetation Belts Distribution Schemes
The lower montane evergreen broadleaf forest belt and middle montane semi-evergreen broadleaf forest belt are considered as montane evergreen broadleaf forest belt in the studies of Xinshi Zhang, Du Zheng and Weilie Chen, and Hang Sun and Zhekun Zhou [29,30,37]. The main reason would be that the most important dominant species of two belts, both the Castanopsis and Cyclobalanopsis species, are considered as evergreen broadleaf species [38]. In addition, the vegetation plot data and physiognomic and phenological data are also insufficient. Therefore, the middle montane semi-evergreen broadleaf forest is doubted since it was first put forward [39]. Based on plots data, our quantitative analyses provided strong evidence for the validity of semi-evergreen broadleaf forests. There were large variations in species composition between the two vertical belts. Furthermore, the dominant species of the semi-evergreen broadleaf forest belt, Cyclobalanopsis lamellose and C. kiukiangensis, had a special deciduous phenological period, which was remarkably different from other Cyclobalanopsis species that dominated the evergreen forests in the subtropical region of eastern China. The special seasonal variation had been observed from 2019 to 2021 (Figure 2G,H). Most of the year, the physiognomy of this belt was evergreen, but during the deciduous period between April and May, the forest was brown because the tree layer shed all leaves in the dozen days before the rain season came. From May to June, the appearance of the forest changed from brown to red because the new leaves were red or brown-red and turned to green again in July. More detailed studies on the ecological and physiological adaptive mechanisms of these species to their environments are needed to explaining this distinct phenology.
In previous studies, the elevation range of 2400–2800 m is considered to be the subalpine hemlock forest belt, needleleaf and broadleaf mixed forest belt, or part of the evergreen needleleaf forest belt [9,13,29,31]. The main reason for the difference was that the division is based on their own experiences, which are limited by the scope of investigation and personal knowledge base at that time. Based on the vegetation plots data, our study showed that 2400–3800 m should be considered as subalpine evergreen needleleaf forest belt, which includes the subalpine hemlock forest sub-belt (2400–2800 m) and subalpine fir forest sub-belt (2800–3800 m).
The lower montane seasonal rainforest belt and lower montane evergreen broadleaf forest belts can be identified by clustering analysis. However, the NMDS ordination showed that there are high compositional similarities among these vegetation plots. The boxplots also showed large similarities in the elevation range. The main reason was that slash-and-burn farming and longtime logging have destroyed too much of the primary vegetation. A large number of secondary forests, with more homogenous species compositions, had grown up in both belts. Most of secondary forests were clustered into the lower montane seasonal rainforest belt, so this group showed a large elevation range.
3.2. The Unique Features of the Vertical Vegetation Belts
The vertical vegetation belts of Yarlung Zangbo Grand Canyon National Nature Reserve are similar to Mount Qomolangma [40]. Both of them are one of the most complete vertical vegetation belts in the world. The main reason is that they are located in the south of the Qinghai-Tibet Plain and are affected by the Indian Ocean monsoon. Meanwhile, both have an elevation range of more than 7000 m.
However, Yarlung Zangbo Grand Canyon National Nature Reserve is more humid than the latter because of the major water vapor channel [11,12]. Although the latitude of the former is 29 degrees 37 s, it is 1 degree 38 s higher than that of the latter. Yarlung Zangbo Grand Canyon National Nature Reserve still has the same basic belt as Mount Qomolangma. This is far beyond the latitude where it should be. Therefore, the tropical seasonal rainforest of Yarlung Zangbo Grand Canyon National Nature Reserve is the northernmost tropical seasonal rainforest in the world. Meanwhile, it is also considered to be the northern boundary of the tropical zone in China [15].
The middle montane semi-evergreen broadleaf forest belt is the unique vertical vegetation belt in Yarlung Zangbo Grand Canyon National Nature Reserve. The species of Cyclobalanopsis which are dominant trees in the semi-evergreen broadleaf forest, shed all their leaves and then grow new leaves within a month before the rainy season. This way is different from the species of Cyclobalanopsis, which were dominant trees in evergreen broadleaf forest belts in East Asian. The latter usually shed their leaves while growing new leaves. The main reason for this difference may be the limitation of rainfall and temperature. In April, which is the end of the dry season, the temperature gradually rises. This is the driest time of the year in Yarlung Zangbo Grand Canyon National Nature Reserve. Deciduous leaves at this time may be an ecological adaptation for the semi-evergreen broadleaf forest to withstand drought stress [39].
3.3. Vegetation Conservation
Vegetation classification can improve the conservation planning, monitoring, and management in the reserve by defining clear objects [41]. The knowledge of the vertical vegetation belts and main vegetation types in Yarlung Zangbo Grand Canyon National Nature Reserve are significantly improved by this study.
The research showed that there are complete vertical vegetation belts and diverse ecosystems. However, the low-elevation primary vegetation, which was seriously disturbed by human activities, has formed a large area of secondary vegetation. At present, only a small primary vegetation remains in valleys and steep places. Therefore, the biodiversity of the region has dropped significantly. As climate change and human activities intensify, the remaining vegetation is also facing a survival crisis [42,43,44,45]. Thus, we recommend that the remaining tropical seasonal rainforest at lower elevations should be protected as early as possible.
Currently, the middle montane semi-evergreen broadleaf forest belt had the highest biodiversity. The main reason is that the conditions of climate here are better and the interference from human activities is less. However, with the improvement of human activity ability, the scope of activity interference has gradually expanded. This belt is suffering from more disturbances than before with grazing, logging, construction, etc. Thus, we recommend that the protection should be enhanced and interference from human activities should be reduced in the middle montane semi-evergreen broadleaf forest belt.
Our study not only showed the vertical vegetation belts and the primary alliance and secondary alliance in each of the vertical belts, but also showed that the main factor affecting vegetation distribution is elevation. As the elevation increases, the average annual temperature gradually decreases. The vegetation type is gradually transitioning from thermophilous lower montane seasonal rainforest belt to cold-tolerating alpine shrubland and meadow belt. Annual rainfall, slope, and aspect were not important as people think in the distribution of vegetation in Yarlung Zangbo Grand Canyon National Nature Reserve [46,47]. By understanding the distribution of vegetation, its composition, and biodiversity patterns, the study provides important theoretical support for the ecological restoration and biodiversity conservation in the reserve [48,49].
The adjustment of the reserve and the construction of national parks are being implemented in China. Yarlung Zangbo Grand Canyon National Nature Reserve is recommended as China’s first national park, but there is still a paucity of information about vegetation [10]. Our research provides a base for the management and conservation of these ecosystems in the reserve.
4. Materials and Methods
4.1. Fieldwork and Data Collection
Vegetation surveys were conducted from May 2019 to July 2020. Along the elevation gradients of Xirang (550 m)–Duoxiongla (4200 m) and Xiranng (550 m)–Galongla (4300 m), the survey was conducted by every 100 m elevation span (Figure 7). In addition, typical vegetation surveys were conducted in other accessible areas, including plenty of hiking trails. The information of 190 plots were provided in Appendix C. The 20 m × 20 m plot was selected for the forest; the 10 m × 10 m plot was selected for shrubland; the 1 m × 1 m plot was selected for herbaceous vegetation. Density, height, and cover values of each species were recorded, averaged, and changed to their relative values to get the importance value index (IVI) for each species [50,51]. All species were identified according to Flora Xizang, Flora Yunnan, and Flora Reipublicae Popularis Sinicae [38,52,53]. Some species are difficult to identify, which were identified by experts of the corresponding family and genus. Based on the plot coordinates, bioclimatic variables for each study site were extracted from climate grids with a spatial resolution of 30 arc-s [54]. The grid data were downloaded from WorldClim (http://www.worldclim.org (accessed on 1 January 2021)). By using the Spearman correlation coefficient, the correlations between 22 environmental variables were calculated. For variables with spearman correlation coefficients greater than 0.4, the most ecologically important factors were chosen to vegetation analyses. Finally, six variables were reserved. They are: elevation, slope, aspect, annual precipitation (Bio12), precipitation of driest month (Bio14), and precipitation seasonality (Bio15).
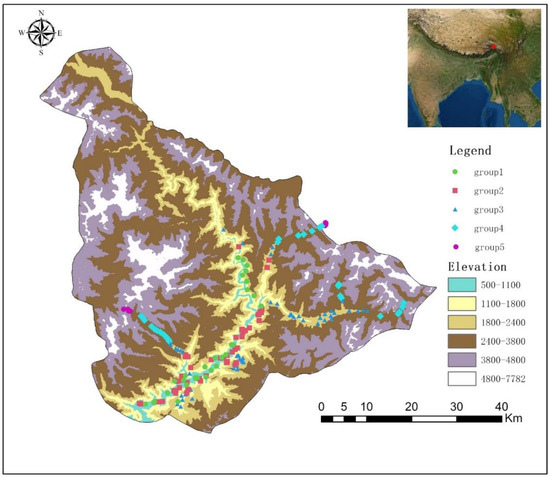
Figure 7.
Locations of surveyed plots in Yarlung Zangbo Grand Canyon National Nature Reserve.
4.2. Statistical Analyses
The primary data from the field surveys were transformed in a matrix of 190 plots × 1416 species, which were log (x + 1) transformed. Alliances were named according to the vegetation classification system of China [55,56]. The matrix of importance value was subjected to Ward’s method cluster analysis based on Bray–Curtis dissimilarity, by using the stat package [57,58]. The value of fusion level and silhouette width was used to evaluate the rationality of clustering results by using the cluster package [59]. For identifying indicator species significantly associated with each vertical vegetation belt, indicator species analysis was performed by using the indicspecies package [60].
To relate the species composition of the accepted groups to environmental variables, Nonmetric Multidimensional Scaling (NMDS) ordination was used based on Bray–Curtis dissimilarity [61]. The lowest stress value was 0.19, which belonged to a two-dimensional configuration. The coordinates of plots were overlaid with the environmental data by using the “envfit” function of the vegan package. The significance of passive vectors was computed using a permutation test with 999 iterations. Bartlett test and Tukey’s honest significant difference test were used to measure the elevation difference between each group in conjunction with ANOVA.
The species richness, Shannon diversity index, Simpson diversity index, and Pielou diversity index were calculated by using the vegan package [61]. The Bartlett test and Kruskal–Wallis test were used to test the diversity differences between five groups. A binomial regression model was used to determine the elevation pattern of species richness by the “lm” function. All analyses were done using R 4.0.3 [58].
We reviewed and summarized previous literature about vertical vegetation belts in Yarlung Zangbo Grand Canyon National Nature Reserve. Based on previous literature and the results of cluster analysis and ordination analysis, we proposed a new scheme for the vertical vegetation belts of the reserve. The new scheme was compared with previous schemes by histogram. Their similarities and differences were discussed.
5. Conclusions
We proposed a new division scheme of vertical vegetation belts in Yarlung Zangbo Grand Canyon National Nature Reserve and discussed differences and similarities with previous schemes. The establishment of the semi-evergreen broadleaf forest was supported in the new scheme. The main factor affecting vegetation distribution is elevation. However, the elevation range of the lower montane seasonal rainforest belt and lower montane evergreen broadleaf forest belt was similar. The main reason is that slash-and-burn farming and longtime logging are greatest and most frequent in the region. Thus, the biodiversity of the region has decreased significantly. Meanwhile, the middle montane semi-evergreen broadleaf forests had the highest biodiversity. Therefore, we recommended that tropical seasonal rainforests and semi-evergreen broadleaf forests should be protected as soon as possible. Based on the distribution of vegetation and the condition of biodiversity, local governments can better formulate conservation strategies to optimize conservation efforts and cope with global climate change.
Author Contributions
Conceptualization: P.-P.W., Z.W., and K.G.; methodology, P.-P.W., C.-C.L., and X.-G.Q.; validation, P.-P.W.; formal analysis, P.-P.W.; investigation, P.-P.W., Z.W., N.-X.J., S.-Q.D., X.-Y.Q., X.-G.Q., C.-C.L., and K.G.; data curation, P.-P.W., Z.W., N.-X.J., and S.-Q.D.; writing—original draft preparation, P.-P.W.; writing—review and editing, P.-P.W., Z.W., N.-X.J., S.-Q.D., X.-Y.Q., X.-G.Q., C.-C.L., and K.G.; visualization, P.-P.W.; supervision, K.G.; pictures, P.-P.W.; project administration, C.-C.L., and K.G.; funding acquisition, C.-C.L., and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by The Second Tibetan Plateau Scientific Expedition and Research Program (STEP), Grant NO. 2019QZKK0301, and “the Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA19050402).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Bosheng Li for his guidance and advice. We thank Xiaohua Jin (Orchidaceae), Xianchun Zhang (Pteridophyta), Bing Liu, Bin Chen, Xiaomei Xia (Ericaceae), Yongpeng Ma (Ericaceae), Mengqi Han (Gesneriaceae), Zhengyu Zuo (Dryopteris), and Shiwei Guo (Begonia & Elatostema) for their help in species identification. We thank Guojun Hua, Shang Qu, Yanqing Guo, Hailei Zheng, and Zhenqi Song, for their help during the field survey. The Xizang Forestry and Grassland Administration is acknowledged for their help and support. We are grateful to the Associate Editor and the anonymous referees for providing valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
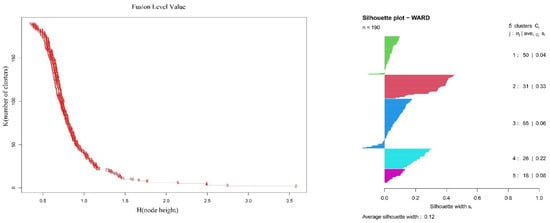
Figure A1.
The plots of fusion level value and silhouette width.
Appendix B

Table A1.
The top 20 indicator species in five vertical vegetation belts.
Table A1.
The top 20 indicator species in five vertical vegetation belts.
| Species Name | Group1 | Group2 | Group3 | Group4 | Group5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IndVal | p | IndVal | p | IndVal | p | IndVal | p | IndVal | p | |
| Altingia excelsa | 0.411 | 0.001 | ||||||||
| Impatiens stenantha | 0.368 | 0.001 | ||||||||
| Phrynium placentarium | 0.339 | 0.001 | ||||||||
| Impatiens namchabarwensis | 0.314 | 0.002 | ||||||||
| Sambucus ebulus | 0.298 | 0.002 | ||||||||
| Mussaenda decipiensis | 0.291 | 0.004 | ||||||||
| Terminalia myriocarpa | 0.284 | 0.006 | ||||||||
| Boehmeria macrophylla var. rotundifolia | 0.279 | 0.004 | ||||||||
| Lagerstroemia minuticarpa | 0.276 | 0.008 | ||||||||
| Blumea balsamifera | 0.275 | 0.004 | ||||||||
| Cordia dichotoma | 0.26 | 0.006 | ||||||||
| Macaranga denticulata | 0.258 | 0.008 | ||||||||
| Trachelospermum jasminoides | 0.253 | 0.007 | ||||||||
| Dichrocephala benthamii | 0.252 | 0.014 | ||||||||
| Polygonum chinense | 0.245 | 0.007 | ||||||||
| Gynostemma pentaphyllum | 0.238 | 0.018 | ||||||||
| Poikilospermum suaveolens | 0.238 | 0.02 | ||||||||
| Equisetum diffusum | 0.231 | 0.035 | ||||||||
| Chrysopogon aciculatus | 0.225 | 0.032 | ||||||||
| Syngonium podophyllum | 0.224 | 0.029 | ||||||||
| Castanopsis indica | 0.901 | 0.001 | ||||||||
| Glochidion hirsutum | 0.462 | 0.001 | ||||||||
| Oplismenus undulatifolius | 0.404 | 0.001 | ||||||||
| Triumfetta cana | 0.368 | 0.001 | ||||||||
| Pteris cretica var. nervosa | 0.333 | 0.001 | ||||||||
| Desmodium sequax | 0.32 | 0.001 | ||||||||
| Colocasia antiquorum | 0.314 | 0.003 | ||||||||
| Strobilanthes dimorphotricha | 0.314 | 0.001 | ||||||||
| Polygonum capitatum | 0.307 | 0.002 | ||||||||
| Saurauia napaulensis | 0.296 | 0.002 | ||||||||
| Elatostema acuminatum | 0.287 | 0.009 | ||||||||
| Circaea cordata | 0.287 | 0.009 | ||||||||
| Chirita pumila | 0.287 | 0.013 | ||||||||
| Dioscorea hispida | 0.287 | 0.009 | ||||||||
| Solena amplexicaulis | 0.287 | 0.006 | ||||||||
| Hedyotis scandens | 0.287 | 0.004 | ||||||||
| Wallichia disticha | 0.257 | 0.006 | ||||||||
| Ophiorrhiza mungos | 0.253 | 0.019 | ||||||||
| Impatiens arguta | 0.25 | 0.017 | ||||||||
| Engelhardtia spicata | 0.249 | 0.009 | ||||||||
| Cyclobalanopsis lamellosa | 0.571 | 0.001 | ||||||||
| Exbucklandia populnea | 0.378 | 0.001 | ||||||||
| Cyclobalanopsis kiukiangensis | 0.367 | 0.001 | ||||||||
| Damnacanthus indicus | 0.361 | 0.001 | ||||||||
| Pholidota articulata | 0.352 | 0.001 | ||||||||
| Ficus sarmentosa | 0.348 | 0.001 | ||||||||
| Disporum bodinieri | 0.342 | 0.001 | ||||||||
| Arisaema concinum | 0.341 | 0.001 | ||||||||
| Ainsliaea latifolia | 0.315 | 0.001 | ||||||||
| Vaccinium kingdom-wardii | 0.31 | 0.001 | ||||||||
| Myrsine semiserrata | 0.306 | 0.002 | ||||||||
| Pinus bhutanica | 0.29 | 0.003 | ||||||||
| Remusatia vivipara | 0.289 | 0.003 | ||||||||
| Toxicodendron hookeri var. microcarpum | 0.288 | 0.001 | ||||||||
| Ilex wilsonii | 0.288 | 0.002 | ||||||||
| Viburnum cylindricum | 0.287 | 0.001 | ||||||||
| Betula cylindrostachya | 0.277 | 0.004 | ||||||||
| Pyrrosia lanceolata | 0.269 | 0.004 | ||||||||
| Tupistra aurantiaca | 0.268 | 0.005 | ||||||||
| Taxillus dalavayi | 0.267 | 0.004 | ||||||||
| Tsuga dumosa | 0.717 | 0.001 | ||||||||
| Abies delavayi var. motuoensis | 0.69 | 0.001 | ||||||||
| Acanthopanax evodiaefolius | 0.49 | 0.001 | ||||||||
| Ribes glaciale | 0.483 | 0.001 | ||||||||
| Lindera obtusiloba | 0.443 | 0.001 | ||||||||
| Pilea notata | 0.434 | 0.001 | ||||||||
| Acer campbellii | 0.426 | 0.001 | ||||||||
| Synotis alata | 0.375 | 0.001 | ||||||||
| Circaea alpina | 0.37 | 0.001 | ||||||||
| Smilacina fusca | 0.359 | 0.001 | ||||||||
| Lonicera tangutica | 0.353 | 0.002 | ||||||||
| Lunathyrium medogense | 0.353 | 0.001 | ||||||||
| Oxalis acetosella subsp. Leucolepis | 0.348 | 0.001 | ||||||||
| Gaultheria trichophylla | 0.347 | 0.001 | ||||||||
| Dysosma tsayuensis | 0.345 | 0.003 | ||||||||
| Acer sp. | 0.335 | 0.001 | ||||||||
| Arisaema rhizomatum | 0.333 | 0.002 | ||||||||
| Arisaema biauriculatum | 0.328 | 0.001 | ||||||||
| Mahonia fortunei | 0.32 | 0.003 | ||||||||
| Euonymus alatus | 0.316 | 0.001 | ||||||||
| Pleurospermum angelicoides | 0.534 | 0.001 | ||||||||
| Dryopteris barbigera | 0.511 | 0.001 | ||||||||
| Viola biflora | 0.486 | 0.001 | ||||||||
| Athyrium attenuatum | 0.482 | 0.001 | ||||||||
| Geranium polyanthes | 0.474 | 0.001 | ||||||||
| Cardamine macrophylla | 0.469 | 0.001 | ||||||||
| Pedicularis lineata | 0.467 | 0.001 | ||||||||
| Polygonum polystachyum | 0.466 | 0.001 | ||||||||
| Rosa sericea | 0.461 | 0.001 | ||||||||
| Rhododendron viridescens | 0.457 | 0.001 | ||||||||
| Ribes orientale | 0.456 | 0.001 | ||||||||
| Polygonum viviparum | 0.445 | 0.001 | ||||||||
| Cirsium eriophoroides | 0.445 | 0.001 | ||||||||
| Gaultheria dolichopoda | 0.444 | 0.001 | ||||||||
| Anaphalis margaritacea var. japonica | 0.44 | 0.001 | ||||||||
| Lonicera angustifolia var. myrtillus | 0.425 | 0.001 | ||||||||
| Rhodiola rosea | 0.416 | 0.001 | ||||||||
| Saxifraga sp. | 0.416 | 0.001 | ||||||||
| Polygonatum verticillatum | 0.408 | 0.001 | ||||||||
| Senecio lingianas | 0.396 | 0.001 | ||||||||
Appendix C

Table A2.
The information of 190 plots.
Table A2.
The information of 190 plots.
| Plot | Elevation | Longitude | Latitude | Aspect | Slope |
|---|---|---|---|---|---|
| CaIn32 | 635 | 95.051 | 29.185 | 153.43 | 25.84 |
| CaIn21 | 718 | 95.054 | 29.188 | 286.02 | 39.18 |
| made3 | 759 | 95.084 | 29.188 | 191.59 | 18.35 |
| CaIn10 | 815 | 95.103 | 29.215 | 247.61 | 20.95 |
| lixi2 | 664 | 95.004 | 29.182 | 202.69 | 13.94 |
| cain | 867 | 95.021 | 29.181 | 136.25 | 28.20 |
| made2 | 1518 | 95.131 | 29.256 | 187.35 | 38.00 |
| CaIn6 | 1557 | 95.129 | 29.253 | 7.41 | 43.13 |
| fise5 | 1415 | 95.14 | 29.252 | 157.28 | 21.22 |
| oxpa | 1472 | 95.156 | 29.269 | 116.68 | 42.57 |
| fiau | 1152 | 95.18 | 29.331 | 292.03 | 20.68 |
| expo8 | 2079 | 95.348 | 29.308 | 193.09 | 20.19 |
| pana | 2029 | 95.354 | 29.303 | 187.59 | 7.19 |
| dibu | 1615 | 95.179 | 29.224 | 85.31 | 14.30 |
| expi | 1621 | 95.182 | 29.228 | 270.00 | 28.07 |
| cyki6 | 1479 | 95.153 | 29.206 | 214.17 | 22.19 |
| CaIn5 | 1677 | 95.14 | 29.187 | 11.85 | 19.02 |
| expo7 | 1843 | 95.168 | 29.21 | 334.33 | 19.59 |
| cyla18 | 1863 | 95.168 | 29.21 | 317.68 | 20.68 |
| cyki1 | 1644 | 95.189 | 29.225 | 227.60 | 14.54 |
| expo6 | 1823 | 95.169 | 29.219 | 2.94 | 36.13 |
| sapo4 | 1679 | 95.178 | 29.226 | 90.90 | 28.07 |
| lipu | 1018 | 95.132 | 29.214 | 321.74 | 28.89 |
| alex19 | 669 | 95.127 | 29.222 | 310.33 | 16.15 |
| temy4 | 814 | 95.147 | 29.232 | 350.39 | 15.35 |
| alex8 | 1200 | 95.155 | 29.255 | 119.05 | 34.46 |
| temy3 | 595 | 94.999 | 29.183 | 203.19 | 31.08 |
| CaIn4 | 631 | 95.069 | 29.187 | 166.07 | 28.21 |
| CaIn3 | 895 | 95.174 | 29.24 | 320.42 | 43.08 |
| alex7 | 853 | 95.134 | 29.222 | 343.30 | 38.06 |
| alex6 | 859 | 95.175 | 29.247 | 234.59 | 22.99 |
| alex5 | 1648 | 95.174 | 29.214 | 52.25 | 18.09 |
| ensp2 | 722 | 95.162 | 29.255 | 123.08 | 33.28 |
| CaIn2 | 758 | 95.169 | 29.27 | 113.19 | 15.93 |
| alex4 | 1115 | 95.206 | 29.263 | 60.26 | 3.84 |
| CaIn1 | 1127 | 95.207 | 29.26 | 326.53 | 16.41 |
| CaIn31 | 1063 | 95.213 | 29.262 | 325.30 | 7.51 |
| CaIn30 | 1063 | 95.213 | 29.262 | 325.30 | 7.51 |
| CaIn29 | 789 | 95.246 | 29.268 | 38.66 | 3.05 |
| CaIn28 | 865 | 95.264 | 29.285 | 320.35 | 10.72 |
| tsdu11 | 2458 | 95.099 | 29.395 | 127.47 | 17.48 |
| tsdu8 | 2361 | 95.103 | 29.391 | 233.13 | 24.62 |
| cyts | 2377 | 95.108 | 29.387 | 232.98 | 34.15 |
| cyki5 | 2308 | 95.111 | 29.381 | 229.35 | 31.82 |
| cyla9 | 2202 | 95.117 | 29.373 | 180.00 | 15.37 |
| cyla8 | 2189 | 95.117 | 29.372 | 58.32 | 12.95 |
| cyla7 | 1990 | 95.135 | 29.358 | 70.11 | 11.76 |
| cyki4 | 1847 | 95.144 | 29.354 | 170.28 | 37.42 |
| expo5 | 1958 | 95.153 | 29.351 | 40.49 | 12.65 |
| cyki3 | 1877 | 95.162 | 29.347 | 82.21 | 21.77 |
| fise4 | 1139 | 95.179 | 29.331 | 127.14 | 9.79 |
| cyla6 | 1224 | 95.172 | 29.329 | 278.13 | 31.74 |
| CaIn27 | 842 | 95.244 | 29.264 | 62.30 | 20.63 |
| CaIn26 | 820 | 95.284 | 29.299 | 278.24 | 31.40 |
| CaIn25 | 820 | 95.279 | 29.325 | 339.53 | 16.59 |
| CaIn24 | 887 | 95.382 | 29.414 | 171.09 | 19.29 |
| figl | 797 | 95.358 | 29.39 | 132.31 | 20.68 |
| alex3 | 1407 | 95.225 | 29.252 | 18.43 | 4.52 |
| alex2 | 1487 | 95.226 | 29.258 | 136.24 | 15.16 |
| cahy2 | 1393 | 95.22 | 29.259 | 306.20 | 30.04 |
| CaIn23 | 1216 | 95.341 | 29.367 | 132.43 | 21.56 |
| CaIn22 | 856 | 95.347 | 29.366 | 115.49 | 16.70 |
| CaIn20 | 746 | 95.319 | 29.335 | 147.45 | 27.42 |
| CaIn19 | 802 | 95.304 | 29.326 | 161.00 | 15.04 |
| fise3 | 1125 | 95.265 | 29.32 | 65.67 | 35.67 |
| cete | 835 | 95.299 | 29.325 | 146.51 | 17.99 |
| lixi1 | 1830 | 95.19 | 29.296 | 188.13 | 32.95 |
| expo4 | 1617 | 95.184 | 29.277 | 61.93 | 15.81 |
| CaIn18 | 1407 | 95.242 | 29.297 | 202.59 | 31.72 |
| lami | 896 | 95.393 | 29.411 | 1.81 | 21.60 |
| temy2 | 849 | 95.387 | 29.41 | 347.59 | 23.10 |
| CaIn17 | 1014 | 95.384 | 29.406 | 316.12 | 16.72 |
| alex1 | 952 | 95.38 | 29.392 | 344.74 | 18.39 |
| cyki2 | 1998 | 95.381 | 29.323 | 108.68 | 31.44 |
| cyki8 | 2107 | 95.37 | 29.32 | 156.25 | 6.49 |
| cyla5 | 1984 | 95.38 | 29.313 | 60.12 | 12.72 |
| cyki7 | 2044 | 95.377 | 29.317 | 49.40 | 8.74 |
| CaIn16 | 2043 | 95.365 | 29.332 | 5.71 | 14.10 |
| cyla4 | 2136 | 95.359 | 29.298 | 303.99 | 12.19 |
| rhde | 1940 | 95.358 | 29.309 | 17.56 | 19.04 |
| coca2 | 1843 | 95.36 | 29.331 | 257.30 | 25.36 |
| alsh | 1084 | 95.362 | 29.356 | 188.91 | 40.70 |
| alex18 | 923 | 95.353 | 29.351 | 174.17 | 39.38 |
| alca | 2435 | 95.36 | 29.287 | 148.67 | 12.64 |
| cyla1 | 2303 | 95.347 | 29.291 | 23.59 | 38.34 |
| ospa | 1308 | 95.324 | 29.313 | 322.22 | 22.86 |
| alex17 | 1365 | 95.322 | 29.309 | 297.83 | 22.75 |
| sapo3 | 824 | 95.323 | 29.332 | 301.90 | 27.48 |
| alex16 | 754 | 95.31 | 29.324 | 326.04 | 13.82 |
| fise2 | 1261 | 95.343 | 29.343 | 287.14 | 25.01 |
| pibh6 | 1579 | 95.184 | 29.224 | 333.43 | 2.13 |
| made1 | 832 | 95.383 | 29.413 | 153.71 | 37.64 |
| alex15 | 947 | 95.38 | 29.416 | 190.00 | 26.72 |
| alex14 | 895 | 95.408 | 29.42 | 261.86 | 34.12 |
| pibh5 | 1997 | 95.349 | 29.303 | 62.10 | 4.58 |
| coca1 | 1812 | 95.359 | 29.33 | 260.86 | 25.29 |
| pibh4 | 1814 | 95.193 | 29.195 | 309.80 | 11.04 |
| alex13 | 1340 | 95.448 | 29.471 | 223.38 | 22.71 |
| caec2 | 1434 | 95.453 | 29.468 | 236.50 | 34.19 |
| alex12 | 1407 | 95.455 | 29.478 | 305.70 | 18.18 |
| CaIn15 | 1343 | 95.453 | 29.489 | 333.43 | 14.62 |
| cyla3 | 2028 | 95.487 | 29.501 | 125.44 | 31.11 |
| pibh3 | 1528 | 95.512 | 29.496 | 43.85 | 16.41 |
| expo3 | 1657 | 95.558 | 29.458 | 28.44 | 12.81 |
| expo2 | 1798 | 95.571 | 29.452 | 357.66 | 42.66 |
| caec1 | 1691 | 95.563 | 29.46 | 199.35 | 18.09 |
| deti | 1797 | 95.444 | 29.442 | 338.11 | 45.16 |
| muba | 876 | 95.444 | 29.462 | 347.08 | 24.98 |
| cahy1 | 1651 | 95.558 | 29.459 | 210.96 | 17.52 |
| lixy | 1785 | 95.59 | 29.454 | 160.01 | 5.57 |
| pibh2 | 1750 | 95.606 | 29.441 | 298.30 | 7.01 |
| saps2 | 1977 | 95.66 | 29.429 | 346.84 | 24.60 |
| pibh1 | 2155 | 95.668 | 29.447 | 180.83 | 40.78 |
| expo1 | 2001 | 95.685 | 29.439 | 323.23 | 24.34 |
| jusi3 | 2336 | 95.686 | 29.459 | 180.00 | 4.29 |
| saps1 | 2151 | 95.721 | 29.462 | 159.37 | 22.49 |
| jusi2 | 2641 | 95.71 | 29.469 | 128.75 | 20.77 |
| powi | 2192 | 95.776 | 29.468 | 189.36 | 21.02 |
| saps4 | 2821 | 95.739 | 29.553 | 296.56 | 8.48 |
| saps3 | 2788 | 95.737 | 29.548 | 218.29 | 5.76 |
| tsdu7 | 2621 | 95.743 | 29.515 | 290.46 | 16.59 |
| tsdu6 | 2605 | 95.747 | 29.508 | 198.43 | 15.46 |
| cyla2 | 2492 | 95.753 | 29.5 | 264.55 | 9.97 |
| temy1 | 826 | 95.441 | 29.461 | 296.38 | 31.35 |
| rhsa3 | 3498 | 94.971 | 29.481 | 81.53 | 11.19 |
| rhsa2 | 3498 | 94.971 | 29.481 | 81.53 | 11.19 |
| rhsa1 | 3498 | 94.971 | 29.481 | 81.53 | 11.19 |
| safl3 | 3613 | 94.966 | 29.481 | 74.95 | 21.86 |
| safl2 | 3613 | 94.966 | 29.481 | 74.95 | 21.86 |
| safl1 | 3613 | 94.966 | 29.481 | 74.95 | 21.86 |
| rhch | 4255 | 94.947 | 29.487 | 9.32 | 15.79 |
| sare4 | 3834 | 94.963 | 29.486 | 144.21 | 48.74 |
| sare3 | 3834 | 94.963 | 29.486 | 144.21 | 48.74 |
| sare2 | 3834 | 94.963 | 29.486 | 144.21 | 48.74 |
| abmo10 | 3233 | 95.009 | 29.463 | 237.65 | 16.48 |
| abmo8 | 3186 | 95.014 | 29.458 | 231.34 | 36.76 |
| abmo7 | 2969 | 95.021 | 29.45 | 231.65 | 19.59 |
| ensp1 | 2023 | 95.493 | 29.655 | 124.87 | 18.53 |
| sapo2 | 1770 | 95.485 | 29.637 | 258.94 | 20.27 |
| cyla17 | 2229 | 95.499 | 29.678 | 106.69 | 27.56 |
| tsdu5 | 2454 | 95.518 | 29.694 | 136.34 | 26.61 |
| tsdu4 | 2602 | 95.522 | 29.703 | 253.55 | 24.52 |
| tsdu3 | 2813 | 95.594 | 29.713 | 187.12 | 15.04 |
| abmo6 | 3007 | 95.614 | 29.716 | 207.47 | 6.70 |
| abmo5 | 3213 | 95.645 | 29.726 | 203.62 | 8.28 |
| fise1 | 1576 | 95.478 | 29.613 | 96.43 | 29.14 |
| abmo4 | 3424 | 95.673 | 29.737 | 154.23 | 15.02 |
| abmo3 | 3589 | 95.681 | 29.741 | 181.07 | 41.72 |
| jusi1 | 3712 | 95.696 | 29.753 | 158.42 | 43.92 |
| CaIn14 | 1721 | 95.138 | 29.179 | 336.80 | 1.82 |
| CaIn13 | 1551 | 95.15 | 29.171 | 71.57 | 12.62 |
| CaIn12 | 1540 | 95.156 | 29.193 | 242.35 | 30.65 |
| cyla16 | 2048 | 95.384 | 29.309 | 7.43 | 5.52 |
| cyla15 | 2034 | 95.382 | 29.31 | 1.00 | 8.53 |
| cyla14 | 2280 | 95.71 | 29.444 | 335.44 | 20.35 |
| cyla13 | 2218 | 95.708 | 29.453 | 305.21 | 9.84 |
| abmo2 | 3273 | 95.976 | 29.488 | 51.73 | 49.16 |
| abmo1 | 3151 | 95.971 | 29.475 | 116.80 | 24.36 |
| abmo9 | 2918 | 95.959 | 29.459 | 111.50 | 16.46 |
| tsdu2 | 2827 | 95.955 | 29.453 | 156.54 | 13.53 |
| tsdu1 | 2626 | 95.888 | 29.447 | 220.03 | 7.75 |
| tsdu10 | 2519 | 95.843 | 29.466 | 194.56 | 28.66 |
| tsdu9 | 2404 | 95.827 | 29.469 | 191.30 | 9.65 |
| cyla12 | 2288 | 95.808 | 29.467 | 198.43 | 18.23 |
| alex11 | 1152 | 95.374 | 29.682 | 223.31 | 11.33 |
| sapo1 | 1723 | 95.372 | 29.646 | 296.20 | 41.27 |
| cace | 1569 | 95.391 | 29.639 | 155.19 | 28.64 |
| rhme12 | 4219 | 95.696 | 29.753 | 136.63 | 31.75 |
| rhme1 | 4130 | 95.698 | 29.752 | 138.57 | 10.69 |
| saan2 | 4000 | 95.698 | 29.747 | 191.18 | 36.14 |
| saan1 | 3907 | 95.695 | 29.746 | 208.51 | 37.53 |
| sare1 | 3789 | 95.695 | 29.744 | 187.76 | 36.50 |
| cyla11 | 2134 | 95.32 | 29.738 | 258.56 | 36.33 |
| cyla10 | 2462 | 95.389 | 29.692 | 225.60 | 28.98 |
| alex10 | 1473 | 95.404 | 29.621 | 287.96 | 17.95 |
| CaIn11 | 1623 | 95.399 | 29.603 | 345.59 | 38.21 |
| alex9 | 1561 | 95.387 | 29.59 | 228.86 | 41.15 |
| CaIn9 | 1505 | 95.402 | 29.569 | 214.00 | 39.84 |
| CaIn8 | 1929 | 95.402 | 29.542 | 343.94 | 48.85 |
| CaIn7 | 1707 | 95.427 | 29.523 | 247.68 | 40.55 |
| povi | 4255 | 94.947 | 29.487 | 9.32 | 15.79 |
| bepu | 4255 | 94.947 | 29.487 | 9.32 | 15.79 |
| amhi | 1253 | 95.17 | 29.331 | 222.39 | 14.54 |
| fise6 | 1314 | 95.171 | 29.337 | 247.14 | 28.68 |
| expo9 | 1754 | 95.163 | 29.345 | 132.31 | 37.05 |
| tsdu12 | 2875 | 95.047 | 29.43 | 174.55 | 9.97 |
| tsdu13 | 2764 | 95.059 | 29.42 | 173.89 | 33.67 |
| tsdu14 | 2703 | 95.067 | 29.416 | 180.00 | 15.37 |
| tsdu15 | 2603 | 95.079 | 29.411 | 121.60 | 7.25 |
| tsdu16 | 2486 | 95.092 | 29.401 | 113.74 | 6.49 |
References
- Fattorini, S.; Di Biase, L.; Chiarucci, A. Recognizing and interpreting vegetational belts: New wine in the old bottles of a von Humboldt’s legacy. J. Biogeogr. 2019, 46, 1643–1651. [Google Scholar] [CrossRef]
- Von Humboldt, A.; Bonpland, A. Essai Sur la Géographie des Plantes; Levrault, Schoell et Compagnie: Paris, France, 1805. [Google Scholar]
- Morueta-Holme, N.; Engemann, K.; Sandoval-Acuna, P.; Jonas, J.D.; Segnitz, R.M.; Svenning, J.-C. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc. Natl. Acad. Sci. USA 2015, 112, 12741–12745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daubenmire, R.F. Vegetational zonation in the Rocky Mountains. Bot. Rev. 1943, 6, 326–393. [Google Scholar] [CrossRef]
- Shu-Qing, Z.; Jing-Yun, F.; Zhan-Jiang, Z.; Biao, Z.; Hai-Hua, S. Composition, structure and species diversity of plant communities along an altitudinal gradient on the northern slope of Mt.Changbai, Northeast China. Biodivers. Sci. 2004, 12, 164–173. [Google Scholar]
- Just, T.H. The vegetation of the Eastern Alps. Ecology 1940, 21, 270–271. [Google Scholar] [CrossRef]
- Van der Plas, G.W.; Rucina, S.M.; Hemp, A.; Marchant, R.A.; Hooghiemstra, H.; Schueler, L.; Verschuren, D. Climate-human-landscape interaction in the eastern foothills of Mt. Kilimanjaro (equatorial East Africa) during the last two millennia. Holocene 2021, 31, 556–569. [Google Scholar] [CrossRef]
- Hemp, A. Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol. 2006, 184, 27–42. [Google Scholar] [CrossRef]
- Bo-Sheng, L. The vertical spectra of vegetation in the MT. Namjabarwa region. Mt. Res. 1984, 2, 174–181. [Google Scholar]
- Lu, Z. Add Himalayas’ Grand Canyon to China’s first national parks. Nature 2021, 592, 353. [Google Scholar]
- Yan, H.R.; Huang, J.P.; He, Y.L.; Liu, Y.Z.; Wang, T.H.; Li, J.M. Atmospheric Water Vapor Budget and Its Long-Term Trend Over the Tibetan Plateau. J. Geophys. Res.-Atmos. 2020, 125, 17. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Lu, M.Q.; Bracken, C.; Chen, H.N. Spatially coherent clusters of summer precipitation extremes in the Tibetan Plateau: Where is the moisture from? Atmos. Res. 2020, 237, 7. [Google Scholar] [CrossRef]
- Li, B. The zonation of vegetation in the MT.Namjagbarwa region. Mt. Res. 1985, 3, 291–298. [Google Scholar]
- Zhu, H. Geographical elements of seed plants suggest the boundary of the tropical zone in China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 16–22. [Google Scholar] [CrossRef]
- Zhu, H. suggestions for the northern boundary of the tropical zone in China. Plant Sci. J. 2018, 36, 893–898. [Google Scholar]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.C.; Cheng, S.Z. The Flora of the Vascular Plants in MT. Namjagbarwa Region; Beijing Science and Technology Press: Beijing, China, 1992; pp. 1–96. [Google Scholar]
- Wu, P.P.; Wang, Z.; Jia, N.X.; Guo, S.W.; Liu, C.C.; Jin, X.H.; Guo, K. Calanthe x yarlungzangboensis, a new natural hybrid in genus Calanthe (Orchidaceae) from China. Phytotaxa 2021, 518, 167–174. [Google Scholar] [CrossRef]
- Bi, W.-X.; Chen, C.-C.; Lin, M.-Y. First record of Jacobsoniidae (Coleoptera) from China with description of a new species of Sarothrias Grouvelle. ZooKeys 2015, 496, 53–60. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Gorochov, A.V. A new genus for a new species of Podoscirtini from southeast Tibet (Orthoptera: Gryllidae; Podoscirtinae; Podoscirtini). Zootaxa 2015, 4033, 259–264. [Google Scholar] [CrossRef]
- Liu, C.; Ya, J.-D.; Tan, Y.-H.; He, H.-J.; Dong, G.-J.; Li, D.-Z. Marsdenia yarlungzangboensis (Apocynaceae, Asclepiadoideae), a new species from Xizang, China. Phytokeys 2019, 130, 85–92. [Google Scholar] [CrossRef]
- Wang, W.-G.; Lang, X.-A.; Yang, L.-L.; Wu, H.; Zhang, S.-Z. Begonia zhongyangiana, a new species of Begonia (Begoniaceae) from western China. Phytotaxa 2019, 407, 51–58. [Google Scholar] [CrossRef]
- Ya, J.-D.; Guo, Y.-J.; Liu, C.; Cai, J.; Dong, G.-J.; Jiang, H.; Li, D.-Z. Bulbophyllum reflexipetalum (Orchidaceae, Epidendroideae, Malaxideae), a new species from Xizang, China. Phytokeys 2019, 130, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Han, H.L.; Kononenko, V.S. Three new species of the genus Araeopteron Hampson, 1893 (Lepidoptera, Erebidae, Boletobiinae) from the Xizang Autonomous Region, China with an updated list of the world species. Zookeys 2021, 1060, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Li, W. A new species of Neoperla (Plecoptera: Perlidae) from Motuo County of Tibet, China and redescription of Neoperla perspicillata Zwick, 1980. Zootaxa 2021, 4964, 169–178. [Google Scholar] [CrossRef]
- Tong, Y.-H.; Zhao, W.-L.; Wang, B.-M.; Liu, E.-D.; Cai, J.; Guo, Y.-J. Vaccinium motuoense (Ericaceae), a new species from Xizang, China. Phytokeys 2021, 181, 105–111. [Google Scholar] [CrossRef]
- Du, C.; Liu, J.; Ye, W.; Liao, S.; Ge, B.; Liu, B.; Ma, J. Annual report of new taxa and new names for Chinese plants in 2020. Biodivers. Sci. 2021, 29, 1011–1020. [Google Scholar] [CrossRef]
- Du, Z.; Wei-Lie, C. A preliminary study on the vertical belts of vegetation of the eastern himalayas. Acta Bot. Sin. 1981, 23, 228–234. [Google Scholar]
- Hang, S.; Zhe-Kun, Z. Seed Plants of the Big Bend Gorge of Yalu Tsangpo in Tibet, E Himalayas; Yunnan Science and Technology Press: Yunan, China, 2002. [Google Scholar]
- Peng, B.; Pu, L.; Bao, H.; Higgitt, D.L. Vertical Zonation of Landscape Characteristics in the Namjagbarwa Massif of Tibet, China. Mt. Res. Dev. 1997, 17, 43–48. [Google Scholar]
- Chi, Y.; Shi, H.H.; Zheng, W.; Sun, J.K.; Fu, Z.Y. Spatiotemporal characteristics and ecological effects of the human interference index of the Yellow River Delta in the last 30 years. Ecol. Indic. 2018, 89, 880–892. [Google Scholar] [CrossRef]
- Han, D.; Gao, C.; Yu, Z.; Yu, X.; Li, Y.; Cong, J.; Wang, G. Late Holocene vegetation and climate changes in the Great Hinggan Mountains, northeast China. Quat. Int. 2019, 532, 138–145. [Google Scholar] [CrossRef]
- Prokop, P.; PLoSkonka, D. Natural and human impact on the land use and soil properties of the Sikkim Himalayas piedmont in India. J. Environ. Manag. 2014, 138, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of Earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Lotterhos, K.E.; Laruson, A.J.; Jiang, L.-Q. Novel and disappearing climates in the global surface ocean from 1800 to 2100. Sci. Rep. 2021, 11, 15535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. The plateau zonality of vegetation in Xizang. Acta Bot. Sin. 1978, 20, 140–149. [Google Scholar]
- Flora Reipublicae Popularis Sinicae. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1959–2004. [Google Scholar]
- Li, B. A semi-evergreen broadleaf forest on the south slope of the eatern hmimalayas. Acta Bot. Sin. 1985, 27, 334–336. [Google Scholar]
- Zhang, J.W.; Jiang, S. A primary study on the vertical vegetation belt of Mt. Jolmo-Lungma (Everest) region and its relationship with horizontal zone. Acta Bot. Sin. 1973, 15, 221–236. [Google Scholar]
- Rodwell, J.S.; Evans, D.; Schaminee, J.H.J. Phytosociological relationships in European Union policy-related habitat classifications. Rend. Lincei-Sci. Fis. E Nat. 2018, 29, 237–249. [Google Scholar] [CrossRef]
- You, Q.L.; Fraedrich, K.; Ren, G.Y.; Pepin, N.; Kang, S.C. Variability of temperature in the Tibetan Plateau based on homogenized surface stations and reanalysis data. Int. J. Climatol. 2013, 33, 1337–1347. [Google Scholar] [CrossRef] [Green Version]
- Duan, A.M.; Xiao, Z.X. Does the climate warming hiatus exist over the Tibetan Plateau? Sci. Rep. 2015, 5, 9. [Google Scholar] [CrossRef]
- Cai, D.L.; You, Q.L.; Fraedrich, K.; Guan, Y.N. Spatiotemporal Temperature Variability over the Tibetan Plateau: Altitudinal Dependence Associated with the Global Warming Hiatus. J. Clim. 2017, 30, 969–984. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, S.; Sachdeva, K.; Joshi, P.K. Vulnerability of forests in the Himalayan region to climate change impacts and anthropogenic disturbances: A systematic review. Reg. Environ. Change 2018, 18, 1783–1799. [Google Scholar] [CrossRef]
- Oorthuis, R.; Vaunat, J.; Hurlimann, M.; Lloret, A.; Moya, J.; Puig-Polo, C.; Fraccica, A. Slope Orientation and Vegetation Effects on Soil Thermo-Hydraulic Behavior. An Experimental Study. Sustainability 2021, 13, 14. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.C.; Ren, H.B.; Ma, K.P.; Yu, M.J.; Sun, I.F.; He, F.L. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.N.; Yang, Q.P.; Zhou, D.Q.; Xu, W.G.; Gao, J.; Wang, Z. How evergreen and deciduous trees coexist during secondary forest succession: Insights into forest restoration mechanisms in Chinese subtropical forest. Glob. Ecol. Conserv. 2021, 25, e01418. [Google Scholar] [CrossRef]
- Qu, S.; Wang, L.C.; Lin, A.W.; Zhu, H.J.; Yuan, M.X. What drives the vegetation restoration in Yangtze River basin, China: Climate change or anthropogenic factors? Ecol. Indic. 2018, 90, 438–450. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. An upland forest continuum in the prairie-forest border region of wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Kunming Institute of Botany, Chinese Academy of Sicence. Flora Yunnan; Science Press: Beijing, China, 2006; Volume 1–21. [Google Scholar]
- Wu, Z.Y. Flora of Tibet; Science Press: Beijing, China, 1987; Volume 1–5. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Guo, K.; Liu, C.C.; Xie, Z.Q.; Li, F.Y.; Franklin, S.B.; Lu, Z.J.; Ma, K.P. China Vegetation Classification: Concept, approach and applications. Phytocoenologia 2018, 48, 113–120. [Google Scholar] [CrossRef]
- Guo, K.; Fang, J.; Wang, G.; Tang, Z.; Xie, Z.; Shen, Z.; Wang, R.; Qiang, S.; Liang, C.; Da, L.; et al. A revised scheme of vegetation classification system of China. Chin. J. Plant Ecol. 2020, 44, 111–127. [Google Scholar] [CrossRef]
- Pakgohar, N.; Rad, J.E.; Gholami, G.; Alijanpour, A.; Roberts, D.W. A comparative study of hard clustering algorithms for vegetation data. J. Veg. Sci. 2021, 32, e13042. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 October 2021).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P.; Gonzalez, J.; Kozlowski, K.; Schubert, E.; et al. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.1. Available online: https://CRAN.R-project.org/package=cluster (accessed on 1 October 2021).
- De Caceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).